Abstract
Background
Abnormalities in dopamine (DA) and brain morphology are observed in several neuropsychiatric disorders. However, it is not fully understood how these abnormalities may relate to one another. For such in vivo findings to be used as biomarkers for neuropsychiatric disease, it must be understood how variability in DA relates to brain structure under healthy conditions. We explored how the availability of striatal DA D2/3 receptors (D2/3R) is related to the volume of subcortical brain structures in a sample of healthy humans. Differences in D2/3R availability measured with an antagonist radiotracer ([11C]‐raclopride) versus an agonist radiotracer ([11C]‐(+)‐PHNO) were examined.
Methods
Data from 62 subjects scanned with [11C]‐raclopride (mean age = 38.98 ± 14.45; 23 female) and 68 subjects scanned with [11C]‐(+)‐PHNO (mean age = 38.54 ± 14.59; 25 female) were used. Subcortical volumes were extracted from T1‐weighted images using the Multiple Automatically Generated Templates (MAGeT‐Brain) algorithm. Partial correlations were used controlling for age, gender, and total brain volume.
Results
For [11C]‐(+)‐PHNO, ventral caudate volumes were positively correlated with BPND in the dorsal caudate and globus pallidus (GP). Ventral striatum (VS) volumes were positively correlated with BPND in the VS. With [11C]‐raclopride, BPND in the VS was negatively correlated with subiculum volume of the hippocampus. Moreover, BPND in the GP was negatively correlated with the volume of the lateral posterior nucleus of the thalamus.
Conclusion
Findings are purely exploratory and presented corrected and uncorrected for multiple comparisons. We hope they will help inform the interpretation of future PET studies where concurrent changes in D2/3R and brain morphology are observed. Hum Brain Mapp 38:5519–5534, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: dopamine, positron emission tomography, D2/3 receptors, raclopride, PHNO, morphology, volume, striatum
INTRODUCTION
Elucidating neurochemical and structural brain changes associated with mental disorders remains a critical challenge for the development of robust “biomarkers” in psychiatry [Perlis, 2011]. However, it remains poorly understood how in vivo differences in neurochemistry relates to variation in brain structure. For example, while it has been demonstrated that persons with schizophrenia have increased endogenous dopamine (DA) levels in the striatum [Abi‐Dargham et al., 2000; Caravaggio et al., 2015b; Kegeles et al., 2010], this has yet to be associated with any of the morphological brain changes observed in this disorder [Ellison‐Wright et al., 2008; Haijma et al., 2013; Rimol et al., 2010; Song et al., 2015; van Erp et al., 2016; Xiao et al., 2015]. Similarly in obesity, while changes in DA D2/3 receptor (D2/3R) availability [Caravaggio et al., 2015c; Dang et al., 2016; Gaiser et al., 2016; Guo et al., 2014; Karlsson et al., 2016; Mawlawi et al., 2001] and brain morphology [Bond et al., 2011; Taki et al., 2008; Veit et al., 2014; Walther et al., 2010] have been observed, these changes have yet to be directly associated with each other [Karlsson, 2016]. Thus, the use of in vivo DA functioning and brain morphology as biomarkers for disease remains hindered insofar as it is not firmly established how they may relate to one another under normal conditions [Strimbu and Tavel, 2010].
While several animal studies have examined the effect of altering DA levels on brain development [Alvarez et al., 2002; Jones et al., 1996; Kalsbeek et al., 1989; Meredith et al., 1995; Pappas et al., 1992; Reinoso et al., 1996], few human studies have examined how in vivo DA functioning, measured with positron emission tomography (PET), relates to brain morphology [Casey et al., 2013; Morales et al., 2015; Werhahn et al., 2006; Woodward et al., 2009]. Using voxel‐based morphology and the antagonist radioligand [18F]‐fallypride, Woodward et al. were the first to examine how variations in brain morphology relates to D2/3R availability in healthy persons [Woodward et al., 2009]. They observed that D2/3R availability within a given brain region was generally positively correlated with the gray matter (GM) density/volume of that region (e.g., within the caudate, thalamus, amygdala, and substantia nigra). This generally suggests that, (i) D2/3R availability may vary positively with the amount of GM, and/or, (ii) differences in D2/3R availability may alter brain structure during development [Woodward et al., 2009]. However, negative correlations [Woodward et al., 2009], and null correlations [Werhahn et al., 2006], have been observed between hippocampal volume and hippocampal D2/3R availability measured with [18F]‐fallypride. Using [18F]‐fallypride, midbrain D2/3R availability was also found to be positively correlated with GM volume in the striatum, prefrontal cortex, insula, hippocampus, and temporal cortex of methamphetamine users, but not healthy controls [Morales et al., 2015]. Finally, using [11C]‐raclopride, it has been demonstrated that striatal DA release in response to amphetamine is negatively correlated with frontal lobe thickness in healthy controls [Casey et al., 2013].
Previous studies have not systematically examined how striatal D2/3R availability is related to subcortical morphology in healthy persons. Moreover, previous studies have only used antagonist radiotracers for D2/3R, that is, [18F]‐fallypride and [11C]‐raclopride. It is generally believed that D2/3R exist in (at least) two conformational states for agonist binding: an active state (D2High) and an inactive state (D2Low) [Seeman, 2011]. In theory, agonist radiotracers—such as [11C]NPA [Narendran et al., 2004], [11C]MNPA [Finnema et al., 2005], and [11C]‐(+)‐PHNO [Willeit et al., 2006]—should preferably bind to “active” D2/3R. Thus, agonist radiotracers may provide a more sensitive and physiologically meaningful estimate of DA release and D2/3R availability, respectively.
[11C]‐(+)‐PHNO is an agonist radiotracer for D2/3R which also has preferential affinity for D3R over D2R [Narendran et al., 2006; Wilson et al., 2005]. This unique property of [11C]‐(+)‐PHNO, ∼20–40 fold selectivity of D3R over D2R [Freedman et al., 1994; Gallezot et al., 2012; Rabiner et al., 2009; Searle et al., 2010; Seeman et al., 1993], results in a differential contribution of D2R and D3R to the [11C]‐(+)‐PHNO signal across different regions of interest (ROIs). The estimated percent of the [11C]‐(+)‐PHNO signal attributed to D3R across ROIs in humans are: substantia nigra (∼100%), globus pallidus (GP, ∼65%), ventral striatum (VS, ∼26%), and dorsal caudate‐putamen (negligible) [Graff‐Guerrero et al., 2010; Searle et al., 2013b; Tziortzi et al., 2011]. Also, as an agonist, baseline [11C]‐(+)‐PHNO binding to D2/3R is more sensitive to competition with endogenous dopamine in vivo in humans [Caravaggio et al., 2014, 2016a; Shotbolt et al., 2012]. Thus, it remains unknown how D2/3R availability measured with an agonist radiotracer, and how D3R availability specifically, may be related to brain morphology.
In the current investigation, we sought to explore how D2/3R availability measured with the antagonist radiotracer [11C]‐raclopride and the agonist radiotracer [11C]‐(+)‐PHNO is related to subcortical brain volume in healthy persons. We specifically examined the volume of (i) striatal subdivisions, (ii) hippocampal subdivisions, (iii) thalamic subdivisions, and (iv) the amygdala. This investigation marks an important first step in elucidating how in vivo differences in dopaminergic functioning may relate to subcortical brain morphology. A better understanding of these relationships will help inform the use of neurochemical and structural brain changes as potential biomarkers for neuropsychiatric disease.
METHODS
Participants
Healthy control data from a previous study were re‐analyzed for the current investigation [Nakajima et al., 2015]. This sample comprises data collected by our laboratory from various PET studies [Caravaggio et al., 2015b; Graff‐Guerrero et al., 2008, 2009; Payer et al., 2014] that were approved by the Research Ethics Board of the Centre for Addiction and Mental Health (CAMH), Toronto. For the current investigation, subjects were included if they provided a T1 structural MRI (1.5T or 3T), and an [11C]‐raclopride or [11C]‐(+)‐PHNO scan. The participants were right‐handed and free of any major medical or psychiatric disorder as determined by clinical interview, the Mini‐International Neuropsychiatric Interview, and electrocardiography. Current or past alcohol abuse was an exclusion criteria. Participants were required to provide a full urine drug screen, and produced a negative urine screen for drugs of abuse and/or pregnancy at inclusion and before the PET scan. This included ethyl alcohol. Before the scan, the aural temperature and blood pressure of the participants were measured to insure they were within normal limits. All participants provided written informed consent and were non‐smokers.
MRI Imaging
Fifty‐one subjects scanned with [11C]‐raclopride and 58 subjects scanned with [11C]‐(+)‐PHNO provided fast spin echo T1‐weighted imaging (fast spoiled gradient echo, TE = 5.3–15 ms, TR = 8.9–12 ms, FOV = 20 cm 3D, 256 × 256, voxel 1.5 mm isotropic, NEX = 1) acquired on a 1.5‐Tesla Sigma‐GE scanner (General Electric Medical Systems, Milwaukee, WI) at Toronto General Hospital (Toronto, Canada). Eleven subjects scanned with [11C]‐raclopride and 11 subjects scanned with [11C]‐(+)‐PHNO underwent MRI fast spin echo T1‐weighted imaging (TR/TE = 30/8 ms, flip angle 45°, field of view 24 cm, 256 × 256 matrix, 124 coronal slices, and slice thickness = 1.0 mm, NEX = 1) acquired on a 3‐Tesla Signa‐GE scanner (GE Discovery MR750 3T; 8‐Channel Head Coil, GE Standard 8HR Brain) at CAMH (Toronto, Canada).
