Abstract
Executive function (EF) refers to a set of cognitive abilities involved in self‐regulated behavior. Given the critical role of EF in cognition, strategies for improving EF have attracted intensive attention in recent years. Previous studies have explored the effects of abacus‐based mental calculation (AMC) training on several cognitive abilities. However, it remains unclear whether AMC training affects EF and its neural correlates. In this study, participants were randomly assigned to AMC or control groups upon starting primary school. The AMC group received 2 h AMC training every week, while the control group did not have any abacus experience. Neural activity during an EF task was examined using functional MRI for both groups in their 4th and 6th grades. Our results showed that the AMC group performed better and faster than the control group in both grades. They also had lower activation in the frontoparietal reigons than the control group in the 6th grade. From the 4th to the 6th grade, the AMC group showed activation decreases in the frontoparietal regions, while the control group exhibited an opposite pattern. Furthermore, voxel‐wise regression analyses revealed that better performance was associated with lower task‐relevant brain activity in the AMC group but associated with greater task‐relevant brain activity in the control group. These results suggest that long‐term AMC training, with calculation ability as its original target, may improve EF and enhance neural efficiency of the frontoparietal regions during development. Hum Brain Mapp 38:5234–5249, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: executive control, child development, training plasticity, abacus‐based mental calculation, functional MRI
INTRODUCTION
Executive function (EF) refers to a variety of cognitive abilities necessary for self‐regulated behavior [Miyake et al., 2000]. Core EF components are working memory (the ability to maintain and manipulate task‐relevant information), inhibition (the ability to control one's behavior or thoughts to override a strong internal predisposition or external lure) and task switching (the ability to flexibly switch between different mental sets) [Miyake et al., 2000]. Performance of tasks that tap one or multiple components of EF has been reported to be associated with a wide range of cognitive activities, such as theory of mind, problem‐solving, and academic achievements [Bull et al., 2008; Hechtman, 2015; Hughes and Ensor, 2007]. EF performance measured during early childhood is even predictive of cognitive outcomes 32 years later [Moffitt et al., 2011]. Moreover, EF deficits have been observed in patients with common psychiatric or neurodevelopmental disorders, such as attention deficit hyperactivity [Biederman et al., 2007]. Given these critical impacts of EF, the numbers of studies regarding EF training [Diamond and Lee, 2011; Klingberg et al., 2005] have increased dramatically during the past 20 years.
A variety of training programs have been shown to improve EF performance [Diamond, 2014; Klingberg et al., 2005; Thorell et al., 2009]. The strongest evidence comes from computerized working memory training, which has been repeatedly found successful for typically developing children and those with EF deficits [Klingberg et al., 2005; Thorell et al., 2009]. The majority of these training programs are constrained by the fact that they are performed in a controlled laboratory setting with relatively short training length ranging from a few hours to several weeks [Diamond and Lee, 2011; Klingberg, 2010]. Such training may be sufficient to improve EF, but transfer gains appear to be narrow and are often restricted to tasks that have high cognitive processing overlaps with the trained tasks [Diamond, 2014; Diamond and Lee, 2011]. Interestingly, long‐term training of specific skills, such as music learning, appears to enhance EF globally [Bialystok and Depape, 2009; Rodrigues et al., 2013]. And the transfer gains can be enhanced when the training period is lengthened [Ho et al., 2003; Schlaug et al., 2005]. Therefore, long‐term training targeted on specific skills may show promise in enhancing EF.
Here, we want to explore whether EF can be improved by long‐term training of abacus‐based mental calculation (AMC), one specific skill of mental calculations. In Asian countries, some primary school students can receive AMC instructions either in school or in extracurricular programs. They first learn to use a physical abacus to perform calculations. After long‐term intensive training, they can perform rapid and precise mental calculations without the use of a physical abacus. A visuospatial strategy resorting to an imaginary abacus has been proposed to underlie this unusual arithmetic ability [Chen et al., 2006; Frank and Barner, 2012; Hanakawa et al., 2003]. Prior studies have shown that AMC users have advantages in a wide range of cognitive tasks, such as mathematics [Frank and Barner, 2012; Wang et al., 2015], numerical processing efficiency [Wang et al., 2013; Yao et al., 2015], and intelligence [Irwing et al., 2008]; all these tasks are related to EF at the cognitive level [Ardila et al., 2000; Bull et al., 2008; Houdé et al., 2010]. Moreover, studies from fMRI have consistently reported activation in frontal and parietal regions for individuals performing AMC [Chen et al., 2006; Hanakawa et al., 2003] and EF tasks [Kim et al., 2012; Owen et al., 2005]. Considering that transfer can occur if the training and transfer tasks engage overlapping cognitive processes and brain regions [Dahlin et al., 2008], it is interesting to explore whether EF and the underlying neural activity can be influenced by long‐term AMC training. However, previous studies examining impacts of AMC training on EF are few in number, and focus on only one component of EF, namely, working memory. For example, several studies reported that AMC training could improve working memory in children and adults [Dong et al., 2016; Lee et al., 2007; Li et al., 2013a; Tanaka et al., 2002]. Only one study provided evidence that three‐year AMC training enhanced the association between task switching and mathematics in children at about 9 years of age [Wang et al., 2015].
This study investigates the effects of AMC training on EF and its underlying brain activity using a dots task adapted from Davidson et al. [2006]. This task includes three conditions. In the congruent condition, participants are required to respond on the same side as the stimulus. In the incongruent condition, participants are required to respond on the opposite side to the stimulus. In the mixed condition, congruent and incongruent trials are randomly presented, where participants are instructed to respond on either the same or opposite side according to the type of the presented stimulus. According to the study of Davidson et al. [2006], task switching is required for the mixed condition to switch between responding on the same side and responding on the opposite side. Inhibition is needed for the incongruent and mixed conditions to suppress the prepotent tendency to respond on the same side as the stimulus. And working memory is needed for all three conditions to remember task rules. Demands on working memory should be greater for the mixed condition than the congruent and incongruent conditions because of remembering two task rules. The mixed condition also needs inhibition to suppress the prepotent tendency to continue responding with the previous task rule for trials that require a switch. Thus, demands on inhibition should be also greater for the mixed condition than the incongruent condition. Therefore, this task can capture multiple components of EF and manipulate different levels of EF demands. In keeping with prior research examining effects of AMC training on working memory [Dong et al., 2016; Li et al., 2013a], we hypothesized that AMC training would improve behavioral performance in the dots task. Moreover, we speculated that AMC training will alter the underlying brain activation. In the literature, brain activation has been reported to either increase [Olesen et al., 2004] or decrease [Garavan et al., 2000] after specific cognitive training. It has been suggested that the increase in activation is likely associated with the implementation of a new behavioral strategy [Bor and Owen, 2007a,2007b; Jonides, 2004], while the decrease in activation may reflect more efficient neural processing [Jonides, 2004; Kelly, 2004]. Therefore, there are at least two predictions for potential brain activation alterations following AMC training. First, brain activation related to EF may increase after AMC training, possibly due to the implementation of a visuospatial imagery strategy. This prediction is consistent with findings from several AMC studies [Hanakawa et al., 2003; Li et al., 2013a; Tanaka et al., 2002]. These studies have consistently reported greater frontoparietal activation in calculation and digit memory tasks for AMC experts relative to matched controls. The authors proposed that these activation increases were likely attributed to the implementation of a visuospatial imagery strategy. Second, brain activation related to EF may decrease after AMC training, possibly resulting from enhanced neural efficiency. Interestingly, our recent work observed that right frontoparietal activation during an n‐back task decreased after 20 days of AMC training in adults, and the decreases in activation were positively correlated with performance gains [Dong et al., 2016]. The findings provide evidence that AMC training may enhance neural efficiency related to EF, which is consistent with the latter prediction.
