Abstract
Magnetic resonance imaging (MRI) studies in humans have reported that the T1‐weighted signal in the cerebral cortex follows an inverted “U” trajectory over the lifespan. Here, we investigated the T1‐weighted signal trajectory from late adolescence to middle adulthood in humans to characterize the age range when mental illnesses tend to present, and efficacy of treatments are evaluated. We compared linear to quadratic predictors of age on signal in 67 healthy individuals, 17–45 years old. We investigated ¼, ½, and ¾ depths in the cortex representing intracortical myelin (ICM), in the superficial white matter (SWM), and in a reference deep white matter tract. We found that the quadratic fit was superior in all regions of the cortex, while signal in the SWM and deep white matter showed no global dependence on age over this range. The signal trajectory in any region followed a similar shape regardless of cortical depth. The quadratic fit was analyzed in 70 cortical regions to obtain the age of maximum signal intensity. We found that visual, cingulate, and left ventromedial prefrontal cortices peak first around 34 years old, whereas motor and premotor areas peak latest at ∼38 years. Our analysis suggests that ICM trajectories over this range can be modeled well in small cohorts of subjects using quadratic functions, which are amenable to statistical analysis, thus suitable for investigating regional changes in ICM with disease. This study highlights a novel approach to map ICM trajectories using an age range that coincides with the onset of many mental illnesses. Hum Brain Mapp 38:3691–3703, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: intracortical myelin, aging, magnetic resonance imaging, cerebral cortex
Abbreviations
- AIC
Akaike Information Criterion
- ARC
Autocalibrating Reconstruction for Cartesian imaging
- BD
bipolar disorder
- DSM
Diagnostic and Statistical Manual
- FOV
field of view
- GLM
general linear model
- GM
grey matter
- ICM
intracortical myelin
- RF
radiofrequency
- SLF
superior longitudinal fasciculus
- SWM
superficial white matter
- WM
white matter
INTRODUCTION
Myelin is a crucial substance in the nervous system that speeds the conduction of neural signals and has been suggested to play an important role in functional plasticity in the grey matter (GM) of the cerebral cortex [Gibson et al., 2014; Xiao et al., 2016]. Multiple studies using high‐resolution magnetic resonance imaging (MRI) and matched histology have shown that it is possible to investigate intracortical myelin (ICM) in vivo using MRI [Bock et al., 2009; Dinse et al., 2015; Eickhoff et al., 2005; Fracasso et al., 2016a, 2016b; Stüber et al., 2014]. Myelin affects many MR tissue properties, and changes in a given MR parameter are taken as a surrogate marker for changes in the ICM content. While several different MR parameters have been demonstrated to effectively map ICM, we use strongly T1‐weighted signal optimized for increased dynamic range within the cerebral cortex [Bock et al., 2013]. We further divide the T1‐weighted image by a proton density‐weighted image to fully remove bias in the ICM map resulting from B1− inhomogeneity and reduce the bias resulting from B1+ inhomogeneity [Marques and Gruetter, 2013]. This optimized protocol can potentially detect significant changes ICM in clinical studies in small cohorts of subjects better than protocols that have not been explicitly optimized for intracortical contrast. Finally, we use this MR contrast as a matched in vivo MRI and histology study in nonhuman primates showed that this image contrast is spatially correlated with the presence of ICM across the entire cortex [Bock et al., 2009] while another study extended this finding to postmortem human brains at 7 T [Fracasso et al., 2016a].
MRI has long been used to visualize the myelin content of the cortex [Clark et al., 1992]. Within the past decade, however, there has been an increased interest in using the MRI signal to study ICM. This includes studies that map the location and boundaries of cortical regions [De Martino et al., 2015; Dinse et al., 2015; Glasser and Van Essen, 2011; Lutti et al., 2014; Sereno et al., 2013], correlate ICM with cognitive measures [Grydeland et al., 2013, 2016; Vidal‐Piñeiro et al., 2016; Westlye et al., 2010b], and investigate changes in ICM in disease populations [Iwatani et al., 2015; Schnack et al., 2016].
Myelination in the cortex develops into adulthood and the age‐related trajectory of myelination differs across brain areas depending on their function [Bartzokis et al., 2001, 2004; Grydeland et al., 2013; Miller et al., 2012]. It has been previously shown that ICM follows an inverted “U” trajectory with normal aging with a rapid increase during childhood development, a slower increase into adulthood, and a decline with advanced age [Bartzokis, 2004; Grydeland et al., 2013]. Using MRI methods that are sensitive to ICM, it is now possible to investigate this trajectory in vivo over the entire cortex.
This study examines ICM in living humans from late adolescence to middle adulthood. Our intent is to characterize healthy ICM trajectories using a framework that allows future disease studies in this crucial age range where many severe mental diseases emerge and are treated in humans [Kessler et al., 2005]. By using T1‐weighted images with strong ICM contrast, we investigate the myelin content of the cortex and explore the best fitting options to map the trajectory with age of intracortical myelin across the cortex. Characterizing the trajectory of ICM over the entire human lifespan requires fitting functions with complex shapes, such as splines [Fjell et al., 2010; Grydeland et al., 2013]. However, we hypothesize that the limited age range we are characterizing in this study can be modeled using parametric linear [Shafee et al., 2015] or quadratic fits, which allows age effects to be incorporated in statistical models using limited numbers of parameters. We also explore whether voxel‐wise trajectories can be averaged over regions‐of‐interest (ROIs) across cortex without losing information. The advantage of investigating trajectories using ROIs is that it reduces the number of statistical tests one needs to compare signal trajectories in healthy and diseased groups across the cortex. Previous work has shown that it is possible to detect variations in signal across cortical depths [Fracasso et al., 2016a; Sereno et al., 2013; Waehnert et al., 2016]. We expand on this to investigate the trajectory of T1‐weighted signal at three cortical depths (¼, ½, and ¾), in the SWM to examine if the effects of aging on this population are depth dependent. This is important to establish whether trajectories in ICM differ over depths of the cortex with differing myeloarchitecture, and whether the SWM follows a trajectory closer to ICM or myelin in deep white matter (WM) tracts. Finally, we explore development patterns by using k‐means clustering to group the signal data based on commonalities in the age of maximum signal intensity across different regions of the cortex. We determine and appropriate number of clusters to best represent and summarize general trends in ICM trajectory with age.
METHODS
Subjects
This study was approved by the Hamilton Integrated Research Ethics Board and informed consent was obtained from each volunteer before enrolment. Images were collected in 67 right‐handed, healthy individuals (30 males, 37 females) aged 17–45. All female subjects were premenopausal. Additional exclusion criteria included: unstable medical or general inflammatory conditions, alcohol/substance abuse within the last year (excluding caffeine or nicotine), past or current history of neurological disorders (including head trauma and migraines), and any MRI contraindications. Participants completed the Structured Clinical Interview for DSM‐IV (SCID‐I) to rule out current or lifetime psychiatric conditions.