Subcortical Volume Analyses
The Multiple Automatically Generated Templates (MAGeT‐Brain) algorithm [Chakravarty et al., 2013; Pipitone et al., 2014] was used to provide fully‐automated segmentation of striatal subdivisions [Chakravarty et al., 2006], hippocampal subdivisions [Pipitone et al., 2014; Winterburn et al., 2013], thalamic subdivisions [Chakravarty et al., 2006, 2008, 2009], and the amygdala [Treadway et al., 2015]. Typically, in a multi‐atlas segmentation approach, manually drawn labels from atlases are warped (or propagated) into subject space by applying transformations estimated from non‐linear image registration. Candidate labels from all atlas images are fused (via probabilistic segmentation techniques) to create a final segmentation. The goal of the MAGeT‐Brain algorithm is to mitigate sources of error from such approaches including: (1) spurious non‐linear registration or resampling errors (including partial volume effects [PVE] in label resampling), and (2) irreconcilable differences in neuroanatomy between the atlas and target images. The MAGeT‐Brain algorithm is a modified multi‐atlas segmentation technique, which uses a limited number of high‐quality manually segmented atlases as an input to reduce bias and enhance segmentation accuracy. MAGeT‐Brain propagates atlas segmentations to a template library, formed from a subset of target images, via transformations estimated by nonlinear image registration. The resulting segmentations are then propagated to each target image and fused using a label fusion method. Specifically, for those subjects who provided a 1.5T MRI, subsets of subjects scanned with [11C]‐raclopride (n = 21) and [11C]‐(+)‐PHNO (n = 21) were used as template libraries through which the final segmentation was bootstrapped. All 11 subjects who provided a 3T MRI were used as a separate template. Templates were chosen based on representative subject characteristics [Schuetze et al., 2016]. Each subject in the template library was segmented through non‐linear atlas‐to‐template registration followed by label propagation, yielding a unique definition of the subdivisions for each of the templates. The bootstrapping of the final segmentations through the template library results in candidate labels produced for each subject and labels are then fused using a majority vote to complete the segmentation process. Non‐linear registration was performed using a version of the Automatic Normalization Tools (ANTS) registration technique [Avants et al., 2008] that is compatible with the minc toolkit (https://github.com/vfonov/mincANTS). Volumes (mm3) from ROIs were averaged across hemispheres. It is important to note that regional BPND values and regional volume values are not derived from images warped into the same space. Namely, the BPND values come from images normalized to MNI space, while the regional volume values do not (labels are propagated into individual subject space, based on a voxel‐voting procedure from a large number of candidate labels, using study sample specific templates). Thus, it is highly unlikely that there is overlap in potential variance from differences in “goodness” of normalization to the same image space (MNI). Importantly, compared to other automated techniques such as FreeSurfer and FSL, MAGeT‐Brain demonstrates the highest correlation with gold‐standard manual segmentation techniques—FreeSurfer and FSL significantly overestimate subcortical volumes compared to MAGeT‐Brain [Makowski et al., in press].
Total Brain Volume Analysis
The procedure for total brain volume (TBV) analysis has been published elsewhere [Plitman et al., 2016b]. TBV was obtained using the Brain Extraction based on non‐local Segmentation Technique (BEaST) method [Eskildsen et al., 2012], which is based on non‐local segmentation in a multi‐resolution framework. Each voxel is labeled based on the similarity of its neighborhood of voxels to all the neighborhoods in a library of pre‐defined priors, and a non‐local means estimator is used to estimate the label at the voxel. Inputs are down‐sampled to a lower resolution, segmentation is performed, and results are propagated up to higher resolutions [Eskildsen et al., 2012]. BEaST is designed to include CSF (in the ventricles, cerebellar cistern, deep sulci, along surface of brain, and brainstem), the brainstem, and cerebellar white matter (WM) and GM in the brain mask, while excluding the skull, skin, fat, muscles, dura, eyes, bone, exterior blood vessels, and exterior nerves.
PET Imaging
Subjects were asked to abstain from food for no less than 90 min prior to PET procedures. The radiosynthesis of [11C]‐raclopride [Wilson et al., 2000] and [11C]‐(+)‐PHNO [Wilson et al., 2005], along with the acquisition of PET images [Graff‐Guerrero et al., 2010], has been described in detail elsewhere. Images were acquired on a high–resolution, head‐dedicated PET camera system (CPS‐HRRT; Siemens Molecular Imaging, USA), which measures radioactivity in 207 brain slices with a thickness of 1.2 mm each. The in‐plane resolution was ∼2.8 mm full‐width at half‐maximum (FWHM). Transmission scans were acquired with the use of a 137Cs (T 1/2 = 30.2 years, energy = 662 KeV) single‐photon point source to provide attenuation correction, and the emission data were acquired in list mode. The raw data were reconstructed by filtered back‐projection. For the [11C]‐raclopride scans (n = 62), the mean radioactivity dose was 9.75(±1.0)mCi, with a specific activity of 1,234.58(±569.11)mCi/µmol, and an injected mass of 3.78(±2.19)µg. [11C]‐raclopride data were acquired for 60 min and redefined into 28 frames (1–5 of 1‐min duration, 6–25 of 2‐min duration, and 26–28 of 5‐min duration). For the [11C]‐(+)‐PHNO scans (n = 68), the mean radioactivity dose was 9.13(±1.46)mCi, with a specific activity of 1,100.91(±394.67)mCi/µmol, and an injected mass of 2.15(±.47)µg. None of the participants included in this sample reported nausea given the [11C]‐(+)‐PHNO injection. [11C]‐(+)‐PHNO data were acquired for 90 min after injection and redefined into 30 frames (1–15 of 1–min duration and 16–30 of 5–min duration).
PET Image Analysis
The ROI‐based analysis for [11C]‐(+)‐PHNO has been described in detail elsewhere [Graff‐Guerrero et al., 2008; Tziortzi et al., 2011]. Time activity curves (TACs) from ROIs were obtained from the dynamic PET images in native space with reference to each subject's co‐registered MRI image. The co‐registration of each subjects MRI to PET space was done using the normalized mutual information algorithm [Studholme et al., 1997] as implemented in SPM2 (SPM2, Wellcome Department of Cognitive Neurology, London; http://www.fil.ion.ucl.ac.uk/spm). The TACs were analyzed using the Simplified Reference Tissue Method (SRTM) [Lammertsma and Hume, 1996] which has been validated for use with [11C]‐(+)‐PHNO [Ginovart et al., 2007]. The cerebellum was used as the reference region to derive a quantitative estimate of binding—binding potential relative to the non‐displaceable compartment (BPND)—as defined by the consensus nomenclature for in vivo imaging of reversibly binding radioligands [Innis et al., 2007]. The basis function implementation of the SRTM [Gunn et al., 1997] was applied to the dynamic PET images to generate parametric voxelwise BPND maps using PMOD (v2.7, PMOD Technologies, Zurich, Switzerland). These images were spatially normalized into MNI brain space by Nearest Neighbor Interpolation with a voxel size fixed in 2 × 2 × 2 mm3 using SPM2. Regional BPND estimates were then derived from ROIs defined in MNI space, except for the hypothalamus and ventral pallidum ROIs. The VS and dorsal striatum (dorsal caudate, hereafter caudate, and dorsal putamen, hereafter putamen) were defined according with the criteria of Mawlawi et al [Mawlawi et al., 2001]. The GP ROI was defined according to the criteria of Tziortzi et al [Tziortzi et al., 2011].
Statistical Analysis
Statistical analyses were conducted using IBM SPSS (v.20; Armonk, NY: IBM Corp) and GraphPad Prism (v.7.0; GraphPad Software, La Jolla California). The relationship between D2/3R availability and subcortical volume was explored using partial correlations (two‐tailed), controlling for age, gender, and TBV. Four exploratory partial correlation matrices were conducted separately for [11C]‐raclopride and [11C]‐(+)‐PHNO (i.e., eight in total). These explored the relationship between BPND in each ROI and volumes of, (i) striatal subdivisions, (ii) hippocampal subdivisions, (iii) thalamic subdivisions, and (iv) the amygdalaBonferroni correction for multiple comparisons was applied to each correlation matrix individually, and relationships surviving this threshold (P < 0.05 ÷ n, where n = # of comparisons) were considered noteworthy. We believe this exploratory, data‐driven approach may help guide future studies in developing potential a priori hypotheses, while minimizing potential type‐II errors.
RESULTS
Data from 62 subjects scanned with [11C]‐raclopride (mean age = 38.98 ± 14.45; 23 female) and 68 subjects scanned with [11C]‐(+)‐PHNO (mean age = 38.54 ± 14.59; 25 female) were used in the study (see Supporting Information Figure 1). The relationships between striatal subregion volume and D2/3R availability measured with [11C]‐raclopride are presented in Table 1. While [11C]‐raclopride BPND in the VS was positively correlated with VS volume (see Fig. 1), this relationship did not survive correction for multiple comparisons. Adding MRI fieldstrength (1.5T versus 3T) as an additional covariate did not significantly alter the strength of this finding (r(56) = 0.30, P = 0.02).
Table 1.
Relationship between [11C]‐raclopride BPND and striatal volume in healthy participants (n = 62), controlling for age, sex, and total brain volume
| [11C]‐raclopride BPND | ||||
|---|---|---|---|---|
| Striatal Volume | Dorsal caudate | Dorsal putamen | Ventral striatum | Globus pallidus |
| Pre‐commissural caudate | 0.12 | 0.02 | 0.003 | 0.02 |
| (0.36) | (0.86) | (0.98) | (0.87) | |
| Post‐commissural caudate | 0.08 | −0.07 | −0.09 | 0.02 |
| (0.57) | (0.61) | (0.52) | (0.88) | |
| Pre‐commissural putamen | 0.15 | 0.16 | 0.08 | −0.06 |
| (0.27) | (0.23) | (0.55) | (0.67) | |
| Post‐commissural putamen | 0.19 | 0.16 | 0.06 | −0.004 |
| (0.15) | (0.24) | (0.68) | (0.94) | |
| Ventral striatum | 0.23 | 0.19 | 0.30 a | −0.02 |
| (0.08) | (0.16) | (0.02) | (0.87) | |
| Globus pallidus | 0.14 | 0.06 | −0.04 | −0.06 |
| (0.29) | (0.67) | (0.76) | (0.65) | |
Data are presented as Pearson product moment partial correlations (r) with P‐values in parentheses.
Significance at P < 0.05 (two‐tailed), uncorrected for multiple comparisons (adjusted P‐threshold < 0.002).
Figure 1.
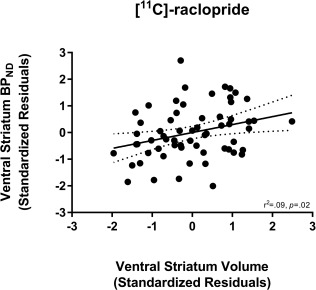
Relationship between [11C]‐raclopride BPND in the ventral striatum (VS) and VS volume. Values represent standardized residuals controlling for age, sex, and total brain volume.
