Abstract
Gamma‐aminobutyric acid (GABA) and glutamate are believed to have inhibitory and exhibitory neuromodulatory effects that regulate the brain's response to sensory perception. Furthermore, frequency‐specific synchronization of neuronal excitability within the gamma band (30–80 Hz) is attributable to a homeostatic balance between excitation and inhibition. However, our understanding of the physiological mechanism underlying gamma rhythms is based on animal models. Investigations of the relationship between GABA concentrations, glutamate concentrations, and gamma band activity in humans were mostly restricted to the visual cortex and are conflicting. Here, we performed a multimodal imaging study combining magnetic resonance spectroscopy (MRS) with electroencephalography (EEG) in the auditory cortex. In 14 healthy subjects, we investigated the impact of individual differences in GABA and glutamate concentration on gamma band response (GBR) following auditory stimulus presentation. We explored the effects of bulk GABA on the GBR across frequency (30–200 Hz) and time (−200 to 600 ms) and found no significant relationship. Furthermore, no correlations were found between gamma peak frequency or power measures and metabolite concentrations (GABA, glutamate, and GABA/glutamate ratio). These findings suggest that, according to MRS measurements, and given the auditory stimuli used in this study, GABA and glutamate concentrations are unlikely to play a significant role in the inhibitory and excitatory drive in the generation of gamma band activity in the auditory cortex. Hum Brain Mapp 38:3975–3987, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: proton magnetic resonance spectroscopy, gamma‐band response, gamma oscillations, EEG, gamma‐aminobutyric acid, auditory system
INTRODUCTION
Rhythmic patterns of action potentials in the brain have been of renewed interest in clinical and cognitive neuroscience [Başar, 2013; Busch et al., 2006; Herrmann et al., 2010; Roux and Uhlhaas, 2014; Uhlhaas and Singer, 2013; Wang, 2010]. Oscillatory rhythms in the gamma‐band, that is, the frequency range from 30 to 80 Hz, play a pivotal role in facilitating neural synchronization [Buzsaki, 2006; Gray et al., 1989; Singer, 1999]. However, the exact mechanisms underlying gamma oscillations are still not understood. Animal and modelling studies have shown that gamma‐aminobutyric acid (GABA)‐mediated inhibition appears to be a key element in the generation of gamma rhythm [Bartos et al., 2007; Buzsáki and Wang, 2012; Wang and Buzsáki, 1996]. The prominent pyramidal interneuron network gamma model [Whittington et al., 2000] states that pyramidal cells receive negative feedback inhibition by GABAergic interneurons, especially those expressing the calcium binding protein parvalbumin (PV) [Sohal et al., 2009]. This induces a transition from the asynchronously to the synchronous firing of pyramidal neurons through a shifting balance between inhibition and excitation [Brunel and Wang, 2003] (for a review, see Buzsáki and Wang [2012]).
Inhibition–excitation balance of cortical cell assemblies ensures that neuronal activity does not become excessive. The neurotransmitters GABA and glutamate are crucial in regulating the inhibition–excitation balance and thus the brain's response to afferent sensory input [Carcea and Froemke, 2013; Metherate, 2012]. Continuously adapting to new challenges and environments requires selective adjustment of pyramidal–interneuron network properties to improve perceptual abilities and behavioral performance [Carcea and Froemke, 2013]. Direct evidence comes from optogenetic studies where individual interneurons are modulated to demonstrate a direct effect on behavioral functions [Kvitsiani et al., 2013; Sohal et al., 2009].
However, the reliable quantification of GABA and glutamate, which are involved in this inhibition–exhibition mechanism in the human brain, is difficult to attain. GABA‐A receptors can be quantified by means of 11C‐flumazenil positron emission tomography (PET) measurements [Frankle et al., 2015]. Recent findings provided a direct relationship between gamma oscillations and GABA‐A receptors in the primary visual cortex [Kujala et al., 2015]. Direct, noninvasive detection of endogenous GABA in vivo in the brain is done by magnetic resonance spectroscopy (MRS). In MRS, the GABA signal is often obscured by more abundant metabolites with similar chemical shifts and specific scanning techniques are needed [Puts and Edden, 2012]. Since the seminal publication of Muthukumaraswamy et al. [2009], the amplitude and peak frequency of gamma oscillations yield great potential as a marker of the modulatory actions of GABA. However, recent evidence from MRS and electrophysiological studies showed ambiguous results and it is still debated whether synchronized neural network oscillations are mediated by GABA in the human brain [Cousijn et al., 2014; Gaetz et al., 2011; Muthukumaraswamy et al., 2009]. Furthermore, the research studies that concentrated on this relationship during sensory processing were restricted to the visual and motor cortex. Investigating this mechanism in the auditory cortex should make an important contribution, all the more so, since the highest densities of GABA‐A receptors in sensory areas have been found in the primary auditory cortex (PAC) [La Fougere et al., 2011; Zilles et al., 2002]). Moreover, several pharmaceutical challenge studies have stated that early auditory processing is modulated by GABA neurotransmission [Kähkönen, 2006]. In this study, we used the loudness dependence of auditory‐evoked potentials (LDAEP), a reliable auditory paradigm well known in psychiatry research [Gallinat et al., 2000; Hegerl and Juckel, 1994; Hensch et al., 2008; Park et al., 2010; Wyss et al., 2013], combined it with MRS measurements. Using the LDAEP paradigm, Schadow et al. [2007] demonstrated that gamma oscillations were highly sensitive to loudness variation, making it a reliable paradigm to investigate gamma oscillations in auditory areas.
This study fills a gap in the literature by addressing the question of whether individual GABA concentrations, measured by MRS, are correlated with auditory gamma band responses in the primary auditory cortex (PAC). These results may help us to understand the mediating effects of endogenous GABA on neural oscillations in sensory processing. Moreover, the neurotransmitter system is highly relevant to investigations of psychiatric disorders. For example, the GABAergic system is altered in patients with mental illness and neuropathologies, such as Huntington's chorea, Parkinson's disease, tardive dyskinesia, epilepsy, schizophrenia, anxiety, major depression, and other behavioral disorders [Beleboni et al., 2004]. Thus, determining the contribution of the GABAergic system in a noninvasive manner has great importance for the etiopathology and pharmacological treatment of these patients.
MATERIALS AND METHODS
Participants
Fifteen healthy subjects were included in the study. The data for 1 subject was dismissed due to poor electroencephalography (EEG) quality; hence, data from 14 subjects were analyzed (male, right‐handed, mean age 27 years ranging from 19 to 31 years, SD 3.26). All subjects were recruited from the academic staff of Forschungszentrum Jülich. Males were chosen because the GABA level varies with the menstrual phase in female subjects [Harada et al., 2011a]. None of the participants, or their first‐degree relatives, had a history of neuropsychiatric disorders as assessed by the Mini International Neuropsychiatry Interview (MINI) [Sheehan et al., 1998]. They reported no history of drug or alcohol abuse, smoking, or metabolic disorders. Subjects were instructed to consume no alcohol or any pharmaceuticals 48 h beforehand and no caffeine 12 h before measurements. Handedness was assessed by the Edinburgh Handedness Inventory [Oldfield, 1971]. All subjects gave written informed consent. This study was approved by the local ethics committee and was carried out in accordance with the Declaration of Helsinki.