Imaging
Images were acquired on a 3 T General Electric scanner (Software Version 22.0) using a 32‐channel receive‐only radiofrequency (RF) coil for the head (MR Instruments) and a transmit RF body coil (GE). All images were acquired with 1 mm isotropic resolution. Images were acquired for anatomical reference, and to produce a T1‐weighted image with optimized intracortical contrast for ICM analysis [Bock et al., 2013; Rowley et al., 2015].
Anatomical reference image
A 3D T1‐weighted whole‐head image was made using a 3D inversion‐recovery gradient echo sequence (GE 3D BRAVO) [Inversion time = 450 ms, TE = 3.2 ms, TR in acquisition block = 8.4 ms, flip angle in acquisition block = 12°, field of view (FOV) = 25.6 × 25.6 × 25.6 cm, linear phase encoding, Autocalibrating Reconstruction for Cartesian imaging (ARC) parallel imaging factor of 2 in the second phase encode direction, Number of averages = 1, time = 5 min 32 s]. This image served as an anatomical reference for image registration.
T1‐weighted image
Another 3D T1‐weighted whole‐head image with strong intracortical contrast was made from four separate images collected with an inversion‐recovery gradient echo sequence (GE 3D BRAVO) [inversion time = 1100 ms, time between end of acquisition block and next 180° pulse (TD) = 1000 ms, TE = 3.2 ms, TR in acquisition block = 8.4 ms, flip angle in acquisition block = 12°, FOV = 24.0 × 10 (selective slab in left/right direction) × 24.0 cm, centric phase encoding, ARC factor 2 in second phase‐encoding direction, Number of averages = 1, time = 5 min 53 s]. Each hemisphere was imaged separately to increase intracortical contrast as this reduced the matrix dimension in the first phase‐encoding direction in the sequence allowing for a shortened acquisition block following the inversion pulse. This is analogous to using multiple segments in a magnetization‐prepared rapidly acquired gradient echo sequence (MP‐RAGE) [Deichmann et al., 2000], where the shorter acquisition block improves contrast. The reduced FOV was applied in this study as a true MP‐RAGE sequence was not available on our scanner. The long TD in the sequence provided increased intracortical contrast [Bock et al., 2013]. Each hemisphere was imaged twice to increase the contrast‐to‐noise ratio. The four images took 24 min in total to acquire.
Each of the four separate images was registered to the anatomical reference image via a 6‐parameter rigid transformation with sinc resampling using the FLIRT tool in FSL [Jenkinson et al., 2012] Version 5.0 (fsl.fmrib.ox.ac.uk/fsl/, RRID:SCR_002823). The magnitude images were then summed to create the final image of the whole head.
Ratio image
A final 3D proton density‐weighted whole‐head image was collected to correct intensity inhomogeneity in the T1‐weighted image. This image was made with a 3D gradient‐echo sequence (GE 3D SPGR) [parameters: TE = 3.1 ms, TR = 7.9 ms, flip angle = 4°, FOV = 24.0 × 17.4 × 24.0 cm, number of averages = 1, time = 5 min 29 s].
The proton‐density weighted image was registered to the T1‐weighted image using a 6‐parameter rigid transform (FSL) and filtered with a 3D median filter with a 5 × 5 × 5 mm kernel size. The T1‐weighted image was then divided by the filtered proton‐density‐weighted image to create the ratio image, which is a strongly T1‐weighted image with B1− and some B1+ inhomogeneities removed [Marques and Gruetter, 2013; van de Moortele et al., 2009; Wang et al., 2005].
Processing
Image processing was performed predominantly in MIPAV v7.0.1 software (http://mipav.cit.nih.gov, RRID:SCR_007371) using the JIST v3.0 (http://www.nitrc.org/projects/jist/, RRID:SCR_008887), TOADS‐CRUISE vR3c (http://www.nitrc.org/projects/toads-cruise/, RRID:SCR_005977), and CBS High‐Res Brain Processing Tools Version v3.0 (http://www.nitrc.org/projects/cbs-tools/, RRID:SCR_009452) plug‐ins, and Amira v5.2 software (Visage Imaging, RRID:SCR_014305).
Processing began by creating a mask of the cerebrum using the T1‐weighted image as an input to the SPECTRE 2010 algorithm [Carass et al., 2011] in MIPAV. This mask identifying the brain and dura matter was used to skull strip the Ratio image. The skull‐striped Ratio image was used as input to the Multiple Object Geometric Deformable Model (MGDM) Multi‐Contrast Brain Segmentation algorithm [Bazin et al., 2014; Bogovic et al., 2013] in MIPAV to generate initial probabilistic labels for tissue classes for each hemisphere of the brain. The probability labels generated for cerebral GM and WM were used as input to the CRUISE algorithm [Han et al., 2004] to generate smoothed, topologically correct labels for the cerebrum, which was performed in each hemisphere separately.
Subcortical structures and ventricles (as identified by the MGDM algorithm) were then removed from the labels for the left and right cerebrums and the remaining labels combined. At this point, all GM segmentations were inspected and manual edits were made to replace missing cortex and to remove remaining dura mater arising from potential errors in the MGDM and CRUISE algorithms to ensure an accurate pial surface. This label for the entire cerebrum without the subcortical structures was then used to mask the Ratio image such that it only contained the cerebral cortex and underlying major white matter tracts.
This corrected, cerebrum‐masked Ratio image was next segmented into two main tissue classes: WM and GM, taking into account lightly and heavily myelinated components of GM [Rowley et al., 2015] using the FANTASM algorithm [Pham, 2001] in MIPAV. This yields a more accurate WM label for ICM analysis than the output from CRUISE. The hemispheres were segmented together to avoid potential hemispheric bias, such that the algorithm used the entire cerebrum for its classification. Following segmentations, labels for each tissue class were split back into left and right hemispheres for subsequent processing. The labels near the GM/WM‐boundary were morphologically processed to remove all WM tissue not connected to the largest WM mass.
The corrected GM labels from CRUISE representing the pial surface, and WM labels from FANTASM representing the GM/WM boundary surface were used as inputs to a volume‐preserving cortical depth model to generate intracortical surfaces at ¼, ½, and ¾ cortical depths to sample the T1‐weighted image signal [Waehnert et al., 2014] (Fig. 1). An additional surface was generated 1mm from the GM/WM boundary into the WM to sample superficial white matter (SWM) intensity. Each surface was registered to the equivalent depth surface generated from the MNI‐152 atlas using a multicontrast multiscale surface registration approach [Tardif et al., 2015]. The signal was first sampled onto the individual subject's surface, which was then deformed to be in register with the MNI‐152 equivalent surface.
