Abstract
Objective
Focal epilepsies, such as temporal lobe epilepsy (TLE), are known to disrupt network activity in areas outside the epileptogenic zone [Tracy et al., 2015]. We devised a measure of temporal instability of resting state functional connectivity (FC), capturing temporal variations of BOLD correlations between brain regions that is less confounded than the “sliding window” approach common in the literature.
Methods
We investigated healthy controls and unilateral TLE patients (right and left seizure focus groups), utilizing group ICA to identify the default mode network (DMN), a network associated with episodic memory, a key cognitive deficit in TLE. Our instability analyses focused on: (1) connectivity between DMN region pairs, both within and between TLE patients and matched controls, (2) whole brain group differences between region pairs ipsilateral or contralateral to the epileptogenic temporal lobe.
Results
For both the whole brain and a more focused analysis of DMN region pairs, temporal stability appears to characterize the healthy brain. The TLE patients displayed more FC instability compared to controls, with this instability more pronounced for the right TLE patients.
Significance
Our findings challenge the view that the resting state signal is stable over time, providing a measure of signal coherence change that may generate insights into the temporal components of network organization. The precuneus was the region within the DMN consistently expressing this instability, suggesting this region plays a key role in large scale temporal dynamics of the DMN, with such dynamics disrupted in TLE, putting key cognitive functions at risk. Hum Brain Mapp 38:528–540, 2017. © 2016 Wiley Periodicals, Inc.
Keywords: functional connectivity instability, dynamic resting‐state fMRI analysis, temporal lobe epilepsy, default mode network, precuneus
Abbreviations
- AAL
Anatomical automatic labeling
- DMN
Default mode network
- FC
Functional connectivity
- FD
Framewise Displacement
- FDR
False discovery rate
- HC
Healthy control
- MTS
Mesial temporal sclerosis
- rsfMRI
Resting state functional MRI
- TLE
Temporal lobe epilepsy
INTRODUCTION
Temporal lobe epilepsy (TLE) is the most frequent form of refractory epilepsy, and is commonly associated with mesial temporal sclerosis (MTS). However, there is a growing body of evidence that brain abnormalities in TLE are not limited to the epileptogenic region, but extend into widespread areas of the ipsilateral and contralateral hemispheres [Gross, 2011; Tracy and Doucet, 2015]. Structural and functional abnormalities both within and outside the epileptogenic zone have been observed [Catenoix et al., 2005; Lieb et al., 1991]. The dynamic nature of functional connectivity (FC) in resting state functional MRI (rsfMRI) studies is emerging as a key component for understanding network behavior of the brain in both healthy and pathologic populations [Damoiseaux, 2012], including epilepsy [Bettus et al., 2010; Bettus et al., 2009; Maccotta et al., 2013; Morgan et al., 2011; Pereira et al., 2010]. Intermittent distortion of normal FC in the form of seizures is a defining feature of epilepsy [Chen et al., 2012; Holmes et al., 2014; Zhang et al., 2009], but the dynamic connectivity changes that occur in the interictal state are unknown.
Most resting‐state work has implicitly or explicitly assumed that FC properties of both healthy and TLE subjects are static in time, differing only in response to external stimulus demands with known temporal characteristics. Recent studies, however, have demonstrated that healthy subjects exhibit differences in connectivity over time in the absence of known changes in stimulus input and timing [Barttfeld et al., 2015; Chang and Glover, 2010; Hansen et al., 2015; Hutchison et al., 2013]. Changes in connectivity can include, but are not limited to, shifts in regional bivariate correlation (i.e., temporal coherence). Integrating and collapsing FC signals over time may obscure important features of network behavior, including the disruptions caused by the accrual and spread of seizures. For example, if aspects of pathological connectivity are transient in time, or are characterized by fluctuations whose magnitude is time varying, then FC measures collapsed over extended time intervals will fail to capture them.
FC dynamics will, of course, vary both temporally and regionally, and do so at several time scales [Honey et al., 2007] [for an example in epilepsy cellular electrophysiology see Kramer et al. (2010)]. Comparable work utilizing the larger temporal time scale of the BOLD signal is just beginning to emerge. Laufs et al. [2014] utilized a sliding window approach, with a hippocampal seed, and found changes in FC were larger in TLE patients compared to controls in several prefrontal areas, precuneus, occipital cortex, and superior frontal gyrus.
In studies of dynamic connectivity, differences between connectivity measures (coherence, correlation) calculated within separate time windows are a natural quantity of interest. Interpreting fluctuations in FC, in particular distinguishing meaningful changes from those due to chance variation, is difficult in fMRI studies due to factors such as low SNR, variation in characteristics of the BOLD signal over time, and other physiological or scanning artifacts. FC measures computed with overlapping sliding windows, especially short windows, exacerbate these issues. For instance, random noise under static correlation conditions (including uncorrelated white noise time series) will induce smooth fluctuations over time in sliding window FC measures [Hutchison et al., 2013; Lindquist et al., 2014; Robinson et al., 2008] which can easily be misinterpreted as meaningful changes in connectivity. The strong statistical dependence between neighboring windows inhibits clear assessment of the significance of changes in FC over time. Recent neuroimaging work demonstrating the problems with sliding window analyses through simulation studies, have advocated alternate approaches. In fields such as climatology and financial time series analysis, for instance, the sliding windows have been largely rejected due to the issues described above. Summary statistics for a sequence of correlations may be useful as a comparative tool, as in Laufs et al. [2014]; however, it is difficult to assign statistical significance to the behavior of the sequences themselves.
In this work, we seek to understand the relative stability of FC over time. To avoid potential pitfalls in sliding window analyses, we propose a simple and novel characterization of instability using non‐overlapping windows to capture meaningful differences between neighboring temporal period using, inter‐regional correlation to assess the magnitude of FC. We directly compare instability between healthy controls (HCs) and TLE patients, first within the default mode network (DMN) and then across the whole brain. We chose the DMN as it is one of the major resting‐state networks [Raichle et al., 2001], implicated in a wide range of processes relevant to TLE, such as episodic memory [Doucet et al., 2014]. More generally, the DMN has been described as abnormal in TLE patients compared to matched HCs [Doucet et al., 2014; Haneef et al., 2012; Zhang et al., 2010]. For the TLE patients, we also explore and report instability findings specific to the epileptogenic hemisphere. A sensitivity analysis of the effects of temporal window length and spatial definition of brain regions reveals that our results are robust to different modeling choices in the spatial and temporal domains.
