Abstract
We employed a novel parametric spider picture set in the context of a parametric fMRI anxiety provocation study, designed to tease apart brain regions involved in threat monitoring from regions representing an exaggerated anxiety response in spider phobics. For the stimulus set, we systematically manipulated perceived proximity of threat by varying a depicted spider's context, size, and posture. All stimuli were validated in a behavioral rating study (phobics n = 20; controls n = 20; all female). An independent group participated in a subsequent fMRI anxiety provocation study (phobics n = 7; controls n = 7; all female), in which we compared a whole‐brain categorical to a whole‐brain parametric analysis. Results demonstrated that the parametric analysis provided a richer characterization of the functional role of the involved brain networks. In three brain regions—the mid insula, the dorsal anterior cingulate, and the ventrolateral prefrontal cortex—activation was linearly modulated by perceived proximity specifically in the spider phobia group, indicating a quantitative representation of an exaggerated anxiety response. In other regions (e.g., the amygdala), activation was linearly modulated in both groups, suggesting a functional role in threat monitoring. Prefrontal regions, such as dorsolateral prefrontal cortex, were activated during anxiety provocation but did not show a stimulus‐dependent linear modulation in either group. The results confirm that brain regions involved in anxiety processing hold a quantitative representation of a pathological anxiety response and more generally suggest that parametric fMRI designs may be a very powerful tool for clinical research in the future, particularly when developing novel brain‐based interventions (e.g., neurofeedback training). Hum Brain Mapp 38:3025–3038, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: magnetic resonance imaging, spider phobia, spiders, subjective anxiety, threat, anxiety disorders, phobic disorders, amygdala, insula, neurofeedback
INTRODUCTION
A significant proportion of the adult population (12–18%) will suffer from an anxiety disorder at least once in their lifetime [Kessler et al., 2005; Wittchen and Jacobi, 2005]. In the past decades, success rates in treatment of anxiety disorders have stagnated [Ost, 2008], leading to an increasing interest in novel therapeutic approaches, such as novel pharmacological, cognitive, and behavioral interventions, as well as neuromodulation approaches applying external (e.g., deep brain stimulation [DBS]/transcranial magnetic stimulation [TMS]) or internal brain stimulation (e.g., neurofeedback guided self‐regulation [Zilverstand et al., 2015]). To define distinct neural targets for the development, evaluation, and optimization of these novel therapeutic approaches, the goal of this study was to tease apart (a) brain regions that show increased activation levels with increasingly intense stimuli in spider phobics and healthy controls alike, indicating a general involvement of these regions in threat monitoring; and (b) brain regions that show an increase in spider phobics only, as expected for regions involved in the quantitative representation of a pathological anxiety response.
Until recently, anxiety provocation studies in patients have mainly used group comparisons and categorical on–off designs to identify which brain regions are activated by high levels of anxiety, while a handful of studies have correlated brain activation levels with subjective anxiety ratings post‐hoc, generally using a region of interest approach. These studies have described a network of brain regions activated by heightened subjective anxiety in patients, including visual areas, the amygdala, insula, thalamus, ventromedial prefrontal cortex, orbitofrontal cortex, anterior cingulate cortex, and dorsolateral prefrontal cortex [Del Casale et al., 2012; Dilger et al., 2003; Goossens et al., 2007; Schienle et al., 2005; Veltman et al., 2004]. A meta‐analysis of studies performed in patients with specific phobias concluded that patients showed consistently increased activation in amygdala, insula, and thalamus [Ipser et al., 2013]. A second meta‐analysis performed across anxiety disorders demonstrated that only two brain regions—amygdala and insula—were consistently activated independent of diagnosis [Etkin and Wager, 2007]. The authors concluded that the amygdala and insula formed the core of a neurobiological anxiety network [Etkin and Wager, 2007]. One of the rare patient studies employing a whole‐brain (rather than region of interest) post‐hoc correlational approach demonstrated that activation levels in visual regions and thalamus were modulated by subjective anxiety levels in spider phobics and healthy participants alike, while activation levels in insula and dorsal anterior cingulate were linked specifically to subjective anxiety in spider phobics [Caseras et al., 2010]. Further, a study conducted in healthy participants investigated the influence of nonpathological fear on brain activation levels through a parametric whole‐brain approach as proposed in this study [Mobbs et al., 2010]. In this study, healthy participants were convinced that a living tarantula spider was placed in the MRI scanner at varying distances to their foot, either approaching or retreating. They were then asked to rate perceived threat. By using this design in healthy participants, this study was able to separate brain networks that encode proximity of the spider from brain regions representing subjective fear during threat monitoring. While proximity modulated brain activation levels in a larger network including the midbrain, amygdala, striatum, insula, and anterior cingulate cortex, threat monitoring was linked to the amygdala [Mobbs et al., 2010].
To tease apart in a patient population which brain regions quantitatively represent an exaggerated anxiety response and which are involved in threat monitoring, we developed a novel parametric spider picture set to use in a whole‐brain parametric functional magnetic resonance imaging (fMRI) anxiety provocation study in spider phobics and control participants. To develop the picture set, spider photographs were systematically manipulated controlling for perceived proximity along several dimensions, as it is well known that reducing the perceived distance to the spider will increase fear [Barlow, 2002]. To ensure that the manipulation affected participants as hypothesized, the stimulus material was validated in an initial behavioral rating study before the stimuli were employed in the fMRI anxiety provocation study. We hypothesized that visual regions and regions involved in threat monitoring in healthy participants, such as the amygdala, would show a parametric modulation of activation levels in both healthy and anxious individuals. Second, we expected that brain regions that have been specifically linked to a pathological anxiety response, such as the insula and dorsal anterior cingulate, would demonstrate a parametric modulation of activation in spider phobics only, indicating a quantitative representation of an exaggerated subjective anxiety response.
MATERIALS AND METHODS
Participants
Twenty‐seven women with high spider fear (selection criterion: ≥75th percentile on the Dutch version of the Spider Phobia Questionnaire [SPQ, Klorman et al., 1974]) and a control group of 27 age‐matched females with low spider fear (selection criterion: ≤25th percentile on the SPQ) were recruited by public advertisement at Maastricht University. The majority of participants were students, with an age range of 18–46 years. Of the 54 women, 40 participated in the behavioral stimulus validation study (see Table Ia for participants' characteristics) and 14 participated in the subsequent fMRI anxiety provocation study (Table Ib). All fMRI participants (1) were diagnosed with spider phobia according to the criteria of The Diagnostic and Statistical Manual of Mental Disorders DSM‐IV TR [American Psychiatric Association, 2000]; (2) did not have other current or previous neuropsychiatric comorbidities as evaluated by means of the Dutch version of the structured clinical interview (Mini International Neuropsychiatric Interview, MINI, [Sheehan et al., 1998]); (3) passed fMRI screening criteria; (4) were right handed (as evaluated by the Edinburgh Inventory [Oldfield, 1971]); (5) were free of psychotropic medication and not currently in treatment for their spider phobia. The fMRI participants were additionally administered the Dutch version Fear of Spider Questionnaire [FSQ [Szymanski, 1995]. All participants received a financial compensation (8 €/hour), and gave their written informed consent prior to the experiment, which was conducted in conformity with the Declaration of Helsinki and approved by the local Medical Ethics Committee at Maastricht University.