Figure 1.
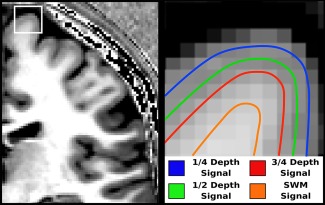
llustration of measured T1‐weighted signal at four depths. Left: 1 mm isotropic T1‐weighted scan with high intracortical myelin contrast. Right: Four depth‐dependent surfaces in and beneath the cortex, which sample the MRI signal. SWM, superficial white matter. [Color figure can be viewed at http://wileyonlinelibrary.com]
Statistics
The MarsAtlas [Auzias et al., 2016] was used to parcellate the cortex into 82 regions‐of‐interest (ROIs) for analysis. The ICBM DTI‐81 Atlas [Mori et al., 2008] was used to define an ROI in the superior longitudinal fasciculus, a deep WM tract that lies near the cortex. Statistical analysis was completed using R (https://www.r-project.org, RRID:SCR_001905) and cortical maps were created using Surfstat (http://www.math.mcgill.ca/keith/surfstat/, RRID:SCR_007081) in Matlab (v R2015a, RRID:SCR_001622). Signal intensity as a function of age was investigated for the ROIs using two general linear models (GLM) to compare linear to quadratic fits. The linear fit used the predictors: Depth + Age + Gender, and the quadratic fit: Depth + Age + Age2 + Gender, where Depth was the cortical depth of the sampling surface. A chi‐square test was used to determine if there was a significant difference between the GLMs, and the Akaike information criterion (AIC) was used to determine which model provided a higher quality fit [Akaike, 1998]. Six ROIs per hemisphere (12 total) were not analyzed due to poor signal intensity profiles arising from topological errors in segmentations. These corresponded to the isthmus of the cingulate, the insula, and regions of occipital and temporal lobes. The remaining 70 regions were used in the analysis to predict temporal shift of the quadratic model of signal intensity with age in all depths. The signal was averaged across each ROI and then fitted with predictors: Age + Age2, to generate coefficients for a quadratic equation in the form: y = Ax 2 + Bx + C. The temporal shift, or the age where the maximum signal occurs is where x = −B/2A, which is obtained by taking the first derivative of the quadratic. The averaged ROI values were also fit with a smoothing spline using ggplot2 in R (using default values of stat_smooth with third‐order polynomial b‐spline, loess fitting, and span = 0.75), where the maximum signal and age at the signal of the fit were calculated. A k‐means clustering algorithm was performed in MATLAB using four clusters to cluster the ROIs based on commonalities in the age of peak signal intensity in the middle depth. This was used to group the signal data to reduce the number of ROIs to show general trends of ICM trajectory with age across the cortex. The number of clusters was decided upon using the elbow method, such that adding additional clusters provided a diminishing change on the summed distance of the points to the centroids.
RESULTS
Linear versus Quadratic Fit
Gender was not found to be a significant predictor in either model in any ROI (P > 0.05) so it was not included for the remainder of the analysis. Quadratic models with age satisfied the conditions in all areas for the GLMs. GLMs also satisfied the conditions in nearly all regions (conditions satisfied if P of the model, normalized residuals tested with the Shapiro–Wilk test and homoscedasticity tests were all <0.05).
A chi‐square test was used to compare fit models in the ROIs for the ½ depth signal and suggested a significant difference between the two general linear models (P < 0.05, Bonferonni corrected for 70 tests). AIC was used to test which model provided a higher quality fit. For all regions, AIC was lower in the quadratic model, signifying that the quadratic fit with Age was better than the linear fit. The two models are compared in Figure 2. A higher degree of variance, as shown by an increase in r 2, is explained by the quadratic model across the cortex relative to the linear model. The P value maps show similar significance for the models across the cortex, except for regions in the visual cortex where the linear model no longer fits significantly (P > 0.05). These maps were also created for the ¼ and ¾ cortical depths, and the quadratic fit universally provided a better data fit. SWM was the only sampling surface where neither the linear nor the quadratic models provided a significant data fit across the brain.
Figure 2.
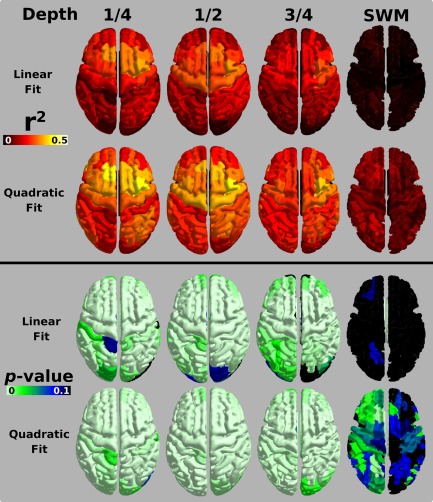
GLM fit metrics mapped across the brain. Top: r 2 values for MARS atlas ROIs with signal for the half‐depth regressed against age (linear) and age2 (quadratic). An increasing yellow hue in the map values indicates more variability is explained by the model fit. Bottom: P value for the fit models. An increasing green hue in the map values depicts a more significant model fit. [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 3 illustrates the quadratic fit to the signal data points in the ROI defining the Caudal Dorsomedial Prefrontal Cortex. T1‐weighted signal across the age span is referenced against the signal found in the superior longitudinal fasciculus (SLF) in deep WM, which was found not to be correlated with age over the range used in this study.
Figure 3.
