Abstract
Testosterone, a male sex hormone, has been suggested to partly explain mixed findings in males and females when investigating behavioral tendencies associated with the MAOA polymorphism. Prior studies indicated that the MAOA polymorphism represents a vulnerability factor for financial risk‐taking and harm avoidance and that testosterone increases human risk‐taking. We therefore assumed an interactive influence of the MAOA polymorphism and testosterone application on decision making and corresponding neural correlates in a risk and reward context. Stratified for the MAOA polymorphism (S =short, L =long), 103 healthy males were assigned to a placebo or testosterone group (double blind, randomized) receiving a topical gel containing 50 mg testosterone. During a functional MRI scan, the participants performed a sequential decision making task. Our results indicate that testosterone and the MAOA polymorphism jointly influence sequential decision making. The MAOA‐S variant was associated with less automatic harm avoidance as reflected in response times on safe decisions. Moreover, after testosterone administration, MAOA‐S carriers were more risk‐taking. Overall activity in the anterior cingulate cortex, anterior insula and inferior frontal gyrus increased with growing risk for losses. In the anterior insula, testosterone administration mitigated this effect solely in MAOA‐S carriers. This might be a reflection of an improved coping during risk‐reward conflicts subsequently modulating risky decision making. While the molecular basis is not well defined so far, our results support the assumption of testosterone as a modulatory factor for previously reported sex differences of behavioral associations with the MAOA‐S variant. Hum Brain Mapp 38:4574–4593, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: testosterone, monoamine oxidase A polymorphism, risk‐taking, humans, gene‐hormone‐interaction
INTRODUCTION
Human decisions in risky situations are driven mainly by the pursuit of reward or the avoidance of punishment. These motivational drivers have been shown to be influenced by genetic variants [Cesarini et al., 2009, 2010] and hormones, such as testosterone [van Honk et al., 2004]. Testosterone can directly or indirectly influence the transcription rate of a gene or interact with metabolites of the encoded enzyme. Such joint mechanisms potentially modulate human brain activity and thereby influence human behavior. Investigating the role of testosterone interacting with genetic variants might be particularly interesting due to its functioning as male sex hormone. There are several genetic polymorphisms for which sex differences have been reported [Perry et al., 2017]. One famous example is the monoamine oxidase‐A‐linked polymorphism (MAOA LPR), which describes the variable length of a 30‐base repeat sequence in the promoter region of the MAOA gene and encodes mitochondrial enzymes [Hotamisligil and Breakefield, 1991] catalyzing serotonin, dopamine and noradrenalin. In humans, a low MAOA activity in cortical and subcortical regions has been related to high trait aggression [Alia‐Klein et al., 2008]. Interestingly, testosterone modulates human brain activity in similar regions (e.g., amygdala) [Höfer et al., 2013]. An interaction of testosterone and the MAOA LPR thus might be reflected in differential brain activation especially in subcortical regions.
On a behavioral level, both testosterone [Carré et al., 2011; Montoya et al., 2012] and the MAOA LPR [Buckholtz and Meyer‐Lindenberg, 2008; Chester et al., 2015] have been associated with a variety of behavioral tendencies tapping into the concept of aggression and risk‐taking. Concerning the MAOA LPR, these associations have been more consistently established in human males supporting a potential interaction with male sex hormones which has been reported for the investigation of aggression in a male sample [Sjöberg et al., 2008].
Testosterone, a male neuroactive steroid hormone has been suggested as a modulating biological substrate in the context of competition, reward and threat [Bos et al., 2012; Carré and Olmstead, 2015; Wu et al., 2016], social and financial risk‐taking [Eisenegger et al., 2017; Stenstrom and Saad, 2011]. Likewise the neural response to threat and reward is influenced by exogenous testosterone. The ventral striatum and the insula seem to be modulated by monetary rewards depending on individual testosterone increases [Hermans et al., 2010; Macoveanu et al., 2016]. The neurobiological mechanism behind the hyperreactivity in the reward network could be a modification of dopamine neurons via androgens increasing the sensitivity for reward [Montoya et al., 2012]. Furthermore, amygdala activity during threat processing seems to be modulated by testosterone administration [e.g., Goetz et al., 2014; Radke et al., 2015; Volman et al., 2011].
While neuroimaging studies did not investigate the role of testosterone on sequential decision making, behavioral studies support its influence. Applying a sequential risk task, the balloon analogue risk task (BART) [Mehta et al., 2015b] found that in combination with low cortisol levels, high endogenous testosterone levels led to more risky decisions in men. Increasing testosterone levels by an estradiol aromatase inhibitor lead to enhanced sequential risk‐taking in the BART [Goudriaan et al., 2010]. While testosterone might causally influence risk‐taking behavior its influence might vary dependent on additional factors including trait dominance, cortisol levels [Carré et al., 2017; Denson et al., 2013] or genetic variance. In particular, as suggested before [Sjöberg et al., 2008], the genetic variation in the promoter region of the MAOA polymorphism might interact with testosterone which might either directly influence the encoding of the enzyme or interact with its substrates.
Such an interaction might explain heterogeneous findings on the MAOA polymorphism: While MAOA‐S carriers take a higher financial risk if this is advantageous for higher rewards [Frydman et al., 2011] others suggest higher reward dependence and increased novelty seeking for MAOA‐L carriers [Shiraishi et al., 2006]. A postulated shift in reward and threat evaluation in MAOA‐S carriers has been related to perturbed cortico‐limbic activation and connectivity [Buckholtz and Meyer‐Lindenberg, 2008]. Especially increased amygdala and insula activation, for example, during facial threat processing have been reported in contrast to diminished recruitment of prefrontal regulatory regions [Buckholtz and Meyer‐Lindenberg, 2008].
The mechanisms beyond an interaction of testosterone and the MAOA LPR are still speculative. However, potential interactions of MAOA and testosterone have been described in animals [Luine et al., 1975; Redmond et al., 1976] and humans [Shih et al., 2011; Sjöberg et al., 2008]. In primates, high endogenous testosterone levels are associated with low MAO activity, and conversely, decreasing testosterone seems to increase MAO activity [Redmond et al., 1976]. In humans, testosterone levels possibly influence the transcription rate of the MAOA gene via binding at androgen response elements or transcription factors Sp1 and R1 in the promoter region [Ou et al., 2006]. Testosterone [Chaudhari and Nampoothiri, 2016] or its aromatized form estradiol [Belelli et al., 2006; Zheng, 2009] might also interact with the MAOA metabolites such as dopamine, serotonin or norepinephrine. These metabolites potentially modulate human behavior [Eisner et al., 2016; Knutson and Greer, 2008; Morris et al., 2015; Schlüter et al., 2016] and specifically decision making [Hu, 2016; Macoveanu, 2014; McCabe et al., 2010; Schultz, 2010; Varazzani et al., 2015]. Instead of suggesting a specific molecular mechanism, our hypotheses are mainly based on previous findings in the behavioral field suggesting more risky and reward seeking decisions in MAOA‐S carriers and with higher testosterone levels. Moreover, we assume that the joint influence of the MAOA LPR and testosterone on decision making takes place on a level of a functional relationship of cortico‐limbic brain systems. Cortico‐limbic‐striatal regions are strongly involved in the decision making process [Floresco et al., 2008] and are modulated by both the MAOA LPR and testosterone [Buckholtz and Meyer‐Lindenberg, 2008; Höfer et al., 2013]. Thus, differential decision making might be reflected by modulated activation in these brain regions.
For this study, sequential risk‐taking and its underlying neural processing was studied by applying the BART [Lejuez et al., 2002]. In this task, increasing monetary reward for inflating a virtual balloon is coupled with increasing risk for losing the collected earnings when the balloon explodes. While a disadvantage of the task is the missing possibility of disentangling risk avoidance and reward seeking tendencies, prior studies demonstrated that exogenous testosterone influences the balance between risk and reward driven decisions in this task. Such a holistic reward‐risk relationship was assumed to be ideal for eliciting a testosterone‐driven increase in risky behavior.
In addition to the holistic nature of the task, the BART is associated with various real‐world risk‐taking behaviors [Hunt et al., 2005] and has been successfully used before to investigate neural processing of risk‐taking behavior [e.g., Rao et al. 2008; Lighthall et al. 2012; Galván et al. 2013]. The decision process an individual here is faced with, involves frontal control circuits including the dorsal anterior cingulate cortex (dACC) and the reward circuit including the striatum and the ventromedial prefrontal cortex (vmPFC) [Rao et al., 2008; Schonberg et al., 2012]. While activation in the ventral striatum is most consistently implicated during reward processing, the anterior insula specifically seems to process potential losses namely the risk associated with a decision [Knutson and Huettel, 2015]. Therefore, the insula is a primary target in the human brain to investigate harm avoidance or risk aversion [Christopoulos et al., 2009; Fukunaga et al., 2012]. In addition both the ventral striatum and the insula have been associated with altered reward processing after pharmacologic testosterone manipulation [Hermans et al., 2010; Macoveanu et al., 2016].
Applying the BART task in a double‐blind placebo‐controlled study, we aimed to investigate the influence of testosterone administration on risk‐taking and its underlying neural correlates. Assuming an influence of the MAOA‐polymorphism on risk‐taking [e.g., Frydman et al. 2011], the goal of this study was to investigate a potential MAOA‐genotype by testosterone interaction during risk‐taking.
We assumed that depending on the MAOA polymorphism, testosterone administration would lead to more risky decisions (1) and altered response times during decision making (2). Neural processing of sequentially increased risk‐taking was expected to correlate with activity in the decision‐making network and specifically with activity in the striatum and the anterior insula which would be modified by testosterone administration and the MAOA polymorphism (3).
METHODS
Sample
From 105 scheduled male participants recruited via online and paper advertisements, 2 had to be excluded because a final security screening revealed an MR incompatibility (metal). Women were excluded because administration of TestimTM (a testosterone gel) is currently not approved in Germany for females. Moreover, the MAOA polymorphism is located on the X chromosome which impedes the assignment of females to either the MAOA‐S or MAOA‐L group. For one participant the genetic analysis failed, which is why he was excluded. All included participants had normal or corrected vision, no MR contraindications, and no history of traumatic brain injury, psychiatric or neurological illness. Participants with high blood pressure, current or previous prostate disease or any known allergic reactions to the testosterone gel (TestimTM) were also excluded. According to the Edinburgh Handedness Inventory [Oldfield, 1971], all participants were right handed. In order to stratify the sample according to treatment (testosterone, placebo) and genotype (MAOA‐L, MAOA‐S), the genotype was determined prior to study participation starting from subject 70. All participants before were randomly allocated to either the testosterone or the placebo group. The present a priori genotyping design enhances statistical power: Aiming at an equal cell size of participants with a certain genotype naturally occurring more seldom genotypes can be chosen from a large pool of potential participants (see Montag and Reuter [2014]; a recent example for successful implementation of this strategy: [Kunz et al., 2015]). Since the distribution of the genotypes was biased toward more MAOA‐L subjects, group sizes differed slightly. Age did not differ significantly between groups (Table 1).
Table 1.