Table 1.
Characteristics of participants of behavioral rating study
| Variables (mean ± SD) | Spider phobics | Control group | P value | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender (female) | n = 20 | n = 20 | |||||||||||||||||||||||||||||
| Age | 22.6 (3.2) | 22.5 (3.3) | 0.88 | ||||||||||||||||||||||||||||
| SPQ | 20.3 (3.4) | 1.8 (1.3) | <0.001 | ||||||||||||||||||||||||||||
|
TABLE IB. Characteristics of participants of fMRI study
| |||||||||||||||||||||||||||||||
SPQ, Spider Phobia Questionnaire; FSQ, Fear of Spider Questionnaire; SCID, Score Structured Clinical Interview DSM IV, Duration since onset of phobia symptoms.
Stimuli
A set of 48 photo stimuli depicting European Spiders in their natural environment was acquired from the photographer Ed Nieuwenhuys (http://ednieuw.home.xs4all.nl/), which we refer to here as the natural spider picture set. Three selected spiders from this set were used to create the parametric spider picture set. The spiders were depicted in four different sizes (covering 1, 3, 5, or 7 degree visual angle at picture size 600 × 600 pixels), with their posture being more orientated towards the viewer with increasing size (“zoom” dimension), thus manipulating perceived proximity in space. Furthermore, the three selected spiders were mounted on variants of four different photographic backgrounds (1, nature; 2, computer keyboard; 3, clothes; 4, face), thus changing the proximity of the spider to the viewer in context (“context” dimension). Both manipulations were hypothesized to evoke gradually increasing levels of anxiety (Fig. 1). All visual manipulations were carried out by a professional graphic designer in Adobe Photoshop (Version 13.0, Adobe Systems Inc., San Jose, U.S.A.) using tools such as shadow cast to ensure that the stimuli were photorealistic. Initially, 16 variants of each of the three selected spiders were created, resulting in the 48‐picture original parametric spider picture set used in the stimulus rating study. For the fMRI study, this picture set was further extended by adding variants of three additional spiders, as well as new backgrounds to generate a total number of 192 parametric spider pictures. Also, a neutral category with 48 pictures depicting only the background variants was added, so that the final parametric spider picture set encompassed 240 pictures.
Figure 1.
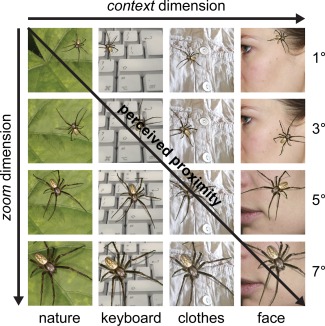
The parametric spider picture set. Shown are example stimuli from the parametric spider picture set. Stimuli were manipulated along the zoom and context dimension. Along the zoom dimension, the spiders increased in size (covering 1°, 3°, 5°, or 7° visual angle occupied at picture size 600 × 600 pixels) and oriented more toward the viewer. Along the context dimension, the spider's context was changed (1. nature, 2. computer keyboard, 3. clothes, 4. face). Both manipulations were hypothesized to gradually increase perceived proximity (in space and context) and thus intensify provoked subjective anxiety. [Color figure can be viewed at http://wileyonlinelibrary.com]
Procedure Stimulus Rating Study
During the behavioral stimulus validation study, all participants rated the stimuli from both the 48‐picture parametric spider picture set and the original 48‐picture natural spider picture set, which were presented in random order. All stimuli were displayed on a computer screen in a quiet room, with the experimenter present in the adjacent room. All stimuli were presented three times, in three different picture sizes (“picture size” dimension: 120 × 120 pixels, 360 × 360 pixels, 600 × 600 pixels). The session duration was 30 min on average (288 trials in total). Each participant was asked to rate each stimulus according to “their initial reaction” on a digital visual analog scale (VAS) anchored with “not fearful at all” and “extremely fearful.” All stimuli were displayed and ratings recorded with the Presentation software (Version 16, Neurobehavioral Systems Inc., Albany, CA, USA).
Analysis Stimulus Rating Study
The subjective anxiety ratings were analyzed in SPSS Statistics (Version 21; IBM, Armonk, NY, USA) using a repeated measures general linear model (GLM). The ratings from the parametric spider picture set were analyzed using linear contrasts for the within subject factors zoom, context, and picture size, with group as a between‐subject factor. The ratings from the natural spider picture set were analyzed with a linear contrast for the within subject factor picture size and group as a between‐subject factor. Effect sizes were estimated using partial eta squared [Cohen, 1973]. To investigate how consistent the findings were across participants, all within‐subject analyses were repeated at the single‐subject level.
Procedure fMRI Study
The 1 h imaging session started with an 8 min anatomical imaging run during which the (generally fMRI naïve) participants watched silent cartoons to reduce anticipatory anxiety, followed by four 11 min functional imaging runs during which the spider stimuli were presented. Before the imaging session, participants were instructed to refrain from movement and attend to all pictures carefully. They were informed that the spider pictures would be presented in blocks of four, with each picture remaining on the screen for 1.5 s (6‐s blocks). The stimuli were selected based on the results of the behavioral validation study to provoke 1 of 5 different anxiety levels (Neutral, Anxiety Level 1, Anxiety Level 2, Anxiety Level 3, and Anxiety Level 4). During each functional imaging run, each condition (anxiety level) was presented six times in random order (30 picture blocks per run in total), with each of the 240 stimuli presented once during the first two imaging runs and once during the last two imaging runs. After each 6 s picture block, participants were asked to indicate their current anxiety level on a 5‐point Likert scale anchored with 0 = “not fearful at all” and 4 = “extremely fearful” using a button box (3 s rating period), followed by a jittered resting period of 10.5–13.5 s (with fixation cross). Stimulus presentation and recording of ratings were programmed in Presentation to be triggered by the scanner. Stimuli were presented via a mirror mounted to the head coil. All subjects from both groups were scanned by the same experimenter in random order.
Image Acquisition fMRI Study
The images were acquired at Maastricht Brain Imaging Centre (Maastricht University) on a 3 T scanner (Magnetom Allegra, Siemens Healthcare, Germany), equipped with standard quadrature birdcage head coil (1‐channel coil). Functional images were acquired with a gradient‐echo T2*‐weighted repeated single‐shot echo‐planar imaging (EPI) sequence that was optimized for imaging of subcortical and prefrontal areas [Deichmann et al., 2003; Domsch et al., 2013; Morawetz et al., 2008; Robinson et al., 2004; Weiskopf et al., 2007]: repetition time = 1500 ms, echo time = 25 ms, flip angle = 71°, slice thickness = 3 mm, 10% gap, in‐plane resolution = 3.5 × 3.5 mm, slice angle = 25°–30°, field of view = 224 × 224 mm2, matrix size = 64 × 64, bandwidth = 1736 Hz/Px, imaging 25 slices per volume (with A/P phase encoding, coronal slice orientation, ascending interleaved order). This ensured full coverage of subcortical structures of interest and prefrontal cortex, while compromising on coverage of the parietal cortex. The aim was to optimize statistical power of the analysis, while keeping a tolerable session length. To monitor for a possible increase in heart rate with increasing anxiety, pulse rate was recorded using Siemens pulse oximeter during all functional imaging runs. Anatomical images were collected with a T1‐weighted sequence based on the Alzheimer's Disease Neuroimaging Initiative (ADNI): repetition time = 2250 ms, echo time = 2.6 ms, flip angle = 9°, field of view = 256 × 256 mm2, 192 slices, voxel size 1 × 1 × 1 mm3, with duration 8:26 min.