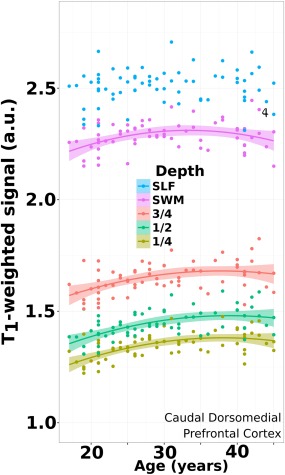
Age trajectory from superficial cortex to deep WM. Data plotted for an ROI (caudal dorsomedial prefrontal cortex) in all subjects where the quadratic model fit the signal data at all depths. This is contrasted against the signal of a volumetric ROI placed in the superior longitudinal fasciculus (SLF) in deep white matter, which demonstrated no significant effect with age over this range. Shaded regions denote the 95% confidence interval. [Color figure can be viewed at http://wileyonlinelibrary.com]
Estimating Maximum Value From Fits
Intercept values and coefficients for Age and Age2 were calculated using the parameters estimated from the general linear model for all ROIs. This equation can be transformed into vertex form:
where x is the Age, h is the age where the maximum T1‐weighted signal occurs, k is the maximal signal value reached, and A describes the shape of the trajectory. The graphical interpretation of h and k is illustrated in Figure 4 in the top left plot, and can be visualized in three additional ROIs at the three cortical depths. Figure 5 maps these values across the cortex both from vertex‐wise fits and from fits of signal averaged over the ROIs. The signal maximum plots (k) resemble previously reported myelin maps derived from MRI [Bock et al., 2013; Dinse et al., 2015; Glasser and Van Essen, 2011; Lutti et al., 2014; Rowley et al., 2015; Waehnert et al., 2016], with high signal intensity in primary cortical areas, and lower intensity in association regions. The age where the signal reaches a maximum in the fit shows that the visual regions, cingulate, and left ventromedial prefrontal cortex peak first, and the premotor and primary motor cortices peak last. Both maps of signal peak age (h) and peak signal value (k) show considerable regional variation, although the two metrics have distinct spatial patterns. The bottom panel allows the visual comparison of h and k values extracted using a smoothing spline as an alternate fit. Over the age range we investigated, the quadratic and smoothing spline fits provide very similar maps of h and k over the cortex. A benefit of describing the trajectory using a quadratic model is the parameters can be incorporated into linear models for performing inferential statistics between populations of subjects. The benefit of a smoothing spline is that it would better capture trajectory if the age range were expanded beyond 17–45 years old [Fjell et al., 2010].
Figure 4.
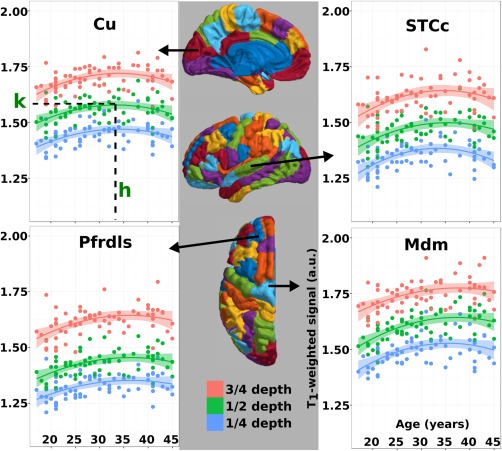
Four sample ROIs displaying the T1‐weighted signal intensity values fit against age. Shading surrounding the quadratic fit line denotes the 95% confidence interval. Top left: cuneus. Parameters extracted from the quadratic for further analysis are highlighted on this plot. “h” is the age where the maximum signal occurs based on the quadratic fit. “k” is the maximal signal achieved in this brain region. Top right: caudal superior temporal cortex. Bottom left: rostral dorsolateral superior prefrontal cortex. Bottom right: dorsomedial motor cortex. [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 5.
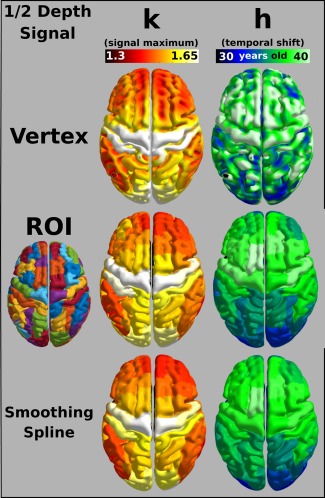
Mapping quadratic parameters across the cortex. Quadratic parameters calculated from the GLM fit shown for vertex‐wise calculations and with signal first averaged across an ROI. Left: The maximum signal reached across the cortex as predicted by the quadratic model or using a smoothing spline (bottom). Right: The temporal shift of the maximum signal value as estimated by the quadratic model, or by the smoothing spline (bottom), is mapped across the cortex. [Color figure can be viewed at http://wileyonlinelibrary.com]
The quadratic fit parameters were investigated across three different cortical depths and are summarized in Figure 6. An interaction term was added to the general linear model between Depth and Age to determine if there was a significant difference in the change of signal across age at the different depths. None of the interaction terms was found to be significant (all P > 0.05). The same maturation trend tracks across the cortex at the three cortical depths with visual areas and cingulate cortex reaching their fitted maximum first, and motor areas achieving their peak last. This suggests that ICM at different depths of the cortex follows the same age‐related trajectory. The lack of a significant fit of either model across the SWM and SLF suggests myelination does not vary with age over the age range we investigated.
Figure 6.
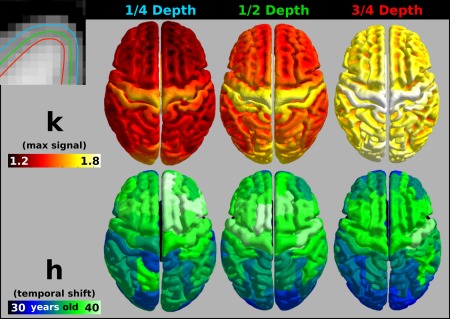
Calculated quadratic parameters across three cortical depths. Top left: Illustration of the cortical sampling surfaces overlaid on the T1‐weighted image. Top row: The maximum signal for each vertex at each depth (voxel‐wise). Bottom row: The temporal shift of the quadratic fit of the signal intensity with age (averaged over ROI). [Color figure can be viewed at http://wileyonlinelibrary.com]
Summarizing Trajectories Across the Cortex Using K‐Means Clustering
The age of the peak signal, h, for the ROIs was analyzed using a k‐means algorithm with four clusters to visualize regional commonalities across the cortex. Four clusters were chosen for our analysis using the elbow method by plotting the summed distance to the centroids against the number of clusters, and selecting the cluster where adding additional clusters provided little increase in explained variance in the data (Supporting Information, Fig. 1). We also found that this number qualitatively best summarized global trends in ROIs across the cortex. The results of the clustering are illustrated in Figure 7. Interestingly, the four clusters localized fairly well to the visual and cingulate cortices, frontal pole and parietal and temporal cortices, prefrontal cortex, and premotor and motor cortices. Signal values were then z‐scored within the cluster to allow a better visualization of a shift along the age axis, and to display the relative variance seen within each cluster. The plot reveals the earliest peaking cluster is the one that includes the visual, cingulate and left ventromedial prefrontal cortices, which reaches its maximum value around age 34. The next cluster to peak contains the frontal pole, parietal, and temporal cortices and occurs around 35 years of age. Subsequently, the cluster containing prefrontal and primary sensory cortices peaks just following 36 years of age, and the cluster containing the motor and premotor areas reach a maximum at age 38.
Figure 7.