METHODS
Subjects
A total of 95 patients with refractory TLE were recruited from the Thomas Jefferson University Comprehensive Epilepsy Center (55 LTLE and 40 RTLE patients). The side of seizure focus was lateralized based using a combination of EEG, MRI, PET, and neuropsychological testing [Sperling et al. 1992]. All patient participants met the following inclusion criteria: unilateral temporal lobe seizure onset through surface video/EEG recordings (i.e., a single unilateral temporal lobe focus); normal MRI or MRI evidence of mesial temporal pathology such as a MTS in the epileptogenic temporal lobe; concordant PET finding of hypometabolism in the temporal lobe (available for most patients), and no patient had a non‐concordant PET. TLE patients were excluded from the study for any of the following: medical illness with central nervous system impact other than epilepsy; prior or current alcohol or illicit drug abuse; extratemporal or multifocal epilepsy; contraindications to MRI; psychiatric diagnosis other than Axis‐I Depressive Disorder; or hospitalization for any Axis I disorder listed in the Diagnostic and Statistical Manual of Mental Disorders, IV. Depressive Disorders were allowed given the high co‐morbidity of depression and epilepsy [Tracy et al., 2007]. Table 1 outlines the demographic and clinical characteristics of the subjects. The Edinburgh handedness scale was used as a measure of handedness [Oldfield, 1971].
Table 1.
Demographic and clinical information of sample
| Right TLE | Left TLE | Controls | |
|---|---|---|---|
| N (Females) | 40 (23) | 55 (32) | 75 (32) |
| Age (M ± SD) | 38.8 ± 12.3 | 41.3 ± 11.8 | 36.5 ± 11.4 |
| Right‐handers | 35 | 50 | 68 |
| Education (M ± SD) | 14.3 ± 2.7 | 14.1 ± 2.3 | 16.4 ± 2.6 |
| MTS | 16 | 23 | N.A. |
| Age of Onset | 21.6 ± 11.6 | 23.7 ± 14.3 | N.A. |
A total of 75 HCs were also recruited from the Thomas Jefferson University community, in order to match the patient participants in age and gender (Table 1). All controls were free of psychiatric or neurological disorders based on a health screening measure.
This study was submitted for approval by the Institutional Review Board for Research with Human Subjects at Thomas Jefferson University and all participants provided a written informed consent.
Imaging Acquisition
All participants were scanned on a 3‐T X‐series Philips Achieva MRI scanner (Amsterdam, the Netherlands). Five minutes of rsfMRI was collected from all participants with a single‐shot echoplanar gradient echo imaging sequence acquiring T2* signals (120 volumes; 34 axial slices; TR = 2.5s, TE = 35 ms; FOV = 256 mm, 128 × 128 data matrix voxels, flip angle = 90°, slice thickness = 4 mm). Participants lay in a foam pad to comfortably stabilize the head, were instructed to remain still, not fall asleep, and keep their eyes closed during the entirety of the scan. Each imaging series started with three discarded scans to allow for signal stabilization. Prior to collection of the T2* images, T1‐weighted images (180 slices) were collected using an MPRage sequence (TR = 640 ms; TE = 3.2 ms, FOV = 256 mm, 256 × 256 data matrix voxels, flip angle = 8°) in positions identical to the functional scans to provide an anatomical reference.
Imaging Processing
All imaging data were preprocessed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8). Slice time correction, image realignment within a session, motion regressor calculation, normalization into a standard template (MNI 152), segmentation (cerebro‐spinal fluid, gray and white matter), and image smoothing was carried out using standard parameters and methods [see Doucet et al. (2015)]. Note, our normalization process was informed by structural scans. These structural scans were registered to the MNI152 template, and the functional data was then placed in this new T1 space. Sources of spurious variance were then removed from the data through linear regression (head motion, cerebro‐spinal fluid and white signals). Finally, fMRI data were temporally filtered at 0.008 < f < 0.1 Hz using the REST Toolbox [Song et al., 2011].
Statistical Analysis
To assess the instability of FC in the resting state, we measured fluctuation over time in the correlation between pairs of brain regions. In this study, we define temporal stability as the degree of change (or lack thereof) of correlation in the rsfMRI signal across the five time periods measured, as captured by our instability index. Changes in bivariate correlation in the resting state signal between neighboring time periods (one and two, two and three, etc) are combined into an overall measure of temporal instability. Because of the variability in network measures computed over short time intervals (time scales on which meaningful changes may occur), we have chosen to restrict our analysis to a relatively simple, robust measure (pairwise correlation), rather than more variable, sensitive global network properties.
We analyzed instability in the TLE and HC groups across two different networks. The first is a group of regions identified as a component derived through group ICA [Jenkinson et al., 2012] closely matching a standard template for the DMN. A total of 8 ROI's emerged as part of the DMN. The second is a whole‐brain analysis of a network of 90 cortical regions segmented through the anatomical automatic labeling (AAL) atlas [Tzourio‐Mazoyer et al., 2002]. For each, a simple measure of the temporal instability of pairwise FC was employed to assess whether TLE patients differed from controls with respect to the degree of instability, and, if so, to identify which pairs of regions showed differences.