Analysis fMRI Study
To evaluate if different levels of anxiety were provoked as hypothesized, the subjective anxiety ratings made during the imaging session were analyzed in SPSS Statistics with a linear contrast for anxiety level and group as a between‐subject factor. To monitor for possible physiological artifacts, pulse rates were computed using a custom made MATLAB tool (R2010a; The MATHWORKS, Natick, MA, USA) and submitted to the same analysis.
All imaging data were analyzed with BrainVoyager (Version QX 2.7, Brain Innovation, Maastricht, The Netherlands). After discarding the first four volumes to account for T1‐saturation effects, the functional data were preprocessed in the following order: (1) interscan slice‐time correction (cubic spline interpolation); (2) 3D rigid‐body motion correction (trilinear detection/sinc interpolation, aligned to the first volume); (3) temporal high‐pass filtering with a GLM Fourier basis set; (4) resampling to 3 × 3 × 3 mm3 voxels (sinc interpolation); and (5) spatial smoothing with an isotropic Gaussian kernel of 6‐mm FWHM. None of the participants moved more than 1.9 mm/degrees in any direction; therefore, none of the data was removed from subsequent analyses. Individual anatomical images were transformed into Talairach space (sinc interpolation). Functional imaging data was aligned to the individual participant's anatomy using an affine transformation (6 degrees of freedom) and manually inspected for quality of registration. Subsequent statistical analyses were performed using a two‐level random‐effects univariate GLM approach [voxelwise linear regression using generalized least squares with AR(2) autocorrelation model], modeling BOLD signal change by convolving the task predictors with a standard two‐gamma hemodynamic response function. Noise artifacts were modeled by including the three translational and three rotational head motion parameters [Hutton et al., 2011; Weissenbacher et al., 2009], a localized estimate of the white matter signal to model scanner artifacts [Jo et al., 2010] and the ventricular signal to estimate physiological artifacts [Birn et al., 2009] in the GLM model. The employed combination of nuisance regressors is recommended to increase specificity and reliability of the results [Guo et al., 2012; Hutton et al., 2011; Weissenbacher et al., 2009].
To compare the parametric approach with a categorical analysis, two different statistical models were employed. First, each of the five task conditions (Neutral, Anxiety Level 1, Anxiety Level 2, Anxiety Level 3, Anxiety Level 4) was modeled by unweighted task regressors. This allowed us to contrast the mean of the four unweighted anxiety conditions with the neutral condition (balanced contrast values: −4, 1, 1, 1, 1), as done in categorical fMRI anxiety provocation studies. Second, for the parametric approach in which the neutral condition was of no interest, the four anxiety conditions were weighted to model a linear increase with provoked anxiety level (balanced contrast values: −3, −1, 1, 3). A conjunction of this parametric contrast with the main contrast of task versus rest (contrast values: 1, 1, 1, 1) was computed; therefore, restricting our search to brain regions whose activation was task‐relevant and parametrically modulated by anxiety level. All resulting maps were statistically thresholded with a voxelwise threshold for statistical significance of α = 0.05 [Forman et al., 1995] while correcting for multiple comparisons using cluster‐size thresholding with a 3D cluster‐level false‐positive rate of α = 0.05 [Forman et al., 1995; Goebel et al., 2006]. Finally, to evaluate the statistical power of both analyses in an exploratory post‐hoc analysis, we computed partial eta‐squared (η p 2, the proportion of the total variability in the dependent variable attributable to an effect) and observed power (statistical power based on the observed effect size) for the 2nd level effects (group effects) in select brain regions, which were of particular importance to the results: thalamus, amygdala, mid insula, dorsal anterior cingulate, and dorsolateral prefrontal cortex (see Supporting Information, Table I). Importantly, the signal of these regions of interest was extracted only from voxels which showed a significant effect in both analyses, except for the dorsolateral prefrontal cortex, where the extent of the region could be solely defined based on the categorical analysis.
RESULTS
Stimulus Rating Study
Results from the behavioral rating study indicated a highly significant group difference in average subjective anxiety level between spider phobic and control participants (across stimuli), with a similar difference for both stimulus sets (natural spider picture set: difference = 43% of the scale, F(1,38) = 56.4, P < 0.001, η p 2 = 0.62; parametric spider picture set: difference = 47% of the scale, F(1,38) = 122.4, P < 0.001, η p 2 = 0.78, Fig. 2). Second, as expected, subjective anxiety increased with greater perceived proximity in both groups. This modulation was linear in both groups and for both stimulus sets. Along the picture size dimension, this increase was larger in the spider phobia group, as indicated by a significant interaction effect (natural spider picture set: interaction group × linear increase: F(1,38) = 5.5, P < 0.05, η p 2 = 0.14; within control group: F(1,19) = 18.9, P < 0.001, η p 2 = 0.53; within spider phobia group: F(1,19) = 38.1, P < 0.001, η p 2 = 0.69, Fig. 2a; parametric spider picture set: interaction group × linear increase: F(1,38) = 4.5, P < 0.05, η p 2 = 0.12; within control group: F(1,19) = 20.9, P < 0.001, η p 2 = 0.55; within spider phobia group: F(1,19) = 39.1, P < 0.001, η p 2 = 0.70, Fig. 2b). This effect was highly consistent at the single‐subject level, as it was significant in 20/20 spider phobics and 16/20 control participants. Along the context and zoom dimensions, both groups also showed a linear increase of subjective anxiety with greater perceived proximity (parametric spider picture set: linear increase with context dimension: F(1,38) = 64.9, P < 0.001, η p 2 = 0.66; Fig. 2c; linear increase with zoom dimension: F(1,38) = 93.3, P < 0.001, η p 2 = 0.73; Fig. 2d). While the interaction effect testing for a larger increase in spider phobics than controls did not reach significance, effects were again highly consistent across participants (significant in 19/20 participants for both groups).
Figure 2.
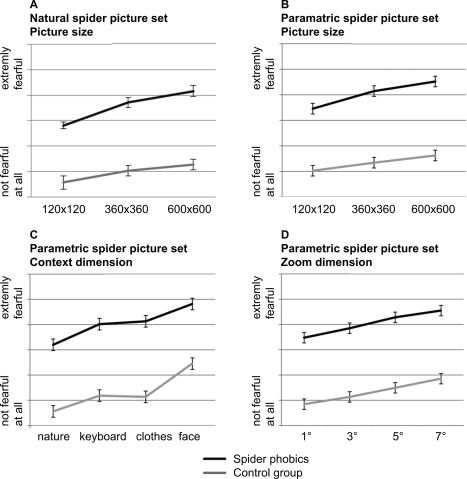
Results from the stimulus rating study. Plotted are subjective anxiety ratings from the behavioral rating study. Stimuli were rated on a visual analogue scale (VAS) anchored by “not fearful at all” and “extremely fearful.” Subjective anxiety increased along all manipulated stimulus dimensions as predicted. There was a highly significant group difference in general anxiety level (A–D) and a significant interaction effect indicating a stronger modulation of subjective anxiety by perceived proximity in the spider phobia group along the picture size dimension (for both stimulus sets, A and B).
