Abstract
Individual differences of brain changes of neural communication and integration in the modular architecture of the human brain network exist for the repeated migraine attack and physical or psychological stressors. However, whether the interindividual variability in the migraine brain connectome predicts placebo response to placebo treatment is still unclear. Using DTI and graph theory approaches, we systematically investigated the topological organization of white matter networks in 71 patients with migraine without aura (MO) and 50 matched healthy controls at three levels: global network measure, nodal efficiency, and nodal intramodule/intermodule efficiency. All patients participated in an 8‐week sham acupuncture treatment to induce analgesia. In our results, 30% (n = 21) of patients had 50% change in migraine days from baseline after placebo treatment. At baseline, abnormal increased network integration was found in MO patients as compared with the HC group, and the increased global efficiency before starting clinical treatment was associated with their following placebo response. For nodal efficiency, significantly increased within‐subnetwork nodal efficiency and intersubnetwork connectivity of the hippocampus and middle frontal gyrus in patients' white matter network were correlated with the responses of follow‐up placebo treatment. Our findings suggested that the trait‐like individual differences in pain‐related maladaptive stress interfered with and diminished the capacity of chronic pain modulation differently, and the placebo response for treatment could be predicted from a prior white matter network modular structure in migraineurs. Hum Brain Mapp 38:5250–5259, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: migraine, brain connectome, acupuncture
INTRODUCTION
Migraine has caused significant individual and societal burden due to recurrent moderate to severe migraine, resulting in disability and even productivity loss [Borsook et al., 2012]. The dynamic communication within and between networks in shaping cognition and behavior that contribute to individual differences in migraine vulnerability and pathophysiology is beginning to be understood [Kucyi and Davis, 2015; Liu et al., 2015]. The neural networks underlying such differences may be closely related to the variations in the biological response to reward anticipation and placebo administration in migraineurs [Kucyi and Davis, 2015]. To potentially improve treatment strategies, further investigations into interindividual variability of structural and functional changes in brain plasticity and its relationship with placebo response are warranted.
The placebo effect is based on expectation and experience, where the latter is a form of learning and has been intensively studied in healthy volunteers by using functional MRI [Amanzio et al., 2013]. Researchers pointed out that the pattern of brain activity during the anticipation and experience of pain could predict individual differences in placebo‐induced changes in pain processing in healthy subjects [Kong et al., 2009; Lu et al., 2010; Wager et al., 2011; Watson et al., 2009]. However, there is little knowledge about the predictive role of the brain in chronic pain patients. Since the individual's treatment expectations and beliefs are altered in migraine due to the patient's particular migraine experience, the brain circuitry for psychologically induced placebo hypoalgesia may differ from that in healthy subjects.
Kucyi et al. [2015] have recently proposed a theoretical model describing neural networks that comprise the pain connectome and mechanisms underlying individual differences in the intrinsic dynamics of chronic pain [Kucyi and Davis, 2015]. The authors argue that the different interactions between pain‐related brain subnetworks may allow different information communication and produce different therapeutic effects [Kucyi and Davis, 2015], even physiological responses to psychological aspects of treatment [Hashmi et al., 2014]. From a perspective of information segregation and integration, graph theoretical analysis has become a powerful tool to investigate the topological properties of brain networks [Bullmore and Bassett, 2011; Bullmore and Sporns, 2009; He and Evans, 2010; Sporns, 2011]. The investigation of the segregated and integrated processing over distributed brain circuits in the migraineurs' brain network will significantly advance our understanding of how the dynamic pain connectome is shaping individual differences of patients and interindividual variability in pain relief with placebo treatment.
Here, we used diffusion tensor imaging (DTI) tractography and graph theory approaches to systematically investigate pathological white matter (WM) network reorganization in patients with migraine without aura (MO). We hypothesized that long‐term migraine would alter the topological organization of the whole brain white matter network in MO patients, and the abnormal intermediate modular architecture would predict placebo response before starting clinical treatment. To induce analgesia, all patients participated in an 8‐week sham acupuncture treatment. Placebo responders were defined as patients with a reduction in migraine days of at least 50% [Meissner et al., 2013]. In the current study, MO patients were subdivided (50% change in migraine days from baseline) into recovering (MOr) and persisting (MOp) patients [Linde et al., 2016; Tfelt‐Hansen et al., 2012].
METHODS
All research procedures were approved by the Medical Ethics Committee of the First Affiliated Hospital of the Medical College in Xi'an Jiaotong University and were conducted in accordance with the Declaration of Helsinki. All subjects gave written, informed consent after the experimental procedures had been fully explained.
Participants
Inclusion criteria for the migraine patient group were according to the ICHD‐III‐beta MO (2013): (1) migraine attacks last 4–72 h (untreated or unsuccessfully treated); (2) featuring at least two of the following characteristics: unilateral location, pulsating quality, moderate to severe pain intensity and aggravation by causing avoidance of routine physical activity; (3) there is nausea and/or vomiting, or photophobia and phonophobia during migraine. Exclusion criteria were: (1) any physical illness such as a brain tumor, hepatitis, or epilepsy as assessed according to clinical evaluations and medical records; (2) existence of other comorbid chronic pain conditions (e.g., tension type migraine, fibromyalgia, etc.); (3) existence of a neurological disease or psychiatric disorder; (4) pregnancy; (5) use of prescription medications within the last month; (6) alcohol, nicotine, or drug abuse; and (7) claustrophobia.
