Abstract
Despite over 400 peer‐reviewed structural MRI publications documenting neuroanatomic abnormalities in bipolar disorder and schizophrenia, the confounding effects of head motion and the regional specificity of these defects are unclear. Using a large cohort of individuals scanned on the same research dedicated MRI with broadly similar protocols, we observe reduced cortical thickness indices in both illnesses, though less pronounced in bipolar disorder. While schizophrenia (n = 226) was associated with wide‐spread surface area reductions, bipolar disorder (n = 227) and healthy comparison subjects (n = 370) did not differ. We replicate earlier reports that head motion (estimated from time‐series data) influences surface area and cortical thickness measurements and demonstrate that motion influences a portion, but not all, of the observed between‐group structural differences. Although the effect sizes for these differences were small to medium, when global indices were covaried during vertex‐level analyses, between‐group effects became nonsignificant. This analysis raises doubts about the regional specificity of structural brain changes, possible in contrast to functional changes, in affective and psychotic illnesses as measured with current imaging technology. Given that both schizophrenia and bipolar disorder showed cortical thickness reductions, but only schizophrenia showed surface area changes, and assuming these measures are influenced by at least partially unique sets of biological factors, then our results could indicate some degree of specificity between bipolar disorder and schizophrenia. Hum Brain Mapp 38:3757–3770, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: schizophrenia, bipolar disorder, neuroanatomy, neuroimaging, structural MRI, head motion, neuroanatomic specificity
INTRODUCTION
Structural MRI has been used to document subtle neuroanatomic abnormalities in affective and psychotic disorders in over 440 peer‐reviewed articles. Typically, the goal of these experiments is to identify between‐group differences of specific features from individual images to infer neuronal abnormalities in vivo. Yet, as the practice of psychiatric neuroimaging matures, there are increasing concerns that the field deemphasizes alternative hypotheses, particularly those associated with methodological or biological confounds, in favor of strict pathobiological interpretations [Van Haren et al., 2013; Weinberger and Radulescu, 2016]. In such an environment, the potential for confirmation bias increases, limiting strong inference [Platt, 1964] and reducing the possibility for developing truly informative models of psychopathology [Carpenter et al., 1993]. In this context, we exploit a large, homogeneous structural MRI data set to (1) examine possible effects of in scanner head motion, one of the better studied confounds, and (2) consider the regional specificity of neuroanatomic abnormalities in schizophrenia and bipolar disorder. Our goal is to test these two alternative hypotheses for commonly observed regional brain changes in hopes of spurring a critical review of psychiatric neuroimaging and prompt novel insights into psychopathology.
A myriad of biological and methodological factors could impact interpretation of between‐group structural MRI differences, including, but not limited to hydration [Duning et al., 2005], body mass [Drgon et al., 2010], lithium usage [Cousins et al., 2013], and exercise [Erickson et al., 2011]. However, the influences of head motion on estimating neuroanatomic features is the most thoroughly investigated to date [Alexander‐Bloch et al., 2016; Pardoe et al., 2016; Reuter et al., 2015]. Motion during scan acquisition can lead to image artifacts including shading, blurring, and geometric distortions [Bellon et al., 1986; Morelli et al., 2011; Wood and Henkelman, 1985]. The effects of motion are particularly important for delineating boundaries between gray and white matter in brain [Satterthwaite et al., 2012], as this boundary directly influences derived neuroanatomic measurements of cortical thickness and volume. Reuter et al. [2015] invited 12 healthy subjects to vary their level of head motion during serial acquisitions within the same imaging session. Despite using standard quality control methods to exclude overtly artefactual scans, even minimal head motion was associated with reduced gray matter volume and cortical thickness estimates comparable to annual atrophy rates among individuals with neurodegenerative diseases [Reuter et al., 2015]. Alexander‐Bloch et al. [2016] and Pardoe et al. [2016] independently reported that motion measured during resting state functional MRI time‐series can be used as a proxy for head motion during structural scans. Using this quantitative index, these groups found that even subtle motion leads to reduced cortical thickness estimates in an anatomically heterogeneous pattern: increased cortical thickness in occipital lobe and in the most anterior extent of the prefrontal cortex and decreased thickness almost everywhere else [Alexander‐Bloch et al., 2016; Pardoe et al., 2016]. This pattern was generally consistent across different samples, including children and adults and multiple patient populations, and was relatively independent of image analysis pipelines applied [Alexander‐Bloch et al., 2016; Pardoe et al., 2016].
While head motion is often considered a confound for neuroanatomic and connectivity studies [Power et al., 2012; Satterthwaite et al., 2012; Van Dijk et al., 2012], there is growing evidence that the ability to stay perfectly still for an extended period is a biologically meaningful trait. Head movement appears to be relatively stable over time in adult samples [Couvy‐Duchesne et al., 2014; Van Dijk et al., 2012; Zeng et al., 2014], decreases with development [Satterthwaite et al., 2012], and may be related to behavioral measures of impulsivity [Hodgson et al., 2016; Kong et al., 2014]. Furthermore, head motion is heritable [Couvy‐Duchesne et al., 2014] and genetically correlated with body mass index [Hodgson et al., 2016]. Regardless of its origin, as head motion influences MR‐based neuroanatomic measures and varies between groups, it could bias analyses designed to delineate structural brain changes in affective and psychotic disorders. Regrettably, very few studies have systematically included estimated motion when comparing groups. Thus, it remains unclear how accounting for head motion will influence putative structural MRI differences between individuals with schizophrenia, individuals with bipolar disorder, and healthy comparison subjects.
A second key issue when considering structural MRI changes in bipolar disorder and schizophrenia is the observed pattern of regional neuroanatomic abnormalities. Unfortunately, image analysis pipelines focused on different levels of analyses (e.g., voxel/vertex or region of interest, ROI) tend to highlight dissimilar imaging features [Alexander‐Bloch et al., 2016; Asami et al., 2012; Giuliani et al., 2005], further confusing the importance of individual brain regions for the pathobiology of bipolar disorder and schizophrenia. ROI based meta‐analyses provide evidence for decreased brain size, enlarged ventricles, and reduced hippocampal volume in both illnesses [Arnone et al., 2009; Hibar et al., 2016; McDonald et al., 2004; van Erp et al., 2016; Van Horn and McManus, 1992; Wright et al., 2000]. Ventricular enlargement may simply be the easiest feature to glean from neuroanatomic scans and regional specificity using ROI methods may emerge from large‐scale meta‐analyses using modern analytic methods [Hibar et al., 2016; van Erp et al., 2016]. In contrast to data syntheses based on ROIs, voxel‐based meta‐analyses in schizophrenia report reduced gray matter density relative to control subjects in a distributed network of regions, including bilateral insular/inferior frontal cortex, anterior cingulate gyrus/medial frontal cortex, superior temporal and parahippocampal gyri, postcentral gyrus, thalamus, and amygdala [Bora et al., 2011; Ellison‐Wright and Bullmore, 2010; Ellison‐Wright et al., 2008; Glahn et al., 2008]. Similar meta‐analyses conducted in bipolar disorder observe more localized gray matter density reductions in bilateral insular cortex and anterior cingulate [Bora et al., 2010; Ellison‐Wright and Bullmore, 2010]. Despite the anatomic heterogeneity between these syntheses, specific regional neuroanatomical patterns are the cornerstone for important and compelling neurobiological models of psychotic and affective disorders [e.g., Gaser et al., 2004; Strakowski et al., 2005; Weinberger, 1987]. Yet, the validity of these theoretical models is at least partially dependent upon the certainty that a specific brain region is involved in bipolar disorder or schizophrenia. To date, few imaging studies systematically condition between‐group contrasts on global indices, leaving doubts about the relative importance of a specific region relative to more pervasive brain‐wide changes. Failing to account for global changes could lead to erroneous conclusions about the specific involvement of particular brain regions in the development of the disorder.