Moreover, although there is sufficient evidence that training can improve EF performance [Diamond, 2014; Diamond and Lee, 2011], and alter underlying brain activity [Dahlin et al., 2008; Jolles et al., 2010], few studies have explored effects of training against the background of EF development. Previous studies have shown that task performance in a wide range of EF measures improves rapidly in preschool and early school children [Anderson, 2002; Van der Ven et al., 2012], and continues to improve in adolescents and young adults [Anderson et al., 2001; Davidson et al., 2006], indicating that EF has a protracted developmental trajectory. At the neural level, frontal and parietal regions that underlie EF also experience prolonged developmental changes that may extend into adulthood. Structural changes include decreases in grey matter volumes [Sowell et al., 1999], increases in white matter volumes [Giedd, 2004], and decreases in regional cortical thickness [Kharitonova et al., 2013]. There are also functional changes such as increases [Rubia et al., 2006] and decreases [Ordaz et al., 2013; Tamm et al., 2002] in task‐state brain activation, and increases in long‐range functional connectivity and decreases in short‐range functional connectivity [Fair et al., 2007, 2012]. Although the precise contribution of each of these neural changes to EF remains unclear, a commonly accepted explanation is that the protracted development of EF is closely tied to the relatively prolonged maturation of frontal and parietal regions [Anderson, 2001; Best and Miller, 2010]. Particularly, the age of 10–12 years old, a transitional period from middle childhood to preadolescence, is a critical stage when several aspects of EF begin to show signs of maturity [Anderson, 2002; Best and Miller, 2010; Klimkeit et al., 2004] but structural and functional properties of frontal and parietal regions are still continuing to develop [Fair et al., 2007; Giedd, 2004]. A developmental perspective about how AMC training affects EF and its underlying brain activity at this transitional period may provide further insight into mechanisms of academic skill training underlying EF.
Therefore, this study used a longitudinal design to investigate impacts of AMC training on EF and underlying brain activity from a developmental perspective. Seventy‐two children were randomly assigned to AMC and control groups at the start of primary school. A go/no‐go task, an intelligence test and two parent‐report questionnaires were administered to ensure that both groups were comparable in terms of inhibition, intelligence, and behavioral traits (six aspects of mastery motivation and three aspects of behavioral problems) before the training began. Inhibition is a core component of EF and has moderate relationships with other EF components [Miyake et al., 2000]. Children's intelligence and behavioral traits are also critical for EF and the underlying brain development [Ardila et al., 2000; Clark et al., 2002; Gray et al., 2005; Jennings, 2002; Low and Webster, 2016]. It is crucial to control for these factors so as to help account for their influence when studying effects of AMC training. The AMC group was trained intensively from the 1st to the 6th grade while the control group did not have any experiences with abacus operation. Neural activation during the dots task was assessed using fMRI for both groups in the 4th (children aged about 10 years old) and 6th grades (children aged about 12 years old). In this study, we predicted that (1) children with AMC training would perform better than their peers in the dots task; (2) children with AMC training would exhibit greater brain activation related to EF if the training helped them generate a new behavioral strategy to perform the task better; or they would exhibit lower brain activation related to EF if the training enhanced neural processing efficiency related to EF.
METHODS
Ethics Statement
The study was approved by Zhejiang University in China. All procedures followed were in compliance with the guidelines of the Helsinki Declaration. All subjects and their parents (or other guardians) provided written informed consents.
Participants
A total of 72 children were recruited from a single district within Qiqihar city. They were from urban families, had normal or corrected‐to‐normal visual acuity, and had no reports of hearing loss, psychiatric disorder, or neurological disease, or special educational assistance requirements. Twenty‐one children were excluded from the final statistical analyses. Reasons for attrition are as follows: one transferred to another school, two were absent from school for almost half a year, three did not complete all the measures in the 1st grade, ten were absent from the fMRI scans, and five had excessive head motion during fMRI (exceeding 2.0 mm in displacement or 2° in rotation). Consequently, 51 children constituted the final sample. They had intelligence in the average range (Combined Raven scores 82–126). And none of them failed (a score of <60 points on a 100‐point scoring system) at any of the final academic tests from the 1st to the 6th grade, indicating no children with academic underachievement in this study.
Training and Assessment Procedures
At the start of primary school, the children were randomly assigned into two groups. Both groups were taught based on the same primary school curriculum. Additionally, the AMC group received 2 h per week of AMC training from the 1st to the 6th grade. They were instructed together in a classroom by an experienced AMC teacher. They first learned the principles of abacus operation and then performed calculations by manipulating the beads on a physical abacus. Finally, they performed rapid and precise calculations by visualizing an imaginary abacus. The control group, during the same period, received extra learning on such materials as reading, traditional calculation, simple geometry, or some activities such as sports.
Three testing sessions were administered in the longitudinal study (Table 1). In the first testing session, Combined Raven Test for Rural China [Li et al., 1988], go/no‐go task, Dimensions of Mastery Questionnaires [Morgan et al., 2009], and Early School Behavior Rating Scale [Pianta and Caldwell, 1991] were conducted at the first month of the 1st grade. In the second and third testing sessions, fMRI data were acquired while participants completed an EF task. These two testing sessions were conducted at the fourth month of the 4th grade and the third month of the 6th grade, respectively. Each of the three testing sessions was completed within 2 weeks. Because the training was suspended during weeks with testing sessions, academic examinations, vacations, and other inevitable circumstances, the actual length of AMC training for the second and third testing sessions were 26 and 40 months, respectively. To ensure the quality of training, AMC children were required to participate in the qualification examination held by the Chinese Abacus and Mental Arithmetic Association. There are 10 levels of assessments. Level 10 is the easiest, while Level 1 is the most difficult. Each level contains 10 serial addition/subtraction and 10 multiplication/division tasks. Participants were considered to pass each level if they were able to use the AMC method to correctly solve eight of the addition/subtraction problems and eight of the multiplication/division problems in 5 min. The examinations indicated that all children in the AMC group passed at least level 7 at the second testing session and at least level 2 at the third testing session.
Table 1.
Testing procedure in the longitudinal study
| The first testing session | The second testing session | The third testing session | |
|---|---|---|---|
| Time | The first month of the 1st grade | The fourth month of the 4th grade | The third month of the 6th grade |
| Measures | Raven test | The dots task | The dots task |
| Go/no‐go task | Functional MRI | Functional MRI | |
| Dimensions of Mastery Questionnaires | |||
| Early School Behavior Rating Scale | |||
| Length of AMC training | 0 | 26 months | 40 months |
Measures in the First Testing Session
Combined Raven Test
The Combined Raven Test for Rural China [Li et al., 1988] was used to assess children's general intelligence. It includes the Raven's Colored Progressive Matrices (A, AB, B subsets) and the final three parts of the Standard Progressive Matrices (C, D, and E subsets). Children were given 40 min to complete the test. A T score was computed according to the Chinese adapted city norm [Wang and Ming, 2007].
Go/no‐go task
An animal version was developed. Children were instructed to press the spacebar for all the animal pictures (nontarget stimuli) except for the orangutans (target stimuli). All the five pictures of orangutans were presented individually for each child before the task, making sure that they had no difficulty in identifying the targets. Each stimulus appeared on the screen for 500 ms, followed by a stimulus interval of 1100–1200 ms. Children completed one practice block (12 trials) and two experimental blocks (70 trials per block). A ratio of 20% target stimuli was maintained.
Dimensions of Mastery Questionnaires
The Dimensions of Mastery Questionnaires [Morgan et al., 2009] was used to assess children's mastery motivation. It was translated into Chinese by Wang et al. [2009], and the Cronbach's alpha for parents' rating was 0.82 for Chinese children [Morgan et al., 2012]. There are 8 items (e.g., likes physical activities and tries to do them well) for gross motor persistence, 6 items (e.g., enjoys talking with adults and tries to keep them interested) for social persistence with adults, 6 items (e.g., tries hard to make friend with other kids) for social persistence with children, 6 items (e.g., shows excitement when things are done successfully) for mastery pleasure, 5 items (e.g., very sad after failing to complete things he or she tried hard to do) for negative reactions to failure, and 5 items (e.g., solves problems quickly) for general competence. Parents rated how typical each behavior was for their children using a 5‐point rating scale, with 1 indicating not at all typical and 5 as very typical. Each dimension of mastery motivation was calculated as the average of the corresponding items.
Early School Behavior Rating Scale
The Early School Behavior Rating Scale [Pianta and Caldwell, 1991] was used to assess children's behavioral problems. The scale has been translated into Chinese with a Cronbach's alpha of 0.87 [Zhang, 2011]. There are 9 items (e.g., has poor attention span or lacks concentration) for conduct problems, 18 items (e.g., is afraid of new situations or strangers) for internalizing/anxiety problems, and 16 items (e.g., helps around the house) for competence problems. Parents rated how typical each behavior was for their children using a 4‐point rating scale, with 1 indicating hardly ever and 4 as almost always. Each aspect of behavioral problems was computed as the average of the corresponding items.