Personality questionnaires (mean ± SEM) for impulsivity and risk
| MAOA‐L | MAOA‐S | |||||||
|---|---|---|---|---|---|---|---|---|
| PL | T | PL | T | MAOA | T/PL | interaction | ||
| (26) | (28) | (22) | (25) | |||||
| Age | 24.15 ± 3.61 | 24.25 ± 4.23 | 23.73 ± 3.91 | 24.24 ± 3.60 | P= .784 | P= .694 | P= .794 | |
| BISBAS | BAS | 41.71 ± 3.32 | 39.56± 5.58 | 36.71 ± 5.83 | 39.00 ± 5.43 | P= .010 a | P= .948 | P=.039 a |
| Drive | 12.54 ± 1.99 | 12.00 ± 2.27 | 11.10 ± 2.30 | 11.67 ±2.35 | P= .057 | P= .974 | P= .231 | |
| Fun | 12.29 ± 1.71 | 11.32 ± 2.01 | 10.38 ± 2.16 | 11.33 ± 1.49 | P= .015 a | P= .980 | P= .014 a | |
| Reward | 16.88 ± 1.94 | 16.24 ± 2.37 | 15.24 ± 2.32 | 16.00 ± 2.57 | P= .053 | P= .895 | P= .147 | |
| BIS | 19.42 ± 2.70 | 18.28 ± 3.17 | 18.43 ± 2.18 | 20.50 ± 2.86 | P= .285 | P= .417 | P=.006 a | |
| BIS11 | 77.13 ± 11.14 | 79.11 ± 6.70 | 73.26 ± 7.40 | 76.00 ± 6.81 | P= .035a | P= .816 | P=.152 | |
| AQ | AQ Total | 8.69 ± 1.52 | 9.32 ± 1.42 | 8.84 ± 1.58 | 8.70 ± 1.67 | P= .447 | P= .421 | P= .211 |
| Anger | 1.80 ± .48 | 2.05 ± .56 | 1.92 ± .48 | 1.85 ± .53 | P= .677 | P= .383 | P= .113 | |
| Hostility | 2.00 ± .54 | 2.14 ± .52 | 2.04 ± .60 | 1.97 ± .48 | P= .557 | P= .795 | P= .315 | |
| Verbal | 2.81 ± .47 | 2.86 ± .41 | 2.67 ± .47 | 2.67 ± .62 | P= .105 | P= .792 | P= .811 | |
| Physical | 8.69 ± 1.52 | 9.32 ± 1.42 | 8.84 ± 1.58 | 8.70 ± 1.67 | P= .447 | P= .421 | P= .211 | |
Tests that significantly differed between groups are highlighted in bold letters.
The BISBAS scales were only applied to 93 subjects since the questionnaire was not directly available at the start of the data assessment.
Compliance with Ethical Standards
Experimental procedures performed in the current study were in accordance with the Helsinki declaration (1964) and its later amendments or comparable ethical standards and approved by the Ethics Committee of the Medical Faculty of the RWTH Aachen University. All participants included in the study gave oral and written informed consent. They received a fixed financial compensation of 70 Euros for participation. In addition, monetary rewards of the tasks were disbursed (between 15 and 35 Euro depending on the individual performance).
Procedure
The experiment was part of a large study, which included several behavioral and fMRI tasks, for example, Wagels et al. [2017], investigating aggression and risk‐taking behavior. The whole experiment was conducted at one day starting in the early afternoon (between 12.00 and 14.00) and lasting 6–7 h in total. The first part of the experiment included baseline measurements (T1) of mood, cortisol and testosterone blood levels and filling in of several questionnaires (Table 1) to assess individual differences in trait measures related to emotion, impulsivity and aggression (1.5 h). The application of the 5 g TestimTM gel, corresponding to 50 mg testosterone, followed directly after taking the first blood sample. The testosterone gel was applied on the upper part of the participants' back and shoulders in form of a colorless and odorless transdermal cream. The placebo could not be differentiated visually from the testosterone cream and was applied correspondingly, ensuring a double blind procedure. As soon as the cream was absorbed, participants were allowed to dress and carry on with the study procedure as described above. A second blood sample was assessed before the fMRI session (T2: ∼210 min) and two further samples were assessed after each fMRI task (T3: + ∼270 min; T4: + ∼300). Directly before entering the scanner, participants received written task instructions including visual examples. The BART was conducted as second task (and lasted about 25 to 30 min; self‐paced task). Before and after the task, mood levels (Table 1) were assessed via computerized versions of the Positive and Negative Affect Scale (PANAS) [Watson et al. 1988] which could be answered on a 5 point visual analogue scale by shifting a response frame with a fMRI compatible keyboard.
Questionnaire Section
Trait impulsivity was assessed via the Baratt Impulsiveness Scale (BIS11 [Patton et al., 1995]) and approach/avoidance motivation via the Behavioral Inhibition and Activation Scales (BISBAS [Carver and White, 1994]). In addition, we assessed characteristics for trait aggression via the Buss and Perry Aggression Questionnaire (AQ [Buss and Perry, 1992]).
Task
In order to investigate neural correlates of risk‐taking, we adapted the BART [Lejuez et al., 2002] for fMRI (compare Fig. 1). In the task, participants could inflate simulated balloons by pressing a button with their second finger (index‐finger) of their right hand in order to receive a monetary reward (‘inflate event’). Increasing reward simultaneously was linked to increasing risk to lose the accumulated reward within one trial (= one balloon) as the balloon could explode (‘explosion event’). While the explosion point varied between trials, theoretically a balloon could be inflated maximally 12 times before exploding. The probability of explosions and the amount of money which participants could earn was adapted from Rao et al. [2008] and was unknown to participants. Participants could choose to stop the inflation by pressing a key with their middle finger in order to save the accumulated reward of the trial (‘cash‐out event’). The current and the total amount of money were presented as a visual feedback after the decision to cash‐out (‘reward feedback’). In total participants could inflate 33 green balloons. In addition, participants had to inflate orange ‘control balloons’ which would neither explode nor entail a monetary reward (27 balloons in total). Participants in this condition were instructed to inflate the balloon until it disappeared from the screen. The maximal size of the orange balloon varied between trials reflecting the same distribution as the underlying probability structure programmed for the green balloon (maximum 12 pumps). This procedure was adapted from others [Qu et al., 2015; Schonberg et al., 2012]. Please note that despite of a lower number of trials for the orange balloon, the number of events during the green and orange balloon were highly comparable: M (green) = 163.84 ± 17.38; M (orange) = 163.61 ± 18.62, t(90)= .10, p = .921. The important difference between the green and orange balloon was that for green balloons participants had to make a decision, while for the orange balloons only visual and motoric requirements were comparable with the green balloon condition. Participants did not make any decision and were not exposed to any risk or reward in this condition. For the fMRI analysis this condition thus served as high level baseline. The order in which trials were presented was pseudorandomized following the same pattern for each participant. Within one trial (one balloon) the inter‐stimulus interval (black screen between balloons within a trial) was jittered with 1.5–3.5 s. Between trials there was a jittered inter trial interval of 1–12 s presenting a baseline in form of a fixation cross.
Figure 1.
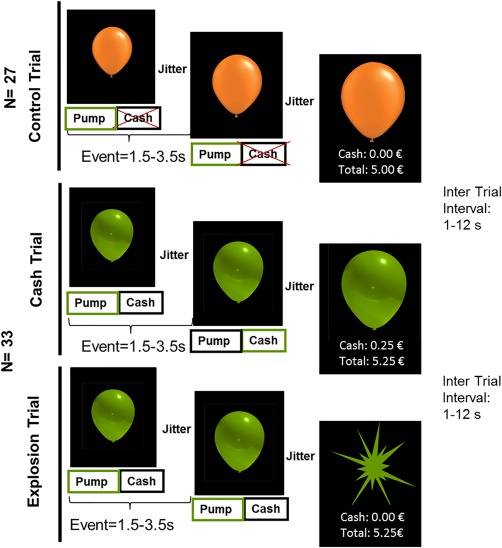
Time course of the paradigm: The upperpart presents control balloons parametrically increasing without risk or reward assignment. Green balloons were experimentally manipulated with a risk/reward assignment and could result in a cash (middle) or explosion (bottom) trial. [Color figure can be viewed at http://wileyonlinelibrary.com]
Since the balloon sizes were coupled with a fixed potential reward and a fixed explosion probability (risk), the same risk and reward values could be assigned to the orange control balloon. However, since they were not actually coupled with a risk or reward, we would not expect an influence of these arbitrary levels, thereby providing an ideal control condition for the risk modulation.
Genotyping
While participants were randomly assigned to placebo or testosterone, they were stratified by the MAOA VNTR polymorphism. Due to a ∼40:60 prevalence of MAOA‐S and MAOA‐L carriers 70 participants took part in the study including the genotyping at the beginning of the measurement. In order to keep a preferably balanced group size of MAOA‐type/treatment group, all further participants had to provide buccal mucosa cell samples prior to participation. DNA from buccal mucosa cell samples was analyzed in a collaborate laboratory (Molecular Psychology, Ulm, Germany). Genomic DNA was extracted from buccal cells using the MagNAPure 96 System and a commercial extraction kit (MagNa Pure 96 DNA Kit; Roche Diagnostics, Mannheim, Germany). Amplification of the MAOA VNTR sequence was performed via polymerase chain reaction PCR (30 s at 95°C, 30 s at 58.4°C, 60 s at 72°C for 30 cycles with an initial denaturation step of 5 min at 95°C and a final elongation step of 5 min at 72°C). PCR products were separated by electrophoresis on a 2% high resolution agarose gel and visualized by ethidium bromide staining. All gel electrophoresis runs were performed with controls of different repeat variants. The repeat numbers of the controls and ambiguous samples were checked by matters of fragment length analysis using FAM‐labeled forward primers.
Opening primer sequences for the 30‐bp VNTR in the promoter region of the MAOA region frame were: forward, 5′ACAGCCTGACCGTGGAGAAG‐3′; and reverse, 5′‐GAACGG ACGCTCCATTCGGA‐3′ [Sabol et al., 1998].
Different to the findings of Sabol et al. [1998], we did not find a 3.5 repeat variant. Instead of the 3.5 (3 repeats + 15bp) we detected a 3a variant (3 repeats + 18 bp) variant consistent with Deckert et al. [1999]. The term ‘3a’ is used in the Deckert et al. publication.
Alleles with repeat sizes 3R (314 bp), 3a R (332 bp) and 4R (344 bp) were included, with the most common being the 3R (314 bp) and 4R (344 bp) alleles.
Hormonal Assessment and Analysis
Testosterone levels were assessed via serum samples at four time points during the study (compare Fig. 2) and analyzed using immunologic in vitro quantitative determination of testosterone (Electrochemiluminescence immunoassay, ECLIA; Roche® Diagnostics GmbH, Mannheim, Germany, http://www.roche-diagnostics.com). Functional sensitivity and specificity of the applied method was 0.087–52.0 nmol/L. Intra‐ and interassay variabilities of testosterone were below 3%.
Figure 2.
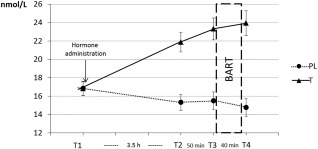
Hormone levels are presented separately for the testosterone (T) and placebo (PL) group. Mean raw levels and standard errors are indicated. Testosterone levels were significantly higher after applying the testosterone gel in the testosterone group (see T2, T3 and T4). The BART was performed between T3 and T4.
A repeated measures ANOVA was performed comparing testosterone levels as a function of time by hormone application and by genotype. The within subject factor time had four levels (T1‐T4) describing the baseline prior hormonal treatment (T1), the serum levels prior to the scanning session (T2), before the BART (T3) and after the BART (T4). Between subject factors were treatment group (testosterone, placebo) and genotype (MAOA‐L, MAOA‐S). In case of a violation of the sphericity assumption Greenhouse Geisser corrections were used. Results for post hoc tests are based on a Bonferroni adjusted alpha level.
Behavioral Analysis
Behavioral data were analyzed via IBM SPSS statistics 21. The behavioral analysis included the measurement of adjusted inflations (successful inflations) and number of explosions, since these measures are the most validated parameters for risk‐taking within the BART [Lejuez et al., 2002]. In order to follow up task characteristics in more detail, the number of successful inflations (the number of inflations in trials that were terminated with a ‘cash out’ and therefore reflected the participants decision for maximal inflations, compare ‘adjusted pumps’ in Lejuez et al. [2002]) and the number of inflations in explosion trials (in which the inflation number was influenced by the programmed structure of the task) were modeled as separate conditions. To calculate group and condition differences, a repeated measures ANOVA with fixed effects was performed including two between group factors (genotype: MAOA‐L vs MAOA‐S, treatment: testosterone versus placebo) and the within‐subject factor condition (cash trials versus explosion trials).
