Abstract
The ability to mentally design and evaluate series of future actions has often been studied in terms of planning abilities, commonly using well‐structured laboratory tasks like the Tower of London (ToL). Despite a wealth of studies, findings on the specific localization of planning processes within prefrontal cortex (PFC) and on the hemispheric lateralization are equivocal. Here, we address this issue by integrating evidence from two different sources of data: First, we provide a systematic overview of the existing lesion data on planning in the ToL (10 studies, 211 patients) which does not indicate any evidence for a general lateralization of planning processes in (pre)frontal cortex. Second, we report a quantitative meta‐analysis with activation likelihood estimation based on 31 functional neuroimaging datasets on the ToL. Separate meta‐analyses of the activation patterns reported for Overall Planning (537 participants) and for Planning Complexity (182 participants) congruently show bilateral contributions of mid‐dorsolateral PFC, frontal eye fields, supplementary motor area, precuneus, caudate, anterior insula, and inferior parietal cortex in addition to a left‐lateralized involvement of rostrolateral PFC. In contrast to previous attributions of planning‐related brain activity to the entire dorsolateral prefrontal cortex (dlPFC) and either its left or right homolog derived from single studies on the ToL, the present meta‐analyses stress the pivotal role specifically of the mid‐dorsolateral part of PFC (mid‐dlPFC), presumably corresponding to Brodmann Areas 46 and 9/46, and strongly argue for a bilateral rather than lateralized involvement of the dlPFC in planning in the ToL. Hum Brain Mapp 38:396–413, 2017. © 2016 Wiley Periodicals, Inc.
Keywords: prefrontal cortex, planning, lesion studies, functional neuroimaging, meta‐analysis, activation likelihood estimation, Tower of London Task
INTRODUCTION
The functional anatomy of planning processes in the prefrontal cortex (PFC) and the concomitant question of hemispheric lateralization have been a matter of interest since the seminal study of Shallice [1982] on the Tower of London (ToL) task. In that study, patients with left anterior brain lesions were found to have substantial decrements in planning accuracy and increased initial thinking times when compared to patients with right anterior lesions as well as with left and right posterior lesions [Shallice, 1982]. Lateralization of planning processes in the ToL was thereafter addressed in several neuropsychological studies with brain‐lesioned patients. But as summarized in Box 1, effects of lateralization of (pre)frontal lesions on planning accuracy (in terms of optimal solutions within the minimum number of moves) and initial thinking times could not be found in any of these subsequent studies (Table B1) [see also Sullivan et al., 2009].
Box 1. Overview on prefrontal lateralization of planning ability in lesion studies.
In addition to the ALE analyses presented in the main text, we conducted a literature search for studies on the Tower of London task in patients with frontal brain lesions. The aim was to provide another approach particularly on the question of hemispheric lateralization of human planning. To identify studies that compared right against left frontal lesioned patients, all studies from the original literature search were rescanned. Identified studies were checked for cross references of further suited studies. The literature research revealed 13 studies in total that examined the ToL task (cf., Fig. 1) in patients with frontal brain lesions. However, note that not all of these reported patient samples were independent, as some studies reported (partly) overlapping samples (indicated in Table B1) [cf. Owen et al., 1990, and Pantelis et al., 1997]. Furthermore, descriptive statistics or verbatim information on the comparisons of interest here (frontal lesion patients vs. controls, left‐ vs. right frontal lesion patients) were not at all available for some studies [Cockburn, 1995; Levin et al., 1994; Shallice et al., 1989] whereas many of the remaining studies did not differentiate between left and right frontal lesions [Anderson et al., 2002; Andrés and Van der Linden, 2001; Andrews et al., 2014; Jacobs and Anderson, 2002; Pantelis et al., 1997; Shum et al., 2009].
In consequence, demographic and performance data from 10 studies reporting effects of (pre)frontal lesions on planning ability was extracted (see Table B1 for an overview). To allow for comparison of effect sizes between studies, if descriptive and/or inferential statistics were reported, Hedges' g was calculated [Hedges and Olkin, 1985]. If no statistical characteristics but only verbatim descriptions of observed results were reported in the original studies (e.g., Shallice, 1982: “the left anterior group was the slowest of all”, p. 205) effects were listed descriptively (e.g. le > ri).
Given the small overall number of studies together with the heterogeneity of reported information, a formal assessment in terms of a meta‐analysis was not feasible. A brief overview and summary of the results is therefore listed below.
Frontally Lesioned Patients Compared to Healthy Controls
A total of six studies reported a reduced planning accuracy in patients compared to healthy controls [significant: Andrews et al., 2014, Owen et al., 1990, 1995, Shum et al., 2009; not significant: Jacobs and Anderson, 2002, Pantelis et al., 1997] whereas only one study [Anderson et al., 2002] reported an opposite pattern (significantly higher accuracy in frontally lesioned patients). An increased number of executed moves as another index of impaired planning was observed for frontally lesioned patients in three studies [significant: Carlin et al., 2000, Owen et al., 1990; not significant: Pantelis et al., 1997]. Although not significant, one study [Andrés and Van der Linden, 2001] found effects in the other direction (decreased number of executed moves).
A prolonged initial thinking (or pre‐planning) time for lesioned patients was observed in four studies [significant: Pantelis et al., 1997; not significant: Andrés and Van der Linden, 2001, Jacobs and Anderson, 2002, Shum et al., 2009]. One study reported a (not significant) reduction in initial thinking time [Carlin et al., 2000]. For another three studies, no information on the direction of effects on initial thinking time was available [Andrews et al., 2014, Owen et al., 1990, 1995]. The movement execution time of patients was prolonged in four studies [all significant: Andrés and Van der Linden, 2001, Carlin et al., 2000, Owen et al., 1990, Pantelis et al., 1997].
Left Frontally Lesioned Compared to Right Frontally Lesioned Patients
With respect to the hemispheric lateralization of planning in well‐structured tower tasks, none of the studies reported any significant findings, but descriptions of the effects' directions. A total of two studies observed a lower accuracy in left lesioned versus right lesioned patients [Carlin et al., 2000, Shallice, 1982] and another two studies [Owen et al., 1990, 1995] reported non‐significant results without specifying the direction. The number of executed moves was increased for left lesioned versus right lesioned patients in one study [Carlin et al., 2000], whereas one study reported [Owen et al., 1990] non‐significant effects without specifying the direction.
Initial thinking time was increased for left lesioned patients in one study [Shallice, 1982] and for right lesioned patients in one study [Carlin et al., 2000], another two studies [Owen et al., 1990, 1995] reported non‐significant effects without specifying the direction. Movement execution time was prolonged for left lesioned patients in one study [Carlin et al., 2000]; again one study [Owen et al., 1990] reported non‐significant results without specifying the direction.
Taken together and summarized in Table B2, comparing frontally lesioned patients to healthy controls often results in a reduced planning accuracy and, occasionally, in an increased number of executed moves, while initial thinking time and movement execution time are prolonged in some but not all cases. Neither a clear pattern nor any significant findings emerges for comparing left and right frontally lesioned patients in common performance and latency measures of planning in well‐structured tasks. However, when interpreting these results, it has to be borne in mind that the studies mostly consisted of small sample sizes and presumably comprised a heterogeneous collection of etiologies (e.g., stroke, tumor, trauma) with different courses of disease and brain plasticity.
Table B1.
Overview of studies on the Tower of London task investigating planning ability in frontally lesioned patients
| Study | Sample | Task | Respected Effects | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Sex (m/f) | Age M±SD (years) | Tower Type | Minimum Moves | Accuracy | Number of Executed Moves | Initial Thinking Time | Movement Execution Time | ||||||||
| Pat/NC | le/ri/bi | Pat | NC | Pat | NC | Pat vs. NC | le vs. ri | Pat vs. NC | le vs. ri | Pat vs. NC | le vs. ri | Pat vs. NC | le vs. ri | |||
| Anderson et al. [2002] | 20/80 | 15/5 | 43/37 | 11.4± 2.9 | 9.4 ± 2.8 | ToL | 2–5 | 0.11 | ||||||||
| Andrés and Van der Linden [2001] | 13/13 | 6/4/3 | 13/0 | 13/0 | 33.2 ± 13.8 | 32.8 ± 13.2 | ToL | 3 | −0.35 | 0.41 | 0.69 | |||||
| 5 neutral | −0.06 | 0.71 | 1.31** | |||||||||||||
| 5 facilitating | −0.16 | 0.56 | 0.33 | |||||||||||||
| 5 misleading | −0.42 | 0.18 | 0.49 | |||||||||||||
| Andrews et al. [2014] | 14/40 | 7/4/3 | 7/7 | 24/16 | 64.29 ± 8.82 | 68.28 ± 12.16 | ToL 4‐D | 2–9 | −1.02 ** | n.s. | ||||||
| Carlin et al. [2000] | 14/15 | 7/5/2 | 10/4 | 11/4 | 59.9 ± 12.6 | 57.5 ± 16.6 | ToL | 2–5 | −0.23 | 0.69 * | 0.19 | −0.06 | −0.14 | 0.87 ** | 0.14 | |
| 2–3 | −0.39 | −0.21 | −0.26 | |||||||||||||
| 4–5 | 0.71 | −0.22 | 0.94† | |||||||||||||
| Jacobs and Anderson [2002] | 31/38 | 12/9/10 | 19/12 | 19/19 | 11.3 ± 2.8 | 10.8 ± 2.7 | ToL | 2–5 | −0.18 | 0.14 | ||||||
| Owen et al. [1990]#1 | 26/26 | 8/15/3 | 14/12 | 14/12 | 43.0 ± 18.8 | 44.6 ± 17.7 | ToL (SoC) | 2–5 | −0.63 * | n.s. | .59 * | n.s. | n.s. | n.s. | 0.60 * | n.s. |
| Pantelis et al. [1997]#1 | 26/31 | 8/15/3 | 15/11 | 18/13 | 41.73 ± 19.1 | 47.48 ± 11.6 | ToL (SoC) | 2 | 0.24 | −0.24 | 0.27 | 0.59† | ||||
| 3 | −0.18 | 0.19 | 0.58† | 0.55† | ||||||||||||
| 4 | −0.17 | 0.12 | 0.54† | 0.59† | ||||||||||||
| 5 | −0.34 | 0.29 | 0.27 | 0.64† | ||||||||||||
| Owen et al. [1995] | 17/10 | 5/10/2 | 9/8 | 7/10 | 46.5 ± 18.1 | 44.1 ± 13.3 | ToL (SoC) | 1–5 | −1.28 *** | n.s. | n.s. | n.s. | ||||
| Shallice [1982] | 61/20 | ToL | 2–5 | le < ri | le > ri | |||||||||||
| Shum et al. [2009] | 15/15 | ToL 4‐D | 2–9 | −0.82 * | 0.04 | |||||||||||
| 2–5 | −0.39 | 0.45 | ||||||||||||||
| 6–9 | −0.80 * | −0.05 | ||||||||||||||
Note. If descriptive statistics were available, the here reported effect sizes were computed as Hedges' g [Hedges and Olkin, 1985] allowing comparisons across studies. Otherwise effects were reported verbatim (e.g., le > ri, n.s.). Significant results are highlighted in bold font (*, P < 0.05; ** P < 0.01. ***; P < 0.001; n.s., not significant). Stars (*) indicate P‐values reported by the authors whereas crosses (†) indicate posterior tests of significance (Students t‐test for independent groups and unequal sample sizes) by the authors of the present study based on the available data. Superscripts starting with hashtags followed by numbers indicate partly or fully overlapping samples between the studies. Abbreviations: N, sample size; m, male; f, female; Pat, patients; NC, normal controls; le, left frontal lesion; ri, right frontal lesion; bi, bilateral frontal lesion; ToL, Tower of London; SoC, Stockings of Cambridge; 4‐D, four‐disc version.