Stimuli and Procedure
EEG responses to auditory stimuli were recorded in a Faraday‐shielded room with the subject in a supine position with their eyes open. They were asked to stay relaxed and avoid facial muscle movements throughout the recording. The auditory stimuli were sinusoidal tones (1000 Hz, 40 ms duration with 10 ms rise and fall time) generated by a digital signal processor (Multi I/O Processor RX8, TDT System 3, Tucker–Davis Technologies, Alachua, USA) and were presented binaurally through earphones with plastic tubes and ear plugs inserted into the outer ear canals. Two programmable attenuators for the left and right ear (PA5, TDT System 3, Tucker–Davis Technologies, Alachua, USA) were used to present the tones at 60, 70, 80, 90, and 100 dB sound pressure level (SPL) in a pseudorandomized order with not more than two equal levels following each other and pseudorandom stimulus onset asynchrony between 2 and 3 s in steps of 17 ms. A system‐specific constant time delay of 20 ms relative to the stimulus onset was taken into account and later subtracted in the analysis. Subjects were simultaneously shown a silent movie for distraction. 1H‐MRS and EEG measurements for each subject were taken on the same day, although individual GABA concentrations are relatively stable and do not vary more than 6% within a day or over a period of week [Cai et al., 2012].
EEG Methods
EEG data were recorded from a 32‐channel MR compatible EEG system using Brain Vision Recorder (Brain Products, Gilching, Germany). The EEG cap (BrainCap MR, EasyCap GmbH, Breitbrunn, Germany) consisted of 31 scalp electrodes distributed according to the 10–20 system using Cz as reference and Fz as ground and one additional electrode, which was attached to the subjects' back for recording the electrocardiogram (ECG). Data were recorded with a sampling rate of 1000 Hz and band‐pass filtered between 0.1 and 1000 Hz. Electrode impedances were maintained below 10 kΩ prior to data acquisition. EEG preprocessing and averaging was performed using Brain Vision Analyzer (Version 2.02, Brain Products, Gilching, Germany). The EEG data were downsampled to a rate of 250 Hz, rereferenced to an average reference, and filtered offline with a lower cutoff of 0.16 Hz (12 dB/octave, zero phase). Continuous data were visually inspected and excessive muscle activity was rejected. Independent component analysis (ICA, extended infomax; Lee et al. [1999]) was applied to remove eye artefacts. The first responses of each of the five intensities were excluded to reduce short‐term habituation effects. Trials with amplitudes exceeding ±100 μV were automatically rejected. All data were analyzed by the same trained operator. Thereafter, data were segmented into epochs from 200 ms before, to 1000 ms after, stimulus onset and averaged across conditions. A baseline correction was calculated from the prestimulus interval −200 to −10 ms.
Based on the grand average, a dipole model was computed in Brain Electrical Source Analysis (BESA, Version 6.1, MEGIS, Gräfelfing, Germany). The best fit was reached with three regional sources: each in the left and right primary auditory cortex, and one in the premotor cortex. The variance explained by the three‐dipole model within the fitted N1‐P2 interval (70–270 ms) was on average 99.1%. Activation in the premotor cortex is in accordance with data shown in a recent MEG whole‐brain analysis in this time interval [Wyss et al., 2014].
The model was applied to the individual data sets and single‐trial source waveforms from the auditory cortices were exported to Brain Vision Analyzer for subsequent single‐trial time–frequency analysis using the MNE‐Python software package [Gramfort et al., 2013, 2014].
For the analysis of total gamma‐band response activity, a Morlet‐based complex wavelet transform with a width of 12 cycles per frequency bin was employed from 30 to 200 Hz (1 Hz linear steps) to examine frequency based band variations across time (for details refer to Cohen [2014]). The Unit Energy Normalization was applied to normalize the wavelet function in such a way that all frequency levels had the same energy value. Total power refers to averaged single‐trial time–frequency maps and includes evoked and induced power. This power measure was chosen here to be comparable with the studies of Cousijn et al. [2014] and Muthukumaraswamy et al. [2013]. Moreover, to prevent from bias of unequal number of trials across experimental conditions in each subject, we randomly selected trials in order to get the same amount of trials (n = 78) throughout analysis. Before statistical analysis power was normalized by conversion to a z score with the mean and variance determined from the prestimulus period ranging from −200 to −50 ms.
For the correlational analysis, individual peak frequency for each subject was defined based on the highest peak in the statistically significant total gamma power increase (60 vs 100 dB) in a time window of interest. The window of interest (−2 and 248 ms) was defined based on statistical significance in this contrast. Additionally, the maximum and the mean of the relative GBR (relative to baseline in z scores) across the time window of interest were computed for each subject.
MRS Methods
Single‐voxel 1H‐MRS was performed on a 3 T scanner (Trio, Siemens, Erlangen, Germany) equipped with a 32‐channel phase array coil for receive and a birdcage body coil for transmit. Anatomical T1‐weighted images were acquired for voxel placement with a Magnetization Prepared RApid Gradient Echo (MP‐RAGE) sequence (TR = 2.0 s, TE = 3.03 ms, TI = 900 ms, echo train duration = 700 ms, FOV = 176 × 232 × 256 mm, matrix size = 232 × 256, 176 sagittal slices of 1 mm slice thickness, GRAPPA acceleration factor 2 in the phase encode direction with 24 reference line, flip angle = 9°). To avoid head movements, each subject's head was held firmly in place during the scanning procedure with vacuum cushions and sponge pads.
The spectroscopic voxel (size = 2.5 × 2.5 × 2.5 cm3) was placed by a trained operator at the left and right transverse temporal gyrus of Heschl (Brodmann area 41, primary auditory cortex) on the superior temporal plane. The position of the voxel was reviewed by a second trained operator. The voxel included portions of insula, parietal, and frontal operculum. Figure 1 illustrates the voxel position and representative spectra. The concentration of the metabolites was assessed using a standard point resolved spectroscopy (PRESS) sequence optimized for GABA measurement (TE1 = 14 ms, TE = 105 ms, TR = 2.5 s, NA = 128, RF pulse centered at 2.4 ppm, 16‐step phase cycling). The method has been published in detail elsewhere [Napolitano et al., 2013]. Before the spectroscopy measurements, the static magnetic field was homogenized by running FASTESTMAP [Gruetter and Tkáč, 2000] iteratively to ensure that the full‐width at half‐maximum (FWHM) of the reference water peak was below 0.05 ppm.
Figure 1.