To measure instability, the 5 minute scanning window was divided into 5 non‐overlapping windows of 24 TRs each. Previous studies found that a window length of 30∼60s is efficient for capturing cognitive status, providing a good trade‐off between the ability to resolve dynamics and the quality of connectivity estimation [Allen et al., 2014; Shirer et al., 2012]. Selection of window length requires a somewhat arbitrary choice by the researcher, but can be guided by considerations of power and validated using a sensitivity analysis. Relatively shorter window lengths have the advantage of greater homogeneity in FC within the window, which will tend to increase the magnitude of differences between neighboring windows when the true underlying FC changes, and thus ability of the instability index to capture true fluctuations. The disadvantage of relatively shorter windows is increased random sampling variability of the computed correlation coefficient due to the small number of TRs used to compute it, increasing the noise in the instability index and decreasing the power to detect between group‐differences where they exist. Based on these considerations, our goal was to choose the shortest window length that would not unduly increase the variability of the sample correlation coefficient. The standard error of the sample correlation coefficient decreases nonlinearly with the number of TRs used to compute it. For example, for independent and identically distributed (i.i.d) data, an increase in sample size from 18 to 24 will decrease the standard error of the correlation coefficient by 21%, but an increase of the sample size from 24 to 30 will only decrease the SE by 11%, and an increase from 30 to 36 will decrease the SE by 9%. Because of the sharp increase in the SE of the correlation coefficient between below 24 TRs, windows of length 24 were selected. To assess the sensitivity of this choice, we compared the results to analyses from window lengths of 20 and 30 and Bayesian information criterion [BIC; Schwarz (1978)] values were computed for each window length. In short, our window length was chosen to obtain a good balance between sufficient BOLD signal, and a sufficient number of discrete time points to capture any bona fide process of connectivity change between brain regions.
Correlations between pairs of regions were computed in each window. For a particular subject i and a given pair of regions, the degree of fluctuation in correlations over time (K = instability index) was given by
where ρt is the correlation between the pair of regions over time window t, t = 1, … 5. This index is an overall metric of the changes in correlation across neighboring windows. Larger values indicate more instability in FC over time. A schematic for the measure of instability is shown in Figure 1.
Figure 1.
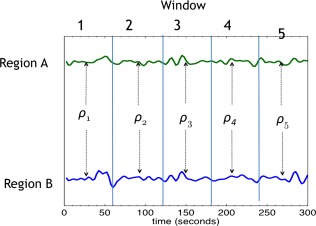
Bivariate correlation coefficients are computed over non‐overlapping time windows of 24 TRs (60s). The squared differences between neighboring windows are then summed to create an index of correlation instability. [Color figure can be viewed at http://wileyonlinelibrary.com.]
Residual head motion will cause fluctuations in FC [Van Dijk et al., 2012], and is, therefore, important to properly address in studies of dynamic FC. Accordingly, all cases with more than 2 mm head motion were discarded. To further address the micromotion components, we calculated Framewise Displacement (FD) and the numbers of volumes that have a FD over 0.5 are minimal for both groups (3.3% of volumes for TLE; 3.7% for controls). Also, head motion parameters were included in the design matrix, removing motion‐related variance from the resting state signal utilized in our analyses. Beyond these standard realignment correction procedures, we analyzed the relationship between estimated head motion parameters and instability (see Supporting Information Data, part 1). Another important consideration is the possibility that interictal discharges occurred during each data window, a factor that could influence the BOLD signal. It is important to note that any such interictal activity during the resting state period would not be systematic across subjects, and, thus, would cancel out, not resulting in any systematic correlation with our instability index. As a precaution, however, we did check our EEG summary documents to classify the TLE patients as having interictal activity or not, and found no relation to our instability index.
Lastly, we assessed linear relationships between our instability index and covariates including education, the presence of MTS, age of seizure onset, chronological age, disease duration, handedness, type of seizure medication (sodium channel versus non‐sodium channel blocker, or both), and full scale IQ.
DMN Analysis
We performed a group ICA analysis using the MELODIC procedure in the software package FSL [Smith et al., 2004] by temporally concatenating the time courses of all subjects (patients and HCs) and computed a single set of components for the entire group of subjects using this 2‐D matrix. In this method, temporal activity across each component is correlated within subjects, but the temporal structure for a given component need not be consistent across subjects. The component best matching a standard template of the DMN was selected using a z‐score matching procedure [Greicius et al., 2009], and is shown in Figure 2.
Figure 2.
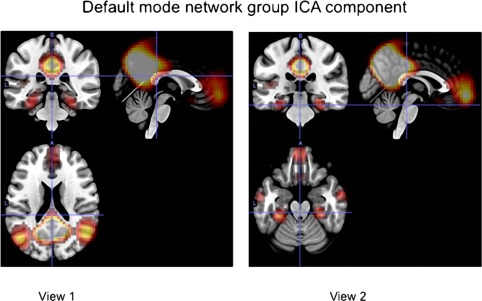
The group component best matching the DMN component. [Color figure can be viewed at http://wileyonlinelibrary.com.]
Using the DMN component, spatial regions within the component were determined by selecting voxels that exceeded a threshold of 50% (posterior probability) for inclusion in the component, then subdividing this set of voxels into spatially distinct regions.
Spatial segmentation of the DMN was performed first by identifying spatially disjoint regions, and then by manually separating a large connected region consisting of the precuneus, and left and right inferior parietal regions, into three sub‐divisions. We also excluded regions that were less than 10 voxels in size, with the largest excluded region consisting of 6 voxels. This yielded a total of 11 regions. For other details on the spatial segmentation of the DMN, see Supporting Information Data, part 2.
Regions specific to each subject were created by combining the segmented component map with subject‐specific components identified by dual regression [see Beckmann et al., 2009 for a complete description of the procedure]. The group‐level average map of the DMN was then applied as a mask to each subject‐specific component map to create a unique set of regions for each subject while restricting regions of interest to those showing spatial consistency across subjects. Due to individual differences, some regions of the whole‐group mask did not overlap with the subject‐specific masks. Regions that did not have an overlap of at least 10 voxels in at least 80% of subjects were excluded, leaving 8 regions. Differences between groups in the average number of overlapping voxels for each region were evaluated to rule out region size as a potential confounder, and no such group differences were found. A goodness of fit statistic [average of z‐scores from dual regression results within DMN template minus average of z‐scores outside DMN template, Greicius et al., 2004] show a small difference between TLE patients and controls, however the difference is minimal and does not induce a difference in the number of voxels included in the subject‐specific components after thresholding, as described above (see details in Supporting Information data, part 2).
For each subject, the instability index was calculated for each of the 28 pairs between the 8 DMN regions. Differences between patients and controls, and between LTLE and RTLE patients were assessed for each pair of regions. Individual pairs were tested for group differences in instability, and the results over all pairs were corrected for multiple comparisons using a false discovery rate (FDR) [Benjamini and Hochberg, 1995] procedure.
