Abstract
Mindfulness has been shown to reduce stress, promote health, and well‐being, as well as to increase compassionate behavior toward others. It reduces distress to one's own painful experiences, going along with altered neural responses, by enhancing self‐regulatory processes and decreasing emotional reactivity. In order to investigate if mindfulness similarly reduces distress and neural activations associated with empathy for others' socially painful experiences, which might in the following more strongly motivate prosocial behavior, the present study compared trait, and state effects of long‐term mindfulness meditation (LTM) practice. To do so we acquired behavioral data and neural activity measures using functional magnetic resonance imaging (fMRI) during an empathy for social pain task while manipulating the meditation state between two groups of LTM practitioners that were matched with a control group. The results show increased activations of the anterior insula (AI) and anterior cingulate cortex (ACC) as well as the medial prefrontal cortex and temporal pole when sharing others' social suffering, both in LTM practitioners and controls. However, in LTM practitioners, who practiced mindfulness meditation just prior to observing others' social pain, left AI activation was lower and the strength of AI activation following the mindfulness meditation was negatively associated with trait compassion in LTM practitioners. The findings suggest that current mindfulness meditation could provide an adaptive mechanism in coping with distress due to the empathic sharing of others' suffering, thereby possibly enabling compassionate behavior. Hum Brain Mapp 38:4034–4046, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: mindfulness meditation, social pain, vicarious embarrassment, empathy, anterior insula
INTRODUCTION
During social interactions we attempt to make sense of others' states like their sensations, emotions or thoughts in order to predict and understand their behavior and react accordingly. One route to understanding others is to empathically share their states and feelings, be it physical pain [Singer et al., 2004], social pain, such as social exclusion [Beeney et al., 2011; Masten et al., 2011; Novembre et al., 2013] and embarrassment [Krach et al., 2011, 2015; Müller‐Pinzler et al., 2016; Paulus et al., 2015b], or joy [Mobbs et al., 2009]. Here, negative affective experiences like social exclusion and embarrassment are considered to be a form of social pain. This relatively new construct of social pain has been established to emphasize the conceptual overlap of the distress and affective arousal with physical pain, which is thought to signal a threat for our bodily integrity. In this line, affective experiences in the domain of social pain serve a similar function, however signaling threats to our social integrity, a core aspect of the embarrassment experience [Eisenberger et al., 2003; Keltner, 1995; Macdonald and Leary, 2005; Müller‐Pinzler et al., 2015]. Neuroimaging and pharmacological studies support this conceptual overlap indicating some common pathways involved in processing both social and physical pain [Dewall et al., 2010; Kross et al., 2011; Paulus et al., 2015b]. It has been suggested, however, that sharing others' negative affective experiences or social pain might also lead to feelings of empathic distress in the observer [Batson, 2009]. Recent studies showed that empathic distress alters social decision making [Sarlo et al., 2014] and increases the egocentric desire to reduce one's own distress [Batson, 1991; Eisenberg et al., 2010]. While empathy is typically positively associated with prosocial behavior and increases the motivation to help the other person in pain [Eisenberg and Miller, 1987] the experience of intense negative affect might also have the opposite consequences and shift the individuals attention toward the own distress [Hoffmann, 2008]. In contrast to the experience of empathy and sympathy, distress has been shown to evoke egoistic motivations to reduce one's own aversive arousal [Batson et al., 1987] and can reduce prosocial helping behavior [Eisenberg et al., 1989; Eisenberg and Fabes, 1990]. Hence, empathizing with others' negative emotions and especially feeling empathic distress may not necessarily have prosocial consequences.
Mindfulness meditation, typically described as a state of “non‐judgmental attention to experiences in the present moment,” is often applied with the purpose to counteract these tendencies, reduce stress and promote health and well‐being [Holzel et al., 2011; Kabat‐Zinn, 1982]. As a psychotherapeutic intervention mindfulness has been shown to produce beneficial effects on mental and physical health [Grossman et al., 2004] as well as on clinical measures and stress‐related symptoms in healthy subjects [Khoury et al., 2015]. Mechanisms involved during mindfulness are assumed to enhance self‐regulatory processes and cognitive reappraisal, as well as decrease emotional reactivity, as shown in depression [Teasdale et al., 1995] or as discussed for physiological reactivity [Holzel et al., 2011; Khoury et al., 2015], thereby reducing distress in response to one's own painful experiences and expectedly also in response to others' suffering. Although compassion meditation has a more specific focus on feelings of positivity and happiness, while mindfulness meditation focuses on non‐judgmentally becoming fully aware of the present moment, recent research has provided evidence that mindfulness meditation in itself enhanced compassion [Wallmark et al., 2013] and compassionate responses to suffering [Condon et al., 2013; Lim et al., 2015]. Other studies also suggest that the practice of mindfulness encourages the development of compassionate emotions [Birnie et al., 2010]. In addition, some Buddhist traditions support the idea that compassion emerges from or is a synonym of pure awareness, which is the goal of the practice of mindfulness [Nakagawa, 2009]. Therefore, mindfulness meditation might inherently impact affective and empathic reactions via processes comprising compassionate responding, which has been found to be a strong predictor of prosocial behavior [Sprecher, 2005].
In this line, studies showed that mindfulness based trainings lead to more engagement in empathy [Lamothe et al., 2016; Shapiro et al., 1998], but less experience of personal distress when confronted with other's suffering [Dekeyser et al., 2008]. Similar effects were found for short term meditation trainings specifically involving exercises and ideas from compassion meditation with enhanced sympathetic concern for, and reduced aversion to the suffering of others on the level of emotion expressions [Rosenberg et al., 2015]. Here, we ask, what the effect of mindfulness meditative practice is on the empathic sharing of a specific form of social pain, namely the experience of embarrassment on behalf of others.
To date, there is a large body of literature which specifically focuses on the neural correlates of empathy for others' physical and social pain. Understanding the threat to another's social integrity is a rather complex process and it is assumed that mainly two distinct but interacting routes are involved enabling humans to understand another's condition [Keysers and Gazzola, 2007; Paulus et al., 2013b; Waytz and Mitchell, 2011]. One of them is referred to as “mentalizing” and involves mental projections of oneself into another person's perspective, reflections about their behavior, and it typically engages brain regions in the temporal lobe and temporo‐parietal junction, the medial prefrontal cortex (mPFC), and the precuneus [Frith and Frith, 2003]. Previous studies showed that these mentalizing areas were recruited when making sense of others' socially painful situations [Müller‐Pinzler et al., 2016; Paulus et al., 2017; Paulus et al., 2015b]. The second route involves so‐called “mirroring” or “sharing” and is assumed to be a direct mapping of others' sensory or affective states and actions on one's own neural system predominantly through sensory and motor streams. When we share others' experiences, the same regions, which are involved in the first‐hand experience, show increased activation [among others the anterior insula (AI) and anterior cingulate cortex (ACC)], leading to the so‐called “shared circuits” hypothesis [Eisenberger et al., 2003; Engen and Singer, 2013; Krach et al., 2011; Kross et al., 2011; Singer et al., 2004]. Activations within these regions are supposed to map the conscious negative affective experience and interoceptive awareness of physiological arousal [Craig, 2003] regardless of it being caused by one's own or another's suffering. The empathic responses of these circuits show strong modulations not only by intra‐individual variation in empathic resonance [Kanske et al., 2015], but also inter‐individual differences in trait empathy [Krach et al., 2011; Singer et al., 2004] with individuals scoring higher in trait empathy showing increased activation in AI and ACC. Interoceptive deficits related to alexithymia are also associated with decreased responses of empathy related brain regions [Bird et al., 2010]. Other studies showed that empathy related neural activations can be modulated by appraisal processes and assumptions about the observer's state of mind [Lamm et al., 2007a, 2007b], suggesting that empathic responses and behavioral reactions to others' suffering are highly variable and can be willfully influenced by cognitive and motivational processes.