To begin to address some of the concerns regarding head motion and regional neuroanatomical specificity, this study analyzed structural MRI scans from a cohort of 823 individuals who participated in studies at the Olin Neuropsychiatric Research Center (ONRC), Institute of Living, Hartford Hospital. We investigate the following hypotheses: (1) a portion of the observed neuroanatomical abnormalities between individuals with schizophrenia or bipolar disorder and comparison subjects is influenced by head motion; and (2) conditioning on global estimates of cortical thickness or surface area, respectively, will fundamentally alter regional abnormalities.
METHODS
Subjects
Volunteers from various research studies conducted at the ONRC were included in the current analysis, including 227 individuals with bipolar I disorder (86% with psychotic symptoms), 226 individuals with schizophrenia or schizoaffective disorder (n = 49), and 370 demographically matched healthy comparison subjects (see Table 1 for demographic information). Patients were recruited in the Hartford area through inpatient services, outpatient clinics, and community mental health services. To be included in the study, patients met diagnostic criteria for either bipolar I disorder or schizophrenia using the Structured Clinical Interview (SCID) for DSM‐IV [Association, 1994]. Comorbid anxiety disorders and/or substance abuse (remitted for at least 6 months prior to this study) diagnoses were allowed to increase representativeness of the samples. Thirty‐six percent of individuals with bipolar disorder and 28% of individuals with schizophrenia/schizoaffective disorder were not taking medications at the time of scanning. More individuals with schizophrenia were prescribed typical (0% bipolar vs. 10% schizophrenia, P = 7.0 × 10−6) and atypical (33 vs. 56%, P = 7.0 × 10−6) antipsychotics than individuals with bipolar disorder. In contrast, more individuals with bipolar disorder were prescribed lithium (13% bipolar vs. 2% schizophrenia, P = 4.0 × 10−6) and mood stabilizes (32 vs. 19%, P = 2.8 × 10−3) relative to individuals with schizophrenia. Patient groups did not differ in terms of prescription rates of antidepressants (38 vs. 34%, P = 4.0 × 10−1), anxiolytics (20 vs. 20%, P = 8.9 × 10−1) or stimulants (3 vs. 3%, P = 7.8 × 10−1) at the time of assessment. On average, individuals with schizophrenia were moderately symptomatic at the time of assessment (PANSS positive score 16.31 ± 5.33 [range 7–38]; PANSS negative score 14.70 ± 5.47 [7–30]). Similarly, individuals with bipolar disorder showed moderate depressive (MADRS 10.29 ± 10.32 [0–43] or HAMD 5.91 ± 6.68 [0–29]), manic (YMRS 4.70 ± 5.86 [0–27]) or psychotic (PANSS positive score = 12.41 ± 4.30 [7–24]) symptoms at the time of scanning.
Table 1.
Sample demographic, motion, and global anatomic measurements
| Bipolar Disorder (BP; n = 227) | Healthy Controls (CT; n = 370) | Schizophrenia (SC; n = 226) | BP vs. CTa | SC vs. CTa | BP vs. SCa | Agea | Sexa | |
|---|---|---|---|---|---|---|---|---|
| % Female | 61% | 57% | 40% | 9.8 × 10−1 | 2.0 × 10−3 | 8.1 × 10−4 | ||
| Age | 35.51 (13) | 33.84 (14) | 36.26 (13) | 8.4 × 10−1 | 3.4 × 10−1 | 1.0 | ||
| % Left Handed | 12% | 8% | 15% | 9.9 × 10−1 | 2.2 × 10−1 | 1.0 | ||
| % African American | 12% | 23% | 18% | 1.7 × 10−1 | 8.7 × 10−1 | 9.7 × 10−1 | ||
| Education | 14.21 (2) | 14.62 (2) | 12.84 (2) | 6.4 × 10−1 | 6.7 × 10−5 | 3.4 × 10−4 | ||
| Estimate Head Motionb, c | 0.282 (0.17) | 0.283 (0.16) | 0.327 (0.24) | 1.0 | 7.7 × 10−2 | 4.7 × 10−1 | 5.0 × 10−4 | 1.0 |
| Global Surface Areac | 1.661 (0.18) | 1.667 (0.19) | 1.635 (0.21) | 1.0 | 5.0 × 10−4 | 5.0 × 10−4 | 5.0 × 10−4 | 5.0 × 10−4 |
| Global Cortical Thicknessc | 2.481 (0.11) | 2.502 (0.13) | 2.452 (0.12) | 2.2 × 10−1 | 1.0 × 10−3 | 8.2 × 10−1 | 5.0 × 10−4 | 1.0 |
P‐value from the statistical test, FWER‐adjusted for the five variables and the six possible pairwise group comparisons for each in the upper part of the table, and for the four different variables and 10 possible comparisons for each in the lower part of the table.
Head motion estimated with frame‐displacement method using time‐series data.
Covarying for age, sex, and MRI sequence.
Healthy comparison subjects were recruited through community advertising and flyers. Eligible comparison subjects did not meet criteria for an Axis I mood or psychotic disorder, as assessed by SCID‐NP, nor did they meet criteria for a current substance misuse disorder (remitted for at least 6 months prior to this study). Finally, healthy comparison subjects did not have a first‐degree relative with bipolar disorder or schizophrenia. Exclusion criteria for all subjects included history of a major medical or neurological condition, including epilepsy, migraine, or head trauma with loss of consciousness; MR contraindications; mental retardation; or inability to consent. All volunteers provided signed informed consent on forms approved by the Hartford Hospital and Yale University institutional review boards.
MRI Acquisition
The same research dedicated Siemens Magnetom Allegra 3T scanner, with the same head coil, was used for all subjects. All subjects received similar structural MRI sequences and identical resting state series. T1‐weighted images were collected using four comparable MPRAGE sequences (see Supporting Information Table S1 for acquisition details). Despite the comparability of these scans, sequence was included as nuisance variable all analyses.
Each subject underwent a resting state functional MRI sequence using identical sequences. Functional images were collected with axial slices parallel to the anterior‐posterior commissure (AC‐PC) using a spin‐echo, echo‐planar sequence (repetition time/echo time = 1500/27 ms, flip angle = 60°, field of view = 22 × 22 cm, acquisition matrix = 64 × 64, voxel size = 3.4 × 3.4 × 4 mm), ensuring whole brain coverage (29 slices/volume, interslice gap = 1 mm). Functional data collection lasted 5.25 min, resulting in 210 volumes. While these data are not directly analyzed in the current manuscript, they provide an index of in‐scanner head motion. Specifically, mean frame‐wise displacement (FD) [Power et al., 2012], was used to index head motion (see Fig. 1A).
Figure 1.