In order to investigate the difficulty to make a decision, response times for cash events and inflate events were compared as parametrically modulated by the risk level. Therefore a mixed model was performed including the repeated measures factor risk level (2–6) and condition (cash versus inflate) as well as the two between group factors (genotype, treatment). Due to the sequential nature of the task, inflation events naturally decreased with increasing risk levels whereas the highest number of cash outs would appear at a medium risk level. In order to ensure a valid number of responses included in the model, response times were only evaluated for risk levels in which more than 80% of the participants chose both inflations and cash outs (risk level 3–6 = explosion probability of 6.3; 14.6; 23.9; 31.3).
Since significant differences regarding impulsivity (BIS11) and activation/inhibition systems (BAS, BIS) were detected, additional analyses were performed. In order to follow up differences in impulsivity and activation/inhibition motivation within genotype groups, their influence on behavior was tested via mediation analyses with the PROCESS tool implemented in SPSS. We tested (a) if the BIS11 would mediate the association of genotype and reaction time during cash outs (b) if the BIS11 would mediate the association of genotype and successful inflations (c) if the BAS would mediate the association of genotype and reaction time during cash outs (d) if the BAS would mediate the association of genotype and successful inflations (e) if the BIS would mediate the association of genotype and reaction time during cash outs (f) if the BIS would mediate the association of genotype and successful inflations (for results, see Supporting Information).
Mood levels (positive and negative affect) were compared in a repeated measure ANOVA (pre, post task) including genotype and treatment group as between subject factors.
fMRI Data Acquisition and Analyses
Imaging data were acquired using a Siemens 3T Prisma scanner (Siemens AG; Erlangen, Germany) equipped with a 12‐channel head matrix coil located in the Department of Psychiatry, Psychotherapy and Psychosomatics, RWTH Aachen University. The BART was performed as the second fMRI paradigm within the study followed by a resting state measurement and an anatomical run. A time series of approximately 830 functional images per participant was acquired, using a spin‐echo EPI sequence with the following acquisition parameters: TR = 2000 ms; TE = 30 ms, flip angle = 77°, FOV = 192 × 192 mm2, matrix size = 64 × 64 mm, 36 slices, slice thickness = 3.1 mm, voxel size = 3 × 3 × 3 mm³, interleaved ascending, slice gap 0.8 mm. Functional scans lasted 25–30 min. Structural scans were acquired using a T1‐weighted MPRAGE sequence with the following acquisition parameters: TR = 2300, TE = 2.98 ms, flip angle = 9°, FOV = 256 × 256 mm, 176 slices, voxel size = 1 mm³, interleaved, distance factor: 50%.
The analysis of the functional imaging data was performed with SPM8 software (http://www.fil.ion.ucl.ac.uk/spm/). The realignment of the time‐series of images followed a two‐pass procedure using the first image (first pass) and the mean image (second pass) as references. Low‐frequency drifts were removed using a high‐pass filter at 128 s. Each anatomical scan was coregistered according to its mean EPI scan and subsequently used to determine spatial normalization parameters by means of the unified segmentation approach. These normalization parameters were applied to the functional scans, thus transforming the time‐series into the standard space defined by the Montreal Neurological Institute (MNI). During normalization, all images were resampled to a voxel size of 2 × 2 × 2 mm³. Afterwards, images were smoothed using an isotropic Gaussian kernel of 8 mm full‐width‐at‐half‐maximum.
Individual time‐series were analyzed (first level) within the framework of the general linear model (GLM). Three main regressors were specified to model the time‐series of each individual. The regressors modelled the decision events including inflate events for the green (experimental) and orange (control) balloon and cash events (only green balloon). For each decision event, a parametric modulator (PM) representing the increasing explosion risk was added similar to Schonberg et al. [2012]. In the control condition, for the orange balloon, only inflate events were modelled. The same parametric modulator was added in this control condition, however, without accounting for the risk/reward factor. This was possible because for the green balloon risk and reward values were collapsed with a specific balloon size. In the orange balloon, the corresponding balloon size was not coupled with the psychological risk or reward factor since participants knew that they would not receive a reward and there was no risk of explosion. In this way, the same values in the orange balloon represented a baseline for measuring brain activity corresponding to increasing balloons without risk and reward context. In addition, the two feedback events were modelled separately including the presentation of the current and total amount of won money (after successful cash outs or an explosion event). Finally, the inter stimulus interval as well as motion parameters were modelled as regressors of no interest. We modelled the interstimulus interval separately, because this likely would capture the uncertainty about the outcome after the decision but not the decision to choose a risky or safe option. The inter trial interval was not modelled as a regressor and, therefore, provided a low level baseline.
For the second level analysis, two separate GLMs were fitted using a full factorial design. Corresponding to the behavioral analyses, both GLMs included two between subject factors (hormone group, genotype). The within subject factor in the first model was risk (PM risk vs PM control). Therefore, the parametric modulation of inflations of the green balloon reflecting the increasing risk and of the orange balloon (control balloon with corresponding risk values of the green balloon at the respective balloon size) was used. The second GLM included the within‐subject factor decision (inflate versus cash). As described above, parametrically modulated brain maps were included for cash out events and inflate events of the green balloon. If not reported otherwise, a threshold of P =.05 corrected for multiple comparisons at voxel level (FWE) was applied.
In addition, small volume correction (SV) was performed in specified regions of interest representing risk processing. Referring to the risk matrix [Knutson and Huettel, 2015], especially the insula and the ventral striatum would be of high interest. These regions were also found in a recent meta‐analysis [Bartra et al., 2013].Creating spheres with a radius of 10 mm, we used the coordinates of the mentioned meta‐analysis that were found for the relatively low subjective value: R anterior insula (x = 40; y =22; z=− 6), L anterior insula (x = 40; y = 22; z = − 6), R ventral striatum (x = 12; y =10; z= − 2), L ventral striatum (x = −12; y =4; z = 2).
Interactions were further disentangled by post hoc tests via extracting the mean activity of the significant cluster using a Matlab function which calculates the mean beta value within a defined ROI (cluster). The function provides a subject specific mean beta value for each condition that was set up in the specific second level model. Within the insula ROI we also formally tested if the insula reactivity in the experimental condition would mediate the association of testosterone and risk‐taking and if this depended on the MAOA LPR. Therefore, a mediated moderation model was set up using model 8 of the PROCESS tool of SPSS (the conceptual model is presented in Fig. 7a). In addition, mean activation of this cluster was correlated with the inflation rate and scores of personality traits (impulsivity, BIS11 and behavioral inhibition/activation, BIS/BAS).
Figure 7.
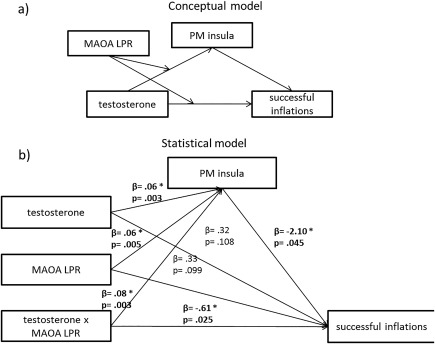
(a) Conceptual model and (b) statistical model of the mediated moderation.
RESULTS
Excluded Participants
In total, 103 participants underwent the described study procedure performing the BART. One participant was excluded since the MAOA‐type could not be determined. The behavioral analysis of the BART task therefore included 102 participants. Serum levels could not be assessed at T3 for 8 participants and at T4 for 12. Consequently, the repeated measures ANOVA comparing testosterone levels only included 88 participants in total. An additional analysis comparing T1 and T2 including all participants showed similar results considering significance and is therefore not reported separately. For the fMRI analysis, 11 participants had to be excluded because of excessive head movement (>5 mm) during the paradigm resulting in the following group sizes: (placebo/MAOA‐L: 25, placebo/MAOA‐S: 18, testosterone/MAOA‐L: 24, testosterone/MAOA‐S: 23). As mentioned in the Methods section, the a priori genotyping design led to comparably equivalent participant numbers in all groups. Results of the behavioral analyses when including only subjects that were included in the fMRI analysis were highly comparable with the results of the whole sample and are therefore not reported separately.
Group Comparisons Trait Characteristics and Mood Levels
We compared various trait characteristics of the groups in order to identify possible additional influential factors. Aggression traits were neither significantly different between MAOA‐L and MAOA‐S carriers nor between testosterone or placebo treated groups. In contrast, trait characteristics of impulsivity (BIS11) and approach‐avoidance motivation (BISBAS) differed between MAOA‐L and MAOA‐S carriers (Table 2). MAOA‐L carriers were more driven by their behavioral activation system (BAS) and they were more impulsive (BIS11) than MAOA‐S carriers. The difference in the activation system mainly emerged in the placebo group (MAOA‐L carriers in the placebo group were activated by ‘Fun’ responsiveness more than any other group).
Table 2.
Explosion risk and actual monetary reward at each inflation level
|
Number of Inflation (n) |
Explosion Risk (%) |
Monetary Reward (Cent) |
|---|---|---|
| 1 | 0 | 0 |
| 2 | 2.1 | 5 |
| 3 | 4.2 | 15 |
| 4 | 6.3 | 25 |
| 5 | 14.6 | 55 |
| 6 | 23.9 | 95 |
| 7 | 31.3 | 145 |
| 8 | 43.8 | 205 |
| 9 | 56.3 | 275 |
| 10 | 68.8 | 345 |
| 11 | 79.2 | 425 |
| 12 | 89.6 | 515 |
Interestingly, the inhibition system was also higher in MAOA‐L carriers in the placebo group and moreover in MAOA‐S carriers in the testosterone group. In order to follow‐up these group differences and their potential indirect effect on the current investigation towards risk‐taking and its neural processing, several additional analyses (mediation and correlation analyses) were performed (see Supporting Information). These analyses unambiguously demonstrated that neither genotype nor treatment effects could be explained by differences in impulsivity or activation/inhibition (BISBAS) motivation.
Mood Levels
Comparisons of positive affect pre and post measurement did not differ significantly. MAOA‐L and MAOA‐S carriers as well as testosterone and placebo group did not differ significantly either. There were no interactions of mood and genotype or treatment group (all ps >.05). Concerning negative affect, there was a significant difference between pre and post measurement, F(1,95) = 81.92, P < .001, np 2 = .463. This effect demonstrated that negative affect was reduced after the BART (M pre = 1.75 ± .066; M post = 1.30 ± .039). All other group effects or interactions were not significant (all Ps > .05).
Hormone Levels
Due to skewed testosterone values, the analysis was performed using log‐transformed values as suggested by others [Sollberger et al., 2016]. As expected, there was a main effect of treatment group, F(1, 86) = 18.60, P = .001, np 2= .178 with higher testosterone levels in the testosterone group. Testosterone levels also significantly differed comparing different time points, F(1.46, 123.45)= 4.71, P=.020, np 2= .052. Detailed characteristics of these two main effects, were overlaid by an interaction of treatment group‐by‐time, F(1.46, 123.45) = 41.54, P < .001, np 2= .326. The interaction demonstrated an increase of testosterone levels in the testosterone group from T1 to T2 and from T2 to T3 (all Ps <.001). T 3 and T4 did not differ significantly in the testosterone group. In the placebo group there was a significant reduction in the testosterone levels from T1 to T2 and from T3 to T4 (all Ps<.05) while T2 and T3 did not differ significantly. Comparing via time, testosterone levels were significantly higher in the testosterone group at T2, T3 and T4 (all Ps<.001), but did not differ from the placebo group at T1 (P =.521), compare Figure 2. There neither was a main effect of the genotype nor an interaction with the treatment group or time. Overall, the analysis of testosterone levels therefore confirmed a successful manipulation of the testosterone level in the testosterone group independent of the genotype.