Along these lines, a few studies investigated the impact of meditation training on responses to others' suffering and the related neural correlates [Klimecki et al., 2013, 2014]. Although these studies implemented short‐term meditation training that focused on compassion rather than mindfulness, they might provide an important reference for our understanding of empathic responding. This is supported by studies showing that both, mindfulness as well as compassion, are assumed to go along with increased empathy as well as decreased personal distress responses to others' suffering [Dekeyser et al., 2008; Lamothe et al., 2016]. These studies showed that short‐term compassion meditation training increased positive affect in response to others' suffering along with activation of the medial orbitofrontal cortex and ventral striatum, regions typically associated with reward processing [Knutson et al., 2000]. Activations in AI and ACC, potentially mapping shared negative affect and physiological arousal, were reduced while observing others' suffering [Klimecki et al., 2014]. Mindfulness training has been shown to decrease the experience of one's own bodily pain [Haase et al., 2016; Zeidan et al., 2011]. Similarly, subsequent to mindfulness training higher non‐reactivity to own inner (affective) experiences as one aspect of mindfulness was associated with decreased responding of the AI, potentially mapping a mechanism of reduced vulnerability to negative affect [Paul et al., 2013]. However, while some studies found decreased activations of AI and ACC during mindfulness meditation [Ives‐Deliperi et al., 2011], others support the notion that heightened awareness of interoceptive and bodily signals during a state of mindfulness is accompanied by increased activations of the AI [Holzel et al., 2011]. Therefore, the role of the AI especially in response to others' suffering as a consequence of mindfulness training does not seem to be thoroughly clear. Effects of short‐term trainings might also be of limited duration. Therefore, studies on experienced meditators, who are used to practice mindfulness meditation throughout their lives, might provide additional and valuable insights.
Similar to the effects found after mindfulness training, neuroimaging studies on long‐term meditation (LTM) practitioners showed behavioral effects of reduced pain experiences and increased activations of the AI and ACC during the experience of own bodily pain [Gard et al., 2012; Lutz et al., 2013] and merely while being in a state of mindfulness [Fox et al., 2016]. Thereby, altered functioning of the AI was speculated to be based on structural changes of the insula cortex [Fox et al., 2014]. However, study designs varied in several aspects, for example, in how (visual vs. auditory) and if stimuli were presented and when (during vs. after meditation) brain activation was measured. Moreover, many studies assessed effects of mindfulness meditation training on the experience of one's own bodily pain [Gard et al., 2012; Haase et al., 2016; Lutz et al., 2013; Zeidan et al., 2011]. Concentrating on one's own (inner) sensations in a state of mindfulness in contrast to focusing on a social target in pain and experiencing (reduced) personal distress might elicit greatly differential neural activation patterns. We, therefore, assume that comparisons across studies and general conclusions are still limited. Thus, the questions remain how, first, mindfulness meditation affects the experience of socially painful situations and second how empathizing with others' painful states is affected by meditation. Since many traditional meditation practices aim to counteract self‐centered tendencies, decrease egocentric attitudes toward our external world, and increase other‐oriented thinking [see Lutz et al., 2008], it is of great interest to further unravel effects on empathic sharing of others' socially painful experiences.
The aim of this study is therefore to characterize how long‐term mindfulness meditation, which is associated with increased self‐regulation and decreased distress [Holzel et al., 2011], modulates empathy for others' social pain including the related neural activity in the AI. Specifically, we wanted to test if a change in AI activation, which has been shown after short‐term compassion meditation trainings [Klimecki et al., 2014], might be evident in LTM practitioners of mindfulness meditation when confronted with others' social suffering. To this end, we applied a validated empathy for social pain paradigm, during which stimuli are presented that depict social targets in a wide variety of potentially embarrassing and unpleasant social situations [Krach et al., 2011; Müller‐Pinzler et al., 2012; Paulus et al., 2013a, 2015a, 2015b]. Furthermore, it is an open question if LTM induces outlasting dispositional changes, which affect the perception of social situations in general, or if current meditation practice activates a distinctive state, which only affects social perceptions momentarily. We therefore specifically compared state versus trait effects of mindfulness meditation practice while maintaining the subjects' focus on the suffering individual.
MATERIALS AND METHODS
Ethics Statement
We confirm that the research has been conducted in compliance with the ethical guidelines of the American Psychological Association (APA). The study protocol was approved by the Marburg ethics committee at the local faculty of medicine and all subjects gave written informed consent.
Participants
Overall, the study included N = 51 participants. None of them were diagnosed with neurological or psychiatric disorders (present and past), current alcohol or drug abuse, use of psychiatric medications (present and past), anatomical brain abnormalities (e.g., lesions, strokes, etc.). All participants were fluent in German, and had normal or corrected‐to‐normal vision. N = 32 of the participants were experienced LTM practitioners, the others were control subjects (CON), who reported no meditation experience (n = 19, 10 male, aged 42–63 years; M = 50.37; SD = 5.44; see Table 1 for all participant characteristics). LTM practitioners were recruited from Buddhist and Zen centers across Germany and were regularly practicing meditation (M = 32.10 min/day, SD = 23.63). LTM practitioners were randomly assigned to two groups and were either instructed to actively meditate prior to the task (MED_ON, n = 16, 11 male, aged 36–64 years; M = 49.69; SD = 8.90) or rest in the scanner for the same amount of time (MED_OFF, n = 16, 9 male, aged 40–60 years; M = 52.69; SD = 6.61). Years of meditation experience ranged between 5 and 38 years and there was no difference between the two LTM groups (F (1,30) = 0.48, P = 0.494). The three groups did also not differ in terms of sex (Chi2 = 0.995, P = 0.608), age, and IQ (see Table 1). In order to control for differences in the exact meditation styles that meditators were practicing on a regularly basis, we asked participants to indicate the meditation style that they had expertise in. In the following, a meditation expert categorized these meditation styles in Zen meditation, Vipassana meditation, and other types of mindfulness meditation (see Supporting Information Table S1). While about half of the meditators indicated that they practiced one specific mindfulness meditation style (n = 17) the others were practicing two or more different types of mindfulness meditation (n = 15) and distributions did not differ between the two groups in this regard (Chi2 = 0.15, P = 0.928). Frequencies of Zen, Vipassana, and mindfulness practice of other styles did not differ significantly between groups (Zen: Chi2 = 2.03, P = 0.154; Vipassana: Chi2 = 0.00, P > 0.999; other: Chi2 = 2.33, P = 0.127).
Table 1.
Means and standard deviations for sample characteristics and empathy for embarrassment ratings
| CON | MED_OFF | MED_ON | F | P | η2 | ||||
|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | ||||
| Age | 50.37 | 5.44 | 52.69 | 6.61 | 49.69 | 8.90 | 0.81 | 0.452 | 0.03 |
| IQ | 126.11 | 14.20 | 128.75 | 11.14 | 123.50 | 12.31 | 0.68 | 0.511 | 0.03 |
| CLS | 4.17 | 0.82 | 5.05 | 0.65 | 4.95 | 0.59 | 8.39 | 0.001 | 0.26 |
| IRI Fantasy | 3.36 | 0.42 | 3.39 | 0.43 | 3.42 | 0.33 | 0.12 | 0.884 | 0.01 |
| IRI Perspective | 3.61 | 0.51 | 3.72 | 0.36 | 3.63 | 0.48 | 0.29 | 0.749 | 0.01 |
| IRI Empathy | 3.54 | 0.52 | 3.48 | 0.39 | 3.63 | 0.38 | 0.42 | 0.660 | 0.02 |
| IRI Distress | 2.86 | 0.44 | 2.56 | 0.54 | 2.56 | 0.59 | 1.88 | 0.164 | 0.07 |
| Years of Meditation | 17.69 | 11.32 | 15.31 | 7.76 | 0.48 | 0.494 | 0.02 | ||
| Meditation per day (min) | 38.26 | 30.23 | 25.94 | 14.02 | 2.19 | 0.150 | 0.07 | ||
| EE | 4.01 | 0.48 | 3.71 | 0.57 | 3.79 | 0.60 | 1.36 | 0.266 | 0.05 |
| NEUT | 1.05 | 0.10 | 1.06 | 0.09 | 1.12 | 0.22 | 1.17 | 0.318 | 0.05 |
Note. M = mean. SD = standard deviation. CON = control group (10 m/9 f). MED_OFF = group resting before the social pain task (9 m/7 f). MED_ON = group meditating before the social pain task (11 m/5 f). CLS = Compassionate Love Scale. IRI = Interpersonal Reactivity Index, split into four subscales. EE = empathic embarrassment rating during scanning. NEUT = empathic embarrassment rating during scanning. F‐values, P‐values, and η2‐values refer to one‐way ANOVAs comparing variables across the three or two groups, respectively.
