Abstract
The aim of this study was to apply recently developed automated fiber segmentation and quantification methods using diffusion tensor imaging (DTI) and DTI‐based deterministic and probabilistic tractography to access local and global diffusion changes in blast‐induced mild traumatic brain injury (bmTBI). Two hundred and two (202) male active US service members who reported persistent post‐concussion symptoms for more than 6 months after injury were recruited. An additional forty (40) male military controls were included for comparison. DTI results were examined in relation to post‐concussion and post‐traumatic stress disorder (PTSD) symptoms. No significant group difference in DTI metrics was found using voxel‐wise analysis. However, group comparison using tract profile analysis and tract specific analysis, as well as single subject analysis using tract profile analysis revealed the most prominent white matter microstructural injury in chronic bmTBI patients over the frontal fiber tracts, that is, the front‐limbic projection fibers (cingulum bundle, uncinate fasciculus), the fronto‐parieto‐temporal association fibers (superior longitudinal fasciculus), and the fronto‐striatal pathways (anterior thalamic radiation). Effects were noted to be sensitive to the number of previous blast exposures, with a negative association between fractional anisotropy (FA) and time since most severe blast exposure in a subset of the multiple blast‐exposed group. However, these patterns were not observed in the subgroups classified using macrostructural changes (T2 white matter hyperintensities). Moreover, post‐concussion symptoms and PTSD symptoms, as well as neuropsychological function were associated with low FA in the major nodes of compromised neurocircuitry. Hum Brain Mapp 38:352–369, 2017. © 2016 Wiley Periodicals, Inc.
Keywords: mild traumatic brain injury, blast injury, cognition, concussion, post‐traumatic stress disorder, diffusion tensor imaging, tractography, neurocircuitry
INTRODUCTION
Traumatic brain injury (TBI), frequently through blast, is a common combat injury in those serving in Operation Enduring Freedom in Afghanistan and Operation Iraqi Freedom (OEF/OIF), with mild TBI (mTBI) being the most common among this group [Bhattacharjee, 2008; Ling et al., 2009; Taber et al., 2006; Warden, 2006]. This injury mechanism is in contrast to the civilian population where the majority of traumatic brain injuries are due to automobile accidents and falls [Langlois et al., 2006]. Blast TBI (bTBI) is categorized by injuries resulting from the different physical aspects of the blast phenomena [Cernak, 2010; Chen et al., 2009], such as the shockwaves generated by an explosion blast, secondary injury by displaced objects accelerated by the energy of explosion, blast wind injury and the thermal properties of the blast [Chen et al., 2009]. Blast‐induced TBI can manifest at all levels of severity and types of injury, with the level of bTBI severity based primarily on the duration of altered mental status.
Traumatic axonal injury [Kumar et al., 2009; Langlois et al., 2006; Parizel et al., 1998], or axonal stretch [Chatelin et al., 2011; Garman et al., 2011], is a hallmark of more severe TBI and conventionally refers to white matter (WM) damage arising from torsional forces generated by the sudden rotational acceleration/deceleration forces (“bobblehead” effects) of an impact head injury [Parizel et al., 1998, 2005; Shaw, 2002]. However, results from experimental studies using animal models of blast exposure have demonstrated that direct blast shockwave is capable of penetrating the calvarium [Chavko et al., 2007] and can induce high strain rates leading to structural deficits such as axonal membrane disruption [Connell et al., 2011], myelin disruption, and neuronal death [Cernak et al., 2001; Saljo et al., 2002], as well as altered brain function [Robinson et al., 2015].
Diffusion tensor imaging (DTI) measures the diffusion behavior of water molecules and is sensitive to differences related to the microstructure of brain nerve tissues. DTI yields estimates of the main direction of axon fibers with reasonably good spatial resolution [Basser et al., 1994; Basser and Jones, 2002]. It also provides a unique insight into the microstructure of various tissues. Within the brain, DTI can be used to quantify white matter integrity and extract white matter features for visualization such as tractography [Basser et al., 2000]. Neuronal tissue is a fibrillar structure consisting of highly oriented and tightly packed axons that are surrounded by glial cells. Thus, the organized bundles of neuronal tissue restrict the movement of water molecules on a micrometric scale to a greater extent in the direction perpendicular [radial diffusivity (RD)] than parallel [axial diffusivity (AD)] to the axonal orientation. Several recent studies have investigated the role of diffusion MR and shown promising results in detecting microstructural changes in mild TBI [Kasahara et al., 2012; Matsushita et al., 2011; Mayer et al., 2010].
Our previous study shows that central and superior–inferiorly oriented fibers such as the projection fibers interconnecting cortico‐subcortical regions, for example, centrum semiovale, superior corona radiata, cerebral peduncles/corticospinal tracts, cingulum bundles, precuneal white matter, fornix, cerebellar peduncles, and brainstem fibers, are more vulnerable to blast injury [Yeh et al., 2014]. These fibers form the substrate for information transfer between brain regions and are central to our understanding of brain function. However, one recent study failed to find a significant group difference of DTI metrics between mild/moderate bTBI patients and controls [Levin et al., 2010]. This discrepancy might be due to the timing post‐injury and heterogeneous mechanisms of injury in military TBI. Regional quantitative tractography has been used to reveal microstructural white matter changes over the long association fibers in civilian mild TBI patients without associated findings in routine structural MRI exams [Brandstack et al., 2013]. Although diffusion MRI offers a non‐invasive quantitative measure related to WM microstructure, there is no consensus on how DTI should be analyzed, and it is unclear how post‐processing would affect the results. Diffusion MRI tractography has an inherent limitation in estimating local fiber orientation, particularly long‐range anatomical pathways. Additionally, anatomical accuracy in fiber tracking is highly dependent on the parameters of tractography algorithms [Thomas et al., 2014]. Deterministic tractography based on a single tensor model has a better specificity (it is less likely to identify spurious pathways), but is suboptimal in estimating local fiber orientation in locations where fibers cross. On the contrary, probabilistic tractography, which uses a more liberal visual threshold, would be more appropriate if the goal is to reduce the likelihood of missing salient pathways [Thomas et al., 2014]. Nevertheless, when constructing the known pathways, using a priori anatomical knowledge to constrain tractography can reduce the occurrence of false‐positive trajectories of WM tracts.
On the other hand, white matter hyperintensities (WMHs) have been noted to occur with increased frequency in TBI cases [Riedy et al., 2016]. WMHs‐associated tissue changes (see for [Gouw et al., 2011] for review) may take place long before the manifestation of cognitive dysfunction [Hoge et al., 2008b]. Nevertheless, the impact of previous military‐related blast exposure(s) and the effect of time since injury on white matter structural changes in chronic mTBI; and the relationship between disrupted white matter tracts and affected domains of cognitive function is unclear.
The objectives of this study are to: (1) assess regional and global WM microstructural changes in chronic mild bTBI service members using different DTI reconstruction methods and tractography algorithms; (2) evaluate the effect of time since injury on WM microstructural changes in chronic mTBI; and (3) evaluate their associations with the number of previous blast exposures, clinical symptoms and neuropsychological function. We hypothesize that the parasagittal white matter fibers, such as fronto‐parieto‐temporal, thalamo‐(sub)cortico‐thalamic, and inter‐hemispheric pathways, are particularly vulnerable to blast injury in military‐related operations with a faster aging trajectory of WM integrity in high blast‐exposed group than low blast‐exposed group. Additionally, the compromised neurocircuitry has significant effects on the reported symptoms and neuropsychological functions of military TBI patients.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board of The Walter Reed National Military Medical Center (WRNMMC), Bethesda, Maryland. Written informed consent was obtained from all the subjects before participation.
Experimental Design
Study participants were not randomized or blinded in this observational study.
Participants
Two hundred and two (202) male blast‐related mild TBI participants (mean age ± standard deviation = 31.9 ± 7.3 years), who were US military personnel and had been exposed to military‐related blast(s) and reported persistent postconcussion symptoms (Silverberg, Lange et al. 2013) that interfered with full military functioning, were treated at National Intrepid Center of Excellence (NICoE), WRNMMC and recruited into the study. In general, admission to the NICoE intensive treatment program requires the presence of persistent symptoms for at least 6 months and a history of TBI. Prior to admission, all subjects have received a careful review of their medical history in the military electronic medical record to document ongoing difficulties and relevant exposure history. Subjects included here were even more remote from point of injury (576.0 ± 238.9 days). Forty (40) male nonTBI controls were also recruited (mean age ± standard deviation = 33.5 ± 8.6 years), who were military personnel on active duty but had not been previously deployed. Handedness information was only available for 100 TBI participants (87 right dominance, 11 left dominance, and 2 ambidextrous) and 30 nonTBI (29 right handed and 1 ambidextrous). The control subjects were military health care beneficiaries who were recruited through advertisements at the hospital. Control subjects recruited for this study were free from TBI, previous diagnosis of psychiatric condition such as psychosis, head injuries or neurological injuries or conditions (e.g., stroke, multiple sclerosis, spinal cord injury) and between the ages of 18 and 60. Subjects were asked to report relevant health conditions and queried for TBI history. As needed, previous health care contacts were reviewed in the military electronic medical record by one of the study physicians (G. R.). The control subjects were selected from a larger group of 67 controls. Eighteen of those controls were rejected based on their sex, two had incomplete data, four were rejected because of incidental findings on the scans (e.g., Chiari malformation), and three were rejected because of evidence of prior TBI or significant blast exposure.
All patients had sustained a blast injury and agreed to the use of their clinical and neuroimaging data for research purposes. The presence of a “blast‐related” injury was defined as a TBI that occurred in which a blast was the cause of the injury (e.g., an explosion causing a vehicle crash; a blast causing one to fall over and strike his head). Although blast was an important component of injury in all of the “blast” cases, these were not primary blast cases; usually a mechanical mechanism (e.g., fall) was also involved.
Post‐hoc diagnosis of TBI was based on a routine comprehensive clinical screening evaluation undertaken by medical/health‐care professionals at the NICoE, WRNMMC. Diagnosis of TBI is based on the presence and duration of loss of consciousness (LOC), presence and duration of post‐traumatic amnesia (PTA), duration of alteration of consciousness, and neuroradiological scans. Self‐reported symptoms, such as post‐concussion and PTSD symptoms, are routinely obtained during the TBI evaluation but are not used for diagnostic or classification purposes.