However, effects of prefrontal lateralization may have been attenuated in lesion studies due to several methodological reasons: The number of lesion studies on the topic is small and their sample sizes are mostly limited as well. Furthermore, there is commonly a high variability in extent, localization, and focality of lesions in PFC and beyond, as well as a high variability in functional impairments given heterogeneous etiologies and time courses of brain plasticity and reorganization following brain injuries [cf., Rorden and Karnath, 2004]. Hence, the plethora of neuroimaging studies in healthy volunteers on the ToL may represent a more homogeneous foundation for examining the functional anatomy of planning processes and for addressing questions concerning the localization and lateralization in the PFC. Most notably in this respect, although planning is commonly associated with the mid‐dorsolateral prefrontal cortex (dlPFC) [cf., Unterrainer and Owen, 2006], the surprisingly wide‐spread distribution of prefrontal peak voxels derived from 31 neuroimaging studies on the ToL (see Fig. 3) does not imply a clear focus of planning‐related neural activation within mid‐dlPFC and thus runs counter to this common tenet. The term “dlPFC” is in fact used to refer to an extended prefrontal area that covers parts of the middle and superior frontal gyri and thus includes the functionally different, Brodmann areas (BA) 9, 9/46, 46, and parts of 8 and 10 [Petrides, 2005]. Therefore, the precise spatial localization of planning processes within the dlPFC is highly relevant for correctly identifying the neural underpinnings of planning processes. That is, adding to the unresolved issue of a possible lateralization of PFC involvement in planning (cf., Table B1) [see also Cazalis et al., 2003; Kaller et al., 2011b], the often assumed association with its mid‐dorsolateral part [e.g., Unterrainer and Owen, 2006] remains to be empirically confirmed by a quantitative examination of activation coordinates across studies.
Figure 3.
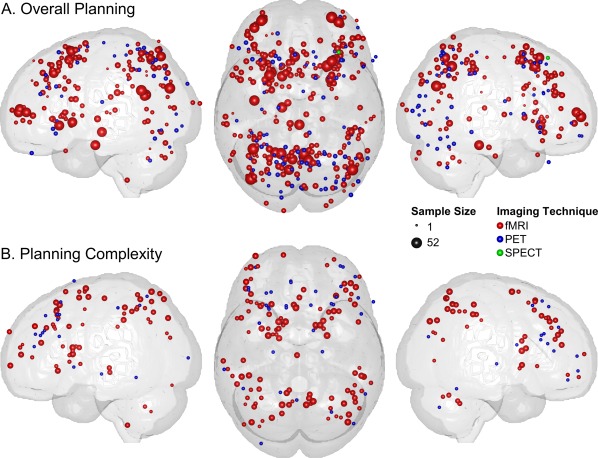
Spatial distributions of activation foci from ToL neuroimaging studies included in the present ALE meta‐analyses on (A) Overall Planning and (B) Planning Complexity (see Methods section for further details). Individual foci are marked by spheres. The sizes and the colors of the spheres refer to the underlying sample sizes and the used brain imaging techniques. The renderings for the lateral views only comprise the activation foci for the respective hemisphere, whereas the topview renderings represent all foci.
Quantitative meta‐analyses have a long history in clinical research [O'Rourke, 2007] but have also become increasingly popular in functional neuroimaging [e.g., Eickhoff et al., 2006; Farrell et al., 2005; Price et al., 2005; Wager et al., 2004; Wager and Smith, 2003], as they allow assessing brain‐behavior relationships independent of study‐specific confounds such as characteristics of samples and experimental procedures, task implementation, imaging parameters and artefacts, preprocessing, statistical thresholds, and correction mechanisms. As one of the most common algorithms for coordinate‐based meta‐analyses, activation likelihood estimations (ALE) are computed for the distribution of reported activation peaks of all included studies and compared against random distributions to account for the spatial nature of neuroimaging results [Eickhoff et al., 2009; Laird et al. 2005; Turkeltaub et al., 2012].
In the present study, we therefore adopted this meta‐analytic ALE approach with the aim of examining the functional anatomy of planning processes and particularly with the aim of resolving the open questions on the specific localization and the hemispheric lateralization of PFC involvement. To this end, the meta‐analysis was based on functional neuroimaging studies using the ToL, as it constitutes the most frequently applied experimental planning paradigm in clinical and cognitive neuroscience [Kaller et al., 2011a].
METHODS
Study Selection
The web‐hosted databases PubMed (http://www.ncbi.nlm.nih.gov/pubmed), ISI Web of Knowledge (http://www.webofknowledge.com), Science Direct (http://www.sciencedirect.com) and PsycINFO (http://www.apa.org/psycinfo) were searched using pair‐wise combinations of the key term ToL with the following terms: fMRI, functional magnetic resonance imaging, PET, positron emission tomography, SPECT, single photon emission computed tomography, NIRS, near infrared spectroscopy, EEG, electroencephalography, planning, problem solving, brain imaging, functional imaging, activity. In addition to these brain‐imaging specific search terms, the key term ToL was also combined with the broader terms planning and problem solving to maximize chances for detecting all relevant studies. The present literature search included all studies published until December 2014.
Figure 1.
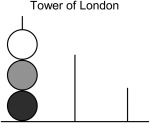
Illustration of the ToL disc‐transfer task. The tower configuration consists of three balls placed on three rods of different heights. The start configuration of the balls has to be transformed into a given goal configuration while considering several rules. The major rules for transformation are identical for the two tasks: Only one ball can be moved at once, a ball must not be placed beside a rod, and only the topmost ball of each rod can be moved. The three balls differ in their color and the rods in their length and capacity for holding balls. The left rod can hold up to three balls, the middle rod up to two balls, and the right rod only one ball. Subjects are commonly instructed to solve a given problem in the minimum number of moves. Planning ahead is hence required for efficient problem solving in terms of attaining the optimal solution.
As illustrated in the flow chart in Figure 2, 636 individual reports were identified. During the subsequent selection process, abstracts and/or manuscripts were screened and 565 studies excluded as not relevant for the topic. Of the remaining 71 functional imaging reports, another 40 studies did not qualify for the present meta‐analysis. A flowchart of the selection process is provided in Figure 2.
Figure 2.
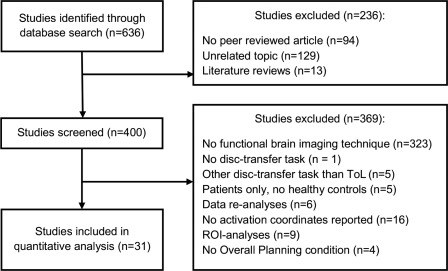
Flow chart illustrating the step‐wise study selection procedure.
The final selection consisted of 31 independent datasets that were published in 31 neuroimaging papers1 (Table 1). All included studies used three‐ball versions of the ToL with either three differently sized pegs (n = 16) [cf., Shallice, 1982] or three equally sized pegs (n = 3) [cf. Ward and Allport, 1997], or the Stockings of Cambridge (SoC) version with differently sized pockets (n = 10) [cf. Owen et al., 1990]. Furthermore, studies differed with respect to the response modes (cf., solution execution in Table 1): In some studies, subjects were asked to solve the problems by executing the individual moves (n = 8), whereas in other studies, subjects only had to indicate the minimum number required for the optimal solution of a given problem without actually executing these moves (n = 24).2 However, assuming that planning ahead was required for both types of response modes, these were not further differentiated in the subsequent analyses.
Table 1.