General Procedure
In order to distinguish effects of enduring trait characteristics of LTM practitioners from effects of temporary state changes elicited by the preceding meditation practice on empathy for others' social pain, we manipulated the meditation state in a between‐subjects design. Half of the LTM practitioners were randomly assigned to actively meditate for eight minutes before the task (MED_ON group) while the other half had a rest period of equal length prior to the task (MED_OFF group). The meditation and resting period was conducted in the MRI just prior to the empathy for embarrassment experiment. The MED_ON group had the instruction to perform a mindfulness meditation, which typically involves paying “non‐judgmental attention to experiences in the present moment” [Kabat‐Zinn, 1982] and the MED_OFF group was explicitly instructed not to meditate but rest. The CON group was instructed to rest for the same time as the MED_OFF group did.
After providing written informed consent participants were carefully instructed about the experimental procedure. Participants received two example trials of the empathy for embarrassment paradigm for practice that were not displayed during the fMRI session and were asked to mentally visualize the displayed situations as vividly as possible (for the description of the paradigm and stimuli see fMRI paradigm and stimuli). After completion of the fMRI paradigm, participants were asked to fill out the Interpersonal Reactivity Scale [IRI; Davis, 1980] and the Compassionate Love Scale [CLS; Sprecher, 2005] in order to assess their trait levels of empathy as well as compassion.
fMRI Paradigm and Stimuli
During the empathy for embarrassment paradigm all groups viewed the same set of 20 previously validated hand‐drawn sketches depicting embarrassing situations and 10 neutral sketches (NEUT) serving as control situations. The embarrassment situations displayed a social target while he or she was violating a social norm in public and threatened his or her social integrity. Importantly, in 10 of these situations the target was aware about the norm transgression, thus experiencing embarrassment him‐ or herself (EE). From the observers' perspective these situations conform to the definition of empathy, as the social target and the observer share a similar affective state, that is, the embarrassment in response to the norm transgression. These trials were thus included for the purpose of the present analysis of empathic embarrassment. The other ten situations depicted protagonists in embarrassing situation, who were unaware about the norm violation during the situation, thus not experiencing embarrassment themselves. These situations were excluded from the present analyses, since they are conceptually different from true empathic experience as we have previously argued [Paulus et al., 2013b]. Neutral control stimuli displayed the social target in a similar public context, however, without violating socially normative standards. For clarification each sketch was accompanied by a two‐sentence description of the current situation (e.g., “You are at the grocery store: You observe a woman at the cashier who is realizing that she cannot pay her purchase…,” see Fig. 1).
Figure 1.
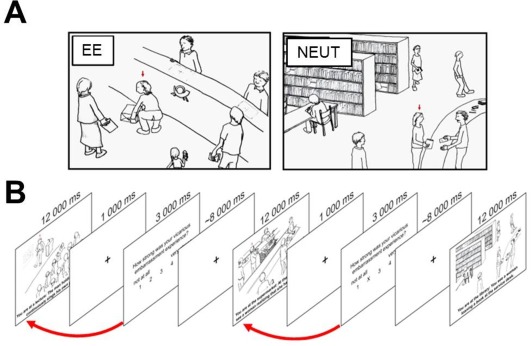
A. Stimuli used in the fMRI experiment. EE situations depicted social targets in embarrassing situations, potentially eliciting empathic embarrassment in the observer. Ten sketches displayed non‐norm‐violating control situations (NEUT). Stimuli were presented together with two sentences describing the situation below the sketches [e.g., You are at a post‐office: you observe a women's trouser ripping while she bends down to lift a package… (EE)]. B. Sequence of the experimental paradigm. Red arrows indicate that subjective ratings of the protagonist's experience of embarrassment correspond to the preceding embarrassment situation. [Color figure can be viewed at http://wileyonlinelibrary.com]
In the MRI, stimuli were presented on an LCD screen with Presentation 12.1 software package (Neurobehavioral Systems, Albany, CA) and the participants were able to see them via a mirror placed on the head coil. All sketches were presented for 12 seconds together with the description of the situation. The text was presented in a black 24‐point non‐serif font (Arial) on a white background in two to three rows below the sketches. The stimulus presentation was followed by a blank screen for 1 second and a subsequent rating period of 3 seconds. In order to focus the participants' attention on the social target's state not on their own feelings, participants were asked to evaluate the intensity of the social target's experience of embarrassment during the preceding situation after each stimulus presentation (“How strongly did the person you were observing experience embarrassment?”). Responses were made on a scale ranging from 1 (“not at all”) to 5 (“very strong”) using a button press of the right hand. A jittered low‐level baseline showing a fixation cross for an average of 8 seconds was interleaved between the rating period and the following trial. Stimuli were presented in a pseudo‐randomized order, ensuring that no type of situation was immediately repeated and there were no accumulations of one specific type of situations during the fMRI measurement. The total experiment lasted 12.14 minutes.
Data Acquisition
Participants were scanned at 3T (Siemens Trio, Erlangen). A BOLD sensitive echo planar imaging (EPI) sequence was used for acquisition of functional volumes during the experiment (TR = 2.2 s, TE = 30 ms, flip angle = 90°, 36 ascending slices, slice thickness = 3 mm, 10% gap, FoV = 192 mm, matrix 64 × 64).
Data Analysis
Behavioral data
Data were analyzed with IBM SPSS Statistics for Windows, Version 22.0 (IBM Corp., 2013, Armonk, NY). Overall, 0.39% of the participants' ratings of the social target's embarrassment were missing and missing values were replaced by the subject and condition specific average. Each participant's ratings were averaged within the empathy for embarrassment and neutral situations. Averaged ratings of embarrassment were then analyzed using analysis of variance (ANOVA) with Group as a between‐subject factor (CON, MED_ON, and MED_OFF) and Condition as a within‐subject factor [empathy for embarrassment (EE) and neutral (NEUT)]. Paired t‐tests comparing the EE condition with the NEUT for each group separately were implemented to test for group specific empathy effects. Additionally, the comparison of the MED_OFF versus CON group for the empathy effect ([CON_EE‐CON_NEUT] vs. [MED_OFF_EE‐MED_OFF_NEUT]) allowed testing for trait effects of LTM on empathy while controlling for state effects. The comparison of the MED_ON versus MED_OFF group tests the state effects of meditation on empathy controlling for the trait effects of LTM that are common to both LTM groups ([MED_OFF_EE‐MED_OFF_NEUT] vs. [MED_ON_EE‐MED_ON_NEUT]).
Functional MRI data
FMRI data was analyzed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm). The first four images were dummy scans and were discarded from further analyses. The remaining 331 EPI volumes were corrected for timing differences of the slice acquisitions, motion‐corrected, and spatially normalized to the standard template of the Montreal Neurological Institute (MNI) using the EPI template. The normalized volumes were resliced with a voxel size of 2 × 2 × 2 mm, smoothed with an 8 mm full‐width half‐maximum isotropic Gaussian kernel, and high‐pass filtered at 1/256 Hz to remove drifts.