TBI patients were diagnosed as mild TBI based on duration of LOC, duration of PTA, alteration in mental status at the time of injury, and presence of focal neurological deficit, and consistent with DoD/VA standards [Cifu et al., 2009], namely that mild TBI was defined as alteration of consciousness and (if present) LOC less than 30 minutes or PTA less than 24 hours and no radiologic abnormalities were noted on conventional imaging. All of the patients had blast injuries, caused by either improvised explosive device (IED) or rocket attacks. One hundred and twelve (112) TBI patients had multiple episodes of blast injury with a median number of two blast exposures among all TBI participants. The days elapsed since the injury ranged from 185 to 1074 days (mean days ± standard deviation = 576.0 ± 238.9 days, median = 560 days). One hundred and sixty (160) mTBI patients were diagnosed with comorbid PTSD, anxiety and/or depression based on medical records (Supporting Information I.A). Two nonTBI participants had previously been diagnosed depression and/or anxiety based on the self‐reported screening and medical records.
Military blast exposure in mTBI
To investigate the potential effects of white matter microstructural changes due to blast exposure, TBI participants were further divided into two groups, high blast exposure group having three or more previous blast events, and low blast exposure group having one or two blast exposures.
Post‐concussion and PTSD symptoms
TBI patients were administered the Neurobehavioral Symptom Inventory (NSI) [Cicerone and Kalmar, 1995] and PTSD Check List‐Civilian Version (PCLC) [Blanchard et al., 1996] at the time of enrollment/MR scan in the study. The NSI [Cicerone and Kalmar, 1995] is a 22‐item self‐report of post‐concussion symptoms, including the severity/presence of headache, balance problems, nausea, fatigue, sensitivity to noise, irritability, sadness, nervousness, visual problems, and difficulty concentrating and remembering. The PCLC [Blanchard et al., 1996] is a 17‐item self‐report measure of PTSD symptoms, designed specifically to address Category B, C, and D symptom criteria of the DSM‐IV [APA, 1994] for PTSD (Supporting Information I.A.1). A cutoff PCLC score of 50 [Mcdonald and Calhoun, 2010] was used to classify whether the participants had PTSD or not. The Validity‐10 scale was used for validity assessment of NSI. For those participants with non‐valid response, data of self‐report symptoms and neuropsychological testing were discarded; but neuroimaging data were still used for further analyses.
Table 1 lists demographics of the participants, and injury severity characteristics and descriptive summary of NSI and PCLC scores.
Table 1.
Demographics of all participants
| Controls (40 males) | mTBI (202 males) | Statistical inference | |
|---|---|---|---|
| Years of age | 33.5 ± 8.6; [19, 50] | 31.9 ± 7.3; [20, 50] | t = 1.21, P = 0.29 |
| Years of education | 16.8 ± 3.5; [12, 24] | 13.8 ± 2.1; [11, 20] | t = 5.12, P < 0.0001 |
| Days since the major blast event | – | 576.0 ± 238.9; [185, 1074] | – |
| Percentage right‐handed | 100% | 89.19% | Pearson chi‐square = 2.3, P = 0.31 |
| Number of blast exposures | – | 2.6 ± 2.3; [1, 19] | – |
| Percentage of Microhemorrhages | 0% | 1.98% (4 TBI patients) | – |
| Number of WMHs | 1.3 ± 2.9; [0, 14] (14 (34.14%) of controls) | 5.1 ± 14.5; [0, 99] (98 (48.51%) of mTBI participants) | t = 3.42, P = 0.007 |
| NSI total | 5.1 ± 7.8; [0, 32] | 31.2 ± 13.7; [3, 68] | t = 14.33, P < 0.0001 |
| PCLC total | 20.9 ± 7.8; [17, 57] | 51.7 ± 16.2; [19, 85] | t = 10.90, P < 0.0001 |
Numbers are shown as mean ± SD, and [] is the range.
NSI, Neurobehavioral Symptom Inventory; PCLC, PTSD Check List‐Civilian Version; WMHs, white matter hyperintensities.
MR Acquisition
MR Imaging was performed on a 3T 750 MRI scanner (General Electric, Milwaukee, WI) with a 32‐channel head coil (MR Instruments, Minnetonka, MN). In addition to diffusion‐weighted imaging (DWI), a series of MRI data, including structural MRI, dynamic contrast susceptibility, fMRI, and MR spectroscopic imaging, were acquired in a ninety minute session for all of the participants. DWI was acquired with a single shot dual spin‐echo echo planar imaging sequence with slice‐selection gradient reversal and peripheral cardiac‐gating [TR/TE ∼ 10,000/90 ms, FOV = 256 × 256 mm2, matrix = 128 × 128, voxel size 2 × 2 × 2 mm3, b = 1,000 s/mm2, 48 noncollinear diffusion gradient directions plus 7 volumes of non‐diffusion weighted (b0) evenly distributed among diffusion‐weighted volumes]. Fluid attenuated inversion recovery (FLAIR) imaging was used to assess presence of T2 WMHs.
DTI Processing and Analysis
Preprocessing
Preprocessing of DTI data included correction of EPI geometric distortion using B0 fieldmap [Jezzard and Balaban, 1995], correction of motion and eddy current artifacts and digital brain extraction (skull stripping) using software from the FSL toolkit [Smith et al., 2004]. DTI scalar images, for example, fractional anisotropy (FA) and mean diffusivity (MD), were created using either a simple least squares fit for single tensor reconstruction method or a robust estimation of tensors by outlier rejection through an iterative re‐weighting process [Chang et al., 2005] (Supporting Information I.B.1).
Voxel‐wise analysis of diffusion metrics
Spatial normalization of whole brain DTI metrics was carried out by using high‐dimensional tensor‐based image registration [Zhang et al., 2005] (Supporting Information I.B.2).
Tract specific analysis
To perform tract specific statistical mapping analysis, deformable geometric medial models were used to model the continuous medial representations (cm‐reps) of individual sheet‐like white matter structures [Yushkevich et al., 2008]. Under the framework of modeling average tensor‐based features along directions perpendicular to the tracts using cm‐reps, a generic atlas of six major white matter tracts [Yushkevich et al., 2008] was used for the spatial normalization of white matter tracts and tract specific statistical analysis across all participants. The six major fiber tracts include the corpus callosum, bilateral corticospinal tracts (CST), inferior longitudinal fasciculi (ILF), superior longitudinal fasciculi (SLF), inferior fronto‐occipital fasciculi (IFOF), and uncinate fasciculi (UNC).
For whole brain spatial analysis, voxel‐wise analyses of diffusion metrics across the group of study participants were carried out only on the voxels with FA ≥ 0.20, in order to avoid gray matter which typically has FA between 0.1 and 0.2 [Mori and van Zijl, 2002] and to minimize mis‐registration caused by large ventricles and/or brain atrophy. The images of spatially normalized whole brain DTI metrics reconstructed using high‐dimensional tensor warping before statistical analysis.
Fiber tracking and segmentation
Two automated fiber segmentation and quantification methods, Automated Fiber Quantification (AFQ) uses the deterministic streamlines algorithm [Basser et al., 2000; Mori et al., 1999] while TRActs Constrained by UnderLying Anatomy (TRACULA) uses ball‐and‐stick probabilistic tracking [Jbabdi et al., 2007] were implemented to assess the integrity of major white matter tracts.
Automated fiber quantification (AFQ) [Yeatman et al., 2012]
AFQ uses a deterministic streamlines tracking algorithm [Basser et al., 2000; Mori et al., 1999] and waypoint ROI‐based fiber tact segmentation [Wakana et al., 2007]. Tract refinement based on a standard fiber tract probability map [Wakana et al., 2007] was then used to identify twenty major tracts. The 1D profiles of weighted mean FA of each fiber tract along the 1D path were calculated for tract profile analysis (Supporting Information I.B.3).
TRActs constrained by underlying anatomy (TRACULA) [Yendiki, 2011]
TRACULA is based on the Bayesian framework for global probabilistic tractography [Jbabdi et al., 2007]. It utilizes prior information on the anatomy of the white matter pathways from a set of training subjects. The average weighted mean DTI metrics of the whole white matter tracts were calculated for ROI analysis (Supporting Information I.B.4).
Segmented and labeled fiber tracts using AFQ or TRACULA included anterior thalamic radiation (ATR), cingulum–cingulate gyrus (supracallosal) bundle (CCG), cingulum–angular (infracallosal, parahippocampal) bundle (CAB), corpus callosum–forceps major (FMAJ), and corpus callosum–forceps minor (FMIN), CST, superior longitudinal fasciculus–parietal bundle (SLFp), superior longitudinal fasciculus–temporal bundle (SLFt, TRACULA method)/arcuate fasciculus (ARC) (AFQ method), ILF, UNC, and IFOF (only for AFQ method) (Supporting Information Fig. 1).
Neuropsychological Testing
Selected neuropsychological tests were administered to mTBI participants depending on clinical judgments. Neuropsychological measures included a comprehensive battery of tasks that were conceptually grouped into neuropsychological domains in order to facilitate data reduction. The neuropsychological domains and their associated measures included seven major neuropsychological domains (overall intelligence, fine‐motor skills, attention, language, visual‐spatial, memory, and executive function) (Supporting Information I.C). Age‐based standard scores were calculated for all of the tests for data analyses.
Statistical Analyses
Statistical analyses of whole brain voxel‐wise analyses were performed on 1 mm3 isotropic voxels using standard neuroimaging analysis packages (FSL, AFNI, and MRTRIX3). The summary statistics (Table 1) and the ROI analysis were carried out using SAS (Cary, NC, version 9.2).
Group comparisons of FA
Whole brain voxel‐wise analysis
For both whole brain voxel‐wise and following tract specific analysis, general linear model (GLM) analyses evaluated the local group differences, that is, the level of two groups (nonTBI vs. TBI) of measured FA within the white matter tracts after regressing out the effects of age. Education level was not included as a covariate in GLM due to missing data of some participants, and the incapability of handling missing data by GLM. For voxel‐wise analysis, statistical inference P‐values were corrected for multiple comparisons with controlled family‐wise error (FWE) rate set at 5% based on the calculated t‐statistic images, with false discovery rate (FDR) set at rates of 5%, 6%, 7%, 8%, 9%, and 10%, based on the uncorrected P‐values [Benjamini and Hochberg, 1995]. For controlling FWE, permutation methods [Nichols and Holmes, 2002] followed by threshold‐free cluster enhancement (TFCE) approaches [Smith and Nichols, 2008] with corrected P ≤ 0.05 considered as significantly different between groups.