The relationships between striatal subregion volume and D2/3R availability measured with [11C]‐(+)‐PHNO are presented in Table 2 and Figure 2. Several positive correlations emerged and survived correction for multiple comparisons. Notably, (i) BPND in both the dorsal caudate and GP was correlated with ventral caudate volume and (ii) BPND in the VS was correlated with VS volume. Adding MRI tesla strength as an additional covariate did not significantly alter the strength of any of the aforementioned correlations: ventral caudate volume with BPND in the dorsal caudate (r(62) = 0.40, P = 0.001) and GP (r(62) = 0.38, P = 0.002); VS volume with VS BPND (r(62) = 0.39, P = 0.001).
Table 2.
Relationship between [11C]‐(+)‐PHNO BPND and striatal volume in healthy participants (n = 69), controlling for age, sex, and total brain volume
| [11C]‐(+)‐PHNO BPND | |||||
|---|---|---|---|---|---|
| Striatal volume | Substantia nigra | Dorsal caudate | Dorsal putamen | Ventral striatum | Globus pallidus |
| Pre‐commissural caudate | −0.05 | 0.26 a | 0.17 | 0.21 | 0.11 |
| (0.69) | (0.04) | (0.17) | (0.10) | (0.38) | |
| Post‐commissural caudate | −0.02 | 0.40 c | 0.32 a | 0.01 | 0.38 c |
| (0.90) | (0.001) | (0.01) | (0.92) | (0.002) | |
| Pre‐commissural putamen | 0.14 | 0.03 | 0.24 | 0.26 a | 0.11 |
| (0.26) | (0.79) | (0.05) | (0.04) | (0.39) | |
| Post‐commissural putamen | 0.22 | 0.05 | 0.23 | 0.09 | 0.30 a |
| (0.08) | (0.72) | (0.07) | (0.49) | (0.02) | |
| Ventral striatum | 0.16 | −0.02 | −0.005 | 0.39 c | −0.01 |
| (0.22) | (0.87) | (0.97) | (0.001) | (0.93) | |
| Globus pallidus | 0.22 | 0.26 a | 0.24 | 0.18 | 0.36 b |
| (0.09) | (0.04) | (0.06) | (0.15) | (0.004) | |
Data are presented as Pearson product moment partial correlations (r) with P‐values in parentheses.
Significance at P < 0.05 (two‐tailed), uncorrected for multiple comparisons (adjusted P‐threshold < 0.002).
Significance at P < 0.01 (two‐tailed), uncorrected for multiple comparisons (adjusted P‐threshold < 0.002).
Survives correction for multiple comparisons (adjusted P‐threshold < 0.002).
Figure 2.
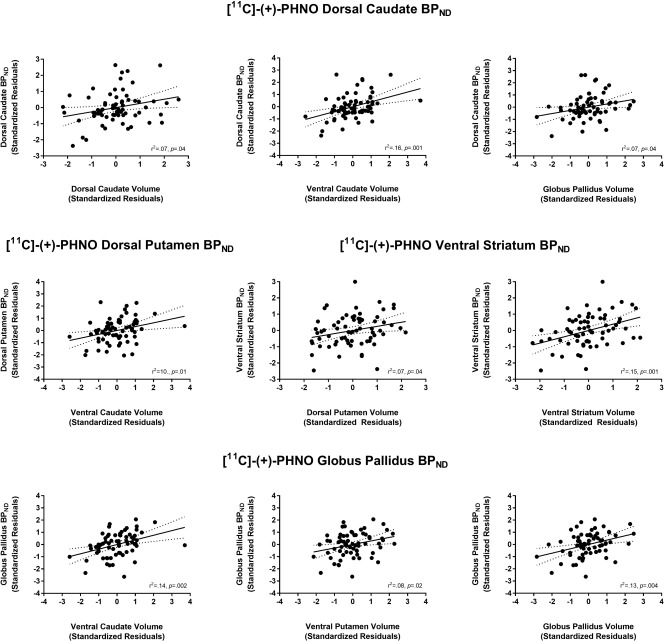
Relationships between [11C]‐(+)‐PHNO BPND in several regions of interest with striatal volumes. Values represent standardized residuals controlling for age, sex, and total brain volume.
The relationships between hippocampal subregion volume and D2/3R availability measured with [11C]‐raclopride and [11C]‐(+)‐PHNO are presented in Table 3 and Table 4, respectively. A negative correlation between [11C]‐raclopride BPND in the VS and subiculum volume was observed (see Fig. 3). However, this did not survive correction for multiple comparisons. Adding MRI tesla strength as an additional covariate did not significantly alter the strength of any of the aforementioned correlations: VS BPND with subiculum volume (r(56) = −0.27, P = 0.04).
Table 3.
Relationship between [11C]‐raclopride BPND and hippocampal volume in healthy participants (n = 62), controlling for age, sex, and total brain volume
| [11C]‐raclopride BPND | ||||
|---|---|---|---|---|
| Hippocampal volume | Caudate | Putamen | Ventral striatum | Globus pallidus |
| CA1 | −0.14 | −0.17 | −0.26 | 0.004 |
| (0.29) | (0.21) | (0.05) | (0.98) | |
| Subiculum | 0.003 | −0.15 | –0.29 a | −0.02 |
| (0.98) | (0.25) | (0.03) | (0.90) | |
| CA4/Dentate gyrus | −0.05 | −0.02 | −0.08 | 0.13 |
| (0.72) | (0.86) | (0.56) | (0.32) | |
| CA2/CA3 | −0.06 | 0.02 | −0.06 | 0.09 |
| (0.67) | (0.88) | (0.64) | (0.48) | |
| Stratum radiatum | −0.08 | −0.13 | −0.24 | 0.05 |
| (0.54) | (0.33) | (0.07) | (0.68) | |
Data are presented as Pearson product moment partial correlations (r) with P‐values in parentheses.
Significance at P < 0.05 (two‐tailed), uncorrected for multiple comparisons (adjusted P‐threshold < 0.003).
Table 4.
Relationship between [11C]‐(+)‐PHNO BPND and hippocampal volume in healthy participants (n = 69), controlling for age, sex, and total brain volume
| [11C]‐(+)‐PHNO BPND | |||||
|---|---|---|---|---|---|
|
Hippocampal volume |
Substantia nigra | Caudate | Putamen | Ventral striatum | Globus pallidus |
| CA1 | 0.04 | 0.03 | 0.03 | −0.11 | 0.11 |
| (0.76) | (0.79) | (0.79) | (0.37) | (0.38) | |
| Subiculum | 0.13 | 0.19 | 0.10 | 0.05 | 0.12 |
| (0.29) | (0.13) | (0.44) | (0.71) | (0.35) | |
| CA4/Dentate gyrus | 0.13 | 0.09 | 0.09 | 0.11 | 0.04 |
| (0.30) | (0.48) | (0.49) | (0.38) | (0.76) | |
| CA2/CA3 | 0.13 | −0.14 | −0.08 | 0.01 | 0.10 |
| (0.30) | (0.26) | (0.55) | (0.91) | (0.41) | |
| Stratum radiatum | 0.04 | −0.06 | −0.03 | −0.11 | 0.05 |
| (0.74) | (0.66) | (0.79) | (0.38) | (0.72) | |
Data are presented as Pearson product moment partial correlations (r) with P‐values in parentheses.
Figure 3.
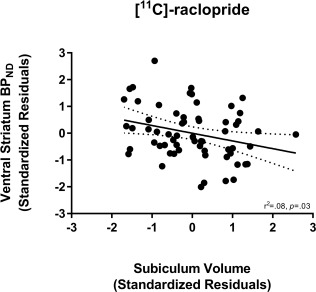
Relationship between [11C]‐raclopride BPND in the ventral striatum (VS) and subiculum volume. Values represent standardized residuals controlling for age, sex, and total brain volume.
The relationships between thalamic subregion volume and D2/3R availability measured with [11C]‐raclopride and [11C]‐(+)‐PHNO and are presented in Table 5 and Table 6, respectively. While several positive and negative correlations emerged (see Fig. 4), none survived correction for multiple comparisons. Adding MRI tesla strength as an additional covariate did not significantly alter the strength of these correlations (data not shown).
Table 5.
Relationship between [11C]‐raclopride BPND and thalamus volume in healthy participants (n = 62), controlling for age, sex, and total brain volume
| [11C]‐raclopride BPND | ||||
|---|---|---|---|---|
| Thalamus volume | Caudate | Putamen | Ventral striatum | Globus pallidus |
| Lateral geniculate nucleus (LGN) | −0.21 | –0.30 a | −0.26 | −0.14 |
| (0.13) | (0.02) | (0.05) | (0.30) | |
| Medial geniculate nucleus (MGN) | −0.06 | −0.11 | −0.12 | −0.12 |
| (0.68) | (0.40) | (0.35) | (0.36) | |
| Anterior nuclei | −0.14 | 0.11 | 0.29 a | 0.09 |
| (0.30) | (0.41) | (0.03) | (0.48) | |
| Central nuclei | 0.02 | −0.18 | −0.24 | −0.17 |
| (0.89) | (0.18) | (0.07) | (0.19) | |
| Lateral dorsal | −0.04 | 0.10 | 0.15 | −0.06 |
| (0.78) | (0.46) | (0.27) | (0.64) | |
| Lateral posterior | −0.15 | −0.07 | −0.09 | –0.36 a |
| (0.25) | (0.58) | (0.50) | (0.006) | |
| Medial dorsal | 0.02 | 0.01 | −0.06 | −0.06 |
| (0.86) | (0.93) | (0.68) | (0.67) | |
| Pulvinar | −0.09 | −0.09 | −0.13 | −0.08 |
| (0.51) | (0.52) | (0.31) | (0.55) | |
| Ventral anterior nucleus (VAN) | −0.06 | 0.08 | 0.09 | 0.04 |
| (0.65) | (0.53) | (0.49) | (0.78) | |
| Ventral lateral nucleus (VLN) | −0.03 | −0.001 | −0.04 | −0.14 |
| (0.81) | (0.99) | (0.79) | (0.30) | |
| Ventral posterior nucleus (VPN) | −0.03 | −0.17 | −0.18 | −0.25 |
| (0.82) | (0.19) | (0.18) | (0.06) | |
Data are presented as Pearson product moment partial correlations (r) with P‐values in parentheses.
