Abstract
Background
Cognitive and neuroimaging changes under chronic high‐altitude exposure have never been followed up and dynamically assessed.
Objectives
To investigate the cognitive and brain structural/functional alterations associated with chronic high‐altitude exposure.
Methods
Sixty‐nine college freshmen that were immigrating to Tibet were enrolled and followed up for two years. Neuropsychological tests, including verbal/visual memory and simple/recognition reaction time, were utilized to determine whether the subjects' cognitive function had changed in response to chronic high‐altitude exposure. Structural magnetic resonance imaging (MRI) and resting‐state functional MRI (rs‐fMRI) were used to quantify brain gray matter (GM) volumes, regional homogeneity (ReHo) and functional connectivity (FC) alterations before and after exposure. Areas with changes in both GM and ReHo were used as seeds in the inter‐regional FC analysis.
Results
The subjects showed significantly lower accuracy in memory tests and longer reaction times after exposure, and neuroimaging analysis showed markedly decreased GM volumes and ReHo in the left putamen. FC analysis seeding of the left putamen showed significantly weakened FC with the superior temporal gyrus, anterior/middle cingulate gyrus and other brain regions. In addition, decreased ReHo was found in the superior temporal gyrus, superior parietal lobule, anterior cingulate gyrus and medial frontal gyrus, while increased ReHo was found in the hippocampus. Differences in ReHo/FC before and after high‐altitude exposure in multiple regions were significantly correlated with the cognitive changes.
Conclusion
Cognitive functions such as working memory and psychomotor function are impaired during chronic high‐altitude exposure. The putamen may play an important role in chronic hypoxia‐induced cognitive impairment. Hum Brain Mapp 38:3865–3877, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: high‐altitude exposure, cognition, magnetic resonance imaging
INTRODUCTION
Tibet, located in Southwest China, is the highest plateau area in the world. According to the Chinese national census in 2010, over 260,000 people had immigrated into Tibet from low‐altitude areas for occupational or economic opportunities and this figure continues to grow. The new immigrants in Tibet suffer from stress associated with high‐altitude exposure. The potential influence of high‐altitude exposure on the immigrating population has raised increasing concern nationwide, especially in those with an intent to immigrate to this region.
At high altitudes, hypoxia combined with other physiological stressors, such as hypobaria, cold, ultraviolet rays and dehydration, contributes to a decline in cognitive function [Virues‐Ortega et al., 2004]. Acute hypoxia exposure for several weeks during a short‐term expedition to high‐altitude areas causes a decline in working memory, learning ability, attention and concentration [Wang et al., 2013; Yan, 2014]. For example, Virués‐Ortega reported that impairments in codification and short‐term memory are especially noticeable above an altitude of 6,000 m [Virues‐Ortega et al., 2004]. Furthermore, McGuire et al. demonstrated that United States Air Force (USAF) U‐2 pilots with occupational exposure to repeated hypobaria had a lower neurocognitive performance than pilots without repeated hypobaric exposure [Hellmann‐Regen et al., 2014; McGuire et al., 2012, 2014a, 2014b]. Although cognitive and neuroimaging changes at high altitudes have been reported previously, the data supporting these conclusions have been principally derived from observations associated with an acute ascent to higher altitude areas. In contrast, there are fewer studies on the cognitive impairments associated with chronic exposure to high‐altitude hypoxia. Furthermore, these studies commonly compared high‐altitude indigenous residents or immigrants with age‐matched sea‐level residents; in this case, when assessing neuropsychological/cognitive differences, the demographic, cultural and socioeconomic differences between the populations may lead to significant bias. The influence of chronic high‐altitude exposure on cognitive function in high‐altitude immigrants has yet to be dynamically assessed and fully confirmed.
In previous studies, neuroimaging studies based on anatomical T1‐weighted magnetic resonance imaging (MRI) and resting state functional MRI (rs‐fMRI) have been widely utilized to assess structural and functional modifications, respectively. Anatomical T1‐weighted MRI is routinely used for analysis of voxel‐based morphology (VBM) to explore possible changes in the volume of the gray matter (GM), white matter and cerebrospinal fluid (CSF) [Ashburner and Friston, 2000]; in turn, rs‐fMRI signal fluctuations reflect the intrinsic functional organization and the endogenous neurophysiological process of the human brain, providing pertinent information on core mechanisms of neuropsychological changes [Gusnard et al., 2001]. In recent years, structural and functional MRI analyses have reported several compensatory differences in brain structure and function under high‐altitude hypoxia by comparing high‐altitude‐exposed subjects with age‐matched sea‐level controls. [Yan et al., 2010; Zhang et al., 2010] However, these studies, whose sample sizes were relatively small, have reported conflicting results. For example, Foster et al. observed a GM reduction after a 3‐week exposure to a 5,500‐m altitude, while the white matter was unchanged [Foster et al., 2015]. However, McGuire et al. demonstrated that occupational exposure to hypobaria is associated with subcortical white matter hyperintensities [McGuire et al., 2012]. Furthermore, a before‐after comparison of the brain structure and function of immigrants under chronic high‐altitude exposure has never been carried out before.
Herein, we performed a panel study on a young healthy high‐altitude immigrant population, allowing us to clarify the cognitive changes as well as the brain structural and functional alterations associated with chronic high‐altitude exposure. We hypothesized that modified GM, regional homogeneity (ReHo) and functional connectivity (FC) in certain brain regions would be revealed after chronic high‐altitude exposure and that these changes may be associated with decreased cognitive function. To the best of our knowledge, this is the first panel study to address potential cognitive and neuroimaging changes in high‐altitude immigrants, along with the neural structural and functional bases for the neuropsychological impairments associated with chronic high‐altitude exposure.
MATERIALS AND METHODS
Study Design and Subjects
The protocol was approved by the Ethics Committee of the Medical Faculty of Fourth Military Medical University (Registry no. KY20143344‐1), and all studies were conducted in accordance with the ethical principles for medical research involving human subjects as defined in the Declaration of Helsinki.
In July 2014, 120 high school graduates from Shaanxi Province were admitted to Tibet University for a four‐year higher education period in Lhasa (average altitude: 3,658 m), China. We contacted all of the admitted graduates and their parents via telephone and mail to invite them to participate in our study. Of the 120 subjects initially invited, 70 subjects (total response rate, 58.3%) agreed to participate. The subjects were screened for the following criteria: (1) 17–20 years of age; (2) altitude of permanent residence less than 900 m; (3) no high‐altitude exposure (≥2,500 m) in the past; (4) no tobacco or recreational drug use; (5) no history of taking medications that might affect cognitive function or carbonic anhydrase activity; (6) no chronic or genetic diseases; and (7) a willingness to participate in the study and sign the informed consent form (ICF). As a result, one subject was excluded due to previous high‐altitude exposure, and a total of 69 subjects were recruited for further study. Considering the significant time commitment associated with MRI, we asked the participants to decide if they wished to attend the MRI sessions and offered two versions of the ICF.