Overview of the functional neuroimaging datasets included in the ALE meta‐analyses
| Sample | Task | Number of Reported Activation Foci | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Data set # | Study | Imaging technique | N | Sex (m/f) | Mean age (years) | ToL version | Minimum number of movesa | Solution execution | Design | Overall planning | Planning complexity |
| 1.1 | Baker et al. [1996] | PET | 6 | 5/1 | 31 | SoC | 1–6 moves | no | B | 20 | |
| 1.2 | Elliott et al. [1997] | PET | 6 | 5/1 | 31 | SoC | 2–3 vs. 4–5 moves | no | B | 8 | |
| 2 | Beauchamp et al. [2003] | PET | 12 | 6/6 | 56.8 | SoC | 3–5 moves | yes | B | 11 | |
| 3 | Boghi et al. [2006] | fMRI | 18 | 9/9 | 35.9 | WATT | 1–3 vs. 4–6 moves | no | n.r. | 14 | 8 |
| 4 | Campbell et al. [2009] | fMRI | 1 | 1/0 | 21 | Original ToL | n.r. | yes | n.r. | 18 | |
| 5 | Cohen et al. [2015] | fMRI | 17 | 15/2 | 20.9 | WATT | 1–7 moves | no | E | 13 | |
| 6 | Dagher et al. [1999] | PET | 6 | 2/4 | 58.6 | SoC | 1–5 moves | yes | B | 29 | 22 |
| 7 | de Ruiter et al. [2009] | fMRI | 19 | 19/0 | 34.1 | Original ToL | 1–5 moves | no | E | 9 | 9 |
| 8 | de Ruiter et al. [2011] | fMRI | 15 | 0/15 | 58.2 | Original ToL | 1–5 moves | no | E | 17 | |
| 9 | den Braber et al. [2008] | fMRI | 12 | 5/7 | 32.8 | Original ToL | 1–5 moves | no | E | 13 | 11 |
| 10 | Desco et al. [2011] | fMRI | 14 | 9/5 | 13.4 | SoC | 2–5 moves | no | B | 21 | |
| 11 | Fallon et al. [2013] | fMRI | 52 | 23/29 | 64.3 | SoC | 2–4 moves | no | E | 7 | |
| 12 | Goethals et al. [2004] | SPECT | 10 | 6/4 | 24 | Original ToL | 2–6 moves | yes | B | 1 | |
| 13 | Huyser et al. [2010] | fMRI | 25 | 9/16 | 13.7 | Original ToL | 1–5 moves | no | E | 14 | 10 |
| 14 | Just et al. [2007] | fMRI | 18 | 15/3 | 24.5 | SoC | 1–3 moves | no | B | 13 | |
| 15 | Kempton et al. [2011] | fMRI | 10 | 5/5 | 16.8 | SoC | 2–4 moves | no | E | 8 | |
| 16 | Lazeron et al. [2000] | fMRI | 9 | 5/4 | 22 | Original ToL | 2–7 moves | no | B | 10 | |
| 17 | Lazeron et al. [2004] | fMRI | 18 | 12/4 | 36.6 | Original ToL | 2–4, 6–8 moves | no | B | 8 | |
| 18 | Liemburg et al. [2015] | fMRI | 20 | 14/6 | 31.1 | Original ToL | 1–2 vs. 3–5 moves | no | B | 6 | 3 |
| 19.1 | Owen et al. [1996] | PET | 12 | 6/6 | 41.4 | SoC | 3–5 moves | yes | B | 26 | |
| 19.2 | Owen et al. [1998] | PET | 6 | 4/2 | 57.7 | SoC | 3 vs. 4–5 moves | yes | B | 1 | |
| 20 | Rasmussen et al. [2006] | fMRI | 8 | 8/0 | 25 | Original ToL | 3–5 moves | yes | B | 25 | 9 |
| 21 | Rowe et al. [2001] | PET | 10 | 10/0 | 27 | SoC | 0–9 moves | yes, no | B | 17 | |
| 22.1 | Schall et al. [2003] | PET | 6 | 5/1 | 33.8 | WATT | 1–7 moves | no | B | 6 | |
| 22.2 | fMRI | 6 | 5/1 | 31 | WATT | 1–7 moves | no | B | 8 | 3 | |
| 23 | Schöepf et al. [2011] | fMRI | 28 | 12/16 | 27.3 | Original ToL | n.r. | no | B | 9 | |
| 24 | Spreng et al. [2010] | fMRI | 20 | 3/17 | 21.3 | Original ToL | 3–7 moves | no | E | 20 | |
| 25 | Stokes et al. [2011] | fMRI | 47 | 19/28 | 35.7 | SoC | 3–5 moves | no | B | 8 | |
| 26 | van den Heuvel et al. [2003] | fMRI | 22 | 11/11 | 29.9 | Original ToL | 1–5 moves | no | E | 18 | 26 |
| 27 | van den Heuvel et al. [2013] | fMRI | 11 | 4/7 | 25.5 | Original ToL | 1–5 moves | no | E | 20 | 25 |
| 28.1 | van't Ent et al. [2014] | fMRI | 46 | 13/33 | 36.9 | Original ToL | 1–5 moves | no | E | 8 | |
| 28.2 | fMRI | 45 | 13/32 | 36.9 | Original ToL | 1–5 moves | no | E | 3 | ||
| 29 | Wagner et al. [2006] | fMRI | 17 | 9/8 | 27.5 | Original ToL | 2–5 moves | no | E | 10 | |
Note. Listed are reference numbers and respective studies with author and year of all 31 studies that were included in the ALE analysis on Overall Planning or Planning Complexity. Moreover, the respective imaging technique, the sample size (overall and separated for gender), mean age of the sample, and the amount of reported activation foci are listed. SoC, Stocking of Cambridge. WATT, Ward and Allport Tower Task.
The minimum number of moves states the all problem difficulties that were utilized in the studies. Moreover, if there is solely a range listed (e.g., 1–5 moves) the reported planning complexity analysis was parametrical whereas if there are two numbers or ranges listed separated by a “vs.” (e.g., 1–3 vs. 4–6 moves) the reported planning complexity analyses was categorical. n.r., not reported; B, block design; E, event‐related design.
Table B2.
Overview on the frequency and direction of effects on planning performance across lesion studies
| Number of studies overall (number of studies with significant effects or trends) | |||
|---|---|---|---|
| Pat vs. NC | Pat < NC | Pat > NC | No direction reported |
| Accuracy | 6 (4) | 1 (0) | 0 |
| Executed moves | 3 (2) | 1 (0) | 0 |
| ITT | 4 (1) | 1 (0) | 3 |
| MET | 4 (4) | 0 (0) | 0 |
| Left vs. Right Frontal | Left < Right | Left > Right | No direction reported |
|---|---|---|---|
| Accuracy | 2 (1) | 0 (0) | 2 |
| Executed moves | 1 (0) | 0 (0) | 1 |
| ITT | 1 (0) | 1 (1) | 2 |
| MET | 1 (0) | 0 (0) | 1 |
Abbreviations: Pat, patients; NC, normal controls; ITT, initial thinking time; MET, movement execution time.
Meta‐Analyses on Overall Planning and Planning Complexity
In the majority of functional brain imaging studies using the ToL task, planning‐related neural activation is identified by either contrasting planning with a baseline condition or by experimentally manipulating planning complexity with factorial or parametric designs [see overview in Kaller et al., 2011b]. In consequence, in the present ALE study separate meta‐analyses were conducted for Overall Planning (compared with a baseline) and for Planning Complexity.
The meta‐analysis on Overall Planning comprised independent 29 datasets with the following control conditions (see Table 1): counting balls on screens (n = 12); zero‐move problems with identical start and goal states (n = 5; including one study in which additionally appearing yellow rings had to be touched); ball detection and selection of a blinking ball while constantly tapping the screen (n = 1); one‐move problems (n = 2); problem displayed but subjects were requested not to solve it (n = 1); examiner showed the solution and participants had to simply copy moves (n = 2; including one study where in an additional control condition empty SoC pockets were presented); counting vowels of words written on the ToL balls (n = 1); scrambled, unrecognizable picture of the ToL (n = 1); an empty screen or a fixation cross (n = 4).
For the meta‐analysis on Planning Complexity, independent 14 datasets were identified (see Table 1). In 10 of these, problem difficulty was modeled as a parametric modulation in terms of the minimum number of moves to solution. In the remaining four datasets, ToL problems were divided into easy versus difficult problems so as to run comparisons for these two types of problems.
ALE Approach
ALE is a method to perform quantitative meta‐analysis on functional brain imaging results based on all local activation maxima (resp. foci) [Eickhoff et al., 2009]. The algorithm treats the local maxima not as individual points in space but as spatial probability distributions, so that an activation probability can be computed for every voxel. To create a null distribution for statistical evaluations, random foci maps are generated based on the same amount of foci as inserted into the meta‐analysis. Subsequently, the probability map resulting from the meta‐analysis is compared with the random map so as to calculate q values of the false discovery rate (FDR) [Eickhoff et al., 2009].
Present analyses were performed with GingerALE 2.3.2 (http://www.brainmap.org) using the approach of Turkeltaub et al. [2012] to minimize within‐experiment and within‐group effects on the overall estimation. Coordinate‐based ALE meta‐analyses were conducted in Montreal Neurological Institute (MNI) stereotactic standard space. Coordinates of brain activation foci reported in Talairach standard space [Talairach and Tournoux, 1988] were converted into MNI space using the icbm2tal_spm algorithm [Lancaster et al., 2007] implemented in GingerALE 2.3.2. All activation foci reported in the included studies were double‐checked for plausibility and congruence with the provided anatomical labels. Observed divergences between signs of x‐coordinates and reported hemisphere were corrected by reversing the sign before entering the coordinates in the ALE meta‐analyses. This correction concerned 13 foci from three studies.3
GingerALE automatically determines full‐width‐at‐half‐maximum (FWHM) values, so that no additional FWHM filter was applied here, which conforms to the suggested default analysis procedure (http://www.brainmap.org). For masking outliers, the less conservative approach implemented in GingerALE 2.3.2 was selected. ALE analyses were computed with a FDR of P < 0.01 as cluster‐forming threshold and P < 0.05 for cluster‐level inference as recommended in the manual.
RESULTS
The Functional Anatomy of Planning Processes
Meta‐analysis of neural activation patterns for overall planning
Of the 31 published neuroimaging papers on the ToL suitable for ALE meta‐analysis, 29 independent datasets from 28 studies were included in the analysis on Overall Planning, comprising 537 normal subjects (48.8% male) with a mean age (± standard deviation, SD) of 32.4 ± 13.1 years. Adjusted for sample size, the weighted mean age was 35.0 years. The analysis was based on a total of 391 activation foci (Fig. 3A), while another 20 foci (5.1%) were disregarded due to a location outside of the applied ALE brain mask (see above).
As illustrated in Figure 4A, results of the ALE meta‐analysis on Overall Planning activation with a cluster level threshold of P = 0.05 and a FDR‐threshold of q < 0.01 revealed bilateral contributions of mid‐dlPFC, which were however more pronounced for its right ([41 36 30], 3,776 mm3) than for its left homolog ([−41 32 31], 2,736 mm3). Results further yielded bilateral involvement of the frontal eye fields (FEFs), caudate, anterior insula, inferior parietal lobule (IPL), supplementary motor area (SMA), precuneus, and rostrolateral prefrontal cortex (rlPFC). Lateralized activation was found for right inferior occipital gyrus, right inferior temporal gyrus, and left posterior cingulate. A detailed overview on the contributions of the individual studies underlying these results is provided in Table 2.
Figure 4.
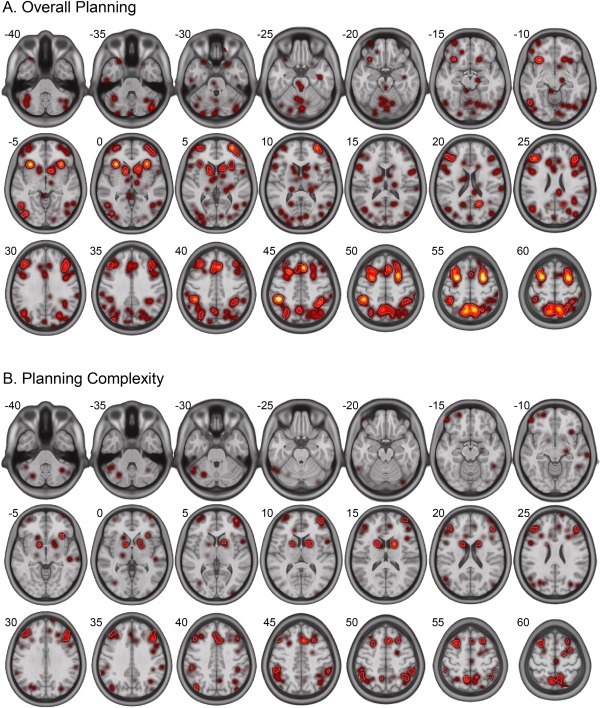
Spatial distribution of resulting activation patterns for the ALE meta‐analyses on (A) Overall Planning and (B) Planning Complexity. White contours reflect the applied FDR threshold of P < 0.01. Numbers besides the axial slices refer to z‐coordinates in MNI‐space.