Empathic embarrassment related activation
Statistical analysis was performed in a two‐level, mixed‐effects procedure. The fixed‐effects generalized linear model (GLM) on the first level included four epoch regressors modeling hemodynamic responses to the empathy for embarrassment situations (1), the vicarious embarrassment situations not included in the current analyses (1), neutral situations (1), and the rating phase (1) with the abovementioned stimulus durations. The embarrassment ratings after each social pain situation were entered as parametric modulators within both of the embarrassment conditions separately to explain additional variance in neural activation on the within‐subject level. Six additional regressors modeling head movement parameters were included as regressors of no interest.
Beta‐maps of activation in the empathy for embarrassment and the neutral situations were analyzed on the second level. The second‐level analysis of activation differences was conducted with a random‐effects GLM. The GLM contained one factor for the three levels of Group (CON, MED_ON, MED_OFF) and a second factor for the two dependent levels of EE versus NEUT situations. In order to identify brain regions that are involved when experiencing empathy for embarrassment we conducted a conjunction analysis and contrasted the EE against the NEUT condition across all groups ([CON_EE–CON_NEUT] ∩ [MED_ON_EE–MED_ON_NEUT] ∩ [MED_OFF_EE–MED_OFF_NEUT]). Effects of LTM on the trait level were tested by contrasting empathy effects (EE vs. NEUT) for MED_OFF versus CON. Effects of preceding meditation practice on the current state, controlling for trait effects, were tested by comparing empathy effects for MED_ON versus MED_OFF. We investigated down‐regulations of empathy related neural networks (NEUT‐EE) and additionally contrasted EE‐NEUT in order to test for up‐regulations. Both comparisons were first examined within the whole brain and additionally within a functional left AI ROI, which was derived from a previous study on vicarious embarrassment [Krach et al., 2011]. In order to control for differences in meditation style that participants practiced on a regularly basis we set up a random‐effects GLM with one factor containing the difference between levels of EE versus NEUT situations for the three levels of Group (CON, MED_ON, MED_OFF) and meditation style as a covariate across groups coded as Zen experience only, Vipassana experience only and experience in both meditation styles. By this, all comparisons between the two meditation groups were repeated while controlling for meditation style. All analyses were thresholded at P < 0.05 and corrected for multiple comparisons using the respective family‐wise error correction as implemented in SPM8.
In order to investigate how differences in trait level compassion, assessed via the CLS, modulate activations of the AI, Pearson correlations between measures were calculated within each group separately. AI activations were extracted from the peak voxel of the contrast comparing empathy effects for the MED_ON versus MED_OFF group ([MED_ON_EE‐MED_ON_NEUT] − [MED_OFF_EE‐MED_OFF_NEUT]) within the functional AI ROI (x = −44, y = 22, z = 2 mm). In order to account for large variability of meditation experience (in daily practice hours and years of meditation experience) within groups Spearman correlations were calculated to test associations with AI activation.
RESULTS
Behavioral Data
Participants' self‐reports of empathic embarrassment were significantly stronger for EE situations compared with NEUT situations across all groups (F (1,48) = 1215.77, P < 0.001, η2 = 0.962). This effect was also present within each group (CON: t (18) = 25.91, P < 0.001, d = 10.05; EE: M = 4.01; SD = 0.48; NEUT: M = 1.05; SD = 0.10; MOD_OFF: t (15) = 18.11, P < 0.001, d = 7.79; EE: M = 3.71; SD = 0.57; NEUT: M = 1.06; SD = 0.09; MED_ON t (15) = 17.43, P < 0.001, d = 5.72; EE: M = 3.79; SD = 0.60; NEUT: M = 1.12; SD = 0.22). There was no Group × Condition interaction (F (2,48) = 1.65, P = 0.203, η2 = 0.064), no meditation state effect (t (30) = 0.10 P = 0.925, d = 0.034), and no meditation trait effect (t (33) = 1.67, P = 0.104, d = 0.567) on empathic embarrassment ratings during EE compared with NEUT situations.
The LTM groups did not differ in terms of trait compassion as assessed via the CLS (t (30) = 0.45 P = 0.657, d = 0.16) but showed higher levels compared with the CON group (t (49) = 4.11 P < 0.001, d = 1.19). Groups did not differ in terms of trait empathy as assessed with the IRI (see Table 1).
Neuroimaging Data
Common activations across groups
In accordance with our previous studies, we found increased activations of the ACC and the left AI across all groups during EE. Additionally, a region within the mPFC also typically recruited during mentalizing (see Supporting Information Fig. S2) showed increased activity. Together with activations of the caudate nucleus, SMA, as well as a region within the thalamus/brainstem the findings replicate the typical activation patterns in response to empathic embarrassment [P < 0.05, FWE corrected for the whole brain; see Fig. 2 and Table 2; Paulus et al., 2015b]. An additional analysis showed that activation within these areas was specifically associated with vicarious embarrassment ratings varying between all of the presented social situations on the within subject level (see Supporting Information Results and Figures).
Figure 2.
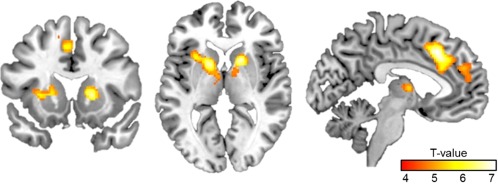
Results of the conjunction analysis of empathic embarrassment (EE) versus neutral (NEUT) situations across groups ([CON_EE–CON_NEUT] ∩ [MED_ON_EE–MED_ON_NEUT] ∩ [MED_OFF_EE–MED_OFF_NEUT]) are depicted family‐wise‐error corrected for the whole brain. For further results see also Table II. [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 2.
Activation during empathic embarrassment compared with neutral situations
| Anatomical region | Cyto area | Side | MNI coordinates | Cluster size | T | P | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Conjunction EE>NEUT | ||||||||
| Cingulate cortex | L/R | 1,188 | ||||||
| Middle cingulate cortex | −6 | 24 | 36 | 6.95 | <0.001 | |||
| Middle frontal gyrus | −20 | 44 | 32 | 5.67 | 0.001 | |||
| Middle cingulate cortex | 6 | 24 | 34 | 5.29 | 0.004 | |||
| Caudate nucleus/putamen | R | 281 | ||||||
| Putamen | 16 | 12 | 4 | 6.77 | <0.001 | |||
| Caudate nucleus | 18 | 8 | 12 | 5.96 | <0.001 | |||
| Thalamus | Th‐Prefrontal | 14 | −2 | 6 | 4.83 | 0.023 | ||
| Anterior insula/putamen | L | 514 | ||||||
| Putamen | −18 | 8 | 6 | 6.70 | <0.000 | |||
| Anterior insula | −28 | 14 | 4 | 5.82 | 0.001 | |||
| Thalamus | Th‐Prefrontal | −10 | −2 | 6 | 5.61 | 0.001 | ||
| Inferior parietal | L | 278 | ||||||
| Inferior parietal lobule | PFt (IPL) | −48 | −36 | 46 | 5.57 | 0.002 | ||
| Supramarginal gyrus | PFt (IPL) | −58 | −30 | 34 | 5.56 | 0.002 | ||
| Postcentral gyrus | Area 3b | −40 | −30 | 54 | 5.06 | 0.010 | ||
| Superior frontal | L | 126 | ||||||
| Superior frontal gyrus | −24 | −6 | 56 | 5.47 | 0.002 | |||
| Posterior‐medial frontal | −16 | 4 | 64 | 5.20 | 0.006 | |||
Note. Results for the conjunction analysis of empathic embarrassment (EE) versus neutral (NEUT) situations across groups ([CON_EE–CON_NEUT] ∩ [MED_ON_EE–MED_ON_NEUT] ∩ [MED_OFF_EE–MED_OFF_NEUT]). The Cyto area column indicates the assigned cytoarchitectonical area derived from the SPM ANATOMY toolbox v2.2b if available [Eickhoff et al., 2005]. All P‐values are family wise error corrected for the whole brain. Clusters with less than 10 voxels are not reported.