Our interest is in using the instability index to study the dynamics of bivariate connectivity. In interpreting observed between‐group differences in the instability index, we sought to exclude the possibility that these differences were explained by phenomena other than changes in the FC strength between regions. To this end we examined two rival explanations. First, when the true correlation coefficient between regions is closer to 0, its sampling variance is increased, causing larger fluctuations between time windows even if the true correlation remains constant. This excess variance reflects the magnitude of the correlation rather than its instability. To test for this, we compared the mean correlation over the entire scanning period for each pair, and compared this between groups, thereby testing whether a difference in overall correlation explains any difference in instability. Second, we looked for differences between groups in the stability of the BOLD signal variance. Changes in the variances of the individual time series will be reflected in the correlation coefficient. We examined variance stability using a formula identical to the one used for calculating our main instability measure. Variance instability for each region in the DMN was then compared between groups to assess whether differences in variance instability could account for differences in correlation instability. If neither the overall correlation magnitude, nor the instability of the region‐wide BOLD signal variance differed between groups, we can conclude that differences in the instability index reflect differences in the magnitude of the regional connectivity over time. For discussion of other confounds related to instability see Supporting Information Data, part 3.
Whole Brain Analysis
Instability was also measured for pairs of the 90 regions across the brain. Instability analysis was restricted to pairs of regions showing high degrees of FC. For each subject, a correlation matrix was computed across regions using the entire scanning interval. The arithmetic mean of these matrices across subjects was computed and thresholded at the α = 0.01 significance level (r = 0.47). Overall instability was compared between subjects based on group (HC, RTLE, LTLE), as well as between regions within subjects. Overall instability was measured by averaging across all pairs within a subject. The differences in mean instability between HC, RTLE and LTLE patients were assessed using ANOVA.
In addition to overall instability, differences between RTLE and LTLE patients, and within‐subjects differences between pairs of regions that are ipsilateral to the epileptogenic zone, contralateral to the epileptogenic zone, or bilateral, were also analyzed. For LTLE and RTLE, ipsilateral and contralateral pairs involve regions in the same or opposite hemisphere as the seizure focus, and bilateral pairs have a region in each hemisphere. To account for the correlation between repeated measures of instability within subjects, and to model variation in overall instability between subjects, a mixed‐effects regression model was used with group (LTLE, RTLE), pair location (ipsilateral, contralateral, bilateral) as predictors. A random effect describing variability at the subject level was included as a random intercept, and the correlation between pairs within a subject was assumed to be unstructured. The mixed‐effects model was fit using the “lme4” package in the statistical programming language R. The effects associated with patient group (RTLE vs LTLE), and region pair classification with respect to epileptogenic side, as described by the fixed effect parameters in the mixed‐effects regression model, were estimated along with associated confidence intervals and tested for statistical significance. In separate models, the effects associated with disease duration (i.e., years since epilepsy diagnosis), exposure to seizure medication, and the presence of MTS (i.e., presence/absence) were adjusted for by adding these variables as covariates to the above regression model.
RESULTS
DMN Analysis
Using group probabilistic ICA, 11 spatial components were derived from the data. Of these, a component best matching the DMN was selected, and subdivided into spatial regions using the procedure described in the methods section, resulting in 8 regions consistently associated with the DMN component across subjects. These regions were precuneus, left and right inferior parietal, left and right middle temporal, left and right middle frontal, and medial frontal (Figure 2).
For each pair of regions, instability was calculated for each subject. After assessing each of the 28 pairs of regions for differences between groups and correcting for multiple comparisons (FDR procedure), we found 5 of 28 pairs of regions that show significant differences between HC and TLE (right and left combined) (see Figure 3). Four other pairs showed differences that were significant at the 0.05 level before FDR correction. In the case of each significant difference, the TLE group was, on average, more unstable than the HC group. There were no significant differences between RTLE and LTLE patients after FDR correction.
Figure 3.
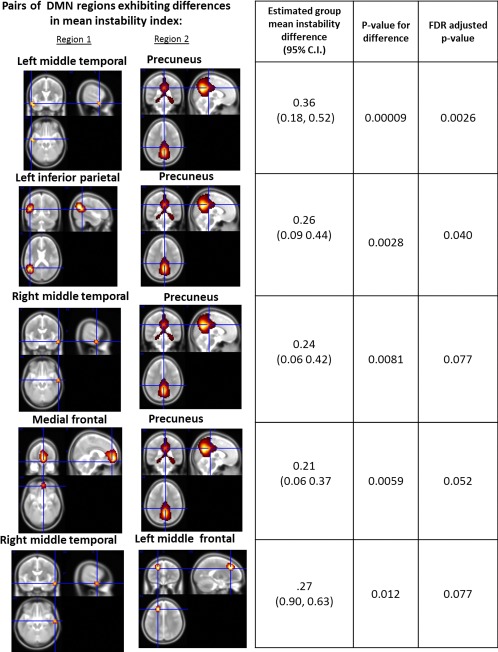
Pairs of DMN regions that showed significant group‐level difference in correlation instability. Columns from left to right show the two regions comprising the pair, the estimated difference in mean stability between TLE patients (left and right combined) and HCs with associated 95% confidence interval, unadjusted P‐value for the test of the null hypothesis of no difference in instability, and FDR adjusted P‐value. [Color figure can be viewed at http://wileyonlinelibrary.com.]
The pairs that showed significant differences after FDR correction were various combinations of five regions: precuneus, left and right middle temporal lobes, medial frontal, and left middle frontal regions. Results for these five pairs are shown in Figure 3. The precuneus was the most common region emerging from this analysis. As an example of the instability involving this and other regions in TLE, Figure 4 provides a graphical depiction of the change (i.e., variance) of the difference in correlations as one moves across the time intervals of our study. One can see when comparing the first to second periods (1–2), second the third (2–3), etc., the difference in the correlation between the successive periods is consistently higher in the two patient groups compared to controls.
Figure 4.