Tractography‐based analysis
Tract specific analysis
GLM analysis followed by permutation test was used to evaluate the local group differences of measured FA across the white matter tracts after regressing out the effects of age. Significance was set at P ≤ 0.05.
Tract profile analysis
For AFQ, generalized linear models [fitglm in 2014 MATLAB® (http://www.mathworks.com) Statistics and Machine Learning Toolbox] with age as a covariate were used to compare average FA values along 1D tract profiles between TBI and control groups. False discovery rate (FDR) [Benjamini and Hochberg, 1995] at rates of 5%, 6%, 7%, 8%, 9%, and 10%, and permutation test at a 5% of FWE were used to control Type I error by correcting for multiple comparisons.
Additional subgroup, including 37 nonTBI controls (age = 33.16 ± 8.73 years, education = 16.8 ± 3.6 years) and 163 mTBI patients (age = 33.90 ± 7.33 years, education = 13.8 ± 2.0 years) who had the information of education level, was analyze separately by adding education in years as a covariate, in addition to age, in the linear model. Another subgroup with matched years of education and age but a smaller size (19 nonTBI controls vs. 162 mTBI patients: age = 29.20 ± 7.30 vs. 31.96 ± 7.31 years, education = 14.3 ± 1.7 vs. 13.8 ± 2.0 years; t test, P = 0.13 and 0.45, respectively) was also analyzed to consider education level as a confounder in white matter integrity [Teipel et al., 2009].
Region of interest (ROI) analysis
The mean FA calculated from TRACULA was used for ROI analysis. To account for correlated responses between and within subjects and white matter tracts, linear mixed model (LME) (MIXED procedures, SAS institute) using unstructured multivariate correlated errors model based on repeated measures analysis of covariance was applied to test group mean difference, with covariates of age and years of education. Education level was included as a covariate in the LME because MIXED is capable of handling missing data. While comparing the least squares means of group fixed effects, Bonferroni correction was used to adjust P values for multiple comparisons of FA differences of white matter tracts between group (corrected P ≤ 0.05).
Associations between DTI metrics and post‐concussive symptom, PTSD symptom, time post‐injury, WMHs, and neuropsychological function
Pearson partial correlation accounting for age effect was used to assess associations between regional white matter integrity (FA and MD of the tracts segmented using TRACULA) and post‐concussive symptom (NSI score), PTSD symptoms (PCLC score), number of whole brain WMHs, days since most severe blast injury and NP test scores in mTBI patients. Interaction terms including blast group × days since blast injury and WMHs group × days since blast injury were added in the MIXED modeling to test whether the association was significantly different between blast groups and between WMHs group, respectively. Among them 165 mTBI patients had completed self‐report questionnaires for post‐concussive symptoms (NSI), and 163 mTBI patients for PCLC. The other fourteen mTBI participants had finished partial PCLC questionnaire; however, if the sum of the completed PCLC items had a score of 50 or above, the participants was classified as mTBI with PTSD.
Bonferroni method was used to correct multiple comparisons with adjusted P ≤ 0.05.
Single Subject Analysis
Tract profile analysis using automated fiber quantification (AFQ)
For individual level inference using AFQ, standardized tract profiles were created by calculating the mean and standard deviation of FA values at each node of each tract in the control samples. Confidence intervals of 5th and 95th percentile for each tract of each mTBI patient were then quantified. FA values larger than 95th or smaller than 5th percentiles with more than three consecutive nodes were considered abnormal.
RESULTS
Participant Demographics
Age did not differ significantly between groups (nonTBI vs. TBI). The TBI group had a lower mean education level (in years) than the nonTBI group (13.8 ± 2.0 vs. 16.8 ± 3.6 years, P < 0.0001). However, NSI and PCLC scores significantly differed between TBI and HC groups with mean total NSI and PCLC scores significantly greater in TBI patients (NSI: 31.2 ± 13.7 in the range of 3–68 for TBI vs. 5.1 ± 7.8 in the range of 0–32 for nonTBI; PCLC: 51.7 ± 16.2 in the range of 19–85 for TBI vs. 20.9 ± 7.8 in the range of 17–57 for nonTBI). Based on the cutoff PCLC score of 50, ninety‐six (96) mTBI participants were classified as mTBI with PTSD and eighty‐one (81) mTBI participants as mTBI without PTSD. One nonTBI participant with a PCLC score of 57 and the other nonTBI participant with a PCLC of 41 were those who had a past history of depression and/or anxiety. NSI total score and PCLC total score were highly correlated with each other (r = 0.83, P < 0.0001). Four of mTBI participants had microhemorrhages revealed by susceptibility‐weighted imaging. Ninety‐eight (98) of 202 mTBI patients and fourteen (14) of 40 nonTBI participants had one or more foci of T2 WMHs demonstrated on FLAIR ((both evaluated by G. R.) (Table 1).
To examine the effect of blast exposure on white matter microstructural changes, TBI patients were further divided into two subgroups, high blast‐exposed group (three or more blasts, 48 participants) and low blast‐exposed group (fewer than three blasts, 154 participants).
To examine the possibility of interaction effect of WMHs on white matter microstructural changes, TBI patients were split into two groups, high WMHs (four or more total WMHs, 53 mTBI participants) and low WMHs group (fewer than four WMHs, 149 mTBI participants).
Table 2 summarizes the demographics, post‐concussion and PTSD symptoms and the findings of structural MRI of mTBI subgroups.
Table 2.
Demographics of mTBI participants based on the history of previous exposures
| High blast exposures (≥3 blasts, 48 males) | Low blast exposures (≤2, 154 males) | Statistical inference | |
|---|---|---|---|
| Years of age | 33.5 ± 6.6; [24, 49] | 31.4 ± 7.4; [20, 50] | t = 1.83, P = 0.07 |
| Years of education | 14.2 ± 2.0; [12; 18] | 13.6 ± 2.0; [11, 20] | t = 1.54, P = 0.13 |
| Days since the major blast event | 595.7 ± 225.8; [192, 1070] | 556.4 ± 244.1 [107, 1074] | t = 0.76, P = 0.43 |
| Percentage right‐handed | 88% | 88% | – |
| Number of blast exposures | 4.5 ± 2.6[3, 19] | 1.3 ± 0.6 [1, 2] | t = 8.34, P < 0.0001 |
| Number of participants with microhemorrhages | 2 | 2 | – |
| Number of WMHs | 4.7 ± 12.7; [0, 84] (45.83%) | 5.2 ± 14.9 [0, 99] (51.28%) | t = 0.24, P = 0.8 |
| NSI total | 30.4 ± 13.0; [3, 55] | 31.5 ± 13.9; [4, 68] | t = 0.47, P = 0.64 |
| PCLC total | 53.6 ± 16.3; [19, 84] | 51.2 ± 16.2; [21,85] | t = 0.82, P = 0.42 |
Numbers are shown as mean ± SD, and [] is the range.
NSI, Neurobehavioral Symptom Inventory; PCLC, PTSD Check List‐Civilian Version; WMHs, white matter hyperintensities.
Group Comparisons (nonTBI vs. TBI)
Voxel‐wise analysis
Four DTI metrics, that is, FA, MD, RD, and AD, of 202 mTBI were compared with those of 40 controls using GLM and permutation test through high dimensional tensor warping followed by voxel‐wise comparison. No significant group differences between mTBI and nonTBI were found among any of the four DTI metrics after correcting multiple comparisons, using both TFCE algorithm for controlling FWE at 5%, and using FDR at rates of 5%, 6%, 7%, 8%, 9%, and 10%.
Tractography‐based analysis
Tract specific analysis (TSA)
Supporting Information Figure 2 shows t‐statistics using TSA with cm‐reps to compare FA between mTBI and controls. The comparison of mTBI to nonTBI controls found the low FA clusters over the left SLF at the frontal region in the mTBI group (corrected P ≤ 0.05 at 5% FWE) (Supporting Information Fig. 3A). When mTBI was divided into blast subgroups, the high blast‐exposed group had clusters with lower FA than low blast‐exposed group and controls in the left uncinate fasciculus at the temporal region (Supporting Information Fig. 3B and the left SLF). When mTBI was divided into PTSD subgroups, mTBI with PTSD had clusters with lower FA over the left UNC than the nonTBI controls (corrected P ≤ 0.05 at 5% FWE), but there was no difference between mTBI with PTSD and mTBI without PTSD.
Tract profile analysis using Automated Fiber Quantification (AFQ)
Compared with the 40 controls, tract FA profiles using AFQ method revealed that the mTBI patients as a group had lower FA over the regions of the left CAB [corrected P ≤ 0.05, FDR = 0.06 (Fig. 1A), FWE = 0.05 (t ≥ 2.80) (Supporting Information Fig. 4A)]. The results of additional subgroup analyses, that is, 37 nonTBI versus 163 mTBI with education as a covariate in the model, and 19 nonTBI versus 162 mTBI with matched education level, also found difference of FA tract profile over the left CAB between mTBI and control (FDR = 0.10 and 0.12, respectively, results not shown), but less significant as compared with the result including all participants. For the PTSD subgroups, the mTBI with PTSD had lower FA over the left CAB than the nonTBI [FDR = 0.06 (Supporting Information Fig. 4B), FWE = 0.05], but higher FA over the left CAB (FDR = 0.08, FWE = 0.05) than that of the mTBI without PTSD. For the blast subgroups, the high blast‐exposed group had lower FA than the low blast‐exposed group over the forceps major (FWE = 0.05), the left ATR (FDR = 0.08) (Fig. 1B) and the right IFOF [FDR = 0.1 (Fig. 1C), FWE = 0.05]. The low blast‐exposed group had lower FA than the controls over the left CAB (FDR = 0.08, FWE = 0.05) and the right ILF (FWE = 0.05). While combining PTSD and blast subgroups, the high blast‐exposed mTBI patients with PTSD had lower FA than the high blast‐exposed mTBI patients without PTSD over the right CAB [FDR = 0.05 (Fig. 1D), FWE = 0.05], but higher FA than the low blast‐exposed patients without PTSD over the left ARC [FDR = 0.08 (Supporting Information Fig. 4C), FWE = 0.05].
Figure 1.