A total of 196 acupuncture‐naïve episodic MO were recruited from Xi'an Jiaotong University for the study of acupuncture hypoalgesia between March 2014 and October 2015. All patients were right‐handed college students and naïve to acupuncture treatment. Fifty right‐handed healthy controls (HC) were recruited. The controls neither had any migraine days per year nor had family members who suffered regularly from migraine or other headaches.
Acupuncture Treatment
Acupuncture, as a traditional Chinese medicine for the treatment of many pain conditions, is widely used for preventing migraine attacks although its effectiveness has not yet been fully understood [Klaus et al., 2005; Melchart et al., 1999]. Because of the elaborate and impressive treatment rituals of acupuncture, patients may have greater expectation and inextricable placebo responses to the treatments [Diederich and Goetz, 2008; Pollo et al., 2001; Zubieta and Stohler, 2009]. In our study, an 8‐week double‐blinded, randomized, placebo‐controlled acupuncture was used. All patients were randomly assigned into the traditional acupuncture group and sham acupuncture group, and they were blinded to which treatment they received. The duration of the study per patient was 16 weeks: 4 weeks before the MRI scan or baseline; 8 weeks of treatment; and 4 weeks of follow‐up. Before the acupuncture treatment, all patients received the MRI scan during the interictal phase of migraine. All women were not scanned in their menstrual phase. In the current study, we focused on the data from the sham acupuncture group (n = 98) to investigate the placebo response in migraineurs. The flow chart of patient inclusion is shown in the Supporting Information.
Eight weeks of acupuncture treatment consisting of 24 sessions and 30 min of duration (3 sessions per week) followed a similar experimental model in Linde et al.'s [2005] study [Linde et al., 2005]. Specifically, all patients were treated bilaterally at what are called basic points, including Du Mai governing vessel 20, Liver 3, San Jiao 3 or 5, and extra point Taiyang [Linde et al., 2005]. Sham acupuncture was performed by using Streitberger needles [Streitberger and Kleinhenz, 1998] at non‐acupoints focused near real acupoints (approximately 2–3 cm laterally). The blunt Streitberger needle is retracted into its sheath when pressed against the skin surface, which has been well validated as a sham acupuncture device [Hashmi et al., 2014]. After the 8 weeks of acupuncture treatment, patients were asked whether they thought that they had received traditional acupuncture or sham acupuncture by using a credibility questionnaire. “De‐qi” or needling sensation, is the constellation of sensations a person feels during acupuncture needle manipulation, which is considered to be closely related to clinical efficacy in Traditional Chinese Medicine [Hui et al., 2005]. In our study, de‐qi was avoided in all 24 sessions of the sham acupuncture treatment. After 8 weeks of acupuncture treatment, the success of blinding was tested using a credibility questionnaire: “When you volunteered for the trial, you were informed that you had an equal chance of receiving traditional acupuncture or sham acupuncture. Which acupuncture do you think you received?” For all patients, they were allowed to take pirprofen when their migraine was difficult to endure. Detailed information about the patients' drug intake was recorded.
Outcome Measurement
During the 4 weeks before and after acupuncture treatment, patients carefully rated the average pain intensity of the attacks (0–10 scale, 10 being the most intense pain imaginable), migraine days and migraine attack duration (hours). The Zung Self‐Rating Anxiety Scale (SAS) and Zung Self‐Rating Depression Scale (SDS) were also used to quantify anxiety/depression‐related symptoms of the patients. Analysis of migraine diaries was performed by 2 blinded evaluators. The primary outcome measure was the difference in the number of days with migraine [Linde et al., 2005, 2016; Tfelt‐Hansen et al., 2012]. Patients who had reduced migraine days by at least 50% were considered to have an effective treatment.
Imaging Acquisition
This experiment was carried out in a 3.0 Tesla Signa GE scanner with an 8‐channel phase array head coil. For each subject, a high‐resolution structural image was acquired by using a three‐dimensional MRI sequence with a voxel size of 1 mm × 1 mm × 1 mm using an axial Fast Spoiled Gradient Recalled sequence (FSGR) with the following parameters: repetition time (TR) =1,900 ms; echo time (TE) =2.26 ms; data matrix = 256 × 256; field of view (FOV) = 256 mm × 256 mm.
DTI was obtained with a single‐shot echo‐planar imaging sequence. The diffusion sensitizing gradients were applied along 30 noncollinear directions (b = 1,000 s/mm2) with five acquisitions without diffusion weighing (b = 0 s/mm2). The imaging parameters were 75 continuous axial slices with a slice thickness of 2 mm and no gap, field of view (FOV)=256; TR = 9,400 ms; TE = 84 ms; matrix size = 128 × 128, resulting in 2 mm isotropic voxels.