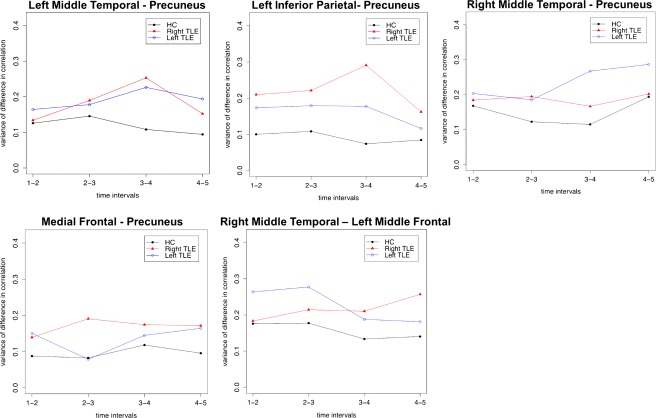
The variance of differences in correlation from one time interval to the next, var (ρ t − ρ t‐1), for pairs showing significant differences in instability. Note, the y‐axis plots variability across the time windows for each group. [Color figure can be viewed at http://wileyonlinelibrary.com.]
As noted, we assessed linear relationships between our instability index and covariates including education, presence of MTS, age of seizure onset, chronological age, disease duration, handedness, seizure medication, and full scale IQ. When instability was averaged across pairs within the DMN or across the brain, it showed no significant relationship with disease duration. However, for two DMN region pairs (those with the largest between‐group differences in instability), there was evidence of an association between instability and disease duration. These pairs involved the precuneus and left inferior parietal (P = 0.003), and the precuneus and left middle temporal (P = 0.03) cortices, with higher levels of instability associated with longer disease duration. These correlations appeared to be a general effect, as the correlations for left and right TLE were essentially equivalent. There was no significant relationship between chronological age and mean instability, either averaged over all cortical pairs (P = 0.76), or over all pairs within the DMN (P = 0.57). When region pairs within the DMN were assessed for their relationship to age, instability in the precuneus and left inferior parietal pair showed a significant positive relationship with age (P = 0.01), but other pairs did not. Adjusting for age effects did not affect the primary finding involving the observed between‐group difference in instability. For handedness, age of seizure onset, full scale IQ, education, and seizure medication, there was no significant linear association with instability, either in the DMN or whole brain analyses.
Whole Brain Analysis
Instability was analyzed across the brain between pairs of 90 cortical regions. After excluding pairs that did not exhibit across all subjects a highly significant mean correlation (using an α = 0.01 threshold), 253 out of a possible 4005 pairs were retained for each subject. An adjacency matrix displaying mean instability for patients and controls is shown in Figure 5.
Figure 5.
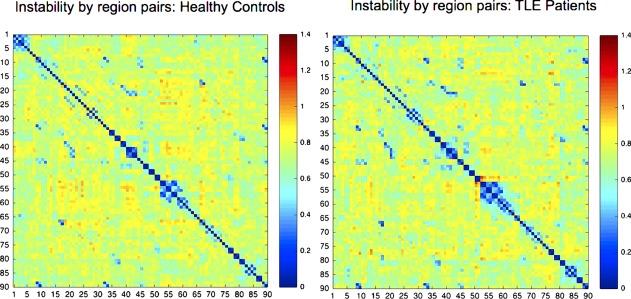
Adjacency matrix for instability measure across all connections analyzed (90 by 90 ROI's). [Color figure can be viewed at http://wileyonlinelibrary.com.]
An overall measure of instability was created for each subject by averaging the instability index across all 253 pairs. Average instability was significantly higher (P = 0.0002) in both RTLE and LTLE groups vs. HCs, with larger differences observed between HC and RTLE, than between HC and LTLE. There was weaker evidence for an overall difference between LTLE and RTLE patients (P = 0.08).
Variation in instability across individual region‐pairs was also analyzed within RTLE and LTLE patients. Utilizing a mixed effects regression model with instability at each region pair as the outcome, differences in instability were also analyzed with respect to whether the pair was located in the epileptogenic or contralateral non‐epileptogenic hemisphere, or crossed hemispheres (bilateral pair). Because of the skewed distribution of instability for individual pairs, a normalized log transformation was applied to the instability index. Ipsilateral and contralateral pairs were found to be significantly more unstable than bilateral pairs. However, no significant differences were found between ipsilateral and contralateral pairs. We then examined whether controlling for subject‐level differences in duration of the epilepsy disease (time since diagnosis), presence of MTS, or age of onset would alter the above effect, and they did not. These results are shown in Table 2.
Table 2.
Estimated unique effects on log instability associated with group, age of onset, ipsilaterality to epileptigenic zone, contralaterality to epileptogenic zone, disease duration, and MTS status
| Effect | Estimate | Standard error | 95% Confidence interval |
|---|---|---|---|
| Intercept | −1.36 | 0.055 | (−1.45, −1.23) |
| Group: left‐right | −0.063 | 0.048 | (−1.56, 0.032) |
| Epilepsy duration | 0.00016 | 0.0019 | (−0.0048, 0.0022) |
| Ipsilateral vs bilateral | 0.135 | 0.019 | (0.098, 0.173)a |
| Contralateral vs bilateral | 0.142 | 0.019 | (0.102, 0.172)a |
| Ipsilateral vs contralateral | −0.0072 | 0.02 | (−0.044, 0.03) |
| MTS | −0.036 | 0.053 | (−0.14, 0.068) |
| Age of onset | −0.0013 | 0.0018 | (−0.005, 0.002) |
Significantly different from 0 at 0.00001 level.
Sensitivity Analysis
We evaluated the robustness of our results with respect to the choice of window length. The results using 5 windows of 24 TRs each were compared to those using 6 and 4 windows with 20 and 30 TRs each, respectively. In the DMN, increased instability in the TLE group versus controls was also observed in using windows of length 20 and 30, although the statistical significance was reduced in 20 TR windows (P‐value = 0.11 for group differences averaged over all DMN pairs) due to increased within‐group variance. As expected, the both the magnitude of the mean difference in instability and the within‐group instability variances were largest for the 20 TR windows, and decreased as window length increased. Similar results were observed in the whole‐brain analysis. The results presented for 24 TR windows were judged to have the best balance of mean and variance, reflected in the highest effect sizes. As a more objective criteria, BIC values are computed for the models with 20, 24, and 30 TR windows. BIC is computed assuming multivariate normality of the spatially averaged BOLD values in the 8 DMN ROI, with covariance matrices assumed to be homogenous within non‐overlapping windows of differing length. We find that of the three potential window values, the BIC value for 24 TRs was the lowest (1,010,011.3 for 24 TRs vs 1,010,365.6 and 1,010,445.3 for 30 and 20 TRs respectively), which validates our more subjective assessment based on observed variability and effect sizes.