Between group comparisons: ROI analyses
Comparisons between the LTM and CON groups showed that there were no significant effects of LTM on the trait level corrected within the ROI in the left AI. In contrast, we did find specific effects of preceding meditation on the current state. LTM practitioners, who had meditated just prior to being exposed to others' embarrassing situations (MED_ON), showed decreased activation of the left AI (t (96) = 3.10, P = 0.020, FWE corrected within left AI ROI; x = −44, y = 22, z = 2 mm) compared with the MED_OFF group, indicating a down‐regulation of AI activity during empathic embarrassment (see Fig. 3). Controlling for meditation style did not change the finding of lower AI activation in the MED_ON versus MED_OFF group in response to EE versus NEUT situations substantially (t (47) = 3.10, P = 0.027, FWE corrected within left AI ROI; x = −44, y = 22, z = 0 mm).
Figure 3.
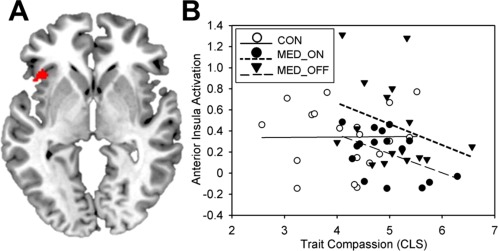
A. Decreased activation of the left AI in the MED_ON versus MED_OFF group during empathic embarrassment (EE) versus neutral situations (NEUT) indicating a down‐regulation of shared circuits activation in response prior meditation ([MED_ON_EE–MED_ON_NEUT]<[MED_OFF_EE–MED_OFF_NEUT]). Results are depicted uncorrected for displaying purposes within the left AI ROI. B. Correlation plots for Compassionate Love (CLS) scores and activation of the left anterior insula for control group (CON; r (17) = 0.01, P = 0.484), the meditation group (MED_ON; r (14) = −0.49, P = 0.028), and the rest group (MED_OFF; r (14) = −0.31, P = 0.119). Parameter estimates of the left AI are derived from the peak voxel for the contrast [MED_ON_EE–MED_ON_NEUT]<[MED_OFF_EE–MED_OFF_NEUT] (x = −44, y = 22, z = 2 mm). [Color figure can be viewed at http://wileyonlinelibrary.com]
Between group comparisons: whole brain analyses
Similarly, comparisons between the LTM and CON groups showed no significant effects of trait LTM in the whole brain analysis. Neither was there an increase of activation in LTM practitioners (MED_OFF) compared with the CON group, nor a down‐regulation of any brain region as indicated by a whole‐brain analysis (P < 0.05, FWE corrected) even at more lenient thresholds (P < 0.001, uncorrected).
There was no additional up‐regulation or down‐regulation associated with the current meditation state corrected on the whole brain level (P < 0.05, FWE corrected). On a more lenient threshold there was an additional down‐regulation for the MED_ON compared with the MED_OFF group in the middle cingulate cortex in response to EE versus NEUT situations (t (96) = 3.98, P < 0.001, k > 50; x = 2, y = 0, z = 28 mm). When controlling for meditation style we found similar results (t (47) = 4.17, P < 0.001, k > 50; x = 2, y = 2, z = 28 mm).
Associations of neural activation and trait measures
Correlation analyses showed a negative correlation of left AI activation and trait compassion (r (14) = −0.49, P = 0.028) for the MED_ON group (within the region showing decreased activation in the MED_ON vs. MED_OFF group; x = −44, y = 22, z = 2 mm, see Fig. 3). The MED_OFF group also showed a negative association with CLS, which however was not significant (r (14) = −0.31, P = 0.119; pooled partial correlation across LTM groups controlled for group: r (29) = −0.36, P = 0.047). There was no association of CLS scores and left AI activation in the CON group (r (17) = 0.01, P = 0.484). Further, years of meditation experience did not correlate significantly with AI activation (MED_ON: r (14) = 0.06, P = 0.416; MED_OFF: r (14) = −0.13, P = 0.312). Daily meditation practice showed no association with left AI activation in the MED_OFF group (r (14) = 0.15, P = 0.295). In comparison there was a stronger negative association of daily meditation practice and left AI activation in the MED_ON group (trend‐wise effects; r (14) = −0.42, P = 0.053; MED_ON vs. MED_OFF: z = 1.51, P = 0.065). The negative association of daily meditation practice and left AI activation was also stronger compared with the correlation of the years of meditation practice and AI activation in the MED_ON group (trend: z = 1.29, P = 0.099).
DISCUSSION
In the present study we examined how LTM modulates the experience of empathic embarrassment on behalf of others' flaws, failures or norm violations and its processing on the neural systems level. We therefore tested a sample of LTM practitioners, who had been practicing various mindfulness meditation techniques for many years prior to our study, and have compassionate characteristics, as indicated by higher trait compassion levels. As previous studies already showed [Krach et al., 2011; Paulus et al., 2015b] empathic embarrassment triggered activity within areas of the mentalizing network, that have been related to the evaluation of the social target's mental state and image threats during the embarrassing incident [Müller‐Pinzler et al., 2016; Paulus et al., 2015b], in control subjects as well as LTM practitioners. Similarly, activations of the AI and ACC were increased as a measure for an empathic sharing of others' embarrassing moments. There were no differences in the neural response between the groups of LTM practitioners and controls that could be related to trait differences. LTM practitioners, however, who meditated just prior to the task, showed decreased activations of the left AI in response to empathic embarrassment. This finding rather argues for state‐related changes in empathic responding. Additionally, left AI activation was correlated negatively with trait compassion and meditation experience specifically in the group of LTM practitioners, who meditated before the task.
Noteworthy, the activation decrease in the left AI when LTM practitioners engaged in a meditative state just before completing the empathic embarrassment paradigm is in line with earlier studies showing a down‐regulation of AI activation after mindfulness training in response to own painful experiences [Haase et al., 2016] and negative affective stimuli [Paul et al., 2013] and also after short‐term compassion meditation training more specifically in response to another's suffering [Klimecki et al., 2014]. The AI is a brain region that is assumed to be implicated in processing of physiological arousal and the conscious experience of one's internal bodily and affective states [Craig, 2003; Critchley et al., 2004]. Earlier studies on empathic embarrassment showed that the intensity of subjective experiences of vicarious embarrassment on behalf of another person is significantly associated with AI activations [Paulus et al., 2015b]. This points to the AI's role in generating an interoceptive representation while affectively and empathically sharing another's embarrassing moments. Previous studies investigated structural changes in LTM practitioners and have suggested increased thickness [Lazar et al., 2005], increased cortical folding complexity [Luders et al., 2012], as well as increased connectivity of white matter within the insula cortex in meditators [Laneri et al., 2016]. These structural changes might constitute the architecture that underlies altered functioning of the AI cortex in LTM practitioners.
With this study we could show that LTM practitioners show reduced empathic sharing of the another's suffering on the neural systems level when engaged in a meditative state prior to observing others' socially painful situations. Enduring trait changes and specifically increases in compassionate responding are an explicit goal of most meditation traditions and mindfulness meditation is associated with increased self‐compassion, and kindness [Holzel et al., 2011] as well as compassionate behavior toward others [Condon et al., 2013; Lim et al., 2015]. The aim is to keep one's negative feelings and thoughts in a “balanced awareness instead of over‐identifying with them” [Neff, 2003], which might be a mechanism to reduce distress in response to one's own but also another's negative feeling states [Holzel et al., 2011]. Improving the regulation of painful thoughts, emotions, and body sensations, no matter if they are one's own or shared experiences of another, and developing compassionate feelings instead might be a mechanism associated with altered insula functioning [Fox et al., 2016; Holzel et al., 2011]. This is supported by the negative correlation of trait compassion and left AI activation in the LTM group, which meditated just prior to the experiment. Our finding suggests that a more compassionate mindset during meditation might reduce the experience of empathic distress and interoceptive sharing of the other's suffering.