Data Preprocessing
DTI data preprocessing was carried out using FMRIB's Diffusion Toolbox (FDT) 2.0 and parts of the FMRIB software Library (FSL) 4.1.9 (Oxford Centre for Functional Magnetic Resonance Imaging of the Brain Software Library, http://www.fmrib.ox.ac.uk/fsl/). The five b0 volumes were averaged. All datasets were processed using the following steps: distortion correction, eddy‐currents and head motion correction, echo planar imaging distortion correction, estimation of the diffusion tensor, and calculation of fractional anisotropy (FA). White matter pathways were reconstructed using streamline tractography [Mori and van Zijl, 2002], and a large sample of reconstructable fiber tracts of the brain network were obtained.
Network Construction
In this study, white matter brain networks were constructed at the macroscale with nodes representing brain regions and edges representing fibers between brain regions [Kang et al., 2017]. To define the network nodes, the automated anatomical labeling (AAL) atlas was applied (Supporting Information Table SI) [Tzourio‐Mazoyer et al., 2002]. The structural connection between nodes i and j was defined if a set of fibers (≥3) existed between these two regions in the total collection of the reconstructed fiber tracts [Shu et al., 2011, 2015]. The un‐weighted binary networks were constructed in all subjects.
Network Metrics
The global graph metrics were calculated to investigate possible differences in the overall network topology between patients and controls. The global metrics included: clustering coefficient (C), shortest path length (L), small‐worldness (SW), global efficiency (GE), local efficiency (LE), network modularity, intramodule connectivity, and intermodule connectivity (Supporting Information). For nodal centrality, we calculated nodal efficiency, normalized within‐module nodal efficiency, and participation coefficient (Supporting Information).
Statistical Analysis
To test the group differences in subjects' basic information (age, years of education, height, weight, temperature, blood pressure, and heart rate), we analyzed the data with one‐way ANOVAs. Post hoc pairwise comparisons were then performed using two sample t test. The numbers of males and females were analyzed using a χ 2 test. To investigate the group differences in patients' migraine activity before and after placebo treatment, two‐way ANOVAs was applied.
To assess the statistical significance of graph metrics between patients and controls, nonparametric permutation tests were applied (permutation number =5,000). The threshold for statistical significance was P < 0.005. To further characterize the structural and functional alterations found in MO, a regression analysis was performed for clinical variables.
RESULTS
Participant Demographic and Migraine Activity
In the current study, a 12‐week double‐blinded, randomized, placebo‐controlled acupuncture was used, and we only focused on the data from the sham acupuncture group. For the sham acupuncture group, twelve patients were dropped due to discomfort or technical failures. Another fifteen patients were excluded for incomplete clinical and neuroimaging data. The remaining 71 patients completed the study. After the treatment sessions, patients rated the credibility of acupuncture and sham acupuncture very highly and almost identically (P > 0.05, χ2 test). Migraine patients were subdivided (50% change in migraine days from baseline to post‐treatment) into MOr (n = 21) and MOp (n = 50) patients. At baseline, there were no between group differences among HC, MOp, and MOr group for the subjects' basic information (Table 1). No patients took drugs during the whole experiment based on their records (see Supporting Information).
Table 1.
Participant demographic
| Information | Baseline (mean ± SE) | |||
|---|---|---|---|---|
| HC(n = 50) | MOp (n = 50) | MOr (n = 21) | P values | |
| Age (years) | 22 ± 0.28 | 21.9 ± 0.31 | 22.1 ± 0.55 | >0.05 |
| Education (years) | 15.4 ± 0.3 | 15.2 ± 0.26 | 15.5 ± 0.32 | >0.05 |
| Height (cm) | 161.2 | 159.8 ± 1.06 | 162.0 ± 2.03 | >0.05 |
| Weight (kg) | 52.7 ± 1.19 | 51.8 ± 1.37 | 54.1 ± 1.80 | >0.05 |
| Temperature | 36.7 ± 0.08 | 36.8 ± 0.06 | 36.7 ± 0.09 | >0.05 |
| Blood pressure | 107.9 ± 1.1/71.6 ± 1.1 | 108 ± 1.1/72.6± 1.09 | 107.6 ± 1.69/71.3± 1.46 | >0.05 |
| Heart rate | 77.2 ± 0.9 | 76.6 ± 0.82 | 78.2 ± 1.08 | >0.05 |
The comparisons of the basic information were performed among the three groups (HC, MOp, and MOr) using one‐way ANOVAs. Post hoc pairwise comparisons were then performed using two sample t test. P < 0.05 was considered significant.
In our results, patients in the MOp and MOr group had similar migraine activity and mood characteristics at baseline. As compared with the MOp group, patients in the MOr group showed significantly decreased number of days with migraine after 8‐weeks sham acupuncture treatment (Table 2, two‐way ANOVA, P < 0.01).
Table 2.