The general consistency of results between the DMN analysis, which uses an ICA‐based parcellation, and the anatomical‐atlas based whole brain analysis, also reflects the robustness of our results with respect to modeling choices in the spatial as well as temporal domain.
To assess whether differences in correlation instability could be explained by differences in the magnitude of correlation over the entire scanning interval (via differences in sampling variability, as explained in the statistical methods section), correlation over the entire time period was compared between groups for each pair of regions. After FDR correction, two pairs out of 28 showed significant differences in overall correlation, with one pair (the precuneus and left middle temporal lobe) identified as having significant differences in instability. Averaged across subjects, the difference in correlation for this pair was 0.46 in the TLE group vs 0.58 in the HC group, corresponding to a difference in standard errors for the sample correlations of 0.189 vs. 0.174, respectively, within each of the 24 TR windows. While this difference could account for some of the increased instability in the TLE group for the precuneus ‐ left middle temporal pair, we believe that the general inconsistency when comparing the instability and overall correlation measures indicates that the instability index is likely capturing an effect distinct from any group difference related to sampling variability or more general whole brain effects.
Differences in the stability of variance between groups was also examined, with no significant differences evident (e.g., HC vs. TLE; LTLE vs. RTLE) for any of the 8 DMN regions. This indicates that the observed correlation instability reflects fluctuations in the connections between regions, rather than fluctuations in the variance of the individual region time series.
Comparison to Static FC Analysis
The results on between‐group differences in overall (static correlation) described above highlight both the utility distinctiveness of our instability index in terms of understanding group differences in the behavior of brain networks. Without this information, the dynamic change occurring in some but lacking in other pairwise connections would have been invisible, both with reference to our DMN and whole brain data. Within the DMN, we see some evidence of reduced overall FC in the TLE patient group in a subset of region pairs, which differs from the subset of region pairs exhibiting differences in instability. For the 28 pairs, nine showed group differences in overall correlation that were significant at the 0.05, level. After FDR correction, two pairs out of 28 showed significant differences in overall correlation, with one pair (the precuneus and left middle temporal lobe) also identified as having significant differences in instability (one additional pair of the five showing instability differences also showed a mean difference at the 0.05 level before FDR correction). Thus, the complementary rather than redundant information provided by the instability analysis, compared to overall correlation data, contributes additional information in understanding FC characteristics in our TLE patients.
Analysis of Head Motion as a Confounder
We tested whether head motion was a potential confounder in any observed relationship between clinical status (patient vs. control) and instability. Head motion was characterized in two ways: maximum absolute displacement over the 120 TR scanning period for each of 6 motion measures (displacement and rotation in three dimensions), and the average absolute displacement over the scanning period of the 6 motion parameters. The squared maximum and squared average parameters were also included in the analysis. In the DMN, neither maximum nor average head motion was significantly associated with overall (averaged across region pairs) instability. In the whole‐brain analysis, regression analyses revealed that averaged across all pairs, a weak (R 2 = 0.07, adjusted R 2 = 0.03 for maximum displacement, R 2= 0.08, adjusted R 2 = 0.05 for mean displacement) relationship emerged between the instability index and head motion parameters. There was no evidence, however, of an association between clinical status and head motion parameters (P = 0.18), and the instability differences between groups were unaffected by adjusting for motion parameters as covariates in multiple regression models involving either the DMN or the whole brain analyses. Thus, while there is some weak association between instability and head motion, it is not a confounder for the relationship between group status and instability and, therefore, does not explain our observed differences between groups.
DISCUSSION
We report data on a simple, novel measure of instability, one that uses non‐overlapping time windows, thereby avoiding the problem of temporal dependence and autocorrelation across sequential windows. With regard to both the whole brain and a more focused analysis of the DMN, we found that temporal stability appears to characterize the healthy brain. TLE patients, as a whole, displayed greater instability than matched, HCs, and no differences in this regard emerged when comparing the right and left TLE groups. In our analysis of the whole brain, both TLE groups showed higher overall levels of instability relative to controls, with this difference larger for the right TLE group. In our DMN analysis, we found both TLE groups displayed greater FC instability over time compared to HCs. The precuneus appeared to be the region within the DMN consistently expressing this instability in its relationship with other regions. A sensitivity analysis of the effects of temporal window length and the fact that our results are consistent across regional and whole brain analyses, suggests that our modeling choices were robust in both spatial and temporal domains. Importantly, these FC effects were not related to group differences in chronological age, gender, handedness, the presence of MTS, seizure medication, the overall magnitude of the correlation across the measured time period, nor, lastly, the fluctuations in the variance of the individual time series.
This finding for the precuneus raises the possibility that this structure plays a key role in the large scale temporal dynamics of the DMN. Our data suggest that regardless of the side of the epilepsy, the precuneus displays instability in the setting of both left and right epileptogenic regions compared to controls (i.e., our precuneus/middle temporal cortex finding). This implies that when the FC of this major DMN hub is disrupted by TLE, the disruption is not strictly limited to the epileptogenic hemisphere. Moreover, instability in some of these precuneus connections was associated with disease duration, raising the possibility that connections to this key brain and DMN hub may become more temporally unstable as the seizures of TLE become more intractable and chronic. It is worth noting that our instability finding is consistent with diffusion tensor white matter showing that TLE patients express abnormalities in precuneus regions and precuneus‐to‐mesial‐temporal tracts [Campos et al., 2015; Liao et al., 2011; Nazem‐Zadeh et al., 2016]. This may suggest that the instability we found associated with the precuneus potentially stems from white matter deficits.
The instability of connections involving precuneus is broadly consistent with the findings of Laufs et al. [2014], who in an analysis of regions showing higher BOLD signal variance in TLE vs HC subjects found greater fluctuations in connectivity between left hippocampus (the seed region) and precuneus. We provide, however, a more complete description of differences in FC stability across the brain and more carefully account for factors such as education, age of onset, chronological age, disease duration, handedness, seizure medication, full scale IQ, MTS, and brain location with respect to the epileptogenic hemisphere. While the creation of non‐overlapping windows may reduce statistical power if the windows do not accurately reflect the time scale of true fluctuations, our results are not subject to the potential misinterpretation associated with sliding window analyses [Lindquist et al., 2014].