Significance at P < 0.05 (two‐tailed), uncorrected for multiple comparisons (adjusted P‐threshold < 0.001).
Table 6.
Relationship between [11C]‐(+)‐PHNO BPND and thalamus volume in healthy participants (n = 67), controlling for age, sex, and total brain volume
| [11C]‐(+)‐PHNO BPND | |||||
|---|---|---|---|---|---|
| Thalamus volume | Substantia nigra | Caudate | Putamen | Ventral striatum | Globus pallidus |
| Lateral geniculate nucleus (LGN) | 0.07 | −0.003 | −0.07 | −0.09 | 0.02 |
| (0.57) | (0.98) | (0.58) | (0.49) | (0.87) | |
| Medial geniculate nucleus (MGN) | 0.11 | 0.002 | −0.09 | 0.02 | −0.11 |
| (0.39) | (0.99) | (0.48) | (0.91) | (0.37) | |
| Anterior nuclei | −0.02 | −0.001 | 0.03 | 0.19 | −0.12 |
| (0.90) | (0.99) | (0.83) | (0.13) | (0.33) | |
| Central nuclei | 0.26 a | −0.10 | −0.01 | 0.19 | −0.08 |
| (0.04) | (0.42) | (0.93) | (0.12) | (0.52) | |
| Lateral dorsal | −0.08 | 0.09 | 0.05 | 0.14 | −0.005 |
| (0.52) | (0.48) | (0.68) | (0.27) | (0.97) | |
| Lateral posterior | −0.07 | −0.03 | −0.05 | 0.05 | −0.19 |
| (0.60) | (0.85) | (0.70) | (0.69) | (0.14) | |
| Medial dorsal | −0.07 | −0.20 | 0.03 | 0.16 | −0.07 |
| (0.56) | (0.12) | (0.81) | (0.20) | (0.58) | |
| Pulvinar | 0.02 | −0.10 | 0.04 | −0.09 | 0.06 |
| (0.90) | (0.45) | (0.77) | (0.50) | (0.62) | |
| Ventral anterior nucleus (VAN) | −0.03 | −0.02 | 0.02 | 0.26 a | −0.13 |
| (0.83) | (0.86) | (0.86) | (0.04) | (0.30) | |
| Ventral lateral nucleus (VLN) | −0.04 | −0.03 | 0.02 | 0.18 | −0.14 |
| (0.73) | (0.82) | (0.87) | (0.14) | (0.27) | |
| Ventral posterior nucleus (VPN) | 0.06 | 0.03 | 0.03 | −0.02 | −0.05 |
| (0.62) | (0.84) | (0.79) | (0.86) | (0.67) | |
Data are presented as Pearson product moment partial correlations (r) with P‐values in parentheses.
Significance at P < 0.05 (two‐tailed), uncorrected for multiple comparisons (adjusted P‐threshold < 0.001).
Figure 4.
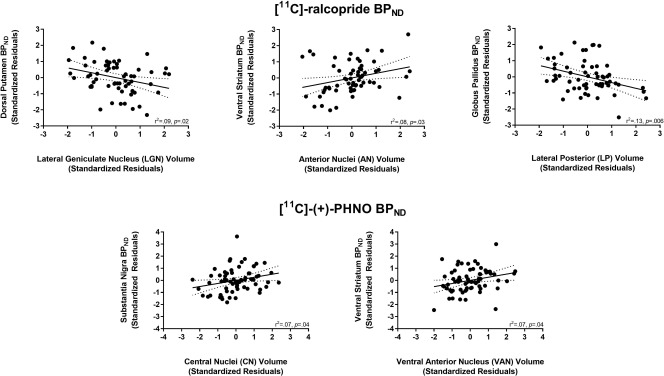
Relationship between [11C]‐raclopride and [11C]‐(+)‐PHNO BPND in several regions of interest with thalamic volumes. Values represent standardized residuals controlling for age, sex, and total brain volume.
For [11C]‐raclopride, amygdala volume was not significantly correlated with BPND in the caudate (r(57) = −0.11, P = 0.45), putamen (r(57) = −0.06, P = 0.65), VS (r(57) = −0.11, P = 0.40), nor the GP (r(57) = 0.11, P = 0.41). Adding MRI tesla strength as an additional covariate did not significantly alter the strength of these correlations: caudate (r(56) = −0.11, P = 0.40), putamen (r(56) = −0.05, P = 0.73), VS (r(56) = −0.09, P = 0.52), and GP (r(56) = 0.14, P = 0.23). For [11C]‐(+)‐PHNO, amygdala volume was not significantly correlated with BPND in the SN (r(63) = 0.15, P = 0.24), caudate (r(63) = 0.14, P = 0.26), putamen (r(63) = 0.13, P = 0.30), VS (r(63) = 0.05, P = 0.72), nor the GP (r(63) = 0.12, P = 0.34). Adding MRI tesla strength as an additional covariate did not significantly alter the strength of these correlations: SN (r(62) = 0.15, P = 0.25), caudate (r(62) = 0.14, P = 0.26), putamen (r(62) = 0.12, P = 0.35), VS (r(62) = 0.05, P = 0.68), and GP (r(62) = 0.12, P = 0.36).
DISCUSSION
This investigation is the first to examine how subcortical brain morphology is related to striatal D2R and D3R availability as measured with both an antagonist and agonist radiotracer. First with the antagonist [11C]‐raclopride, D2/3R availability within striatal subregions was generally not significantly correlated with the volume of those regions. This is in contrast to the findings of Woodward et al., who used the antagonist radiotracer [18F]‐fallypride [Woodward et al., 2009]. One exception was the VS, for which there was a positive correlation that did not survive correction for multiple comparisons. However, with the agonist [11C]‐(+)‐PHNO, BPND within striatal subregions was generally correlated with the volume of those regions, with the exception of the putamen. Notably, this was especially true for the VS, which survived correction for multiple comparisons.
One potential interpretation of this finding is a causal, biological one. Namely, that the availability of functional D2/3R binding sites (i.e., D2High) is positively related to striatal size, while the availability of total binding sites (i.e., D2High + D2Low) is not. As [11C]‐(+)‐PHNO is more sensitive to endogenous DA at baseline [Caravaggio et al., 2016a], an alternative interpretation is that persons with less endogenous DA (and therefore more radiotracer binding) have larger striatal volumes. Another potential interpretation is a methodological one. Namely, that subtle individual variations in striatal size which persist after normalization may influence D2/3R availability measures, that is, a partial volume effect (PVE). The PVE is a phenomenon whereby the apparent concentration of a radiotracer decreases as the size of the ROI approaches the instrument resolution (FWHM) [Hoffman et al., 1979]. We suggest it is unlikely that our findings are due to potential PVE. First, PVEs are most pronounced for ROIs 2–3 times smaller than the FWHM [Rousset et al., 1998]. It is also a concern for studies of aging, where certain ROIs are known to decrease in size with age. However, the PVE is minimized using the resolution of the CPS‐HRRT, and we have not observed significant PVE using other radiotracers in healthy persons with this scanner [Nakajima et al., 2015; Uchida et al., 2011]. Moreover, it is unclear why [11C]‐(+)‐PHNO BPND in the striatum would be more susceptible to PVE than [11C]‐raclopride, acquired on the same scanner.
Unlike Woodward et al. [2009], we controlled for differences in age, gender, and TBV. Thus, our findings suggest that after considering these factors, subtle variations in striatal structure may be related to D2/3R availability measured with [11C]‐(+)‐PHNO, but not [11C]‐raclopride. This finding is generally important for [11C]‐(+)‐PHNO studies using cross‐sectional or between group designs. For example, it will be important to determine whether increased [11C]‐(+)‐PHNO BPND in obese persons corresponds with concordant increases in striatal volumes [Caravaggio et al., 2015c; Gaiser et al., 2016].
Notably, [11C]‐(+)‐PHNO BPND in the dorsal caudate and GP were both correlated with ventral caudate volume. As the dorsal and ventral caudate border each other, it could be argued that this relationship is being driven by spill‐over effects. However, with such an interpretation it would be harder to explain the relationship between GP BPND and ventral caudate volume. Notably, ∼65% of the [11C]‐(+)‐PHNO BPND signal from the GP reflects D3R versus D2R [Tziortzi et al., 2011]. While the dorsal caudate and putamen have negligible D3R expression, the ventral caudate and putamen express D3R (∼1/3rd) [Seeman et al., 2006]. It may be possible that neurons which express D3R in the ventral caudate project to those neurons in the internal segment of the GP (GPi) which express D3R [Gurevich and Joyce, 1999]. This in turn could potentially drive the relationship between [11C]‐(+)‐PHNO BPND in the GP and ventral caudate volume. However, this is speculative and requires substantiation from ex vivo work.
We also explored how D2/3R availability in the striatum was related to the volume of other subcortical structures: the hippocampus, thalamus, and amygdala. While none of these correlations survived correction for multiple comparisons, a few notable relationships emerged. First, with [11C]‐raclopride there was a negative correlation between VS BPND and subiculum volume of the hippocampus. The subiculum, which is the main output structure of the hippocampus, projects densely to the nucleus accumbens of the VS [Chase et al., 2015; Groenewegen et al., 1987; Kelley and Domesick, 1982; Lopes da Silva et al., 1984]. It will be important for future in vivo and ex vivo work to elucidate the relationship between the structure and neurochemistry of this pathway. [11C]‐raclopride BPND in the GP was also negatively correlated with the volume of the lateral posterior nucleus of the thalamus. This large nucleus lies medial to the dorsal lateral geniculate nucleus and receives inputs mainly from the superior colliculus—projecting to primary and secondary visual areas [Puelles et al., 2012]. The superior colliculus plays a large role in integrating sensory motor information and, in particular, the generation of saccadic eye movements [Gandhi and Katnani, 2011]. Interestingly, while the GP is involved in motor generation and inhibition in general, the external segment of the GP (GPe) in particular may play an integral role in the generation and suppression of antisaccadic eye movements [Yoshida and Tanaka, 2016]. As the majority of the [11C]‐raclopride BPND in the GP reflects D2R in the external segment, our exploratory finding may point to a role for these receptors in saccadic eye movements. While highly speculative, we surmise that future PET studies should examine the relationship between the availability of D2R in the GPe and D3R in the GPi and saccadic eye movements—measured with [11C]‐raclopride and [11C]‐(+)‐PHNO, respectively.
