Abstract
Childhood maltreatment is associated with alterations in neural architecture that potentially put these children at increased risk for psychopathology. Alterations in white matter (WM) tracts have been reported, however no study to date has investigated WM connectivity in brain networks in maltreated children to quantify global and local abnormalities through graph theoretical analyses of DTI data. We aimed for a multilevel investigation examining the DTI‐based structural connectome and its associations with basal cortisol levels of 25 children with documented maltreatment experiences before age 3, and 24 matched controls (age: 10.6 ± 1.75 years). On the global and lobar level, maltreated children showed significant reductions in global connectivity strength, local connectivity and increased path length, suggesting deviations from the small‐world network architecture previously associated with psychopathology. Reductions in global connectivity were associated with placement instability, attenuated cortisol secretion and higher levels of internalizing and externalizing behaviours. Regional measures revealed lower connectivity strength especially in regions within the ventromedial prefrontal cortex (vMPFC) in maltreated children. These findings show that childhood maltreatment is associated with systemic global neurodevelopmental alterations in WM networks next to regional alterations in areas involved in the regulation of affect. These alterations in WM organization could underlie global functional deficits and multi‐symptom patterns frequently observed in children with maltreatment experiences. Hum Brain Mapp 38:855–868, 2017. © 2016 Wiley Periodicals, Inc.
Keywords: child maltreatment, connectome, cortisol, diffusion tensor imaging, white matter
Abbreviations
- ANOVA
Analyses of variance
- CBCL
Child‐behavior‐checklist for children
- DWI
Diffusion‐weighted images
- FA
Fractional anisotropy
- FAS
Fetal alcohol syndrome
- FDR
False discovery rate
- GNS
General node strength
- GR
Glucocorticoid‐receptor
- HPA
Hypothalamic‐pituitary‐adrenal
- IFOF
Inferior fronto‐occipital fasciculus
- MANOVA
Multivariate analyses of variance
- NS
Node strength
- PTSD
Post‐traumatic stress disorder
- ROI
Region‐of‐interest
- UNS
Undirected node strength
- vMPFC
Ventromedial prefrontal cortex
- WM
White matter
INTRODUCTION
Childhood maltreatment is a major risk factor for children's social, emotional and neural development, rendering these children at high risk for a range of adverse outcomes at all stages of development [MacMillan and Fleming, 2014]. Indeed, childhood maltreatment has been associated with poorer educational and economic attainment [Zielinski, 2009], physical and psychological well‐being [Hanson et al., 2015a, 2015b; Nusslock and Miller, 2016], as well as impaired social cognition [Flynn et al., 2014; Puetz et al., 2014; Puetz et al., 2016a]. There is increasing consensus among researchers that severe stress in the form of childhood maltreatment triggers a cascade of neurobiological changes in the developing brain via the hypothalamic‐pituitary‐adrenal (HPA) axis [Lupien et al., 2009]. Chronic secretion of the glucocorticoid stress hormone cortisol by the adrenals, in response to stress, can affect dendritic and axonal development in glucocorticoid‐receptor (GR) rich areas such as the prefrontal cortex and the limbic system [Bambico et al., 2013; Helmeke et al., 2008; Muhammad and Kolb, 2011]. Excess exposure to glucocorticoids can ultimately alter neural structure and subsequent function in the prenatal and postnatal phase [Lupien et al., 2009; Murmu et al., 2006]. Considering the ability of the prefrontal and limbic structures to influence the organism's ability to cope with, and recover from stress [Carlyle et al., 2012; Sanchez, 2006; Sapolsky et al., 2000], it is not surprising that neuroimaging studies have documented long‐lasting structural changes in these brain areas associated with stress‐related disorders, such as depression and anxiety [Etkin and Wager 2007], especially in individuals who have been maltreated in childhood [Hanson et al., 2015a, 2015b; van Harmelen et al., 2010; Teicher et al., 2014; Dannlowski et al., 2012; Kaufman and Charney, 2001; Kelly et al., 2013; DeBrito et al., 2013; Teicher and Samson, 2013].
Specifically, structural and functional MRI studies have demonstrated volumetric and functional alterations predominantly in the prefrontal cortex (including anterior cingulate; [Kelly et al., 2013; Teicher and Samson, 2013; Mueller et al., 2010; van Harmelen et al., 2010; van Harmelen et al., 2013; Puetz et al., 2014] and subcortical structures such as the amygdala and the hippocampus [Maheu et al., 2010; McCrory et al., 2011, 2013]. However, the impact of childhood maltreatment on white matter (WM) development and hence, on the inter‐ and intra‐hemispheric communication, has received less attention and remains an area of considerable need for research.
Studies investigating WM integrity in individuals who have experienced maltreatment have predominantly demonstrated reduced fractional anisotropy (FA) values in WM tracts either originating or terminating in the frontal lobe, e.g., the uncinate fasciculus connecting the orbitofrontal and temporal lobe; [Eluvathingal et al., 2006; Hanson et al., 2015a; Govindan et al., 2010], the superior longitudinal fasciculus connecting the association cortices with the frontal lobe, including the arcuate, involved in language functions [Choi et al., 2009; Govindan et al., 2010; Hanson et al., 2013; Huang et al., 2012] and the inferior fronto‐occipital fasciculus (IFOF) as well as those fiber bundles connecting the two hemispheres [Bick et al., 2015; Huang et al., 2012; Jackowski et al., 2009]. Importantly, most of these studies demonstrated a dose‐effect relationship with measures indexing the severity of adversity, such that children who endured longer periods of adversity [Govindan et al., 2010; Sheridan et al., 2012], and higher levels of verbal and domestic abuse [Choi et al., 2009, 2012] presented with greater WM alterations. Such WM alterations have been shown to predict internalizing symptomatology [Hanson et al., 2015b] and substance use disorder [Huang et al., 2012]. Recently Hanson et al. [2015b] investigated a large sample of adult university students who experienced maltreatment in childhood and found an association between lower WM integrity in the uncinate fasciculus and higher internalizing symptomatology. Importantly, the authors also found an association between lower WM integrity in the uncinate at baseline and higher internalizing symptoms after subsequent experience of current life stress, suggesting that maltreatment associated changes in WM can also increase vulnerability to future life stress.
However, most of these studies employed a region‐of‐interest (ROI) approach, in which the investigation is confined to fiber bundles defined a‐priori. While this approach is advantageous for several reasons (e.g., controlling for Type I error by limiting the number of statistical tests), the ROI approach only yields limited information about connectivity and WM integrity of isolated and predefined tracts but cannot provide a global quantification of network integrity. However, maltreatment experiences can be very heterogeneous, leading to heterogeneous behavioral phenotypes, and are therefore thought to affect a distributed network on the systems level (global and lobar) that is not solely limited to single fiber tracts but might also affect the large‐scale topological organization of brain networks.
Considering the increasing scientific interest in characterizing the interaction between brain regions, the present study sought to close the gap by quantifying global brain network integrity in addition to complementary regional measures of connectivity of the network. In order to investigate whole brain WM networks on global and regional scales, we applied graph theoretical analyses to DTI data and aimed for a multilevel investigation including indices of the maltreatment experience, indices of internalizing and externalizing psychopathology and basal salivary cortisol measures as a marker of a (dys)regulated stress‐response system in these analyses.