EF Task and fMRI in the Second and Third Testing Sessions
The dot task was adapted from the study of Davidson et al. [2006]. In each trial, a stripped or gray dot appeared on the right or left of a cross (Fig.1A). Children were instructed to press the side‐congruent button for one type of dots and the side‐incongruent button for the other type of dots. The task included three conditions. In the congruent condition, only one type of dots was presented and children should press side‐congruent buttons. In the incongruent condition, only the other type of dots was presented and children should press side‐incongruent buttons. In the mixed condition, two types of dots were presented randomly, and children were asked to press the side‐congruent or side‐incongruent buttons according to which types of dots were presented. For half children in both groups, stripped dots indicated side‐congruent while gray dots indicated side‐incongruent; for the other half in both groups, the rules were reversed.
Figure 1.
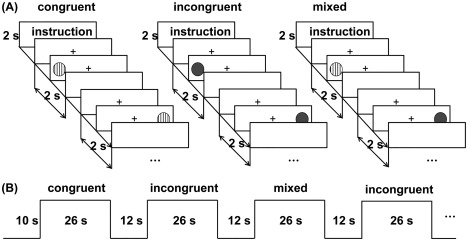
Experimental design of the EF task. (A) Three task conditions. For each task condition, a 2‐s task rule was presented, followed by twelve 2‐s trials. (B) Block design for the fMRI. In each block, a 26‐second task condition was presented, followed by a 12‐s fixation slide.
A block design was used, consisting of two sessions with nine blocks in each (three congruent, three incongruent, and three mixed). Every block had a 26 s task condition, followed by a 12 s fixation slide (Fig.1B). An instruction was presented for 2 s for each task condition, followed by twelve 2 s trials. In each trial, a cross was presented for 500 ms; subsequently, a stimulus was presented for 750 ms or terminated if participants gave a response, and finally, a blank interval followed for a variable duration so that each trial lasted for 2 s. The order of task conditions was randomly determined but fixed within participants. The 12 s fixation slide was used as a baseline condition, in which only a cross was presented on the screen. Participants were instructed to relax and look at the cross. There were also 10 s prescans performed before each session to obtain a stable signal. Thus, the scanning duration for each session was 352 s. Before the first session, participants were given three blocks (one block for each condition) of practice trials to make sure that they understood the task. During the interval between the two sessions, participants were allowed to rest for ∼1 min to diminish the effects of fatigue.
fMRI Acquisition and Preprocessing
Stimuli were presented with E‐Prime software (http://www.pstnet.com/eprime.cfm). Participants viewed the screen through a mirror attached to the head coil and made response by pressing buttons with their right or left index finger. All images were acquired on a 1.5 T Philips MRI scanner with a standard eight‐channel head coil in the First Hospital of Qiqihar. For each child, task‐state fMRI data were obtained using a T2*‐weighted single‐shot echo‐planner imaging sequence (TR = 2,000 ms, TE = 50 ms, flip angle = 90°, matrix size = 64 × 64, FOV = 230 × 230 mm, slice thickness/gap = 5 mm/0.8 mm, and slice numbers = 22 with interleaved acquisition). These images were set obliquely and parallel to the anterior and posterior commissure line. Structural MRI data were collected using a 3D fast‐field echo sequence (TR = 2,500 ms, TE = 4.6 ms, flip angle = 15°, matrix size = 256 × 256, FOV = 256 × 256 mm, voxel size = 1 × 1 × 1 mm3, and slice numbers = 150 in the sagittal plane).
The five prescans were discarded due to instability of initial MR signals. Then image preprocessing was performed using SPM8 with standard procedure (http://www.fil.ion.ucl.ac.uk/spm). It included reorientation, slice‐time correction, and motion correction. And structural images were coregistered to the mean functional images and were subsequently segmented. With the registration parameters obtained, functional images were transformed into MNI space with a resampling voxel size (3 × 3 × 3 mm3) and spatially smoothed with a 6 mm FWHM Gaussian kernel. Before entering the data into the general linear model, a high‐pass filter with a cutoff frequency of 1/128 Hz was applied. For each task condition, a box car model convolved with the hemodynamic response function was modeled. Subject‐specific movement parameters obtained from the realignment procedure were included as covariates. Six contrasts of interest were obtained by producing t statistical parameter maps for each participant: (1) congruent versus baseline; (2) incongruent versus baseline; (3) mixed versus baseline. Then their contrast values were submitted into second‐level analysis.
Behavioral and fMRI Analyses
Performance in the go/no‐go task was indexed by error rates for nontargets, accuracy for targets, and mean reaction time (RT) for targets. Group differences on age, gender, and each measure in the first testing session were assessed using independent samples t test and Chi‐square test. Performance in the dot task was indexed by accuracy and mean RT of correct trials in each condition. Trials with RT shorter than 200 ms were excluded as they were considered as anticipatory response [Davidson et al., 2006]. A 3 × 2 × 2 repeated measures analysis of variance (ANOVA), with Group (AMC or control) as a between‐subject factor, Condition (congruent, incongruent or mixed) and Grade (4th or 6th grade) as within‐subject factors, was computed separately for RT and accuracy in the dot task. Post‐hoc tests with Bonferroni correction were conducted for effects of interest.
For imaging data, in the first step, a task mask was created to restrict our search space to brain regions with increased activity in the dots task. Activation maps of each task condition relative to the baseline condition were generated separately for each grade using one‐sample t tests. Correction for multiple comparisons was accomplished by AlphaSim method (http://afni.nih.gov/afni/docpdf/AlphaSim.pdf) in the resting‐state fMRI Data Analysis Toolkit (http://www.restfmri.net). The parameters were set as the follows: voxel‐wise threshold of P < 0.05, 1000 simulations, two‐sided, 6 mm FWHM, with the gray matter mask (SPM apriori/gray.nii thresholded at 0.25). A corrected significance level of P < 0.05 could be achieved with a minimal cluster size of 164 voxels. An “or” combination of all activation maps were created as a task mask.
In the second step, a 2 (Group: AMC or control) × 3 (Condition: congruent, incongruent or mixed) × 2 (Grade: 4th or 6th grade) factorial ANOVA was conducted. Search space was restricted to voxels in the task mask. AlphaSim corrected significance level of P < 0.05 was obtained by a voxel‐wise threshold of P < 0.05 and a minimal cluster size of 59 voxels. To further visualize effects of interest, beta values were extracted from significant clusters. Post‐hoc tests were conducted with Bonferroni correction.
Finally, brain–behavior correlations were examined separately for each grade and each group using voxel‐wise regression analyses. Given the large number of possible analyses, we chose to only analyze the mixed vs. baseline contrast which captured all three components of EF. Each participant's mixed versus baseline contrast values were entered into the regression models, with accuracy/RT in the mixed condition, age and Raven scores as covariates. It allowed us to investigate the relationships between neural response and EF performance while controlling for age and intelligence. AlphaSim correction was also applied for voxels within the gray matter mask. Cytoarchitectonic probabilistic maps via the SPM anatomy toolbox [Eickhoff et al., 2005] were used to label brain activity in the intraparietal sulcus (IPS) [Choi et al., 2006].
RESULTS
Behavioral Results
Sample characteristics and behavioral measures in the baseline testing session are presented in Table 2. There were no significant group differences in these variables.
Table 2.
Sample characteristics and baseline measures of both groups
| AMC | Control | Group differences | ||
|---|---|---|---|---|
| Gender | (Boy, total) | (12, 26) | (14, 25) | X 2 = 0.49, P = 0.48 |
| Age | ||||
| The first testing session | 6.85 (0.53) | 6.99 (0.41) | t (49) = 1.05, P = 0.30 | |
| The second testing session | 10.07 (0.51) | 10.23 (0.41) | ||
| The third testing session | 11.99 (0.51) | 12.17 (0.42) | ||
| Intelligence | 103.88 (11.80) | 102.44 (10.58) | t (49) = 0.46, P = 0.65 | |
| Go/no‐go task | ||||
| Percentage of false alarms | 0.30 (0.16) | 0.34 (0.13) | t (49) = 1.01, P = 0.32 | |
| Percentage of hits | 0.94 (0.04) | 0.94 (0.03) | t (49) = 0.01, P = 0.99 | |
| RT of hits | 573 (77) | 549 (72) | t (49) = 1.14, P = 0.26 | |
| Dimensions of Mastery Questionnaires | ||||
| Gross motor persistence | 3.56 (0.59) | 3.72 (0.52) | t (49) = 1.06, P = 0.30 | |
| Social persistence with adults | 3.91 (0.65) | 4.01 (0.68) | t (49) = 0.52, P = 0.60 | |
| Social persistence with children | 4.08 (0.65) | 4.27 (0.63) | t (49) = 1.02 P = 0.31 | |
| Mastery pleasure | 3.92 (0.51) | 4.04 (0.59) | t (49) = 0.76, P = 0.45 | |
| Negative reactions to failure | 3.34 (0.69) | 3.56 (0.54) | t (49) = 1.27, P = 0.21 | |
| General competence | 3.44 (0.70) | 3.46 (0.59) | t (49) = 0.10, P = 0.92 | |
| Early School Behavior Rating Scale | ||||
| Conduct problems | 1.55 (0.18) | 1.62 (0.24) | t (49) = 1.12, P = 0.27 | |
| Internalizing/anxiety problems | 1.81 (0.23) | 1.74 (0.27) | t (49) = 1.04, P = 0.30 | |
| Competence problems | 2.74 (0.35) | 2.76 (0.42) | t (49) = 0.18, P = 0.86 |
Mean values and standard deviation are presented for these variables.