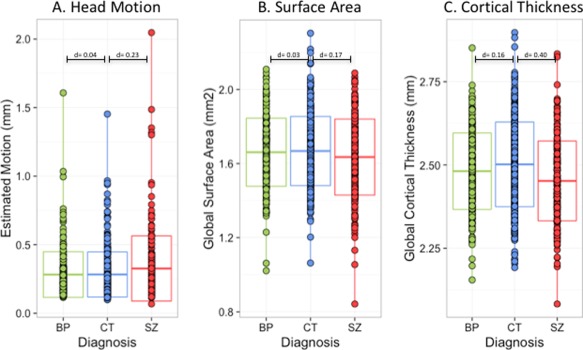
Head Motion, Global Surface Area and Global Cortical Thickness Distributions by Diagnostic Group. Panel A depicts the distribution of head motion, estimated with a frame‐displacement technique during resting state functional MRI, to be used as a proxy for motion during structural scanning. Individuals with schizophrenia (n = 226) had higher levels of head movement when compared with either individuals with bipolar disorder (n = 227) or healthy volunteers (n = 370). Panel B shows the distribution of the average or global surface area across cortex for the three diagnostic groups. Panel C portrays the distribution of global cortical thickness across cortex for the three diagnostic groups. Individuals with schizophrenia had reduced surface area and cortical thickness compared with other groups. [Color figure can be viewed at http://wileyonlinelibrary.com]
Surface‐Based Image Analysis
Cortical surface representations were generated with FreeSurfer 5.3.0 (http://surfer.nmr.mgh.harvard.edu/) [Dale et al., 1999; Fischl et al., 1999]. Briefly, this processing includes alignment and averaging multiple T1‐weighted images, removal of nonbrain tissue, automated Talairach transformation, intensity normalization, segmentation of white matter, tessellation of the gray matter/white matter tissue boundary, automated topology correction, and surface deformation to optimally define the gray/white interface and the pia mater boundary for each subject separately. This method uses both intensity and continuity information from the three‐dimensional image to produce representations of cortical thickness, surface area and cortical volume at sub‐voxel resolution [Reuter et al., 2010]. Maps are created using spatial intensity gradients across tissue classes. To improve matching of homologous regions and signal‐to‐noise ratio, thickness, and area maps were smoothed using a 10 mm full‐width half‐maximum Gaussian kernel. All surface models in our study were visually inspected and, if necessary, corrected for accuracy. Subjects who had excessive pial/white matter surface segmentation errors were excluded.
Cortical thickness was measured as the distance between white and pial surfaces at every vertex [Fischl and Dale, 2000] using a procedure validated against histological analysis [Rosas et al., 2002] and manual measurements [Kuperberg et al., 2003; Salat et al., 2004]. Cortical surface area was measured at each vertex in native geometry, and interpolated to a common grid using a procedure that preserves the amount of area at local, regional, and global levels [Winkler et al., 2012]. Global cortical surface area was calculated as the sum of the areas of all vertices across both hemispheres. Global cortical thickness was calculated as a weighted average, using the corresponding area as the weighting factor.
Statistical Analysis
Between‐group comparisons were modeled at each vertex of the surface‐based representation of the brain using the general linear model, with cortical thickness and surface area as dependent variables, and diagnostic group (healthy comparison, schizophrenia, and bipolar disorder) as independent variables. In all analyses, nuisance variables were age, sex, and image acquisition protocol. Head motion, as estimated via FD, was included as a nuisance variable in indicated analyses. Separate models either including or excluding global average thickness and global cortical surface area were employed; these models serve to two test different hypotheses: one simply about group differences at every vertex, another about group differences at every vertex that are not already explained by an eventual global group difference affecting (on average) the whole cortex. Since the model is fitted separately for each vertex, global effects can be considered regardless of their strength at a given location (see Peelle et al., [2012] for a similar reasoning in the context of voxel‐based morphometry). All the statistical analyses were performed using PALM (Permutation Analysis of Linear Models) [Winkler et al., 2014]. Threshold free cluster enhancement [Smith and Nichols, 2009] was used as the test statistic, and 500 permutations were performed and further enhanced by the fit of a generalized Pareto distribution (GPD) to the tail of the permutation distribution of the maximum statistic [Winkler et al., 2016], from which family wise error‐rate (FWER) adjusted P‐values were obtained. P‐values were considered significant if <0.05, corrected for the multiplicity of vertices across both hemispheres, and corrected for all the six pairwise group comparisons among the three diagnostic groups. To avoid the possibility that conclusions would be driven by imbalance among protocols, side analyzes using only either of the two protocols with the largest sample sizes were also performed; the results of these analyses are shown in the Supporting Information.
The statistical analysis of the non‐vertex data used a similar procedure: Between‐group comparisons of global indices of surface area, cortical thickness, and FD motion estimates were performed using PALM, with diagnostic group (healthy comparison, schizophrenia, and bipolar disorder), age, sex, and image acquisition protocol as independent variables. The number of permutations was set as 1000, with P‐values refined using a GPD. FWER‐correction was performed across all analyses within two sets, one for sample demographics, another for the hypotheses related to the association of the diagnostic categories, age, and sex, with the global imaging indices.
RESULTS
Sample
The schizophrenia sample had fewer females than either the bipolar (P FWER = 8.1 × 10−4) or healthy comparison (P FWER = 2.0 × 10−3) samples, whose sex distribution did not differ (P FWER = 9.8 × 10−1; see Table 1). On average, the sample was 34.97 ± 13.46 years [range 18–65], and age (F 2,820 = 2.54, P FWER = 3.3 × 10−1), handedness (F 2,820 = 2.74, P FWER = 2.8 × 10−1), and proportion of African‐Americans (F 2,820 = 3.10, P FWER = 2.1 × 10−1) did not significantly differ between groups. Educational level was significantly lower for schizophrenia than for bipolar (P FWER = 3.4 × 10−4) and controls (P FWER = 6.7 × 10−5), but not significantly different than between bipolar and control subjects (P FWER = 6.4 × 10−1).
Between Group Head Motion Differences
As described below, head motion was estimated using a frame displacement technique [Power et al., 2012] with time‐series data. Individuals with schizophrenia had higher levels of estimated head motion than healthy comparison subjects (Cohen's d = 0.25; t = 2.78, P FWER = 7.7 × 10−2; see Fig. 1A). These differences persisted when outliers were removed. In contrast, individuals with bipolar disorder and healthy subjects did not differ in terms of motion (d = 0.04, t = 0.46, P FWER = 1.0; Fig. 1A). Estimated motion did not differ between the bipolar and schizophrenia groups (d = 0.20, t = 2.0, P FWER = 4.7 × 10−1). For this and all subsequent analyses, age, sex, and MRI sequence were included as nuisance variables. With regard to the covariates, age (d = 0.02, t = 6.26, P FWER = 5.0 × 10−4) was significantly associated with estimated motion, whereas sex (d = 0.03, t = 0.88, P FWER = 1.0) and acquisition sequence (F 2,820 = 1.43, P FWER = 4.2 × 10−1) were not.
Effects of Motion on Neuroanatomic Measures
At the vertex‐level, across all subjects increased head motion was associated with diffuse decreases in surface area in frontal, temporal, and occipital cortex (see Fig. 2). However, a higher level of motion was not associated with increased surface area. Increased head motion was associated with lower cortical thickness in the frontal, temporal, and parietal cortex, and larger estimated thickness in occipital and anterior prefrontal cortex (Fig. 2). Analyses were repeated for each group separately, resulting in similar patterns of results (Supporting Information Figure S1–S3). These vertex‐level results are consistent with previously published findings [Alexander‐Bloch et al., 2016; Pardoe et al., 2016].
Figure 2.