A graph theoretical framework enables the investigation of a complex and interacting brain network by characterizing the different brain regions as nodes and the WM tracts that were reconstructed through probabilistic fiber tracking as connections between the nodes, or edges [Hagmann et al., 2007; Rubinov and Sporns, 2010]. Within this framework, several network properties can be explored that indicate the degree of functional segregation (e.g., transitivity), integration (characteristic path length) and resilience (e.g., assortativity) of the network [Newman, 2002; Rubinov and Sporns, 2010]. This approach has yielded invaluable novel insights in the study of normative brain development [Supekar et al., 2009], as well as in neuropsychiatric [Bassett et al., 2008; Bai et al., 2012; Finn et al., 2014; van den Heuvel et al., 2010] and neurological disorders [Bernhardt et al., 2011; Kim et al., 2014]. In fact, deviations from the small world brain architecture, which is considered to be the most efficient network organization due to its dense local clusters of nodes connected by short paths facilitating quick information processing [Sporns, 2011], are a hallmark feature of several neurodevelopmental, psychiatric and neurological disorders [Menon, 2011]. A recent study by Teicher et al. [2014] conducted in a large sample of adults with maltreatment experiences used intraregional correlations in measures of cortical thickness to quantify measures of connectedness derived from gray matter volume indices. The authors were the first to report marked reductions in measures of connectedness in regions involved in emotion regulation (e.g., anterior cingulate, inferior temporal gyrus) in the sample of maltreated subjects. However, delineating the network based on measures of gray matter volume/cortical thickness yields (a) only cortical networks and (b) does not yield information about anatomical WM network organization.
To our knowledge, this is the first study to date to investigate the large‐scale WM network organization in the brains of children with maltreatment experiences, by applying graph theoretical analyses to DTI data to quantify the structural connectome. This investigation fills an important gap in the literature, as it remains unclear if global WM network architecture is altered after childhood maltreatment.
Here, we investigated the brain connectome on (a) global, (b) lobar and (c) regional levels in 25 children with a documented history of childhood maltreatment and 24 well‐matched controls. We did not make directional predictions in relation to the specific parameters assortativity, transitivity and characteristic path length in these circuits, for the following reasons. No previous studies have tried to quantify the entire WM connectome by applying graph theoretical approaches, constraining an empirical basis from which to inform directional hypotheses. In contrast, building on previous work discussed above [Hanson et al., 2015a, 2015b; van Harmelen et al., 2010; Teicher et al., 2014], we hypothesized that (a) due to the timing of adversity during critical cortical maturation periods (birth to 3 years), children with a history of maltreatment would show global reductions in brain network connectivity, consistent with a systemic and global influence of adversity on WM development, (b) based on previous findings showing structural and functional alterations as well as increased stress‐related vulnerability in the PFC and temporal lobe, we expected group differences in lobar and regional parameters of WM integrity in these a‐priori regions and, (c) global structural network alterations would be associated with indices of HPA‐functioning (basal morning cortisol) as well as measures related to the maltreatment experience and internalizing psychopathology as shown previously by Hanson et al. [2015b].
MATERIALS AND METHODS
Participants
For this study, we recruited 25 children with a history of maltreatment (mean age = 10.6, SD = 1.75 years; n = 12 female) who were permanently separated from their biological parents and placed into long‐term foster‐ or adoptive families before their 3rd year of life (mean age of separation = 1.59; SD = 1.05 years). This cut‐off of separation between birth and three years was chosen based on research showing that the first three years of life can be a sensitive period for attachment formation and to obtain a sample of children with early life stress that is circumscribed in the duration of adversity. Only children in permanent care (n = 16 adopted; n = 9 permanent foster care) were included to minimize the likelihood that children differed in exposure to present socioeconomic or psychological stressors related to placement instability. The children had lived on average for 9.14 years (SD = 2.24 years) in their permanent families at the time point of testing and experienced between 0‐4 transitory placements before permanent placement. Both groups reported generally high and comparable levels of relationship quality with their parents and caregivers on a self‐report questionnaire [EBF‐KJ, Titze et al., 2005] assessing the child‐caregiver relationship from the child's perspective [t(44)=1.12, P = 0.27].
A comparison group of 26 participants (mean age in years = 10.41, SD = 1.64, n = 12 female) was recruited, but 2 subjects were excluded as their T1 images could not be processed, leaving a final sample of n = 24 in the control group. Subjects had never been separated from their biological parents for prolonged periods of time, had never been in contact with social services with regard to the child's well‐being and were closely matched on age [t(47) = −0.38, P = 0.708)], sex [χ2(1)=0.02, P = 0.889], BMI [t(46)=0.77, P = 0.443)], IQ [(t(47)=1.35, P = 0.184)] and socioeconomic status [t(44)=1.46, P = 0.151]. Ethnicity in the sample was as follows ‐ Controls: Caucasian 87.5%; other: 12.5%; MT group: Caucasian: 56%; other: 44%. Functional MRI data from an overlapping sample sample has been published previously [Puetz et al., 2014]. Demographic information is presented in Table 1.
Table 1.
Sample demographics
| MT Group (n=25) | Control Group (n=24) | |||||
|---|---|---|---|---|---|---|
| Measure | Mean | sd | Mean | sd | P | |
| Age at assessment (y) | 10.60 | 1.75 | 10.42 | 1.64 | 0.71 | |
| Range | 8–14 | 7–13 | ||||
| WASI‐IQ (4‐subscale) | 101.48 | 10.56 | 104.42 | 9.49 | 0.18 | |
| SESa | 2.50 | 0.83 | 2.87 | 0.97 | 0.14 | |
| Relationship quality (EBF‐KJ) | 68.08 | 1.73 | 70.86 | 8.03 | 0.27 | |
| CBCLb Externalizing | 64.44 | 10.02 | 52.09 | 9.58 | <.001 | |
| CBCLb Internalizing | 60.16 | 8.53 | 51.78 | 7.47 | 0.001 | |
| n | % | n | % | P | ||
| Gender (female) | 12 | 48% | 12 | 50% | 0.89 | |
| Nationality | ||||||
| Caucasian | 14 | 56% | 21 | 88% | 0.00 | |
| Medicationc | ||||||
| None | 23 | 84% | 23 | 96% | 0.17 | |
| FAS‐Score | ||||||
| No features | 21 | 84% | / | / | ||
| Mild‐Moderate | 4 | 16% | / | / | ||
| n | % | n | % | |||
| Maltreatment subtype | ||||||
| Emotional or physical neglect | 16 | 64% | / | / | ||
| Abandonment | 6 | 24% | / | / | ||
| Physical abuse | 2 | 8% | / | / | ||
| Domestic Violence | 1 | 4% | / | / | ||
| Mean | sd | Mean | sd | |||
| Age at separation (mo) | 12.71 | 12.65 | / | / | ||
| Range | 0.25–39 | / | / | |||
| Demographics | ||||||
| Time spent in permanent family | 109.76 | 26.98 | 125.04 | 19.68 | ||
| Number of placements | 1.72 | 0.79 | 0 | 0 | ||
| Range | 0–4 | 0–0 | ||||
SES= Socioeconomic status.
CBCL = Child Behaviour Checklist T‐scores (Achenbach and Edelbrock, 1991).
Medication n = 3 MT, discontinued 24 h before assessment.
Exclusion criteria for the study were (a) present or past neurological disorder or brain injury, (b) IQ less than 85 [measured with the 4‐subscale version of the Wechsler Abbreviated Scales of Intelligence; Wechsler, 1999], (c) current pharmacological treatment (stimulant medication discontinued in n = 3 subjects 48 h before scanning) or (d) MRI contraindications (e.g., braces or ferromagnetic implants; claustrophobia).
Inspection of all available case records including medical records and a semi‐structured biographical interview [Groh, 2010] conducted with the children's caregivers about pre‐adoption circumstances (e.g., type of maltreatment) revealed that 64% (n = 16) children had experienced emotional and physical neglect, 8% physical abuse (n = 2), 24% abandonment (n = 6) and 4% witnessed severe domestic violence (n = 1). After separation from their biological parents, 14 children (66%) experienced intermediate secondary placements (ranging from 1 to 4) for a maximum of 12 months before entering a stable placement on average with 1.43 years (SD = 1.06). None of the children lived in the care of kin.