Table 2.
Overview of significant ALE clusters for overall planning and planning complexity
| ALE meta‐analysis area | Lateralization | Cluster extent | Weighted centroid | Number of foci | Dataset (Reference #) | |||
|---|---|---|---|---|---|---|---|---|
| (mm3) | x | y | z | |||||
| Overall Planning | dlPFC | Left | 2736 | −40.6 | 31.8 | 30.6 | 10 | 1.1, 2, 7, 9, 13, 14, 19.1, 20, 24, 27 |
| Right | 3776 | 41.4 | 35.5 | 29.9 | 13 | 1.1, 7, 8, 9, 13, 17, 20, 23, 24, 26, 27 | ||
| FEF | Left | 7552 | −26.2 | 8.7 | 57.1 | 26 | 1.1, 2, 3, 6, 8, 9, 10, 13, 14, 15, 16, 17, 20, 21, 22.1, 24, 25, 26, 27, 28.1, 29 | |
| Right | 7160 | 29.3 | 11.3 | 55.2 | 21 | 1.1, 3, 6, 8, 9, 10, 11, 13, 14, 15, 19.1, 20, 21, 23, 24, 25, 26, 28.1, 29 | ||
| SMA/pre‐SMA | Bilateral | 3984 | −1.9 | 25.8 | 43.9 | 13 | 1.1, 3, 7, 8, 10, 13, 14, 19.1, 20, 24, 25, 26, 29 | |
| IPL | ||||||||
| Left | 2200 | −38.2 | −45.6 | 44.9 | 7 | 2, 7, 10, 15, 19.1, 20 | ||
| Right | 4376 | 46.3 | −39.8 | 47.2 | 15 | 3, 4, 6, 7, 8, 13, 15, 18, 19.1, 20, 24, 26, 27, 29 | ||
| Precuneus | Bilateral | 13664 | 0.0 | −61.5 | 57.3 | 43 | 1.1, 2, 6, 7, 8, 9, 10, 11, 13, 14, 15, 17, 18, 1.9, 20, 21, 23, 25, 26, 27, 28.1, 28.1, 29 | |
| Right | 1512 | 32.9 | −72.1 | 42.5 | 8 | 1.1, 6, 9, 19.1, 21, 26 | ||
| Caudate | Left | 1776 | −12.2 | 14.3 | 1.0 | 7 | 4, 7, 9, 13, 26, 27, 28.1 | |
| Right | 1232 | 13.9 | 10.1 | 2.6 | 5 | 2, 7, 9, 26, 27 | ||
| Anterior insula | Left | 2000 | −31.0 | 23.8 | −0.9 | 7 | 6, 13, 14, 20, 24, 25, 27 | |
| Right | 2784 | 33.1 | 24.3 | −4.2 | 10 | 1.1, 13, 14, 20, 21, 24, 25, 26, 27, 29 | ||
| rlPFC | Left | 2600 | −35.3 | 55.8 | 5.6 | 9 | 8, 9, 13, 14, 20, 25, 28.1, 28.1, 29 | |
| Right | 648 | 33.7 | 57.6 | 3.8 | 4 | 9, 11, 23, 25 | ||
| Posterior cingulate | Left | 792 | −17.1 | −58.2 | 21.5 | 3 | 8, 11, 28.1 | |
| Inferior Occipital Gyrus | Right | 656 | 43.3 | −79.2 | −4.1 | 3 | 19.1, 24 | |
| Inferior Temporal Gyrus | Right | 624 | 52.1 | −62.1 | −5.7 | 2 | 8, 23 | |
| Planning Complexity | dlPFC | Left | 2832 | −43.2 | 32.8 | 30.9 | 8 | 6, 7, 9, 1.2, 20, 26, 27 |
| Right | 1808 | 42.2 | 38.7 | 27.3 | 6 | 5, 6, 7, 9, 26 | ||
| 512 | 46.0 | 26.1 | 42.8 | 2 | 18, 27 | |||
| FEF | Left | 1728 | −24.8 | −0.9 | 65.2 | 5 | 9, 13, 26, 27 | |
| 1176 | −25.0 | 22.5 | 53.3 | 5 | 3, 6, 18, 26 | |||
| Right | 1576 | 29.6 | 17.5 | 55.9 | 5 | 3, 5, 6, 26, 27 | ||
| 400 | 22.8 | −.5 | 66.0 | 2 | 9, 13 | |||
| SMA/pre‐SMA | Bilateral | 2504 | −3.5 | 24.5 | 45.6 | 7 | 3, 5, 7, 13, 22.1, 26, 27 | |
| IPL | Left | 808 | −53.8 | −40.7 | 48.8 | 3 | 7, 26, 27 | |
| 728 | −40.5 | −51.9 | 51.0 | 3 | 5, 9, 27 | |||
| Right | 1792 | 51.2 | −42.8 | 46.7 | 7 | 3, 7, 9, 20, 26, 27 | ||
| Precuneus | Left | 1832 | −11.1 | −57.7 | 60.9 | 4 | 5, 7, 13, 26 | |
| Right | 1848 | 8.3 | −59.3 | 58.4 | 5 | 7, 13, 26, 27 | ||
| Right | 504 | 42.4 | −74.8 | 39.3 | 2 | 13, 26 | ||
| Caudate | Left | 2432 | −16.4 | 7.5 | 11.1 | 8 | 6, 7, 9, 13, 27 | |
| Right | 1184 | 18.3 | 6.8 | 15.6 | 3 | 7, 13, 26 | ||
| 520 | 14.1 | 5.2 | −2.1 | 2 | 26, 27 | |||
| Anterior insula | Left | 504 | −32.9 | 23.1 | −4.5 | 2 | 26, 27 | |
| rlPFC | Left | 1176 | −40.0 | 55.5 | 10.4 | 5 | 9, 1.2, 26, 27 | |
Note. Results are reported at a threshold of FDR P < 0.01 and a cluster extent of 50 mm3. The number of foci contains the number of individual activation foci that contributed to the respective cluster. Dataset (Reference #) refers to the respective column in Table 1. Differences between the number of foci and the number of listed datasets are due to multiple foci of single studies within the same brain area. dlPFC, dorsolateral prefrontal cortex; FEF, frontal eye fields; SMA, supplementary motor area; IPL, inferior parietal lobule; rlPFC, rostrolateral prefrontal cortex.
Meta‐analysis of neural activation patterns for planning complexity
The ALE meta‐analysis on Planning Complexity was conducted on 14 datasets of 13 studies that comprised 182 normal subjects (63.2% male) with a mean age (± SD) of 32.9 ± 12.2 years and a weighted mean age of 29.8 years. The analysis was based on a total of 154 activation foci (Fig. 3B), while another seven foci (4.5%) were disregarded given a location outside the applied ALE brain mask (see above). Results for the cluster level threshold of P = 0.05 and the FDR‐threshold with q < 0.01 yielded a bilateral lateralization for mid‐dlPFC with a more pronounced activation extent in left ([‐43 33 31], 2,832 mm3) compared to right mid‐dlPFC ([42 39 27], 1,808 mm3) (Fig. 4B). In addition, bilateral involvement was found for the FEFs, precuneus, SMA, IPL, and caudate as well as a left lateralization for the rlPFC and anterior insula. Detailed results as well as the individual contributing studies are listed in Table 2.
The Anatomical Localization of Planning Processes in the dlPFC
The localization of planning processes within left and right dlPFC and, in particular, the empirical validation of their putative assignment to the mid‐dlPFC [Unterrainer and Owen, 2006] as a circumscribed cytoarchitectural structure in terms of BA 46 (and 9/46) [cf., Rajkowska and Goldman‐Rakic, 1995a, 1995b] were main objectives of the present meta‐analysis. However, as of yet, probabilistic maps for BA 46 are not available at the voxel level of a stereotactic standard space. We therefore projected the spatial distribution of BA 46 estimated from the five post‐mortem brains investigated by Rajkowska and Goldman‐Rakic [1995b] onto the renderings of the mid‐dlPFC activation for Planning Overall and Planning Complexity (Fig. 5). The resulting overlap indicates that planning is indeed specifically associated with activation of a spatially circumscribed part in the center of the middle frontal gyrus presumably corresponding to BA 46. Furthermore, the overlay with the cytoarchitectural projections of BA 46 suggests that the activation in mid‐dlPFC for overall planning and particularly for planning complexity slightly extends in the caudal direction presumably corresponding to BA 9/46 [cf., Petrides, 2005; Petrides and Pandya, 1999].
Figure 5.
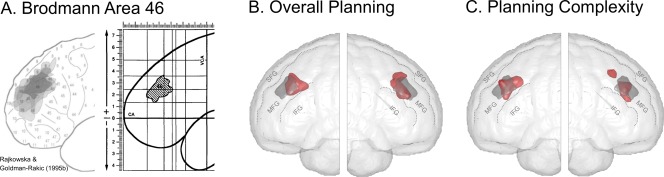
(A) BA 46 distribution maps in five human post‐mortem brain dissections (left) and their resulting overlay in the Talairach and Tournoux [1988] space. Reproduced and adapted from Rajkowska and Goldman‐Rakic, Cereb Cortex, 1995b, 5, 323–337, reproduced by permission (B + C) Overlay of BA 46 as described by Rajkowska and Goldman‐Rakic [1995a, 1995b] (gray) and the activation cluster in dlPFC (red) resulting from the ALE meta‐analyses for (B) Overall Planning and (C) Planning Complexity (rendered at a FDR‐correction of P < 0.01). IFG, inferior frontal gyrus; MFG, middle frontal gyrus; SFG, superior frontal gyrus.
The Hemispheric Lateralization of Planning Processes in the PFC and beyond
ALE contrast analysis
The above reported lateralization of mid‐dlPFC activation during planning was so far assessed only at a descriptive level and revealed larger activation extents for left and right mid‐dlPFC for analyses of Overall Planning and Planning Complexity, respectively (see The Functional Anatomy of Planning Processes section and The Anatomical Localization of Planning Processes in the dlPFC section).