However, LTM practitioners are still able to recognize the target's social pain as indicated by the ratings for the affective experience of the social target, which did not differ from the control participants' ratings. In response to a meditative state LTM practitioners might be relying on mentally projecting themselves in the social target's position and simulating the other's mental state in order to make sense of his or her emotions [see Paulus et al., 2013b; Waytz and Mitchell, 2011]. While there are typically two streams of information processing, namely mentalizing and sharing [Keysers and Gazzola, 2007; Waytz and Mitchell, 2011], which according to our results might be recruited by all of the participants, the potential down‐regulation of AI activation in the MED_ON group suggests that meditators might less strongly rely on interoceptively sharing the other person's negative affect following a meditative state. However, while down‐regulating shared arousal, mentalizing about the other person's state of mind might still provide the information to make sense of the social situation and to understand the social integrity threat [see also Paulus et al., 2015b]. In doing so, meditators might be able to cultivate positive feelings of compassion for the social target [Klimecki et al., 2013] instead of strongly interoceptively sharing the other's distress. Mental projection hereby is assumed to recruit mentalizing areas like the mPFC [Frith and Frith, 2003; Waytz and Mitchell, 2011], which showed similarly enhanced activations in controls and LTM practitioners when observing others' social pain. Fully perceiving and immersing themselves into the social situation while they do not engage in an intense interoceptive sharing of the other's painful experience might enable LTM practitioners to more strongly engage in prosocial behaviors in response to a meditative state [Leiberg et al., 2011]. However, we did not assess the participants' own affective states and positive emotions during the embarrassment paradigm because we aimed to maintain a non‐egocentric focus on the social target's suffering. Hence, further studies are needed to assess the LTM's affective state and its direct associations with AI and ACC activity as well as to investigate behavioral consequences during social situations.
Trait effects of LTM alone did not impact activations of the AI, when LTM practitioners did not meditate prior to the paradigm. This lack of significant differences in neural activation does not necessarily prove the absence of differences between groups and should be considered with caution since the non‐finding could be related to statistical power. Nevertheless, the trend‐wise positive correlation of the daily amount of meditation practice and left AI activation, which was only present when LTM practitioners meditated prior to the experiment, supports the notion that activation differences might indeed be more specifically associated with the state of mindfulness meditation. Noteworthy, the actual plain years of meditation experience were not associated with decreased activations of the AI; it was the magnitude of daily practice that had a stronger impact on the participants' neural responding to others' social suffering.
Daily practice of LTM might thus not induce outlasting dispositions or changes of neural functioning related to empathic responding, which automatically alter the processing and appraisal of social situations. It, however, seems to enable individuals to willfully engage in meditation practice and by doing so activate a distinctive mental state that alters their AI activation in response to other human beings' social pain. The results might indicate that interoceptive sharing of others' social suffering is reduced only by this state of mindfulness and nonjudgmental attention to experiences in the present moment, which is associated with decreased levels of distress [Khoury et al., 2015], as well as potentially a compassionate mindset. While there are studies speaking in favor of more persistent effects of meditation like structural changes of the AI [Laneri et al., 2016; Lazar et al., 2005], and previous longitudinal training studies did find outlasting effects of, for example, altered amygdala activity in response to affective stimuli transferring to non‐meditative states [Desbordes et al., 2012], most studies did not directly investigate state versus trait effects [for further discussion see Fox et al., 2016]. Nevertheless, there are other findings pointing toward specific meditation state effects that do not manifest in the absence of any meditative practice at least for specific tasks or circumstances like effects on somatosensory brain representations of interoceptive states [see Lutz et al., 2009]. This might indicate that meditation effects do not necessarily have to be persistent and automatic but might require willful engagement in meditation practice in order to manifest in neural activation differences.
The discrepancy between study findings might be task specific, for example, due to rather subtle effects of LTM expertise during processing of highly complex social situations as presented in form of pictorial stimuli eliciting embarrassment compared with tasks involving the experience of own bodily pain, which might induce rather strong effects. However, outlasting effects that are independent of the meditation state might also have been facilitated by the way short‐term meditation trainings were implemented in some of the previous studies (e.g., training and test in the same study context) or by the way participants were asked to rate their own affect in response to affective stimuli instead of solely focusing on the social target.
Nevertheless, we did find a negative correlation of trait compassion and AI activation in the MED_ON group, which was not present in the CON group, indicating the importance of trait differences for regulating the AI's response. At the same time the higher trait compassion scores in LTM practitioners compared with controls reflect that LTM practitioners exhibit a greater propensity to experience intense feelings of compassion, likely induced by long‐term practice in increasing altruistic attitudes toward their external world. It has to be considered, however, that differences in trait compassion are not necessarily acquired by meditation training, although the goal of many meditation traditions is to install permanent trait changes [Fox et al., 2016]. Nevertheless, both findings might point to the assumption that the down‐regulation of AI activation is related to the participants' learned ability to experience compassion for other human beings and suggest enduring underlying changes in mental functioning in LTM practitioners. Trait compassion and state meditation practice might interact in a way that inter‐individual differences in trait compassion codetermine the effectiveness of state meditation practice for the modulation of neural activation. We did not assess, however, if state meditation would affect responses in novices in a similar way. In order to further support our assumptions studies on novices will be needed.
Our results seem to point toward an effect of state meditation, which might alter neural processing when meditators willfully engage in meditation practice. In addition, trait characteristics of experienced meditators might gain specific importance with meditators higher in trait compassion showing a stronger down‐regulation of AI activation during state meditation. However, the absence of significant differences in AI activation for the meditation trait condition (MED_OFF) does not necessarily mean that there are no such meditation trait effects. The positive but non‐significant correlation of trait compassion and AI activation in the MED_OFF group might even point toward a potential effect of meditation specific traits that might not be detected due to insufficient power. However, this is rather speculative and future studies are needed in order to draw further conclusions.
Earlier studies could already show that meditation has beneficial effects on mood and health [Fredrickson et al., 2008; Pace et al., 2009], it increases prosocial behavior [Leiberg et al., 2011] and reduces sharing of negative affect in trained meditation novices [Klimecki et al., 2014]. A willful down‐regulation of shared circuit activations in LTM practitioners might provide a potentially powerful long‐term coping mechanism of decreased sharing of others' socially or physically painful experiences during social interactions while remaining fully aware of the situation and its impact on the social target. This might specifically affect individuals who are routinely confronted with other human beings that suffer from physical or mental illnesses, like people working in caretaking provisions. While the specificity and applicability of meditation practice on a daily basis needs to be further investigated (e.g., longitudinal studies), mindfulness and cultivating feelings of compassion might provide potential mechanisms to induce state changes that facilitates coping with distressing social situations of our daily lives.