This study has been conducted in accordance with the Declaration of Helsinki and was approved by the local ethics committee at the RWTH Aachen. All children and their legal caregivers gave written informed assent and consent to participate in this study.
Clinical Assessments
The sample was assessed for present and lifetime mental disorders, according to DSM‐IV criteria, with a semi‐structured diagnostic interview conducted with the children and their caregivers [Groh, 2010; Unnewehr, Schneider, and Margraf, 1995]. The sample was free of current or past affective disorders, post‐traumatic stress disorder (PTSD), substance abuse, psychosis, any neurological disorder or pervasive developmental disorders such as autism. However, nine participants in the MT group fulfilled DSM‐IV criteria for ADHD (n = 6) and/or Dyslexia/Dyscalculia (n = 1), Enuresis (n = 1) or conduct disorder (n = 1). Within the control group, 1 child fulfilled DSM‐IV criteria for ADHD and three children for Dyslexia/Dyscalculia. All analyses were re‐run excluding the subjects with a diagnosis of ADHD and all results remained significant, thus those subjects were included in the final analyses.
Additionally, a dimensional measure of symptom severity and behavioral problems was obtained via the caregiver version of the Child‐Behavior Checklist for youth between 4 and 18 years of age (CBCL; Achenbach and Edelbrock, 1991).
Finally, previous research shows that children in care present with an increased risk for fetal alcohol syndrome (FAS) of up to 15% above the general population [Astley and Kinzel, 2002] and FAS can impact significantly on WM integrity [Sowell et al., 2008]. We therefore included the FAS Facial Photographic Screening Tool (Version 1.0.0) to measure the magnitude of the FAS facial phenotype expression in the MT group.
In the MT group, four children (16%) were identified to show mild to moderate features versus 21 without FAS features (84%). Analyses were run with and without these children and FAS features had no significant effect on the results, so the final analyses included these four children.
Neuroendocrine Assessments
Diurnal salivary cortisol levels were acquired in an overlapping sample of children in order to investigate associations between neuroendocrine alterations and stress‐related psychopathology after childhood maltreatment [Puetz et al., 2016b]. Morning cortisol secretion of saliva was measured in the morning between 7 and 9 AM and 30 minutes after awakening. A complete description of salivary cortisol assessment in this sample can be found elsewhere [Puetz et al., 2016b].
DTI/MRI‐Acquisition Protocol
All data was collected on a Siemens 3T MAGNETOM Trio MRI scanner (Siemens, Erlangen, Germany) with a 32‐channel head coil. Using foam padding to adjust the participant's head within the coil reduced participant's motion. T 1 –weighted anatomical images were obtained with a product MP‐Rage sequence (TE= 2.96 ms; TR= 2250 ms; TI= 900 ms; matrix= 256 × 256 mm2; number of slices: 176; slice thickness: 1 mm; flip angle: 7°; Voxel size: 1 mm3).
Diffusion Tensor Images were acquired using a spin‐echo, single‐shot EPI pulse sequence covering the whole brain with the following parameters: TR= 8.3; TE = 85.0; flip angle: 90°; slices = 65; slice thickness: 2.4 mm; matrix = 96 × 96; Voxel: 2 × 2; b‐values between 0 and 1,000 s/mm2, 30 non‐collinear gradient directions during a scan time of approximately 6 minutes.
DTI Preprocessing
All subject's diffusion‐weighted images (DWI) were visually inspected to detect artifacts such as signal dropouts, geometric distortions and missing slices [Tournier, Mori, and Leemans, 2011]. DWIs were then corrected for motion artifacts and distortions using the motion correction tool Tortoise [Pierpaoli et al., 2010], which corrects for motion artifacts and eddy distortion in each DW image by applying a quadratic deformation model to a structural target image, where all deformations are calculated and then combined to reduce errors from interpolation post‐registration.
In order to improve the co‐registration results, all T1 images were skull‐stripped to remove the skull and neck from the brain using the Freesurfer Tool BET [Jenkinson et al., 2005].
Whole Brain Connectivity
The T1 image of each subject was parcellated into 95 regions of interest (ROI) of the Desikan atlas [Desikan et al., 2006] using FreeSurfer [Dale et al., 1999]. The collection of voxels in the WM‐grey matter boundary in each ROI was used as a seed region for probabilistic tracking to the remaining 94 ROIs. FSL's bedpostx and probtrackx were run using default parameters and a symmetric 95x95 matrix of undirected weighted connectivity measures was created for each subject. Each element of the matrix followed the formula , where represents the number of fibers reaching ROI j when seeded from ROI i, denotes the number of fibers seeded in ROI i and is the surface area of ROI i. Because the result of probabilistic tracking will differ in any two trials, the matrix was then symmetrized by averaging the entry with the entry . For a more detailed description, please see [Ingalhalikar et al., 2014].
The lobar networks (frontal, temporal, parietal, occipital) were generated by excluding connections with any of the other lobes and including only connections within the respective lobe.
ROI analyses
Based on the extant literature showing structural and functional abnormalities in the frontal and temporal lobes in children with MT [Maheu et al., 2010; McCrory et al., 2011; Mueller et al., 2010] as well as our previous work suggesting altered functional connectivity within fronto‐cingulate networks [Puetz et al., 2014], complementary ROI analyses were carried out on individual nodes in the frontal and temporal lobes. Measures for left and right were highly correlated and therefore collapsed to reduce multiple comparisons (rLRfrontal = 0.95, P < 0.001; rLRtemporal = 0.91, P < 0.001).
Values for global‐, lobar and regional (node‐wise) connectivity were extracted to investigate between‐group differences in SPSS (IBM SPSS Statistics Version 19; SPSS Inc., Chicago, IL). Values for general node strength (GNS; see below) were extracted to investigate relationships with maltreatment related variables as well as basal cortisol secretion for all participants. In order to correct for multiple comparisons whilst increasing our power in light of the number of comparisons, all effects were tested on whether they survived a false discovery rate (FDR) threshold of q = 0.05. The threshold was set according to the procedure by Benjamini and Hochberg [Benjamini and Hochberg, 1995].
Global Network Measures
We analyzed the resulting connectivity network on global, lobar and regional (i.e., node‐wise) levels. Utilizing all the connections in the matrix, we calculated global network measures of node strength (NS), transitivity, assortativity and characteristic path length, which we refer to as the global network measures that were applied to the whole connectome and four lobes.1 A brief summary of each measure as defined by [Rubinov and Sporns, 2010] follows.
Node strength (NS) – In order to quantify overall node strength of the whole network (GNS) and per lobe (LNS) two measures were derived from the node‐wise measure undirected node strength (UNS). For each node in the network, we calculated UNS as the sum of the connectivity values involving that node, resulting in 95 regional measures, and the GNS as the average of those 95 measures, i.e., the sum of all edge weights within the network. GNS was calculated to quantify overall connectivity strength. The same measure was calculated for the frontal, parietal, temporal and occipital lobe (LNS) in order to quantify connectivity strength within each of the 4 lobes separately.
Transitivity ‐ The transitivity of a network is an edge‐weighted quantification of the proportion of complete triangles in the network, and is a measure of local connectivity.
Assortativity ‐ Networks with a positive assortativity coefficient have a core of mutually interconnected high‐degree (making many connections) hubs, while networks with a negative assortativity coefficient have widely distributed high‐degree hubs.
Characteristic path length ‐ The characteristic path length is the average shortest weighted path length between all pairs of nodes in the network. This measure reflects the degree to which the regions communicate, with shorter path length reflecting higher efficiency.
Regional (Node‐Wise) Network Measures
Complementary node‐wise measures were calculated for the individual nodes within our regions of interest (i.e., frontal and temporal lobe) in order to investigate group differences in specific individual nodes.