Our analyses focused on the epileptogenic hemispheres revealed that region pairs strictly unilateral in their connectivity were more unstable than bilateral pairs. This effect did not appear related to MTS, nor left or right TLE status. The reason for this is unclear, but it may suggest that communication involving both hemispheres provides temporal stability to the DMN system in a way that solely unilateral connectivity does not. Since this was the case both for the healthy and epileptogenic hemispheres, the implication is that disease‐related temporal instability in functional connections are more likely to normalize when larger areas of cortex provide input to the functional network.
With regard to the overall instability observed in TLE patients relative to controls, both in our DMN and whole brain analyses, it is unclear whether this arises from pathologic mechanisms directly related seizure impact (i.e., seizure spread) or a reactive, protective, and adaptive mechanism (however flawed it may be), to prevent loss of functional integrity [Tracy et al., 2014]. Unfortunately, in this study, we lack correlations with cognitive performance variables to test the degree to which the instability we observed is functionally adaptive. Certainly, the link between the DMN and episodic memory [Doucet et al., 2014] raises questions as to whether the well‐known memory disorder in TLE [Hermann et al., 2008; Lin et al., 2012] is related to temporal instability of FC within the DMN, particularly driven by the precuneus.
Importantly, in this work we present a new measure of FC that captures temporal variations in the BOLD signal between regions, without relying on the problematic “sliding window” approach that is common in the literature. We should emphasize that most previous studies have presumed the resting state signal is stable over time. Our findings, as well as others, are challenging this view. For instance, without this information, the dynamic connectivity present in some, but absent in the other regions pairs we examined would have been invisible. By providing a measure of signal coherence change over time we hope to generate insights into the temporal components of network organization (i.e., stability), with the hope that this will yield important clinical applications. For instance, in the setting of epilepsy, the data we report here may have direct relevance for modeling the effects of the inter‐ictal period in our epilepsy patients, a period of potential epileptiform activity whose impact on cognition and behavior has been particularly difficult to systematically characterize.
Conflict of interests
None of the authors have any conflict of interest to disclose.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The authors thank Dr. Dorian Pustina for his participation in the data acquisition.
REFERENCES
- Allen EA, Damaraju E, Plis SM, Erhardt EB, Eichele T, Calhoun VD (2014): Tracking whole‐brain connectivity dynamics in the resting state. Cereb Cortex 24:663–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barttfeld P, Uhrig L, Sitt JD, Sigman M, Jarraya B, Dehaene S (2015): Signature of consciousness in the dynamics of resting‐state brain activity. Proc Natl Acad Sci 112:887–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Mackay CE, Filippini N, Smith SM (2009): Group comparison of resting‐state FMRI data using multi‐subject ICA and dual regression. Neuroimage 47:S148. [Google Scholar]
- Benjamini Y, Hochberg Y (1995): Controlling the false discovery rate: A practical and powerful approach to multiple testing. J Roy Stat Soci Ser B (Method) 289–300. [Google Scholar]
- Bettus G, Guedj E, Joyeux F, Confort‐Gouny S, Soulier E, Laguitton V, Cozzone PJ, Chauvel P, Ranjeva JP, Bartolomei F, Guye M (2009): Decreased basal fMRI functional connectivity in epileptogenic networks and contralateral compensatory mechanisms. Hum Brain Mapp 30:1580–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettus G, Bartolomei F, Confort‐Gouny S, Guedj E, Chauvel P, Cozzone PJ, Ranjeva JP, Guye M (2010): Role of resting state functional connectivity MRI in presurgical investigation of mesial temporal lobe epilepsy. J Neurol Neurosurg Psychiatry 81:1147–1154. [DOI] [PubMed] [Google Scholar]
- Campos BM, Coan AC, Beltramini GC, Liu M, Yassuda CL, Ghizoni E, Beaulieu C, Gross DW, Cendes F (2015): White matter abnormalities associate with type and localization of focal epileptogenic lesions. Epilepsia 56:125–132. [DOI] [PubMed] [Google Scholar]
- Catenoix H, Magnin M, Guenot M, Isnard J, Mauguiere F, Ryvlin P (2005): Hippocampal‐orbitofrontal connectivity in human: An electrical stimulation study. Clin Neurophysiol 116:1779–1784. [DOI] [PubMed] [Google Scholar]
- Chang C, Glover GH (2010): Time–frequency dynamics of resting‐state brain connectivity measured with fMRI. Neuroimage 50:81–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Wu X, Lui S, Wu Q, Yao Z, Li Q, Liang D, An D, Zhang X, Fang J, Huang X, Zhou D, Gong QY (2012): Resting‐state fMRI study of treatment‐naive temporal lobe epilepsy patients with depressive symptoms. Neuroimage 60:299–304. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS (2012): Resting‐state fMRI as a biomarker for Alzheimer's disease? Alzheimers Res Ther 4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet GE, Skidmore C, Evans J, Sharan A, Sperling MR, Pustina D, Tracy JI (2014): Temporal lobe epilepsy and surgery selectively alter the dorsal, not the ventral, default‐mode network. Front Neurol 5: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet GE, Sharan A, Pustina D, Skidmore C, Sperling MR, Tracy JI (2015): Early and late age of seizure onset have a differential impact on brain resting‐state organization in temporal lobe epilepsy. Brain Topography 28:113–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius, M.D. , Srivastava, G. , Reiss, A.L. , Menon, V. (2004) Default‐mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A, 101:4637–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF (2009): Resting‐state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex 19:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross DW (2011): Diffusion tensor imaging in temporal lobe epilepsy. Epilepsia 52: 32–34. [DOI] [PubMed] [Google Scholar]