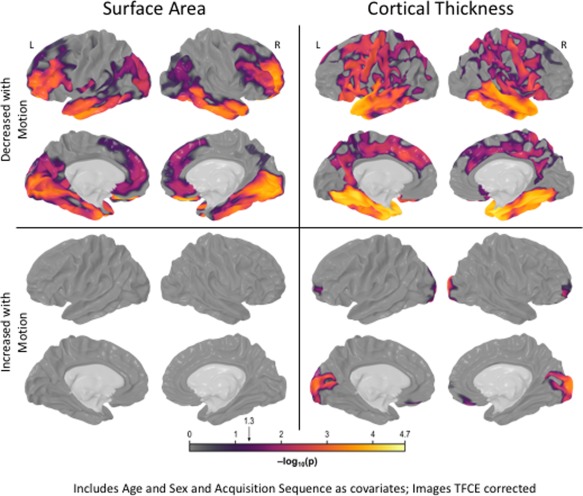
Main Effect of Head Motion on Surface Area and Cortical Thickness Measurments. Across all subjects (n = 823), increased head motion was associated with diffuse decreases in vertex‐level surface area measurements in frontal, temporal, and occipital cortex. Similarly, increased head motion was associated with reduced cortical thickness measurements in frontal, temporal, and parietal cortex, and larger estimated thickness in occipital and anterior prefrontal cortex. Results are strikingly consistent with previously findings. [Color figure can be viewed at http://wileyonlinelibrary.com]
Between Group Surface Area Differences
No significant cortical surface area differences were observed when contrasting individuals with bipolar disorder and healthy comparison subjects (Fig. 3, top row). In contrast, individuals with schizophrenia showed pervasive surface area reductions, particularly in medial and lateral frontal lobe, medial and lateral temporal lobe, and superior parietal cortex when contrasted with healthy subjects (Fig. 3, top row). Differences between individuals with bipolar disorder and those with schizophrenia largely resemble the schizophrenia versus healthy subjects comparison, though somewhat attenuated. Including estimated motion as a covariate increased the significance of regions with a pronounced group difference and attenuated regions with more moderate effects (Fig. 3, middle row).
Figure 3.
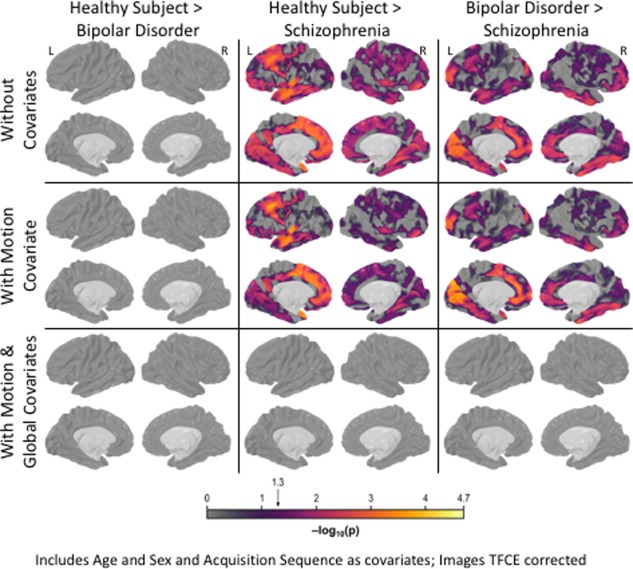
Between‐Group Surface Area Differences. The figure portrays statistically significant between‐group surface area differences for bipolar, schizophrenia and healthy groups. No significant differences were observed when contrasting bipolar disorder and healthy comparison groups, regardless of covariates included. When age, sex and MR sequence were included as covariates, individuals with schizophrenia showed pervasive surface area deficits relative to healthy comparison subjects, particularly in medial and lateral frontal lobes, medial and lateral temporal lobes and superior parietal cortex (top row). These group level differences remained, thought became more concentrated, when estimated head motion was included with other covariates (middle row). However, schizophrenia‐healthy differences were no longer significate when global surface area was included with all prior covariates (bottom row). Differences between bipolar disorder and schizophrenia largely resemble the schizophrenia vs. healthy volunteer contrast, though were somewhat attenuated. [Color figure can be viewed at http://wileyonlinelibrary.com]
Individuals with schizophrenia had lower measured global surface area when compared with healthy (d = 0.42; t = 4.70, P FWER = 5.0 × 10−4; Fig. 1B) and bipolar subjects (d = 0.47; t = 4.62, P FWER = 5.0 × 10−4). Global surface area did not differ between bipolar and healthy subjects (d = 0.05; t = 0.57, P FWER = 1.0; Fig. 1B). When the global surface area index was included as a covariate, no between‐group differences remained statistically significant (Fig. 3, lower row).
Between Group Cortical Thickness Differences
Individuals with bipolar disorder exhibited localized cortical thinning in anterior cingulate and lateral inferior frontal gyrus (Fig. 4, top row). When contrasted with healthy subjects, individuals with schizophrenia showed cortical thickness reductions in limbic, lateral temporal and frontal regions (Fig. 4, top row). Relative to individuals with bipolar disorder, schizophrenia subjects had reduced cortical thickness in a focal region of the most inferior portion of right sensory cortex. Including estimated motion as a covariate increased the significance of regions with a pronounced group difference and attenuated regions with more moderate effects (Fig. 4, middle row).
Figure 4.
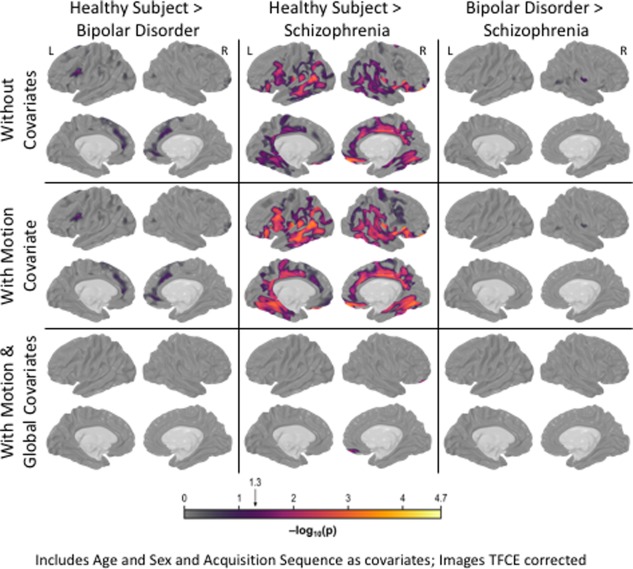
Between‐Group Cortical Thickness Differences. The figure portrays statistically significant between‐group cortical thickness differences for bipolar, schizophrenia and healthy groups. Individuals with bipolar disorder exhibited localized cortical thinning in anterior cingulate and lateral inferior frontal gyrus while covering for age, sex, and MR sequence (top row). Although results did not fundamentally change when motion correction was included with other covariates (middle row), including a global cortical thickness estimate ablated group differences (bottom row). When age, sex, and MR sequence were included as covariates, individuals with schizophrenia showed pervasive cortical thickness deficits relative to healthy comparison subjects, particularly in limbic, lateral temporal and frontal regions (top row). These group‐differences remained, thought became more concentrated, when estimated head motion was included with other covariates (middle row). Including a global cortical thickness covariate (with prior covariates) as small portion of medial orbitofrontal gyrus differed between groups (bottom row). Relative to individuals with bipolar disorder, schizophrenia subjects had reduced cortical thickness in a focal region of the most inferior portion of right sensory cortex (top row). This pattern of results was not fundamentally altered when incorporating the motion correction covariate (middle row), but was ablated when a global index was covaried (bottom row). [Color figure can be viewed at http://wileyonlinelibrary.com]
When compared with healthy volunteers, individuals with schizophrenia had significantly reduced global cortical thickness measures (d = 0.38; t = 4.27, P FWER = 1.0 × 10−3; Fig. 1C). In contrast, global cortical thickness did not differ between bipolar and healthy subjects did not differ (d = 0.22; t = 2.36, P FWER = 2.2 × 10−1; Fig. 1C). Individuals with bipolar disorder and schizophrenia did not differ (d = 0.16; t = 1.56, P FWER = 8.2 × 10−1). When global cortical thickness was included as a covariate, group differences between individuals with bipolar disorder and healthy subjects and between individuals with bipolar disorder and those with schizophrenia were removed (Fig. 4, bottom row). Yet, even with the global covariate, individuals with schizophrenia had reduced cortical thickness in medial orbitofrontal gyrus relative to healthy subjects (Fig. 4, bottom row, middle panel).