AFQ tract profile analyses of FA showing significant group difference, with lower FA in mTBI group over the parahippocampal part of the left cingulum bundle (corrected P ≤ 0.05, FDR = 0.05) than non‐TBI controls (A); lower FA in high blast‐exposed group than low blast‐exposed over the left anterior thalamic radiation (FDR = 0.08) (B), and the left inferior fronto‐occipital fasciculus (FDR = 0.10) (C); lower FA over the right CAB (FDR = 0.08) in the high blast‐exposed mTBI with PTSD than the high blast‐exposed mTBI without PTSD (D).
Single subject analysis using tract profile analysis
The comparison of mTBI to controls using AFQ tract profile single subject analysis found abnormal FA profiles (either smaller or larger than 5th percentiles of controls at more than three consecutive nodes) mainly at the FMAJ, ATR, CST, CCG, CAB, SLF/ARC, and ILF. Examples of low FA profiles compared with the mean of controls in two TBI patients are shown in Figure 2.
Figure 2.
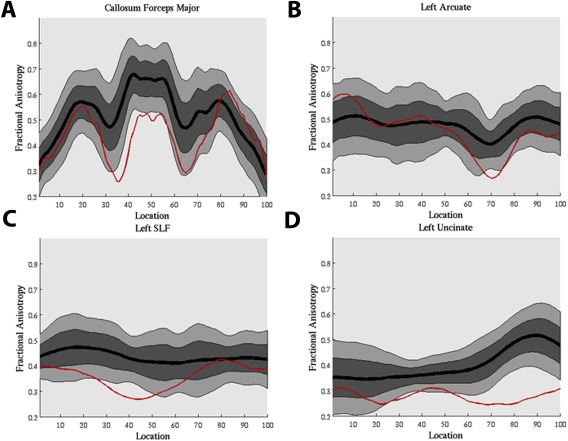
Examples of single subject tract profile analysis using AFQ, identified by FA values smaller than the 5th percentiles of control participants, with larger than 3 consecutive nodes. A mild blast TBI patient (male, 30 years old, 710 days post‐injury) injured by grenade and mortar blast with complaints of headaches, tinnitus, vision, sleep, attention, irritability and cognition. Low FA were found at forceps major (A) and superior longitudinal fasciculus–temporal/arcuate fasciculus (B). A mild blast TBI patient (male, 52 years old, 2,454 days post‐injury) injured by mortar blast and fall with complaints of hearing loss, depression, anxiety, memory loss, and poor cognition. Low FA were found at left superior longitudinal fasciculus (C) and left uncinate fasciculus (D). [Color figure can be viewed at http://wileyonlinelibrary.com.]
Tract ROI analysis using TRActs Constrained by UnderLying Anatomy (TRACULA)
For the ROI analysis of global value of FA between TBI and controls by comparing the estimated mean values in whole white matter tracts using mixed modeling with age and years of education as covariates, there is no significant group difference among the DTI metrics (FA, MD, AD, and RD) between TBI and nonTBI after correcting for multiple comparisons, but TBI had lower FA than nonTBI over the right CAB at the margin of statistical significance (Bonferroni and FDR corrected P = 0.0526). However, this significance did not survive the multiple comparisons correction when mTBI was divided into groups with or without PTSD. For the blast subgroups, the high blast‐exposed group had lower FA than the controls over the right CAB (mean FA ± standard error = 0.275 ± 0.004 vs. 0.300 ± 0.006, corrected P = 0.0051, both Bonferroni and FDR). When mTBI patients was further grouped according to the presence of PTSD and the severity of previous blast exposures, the mTBI patients with PTSD and high blast exposures tended to have the lowest FA of right CAB (uncorrected P = 0.0016, corrected P = 0.1167).
Interaction between blast exposure, WMHs, and aging on DTI measures
To further contrast the interaction effect of blast exposures and aging on white matter integrity, the blast × age interaction was added to the model. Significant (P ≤ 0.05) blast exposure and age interaction effects on FA measure were observed over the cingulate part of bilateral cingulum bundles with the high blast‐exposed group having a faster regional aging trajectory, that is, higher negative slope of the group and age interaction term (left: −2.60E‐4 ± 5.97E‐4, right: −2.63E‐4 ± 5.73E‐4) toward reduced white matter integrity (lower FA) than the low blast‐exposed group (left: −4.20E‐4 ± 5.73E‐4, right: −8.20E‐4 ± 2.92E‐4), and the control group (left: 2.36E‐4 ± 5.94E‐4, right: 3.67E‐4 ± 6.27E‐4) (high blast vs. control in the left CCG and the right CCG: t = 3.37, 3.53, P = 0.02, 0.03, and d = 0.74, 0.77, respectively) (Fig. 3A).
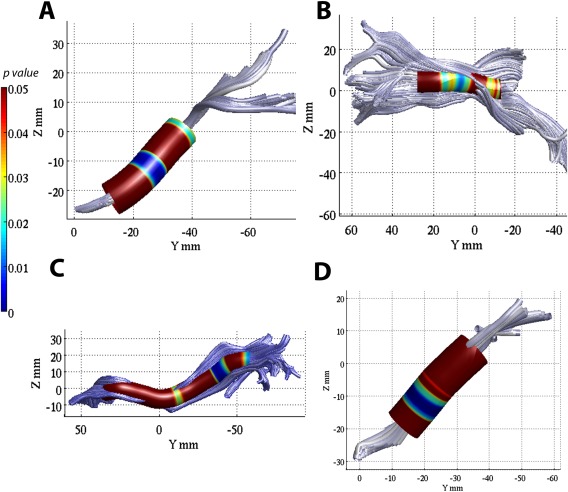
Figure 3.
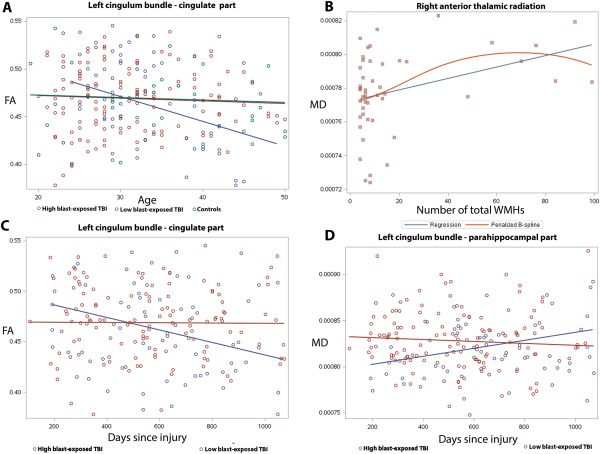
Results of the interaction between blast exposure and aging on cross‐sectional FA (A), the association between MD and WMHs (B), and the interaction between blast exposure and time since most severe blast injury (C, D) on DTI measures using TRACULA ROI analyses. Color‐coding with blue, red and green corresponds to high blast‐exposed, low blast‐exposed and controls, respectively. (A) Linear models of mean FA versus age in the cingulate part of left cingulum bundle that revealed a more rapid regional cross‐sectional aging trajectory toward reduced white matter integrity (reduced FA) in the high blast‐exposed group (at least 3 blast exposures) than low blast‐exposed (1 or 2 blast exposures) and controls (P < 0.05). (B) Linear (blue) and B‐spline (red) models of mean MD of the right anterior thalamic radiation and the number of brain WMHs in the mTBI participants with large number of WMHs (larger than 3 WMHs in total) (P < 0.05). (C, D) Regional mean FA of the cingulate part of left cingulum bundle (C) and mean MD of the left parahippoocampal part of the left cingulum bundle (D) in mTBI patients shows that the trajectories toward reduced white matter tissue integrity, either decreased FA (C) or increased MD (D) with increasing time since blast injury, were unique to previous blast exposures.
After correcting for multiple comparisons, there was no regional FA difference of any TRACULA segmented tracts among the mTBI subgroups, for example, high incidence versus low incidence of blast exposures or high number versus low number of WMHs groups. There was also no MD difference, except that the high WMHs group as a group had a higher MD (mean ± standard error = 88.89E‐5 ± 0.48E‐5) over the FMAJ than the low MWHs group (86.97E‐5 ± 0.29E‐5) and the control group (87.75E‐5 ± 0.65E‐5) (t = 7.08 and 4.93, and d = 0.41 and 0.31, respectively, adjusted P < 0.05).
In addition, MD of the right ATR in the high WMHs group was significantly correlated with the number of WMHs (r = 0.51, adjusted P = 0.004, Fig. 3B), but no significant correlation between FA and the number WMHs was found among the 18 segmented tracts. Furthermore, there was no significant correlation between the number of blasts and the number of WMHs, nor was there significant two‐way interaction effect of WMHs and age on FA or MD when the WMHs * age interaction was included in the model.
Post‐Concussion and PTSD Symptoms, and Time Post‐Injury and FA
Using ROI analysis of the segmented major white matter tracts (TRACULA), a higher score in the NSI sensory domain (more reported sensory symptoms) was associated with lower FA of the left SLFt (r = −0.25, adjusted P = 0.02) after correcting multiple comparisons. Higher total PCLC score (more reported PTSD symptoms) in mTBI patients was associated with lower FA of the right CAB (r = −0.25, adjusted P = 0.02).
Time since the main episode of blast exposure injury was not found significantly correlated with FA or MD metrics in the larger group of mTBI patients. However, when dividing mTBI into high blast and low blast exposure groups, the high blast‐exposed mTBI patients' regional mean FA of the CCG was inversely associated with days since injury (r = −0.30, adjusted P = 0.04), but no significant association of regional mean FA and time since blast injury was seen in the low blast exposure group (Fig. 3C). Regional mean MD of the right ILF, right UNC and the CAB (Fig. 3D) increased as time progressed since injury in the high blast‐exposed mTBI patients (r = 0.38, 0.37, 0.31, respectively, adjusted P < 0.05), but not in the low blast‐exposed patients.
Neuropsychological Function and White Matter Integrity
Table 3 summarizes the results of NP in mTBI patients and their correlations with the mean FA of major white matter tracts. All of the P‐values of significant correlations were corrected using Bonferroni method (corrected P ≤ 0.05). Significance showed that integrity of UNC was important for memory function, CCG for attentional control and cognitive speed, CAB for set‐shifting, UNC and CST for response inhibition control; and SLF was critical for overall executive function, inhibition control and fine‐motor skills (Supporting Information II.A). For example, Category Fluency of the Dellis–Kaplan Executive Function System (D‐KEFS) Verbal Fluency score positively correlated with FA of the left CCG (r = 0.63, P = 0.008; Fig. 4A); FA values of right UNC correlated positively with the Information score (r = 0.56, P = 0.0019) and Full scale IQ of the Wechsler Adult Intelligence Scale, 4th Ed (WAIS‐IV) (r = 0.50, P = 0.026; Fig. 4B); and Total Achievement score of the D‐KEFS correlated with the FA of temporal branch of the right SLF (r = 0.58, P = 0.037).