To examine associations between AMC training and EF performance, repeated measures ANOVA with Group (AMC or control), Condition (congruent, incongruent, or mixed), and Grade (4th or 6th) as factors were conducted separately for RT and accuracy in the dots task. For accuracy, we observed significant main effects of Group (F(1, 49) = 10.62, P < 0.01, partial η 2 = 0.18), Condition (F(2, 98) = 99.33, P < 0.001, partial η 2 = 0.67) and Grade (F(1, 49) = 58.11, P < 0.001, partial η 2 = 0.54). The effect of Group was driven by a higher accuracy in the AMC group than the control group, the effect of Condition was due to decreased accuracy with increasing EF demands, and the effect of Grade was accounted for by increased accuracy from the 4th to the 6th grade. We also observed a significant interaction between Group and Condition (F(2, 98) = 5.81, P < 0.01, partial η 2 = 0.11). Post‐hoc comparisons between AMC and control groups showed a higher accuracy in the AMC group than the control group in the incongruent (P < 0.01) and mixed (P < 0.05) conditions in the 4th grade and in the mixed condition in the 6th grade (P < 0.01), but no group differences in other conditions. There was also a significant interaction between Condition and Grade (F2, 98) = 37.24, P < 0.001, partial η 2 = 0.43). Post‐hoc comparisons between the 4th and the 6th grade showed that accuracy differences between the three task conditions decreased significantly from the 4th to the 6th grade (P < 0.01). For RT, similar repeated measures ANOVA revealed significant main effects of Group (F(1, 49) = 7.73, P < 0.01, partial η 2 = 0.14), Condition (F(2, 98) = 426.78, P < 0.001, partial η 2 = 0.90), and Grade (F(1, 49) = 102.62, P < 0.001, partial η 2 = 0.68). The effect of Group was driven by shorter RTs in the AMC group than the control group, the effect of Condition was due to increased RTs with increasing EF demands, and the effect of Grade was accounted for by decreased RTs from the 4th to the 6th grade. There was also a significant interaction between Condition and Grade (F(2, 98) = 5.09, P = 0.01, partial η 2 = 0.09). Post‐hoc comparisons between the 4th and the 6th grade showed that RT differences between the incongruent and mixed conditions decreased significantly from the 4th to the 6th grade (P < 0.01). Although there were no significant interactions between Group and other factors (P > 0.21), we also conducted post‐hoc comparisons between AMC and control groups in each task condition and each grade. The results showed that the AMC group was faster than the control group in all the comparisons (P < 0.05) except the congruent condition in the 6th grade, in which the AMC group only had a tendency of shorter RTs (P = 0.11). Therefore, AMC children performed better and faster than control children in the dots task, especially in the incongruent and mixed condition, which tapped multiple EF components. Performance in this task is presented in Fig. 2.
Figure 2.
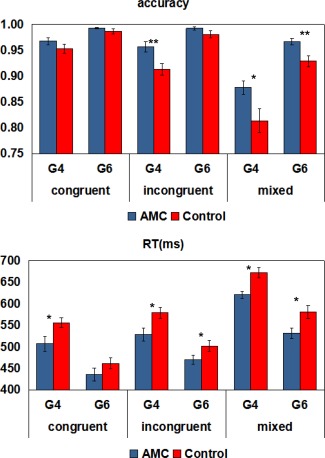
Mean accuracy and RT in the EF task. G4, 4th grade; G6, 6th grade. Error bars indicate one standard error of the mean. * p Bonferroni corrected < 0.05; ** p Bonferroni corrected < 0.01. [Color figure can be viewed at http://wileyonlinelibrary.com]
Effects of AMC Training on Task‐Related Brain Activity
The activation maps for three task conditions against the baseline condition showed robust activation in the superior/middle/inferior frontal gyrus (SFG/MFG/IFG), precentral gyrus (PG), supplementary motor area (SMA), superior/inferior parietal lobules/intraparietal sulcus (SPL/IPL/IPS), insula, caudate, putamen, thalamus, and cerebellum (Fig. 3). These regions have been consistently reported to be activated in individuals performing EF tasks [Kim et al., 2012; Owen et al., 2005; Rubia et al., 2006].
Figure 3.
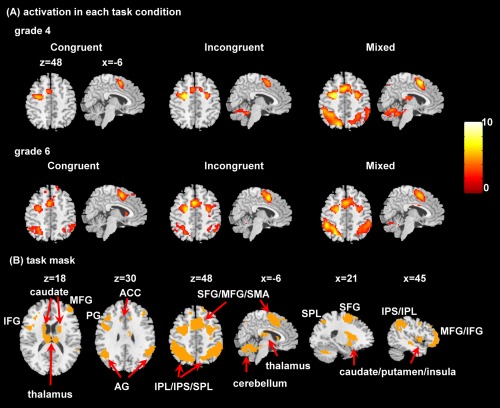
Activated areas during the EF task. (A) Activation maps for each task condition and each grade. All images are thresholded at p < 0.05 using Alphasim correction. (B) The task mask (orange) defined by the combination of all the above activation maps. The left side of the axial slices corresponds to the left side of the brain. SFG, superior frontal gyrus; MFG, middle frontal gyrus; IFG, inferior frontal gyrus; SMA, supplementary motor area; PG, precentral gyrus; AG, angular gyrus; ACC, anterior cingulate cortex; SPL, superior parietal lobule; IPL, inferior parietal lobule; IPS, intraparietal sulcus. [Color figure can be viewed at http://wileyonlinelibrary.com]
A 2 (Group: AMC or control) × 3 (Condition: congruent, incongruent or mixed) × 2 (Grade: 4th or 6th grade) factorial ANOVA detected a significant interaction between Group and Grade in the right IPS/IPL and the right SFG/SMA (Table 3 and Fig. 4A). We further extracted beta values from the two clusters and conducted post‐hoc comparisons. In the right IPS/IPL, the AMC group showed lower activation than the control group in the incongruent (P < 0.01) and mixed (P < 0.01) conditions in the 6th grade. The control group showed significant activation increases from the 4th to the 6th grade in all the three conditions (congruent: P < 0.05; incongruent: P < 0.01; mixed: P < 0.05), which were not detected for the AMC group. In the right SFG/SMA, the AMC group also showed lower activation than the control group in the incongruent (P < 0.05) and mixed (P < 0.01) conditions in the 6th grade. The control group showed significant activation increases from the 4th to the 6th grade in the incongruent (P < 0.05) and mixed (P < 0.05) conditions, whereas the AMC group showed a marginally activation decrease from the 4th to the 6th grade in the mixed condition (P = 0.06). The 2 (Group) × 3 (Condition) × 2 (Grade) factorial ANOVA also detected a significant interaction between Group, Condition, and Grade in the left PG/MFG (Table 3 and Fig. 4B). Post‐hoc comparisons showed that the AMC group had lower activation than the control group in the incongruent (P < 0.05) and mixed (P < 0.001) conditions in the 6th grade. Moreover, in the mixed condition, the AMC group showed a significant activation decrease from the 4th to the 6th grade (P < 0.001), whereas the control group showed a marginally activation increase from the 4th to the 6th grade (P = 0.07).
Table 3.
Significant clusters for effects of interest in the factorial ANOVA
| Significant effects of interest | Cluster size | F value | MNI coordinates | Anatomical region | BA |
|---|---|---|---|---|---|
| Group ×Grade | 136 | 10.28 | (48, −45, 39) | Right IPS/IPL | 40, 7 |
| 65 | 8.47 | (18, 0, 60) | Right SFG/SMA | 6 | |
| Group ×Condition × Grade | 117 | 7.22 | (−27, 0, 45) | Left PG/MFG | 6 |
Thresholds were set at P < 0.05, AlphaSim corrected.