Behavioral Results
Analyzing the mean number of inflations separately for explosion trials or cash trials (Fig. 3) there was an main effect of condition (F (1,97) = 72.22, P < .001, np 2= .427), with a higher mean in successful cash trials than explosion trials (M cash = 5.75 ± .062; M explosion = 5.27 ± .039). There was no main effect of treatment (F (1,97) = .002, P = .964, np 2< .001) or genotype (F (1,97) = .069, P = .793, np 2= .001) and neither interaction of condition and treatment or condition and genotype (all P > .50). However, the interaction of treatment and genotype was significant, F (1,97) = 6.30, P = .014, np 2= .061. Bonferroni corrected post‐hoc tests demonstrated a significantly higher number of inflations in MAOA‐S carriers than in MAOA‐L carriers in the testosterone group, F (1,97) = 4.05, P = .047, np 2= .040, but not in the placebo group, F (1,97) = 2.40, P = .1245, np 2= .024. Further a significant three way interaction of treatment, genotype and condition emerged, F (1,97) = 8.24, P = .005, np 2= .078. The interaction demonstrated that the different number of inflations of MAOA‐L and MAOA‐S carriers in the testosterone group was driven by the cash trials (successful inflations), F (1,97) = 6.08, P = .015, np 2 = .059 not by explosion trials (computer controlled), F (1,97) = .36, P = .552, np 2 = .004. For MAOA‐S, but not MAOA‐L carriers, the mean number of successful inflations was higher in the testosterone group than in the placebo group, F (1,97) = 5.19, P = .025, np 2=. 051. Moreover, within the testosterone group, the mean number of successful inflations was higher in MAOA‐S carriers than MAOA‐L carriers F (1,97) = 4.19, P = .043, np 2= .041 (Fig. 3).
Figure 3.
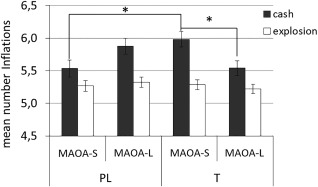
Mean number of successful inflations during cash trials and unsuccessful inflations in explosion trials separately for treatment group (PL, T) and genotype (MAOA‐L, MAOA‐S).
The analysis of response times demonstrated a main effect of the risk level, F (3,86.50) = 4.98, P = .003, and of decision (cash outs, inflations), F (1,93.93) = 15.77, P< .001. In addition, there was an interaction of risk and decision, F (3,87.56) = 15.01, P< .001. While response times were shorter for inflations than cash outs in the lower risk levels (3 and 4, P<.001) there was no significant difference in the response times for the fifth balloon (P = .262) and a trend for longer response times for inflations at risk level 6 (P = .074). Summarizing the results, a characteristic pattern of cash outs and inflations can be described (Fig. 4): For inflations, response times monotonously increased. In contrast, response times for cash outs decreased with increasing risk but did not differ significantly at any risk level
Figure 4.
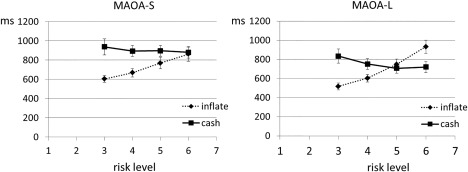
Reaction times (mean ± SEM) for decisions in risk trials are presented with regard to the current risk level separately for MAOA‐S and MAOA‐L carriers.
Neither treatment, F (1, 95.50) = .003, P = .956 nor genotype, F (1,95.50) = 2.40, P = .124 were significantly influencing response times directly. However, there was an interaction of decision and genotype, F (1, 93.93) = 4.55, P = .036. Post‐hoc analyses demonstrated that MAOA‐L and MAOA‐S carriers only differed in cash trials (Fig. 4), F (1, 99.21) = 5.32, P=.023) but not in the inflate trials, F (1, 88.06) =.17, P = .683. In MAOA‐S carriers, response times for cash trials were significantly longer than response times for inflates, F (1, 93.83) = 17.42, P< .001. In MAOA‐L carriers, response times of cash and inflate trials did not differ, F (1, 94.02) =1.816, P< .181.There were no further main effects or interactions.
fMRI Results: Inflations
The parametric modulation of risk in inflate events (similarly performed by Schonberg et al. [2012]) contrasted to the control events showed that bilateral anterior insula, bilateral inferior frontal gyrus, anterior and middle cingulate cortex and the dorsal striatum correlated with increasing risk for inflations (see Table 2, Fig. 5).
Figure 5.
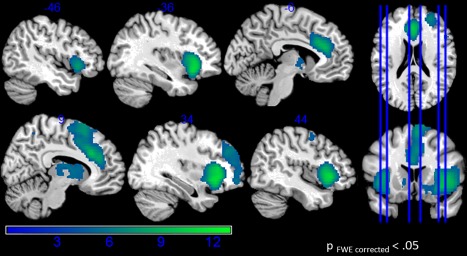
Parametric modulation of the risk level (balloon size) for inflation events for green (risk level) > orange (control level). [Color figure can be viewed at http://wileyonlinelibrary.com]
In contrast, testing the parametrically increasing risk level as a factor between groups revealed no significant effects of treatment or genotype and no interaction at a FWE corrected threshold. Applying a small volume correction for specific regions of interest (anterior insula, striatum) there was a significant cluster in the right anterior insula with a local maximum (k = 29 voxel) at [38 14 −2] testing the interaction of genotype and treatment (F(1,172)= 13.52, P<.05, FWE corrected). Bonferroni adjusted post hoc comparisons of the mean activity (extracted beta values) in this anterior insula cluster (Fig. 6) demonstrated that testosterone administration blunted the risk related activity in MAOA‐S carriers (F(1, 86) = 7.45, P=.008, np 2 = .080) but had no significant influence on MAOA‐L carriers (F(1, 86) = 2.19, P=.142, np 2 = .025). Insula activity in the placebo group was lower for MAOA‐L carriers than for MAOA‐S carriers (F(1, 86) = 9.34, P=.004, np 2 = .091). In contrast, in the testosterone group, there was no significant difference between MAOA‐L and MAOA‐S carriers (F(1, 86) = 2.91, P=.092, np 2 = .033). Post hoc tests also indicated that the effect was driven by the experimental balloon as there was a significant interaction of balloon color, treatment group and genotype (F(1, 86) = 8.84, P=.004, np 2 = .093). This underlined the previously described effects, as MAOA‐L and MAOA‐S carriers only differed in the experimental condition (green balloon, active risk) in the placebo group (F(1, 86) = 9.948, P=.003, np 2 = .099). Likewise, testosterone administration only affected the active risk in MAOA‐S carriers (F(1, 86) = 10.18, P=.002, np 2 = .106).
Figure 6.
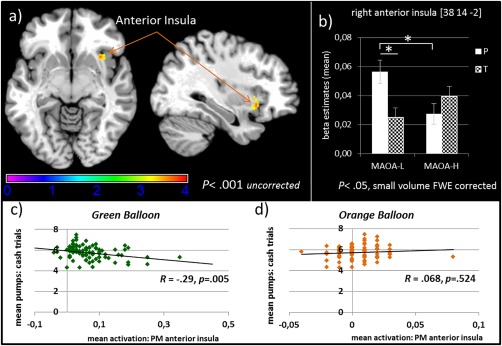
(a) Parametric modulation of the risk level (contrasting risk balloon versus control balloon) shows increased activation in the right anterior insula. Brain activation is presented at an uncorrected threshold of P <.001 for visualization purposes. (b) Beta estimates for treatment and genotype (mean of the significant small volume corrected cluster, k = 29 voxel) in the anterior insula during risk modulated inflations (c) Correlation of the mean number of cash outs with the parametrically modulated insula activation during inflations in the risk balloon (d) and in the control balloon. [Color figure can be viewed at http://wileyonlinelibrary.com]
The full statistical model of the mediated moderation (PROCESS model 8) is shown in Figure 7b. Most interestingly, the highest order interaction was significant (β = ‐.17, CI [‐.438; ‐.003]) demonstrating that the conditional indirect effect of testosterone on risk‐taking was moderated by the MAOA LPR: In MAOA‐S carriers the insula activity significantly mediated the relationship of testosterone administration on the number of inflations in cash trial (β = ‐.13, CI [.001; .337]). However, in MAOA‐L carriers this was not the case (β = ‐.04, CI [‐.137; .021]).
A follow‐up analysis with the extracted mean activation in the anterior insula of the parametric modulation for inflations revealed a negative correlation with the mean number of cash outs in the green balloon (R = ‐.29, P=.005) but not in the orange balloon (R=.068, P=.524). Concerning trait scores for impulsivity and inhibition/approach motivation, no significant correlation with either inflation in the risk condition (green) or in the control condition (orange) emerged (all Ps>.10).
fMRI Result: Decision
The parametric modulation of risk depending on the decision (cash out, inflation) revealed a network of occipital and medial prefrontal regions (Fig. 8). Moreover limbic regions including hippocampus, amygdala and the striatum were activated more strongly with increasing risk levels in cash than inflate events. Contrarily, there was no increased activation for inflate trials contrasted to cash trials modulated by the corresponding risk level. Details are presented in Table 3. After correction for multiple comparisons (FWE), there were no further group effects or interactions.
Figure 8.
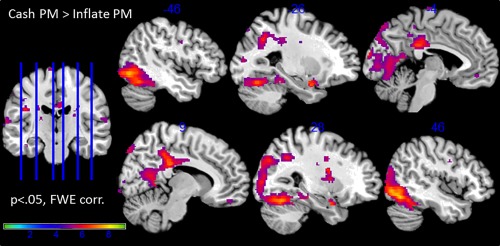
Brain regions that significantly correlated with the risk level in cash > inflate events (corrected for multiple comparisons). [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 3.
MNI coordinates of peak voxels within significant clusters of the parametric modulation of inflations in green (experimental) > orange (control)
| Side | Region | x | y | Z | T | K |
|---|---|---|---|---|---|---|
| R | dorsal ACC | 2 | 26 | 24 | 11.60 | 4426 |
| R | Posterior‐Medial Frontal | 6 | 16 | 58 | 8.74 | |
| R | Superior Frontal Gyrus | 20 | 2 | 72 | 7.56 | |
| R | Anterior Insula | 40 | 24 | −2 | 13.27 | 3374 |
| R | Thalamus | 6 | −14 | 12 | 7.19 | |
| R | Caudate Nucleus | 12 | 10 | −6 | 6.43 | |
| R | Putamen | 18 | 12 | −8 | 5.77 | |
| L | IFG (p. Orbitalis) | −34 | 24 | −10 | 12.20 | 1613 |
| R | Middle Frontal Gyrus | 28 | 48 | 20 | 7.70 | 1380 |
| L | Pallidum | −10 | 6 | −4 | 8.14 | 316 |
| L | Caudate Nucleus | −12 | 4 | 14 | 6.77 | |
| L | Pallidum | −8 | 2 | −6 | 6.63 | |
| L | ventral Striatum | −2 | −4 | −12 | 6.62 | |
| L | Superior Parietal Lobule | −26 | −54 | 60 | 6.24 | 278 |
| L | Precentral Gyrus | −26 | −16 | 60 | 5.89 | 181 |
| R | Precentral Gyrus | 44 | −2 | 50 | 5.62 | 70 |
| R | Precuneus | 12 | −58 | 58 | 5.30 | 42 |
| L | Posterior‐Medial Frontal | −12 | 2 | 46 | 5.27 | 17 |
*All reported clusters are corrected for multiple comparisons (FWE corrected at voxel level threshold, P= .05).
Table 4.