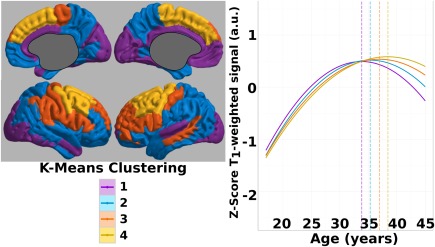
K‐means clustering on peak age signal. Left: K‐means clustering of ROIs into four clusters based on the age when the maximal T1‐weighted signal is reached. Right: The signal values in all ROIs within each cluster were averaged, and then Z‐scores were calculated within each cluster to visualize the signal trajectory. Plotted solid lines highlight the quadratic fit for the half cortical depth signal. Temporal shift (vertical dotted line) increases from cluster 1 to cluster 4. [Color figure can be viewed at http://wileyonlinelibrary.com]
DISCUSSION
We have demonstrated that the T1‐weighted signal in cortical regions follows a trajectory that can be consistently modeled as a quadratic function of age over all regions of the cortex in subjects aged 17–45. Furthermore, we show that age‐related trajectories can be observed in a relatively small cohort of healthy controls making the technique suitable for future clinical studies. If one takes changes in T1‐weighted MRI signal to represent changes in ICM, then one can summarize ICM trajectories using two estimates from the fit: signal peak age (h) and peak signal value (k). The map of peak signal value (k) over the cortex corresponds well with maps from a number of studies of MR parameters thought to be correlated with ICM amounts. The map of signal peak age (h) paints an important picture of how ICM develops from late adolescence, through early and into middle adulthood in humans. ICM development is important to examine in this range, as it coincides with the age of onset of many psychiatric disorders and subsequent treatment, such that developing atlases depicting h in large numbers of subjects would create a useful comparative data set.
Overall, we found that the trajectory in T1‐weighted signal with age was similar over the depths of the cortex we investigated (¼, ½, and ¾ depths), despite the fact that myelin amounts differ significantly over the cortical layers. Specifically, the density of myelinated fibers is much higher in the deepest layers of the cortex (Layers IV–VI) relative to the shallower layers. This can be seen in our maps of the peak signal intensity, k, which increases in value over all regions when moving from the ¼ to the ¾ depth, which compares to the three cortical depth T1 maps reported by Waehnert et al. [2016]. This depth‐dependent analysis is dependent on accurate GM and WM surfaces to define the appropriate intracortical surfaces. We manually inspected all GM segmentations and made corrections where required to ensure accurate tissue classification. Accurate segmentations decrease the observed variance and help to increase statistical power, which would be increasingly important when trying to compare different populations. The lack of depth dependence in our age trajectories suggests that ICM is gained and lost globally in the same manner over all depths of the cortex, regardless of their underlying myeloarchitecture. We also investigated the T1‐weighted signal in the SWM representing myelinated short association fibers, and found no correlation with age, which suggests that the myelin in SWM matures before ICM and is thus similar to myelinated long association fibers in deep WM tracts.
In Figure 5, we show the maps of k and h calculated from quadratic fits to vertex‐wise trajectories and from fits to trajectories averaged first in 70 ROIs. There is little loss in specificity in the k ROI maps and some loss of specificity in the h ROI maps; however, it would be advantageous in future studies comparing k and h between healthy and diseased groups using ROIs because the number of statistical comparisons is reduced versus vertex‐wise studies.
It was previously shown that over an age range of 18–35 years old, the T1‐weighted/T2‐weighted signal follows a linear trajectory with age [Shafee et al., 2015]. Our age range is extended to 45 years old, revealing a middle‐adulthood plateau in the signal trajectory; thus, we found that a quadratic model fit our T1‐weighted signal significantly better than a linear model. If the age range of subjects extends from childhood to late adulthood, however, then simple linear or quadratic functions no longer accurately fit the T1‐weighted signal trajectory with age in all regions. This is because a quadratic fit fails to capture steep increases in ICM in the early period of neurodevelopment [Barkovich et al., 1988; Brody et al., 1987], the middle age plateau, and sharp declines during neurodegeneration in old age[Vidal‐Piñeiro et al., 2016; Westlye et al., 2010a, 2010b]. In fact, Fjell et al. [2010] have warned of the interpretation of quadratic fits to signal data over the entire lifespan as a large signal decrease in old age biases the fit and the following calculated metrics derived from the fit. The ICM trajectory over the entire lifespan is undeniably better fit with functions comprising greater numbers of parameters, such as splines, which can better capture the shape. As such, previous MRI studies of ICM trajectories over a broader human age range have used spline functions to describe age effects. Although splines fit data well over the entire life span, their use makes regional or group–group comparisons difficult, and statistically expensive due to their piece‐wise nature; therefore, it is preferable to use a simpler fit function if it is warranted. Here we have displayed that from the age range of 17–45, we collect very similar results using fewer fitting parameters.
In comparing our results to other work on cortical development and maturation, a previous study using T1‐weighted MRI in subjects aged 8–85 found a peak age roughly between 20 and 30 years of age by fitting the signal data with splines [Westlye et al., 2010b]. Despite the differences in imaging, data fitting, and subject demographics, the development pattern was consistent with our findings with visual areas peaking earliest, cingulate cortices following shortly after, and motor and premotor areas peaking latest. In our study, where age was limited and did not include childhood development or neurodegeneration, we have illustrated a framework that allows for statistical comparisons of trajectories between regions based on quadratic fitting and a simple comparison of h. We did not see any significant regional differences between cortical depths in estimated peak myelin age at the resolution we used for imaging. This suggests that myelination over the different depths of the cortex follows a common trajectory with age, although this finding could be reinforced using higher resolution imaging more specific to the fine structural layering of the cortex.
The increase in T1‐weighted signal we observed over late adolescence and early adulthood may be tracking an increase in myelin density per voxel—this could arise from either an increase in the number of myelinated axons in the cortex or an increase in the caliber of existing sheaths. There is no comprehensive data in humans describing which features of myeloarchitecture in specific cortical regions change over the lifespan. In rhesus monkeys, however, it has been shown that oligodendrocytes show greater change with age than neurons [Peters et al., 2000], and although cortical axons do not change in size with age, the myelin sheath surrounding them increases in diameter [Peters et al., 2001]. Thus, the increase in T1‐weigthed signal we observe could be reflecting increases in sheath thickness. The peak age observed in our data could reflect a turning point where myelin starts to degrade faster than it is produced. Our calculated peak age agrees with a previous study in humans looking at peak glucose consumption in the brain with age [Pardo et al., 2007]. While myelin is known to be important in providing energy to the axons they insulate [Fünfschilling et al., 2013; Lee et al., 2013; Morrison et al., 2013; Rinholm et al., 2011], it also requires large amounts of energy to build and maintain [Harris and Attwell, 2012], such that a slowing of myelin production would coincide with a reduction in glucose consumption. Xi et al. [1999] previously reported a significant decrease in conduction velocity of pyramidal tract neurons in aged cats, compared to young controls. As myelin is important in speeding the conduction of neural signals, this finding could be reporting a decrease in the quantity or quality of myelin on those neurons. These studies suggest that the T1‐weighted signal trajectories are reporting ICM amounts over the age range we investigated; however, more histology work is needed to confirm how the specific features of myeloarchitecture evolve with age in the human cerebral cortex.