To statistically contrast left and right mid‐dlPFC activations at the voxel level, we further transformed the respective foci into a common space. To this end, we used the mid‐dlPFC activations centers (or weighted centroids) derived from the Overall Planning meta‐analysis. To determine the activation extents, inspection of the distance histograms showed that activation foci from individual studies clustered within a range of 20 mm from the weighted centroids. In consequence, all activation foci within this distance were included in the ALE contrast analysis and transformed into a common space by subtracting the weighted centroids. In total, 13 coordinates from the left and 15 from the right hemisphere were included.
Performing an ALE contrast meta‐analysis revealed no lateralization effects for left versus right mid‐dlPFC on the voxel level. A control analysis in which the coordinates from the right hemisphere were mirrored (in x‐direction) with respect to the right centroid (so as to account for the brain surface's convexity) did also not yield any lateralization effects. Note however that both analyses were highly unlikely to have sufficient statistical power to detect (subtle) differences given the relatively small number of analyzable studies and activation foci.
Exploration of systematic differences between studies
To assess whether systematic methodological differences may account for lateralization differences between studies, all studies included in the ALE meta‐analyses for Overall Planning 4 were divided into four groups according to the presence of planning‐related activations in left mid‐dlPFC (yes/no) and right mid‐dlPFC (yes/no) thus resulting in a 2 × 2 contingency table (cf., Table 2). This yielded 16 studies without any activation peaks in the mid‐dlPFC, another three studies with exclusively left and three studies with exclusively right mid‐dlPFC activation peaks, and seven studies with bilateral mid‐dlPFC activation peaks. Analyses of potential between‐study differences concerned the subjects' age and sex ratio and the average and maximum of the applied problem's minimum moves which were entered as dependent variables in separate ANOVAs with subdivisions of studies according to left and right dlPFC activation patterns as between‐study factor. However, no significant between‐study differences could be observed for any of these variables (for detailed results, see Supporting Information Table S1). But given that two of the four groups only comprised three studies the negative findings may be due to insufficient power.
Further explorative analyses concerned study characteristics such as study design (block vs. event‐related), imaging technique (fMRI vs. PET), modeling approach (categorical vs. parametric), solution execution (moves executed vs. moves not executed), and ToL version (original ToL version vs. SOC) which were nominally coded and hence entered together with the 2 × 2 study subdivision (see above) into analyses of multi‐dimensional contingency tables. Notably, these analyses suffered heavily from zero‐frequency cells and most cells with a frequency lower than five which may result in severe distortions. However, the analyses appear to indicate some important differences: In studies applying the original ToL version, the occurrence of right dlPFC activation was balanced (irrespective of left dlPFC activation), whereas none of the studies applying the SOC found activation in right dlPFC (see Supporting Information Table S2). Moreover, the study design (block vs. event‐related) also appeared to affect the probability of right‐lateralized activation (see Supporting Information Table S4). In studies with event‐related designs activation of right dlPFC was slightly more frequent, whereas in studies with block designs the number of studies without reporting activation of right dlPFC was four times higher than that of studies reporting activation in right dlPFC. However, these effects of ToL version and study design were not independent (χ21 = 5.882, P = 0.015, see also Supporting Information Table S8), as studies applying the SOC more often used block design and studies applying the original ToL more often used event‐related designs. Neither the comparison of imaging techniques nor modeling approach nor solution execution yielded any significant differences (see Supporting Information Tables S2).
Whole‐brain lateralization analyses
To examine lateralization effects beyond the (mid‐)dlPFC, two additional whole‐brain ALE contrast analyses were performed separately across all foci from Overall Planning and from Planning Complexity. For Overall Planning, two areas were found to have stronger activation on the right hemisphere than on the left hemisphere, namely the IPL and premotor cortex (see Supporting Information S2 for detailed results). For Planning Complexity, a stronger activation was observed in the left compared to the right SMA.
DISCUSSION
The present ALE meta‐analyses on 29 neuroimaging datasets using the ToL task yielded two major findings on the specific involvement of the dlPFC in human planning. First of all, the results corroborated that planning‐related activation in dlPFC is localized in circumscribed neural assemblies in the mid‐dorsolateral part of the PFC [cf., Unterrainer and Owen, 2006], presumably corresponding to BA 46 [Petrides, 2005; Petrides and Pandya, 1999; Rajkowska and Goldman‐Rakic, 1995a, 1995b] and possibly to caudally adjacent parts of BA 9/46 [Petrides, 2005; Petrides and Pandya, 1999]. Second, results clearly emphasized the importance of bilateral mid‐dlPFC for planning in tasks like the ToL.
The Localization of Planning Processes within the Mid‐dlPFC
The present study revealed the involvement of the bilateral dlPFC in well‐structured planning. Moreover, instead of the extended area of the dlPFC that is referred to in most ToL studies, mainly comprising BA 9, 9/46, and 46 [cf., Petrides, 2005, 2013; Petrides and Pandya, 1999], but sometimes also parts of BA 6, 8, and 10, the middle part of the dlPFC (mid‐dlPFC) emerged as the essential sub‐region for planning in the present meta‐analysis. Importantly, the present meta‐analyses particularly emphasize the functional distinction between BA 9 and BA 46 (see Fig. 5) in that overlapping the present results with the human cytoarchitectonic maps from Rajkowska and Goldman‐Rakic [1995b] suggests planning‐related brain activity in mid‐dlPFC to be confined to BA 46 (Fig. 5) and, if following the nomenclature by Petrides [2005, 2013], extending slightly into caudally adjacent parts of BA 9/46.
Cytoarchitectonically, the mid‐dlPFC is a brain area with a relatively large layer thickness and, on average, with a high neuronal packing density [Rajkowska and Goldman‐Rakic, 1995a]. The layers of BA 46 and BA9/46 are characterized by a well‐developed internal granular layer (IV) [Fuster, 2015; Petrides, 2005, Petrides et al., 2012] and, for BA 46, an external pyramidal layer (III) with only small to medium pyramidal neurons [Petrides, 2005; Petrides and Pandya, 1994, 1999; Petrides et al., 2012]. By contrast, BA 9 has a poorly developed layer IV with lower neuronal density than BA 46 and 9/46 and comprises large pyramidal neurons in layer III [Petrides, 2005; Petrides et al., 2012; Petrides and Pandya, 1999]. In terms of connectivity based on tracer studies in monkeys [e.g., Petrides and Pandya, 1999, 2002; Schmahmann and Pandya, 2006; for a review, see Yeterian et al., 2012], the three areas share a common basic pattern in that they are connected with each other and with other prefrontal and frontal areas (mainly BA 6, 8, 10, 45, and 47), multimodal temporal areas, the anterior and posterior cingulate, and receive afferents from the retrosplenial cortex. Critically although, BA 46 and 9/46, but not BA 9, are reciprocally connected to medial as well as inferior and superior lateral parietal areas and send efferents to the retrosplenial cortex [Petrides, 2005; Petrides et al., 2012; Petrides and Pandya, 1999, 2006]. Thus, BA46 and 9/46 are distinguishable from BA 9 by their strong connection to heteromodal parietal areas that were also found here to form part of the planning network (cf. Fig. 4, Table 2), such as the IPL and precuneus (see below), as well as a reciprocal connection to the retrosplenial cortex. Given that Brodmann's [1908] and Walker's [1940] descriptions of area 46 in humans and macaque monkeys, respectively, suggest a very similar cytoarchitecture [Petrides, 2005], it is likely that not only the functional properties of BA 46 and BA 9/46 but also their structural connections share a substantial amount of commonalities in both species and give hence rise to comparable cognitive functions [cf., Petrides, 2005, Petrides and Pandya, 1999]. Thus, considering this connectivity pattern, it has been argued that BA 6 and 8 subserve the simple maintenance of visuo‐spatial working memory contents, whereas the higher‐order monitoring and manipulation of these representations is subserved by BA 46 and 9/46, that is, the mid‐dorsolateral PFC [Petrides and Pandya, 1994, 1999; Petrides 2005].
In sum, when considering the cytoarchitecture and anatomical connections of the dlPFC, current results can be regarded as reflecting bilateral activity of the mid‐dlPFC, specifically located in BA 46, during human planning, which in turn represents a prototypical example of goal‐directed higher‐order cognitive processes in the visuo‐spatial domain.
The Role of the Left and Right Mid‐dlPFC in Planning
The present meta‐analysis demonstrated the involvement of the bilateral mid‐dlPFC in planning, which runs contrary to the tenet of a strictly lateralized involvement of either left or right mid‐dlPFC. Furthermore, a relative lateralization suggesting a stronger activation of left over right mid‐dlPFC or vice versa could not be found (see The Hemispheric Lateralization of Planning Processes in the PFC and beyond section) but has to be interpreted with caution given the small number of studies underlying this comparison.
Furthermore, as not all studies included in the meta‐analysis have uniformly reported bilateral dlPFC activation, the question remains whether methodological differences between the studies may have driven the differences concerning unilateral and bilateral dlPFC involvement during planning. Explorative analyses indicated two (partially confounded) between‐study differences entailing stronger contributions of left compared to right dlPFC, namely the tower version (SoC vs. original ToL) and the task design (block vs. event‐related presentation and modeling of hemodynamic responses). It could hence be that block designs are generally less effective to detect right dlPFC activation during planning. This interpretation is supported by recent comparisons between stimulus‐locked and response‐locked modeling approaches for fMRI data on the ToL showing that activation in left mid‐dlPFC was elicited particularly early during the planning phase and for a longer period of time, whereas activation in right mid‐dlPFC occurred later in the planning phase and for a shorter period of time [Ruh et al., 2012] (see also below). Activation of right mid‐dlPFC during planning seems hence more likely to be attenuated by coarse block‐type modeling of the hemodynamic response across several problem items. In this regard, one might further question whether the common modeling approach—be it event‐ or block‐related—of convolving a canonical hemodynamic response function across the duration of one or several problem items is appropriate at all for fitting hemodynamic changes during complex cognitive task such as planning. That is, this approach goes along with the implicit assumption of a continuous neural activation during the totally modeled complete course of cognitive processing, which may not equally hold for all brain areas involved and all cognitive processes modeled. That is, modeling approaches using sets of finite impulse response basis functions, which allow for a closer and area‐specific fit to the actual course of the hemodynamic response during longer intervals of cognitive processing, may constitute a promising modeling alternative in future studies [e.g., Kaller et al., 2011a, 2011b, Ruh et al., 2012].