Baseline investigation, neuropsychological and MRI measures were performed in July 2014 in Xi'an (altitude 466 m), China. The subjects were instructed to complete a set of questionnaires comprising high‐altitude exposure history, medical history, academic performance and sociodemographic information including parental education, vocation and socioeconomic status. The follow‐up investigation and neuropsychological measures were performed in June 2015 and May 2016 in Lhasa. During the follow‐up investigation, the subjects were instructed to complete a questionnaire comprising the duration of high‐altitude exposure and experience in mountain climbing and low‐altitude visits, as well as any medical events that have occurred during the study period. The follow‐up MRI measures were performed in 2016 at the same time as the other measures. During the baseline and follow‐up investigations, physical examinations were performed to assess the subjects' general health condition.
Neuropsychological Tests
The neuropsychological test battery consisted of the following four tests: (1) verbal memory test, which measures working memory for words, testing immediate verbal memory and delayed verbal memory; (2) visual memory test, which measures working memory for figures and shapes, testing immediate visual memory and delayed visual memory; (3) simple reaction time test, which assesses psychomotor function in response to a simple stimulus, testing visual simple reaction time and auditory simple reaction time; (4) recognition (go/no‐go) reaction time test, which measures psychomotor function in response to target/distractive stimuli, testing visual recognition reaction time and auditory recognition reaction time. All tests were performed using CNS Vital Signs (http://www.cnsvs.com/) [Gualtieri and Johnson, 2006].
MRI Image Acquisition
The baseline and follow‐up magnetic resonance images were acquired with a General Electric Discovery MR750 3.0T (General Electric Co. Ltd., CT) in Xijing Hospital of the Fourth Military Medical University and the General Hospital of Tibet Military Region, respectively. To correlate the neuropsychological test results with neuroimaging data, MRI measurements were performed immediately after the completion of the cognitive tests.
To ensure the comparability of the images, we strictly used the same scanner parameters. Standard T1‐weighted 3D anatomical data were acquired with the 3D magnetization‐prepared rapid gradient echo (3D MPRAGE) sequence (repetition time: 2,530 ms; echo time: 3.5 ms; flip angle: 7°; field of view: 256 mm × 256 mm; matrix: 256 × 256; slice thickness: 1 mm; section gap: 0 mm; number of slices: 192, voxel size = 1 × 1 × 1 mm3).
Rs‐fMRI data were acquired with an echo planar imaging (EPI) sequence (repetition time: 2,000 ms; echo time: 30 ms; flip angle: 90°; field of view: 220 mm × 220 mm; acquisition/reconstruction matrix: 128 × 128; slice thickness: 4 mm; section gap: 0.6 mm; number of slices: 30, voxel size = 1 × 1 × 1 mm3, scam time: 6 min; total volumes: 180) covering the entire brain. A custom‐built head coil cushion and earplugs were used to minimize head motion and dampen scanner noise. During data acquisition, subjects were asked to remain alert with eyes closed and to keep their head still.
Structural MRI Analysis
Structural MRI data were analyzed using VBM in Statistical Parametric Mapping software (SPM8, Wellcome Department of Cognitive Neurology, London, UK; http://www.fil.ion.ucl.ac.uk/spm/) in MATLAB R2014a (MathWorks, Natick, MA) [Matsuda et al., 2012]. Data processing steps were performed according to Ashburner [Schrouff et al., 2013]. We applied Diffeomorphic Anatomical Registration through Exponentiated Lie Algebra (DARTEL), which is implemented as a toolbox for SPM8 and enables the creation of a set of group‐specific templates. The brain images were segmented, normalized and modulated with these templates. The performance of the DARTEL toolbox on non‐linear registration algorithms is superior to that of other similar toolboxes. The registered images were then transformed to Montreal Neurological Institute (MNI) space, and finally, the normalized and modified images were smoothed with a 6‐mm full‐width at half‐maximum (FWHM) Gaussian kernel.
Rs‐fMRI Preprocessing and Analysis
Rs‐fMRI data preprocessing was then performed with the SPM8 and Data Processing Assistant for Resting‐state fMRI Advanced (DPARSFA) tools [Chao‐Gan and Yu‐Feng, 2010]. Prior to preprocessing, the first 10 volumes were discarded to reach a steady‐state magnetization and allow the participants to adapt to the scanning noise. For each subject, the fMRI data were first realigned to correct for head motion (exclusion criteria: > 2.5‐mm translation and/or > 3.0° rotation). The standard MNI template provided by SPM was further utilized for normalization and segmentation, resampling to 3 mm × 3 mm × 3 mm voxels and each participant's brain image was segmented into GM, white matter and CSF. Furthermore, temporal bandpass filtrating (0.01 to 0.08 Hz) was applied to the data in temporal frequency space to minimize low‐frequency signal drift and high‐frequency variations due to cardiac and respiratory effects. In addition to the global mean signal, six motion parameters and the CSF and white matter signals were also removed by linear model to reduce the effects of head motion and non‐neuronal BOLD fluctuations.
The voxel‐based comparison of ReHo was conducted in REST V 2.0 (http://restfmri.net/forum/REST_V2.0) [Cordova‐Palomera et al., 2015; Song et al., 2011; Yan et al., 2016]. The computation of ReHo at rest was spatially smoothed with a 4 mm × 4 mm × 4 mm FWHM Gaussian kernel, as previously described. Briefly, Kendall's coefficient of concordance (KCC) for each voxel in the brain was calculated voxel‐wise by applying a cluster size of 26 voxels. For standardization purposes, each individual ReHo map was divided by that subject's global mean brain KCC value to minimize inter‐individual variability for statistical analysis within the whole‐brain mask. The results are shown at a statistical significance level of P < 0.05 with an AlphaSim correction (with a combination of a threshold of P < 0.01 and a minimum cluster size of 30 voxels).