There are several limitations to the current investigation. First, it has been noted that the injected mass of [11C]‐(+)‐PHNO is not within ideal radiotracer conditions (i.e., <1.5 ng/kg) [Gallezot et al., 2012]. The specific activity required to obtain tracer conditions is not possible with the available radiosynthesis method. While this limitation is currently unavoidable, we attempt to minimize this technical limitation by aiming to control the injected mass of the radiotracer to ≤2.5 µg (≤0.02 µg/kg), as previously used [Mizrahi et al., 2009; Rabiner and Laruelle, 2010; Searle et al., 2013a]. Importantly, our sample (mean injected mass: 2.15 ± 0.47µg) did not include any incidence of side effects associated with high [11C]‐(+)‐PHNO injected mass, such as nausea/vomiting [Mizrahi et al., 2009; Rabiner and Laruelle, 2010]. Second, it has been suggested that [11C]‐(+)‐PHNO BPND in D3R‐rich regions is underestimated if SRTM quantification is used in conjunction with 90 min of data acquisition [Girgis et al., 2011]. Thus, using arterial plasma‐based kinetic models following 120 min of emission data is more ideal for quantifying [11C]‐(+)‐PHNO BPND in D3R‐rich regions [Girgis et al., 2011]. Moreover, use of arterial plasma‐based kinetic models would circumvent limitations associated with using reference tissue methods, namely concerns about specific binding to D3R in cerebellar reference tissue [Searle et al., 2013b]. Future [11C]‐(+)‐PHNO studies would benefit from examining the relationship between D2/3R availability and brain morphology using plasma‐based modeling and 120 mine emission data. This study was retrospective, reanalyzing previously collected PET data. Thus, other relevant measures such as cognition were not available. Moreover, it would be important to determine how longitudinal changes in D2/3R availability with age may be associated with age‐related changes in brain morphology in healthy persons and persons with neuropsychiatric disorders. Moreover, it will be important to determine how brain morphology changes and striatal D2/3R availability changes relate to each other in addiction. For example, while we did not include heavy drinkers in our study, it would be important to determine how the frequency of alcohol use affects the relationship between in vivo brain morphology measures and DA measures. Finally, it will be important for future studies to examine how brain morphology is related to the in vivo status of other neurochemical systems. For example, increased striatal glutamate levels measured with proton magnetic resonance spectroscopy (1H‐MRS) have also been observed in patients with first‐episode psychosis [Plitman et al., 2016a]. In turn, striatal glutamate levels were found to be negatively correlated with dorsal caudate volumes in these patients [Plitman et al., 2016b]. Given the potential interactions between striatal DA and glutamate [Caravaggio et al., 2016b; Stone et al., 2007], it can be inferred that the dopaminergic changes observed in schizophrenia may also be related changes in brain morphology. However, without direct evidence this remains merely conjuncture.
While our participants were not taking medications for any serious medical condition, we did not record oral contraceptive use in our female participants. While some [11C]‐raclopride PET studies have observed no effect of menstrual cycle on baseline D2/3R availability in humans [Farde et al., 1995; Nordström et al., 1998], it is currently unknown whether this may effect [11C]‐(+)‐PHNO BPND. Future studies are required to examine the potential interacting effects between menstrual cycle, contraceptive use, and the availability of D2/3R using agonist radiotracers in females. Several lines of indirect evidence suggest that striatal DA functioning may differ between men and women. For example, women may be more vulnerable to the reinforcing effects of drugs of abuse [Becker and Hu, 2008; Fattore et al., 2007; Lynch et al., 2002; Nolen‐Hoeksema, 2004]. However, PET studies in humans have provided conflicting results as to whether there are sex differences in amphetamine‐induced striatal dopamine release [Munro et al., 2006; Riccardi et al., 2006, 2011]. Moreover, some studies suggest that DA transporter availability may not differ between gender, nor across the menstrual cycle [Best et al., 2005]. However, other studies suggests that women may have greater DA transporter functioning in the dorsal striatum [Andersen et al., 2012; Kaasinen et al., 2015; Lee et al., 2015; Wong et al., 2012], as well as greater DA synthesis capacity in the caudate [Laakso et al., 2002]. While we used sex as a covariate in our main analyses, there may be important sex differences between D2/3R availability and brain morphology. For the purposes of transparency, in Supporting Information A, we re‐conduct all our exploratory analyses within males and females separately. However, we did not sufficiently sample a large, nor equally matched number of males and females. Thus, interpretation of these data warrants caution and requires replication by sufficiently powered samples in the future.
Elucidating the relationship between metabolic health and striatal D2/3R availability remains an on‐going and exciting field of exploration in PET [Caravaggio et al., 2015a, 2015c; Dang et al., 2016; Gaiser et al., 2016; Horstmann, 2017]. It is beyond the scope of the current manuscript to review all these conflicting PET findings; considering all the different radioligands used for D2/3R, each with their own unique in vivo binding characteristics. Unfortunately, in our current sample, body mass index (BMI) was not collected for all the subjects. Moreover, other relevant markers of metabolic health, such as lipid profiles and insulin resistance, were not collected. In Supporting Information B, we provide the demographics of the subjects who provided BMI. For these subjects, we also re‐ran all the analyses of the current investigation using BMI as an additional covariate. Notably, only four subjects scanned with [11C]‐raclopride and three subjects scanned with [11C]‐(+)‐PHNO had BMI's within the moderately obese range (30–35). This is unsurprising as having a co‐morbid medical condition, such as diabetes or heart disease, was an exclusion criteria for being scanned in this retrospective dataset. Thus, we warrant caution when interpreting these supplementary results. We believe our sample is inadequate to address how obesity modulates D2/3R availability and brain morphology; we present this Supporting Information for the purposes of transparency, and for guiding future studies which are better poised to address this important topic. Related to metabolic syndrome, several lines of evidence suggest that low‐grade systemic inflammation may have an impact on cognition and brain structure [Marsland et al., 2015; Minihane et al., 2015]. While we have tried to screen “healthy” participants, we did not collect peripheral markers of inflammation. Future studies should examine how peripheral inflammatory markers—such as cytokines, leukocytes, and C‐reactive protein levels—relates to concurrent changes in brain morphology and D2/3R availability; in healthy persons, persons with neuropsychiatric disorders, and persons with metabolic diseases.
In our sample, PET scans were collected at various times of day. Moreover, we did not record when was the last meal participants had before their PET scans and were only suggested to abstain from food for no less than 90 min prior to PET. To our knowledge, DA release in response to food intake has only been examined with [11C]‐raclopride, and methylphenidate was required to be co‐administered to see a significant change in BPND [Volkow et al., 2002; Wang et al., 2011]. However, given the increased sensitivity of [11C]‐(+)‐PHNO to DA release, this tracer may be able to quantify DA release in response to food receipt without the co‐administration of methylphenidate. This can be examined by future [11C]‐(+)‐PHNO studies. With regards to the current investigation, it is noteworthy that the time of day of the scan did not correlate with the BPND of [11C]‐(+)‐PHNO (SN: r = 0.09, P = 0.49; Caudate: r = 0.10, P = 0.43; Putamen: r = 0.009, P = 0.95; VS: r = 0.20, P = 0.11; GP: r = −0.15, P = 0.21) nor of [11C]‐raclopride (Caudate: r = 0.11, P = 0.41; Putamen: r = 0.11, P = 0.40; VS: r = 0.12, P = 0.36; GP: r = 0.03, P = 0.85). Moreover, the month of scan acquisition was not correlated with the BPND of [11C]‐ (+)‐PHNO (SN: r = −0.02, P = .86; Caudate: r = −0.07, P = 0.57; Putamen: r = 0.06, P = 0.63; VS: r = 0.04, P = 0.74; GP: r = 0.13, P = 0.29) nor of [11C]‐raclopride (Caudate: r = 0.02, P = 0.90; Putamen: r = −0.04, P = 0.79; VS: r = 0.04, P = 0.78; GP: r = −0.08, P = 0.53). While the time of the last meal is not routinely collected across DA PET scans, this may be an important consideration for the future.
In sum, we examined how striatal D2/3R availability, measured with the antagonist [11C]‐raclopride and the agonist [11C]‐(+)‐PHNO, was related to the volume of several subcortical structures in healthy controls. Such an exploration will, (1) aid the interpretation of future PET findings and (2) help elucidate the relationships between brain chemistry, structure, and function which could be used as potential biomarkers for neuropsychiatric disease. Extension of this work to understand how abnormal in vivo DA functioning relates to brain structure and function in disorders like schizophrenia and food addiction are highly warranted.
Conflict of Interest
The authors have no potential conflicts of interest to declare in relation to the current study.