As an alternative interpretation, the effects of tower version (which were however associated with the aforementioned effects of task design), may further indicate structural differences between the ToL versions and/or the applied problem sets [e.g., Kaller et al., 2011a, 2011b and Newman et al., 2009] (see also below for effects of problem structure on neural activation patterns). In this regard, the SoC versions compared to the original ToL versions may have used problems that tap differently into specific planning processes that are performed by the left and right dlPFC. However, these potentional explanations warrant caution, as the two effects of between‐study differences on dlPFC lateralization during planning were not independent and cannot be unambiguously interpreted.
Taken together, present results clearly demonstrate that the left and right mid‐dlPFC are jointly activated both during planning in general and as a function of the complexity of the ToL problems to be solved in particular. However, the finding of a bilateral activation does not preclude that the left and right mid‐dlPFC subserve differential roles during the overall planning process. This raises the question as to what these supposed differential roles might be.
Newman et al. [Newman et al., 2003, 2009; Newman and Pittman, 2007] systematically manipulated structural problem parameters of the ToL. They conclusionded that the left mid‐dlPFC is responsible for control processes as well as the representation and maintenance of general task demands, whereas the right mid‐dlPFC is associated with integration, manipulation, and maintenance of information in working memory that serve to formulate a strategy for solving a given problem [Newman et al., 2009; cf. Newman and Pittman, 2007].
Kaller et al. [2011b] examined the functional lateralization of the mid‐dlPFC using a comparable approach of manipulating structural problem parameters of the ToL. Foundation of the experiment was the concept that the overall planning process dissociates into two separable phases, an early internalization phase (transfer of the start and goal into working memory) and the subsequent core planning phase, where the start is mentally manipulated until its representation matches the goal [cf., Newell and Simon, 1972]. It was found that the internalization phase elicited stronger activation of the left mid‐dlPFC, whereas the core planning phase elicited stronger activation in the right mid‐dlPFC [Kaller et al., 2011b]. Moreover, the data revealed that the left mid‐dlPFC activated earlier during the time course of each trial and that internalization processes occured only initially [Ruh et al., 2012]. In contrast, the right mid‐dlPFC activated especially during the end of the planning phase and the core planning process was found solely here [Ruh et al., 2012].
In a similar focus on the time course of cognitive sub‐processes during planning, Byrd et al. [2011] examined the temporal course of the planning phase which was separated into three parts. In the first part bottom‐up stimuli processing was observed [Byrd et al., 2011]. In the second part, the stimuli were being processed by the (right) dlPFC. During the last part of the planning phase, again a left dlPFC activation was reported.
In interpreting the present findings, it has to be noted that the mid‐dlPFC is found to be involved in a great variety of tasks as a part of the multiple demand (MD) network [Duncan, 2010; Duncan and Owen, 2000]. That is, the very specificity of planning processes that is ascribed to the left and right dlPFC fades in view of their general involvement in complex cognitive tasks. Yet, this study aimed at delineating the neural basis of planning by conducting a meta‐analysis on functional imaging studies using the ToL. Thus, a characterization of the role of the mid‐dlPFC in planning was the focus of this work, not a characterization of the range of cognitive processes requiring (mid‐)dlPFC involvement. Notwithstanding this, a complete understanding of dlPFC functioning would greatly benefit from future meta‐analyses complementing the present one that explicitly investigate the range of cognitive processes drawing on the (mid‐)dlPFC. In this regard, a characterization of the precise cognitive and neural processes underlying human planning could then be conceptualized as specific examples of more general, domain‐independent cognitive processes subserved by the dlPFC in giving rise to higher‐order human cognition.
It has also to be noted that well‐structured tasks like the ToL exert different demands on planning ahead than problems in real‐life situations [e.g., Burgess, 2000; Goel, 2010]. Hence, the here established significance of bilateral mid‐dlPFC for planning in the ToL does not necessarily extrapolate to planning ahead solutions to real‐world problems.
Finally, the preceding sections emphasized the crucial role of the mid‐dlPFC for planning. However, not all included studies found activation that contributed to the mid‐dlPFC clusters, which seems to argue against this critical role of the mid‐dlPFC. Possible reasons for this discrepancy are discussed in the following. In the present meta‐analysis, the clusters with the largest effect sizes and the highest number of contributing activation foci (cf., Table 2) were found in the FEFs (and the precuneus). For ALE analyses, the peak voxel of a given significant cluster is taken as representation of the underlying activation pattern. However, if activations of multiple functionally distinct regions form one large cluster, then the peak of activation does not necessarily constitute an adequate representation of all areas within that large cluster. Given that the FEFs and the dlPFC are located relatively close to each other, the dlPFC―although significantly activated at the voxel level―might not have contributed an activation focus to the meta‐analysis, as the activation peak of its cluster lay in the FEFs. Indeed, connected activation clusters of the FEFs and dlPFC can be found in some of the studies that did not contribute a dlPFC activation focus [e.g., Campbell et al., 2009; Dagher et al., 1999; van't Ent et al., 2014]. Another reason might be the issue of varying significance thresholds, which are chosen rather arbitrarily and differ between studies [Lieberman and Cunningham, 2009]. To be specific, in several of the studies not contributing to the dlPFC activation of the ALE analyses, dlPFC clusters are nonetheless visible (in illustrations at liberal thresholds) that, however, do not pass the given significance threshold of that particular study [e.g., Boghi et al., 2006; Desco et al., 2011; Lazeron et al., 2000; Liemburg et al., 2015; Rowe et al., 2001; Schall et al., 2003; Wagner et al., 2006]. To appropriately address this widely recognized issue of threshold effects, it was recently suggested that in imaging studies all activation independent of significance should be depicted [Allen et al., 2012]. In sum, owing to these methodological considerations, the fact that not all studies included in the meta‐analyses contributed foci of dlPFC activation (i) does not necessarily imply that the dlPFC was not involved during planning in these studies at all and (ii) does not generally argue against the pivotal role of the dlPFC for planning ability.
The Functional Anatomy of Planning Processes beyond (Mid)‐DLPFC
Concurring with the known anatomy and connectivity of BA46 and BA 9/46, the present ALE meta‐analyses revealed a planning‐related network of brain regions comprising prefrontal, premotor, parietal, and insular regions as well as the caudate nucleus. In terms of functional networks, these areas substantially overlap with recent conceptions of fronto‐parietal networks that underlie the executive control of complex behavior. For instance, the dlPFC and rPFC, IPL, the anterior insula, and the caudate nucleus can be assigned to a circumscribed fronto‐parietal control network which is engaged in a variety of tasks that demand controlled information processing [Vincent et al., 2008]. In line with the network's role in planning performance, these tasks can be summarized as taxing higher‐order, adaptive top‐down control functions [Dosenbach et al., 2008, 2007; Fair et al., 2007]. Moreover, large parts of these fronto‐parietal networks can also be ascribed to the MD network [Duncan, 2010; Duncan and Owen, 2000], namely the inferior frontal sulcus, anterior insula, the pre‐SMA, and rostral PFC. The MD network is observed in a great variety of functional imaging studies and is characterized by its involvement in fluid intelligence and other complex, goal‐directed activities [see Duncan, 2013, for dedicated review].
Remaining areas of the present analysis, namely the FEFs, posterior parietal cortex, intraparietal sulcus, superior parietal lobule and also the pre‐SMA emerge as main parts of the dorsal attention network [Vincent et al., 2008] which is assumed to be involved in spatial attention, eye movements, and hand‐eye coordination and, hence, concurs well with the attentional as well as visuo‐spatial processes induced by performing the ToL.
There have also been attempts to ascribe specific function to the single areas rather than consider them a network. Cognitive processing within rlPFC is assumed to operate on a higher‐order level in that the rlPFC is responsible not for devising or executing moves of the ToL, but rather for selecting, evaluating, and monitoring sequences of moves [Baker et al., 1996; Boghi, et al., 2006; Elliot et al., 1997; Rasmussen et al., 2006; Schöpf et al., 2011; cf. Van den Heuvel et al., 2003; Wagner et al., 2006]. The FEFs are also a located in the frontal lobe and are thought to control eye‐movements and attention [Baker et al., 1996; Boghi, et al., 2006; Dagher et al., 1999; den Braber et al, 2008; Desco et al, 2011]. The pre‐SMA is assumed to also manage attention during planning [Baker et al, 1996; Boghi, et al., 2006].
The present analysis further confirmed the contribution of parietal areas. The superior parietal lobule and the precuneus are relatively uniformly associated with visuo‐spatial processing and attentional representation, that is, with the visuo‐spatial working memory component of planning [Baker et al., 1996; Beauchamp et al., 2003; Boghi et al., 2006; de Ruiter et al., 2011; den Braber et al., 2008; Desco et al., 2011; Rasmussen et al., 2006; Wagner et al., 2006].
The insula and the nucleus caudate are both mostly neglected in considerations of individual area. For the insula, the automatic sequencing of moves [Baker et al., 1996] but also the salience processing and shifting between the default mode and the dorsal attention network [Schöpf et al., 2011] were proposed. The caudate forms part of fronto‐striatal processing loops subserving executive functions [Alexander, 1986] and was specifically ascribed to be involved in the selection of appropriate responses and their monitoring during performance of the ToL [Dagher et al., 1999].
CONCLUSION
The present quantitative meta‐analytic evidence empirically substantiates the common notion of the crucial contribution of the dlPFC to planning in the ToL and further pinpoints it to be specifically focused on its mid‐dorsolateral part. Furthermore, instead of a unilateral involvement, results highlight the bilateral contribution of left and right mid‐dlPFC to planning on the ToL. In this regard, findings from previous studies converge on suggesting that the differential involvement of the left and right mid‐dlPFC may be invoked by specific sub‐processes of planning. However, the present methodological approach does not allow addressing this assumed process‐dependent relative lateralization. Therefore, future studies have to explicitly investigate and identify these sub‐processes so as to disentangle the differential roles of the left and right mid‐dlPFC in human planning and thereby contribute to a comprehensive understanding of the general role of the PFC in complex cognitive functions. Apart from the mid‐dlPFC, a range of further frontal (e.g., rostrolateral PFC, FEF, SMA), parietal (e.g., IPL, precuneus), and opercular regions (anterior insula) are significantly involved in planning performance. In this respect, extending the focus of the present meta‐analyses on mid‐dlPFC toward its interaction with these other planning‐associated brain areas may constitute a promising avenue for further research on the neural basis of complex cognition.
Supporting information
Supporting Information
ACKNOWLEDGMENT
None of the authors report any conflict of interest.