FC was examined with a seed‐voxel correlation approach in DPARSFA. The left putamen, with MNI coordinates X = −27.5, Y = 7.5 and Z = 3 and a 6‐mm radius, where both the GM volume and ReHo value had been altered, was identified as the seed. A voxel‐wise correlation was then computed between the time series of the seed reference and those of all brain voxels outside of the seed region. A Fisher r‐to‐Z transformation was used to transform the correlation coefficients to Z values. First, we conducted within‐group comparisons of z‐transformed FC values to verify the FC regions of the region of interest (ROI) seed region in participants before and after exposure. Next, correlation maps were computed according to standard practice using a time series extracted from the ROI by averaging over all included voxels. Then, we conducted voxel‐wise, paired comparisons of the z‐transformed FC values to identify the regions showing significant alterations in FC with the ROI seed region. To control for multiple comparisons, within‐group whole brain results and paired comparison results were controlled with an FDR correction of P < 0.001 and a cluster size threshold of five voxels. The REST View based on Xjview and the [Xia et al., 2013] (http://www.nitrc.org/projects/bnv/) Matlab toolbox was used to visualize the FC maps and alterations.
Statistical Analysis
Friedman test and post hoc Nemenyi test were applied to assess the changes in the neuropsychological parameters over time. Spearman correlation coefficients were separately computed to examine the correlations between GM/ReHo/FC alterations (the differences between the parameters before exposure and those after exposure for each individual) and neuropsychological performance changes. All statistical analyses were performed with R 3.1.3 (https://www.r-project.org/). P < 0.05 was considered statistically significant.
RESULTS
Demographic Information and High‐Altitude Exposure
The procedures for subject enrollment and follow‐up are shown in Figure 1. None of the 49 subjects participating in the MRI sessions was lost to follow‐up.
Figure 1.
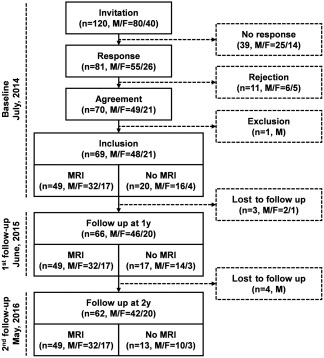
Flow chart of the subject enrollment and follow‐up in this study.
The average age of the subjects (M: 48; F: 21) was 18.2 ± 0.3 (range: 17.5–19.1) at the baseline timepoint. All subjects had just completed the 3‐year high school curriculum and took part in the college entrance examination. Their academic performance in the exams was quite similar (513.3 ± 13.6, range: 483–544); thus, all were admitted to the same college. Most of the subjects came from rural areas (54, 78.26%), and their family yearly incomes were between RMB 50,000 and 100,000 yuan (40, 57.97%). For the majority of the subjects, the paternal and maternal educational years were below 12 (11.7 ± 2.1; 10.6 ± 1.9). In the first year of follow‐up, the average cumulative high‐altitude exposure time of the subjects was 270.0 ± 7.4 d (range: 257 d–299 d). In the second year, the cumulative exposure time increased to 280.8 ± 7.3 d (range: 268 d–314 d). In summary, the high school graduates in this study, who immigrated into Tibet from less developed rural areas, were quite representative of the population immigrating to Tibet.
Cognitive Function Impairment
All of the subjects who participated in the baseline and follow‐up studies completed the neuropsychological tests. The neuropsychological changes are noted in Table 1.
Table 1.
Descriptive values (median/IQR) and statistics of the altered cognitive performance during high‐altitude exposure
| Cognitive performance | Exposure time | Friedman Statistic | P | ||
|---|---|---|---|---|---|
| 0 y | 1 y | 2 y | |||
| IVBM | 28.00 (2.00) | 28.00 (3.25) | 27.50 (4.00) | 9.074 | 0.011 |
| IVIM | 24.00 (4.00) | 22.50 (4.00) | 22.50 (3.50) | 7.492 | 0.024 |
| DVBM | 28.00 (2.00) | 27.00 (3.00) | 26.00 (5.00) | 29.276 | 0.000 |
| DVIM | 22.00 (4.00) | 21.00 (4.00) | 20.00 (4.00) | 16.126 | 0.000 |
| VSRT | 0.196 (0.170) | 0.263 (0.068) | 0.323 (0.091) | 44.637 | 0.000 |
| ASRT | 0.106 (0.141) | 0.281 (0.083) | 0.343 (0.085) | 97.142 | 0.000 |
| VRRT | 0.392 (0.123) | 0.465 (0.162) | 0.507 (0.185) | 34.930 | 0.000 |
| ARRT | 0.377 (0.132) | 0.478 (0.184) | 0.529 (0.164) | 51.803 | 0.000 |
IVBM, immediate verbal memory; IVIM, immediate visual memory; DVBM, delayed verbal memory; DVIM, delayed visual memory; ST, Stroop test; VSRT, visual simple reaction time; ASRT, auditory simple reaction time; VRRT, visual recognition reaction time; ARRT, auditory recognition reaction time.
Decreased Accuracy in the Verbal/Visual Memory Test
In the immediate verbal memory test, the subjects' performance varied significantly over the exposure time (Friedman statistic = 9.074, P = 0.011). However, only the difference between year two (2 y; median = 27.50, IQR = 4.00) and baseline (median = 28.00, IQR = 2.00) was statistically significant in the post hoc tests (P = 0.025). A significant difference was also observed among the number of correct responses obtained in the immediate visual memory test (Friedman statistic = 7.492, P = 0.024). However, no pairwise difference between different time points was identified in post hoc multiple comparisons (baseline: median = 24.00, interquartile range (IQR)=4.00; year one (1 y): median = 22.50, IQR = 4.00; 2 y: median = 22.50, IQR = 3.50).
Furthermore, the number of correct responses in the delayed verbal/visual memory tests was significantly different over the exposure time (Friedman statistic = 29.276, P = 0.000; Friedman statistic = 16.126, P = 0.000, respectively). In the delayed verbal memory test, post hoc tests established that the number of correct responses obtained at 1 y (median = 27.00, IQR = 3.00) and 2 y (median = 26.00, IQR = 5.00) were significantly lower than at baseline (median = 28.00, IQR = 2.00; P = 0.005 & 0.000, respectively), but the numbers of correct responses at 1 y and 2 y were not different from one another. Similarly, in the delayed visual memory test, post hoc tests established that the numbers of correct responses obtained at 1 y (median = 21.00, IQR = 4.00) and 2 y (median = 20.00, IQR = 4.00) were significantly lower than at baseline (median = 22.00, IQR = 4.00; P = 0.007 & 0.002, respectively), but the numbers of correct responses at 1 y and 2 y were not different from one another.
Prolonged Responding Time in the Visual/Auditory Reaction Time Test
The subjects' visual/auditory simple reaction times were significantly prolonged after high‐altitude exposure (Friedman statistic = 44.636, P = 0.000; Friedman statistic = 97.142, P = 0.000, respectively). Post hoc analysis showed that the visual simple reaction times measured at 1 y (median = 0.263, IQR = 0.068) were significantly longer than those at baseline (median = 0.196, IQR = 0.170; P = 0.009), while the reaction times at 2 y (median = 0.323, IQR = 0.091) were longer than those at 1 y (P = 0.002). Similarly, the auditory simple reaction times measured at 1 y (median = 0.281, IQR = 0.083) were significantly longer than those at baseline (median = 0.106, IQR = 0.141; P = 0.000), while the reaction times at 2 y (median = 0.343, IQR = 0.085) were longer than those at 1 y (P = 0.005).