Headache activity and mood characteristics
| Information | Visit 1 | Visit 2 | Visit 2 versus Visit 1 | Visit 1 | Visit 2 | Visit 2 versus Visit 1 | (P2‐P1) versus |
|---|---|---|---|---|---|---|---|
| MOp (n = 50) | MOp | P value | MOr (n = 21) | MOr | P value | (r2‐r1) P value | |
| Disease duration (mh) | 64.3 ± 8.01 | — | — | 47.7 ± 5.09 | — | — | — |
| Headache days | 5.9 ± 0.9 | 6.45 ± 1.19 | 0.29 | 8.8 ± 1.94 | 3.1 ± 0.75 | <0.05 | <0.05 |
| VAS | 5.5 ± 0.3 | 4.3 ± 0.33 | <0.05 | 5.5 ± 0.27 | 3.15 ± 0.40 | <0.05 | 0.34 |
| Average duration of a migraine attack (hours) | 7.09 ± 0.93 | 4.3 ± 0.51 | 0.18 | 8.6 ± 1.6 | 4.0 ± 0.95 | <0.05 | 0.1 |
| SAS | 47.25 ± 1.87 | 42.2 ± 2.2 | 0.1 | 44.4 ± 2.16 | 35.0 ± 1.56 | <0.05 | <0.05 |
| SDS | 48.3 ± 2.35 | 43.2 ± 2.4 | 0.14 | 40.9 ± 2.57 | 37.0 ± 2.69 | >0.05 | 0.43 |
The comparisons of the headache activity between Visit 1 and Visit 2 were performed using two sample t test within MOp and MOr group. P < 0.05 was considered significant.
The group differences in patients' migraine activity before and after placebo treatment ((P2‐P1) vs. (r2‐r1)) were investigated using two way ANOVA. P < 0.05 was considered significant.
Global Network Measures in MO Patients
All individual brain white matter network had a small‐world network organization with higher local efficiency (normalized C > 1) but approximately equal global efficiency (normalized L ≈ 1) than matched random networks (Table 3). As compared with the HC group, patients with MO had significant higher global efficiency, local efficiency and lower shortest path length. Moreover, controlling the treatment credibility, SAS, SDS, and disease duration, the global (r = −0.38, P = 0.02) and local efficiency (r = −0.42, P = 0.01) in patients' structural network at baseline were negatively correlated with the changes in migraine days after placebo treatment, and the positive correlation was found in the shortest path length (r = 0.39, P = 0.02) (Table 3).
Table 3.
Comparisons of the global network measures between the HC and MO groups
| Network Metrics | HC (n=50) | MO (n=71) | Difference | Correlation |
|---|---|---|---|---|
| P1 values | P2 values | |||
| small‐worldness, mean (SE) | 2.03 (0.01) | 2 (0.02) | >0.05 | >0.05 |
| clustering coefficient (C), mean (SE) | 0.61 (0.002) | 0.61 (0.003) | >0.05 | >0.05 |
| normalized C, mean (SE) | 2.15 (0.02) | 2.09 (0.02) | >0.05 | >0.05 |
| shortest path length (L), mean (SE) | 1.82 (0.005) | 1.66 (0.006) | 0.003 | 0.02 |
| normalized L, mean (SE) | 1.06 (0.002) | 1.05 (0.002) | >0.05 | 0.03 |
| global efficiency, mean (SE) | 0.6 (0.002) | 0.79 (0.002) | 0.001 | 0.02 |
| local efficiency, mean (SE) | 0.76 (0.0001) | 0.84 (0.002) | 0.02 | 0.01 |
Two‐sample t test was used to investigate the between group differences. P1 < 0.05 was considered significant.
Pearson correlation was used to test the relationship between network metrics and placebo response. P2 < 0.05 was considered significant.
Intramodule and Intermodule Integration in MO Patients
Based on the group‐average network of HC, we identified six modules (Q = 0.47, Supporting Information Table SII). One module comprised of regions of the bilateral medial cortex and anterior cingulate cortex (anterior default mode network, aDMN; red in the Fig. 1), and one module included the posterior cingulate cortex, precuneus, and hippocampus (posterior default mode network, pDMN) (light green in the Fig. 1). Two contralaterally matched modules were localized to the dorsolateral prefrontal cortices, insula, and striatum of a single hemisphere (M1_right and M1_left, dark blue and red in the Fig. 1). The two remaining modules included contralateral regions of the temporo‐ parietal areas and occipital cortices of a single hemisphere (M2_right and M2_left, yellow and light blue in the Fig. 1). Our findings were consistent with the results of a previous brain structural network analysis [Hagmann et al., 2008].
Figure 1.
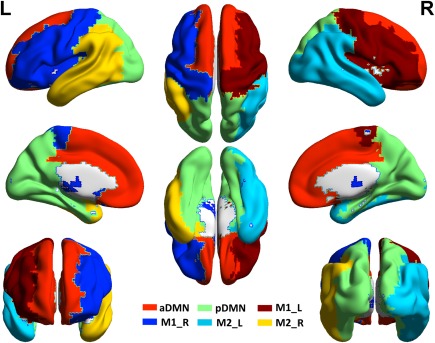
The intermediate modular structure. Six modules were identified for the group‐level network of healthy controls including the anterior default mode network (aDMN, red) and posterior default mode network (pDMN, light green). Two contralaterally matched modules were localized to the dorsolateral prefrontal cortices, insula, and striatum of a single hemisphere (M1_left and M1_right, dark blue and red). The two remaining modules included contralateral regions of temporo‐parietal areas and occipital cortices of a single hemisphere (M2_left and M2_right, yellow and light blue). The results are represented by using the BrainNet viewer [Xia et al., 2013]. [Color figure can be viewed at http://wileyonlinelibrary.com]
On the basis of the above modular architecture, we found that the intramodule and intermodule connectivities were significantly different in the pDMN between MO and HC groups. Compared with controls (P < 0.005, nonparametric permutation tests), significant between group differences of intramodule connectivity were found in the pDMN and M1_right, and the pDMN‐aDMN and pDMN‐M1_right intermodule connectivity showed increased connectivity in the patient group (Fig. 2a). For the comparison between MOp and MOr groups, significantly increased intermodule connectivity between the pDMN and M1_right in MOp were found (P < 0.005, nonparametric permutation tests, Fig. 2b). After controlling the treatment credibility, SAS, SDS, and disease duration, these between‐group differences were still found. These results showed that the migraine attacks had an adverse effect on pathological cortical network organization, which altered the segregation and integration of structural networks in patients.