A post‐hoc analysis comparing the two picture sets within spider phobics demonstrated that the parametric spider picture set covered a 50% larger range of the anxiety rating scale than the natural spider picture set (parametric spider picture set: average standard deviation = 12%: 120 × 120 pixel = 13%, 360 × 360 pixel = 12%, 600 × 600 pixel = 11%; natural spider picture set: average standard deviation = 8%: 120 × 120 pixel = 7%, 360 × 360 pixel = 9%, 600 × 600 pixel = 8%, see Supporting Information, Fig. 1). Importantly, the variance in the ratings was structured as hypothesized (Supporting Information, Fig. 1), confirming that the parametric spider picture set allowed for a more powerful experimental manipulation.
To maximize the effect of the parametric manipulation for the subsequent fMRI study, the 16 stimulus subcategories (4 levels of zoom × 4 different contexts) of the parametric spider picture set were grouped into four categories of provoked anxiety levels based on the results of the behavioral rating study (Anxiety Level 1, Anxiety Level 2, Anxiety Level 3, Anxiety Level 4, Fig. 3). The parametric stimulus set was then extended in the low and high anxiety range (Anxiety Level 1, Anxiety Level 4) to create an equal number of stimuli for all four levels. Finally, a neutral condition was included to allow for a parametric anxiety provocation design with five levels (Neutral, Anxiety Levels 1–4) during imaging.
Figure 3.
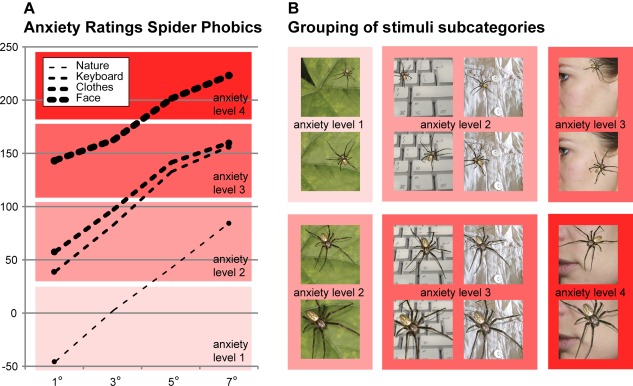
Stimuli used in the fMRI study. Based on the ratings from the phobic participants in the behavioral rating study (shown in A), the 16 stimulus subcategories of the parametric spider picture set were grouped into four different anxiety levels (Anxiety Level 1, Anxiety Level 2, Anxiety Level 3, and Anxiety Level 4), as illustrated by the background colors (B). [Color figure can be viewed at http://wileyonlinelibrary.com]
Parametric fMRI Anxiety Provocation Study
During the imaging session, participants rated their subjective anxiety in 99% of trials (1% missing ratings). The analysis of their anxiety ratings demonstrated a group difference in average anxiety level, which was comparable in size to the difference measured during the behavioral rating study (47% of the scale, F(1,12) = 96.1, P < 0.001, η p 2 = 0.89, Fig. 4). Results further showed that the grouping of the stimuli into five anxiety levels enhanced the behavioral effects such that the phobic group now overall showed a stronger linear increase in subjective anxiety than the control group, indicating an exaggerated anxiety response in this group (interaction effect group × linear increase: F(1,12) = 30.8, P < 0.001, Fig. 4). This interaction effect had a substantial effect size (η p 2 = 0.72). Post‐hoc within group analysis confirmed an exaggerated response in spider phobics, who demonstrated a significant linear increase of subjective anxiety (F(1,6) = 266.3, P < 0.001, η p 2 = 0.98), while control participants only showed a nonsignificant trend (F(1,6) = 3.9, P = 0.09, η p 2 = 0.39).
Figure 4.
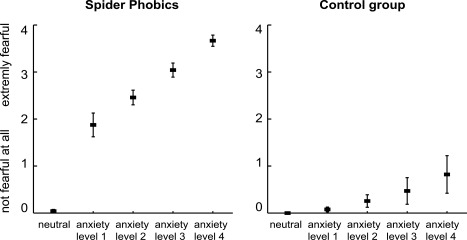
Subjective anxiety during the fMRI study. The subjective anxiety ratings collected during the imaging session show a large group difference in average anxiety level (rated on a 5‐point Likert scale anchored with “not fearful at all” and “extremely fearful”). The grouping of the stimuli enhanced the behavioral effects such that the phobic group now demonstrated a significantly stronger linear increase in subjective anxiety than the control group, indicating an exaggerated anxiety response in this group in the scanner environment.
The analysis of the pulse data showed no evidence that participants' pulse rate was modulated by their anxiety level. There was neither a significant group difference in pulse rate (spider phobia group = 67 beats/min, control group = 69 beats/min; group difference: P = 0.88, interaction effect group × modulation by anxiety level: P = 0.14), nor a significant modulation of pulse by anxiety level across groups (P = 0.98).
The analysis of the fMRI data revealed activation in the expected brain networks. Brain regions activated in spider phobics based on the categorical analysis encompassed midbrain and subcortical areas (periaqueductal gray, thalamus, and striatum), visual input areas (superior colliculi, visual cortex), regions from the hypothesized anxiety network (insula, anterior cingulate), and prefrontal regions (ventrolateral prefrontal cortex, orbitofrontal cortex, dorsolateral prefrontal cortex, Table 2). The parametric approach revealed the same network, further demonstrating that activation levels in spider phobics increased linearly within this network, except within prefrontal regions (orbitofrontal cortex, dorsolateral prefrontal cortex, Table 3). Finally, the parametric, but not the categorical analysis was sensitive enough to detect (parametrically modulated) activation in the amygdala in spider phobics (Table 4). The post‐hoc analysis of statistical power indicated that for the amygdala, the effect size was smaller in the categorical than the parametric analysis (categorical: η p 2 = 0.30, parametric: η p 2 = 0.73), resulting in lower observed statistical power in the categorical approach (categorical: observed power = 0.51; parametric: observed power = 0.99) (Supporting Information, Table I). No brain region showed a linear decrease in activation with increased anxiety level.
Table 2.