Supporting information
Supporting Information BMI
Supporting Information Gender
Supporting Information Figure01
REFERENCES
- Abi‐Dargham A, Rodenhiser J, Printz D, Zea‐Ponce Y, Gil R, Kegeles LS, Weiss R, Cooper TB, Mann JJ, Van Heertum RL, Gorman JM, Laruelle M (2000): Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci USA 97:8104–8109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez C, Vitalis T, Fon EA, Hanoun N, Hamon M, Seif I, Edwards R, Gaspar P, Cases O (2002): Effects of genetic depletion of monoamines on somatosensory cortical development. Neuroscience 115:753–764. [DOI] [PubMed] [Google Scholar]
- Andersen ML, Sawyer EK, Howell LL (2012): Contributions of neuroimaging to understanding sex differences in cocaine abuse. Exp Clin Psychopharmacol 20:2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M, Gee JC (2008): Symmetric diffeomorphic image registration with cross‐correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal 12:26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Hu M (2008): Sex differences in drug abuse. Front Neuroendocrinol 29:36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best SE, Sarrel PM, Malison RT, Laruelle M, Zoghbi SS, Baldwin RM, Seibyl JP, Innis RB, van Dyck CH (2005): Striatal dopamine transporter availability with [123I]β‐CIT SPECT is unrelated to gender or menstrual cycle. Psychopharmacology 183:181–189. [DOI] [PubMed] [Google Scholar]
- Bond DJ, Lang DJ, Noronha MM, Kunz M, Torres IJ, Su W, Honer WG, Lam RW, Yatham LN (2011): The association of elevated body mass index with reduced brain volumes in first‐episode mania. Biol Psychiatry 70:381–387. [DOI] [PubMed] [Google Scholar]
- Caravaggio F, Nakajima S, Borlido C, Remington G, Gerretsen P, Wilson A, Houle S, Menon M, Mamo D, Graff‐Guerrero A (2014): Estimating endogenous dopamine levels at D2 and D3 receptors in humans using the agonist radiotracer [(11)C]‐(+)‐PHNO. Neuropsychopharmacology 39:2769–2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caravaggio F, Borlido C, Hahn M, Feng Z, Fervaha G, Gerretsen P, Nakajima S, Plitman E, Chung JK, Iwata Y, Wilson A, Remington G, Graff‐Guerrero A (2015a): Reduced insulin sensitivity is related to less endogenous dopamine at D2/3 receptors in the ventral striatum of healthy nonobese humans. Int J Neuropsychopharmacol 18:pyv014–pyv014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caravaggio F, Borlido C, Wilson A, Graff‐Guerrero A (2015b): Examining endogenous dopamine in treated schizophrenia using [(1)(1)C]‐(+)‐PHNO positron emission tomography: A pilot study. Clin Chim Acta 449:60–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caravaggio F, Raitsin S, Gerretsen P, Nakajima S, Wilson A, Graff‐Guerrero A (2015c): Ventral striatum binding of a dopamine D2/3 receptor agonist but not antagonist predicts normal body mass index. Biol Psychiatry 77:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caravaggio F, Kegeles LS, Wilson AA, Remington G, Borlido C, Mamo DC, Graff‐Guerrero A (2016a): Estimating the effect of endogenous dopamine on baseline [(11) C]‐(+)‐PHNO binding in the human brain. Synapse (New York, N.Y.) 70:453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caravaggio F, Nakajima S, Plitman E, Gerretsen P, Chung JK, Iwata Y, Graff‐Guerrero A (2016b): The effect of striatal dopamine depletion on striatal and cortical glutamate: A mini‐review. Prog Neuropsychopharmacol Biol Psychiatry 65:49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey KF, Cherkasova MV, Larcher K, Evans AC, Baker GB, Dagher A, Benkelfat C, Leyton M (2013): Individual differences in frontal cortical thickness correlate with the d‐amphetamine‐induced striatal dopamine response in humans. J Neurosci 33:15285–15294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarty MM, Bertrand G, Hodge CP, Sadikot AF, Collins DL (2006): The creation of a brain atlas for image guided neurosurgery using serial histological data. Neuroimage 30:359–376. [DOI] [PubMed] [Google Scholar]
- Chakravarty MM, Sadikot AF, Germann J, Bertrand G, Collins DL (2008): Towards a validation of atlas warping techniques. Med image Anal 12:713–726. [DOI] [PubMed] [Google Scholar]
- Chakravarty MM, Broadbent S, Rosa‐Neto P, Lambert CM, Collins DL (2009): Design, construction, and validation of an MRI‐compatible vibrotactile stimulator intended for clinical use. J Neurosci Methods 184:129–135. [DOI] [PubMed] [Google Scholar]
- Chakravarty MM, Steadman P, van Eede MC, Calcott RD, Gu V, Shaw P, Raznahan A, Collins DL, Lerch JP (2013): Performing label‐fusion‐based segmentation using multiple automatically generated templates. Hum Brain Mapp 34:2635–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase HW, Clos M, Dibble S, Fox P, Grace AA, Phillips ML, Eickhoff SB (2015): Evidence for an anterior‐posterior differentiation in the human hippocampal formation revealed by meta‐analytic parcellation of fMRI coordinate maps: Focus on the subiculum. Neuroimage 113:44–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang LC, Samanez‐Larkin GR, Castrellon JJ, Perkins SF, Cowan RL, Zald DH (2016): Associations between dopamine D2 receptor availability and BMI depend on age. NeuroImage 138:176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison‐Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E (2008): The anatomy of first‐episode and chronic schizophrenia: An anatomical likelihood estimation meta‐analysis. Am J Psychiatry 165:1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskildsen SF, Coupe P, Fonov V, Manjon JV, Leung KK, Guizard N, Wassef SN, Ostergaard LR, Collins DL (2012): BEaST: Brain extraction based on nonlocal segmentation technique. Neuroimage 59:2362–2373. [DOI] [PubMed] [Google Scholar]
- Farde L, Hall H, Pauli S, Halldin C (1995): Variability in D2‐dopamine receptor density and affinity: A PET study with [11C]raclopride in man. Synapse (New York, N.Y.) 20:200–208. [DOI] [PubMed] [Google Scholar]
- Fattore L, Altea S, Fratta W (2007): Sex differences in drug addiction: A review of animal and human studies. Womens Health 4:51–65. [DOI] [PubMed] [Google Scholar]
- Finnema SJ, Seneca N, Farde L, Shchukin E, Sovago J, Gulyas B, Wikstrom HV, Innis RB, Neumeyer JL, Halldin C (2005): A preliminary PET evaluation of the new dopamine D2 receptor agonist [11C]MNPA in cynomolgus monkey. Nucl Med Biol 32:353–360. [DOI] [PubMed] [Google Scholar]
- Freedman SB, Patel S, Marwood R, Emms F, Seabrook GR, Knowles MR, McAllister G (1994): Expression and pharmacological characterization of the human D3 dopamine receptor. J Pharmacol Exp Ther 268:417–426. [PubMed] [Google Scholar]
- Gaiser EC, Gallezot J‐D, Worhunsky PD, Jastreboff AM, Pittman B, Kantrovitz L, Angarita GA, Cosgrove KP, Potenza MN, Malison RT (2016): Elevated dopamine D2/3 receptor availability in obese individuals: A PET imaging study with [11C](+) PHNO. Neuropsychopharmacology 41:3042–3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallezot JD, Beaver JD, Gunn RN, Nabulsi N, Weinzimmer D, Singhal T, Slifstein M, Fowles K, Ding YS, Huang Y, Laruelle M, Carson RE, Rabiner EA (2012): Affinity and selectivity of [(1)(1)C]‐(+)‐PHNO for the D3 and D2 receptors in the rhesus monkey brain in vivo. Synapse (New York, N.Y.) 66:489–500. [DOI] [PubMed] [Google Scholar]
- Gandhi NJ, Katnani HA (2011): Motor functions of the superior colliculus. Annu Rev Neurosci 34:205–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginovart N, Willeit M, Rusjan P, Graff A, Bloomfield PM, Houle S, Kapur S, Wilson AA (2007): Positron emission tomography quantification of [11C]‐(+)‐PHNO binding in the human brain. J Cereb Blood Flow Metab 27:857–871. [DOI] [PubMed] [Google Scholar]
- Girgis RR, Xu X, Miyake N, Easwaramoorthy B, Gunn RN, Rabiner EA, Abi‐Dargham A, Slifstein M (2011): In vivo binding of antipsychotics to D3 and D2 receptors: A PET study in baboons with [11C]‐(+)‐PHNO. Neuropsychopharmacology 36:887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff‐Guerrero A, Willeit M, Ginovart N, Mamo D, Mizrahi R, Rusjan P, Vitcu I, Seeman P, Wilson AA, Kapur S (2008): Brain region binding of the D2/3 agonist [11C]‐(+)‐PHNO and the D2/3 antagonist [11C]raclopride in healthy humans. Hum Brain Mapp 29:400–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff‐Guerrero A, Mamo D, Shammi CM, Mizrahi R, Marcon H, Barsoum P, Rusjan P, Houle S, Wilson AA, Kapur S (2009): The effect of antipsychotics on the high‐affinity state of D2 and D3 receptors: A positron emission tomography study With [11C]‐(+)‐PHNO. Arch Gen Psychiatry 66:606–615. [DOI] [PubMed] [Google Scholar]
- Graff‐Guerrero A, Redden L, Abi‐Saab W, Katz DA, Houle S, Barsoum P, Bhathena A, Palaparthy R, Saltarelli MD, Kapur S (2010): Blockade of [11C](+)‐PHNO binding in human subjects by the dopamine D3 receptor antagonist ABT‐925. Int J Neuropsychopharmacol 13:273–287. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Vermeulen‐Van der Zee E, te Kortschot A, Witter MP (1987): Organization of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport of Phaseolus vulgaris leucoagglutinin. Neuroscience 23:103–120. [DOI] [PubMed] [Google Scholar]
- Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ (1997): Parametric imaging of ligand‐receptor binding in PET using a simplified reference region model. NeuroImage 6:279–287. [DOI] [PubMed] [Google Scholar]
- Guo J, Simmons WK, Herscovitch P, Martin A, Hall KD (2014): Striatal dopamine D2‐like receptor correlation patterns with human obesity and opportunistic eating behavior. Mol Psychiatry 19:1078–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich EV, Joyce JN (1999): Distribution of dopamine D3 receptor expressing neurons in the human forebrain: Comparison with D2 receptor expressing neurons. Neuropsychopharmacology 20:60–80. [DOI] [PubMed] [Google Scholar]