Our study suggests that ICM matures earliest in visual and cingulate cortices, with the rest of the cortex maturing up to 4 years later. While we found ICM to be globally dependent on age, SWM was not. Visual inspection of T1‐weighted/T2‐weighted signal plots by Grydeland et al. [2103] depict a larger plateau in signal at middle age adulthood in the SWM in many regions compared to intracortical plots, reinforcing our findings in this narrowed age range. A study using diffusion tensor imaging (DTI) parameters to investigate SWM from 18 to 74 years of age noted that effects were prominently linear with age, with some quadratic effects, potentially due to a middle‐age plateau [Phillips et al., 2013]. A cross‐sectional examination investigating white matter maturation with MRI‐based DTI showed that fractional anisotropy (FA) increases at least up until 30 years of age [Lebel et al., 2008], while an additional study uncovered data peaks around 30 years of age in white matter DTI measurements [Westlye et al., 2010a]. More recently, Nazeri et al. [2015] found a widespread age association with fractional anisotropy in SWM, but we believe our selected age range was too narrow to uncover this using our method. In humans, a histological investigation on the myelin in the stria of Gennari found a similar peak of myelin into the third decade of life [Lintl and Braak, 1983]. Together, this evidence suggests that the cortex may continue to myelinate after the adjacent WM has finished or plateaued. Also this suggests that a larger age range may be necessary to investigate changes in SWM age trajectories.
We observed a rostrocaudal maturation pattern in the prefrontal cortex (PFC), which was reinforced with our k‐means clustering; regions near the frontal pole belong to the earliest peaking clusters, 1 and 2, regions near the dorsolateral prefrontal cortex peak later in cluster 3, and motor, premotor, and regions in the superior medial prefrontal cortex peak latest and belong to cluster 4 (Fig. 7). This matches a known structural gradient that exists in the rostral–caudal orientation of the PFC, such that in moving from caudodorsal to rostroventral, there is a gradual decrease in the number of fibers, in the caliber of fibers, and in cortical width [Nieuwenhuys, 2012]. There is also an increase in myelination in the rostrocaudal direction [Thiebaut de Schotten et al., 2016]. The functional significance of this gradient was extensively reviewed previously [Badre and D'Esposito, 2009] and it was suggested that hierarchical processing exists in the PFC, with more abstract thoughts being processed rostrally, and more concrete thoughts that are closer to producing a motor output being processed caudally. It is possible that the sequential processing is reflected in the myelination process as the circuits more rostral myelinate to completion before their downstream targets.
CONCLUSION
We have demonstrated that T1‐weighted signal maps the trajectory with age of ICM in healthy young adults using a quadratic model. Considering that this age range coincides with the age of onset of major psychiatric disorders [Kessler et al., 2005], our method may be useful to detect potential changes in ICM trajectories in disorders thought to be involved with abnormalities in myelin/oligodendrocyte maturation such as schizophrenia [Bartzokis et al., 2012, 2009], depression [Lake et al., 2016], and bipolar disorder [Rowley et al., 2015]. It may also be useful to link the development of specific cortical regions with behavioral/cognitive functioning in humans over the range from late adolescence to middle adulthood. Early work has been done to correlate a T1‐/T2‐weighted ratio signal, another contrast correlated with ICM, and intraindividual variability and error processing [Grydeland et al., 2013, 2016]. Such combined studies could shed light on the functional impact of the myelin trajectory, and provide a biological mechanism to support different cognitive processes and patterns [Craik and Bialystok, 2006].
Supporting information
Supporting Information
Conflict of Interest: The authors declare no competing financial interests.
Funding: This project was supported by the Independent Investigator Award, Brain & Behaviour Research Foundation (Dr Frey) and the AFP Innovations Award, Department of Psychiatry and Behavioural Neurosciences, McMaster University (Dr Minuzzi).
Contributor Information
Christopher D. Rowley, Email: rowleycd@mcmaster.ca.
Benicio N. Frey, Email: freybn@mcmaster.ca.
REFERENCES
- Akaike H (1998): Information theory and an extension of the maximum likelihood principle In: Selected Papers of Hirotugu Akaike, Springer Series in Statistics. New York, NY: Springer New York. pp 199–213. [Google Scholar]
- Auzias G, Coulon O, Brovelli A (2016): MarsAtlas: A cortical parcellation atlas for functional mapping. Hum Brain Mapp 37:1573–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, D'Esposito M (2009): Is the rostro‐caudal axis of the frontal lobe hierarchical? Nat Rev Neurosci 10:659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkovich AJ, Kjos BO, Jackson DE, Norman D (1988): Normal maturation of the neonatal and infant brain: MR imaging at 1.5 T. Radiology 166:173–180. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J (2001): Age‐related changes in frontal and temporal lobe volumes in men. A magnetic resonance imaging study. Arch Gen Psychiatry 58:461–465. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=11343525&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- Bartzokis G (2004): Quadratic trajectories of brain myelin content: Unifying construct for neuropsychiatric disorders. Neurobiol Aging 25:49–62. [Google Scholar]
- Bartzokis G, Lu PH, Raven EP, Amar CP, Detore NR, Couvrette AJ, Mintz J, Ventura J, Casaus LR, Luo JS, Subotnik KL, Nuechterlein KH (2012): Impact on intracortical myelination trajectory of long acting injection versus oral risperidone in first‐episode schizophrenia. Schizophrenia Res 140:122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Stewart SB, Oluwadara B, Lucas AJ, Pantages J, Pratt E, Sherin JE, Altshuler LL, Mintz J, Gitlin MJ, Subotnik KL, Nuechterlein KH (2009): In vivo evidence of differential impact of typical and atypical antipsychotics on intracortical myelin in adults with schizophrenia. Schizophrenia Res 113:322–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Sultzer D, Lu PH, Nuechterlein KH, Mintz J, Cummings JL (2004): Heterogeneous age‐related breakdown of white matter structural integrity: Implications for cortical “disconnection” in aging and Alzheimer's disease. Neurobiol Aging 25:843–851. [DOI] [PubMed] [Google Scholar]