Regions activated by a contrast of all anxiety levels (irrespective of level) versus neutral pictures
| Brain region | Brodmann areas (BA) | Spider phobics: anxiety > neutral | Control group: anxiety > neutral | Spider phobics > control group (anxiety > neutral) | |
|---|---|---|---|---|---|
| Cluster threshold | 186 voxels | 45 voxels | 177 voxels | ||
| x/y/z (no of voxels) | x/y/z (no of voxels) | x/y/z (no of voxels) | |||
| Midbrain | |||||
| Brainstem and superior colliculi | L | −8/−27/−3 (95) | −12/−23/−3 (14) | −7/−19/−9 (65) | |
| R | 8/−25/−3 (83) | 14/−26/−3 (7) | 8/−20/−9 (45) | ||
| Visual areas | |||||
| Visual cortex | L | 17, 18, 19, 37 | −45/−71/−7 (804) | −52/−58/7 174) | −21/−87/−5 (312) |
| R | 17, 18, 19, 37 | 30/−75/−12 (678) | 51/−63/4 (59) | 15/−77/−5 (271) | |
| Subcortical areas | |||||
| Thalamus | L | −10/−22/15 (152) | — | −9/−7/12 (60) | |
| R | 6/−11/7 (43) | — | 3/−12/13 (42) | ||
| Striatum | L | −7/10/10 (70) | — | −9/10/12 (61) | |
| R | 8/6/9 (37) | — | 13/13/15 (33) | ||
| Amygdala | L | — | — | — | |
| R | — | — | — | ||
| Medial/temporal areas | |||||
| Anterior insula | L | 13 | −34/16/6 (71) | — | — |
| R | 13 | 39/22/10 (116) | — | — | |
| Mid insula | L | 13 | −34/8/6 (82) | — | — |
| R | 13 | 38/7/0 (20) | — | — | |
| Dorsal anterior cingulate | L | 24, 32 | −9/25/31 (134) | — | — |
| R | 24, 32 | 5/25/32 (113) | — | — | |
| Prefrontal areas | |||||
| Ventrolateral prefrontal cortex | L | 44, 45, 47 | −50/14/1 (47) | — | — |
| R | 44, 45, 47 | 51/22/7 (51) | — | — | |
| Orbitofrontal cortex | L | 10, 11 | −22/43/−3 (114) | — | — |
| R | 10, 11 | 36/59/12 (72) | — | 32/51/12 (115) | |
| Dorsolateral prefrontal cortex | L | 8, 9, 46 | −45/22/28 (124) | — | −50/14/36 (121) |
| R | 8, 9, 46 | 40/22/31 (168) | — | 40/29/31 (111) | |
| Posterior areas | |||||
| Posterior cingulate | L | 23,29 | −7/−35/22 (200) | — | — |
| R | 23,29 | 3/−38/21 (123) | — | — | |
L, left hemisphere; R, right hemisphere.
Talairach coordinates of the most significant voxel and the number of significantly activated functional voxels (3 × 3 × 3 mm3) are reported. Results from whole‐brain random‐effects GLM, P < 0.05 at cluster level, the applied cluster thresholds applied are reported.
Table 3.
Regions activated by parametric anxiety level contrast
| Brain region | Brodmann areas (BA) | Spider phobics: linear increase | Control group: linear increase | Interaction: linear increase in spider phobics only | |
|---|---|---|---|---|---|
| Cluster threshold | 171 voxels | 170 voxels | 152 voxels | ||
| x/y/z (no voxels) | x/y/z (no voxels) | x/y/z (no voxels) | |||
| Midbrain | |||||
| Brainstem and superior colliculi | L | −7/−19/−11 (71) | −2/−26/−2 (44) | — | |
| R | 6/−22/−9 (63) | 3/−25/−3 (66) | — | ||
| Visual areas | |||||
| Visual cortex | L | 18, 19, 37 | −22/−67/−12 (349) | −37/−76/−6 (593) | — |
| R | 18, 19, 37 | 35/−65/−12 (322) | 29/−76/2 (456) | — | |
| Subcortical areas | |||||
| Thalamus | L | −4/−17/6 (107) | −12/28/1 (87) | — | |
| R | 8/−20/9 (118) | 16/−29/3 (123) | — | ||
| Striatum | L | −21/8/9 (77) | — | — | |
| R | 20/3/9 (61) | — | — | ||
| Amygdala | L | −19/−5/−10 (21) | — | — | |
| R | 25/−2/−12 (17) | — | — | ||
| Medial/temporal areas | |||||
| Anterior insula | L | 13 | −36/16/11 (119) | — | — |
| R | 13 | 35/11/15 (101) | — | — | |
| Mid insula | L | 13 | −37/−2/10 (105) | — | −37/4/9 (23) |
| R | 13 | 37/2/6 (101) | — | 37/4/8 (57) | |
| Dorsal anterior cingulate | L | 24, 32 | −8/16/37 (114) | — | −7/16/28 (54) |
| R | 24, 32 | 9/7/36 (77) | — | 5/7/36 (29) | |
| Prefrontal areas | |||||
| Ventrolateral prefrontal | L | 44, 45, 47 | −50/13/4 (23) | — | −52/8/6 (38) |
| R | 44, 45, 47 | 51/21/3 (95) | — | — | |
| Orbitofrontal cortex | L | — | — | — | |
| R | — | — | — | ||
| Dorsolateral prefrontal | L | — | — | — | |
| R | — | — | — | ||
| Posterior areas | |||||
| Posterior cingulate | L | — | — | — | |
| R | — | — | — | ||
L, left hemisphere; R, right hemisphere.
Talairach coordinates of the most significant voxel and the number of significantly activated functional voxels (3 × 3 × 3 mm3) are reported. Results from whole‐brain random‐effects GLM, P < 0.05 at cluster level, the applied cluster thresholds applied are reported.
The categorical group comparison revealed higher activation levels in the spider phobia group in the midbrain, visual, subcortical, and prefrontal regions (Table 2). To analyze which brain regions were involved in threat monitoring, we considered which brain regions showed a parametric modulation in both groups. Midbrain, thalamus, and visual regions demonstrated a clear parametric modulation of activation levels in both phobics and controls (Table 4 and Fig. 5a), with a large effect size for the thalamus across groups (parametric: η p 2 = 0.63, observed power = 0.98, Supporting Information, Table I). In the amygdala and anterior insula, activation levels showed a weak (nonsignificant) linear increase in controls and a significant linear increase in spider phobics (Table 4 and Fig. 5a). However, when formally testing for a group difference, a nonsignificant interaction effect indicated both a linear modulation in phobics and controls for the amygdala and anterior insula (Table 4 and Fig. 5a), with a large effect size for the parametric effect in the amygdala across groups (parametric: η p 2 = 0.73, observed power = 0.99, Supporting Information, Table I). Post‐hoc within‐group analyses further demonstrated that the parametric analysis in these regions in the control group only failed to reach significance because the main contrast detecting task activation remained nonsignificant, but not because the parametric modulation term was nonsignificant. Therefore, only three brain regions—the mid insula, the dorsal anterior cingulate, and the ventrolateral prefrontal cortex—demonstrated a linear increase in activation levels that was specific to spider phobics and supported by a significant interaction effect indicating a linear modulation in this group only (Table 4 and Fig. 5b). This linear modulation of the activation level specifically in the phobia group showed a large effect size (parametric: mid insula η p 2 = 0.80, observed power = 0.98; dorsal anterior cingulate η p 2 = 0.72, observed power = 0.90, Supporting Information, Table I). These three brain regions were therefore the only brain regions that met the criteria for brain regions that hold a quantitative representation of an exaggerated anxiety response. Finally, prefrontal and posterior brain regions such as orbitofrontal cortex, dorsolateral prefrontal cortex, and posterior cingulate showed higher activation levels in spider phobics in the categorical analysis (Table 2), but did not show a significant parametric modulation by anxiety level in either group (Table 4, Fig. 5c, Supporting Information, Table I).