Diagnoses by Motion Interaction
To investigate the relationship between diagnosis and estimated motion, vertex level interaction analyses were conducted. No significant effects were observed for surface area for either group. A significant diagnosis by head motion interaction was observed for the most posterior portion of the left medial orbitofrontal gyrus for bipolar disorder that remained significant after conditioning on global cortical thickness (Fig. 5). Examining the relationship between head motion and cortical thickness for each group separately, it appears that while increased motion is associated with increased cortical thickness in this region for bipolar subjects, the opposite relationship is observed for healthy subjects. A significant diagnosis by head motion interaction was observed for schizophrenia (vs. healthy subjects) surrounding the cuneus and a focal region of the superior parietal gyrus, where increased head motion among schizophrenia subjects was associated with increased thickness in these regions while healthy subjects showed thinner cortex with motion. Finally, a significant diagnosis by head motion interaction was observed in left entorhinal cortex and left parahippocampal gyrus for individuals with schizophrenia versus healthy subject.
Figure 5.
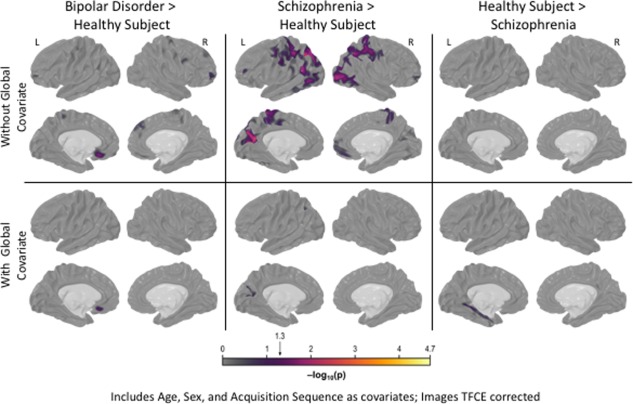
Diagnosis by Head Motion Interaction on Cortical Thickness. The figure depicts statistically significant vertex‐level diagnosis by head motion interactions on cortical thickness measures. An interaction was observed for the most posterior portion of the left medial orbitofrontal gyrus such that increased motion is associated with increased cortical thickness in this region for bipolar subjects, the opposite relationship is observed for healthy subjects (left panel). This interaction remained significant after conditioning on global cortical thickness. A significant diagnosis by head motion interaction was observed for schizophrenia (vs. healthy subjects) surrounding the cuneus and a focal region of the superior parietal gyrus, where increased head motion among schizophrenia subjects was associated with increased thickness in these regions while healthy subjects showed thinner cortex with motion (middle panel). Finally, a significant diagnosis by head motion interaction was observed in left entorhinal cortex and left parahippocampal gyrus for individuals with schizophrenia versus healthy subject (right panel). [Color figure can be viewed at http://wileyonlinelibrary.com]
DISCUSSION
Using a large cohort of individuals scanned on the same research dedicated MRI with broadly similar protocols, we find (1) that head motion influences a portion, but not all, of the putative between‐group structural MRI differences and (2) that between‐group neuroanatomic effects were largely ablated with global surface area or cortical thickness indices were covaried, raising doubts about the regional specificity of structural brain changes in bipolar disorder and schizophrenia.
We replicate earlier reports that head motion influences surface area and cortical thickness measurements [Alexander‐Bloch et al., 2016; Pardoe et al., 2016] and extended these findings by demonstrating that head motion influences the observed between‐group structural differences among individuals with schizophrenia, individuals with bipolar disorder and healthy comparison subjects. To the extent that head motion differs by group (see Fig. 1A), it is not surprising that conditioning on motion has more pronounced effect when comparing individuals with schizophrenia, who tended to move more, compared with either individuals with bipolar disorder or healthy subjects, who tended to move less. Across group contrasts and neuroanatomic measurements (e.g., cortical thickness and surface area), the inclusion of a head motion covariate provides a similar pattern of results. Specifically, areas with a pronounced group difference became more highly significant while those with a more moderate effect became less significant. The inclusion of the head motion covariate appears to improve the certainty around the estimated model parameters rather than the estimates themselves, as the effect size images for these contrasts differed only slightly (see Supporting Information Figure S4 and S5). However, additional work is necessary to determine the accuracy of head motion indices, as even minimal measurement error can influence results [Cochran, 1968], reducing potential gains in incremental validity [Westfall and Yarkoni, 2016].
To further examine the effects of head motion when contrasting psychopathological and comparison samples, we explicitly tested the interaction between these factors. These analyses identified a set of brain regions where anatomic variation (or its measurement) appears to be dependent upon both diagnosis and head motion (see Supporting Information Figure S6). This suggests that either regional anatomical changes associated with diagnosis may render the respective regions more susceptible to movement effects, or that subjects with different diagnoses may move their head in subtly different manners, which on their turn would affect more strongly or weakly different regions during the image acquisition. While there are a number of possible conclusions that could be drawn from these results, among the most conservative is a cautionary note when comparing between group differences in these regions if estimates of head motion are unavailable. Together, these analyses indicate that while head motion clearly influences the measurement of neuroanatomic features in images, cortical thickness and surface area reductions in schizophrenia and cortical thickness reductions in bipolar disorder are not explained by the confounding effects of motion alone. Yet, to the extent that the genetic or neurobiological factors that influence head motion also influence affective or psychotic disorders or closely associated traits (e.g., impulsivity), statistically covarying head motion could bias results by removing important illness‐related variance. As the biological mechanisms that predispose both major mental illness and head motion are largely unknown, determining the extent to which conditioning on head motion biases neuroanatomic results will be difficult to precisely determine.
If differences in T1‐weighted MRI images reflect meaningful neuroanatomic changes in schizophrenia or bipolar disorder, then identifying the location of these alterations is thought to be critical for understanding pathobiology. Unfortunately, there is substantial heterogeneity regarding the regional specificity of MRI‐based neuroanatomic abnormalities in bipolar disorder and schizophrenia, reducing the confidence that any particular region (with the possible exception of enlarged ventricles) is altered in these illnesses. Undoubtedly, some of the heterogeneity in the literature reflects differences in image analyses methods or other technological limitations. However, it is also possible that regional differences are relatively less pronounced (or more difficult to accurately measure) than global abnormalities and that by not adequately controlling for global indices, prior work may have over emphasized neuroanatomic specificity in these illnesses [Elkis et al., 1995; Weinberger et al., 1979]. We found statistically significant global cortical thickness and surface area defects in schizophrenia and lower global cortical thickness in bipolar disorder. Although the effect sizes for these differences are small to medium, when global indices were covaried during vertex‐level analyses, between‐group effects became nonsignificant. These findings raise doubts about the regional specificity of structural brain changes, possible in contrast to functional changes, in affective and psychotic illnesses as measured with current imaging technology. One implication of nonspecific neuroanatomic changes in bipolar disorder and schizophrenia is that the search for genes or other biological mechanisms that predispose the illnesses could have general, rather than regional, influences on brain development or maturation.