- Bazin P‐L, Weiss M, Dinse J, Schäfer A, Trampel R, Turner R (2014): A computational framework for ultra‐high resolution cortical segmentation at 7Tesla. NeuroImage 93:201–209. [DOI] [PubMed] [Google Scholar]
- Bock NA, Hashim E, Janik R, Konyer NB, Weiss M, Stanisz GJ, Turner R, Geyer S (2013): Optimizing T1‐weighted imaging of cortical myelin content at 3. NeuroImage 65:1–12. [DOI] [PubMed] [Google Scholar]
- Bock NA, Kocharyan A, Liu JV, Silva AC (2009): Visualizing the entire cortical myelination pattern in marmosets with magnetic resonance imaging. J Neurosci Met 185:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogovic JA, Prince JL, Bazin P‐L (2013): A multiple object geometric deformable model for image segmentation. Comput Vis Image Underst 117:145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody BA, Kinney HC, Kloman AS, Gilles FH (1987): Sequence of central nervous system myelination in human infancy. I. An autopsy study of myelination. J Neuropathol Exp Neurol 46:283–301. [DOI] [PubMed] [Google Scholar]
- Carass A, Cuzzocreo J, Wheeler MB, Bazin P‐L, Resnick SM, Prince JL (2011): Simple paradigm for extra‐cerebral tissue removal: Algorithm and analysis. NeuroImage 56:1982–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark VP, Courchesne E, Grafe M (1992): In vivo myeloarchitectonic analysis of human striate and extrastriate cortex using magnetic resonance imaging. Cereb Cortex 2:417–424. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Bialystok E (2006): Cognition through the lifespan: Mechanisms of change. Trends Cogn Sci (Regul Ed) 10:131–138. [DOI] [PubMed] [Google Scholar]
- De Martino F, Moerel M, Xu J, van de Moortele P‐F, Ugurbil K, Goebel R, Yacoub E, Formisano E (2015): High‐resolution mapping of myeloarchitecture in vivo: Localization of auditory areas in the human brain. Cereb Cortex 25:3394–3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deichmann R, Good CD, Josephs O, Ashburner J, Turner R (2000): Optimization of 3‐D MP‐RAGE sequences for structural brain imaging. NeuroImage 12:112–127. [DOI] [PubMed] [Google Scholar]
- Dinse J, Härtwich N, Waehnert MD, Tardif CL, Schäfer A, Geyer S, Preim B, Turner R, Bazin PL (2015): A cytoarchitecture‐driven myelin model reveals area‐specific signatures in human primary and secondary areas using ultra‐high resolution in‐vivo brain MRI. NeuroImage 114:71–87. [DOI] [PubMed] [Google Scholar]
- Eickhoff S, Walters NB, Schleicher A, Kril J, Egan GF, Zilles K, Watson JDG, Amunts K (2005): High‐resolution MRI reflects myeloarchitecture and cytoarchitecture of human cerebral cortex. Hum Brain Mapp 24:206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Westlye LT, Østby Y, Tamnes CK, Jernigan TL, Gamst A, Dale AM (2010): When does brain aging accelerate? Dangers of quadratic fits in cross‐sectional studies. NeuroImage 50:1376–1383. [DOI] [PubMed] [Google Scholar]
- Fracasso A, van Veluw SJ, Visser F, Luijten PR, Spliet W, Zwanenburg JJM, Dumoulin SO, Petridou N (2016a): Lines of Baillarger in vivo and ex vivo: Myelin contrast across lamina at 7T MRI and histology. NeuroImage 133:163–175. [DOI] [PubMed] [Google Scholar]
- Fracasso A, van Veluw SJ, Visser F, Luijten PR, Spliet W, Zwanenburg JJM, Dumoulin SO, Petridou N (2016b): Myelin contrast across lamina at 7T, ex‐vivo and in‐vivo dataset. Data in Brief 8:990–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fünfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, Brinkmann BG, Kassmann CM, Tzvetanova ID, Möbius W, Diaz F, Meijer D, Suter U, Hamprecht B, Sereda MW, Moraes CT, Frahm J, Goebbels S, Nave K‐A (2013): Glycolytic oligodendrocytes maintain myelin and long‐term axonal integrity. Nature 485:517–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, Inema I, Miller SE, Bieri G, Zuchero JB, Barres BA, Woo PJ, Vogel H, Monje M (2014): Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 344:1252304–1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Van Essen DC (2011): Mapping human cortical areas in vivo based on myelin content as eevealed by T1‐ and T2‐weighted MRI. J Neurosci 31:11597–11616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grydeland H, Walhovd KB, Tamnes CK, Westlye LT, Fjell AM (2013): Intracortical myelin links with performance variability across the human lifespan: Results from T1‐ and T2‐weighted MRI myelin mapping and diffusion tensor imaging. J Neurosci 33:18618–18630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grydeland H, Westlye LT, Walhovd KB, Fjell AM (2016): Intracortical posterior cingulate myelin content relates to error processing: Results from T1‐ and T2‐weighted MRI myelin mapping and electrophysiology in healthy adults. Cereb Cortex 26:2402–2410. [DOI] [PubMed] [Google Scholar]
- Han X, Pham DL, Tosun D, Rettmann ME, Xu C, Prince JL (2004): CRUISE: Cortical reconstruction using implicit surface evolution. NeuroImage 23:997–1012. [DOI] [PubMed] [Google Scholar]
- Harris JJ, Attwell D (2012): The energetics of CNS white matter. J Neurosci 32:356–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatani J, Ishida T, Donishi T, Ukai S, Shinosaki K, Terada M, Kaneoke Y (2015): Use of T1‐weighted/T2‐weighted magnetic resonance ratio images to elucidate changes in the schizophrenic brain. Brain Behav 5:n/a–n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM (2012): FSL. NeuroImage 62:782–790. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE (2005): Lifetime prevalence and age‐of‐onset distributions of DSM‐IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62:593–602. [DOI] [PubMed] [Google Scholar]
- Lake EMR, Steffler EA, Rowley CD, Sehmbi M, Minuzzi L, Frey BN, Bock NA (2016): Altered intracortical myelin staining in the dorsolateral prefrontal cortex in severe mental illness. Eur Arch Psychiatry Clin Neurosci 1–8. [DOI] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C (2008): Microstructural maturation of the human brain from childhood to adulthood. NeuroImage 40:1044–1055. [DOI] [PubMed] [Google Scholar]
- Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, Liu Y, Tsingalia A, Jin L, Zhang P‐W, Pellerin L, Magistretti PJ, Rothstein JD (2013): Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487:443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintl P, Braak H (1983): Loss of intracortical myelinated fibers: A distinctive age‐related alteration in the human striate area. Acta Neuropathol 61:178–182. [DOI] [PubMed] [Google Scholar]
- Lutti A, Dick F, Sereno MI, Weiskopf N (2014): Using high‐resolution quantitative mapping of R1 as an index of cortical myelination. NeuroImage 93:176–188. [DOI] [PubMed] [Google Scholar]