Figure 5.
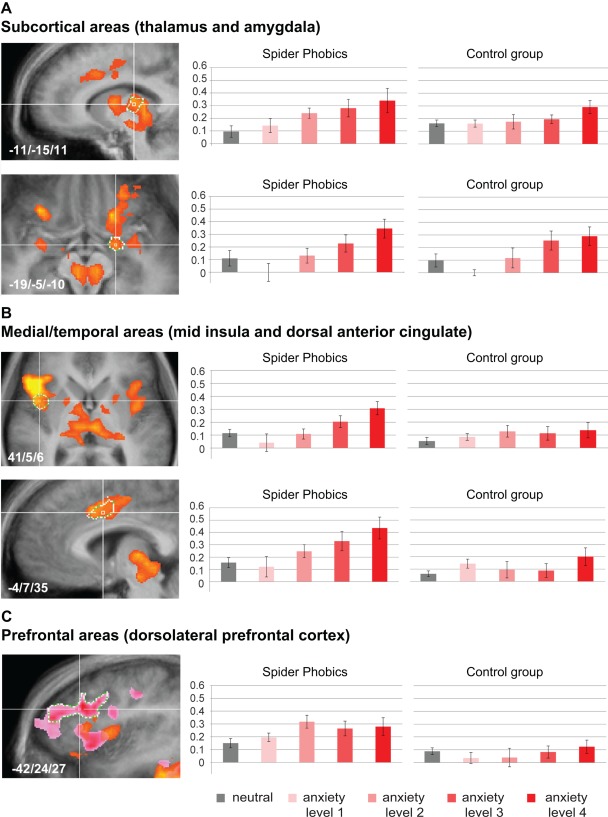
Brain response during the fMRI study. The results from the parametric anxiety fMRI provocation study are depicted (whole‐brain random‐effects GLM, P < 0.05 at cluster level). Voxels showing parametric modulation by anxiety level are depicted in yellow‐orange (A–C, spider phobics), while voxels showing an increased response to spiders versus neutral stimuli in phobic participants in the categorical analysis are depicted for frontal areas (rose‐pink, C). To illustrate effects, beta weights were extracted from regions of interest defined based on the conjunction of both analyses, except for frontal areas where regions were defined based on the categorical analysis (white borders, A–C). The parametric analysis revealed that the midbrain, thalamus, visual regions, and the amygdala demonstrated a linear modulation of activation levels in both groups (A), as hypothesized for regions involved in threat monitoring. Only three brain regions—the mid insula, the dorsal anterior cingulate, and the ventrolateral prefrontal cortex—showed a linear modulation that was unique to the spider phobia group (B), suggesting that these brain regions hold a quantitative representation of an exaggerated anxiety response. In prefrontal brain regions, neither group showed parametric modulation of activation levels (C). [Color figure can be viewed at http://wileyonlinelibrary.com]
DISCUSSION
The goal of this study was to employ a parametric spider picture set in a patient population to tease apart which brain regions are involved in threat monitoring and which hold a quantitative representation of an exaggerated anxiety response. Results from the behavioral rating study demonstrated that it was possible to provoke increasingly higher levels of anxiety with increasingly intense stimuli. All tested stimulus manipulations exhibited large effect sizes and were very consistent across participants. After grouping the stimuli into four anxiety levels for the parametric fMRI anxiety provocation study, this increase in provoked subjective anxiety driven by perceived proximity was stronger in spider phobics than in control participants, indicating an exaggerated anxiety response in spider phobics. The novel parametric spider picture set was successfully used to map the brain network known to be involved in anxiety processing [Del Casale et al., 2012; Dilger et al., 2003; Etkin and Wager, 2007; Goossens et al., 2007; Schienle et al., 2005; Veltman et al., 2004] and threat monitoring [Mobbs et al., 2010]. Importantly, we were able to show that the parametric (but not the categorical) analysis demonstrated that three brain regions—the mid insula, the dorsal anterior cingulate, and the ventrolateral prefrontal cortex—showed a linear modulation by anxiety level in the spider phobia group only, indicating a quantitative representation of an exaggerated anxiety response in these regions. In contrast, other brain regions such as the amygdala, the anterior insula, the midbrain, the thalamus, and the visual cortex showed a linear modulation by perceived proximity in both phobics and control participants, suggesting a functional role in threat monitoring.
These results replicate and extend previous studies which have proposed a role for the amygdala in threat monitoring [Mobbs et al., 2010] and a role for the insula and dorsal anterior cingulate in representing subjective anxiety in phobia [Caseras et al., 2010]. The novel results suggest that threat monitoring involves two processes: first, the visual analysis of potentially threatening stimuli in visual input areas and second, the actual risk estimation, involving the amygdala and anterior insula. These novel results further corroborate previous research in healthy participants showing that the amygdala is involved in rapid, automatic processing of phobia‐related stimuli during initial detection of fear‐relevant stimuli, which is largely independent of attention and awareness [Carlsson et al., 2004; Straube et al., 2006]. They are also consistent with a model suggesting that the anterior insula evaluates how external stimuli will influence bodily states [Paulus and Stein, 2006]. As its main finding, this study identified three brain regions holding a quantitative representation of a pathological anxiety response: the mid insula, the dorsal anterior cingulate, and the ventrolateral prefrontal cortex. Importantly, these three regions have not been found to be directly involved in threat monitoring in previous studies [Mobbs et al., 2010]. Also, hyperactivation of the mid insula during anxiety provocation is seen in phobic patients, but not during fear conditioning in healthy participants [Etkin and Wager, 2007]. The mid insula is further implicated in the representation of bodily states and conscious arousal [Critchley et al., 2001] and the integration of subjective threat evaluations with information on bodily states [Craig, 2011; Grupe and Nitschke, 2013]. Overall, the results therefore suggest that the mid insula holds a primary representation of pathological anxiety levels, while the dorsal anterior cingulate and ventrolateral prefrontal cortex may hold a secondary representation. In concordance with this interpretation, previous evidence suggests that the bodily states represented in the mid insula are remapped in the cingulate cortex for integration with sensory, motor, and attentional inputs [Critchley et al., 2001] and allocation of control and attention [Carter et al., 1999; Paus, 2001; Shenhav et al., 2013]. Finally, the ventrolateral prefrontal cortex is implicated in effortful control of emotion [Ochsner et al., 2004; Phan et al., 2005].
Last, we found that prefrontal brain regions such as the orbitofrontal and dorsolateral prefrontal cortex were active in the spider phobia group but their activity did not parametrically scale with anxiety level. This suggests that these regions did not directly track perceived threat or represent an exaggerated anxiety response; rather, their involvement might be limited to a secondary, higher order cognitive process. Previous literature supports this, by proposing that the orbitofrontal cortex plays a key role in encoding of memory for affective value [Gottfried and Dolan, 2004; Milad et al., 2005; Rolls and Grabenhorst, 2008] while the dorsolateral prefrontal cortex may primarily support the representation of context information and goal setting [D'Esposito et al., 1995; Fuster, 1990; Grupe and Nitschke, 2013].