Despite concerns about the interpretation of structural MRI alterations in psychopathology, neuroanatomic scans are used to further nosological debate, particularly deliberations about the common or unique factors in schizophrenia and bipolar disorder [Altshuler et al., 1998; Ivleva et al., 2013; Mathew et al., 2014; Wang et al., 2015]. By comparing similar MR scans from relatively large samples of individuals with schizophrenia and bipolar disorder, we found reductions in cortical thickness indices in both illnesses, though these effects were less pronounced in bipolar disorder. In contrast, we found no evidence for surface area differences in bipolar disorder and wide‐spread deficits in schizophrenia. It is notable that the vast majority of the individuals with bipolar disorder in this sample have histories of psychosis, suggesting that these between illness differences may not simply reflect the presence or absence of psychotic symptoms. If MRI‐derived measures of cortical thickness and surface area are influenced by at least partially unique sets of biological factors [Panizzon et al., 2009; Winkler et al., 2010], then our results could indicate molecular specificity between bipolar disorder and schizophrenia. Our results are partially consistent with findings from the Thematically Organized Psychosis Research study, which reported lower cortical thickness [Rimol et al., 2010] and lower surface area [Rimol et al., 2012] measures in bipolar disorder and schizophrenia. The partial overlap of findings could reflect methodological variance, differences in sample size or analytical approach. Yet, given the impact of head motion on estimated neuroanatomic measures and the lack of neuroanatomic specificity in affective and psychotic disorders described above, perhaps the most informative comparison between the current and past reports should focus on global indices of cortical thickness and surface area. Consistently reporting these measures could help to focus this area of scientific experimentation and potentially psychiatric nosology.
A strength of our study is that fact images were cted on the same research dedicated scanner with similar structural sequences and identical resting state series. Further, the sample includes individuals with schizophrenia, individuals with bipolar disorder and healthy volunteers and is relatively large numbers for neuroimaging study, providing sufficient power to detect significant group differences. However, several limitations must also be noted. First, we focused on head motion, one of many possible biological/methodological factors that influence structural MRI analyses. However, the potential number of factors that could differ between groups is substantial (e.g., psychotropic medication, body mass, exercise, rates of smoking, drug use) and each will need to be examined in turn [Weinberger and Radulescu, 2016]. Second, we used a measure of head motion derived during a separate sequence as a proxy for motion during the structural scan. While this technique has been applied previously [Alexander‐Bloch et al., 2016; Pardoe et al., 2016], our index of head motion remains a surrogate. Novel technology for directly indexing head motion during structural scans is being developed and may provide additional insight. Third, we applied only a single image analysis pipeline based upon FreeSurfer. Yet, prior experiments found little difference between surface‐based image analysis pipelines on determining the effect of head motion on neuroanatomic features [Alexander‐Bloch et al., 2016; Pardoe et al., 2016]. Finally, we examined the effects of global surface area and cortical thickness through their inclusion in the linear model, rather than computing ratios between local and global measurements. Such ratios tend to introduce systematic error, whereas covarying these measures is less prone to such confounds [Arndt et al., 1991; Nordenskjold et al., 2015]. Nonetheless, additional work designed to facilitate inference about anatomic specificity in the context of global changes is warranted.
In summary, we examined the association of head motion and global variation on observed neuroanatomic abnormalities in schizophrenia and bipolar disorder. While head motion is significantly associated with cortical thickness and surface area, it does not appear to account for all of the between group differences. By covarying global cortical thickness and surface area measurement, we find little evidence for neuroanatomic specificity in affective and psychotic disorders. Although we are not the first to report the nonspecificity of structural brain changes in these illnesses, our findings are contrary to a substantial body of published reports on structural brain changes in schizophrenia and bipolar disorder [Pearlson and Marsh, 1999; Shenton et al., 2001; Strakowski et al., 2005, 2002]. Indeed, taking into account some of the limitations of our study, our findings are generally consistent with Paul Meehls’ conjecture about the ubiquitous nature of the neuronal deficits in these illnesses, namely “that it is something wrong with every single nerve cell at all levels from the sacral cord to the frontal lobes” [Meehl, 1989].
SUPPORTING INFORMATION
Supplementary Tables and Figures as indicated in the text are available in the online version of this article. In addition, a second, larger set of Supporting Information is available for download at the Dryad Digital Repository, and can be found with the DOI: 10.5061/dryad.t2q53.
AUTHOR CONTRIBUTIONS
DCG is responsible for all data, figures and text. JB, GB, TB, RH, and OL were involved with diagnostic and image data collection, curation and management. AMW and NY were primarily responsible for image analysis. KH was involved in non‐image related statistical analyses. DCG was primarily involved in developing the manuscript and defining the research question. MS, MA, TVE, AMW and GP help frame interpretation and commented on manuscripts. All authors approve the content and submission of the paper.
CONFLICTS OF INTERESTS/FINANCIAL DISCLOSURES
The authors do not have financial arrangements or conflicts of interest to disclose.
Supporting information
Supplement Figure 1
Supplement Figure 2
Supplement Figure 3
Supplement Figure 4
Supplement Figure 5
Supplement Figure 6
Supplement Table
ACKNOWLEDGMENTS
The authors thank the participants for volunteering for research.
Financial support for this study was provided by R01 MH080912 (PI: DC Glahn), R01 MH106324 (DC Glahn), R01 MH077945 (GD Pearlson), R01 AA016599 (GD Pearlson), and R37 MH043775 (GD Pearlson). The Olin Neuropsychiatric Research Center is supported, in part, by gifts from Samourkos foundation and the Gengras family fund. AMW received support from the National Research Council of Brazil (CNPq; 211534/2013‐7).