BA, Brodmann area; MNI, Montreal Neurological Institute; IPS, intraparietal sulcus; IPL, inferior parietal lobule; SFG, superior frontal gyrus; SMA, supplementary motor area; PG, precentral gyrus; MFG, middle frontal gyrus.
Figure 4.
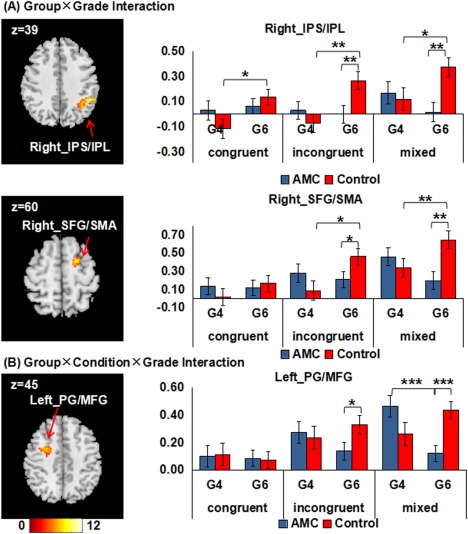
Clusters surviving in the factorial ANOVA. (A) Two clusters that are significant for the Group × Grade interaction effect. (B) One cluster that is significant for the Group × Condition × Grade interaction effect. All images are thresholded at p < 0.05 using Alphasim correction. The left side of the axial slices corresponds to the left side of the brain. The histograms show mean beta value extracted from each cluster. Error bars indicate one standard error of the mean. IPS, intraparietal sulcus; IPL, inferior parietal lobule; SFG, superior frontal gyrus; SMA, supplementary motor area; PG, precentral gyrus; MFG, middle frontal gyrus; G4, 4th grade; G6, 6th grade; * p Bonferroni corrected < 0.05; ** p Bonferroni corrected < 0.01; *** p Bonferroni corrected < 0.001. [Color figure can be viewed at http://wileyonlinelibrary.com]
Brain–Behavior Relationships
Using voxel‐wise regression analyses, we found different patterns of brain–behavior relationships within the two groups (Table 4 and Figs. 5 and 6). In the AMC group, higher accuracy was associated with lower activity in the right insula and right IPL/IPS in the 4th grade and the right SFG and right IPL/IPS in the 6th grade. No relationships were found between brain activity and RT in any grade. In the control group, shorter RT was associated with greater activity in the SMA in the 4th grade, and the left IPL/IPS in the 6th grade. No relationships were found between brain activity and accuracy in any grade.
Table 4.
Significant clusters surviving in the brain–behavior correlations
| Group | Test point | Correlation | Cluster size | T value | MNI coordinates | Anatomical region | BA |
|---|---|---|---|---|---|---|---|
| AMC | 4th grade | Brain–accuracy | 176 | −5.11 | (45, 9, −6) | Right insula | 13, 22 |
| 377 | −4.75 | (54, −48, 42) | Right IPL/IPS | 40, 7 | |||
| Brain–RT | No | ||||||
| 6th grade | Brain–accuracy | 175 | −3.53 | (15, 6, 63) | Right SFG | 6 | |
| 372 | −4.18 | (45, −48, 48) | Right IPL/IPS | 40, 7 | |||
| Brain–RT | No | ||||||
| Control | 4th grade | Brain–accuracy | No | ||||
| Brain–RT | 242 | 5.77 | (9, 18, 42) | SMA | 6, 32, 8 | ||
| 6th grade | Brain–accuracy | No | |||||
| Brain–RT | 231 | 4.32 | (−51, −51, 30) | Left IPL/IPS | 40, 39 |
For the brain–RT correlations, positive T values represent that greater brain activity is associated with shorter RT. Thresholds were set at P < 0.05, AlphaSim corrected.
BA, Brodmann area; MNI, Montreal Neurological Institute; IPS, intraparietal sulcus; IPL, inferior parietal lobule; SFG, superior frontal gyrus; SMA, supplementary motor area.
Figure 5.
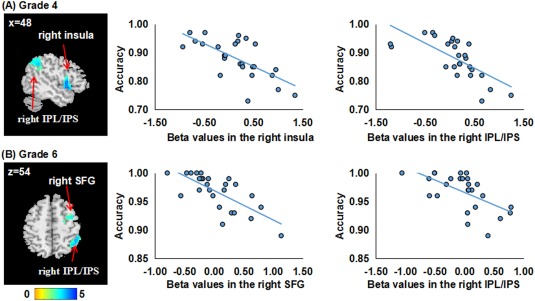
Brain‐behavior correlations in the AMC group. In each row, the left panel displays significant clusters in the AMC group, where higher accuracy is associated with lower brain activity. All images are thresholded at p < 0.05 using Alphasim correction. The left sides of the axial slices correspond to the left side of the brain. The right panel displays scatter distributions between mean beta values extracted from each cluster and accuracy in the mixed condition. IPS, intraparietal sulcus; IPL, inferior parietal lobule; SFG, superior frontal gyrus. [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 6.
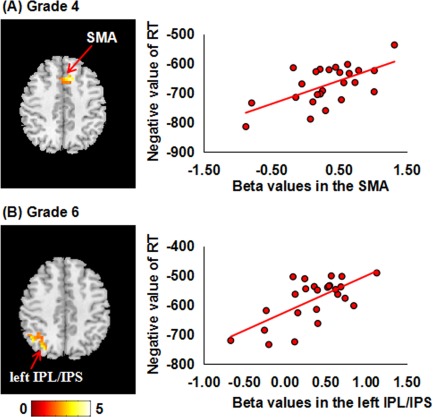
Brain‐behavior correlations in the control group. In each row, the left panel displays significant clusters in the control group, where shorter RT is associated with greater brain activity. All images are thresholded at p < 0.05 using Alphasim correction. The left sides of the axial slices corresponded to the left side of the brain. The right panel displays scatter distributions between mean beta values extracted from each cluster and RT in the mixed condition. SMA, supplementary motor area; IPS, intraparietal sulcus; IPL, inferior parietal lobule. [Color figure can be viewed at http://wileyonlinelibrary.com]
DISCUSSION
This study is the first to examine impacts of AMC training on EF and its neural correlates from a developmental perspective.
As expected, children with AMC training were faster and more accurate than their peers, mainly in the incongruent and mixed conditions. These results add to prior research [Dong et al., 2016; Lee et al., 2007; Li et al., 2013a] demonstrating transferable improvements in EF via a new intervention, namely training children to solve math with AMC method. Our results suggest that effects of AMC training on EF may be not solely limited to enhanced working memory, for the incongruent and mixed conditions were measures that went beyond working memory to also tap inhibition and task switching components of EF. AMC involves multiple cognitive processes, such as translating digital stimuli to mental images, maintaining multiple components of the imaginary abacus in working memory, and inhibiting interference from the environment. These processes are likely to consume executive resources related to task switching, working memory, and inhibition. One possible explanation for our finding is that these cognitive processes taxing subcomponents of EF are trained while performing AMC. Transfer effects may thus occur.
In addition, Diamond [2014] has proposed that effective EF interventions are often those that can continuously challenge EF. The AMC training requires children to perform mental calculations by forming an imaginary abacus and rapidly manipulating the beads on this imaginary abacus. This method is cognitively challenging. To help children develop a high level of AMC skill, the abacus teacher needs to adjust difficulty levels of the training in a step‐by‐step manner. For instance, AMC beginners are allowed to move their fingers so as to aid in moving the imaginary beads. They are instructed to solve relatively easy calculation problems (e.g., a list of 3 or 4 one‐digit numbers) slowly. After a period of practice, they are encouraged to perform AMC as fast as possible without finger movements. During the training, the calculation problem becomes more and more difficult (e.g., a list of 20 three‐digit numbers). Such an adaptive training program of AMC may thus continuously challenge EF, and enhance transfer effects to EF.