- Marques JP, Gruetter R (2013): New developments and applications of the MP2RAGE sequence ‐ Focusing the contrast and high spatial resolution R1 mapping. Ed. Essa Yacoub. PLoS ONE 8:e69294–e69211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DJ, Duka T, Stimpson CD, Schapiro SJ, Baze WB, McArthur MJ, Fobbs AJ, Sousa AMM, Sestan N, Wildman DE, Lipovich L, Kuzawa CW, Hof PR, Sherwood CC (2012): Prolonged myelination in human neocortical evolution. Proc Natl Acad Sci USA 109:16480–16485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Hua K, Faria AV, Mahmood A, Woods R, Toga AW, Pike GB, Neto PR, Evans A, Zhang J, Huang H, Miller MI, van Zijl P, Mazziotta J (2008): Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. NeuroImage 40:570–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison BM, Lee Y, Rothstein JD (2013): Oligodendroglia: Metabolic supporters of axons. Trends Cell Biol 23:644–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazeri A, Chakravarty MM, Rajji TK, Felsky D, Rotenberg DJ, Mason M, Xu LN, Lobaugh NJ, Mulsant BH, Voineskos AN (2015): Superficial white matter as a novel substrate of age‐related cognitive decline. Neurobiol Aging 36:2094–2106. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R (2012): The myeloarchitectonic studies on the human cerebral cortex of the Vogt–Vogt school, and their significance for the interpretation of functional neuroimaging data. Brain Struct Funct 218:303–352. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Lee JT, Sheikh SA, Surerus‐Johnson C, Shah H, Munch KR, Carlis JV, Lewis SM, Kuskowski MA, Dysken MW (2007): Where the brain grows old: Decline in anterior cingulate and medial prefrontal function with normal aging. NeuroImage 35:1231–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Moss MB, Sethares C (2000): Effects of aging on myelinated nerve fibers in monkey primary visual cortex. J Comp Neurol 419:364–376. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C, Killiany RJ (2001): Effects of age on the thickness of myelin sheaths in monkey primary visual cortex. J Comp Neurol 435:241–248. [DOI] [PubMed] [Google Scholar]
- Pham DL (2001): Robust Fuzzy Segmentation of Magnetic Resonance Images. Computer‐Based Medical Systems. [Google Scholar]
- Phillips OR, Clark KA, Luders E, Azhir R, Joshi SH, Woods RP, Mazziotta JC, Toga AW, Narr KL (2013): Superficial white matter: Effects of age, sex, and hemisphere. Brain Connectivity 3:146–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinholm JE, Hamilton NB, Kessaris N, Richardson WD, Bergersen LH, Attwell D (2011): Regulation of oligodendrocyte development and myelination by glucose and lactate. J Neurosci 31:538–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley CD, Bazin P‐L, Tardif CL, Sehmbi M, Hashim E, Zaharieva N, Minuzzi L, Frey BN, Bock NA (2015): Assessing intracortical myelin in the living human brain using myelinated cortical thickness. Front Neurosci 9:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnack HG, van Haren NEM, Nieuwenhuis M, Hulshoff Pol HE, Cahn W, Kahn RS (2016): Accelerated brain aging in schizophrenia: A longitudinal pattern recognition study. AJP 173:607–616. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Lutti A, Weiskopf N, Dick F (2013): Mapping the human cortical surface by combining quantitative T1 with retinotopy. Cereb Cortex 23:2261–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafee R, Buckner RL, Fischl B (2015): Gray matter myelination of 1555 human brains using partial volume corrected MRI images. NeuroImage 105:473–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stüber C, Morawski M, Schäfer A, Labadie C, Wähnert M, Leuze C, Streicher M, Barapatre N, Reimann K, Geyer S, Spemann D, Turner R (2014): Myelin and iron concentration in the human brain: A quantitative study of MRI contrast. NeuroImage 93:95–106. [DOI] [PubMed] [Google Scholar]
- Tardif CL, Schäfer A, Waehnert M, Dinse J, Turner R, Bazin P‐L (2015): Multi‐contrast multi‐scale surface registration for improved alignment of cortical areas. NeuroImage 111:107–122. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Urbanski M, Batrancourt B, Levy R, Dubois B, Cerliani L, Volle E (2016): Rostro‐caudal architecture of the frontal lobes in humans. Cereb Cortex 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Moortele P‐F, Auerbach EJ, Olman C, Yacoub E, Ugurbil K, Moeller S (2009): T1 weighted brain images at 7 Tesla unbiased for Proton Density, T2* contrast and RF coil receive B1 sensitivity with simultaneous vessel visualization. NeuroImage 46:432–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal‐Piñeiro D, Walhovd KB, Storsve AB, Grydeland H, Rohani DA, Fjell AM (2016): Accelerated longitudinal gray/white matter contrast decline in aging in lightly myelinated cortical regions. Hum Brain Mapp 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waehnert MD, Dinse J, Weiss M, Streicher MN, Waehnert P, Geyer S, Turner R, Bazin PL (2014): Anatomically motivated modeling of cortical laminae. NeuroImage 93:210–220. [DOI] [PubMed] [Google Scholar]
- Waehnert MD, Dinse J, Schäfer A, Geyer S, Bazin P‐L, Turner R, Tardif CL (2016): A subject‐specific framework for in vivo myeloarchitectonic analysis using high resolution quantitative MRI. NeuroImage 125:94–107. [DOI] [PubMed] [Google Scholar]
- Wang J, Qiu M, Constable RT (2005): In vivo method for correcting transmit/receive nonuniformities with phased array coils. Magn Reson Med 53:666–674. [DOI] [PubMed] [Google Scholar]
- Westlye LT, Walhovd KB, Dale AM, Bjornerud A, Due‐Tonnessen P, Engvig A, Grydeland H, Tamnes CK, Ostby Y, Fjell AM (2010a): Life‐span changes of the human brain white matter: Diffusion tensor imaging (DTI) and volumetry. Cereb Cortex 20:2055–2068. [DOI] [PubMed] [Google Scholar]
- Westlye LT, Walhovd KB, Dale AM, Bjørnerud A, Due‐Tønnessen P, Engvig A, Grydeland H, Tamnes CK, Østby Y, Fjell AM (2010b): Differentiating maturational and aging‐related changes of the cerebral cortex by use of thickness and signal intensity. NeuroImage 52:172–185. [DOI] [PubMed] [Google Scholar]
- Xi MC, Liu RH, Engelhardt JK, Morales FR, Chase MH (1999): Changes in the axonal conduction velocity of pyramidal tract neurons in the aged cat. Neuroscience 92:219–225. [DOI] [PubMed] [Google Scholar]
- Xiao L, Ohayon D, McKenzie IA, Sinclair‐Wilson A, Wright JL, Fudge AD, Emery B, Li H, Richardson WD (2016): Rapid production of new oligodendrocytes is required in the earliest stages of motor‐skill learning. Nat Neurosci 19:1210–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