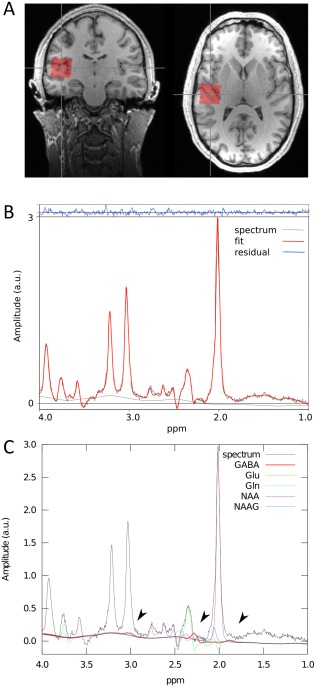
1H‐MRS measurement. Voxel of interest (2.5 cm3) used for magnetic resonance spectroscopy (MRS) positioned in the left and right primary auditory cortex (A). A typical spectrum acquired using the optimized PRESS sequence for a single subject. The fit is shown in red, superimposed on the edited spectrum in black. The high quality of the fit is demonstrated by the small residual remaining after fitting, as shown in blue (B). Corresponding individual fits for selected metabolites are shown. GABA's resonance peaks at 3.00, 2.28, and 1.89 ppm (indicated by arrows) (C). [Color figure can be viewed at http://wileyonlinelibrary.com]
All metabolite concentrations were quantified with LCModel version 6.3‐0I [Provencher, 2001] using a basis set simulated with GAMMA [Smith et al., 1994]. According to this model, the basis set analysis of nonedited spectra included the following metabolites: 2‐hydroxyglutaric acid, alanine (Ala), aspartate (Asp), creatine (Cr), GABA, glucose (Glc), glutamate (Glu), glutamine (Gln), glycerophosphocholine (GPC), glutathione (GSH), lactate (Lac), myoinositol (mI), N‐acetylaspartate (NAA), N‐acetylaspartyglutamate (NAAG), phosphocreatine (PCr), phosphorylcholine (PCh), scyllo‐inositol (sI), and taurine (TAU). All metabolite levels were compensated by the fraction ratios of the grey (GM) and white matters (WM) and cerebrospinal fluid (CSF) in the measurement volume (right: GM 0.49 ± 0.02, WM 0.38 ± 0.03, CSF 0.13 ± 0.04; left: GM 0.51 ± 0.04, WM 0.37 ± 0.03, CSF 0.12 ± 0.04). Spectra were corrected for eddy currents using the water peak for reference [Klose, 1990]. Metabolite concentrations were expressed as concentration ratios to total creatine (tCR), including both phosphocreatine and free creatine [Bogner et al., 2010].
Statistical Analysis
A nonparametric statistical test was applied to time–frequency power estimates for testing differences in the time resolved power spectral densities across different sound pressure levels (SPL). The cluster‐level permutation test [Maris and Oostenveld, 2007], as implemented in MNE‐Python [Gramfort et al., 2013, 2014], was applied to power signals from all SPL measurements to test whether the variance in power between two SPL (e.g., 60 dB vs 100 dB power) conditions is larger than the variation within each SPL condition. For testing variations within the spectrotemporal dynamics of all SPL combinations, F scores were computed over a time window ranging from −200 to 600 ms using a large amount of permutations (105). To identify a significant increase in group power changes across frequencies and time, an independent one‐tailed cluster level F test was computed using a confidence interval (CI) set to 95%. As the cluster level permutation test addresses the multiple comparison problem at the same time [Maris and Oostenveld, 2007], the increased power changes reflect a significance level of P ≤ 0.05.
Multilevel linear models were used to explore the main effects of total GBR (z scores) and condition, metabolite concentration, and their interaction. We used multilevel models to account for similar GBR variances within subjects (i.e., subjects‐specific effects). We conducted a series of linear mixed model analyses for each time point (−200 and 600 ms, in steps of 10 ms) and each frequency (30–200 Hz, steps of 1 Hz). This analysis allows complex relationships to be explored within the frequency and time domain and resting metabolite concentration. The GBR was the dependent variable and the metabolite concentration and condition were the independent variables and subject the random intercept to account for nonindependence of the measurements. The condition was defined by each of the intensity levels (60–100 dB).
To control for partial volume effects, the segmented volume ratios of the voxel (GMWM ratio, CSF) were included as covariates. Significance values were corrected for multiple comparisons by controlling the false discovery rate (FDR). The normal approximation was used to estimate denominator degrees of freedom [Barr, 2013]. 95% confidence intervals (CI) for the regression parameters were calculated. The analyses were done using R (Rstudio, Version 0.98.939).
With reference to studies conducted in this field [Cousijn et al., 2014; Lally et al., 2014; Muthukumaraswamy et al., 2009], we ran additional correlational analyses to investigate whether metabolite concentrations across subjects are correlated with individual peak frequency and maximum/mean of GBR values. Owing to the more pronounced activity pattern of the GBR in higher intensities (Fig. 3A), we used the individual GBR values from contrast analysis (60 vs 100 dB) to investigate the relationship between metabolite concentrations and total GBR measures. Pearson's partial correlation coefficients between GABA and glutamate and these individual GBR measures were calculated and controlled for partial volume effects (GMWM ratio and CSF). This was done with SPSS Statistics 22.
Figure 3.
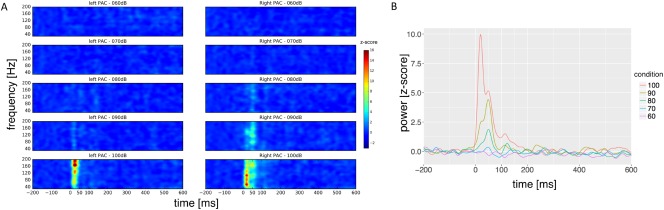
Total gamma‐band activity over different loudness intensities in the primary auditory cortex (A) group total GBR activity increases with higher intensity (60–100 dB). (B) Time courses for total GBR showing main activation before 200 ms. n = 14. [Color figure can be viewed at http://wileyonlinelibrary.com]
RESULTS
1H‐MRS Concentrations
In accordance with the study's hypothesis, only the in vivo concentrations of GABA and glutamate were used for analysis. Metabolites detected with Cramer‐Rao lower bound (CRLB) below 20% indicate reliable quantification. We successfully detected GABA and glutamate in PAC with CRLB well below 20% (Fig. 2). Owing to the limited quality in two of the GABA measurements in the left auditory cortex, we only focused on the right auditory cortex to keep as many subjects in the analysis as possible. Following Harada et al. [2011b], we calculated an inhibitory control index, that is, the values of GABA divided by the values of glutamate. The concentrations of the metabolites of interest in the right hemisphere, summarized in Table 1, showed considerable intersubject variability.
Figure 2.
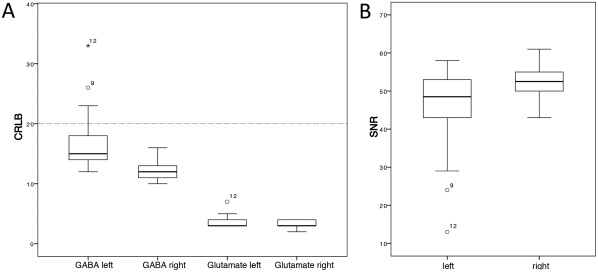
Quality measures of the MRS data. Cramer–Rao lower bound (CRLB) for each metabolite (A) and signal‐to‐noise ratios (SNR) (B) as provided by LCModel are shown for the left and right voxel in the primary auditory cortex. CRLB below 20% indicate reliable quantification. N = 14.
Table 1.
Metabolite concentrations (i.e., metabolite ratios) within the voxel placed in the right primary auditory cortex (mean ± SD)
| Metabolite concentrations | |
|---|---|
| GABA | 0.231 ± 0.029 |
| Glutamate | 2.893 ± 0.232 |
| GABA/glutamate ratio | 0.080 ± 0.008 |
Total Oscillatory Activity
The wavelet analysis revealed an intensity‐dependent increase in auditory‐total GBR. This is illustrated in Figure 3A. A significant positive main effect over all conditions resulted from linear mixed effect models in a looped manner (Table 2). The condition effect was stable for both GABA (estimate = 4.562, t = 0.558, P < 0.01) and glutamate (estimate = 0.021, t = 8.174, P < 0.01) included as predictors in the model. As shown in Figure 4A, significant components were found around 20–90, 100–140, and 200 ms after stimulus presentation, whereas the third component was much more pronounced. Additionally, we found significant power changes in contrast analysis between 100 and 60 dB (mean F value = 6.48, P < 0.05) that looked quite similar than the effect over all intensities (Fig. 4B). However, GBR occurred earlier in the contrast analysis and was even more pronounced at the third component in comparison to the main effect over all intensities. In lower intensity levels, only 100 versus 70 dB reached significance (mean F value = 6.58, P < 0.05). The contrasts 100 versus 80 dB (min P = 0.449) and 100 versus 90 dB (min P = 0.487), the F scores were not significant across subjects.