Figure 2.
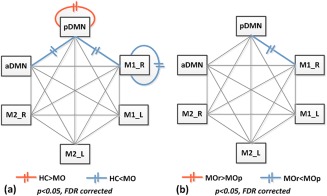
Disrupted intramodule and intermodule structural connectivity between HC and patient groups. The red and blue lines indicate significant between group differences (a. HC vs. MO; b. MOr vs. MOp). [Color figure can be viewed at http://wileyonlinelibrary.com]
Group Comparisons at the Nodal Level in MO Patients
Compared with the HC group, a significantly higher nodal efficiency was found in the middle frontal gyrus (MFG), precentral gyrus, hippocampus, insula, olfactory cortex, and inferior occipital gyrus in the patient group (P < 0.005, nonparametric permutation tests, Fig. 3a). No significant decreased nodal efficiency was found in the patient group.
Figure 3.
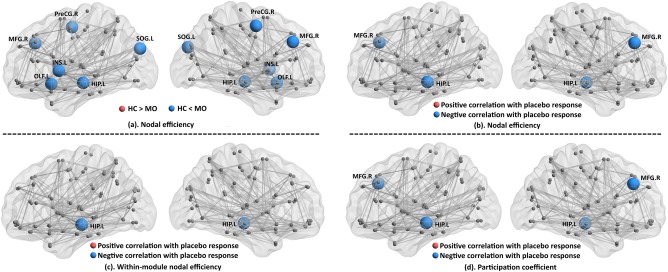
Nodal efficiency was significantly increased in the middle frontal gyrus (MFG), precentral gyrus, hippocampus, insula, olfactory cortex, and inferior occipital gyrus in the patient group as compared with the controls (a). No significant decreased nodal efficiency was found in the patient group. Significant association was found between the changes in migraine days and the nodal efficiency of the hippocampus and MFG (b), within‐module nodal efficiency of the hippocampus (c), and the participation coefficient of the hippocampus and MFG (d). The results are represented by using the BrainNet viewer [Xia et al., 2013]. [Color figure can be viewed at http://wileyonlinelibrary.com]
Correlation between Changes in Migraine Days and Nodal Efficiency
After controlling for treatment credibility, SAS, SDS, and disease duration, significant associations were found between changes in migraine days and nodal efficiency in the MFG and hippocampus (P < 0.05, false discovery rate corrected, Fig.3b).
When considering the within‐module nodal efficiency and nodal participation coefficient between different modules, we found that the changes in migraine days were significantly correlated with the within‐module nodal efficiency of the hippocampus (Fig. 3c) and nodal participation coefficient of the hippocampus and MFG (Fig. 3d).
Individual Differences of Nodal Efficiency in MO Patients
After controlling for treatment credibility, SAS, SDS, and disease duration, the MOp group had higher within‐module nodal efficiency in the hippocampus (Fig. 4a), and no significant lower result was found. For the intermodule connectivity, the MOp group had a higher participation coefficient in the MFG and precentral gyrus, and a lower result was found in the posterior cingulate gyrus (PCG) (Fig. 4b).
Figure 4.
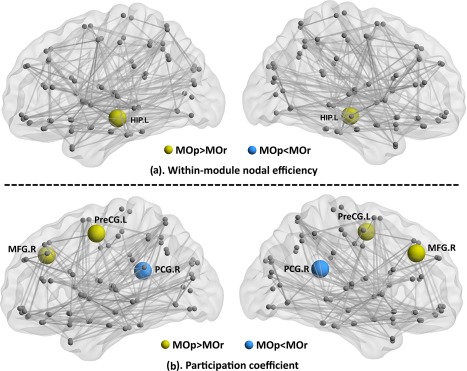
Between group differences of the nodal efficiency between MOr and MOp patients (a. within‐module nodal efficiency; b. participation coefficient). [Color figure can be viewed at http://wileyonlinelibrary.com]
DISCUSSION
The current study investigated whether the topological properties of migraineurs' brain WM network in a pretreatment scan could predict placebo response in an 8‐week sham acupuncture treatment. There were three major findings: (i) long‐term migraine attack associated with the abnormal brain WM network organization, and the global network efficiency was correlated with the following placebo responses; (ii) abnormal intramodule and intermodule integration were found in patients, indicating disrupted information segregation and integration in the patients' structural network; and (iii) the nodal efficiency of the hippocampus and MFG predicted future placebo responses in patients.