REFERENCES
- Alexander‐Bloch A, Clasen L, Stockman M, Ronan L, Lalonde F, Giedd J, Raznahan A (2016): Subtle in‐scanner motion biases automated measurement of brain anatomy from in vivo MRI. Hum Brain Mapp 37:2385–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altshuler LL, Bartzokis G, Grieder T, Curran J, Mintz J (1998): Amygdala enlargement in bipolar disorder and hippocampal reduction in schizophrenia: An MRI study demonstrating neuroanatomic specificity. Arch Gen Psychiatry 55:663–664. [DOI] [PubMed] [Google Scholar]
- Arndt S, Cohen G, Alliger RJ, Swayze VW II, Andreasen NC (1991): Problems with ratio and proportion measures of imaged cerebral structures. Psychiatry Res 40:79–89. [DOI] [PubMed] [Google Scholar]
- Arnone D, Cavanagh J, Gerber D, Lawrie S, Ebmeier K, McIntosh A (2009): Magnetic resonance imaging studies in bipolar disorder and schizophrenia: meta‐analysis. Br J Psychiatry 195:194–201. [DOI] [PubMed] [Google Scholar]
- Asami T, Bouix S, Whitford TJ, Shenton ME, Salisbury DF, McCarley RW (2012): Longitudinal loss of gray matter volume in patients with first‐episode schizophrenia: DARTEL automated analysis and ROI validation. Neuroimage 59:986–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association, A.P. (1994) Diagnostic and Statistical Manual of Mental Disorders. Washington, D.C. American Psychiatric Association. [Google Scholar]
- Bellon EM, Haacke EM, Coleman PE, Sacco DC, Steiger DA, Gangarosa RE (1986): MR artifacts: A review. Am J Roentgenol 147:1271–1281. [DOI] [PubMed] [Google Scholar]
- Bora E, Fornito A, Yucel M, Pantelis C (2010): Voxelwise meta‐analysis of gray matter abnormalities in bipolar disorder. Biol Psychiatry 67:1097–1105. [DOI] [PubMed] [Google Scholar]
- Bora E, Fornito A, Radua J, Walterfang M, Seal M, Wood SJ, Yucel M, Velakoulis D, Pantelis C (2011): Neuroanatomical abnormalities in schizophrenia: A multimodal voxelwise meta‐analysis and meta‐regression analysis. Schizophr Res 127:46–57. [DOI] [PubMed] [Google Scholar]
- Carpenter WT, Jr. , Buchanan RW, Kirkpatrick B, Tamminga C, Wood F (1993): Strong inference, theory testing, and the neuroanatomy of schizophrenia. Arch Gen Psychiatry 50:825–831. [DOI] [PubMed] [Google Scholar]
- Cochran WG (1968): Errors of Measurement in Statistics. Technometrics 10:637–666. [Google Scholar]
- Cousins DA, Aribisala B, Nicol Ferrier I, Blamire AM (2013): Lithium, gray matter, and magnetic resonance imaging signal. Biol Psychiatry 73:652–657. [DOI] [PubMed] [Google Scholar]
- Couvy‐Duchesne B, Blokland GA, Hickie IB, Thompson PM, Martin NG, de Zubicaray GI, McMahon KL, Wright MJ (2014): Heritability of head motion during resting state functional MRI in 462 healthy twins. Neuroimage 102: 424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI (1999): Cortical surface‐based analysis. I. Segmentation and surface reconstruction. Neuroimage 9:179–194. [DOI] [PubMed] [Google Scholar]
- Drgon T, Zhang P‐W, Johnson C, Walther D, Hess J, Nino M, Uhl GR (2010): Genome wide association for addiction: Replicated results and comparisons of two analytic approaches. PloS One 5:e8832‐e8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duning T, Kloska S, Steinstrater O, Kugel H, Heindel W, Knecht S (2005): Dehydration confounds the assessment of brain atrophy. Neurology 64:548–550. [DOI] [PubMed] [Google Scholar]
- Elkis H, Friedman L, Wise A, Meltzer HY (1995): Meta‐analyses of studies of ventricular enlargement and cortical sulcal prominence in mood disorders. Comparisons with controls or patients with schizophrenia. Arch Gen Psychiatry 52:735–746. [DOI] [PubMed] [Google Scholar]
- Ellison‐Wright I, Bullmore E (2010): Anatomy of bipolar disorder and schizophrenia: A meta‐analysis. Schizophr Res 117:1–12. [DOI] [PubMed] [Google Scholar]
- Ellison‐Wright I, Glahn D, Laird A, Thelen S, Bullmore E (2008): The anatomy of first‐episode and chronic schizophrenia: An anatomical likelihood estimation meta‐analysis. Am J Psychiatry 165:1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF (2011): Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A 108:3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM (2000): Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A 97:11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM (1999): Cortical surface‐based analysis. II: Inflation, flattening, and a surface‐based coordinate system. Neuroimage 9:195–207. [DOI] [PubMed] [Google Scholar]
- Gaser C, Nenadic I, Volz HP, Buchel C, Sauer H (2004): Neuroanatomy of “hearing voices”: A frontotemporal brain structural abnormality associated with auditory hallucinations in schizophrenia. Cereb Cortex 14:91–96. [DOI] [PubMed] [Google Scholar]
- Giuliani NR, Calhoun VD, Pearlson GD, Francis A, Buchanan RW (2005): Voxel‐based morphometry versus region of interest: A comparison of two methods for analyzing gray matter differences in schizophrenia. Schizophr Res 74:135–147. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Laird AR, Ellison‐Wright I, Thelen SM, Robinson JL, Lancaster JL, Bullmore E, Fox PT (2008): Meta‐analysis of gray matter anomalies in schizophrenia: Application of anatomic likelihood estimation and network analysis. Biol Psychiatry 64:774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibar DP, Westlye LT, van Erp TG, Rasmussen J, Leonardo CD, Faskowitz J, Haukvik UK, Hartberg CB, Doan NT, Agartz I, Dale AM, Gruber O, Kramer B, Trost S, Liberg B, Abe C, Ekman CJ, Ingvar M, Landen M, Fears SC, Freimer NB, Bearden CE; Costa Rica/Colombia Consortium for Genetic Investigation of Bipolar , E, Sprooten E, Glahn DC, Pearlson GD, Emsell L, Kenney J, Scanlon C, McDonald C, Cannon DM, Almeida J, Versace A, Caseras X, Lawrence NS, Phillips ML, Dima D, Delvecchio G, Frangou S, Satterthwaite TD, Wolf D, Houenou J, Henry C, Malt UF, Boen E, Elvsashagen T, Young AH, Lloyd AJ, Goodwin GM, Mackay CE, Bourne C, Bilderbeck A, Abramovic L, Boks MP, van Haren NE, Ophoff RA, Kahn RS, Bauer M, Pfennig A, Alda M, Hajek T, Mwangi B, Soares JC, Nickson T, Dimitrova R, Sussmann JE, Hagenaars S, Whalley HC, McIntosh AM, Thompson PM, Andreassen OA (2016): Subcortical volumetric abnormalities in bipolar disorder. Mol Psychiatry 21:1710–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson K, Poldrack RA, Curran JE, Knowles EE, Mathias S, Goring HH, Yao N, Olvera RL, Fox PT, Almasy L, Duggirala R, Barch DM, Blangero J, Glahn DC (2016): Shared Genetic Factors Influence Head Motion During MRI and Body Mass Index. Cereb Cortex [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivleva EI, Bidesi AS, Keshavan MS, Pearlson GD, Meda SA, Dodig D, Moates AF, Lu H, Francis AN, Tandon N, Schretlen DJ, Sweeney JA, Clementz BA, Tamminga CA (2013): Gray matter volume as an intermediate phenotype for psychosis: Bipolar‐Schizophrenia Network on Intermediate Phenotypes (B‐SNIP). Am J Psychiatry 170:1285–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong XZ, Zhen Z, Li X, Lu HH, Wang R, Liu L, He Y, Zang Y, Liu J (2014): Individual differences in impulsivity predict head motion during magnetic resonance imaging. PLoS One 9:e104989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, Goff D, West WC, Williams SC, van der Kouwe AJ, Salat DH, Dale AM, Fischl B (2003): Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry 60:878–888. [DOI] [PubMed] [Google Scholar]
- Mathew I, Gardin TM, Tandon N, Eack S, Francis AN, Seidman LJ, Clementz B, Pearlson GD, Sweeney JA, Tamminga CA, Keshavan MS (2014): Medial temporal lobe structures and hippocampal subfields in psychotic disorders: findings from the Bipolar‐Schizophrenia Network on Intermediate Phenotypes (B‐SNIP) study. JAMA Psychiatry 71:769–777. [DOI] [PubMed] [Google Scholar]
- McDonald C, Zanelli J, Rabe‐Hesketh S, Ellison‐Wright I, Sham P, Kalidindi S, Murray RM, Kennedy N (2004): Meta‐analysis of magnetic resonance imaging brain morphometry studies in bipolar disorder. Biol Psychiatry 56:411–417. [DOI] [PubMed] [Google Scholar]