Furthermore, the subjects' visual/auditory recognition reaction times also varied over the exposure time (Friedman statistic = 34.930, P = 0.000; Friedman statistic = 51.803, P = 0.000, respectively). Post hoc analysis showed that the visual recognition reaction times obtained at 1 y (median = 0.465, IQR = 0.162) and 2 y (median = 0.507, IQR = 0.185) were significantly longer than that at baseline (median = 0.392, IQR = 0.123; P = 0.002 & 0.000, respectively). Similarly, the auditory recognition reaction times obtained at 1 y (median = 0.478, IQR = 0.184) and 2 y (median = 0.529, IQR = 0.164) were also significantly longer than that at baseline (median = 0.377, IQR = 0.132; P = 0.000 & 0.000, respectively).
Brain Structural and Functional Alterations
Decreased GM volume in the left putamen
Structural imaging analysis using VBM revealed brain structural alterations after chronic high‐altitude exposure. Decreased GM volume was found in the left putamen before and after exposure (paired t‐test, |t|> 4.90, P < 0.001, P < 0.05 FDR corrected). See details in Figure 2 and Table 2.
Figure 2.
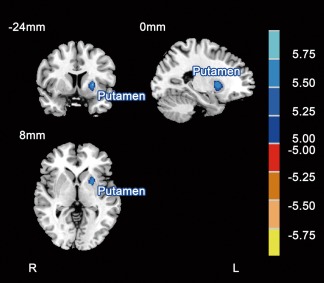
Paired comparisons of regional GM volumes before and after chronic high‐altitude exposure (|t|> 4.90, P < 0.001, P < 0.05 FDR corrected). Compared with that pre‐exposure, the blue areas indicate lower GM volume in the subjects after exposure. The MNI atlas plane of the section is marked in the upper‐left. The color bar on the right indicates the T values. L, left; R, right.
Table 2.
Regions showing significantly lower GM volume after high‐altitude exposure compared with that pre‐exposure
| Brain region | MNI coordinates (mm) | Volume (mm3) | Peak T value | ||
|---|---|---|---|---|---|
| x | y | z | |||
| L Putamen | −25 | 7.5 | 3 | 1,401 | −6.890 |
L, left; R, right; MNI coordinates, the coordinates of the peak point located in the MNI space.
Decreased ReHo in multiple regions and increased ReHo in bilateral hippocampi
Functional imaging analysis based on rs‐fMRI was performed to investigate the ReHo differences before and after high‐altitude exposure. Compared to the ReHo preexposure, the ReHo after exposure was significantly decreased in the bilateral putamen, bilateral superior temporal gyri, bilateral superior parietal lobule, anterior cingulate gyri, medial frontal gyri and anterior cerebellum lobe, while increased ReHo was found in bilateral hippocampi (paired t‐test, |t|> 2.70, P < 0.01, AlphaSim corrected; Fig. 3 and Table 3).
Figure 3.
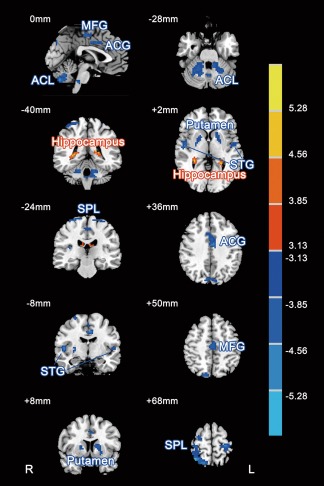
Paired comparisons of regional ReHo before and after chronic high‐altitude exposure (|t|> 2.70, P < 0.01, AlphaSim corrected). Compared with those pre‐exposure, the red areas indicated higher ReHo and blue areas indicated lower ReHo in the subjects after exposure. The slice location is marked in the upper‐left corner in each image. The color bar on the right indicates the T values. ACL, anterior cerebellum lobule; STG, superior temporal gyrus; SPL, superior parietal lobule; ACG, anterior cingulate gyrus; MFG, middle frontal gyrus. L, left; R, right.
Table 3.
Regions showing significantly different ReHo after high‐altitude exposure compared with that pre‐exposure
| Brain region | MNI coordinates (mm) | Volume (mm3) | Peak T value | ||
|---|---|---|---|---|---|
| x | y | z | |||
| L Putamen | −15 | 12 | 9 | 2,511 | −4.323 |
| R Putamen | 24 | −3 | 6 | 1,404 | −4.009 |
| L Superior Temporal Gyrus | −57 | −3 | 6 | 1,404 | −4.898 |
| R Superior Temporal Gyrus | 51 | −9 | 6 | 2,592 | −4.932 |
| L Superior Parietal Lobule | −27 | −27 | 69 | 1,458 | −4.881 |
| R Superior Parietal Lobule | 33 | −57 | 69 | 10,962 | −5.413 |
| Anterior Cingulate Gyrus | −6 | −9 | 39 | 2,619 | −5.136 |
| Middle Frontal Gyrus | 3 | −21 | 54 | 1,485 | −4.374 |
| Cerebellum Anterior Lobe | 15 | −60 | −30 | 15,282 | −5.767 |
| L Hippocampus | −15 | 30 | 18 | 1,917 | 5.359 |
| R Hippocampus | 18 | −30 | 18 | 1,782 | 5.120 |
L, left; R, right; MNI coordinates, the coordinates of the peak point located in the MNI space.
Decreased FC of multiple regions with the ROI seed
A region in the anterior left putamen (X = −27.5, Y = 7.5 and Z = 3 in MNI coordinate with a radius of 6 mm), where both GM volume and ReHo were significantly decreased, was used as the ROI seed to study alterations in the FC with other brain regions.
First, before exposure, numerous regions showed a strong correlation with the left putamen (Fig. 4A), including large clusters of significance centering in the bilateral striatum (including the caudate, putamen and pallidum), thalamus, insula, brainstem, hippocampi, superior temporal gyri, inferior/medial frontal gyri and anterior/middle cingulate gyri and smaller clusters of significance in the bilateral cerebellum, postcentral cortex, precentral cortex, medial frontal gyri and superior motor areas. However, after exposure, the correlations with the left putamen were markedly altered (Fig. 4B). Only several large clusters centering in the bilateral striatum (including caudate, putamen and pallidum) displayed were significantly correlated with the left putamen.
