Abstract
Background:
Previous studies suggest that prenatal exposure to phthalates, ubiquitous synthetic chemicals, may adversely affect neurodevelopment. However, data are limited on how phthalates affect cognition, executive function, and behavioral function into adolescence.
Objective:
We aimed to investigate associations of prenatal phthalate exposure with neurodevelopment in childhood and adolescence in the Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS) study.
Methods:
We examined associations between maternal urinary phthalate metabolite concentrations measured twice during pregnancy and a range of neurodevelopmental outcomes from ages 7 through 16 y in the CHAMACOS birth cohort (). We used age-specific linear regression models and generalized estimating equation models to assess longitudinal effects and examined differences by sex.
Results:
Phthalate metabolites were detected in 88%–100% of samples, depending on the metabolite. Associations of phthalates with neurodevelopmental outcomes were largely null with some noteworthy patterns. Higher prenatal concentrations of metabolites of low-molecular weight phthalates () were associated with more self-reported hyperactivity [, 95% confidence interval (CI): 0.1, 1.4 per 2-fold increase in phthalates], attention problems (, 95% CI: 0.7, 2.2), and anxiety (, 95% CI: 0.0, 1.8) at age 16. We observed sex-specific differences for the sums of high-molecular-weight and di(2-ethylhexyl) metabolites and cognitive outcomes (e.g., for Full-Scale IQ for , 95% CI: , 0.3 and , 95% CI: , 0.3, respectively; for , 95% CI: 0.1, 3.4 and 1.6, 95% CI: 0.0, 3.2, respectively; for both).
Conclusion:
We found predominantly null associations of prenatal phthalates with neurodevelopment in CHAMACOS, and weak associations of phthalates with internalizing and externalizing behaviors in adolescence. No previous studies have examined associations of prenatal phthalate exposure with neurodevelopment into adolescence, an important time for manifestations of effects. https://doi.org/10.1289/EHP5165
Introduction
Phthalates are a group of chemicals used in multiple consumer products (Hauser and Calafat 2005). Low-molecular-weight (LMW) phthalates are used in fragrances, cosmetics, and shampoos (ATSDR 1995, 2001; Harley et al. 2017), and personal care products appear to be a primary source of exposure (Duty et al. 2005; Ejaredar et al. 2015). High-molecular-weight (HMW) phthalates are common plasticizers in products such as food packaging, building materials, medical devices, and toys (Ejaredar et al. 2015; Harley et al. 2017). Phthalates are not covalently bound to the plastics that they soften and can easily leach into the environment (Braun et al. 2013; Meeker et al. 2009). Exposure to phthalates is ubiquitous in the U.S. population (Chopra et al. 2014) and occurs via ingestion, inhalation, and dermal absorption (Adibi et al. 2003; Rudel et al. 2003). A recent examination of five cycles of the National Health and Nutrition Examination Survey (NHANES) found that 10 different phthalate metabolites were detected in of participants between 2001 and 2010 (Zota et al. 2014).
Although phthalates have short half-lives in the body and are rapidly excreted (ATSDR 1995, 2001, 2002; Meeker et al. 2009), humans are chronically exposed from common consumer goods, including plastics, personal care products, and food packaging. Phthalates are endocrine disrupting chemicals (EDCs) that cross the placenta, exposing the developing fetus (Silva et al. 2004; Wittassek et al. 2009). The Chronic Hazard Advisory Panel of the U.S. Consumer Product Safety Commission identified fetal development as the most vulnerable target of toxicity for phthalates (Lioy et al. 2015), and the nervous system may be particularly susceptible due to rapid brain development during the prenatal period. Animal and epidemiologic studies have shown that neurotoxic effects of phthalates may be mediated by antiandrogenic activity (Borch et al. 2006; Weiss 2012), alterations in thyroid function (Boas et al. 2010; Gao et al. 2017), and disruption of brain dopaminergic activity (Bellinger 2013; Tanida et al. 2009).
Several epidemiological studies have evaluated associations between prenatal phthalate exposure and neurodevelopmental outcomes among children followed up to age 10 (Braun et al. 2014, 2017; Engel et al. 2010; Factor-Litvak et al. 2014; Gascon et al. 2015; Kobrosly et al. 2014; Lien et al. 2015; Miodovnik et al. 2011; Shin et al. 2018; Whyatt et al. 2012), with inconsistent findings that vary by phthalate metabolite and child sex (Braun et al. 2013). Although previous studies have reported associations of prenatal urinary phthalate concentrations with poorer cognition (Factor-Litvak et al. 2014), social cognition (Miodovnik et al. 2011), and executive function (Engel et al. 2010) and more internalizing and externalizing behaviors (Engel et al. 2010; Kobrosly et al. 2014; Lien et al. 2015; Whyatt et al. 2012), others have found associations with improved cognitive performance (Braun et al. 2017) or null associations with social cognition outcomes (Braun et al. 2014; Shin et al. 2018).