Undirected node strength (UNS) – see Node Strength (NS) above.
Local Efficiency – Local efficiency of node i is defined as the global efficiency calculated only over paths constructed using the immediate neighbors of i in the graph.
RESULTS
In order to rule out potential confounding effects of variables known to be associated with WM network parameters and maltreatment [Sheridan et al., 2012; Strathearn et al., 2001], two univariate analyses of variance (ANOVA) with 1) head circumference (HC) and 2) total WM volume as the between‐subject factor controlling for age were carried out. Both ANOVAs revealed no significant group‐by‐age interactions or group differences in HC (F 46 < 3.67; P > 0.05) or total WM volume (F 49 > 1.80; P > 0.05), but an expected significant age effect on HC (F 46 = 16.59; P < 0.05) and total WM volume (F 49 > 13.80; P < 0.05). All subsequent whole brain WM network analyses were therefore controlled for age.
Global Measures of Network Strength and Integrity
Multivariate analyses of variance (MANOVA) revealed significant group differences between children in the MT and control group on average whole‐brain general node strength (GNS: F 49 = 9.14, P = 0.004, see Table 2). Post‐hoc analyses showed that children in the MT group had lower GNS as compared to controls across the entire connectome [t(48) = −3.02, P = 0.004]. Between‐group analyses of the other global indices of network integrity (transitivity, assortativity and characteristic path length) indicated a significantly reduced global assortativity [t(48) = −2.9, P = 0.006] and transitivity [t(48) = −2.76, P = 0.008], being lower in the MT group than the control group [all FDR corrected]. No significant between‐group differences emerged for the global measure of characteristic path length (all P > 0.133; see Table 2).
Table 2.
Global measures of network integrity for the whole brain and frontal and temporal lobes (n = 25 MT; n = 24 controls)
| Measure | Region | T | P | FDR‐corrected |
|---|---|---|---|---|
| GNS | Whole‐brain | −3.02 | 0.00 | * |
| Assortativity | −2.90 | 0.01 | * | |
| Transitivity | −2.76 | 0.01 | * | |
| Characteristic path length | 1.53 | 0.13 | / | |
| Assortativity | L&R Frontal | −0.32 | 0.73 | / |
| Transitivity | −1.76 | 0.09 | / | |
| Characteristic path length | 3.66 | 0.00 | * | |
| Assortativity | L&R Temporal | 0.07 | 0.95 | / |
| Transitivity | −1.01 | 0.45 | / | |
| Characteristic path length | 0.57 | 0.76 | / |
Lobar Measures of Network Strength and Integrity
In order to investigate if the individual lobes drove group differences in GNS differentially, analyses were carried out separately for the frontal, temporal, parietal and occipital lobe. Analyses of GNS within the lobes (LNS) revealed significantly lower LNS in the MT group in the frontal‐ [t(48) = −3.00, P = 0.004], parietal‐ [t(48) = −2.69 P = 0.01] and the occipital lobe [t(48) = −3.84, P < 0.001] and temporal lobe [t(48) = −2.38, P = 0.021; all FDR corrected].
We then examined the three measures of network integrity on the lobar level and focused specifically on our a‐priori regions frontal and temporal lobe. Within the frontal lobe, characteristic path length was significantly greater for the MT group [t(48) = −2.73, P = 0.001, FDR corrected]. No significant between group differences were found within the frontal lobe for measures of transitivity or assortativity (all P > 0.09).
No significant group differences were found for any of the indices within the temporal lobe (all P > 0.45). Results for the lobar indices of network integrity are shown in Table 2.
Node‐Wise Measures (Regional Measures)
Frontal lobe regions
We calculated complementary node‐wise measures of the indices for UNS and local efficiency for each individual regional node of the frontal and temporal lobe in order to identify specific anatomical regions with group differences within the frontal and temporal lobe (see Table 3). As expected from the lobar analyses of LNS, between‐group analyses showed that a substantial number of regions within the frontal lobe had lower UNS in the MT group relative to controls (for complete results see Table 3 and Fig. 1). Specific regions showing significantly lower UNS in the MT group relative to controls included the right caudal anterior cingulate cortex [t(48) = −3.51, P = 0.001], the left and right superior frontal gyrus [left: t(48) = −4.15, P < 0.001, right: t(48) = −4.07, P < 0.001], the right frontal pole [t(48) = −4.05; P < 0.001] as well as left and right orbitofrontal gyri (all P < 0.003, see Table 3).
Table 3.
Node‐wise measures of the individual regions of the frontal and temporal lobe (n = 25 MT; n = 24 controls)
| Measure | Region/Node | T | P | FDR‐corrected |
|---|---|---|---|---|
| Frontal lobe | ||||
| Undirected node strength | ||||
| Left superior frontal gyrus | −4.15 | 0.000 | * | |
| Right superior frontal gyrus | −4.07 | 0.000 | * | |
| Right frontal pole | −4.05 | 0.000 | * | |
| Right caudal anterior cingulate | −3.51 | 0.001 | * | |
| Right pars orbitalis | −3.65 | 0.001 | * | |
| Left rostral anterior cingulate gyrus | −3.24 | 0.002 | * | |
| Right lateral orbitofrontal | −3.32 | 0.002 | * | |
| Left lateral orbitofrontal gyrus | −3.18 | 0.003 | * | |
| Left caudal middle frontal gyrus | −3.03 | 0.004 | * | |
| Right rostral middle frontal gyrus | −2.87 | 0.006 | * | |
| Left rostral middle frontal gyrus | −2.83 | 0.007 | * | |
| Left middle orbitofrontal gyrus | −2.69 | 0.010 | * | |
| Left caudal anterior cingulate | −2.67 | 0.011 | * | |
| Right rostral anterior cingulate | −2.49 | 0.016 | * | |
| Right Caudal middle frontal | −2.45 | 0.018 | * | |
| Right middle orbitofrontal | −2.40 | 0.020 | * | |
| Left pars orbitalis | −2.34 | 0.024 | * | |
| Left precentral gyrus | −2.20 | 0.033 | / | |
| Local efficiency | ||||
| Right caudal middle frontal gyrus | −2.7 | 0.010 | * | |
| Right frontal pole | −2.03 | 0.048 | / | |
| Temporal lobe | ||||
| Undirected node strength | ||||
| Right fusiform gyrus | −3.76 | 0.000 | * | |
| Left fusiform gyrus | −3.46 | 0.001 | * | |
| Left parahippocampal gyrus | −3.36 | 0.002 | * | |
| Right parahippocampal gyrus | −2.55 | 0.014 | * | |
| Right entorhinal gyrus | −2.42 | 0.019 | * | |
| Left superior temporal gyrus | −2.36 | 0.023 | * | |
| Left STS | −2.07 | 0.044 | / | |
| Right superior temporal gyrus | −2.06 | 0.045 | / | |
| Local efficiency | ||||
| Left temporal pole | −2.91 | 0.006 | * | |
Figure 1.