- Meehl PE (1989): Schizotaxia revisited. Arch Gen Psychiatry 46:935–944. [DOI] [PubMed] [Google Scholar]
- Morelli JN, Runge VM, Ai F, Attenberger U, Vu L, Schmeets SH, Nitz WR, Kirsch JE (2011): An image‐based approach to understanding the physics of MR artifacts. Radiographics 31:849–866. [DOI] [PubMed] [Google Scholar]
- Nordenskjold R, Malmberg F, Larsson EM, Simmons A, Ahlstrom H, Johansson L, Kullberg J (2015): Intracranial volume normalization methods: Considerations when investigating gender differences in regional brain volume. Psychiatry Res 231:227–235. [DOI] [PubMed] [Google Scholar]
- Panizzon M, Fennema‐Notestine C, Eyler L, Jernigan T, Prom‐Wormley E, Neale M, Jacobson K, Lyons M, Grant M, Franz C, Xian H, Tsuang M, Fischl B, Seidman L, Dale A, Kremen W (2009): Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex 19:2728–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoe HR, Kucharsky Hiess R, Kuzniecky R (2016): Motion and morphometry in clinical and nonclinical populations. Neuroimage 135:177–185. [DOI] [PubMed] [Google Scholar]
- Pearlson GD, Marsh L (1999): Structural brain imaging in schizophrenia: A selective review. Biol Psychiatry 46:627–649. [DOI] [PubMed] [Google Scholar]
- Peelle JE, Cusack R, Henson RN (2012): Adjusting for global effects in voxel‐based morphometry: Gray matter decline in normal aging. Neuroimage 60:1503–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt JR (1964): Strong Inference: Certain systematic methods of scientific thinking may produce much more rapid progress than others. Science 146:347–353. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012): Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Rosas HD, Fischl B (2010): Highly accurate inverse consistent registration: A robust approach. Neuroimage 53:1181–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Tisdall MD, Qureshi A, Buckner RL, van der Kouwe AJ, Fischl B (2015): Head motion during MRI acquisition reduces gray matter volume and thickness estimates. Neuroimage 107:107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimol LM, Hartberg CB, Nesvag R, Fennema‐Notestine C, Hagler DJ Jr., Pung CJ, Jennings RG, Haukvik UK, Lange E, Nakstad PH, Melle I, Andreassen OA, Dale AM, Agartz I (2010): Cortical thickness and subcortical volumes in schizophrenia and bipolar disorder. Biol Psychiatry 68:41–50. [DOI] [PubMed] [Google Scholar]
- Rimol LM, Nesvag R, Hagler DJ Jr., Bergmann O, Fennema‐Notestine C, Hartberg CB, Haukvik UK, Lange E, Pung CJ, Server A, Melle I, Andreassen OA, Agartz I, Dale AM (2012): Cortical volume, surface area, and thickness in schizophrenia and bipolar disorder. Biol Psychiatry 71:552–560. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, van der Kouwe A, Jenkins BG, Dale AM, Fischl B (2002): Regional and progressive thinning of the cortical ribbon in Huntington's disease. Neurology 58:695–701. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Morris JC, Dale AM, Fischl B (2004): Thinning of the cerebral cortex in aging. Cereb Cortex 14:721–730. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Loughead J, Ruparel K, Elliott MA, Hakonarson H, Gur RC, Gur RE (2012): Impact of in‐scanner head motion on multiple measures of functional connectivity: Relevance for studies of neurodevelopment in youth. Neuroimage 60:623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW (2001): A review of MRI findings in schizophrenia. Schizophr Res 49:1–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Nichols TE (2009): Threshold‐free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44:83–98. [DOI] [PubMed] [Google Scholar]
- Strakowski S, Delbello M, Adler C (2005): The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatry 10:105–116. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, Adler CM, DelBello MP (2002): Volumetric MRI studies of mood disorders: do they distinguish unipolar and bipolar disorder? Bipolar Disord 4:80–88. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL (2012): The influence of head motion on intrinsic functional connectivity MRI. Neuroimage 59:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Erp TG, Hibar DP, Rasmussen JM, Glahn DC, Pearlson GD, Andreassen OA, Agartz I, Westlye LT, Haukvik UK, Dale AM, Melle I, Hartberg CB, Gruber O, Kraemer B, Zilles D, Donohoe G, Kelly S, McDonald C, Morris DW, Cannon DM, Corvin A, Machielsen MW, Koenders L, de Haan L, Veltman DJ, Satterthwaite TD, Wolf DH, Gur RC, Gur RE, Potkin SG, Mathalon DH, Mueller BA, Preda A, Macciardi F, Ehrlich S, Walton E, Hass J, Calhoun VD, Bockholt HJ, Sponheim SR, Shoemaker JM, van Haren NE, Hulshoff Pol HE, Ophoff RA, Kahn RS, Roiz‐Santianez R, Crespo‐Facorro B, Wang L, Alpert KI, Jonsson EG, Dimitrova R, Bois C, Whalley HC, McIntosh AM, Lawrie SM, Hashimoto R, Thompson PM, Turner JA (2016): Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry 21:547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haren NE, Cahn W, Hulshoff Pol HE, Kahn RS (2013): Confounders of excessive brain volume loss in schizophrenia. Neurosci Biobehav Rev 37:2418–2423. [DOI] [PubMed] [Google Scholar]
- Van Horn JD, McManus IC (1992): Ventricular enlargement in schizophrenia. A meta‐analysis of studies of the ventricle:brain ratio (VBR). Br J Psychiatry 160:687–697. [DOI] [PubMed] [Google Scholar]
- Wang Z, Meda SA, Keshavan MS, Tamminga CA, Sweeney JA, Clementz BA, Schretlen DJ, Calhoun VD, Lui S, Pearlson GD (2015): Large‐Scale Fusion of Gray Matter and Resting‐State Functional MRI Reveals Common and Distinct Biological Markers across the Psychosis Spectrum in the B‐SNIP Cohort. Front Psychiatry 6:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR (1987): Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry 44:660–669. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Radulescu E (2016): Finding the Elusive Psychiatric “Lesion” With 21st‐Century Neuroanatomy: A Note of Caution. Am J Psychiatry 173:27–33. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Torrey EF, Neophytides AN, Wyatt RJ (1979): Lateral cerebral ventricular enlargement in chronic schizophrenia. Arch Gen Psychiatry 36:735–739. [DOI] [PubMed] [Google Scholar]
- Westfall J, Yarkoni T (2016): Statistically Controlling for Confounding Constructs Is Harder than You Think. PLoS One 11:e0152719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler A, Kochunov P, Blangero J, Almasy L, Zilles K, Fox P, Duggirala R, Glahn D (2010): Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage 53:1135–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Sabuncu MR, Yeo BT, Fischl B, Greve DN, Kochunov P, Nichols TE, Blangero J, Glahn DC (2012): Measuring and comparing brain cortical surface area and other areal quantities. Neuroimage 61:1428–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE (2014): Permutation inference for the general linear model. Neuroimage 92:381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Douaud G, Nichols TE, Smith SM (2016): Faster permutation inference in brain imaging. Neuroimage 141:502–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood ML, Henkelman RM (1985): MR image artifacts from periodic motion. Med Phys 12:143–151. [DOI] [PubMed] [Google Scholar]
- Wright IC, Rabe‐Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET (2000): Meta‐analysis of regional brain volumes in schizophrenia. Am J Psychiatry 157:16–25. [DOI] [PubMed] [Google Scholar]
- Zeng LL, Wang D, Fox MD, Sabuncu M, Hu D, Ge M, Buckner RL, Liu H (2014): Neurobiological basis of head motion in brain imaging. Proc Natl Acad Sci U S A 111:6058–6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Figure 1
Supplement Figure 2
Supplement Figure 3
Supplement Figure 4
Supplement Figure 5
Supplement Figure 6
Supplement Table