Most previous neuroimaging studies about AMC reported that AMC experts exhibited greater frontoparietal activation than their matched peers during complex calculation and digit memory tasks [Hanakawa et al., 2003; Tanaka et al., 2002]. The authors interpreted that AMC experts could utilize a visuospatial imagery strategy for calculation and digit memory, leading to enhanced involvement of frontoparietal areas related to visuospatial processing. However, our fMRI findings found that the AMC group exhibited lower activity of the frontoparietal areas than the control group while performing the incongruent and mixed conditions, providing new evidence of neural plasticity associated with AMC training. In general, activation decreases after cognitive training are thought to be associated with enhanced neural efficiency in the brain network underlying cognitive performance [Kelly et al., 2006], which has been assumed to be associated with efficiency at the neuronal synaptic level [Poldrack, 2000]. Importantly, the frontal and parietal areas are thought to play a general role in executive functioning, such as different types of working memory (e.g. verbal or nonverbal) [Berryhill and Olson, 2008; Owen et al., 2005] and different types of task switching (e.g., perceptual, response, and context switching) [Kim et al., 2012; Philipp et al., 2013]. These areas are even considered to subserve a common cognitive process across a wide range of EF task [Collette et al., 2005]. Interestingly, previous studies have also indicated that the frontal and parietal areas are responsible for visuospatial transformation and processing during the operation of AMC [Chen et al., 2006; Hanakawa et al., 2003; Ku et al., 2012; Tanaka et al., 2012]. It is possible that the visuospatial imagery process during AMC provides an opportunity to enhance neural efficiency of these brain areas. Then the enhanced neural efficiency results in activation decreases in these areas while performing tasks taxing multiple EF components [Jonides, 2004; Kelly et al., 2006].
Previous developmental studies about cognitive control have reported divergent patterns of developmental changes in frontoparietal activation from childhood to adulthood, including monotonically increased activation with age [Klingberg et al., 2002; Olesen et al., 2003; Rubia et al., 2006], monotonically decreased activation with age [Durston et al., 2006; Sheridan et al., 2014; Tamm et al., 2002], and an inverted U‐shaped pattern with age [Brenhouse and Andersen, 2011; Chang et al., 2016; Geier et al., 2009; Somerville et al., 2011], confirming that the relative participation of the frontoparietal network changes with maturity. The divergent findings may result from heterogeneity of task measures, experimental designs, and sample characteristics across studies. Importantly, our data, the first to examine neurodevelopmental processes in both typical control children and AMC‐trained children, showed distinct developmental patterns in the brain activation underlying multiple EF processes. The control group demonstrated significant activation increases or tendencies of activation increases in the frontoparietal regions from the 4th to 6th grade, while the AMC group showed significant activation decreases or tendencies of activation decreases in the similar brain regions from the 4th to 6th grade, mainly in the incongruent and mixed conditions. These results suggest that long‐term AMC training may influence the developmental trajectory of EF‐relevant brain activity. The pattern of activation increases in the control group might reflect the increasing difficulty for control children to perform the task at a more mature level. In contrast, the pattern of activation decreases in the AMC group might be attributed to increased neural efficiency from the 4th to 6th grade. Importantly, previous studies has proposed an inverted U‐shaped developmental pattern for the underlying frontoparietal activation from childhood to adulthood, where brain activation increases in the early stage of development and then decreases at a more mature stage [Brenhouse and Andersen, 2011; Chang et al., 2016]. If the neurodevelopmental changes related to EF follow this pattern, the activation increases in the control group and the activation decreases in the AMC group might indicate that the EF‐relevant neurofunctional network in the AMC group was more mature than that in the control group.
Many developmental studies have reported that greater recruitment of task‐relevant brain activity is associated with better EF performance [Klingberg et al., 2002; Olesen et al., 2003; Rubia et al., 2006]. There are also studies reporting that lower task‐relevant brain activity is related to better EF performance [Somerville et al., 2011]. However, few systematic reviews have discussed why both greater and lower task‐relevant brain activity can be meaningfully linked to EF performance during development. Interestingly, a study by Booth et al. [2004] examined the relationship of cognitive performance to the magnitude of brain activation in children aged between 9 and 12 years old. The study found that better performance in a go/no‐go task was associated with greater activation in the response inhibition brain network, while better performance in a visual search task was related to lower activation in the selective attention brain network. Given that the maturation of the response inhibition network is more prolonged than the maturation of the selective attention network [Booth et al., 2003], the authors speculated that different brain–behavior correlations may indicate different developmental trajectories for the underlying neurocognitive networks. In other words, a positive brain–behavior correlation is likely to reflect an immature neurocognitive network, while a negative brain–behavior correlation tends to indicate a relatively mature neurocognitive network. Interestingly, this study for the first time, detected the dissociation between AMC‐trained children and their peers in brain–behavior associations. Better EF performance was associated with lower brain activation in the AMC group, but was correlated with greater brain activation in the control group. Based on the view of Booth et al. [2004], distinct brain–behavior correlations in this study might also indicate a promotive effect of AMC training on the functional maturation of EF‐relevant neural correlates.
Previous neuroimgaing studies have also combined functional and structural imaging data in one analysis to explain the development of cognitive control [Olesen et al., 2003; Steinbeis et al., 2012]. The findings suggest that developmental‐related differences in both structural and functional properties can contribute unique portions of variance in explaining the development of cognitive control. Interestingly, our previous structural imaging studies have reported decreases in gray matter volumes and increases in white matter tracts for children receiving long‐term AMC training [Hu et al., 2011; Li et al., 2013b]. Taking into account these findings, long‐term AMC training may contribute to EF development from both functional and structural aspects of the brain. Future work in this field can analyze structural and functional data together to further explore the effects of AMC training on the neurodevelopmental processes of EF.
The main limitation of this study was the lack of baseline behavioral or fMRI assessments for the dot task. Although we carefully matched the two groups in age, gender, intelligence, inhibition, and preschool behavioral traits, all of which are critical for EF and the underlying cortical activity [Ardila et al., 2000; Clark et al., 2002; Gray et al., 2005; Jennings, 2002], we could not exclude the existence of pretraining group differences in behavioral performance or brain activation in the dots task. In fact, the current data were collected as parts of a larger study, in which the task‐state fMRI was collected along with resting‐state fMRI, 3D structural MRI, and diffusion tensor imaging scanning sessions in the 1st, 4th, and 6th grades, respectively. Unfortunately, the task‐state fMRI in the 1st grade failed: four children were unable to stay focused on the dots task while receiving fMRI scanning, five children performed at or below chance on the dots task or had excessive head motion. Thus, we determined to give up the task‐state fMRI session for the remaining children. Future experiments should consider stricter baseline control for the task‐state fMRI. In our future work, we would analyze the participants' other imaging data that may correlate with EF. Baseline controls of these data may provide additional insight into pretraining variability. Another limitation of the study was that only one EF task was tested. In the literature, EF task measures are numerous, and different measure types may show different results [Davidson et al., 2006]. It is unclear whether the effects of AMC training are influenced by heterogeneity of EF tasks. Therefore, future research should also consider multiple EF tasks.
CONCLUSION
In sum, this study examined the effects of long‐term AMC training on the development of EF and its underlying brain activity. Our results indicated that long‐term AMC training may serve as an effective method to improve EF performance, and increase neural efficiency in the frontoparietal regions. Besides, from the age of 10 to 12, AMC trained children showed activation decreases in the frontoparietal regions, while control children exhibited an opposite pattern. Furthermore, different patterns of brain–behavior relationships were detected for the two groups, where better performance was associated with lower task‐relevant brain activity in AMC trained children and with greater task‐relevant brain activity in control children. These findings have important implications for our understanding of the role of AMC training in the developmental process of EF, particularly at the neural level. In future work, we would consider stricter baseline controls and to examine how the neural bases subsuming EF are anatomically and functionally interconnected, which may help characterize EF plasticity at a deeper level.
ACKNOWLEDGMENTS
The authors are very grateful to the Chinese Abacus and Mental Arithmetic Association, the Heilongjiang Abacus Association for their kind support. Thanks also to Dr Yunqi Wang for help with language editing, and the children, parents, and teachers of Qiqihar for their participation in the study.