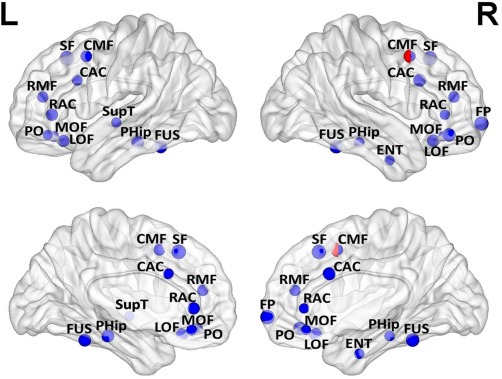
Node measures of the individual regions of the frontal and temporal lobe (n = 25 MT; n = 24 Controls), placed in the brain according to the coordinates of their centroids. Blue spheres represent statistically significantly higher UNS in controls, with asterisks denoting results that survive FDR correction for multiple comparisons. Red spheres represent statistically significantly higher local efficiency in controls. In two nodes with significant results for both node strength and local efficiency, half of each sphere has been depicted. The diameter of each sphere is proportional to the Cohen's d effect size measure (Min: 0.68, Max: 1.21). Nodes were plotted using the BrainNet Viewer software [Xia et al., 2013]. Abbreviations: L = Left; R = Right; RMF= rostral middle frontal gyrus; SF= superior frontal gyrus; CAC= caudal anterior cingulate; CMF= caudal middle frontal gyrus; PC= precentral gyrus; RAC= rostral anterior cingulate; PT= pars triangularis; LOF= lateral orbitofrontal gyrus; TP= temporal pole; SupT= superior temporal gyrus; PHip= parahippocampal gyrus; FUS= fusiform gyrus; BSS=; IC= isthmus cingulate; FP= frontal pole; MOF= medial orbitofrontal gyrus; ENT= entorhinal gyrus; PO= pars orbitalis.
Temporal lobe regions
Analyses of UNS within the individual regions of the temporal lobe indicated lower UNS in the left and right fusiform gyrus (left: t(48) = −3.45, P = 0.001, right: t(48) = −3.76, P < 0.001; see Fig. 1) and left parahippocampal gyrus [t(48) = −3.36, P = 0.002]. Complete results for all nodes are displayed in Table 3.
Relationships Between General Node Strength and Factors Related to the Maltreatment Experience and General Psychopathology
To investigate potential relationships between GNS and factors related to early life stress we conducted a multivariate regression model with the predictors number of placements (ranging from 0‐4), age at separation and time spent in the permanent foster or adoptive family using bootstrapping in the whole sample [Hayes, 2013]. A higher number of transitory placements that a child experienced before final placement significantly predicted lower overall GNS [B = −4.06, t(48) = −2.53; P = 0.015], while age at separation and time spent in the permanent family did not reach significance (both P > 0.30) in the model.
A correlational analysis was carried out for the association between morning cortisol levels and GNS, since cortisol was only available for a subset of children in the sample (n = 40; n = 21 MTs, n = 19 controls). The subset of MT and control children for whom cortisol data was available did not differ from the larger sample on any demographic variables listed in Table 1 (all Ps > 0.11) or within subsample (all Ps < 0.37, except for nationality: P < 0.001). The groups in the subsample differed trend‐level on GNS (p.06).
This analysis revealed a significant positive association, indicating that higher morning cortisol levels were associated with higher GNS (rs = 0.44, P = 0.005).
Finally, exploratory correlational analyses were carried out to investigate relationships between GNS and internalizing and externalizing behavior problems as measured with the child‐behavior‐checklist for children (CBCL). Significant negative relationships between GNS and externalizing and internalizing behaviours emerged, indicating that children with lower GNS displayed more internalizing (r = −0.33, P = 0.02) and externalizing (r = −0.31, P = 0.031) problems as compared to children with higher GNS.
DISCUSSION
In the present study, we closed an important gap in the literature by applying graph theoretical analyses to DTI data to investigate alterations in global and regional parameters of brain connectivity associated with childhood maltreatment. Our data provides evidence suggesting global and specific regional reductions in brain connectivity after childhood maltreatment. Moreover, the decrease in global connectivity was associated with placement instability, altered endocrine functioning and higher levels of internalizing and externalizing behavior.
The finding of reduced GNS in the entire structural connectome, as well as specific reductions in frontal and temporal lobe regions suggests global and region‐specific alterations in WM integrity after childhood maltreatment and is in line with previous findings of regional reductions in WM indices such as FA [Hanson et al., 2015b; Hanson et al., 2013; Govindan et al., 2010], WM volume [Eluvathingal et al., 2006; Mehta et al., 2009; Sheridan et al., 2012] and reductions in overall EEG α‐power which correlates with WM integrity [Marshall et al., 2002; Valdés‐Hernández et al., 2010]. The significant reduction in GNS on the global and individual lobar level support our hypotheses that early adversity, at least within the first three years of life, affects WM development on systemic and focal scales. Within a neurodevelopmental context, this finding is likely due to the uniquely linear developmental trajectories of WM [Giedd et al., 1996; Gogtay et al., 2004], which develops significantly during the first 2 years of life, in an experience‐dependent fashion [Gao et al., 2009].
It is therefore conceivable that increased exposure to glucocorticoids as a response to maltreatment and the number of transitory and unstable placements after removal from the biological family initially affect WM development globally, and specific regional neural structures at different developmental periods. In our sample, we found a significant association between morning hypocortisolism and lower GNS, which is in line with research showing associations between WM connectivity and cortisol based measures of stress‐reactivity in young children [Sheikh et al., 2014] and healthy adults [Hermans et al., 2011]. A recent study by Sheikh et al. [2014] was the first to report a mediating influence of parenting on the negative association between stress‐reactivity (i.e., higher cortisol secretion in response to a stressor) and WM integrity in girls. This provides preliminary support for our finding of global alterations in WM microstructure associated with adverse caregiving and altered neuroendocrine function. However, we can merely establish a correlational relationship between reduced basal cortisol production and reduced WM integrity and we assume that a cascade of adverse events during the first three years of life (before permanent removal from the adverse environment) had a cumulative severity effect on WM development that is still detectable years after the removal of the stressor.
Alternatively, the WM alterations found here could reflect adaptations to the early environment, in that the organism favored the less extensive wiring‐cost associated with high GNS and network resilience in the absence of environmental stimulation. However, findings from graph‐theoretical analyses of developing brain networks comparing children and young adults clearly speak against this account, showing that maturation of brain networks is characterized by a strengthening of long‐range connectivity and stronger indices of small‐world organization, such as shorter path length [Supekar et al., 2009].
In line with this, we found other metrics of large‐scale networks involved in psychopathology to be significantly altered after childhood maltreatment, including reduced assortativity on the global level and increased characteristic path length within the frontal lobe. Reduced assortativity has been associated with decreased clustering and connectivity between the nodes and at the expense of lower wiring cost, the system is less resilient to damage and disruption [Barrat, 2004; Lynall et al., 2010].
Additionally, the finding of increased characteristic path length in the prefrontal lobe has been identified as a marker of dysfunctional brain organization implicated in psychopathology as “a tell‐tale sign of significant global deficits” [Menon, 2011, p.8; Kim et al., 2014]. In fact, this pattern of global network alterations indicating a deviation from the small world‐organization (low GNS, high CP) present in our sample of maltreated children, has been shown before in disorders emerging either in childhood [Dyslexia: Finn et al., 2014; ADHD: Wang et al., 2009; Autism: Shi et al., 2013] or adolescence [Depression: Bai et al., 2012; Leistedt et al., 2009; Schizophrenia: Supekar et al., 2009], but not in adulthood [see e.g. Alzheimer's disease: Wen et al., 2011]. This supports the notion that stress associated with early childhood maltreatment acts also at the neural systems level in addition to the modular level, and that early neurodevelopmental changes in brain organization through stress could underlie global cognitive and emotional alterations, as is commonly observed in pediatric diseases or childhood TBI [Ewing‐Cobbs et al., 1997]. In line with this, we found reductions in GNS to be associated with higher levels of internalizing (and externalizing) pschopathology in our sample, a relationship that has previously been established between lower integrity of the uncinate fasciculus and internalizing symptoms and future vulnerability to future life stress [Hanson et al., 2015b]. However, the exact mechanisms and timing by which and when, stress associated with childhood maltreatment can alter WM organization during neuronal maturation processes, ranges from synaptogenesis over to synaptic pruning and needs further investigation in animal studies.