Figure 4.
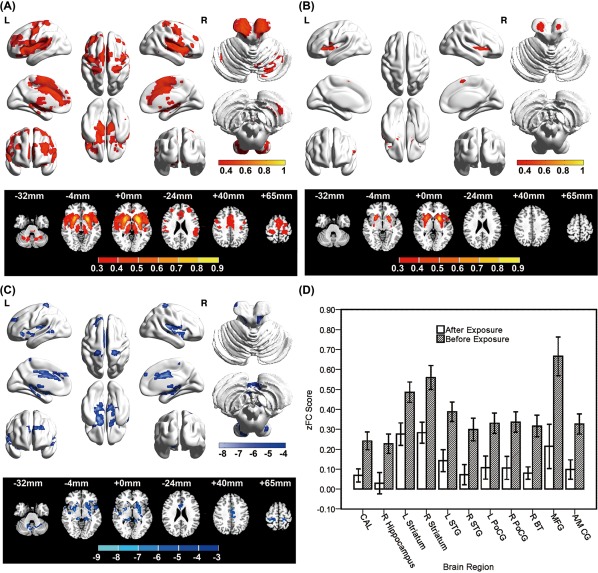
FC maps (A, B) and alterations (C, D) of the FCs with the seed region (left putamen: X=–25.5, Y = 7.5, Z = 3). FC [Fisher z‐transformed correlations, z(r)] averaged over subjects before (A) and after high‐altitude exposure (B). Red indicates the areas with a positive functional correlation with the seed region. (C) Decreased FC clusters with the seed region after paired t‐test analysis contrasting the subjects before versus after exposure. All illustrated clusters were FDR corrected using P < 0.001, cluster size >25 voxels. The MNI axial atlas plane of the section is indicated in each column. Blue indicates the areas showing decreased FC with seed region. (D) Z(r) was evaluated in each individual by averaging over the voxels showing a significant effect listed in Table IV. Bar height indicates the group mean. Error bars indicate the standard error of the mean. CAL, cerebellum anterior lobe; STG, superior temporal gyrus; PoCG, postcentral gyrus; BT, brainstem; MFG, medial frontal gyrus; ACG, anterior cingulate gyrus; MCG, medial cingulate gyrus; L, left; R, right.
Multiple regions exhibited significantly lower FC with the left putamen in the before‐after exposure comparison (Fig. 4C/4D and Table 4). After exposure, subjects were characterized by decreased FC in the bilateral superior temporal gyri, bilateral striatum, bilateral postcentral gyri, anterior/middle cingulate gyri, medial frontal gyri, right cerebellum, right hippocampi and right brainstem.
Table 4.
Clusters showing significant decreased FC with the ROI seed after high‐altitude exposure compared with that pre‐exposure
| Connectivity region | MNI coordinates (mm) | Volume (mm3) | Peak T value | ||
|---|---|---|---|---|---|
| x | y | z | |||
| L Superior Temporal Gyrus | −51 | 9 | −6 | 1,512 | −7.209 |
| R Superior Temporal Gyrus | 42 | −12 | 0 | 6,669 | −6.422 |
| L Striatum/Hippocampus/Parahippocampal Gyrus | −21 | −6 | 0 | 13,905 | −7.641 |
| R Striatum | 24 | 0 | −3 | 2,484 | −7.893 |
| L Postcentral Gyrus | −18 | −33 | 69 | 999 | −6.095 |
| R Postcentral Gyrus | 18 | −36 | 66 | 1,539 | −5.972 |
| Anterior/Middle Cingulate Gyrus | 0 | 9 | 30 | 5,697 | −6.847 |
| Medial Frontal Gyrus | 0 | −60 | −6 | 837 | −5.748 |
| Medial Frontal Gyrus | 0 | −24 | 57 | 1,026 | −5.870 |
| R Cerebellum Anterior Lobe | 12 | −51 | −36 | 1,323 | −6.139 |
| R Hippocampus | 27 | −18 | −15 | 891 | −6.876 |
| R Brainstem | 12 | −33 | −3 | 1,323 | −8.389 |
All P < 0.001, corrected for FDR; L, left; R, right; MNI coordinates, the coordinates of the peak point located in the MNI space.
Significant Correlations between Cognitive Changes and Brain Functional Alterations
To understand the effects of the chronic high‐altitude exposure on brain structure and function, we conducted correlation analyses between the before‐after exposure cognitive changes and the corresponding GM volumes, ReHo and FC alterations in the abovementioned significantly altered regions.
ReHo alterations in the left putamen were positively associated with the accuracy changes in immediate and delayed verbal memory (Fig. 5A; P = 0.000, r = 0.554; P = 0.000, r = 0.514, respectively). In addition, change in the ReHo of the right superior temporal gyrus was negatively associated with auditory simple reaction time changes (P = 0.022, r = −0.326).
Figure 5.
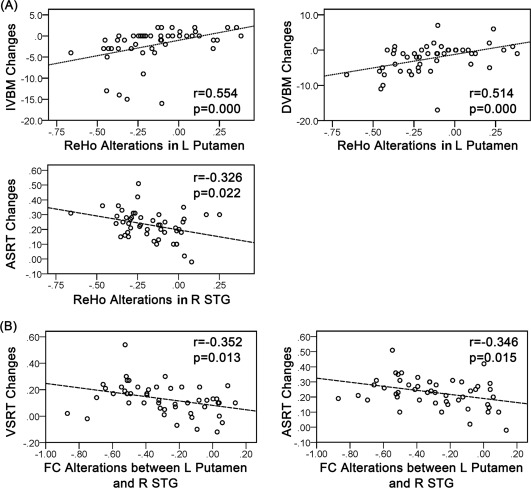
(A) Scatter plots indicating the correlation between the before‐after high‐altitude exposure ReHo alterations and the corresponding cognitive performance changes (P < 0.05). (B) Scatter plots indicating the correlation between the before‐after alterations of the Fisher z‐transformed FC values and the corresponding cognitive performance changes (< 0.05). IVBM, immediate verbal memory; DVBM, delayed verbal memory; VSRT, visual simple reaction time; ASRT, auditory simple reaction time; STG, superior temporal gyrus; MFG, medial frontal gyrus; L, left; R, right.
Among the altered FCs after exposure, the alteration in the FC between the left putamen and right superior temporal gyrus was negatively associated with the changes in visual and auditory simple reaction time (P = 0.013, r = −0.352; P = 0.015 r = −0.346; Fig. 5B). There were no associations between the changes in GM volume and before‐after exposure cognitive performance.