A conceptual limitation of this study is that the interpretation of the functional role of the involved brain regions depends on statistical thresholding. As the sample size of the imaging study was small, this biases the results toward robust effects with a large effect size. Importantly, however, the results demonstrate that select regions, such as the mid insula, the dorsal anterior cingulate, and the ventrolateral prefrontal cortex show such robust effects with large effect sizes, which are specific to the spider phobia group. Another limitation is that, due to limited imaging coverage, the role of parietal regions cannot be further discussed. This constraint was necessary to limit the duration of each imaging session to 60 min. Importantly, with this design, none of the participants reported adverse effects. To control for possible confounding factors and ensure high data quality, we monitored for a possible increase in heart rate with increasing anxiety through recording the pulse rate of all participants during imaging. These data showed that physiological artifacts were unlikely a confounding factor, as the pulse rate was not modulated by provoked anxiety. As an additional precaution, nuisance regressors for modeling motion, physiological and scanner artifacts were added to each statistical model. Finally, a practical limitation of our approach may be that a parametric analysis requires a suited stimulus set, which needs to be validated beforehand. However, we are confident that the presented results show that the additional power of a parametric analysis approach offsets these initial costs, particularly when such a stimulus set can be reused for multiple studies.
Beyond the neuroscientific questions answered in this study, we believe that the results demonstrate the power of parametric designs for studying patient populations. The parametric analysis proved to be more sensitive than the categorical approach, as only this analysis revealed involvement of the amygdala. Moreover, the parametric approach enabled us to further characterize the functional role of the involved networks, by allowing us to tease apart the involvement of brain regions in threat monitoring versus representing an exaggerated anxiety response. The current results, therefore, suggest that parametric designs may be a powerful tool for future clinical research, providing a rich characterization of the involved networks, which may prove essential for developing novel brain‐based interventional approaches (see Zilverstand et al., 2015 for an application of the developed parametric picture set in a neurofeedback training).
CONCLUSIONS
In conclusion, the data presented here provides convincing evidence that parametric fMRI designs can be used to characterize the functional roles of relevant brain networks in patient populations. The current results suggest that only three brain regions—the mid insula, the dorsal anterior cingulate, and the ventrolateral prefrontal cortex—hold a quantitative representation of an exaggerated anxiety response in spider phobics, which is characterized by a linear relationship between activation levels and provoked subjective anxiety. The quantitative nature of this relationship implies that these brain regions may be important targets in novel brain‐based interventions.
Supporting information
Supporting Information Figure 1
Supporting Information Table 1
ACKNOWLEDGMENTS
We would like to thank Ed Nieuwenhuys for providing the high‐quality photographs of European Spiders; Valentin Kemper for his support with optimizing fMRI data acquisition; Armin Heinecke, Judith Eck, and Jan Zimmermann for their very helpful suggestions regarding the analyses; and Anna Konova for help with editing the manuscript.
REFERENCES
- American Psychiatric Association (2000): Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM‐IV‐TR). Arlington, VA, USA: American Psychiatric Association. [Google Scholar]
- Barlow DH (2002): True alarms, false alarms, and learned (conditioned) anxiety In: Barlow DH, editor. Anxiety and Its Disorders: The Nature and Treatment of Anxiety and Panic, 2nd ed New York: The Guilford Press; pp 219–251. [Google Scholar]
- Birn RM, Murphy K, Handwerker DA, Bandettini PA (2009): fMRI in the presence of task‐correlated breathing variations. Neuroimage 47:1092–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson K, Petersson KM, Lundqvist D, Karlsson A, Ingvar M, Ohman A (2004): Fear and the amygdala: Manipulation of awareness generates differential cerebral responses to phobic and fear‐relevant (but nonfeared) stimuli. Emotion 4:340–353. [DOI] [PubMed] [Google Scholar]
- Carter CS, Botvinick MM, Cohen JD (1999): The contribution of the anterior cingulate cortex to executive processes in cognition. Rev Neurosci 10:49–57. [DOI] [PubMed] [Google Scholar]
- Del Casale A, Ferracuti S, Rapinesi C, Serata D, Piccirilli M, Savoja V, Kotzalidis GD, Manfredi G, Angeletti G, Tatarelli R, Girardi P (2012): Functional neuroimaging in specific phobia. Psychiatry Res 202:181–197. http://www.ncbi.nlm.nih.gov/pubmed/22804970. [DOI] [PubMed] [Google Scholar]
- Caseras X, Giampietro V, Lamas a, Brammer M, Vilarroya O, Carmona S, Rovira M, Torrubia R, Mataix‐Cols D (2010): The functional neuroanatomy of blood‐injection‐injury phobia: A comparison with spider phobics and healthy controls. Psychol Med 40:125–134. http://www.ncbi.nlm.nih.gov/pubmed/19435544. [DOI] [PubMed] [Google Scholar]
- Cohen J (1973): Eta‐squared and partial eta‐squared in fixed factor ANOVA designs. Educ Psychol Meas 33:107–112. [Google Scholar]
- Craig A (Bud) (2011): Significance of the insula for the evolution of human awareness of feelings from the body. Ann N Y Acad Sci 1225:72–82. http://www.ncbi.nlm.nih.gov/pubmed/21534994. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ (2001): Neuroanatomical basis for first‐ and second‐order representations of bodily states. Nat Neurosci 4:207–212. http://www.ncbi.nlm.nih.gov/pubmed/11175883. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Detre JA, Alsop DC, Shin RK, Atlas S, Grossman M (1995): The neural basis of the central executive system of working memory. Nature 378:279–281. [DOI] [PubMed] [Google Scholar]
- Deichmann R, Gottfried J, Hutton C, Turner R (2003): Optimized EPI for fMRI studies of the orbitofrontal cortex. Neuroimage 19:430–441. http://linkinghub.elsevier.com/retrieve/pii/S1053811903000739. [DOI] [PubMed] [Google Scholar]
- Dilger S, Straube T, Mentzel H‐J, Fitzek C, Reichenbach JR, Hecht H, Krieschel S, Gutberlet I, Miltner WHR (2003): Brain activation to phobia‐related pictures in spider phobic humans: An event‐related functional magnetic resonance imaging study. Neurosci Lett 348:29–32. http://linkinghub.elsevier.com/retrieve/pii/S0304394003006475. [DOI] [PubMed] [Google Scholar]
- Domsch S, Linke J, Heiler PM, Kroll A, Flor H, Wessa M, Schad LR (2013): Increased BOLD sensitivity in the orbitofrontal cortex using slice‐dependent echo times at 3 T. Magn Reson Imaging 31:201–211. http://www.ncbi.nlm.nih.gov/pubmed/22925606. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD (2007): Functional neuroimaging of anxiety: A meta‐analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 164:1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun M. a, Noll DC (1995): Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster‐size threshold. Magn Reson Med 33:636–647. [DOI] [PubMed] [Google Scholar]