Table 2.
Summary of values from the linear mixed effect models for each time point and each frequency
| Summary: linear mixed effect models for each time point and each frequency | |||||||
|---|---|---|---|---|---|---|---|
| P value, min | Number of pixels with P value, P < 0.05 | Median estimate | Mean estimate | SD estimate | 2.5% CI | 97.5% CI | |
| GABA | 0.000 | 9977 | 62.840 | 115.150 | 216.961 | −133.662 | 604.055 |
| Glutamate | 0.000 | 15768 | −9.338 | −1.778 | 38.898 | −71.549 | 86.454 |
| Condition | 0.000 | 32457 | 0.042 | 0.072 | 0.064 | −0.017 | 0.240 |
| GABA fdr | 0.455 | 0 | – | – | – | – | – |
| Glutamate fdr | 0.068 | 0 | – | – | – | – | – |
| Condition fdr | 0.000 | 7313 | 0.054 | 0.081 | 0.055 | 0.079 | 0.217 |
fdr = false discovery rate corrected.
Figure 4.
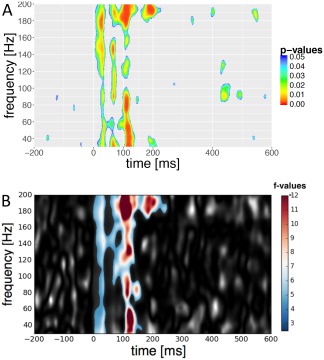
Effect of loudness variation on total gamma‐band activity in the primary auditory cortex (A) Main effect from a linear mixed‐effect model analysis over all conditions (60–100 dB). P values are FDR corrected. (B) Contrast between 60 dB and 100 dB using a nonparametric 2‐sample permutation cluster test. F values are shown in grey for each frequency bin and time point. A single cluster (color coded) was found to be significant (P = 0.001, corrected for multiple comparisons) across subjects (n = 14) and over a time span of 250 ms (−2 to 248 ms). The duration of the significantly increased total GBR power indicates sustained GBR activity over a wide range of frequencies during the 100 dB intensity recordings with its maximum around 120 ms. [Color figure can be viewed at http://wileyonlinelibrary.com]
Neurochemical Correlates of Gamma‐Band Oscillatory Activity
Results for the relationship between metabolite concentration and GBR in each frequency and time point are shown in Table 2. It revealed no significant activation pattern for GABA and glutamate levels after correction for multiple comparisons. No significant effects of the fraction ratios of brain tissue (GMWM ratio and CSF) on the GBR were observed.
Turning now to the experimental evidence on correlational analysis (Fig. 5), no correlations were found between GABA values and gamma‐band peak frequency (r(10) = −0.206, P = 0.521), gamma‐band relative power (vs baseline) maximum (r(10) = −0.166, P = 0.607) or gamma‐band relative power (vs baseline) mean (r(10) = −0.065, P = 0.840), glutamate and gamma‐band peak frequency (r(10) = 0.194, P = 0.545), gamma‐band relative power (vs baseline) maximum (r(10) = −0.056, P = 0.864) or gamma‐band relative power (vs baseline) mean (r(10) = −0.237, P = 0.459). Or GABA/glutamate ratio and gamma‐band peak frequency (r(10) = −0.378, P = 0.226), gamma‐band relative power (vs baseline) maximum (r(10) = −0.219, P = 0.493) or gamma‐band relative power (vs baseline) mean (r(10) = −0.229, P = 0.475).
Figure 5.
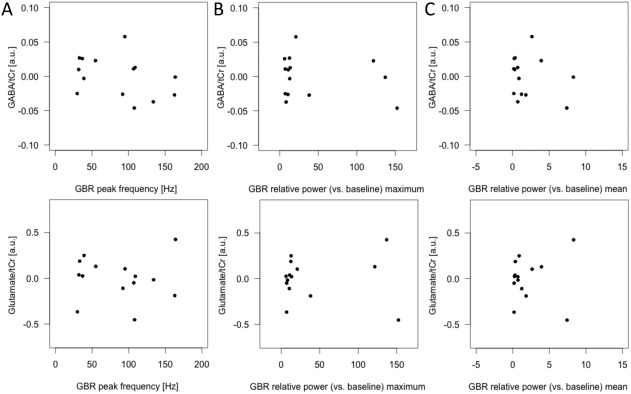
Scatterplots showing the relationship between 1H‐MRS and total GBR measures in response to highest intensity (contrast 100 dB versus 60 dB). No correlations were observed between GABA and glutamate concentrations and GBR peak frequency (A), maximum of the relative GBR (z values) (B), and mean of the relative GBR (z values) based on individual data and across the time window of interest (−2 and 248 ms). tCr = total creatine; a.u. = arbitrary unit; n = 14.
No effects of age have been observed for GABA (r = −0.352, P = 0.198) or glutamate (r = −0.113, P = 0.688) concentrations and gamma‐band peak frequency (r = 0.060, P = 0.838), gamma‐band relative power (vs baseline) maximum (r = −0.212, P = 466) or gamma‐band relative power (vs baseline) mean (r = −0.083, P = 0.779).
DISCUSSION
High‐frequency oscillations may represent the extent of excitatory and inhibitory balance of neural networks [Gonzalez‐Burgos et al., 2015]. It is suggested that this fine‐tuning of cortical excitability has a direct impact on cognitive function and sensory processing [Carcea and Froemke, 2013] and is altered in clinical samples [Frankle et al., 2015; Uhlhaas and Singer, 2013].
Our data clearly showed that GBR is sensitive to characteristics of sensory stimuli in a passive listening task. Increased sound intensity evoked stronger total gamma band responses in the auditory cortex. This is not surprising as a systematic increase of neuronal response with higher intensities is widely supported by EEG, MEG, and fMRI measurements [Mulert et al., 2005; Neukirch et al., 2002; Neuner et al., 2014; Wyss et al., 2014]. Other studies using the same paradigm to study the intensity dependence of auditory evoked potentials showed high interindividual variability [Buchsbaum, 1971] and high retest reliabilities (r = 0.88) of N1/P2 slopes in the auditory cortex after 3 weeks and 1 year, indicating stability of the trait over time [Hegerl et al., 1994]. Not surprisingly, a study by Schadow et al. [2007] compared evoked potentials with gamma band responses in a similar paradigm with a forced‐choice discrimination task and reported that gamma oscillations were more responsive to variation in sound intensity than evoked potentials. However, this intensity effect only reached significance for evoked GBR, not for total GBR, although an effect was visually evident. In contrast to this study, intensity effects for total GBR were significant in our study (Figs. 3B and 4A). Total GBR contains the phase‐locked and non‐phase‐locked information of the GBR.