REFERENCES
- Anderson P (2002): Assessment and development of executive function (EF) during childhood. Child Neuropsychol 8:71–82. [DOI] [PubMed] [Google Scholar]
- Anderson V. a, Anderson P, Northam E, Jacobs R, Catroppa C (2001): Development of executive functions through late childhood and adolescence in an Australian sample. Dev Neuropsychol 20:385–406. [DOI] [PubMed] [Google Scholar]
- Anderson V (2001): Assessing executive functions in children: biological, psychological, and developmental considerations. Pediatr Rehabil 4:119–136. [DOI] [PubMed] [Google Scholar]
- Ardila A, Pineda D, Rosselli M (2000): Correlation between intelligence test scores and executive function measures. Arch Clin Neuropsychol 15:31–36. [PubMed] [Google Scholar]
- Berryhill ME, Olson IR (2008): Is the posterior parietal lobe involved in working memory retrieval? Evidence from patients with bilateral parietal lobe damage. Neuropsychologia 46:1775–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best J, Miller P (2010): A developmental perspective on executive function. Child Dev 81:1641–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialystok E, Depape A‐M (2009): Musical expertise, bilingualism, and executive functioning. J Exp Psychol Hum Percept Perform 35:565–574. [DOI] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Fried R, Doyle AE, Spencer T, Seidman LJ, Gross L, Poetzl K, Faraone SV (2007): Stability of executive function deficits into young adult years: a prospective longitudinal follow‐up study of grown up males with ADHD. Acta Psychiatr Scand 116:129–136. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Trommer BL, Davenport ND, Parrish TB, Gitelman DR, Mesulam M (2004): Brain–behavior correlation in children depends on the neurocognitive network. Hum Brain Mapp 23:99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Lei Z, Trommer BL, Davenport ND, Li W, Parrish TB, Gitelman DR, Mesulam MM (2003): Neural development of selective attention and response inhibition. NeuroImage 20:737–751. [DOI] [PubMed] [Google Scholar]
- Bor D, Owen AM (2007a): Cognitive training: Neural correlates of expert skills. Curr Biol 17:R95–R97. [DOI] [PubMed] [Google Scholar]
- Bor D, Owen AM (2007b): A common prefrontal–parietal network for mnemonic and mathematical recoding strategies within working memory. Cereb Cortex 17:778–786. [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL (2011): Developmental trajectories during adolescence in males and females: A cross‐species understanding of underlying brain changes. Neurosci Biobehav Rev 35:1687–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull R, Espy KA, Wiebe SA (2008): Short‐term memory, working memory, and executive functioning in preschoolers: longitudinal predictors of mathematical achievement at age 7 years. Dev Neuropsychol 33:205–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T‐T, Metcalfe AWS, Padmanabhan A, Chen T, Menon V (2016): Heterogeneous and nonlinear development of human posterior parietal cortex function. NeuroImage 126:184–195. [DOI] [PubMed] [Google Scholar]
- Chen F, Hu Z, Zhao X, Wang R, Yang Z, Wang X, Tang X (2006): Neural correlates of serial abacus mental calculation in children: A functional MRI study. Neurosci Lett 403:46–51. [DOI] [PubMed] [Google Scholar]
- Choi H‐J, Zilles K, Mohlberg H, Schleicher A, Fink GR, Armstrong E, Amunts K (2006): Cytoarchitectonic identification and probabilistic mapping of two distinct areas within the anterior ventral bank of the human intraparietal sulcus. J Comp Neurol 495:53–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C, Prior M, Kinsella G (2002): The relationship between executive function abilities, adaptive behaviour, and academic achievement in children with externalising behaviour problems. J Child Psychol Psychiatry 43:785–796. [DOI] [PubMed] [Google Scholar]
- Collette F, Van Der Linden M, Laureys S, Delfiore G, Degueldre C, Luxen A, Salmon E (2005): Exploring the unity and diversity of the neural substrates of executive functioning. Hum Brain Mapp 25:409–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlin E, Neely AS, Larsson A, Backman L, Nyberg L (2008): Transfer of learning after updating training mediated by the striatum. Science (80‐) 320:1510–1512. [DOI] [PubMed] [Google Scholar]
- Davidson MC, Amso D, Anderson LC, Diamond A (2006): Development of cognitive control and executive functions from 4 to 13 years: Evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia 44:2037–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A (2014): Want to optimize executive functions and academic outcomes? Simple, just nourish the human spirit. Minnesota Symp Child Psychol 205–230. [PMC free article] [PubMed] [Google Scholar]
- Diamond A, Lee K (2011): Interventions shown to aid executive function development in children 4 to 12 years old. Science (80‐) 333:959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S, Wang C, Xie Y, Hu Y, Weng J, Chen F (2016): The impact of abacus training on working memory and underlying neural correlates in young adults. Neuroscience 332:181–190. [DOI] [PubMed] [Google Scholar]
- Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, Casey BJ (2006): A shift from diffuse to focal cortical activity with development. Dev Sci 9:18–20. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K (2005): A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage 25:1325–1335. [DOI] [PubMed] [Google Scholar]
- Fair D. a, Dosenbach NUF, Church J. a, Cohen AL, Brahmbhatt S, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL (2007): Development of distinct control networks through segregation and integration. Proc Natl Acad Sci USA 104:13507–13512. 17679691 [Google Scholar]
- Fair D, Nigg JT, Iyer S, Bathula D, Mills KL, Dosenbach NUF, Schlaggar BL, Mennes M, Gutman D, Bangaru S, Buitelaar JK, Dickstein DP, Di Martino A, Kennedy DN, Kelly C, Luna B, Schweitzer JB, Velanova K, Wang Y‐F, Mostofsky S, Castellanos FX, Milham MP (2012): Distinct neural signatures detected for ADHD subtypes after controlling for micro‐movements in resting state functional connectivity MRI data. Front Syst Neurosci 6:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MC, Barner D (2012): Representing exact number visually using mental abacus. J Exp Psychol Gen 141:134–149. [DOI] [PubMed] [Google Scholar]
- Garavan H, Kelley D, Rosen A, Rao SM, Stein EA (2000): Practice‐related functional activation changes in a working memory task. Microsc Res Tech 51:54–63. [DOI] [PubMed] [Google Scholar]
- Geier CF, Terwilliger R, Teslovich T, Velanova K, Luna B (2009): Immaturities in reward processing and its influence on inhibitory control in adolescence. Cereb Cortex 20:1613–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd J (2004): Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci 1021:77–85. [DOI] [PubMed] [Google Scholar]
- Gray JR, Burgess GC, Schaefer A, Yarkoni T, Larsen RJ, Braver TS (2005): Affective personality differences in neural processing efficiency confirmed using fMRI. Cogn Affect Behav Neurosci 5:182–190. [DOI] [PubMed] [Google Scholar]
- Hanakawa T, Honda M, Okada T, Fukuyama H, Shibasaki H (2003): Neural correlates underlying mental calculation in abacus experts: A functional magnetic resonance imaging study. NeuroImage 19:296–307. [DOI] [PubMed] [Google Scholar]
- Hechtman LA (2015): The Relationship Between Executive Function and Creative Cognition: A Behavioral and Neural Investigation. Northwestern University. [Google Scholar]
- Ho Y‐C, Cheung M‐C, Chan AS (2003): Music training improves verbal but not visual memory: cross‐sectional and longitudinal explorations in children. Neuropsychology 17:439–450. [DOI] [PubMed] [Google Scholar]
- Houdé O, Rossi S, Lubin A, Joliot M (2010): Mapping numerical processing, reading, and executive functions in the developing brain: an fMRI meta‐analysis of 52 studies including 842 children. Dev Sci 13:876–885. [DOI] [PubMed] [Google Scholar]
- Hu Y, Geng F, Tao L, Hu N, Du F, Fu K, Chen F (2011): Enhanced white matter tracts integrity in children with abacus training. Hum Brain Mapp 32:10–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C, Ensor R (2007): Executive function and theory of mind: Predictive relations from ages 2 to 4. Dev Psychol 43:1447–1459. [DOI] [PubMed] [Google Scholar]
- Irwing P, Hamza A, Khaleefa O, Lynn R (2008): Effects of Abacus training on the intelligence of Sudanese children. Pers Individ Dif 45:694–696. [Google Scholar]
- Jennings KD (2002): Mastery motivation and the formation of self‐concept from infancy through early. Mastery Motiv Child Investig Persistence Dev 36. [Google Scholar]
- Jolles DD, Grol MJ, Van Buchem MA, Rombouts SARB, Crone EA (2010): Practice effects in the brain: Changes in cerebral activation after working memory practice depend on task demands. NeuroImage 52:658–668. [DOI] [PubMed] [Google Scholar]
- Jonides J (2004): How does practice makes perfect? Nat Neurosci 7:10–11. [DOI] [PubMed] [Google Scholar]