DISCUSSION
In recent years, encouraged by a series of new policies, an increasing number of Chinese people from less developed rural areas have immigrated to Tibet for business, education or service. Health maintenance of the high‐altitude immigrant population has become a major focus for public health departments in the Chinese government. Thus, we carried out a panel study in a young healthy high‐altitude immigrant population and addressed the cumulative impact of chronic high‐altitude exposure on cognitive function. In addition, brain structural and functional alterations during chronic high‐altitude exposure, together with cognitive changes, were followed to ascertain the neural structural and functional basis of the neuropsychological impairment associated with chronic high‐altitude exposure.
All of the subjects enrolled in this study were below 20 years of age (17.5–19.1). People in this age group, who are in the late period of neurodevelopment and are more vulnerable to high‐altitude hypoxia, constitute the main body of immigrants in Tibet. In addition, most subjects came from less developed rural areas and immigrated to Tibet to acquire higher education. Furthermore, the cumulative high exposure time of the subjects for 12 months (301.6 ± 9.1 d) approximated the average level of the entire immigrant population (312.2 ± 12.5 d) investigated in our previous cross‐sectional survey (not published). Taken together, since the current follow‐up study was based on the typical Chinese high‐altitude immigrant population, the cognitive and neuroimaging assessment acquired in this study may be representative and valuable as a reference for health maintenance in Chinese Tibet immigrants.
The current study, for the first time, revealed that immediate and delayed working memory for figures and shapes was impaired upon chronic high‐altitude exposure. The decreased accuracy in immediate and delayed verbal memory is consistent with previous studies on chronic exposure, which found verbal working memory impairment in other neuropsychological tasks. [Yan et al., 2011]. Furthermore, compared to immediate memories, delayed memories displayed more significant changes upon high‐altitude exposure, further confirming that complex neuropsychological processes are more susceptible to high‐altitude‐related impairments. Both simple and recognition reaction times were prolonged after exposure in contrast to results previously published where high‐altitude residents and controls showed no significant difference in a serial reaction time task [Zhang et al., 2011]. Notably, all of the abovementioned cognitive impairments occurred in the early stages of exposure (before 1 y) with no amelioration during the remaining time in the follow‐up period, indicating that the cognitive changes are irreversible under chronic high‐altitude exposure.
Previous neuroimaging studies have compared high‐altitude indigenous residents with sea‐level control subjects [Yan et al., 2010; Zhang et al., 2013]. In these studies, at a significance level of P < 0.05 with family‐wise error (few) correction, the high‐altitude residents exhibited a lower GM volume in several scattered distributed small clusters throughout the whole brain. In the current study, at a significance level of P < 0.001 with false discovery rate (FDR) correction (which was stricter), we found decreased GM volume in the left putamen of subjects after chronic high‐altitude exposure compared with that pre‐exposure. We inferred that the difference in the results might be caused by the differences in the study designs. To the best of our knowledge, this is the first study to provide direct evidence of GM loss in the putamen after chronic high‐altitude exposure.
According to Zatorre et al., a reduction in adult GM volume may result from anaerobic metabolic byproducts secondary to hypoxia, whereas an increase in GM volume may be related to neurogenesis, gliogenesis, synaptogenesis and/or vascular changes [Zatorre et al., 2012]. We posit that the significant decrease in GM volume in deep brain nuclei noted herein was caused by persistent and severe oxygen deficiency, leading to loss of neurons. As reported in other clinical studies, the left basal ganglia (including the putamen) appears to be more vulnerable to infarction and hypoxia than the right side due to the variance in the intracranial blood supply in the different hemispheres; thus, the loss of GM only in the left putamen is consistent with earlier studies, corroborating that the left side is more sensitive to chronic high‐altitude hypoxia than the right side [Yoo et al., 1998].
In the rs‐fMRI analysis, we found increased ReHo in bilateral hippocampi, partly consistent with previous fMRI studies that reported higher ReHo in the hippocampus, insula and postcentral gyrus among high‐altitude residents [Chen et al., 2016]. ReHo was designed to reflect the similarity of the time series of a given voxel to those of its neighboring voxels [Guo et al., 2014; Zhao et al., 2014]. Increased ReHo in bilateral hippocampi after exposure, indicative of increased brain activity, may result from an increase in microvessel density in these regions, representing a functional adaption mechanism to tolerate chronic hypoxia [Hochachka et al., 1994].
Decreased ReHo has been reported in various neuropsychological disorders as a potential sign of disrupted local functionality [Chen et al., 2012; Liang et al., 2013] but has not been observed in previous studies on high‐altitude exposure. For the first time, we found decreased ReHo after exposure in multiple brain regions, including the bilateral putamen, bilateral superior temporal gyri, bilateral superior parietal lobules, anterior cingulate gyri and medial frontal gyri. The bilateral superior temporal gyri and superior parietal lobules are critical brain regions for sensory perception and interpretation, especially for sound processing. ReHo in these regions was significantly decreased, and the changes in the auditory simple reaction time were negatively correlated with ReHo alterations in the right superior temporal gyrus, suggesting that reduced ReHo in the above regions reflects the weakened function of sensory perception and processing associated with chronic high‐altitude exposure. The anterior cingulate gyrus is an integral part of the limbic system, which controls emotion formation and processing, learning and memory. Abnormalities in the anterior cingulate gyrus have been previously observed in mental disorders such as schizophrenia and depression, while decreased ReHo in the anterior cingulate gyrus has been associated with depression [Xue et al., 2016]. Interestingly, previous studies have reported that acute high‐altitude exposure may lead to depression and bipolar disorder as well as increase the psychological ratings for these disorders; thus, decreased ReHo in the anterior cingulate gyrus may be a neuroimaging signature for emotional changes caused by chronic high‐altitude exposure [Levine, 2015]. Several studies have reported that the medial frontal gyrus is associated with executive mechanisms and decision‐making abilities [Lei et al., 2017]. Therefore, the decreased ReHo in the medial frontal gyrus, reflecting decreased local brain activity in this region, is likely involved in the high‐altitude‐induced impairment in go/no‐go decision making upon high‐altitude exposure.