Table 3.
Neuropsychological test scores (t scores or standard scores) of mTBI participants
| Cognitive domain and measure | Mean ± SD | Significant correlation with regional FA (adjusted P < 0.05)a |
|---|---|---|
| Intelligence | ||
| WAIS‐IV | ||
| Full‐scale IQ | 53.57 ± 7.50 | R UNC |
| Information | 54.79 ± 8.49 | R UNC |
| Symbol Search | 49.99 ± 8.91 | L CABb |
| Global Ability Index | 55.23 ± 7.97 | |
| Perceptual Reasoning Index | 55.48 ± 7.76 | |
| Language | ||
| WAIS‐IV Vocabulary Comprehensive Index | 53.44 ± 7.95 | |
| D‐KEFS Word Reading | 49.98 ± 9.11 | |
| Learning and memory | ||
| CVLT (standard score) | ||
| Short delay cued recall | 0.42 ± 0.83 | |
| Long delay cued recall | 0.35 ± 0.91 | |
| Short delay free recall | 0.47 ± 1.05 | |
| Long delay free recall | 0.29 ± 1.10 | |
| Working memory | ||
| WAIS‐IV Working memory Index | 51.42 ± 8.66 | |
| Attention | ||
| WAIS‐IV Digit Span | 51.25 ± 9.05 | |
| Stroop Color and Word Test | ||
| D‐KEFS Inhibition Switching | 50.12 ± 8.56 | R UNC |
| Executive function/Abstract reasoning | ||
| WCST | ||
| Perseverative Responses | 58.12 ± 11.44 | |
| Overall Achievement | ||
| D‐KEFS Total Achievement | 57.79 ± 8.04 | R SLFt |
| Set shifting | ||
| Trail_making B | 50.34 ± 9.90 | |
| D‐KEFS Category Switch Response | 52.42 ± 11.22 | R CAB |
| D‐KEFS Category Switch Accuracy | 55.13 ± 9.29 | R CAB |
| Verbal Fluency | ||
| D‐KEFS Category Fluency | 59.24 ± 9.16 | L CCG |
| D‐KEFS Letter Fluency | 51.96 ± 11.21 | |
| Inhibitory Control | ||
| CCPT Commission | 52.52 ± 10.71 | |
| D‐KEFS Inhibition | 51.52 ± 8.38 | L CST, R SLFt |
| Self‐monitoring, Planning, Speed of Processing | ||
| D‐KEFS Time‐per‐move ratio | 48.44 ± 5.79 | L CST, L |
| D‐KEFS First‐move Time of the Tower Test | 45.84 ± 9.62 | UNCb |
| D‐KEFS total Rule Violation Ratio | 51.28 ± 1.63 | L CST, L UNC R ATR |
| Visual‐spatial function | ||
| ROCF Recognition Correct Total | 41.11 ± 13.58 | |
| WAIS‐IV Block Design | 54.27 ± 8.01 | |
| BVMT‐R Delayed Recall | 53.98 ± 12.75 | |
| BVMT‐R Learning | 61.70 ± 9.64 | |
| BVMT‐R Total Recall | 49.60 ± 10.84 | |
| Psychomotor speed | ||
| Trail Making A | 50.95 ± 10.28 | |
| Grooved Pegboard Test | ||
| Dominant hand | 49.47 ± 9.90 | R SLFp, R |
| Nondominant hand | 50.05 ± 10.82 | SLFt |
Abbreviations of Neuropsychological tests: BVMT‐R, Brief Visuospatial Memory Test – Revised; CCPT, Conners Continuous Performance Test; CVLT, California Verbal Learning Test; D‐KEFS, Dells‐Kaplan Executive Function System; ROCF, Rey‐Osterrieth Complex Figure; WAIS‐IV, Wechsler Adult Intelligence Scale, 4th Ed; WCST, Wisconsin Card Sorting Test.
Abbreviations of white matter tracts: ATR, anterior thalamic radiation; CST, corticospinal tract; SLFp, superior longitudinal fasciculus–parietal bundle; SLFt, superior longitudinal fasciculus–temporal bundle; UNC, uncinate fasciculus.
Significant partial Pearson correlation between FA of major fiber tracts and neuropsychological scores after controlling age covariate (corrected P ≤ 0.05).
Negative correlation.
Figure 4.
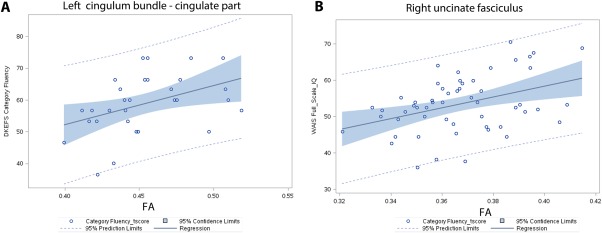
Examples of the results of significant associations between FA in two distinct white matter tracts and cognitive functions in mTBI service members with military‐related blast injury. Cognition functions were displayed as t scores. (A) Correlation of Dells‐Kaplan Executive Function System Category Fluency of Verbal Fluency performance and average FA of the cingulate part of the left cingulum bundle (r = 0.63, P = 0.00). (B) Correlation of Wechsler Adult Intelligence Scale, 4th Ed Full Scale IQ score and average FA of the right uncinate fasciculus (r = 0.50, P = 0.026). [Color figure can be viewed at http://wileyonlinelibrary.com.]
DISCUSSION
Using tract profile analysis and tract specific analysis, we have revealed evidence of white matter injury in chronic blast TBI patients. Due to the heterogeneity of injury mechanisms from blast TBI, it is not surprising to observe heterogeneous and spatially diverse white matter abnormalities [Davenport et al., 2012] reported in different studies [Mac Donald et al., 2011; Petrie et al., 2014; Taber et al., 2014].
Our results show that white matter damages indicated by reduced FA were most prominent in the pathways within the fronto‐limbic and the fronto‐striatal circuits, particularly the fiber tracts in the parasagittal white matter of the cerebral cortex such as the CAB, ATR, SLF and IFOF. These results are similar to our previous report [Yeh et al., 2014] and other reports of chronic changes in military‐related blast mTBI [Mac Donald et al., 2011] using voxel‐wise analysis for group comparison. In addition, newer approaches using tract profile analyses were useful in group comparison and single subject analysis, which pinpoint specific damaged fiber tracts and affected neurocircuitry.
Moreover, the compromised fiber tracts (reduced FA) in the nodes of fronto‐striatal and fronto‐limbic circuits were associated with greater post‐concussion and PTSD symptoms. These findings suggest the networks of the fronto‐parieto‐temporal circuit, fronto‐striatal circuit, and the frontal‐limbic circuit are most vulnerable to military related blast injury, which may also play an important role in the development of neuropsychological symptoms frequently seen in military TBI patients.
Interpretation of Microstructural Changes in Chronic Blast TBI
Group analysis
Blast TBI as described in this work refers to “blast plus impact.” That is, while components of primary blast may be involved, most injuries also involve secondary or tertiary effects, which have features also seen with mechanically induced TBI [Warden, 2006].
Through simulation, it has been shown the dynamic deformation of white matter arising from shock wave propagation can result in axonal stretch and disruption [Besenski, 2002; Chafi et al., 2010]. From a mechanical standpoint, macro and micro interfaces between structures with distinct differences in density and elasticity are particularly vulnerable to damage caused by shock wave propagation observed in blast‐related brain injury. The anatomical locations of low FA in blast‐related mTBI demonstrate that parasagittal frontal white matter, particularly the fronto‐limbic and the fronto‐striatal projection fibers such as CCG/CAB, ATR and FMAJ (Figs. 1 and 2), as well as long association fibers interconnecting the medial frontal, temporal, parietal and occipital lobes (Supporting Information Figs. 3 and 4) are vulnerable to blast injury. These results were consistent with our previous DTI and tractography report in subacute TBI patients [Yeh et al., 2014] that revealed subcortical superior‐inferiorly oriented tracts were particularly vulnerable to blast injury, as well as other reports of chronic changes in mTBI, either military personnel [Mac Donald et al., 2011] or civilian populations [Inglese et al., 2005; Kraus et al., 2007; Lipton et al., 2009]. The anatomical locations of low FA using tract specific analysis and tract profile analysis in this study are also consistent with the results from using mechanical simulation and finite element analysis of brain exposure to blasts, showing that the highest level of axonal shear/strain effects developed in the regions of corpus callosum and corona radiata [Chatelin et al., 2011] in mTBI model.
Secondary brain injury and repair mechanisms such as chronic inflammation and hypermetabolism may sensitize the brain to the subsequent injury [Calabrese et al., 2014], leading to axonal repair, reactive gliosis [Glushakova et al., 2014; Johnson et al., 2013; Kiraly and Kiraly, 2007] or irreversible axonal damage [Gupta and Przekwas, 2013]. Our findings of the inverse relationship between post‐injury duration and FA over the cingulum bundles (Fig. 3C) in TBI patients with previous high blast exposure frequency raise the possibility of Wallerian degeneration which may contribute to the long‐lasting impairment of cognitive performance seen in many chronic TBI patients.
Diffusion MRI tractography‐based analysis, either ROI‐based, tract specific or tract profile analysis, can be very time‐consuming. With larger ROIs, there is a risk of including voxels containing signals from adjacent tissues such as CSF and other tracts and this contamination can be even more pronounced in studying small white matter tracts [Vos et al., 2011]. In addition, ROI‐based approaches can result in loss of information regarding local variations in diffusion parameters [Kvickström et al., 2011], which may explain why no significant group difference between the mTBI group and controls was found using ROI‐based TRACULA. To the best of our knowledge, this is the first report evaluating DTI metrics along white mater tracts in chronic blast mTBI patients as a function of distance from specific anatomical landmarks. It is anticipated that the exact spatial locations of low FA clusters would be slightly different among different tractography‐based analysis methods. Nevertheless, the identified abnormal tracts, such as, the CCG and the SLF in TBI patients were consistent between tract specific analysis and tract profile analysis in our results.