- Haijma SV, Van Haren N, Cahn W, Koolschijn PC, Hulshoff Pol HE, Kahn RS (2013): Brain volumes in schizophrenia: A meta‐analysis in over 18 000 subjects. Schizophr Bull 39:1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman EJ, Huang SC, Phelps ME (1979): Quantitation in positron emission computed tomography: 1. Effect of object size. J Comput Assist Tomogr 3:299–308. [DOI] [PubMed] [Google Scholar]
- Horstmann A (2017): It wasn't me; it was my brain – Obesity‐associated characteristics of brain circuits governing decision‐making. Physiol Behav 176:125–133. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE (2007): Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 27:1533–1539. [DOI] [PubMed] [Google Scholar]
- Jones L, Fischer I, Levitt P (1996): Nonuniform alteration of dendritic development in the cerebral cortex following prenatal cocaine exposure. Cereb Cortex (New York, N.Y.: 1991) 6:431–445. [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Joutsa J, Noponen T, Johansson J, Seppanen M (2015): Effects of aging and gender on striatal and extrastriatal [123I]FP‐CIT binding in Parkinson's disease. Neurobiol Aging 36:1757–1763. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Matthijssen MA, Uylings HB (1989): Morphometric analysis of prefrontal cortical development following neonatal lesioning of the dopaminergic mesocortical projection. Exp Brain Res 78:279–289. [DOI] [PubMed] [Google Scholar]
- Karlsson, H. (2016) Neuroreceptor Availability and Cerebral Morphology in Human Obesity. Turku, Finland: University of Turku. [Google Scholar]
- Karlsson HK, Tuulari JJ, Tuominen L, Hirvonen J, Honka H, Parkkola R, Helin S, Salminen P, Nuutila P, Nummenmaa L (2016): Weight loss after bariatric surgery normalizes brain opioid receptors in morbid obesity. Mol Psychiatry 21:1057–1062. [DOI] [PubMed] [Google Scholar]
- Kegeles LS, Abi‐Dargham A, Frankle WG, Gil R, Cooper TB, Slifstein M, Hwang DR, Huang Y, Haber SN, Laruelle M (2010): Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry 67:231–239. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Domesick VB (1982): The distribution of the projection from the hippocampal formation to the nucleus accumbens in the rat: An anterograde‐ and retrograde‐horseradish peroxidase study. Neuroscience 7:2321–2335. [DOI] [PubMed] [Google Scholar]
- Laakso A, Vilkman H, Bergman J, Haaparanta M, Solin O, Syvalahti E, Salokangas RK, Hietala J (2002): Sex differences in striatal presynaptic dopamine synthesis capacity in healthy subjects. Biol Psychiatry 52:759–763. [DOI] [PubMed] [Google Scholar]
- Lammertsma AA, Hume SP (1996): Simplified reference tissue model for PET receptor studies. NeuroImage 4:153–158. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Ham JH, Lee PH, Sohn YH (2015): Gender differences in age‐related striatal dopamine depletion in Parkinson's disease. J Mov Disord 8:130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes da Silva FH, Arnolds DE, Neijt HC (1984): A functional link between the limbic cortex and ventral striatum: Physiology of the subiculum accumbens pathway. Exp Brain Res 55:205–214. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME (2002): Biological basis of sex differences in drug abuse: Preclinical and clinical studies. Psychopharmacology 164:121–137. [DOI] [PubMed] [Google Scholar]
- Makowski C, Beland S, Kostopoulos P, Bhagwat N, Devenyi GA, Malla AK, Joober R, Lepage M, Chakravarty MM (2017): Evaluating accuracy of striatal, pallidal, and thalamic segmentation methods: Comparing automated approaches to manual delineation. Neuroimage (in press). [DOI] [PubMed] [Google Scholar]
- Marsland AL, Gianaros PJ, Kuan DC, Sheu LK, Krajina K, Manuck SB (2015): Brain morphology links systemic inflammation to cognitive function in midlife adults. Brain Behav Immun 48:195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, Huang Y, Simpson N, Ngo K, Van Heertum R, Laruelle M (2001): Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab 21:1034–1057. [DOI] [PubMed] [Google Scholar]
- Meredith G, Ypma P, Zahm D (1995): Effects of dopamine depletion on the morphology of medium spiny neurons in the shell and core of the rat nucleus accumbens. J Neurosci 15:3808–3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minihane AM, Vinoy S, Russell WR, Baka A, Roche HM, Tuohy KM, Teeling JL, Blaak EE, Fenech M, Vauzour D, McArdle HJ, Kremer BHA, Sterkman L, Vafeiadou K, Benedetti MM, Williams CM, Calder PC (2015): Low‐grade inflammation, diet composition and health: Current research evidence and its translation. Br J Nutr 114:999–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrahi R, Wilson A, Houle S (2009): Side effects profile of [11C]‐(+)‐PHNO in human, a dopamine D2/3 agonist ligand. J Nucl Med 50:1288. [DOI] [PubMed] [Google Scholar]
- Morales AM, Kohno M, Robertson CL, Dean AC, Mandelkern MA, London ED (2015): Gray‐matter volume, midbrain dopamine D2/D3 receptors and drug craving in methamphetamine users. Mol Psychiatry 20:764–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro CA, McCaul ME, Wong DF, Oswald LM, Zhou Y, Brasic J, Kuwabara H, Kumar A, Alexander M, Ye W, Wand GS (2006): Sex differences in striatal dopamine release in healthy adults. Biol Psychiatry 59:966–974. [DOI] [PubMed] [Google Scholar]
- Nakajima S, Caravaggio F, Boileau I, Chung JK, Plitman E, Gerretsen P, Wilson AA, Houle S, Mamo DC, Graff‐Guerrero A (2015): Lack of age‐dependent decrease in dopamine D(3) receptor availability: A [(11)C]‐(+)‐PHNO and [(11)C]‐raclopride positron emission tomography study. J Cereb Blood Flow Metab 35:1812–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendran R, Hwang DR, Slifstein M, Talbot PS, Erritzoe D, Huang Y, Cooper TB, Martinez D, Kegeles LS, Abi‐Dargham A, Laruelle M (2004): In vivo vulnerability to competition by endogenous dopamine: Comparison of the D2 receptor agonist radiotracer (‐)‐N‐[11C]propyl‐norapomorphine ([11C]NPA) with the D2 receptor antagonist radiotracer [11C]‐raclopride. Synapse (New York, N.Y.) 52:188–208. [DOI] [PubMed] [Google Scholar]
- Narendran R, Slifstein M, Guillin O, Hwang Y, Hwang DR, Scher E, Reeder S, Rabiner E, Laruelle M (2006): Dopamine (D2/3) receptor agonist positron emission tomography radiotracer [11C]‐(+)‐PHNO is a D3 receptor preferring agonist in vivo. Synapse (New York, N.Y.) 60:485–495. [DOI] [PubMed] [Google Scholar]
- Nolen‐Hoeksema S (2004): Gender differences in risk factors and consequences for alcohol use and problems. Clin Psychol Rev 24:981–1010. [DOI] [PubMed] [Google Scholar]
- Nordström A‐L, Olsson H, Halldin C (1998): A PET study of D2 dopamine receptor density at different phases of the menstrual cycle. Psychiatry Res 83:1–6. [DOI] [PubMed] [Google Scholar]
- Pappas BA, Murtha SJ, Park GA, Condon KT, Szirtes RM, Laventure SI, Ally A (1992): Neonatal brain dopamine depletion and the cortical and behavioral consequences of enriched postweaning environment. Pharmacol Biochem Behav 42:741–748. [DOI] [PubMed] [Google Scholar]
- Payer DE, Behzadi A, Kish SJ, Houle S, Wilson AA, Rusjan PM, Tong J, Selby P, George TP, McCluskey T, Boileau I (2014): Heightened D3 dopamine receptor levels in cocaine dependence and contributions to the addiction behavioral phenotype: A positron emission tomography study with [11C]‐+‐PHNO. Neuropsychopharmacology 39:311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis RH (2011): Translating biomarkers to clinical practice. Mol Psychiatry 16:1076–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipitone J, Park MT, Winterburn J, Lett TA, Lerch JP, Pruessner JC, Lepage M, Voineskos AN, Chakravarty MM (2014): Multi‐atlas segmentation of the whole hippocampus and subfields using multiple automatically generated templates. Neuroimage 101:494–512. [DOI] [PubMed] [Google Scholar]
- Plitman E, de la Fuente‐Sandoval C, Reyes‐Madrigal F, Chavez S, Gomez‐Cruz G, Leon‐Ortiz P, Graff‐Guerrero A (2016a): Elevated myo‐inositol, choline, and glutamate levels in the associative striatum of antipsychotic‐naive patients with first‐episode psychosis: A proton magnetic resonance spectroscopy study with implications for glial dysfunction. Schizophr Bull 42:415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plitman E, Patel R, Chung JK, Pipitone J, Chavez S, Reyes‐Madrigal F, Gomez‐Cruz G, Leon‐Ortiz P, Chakravarty MM, de la Fuente‐Sandoval C, Graff‐Guerrero A (2016b): Glutamatergic metabolites, volume and cortical thickness in antipsychotic‐naive patients with first‐episode psychosis: Implications for excitotoxicity. Neuropsychopharmacology 41:2606–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puelles, L. , Martinez‐de‐la‐Torre, M. , Ferran, J.‐L. , Watson, C. (2012) Chapter 9 ‐ Diencephalon In: The Mouse Nervous System. San Diego: Academic Press; pp 313–336. [Google Scholar]
- Rabiner EA, Laruelle M (2010): Imaging the D3 receptor in humans in vivo using [11C](+)‐PHNO positron emission tomography (PET). Int J Neuropsychopharmacol 13:289–290. [DOI] [PubMed] [Google Scholar]
- Rabiner EA, Slifstein M, Nobrega J, Plisson C, Huiban M, Raymond R, Diwan M, Wilson AA, McCormick P, Gentile G, Gunn RN, Laruelle MA (2009): In vivo quantification of regional dopamine‐D3 receptor binding potential of (+)‐PHNO: Studies in non‐human primates and transgenic mice. Synapse (New York, N.Y.) 63:782–793. [DOI] [PubMed] [Google Scholar]