The time course of total GBR response showed significant response between −2 and 248 ms. A broad peak in power with a bimodal characteristic was displayed between about 20 and 90 ms followed by a component from around 100 to 140 ms. These temporal activations resembles the ones of evoked responses and are in line with other studies investigating gamma activity in auditory cortex [Crone et al., 2001; Edwards et al., 2005; Ray et al., 2008; Roach and Mathalon, 2008].
Based on the activation pattern shown in Figure 3A, a clear gamma peak at specific frequencies was not evident, but was mainly found along the whole frequency spectrum ranging from 30 to 200 Hz. Gamma band activity includes lower gamma (30–80 Hz) and high gamma (80–150 Hz) [Canolty et al., 2006; Cohen, 2014]. However, a common problem when analyzing high frequencies are some small muscular artefacts that remain present, even in artefact‐free recordings [Muthukumaraswamy, 2013]. In our study, the broadband power responses occurring around 20 ms at highest intensities could be due to muscle activity, although we had rejected large muscle artifacts exceeding 100 μV. This activation might be related to a reflex provoked by loud acoustic stimuli by contracting the orbicularis oculi muscle with the purpose to protect the organism from sudden harms [De Pascalis et al., 2012]. However, the permutation cluster test contrasting 60 vs 100 dB showed highest response at the second component between 100 and 140 ms with a more distributed power spectrum across frequencies. As we used this contrast power values as a basis for the correlational analysis, the influence of muscular contamination was minimized and hence guaranteed a valid definition of individual gamma peak.
The underlying mechanism of gamma oscillations in the human brain is still under investigation. Recent interest has focused on correlations between gamma peak frequency and GABA concentration [Cousijn et al., 2014; Gaetz et al., 2011; Muthukumaraswamy et al., 2009] and showed contradictory results. In this study, we aimed to investigate this correlation in the auditory cortex. It is only recently that MRS studies were capable of measuring GABA or glutamate concentrations in the auditory cortex [Gao et al., 2015; Hugdahl et al., 2015; Kompus et al., 2015]. Limitations in the choice of placement of the MRS voxel were due to the fact that better signal‐to‐noise ratios were achieved in brain regions such as the occipital cortex in close proximity to the MR coil elements [Puts and Edden, 2012]. Accordingly, in this study, the placement of the MRS voxel was not limited by the MR receive coil, as in some studies reviewed by Puts and Edden [2012], because of the good coverage of the primary auditory cortex provided by the 32‐channel whole‐brain receive array coil. Furthermore, by using the PRESS sequence with optimized echo‐time for GABA detection [Napolitano et al., 2013], it allowed us to achieve similar GABA detection quality as in other editing sequences, such as MEGA‐PRESS, but in smaller voxels and shorter measurement time. In general, the use of different editing methods has some effects on data quality, voxel size, or acquisition time and has to be considered when comparing different findings (for detailed methodological information, please refer to reviews by Duarte et al. [2012], Mullins et al. [2014], and Puts and Edden [2012]).
We successfully measured GABA and glutamate levels in the primary auditory cortex. Furthermore, the time–frequency analysis was done using wavelet transform that has some advantages compared to FFT in localizing changes in the frequency characteristics over time [Cohen, 2014]. This allowed us to focus not only on individual peak frequency and peak power, but also on individual oscillatory shifts across time and frequency with high resolution to find a significant individual pattern that is modulated by GABA. To our knowledge, this is the first time that such an analysis has been done in this context. Most other studies have focused on the correlational analysis between peak gamma oscillatory frequency and metabolite conditions [Cousijn et al., 2014; Gaetz et al., 2011; Muthukumaraswamy et al., 2009].
Our results differ from the findings presented in the seminal study by Muthukumaraswamy et al. [2009], where they found that resting GABA concentration is correlated with individual peak frequency of evoked gamma band response. This result attracted significant interest as it opened up a window into how cortical networks function and how interneurons and pyramidal cells are linked. A recent study using PET measurements supports such a link. The authors found that GABA‐A receptor density positively correlated with gamma peak frequency and negatively with gamma amplitude in human primary visual cortex [Kujala et al., 2015].
However, an MRS‐based study with a larger sample size did not replicate such a relationship in the visual cortex [Cousijn et al., 2014]. Similarly, we did not find any relationship between GABA and total GBR when looking at time and frequency in a detailed manner or in correlational analysis. Another study investigated the relationship between gamma oscillations at movement onset in the motor cortex and MRS measurements centered on primary motor areas [Gaetz et al., 2011]. Using beamformer source localization, they found a significant correlation between peak frequency and GABA concentration in the motor cortex. However, only 9 subjects were used in the study.
A possible explanation for the inconsistent findings relating to GABA might be that MRS measures total tissue GABA and cannot differentiate synaptic GABA from cytoplasmic GABA, which is not involved in neurotransmission [Duncan et al., 2014a; Stagg et al., 2011a]. MRS sensitivity is commonly tested by administering selective compounds that modulate synaptic GABA concentration. Indeed, a pharmacological study with tiagabine that works as a GABA reuptake inhibitor at the synapse found no significant increase between baseline and challenge MRS measurements [Myers et al., 2014]. On the other hand, a gabapentin challenge, which increases GABA turnover, did increase bulk GABA concentration up to 55% measured with MRS at 7 T [Cai et al., 2012]. Furthermore, specific transcranial magnetic stimulation (TMS) protocols can provide an index of GABA synaptic activity. However, when combined with MRS, no relationship between these two measurements was found, so that the interpretation of GABA concentrations by means of MRS remains unclear [Stagg et al., 2011b; Tremblay et al., 2013]. Support for a direct relationship between synaptic GABA activity and an MRS measurement comes from a genetic study. Marenco et al. [2011] investigated common genetic variants in ERBB4, which expresses on PV GABAergic cortical interneurons [Fazzari et al., 2010]. The authors reported that significant genotype effects in ERBB4 could predict cortical GABA concentration measured by means of MRS. In summary, these studies indicate that MRS measurements of GABA directly relate to synaptic activity. However, causal evidence on the relationship between MRS measurements and GABA concentration is needed. Furthermore, it has to be kept in mind that multiple mechanisms, for example, cycling rates, at the synaptic level have an impact on the dynamic regulation of neurotransmission [Moss and Smart, 2001]. This makes it difficult to determine the exact physiological mechanisms of any suggested neurobiochemical relationship [Duncan et al., 2014a]. Hence, Flumazenil‐PET measurement is an alternative technique that is deemed to be more suitable than MRS‐based measures to investigate the GABAergic inhibitory drive [Kujala et al., 2015]. On the other hand, it is unresolved how receptor densities are related to relevant aspects of human behavior. However, as the mode of action between GABA‐A receptors and regional change of synaptic GABA concentration is not clear, discrepancies between both quantification techniques are difficult to compare [Duncan et al., 2014b].