Single subject analysis
In contrast to the results using voxel‐wise analysis in single subject analysis, the results using tract profile analysis specified the injured tracts by revealing the low FA profile within the whole tract, either the entire tract (Fig. 2D) or segment(s) within the tract (Fig. 2A–C). FA profile values quantified by tract profile analysis varied substantially within a tract but the shape of the tract FA profile was consistent across subjects (Fig. 2), demonstrating the precision of tract profile analysis for quantifying white matter properties at specific spatial locations on a fiber tract across subjects. There is minimal contamination of FA measurements from crossing or kissing fibers within the central portion of the tract, thus the measured FA values within the main fascicles are more indicative of tract integrity than those from peripheral locations.
Post‐concussion and PTSD symptoms and neurocircuitry affected in TBI
Our findings of significant associations between WM integrity and post‐concussion, and WM integrity and PTSD symptoms were in the affected regions of the neural networks in which the somatic sequelae (SLF sensory/motor pathways) and affective symptoms (cingulum bundle of limbic pathways) have intriguing clinical‐anatomical correlates. The cingulum bundle is one of the locations found to be associated with PTSD [Daniels et al., 2013], which is consistent with the findings of our group comparisons using AFQ tract profile analysis, showing lower FA over the left CAB in mTBI with PTSD than nonTBI controls (Supporting Information Fig. 4B), and lower FA over the right CAB in the high blast‐exposed mTBI patients with PTSD than the high blast‐exposed mTBI patients without PTSD (Fig. 1D). In addition, the finding of lower FA over the left UNC in mTBI with PTSD than the nonTBI revealed by tract specific analysis suggests disrupted WM networks in limbic system, for example, cingulum bundle and uncinate fasciculus, are associated with the PTSD symptoms in comorbid mTBI‐PTSD patients.
The frequent comorbidity of PTSD and TBI is well described in military TBI patients [Belanger et al., 2010; Hoge et al., 2008a; Ruff et al., 2010; Warden, 2006]; and the risk of PTSD might be increased by cognitive dysfunction following injury. Therefore, the compromised integrity of white matter fiber connections of this study can be the combination of comorbid PTSD and TBI, chronic TBI spectrum, as these two separate and distinct diseases share common clinical symptoms. It should be noted as a limitation that self‐report measures were used for post‐concussive and post‐traumatic stress disorder symptoms. Self‐report measures have been shown to correlate weakly with objectively measured deficits in various domains in multiple studies [Drag et al., 2012; French et al., 2014; Spencer et al., 2010]. However, those individuals with evidence of symptom exaggeration on the NSI were excluded from these analyses.
Frequency of blast exposures, WMHs, and aging on white matter integrity
Our findings of significant blast exposure × age interaction on FA of the bilateral cingulum bundles (Fig. 3A) are consistent with the findings of a prior work [Trotter et al., 2015] using voxel‐wise analysis followed by ROI analyses to study chronic TBI veterans, including moderate and severe TBI patients besides mTBI patients of the recent conflicts. In addition, the cingulum bundle in high blast‐exposed individuals, but not in age‐matched low blast‐exposed individuals, had rapid trajectory (Fig. 3C,D) toward reduced WM tissue integrity (FA) with increasing days since most severe blast injury. The findings of high blast‐exposed service members exhibiting both a more rapid cross‐sectional age trajectory and time since major blast trajectories toward reduced white matter tissue integrity than those of low blast‐exposed individuals and controls suggest repeated blast exposures can initiate detrimental process on white matter integrity prior to normal aging [Trotter et al., 2015] and may lead to Wallerian degeneration [Pierpaoli et al., 2001].
The radiological appearances of T2 FLAIR WMHs in mTBI participants were small, round with relatively homogeneous in size (around 2–3 mm3 for each WMH), and mainly located in the deep WM region but not in the periventricular region (not shown). Thus, the number of WMHs would be proportional to the total volume of WMHs, though total WMHs size was not quantified in this study. The prevalence of WMHs of this cohort was around 30% for controls, which is relatively higher than the previous reports for neurologically non‐diseased adults under age 50 [Hopkins et al., 2006] or 15% at the aged of 60 [Ylikoski et al., 1995]; and 50% for mTBI participants, which is consistent with other TBI studies [Bigler et al., 2013; Marquez de la Plata et al., 2007]. Our findings of significant association between the number of total WMHs and MD, but not FA, of the ATR, and difference of MD of the FMAJ between mTBI subgroups of high and low WMHs suggest that underlying pathological changes of WMHs of mTBI is likely due to enlarged perivascular spaces with increased interstitial space, scarring or gliosis. However, no significant association between the number of WMHs and the number of blast exposures might suggest that the WMHs can be unrelated to blast injury directly.
Disrupted subcortical and cortical projections and neuropsychological implications in mTBI
The most common residual deficits in mTBI patients are cognitive speed, attention, memory abilities, and visuoconstruction [Millis et al., 2001], which can cause adverse long‐term neuropsychological outcomes [Vanderploeg et al., 2005]. Reduced memory and attention corresponded with disrupted WM integrity mainly over the regions of the ventral prefrontal WM such as UNC, CAB [Wu et al., 2010], ILF, and genu and splenium of the CC [Bigler, 2013; Niogi et al., 2008], indicating that cognitive deficits from mTBI may be region and task specific [Niogi et al., 2008]. Furthermore, disrupted dorsal prefrontal WM, such as CCG and SLF, impairs frontal top‐down control, verbal working memory [Charlton et al., 2010; Kennedy and Raz, 2009; Palacios et al., 2011] and overall executive function performance [Ashley, 2004].
However, by taking error variance of measurement into account [Bendlin et al., 2008], traditional neuropsychological testing is not sensitive and has limited ability to document ongoing brain function impairment in mTBI (if present) [Heitger et al., 2009]. Therefore, the relationship between neuroimaging biomarkers in mTBI and neuropsychological outcome is not conclusive (see [Bigler, 2013] for review).
Our findings of the association between microstructural variation of the cingulum bundles (CCG, CAB) and the subdomains of D‐KEFS support previous literature reports regarding the importance of anterior cingulum integrity in attentional control and cognitive speed [Kubicki et al., 2009; Nestor et al., 2004], which is essential for executive function, decision‐making and emotion control [Heilbronner and Haber, 2014]. The findings of the associations of the UNC FA with subdomains of WAIS‐IV and inhibition switching performance suggest that damage in the UNC may link to memory dysfunction [von der Heide et al., 2013] and executive dysfunction [Widjaja et al., 2013] such as response inhibition in mTBI. Our findings of the correlation between the SLF FA and the overall executive function and the inhibition response domains of D‐KEFS, as well as Grooved pegboard motor speed support the importance of the integrity of the SLF in the visual‐motor coordination [Skranes et al., 2007], visuospatial processing, speed and manual dexterity.
Limitation
The blast injuries seen in our population reflect “blast‐plus” in which components of the blast wave (primary blast) are combined with traditional mechanical and rotational components. Self‐report measures were used for post‐concussive and PTSD symptoms, and mTBI participants were on the average of 2 years from point of injury; thus, the neuroimaging findings may not be directly related to symptoms caused by blast injury itself. The microstructural alterations in white matter integrity revealed in the study may not be specific to blast mTBI only, but possibly caused by comorbidities such as PTSD. Furthermore, we did not administer neuropsychological testing to the controls and were not able to assess the difference of the correlation between NP testing and DTI measures among TBI and controls. In addition, we are aware of inherent limitation using diffusion tensor tractography to determine trajectories of WM pathways. However, priori anatomical information was applied in this study to constrain tractography, which can greatly reduce the occurrence of false‐positive trajectories of fiber pathways. Future study using advanced diffusion MRI techniques such as high angular resolution diffusion imaging is needed to verify these results. Nevertheless, the results of this study suggest that diffusion MRI tractography can be used as noninvasive biomarkers in assessing affected networks and for a better understanding of neuropathology of blast mTBI.
CONCLUSION
Our findings suggest that the association and projection fibers interconnecting fronto‐parieto‐temporal region, for example, CCG/CAB, SLF, and UNC; and fronto‐subcortical regions, for example, ATR, are particularly vulnerable to military‐related blast injury, where the compromised circuits have significant effects on the functional outcome of chronic mTBI patients. Furthermore, high frequency of blast exposures may deflect negatively normal aging trajectories of white matter integrity. However, longitudinal study with follow‐up scans is needed to validate these findings. Nevertheless, our results suggest the usefulness of diffusion MRI and tractography in assessing white matter changes in chronic blast‐related mTBI.
Disclaimer
The views expressed in this article are those of the author and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, or U.S. Government.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
We thank Rachel Wolfowitz and Jamie Harper for participant recruitment and project coordination.