- Reinoso BS, Undie AS, Levitt P (1996): Dopamine receptors mediate differential morphological effects on cerebral cortical neurons in vitro. J Neurosci Res 43:439–453. [DOI] [PubMed] [Google Scholar]
- Riccardi P, Zald D, Li R, Park S, Ansari MS, Dawant B, Anderson S, Woodward N, Schmidt D, Baldwin R, Kessler R (2006): Sex differences in amphetamine‐induced displacement of [(18)F]fallypride in striatal and extrastriatal regions: A PET study. Am J Psychiatry 163:1639–1641. [DOI] [PubMed] [Google Scholar]
- Riccardi P, Park S, Anderson S, Doop M, Ansari M, Schmidt D, Baldwin R (2011): Sex Differences in the relationship of regional Dopamine release to affect and cognitive function in Striatal and Extrastriatal Regions using PET and [(18)F]Fallypride. Synapse (New York, N.Y.) 65:99–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimol LM, Hartberg CB, Nesvag R, Fennema‐Notestine C, Hagler DJ, Jr. , Pung CJ, Jennings RG, Haukvik UK, Lange E, Nakstad PH, Melle I, Andreassen OA, Dale AM, Agartz I (2010): Cortical thickness and subcortical volumes in schizophrenia and bipolar disorder. Biol Psychiatry 68:41–50. [DOI] [PubMed] [Google Scholar]
- Rousset OG, Ma Y, Evans AC (1998): Correction for partial volume effects in PET: Principle and validation. J Nucl Med 39:904–911. [PubMed] [Google Scholar]
- Schuetze M, Park MTM, Cho IYK, MacMaster FP, Chakravarty MM, Bray SL (2016): Morphological alterations in the thalamus, striatum, and pallidum in autism spectrum disorder. Neuropsychopharmacology 41:2627–2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle G, Beaver JD, Comley RA, Bani M, Tziortzi A, Slifstein M, Mugnaini M, Griffante C, Wilson AA, Merlo‐Pich E, Houle S, Gunn R, Rabiner EA, Laruelle M (2010): Imaging dopamine D3 receptors in the human brain with positron emission tomography, [11C]PHNO, and a selective D3 receptor antagonist. Biol Psychiatry 68:392–399. [DOI] [PubMed] [Google Scholar]
- Searle GE, Beaver JD, Tziortzi A, Comley RA, Bani M, Ghibellini G, Merlo‐Pich E, Rabiner EA, Laruelle M, Gunn RN (2013a): Mathematical modelling of [11C]‐(+)‐PHNO human competition studies. NeuroImage 68:119–132. [DOI] [PubMed] [Google Scholar]
- Searle GE, Beaver JD, Tziortzi A, Comley RA, Bani M, Ghibellini G, Merlo‐Pich E, Rabiner EA, Laruelle M, Gunn RN (2013b): Mathematical modelling of [(1)(1)C]‐(+)‐PHNO human competition studies. Neuroimage 68:119–132. [DOI] [PubMed] [Google Scholar]
- Seeman P (2011): All roads to schizophrenia lead to dopamine supersensitivity and elevated dopamine D2(high) receptors. CNS Neurosci Ther 17:118–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P, Ulpian C, Larsen RD, Anderson PS (1993): Dopamine receptors labelled by PHNO. Synapse (New York, N.Y.) 14:254–262. [DOI] [PubMed] [Google Scholar]
- Seeman P, Wilson A, Gmeiner P, Kapur S (2006): Dopamine D2 and D3 receptors in human putamen, caudate nucleus, and globus pallidus. Synapse (New York, N.Y.) 60:205–211. [DOI] [PubMed] [Google Scholar]
- Shotbolt P, Tziortzi AC, Searle GE, Colasanti A, van der Aart J, Abanades S, Plisson C, Miller SR, Huiban M, Beaver JD, Gunn RN, Laruelle M, Rabiner EA (2012): Within‐subject comparison of [(11)C]‐(+)‐PHNO and [(11)C]raclopride sensitivity to acute amphetamine challenge in healthy humans. J Cereb Blood Flow Metab 32:127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Quan M, Lv L, Li X, Pang L, Kennedy D, Hodge S, Harrington A, Ziedonis D, Fan X (2015): Decreased cortical thickness in drug naive first episode schizophrenia: In relation to serum levels of BDNF. J Psychiatric Res 60:22–28. [DOI] [PubMed] [Google Scholar]
- Stone JM, Morrison PD, Pilowsky LS (2007): Glutamate and dopamine dysregulation in schizophrenia–a synthesis and selective review. J Psychopharmacol (Oxford, England) 21:440–452. [DOI] [PubMed] [Google Scholar]
- Strimbu K, Tavel JA (2010): What are biomarkers? Curr Opin HIV AIDS 5:463–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studholme C, Hill DL, Hawkes DJ (1997): Automated three‐dimensional registration of magnetic resonance and positron emission tomography brain images by multiresolution optimization of voxel similarity measures. Med Phys 24:25–35. [DOI] [PubMed] [Google Scholar]
- Taki Y, Kinomura S, Sato K, Inoue K, Goto R, Okada K, Uchida S, Kawashima R, Fukuda H (2008): Relationship between body mass index and gray matter volume in 1,428 healthy individuals. Obesity (Silver Spring, Md.) 16:119–124. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Waskom ML, Dillon DG, Holmes AJ, Park MT, Chakravarty MM, Dutra SJ, Polli FE, Iosifescu DV, Fava M, Gabrieli JD, Pizzagalli DA (2015): Illness progression, recent stress, and morphometry of hippocampal subfields and medial prefrontal cortex in major depression. Biol Psychiatry 77:285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tziortzi AC, Searle GE, Tzimopoulou S, Salinas C, Beaver JD, Jenkinson M, Laruelle M, Rabiner EA, Gunn RN (2011): Imaging dopamine receptors in humans with [11C]‐(+)‐PHNO: Dissection of D3 signal and anatomy. NeuroImage 54:264–277. [DOI] [PubMed] [Google Scholar]
- Uchida H, Chow TW, Mamo DC, Kapur S, Mulsant BH, Houle S, Pollock BG, Graff‐Guerrero A (2011): Effects of aging on 5‐HT(2A) R binding: A HRRT PET study with and without partial volume corrections. Int J Geriatr Psychiatry 26:1300–1308. [DOI] [PubMed] [Google Scholar]
- van Erp TG, Hibar DP, Rasmussen JM, Glahn DC, Pearlson GD, Andreassen OA, Agartz I, Westlye LT, Haukvik UK, Dale AM, Melle I, Hartberg CB, Gruber O, Kraemer B, Zilles D, Donohoe G, Kelly S, McDonald C, Morris DW, Cannon DM, Corvin A, Machielsen MW, Koenders L, de Haan L, Veltman DJ, Satterthwaite TD, Wolf DH, Gur RC, Gur RE, Potkin SG, Mathalon DH, Mueller BA, Preda A, Macciardi F, Ehrlich S, Walton E, Hass J, Calhoun VD, Bockholt HJ, Sponheim SR, Shoemaker JM, van Haren NE, Hulshoff Pol HE, Ophoff RA, Kahn RS, Roiz‐Santianez R, Crespo‐Facorro B, Wang L, Alpert KI, Jonsson EG, Dimitrova R, Bois C, Whalley HC, McIntosh AM, Lawrie SM, Hashimoto R, Thompson PM, Turner JA (2016): Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry 21:547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit R, Kullmann S, Heni M, Machann J, Häring H‐U, Fritsche A, Preissl H (2014): Reduced cortical thickness associated with visceral fat and BMI. NeuroImage 6:307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Jayne M, Franceschi D, Wong C, Gatley SJ, Gifford AN, Ding YS, Pappas N (2002): “Nonhedonic” food motivation in humans involves dopamine in the dorsal striatum and methylphenidate amplifies this effect. Synapse (New York, N.Y.) 44:175–180. [DOI] [PubMed] [Google Scholar]
- Walther K, Birdsill AC, Glisky EL, Ryan L (2010): Structural brain differences and cognitive functioning related to body mass index in older females. Hum Brain Mapp 31:1052–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Geliebter A, Volkow ND, Telang FW, Logan J, Jayne MC, Galanti K, Selig PA, Han H, Zhu W, Wong CT, Fowler JS (2011): Enhanced striatal dopamine release during food stimulation in binge eating disorder. Obesity (Silver Spring, Md.) 19:1601–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werhahn KJ, Landvogt C, Klimpe S, Buchholz HG, Yakushev I, Siessmeier T, Muller‐Forell W, Piel M, Rosch F, Glaser M, Schreckenberger M, Bartenstein P (2006): Decreased dopamine D2/D3‐receptor binding in temporal lobe epilepsy: An [18F]fallypride PET study. Epilepsia 47:1392–1396. [DOI] [PubMed] [Google Scholar]
- Willeit M, Ginovart N, Kapur S, Houle S, Hussey D, Seeman P, Wilson AA (2006): High‐affinity states of human brain dopamine D2/3 receptors imaged by the agonist [11C]‐(+)‐PHNO. Biol Psychiatry 59:389–394. [DOI] [PubMed] [Google Scholar]
- Wilson AA, Garcia A, Jin L, Houle S (2000): Radiotracer synthesis from [(11)C]‐iodomethane: A remarkably simple captive solvent method. Nucl Med Biol 27:529–532. [DOI] [PubMed] [Google Scholar]
- Wilson AA, McCormick P, Kapur S, Willeit M, Garcia A, Hussey D, Houle S, Seeman P, Ginovart N (2005): Radiosynthesis and evaluation of [11C]‐(+)‐4‐propyl‐3,4,4a,5,6,10b‐hexahydro‐2H‐naphtho[1,2‐b][1,4]oxazin‐9‐ol as a potential radiotracer for in vivo imaging of the dopamine D2 high‐affinity state with positron emission tomography. J Med Chem 48:4153–4160. [DOI] [PubMed] [Google Scholar]
- Winterburn JL, Pruessner JC, Chavez S, Schira MM, Lobaugh NJ, Voineskos AN, Chakravarty MM (2013): A novel in vivo atlas of human hippocampal subfields using high‐resolution 3 T magnetic resonance imaging. Neuroimage 74:254–265. [DOI] [PubMed] [Google Scholar]
- Wong KK, Muller ML, Kuwabara H, Studenski SA, Bohnen NI (2012): Gender differences in nigrostriatal dopaminergic innervation are present at young‐to‐middle but not at older age in normal adults. J Clin Neurosci 19:183–184. [DOI] [PubMed] [Google Scholar]
- Woodward ND, Zald DH, Ding Z, Riccardi P, Ansari MS, Baldwin RM, Cowan RL, Li R, Kessler RM (2009): Cerebral morphology and dopamine D2/D3 receptor distribution in humans: A combined [18F]fallypride and voxel‐based morphometry study. Neuroimage 46:31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Lui S, Deng W, Yao L, Zhang W, Li S, Wu M, Xie T, He Y, Huang X, Hu J, Bi F, Li T, Gong Q (2015): Altered cortical thickness related to clinical severity but not the untreated disease duration in schizophrenia. Schizophr Bull 41:201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A, Tanaka M (2016): Two types of neurons in the primate globus pallidus external segment play distinct roles in antisaccade generation. Cereb Cortex (New York, N.Y.: 1991) 26:1187–1199. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information BMI
Supporting Information Gender
Supporting Information Figure01