We found both significant GM loss and decreased ReHo in the putamen after high‐altitude exposure, suggesting the following: (1) as GM loss was only observed in the left putamen, while decreased ReHo was found bilaterally, the functional abnormality revealed by ReHo may be more extensive than the structural abnormality associated with chronic exposure, and (2) the putamen is more sensitive to chronic hypoxia exposure than other brain regions. Anatomically, the putamen is a portion of the striatum, which projects to the internal pallidal segment and substantia nigra pars reticulata. The main function of the putamen is to regulate movements [Albin et al., 1995]; however, it is also critical in reinforcement learning and category learning. Patients with focal lesions in the basal ganglia (specifically the putamen) due to stroke perform poorly in rule‐based task learning [Graybiel, 1995; Packard and Knowlton, 2002]. Furthermore, the putamen plays a critical role in working memory and cognitive flexibility. For example, Baier et al. reported that in stroke patients, lesions of the left basal ganglia (putamen) render working memory susceptible to irrelevant information [Baier et al., 2010]. In addition, Shu et al. reported that a lesion in the medial area of the left putamen caused learning and memory deficits [Shu et al., 2009]. Yen Yu et al. observed elevated BOLD responses in the left putamen in response to stimuli reflecting D2 stimulation of striatal spiny neurons or prefrontal afferents in layer V [Yu et al., 2013], with a possible consequence of inhibition of the default “No‐Go” indirect pathway in go/no‐go tasks. Herein, the structural and functional abnormalities noted in the putamen may be the key to memory and psychomotor impairment under chronic high‐altitude exposure, which was further corroborated by the significant positive correlation between the decrease in immediate/delayed verbal memory accuracy and the ReHo alterations in the putamen.
In addition to GM loss and decreased ReHo, for the first time, we demonstrated weakened FC between the putamen and other brain regions, such as the bilateral superior temporal gyri, bilateral striatum, bilateral postcentral gyri, anterior/middle cingulate gyri, medial frontal gyri, right cerebellum, right hippocampus and right brainstem, after high‐altitude exposure. The reduction in FCs with the putamen in the superior temporal gyrus, medial frontal gyrus and right hippocampus have yet to be noted in previous fMRI studies. As described above, these regions play an important role in sensory processing, executive function, memory and other vital cognitive functions. The corresponding FC impairments found in this study suggest that the structural and functional abnormality in the putamen had “weakened” the connections between the putamen and other cognition‐related regions, representing a novel finding in brain injury caused by chronic high‐altitude exposure. Interestingly, the alteration in the FC between the right superior temporal gyrus and putamen was negatively correlated with visual/auditory simple reaction time changes, suggesting that the impaired psychomotor function after high‐altitude exposure may be related to this alteration in FC.
Some limitations of the study must be recognized. First, the cognitive and neuroimaging changes identified in this panel study were not further confirmed by comparisons with sea‐level control subjects. However, according to another study we have performed (in preparation), the cognitive and neuroimaging changes identified herein were not observed in a sea‐level college student cohort. Second, a two‐year follow‐up may not be sufficient to assess the dynamic cognitive and neuroimaging changes that occur upon chronic high‐altitude exposure. The following questions remain unclear: whether the changes upon chronic high‐altitude exposure are reversible after more time in the high‐altitude environment and whether the psychological impairments and neuroimaging changes improve or dissipate over time when the subjects leave the high‐altitude environment and return to sea level. We will continue to follow these subjects for another four years in search of these answers.
CONCLUSION
We carried out a panel study on a young healthy high‐altitude immigrant population to assess the cognitive and neuroimaging changes associated with chronic high‐altitude exposure. A series of cognitive functions were identified to be impaired during high‐altitude exposure, such as working memory and psychomotor function. Structural MRI and rs‐fMRI analyses provided direct evidence of GM loss and decreased regional ReHo in the putamen after chronic high‐altitude exposure. Compared with pre‐exposure, the putamen also displayed weakened FC with other cognition‐related brain regions, providing corroborating neuroimaging evidence for the impairment in working memory and psychomotor function. Taken together, the negative impact of chronic high‐altitude exposure on human cognitive function should be considered in the health maintenance of high‐altitude immigrants. A dysfunctional putamen may serve as the structural and functional basis for the neuropsychological impairment associated with chronic high‐altitude exposure.
Competing financial interests: The authors declare they have no actual or potential competing financial interests.
Contributor Information
Hong Yin, Email: yinhong@fmmu.edu.cn.
Jingyuan Chen, Email: jy_chen@fmmu.edu.cn.
Wenjing Luo, Email: luowenj@fmmu.edu.cn.
REFERENCES
- Albin RL, Young AB, Penney JB (1995): The functional anatomy of disorders of the basal ganglia. Trends Neurosci 18:63–64. [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (2000): Voxel‐based morphometry–the methods. NeuroImage 11:805–821. [DOI] [PubMed] [Google Scholar]
- Baier B, Karnath HO, Dieterich M, Birklein F, Heinze C, Muller NG (2010): Keeping memory clear and stable–the contribution of human basal ganglia and prefrontal cortex to working memory. J Neurosci 30:9788–9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao‐Gan Y, Yu‐Feng Z (2010): DPARSF: A MATLAB toolbox for “pipeline” data analysis of resting‐state fMRI. Front Syst Neurosci 4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HJ, Zhu XQ, Yang M, Liu B, Zhang Y, Wang Y, Teng GJ (2012): Changes in the regional homogeneity of resting‐state brain activity in minimal hepatic encephalopathy. Neurosci Lett 507:5–9. [DOI] [PubMed] [Google Scholar]
- Chen J, Fan C, Li J, Han Q, Lin J, Yang T, Zhang J (2016): Increased intraregional synchronized neural activity in adult brain after prolonged adaptation to high‐altitude hypoxia: A resting‐state fMRI study. High Alt Med Biol 17:16–24. [DOI] [PubMed] [Google Scholar]
- Cordova‐Palomera A, Tornador C, Falcon C, Bargallo N, Nenadic I, Deco G, Fananas L (2015): Altered amygdalar resting‐state connectivity in depression is explained by both genes and environment. Hum Brain Mapp 36:3761–3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster GE, Davies‐Thompson J, Dominelli PB, Heran MK, Donnelly J, duManoir GR, Ainslie PN, Rauscher A, Sheel AW (2015): Changes in cerebral vascular reactivity and structure following prolonged exposure to high altitude in humans. Physiol Rep 3:e12647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM (1995): Building action repertoires: Memory and learning functions of the basal ganglia. Curr Opin Neurobiol 5:733–741. [DOI] [PubMed] [Google Scholar]