MNI coordinates of peak voxels within significant clusters of the parametric modulation of cash > inflation
| Cluster | Maximum | Region | x | y | z | T | k |
|---|---|---|---|---|---|---|---|
| cash > inflation PM | |||||||
| cluster 1 | 1 | R Fusiform Gyrus | 40 | −62 | −16 | 8.71 | 15572 |
| 2 | L Fusiform Gyrus | −36 | −62 | −20 | 8.63 | ||
| 3 | L Inferior Occipital Gyrus | −48 | −72 | −8 | 8.02 | ||
| 4 | R Inferior Occipital Gyrus | 40 | −76 | −14 | 7.95 | ||
| 5 | R Cerebelum (VI) | 36 | −54 | −24 | 7.70 | ||
| 6 | R Middle Occipital Gyrus | 36 | −90 | 0 | 7.67 | ||
| 7 | R Inferior Temporal Gyrus | 48 | −68 | −8 | 7.57 | ||
| cluster 2 | 1 | L Superior Parietal Lobule | −8 | −84 | 50 | 6.52 | 488 |
| 2 | L Cuneus | −4 | −86 | 36 | 5.49 | ||
| cluster 3 | 1 | L Rolandic Operculum | −62 | 4 | 2 | 6.04 | 320 |
| 2 | L Superior Temporal Gyrus | −66 | −20 | 8 | 5.72 | ||
| cluster 4 | L Precentral Gyrus | −40 | −6 | 32 | 6.06 | 229 | |
| cluster 5 | R Rolandic Operculum | 56 | −10 | 10 | 5.53 | 209 | |
| 2 | R Superior Temporal Gyrus | 66 | −26 | 14 | 5.53 | ||
| cluster 6 | 1 | L Amygdala | −26 | 2 | −24 | 6.76 | 166 |
| 2 | L Putamen | −32 | −8 | −4 | 5.37 | ||
| cluster 7 | R Putamen | 28 | −2 | 8 | 5.91 | 149 | |
| cluster 8 | R SupraMarginal Gyrus | 56 | −28 | 34 | 5.91 | 56 | |
| cluster 9 | R Rectal Gyrus | 8 | 44 | −22 | 5.52 | 46 | |
| cluster 10 | R Amygdala | 26 | 2 | −24 | 7.14 | 46 | |
| cluster 11 | L Mid Orbital Gyrus | −10 | 44 | −12 | 5.29 | 43 | |
| cluster 12 | L Postcentral Gyrus | −56 | −12 | 46 | 5.09 | 40 | |
| cluster 13 | L Paracentral Lobule | −8 | −20 | 78 | 5.47 | 37 | |
| cluster 14 | L Middle Orbital Gyrus | −22 | 36 | −16 | 5.33 | 25 | |
| cluster 15 | R Precentral Gyrus | 50 | 0 | 30 | 5.19 | 16 | |
| cluster 16 | R IFG (p. Triangularis) | 40 | 30 | 14 | 5.38 | 15 | |
| cluster 17 | L Fusiform Gyrus | −38 | −20 | −22 | 5.64 | 15 | |
| cluster 18 | L Fusiform Gyrus | −36 | −10 | −30 | 5.47 | 12 | |
| cluster 19 | L Thalamus | −20 | −20 | 14 | 5.04 | 11 | |
DISCUSSION
The goal of this study was to investigate the interaction of testosterone administration and the MAOA LPR on decision‐making during sequential risk‐taking with increasing rewards; and its neural processing. Confirming our assumption of a potential interaction, MAOA‐S carriers were found to be more risk‐taking/reward seeking when receiving testosterone, but MAOA‐L carriers were not. Complementary, BOLD activity in the anterior insula, a core region of risk processing, was positively related to increasing risk and reward levels, and moreover reflected the gene‐hormone interaction: An attenuated increase during sequentially ascending risk/reward levels was found in MAOA‐S carriers after testosterone administration and in MAOA‐L carriers in the placebo group. This matched with higher risk‐taking in these groups during the BART. An additional finding referred to response times during the decision making process. As expected, response times were longer with increasing risk and reward values when participants decided to inflate the balloon. In contrast, when participants decided to stop the inflation by choosing the safe option, the risk‐reward level did not influence the response time. Most interestingly, the stop response in MAOA‐S carriers was slower, independent of the treatment group, potentially indicating a reduced tendency of harm avoidance. Due to the rather complicated interaction of the MAOA LPR and testosterone administration, we will discuss the findings on a behavioral level first, and in the second part we will outline the potential neural mechanisms. Since this study did not include molecular measures, mechanisms that are underlying the gene‐hormone interaction are only presaged as potential functions and may serve as hypotheses for future studies.
Influence of MAOA and Testosterone on Risky Decision Making
In this study, a pharmacological challenge, known to enhance aggressive responses and risk‐taking [Apicella et al., 2014; Carré et al., 2017; Eisenegger et al., 2017; Stanton et al., 2011; Stenstrom and Saad, 2011], triggered an elevated risk and reward preference in MAOA‐S carries. In contrast to fluctuating hormonal levels, the genetic polymorphism is a temporally stable characteristic and may therefore transport a more general risk attitude. MAOA‐S carriers for instance might be less prone to harm avoidance [Buckholtz et al., 2008] which likely increases the probability of enhanced risk‐taking. In line with others, we observed a genetic influence on personality traits (activation‐inhibition system and impulsivity) like impulsivity or novelty seeking. Our post‐hoc analyses however, do not suggest that these trait differences would explain the differential risk‐taking preferences in the BART. In contrast, although traits for impulsivity and approach tendencies were found to be differently associated with MAOA‐S and MAOA‐L, these personality traits did not correlate with the decision making behavior in the current task. In the current context, impulsivity and approach tendency thus seem to be related to the MAOA polymorphism independently and are thereby a possible separate factor as suggested in model of gene‐environment interactions [Holz et al., 2016]. The genetic variation may influence other personality traits as well or bias learning and attention [Lundwall and Rasmussen, 2016; Ma et al., 2016] which in turn might lead to differential decision strategies in MAOA‐L or MAOA‐S carriers [Frydman et al., 2011]. Nevertheless, one of the most famous findings in research on the MAOA polymorphism is its known interaction with the environment [Beitchman et al., 2004; Meyer‐Lindenberg et al., 2006]. MAOA‐S carriers may thus be more vulnerable for increased risk‐taking—but only under specific conditions.
A specific condition might be a currently elevated level of testosterone. Based on prior findings [Mehta et al., 2015b], we assumed that testosterone could modulate decision making in the context of increasing risk and reward behavior, thereby adding to the genetic vulnerability. A causal influence of testosterone on risk‐taking/reward‐seeking [Goudriaan et al., 2010; van Honk et al., 2004; Mehta et al., 2015b] thus could be salient in the genetically vulnerable group—MAOA‐S carriers. In these persons, the anxiolytic and dominance enhancing potential of testosterone [Mazur and Booth 1998; Terburg and van Honk 2013; Enter et al. 2014] as well as its effect on competitiveness [Eisenegger et al., 2017] might interact with genetic dispositions. Critically however, testosterone administration does not improve profit‐maximization under every condition. During bluff poker, testosterone administration for example led to disadvantageous decisions, if this increased social reputation [van Honk et al., 2016]. Boosted aggressiveness following testosterone application was observed only in dominant males [Carré et al., 2017]. Thus, our findings add to previous studies that find the testosterone effect depending on situational or individual differences. Since testosterone had a stronger effect on MAOA‐S carriers as shown by others [Sjöberg et al., 2008], the genetic polymorphism might transfer an important individual trait.
Each decision a participant made was combined with a potential rewarding outcome and a potential risk of losing the so far collected reward. Thus both a modulated reward seeking behavior as well as a reduced avoidance of potential losses might have influenced participants' decisions. An altered enjoyment of the rewarding outcome as associated with increased testosterone [Mehta et al., 2015a] or a reduced impulse control during risky decisions [Mehta et al., 2015b; Mehta and Beer, 2010] are putative mechanisms. However, as discussed in the following, testosterone administration did not seem to influence impulsive responses as observed via response times. The MAOA‐S carriers moreover were not characterized by reduced prefrontal engagement as associated with low control mechanisms.
Urgency for a Decision: No Automatic Stop Response in MAOA‐S Carriers
In addition to the observed risk‐taking scores, response times before decisions revealed an informative pattern as the time for an inflation or stop decision clearly differed. Naturally, persons who discontinued the inflations would perceive a high risk—if not, they would not have cashed out. As a result, the response time before a cash‐out likely would be independent of the ‘real’ risk level and mirror the need to protect rewards and to avoid a potential loss. In contrast, during inflations constantly increasing response times for higher risk levels might reflect enhanced response conflict, ambivalence and the need for evaluating the option, because the decision becomes more significant.
While testosterone administration itself did not affect response times, MAOA‐L and MAOA‐S carriers differed in their response pattern. Both showed increasing response times for higher risk during inflations, but before a cash‐out, MAOA‐L participants reacted faster. Especially interesting is the observation that response times for cash‐outs and inflations in MAOA‐S carriers seemed to converge, but in MAOA‐L participants, cash‐out decisions were even faster than risky decisions under high risk. This behavioral difference might be an indication for different approaches in the sequential risk task. Faster decisions as observed for MAOA‐L carriers might reflect a more automatic and protective strategy [Sanfey et al., 2006]. Automatic switching towards the safe option thus might indicate an anxiety driven behavior. In mice, low MAOA enzyme activity has been associated with perseverative and less anxiety‐like behaviors [Bortolato et al., 2011]. Concerning humans, the MAOA‐L variant is associated with panic disorder in females [Reif et al., 2014], whereas the MAOA‐S variant is associated with less harm avoidance in males [Buckholtz et al., 2008]. It is plausible to assume that longer response times before cash‐outs reflect the difficulty to change one's behavior in response to punishment cues (large balloon = high explosion risk). MAOA‐S carriers thus seem to be less reactive towards punishment not being driven by an automatic avoidance of high risk. While this might enable MAOA‐S carriers to make more optimal choices when high risk is rewarding [Frydman et al., 2011], it also may impede their decision making process with high risk‐reward conflict. As a result MAOA‐S carriers in those high conflict situations are potentially more vulnerable for additional influencing factors (as, e.g., enhanced testosterone levels).
Neural Processing of Sequential Risk‐Taking
Sequential risk‐taking in the BART elicited activation in large parts of the salience network including anterior insula, anterior cingulate cortex and the striatum, replicating previous findings [Fukunaga et al., 2012; Lighthall et al., 2012; Rao et al., 2008]. Importantly, the study confirms that activity in the salience network and the reward circuit correlates with increasing risk [Macoveanu et al., 2013; Macoveanu et al., 2016; Schonberg et al., 2012] when participants decided on the risky option. Specifically, our findings are in line with a meta‐analysis on anticipated risk processing and risky decision making [Mohr et al., 2010]. As a sequential decision making task, the BART elicited increased activity in the anterior insula and the ACC. Increasing activation with gradient risk found in the anterior insula, ACC, striatum and thalamus may represent the neural signal for a subjective value associated with the current alternatives [Bartra et al., 2013].
Contrasting the risky decision with the stop decision, revealed increasing activity in occipital and temporal structures with ascending risk/reward levels. In particular, limbic structures including bilateral amygdala and hippocampus, the ventral striatum and the medial orbitofrontal cortex were activated more strongly. While the striatum and the medial orbitofrontal cortex may represent the predicted reward value [Diekhof et al., 2012], heightened amygdala activation has been found for safe choices in gain frames (but for risky choices in loss frames) [De Martino et al., 2006]. Amygdala activation in this context has been associated with an emotionally based decision process in contrast to an analytic decision process. Neither testosterone administration nor genotype had a modulatory effect on comparing neural activity related to the individual decisions. Therefore, the ultimate goal of the study was to identify a potential neural process which could explain the behavioral differences observed for risk selection and response time tendencies. Since there were no differences in the decision related contrast, the behavioral differences might be explained rather by a shift in the evaluation of the risk itself as discussed in the following.