Considering that early neurodevelopmental changes can influence subsequent neurodevelopmental processes, the network alterations found in our present sample can potentially increase vulnerability to a wide range of psychopathology in adolescence, consistent with the multi‐symptom profiles observed in victims of childhood maltreatment [Lawrence et al., 2006].
In our sample, the analyses of UNS showed significantly lower node strength in the anterior and dorsal anterior cingulate cortex (aACC/dACC), the ventromedial prefrontal cortex and the orbitofrontal cortex (OFC), all of which have been implicated in structural and functional neuroimaging studies of childhood maltreatment [De Brito et al., 2013; Hanson et al., 2013; Kelly et al., 2013; Puetz et al., 2014]. Importantly, the vmPFC has intensive connections with the temporal lobe, specifically the amygdala, exerting top‐down control in negative affect regulation and fear extinction [Phelbs et al., 2004]. Reduced local efficiency within the vmPFC could give rise to heightened amygdala reactivity observed in maltreated children [McCrory et al., 2011; McCrory et al., 2013; Maheu et al., 2010; Tottenham et al., 2010].
Whilst our hypotheses regarding global alterations of the large‐scale network, as well as lobar and regional alterations of the frontal network were supported, we did not find evidence for significant alterations of lobar alterations in the temporal lobe, as suggested by previous fMRI studies [e.g. Maheu et al., 2010]. One possible explanation is the heterogeneity in samples, with previous studies reporting e.g. on children in institutions with comorbid psychiatric disorders or more recent experiences of maltreatment, while all of the children in our study were permanently removed from the adverse caregiving environment before age 3. In line with this, it is noteworthy that at least at the time point of participation in the study, the current sample did not present with affective disorders and could thus be considered relatively well functioning, potentially limiting generalizability to youth more affected by early adversity.
In the present sample, the subjects were free of affective disorders and assessed before entering puberty, so we were not able to investigate potential relationships between WM organization and psychopathology during a time of substantial neuronal reorganization and vulnerability. Future longitudinal studies are needed that elucidate potential associations between WM organization and psychopathology before and after puberty.
However, a number of limitations need to be kept in mind with respect to the current study. First, the incomplete biographical records before removal from the biological family make it impossible to rule out potential genetic and prenatal influences on WM integrity or environmental factors such as malnutrition. However, there is evidence from animal studies that the impact of maltreatment on brain development persists even after controlling for environmental and genetic factors [Malter Cohen et al., 2013] and in this carefully matched sample, the indices of malnutrition that were available to us, i.e., height, weight, BMI and importantly HC and total WM volume, did not differ between the groups thereby ruling out potentially confounding effects of these parameters. In addition, since the children in our sample did not report themselves on maltreatment experiences via self‐report measures, but control group status was ascertained via the absence of contact with social services, we cannot rule out that undetected child abuse in the control group affected the findings. However, we consider the chances of undetected maltreatment unlikely as we carefully assessed past and present trauma in the PTSD section of the clinical interview (K‐DIPS, child‐ and parent interview), and found no indications for the presence of trauma within the control group. Furthermore, previous research has suggested gender differences in WM organization [Ingalhalikar et al., 2014], which we were unable to investigate considering the sample size. Future studies should also employ a longitudinal approach considering that WM development continues well after the age‐range that was investigated in the present study.
Keeping these limitations in mind, the present study closed an important gap in the existing literature by demonstrating global and complementary local brain network alterations after childhood maltreatment, that are consistent with a less efficient neural organization and neural communication that might underlie dysfunctional processing of affective, cognitive and sensory information previously shown in fMRI studies. We hope these new insights open up new avenues of research into large‐scale network organizations in maltreated children
ACKNOWLEDGEMENTS
The authors thank the families who participated in this study as well as M. Zvyagintsev and W. Grodd at University Hospital RWTH Aachen for their invaluable technical advice regarding the implementation of the sequence.
Conflict of interest: K.K. has received speaking fees from Lilly, Novartis, Medice and Shire. She has an investigator‐initiated trial (IIT) with Vivor AG on the effects of polyunsaturated fatty acids on brain maturation in children with and without ADHD. All other authors report no potential conflicts of interest.
Footnotes
The measures of assortativity and transitivity utilized a binarized version of the connectivity matrix. The remaining global measures of modularity and characteristic path length were calculated using the weighted, undirected variation of the formula.
REFERENCES
- Achenbach TM, Edelbrock C (1991): Manual for the Child Behavior Checklist and 199Z Profile. Burlington, VT: University of Vermont Department of Psychiatry. [Google Scholar]
- Astley SJ, Kinzel J. 2002. Fetal Alcohol Syndrome Facial Photographic Analysis Software. Seattle (WA): University of Washington; [Google Scholar]
- Bai F, Shu N, Yuan Y, Shi Y, Yu H, Wu D, Wang J, Xia M, He Y, Zhang Z (2012): Topologically convergent and divergent structural connectivity patterns between patients with remitted geriatric depression and amnestic mild cognitive impairment. J Neuroscie 32:4307–4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambico FR, Lacoste B, Hattan PR, Gobbi G (2013): Father absence in the monogamous california mouse impairs social behavior and modifies dopamine and glutamate synapses in the medial prefrontal cortex. Cereb Cortex 25:1163–1175. [DOI] [PubMed] [Google Scholar]
- Barrat A (2004): The architecture of complex weighted networks. Proc Natl Acad Sci USA 101:3747–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Bullmore E, Verchinski BA, Mattay VS, Weinberger DR, Meyer‐Lindenberg A (2008): Hierarchical organization of human cortical networks in health and schizophrenia. J Neurosci 28:9239–9248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y (1995): Controlling the false discovery rate: A practical and powerful approach to multiple testing. J Roy Stat Soc Ser B (Method) 289–300. [Google Scholar]
- Bernhardt BC, Chen Z, He Y, Evans AC, Bernasconi N (2011): Graph‐theoretical analysis reveals disrupted small‐world organization of cortical thickness correlation networks in temporal lobe epilepsy. Cereb Cortex 21:2147–2157. [DOI] [PubMed] [Google Scholar]
- Bick J, Zhu T, Stamoulis C, Fox NA, Zeanah C, Nelson CA (2015): Effect of early institutionalization and foster care on long‐term white matter development: A randomized clinical trial. JAMA Pediatr 169:211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli C, Walker L, Irfanoglu MO, Barnett A, Basser P, Chang LC, Koay C, Pajevic S, Rohde G, Sarlls J, Wu M (2010): TORTOISE: An integrated software package for processing of diffusion MRI data Title In: ISMRM 18th annual meeting. Stockholm, Sweden. [Google Scholar]