- Gualtieri CT, Johnson LG (2006): Reliability and validity of a computerized neurocognitive test battery, CNS Vital Signs. Arch Clin Neuropsychol 21:623–643. [DOI] [PubMed] [Google Scholar]
- Guo J, Chen N, Li R, Wu Q, Chen H, Gong Q, He L (2014): Regional homogeneity abnormalities in patients with transient ischaemic attack: A resting‐state fMRI study. Clin Neurophysi 125:520–525. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME, Raichle ME (2001): Searching for a baseline: Functional imaging and the resting human brain. Nat Rev Neurosci 2:685–694. [DOI] [PubMed] [Google Scholar]
- Hellmann‐Regen J, Hinkelmann K, Regen F, McGuire SA, Antonio S (2014): White matter hyperintensities on MRI in high‐altitude U‐2 pilots. Neurology 82:1102–1103. [DOI] [PubMed] [Google Scholar]
- Hochachka PW, Clark CM, Brown WD, Stanley C, Stone CK, Nickles RJ, Zhu GG, Allen PS, Holden JE (1994): The brain at high altitude: Hypometabolism as a defense against chronic hypoxia? J Cereb Blood Flow Metab 14:671–679. [DOI] [PubMed] [Google Scholar]
- Lei Y, Su J, Jiang H, Guo Q, Ni W, Yang H, Gu Y, Mao Y (2017): Aberrant regional homogeneity of resting‐state executive control, default mode, and salience networks in adult patients with moyamoya disease. Brain Imaging Behav 11:176‐184. [DOI] [PubMed] [Google Scholar]
- Levine BD (2015): Going high with heart disease: The effect of high altitude exposure in older individuals and patients with coronary artery disease. High Alt Med Biol 16:89–96. [DOI] [PubMed] [Google Scholar]
- Liang X, Wen J, Ni L, Zhong J, Qi R, Zhang LJ, Lu GM (2013): Altered pattern of spontaneous brain activity in the patients with end‐stage renal disease: A resting‐state functional MRI study with regional homogeneity analysis. PLoS One 8:e71507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H, Mizumura S, Nemoto K, Yamashita F, Imabayashi E, Sato N, Asada T (2012): Automatic voxel‐based morphometry of structural MRI by SPM8 plus diffeomorphic anatomic registration through exponentiated lie algebra improves the diagnosis of probable Alzheimer Disease. AJNR Am J Neuroradiol 33:1109–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire SA, Sherman PM, Brown AC, Robinson AY, Tate DF, Fox PT, Kochunov PV (2012): Hyperintense white matter lesions in 50 high‐altitude pilots with neurologic decompression sickness. Aviat Space Environ Med 83:1117–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire SA, Sherman PM, Wijtenburg SA, Rowland LM, Grogan PM, Sladky JH, Robinson AY, Kochunov PV (2014a): White matter hyperintensities and hypobaric exposure. Ann Neurol 76:719–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire SA, Tate DF, Wood J, Sladky JH, McDonald K, Sherman PM, Kawano ES, Rowland LM, Patel B, Wright SN, Hong E, Rasmussen J, Willis AM, Kochunov PV (2014b): Lower neurocognitive function in U‐2 pilots: Relationship to white matter hyperintensities. Neurology 83:638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ (2002): Learning and memory functions of the Basal Ganglia. Ann Rev Neurosci 25:563–593. [DOI] [PubMed] [Google Scholar]
- Schrouff J, Rosa MJ, Rondina JM, Marquand AF, Chu C, Ashburner J, Phillips C, Richiardi J, Mourao‐Miranda J (2013): PRoNTo: Pattern recognition for neuroimaging toolbox. Neuroinformatics 11:319–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu SY, Song C, Wu Y, Mo L, Guo Z, Liu SH, Bao X (2009): Learning and memory deficits caused by a lesion in the medial area of the left putamen in the human brain. CNS Spectr 14:473–476. [DOI] [PubMed] [Google Scholar]
- Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, He Y, Yan CG, Zang YF (2011): REST: A toolkit for resting‐state functional magnetic resonance imaging data processing. PLoS One 6:e25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virues‐Ortega J, Buela‐Casal G, Garrido E, Alcazar B (2004): Neuropsychological functioning associated with high‐altitude exposure. Neuropsychol Rev 14:197–224. [DOI] [PubMed] [Google Scholar]
- Wang J, Ke T, Zhang X, Chen Y, Liu M, Chen J, Luo W (2013): Effects of acetazolamide on cognitive performance during high‐altitude exposure. Neurotoxicol Teratol 35:28–33. [DOI] [PubMed] [Google Scholar]
- Xia M, Wang J, He Y (2013): BrainNet Viewer: A network visualization tool for human brain connectomics. PLoS One 8:e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue S, Wang X, Wang W, Liu J, Qiu J (2016): Frequency‐dependent alterations in regional homogeneity in major depression. Behav Brain Res 306:13–19. [DOI] [PubMed] [Google Scholar]
- Yan CG, Wang XD, Zuo XN, Zang YF (2016): DPABI: Data processing & analysis for (resting‐state) brain imaging. Neuroinformatics 14:339–351. [DOI] [PubMed] [Google Scholar]
- Yan X (2014): Cognitive impairments at high altitudes and adaptation. High Alt Med Biol 15:141–145. [DOI] [PubMed] [Google Scholar]
- Yan X, Zhang J, Shi J, Gong Q, Weng X (2010): Cerebral and functional adaptation with chronic hypoxia exposure: A multi‐modal MRI study. Brain Res 1348:21–29. [DOI] [PubMed] [Google Scholar]
- Yan X, Zhang J, Gong Q, Weng X (2011): Prolonged high‐altitude residence impacts verbal working memory: An fMRI study. Exp Brain Res 208:437–445. [DOI] [PubMed] [Google Scholar]
- Yoo KM, Shin HK, Chang HM, Caplan LR (1998): Middle cerebral artery occlusive disease: The New England Medical Center Stroke Registry. J Stroke Cerebrovasc Dis 7:344–351. [DOI] [PubMed] [Google Scholar]
- Yu Y, FitzGerald TH, Friston KJ (2013): Working memory and anticipatory set modulate midbrain and putamen activity. J Neurosci 33:14040–14047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ, Fields RD, Johansen‐Berg H (2012): Plasticity in gray and white: Neuroimaging changes in brain structure during learning. Nat Neurosci 15:528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Yan X, Shi J, Gong Q, Weng X, Liu Y (2010): Structural modifications of the brain in acclimatization to high‐altitude. PLoS One 5:e11449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Liu H, Yan X, Weng X (2011): Minimal effects on human memory following long‐term living at moderate altitude. High Alt Med Biol 12:37–43. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhang H, Li J, Chen J, Han Q, Lin J, Yang T, Fan M (2013): Adaptive modulation of adult brain gray and white matter to high altitude: Structural MRI studies. PLoS One 8:e68621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Liu J, Zhang F, Dong X, Peng Y, Qin W, Wu F, Li Y, Yuan K, von Deneen KM, Gong Q, Tang Z, Liang F (2014): Effects of long‐term acupuncture treatment on resting‐state brain activity in migraine patients: A randomized controlled trial on active acupoints and inactive acupoints. PLoS One 9:e99538. [DOI] [PMC free article] [PubMed] [Google Scholar]