Supporting information
Supporting Information
REFERENCES
- Batson CD (1991): The Altruism Question: Toward a Social‐Psychological Answer. Hillsdale, NJ: Erlbaum. [Google Scholar]
- Batson CD (2009): These things called empathy: Eight related but distinct phenomena In: Decety J, Ickes W, editors. The Social Neuroscience of Empathy. Cambridge: MIT press; pp 3–15. [Google Scholar]
- Batson CD, Fultz J, Schoenrade PA (1987): Distress and empathy: Two qualitatively distinct vicarious emotions with different motivational consequences. J Pers 55:19–39. [DOI] [PubMed] [Google Scholar]
- Beeney JE, Franklin RG, Levy KN, Adams RB (2011): I feel your pain: Emotional closeness modulates neural responses to empathically experienced rejection. Soc Neurosci 6:369–376. [DOI] [PubMed] [Google Scholar]
- Bird G, Silani G, Brindley R, White S, Frith U, Singer T (2010): Empathic brain responses in insula are modulated by levels of alexithymia but not autism. Brain 133:1515–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnie K, Speca M, Carlson LE (2010): Exploring self‐compassion and empathy in the context of mindfulness‐based stress reduction (MBSR). Stress Heal 26:359–371. [Google Scholar]
- Condon P, Desbordes G, Miller WB, DeSteno D (2013): Meditation increases compassionate responses to suffering. Psychol Sci 24:2125–2127. [DOI] [PubMed] [Google Scholar]
- Craig ADB (2003): Interoception: The sense of the physiological condition of the body. Curr Opin Neurobiol 13:500–505. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ (2004): Neural systems supporting interoceptive awareness. Nat Neurosci 7:189–195. [DOI] [PubMed] [Google Scholar]
- Davis MH (1980): A multidimensional approach to individual differences in empathy. JSAS Cat Sel Doc Psychol 10:85. [Google Scholar]
- Dekeyser M, Raes F, Leijssen M, Leysen S, Dewulf D (2008): Mindfulness skills and interpersonal behaviour. Pers Individ Dif 44:1235–1245. [Google Scholar]
- Desbordes G, Negi LT, Pace TWW, Wallace BA, Raison CL, Schwartz EL (2012): Effects of mindful‐attention and compassion meditation training on amygdala response to emotional stimuli in an ordinary, non‐meditative state. Front Hum Neurosci 6:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewall CN, Macdonald G, Webster GD, Masten CL, Baumeister RF, Powell C, Combs D, Schurtz DR, Stillman TF, Tice DM, Eisenberger NI (2010): Acetaminophen reduces social pain: Behavioral and neural evidence. Psychol Sci 21:931–937. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Miller PA (1987): The relation of empathy to prosocial and related behaviors. Psychol Bull 101:91–119. [PubMed] [Google Scholar]
- Eisenberg N, Fabes RA (1990): Empathy: Conceptualization, measurement, and relation to prosocial behavior. Motiv Emot 14:131–149. [Google Scholar]
- Eisenberg N, Fabes RA, Miller PA, Fultz J, Shell R, Mathy RM, Reno RR (1989): Relation of sympathy and personal distress to prosocial behavior: A multimethod study. J Pers Soc Psychol 57:55–66. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Eggum ND, Di Giunta L (2010): Empathy‐related responding: Associations with prosocial behavior, aggression, and intergroup relations. Soc Issues Policy Rev 4:143–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD (2003): Does rejection hurt? An FMRI study of social exclusion. Science (80) 302:290–292. [DOI] [PubMed] [Google Scholar]
- Engen HG, Singer T (2013): Empathy circuits. Curr Opin Neurobiol 23:275–282. [DOI] [PubMed] [Google Scholar]
- Fox KCR, Nijeboer S, Dixon ML, Floman JL, Ellamil M, Rumak SP, Sedlmeier P, Christoff K (2014): Is meditation associated with altered brain structure? A systematic review and meta‐analysis of morphometric neuroimaging in meditation practitioners. Neurosci Biobehav Rev 43:48–73. [DOI] [PubMed] [Google Scholar]
- Fox KCR, Dixon ML, Nijeboer S, Girn M, Floman JL, Lifshitz M, Ellamil M, Sedlmeier P, Christoff K (2016): Functional neuroanatomy of meditation: A review and meta‐analysis of 78 functional neuroimaging investigations. Neurosci Biobehav Rev 65:208–228. [DOI] [PubMed] [Google Scholar]
- Fredrickson BL, Cohn MA, Coffey KA, Pek J, Finkel SM (2008): Open hearts build lives: Positive emotions, induced through loving‐kindness meditation, build consequential personal resources. J Pers Soc Psychol 95:1045–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith U, Frith CD (2003): Development and neurophysiology of mentalizing. Philos Trans R Soc Lond B Biol Sci 358:459–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard T, Holzel BK, Sack a. T, Hempel H, Lazar SW, Vaitl D, Ott U (2012): Pain attenuation through mindfulness is associated with decreased cognitive control and increased sensory processing in the brain. Cereb Cortex 22:2692–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman P, Niemann L, Schmidt S, Walach H (2004): Mindfulness‐based stress reduction and health benefits. J Psychosom Res 57:35–43. [DOI] [PubMed] [Google Scholar]
- Haase L, Thom NJ, Shukla A, Davenport PW, Simmons AN, Stanley E. a, Paulus MP, Johnson DC (2016): Mindfulness‐based training attenuates insula response to an aversive interoceptive challenge. Soc Cogn Affect Neurosci 11:182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann ML (2008): Empathy and Prosocial Behavior In: Lewis M, Haviland‐Jones JM, Feldman Barrett L, editors. Handbook of Emotions, Vol. 3, 3rd ed. New York: Wiley; pp 440–456. [Google Scholar]
- Holzel BK, Lazar SW, Gard T, Schuman‐Olivier Z, Vago DR, Ott U (2011): How does mindfulness meditation work? Proposing mechanisms of action from a conceptual and neural perspective. Perspect Psychol Sci 6:537–559. [DOI] [PubMed] [Google Scholar]
- Ives‐Deliperi VL, Solms M, Meintjes EM (2011): The neural substrates of mindfulness: An fMRI investigation. Soc Neurosci 6:231–242. [DOI] [PubMed] [Google Scholar]
- Kabat‐Zinn J (1982): An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: Theoretical considerations and preliminary results. Gen Hosp Psychiatry 4:33–47. [DOI] [PubMed] [Google Scholar]
- Kanske P, Böckler A, Trautwein F‐M, Singer T (2015): Dissecting the social brain: Introducing the EmpaToM to reveal distinct neural networks and brain–behavior relations for empathy and Theory of Mind. Neuroimage 122:6–19. [DOI] [PubMed] [Google Scholar]
- Keltner D (1995): Signs of appeasement: Evidence for the distinct displays of embarrassment, amusement, and shame. J Pers Soc Psychol 68:441–454. [Google Scholar]
- Keysers C, Gazzola V (2007): Integrating simulation and theory of mind: From self to social cognition. Trends Cogn Sci 11:194–196. [DOI] [PubMed] [Google Scholar]
- Khoury B, Sharma M, Rush SE, Fournier C (2015): Mindfulness‐based stress reduction for healthy individuals: A meta‐analysis. J Psychosom Res 78:519–528. [DOI] [PubMed] [Google Scholar]
- Klimecki OM, Leiberg S, Lamm C, Singer T (2013): Functional neural plasticity and associated changes in positive affect after compassion training. Cereb Cortex 23:1552–1561. [DOI] [PubMed] [Google Scholar]
- Klimecki OM, Leiberg S, Ricard M, Singer T (2014): Differential pattern of functional brain plasticity after compassion and empathy training. Soc Cogn Affect Neurosci 9:873–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Westdorp a, Kaiser E, Hommer D (2000): FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage 12:20–27. [DOI] [PubMed] [Google Scholar]
- Krach S, Cohrs JC, de Echeverría Loebell NC, Kircher T, Sommer J, Jansen A, Paulus FM (2011): Your flaws are my pain: Linking empathy to vicarious embarrassment. PLoS One 6:e18675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krach S, Kamp‐Becker I, Einhäuser W, Sommer J, Frässle S, Jansen A, Rademacher L, Müller‐Pinzler L, Gazzola V, Paulus FM (2015): Evidence from pupillometry and fMRI indicates reduced neural response during vicarious social pain but not physical pain in autism. Hum Brain Mapp 36:4730–4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kross E, Berman MG, Mischel W, Smith EE, Wager TD (2011): Social rejection shares somatosensory representations with physical pain. Proc Natl Acad Sci U S A 108:6270–6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Batson CD, Decety J (2007a): The neural substrate of human empathy: Effects of perspective‐taking and cognitive appraisal. J Cogn Neurosci 19:42–58. [DOI] [PubMed] [Google Scholar]