Footnotes
Note in this respect that Elliot et al. [1997] reported a reanalysis of the data of Baker et al. [1996], whereas Owen et al. [1998] reported a reanalysis of the data of Owen et al. [1996]. However, as the analyses on Overall Planning in the original studies were extended in both follow‐up studies by analyses of Planning Complexity, this additional information was included in the present meta‐analysis. Schall et al., [2003] and van't Ent et al. [2014] reported two independent samples each. Den Braber et al. [2008] examined twins where one twin suffered obsessive‐compulsive symptoms. Only the reported activations of the healthy twins were included in the present ALE analyses. De Ruiter et al. [2009] examined problem gamblers, smokers, and healthy controls. They reported only the activation foci across all groups, as they did not find interactions between groups and experimental conditions. Therefore, the reported foci of the main effect of planning versus baseline were included; however, solely the sample size and description of the healthy controls were used.
Studies with versus without solution execution (cf., Table 1) sum up to 32, as one study applied both response modes in two different runs [Rowe et al. 2001] (reported coordinates refer to a collapsed analysis across runs).
The x‐coordinates of 10 activation foci from Lazeron et al. [2000], one activation focus from Cohen et al. [2014], and two foci from Schoepf et al. [2011] were accordingly corrected.
Note that between‐study analyses on Planning Complexity were not carried out due to the limited number of data sets.
Contributor Information
Kai Nitschke, Email: kai.nitschke@uniklinik-freiburg.de.
Christoph P. Kaller, Email: christoph.kaller@uniklinik-freiburg.de.
REFERENCES
- Allen EA, Erhardt EB, Calhoun VD (2012): Data visualization in the neurosciences: Overcoming the curse of dimensionality. Neuron 74:603–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson VA, Anderson P, Northam E, Jacobs R, Mikiewicz O (2002): Relationships between cognitive and behavioral measures of executive function in children with brain disease. Child Neuropsychol 8:231–240. [DOI] [PubMed] [Google Scholar]
- Andrés P, Van der Linden M (2001): Supervisory attentional system in patients with focal frontal lesions. J Clin Exp Neuropsychol 23:225–239. [DOI] [PubMed] [Google Scholar]
- Andrews G, Halford GS, Chappell M, Maujean A, Shum DHK (2014): Planning following stroke: A relational complexity approach using the Tower of London. Front Hum Neurosci 8(December):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE (1986): Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9:357–381. [DOI] [PubMed] [Google Scholar]
- Baker SC, Rogers RD, Owen AM, Frith CD, Dolan RJ, Frackowiak RS, Robbins TW (1996): Neural systems engaged by planning: A PET study of the Tower of London task. Neuropsychologia 34:515–526. [DOI] [PubMed] [Google Scholar]
- Beauchamp MH, Dagher A, Aston JA, Doyon J (2003): Dynamic functional changes associated with cognitive skill learning of an adapted version of the Tower of London task. NeuroImage 20:1649–1660. [DOI] [PubMed] [Google Scholar]
- Boghi A, Rasetti R, Avidano F, Manzone C, Orsi L, D'Agata F, Caroppo P, Bergui M, Rocca P, Pulvirenti L, Bradac GB, Bogetto F, Mutani R, Mortara P (2006): The effect of gender on planning: An fMRI study using the Tower of London task. NeuroImage 33:999–1010. [DOI] [PubMed] [Google Scholar]
- Brodmann K (1908): Beiträge zur histologischen Lokalisation der Grosshirnrinde. VI. Mitteilung: Die Cortexgliederung des Menschen. J Psychol Neurol 10:231–246. [Google Scholar]
- Burgess PW (2000): Strategy application disorder: The role of the frontal lobes in human multitasking. Psychol Res 63:279–288. [DOI] [PubMed] [Google Scholar]
- Byrd DL, Case KH, Berg WK (2011): Planning: Fixed‐foreperiod event‐related potentials during the Tower of London task. Neuropsychologia 49:1024–1032. [DOI] [PubMed] [Google Scholar]
- Campbell Z, Zakzanis KK, Jovanovski D, Joordens S, Mraz R, Graham SJ (2009): Utilizing virtual reality to improve the ecological validity of clinical neuropsychology: An fMRI case study elucidating the neural basis of planning by comparing the Tower of London with a three‐dimensional navigation task. Appl Neuropsychol 16:295–306. [DOI] [PubMed] [Google Scholar]
- Carlin D, Bonerba J, Phipps M, Alexander G, Shapiro M, Grafman J (2000): Planning impairments in frontal lobe dementia and frontal lobe lesion patients. Neuropsychologia 38:655–665. [DOI] [PubMed] [Google Scholar]
- Cazalis F, Valabregue R, Pelegrini‐Issac M, Asloun S, Robbins TW, Granon S (2003): Individual differences in prefrontal cortical activation on the Tower of London planning task: Implication for effortful processing. Eur J Neurosci 17:2219–2225. [DOI] [PubMed] [Google Scholar]
- Cockburn J (1995): Performance on the Tower of London test after severe head injury. J Int Neuropsychol Soc 1:537–544. [DOI] [PubMed] [Google Scholar]
- Cohen M, Johnston P, Ehlkes T, Fulham R, Ward P, Thienel R, Rassera P, Carrd V, Bakera A, Schall U (2014): Functional magnetic resonance brain imaging of executive cognitive performance in young first‐episode schizophrenia patients and age‐matched long‐term cannabis users. Neurol Psychiatry Brain Res 21:51–63. [Google Scholar]
- Dagher A, Owen AM, Boecker H, Brooks DJ (1999): Mapping the network for planning: A correlational PET activation study with the Tower of London task. Brain 122:1973–1987. [DOI] [PubMed] [Google Scholar]
- de Ruiter MB, Reneman L, Boogerd W, Veltman DJ, van Dam FSAM, Nederveen AJ, Boven E, Schagen SB (2011): Cerebral hyporesponsiveness and cognitive impairment 10 years after chemotherapy for breast cancer. Hum Brain Mapp 32:1206–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruiter MB, Veltman DJ, Goudriaan AE, Oosterlaan J, Sjoerds Z, van den Brink W (2009): Response perseveration and ventral prefrontal sensitivity to reward and punishment in male problem gamblers and smokers. Neuropsychopharmacology 34:1027–1038. [DOI] [PubMed] [Google Scholar]
- den Braber A, , van ‘t Ent D , Blokland GAM, van Grootheest DS, Cath DC, Veltman DJ, de Ruiter MB, Boomsma DI (2008): An fMRI study in monozygotic twins discordant for obsessive‐compulsive symptoms. Biol Psychol 79:91–102. [DOI] [PubMed] [Google Scholar]
- Desco M, Navas‐Sanchez FJ, Sanchez‐Gonzalez J, Reig S, Robles O, Franco C, Guzmán‐De‐Villoria JA, García‐Barreno P, Arango C (2011): Mathematically gifted adolescents use more extensive and more bilateral areas of the fronto‐parietal network than controls during executive functioning and fluid reasoning tasks. NeuroImage 57:281–292. [DOI] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Cohen AL, Schlaggar BL, Petersen SE (2008): A dual‐networks architecture of top‐down control. Trends Cogn Sci 12:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE (2007): Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA 104:11073–11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J (2010): The multiple‐demand (MD) system of the primate brain: Mental programs for intelligent behaviour. Trends Cogn Sci 14:172–179. [DOI] [PubMed] [Google Scholar]
- Duncan J (2013): The structure of cognition: Attentional episodes in mind and brain. Neuron 80:35–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J, Owen AM (2000): Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci 23:475–483. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Amunts K, Mohlberg H, Zilles K (2006): The human parietal operculum. II. Stereotaxic maps and correlation with functional imaging results. Cereb Cortex 16:268–279. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT (2009): Coordinate‐based activation likelihood estimation meta‐analysis of neuroimaging data: A random‐effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp 30:2907–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Baker SC, Rogers RD, O'Leary DA (1997): Prefrontal dysfunction in depressed patients performing a complex planning task: A study using positron emission tomography. Psychol Med 27:931–942. [DOI] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NUF, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL (2007): Development of distinct control networks through segregation and integration. Proc Natl Acad Sci USA 104:13507–13512. 17679691 [Google Scholar]
- Fallon SJ, Hampshire A, Williams‐Gray CH, Barker RA, Owen AM (2013): Putative cortical dopamine levels affect cortical recruitment during planning. Neuropsychologia 51:2194–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell MJ, Laird AR, Egan GF (2005): Brain activity associated with painfully hot stimuli applied to the upper limb: A meta‐analysis. Hum Brain Mapp 25:129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster, J. M. (2015). The Prefrontal Cortex, 5th ed London: Academic Press. [Google Scholar]
- Goel V (2010): Neural basis of thinking: Laboratory problems versus real‐world problems. Wiley Interdiscip Rev: Cogn Sci 1:613–621. [DOI] [PubMed] [Google Scholar]
- Goethals I, Audenaert K, Jacobs F, van de Wiele C, Pyck H, Ham H, Vandierendonck A, van Heeringen C, Dierckx R (2004): Application of a neuropsychological activation probe with SPECT: The 'Tower of London' task in healthy volunteers. Nuclear Med Commun 25:177–182. [DOI] [PubMed] [Google Scholar]
- Hedges LV, Olkin I (1985): Statistical Methods for Meta‐Analysis. Orlando, FL: Academic Press. [Google Scholar]
- Huyser C, Veltman DJ, Wolters LH, de Haan É, Boer F (2010): Functional magnetic resonance imaging during planning before and after cognitive‐behavioral therapy in pediatric obsessive‐compulsive disorder. J Am Acad Child Adolesc Psychiatry 49:1238–1248. [DOI] [PubMed] [Google Scholar]
- Jacobs R, Anderson V (2002): Planning and problem solving skills following focal frontal brain lesions in childhood: Analysis using the Tower of London. Child Neuropsychol 8:93–106. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ (2007): Functional and anatomical cortical underconnectivity in autism: Evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cereb Cortex 17:951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaller CP, Rahm B, Köstering L, Unterrainer JM (2011a): Reviewing the impact of problem structure on planning: A software tool for analyzing tower tasks. Behav Brain Res 216:1–8. [DOI] [PubMed] [Google Scholar]
- Kaller CP, Rahm B, Spreer J, Weiller C, Unterrainer JM (2011b): Dissociable contributions of left and right dorsolateral prefrontal cortex in planning. Cereb Cortex 21:307–317. [DOI] [PubMed] [Google Scholar]