Migraine is highly subjective, and thus, the underlying balance between an individual's ascending nociceptive/descending pain‐modulatory pathways may cause different pain experiences from one individual to another [Liu et al., 2015]. According to a new systematic review, placebo clinical trials to prevent migraine had a response rate of 0–56% [Meissner et al., 2013]. Such variability in treatment success may be directly linked with the variation of the individual's brain network architecture in migraineurs. In our results, global network metrics of the WM network were differentially altered across subject groups, such that the average shortest path length was lower and global network efficiency was higher in MO patients. The global efficiency and average shortest path length between all pairs of network nodes is the most commonly used measure of network integration with shorter paths implying stronger potential for integration [Watts and Strogatz, 1998]. Hence, our findings may indicate excessive network integration in MO patients. We inferred that abnormal increased WM network integration in the brain of migraine patients may have more ability to rapidly combine specialized information from different brain circuits, which deliver pain‐related information faster and effectively and disseminate a large proportion of migraine activity. In our findings, the network efficiency was negatively correlated with the changes in migraine days after placebo treatment, which further suggested that patients with abnormal higher WM network integration may diminish the capacity of pain modulatory networks in translating psychological signals into analgesia. We provide the evidence that topological property of the white matter network observed in a pretreatment scan was predictive of subsequent psychologically mediated placebo response in migraine patients.
Prior studies noted the importance of modular structure in biological networks, which may arise from natural selection or evolutionary constraint for adaption or maladaption to environmental demands [He et al., 2009; Hintze and Adami, 2008; Liu et al., 2012]. In our findings, we observed significant differences of intramodule and intermodule connectivity in the hippocampus between HC and MO groups, which were also correlated with the responses of follow‐up placebo treatment in patients. The hippocampus is known to be involved in memory and learned behavior [van der Flier and Scheltens, 2009], and has been implicated as an important site for glucocorticoid negative feedback regulation of the hypothalamic‐pituitary‐adrenal axis that controls reactions to stress [Gerritsen et al., 2011]. Research has consistently shown that structural and functional changes in the hippocampus are involved in chronic pain, possibly due to poor adaptation over time resulting in damage with repeated stressful pain attacks and/or by failure to shut off the response after the challenge is gone [Baliki and Apkarian, 2015]. As we know, frequent migraine attacks associated with a number of physiological and emotional stressors may cause abnormal glucocorticoid release [Ising and Braun, 2000] and increase excitatory amino acid activity [Flier et al., 1998], which in turn may result in deleterious effects on the hippocampus [Maleki et al., 2013]. In the current study, we found that excessive network integration of the hippocampus in patients was negatively correlated with placebo treatment outcomes. Hence, we inferred that abnormally increased hippocampus integration in the WM network may be a predisposing factor for less responsiveness to treatments, more sensitivity to stressors, and be an overall contribution to the development and maintenance of a persistent migraine. The higher level of the within‐module nodal efficiency of the hippocampus in the MOp group as compared with the MOr group may support our inferences.
Recently, insights from resting‐state functional MR imaging reported that the extent of functional connectivity of the MFG could predict psychologically conditioned analgesic responses to acupuncture treatment [Hashmi et al., 2014] and identify placebo responders in a 2‐week single‐blinded placebo pill trial [Tétreault et al., 2016] in chronic knee osteoarthritis (OA) pain patients. In our findings, the participation coefficient of the MFG in the patients' structural network was associated with changes in migraine days and exhibited significant differences between MOr and MOp patients. As the participation coefficient measures the diversity of intermodular connections of the individual nodes [Liang et al., 2015], the higher level of the MFG indicated stronger intermodular connections that were distributed across multiple different modules, thereby having a stronger functional integration. Our results further supported that excessive network integration could affect placebo analgesia.
Based on the global workspace theory, local elements of the nervous system are active in parallel and process information in an unconscious manner [Kitzbichler et al., 2011]. For the consciously attended stimulus, it could be suddenly replaced by the ignition of a globally synchronized system [Dehaene and Changeux, 2005]. Migraine could be considered as a persistent presence of an unconditioned stimulus. Thus, pain‐related brain regions may be active with high frequencies, not acting as separate cortical subsystems but instead operating as a global synchronization participating in a diverse set of brain circuits. It may consolidate the abnormal interaction across multiple pain‐related brain networks. In our findings, MOp patients exhibited higher local network integration in the hippocampus and MFG as compared to MOr patients. We inferred that such trait‐like individual differences of brain structural network organization were associated with and diminished the capacity of chronic pain modulation differently, and excessive network integration may have susceptibility to persistent headache and diminished response for placebo treatment.
There are several issues that should be addressed. First, sham acupuncture treatment has been previously reported to be effective in migraine prophylaxis, even superior to oral placebo medications [Klaus et al., 2005]. It suggests that sham acupuncture could be an effective treatment, and not just a “pure placebo.” Some studies suggested that part of the enhanced placebo effect may be due to the physiological effects of skin injury during the stimulation [Lund and Lundeberg, 2006]. In our study, to make sham acupuncture be a “pure placebo,” sham acupuncture was performed by using Streitberger needles at non‐acupoints located near real acupoints. However, our findings still need to be proved further. Next, different parcellation strategies for network construction may result in distinct topological architecture. Our network had low‐resolution ROIs (n = 90) covering the entire cerebral cortex, which were used in several published studies. Future studies still need to consider the effect that other specific parcellation approaches have on graph analytical findings.