- Fuster JM (1990): Prefrontal cortex and the bridging of temporal gaps in the perception‐action cycle. Ann N Y Acad Sci 608:318–336. http://www.ncbi.nlm.nih.gov/pubmed/2127512. [DOI] [PubMed] [Google Scholar]
- Goebel R, Esposito F, Formisano E (2006): Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: From single‐subject to cortically aligned group general linear model analysis and self‐organizing group independent component analysis. Hum Brain Mapp 27:392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens L, Schruers K, Peeters R, Griez E, Sunaert S (2007): Visual presentation of phobic stimuli: Amygdala activation via an extrageniculostriate pathway?. Psychiatry Res 155:113–120. http://www.ncbi.nlm.nih.gov/pubmed/17499485. [DOI] [PubMed] [Google Scholar]
- Gottfried J. a, Dolan RJ (2004): Human orbitofrontal cortex mediates extinction learning while accessing conditioned representations of value. Nat Neurosci 7:1144–1152. http://www.ncbi.nlm.nih.gov/pubmed/15361879. [DOI] [PubMed] [Google Scholar]
- Grupe DW, Nitschke JB (2013): Uncertainty and anticipation in anxiety: An integrated neurobiological and psychological perspective. Nat Rev Neurosci 14:488–501. http://www.ncbi.nlm.nih.gov/pubmed/23783199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo CC, Kurth F, Zhou J, Mayer E. a, Eickhoff SB, Kramer JH, Seeley WW (2012): One‐year test‐retest reliability of intrinsic connectivity network fMRI in older adults. Neuroimage 61:1471–1483. http://www.ncbi.nlm.nih.gov/pubmed/22446491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton C, Josephs O, Stadler J, Featherstone E, Reid A, Speck O, Bernarding J, Weiskopf N (2011): The impact of physiological noise correction on fMRI at 7 T. Neuroimage 57:101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipser JC, Singh L, Stein DJ (2013): Meta‐analysis of functional brain imaging in specific phobia. Psychiatry Clin Neurosci 67:311–322. [DOI] [PubMed] [Google Scholar]
- Jo HJ, Saad ZS, Simmons WK, Milbury L. a, Cox RW (2010): Mapping sources of correlation in resting state FMRI, with artifact detection and removal. Neuroimage 52:571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE (2005): Prevalence, severity, and comorbidity of 12‐month DSM‐IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62:617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klorman R, Weerts TC, Hastings JE, Melamed BG, Lang PJ (1974): Psychometric description of some specific‐fear questionnaires. Behav Ther 5:401–409. [Google Scholar]
- Milad MR, Quinn BT, Pitman RK, Orr SP, Fischl B, Rauch SL (2005): Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proc Natl Acad Sci 102:10706–10711. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1180773&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Yu R, Rowe JB, Eich H, Feldmanhall O, Dalgleish T (2010): Neural activity associated with monitoring the oscillating threat value of a tarantula. Proc Natl Acad Sci 107:20582–20586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawetz C, Holz P, Lange C, Baudewig J, Weniger G, Irle E, Dechent P (2008): Improved functional mapping of the human amygdala using a standard functional magnetic resonance imaging sequence with simple modifications. Magn Reson Imaging 26:45–53. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JDE, Gross JJ (2004): For better or for worse : Neural systems supporting the cognitive down‐ and up‐regulation of negative emotion. Neuroimage 23:483–499. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Ost L‐G (2008): Cognitive behavior therapy for anxiety disorders: 40 years of progress. Nord J Psychiatry 62:5–10. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB (2006): An insular view of anxiety. Biol Psychiatry 60:383–387. http://www.ncbi.nlm.nih.gov/pubmed/16780813. [DOI] [PubMed] [Google Scholar]
- Paus T (2001): Primate anterior cingulate cortex: Where motor control, drive and cognition interface. Nat Rev Neurosci 2:417–424. http://www.ncbi.nlm.nih.gov/pubmed/11389475. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald D. a, Nathan PJ, Moore GJ, Uhde TW, Tancer ME (2005): Neural substrates for voluntary suppression of negative affect: A functional magnetic resonance imaging study. Biol Psychiatry 57:210–219. http://www.ncbi.nlm.nih.gov/pubmed/15691521. [DOI] [PubMed] [Google Scholar]
- Robinson S, Windischberger C, Rauscher A, Moser E (2004): Optimized 3 T EPI of the amygdalae. Neuroimage 22:203–210. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Grabenhorst F (2008): The orbitofrontal cortex and beyond: From affect to decision‐making. Prog Neurobiol 86:216–244. http://www.ncbi.nlm.nih.gov/pubmed/18824074. [DOI] [PubMed] [Google Scholar]
- Schienle A, Schäfer A, Walter B, Stark R, Vaitl D (2005): Brain activation of spider phobics towards disorder‐relevant, generally disgust‐ and fear‐inducing pictures. Neurosci Lett 388:1–6. http://www.ncbi.nlm.nih.gov/pubmed/16046064. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC (1998): The Mini‐International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. J Cinical Psychiatry 59 (Suppl 2):22–57. [PubMed] [Google Scholar]
- Shenhav A, Botvinick MM, Cohen JD (2013): The expected value of control: An integrative theory of anterior cingulate cortex function. Neuron 79:217–240. http://www.ncbi.nlm.nih.gov/pubmed/23889930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube T, Mentzel H‐J, Miltner WHR (2006): Neural mechanisms of automatic and direct processing of phobogenic stimuli in specific phobia. Biol Psychiatry 59:162–170. [DOI] [PubMed] [Google Scholar]
- Szymanski J (1995): Fear of spiders questionnaire. J Behav Ther Exp Psychiatry 26:31–34. [DOI] [PubMed] [Google Scholar]
- Veltman DJ, Tuinebreijer WE, Winkelman D, Lammertsma A. a, Witter MP, Dolan RJ, Emmelkamp PMG (2004): Neurophysiological correlates of habituation during exposure in spider phobia. Psychiatry Res 132:149–158. http://www.ncbi.nlm.nih.gov/pubmed/15598549. [DOI] [PubMed] [Google Scholar]
- Weiskopf N, Hutton C, Josephs O, Turner R, Deichmann R (2007): Optimized EPI for fMRI studies of the orbitofrontal cortex: Compensation of susceptibility‐induced gradients in the readout direction. magma 20:39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenbacher A, Kasess C, Gerstl F, Lanzenberger R, Moser E, Windischberger C (2009): Correlations and anticorrelations in resting‐state functional connectivity MRI: A quantitative comparison of preprocessing strategies. Neuroimage 47:1408–1416. [DOI] [PubMed] [Google Scholar]
- Wittchen H‐U, Jacobi F (2005): Size and burden of mental disorders in Europe–a critical review and appraisal of 27 studies. Eur Neuropsychopharmacol 15:357–376. [DOI] [PubMed] [Google Scholar]
- Zilverstand A, Sorger B, Sarkheil P, Goebel R (2015): fMRI neurofeedback facilitates anxiety regulation in females with spider phobia. Front Behav Neurosci 9:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figure 1
Supporting Information Table 1