The Influence of Testosterone and MAOA Polymorphism on Decision Making
MAOA‐S carriers who received exogenous testosterone were more risk‐taking than MAOA‐S subjects in the placebo group. This interaction was reflected similarly in the neural signal of the anterior insula during risk processing. Under testosterone administration the insula response to increasing risk was blunted in MAOA‐S carriers compared to MAOA‐L carriers. In contrast, in the placebo group, the insula response towards increasing risk levels was mitigated in MAOA‐L carriers compared to MAOA‐S carriers (which would reversely fit the behavioral pattern for risk choices, with the limitation, that the difference in the placebo group was not significant for behavioral data). In other words, participants with higher risk preferences (MAOA‐L/placebo and MAOA‐S/testosterone) would have a blunted neural signal in the anterior insula with increasing riskiness. Actually, the negative association of the mean number of risky decisions with the insula signal during risk processing further underlined this interpretation. Whereas insula activation increased with higher risk levels, for participants that chose to inflate balloons more often this increase was blunted. As a higher number of inflations in the BART has been associated with risk preference [Lejuez et al., 2002], we assume that a high risk preference is reflected by attenuated insula activation likely reflecting a shift from a negative evaluation of risk towards the potential gains. However, this assumption has to be confirmed in future studies since within the current task reward and risk simultaneously increase and a differentiation of the neural signal is not possible.
Previous research shows that the BOLD signal in the anterior insula conveys both individual risk attitudes and current information about uncertainty [Singer et al., 2009]. As part of the salience network, the insula modulates decision behavior by predicting risk and risk prediction error [Bossaerts, 2010; Preuschoff et al., 2008]. During decision making, functions like performance monitoring, error processing as well as the perception of autonomic responses have been assigned to the anterior insula. While the superior anterior insula has been associated mainly with response conflict and uncertainty, the inferior part particularly seems to process post response errors (for an overview, compare Ullsperger et al. [2010]).
Besides processing current information about risk, individual preferences modulate the activity. Loss aversion, a tendency to overweight potential losses compared to gains, has been associated with gray matter volume reductions in the insula repeatedly [Markett et al., 2016, 2014]. In choice tasks, harm avoidance has been associated with elevated insular activity [Paulus et al., 2003; Votinov et al., 2013, 2010] and risk preferences with blunted insula activity [Kuhnen and Knutson, 2005]. Similar, higher insula activity in risk avoiding than risk seeking subjects has been observed during risk prediction indicating an overestimation of risk [Rudorf et al., 2012]. Attenuated insular activity in turn might be a sign for a reduced subjective evaluation of threat, danger and risk. In conclusion, the anterior insula may modulate an individuals' decision‐making via updating current risk information depending on the subjective risk evaluation.
Due to the parallel function of updating current risk information and transferring a dispositional risk attitude, the observed insula activity may be a perfect model for the interaction of testosterone and the MAOA polymorphism. While the genetic variation likely transfers trait characteristics such as a risk preference or aversion, fluctuating hormone levels may influence the evaluation of a current situation. Testosterone for instance has been related to anxiolytic and dominance enhancing effects in animals and humans [Aikey et al., 2002; Terburg and van Honk, 2013]. Moreover, testosterone administration in humans may reduce acute stress responses towards threat [Hermans et al., 2007, 2006].
Two alternative pathways could be imagined here: On the one hand, testosterone may increase the subjective feeling for gains. On the other hand, it may decrease the estimation of losses, thereby shifting the balance between punishment sensitivity and reward dependence [van Honk et al., 2004]. This shift likely underlies the enhanced risk preferences associated with elevated testosterone levels [Carney and Mason, 2010; Eisenegger et al., 2017; Ronay and von Hippel, 2010; Stanton et al., 2011; Stenstrom and Saad, 2011]. Due to the nature of the BART, it is not possible to disentangle risk aversion and reward seeking related to insula activity. A prior study, however, reporting heightened insula activity during risky gambling associated with elevated circulating testosterone levels suggests that the rewarding value might be the driving factor [Macoveanu et al., 2016]. In contrast to this study which investigated the evaluation of potential risk versus reward, Macoveanu and colleagues investigated the processing of the outcome. Assuming, that testosterone primarily shifts the balance of risk and reward processing, both heightened insula activation during reward processing and attenuated insula responsiveness during risk evaluation might reflect a behavioral imbalance at the brain level. Even though an imbalance in both direction is reasonable, the here observed pattern of the insula reflects the behavioral pattern of increased risk‐taking in MAOA‐S carriers after testosterone administration as similarly reported by others [Paulus et al., 2003]. Divergent behavioral and neural findings may by result of non‐directed shifts in such a disturbed risk‐reward balance as observed via modified reward‐related brain activity after testosterone administration [Hermans et al., 2010]. Besides form possible contextual influences that have also been observed by others [Radke et al., 2015], the genetic make‐up might influence the effect testosterone has, both on a behavioral and neural level. Future studies might demonstrate if the here observed finding is specific for the risk or reward evaluation preceding a decision or if the MAOA LPR also influences outcome processing resulting in inverse activation patterns.
Modulation of Dopamine, Noradrenalin and Serotonin as Potential Mechanisms
To date, a biological model explaining the mechanism of the MAOA polymorphism on human behavior and neural signaling is still missing. Notably however, major substrates of MAOA are serotonin, dopamine and norepinephrine which have been shown to interact with testosterone [Lewis and Dluzen, 2008; Macoveanu et al., 2016; Morris et al., 2015; Purves‐Tyson et al., 2014]. Interestingly, catecholamines are suggested as potential neuromodulators of insula activity [Knutson and Greer, 2008] and thereby might influence human decision making. During decision making, neurotransmitters have been proposed to operate on different levels [Doya, 2008] modulating reward expectation (dopamine), the urgency for direct reward (serotonin) and exploration during uncertain situations (norepinephrine). Here, the enhanced decision for risky but potentially rewarding outcomes in MAOA‐S carriers after testosterone administration could be the result of such modulated neurotransmitter levels.
One mechanism that has been discussed in the context of aggression, fear and anxiety is the imbalance of serotonin and testosterone (or estradiol) modulating behavior [Birger et al., 2003; Montoya et al., 2012; Pavlov et al., 2012; Rosell and Siever, 2015]. Imaging studies mainly emphasize the role of the amygdala and its connectivity with the prefrontal cortex as a neural correlate of these brain processes [Radke et al., 2015; Rosell and Siever, 2015; Summers et al., 2000]. However, as discussed earlier, during outcome processing after rewarding choices increased testosterone levels have been related to enhanced insular activity [Macoveanu et al., 2016].
Testosterone as Explanatory Variable for Sex Differences
This study contributes to previous suggestions of testosterone being a mediating factor for the inconsistent findings across males and females associating the MAOA LPR with impulsive and aggressive behaviors [Åslund et al., 2011; Perry et al., 2017]. In general, both activational and organizational effects of testosterone may interact with the MAOA LPR mediating such sex differences [Arnold, 2009]. Organizational effects are related to permanent structural changes in the brain that are induced by testosterone during early development (e.g., prenatal) and might interact with the genetic make‐up. However, the pharmacological administration enabled us to study activational effects of circulating testosterone. Since in adult males the testosterone level is more than 10 times higher than in adult females, circulating testosterone levels could be a source target for sex differences. Inversely, applying testosterone in females might dissolve these sex differences. Moreover, estradiol, the aromatized form of testosterone and the main female sex hormone could transfer these differences via interactions with the MAOA enzyme or its substrates [Kranz et al., 2015; Zheng, 2009].
As a side note, it should be added that the MAOA polymorphism is X linked and thereby has two copies in females but only one in males. Furthermore, the sex determining region Y (SRY), an exclusively male factor, seems to activate the MAOA promoter and enzymatic activity [Shih et al., 2011; Wu et al., 2009]. Finally, sex differences on the molecular level might stem from different methylation of the MAOA promoter as suggested by others [Philibert et al., 2008]. Due to many possible influential factors investigating males and females would be optimal and is needed for further conclusions.
LIMITATIONS AND STRENGTHS
The current findings were obtained within a selective, homogenous sample of healthy young men. Investigating such a homogenous group allowed us to control for additional influences, thereby ensuring an improved interpretation. In light of the sex and age‐related differences in hormones, it remains to be investigated whether the current results can be replicated in female or older samples. Moreover, while observing effects on behavior and BOLD activity, we cannot draw firm conclusions about mechanisms of MAOA substrates which likely mediate brain activity. Therefore, the current findings underline the importance for further studies on the biochemical process underlying risk processing in dependence of the genetic variation. Finally, the sequential risk task does not allow for a specific interpretation of different processes regarding reward and risk processing. While the holistic nature of the task provided an improved capture of real life processes [Lejuez et al., 2002], it would also be interesting to investigate if testosterone has a selective influence on either risk or reward perception.
CONCLUSIONS
This study demonstrated a gene‐hormone interaction both on a behavioral and neural level on risk‐taking in young men. MAOA‐S carriers showed attenuated automatic avoidance tendencies as reflected via response times on cash‐outs. Actually, that did not directly affect the decision itself. While under placebo MAOA‐S and MAOA‐L carriers did not differ concerning their riskiness during the BART, testosterone administration promoted risk‐taking in MAOA‐S carriers. Interestingly, the BOLD signal in the anterior insula monitored during sequential risk processing reflected this behavioral pattern. While higher risk levels were associated with increased insula activity, this signal was blunted in participants that were more risk‐taking. Likely, this reflects decreased arousal or stress under uncertainty, leading to a reduced avoidance behavior or promoting the decision for the risk option. Our results underline the role of automatic tendencies, which might have a genetic origin but likely are modulated by situational factors, as hormones. Importantly, the risk promoting effect of testosterone likewise might be evident only in participants that do not show automatic harm avoidance. Considering that norepinephrine and dopamine, two major substrates of MAOA, may drive risk‐related activity in the anterior insula, these neurotransmitters may mediate the gene‐hormone interaction. Future studies are needed to test the underlying neurochemical processes of this gene‐hormone interaction.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
We thank the laboratory (Labordiagnostisches Zentrum, LDZ, Aachen) for blood serum analyses and Dr. Hansen for his consultation. In addition, we want to thank the pharmacy of the University Hospital RWTH Aachen, especially Ms. Grise, for providing placebo gel and blinding and coding the tubes for the double‐blind study setting. For their assistance during measurement, we also want to thank Laura Westerhoff and Despina Panagiotidis. For the programming of the fMRI task, we want to thank Paul Mols founded by the IZKF Aachen, Brian imaging facility. Christian Montag is funded by a Heisenberg grant awarded to him by the German Research Foundation (DFG, MO2363/3–1). The funding sources had no role in study design, in collection, analysis, and interpretation of data, in the writing of the report, and in the decision to submit the article for publication. There are no conflicts of interest.