- Kempton MJ, Ettinger U, Foster R, Williams SCR, Calvert GA, Hampshire A, Zelaya FO, O'Gorman RL, McMorris T, Owen AM, Smith MS (2011): Dehydration affects brain structure and function in healthy adolescents. Hum Brain Mapp 32:71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, Turkeltaub PE, Kochunov P, Fox PT (2005): ALE meta‐analysis: Controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp 25:155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas‐Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT (2007): Bias between MNI and talairach coordinates analyzed using the ICBM‐152 brain template. Hum Brain Mapp 28:1194–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazeron RH, Rombouts S, Machielsen WC, Scheltens P, Witter MP, Uylings HB, Barkhof F (2000): Visualizing brain activation during planning: The Tower of London test adapted for functional MR imaging. Am J Neuroradiol 21:1407–1414. [PMC free article] [PubMed] [Google Scholar]
- Lazeron RH, Rombouts S, Scheltens P, Polman CH, Barkhof F (2004): An fMRI study of planning‐related brain activity in patients with moderately advanced multiple sclerosis. Mult Scler 10:549–555. [DOI] [PubMed] [Google Scholar]
- Levin HS, Mendelsohn D, Lilly MA, Fletcher JM, Culhane KA, Chapman SB, Harward H, Kusnerik L, Bruce D, Eisenberg HM (1994): Tower of London performance in relation to magnetic resonance imaging following closed head injury in children. Neuropsychology 8:171–179. [Google Scholar]
- Lieberman MD, Cunningham WA (2009): Type I and Type II error concerns in fMRI research: Re‐balancing the scale. Soc Cogn Affect Neurosci 4:423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liemburg EJ, Dlabac‐De Lange J. J L a S, Bais L, Knegtering H, van Osch MJP, Renken RJ, Aleman A (2015): Neural correlates of planning performance in patients with schizophrenia—Relationship with apathy. Schizophr Res 161:367–375. [DOI] [PubMed] [Google Scholar]
- Newell A, Simon HA (1972): Human Problem Solving. Englewood Cliffs, NJ: Prentice‐Hall. [Google Scholar]
- Newman SD, Carpenter PA, Varma S, Just MA (2003): Frontal and parietal participation in problem solving in the Tower of London: fMRI and computational modeling of planning and high‐level perception. Neuropsychologia 41:1668–1682. [DOI] [PubMed] [Google Scholar]
- Newman SD, Greco JA, Lee D (2009): An fMRI study of the Tower of London: A look at problem structure differences. Brain Res 1286:123–132. [DOI] [PubMed] [Google Scholar]
- Newman SD, Pittman G (2007): The Tower of London: A study of the effect of problem structure on planning. J Clin Exp Neuropsychol 29:333–342. [DOI] [PubMed] [Google Scholar]
- O'Rourke K (2007): An historical perspective on meta‐analysis: Dealing quantitatively with varying study results. J R Soc Med 100:579–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, Downes JJ, Sahakian BJ, Polkey CE, Robbins TW (1990): Planning and spatial working memory following frontal‐lobe lesions in man. Neuropsychologia 28:1021–1034. [DOI] [PubMed] [Google Scholar]
- Owen AM, Doyon J, Dagher A, Sadikot A, Evans AC (1998): Abnormal basal ganglia outflow in Parkinson's disease identified with PET. Implications for higher cortical functions. Brain 121:949–965. [DOI] [PubMed] [Google Scholar]
- Owen AM, Doyon J, Petrides M, Evans AC (1996): Planning and spatial working memory: A positron emission tomography study in humans. Eur J Neurosci 8:353–364. [DOI] [PubMed] [Google Scholar]
- Owen A, Sahakian B, Semple J, Polkey C, Robbins T (1995): Visuo‐spatial short‐term recognition memory and learning after temporal lobe excisions, frontal lobe excisions or amygdalo‐hippocampectomy in man. Neuropsychologia 33:1–24. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Barnes TRE, Nelson HE, Tanner S, Weatherley L, Owen AM, Robbins TW (1997): Frontal‐striatal cognitive deficits in patients with chronic schizophrenia. Brain 120:1823–1843. [DOI] [PubMed] [Google Scholar]
- Petrides M (2005): Lateral prefrontal cortex: Architectonic and functional organization. Philos Trans R Soc Lond Ser B Biol Sci 360:781–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M (2013): The mid‐dorsolateral prefronto‐parietal network and the epoptic process In: Stuss DT, Knight RT, editors. Principles of Frontal Lobe Function, 2th ed. Oxford: Oxford University Press; pp. 79–89. [Google Scholar]
- Petrides M, Pandya DN (1994): Comparative architectonic analysis of the human and the macaque frontal cortex In: Boller F, Grafman J, editors. Handbook of Neuropsychology, Vol. 9 Amsterdam: Elsevier; pp. 17–58. [Google Scholar]
- Petrides M, Pandya DN (1999): Dorsolateral prefrontal cortex: Comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur J Neurosci 11:1011–1036. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN (2002): Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur J Neurosci 16:291–310. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN (2006): Efferent association pathways originating in the caudal prefrontal cortex in the macaque monkey. J Comp Neurol 498:227–251. [DOI] [PubMed] [Google Scholar]
- Petrides M, Tomaiuolo F, Yeterian EH, Pandya DN (2012): The prefrontal cortex: Comparative architectonic organization in the human and the macaque monkey brains. Cortex 48:46–57. [DOI] [PubMed] [Google Scholar]
- Price CJ, Devlin JT, Moore CJ, Morton C, Laird AR (2005): Meta‐analyses of object naming: Effect of baseline. Hum Brain Mapp 25:70–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Goldman‐Rakic PS (1995a): Cytoarchitectonic definition of prefrontal areas in the normal human cortex: I. Remapping of areas 9 and 46 using quantitative criteria. Cereb Cortex 5:307–322. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Goldman‐Rakic PS (1995b): Cytoarchitectonic definition of prefrontal areas in the normal human cortex: II. Variability in locations of areas 9 and 46 and relationship to the Talairach Coordinate System. Cereb Cortex 5:323–337. [DOI] [PubMed] [Google Scholar]
- Rasmussen IA, Antonsen IK, Berntsen EM, Xu J, Lagopoulos J, Haberg AK (2006): Brain activation measured using functional magnetic resonance imaging during the Tower of London task. Acta Neuropsychiatrica 18:216–225. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath HO (2004): Using human brain lesions to infer function: A relic from a past era in the fMRI age? Nat Rev Neurosci 5:813–819. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Owen AM, Johnsrude IS, Passingham RE (2001): Imaging the mental components of a planning task. Neuropsychologia 39:315–327. [DOI] [PubMed] [Google Scholar]
- Ruh N, Rahm B, Unterrainer JM, Weiller C, Kaller CP (2012): Dissociable stages of problem solving (II): First evidence for process‐contingent temporal order of activation in dorsolateral prefrontal cortex. Brain Cogn 80:170–176. [DOI] [PubMed] [Google Scholar]
- Schall U, Johnston P, Lagopoulos J, Juptner M, Jentzen W, Thienel R, Dittmann‐Balçar A, Bender S, Ward PB (2003): Functional brain maps of Tower of London performance: A positron emission tomography and functional magnetic resonance imaging study. NeuroImage 20:1154–1161. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN (2006): Fiber Pathways of the Brain. Oxford: Oxford University Press. [Google Scholar]
- Schöpf V, Windischberger C, Robinson S, Kasess CH, Fischmeister FP, Lanzenberger R, Albrecht J, Kleemann AM, Kopietz R, Wiesmann M, Moser E (2011): Model‐free fMRI group analysis using FENICA. NeuroImage 55:185–193. [DOI] [PubMed] [Google Scholar]
- Shallice T (1982): Specific impairments of planning. Philos Trans R Soc B Biol Sci 298:199–209. [DOI] [PubMed] [Google Scholar]
- Shallice T, Burgess PW, Schon F, Baxter DM (1989): The origins of utilization behaviour. Brain 112:1587–1598. [DOI] [PubMed] [Google Scholar]
- Shum D, Gill H, Banks M, Maujean A, Griffin J, Ward H (2009): Planning ability following moderate to severe traumatic brain injury: Performance on a 4‐disk version of the Tower of London. Brain Impair 10:320–324. [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL (2010): Default network activity, coupled with the frontoparietal control network, supports goal‐directed cognition. NeuroImage 53:303–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes PRA, Rhodes RA, Grasby PM, Mehta MA (2011): The effects of The COMT val(108/158) met polymorphism on BOLD activation during working memory, planning, and response inhibition: A role for the posterior cingulate cortex?. Neuropsychopharmacology 36:763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JR, Riccio CA, Castillo CL (2009): Concurrent validity of the tower tasks as measures of executive function in adults: A meta‐analysis. Appl Neuropsychol 16:62–75. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988): Co‐Planar Stereotaxic Atlas of the Human Brain. Stuttgart: Thieme. [Google Scholar]
- Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P (2012): Minimizing within‐experiment and within‐group effects in activation likelihood estimation meta‐analyses. Hum Brain Mapp 33:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterrainer JM, Owen AM (2006): Planning and problem solving: From neuropsychology to functional neuroimaging. J Physiol Paris 99:308–317. [DOI] [PubMed] [Google Scholar]
- Van den Heuvel OA, Groenewegen HJ, Barkhof F, Lazeron RH, van Dyck R, Veltman DJ (2003): Frontostriatal system in planning complexity: A parametric functional magnetic resonance version of Tower of London task. NeuroImage 18:367–374. [DOI] [PubMed] [Google Scholar]
- Van den Heuvel OA, van Gorsel HC, Veltman DJ, van der Werf YD (2013): Impairment of executive performance after transcranial magnetic modulation of the left dorsal frontal‐striatal circuit. Hum Brain Mapp 34:347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van't Ent D, den Braber A, Rotgans E, de Geus EJC, de Munck JC (2014): The use of fMRI to detect neural responses to cognitive interference and planning: Evidence for a contribution of task related changes in heart rate? J Neurosci Methods 229:97–107. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL (2008): Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol 100:3328–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AE (1940): A cytoarchitectural study of the prefrontal areas of the macaque monkey. J Comp Neurol 73:59–86. [Google Scholar]
- Wager TD, Jonides J, Reading S (2004): Neuroimaging studies of shifting attention: A meta‐analysis. NeuroImage 22:1679–1693. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE (2003): Neuroimaging studies of working memory: A meta‐analysis. Cogn Affect Behav Neurosci 3:255–274. [DOI] [PubMed] [Google Scholar]
- Wagner G, Koch K, Reichenbach JR, Sauer H, Schloesser RGM (2006): The special involvement of the rostrolateral prefrontal cortex in planning abilities: An event‐related fMRI study with the Tower of London paradigm. Neuropsychologia 44:2337–2347. [DOI] [PubMed] [Google Scholar]
- Ward G, Allport A (1997): Planning and problem solving using the five disc Tower of London task. Q J Exp Psychol 50A 49–78. [Google Scholar]
- Yeterian EH, Pandya DN, Tomaiuolo F, Petrides M (2012): The cortical connectivity of the prefrontal cortex in the monkey brain. Cortex 48:68–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