To our knowledge, no studies have examined the associations of prenatal phthalate exposure with neurodevelopment measured longitudinally during childhood and into adolescence—information that is important for evaluating the potential long-term impact of these chemicals. We examined associations of prenatal phthalate exposure with cognition, executive function, and behavior outcomes assessed from ages 7 through 16 y in the Center for the Health Assessment of Mother and Children of Salinas (CHAMACOS) birth cohort study. We hypothesized that higher prenatal phthalate concentrations would be associated with adverse neurodevelopment during childhood and adolescence.
Methods
Study Population
CHAMACOS is a longitudinal birth cohort study examining the health effects of prenatal and early life environmental exposures among Mexican-American children in California’s Salinas Valley. Subject recruitment and procedures for CHAMACOS have been described elsewhere (Eskenazi et al. 2004, 2006). Briefly, eligible pregnant women (i.e., , gestation, Spanish- or English-speaking, qualified for low-income health insurance, and planning to deliver at the county hospital) were recruited in community clinics between September 1999 and December 2000. Of the 601 women initially enrolled, 527 (88%) remained in the study and delivered a live-born singleton.
We conducted interviews with mothers at two prenatal study visits ( and 26 wk gestation), shortly after delivery, and when children were age 6 months, and 1, 2, 3.5, 5, 7, 9, 10.5, 12, 14, and 16 y. We also assessed CHAMACOS children’s growth and development from ages 6 months to 16 y. We restricted current analyses to children born to mothers with measures of prenatal urinary phthalate metabolites who completed at least one neurodevelopmental assessment at age 7 (), 9 (), 10.5 (), 12 (), 14 (), or 16 y (). In total, 334 children (56% of the initial cohort) had prenatal phthalate data and completed at least one assessment included in these analyses (total sample size exceeds sample size at any individual time point because some participants only completed tests at one time point; 334 unique participants provided usable data from at least one visit).
The University of California Berkeley Committee for the Protection of Human Subjects approved all study activities, and we obtained written informed consent from all mothers. We also obtained child verbal assent at age 7, 9, and 10.5 y, and child written assent at age 12, 14, and 16 y. The Centers for Disease Control and Prevention (CDC) deferred to the University of California Berkeley IRB as the IRB of record.
Neurodevelopmental Outcomes
Bilingual, bicultural psychometricians, trained and supervised by a clinical neuropsychologist, administered neuropsychological tests and computer-based tasks in the child’s dominant language in a quiet room free from distraction. We obtained additional information on children’s behavior across multiple settings with rating scales administered to the parent at all study visits (7, 9, 10.5, 12, 14 and 16 y), the teacher at the 7-y visit, and the child (self-report) at the 10.5-, 14-, and 16-y visits. Outlined below are the instruments we used to assess cognition, executive function, social cognition, and behavior in CHAMACOS children.
Executive Function
Behavior Rating Inventory of Executive Function (BRIEF) (Gioia et al. 2000).
Parents of CHAMACOS children completed the BRIEF when their children were age 7, 9, and 12 y. Teachers completed the BRIEF when children were 7 y old. We examined age- and sex-standardized scores (T-scores; , ) across two indices (the Behavioral Regulation Index and the Metacognition Index) and one summary score (Global Executive Composite).
NEPSY tower (Korkman et al. 1998).
At age 9, children completed the NEPSY tower, which assesses planning, monitoring, self-regulation, and problem solving and yields a single scaled score (, ).
Wisconsin Card Sort Task-64: Computer version 2 – research edition (WCST) (Heaton 2000).
At ages 9 and 12 y, children completed the WCST, a computerized test that measures skills around strategic planning, ability to shift cognitive strategies, and impulse control. We examined T-scores for errors and perseverative errors (, ).
Cognition
Weschler Intelligence Scale for Children, fourth edition (WISC-IV) (Weschler 2003).
Study staff administered the WISC-IV at the 7- and 10.5-y study visits. Children were administered the WISC-IV in English (33.3% of participants) or Spanish (66.7% of participants) at age 7 y. All children were tested in English at age 10.5 y. We calculated scores for Full-Scale IQ (FSIQ) and four subscales: Verbal Comprehension (VCIQ), Perceptual Reasoning (PRIQ), Working Memory (WMIQ), and Processing Speed (PSIQ). We standardized scores against U.S. population-based norms for English- and Spanish-speaking children (, ).
Social Cognition
Evaluación neuropsicológica del niño (ENI) (Matute et al. 2007).
Children completed the ENI at age 9 y. In this test, children were shown eight different photographs and scored on how well they identified the mental state of others (e.g., happy, sad, angry, scared). Participants received a score of 1 for correctly identifying the expression and a score of 0 for incorrectly identifying the expression ().
NEPSY-II Affect Recognition subtest (Korkman et al. 2007).
Children completed the NEPSY-II Affect Recognition at age 12 y. In this test, children had to: a) identify whether two photographs depicted faces with the same affect; b) select two faces with the same affect from three to four photographs; c) select one of four faces that depicted the same affect as the face at the top of the page; and d) view a photograph of a face briefly and select two faces that depicted the same affect as the face previously viewed. Participants received a score of 1 for correctly identifying the expression and a score of 0 for incorrectly identifying the expression ().