- Kelly AMC (2004): Human functional neuroimaging of brain changes associated with practice. Cereb Cortex 15:1089–1102. [DOI] [PubMed] [Google Scholar]
- Kelly C, Foxe JJ, Garavan H (2006): Patterns of normal human brain plasticity after practice and their implications for neurorehabilitation. Arch Phys Med Rehabil 87:20–29. [DOI] [PubMed] [Google Scholar]
- Kharitonova M, Martin RE, Gabrieli JDE, Sheridan MA (2013): Cortical gray‐matter thinning is associated with age‐related improvements on executive function tasks. Dev Cogn Neurosci 6:61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Cilles SE, Johnson NF, Gold BT (2012): Domain general and domain preferential brain regions associated with different types of task switching: A Meta‐Analysis. Hum Brain Mapp 33:130–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimkeit EI, Mattingley JB, Sheppard DM, Farrow M, Bradshaw JL (2004): Examining the development of attention and executive functions in children with a novel paradigm. Child Neuropsychol 10:201–211. [DOI] [PubMed] [Google Scholar]
- Klingberg T (2010): Training and plasticity of working memory. Trends Cogn Sci. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Fernell E, Olesen PJ, Johnson M, Gustafsson P, Dahlström K, Gillberg CG, Forssberg H, Westerberg H (2005): Computerized training of working memory in children with ADHD‐a randomized, controlled trial. J Am Acad Child Adolesc Psychiatry 44:177–186. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Forssberg H, Westerberg H (2002): Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. J Cogn Neurosci 14:1–10. [DOI] [PubMed] [Google Scholar]
- Ku Y, Hong B, Zhou W, Bodner M, Zhou YD (2012): Sequential neural processes in abacus mental addition: An EEG and fMRI case study. PLoS One 7: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Lu M, Ko H (2007): Effects of skill training on working memory capacity. Learn Instr 17:336–344. [Google Scholar]
- Li D, Hu KD, Chen GP, Jin Y, Li M (1988): The testing results report on the combined Raven's test in Shanghai. Psychol Sci 4:27–31. [Google Scholar]
- Li Y, Hu Y, Zhao M, Wang Y, Huang J, Chen F (2013a): The neural pathway underlying a numerical working memory task in abacus‐trained children and associated functional connectivity in the resting brain. Brain Res 1539:24–33. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang Y, Hu Y, Liang Y, Chen F (2013b): Structural changes in left fusiform areas and associated fiber connections in children with abacus training: Evidence from morphometry and tractography. Front Hum Neurosci 7: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low JA, Webster L (2016): Attention and executive functions as mediators of attachment and behavior problems. Soc Dev 25:646–664. [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD (2000): The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cogn Psychol 41:49–100. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Arseneault L, Belsky D, Dickson N, Hancox RJ, Harrington H, Houts R, Poulton R, Roberts BW, Ross S (2011): A gradient of childhood self‐control predicts health, wealth, and public safety. Proc Natl Acad Sci 108:2693–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan GA, Busch‐Rossnagel NA, Barrett KC, Wang J (2009): The Dimensions of Mastery Questionnaire (DMQ): a manual about its development, psychometrics, and use. Fort Collins Color State Univ [Google Scholar]
- Morgan GA, Wang J, Liao H‐F, Xu Q (2012): Using the Dimensions of Mastery Questionnaire (DMQ) to assess mastery motivation of English‐and Chinese‐speaking children. Handb Self‐Regulat Process Dev New Dir Int Perspect 305. [Google Scholar]
- Olesen PJ, Westerberg H, Klingberg T (2004): Increased prefrontal and parietal activity after training of working memory. Nat Neurosci 7:75–79. [DOI] [PubMed] [Google Scholar]
- Olesen PJ, Nagy Z, Westerberg H, Klingberg T (2003): Combined analysis of DTI and fMRI data reveals a joint maturation of white and grey matter in a fronto‐parietal network. Cogn Brain Res 18:48–57. [DOI] [PubMed] [Google Scholar]
- Ordaz SJ, Foran W, Velanova K, Luna B (2013): Longitudinal growth curves of brain function underlying inhibitory control through adolescence. J Neurosci 33:18109–18124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E (2005): N‐back working memory paradigm: A meta‐analysis of normative functional neuroimaging studies. Hum Brain Mapp 25:46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipp AM, Weidner R, Koch I, Fink GR (2013): Differential roles of inferior frontal and inferior parietal cortex in task switching: Evidence from stimulus‐categorization switching and response‐modality switching. Hum Brain Mapp 34:1910–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pianta RC, Caldwell CB (1991): A measure of young children's problem and competence behaviors: The early school behavior scale. J Psychoeduc Assess. [Google Scholar]
- Poldrack RA (2000): Imaging brain plasticity: Conceptual and methodological issues — A theoretical review. NeuroImage 12:1–13. [DOI] [PubMed] [Google Scholar]
- Rodrigues AC, Loureiro MA, Caramelli P (2013): Long‐term musical training may improve different forms of visual attention ability. Brain Cogn 82:229–235. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, Brammer M (2006): Progressive increase of frontostriatal brain activation from childhood to adulthood during event‐related tasks of cognitive control. Hum Brain Mapp 27:973–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaug G, Norton A, Overy K, Winner E (2005): Effects of music training on the child's brain and cognitive development. Ann N Y Acad Sci 1060:219–230. [DOI] [PubMed] [Google Scholar]
- Sheridan M, Kharitonova M, Martin RE, Chatterjee A, Gabrieli JDE (2014): Neural substrates of the development of cognitive control in children ages 5–10 years. J Cogn Neurosci 26:1840–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Hare TA, Casey BJ (2011): Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. J Cogn Neurosci 23:2123–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Sowell ER, Thompson PM, Thompson PM, Holmes CJ, Holmes CJ, Batth R, Batth R, Jernigan TL, Jernigan TL, Toga a. W, Toga a. W (1999): Localizing age‐related changes in brain structure between childhood and adolescence using statistical parametric mapping. NeuroImage 9:587–597. [DOI] [PubMed] [Google Scholar]
- Steinbeis N, Bernhardt BC, Singer T (2012): Impulse control and underlying functions of the left DLPFC mediate age‐related and age‐independent individual differences in strategic social behavior. Neuron 73:1040–1051. [DOI] [PubMed] [Google Scholar]
- Tamm L, Menon V, Reiss AL (2002): Maturation of brain function associated with response inhibition. J Am Acad Child Adolesc Psychiatry 20:1231–1238. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Michimata C, Kaminaga T, Honda M, Sadato N (2002): Superior digit memory of abacus experts: an event‐related functional MRI study. Neuroreport 13:2187–2191. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Seki K, Hanakawa T, Harada M, Sugawara SK, Sadato N, Watanabe K, Honda M (2012): Abacus in the brain: A longitudinal functional MRI study of a skilled abacus user with a right hemispheric lesion. Front Psychol 3: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorell LB, Lindqvist S, Nutley SB, Bohlin G, Klingberg T (2009): Training and transfer effects of executive functions in preschool children. Dev Sci 12:106–113. [DOI] [PubMed] [Google Scholar]
- Van der Ven SHG, Kroesbergen EH, Boom J, Leseman PPM (2012): The development of executive functions and early mathematics: A dynamic relationship. Br J Educ Psychol 82:100–119. [DOI] [PubMed] [Google Scholar]
- Wang C, Geng F, Yao Y, Weng J, Hu Y, Chen F (2015): Abacus training affects math and task switching abilities and modulates their relationships in Chinese children. Ed. Xuchu Weng. PLoS One 10:e0139930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Ming DI (2007): A report on the third revision of Combined Raven's Test(CRT‐C3) for children in China. Chinese J Clin Psychol. [Google Scholar]
- Wang J, Morgan GA, Barrett KC, Xu QM (2009): Comparison of American and Chinese parents' perceptions of their preschool and school‐aged children's mastery motivation. In: Poster presented at the Biannual Meeting of the Society for Research in Child Development. Denver, CO.
- Wang Y, Geng F, Hu Y, Du F, Chen F (2013): Numerical processing efficiency improved in experienced mental abacus children. Cognition 127:149–158. [DOI] [PubMed] [Google Scholar]
- Yao Y, Du F, Wang C, Liu Y, Weng J, Chen F (2015): Numerical processing efficiency improved in children using mental abacus: ERP evidence utilizing a numerical Stroop task. Front Hum Neurosci 9:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X (2011): Parent‐child and teacher‐child relationships in Chinese preschoolers: The moderating role of preschool experiences and the mediating role of social competence. Early Child Res Q 26:192–204. [Google Scholar]