- Carlyle BC, Duque A, Kitchen RR, Bordner KA, Coman D, Doolittle E, Papademetris X, Hyder F, Taylor JR, Simen AA (2012): Maternal separation with early weaning: A rodent model providing novel insights into neglect associated developmental deficits. Dev Psychopathol 24:1401–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Jeong B, Rohan ML, Polcari AM, Teicher MH (2009): Preliminary evidence for white matter tract abnormalities in young adults exposed to parental verbal abuse. Biol Psychiatry 65:227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Jeong B, Polcari A, Rohan ML, Teicher MH (2012): Reduced fractional anisotropy in the visual limbic pathway of young adults witnessing domestic violence in childhood. NeuroImage 59:1071–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI (1999): Cortical surface‐based analysis. I. Segmentation and surface reconstruction. NeuroImage 9:179–194. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, Domschke K, Hohoff C, Ohrmann P, Bauer J, Lindner C (2012): Limbic scars: Long‐term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry 71:286–293. [DOI] [PubMed] [Google Scholar]
- De Brito SA, Viding E, Sebastian CL, Kelly PA, Mechelli A, Maris H, McCrory EJ (2013): Reduced orbitofrontal and temporal grey matter in a community sample of maltreated children. J Child Psychol Psychiatry 54:105–112. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS (2006): An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 31:968–980. [DOI] [PubMed] [Google Scholar]
- Eluvathingal TJ, Chugani HT, Behen ME, Juhász C, Muzik O, Maqbool M, Chugani DC, Makki M (2006): Abnormal brain connectivity in children after early severe socioemotional deprivation: A diffusion tensor imaging study. Pediatrics 117:2093–2100. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD (2007): Functional Neuroimaging of Anxiety: A Meta‐Analysis of Emotional Processing in PTSD, Social Anxiety Disorder, and Specific Phobia. Am J Psychiatry 164:1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing‐Cobbs L, Fletcher JM, Levin HS, Francis DJ, Davidson K, Miner M (1997): Longitudinal neuropsychological outcome in infants and preschoolers with traumatic brain injury. J Int NeuropsychSoc 3:581–91. [PubMed] [Google Scholar]
- Finn ES, Shen X, Holahan JM, Scheinost D, Lacadie C, Papademetris X, Shaywitz SE, Shaywitz BA, Constable RT (2014): Disruption of functional networks in dyslexia: A whole‐brain, data‐driven analysis of connectivity. Biol Psychiatry 76:397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn M, Cicchetti D, Rogosch F (2014): The prospective contribution of childhood maltreatment to low self‐worth, low relationship quality, and symptomatology across adolescence: A developmental‐organizational perspective. Dev Psychol 50:2165–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Lin W, Chen Y, Gerig G, Smith JK, Jewells V, Gilmore JH (2009): Temporal and spatial development of axonal maturation and myelination of white matter in the developing brain. Am J Neuroradiol 30:290–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, Vauss YC, Rapoport JL (1996): Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: Ages 4‐18 years. J Comp Neurol 366:223–230. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, Herman DH, Clasen LS, Toga AW, Rapoport JL (2004): Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA 101:8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindan RM, Behen ME, Helder E, Makki MI, Chugani HT (2010): Altered water diffusivity in cortical association tracts in children with early deprivation identified with Tract‐Based Spatial Statistics (TBSS). Cereb Cortex 20:561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh EM. 2010. Die psychische Belastung bei Pflegekindern: Vorhersage ihrer posttraumatischen Symptomatik und Adaption eines neuen Instruments zur Erfassung ihrer allgemeinen psychischen Belastung. [Google Scholar]
- Hagmann P, Kurant M, Gigandet X, Thiran P, Wedeen VJ, Meuli R, Thiran JP (2007): Mapping human whole‐brain structural networks with diffusion MRI. PloS One 2:e597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Adluru N, Chung MK, Alexander AL, Davidson RJ, Pollak SD (2013): Early neglect is associated with alterations in white matter integrity and cognitive functioning. Child Dev 84:1566–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Nacewicz BM, Sutterer MJ, Cayo AA, Schaefer SM, Rudolph KD, Shirtcliff EA, Pollak SD, Davidson RJ (2015a): Behavioral problems after early life stress: Contributions of the hippocampus and amygdala. Biol Psychiatry 77:314–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Knodt AR, Brigidi BD, Hariri AR (2015b): Lower structural integrity of the uncinate fasciculus is associated with a history of child maltreatment and future psychological vulnerability to stress. Dev Psychopathol 27:1611–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF ( 2013): Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression‐Based Approach. New York, NY: Guilford Press. [Google Scholar]
- Helmeke C, Ovtscharoff W, Poeggel G, Braun K (2008): Imbalance of immunohistochemically characterized interneuron populations in the adolescent and adult rodent medial prefrontal cortex after repeated exposure to neonatal separation stress. Neuroscie 152:18–28. [DOI] [PubMed] [Google Scholar]
- Hermans EJ, van Marle HJ, Ossewaarde L, Henckens MJ, Qin S, van Kesteren MT, Schoots VC, Cousijn H, Rijpkema M, Oostenveld R, Fernández G (2011): Stress‐related noradrenergic activity prompts large‐scale neural network reconfiguration. Science 334:1151–1153. [DOI] [PubMed] [Google Scholar]
- Huang H, Gundapuneedi T, Rao U (2012): White matter disruptions in adolescents exposed to childhood maltreatment and vulnerability to psychopathology. Neuropsychopharmacology 37:2693–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingalhalikar M, Smith A, Parker D, Satterthwaite TD, Elliott MA, Ruparel K, Hakonarson H, Gur RE, Gur RC, Verma R (2014): Sex differences in the structural connectome of the human brain. Proc Natl Acad Sci USA 111:823–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackowski AP, de Araújo CM, de Lacerda ALT, Mari JJ, Kaufman J (2009): Neurostructural imaging findings in children with post‐traumatic stress disorder: Brief review. Psychiatry Clin Neurosci 63:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Pechaud M, Smith S. 2005. BET2: MR‐based estimation of brain, skull and scalp surfaces In: Eleventh annual meeting of the organization for human brain mapping. [Google Scholar]
- Kaufman J, Charney D (2001): Effects of early stress on brain structure and function: Implications for understanding the relationship between child maltreatment and depression. Dev Psychopathol 13:451–471. [DOI] [PubMed] [Google Scholar]
- Kelly PA, Viding E, Wallace GL, Schaer M, De Brito SA, Robustelli B, McCrory EJ (2013): Cortical thickness, surface area, and gyrification abnormalities in children exposed to maltreatment: Neural markers of vulnerability? Biol Psychiatry 74:845–852. [DOI] [PubMed] [Google Scholar]
- Kim J, Parker D, Whyte J, Hart T, Pluta J, Ingalhalikar M, Coslett HB, Verma R (2014): Disrupted structural connectome is associated with both psychometric and real‐world neuropsychological impairment in diffuse traumatic brain injury. J Int Neuropsychol Soc 20:887–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence CR, Carlson EA, Egeland B (2006): The impact of foster care on development. Dev Psychopathol 18:57–76. [DOI] [PubMed] [Google Scholar]
- Leistedt SJJ, Coumans N, Dumont M, Lanquart JP, Stam CJ, Linkowski P (2009): Altered sleep brain functional connectivity in acutely depressed patients. Hum Brain Mapp 30:2207–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C (2009): Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci 10:434–445. [DOI] [PubMed] [Google Scholar]
- Lynall ME, Bassett DS, Kerwin R, McKenna PJ, Kitzbichler M, Muller U, Bullmore E (2010): Functional connectivity and brain networks in schizophrenia. J Neurosci 30:9477–9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan H, Fleming J (2014): Childhood abuse and lifetime psychopathology in a community sample. Am J Psychiatry 158:1878–1883. [DOI] [PubMed] [Google Scholar]
- Maheu FS, Dozier M, Guyer AE, Mandell D, Peloso E, Poeth K, Jenness J, Lau JY, Ackerman JP, Pine DS, Ernst M (2010): A preliminary study of medial temporal lobe function in youths with a history of caregiver deprivation and emotional neglect. Cogn Affect Behav Neurosci 10:34–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malter Cohen M, Jing D, Yang RR, Tottenham N, Lee FS, Casey BJ (2013): Early‐life stress has persistent effects on amygdala function and development in mice and humans. Proc Natl Acad Sci USA 110:18274–18278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Henze D, Hirase H (2002): Hippocampal pyramidal cell‐interneuron spike transmission is frequency dependent and responsible for place modulation of interneuron discharge. J Neuroscie 22:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory EJ, De Brito SA, Sebastian CL, Mechelli A, Bird G, Kelly P, Viding E (2011): Heightened neural reactivity to threat in child victims of family violence. Curr Biol 21:R947–R948. [DOI] [PubMed] [Google Scholar]