- Lamm C, Nusbaum HC, Meltzoff AN, Decety J (2007b): What are you feeling? Using functional magnetic resonance imaging to assess the modulation of sensory and affective responses during empathy for pain. PLoS One 2:e1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamothe M, Rondeau É, Malboeuf‐Hurtubise C, Duval M, Sultan S (2016): Outcomes of MBSR or MBSR‐based interventions in health care providers: A systematic review with a focus on empathy and emotional competencies. Complement Ther Med 24:19–28. [DOI] [PubMed] [Google Scholar]
- Laneri D, Schuster V, Dietsche B, Jansen A, Ott U, Sommer J (2016): Effects of long‐term mindfulness meditation on brain's white matter microstructure and its aging. Front Aging Neurosci 7:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar SW, Kerr CE, Wasserman RH, Gray JR, Greve DN, Treadway MT, McGarvey M, Quinn BT, Dusek JA, Benson H, Rauch SL, Moore CI, Fischl B (2005): Meditation experience is associated with increased cortical thickness. Neuroreport 16:1893–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiberg S, Klimecki O, Singer T (2011): Short‐term compassion training increases prosocial behavior in a newly developed prosocial game. PLoS One 6:e17798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim D, Condon P, DeSteno D (2015): Mindfulness and compassion: An examination of mechanism and scalability. Ed. J. David Creswell. PLoS One 10:e0118221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Kurth F, Mayer EA, Toga AW, Narr KL, Gaser C (2012): the unique brain anatomy of meditation practitioners: Alterations in cortical gyrification. Front Hum Neurosci 6:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Brefczynski‐Lewis J, Johnstone T, Davidson RJ (2008): Regulation of the neural circuitry of emotion by compassion meditation: Effects of meditative expertise. PLoS One 3:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Greischar LL, Perlman DM, Davidson RJ (2009): BOLD signal in insula is differentially related to cardiac function during compassion meditation in experts vs. novices. Neuroimage 47:1038–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, McFarlin DR, Perlman DM, Salomons TV, Davidson RJ (2013): Altered anterior insula activation during anticipation and experience of painful stimuli in expert meditators. Neuroimage 64:538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald G, Leary MR (2005): Why does social exclusion hurt? The relationship between social and physical pain. Psychol Bull 131:202–223. [DOI] [PubMed] [Google Scholar]
- Masten CL, Morelli S. a, Eisenberger NI (2011): An fMRI investigation of empathy for “social pain” and subsequent prosocial behavior. Neuroimage 55:381–388. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Yu R, Meyer M, Passamonti L, Seymour B, Calder AJ, Schweizer S, Frith CD, Dalgleish T (2009): A key role for similarity in vicarious reward. Science (80) 324:900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller‐Pinzler L, Paulus FM, Stemmler G, Krach S (2012): Increased autonomic activation in vicarious embarrassment. Int J Psychophysiol 86:74–82. [DOI] [PubMed] [Google Scholar]
- Müller‐Pinzler L, Gazzola V, Keysers C, Sommer J, Jansen A, Frässle S, Einhäuser W, Paulus FM, Krach S (2015): Neural pathways of embarrassment and their modulation by social anxiety. Neuroimage 119:252–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller‐Pinzler L, Rademacher L, Paulus FM, Krach S (2016): When your friends make you cringe: Social closeness modulates vicarious embarrassment‐related neural activity. Soc Cogn Affect Neurosci 11:466–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa Y (2009): Awareness and Compassion for the Education of Enlightenment. New York: Springer: pp 593–609. [Google Scholar]
- Neff KD (2003): The development and validation of a scale to measure self‐compassion. Self Identity 2:223–250. [Google Scholar]
- Novembre G, Zanon M, Silani G (2013): Empathy for social exclusion involves the sensory‐discriminative component of pain: A within‐subject fMRI study. Soc Cogn Affect Neurosci 10:153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TWW, Negi LT, Adame DD, Cole SP, Sivilli TI, Brown TD, Issa MJ, Raison CL (2009): Effect of compassion meditation on neuroendocrine, innate immune and behavioral responses to psychosocial stress. Psychoneuroendocrinology 34:87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul NA, Stanton SJ, Greeson JM, Smoski MJ, Wang L (2013): Psychological and Neural Mechanisms of Trait Mindfulness in Reducing Depression Vulnerability Running Title: Mindfulness in Protection from Negative Bias. Soc Cogn Affect Neurosci 8:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus FM, Kamp‐Becker I, Krach S (2013a): Demands in reflecting about another's motives and intentions modulate vicarious embarrassment in autism spectrum disorders. Res Dev Disabil 34:1312–1321. [DOI] [PubMed] [Google Scholar]
- Paulus FM, Müller‐Pinzler L, Westermann S, Krach S (2013b): On the distinction of empathic and vicarious emotions. Front Hum Neurosci 7:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus FM, Krach S, Blanke M, Roth C, Belke M, Sommer J, Müller‐Pinzler L, Menzler K, Jansen A, Rosenow F, Bremmer F, Einhäuser W, Knake S (2015a): Fronto‐insula network activity explains emotional dysfunctions in juvenile myoclonic epilepsy: Combined evidence from pupillometry and fMRI. Cortex 65:219–231. [DOI] [PubMed] [Google Scholar]
- Paulus FM, Müller‐Pinzler L, Jansen A, Gazzola V, Krach S (2015b): Mentalizing and the role of the posterior superior temporal sulcus in sharing others' embarrassment. Cereb Cortex 25:2065–2075. [DOI] [PubMed] [Google Scholar]
- Paulus FM, Müller‐Pinzler L, Stolz D, Mayer A, Rademacher L, Krach S (2017): Laugh or cringe? Common and distinct processes of reward‐based “Schadenfreude” and empathy‐based “Fremdscham.” Neuropsychologia doi: 10.1016/j.neuropsychologia.2017.05.030. [DOI] [PubMed]
- Rosenberg EL, Zanesco AP, King BG, Aichele SR, Jacobs TL, Bridwell DA, MacLean KA, Shaver PR, Ferrer E, Sahdra BK, Lavy S, Wallace BA, Saron CD (2015): Intensive meditation training influences emotional responses to suffering. Emotion 15:775–790. [DOI] [PubMed] [Google Scholar]
- Sarlo M, Lotto L, Rumiati R, Palomba D (2014): If it makes you feel bad, don't do it! Egoistic rather than altruistic empathy modulates neural and behavioral responses in moral dilemmas. Physiol Behav 130:127–134. [DOI] [PubMed] [Google Scholar]
- Shapiro SL, Schwartz GE, Bonner G (1998): Effects of mindfulness‐based stress reduction on medical and premedical students. J Behav Med 21:581–599. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD (2004): Empathy for pain involves the affective but not sensory components of pain. Science (80) 303:1157–1162. [DOI] [PubMed] [Google Scholar]
- Sprecher S (2005): Compassionate love for close others and humanity. J Soc Pers Relat 22:629–651. [Google Scholar]
- Teasdale JD, Segal Z, Williams JMG (1995): How does cognitive therapy prevent depressive relapse and why should attentional control (mindfulness) training help?. Behav Res Ther 33:25–39. [DOI] [PubMed] [Google Scholar]
- Wallmark E, Safarzadeh K, Daukantaitė D, Maddux RE (2013): Promoting altruism through meditation: An 8‐week randomized controlled pilot study. Mindfulness (N Y) 4:223–234. [Google Scholar]
- Waytz A, Mitchell JP (2011): Two mechanisms for simulating other minds: Dissociations between mirroring and self‐projection. Curr Dir Psychol Sci 20:197–200. [Google Scholar]
- Zeidan F, Martucci KT, Kraft RA, Gordon NS, McHaffie JG, Coghill RC (2011): Brain mechanisms supporting the modulation of pain by mindfulness meditation. J Neurosci 31:5540–5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