REFERENCES
- Aikey JL, Nyby JG, Anmuth DM, James PJ (2002): Testosterone rapidly reduces anxiety in male house mice (Mus musculus). Horm Behav 42:448–460. [DOI] [PubMed] [Google Scholar]
- Alia‐Klein N, Goldstein RZ, Kriplani A, Logan J, Tomasi D, Williams B, Telang F, Shumay E, Biegon A, Craig IW, Henn F, Wang G‐J, Volkow ND, Fowler JS (2008): Brain monoamine oxidase A activity predicts trait aggression. J Neurosci 28:5099–5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella CL, Dreber A, Mollerstrom J (2014): Salivary testosterone change following monetary wins and losses predicts future financial risk‐taking. Psychoneuroendocrinology 39:58–64. [DOI] [PubMed] [Google Scholar]
- Arnold AP (2009): The organizational–activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm Behav 55:570–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Åslund C, Nordquist N, Comasco E, Leppert J, Oreland L, Nilsson KW (2011): Maltreatment, MAOA, and delinquency: sex differences in gene–environment interaction in a large population‐based cohort of adolescents. Behav Genet 41:262–272. [DOI] [PubMed] [Google Scholar]
- Bartra O, McGuire JT, Kable JW (2013): The valuation system: A coordinate‐based meta‐analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage 76:412–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitchman JH, Mik HM, Ehtesham S, Douglas L, Kennedy JL (2004): MAOA and persistent, pervasive childhood aggression. Mol Psychiatry 9:546–547. [DOI] [PubMed] [Google Scholar]
- Belelli D, Herd MB, Mitchell EA, Peden DR, Vardy AW, Gentet L, Lambert JJ (2006): Neuroactive steroids and inhibitory neurotransmission: Mechanisms of action and physiological relevance. Neuroscience 138:821–829. [DOI] [PubMed] [Google Scholar]
- Birger M, Swartz M, Cohen D, Alesh Y, Grishpan C, Kotelr M (2003): Aggression: the testosterone‐serotonin link. Isr Med Assoc J 5:653–658. [PubMed] [Google Scholar]
- Bortolato M, Chen K, Godar SC, Chen G, Wu W, Rebrin I, Farrell MR, Scott AL, Wellman CL, Shih JC (2011): Social deficits and perseverative behaviors, but not overt aggression, in MAO‐A hypomorphic mice. Neuropsychopharmacology 36:2674–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos PA, Panksepp J, Bluthé R‐M, Honk J. v (2012): Acute effects of steroid hormones and neuropeptides on human social–emotional behavior: A review of single administration studies. Front Neuroendocrinol 33:17–35. [DOI] [PubMed] [Google Scholar]
- Bossaerts P (2010): Risk and risk prediction error signals in anterior insula. Brain Struct Funct 214:645–653. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Callicott JH, Kolachana B, Hariri AR, Goldberg TE, Genderson M, Egan MF, Mattay VS, Weinberger DR, Meyer‐Lindenberg A (2008): Genetic variation in MAOA modulates ventromedial prefrontal circuitry mediating individual differences in human personality. Mol Psychiatry 13:313–324. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Meyer‐Lindenberg A (2008): MAOA and the neurogenetic architecture of human aggression. Trends Neurosci 31:120–129. [DOI] [PubMed] [Google Scholar]
- Buss AH, Perry M (1992): The aggression questionnaire. J Pers Soc Psychol 63:452–459. [DOI] [PubMed] [Google Scholar]
- Carney DR, Mason MF (2010): Decision making and testosterone: When the ends justify the means. J Exp Soc Psychol 46:668–671. [Google Scholar]
- Carré JM, Geniole SN, Ortiz TL, Bird BM, Videto A, Bonin PL (2017): Exogenous testosterone rapidly increases aggressive behavior in dominant and impulsive men. Biol Psychiatry 82:249–256. [DOI] [PubMed] [Google Scholar]
- Carré JM, McCormick CM, Hariri AR (2011): The social neuroendocrinology of human aggression. Psychoneuroendocrinology 36:935–944. [DOI] [PubMed] [Google Scholar]
- Carré JM, Olmstead NA (2015): Social neuroendocrinology of human aggression: Examining the role of competition‐induced testosterone dynamics. Neuroscience 286C:171–186. [DOI] [PubMed] [Google Scholar]
- Carver CS, White TL (1994): Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. J Pers Soc Psychol 67:319–333. [Google Scholar]
- Cesarini D, Dawes CT, Johannesson M, Lichtenstein P, Wallace B (2009): Genetic Variation in Preferences for Giving and Risk Taking. MIT Press. [Google Scholar]
- Cesarini D, Johanneson M, Lichtenstein P, Sandewall Ö, Wallace B (2010): Genetic variation in financial decision‐making. J Finance 65:1725–1754. [Google Scholar]
- Chaudhari NK, Nampoothiri LP (2016): Neurotransmitter alteration in a testosterone propionate‐induced polycystic ovarian syndrome rat model. Horm Mol Biol Clin Investig. http://www.degruyter.com/view/j/hmbci.ahead-of-print/hmbci-2016-0035/hmbci-2016-0035.xml [DOI] [PubMed] [Google Scholar]
- Chester DS, DeWall CN, Derefinko KJ, Estus S, Peters JR, Lynam DR, Jiang Y (2015): Monoamine oxidase A (MAOA) genotype predicts greater aggression through impulsive reactivity to negative affect. Behav Brain Res 283:97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopoulos GI, Tobler PN, Bossaerts P, Dolan RJ, Schultz W (2009): Neural correlates of value, risk, and risk aversion contributing to decision making under risk. J Neurosci 29:12574–12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martino B, Kumaran D, Seymour B, Dolan RJ (2006): Frames, biases, and rational decision‐making in the human brain. Science 313:(80‐) 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckert J, Catalano M, Syagailo YV, Bosi M, Okladnova O, Bella Di D, NMM , Maffei P, Franke P, Fritze J, Maier W, Propping P, Beckmann H, Bellodi L, Lesch KP (1999): Excess of high activity monoamine oxidase A gene promoter alleles in female patients with panic disorder. Hum Mol Genet 8:621–624. [DOI] [PubMed] [Google Scholar]
- Denson TF, Mehta PH, Ho Tan D (2013): Endogenous testosterone and cortisol jointly influence reactive aggression in women. Psychoneuroendocrinology 38:416–424. [DOI] [PubMed] [Google Scholar]
- Diekhof EK, Kaps L, Falkai P, Gruber O (2012): The role of the human ventral striatum and the medial orbitofrontal cortex in the representation of reward magnitude – An activation likelihood estimation meta‐analysis of neuroimaging studies of passive reward expectancy and outcome processing. Neuropsychologia 50:1252–1266. [DOI] [PubMed] [Google Scholar]
- Doya K (2008): Modulators of decision making. Nat Neurosci 11:410–416. [DOI] [PubMed] [Google Scholar]
- Eisenegger C, Kumsta R, Naef M, Gromoll J, Heinrichs M (2017): Testosterone and androgen receptor gene polymorphism are associated with confidence and competitiveness in men. Horm Behav. 92:93–102. [DOI] [PubMed] [Google Scholar]
- Eisner P, Klasen M, Wolf D, Zerres K, Eggermann T, Eisert A, Zvyagintsev M, Sarkheil P, Mathiak KA, Zepf F, Mathiak K (2016): Cortico‐limbic connectivity in MAOA ‐L carriers is vulnerable to acute tryptophan depletion. Hum Brain Mapp 38:1622–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enter D, Spinhoven P, Roelofs K (2014): Alleviating social avoidance: effects of single dose testosterone administration on approach‐avoidance action. Horm Behav 65:351–354. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Onge JR, St Ghods‐Sharifi S, Winstanley CA (2008): Cortico‐limbic‐striatal circuits subserving different forms of cost‐benefit decision making. Cogn Affect Behav Neurosci 8:375–389. [DOI] [PubMed] [Google Scholar]
- Frydman C, Camerer C, Bossaerts P, Rangel A (2011): MAOA‐L carriers are better at making optimal financial decisions under risk. Proc Biol Sci 278:2053–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga R, Brown JW, Bogg T (2012): Decision making in the Balloon Analogue Risk Task (BART): anterior cingulate cortex signals loss aversion but not the infrequency of risky choices. Cogn Affect Behav Neurosci 12:479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galván A, Schonberg T, Mumford J, Kohno M, Poldrack RA, London ED (2013): Greater risk sensitivity of dorsolateral prefrontal cortex in young smokers than in nonsmokers. Psychopharmacology (Berl) 229:345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz SMM, Tang L, Thomason ME, Diamond MP, Hariri AR, Carré JM (2014): Testosterone rapidly increases neural reactivity to threat in healthy men: a novel two‐step pharmacological challenge paradigm. Biol Psychiatry 76:324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudriaan AE, Lapauw B, Ruige J, Feyen E, Kaufman J‐M, Brand M, Vingerhoets G (2010): The influence of high‐normal testosterone levels on risk‐taking in healthy males in a 1‐week letrozole administration study. Psychoneuroendocrinology 35:1416–1421. [DOI] [PubMed] [Google Scholar]
- Hermans EJ, Bos PA, Ossewaarde L, Ramsey NF, Fernández G, van Honk J (2010): Effects of exogenous testosterone on the ventral striatal BOLD response during reward anticipation in healthy women. Neuroimage 52:277–283. [DOI] [PubMed] [Google Scholar]
- Hermans EJ, Putman P, Baas JM, Gecks NM, Kenemans JL, van Honk J (2007): Exogenous testosterone attenuates the integrated central stress response in healthy young women. Psychoneuroendocrinology 32:1052–1061. [DOI] [PubMed] [Google Scholar]
- Hermans EJ, Putman P, Baas JM, Koppeschaar HP, van Honk J (2006): A single administration of testosterone reduces fear‐potentiated startle in humans. Biol Psychiatry 59:872–874. [DOI] [PubMed] [Google Scholar]
- Höfer P, Lanzenberger R, Kasper S (2013): Testosterone in the brain: Neuroimaging findings and the potential role for neuropsychopharmacology. Eur Neuropsychopharmacol 23:79–88. [DOI] [PubMed] [Google Scholar]
- Holz N, Boecker R, Buchmann AF, Blomeyer D, Baumeister S, Hohmann S, Jennen‐Steinmetz C, Wolf I, Rietschel M, Witt SH, Plichta MM, Meyer‐Lindenberg A, Schmidt MH, Esser G, Banaschewski T, Brandeis D, Laucht M (2016): Evidence for a sex‐dependent MAOA× childhood stress interaction in the neural circuitry of aggression. Cereb Cortex 26:904–914. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Breakefield XO (1991): Human monoamine oxidase A gene determines levels of enzyme activity. Am J Hum Genet 49:383–392. [PMC free article] [PubMed] [Google Scholar]
- Hu H (2016): Reward and aversion. Annu Rev Neurosci 39:297–324. [DOI] [PubMed] [Google Scholar]
- Hunt MK, Hopko DR, Bare R, Lejuez CW, Robinson EV (2005): Construct validity of the balloon analog risk task (BART): associations with psychopathy and impulsivity. Assessment 12:416–428. [DOI] [PubMed] [Google Scholar]
- Knutson B, Greer SM (2008): Anticipatory affect: neural correlates and consequences for choice. Philos Trans R Soc London B Biol Sci 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Huettel SA (2015): The risk matrix. Curr Opin Behav Sci 5:141–146. [Google Scholar]
- Kranz GS, Wadsak W, Kaufmann U, Savli M, Baldinger P, Gryglewski G, Haeusler D, Spies M, Mitterhauser M, Kasper S, Lanzenberger R (2015): High‐dose testosterone treatment increases serotonin transporter binding in transgender people. Biol Psychiatry 78:525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnen CM, Knutson B (2005): The neural basis of financial risk taking. Neuron 47:763–770. [DOI] [PubMed] [Google Scholar]
- Kunz L, Schröder TN, Lee H, Montag C, Lachmann B, Sariyska R, Reuter M, Stirnberg R, Stöcker T, Messing‐Floeter PC, Fell J, Doeller CF, Axmacher N (2015): Reduced grid‐cell–like representations in adults at genetic risk for Alzheimer's disease. Science 80:350. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, Strong DR, Brown RA (2002): Evaluation of a behavioral measure of risk taking: The Balloon Analogue Risk Task (BART). J Exp Psychol Appl 8:75–84. [DOI] [PubMed] [Google Scholar]
- Lewis C, Dluzen DE (2008): Testosterone enhances dopamine depletion by methamphetamine in male, but not female, mice. Neuroscience Letters Vol. 448: [DOI] [PubMed] [Google Scholar]
- Lighthall NR, Sakaki M, Vasunilashorn S, Nga L, Somayajula S, Chen EY, Samii N, Mather M (2012): Gender differences in reward‐related decision processing under stress. Soc Cogn Affect Neurosci 7:476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN, Khylchevskaya RI, McEwen BS (1975): Effect of gonadal steroids on activities of monoamine oxidase and choline acetylase in rat brain. Brain Res 86:293–306. [DOI] [PubMed] [Google Scholar]