REFERENCES
- APA (1994): Diagnostic and Statistical Manual of Mental Disorders Fourth Edition. Washington, DC: American Psychiatric Association. [Google Scholar]
- Ashley MJ (2004): Traumatic Brain Injury: Rehabilitative Treatment and Case Management, 2nd ed. Boca Raton: CRC Press. [Google Scholar]
- Basser PJ, Jones DK (2002): Diffusion‐tensor MRI: Theory, experimental design and data analysis ‐ a technical review. NMR Biomed 15:456–467. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D (1994): Estimation of the effective self‐diffusion tensor from the NMR spin echo. J Magn Reson B 103:247–254. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A (2000): In vivo fiber tractography using DT‐MRI data. Magn Reson Med 44:625–632. [DOI] [PubMed] [Google Scholar]
- Belanger HG, Kretzmer T, Vanderploeg RD, French LM (2010): Symptom complaints following combat‐related traumatic brain injury: Relationship to traumatic brain injury severity and posttraumatic stress disorder. J Int Neuropsychol Soc 16:194–199. [DOI] [PubMed] [Google Scholar]
- Bendlin BB, Ries ML, Lazar M, Alexander AL, Dempsey RJ, Rowley HA, Sherman JE, Johnson SC (2008): Longitudinal changes in patients with traumatic brain injury assessed with diffusion‐tensor and volumetric imaging. Neuroimage 42:503–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y (1995): Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Ser B 57:289–300. [Google Scholar]
- Besenski N (2002): Traumatic injuries: Imaging of head injuries. Eur Radiol 12:1237–1252. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee Y (2008): Neuroscience. Shell shock revisited: Solving the puzzle of blast trauma. Science (New York, N.Y.) 319:406–408. [DOI] [PubMed] [Google Scholar]
- Bigler ED (2013): Neuroimaging biomarkers in mild traumatic brain injury (mTBI). Neuropsychol Rev 23:169–209. [DOI] [PubMed] [Google Scholar]
- Bigler ED, Abildskov TJ, Petrie J, Farrer TJ, Dennis M, Simic N, Taylor HG, Rubin KH, Vannatta K, Gerhardt CA, Stancin T, Owen Yeates K (2013): Heterogeneity of brain lesions in pediatric traumatic brain injury. Neuropsychology 27:438–451. [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Jones‐Alexander J, Buckley TC, Forneris CA (1996): Psychometric properties of the PTSD Checklist (PCL). Behav Res Ther 34:669–673. [DOI] [PubMed] [Google Scholar]
- Brandstack N, Kurki T, Tenovuo O (2013): Quantitative diffusion‐tensor tractography of long association tracts in patients with traumatic brain injury without associated findings at routine MR imaging. Radiology 267:231–239. [DOI] [PubMed] [Google Scholar]
- Calabrese E, Du F, Garman RH, Johnson GA, Riccio C, Tong LC, Long JB (2014): Diffusion tensor imaging reveals white matter injury in a rat model of repetitive blast‐induced traumatic brain injury. J Neurotrauma 31:938–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernak I (2010): The importance of systemic response in the pathobiology of blast‐induced neurotrauma. Front Neurol 1:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernak I, Wang Z, Jiang J, Bian X, Savic J (2001): Ultrastructural and functional characteristics of blast injury‐induced neurotrauma. J Trauma 50:695–706. [DOI] [PubMed] [Google Scholar]
- Chafi MS, Karami G, Ziejewski M (2010): Biomechanical assessment of brain dynamic responses due to blast pressure waves. Ann Biomed Eng 38:490–504. [DOI] [PubMed] [Google Scholar]
- Chang LC, Jones DK, Pierpaoli C (2005): RESTORE: Robust estimation of tensors by outlier rejection. Magn Reson Med 53:1088–1095. [DOI] [PubMed] [Google Scholar]
- Charlton RA, Barrick TR, Lawes INC, Markus HS, Morris RG (2010): White matter pathways associated with working memory in normal aging. Cortex 46:474–489. [DOI] [PubMed] [Google Scholar]
- Chatelin S, Deck C, Renard F, Kremer S, Heinrich C, Armspach JP, Willinger R (2011): Computation of axonal elongation in head trauma finite element simulation. J Mech Behav Biomed Mater 4:1905–1919. [DOI] [PubMed] [Google Scholar]
- Chavko M, Koller WA, Prusaczyk WK, McCarron RM (2007): Measurement of blast wave by a miniature fiber optic pressure transducer in the rat brain. J Neurosci Methods 159:277–281. [DOI] [PubMed] [Google Scholar]
- Chen YC, Smith DH, Meaney DF (2009): In‐vitro approaches for studying blast‐induced traumatic brain injury. J Neurotrauma 26:861–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicerone KD, Kalmar K (1995): Persistent postconcussion syndrome: The structure of subjective complaints after mild traumatic brain injury. J Head Trauma Rehabil 10:1–7. [Google Scholar]
- Cifu D, Hurley R, Peterson M, Cornis‐Pop M, Rikli PA, Ruff RL, Scott SG, Sigford BJ, Silva KA, Tortorice K, Vanderploeg RD, Withlock W, Bowles A, Cooper D, Drake A, Engel C (2009): Clinical practice guideline: Management of concussion/mild traumatic brain injury. J Rehabil Res Dev 46:CP1. [PubMed] [Google Scholar]
- Connell S, Gao J, Chen J, Shi R (2011): Novel model to investigate blast injury in the central nervous system. J Neurotrauma 28:1229–1236. [DOI] [PubMed] [Google Scholar]
- Daniels JK, Lamke J‐P, Gaebler M, Walter H, Scheel M (2013): White matter integrity and its relationship to PTSD and childhood trauma: A systematic review and meta‐analysis. Depress Anxiety 30:207–216. [DOI] [PubMed] [Google Scholar]
- Davenport ND, Lim KO, Armstrong MT, Sponheim SR (2012): Diffuse and spatially variable white matter disruptions are associated with blast‐related mild traumatic brain injury. Neuroimage 59:2017–2024. [DOI] [PubMed] [Google Scholar]
- Drag LL, Spencer RJ, Walker SJ, Pangilinan PH, Bieliauskas LA (2012): The contributions of self‐reported injury characteristics and psychiatric symptoms to cognitive functioning in oef/oif veterans with mild traumatic brain injury. J Int Neuropsychol Soc 18:576–584. [DOI] [PubMed] [Google Scholar]
- French LM, Lange RT, Brickell TA (2014): Subjective cognitive complaints and neuropsychological test performance following military‐related traumatic brain injury. J Rehabil Res Dev 51:933–950. [DOI] [PubMed] [Google Scholar]
- Garman RH, Jenkins LW, Switzer RC, Bauman RA, Tong LC, Swauger PV, Parks SA, Ritzel DV, Dixon CE, Clark RSB, Bayir H, Kagan V, Jackson EK, Kochanek PM (2011): Blast exposure in rats with body shielding is characterized primarily by diffuse axonal injury. J Neurotrauma 28:947–959. [DOI] [PubMed] [Google Scholar]
- Glushakova OY, Johnson D, Hayes RL (2014): Delayed increases in microvascular pathology after experimental traumatic brain injury are associated with prolonged inflammation, blood‐brain barrier disruption, and progressive white matter damage. J Neurotrauma 31:1180–1193. [DOI] [PubMed] [Google Scholar]
- Gouw AA, Seewann A, van der Flier WM, Barkhof F, Rozemuller AM, Scheltens P, Geurts JJG (2011): Heterogeneity of small vessel disease: A systematic review of MRI and histopathology correlations. J Neurol Neurosurg Psychiatry 82:126–135. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Przekwas A (2013): Mathematical models of blast‐induced TBI: Current status, challenges, and prospects. Front Neurol 4:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Heide RJ, Skipper LM, Klobusicky E, Olson IR (2013): Dissecting the uncinate fasciculus: Disorders, controversies and a hypothesis. Brain 136:1692–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronner SR, Haber SN (2014): Frontal cortical and subcortical projections provide a basis for segmenting the cingulum bundle: Implications for neuroimaging and psychiatric disorders. J Neurosci 34:10041–10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitger MH, Jones RD, MacLeod AD, Snell DL, Frampton CM, Anderson TJ (2009): Impaired eye movements in post‐concussion syndrome indicate suboptimal brain function beyond the influence of depression, malingering or intellectual ability. Brain 132:2850–2870. [DOI] [PubMed] [Google Scholar]
- Hoge CW, Castro CA, Messer SC, McGurk D, Cotting DI, Koffman RL (2008a): Combat duty in Iraq and Afghanistan, mental health problems and barriers to care. US Army Med Dep J 7–17. [PubMed] [Google Scholar]
- Hoge CW, McHurk D, Thomas JL, Cox AL, Engel CC, Castro CA (2008b): Mild traumatic brain injury in u.s. soldiers returning from Iraw. N Engl J Med 358:453–463. [DOI] [PubMed] [Google Scholar]
- Hopkins RO, Beck CJ, Burnett DL, Weaver LK, Victoroff J, Bigler ED (2006): Prevalence of white matter hyperintensities in a young healthy population. J Neuroimaging 16:243–251. [DOI] [PubMed] [Google Scholar]
- Inglese M, Makani S, Johnson G, Cohen BA, Silver JA, Gonen O, Grossman RI (2005): Diffuse axonal injury in mild traumatic brain injury: A diffusion tensor imaging study. J Neurosurg 103:298–303. [DOI] [PubMed] [Google Scholar]
- Jbabdi S, Woolrich MW, Andersson JL, Behrens TE (2007): A Bayesian framework for global tractography. Neuroimage 37:116–129. [DOI] [PubMed] [Google Scholar]
- Jezzard P, Balaban RS (1995): Correction for geometric distortion in echo planar images from B0 field variations. Magn Reson Med 34:65–73. [DOI] [PubMed] [Google Scholar]
- Johnson VE, Stewart W, Smith DH (2013): Axonal pathology in traumatic brain injury. Exp Neurol 246:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara K, Hashimoto K, Abo M, Senoo A (2012): Voxel‐ and atlas‐based analysis of diffusion tensor imaging may reveal focal axonal injuries in mild traumatic brain injury ‐ comparison with diffuse axonal injury. Magn Reson Imaging 30:496–505. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Raz N (2009): Aging white matter and cognition: Differential effects of regional variations in diffusion properties on memory, executive functions, and speed. Neuropsychologia 47:916–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiraly M, Kiraly SJ (2007): Traumatic brain injury and delayed sequelae: A review–traumatic brain injury and mild traumatic brain injury (concussion) are precursors to later‐onset brain disorders, including early‐onset dementia. ScientificWorldJournal 7:1768–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM (2007): White matter integrity and cognition in chronic traumatic brain injury: A diffusion tensor imaging study. Brain 130:2508–2519. [DOI] [PubMed] [Google Scholar]
- Kubicki M, Niznikiewicz M, Connor E, Ungar L, Nestor PG, Bouix S, Dreusicke M, Kikinis R, McCarley RW, Shenton ME (2009): Relationship between white matter integrity, attention, and memory in schizophrenia: A diffusion tensor imaging study. Brain Imaging Behav 3:191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Husain M, Gupta RK, Hasan KM, Haris M, Agarwal AK, Pandey CM, Narayana PA (2009): Serial changes in the white matter diffusion tensor imaging metrics in moderate traumatic brain injury and correlation with neuro‐cognitive function. J Neurotrauma 26:481–495. [DOI] [PubMed] [Google Scholar]