The generalizability of these results is subject to certain limitations. Our data acquisition strategy only allowed conclusions about resting GABA concentrations to be drawn; however, more elegant would have been to measure before, during, and after the auditory stimulation paradigm, particularly as some effects from scanner noise on metabolite concentration have been reported [Richards et al., 1997]. Another important point to discuss here is the possible modulation effect of attention on gamma band response. Subjects were instructed to watch a silent movie while hearing the tones. Bottom–up attentional processes, as in this case, are stimuli driven and have less impact on gamma band responses than top–down attention [Debener et al., 2003]. Thus, as seen in Figure 3A,B, total power represented by induced and evoked power is provoked by higher intensities; however, the signal might have increased by a task involving selective attention to tones. Moreover, another point that could have led to more robust response is a prolonged stimuli presentation (>40 ms), as it has been shown in visual cortex that longer stimuli allow to differentiate more clearly between onset and offset peaks [Busch et al., 2006]. Furthermore, the voxel under investigation contained a relatively large volume of 2.5 × 2.5 × 2.5 cm3 and included not only primary auditory cortex but also portions of insula, parietal, and frontal operculum. State‐of‐the‐art MRS‐based GABA measurement methods do not allow satisfying signal‐to‐noise ratios in measuring the tiny spectrum within small volumes, for example, primary auditory cortex [Puts and Edden, 2012]. Compared to other studies required even larger volumes (4 × 3 × 3 cm3) using the MEGA‐PRESS sequence in auditory cortex [Gao et al., 2015], we managed to reduce voxel size with optimized parameters. Studies of morphology in primary auditory cortex state that the sizes vary in individuals between 0.5 and 3 cm3 [Penhune et al., 1996]. However, recently published studies measuring GABA in other brain areas used similar volume sizes (e.g., Gaetz et al. [2011] and Muthukumaraswamy et al. [2009] used 3 × 3 × 3 cm3; Cousijn et al. [2014] used 2 × 2 × 2 cm3). Furthermore, segmentation of brain tissue within the volume (GM/WM/CSF) is substantial to differentiate the exact origin of the signal [De Graaf, 2013]. To control for partial volume effects, we added the ratios as covariates in statistics. Relatively robust segmentation ratios across subjects have been characterized by low standard deviation in our sample. However, comparability with other studies is restricted by different fraction ratios.
CONCLUSION
The present results, although limited by the small sample size of the study, are significant in at least two major respects. First, MRS GABA with CRLB below 20% and total GBR showing a clear intensity dependency was successfully measured in the auditory cortex. Second, we could neither detect a significant association between GABA concentrations assessed by MRS and total GBR across each frequency and time point nor GBR features such as peak frequency and power in the auditory cortex. Overall, the findings of this study complement those of earlier studies and shed light on the relationship between excitatory and inhibitory networks that are of relevance for various neurological and psychiatric disorders.
ACKNOWLEDGMENTS
The authors thank Frank Boers and Andrea Muren for their help with data acquisition.
No funding body was involved in the design of experiment, data collection and analysis, interpretation of the results, or preparation and submission of the manuscript. The authors declare that they have no conflict of interest.
Authors' contributions
C.W., I.N., and W.K. designed research; C.W. and D.H.Y.T. performed research; D.H.Y.T., M.K., J.D., and J.N.S. contributed new reagents/analytic tools; C.W., M.K., D.H.Y.T., and R.A. analyzed data; C.W., N.J.S., and I.N. wrote the article.
REFERENCES
- Barr DJ (2013): Random effects structure for testing interactions in linear mixed‐effects models. Front Psychol 4:328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P (2007): Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci 8:45–56. [DOI] [PubMed] [Google Scholar]
- Başar E (2013): A review of gamma oscillations in healthy subjects and in cognitive impairment. Int J Psychophysiol 90:99–117. [DOI] [PubMed] [Google Scholar]
- Beleboni RO, Carolino ROG, Pizzo AB, Castellan‐Baldan L, Coutinho‐Netto J, dos Santos WF, Coimbra NC (2004): Pharmacological and biochemical aspects of GABAergic neurotransmission: Pathological and neuropsychobiological relationships. Cell Mol Neurobiol 24:707–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogner W, Gruber S, Doelken M, Stadlbauer A, Ganslandt O, Boettcher U, Trattnig S, Doerfler A, Stefan H, Hammen T (2010): In vivo quantification of intracerebral GABA by single‐voxel 1H‐MRS—How reproducible are the results? Eur J Radiol 73:526–531. [DOI] [PubMed] [Google Scholar]
- Brunel N, Wang X‐J (2003): What determines the frequency of fast network oscillations with irregular neural discharges? I. Synaptic dynamics and excitation‐inhibition balance. J Neurophysiol 90:415–430. [DOI] [PubMed] [Google Scholar]
- Buchsbaum M (1971): Individual differences in stimulus intensity response. Psychophysiology 8:600–611. [DOI] [PubMed] [Google Scholar]
- Busch NA, Schadow J, Fründ I, Herrmann CS (2006): Time‐frequency analysis of target detection reveals an early interface between bottom‐up and top‐down processes in the gamma‐band. Neuroimage 29:1106–1116. [DOI] [PubMed] [Google Scholar]
- Buzsaki G (2006): Rhythms of the Brain. Oxford University Press. [Google Scholar]
- Buzsáki G, Wang X‐J (2012): Mechanisms of gamma oscillations. Annu Rev Neurosci 35:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai K, Nanga RP, Lamprou L, Schinstine C, Elliott M, Hariharan H, Reddy R, Epperson CN (2012): The impact of gabapentin administration on brain GABA and glutamate concentrations: A 7T (1)H‐MRS study. Neuropsychopharmacology 37:2764–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, Berger MS, Barbaro NM, Knight RT (2006): High gamma power is phase‐locked to theta oscillations in human neocortex. Science 313:1626–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carcea I, Froemke RC (2013): Cortical plasticity, excitatory‐inhibitory balance, and sensory perception. Prog Brain Res 207:65–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX (2014): Complex Morlet Wavelets and Extracting Power and Phase. Analyzing Neural Time Series Data: Theory and Practice. Cambridge, Massachusetts: MIT Press. [Google Scholar]
- Cousijn H, Haegens S, Wallis G, Near J, Stokes MG, Harrison PJ, Nobre AC (2014): Resting GABA and glutamate concentrations do not predict visual gamma frequency or amplitude. Proc Natl Acad Sci USA 111:9301–9306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone NE, Boatman D, Gordon B, Hao L (2001): Induced electrocorticographic gamma activity during auditory perception. Clin Neurophysiol 112:565–582. [DOI] [PubMed] [Google Scholar]
- De Graaf RA (2013): In Vivo NMR Spectroscopy: Principles and Techniques: John Wiley & Sons. [Google Scholar]
- De Pascalis V, Cozzuto G, Russo E (2012): Effects of personality trait emotionality on acoustic startle response and prepulse inhibition including N100 and P200 event‐related potential. Clin Neurophysiol 124:292–305. [DOI] [PubMed] [Google Scholar]