- McCrory E, De Brito SA, Viding E (2012): The link between child abuse and psychopathology: A review of neurobiological and genetic research. J R Soc Med 105:151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory EJ, De Brito SA, Kelly PA, Bird G, Sebastian CL, Mechelli A, Samuel S, Viding E (2013): Amygdala activation in maltreated children during pre‐attentive emotional processing. Br J Psychiatry 202:269–276. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Golembo NI, Nosarti C, Colvert E, Mota A, Williams SC, Rutter M, Sonuga‐Barke EJ (2009): Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: The English and Romanian Adoptees study pilot. J Child Psychol Psychiatry 50:943–951. [DOI] [PubMed] [Google Scholar]
- Menon V (2011): Large‐scale brain networks and psychopathology: A unifying triple network model. Trends Cogn Sci 15:483–506. [DOI] [PubMed] [Google Scholar]
- Mueller SC, Maheu FS, Dozier M, Peloso E, Mandell D, Leibenluft E, Pine DS, Ernst M (2010): Early‐life stress is associated with impairment in cognitive control in adolescence: An fMRI study. Neuropsychologia 48:3037–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad A, Kolb B (2011): Maternal separation altered behavior and neuronal spine density without influencing amphetamine sensitization. Behav Brain Res 223:2–16. [DOI] [PubMed] [Google Scholar]
- Murmu MS, Salomon S, Biala Y, Weinstock M, Braun K, Bock J (2006): Changes of spine density and dendritic complexity in the prefrontal cortex in offspring of mothers exposed to stress during pregnancy. Eur J Neurosci 24:1477–1487. [DOI] [PubMed] [Google Scholar]
- Newman M (2002): Assortative mixing in networks. Phys Rev Lett 89:208701. [DOI] [PubMed] [Google Scholar]
- Nusslock R, Miller GE (2016): Early‐life adversity and physical and emotional health across the lifespan: A neuro‐immune network hypothesis. Biol Psychiat 80:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE (2004): Extinction learning in humans: role of the amygdala and vmPFC. Neuron 43:897–905. [DOI] [PubMed] [Google Scholar]
- Puetz VB, Kohn N, Dahmen B, Zvyagintsev M, Schüppen A, Schultz RT, Heim CM, Fink GR, Herpertz‐Dahlmann B, Konrad K (2014): Neural response to social rejection in children with early separation experiences. J Am Acad Child Adolesc Psychiatry 53:1328–1337.e8. [DOI] [PubMed] [Google Scholar]
- Puetz VB, Viding E, Palmer A, Kelly PA, Lickley R, Koutoufa I, Sebastian CL, McCrory EJ (2016a): Altered neural response to rejection‐related words in children exposed to maltreatment. J Child Psychol Psychiatr 57:1165–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puetz VB, Zweerings J, Dahmen B, Ruf C, Scharke W, Herpertz‐Dahlmann B, Konrad K (2016b): Multidimensional assessment of neuroendocrine and psychopathological profiles in maltreated youth. J Neural Transm 123:1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, Sporns O (2010): Complex network measures of brain connectivity: Uses and interpretations. NeuroImage 52:1059–1069. [DOI] [PubMed] [Google Scholar]
- Sanchez MM (2006): The impact of early adverse care on HPA axis development: Nonhuman primate models. Horm Behav 50:623–631. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU (2000): How Do Glucocorticoids Influence Stress Responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21:55–89. [DOI] [PubMed] [Google Scholar]
- Sheikh HI, Joanisse MF, Mackrell SM, Kryski KR, Smith HJ, Singh SM, Hayden EP (2014): Links between white matter microstructure and cortisol reactivity to stress in early childhood: Evidence for moderation by parenting. NeuroImage: Clin 6:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan MA, Fox NA, Zeanah CH, McLaughlin KA, Nelson CA (2012): Variation in neural development as a result of exposure to institutionalization early in childhood. Proc Natl Acad Sci USA 109:12927–12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Wang L, Peng Z, Wee CY, Shen D (2013): Altered modular organization of structural cortical networks in children with autism. PLoS One 8:e63131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Johnson A, Kan E, Lu LH, Van Horn JD, Toga AW, O'Connor MJ, Bookheimer SY (2008): Mapping white matter integrity and neurobehavioral correlates in children with fetal alcohol spectrum disorders. J Neurosci 28:1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O (2011): The human connectome: A complex network. Ann N Y Acad Sci 1224 :109–125. [DOI] [PubMed] [Google Scholar]
- Strathearn L, Gray PH, O'Callaghan MJ, Wood DO (2001): Childhood neglect and cognitive development in extremely low birth weight infants: A prospective study. Pediatrics 108:142–151. [DOI] [PubMed] [Google Scholar]
- Supekar K, Musen M, Menon V (2009): Development of large‐scale functional brain networks in children. PLoS Biol 7:1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Samson JA (2013): Childhood maltreatment and psychopathology: A case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. Am J Psychiatry 170:1114–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Anderson CM, Ohashi K, Polcari A (2014): Childhood maltreatment: Altered network centrality of cingulate, precuneus, temporal pole and insula. Biol Psychiatry 76:297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titze K, Wollenweber S, Nell V, Lehmkuhl U (2005): Elternbeziehung aus Sicht von Kindern, Jugendlichen und Klinikern. Entwicklung und klinische Validierung des Elternbild‐Fragebogens (EBF‐KJ). Praxis Kinderpsychol Kinderpsychiatr 54:126–143. [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, Millner A, Galvan A, Davidson MC, Eigsti IM, Thomas KM (2010): Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Developmental science 13:46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier JD, Mori S, Leemans A (2011): Diffusion tensor imaging and beyond. Magnet Reson Med 65:1532–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unnewehr S, Schneider S, Margraf J. 1995. Kinder DIPS – Diagnostisches Interview bei psychischen Störungen im Kindes‐ und Jugendalter. Heidelberg, Germany: Springer. [Google Scholar]
- Valdés‐Hernández PA, Ojeda‐González A, Martínez‐Montes E, Lage‐Castellanos A, Virués‐Alba T, Valdés‐Urrutia L, Valdes‐Sosa PA (2010): White matter architecture rather than cortical surface area correlates with the EEG alpha rhythm. NeuroImage 49:2328–2339. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Mandl RCW, Stam CJ, Kahn RS, Hulshoff Pol HE (2010): Aberrant frontal and temporal complex network structure in schizophrenia: A graph theoretical analysis. J Neuroscie 30:15915–15926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Harmelen AL, van Tol MJ, van der Wee NJ, Veltman DJ, Aleman A, Spinhoven P, van Buchem MA, Zitman FG, Penninx BW, Elzinga BM (2010): Reduced medial prefrontal cortex volume in adults reporting childhood emotional maltreatment. Biol Psychiat 68:832–838. [DOI] [PubMed] [Google Scholar]
- van Harmelen AL, van Tol MJ, Demenescu LR, van der Wee NJ, Veltman DJ, Aleman A, van Buchem MA, Spinhoven P, Penninx BW, Elzinga BM (2013): Enhanced amygdala reactivity to emotional faces in adults reporting childhood emotional maltreatment. Soc Cogn Affect Neurosci 8:p362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhu C, He Y, Zang Y, Cao Q, Zhang H, Zhong Q, Wang Y (2009): Altered small‐world brain functional networks in children with attention‐deficit/hyperactivity disorder. Hum Brain Mapp 30:638–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. 1999. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation and Harcourt Brace. [Google Scholar]
- Wen W, He Y, Sachdev P (2011): Structural brain networks and neuropsychiatric disorders. Curr Opin Psychiatry 24:219–225. [DOI] [PubMed] [Google Scholar]
- Xia M, Wang J, He Y (2013): BrainNet viewer: A network visualization tool for human brain connectomics. PLoS ONE 8:e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski DS (2009): Child maltreatment and adult socioeconomic well‐being. Child Abuse Negl 33:666–678. [DOI] [PubMed] [Google Scholar]