In summary, the current study falls within the general effort of using neuroimaging technology with the brain connectome to predict future placebo response in patients with migraine. We provided evidence that the placebo response could be identified a priori in migraine patients, and suggested that specific topological properties of the brain structural network underlie the clinical placebo effect.
Conflict of interest
None declared.
Supporting information
Supporting Information Figure 1.
Supporting Information
Contributor Information
Jie Tian, Email: tian@ieee.org.
Ming Zhang, Email: profzmmri@gmail.com.
REFERENCES
- Amanzio M, Benedetti F, Porro CA, Palermo S, Cauda F (2013): Activation likelihood estimation meta‐analysis of brain correlates of placebo analgesia in human experimental pain. Hum Brain Mapp 34:738–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Apkarian AV (2015): Nociception, pain, negative moods, and behavior selection. Neuron 87:474–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsook D, Maleki N, Becerra L, Mcewen B (2012): Understanding migraine through the lens of maladaptive stress responses: A model disease of allostatic load. Neuron 73:219–234. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Sporns O (2009): Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10:186–198. [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Bassett DS (2011): Brain graphs: Graphical models of the human brain connectome. Annu Rev Clin Psychol 7:113–140. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Changeux JP (2005): Ongoing spontaneous activity controls access to consciousness: A neuronal model for inattentional blindness. Plos Biol 3:e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diederich NJ, Goetz CG (2008): The placebo treatments in neurosciences New insights from clinical and neuroimaging studies. Neurology 71:677–684. [DOI] [PubMed] [Google Scholar]
- Flier JS, Underhill LH, McEwen BS (1998): Protective and damaging effects of stress mediators. New Engl J Med 338:171–179. [DOI] [PubMed] [Google Scholar]
- Gerritsen L, Comijs HC, van der Graaf Y, Knoops AJ, Penninx BW, Geerlings MI (2011): Depression, hypothalamic pituitary adrenal axis, and hippocampal and entorhinal cortex volumes—The SMART Medea study. Biol Psychiatry 70:373–380. [DOI] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O (2008): Mapping the structural core of human cerebral cortex. PLoS Biol 6:e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashmi JA, Kong J, Spaeth R, Khan S, Kaptchuk TJ, Gollub RL (2014): Functional network architecture predicts psychologically mediated analgesia related to treatment in chronic knee pain patients. J Neurosci 34:3924–3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Evans A (2010): Graph theoretical modeling of brain connectivity. Curr Opin Neurol 23:341–350. [DOI] [PubMed] [Google Scholar]
- He Y, Wang J, Wang L, Chen ZJ, Yan C, Yang H, Tang H, Zhu C, Gong Q, Zang Y (2009): Uncovering intrinsic modular organization of spontaneous brain activity in humans. PLoS One 4:e5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintze A, Adami C (2008): Evolution of complex modular biological networks. PLoS Comput Biol 4:e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui KK, Liu J, Marina O, Napadow V, Haselgrove C, Kwong KK, Kennedy DN, Makris N (2005): The integrated response of the human cerebro‐cerebellar and limbic systems to acupuncture stimulation at ST 36 as evidenced by fMRI. Neuroimage 27:479–496. [DOI] [PubMed] [Google Scholar]
- Ising H, Braun C (2000): Acute and chronic endocrine effects of noise: Review of the research conducted at the Institute for Water, Soil and Air Hygiene. Noise Health 2:7–24. [PubMed] [Google Scholar]
- Kang L, Liu L, Qin Y, Dun W, Xu X, Liu J, Ming Z (2017): Abnormal rich club organization and impaired correlation between structural and functional connectivity in migraine sufferers. Brain Imaging Behav 11:526–540. [DOI] [PubMed] [Google Scholar]
- Kitzbichler MG, Henson RNA, Smith ML, Nathan PJ, Bullmore ET (2011): Cognitive effort drives workspace configuration of human brain functional networks. J Neurosci 31:8259–8270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus L, Andrea S, Susanne J, Andrea H, Benno B, Claudia W, Stephan W, Volker P, Hammes MG, Wolfgang W (2005): Acupuncture for patients with migraine: A randomized controlled trial. JAMA 106:2118–2125. [DOI] [PubMed] [Google Scholar]
- Kong J, Kaptchuk TJ, Polich G, Kirsch I, Vangel M, Zyloney C, Rosen B, Gollub R (2009): Expectancy and treatment interactions: A dissociation between acupuncture analgesia and expectancy evoked placebo analgesia. Neuroimage 45:940–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucyi A, Davis KD (2015): The dynamic pain connectome. Trends Neurosci 38:86–95. [DOI] [PubMed] [Google Scholar]