- Debener S, Herrmann CS, Kranczioch C, Gembris D, Engel AK (2003): Top‐down attentional processing enhances auditory evoked gamma band activity. Neuroreport 14:683–686. [DOI] [PubMed] [Google Scholar]
- Duarte JM, Lei H, Mlynarik V, Gruetter R (2012): The neurochemical profile quantified by in vivo 1 H NMR spectroscopy. Neuroimage 61:342–362. [DOI] [PubMed] [Google Scholar]
- Duncan NW, Wiebking C, Muñoz‐Torres Z, Northoff G (2014a): How to investigate neuro‐biochemical relationships on a regional level in humans? Methodological considerations for combining functional with biochemical imaging. J Neurosci Methods 221:183–188. [PubMed] [Google Scholar]
- Duncan NW, Wiebking C, Northoff G (2014b): Associations of regional GABA and glutamate with intrinsic and extrinsic neural activity in humans ‐ A review of multimodal imaging studies. Neurosci Biobehav Rev 47:36–52. [DOI] [PubMed] [Google Scholar]
- Edwards E, Soltani M, Deouell LY, Berger MS, Knight RT (2005): High gamma activity in response to deviant auditory stimuli recorded directly from human cortex. J Neurophysiol 94:4269–4280. [DOI] [PubMed] [Google Scholar]
- Fazzari P, Paternain AV, Valiente M, Pla R, Lujan R, Lloyd K, Lerma J, Marin O, Rico B (2010): Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature 464:1376–1380. [DOI] [PubMed] [Google Scholar]
- Frankle WG, Cho RY, Prasad KM, Mason NS, Paris J, Himes ML, Walker C, Lewis DA, Narendran R (2015): In vivo measurement of GABA transmission in healthy subjects and schizophrenia patients. Am J Psychiatry 172:1148–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetz W, Edgar JC, Wang D, Roberts TP (2011): Relating MEG measured motor cortical oscillations to resting γ‐aminobutyric acid (GABA) concentration. Neuroimage 55:616–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallinat J, Bottlender R, Juckel G, Munke‐Puchner A, Stotz G, Kuss HJ, Mavrogiorgou P, Hegerl U (2000): The loudness dependency of the auditory evoked N1/P2‐component as a predictor of the acute SSRI response in depression. Psychopharmacology (Berl) 148:404–411. [DOI] [PubMed] [Google Scholar]
- Gao F, Wang G, Ma W, Ren F, Li M, Dong Y, Liu C, Liu B, Bai X, Zhao B, Edden RAE (2015): Decreased auditory GABA + concentrations in presbycusis demonstrated by edited magnetic resonance spectroscopy. Neuroimage 106:311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez‐Burgos G, Cho RY, Lewis DA (2015): Alterations in cortical network oscillations and parvalbumin neurons in schizophrenia. Biol Psychiatry 77:1031–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramfort A, Luessi M, Larson E, Engemann DA, Strohmeier D, Brodbeck C, Goj R, Jas M, Brooks T, Parkkonen L (2013): MEG and EEG data analysis with MNE‐Python. Front Neurosci 7:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramfort A, Luessi M, Larson E, Engemann DA, Strohmeier D, Brodbeck C, Parkkonen L, Hämäläinen MS (2014): MNE software for processing MEG and EEG data. Neuroimage 86:446–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray CM, Konig P, Engel AK, Singer W (1989): Oscillatory responses in cat visual cortex exhibit inter‐columnar synchronization which reflects global stimulus properties. Nature 338:334–337. [DOI] [PubMed] [Google Scholar]
- Gruetter R, Tkáč I (2000): Field mapping without reference scan using asymmetric echo‐planar techniques. Magn Reson Med 43:319–323. [DOI] [PubMed] [Google Scholar]
- Harada M, Kubo H, Nose A, Nishitani H, Matsuda T (2011a): Measurement of variation in the human cerebral GABA level by in vivo MEGA‐editing proton MR spectroscopy using a clinical 3 T instrument and its dependence on brain region and the female menstrual cycle. Hum Brain Mapp 32:828–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada M, Taki MM, Nose A, Kubo H, Mori K, Nishitani H, Matsuda T (2011b): Non‐invasive evaluation of the GABAergic/glutamatergic system in autistic patients observed by MEGA‐editing proton MR spectroscopy using a clinical 3 tesla instrument. J Autism Dev Disord 41:447–454. [DOI] [PubMed] [Google Scholar]
- Hegerl U, Gallinat J, Mrowinski D (1994): Intensity dependence of auditory evoked dipole source activity. Int J Psychophysiol 17:1–13. [DOI] [PubMed] [Google Scholar]
- Hegerl U, Juckel G (1994): Auditory‐evoked dipole source activity ‐ Indicator of central serotonergic dysfunction in psychiatric‐patients. Pharmacopsychiatry 27:75–78. [DOI] [PubMed] [Google Scholar]
- Hensch T, Herold U, Diers K, Armbruster D, Brocke B (2008): Reliability of intensity dependence of auditory‐evoked potentials. Clin Neurophysiol 119:224–236. [DOI] [PubMed] [Google Scholar]
- Herrmann CS, Fründ I, Lenz D (2010): Human gamma‐band activity: A review on cognitive and behavioral correlates and network models. Neurosci Biobehav Rev 34:981–992. [DOI] [PubMed] [Google Scholar]
- Hugdahl K, Craven AR, Nygård M, Løberg E‐M, Berle JØ, Johnsen E, Kroken R, Specht K, Andreassen OA, Ersland L (2015): Glutamate as a mediating transmitter for auditory hallucinations in schizophrenia: A 1H MRS study. Schizophr Res 161:252–260. [DOI] [PubMed] [Google Scholar]
- Kähkönen S (2006): Magnetoencephalography (MEG): A non‐invasive tool for studying cortical effects in psychopharmacology. Int J Neuropsychopharmacol 9:367–372. [DOI] [PubMed] [Google Scholar]
- Klose U (1990): In vivo proton spectroscopy in presence of eddy currents. Magn Reson Med 14:26–30. [DOI] [PubMed] [Google Scholar]
- Kompus K, Westerhausen R, Craven AR, Kreegipuu K, Põldver N, Passow S, Specht K, Hugdahl K, Näätänen R (2015): Resting‐state glutamatergic neurotransmission is related to the peak latency of the auditory mismatch negativity (MMN) for duration deviants: An 1H‐MRS‐EEG study. Psychophysiology 52:1131–1139. [DOI] [PubMed] [Google Scholar]
- Kujala J, Jung J, Bouvard S, Lecaignard F, Lothe A, Bouet R, Ciumas C, Ryvlin P, Jerbi K (2015): Gamma oscillations in V1 are correlated with GABA(A) receptor density: A multi‐modal MEG and Flumazenil‐PET study. Sci Rep 5:16347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvitsiani D, Ranade S, Hangya B, Taniguchi H, Huang JZ, Kepecs A (2013): Distinct behavioural and network correlates of two interneuron types in prefrontal cortex. Nature 498:363–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Fougere C, Grant S, Kostikov A, Schirrmacher R, Gravel P, Schipper HM, Reader A, Evans A, Thiel A (2011): Where in‐vivo imaging meets cytoarchitectonics: The relationship between cortical thickness and neuronal density measured with high‐resolution [(18)F]flumazenil‐PET. Neuroimage 56:951–960. [DOI] [PubMed] [Google Scholar]
- Lally N, Mullins PG, Roberts MV, Price D, Gruber T, Haenschel C (2014): Glutamatergic correlates of gamma‐band oscillatory activity during cognition: A concurrent ER‐MRS and EEG study. Neuroimage 85 Pt 2:823–833. [DOI] [PubMed] [Google Scholar]
- Lee TW, Girolami M, Sejnowski TJ (1999): Independent component analysis using an extended infomax algorithm for mixed subgaussian and supergaussian sources. Neural Comput 11:417–441. [DOI] [PubMed] [Google Scholar]
- Marenco S, Geramita M, van der Veen JW, Barnett AS, Kolachana B, Shen J, Weinberger DR, Law AJ (2011): Genetic association of ErbB4 and Human Cortical GABA levels in‐vivo. J Neurosci 31:11628–11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris E, Oostenveld R (2007): Nonparametric statistical testing of EEG‐and MEG‐data. J Neurosci Methods 164:177–190. [DOI] [PubMed] [Google Scholar]