- Haneef Z, Lenartowicz A, Yeh HJ, Engel J, Stern JM (2012): Effect of lateralized temporal lobe epilepsy on the default mode network. Epilepsy Behav 25:350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen EC, Battaglia D, Spiegler A, Deco G, Jirsa VK (2015): Functional connectivity dynamics: Modeling the switching behavior of the resting state. NeuroImage 105:525–535. [DOI] [PubMed] [Google Scholar]
- Hermann B, Seidenberg M, Jones J (2008): The neurobehavioural comorbidities of epilepsy: Can a natural history be developed? Lancet Neurol 7:151–160. [DOI] [PubMed] [Google Scholar]
- Holmes M, Folley BS, Sonmezturk HH, Gore JC, Kang H, Abou‐Khalil B, Morgan VL (2014): Resting state functional connectivity of the hippocampus associated with neurocognitive function in left temporal lobe epilepsy. Hum Brain Mapp 35:735–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey CJ, Kötter R, Breakspear M, Sporns O (2007): Network structure of cerebral cortex shapes functional connectivity on multiple time scales. Proc Natl Acad Sci 104:10240–10245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, Womelsdorf T, Allen EA, Bandettini PA, Calhoun VD, Corbetta M, Della Penna S, Duyn JH, Glover GH, Gonzalez‐Castillo J (2013): Dynamic functional connectivity: Promise, issues, and interpretations. Neuroimage 80:360–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM (2012): Fsl. Neuroimage 62:782–790. [DOI] [PubMed] [Google Scholar]
- Kramer MA, Eden UT, Kolaczyk ED, Zepeda R, Eskandar EN, Cash SS (2010): Coalescence and fragmentation of cortical networks during focal seizures. J Neurosci 30:10076–10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs H, Rodionov R, Thornton R, Duncan JS, Lemieux L, Tagliazucchi E (2014): Altered fMRI connectivity dynamics in temporal lobe epilepsy might explain seizure semiology. Front Neurol 5: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W, Zhang Z, Pan Z, Mantini D, Ding J, Duan X, Luo C, Wang Z, Tan Q, Lu G, Chen H (2011): Default mode network abnormalities in mesial temporal lobe epilepsy: A study combining fMRI and DTI. Hum Brain Mapp 32:883–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieb JP, Dasheiff RM, Engel J Jr (1991): Role of the frontal lobes in the propagation of mesial temporal lobe seizures. Epilepsia 32:822–837. [DOI] [PubMed] [Google Scholar]
- Lin JJ, Mula M, Hermann BP (2012): Uncovering the neurobehavioural comorbidities of epilepsy over the lifespan. Lancet 380:1180–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist MA, Xu Y, Nebel MB, Caffo BS (2014): Evaluating dynamic bivariate correlations in resting‐state fMRI: A comparison study and a new approach. Neuroimage 101:531–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccotta L, He BJ, Snyder AZ, Eisenman LN, Benzinger TL, Ances BM, Corbetta M, Hogan RE (2013): Impaired and facilitated functional networks in temporal lobe epilepsy. Neuroimage Clin 2:862–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan VL, Rogers BP, Sonmezturk HH, Gore JC, Abou‐Khalil B (2011): Cross hippocampal influence in mesial temporal lobe epilepsy measured with high temporal resolution functional magnetic resonance imaging. Epilepsia 52:1741–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazem‐Zadeh MR, Bowyer SM, Moran JE, Davoodi‐Bojd E, Zillgitt A, Weiland BJ, Bagher‐Ebadian H, Mahmoudi F, Elisevich K, Soltanian‐Zadeh H (2016): MEG Coherence and DTI Connectivity in mTLE. Brain Topography 29:598–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Pereira FR, Alessio A, Sercheli MS, Pedro T, Bilevicius E, Rondina JM, Ozelo HF, Castellano G, Covolan RJ, Damasceno BP, Cendes F (2010): Asymmetrical hippocampal connectivity in mesial temporal lobe epilepsy: Evidence from resting state fMRI. BMC Neurosci 11:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001): A default mode of brain function. Proceedings of the National Academy of Sciences 98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson LF, de La Peña VH, Kushnir Y (2008): Detecting shifts in correlation and variability with application to ENSO‐monsoon rainfall relationships. Theoretical Appl Climatol 94:215–224. [Google Scholar]
- Schwarz GE (1978): Estimating the dimension of a model. Ann Stat 6:461–464. [Google Scholar]
- Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD (2012): Decoding subject‐driven cognitive states with whole‐brain connectivity patterns. Cereb Cortex 22:158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen‐Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM (2004): Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23: S208–S219. [DOI] [PubMed] [Google Scholar]
- Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, He Y, Yan CG, Zang YF (2011): REST: A toolkit for resting‐state functional magnetic resonance imaging data processing. PLoS One 6: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling MR, O'Connor MJ, Saykin AJ, Phillips CA, Morrell MJ, Bridgman PA, French JA, Gonatas N (1992): A noninvasive protocol for anterior temporal lobectomy. Neurology 42:416–422. [DOI] [PubMed] [Google Scholar]
- Tracy JI, Doucet GE (2015): Resting‐state functional connectivity in epilepsy: Growing relevance for clinical decision making. Curr Opin Neurol 28:158–165. [DOI] [PubMed] [Google Scholar]
- Tracy JI, Johnson V, Sperling MR, Cho R, Glosser D (2007): The association of mood with quality of life ratings in epilepsy. Neurology 68:1101–1107. [DOI] [PubMed] [Google Scholar]
- Tracy JI, Pustina D, Doucet GE, Osipowicz K (2014): Seizure induced plasticity and cognitive reorganization in epilepsy In: Tracy J, Hampstead B, Sathian K, editors. Plasticity of Cognition in Neurologic Disorders. New York: Oxford University Press; pp 29–60. [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15:273–289. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL (2012): The influence of head motion on intrinsic functional connectivity MRI. Neuroimage 59:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Lu G, Zhong Y, Tan Q, Yang Z, Liao W, Chen Z, Shi J, Liu Y (2009): Impaired attention network in temporal lobe epilepsy: A resting FMRI study. Neurosci Lett 458:97–101. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Lu G, Zhong Y, Tan Q, Liao W, Wang Z, Wang Z, Li K, Chen H, Liu Y (2010): Altered spontaneous neuronal activity of the default‐mode network in mesial temporal lobe epilepsy. Brain Res 1323:152–160. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