- Liang X, He Y, Salmeron BJ, Gu H, Stein EA, Yang Y (2015): Interactions between the salience and default‐mode networks are disrupted in cocaine addiction. J Neurosci 35:8081–8090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linde K, Streng A, Jürgens S, Hoppe A, Brinkhaus B, Witt C, Wagenpfeil S, Pfaffenrath V, Hammes MG, Weidenhammer W (2005): Acupuncture for patients with migraine: A randomized controlled trial. JAMA 293:2118–2125. [DOI] [PubMed] [Google Scholar]
- Linde K, Allais G, Brinkhaus B, Fei Y, Mehring M, Vertosick EA, Vickers A, White AR (2016): Acupuncture for the prevention of episodic migraine. Cochrane DB Syst Rev 6:CD001218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhao L, Li G, Xiong S, Nan J, Li J, Yuan K, Dong M, Liang F, Qin W, Tian J (2012): Hierarchical alteration of brain structural and functional networks in female migraine sufferers. PLoS One 7:e51250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhao L, Lei F, Zhang Y, Yuan K, Gong Q, Liang F, Tian J (2015): Disrupted resting‐state functional connectivity and its changing trend in migraine suffers. Hum Brain Mapp 36:1892–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H‐C, Hsieh J‐C, Lu C‐L, Niddam DM, Wu Y‐T, Yeh T‐C, Cheng C‐M, Chang F‐Y, Lee S‐D (2010): Neuronal correlates in the modulation of placebo analgesia in experimentally‐induced esophageal pain: A 3T‐fMRI study. Pain 148:75–83. [DOI] [PubMed] [Google Scholar]
- Lund I, Lundeberg T (2006): Are minimal, superficial or sham acupuncture procedures acceptable as inert placebo controls? Acupunct Med 24:13–15. [DOI] [PubMed] [Google Scholar]
- Maleki N, Becerra L, Brawn J, McEwen B, Burstein R, Borsook D (2013): Common hippocampal structural and functional changes in migraine. Brain Struct Funct 218:903–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner K, Fässler M, Rücker G, Kleijnen J, Hróbjartsson A, Schneider A, Antes G, Linde K (2013): Differential effectiveness of placebo treatments: A systematic review of migraine prophylaxis. JAMA Inter Med 173:1941–1951. [DOI] [PubMed] [Google Scholar]
- Melchart D, Linde K, Fischer P, White A, Allais G, Vickers A, Berman B (1999): Acupuncture for recurrent headaches: A systematic review of randomized controlled trials. Cephalalgia 19:779–786. [DOI] [PubMed] [Google Scholar]
- Mori S, van Zijl P (2002): Fiber tracking: Principles and strategies–a technical review. NMR Biomed 15:468–480. [DOI] [PubMed] [Google Scholar]
- Pollo A, Amanzio M, Arslanian A, Casadio C, Maggi G, Benedetti F (2001): Response expectancies in placebo analgesia and their clinical relevance. Pain 93:77–84. [DOI] [PubMed] [Google Scholar]
- Shu N, Liu Y, Li K, Duan Y, Wang J, Yu C, Dong H, Ye J, He Y (2011): Diffusion tensor tractography reveals disrupted topological efficiency in white matter structural networks in multiple sclerosis. Cereb Cortex 21:2565–2577. [DOI] [PubMed] [Google Scholar]
- Shu N, Li X, Ma C, Zhang J, Chen K, Liang Y, Chen Y, Zhang Z (2015): Effects of APOE promoter polymorphism on the topological organization of brain structural connectome in nondemented elderly. Hum Brain Mapp 36:4847–4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O (2011): The human connectome: A complex network. Ann N Y Acad Sci 1224:109–125. [DOI] [PubMed] [Google Scholar]
- Streitberger K, Kleinhenz J (1998): Introducing a placebo needle into acupuncture research. Lancet 352:364–365. [DOI] [PubMed] [Google Scholar]
- Tétreault P, Mansour A, Vachonpresseau E, Schnitzer TJ, Apkarian AV, Baliki MN (2016): Brain connectivity predicts placebo response across chronic pain clinical trials. Plos Biol 14:e1002570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tfelt‐Hansen P, Pascual J, Ramadan N, Dahlöf C, D'Amico D, Diener HC, Hansen JM, Lanteri‐Minet M, Loder E, Mccrory D (2012): Guidelines for controlled trials of drugs in migraine: Third edition. A guide for investigators. Cephalalgia 32:6. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15:273–289. [DOI] [PubMed] [Google Scholar]
- van der Flier WM, Scheltens P (2009): Alzheimer disease: Hippocampal volume loss and Alzheimer disease progression. Nat Rev Neurol 5:361–362. [DOI] [PubMed] [Google Scholar]
- Wager TD, Atlas LY, Leotti LA, Rilling JK (2011): Predicting individual differences in placebo analgesia: Contributions of brain activity during anticipation and pain experience. J Neurosci 31:439–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson A, El‐Deredy W, Iannetti GD, Lloyd D, Tracey I, Vogt BA, Nadeau V, Jones AK (2009): Placebo conditioning and placebo analgesia modulate a common brain network during pain anticipation and perception. Pain 145:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts DJ, Strogatz SH (1998): Collective dynamics of ‘small‐world’networks. Nature 393:440–442. [DOI] [PubMed] [Google Scholar]
- Xia M, Wang J, He Y (2013): BrainNet Viewer: A network visualization tool for human brain connectomics. PLoS One 8:e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta JK, Stohler CS (2009): Neurobiological mechanisms of placebo responses. Ann N Y Acad Sci 1156:198–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figure 1.
Supporting Information