- Metherate R (2012): Modulatory mechanisms controlling auditory processing In: Trussell LO, Popper AN, Fay RR, editors. Synaptic Mechanisms in the Auditory System. New York: Springer; pp 187–202. [Google Scholar]
- Moss SJ, Smart TG (2001): Constructing inhibitory synapses. Nat Rev Neurosci 2:240–250. [DOI] [PubMed] [Google Scholar]
- Mulert C, Jäger L, Propp S, Karch S, Störmann S, Pogarell O, Möller HJ, Juckel G, Hegerl U (2005): Sound level dependence of the primary auditory cortex: Simultaneous measurement with 61‐channel EEG and fMRI. Neuroimage 28:49–58. [DOI] [PubMed] [Google Scholar]
- Mullins PG, McGonigle DJ, O'Gorman RL, Puts NA, Vidyasagar R, Evans CJ, Edden RA (2014): Current practice in the use of MEGA‐PRESS spectroscopy for the detection of GABA. Neuroimage 86:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy SD (2013): High‐frequency brain activity and muscle artifacts in MEG/EEG: A review and recommendations. Front Hum Neurosci 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Edden RE, Jones DK, Swettenham JB, Singh KD (2009): Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc Natl Acad Sci USA 106:8356–8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers JF, Evans CJ, Kalk NJ, Edden RA, Lingford‐Hughes AR (2014): Measurement of GABA using J‐difference edited 1H‐MRS following modulation of synaptic GABA concentration with tiagabine. Synapse 68:355–362. [DOI] [PubMed] [Google Scholar]
- Napolitano A, Kockenberger W, Auer DP (2013): Reliable gamma aminobutyric acid measurement using optimized PRESS at 3 T. Magn Reson Med 69:1528–1533. [DOI] [PubMed] [Google Scholar]
- Neukirch M, Hegerl U, Kötitz R, Dorn H, Gallinat U, Herrmann WM (2002): Comparison of the amplitude/intensity function of the auditory evoked N1m and N1 components. Neuropsychobiology 45:41–48. [DOI] [PubMed] [Google Scholar]
- Neuner I, Kawohl W, Arrubla J, Warbrick T, Wyss C, Hitz K, Boers F, Shah JN (2014): Cortical signal variation in the processing of rising sound pressure levels: A combined event‐related potentials and fMRI study. PLoS One 9:e109216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: The edinburgh inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Park YM, Lee SH, Kim S, Bae SM (2010): The loudness dependence of the auditory evoked potential (LDAEP) in schizophrenia, bipolar disorder, major depressive disorder, anxiety disorder, and healthy controls. Prog Neuropsychopharmacol Biol Psychiatry 34:313–316. [DOI] [PubMed] [Google Scholar]
- Penhune VB, Zatorre RJ, MacDonald JD, Evans AC (1996): Interhemispheric anatomical differences in human primary auditory cortex: Probabilistic mapping and volume measurement from magnetic resonance scans. Cereb Cortex 6:661–672. [DOI] [PubMed] [Google Scholar]
- Provencher SW (2001): Automatic quantitation of localized in vivo1H spectra with LCModel. NMR Biomed 14:260–264. [DOI] [PubMed] [Google Scholar]
- Puts NAJ, Edden RAE (2012): In vivo magnetic resonance spectroscopy of GABA: A methodological review. Prog Nucl Magn Reson Spectrosc 60:29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Niebur E, Hsiao SS, Sinai A, Crone NE (2008): High‐frequency gamma activity (80‐150Hz) is increased in human cortex during selective attention. Clin Neurophysiol 119:116–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards T, Gates G, Gardner J, Merrill T, Hayes C, Panagiotides H, Serafini S, Rubel E (1997): Functional MR spectroscopy of the auditory cortex in healthy subjects and patients with sudden hearing loss. Am J Neuroradiol 18:611–620. [PMC free article] [PubMed] [Google Scholar]
- Roach BJ, Mathalon DH (2008): Event‐related EEG time‐frequency analysis: An overview of measures and an analysis of early gamma band phase locking in schizophrenia. Schizophr Bull 34:907–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux F, Uhlhaas PJ (2014): Working memory and neural oscillations: Alpha–gamma versus theta–gamma codes for distinct WM information? Trends Cogn Sci 18:16–25. [DOI] [PubMed] [Google Scholar]
- Schadow J, Lenz D, Thaerig S, Busch NA, Fründ I, Herrmann CS (2007): Stimulus intensity affects early sensory processing: Sound intensity modulates auditory evoked gamma‐band activity in human EEG. Int J Psychophysiol 65:152–161. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC (1998): The mini‐international neuropsychiatric interview (MINI): The development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. J Clin Psychiatry 59:22–33. [PubMed] [Google Scholar]
- Singer W (1999): Neuronal synchrony: A versatile code for the definition of relations? Neuron 24:49–65. [DOI] [PubMed] [Google Scholar]
- Smith SA, Levante TO, Meier BH, Ernst RR (1994): Computer simulations in magnetic resonance. An object‐oriented programming approach. J Mag Reson A 106:75–105. [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K (2009): Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459:698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Bachtiar V, Johansen‐Berg H (2011a): What are we measuring with GABA magnetic resonance spectroscopy? Commun Integr Biol 4:573–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Bestmann S, Constantinescu AO, Moreno LM, Allman C, Mekle R, Woolrich M, Near J, Johansen‐Berg H, Rothwell JC (2011b): Relationship between physiological measures of excitability and levels of glutamate and GABA in the human motor cortex. J Physiol 589:5845–5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay S, Beaulé V, Proulx S, De Beaumont L, Marjańska M, Doyon J, Pascual‐Leone A, Lassonde M, Théoret H (2013): Relationship between transcranial magnetic stimulation measures of intracortical inhibition and spectroscopy measures of GABA and glutamate+ glutamine. J Neurophysiol 109:1343–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W (2013): High‐frequency oscillations and the neurobiology of schizophrenia. Dialogues Clin Neurosci 15:301–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X‐J (2010): Neurophysiological and computational principles of cortical rhythms in cognition. Physiol Rev 90:1195–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X‐J, Buzsáki G (1996): Gamma oscillation by synaptic inhibition in a hippocampal interneuronal network model. J Neurosci 16:6402–6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Kopell N, Ermentrout B, Buhl EH (2000): Inhibition‐based rhythms: Experimental and mathematical observations on network dynamics. Int J Psychophysiol 38:315–336. [DOI] [PubMed] [Google Scholar]
- Wyss C, Boers F, Kawohl W, Arrubla J, Vahedipour K, Dammers J, Neuner I, Shah JN (2014): Spatiotemporal properties of auditory intensity processing in multisensor MEG. Neuroimage 102:465–473. [DOI] [PubMed] [Google Scholar]
- Wyss C, Hitz K, Hengartner MP, Theodoridou A, Obermann C, Uhl I, Roser P, Grunblatt E, Seifritz E, Juckel G, Kawohl W (2013): The loudness dependence of auditory evoked potentials (LDAEP) as an indicator of serotonergic dysfunction in patients with predominant schizophrenic negative symptoms. PLoS One 8:e68650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilles K, Palomero‐Gallagher N, Grefkes C, Scheperjans F, Boy C, Amunts K, Schleicher A (2002): Architectonics of the human cerebral cortex and transmitter receptor fingerprints: Reconciling functional neuroanatomy and neurochemistry. Eur Neuropsychopharmacol 12:587–599. [DOI] [PubMed] [Google Scholar]