- Lundwall RA, Rasmussen CG (2016): MAOA influences the trajectory of attentional development. Front Hum Neurosci 10:424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma R, Jia H, Yi F, Ming Q, Wang X, Gao Y, Yi J, Yao S (2016): Electrophysiological responses of feedback processing are modulated by MAOA genotype in healthy male adolescents. Neurosci Lett 610:144–149. [DOI] [PubMed] [Google Scholar]
- Macoveanu J (2014): Serotonergic modulation of reward and punishment: Evidence from pharmacological fMRI studies. Brain Res 1556:19–27. [DOI] [PubMed] [Google Scholar]
- Macoveanu J, Henningsson S, Pinborg A, Jensen P, Knudsen GM, Frokjaer VG, Siebner HR (2016): Sex‐steroid hormone manipulation reduces brain response to reward. Neuropsychopharmacology 41:1057–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macoveanu J, Rowe JB, Hornboll B, Elliott R, Paulson OB, Knudsen GM, Siebner HR (2013): Serotonin 2A receptors contribute to the regulation of risk‐averse decisions. Neuroimage 83:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markett S, Heeren G, Montag C, Weber B, Reuter M (2016): Loss aversion is associated with bilateral insula volume. A voxel based morphometry study. Neurosci Lett 619:172–176. [DOI] [PubMed] [Google Scholar]
- Markett S, Voigt G, Montag C, Weber B, Reuter M (2014): Individual differences in during decision making under uncertainty: Loss aversion covaries with insular grey matter volume. Pers Individ Dif 60. [Google Scholar]
- Mazur A, Booth A (1998): Testosterone and dominance in men. Behav Brain Sci 21:353–363. [PubMed] [Google Scholar]
- McCabe C, Mishor Z, Cowen PJ, Harmer CJ (2010): Diminished neural processing of aversive and rewarding stimuli during selective serotonin reuptake inhibitor treatment. Biol Psychiatry 67:439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P, Welker KM, Zilioli S, Carré JM (2015b): Testosterone and cortisol jointly modulate risk‐taking. Psychoneuroendocrinology 56:88–99. [DOI] [PubMed] [Google Scholar]
- Mehta PH, Beer J (2010): Neural mechanisms of the testosterone–aggression relation: the role of orbitofrontal cortex. J Cogn Neurosci 22:2357–2368. [DOI] [PubMed] [Google Scholar]
- Mehta PH, Snyder NA, Knight EL, Lassetter B (2015a): Close versus decisive victory moderates the effect of testosterone change on competitive decisions and task enjoyment. Adapt Hum Behav Physiol 1:291–311. [Google Scholar]
- Meyer‐Lindenberg A, Buckholtz JW, Kolachana B, R Hariri A, Pezawas L, Blasi G, Wabnitz A, Honea R, Verchinski B, Callicott JH, Egan M, Mattay V, Weinberger DR (2006): Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc Natl Acad Sci U S A 103:6269–6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr PNC, Biele G, Heekeren HR (2010): Neural processing of risk. J Neurosci 30:6613–6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montag C, Reuter M (2014): Disentangling the molecular genetic basis of personality: From monoamines to neuropeptides. Neurosci Biobehav Rev 43:228–239. [DOI] [PubMed] [Google Scholar]
- Montoya ER, Terburg D, Bos PA, van Honk J (2012): Testosterone, cortisol, and serotonin as key regulators of social aggression: A review and theoretical perspective. Motiv Emot 36:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RW, Purves‐Tyson TD, Weickert CS, Rothmond D, Lenroot R, Weickert TW (2015): Testosterone and reward prediction‐errors in healthy men and men with schizophrenia. Schizophr Res 168:649–660. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Ou X‐M, Chen K, Shih JC (2006): Glucocorticoid and androgen activation of monoamine oxidase A is regulated differently by R1 and Sp1. J Biol Chem 281:21512–21525. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES (1995): Factor structure of the barratt impulsiveness scale. J Clin Psychol 51:768–774. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB (2003): Increased activation in the right insula during risk‐taking decision making is related to harm avoidance and neuroticism. Neuroimage 19:1439–1448. [DOI] [PubMed] [Google Scholar]
- Pavlov KA, Chistiakov DA, Chekhonin VP (2012): Genetic determinants of aggression and impulsivity in humans. J Appl Genet 53:61–82. [DOI] [PubMed] [Google Scholar]
- Perry LM, Goldstein‐Piekarski AN, Williams LM (2017): Sex differences modulating serotonergic polymorphisms implicated in the mechanistic pathways of risk for depression and related disorders. J Neurosci Res 95:737–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philibert RA, Gunter TD, Beach SRH, Brody GH, Madan A (2008): MAOA methylation is associated with nicotine and alcohol dependence in women. Am J Med Genet Part B Neuropsychiatr Genet 147B:565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuschoff K, Quartz SR, Bossaerts P (2008): Human insula activation reflects risk prediction errors as well as risk. J Neurosci 28:2745–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves‐Tyson TD, Owens SJ, Double KL, Desai R, Handelsman DJ, Weickert CS (2014): Testosterone induces molecular changes in dopamine signaling pathway molecules in the adolescent male rat nigrostriatal pathway. Ed. Joohyung Lee. PLoS One 9:e91151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, Galvan A, Fuligni AJ, Lieberman MD, Telzer EH (2015): Longitudinal changes in prefrontal cortex activation underlie declines in adolescent risk taking. J Neurosci 35:11308–11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke S, Volman I, Mehta P, van Son V, Enter D, Sanfey A, Toni I, de Bruijn ERA, Roelofs K (2015): Testosterone biases the amygdala toward social threat approach. Sci Adv 1:e1400074–e1400074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao H, Korczykowski M, Pluta J, Hoang A, Detre JA (2008): Neural correlates of voluntary and involuntary risk taking in the human brain: an fMRI Study of the Balloon Analog Risk Task (BART). Neuroimage 42:902–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond EDJ, Baulu J, Murphy DL, Loriaux LD, Zeigler MG, Lake RC (1976): The effects of testosterone on plasma and platelet monoamine oxidase (MAO) and plasma dopamine‐[beta]‐hydroxylase (DBH) activities in the male rhesus monkey. J Biobehav Med 38:315–326. [DOI] [PubMed] [Google Scholar]
- Reif A, Richter J, Straube B, Höfler M, Lueken U, Gloster AT, Weber H, Domschke K, Fehm L, Ströhle A, Jansen A, Gerlach A, Pyka M, Reinhardt I, Konrad C, Wittmann A, Pfleiderer B, Alpers GW, Pauli P, Lang T, Arolt V, Wittchen H‐U, Hamm A, Kircher T, Deckert J (2014): MAOA and mechanisms of panic disorder revisited: from bench to molecular psychotherapy. Mol Psychiatry 19:122–128. [DOI] [PubMed] [Google Scholar]
- Ronay R, von Hippel W (2010): Power, testosterone, and risk‐taking. J Behav Decis Mak 23:473–482. [Google Scholar]
- Rosell DR, Siever LJ (2015): The neurobiology of aggression and violence. CNS Spectr 20:254–279. [DOI] [PubMed] [Google Scholar]
- Rudorf S, Preuschoff K, Weber B (2012): Neural correlates of anticipation risk reflect risk preferences. J Neurosci 32:16683–16692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabol SZ, Hu S, Hamer D (1998): A functional polymorphism in the monoamine oxidase A gene promoter. Hum Genet 103:273–279. [DOI] [PubMed] [Google Scholar]
- Sanfey AG, Loewenstein G, McClure SM, Cohen JD (2006): Neuroeconomics: cross‐currents in research on decision‐making. Trends Cogn Sci 10:108–116. [DOI] [PubMed] [Google Scholar]
- Schlüter T, Winz O, Henkel K, Eggermann T, Mohammadkhani‐Shali S, Dietrich C, Heinzel A, Decker M, Cumming P, Zerres K, Piel M, Mottaghy FM, Vernaleken I (2016): MAOA‐VNTR polymorphism modulates context‐dependent dopamine release and aggressive behavior in males. Neuroimage 125:378–385. [DOI] [PubMed] [Google Scholar]
- Schonberg T, Fox CR, Mumford JA, Congdon E, Trepel C, Poldrack RA (2012): Decreasing ventromedial prefrontal cortex activity during sequential risk‐taking: An FMRI investigation of the balloon analog risk task. Front Neurosci 6:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W (2010): Dopamine signals for reward value and risk: basic and recent data. Behav Brain Funct 6:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih JC, Wu JB, Chen K (2011): Transcriptional regulation and multiple functions of MAO genes. J Neural Transm 118:979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi H, Suzuki A, Fukasawa T, Aoshima T, Ujiie Y, Ishii G, Otani K (2006): Monoamine oxidase A gene promoter polymorphism affects novelty seeking and reward dependence in healthy study participants. Psychiatr Genet 16:55–58. [DOI] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K (2009): A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci 13:334–340. [DOI] [PubMed] [Google Scholar]
- Sjöberg RL, Ducci F, Barr CS, Newman TK, Dell'Osso L, Virkkunen M, Goldman D (2008): A non‐additive interaction of a functional MAO‐A VNTR and testosterone predicts antisocial behavior. Neuropsychopharmacology 33:425–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollberger S, Ehlert U (2016): How to use and interpret hormone ratios. Psychoneuroendocrinology 63:385–397. [DOI] [PubMed] [Google Scholar]
- Stanton SJ, Liening SH, Schultheiss OC (2011): Testosterone is positively associated with risk taking in the Iowa Gambling Task. Horm Behav 59:252–256. [DOI] [PubMed] [Google Scholar]
- Stenstrom E, Saad G (2011): Testosterone, financial risk‐taking, and pathological gambling. J Neurosci Psychol Econ 4:254–266. [Google Scholar]
- Summers CH, Larson ET, Ronan PJ, Hofmann PM, Emerson AJ, Renner KJ (2000): Serotonergic responses to corticosterone and testosterone in the limbic system. Gen Comp Endocrinol 117:151–159. [DOI] [PubMed] [Google Scholar]
- Terburg D, van Honk J (2013): Approach‐avoidance versus dominance‐submissiveness: a multilevel neural framework on how testosterone promotes social status. Emot Rev 5:296–302. [Google Scholar]
- Ullsperger M, Harsay HA, Wessel JR, Ridderinkhof KR (2010): Conscious perception of errors and its relation to the anterior insula. Brain Struct Funct 214:629–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Honk J, Schutter DJLG, Hermans EJ, Putman P, Tuiten A, Koppeschaar H (2004): Testosterone shifts the balance between sensitivity for punishment and reward in healthy young women. Psychoneuroendocrinology 29:937–943. [DOI] [PubMed] [Google Scholar]
- van Honk J, Will G‐J, Terburg D, Raub W, Eisenegger C, Buskens V (2016): Effects of testosterone administration on strategic gambling in poker play. Sci Rep 6:18096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varazzani C, San‐Galli A, Gilardeau S, Bouret S (2015): Noradrenaline and dopamine neurons in the reward/effort trade‐off: a direct electrophysiological comparison in behaving monkeys. J Neurosci 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volman I, Toni I, Verhagen L, Roelofs K (2011): Endogenous testosterone modulates prefrontal‐amygdala connectivity during social emotional behavior. Cereb Cortex 21:2282–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Votinov M, Aso T, Koganemaru S, Fukuyama H, Mima T (2013): Transcranial direct current stimulation changes human endowment effect. Neurosci Res 76:251–256. [DOI] [PubMed] [Google Scholar]
- Votinov M, Mima T, Aso T, Abe M, Sawamoto N, Shinozaki J, Fukuyama H (2010): The neural correlates of endowment effect without economic transaction. Neurosci Res 68:59–65. [DOI] [PubMed] [Google Scholar]
- Wagels L, Radke S, Goerlich KS, Habel U, Votinov M (2017): Exogenous testosterone decreases men's personal distance in a social threat context. Horm Behav 90:75–83. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A (1988): Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol 54:1063–1070. [DOI] [PubMed] [Google Scholar]
- Wu JB, Chen K, Li Y, Lau Y‐FC, Shih JC (2009): Regulation of monoamine oxidase A by the SRY gene on the Y chromosome. faseb J 23:4029–4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Liu J, Qu L, Eisenegger C, Clark L, Zhou X (2016): Single dose testosterone administration reduces loss chasing in healthy females. Psychoneuroendocrinology 71:54–57. [DOI] [PubMed] [Google Scholar]
- Zheng P (2009): Neuroactive steroid regulation of neurotransmitter release in the CNS: Action, mechanism and possible significance. Prog Neurobiol 89:134–152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