- Kvickström P, Eriksson B, van Westen D, Lätt J, Elfgren C, Nilsson C (2011): Selective frontal neurodegeneration of the inferior fronto‐occipital fasciculus in progressive supranuclear palsy (PSP) demonstrated by diffusion tensor tractography. BMC Neurol 11:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois JA, Rutland‐Brown W, Wald MM (2006): The epidemiology and impact of traumatic brain injury: A brief overview. J Head Trauma Rehabil 21:375–378. [DOI] [PubMed] [Google Scholar]
- Levin HS, Wilde E, Troyanskaya M, Petersen NJ, Scheibel R, Newsome M, Radaideh M, Wu T, Yallampalli R, Chu Z, Li X (2010): Diffusion tensor imaging of mild to moderate blast‐related traumatic brain injury and its sequelae. J Neurotrauma 27:683–694. [DOI] [PubMed] [Google Scholar]
- Ling G, Bandak F, Armonda R, Grant G, Ecklund J (2009): Explosive blast neurotrauma. J Neurotrauma 26:815–825. [DOI] [PubMed] [Google Scholar]
- Lipton ML, Gulko E, Zimmerman ME, Friedman BW, Kim M, Gellella E, Gold T, Shifteh K, Ardekani BA, Branch CA (2009): Diffusion‐tensor imaging implicates prefrontal axonal injury in executive function impairment following very mild traumatic brain injury. Radiology 252:816–824. [DOI] [PubMed] [Google Scholar]
- Mac Donald CL, Johnson AM, Cooper D, Nelson EC, Werner NJ, Shimony JS, Snyder AZ, Raichle ME, Witherow JR, Fang R, Flaherty SF, Brody DL (2011): Detection of blast‐related traumatic brain injury in U.S. military personnel. N Engl J Med 364:2091–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez de la Plata C, Ardelean A, Koovakkattu D, Srinivasan P, Miller A, Phuong V, Harper C, Moore C, Whittemore A, Madden C, Diaz‐Arrastia R, Devous M Sr. (2007): Magnetic resonance imaging of diffuse axonal injury: Quantitative assessment of white matter lesion volume. J Neurotrauma 24:591–598. [DOI] [PubMed] [Google Scholar]
- Matsushita M, Hosoda K, Naitoh Y, Yamashita H, Kohmura E (2011): Utility of diffusion tensor imaging in the acute stage of mild to moderate traumatic brain injury for detecting white matter lesions and predicting long‐term cognitive function in adults. J Neurosurg 115:130–139. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Ling J, Mannell MV, Gasparovic C, Phillips JP, Doezema D, Reichard R, Yeo RA (2010): A prospective diffusion tensor imaging study in mild traumatic brain injury. Neurology 74:643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcdonald SD, Calhoun PS (2010): Clinical Psychology Review The diagnostic accuracy of the PTSD Checklist: A critical review. Clin Psychol Rev 30:976–987. [DOI] [PubMed] [Google Scholar]
- Millis SR, Rosenthal M, Novack TA, Sherer M, Nick TG, Kreutzer JS, High WM, Ricker JH (2001): Long‐term neuropsychological outcome after traumatic brain injury. J Head Trauma Rehabil 16:343–355. [DOI] [PubMed] [Google Scholar]
- Mori S, van Zijl PC (2002): Fiber tracking: Principles and strategies ‐ a technical review. NMR Biomed 15:468–480. [DOI] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, van Zijl PC (1999): Three‐dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol 45:265–269. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Kubicki M, Gurrera RJ, Niznikiewicz M, Frumin M, McCarley RW, Shenton ME (2004): Neuropsychological correlates of diffusion tensor imaging in schizophrenia. Neuropsychology 18:629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP (2002): Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum Brain Mapp 15:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niogi SN, Mukherjee P, Ghajar J, Johnson CE, Kolster R, Lee H, Suh M, Zimmerman RD, Manley GT, McCandliss BD (2008): Structural dissociation of attentional control and memory in adults with and without mild traumatic brain injury. Brain 131:3209–3221. [DOI] [PubMed] [Google Scholar]
- Palacios EM, Fernandez‐Espejo D, Junque C, Sanchez‐Carrion R, Roig T, Tormos JM, Bargallo N, Vendrell P (2011): Diffusion tensor imaging differences relate to memory deficits in diffuse traumatic brain injury. BMC Neurol 11:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parizel PM, Ozsarlak, Van Goethem JW, van den Hauwe L, Dillen C, Verlooy J, Cosyns P, De Schepper AM (1998): Imaging findings in diffuse axonal injury after closed head trauma. Eur Radiol 8:960–965. [DOI] [PubMed] [Google Scholar]
- Parizel PM, Van Goethem JW, Ozsarlak O, Maes M, Phillips CD (2005): New developments in the neuroradiological diagnosis of craniocerebral trauma. Eur Radiol 15:569–581. [DOI] [PubMed] [Google Scholar]
- Petrie EC, Cross DJ, Yarnykh VL, Richards T, Martin NM, Pagulayan K, Hoff D, Hart K, Mayer C, Tarabochia M, Raskind MA, Minoshima S, Peskind ER (2014): Neuroimaging, behavioral, and psychological sequelae of repetitive combined blast/impact mild traumatic brain injury in Iraq and Afghanistan war veterans. J Neurotrauma 31:425–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli C, Barnett A, Pajevic S, Chen R, Penix LR, Virta A, Basser P (2001): Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. Neuroimage 13:1174–1185. [DOI] [PubMed] [Google Scholar]
- Riedy G, Senseney JS, Liu W, Ollinger J, Sham E, Krapiva P, Patel JB, Smith A, Yeh P‐H, Graner J, Nathan D, Caban J, French LM, Harper J, Eskay V, Morissette J, Oakes TR (2016): Findings from structural mr imaging in military traumatic brain injury. Radiology 279:207–215. [DOI] [PubMed] [Google Scholar]
- Robinson ME, Lindemer ER, Fonda JR, Milberg WP, Mcglinchey RE, Salat DH (2015): Close‐range blast exposure is associated with altered functional connectivity in veterans independent of concussion symptoms at time of exposure. Hum Brain Mapp 36:911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff RL, Riechers RG, Ruff SS (2010): Relationships between mild traumatic brain injury sustained in combat and post‐traumatic stress disorder. F1000 Med Rep 2:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saljo A, Bao F, Shi J, Hamberger A, Hansson HA, Haglid KG (2002): Expression of c‐Fos and c‐Myc and deposition of beta‐APP in neurons in the adult rat brain as a result of exposure to short‐lasting impulse noise. J Neurotrauma 19:379–385. [DOI] [PubMed] [Google Scholar]
- Shaw NA (2002): The neurophysiology of concussion. Prog Neurobiol 67:281–344. [DOI] [PubMed] [Google Scholar]
- Skranes J, Vangberg TR, Kulseng S, Indredavik MS, Evensen KAI, Martinussen M, Dale AM, Haraldseth O, Brubakk AM (2007): Clinical findings and white matter abnormalities seen on diffusion tensor imaging in adolescents with very low birth weight. Brain 130:654–666. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE (2008): Threshold‐free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44:83–98. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen‐Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM (2004): Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23 Suppl 1:S208–S219. [DOI] [PubMed] [Google Scholar]
- Spencer RJ, Drag LL, Walker SJ, Bieliauskas LA (2010): Self‐reported cognitive symptoms following mild traumatic brain injury are poorly associated with neuropsychological performance in OIF/OEF veterans. J Rehabil Res Dev 47:521–530. [DOI] [PubMed] [Google Scholar]
- Taber KH, Warden DL, Hurley RA (2006): Blast‐related traumatic brain injury: What is known? J Neuropsychiatry Clin Neurosci 18:141–145. [DOI] [PubMed] [Google Scholar]
- Taber KH, Hurley RA, Haswell CC, Rowland JA, Hurt SD, Lamar CD, Morey RA (2014): White matter compromise in veterans exposed to primary blast forces. J Head Trauma Rehabil 30:E15–E25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teipel SJ, Meindl T, Wagner M, Kohl T, Bürger K, Reiser MF, Herpertz S, Möller H‐J, Hampel H (2009): White matter microstructure in relation to education in aging and Alzheimer's disease. J Alzheimers Dis 17:571–583. [DOI] [PubMed] [Google Scholar]
- Thomas C, Ye FQ, Irfanoglu MO, Modi P, Saleem KS, Leopold DA (2014): Anatomical accuracy of brain connections derived from diffusion MRI tractography is inherently limited. Proc Natl Acad Sci U S A 111:16574–16579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotter BB, Robinson ME, Milberg WP, McGlinchey RE, Salat DH (2015): Military blast exposure, ageing and white matter integrity. Brain 138:2278–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderploeg RD, Curtiss G, Belanger HG (2005): Long‐term neuropsychological outcomes following mild traumatic brain injury. J Int Neuropsychol Soc 11:228–236. [DOI] [PubMed] [Google Scholar]
- Vos SB, Jones DK, Viergever MA, Leemans A (2011): Partial volume effect as a hidden covariate in DTI analyses. Neuroimage 55:1566–1576. [DOI] [PubMed] [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, Hua K, Zhang J, Jiang H, Dubey P, Blitz A, van Zijl P, Mori S (2007): Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage 36:630–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden D (2006): Military TBI during the Iraq and Afghanistan wars. J Head Trauma Rehabil 21:398–402. [DOI] [PubMed] [Google Scholar]
- Widjaja E, Skocic J, Go C, Snead OC, Mabbott D, Smith ML (2013): Abnormal white matter correlates with neuropsychological impairment in children with localization‐related epilepsy. Epilepsia 54:1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TC, Wilde EA, Bigler ED, Yallampalli R, McCauley SR, Troyanskaya M, Chu Z, Li X, Hanten G, Hunter JV, Levin HS (2010): Evaluating the relationship between memory functioning and cingulum bundles in acute mild traumatic brain injury using diffusion tensor imaging. J Neurotrauma 27:303–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman JD, Dougherty RF, Myall NJ, Wandell BA, Feldman HM (2012): Tract profiles of white matter properties: Automating fiber‐tract quantification. PLoS One 7:e49790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh PH, Wang B, Oakes TR, French LM, Pan H, Graner J, Liu W, Riedy G (2014): Postconcussional disorder and PTSD symptoms of military‐related traumatic brain injury associated with compromised neurocircuitry. Hum Brain Mapp 35:2652–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yendiki A (2011): Automated probabilistic reconstruction of white‐matter pathways in health and disease using an atlas of the underlying anatomy. Front Neuroinform 5:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylikoski A, Erkinjuntti T, Raininko R, Sarna S, Sulkava R, Tilvis R (1995): White matter hyperintensities on MRI in the neurologically nondiseased elderly. Analysis of cohorts of consecutive subjects aged 55 to 85 years living at home. Stroke 26:1171–1177. [DOI] [PubMed] [Google Scholar]
- Yushkevich PA, Zhang H, Simon TJ, Gee JC (2008): Structure‐specific statistical mapping of white matter tracts. Neuroimage 41:448–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Yushkevich PA, Gee JC (2005): Deformable registration of diffusion tensor MR images with explicit orientation optimization. Med Image Comput Comput Assist Interv 8:172–179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