Social Responsiveness Scale, version 2 (SRS-2) (Constantino and Gruber 2012).
At the 14-y visit, parents completed the SRS-2, a 65-item rating scale developed to assess quantitative traits related to Autism Spectrum Disorder (ASD) in population-based samples (Constantino and Gruber 2012). Parents were asked the frequency that their child exhibited specific behaviors, such as avoiding social behavior with peers or adults, over the previous 6 months (, , , and ), and we computed sex-standardized SRS total T-scores (, ).
Attention and Behavior
Behavior Assessment System for Children, second edition (BASC-2) and Self-Report of Personality (SRP) (Reynolds and Kamphaus 2004).
Parents completed the BASC-2 when children were age 7, 10.5, 14, and 16 y. Teachers completed the BASC-2 when children were 7 y old. Children completed specific scales of the BASC-2 Self-Report of Personality (SRP) at 10.5 and 14 years of age and completed a full SRP at 16 years of age. We examined parent- and teacher-reported scores from four individual scales (hyperactivity, attention problems, depression, and anxiety) and two composite scales (internalizing and externalizing problems). We examined self-reported scores for the internalizing problems composite scale (there was no externalizing composite score for the SRP) and the hyperactivity, attention problems, depression, and anxiety subscales. BASC-2 data were examined as age- and sex-standardized T-scores (, ).
Conners’ Attention Deficit Hyperactivity Disorder (ADHD)/DSM-IV Scales, parent versions (CADS) (Conners 2001).
Parents completed the CADS when children were ages 7, 9, and 12 y old. Teachers completed the CADS at the 7-y study visit. We computed age- and sex-standardized T-scores for the four CADS subscales (Conners’ ADHD index, and DSM-IV-based inattentive, hyperactive/impulsive subscales, and total ADHD) (, ).
Conners’ Continuous Performance Test, version 5 (CPT II) (Conners and MHS Staff 2000).
At 9 and 12 years of age, children completed the CPT-II, a computerized test that assesses hit rate, accuracy, and impulse control. We examined sex- and age-standardized T-scores (, ) for errors of commission (false positives), errors of omission (false negatives), and continuous ADHD Confidence Index score. The ADHD Confidence Index score indicates the probability that children are correctly classified as having clinical ADHD.
Phthalate Exposure Assessment
Mothers provided spot urine samples in polypropylene containers at each of the two prenatal study visits ( and 26 wk gestation) (). After collection, study staff aliquoted urine into glass vials and stored them at until shipment on dry ice to the CDC for analysis. Urine samples were analyzed with online solid phase extraction–isotope-dilution high-performance liquid chromatography–electrospray ionization-tandem mass spectrometry (Silva et al. 2007) to quantify concentrations of 11 phthalate metabolites from eight parent compounds: monoethyl phthalate [MEP, metabolite of diethyl phthalate (DEP)]; mono-n-butyl phthalate [MBP, metabolite of di-n-butyl phthalate (DnBP)]; monoisobutyl phthalate [MiBP, metabolite of diisobutyl phthalate (DiBP)]; monobenzyl phthalate [MBzP, metabolite of butylbenzyl phthalate (BBzP)]; four metabolites of di(2-ethylhexyl) phthalate (DEHP): [mono(2-ethylhexyl) phthalate (MEHP), mono (2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono(2-ethyl-5-oxohexyl) phthalate (MEOHP), and mono(2-ethyl-5-carboxypentyl) phthalate (MECCP)]; mono(carboxyoctyl) phthalate [MCOP, metabolite of diisononyl phthalate (DiNP)]; mono(carboxynonyl) phthalate [MCNP, metabolite of diisodecyl phthalate (DiDP)]; and mono-3-carboxypropyl phthalate (MCPP, metabolite of several HMW phthalates and a minor metabolite of DBP). Quality control procedures included the use of laboratory, calibration standards, and quality controls of high and low concentrations. Limits of detection (LODs) ranged from 0.2 to for individual metabolites.
Covariate Data Collection
Study staff administered structured questionnaires to mothers at each study visit to collect information on a variety of factors, including maternal and child demographics; maternal parity; prenatal diet and smoking, alcohol and illicit drug use; mode of delivery; duration of breastfeeding; parental marital status, maternal education, and employment; and household income. We administered the Peabody Picture Vocabulary Test (PPVT)-Revised (Dunn and Dunn 1981) to estimate maternal receptive vocabulary (6-month and 9-y study visits), the Center for Epidemiologic Studies Depression Scale (CES-D) (Radloff 1977) to assess maternal depression (1-, 3-, 7-, and 9-y visits), and the Home Observation for the Measurement of the Environment-Short Form (HOME-SF) (Caldwell and Bradley 1984) to assess enrichment in the home (6-month, 1-, 2-, 3.5-, 7-, 9-, and 10.5-y visits). We abstracted medical information, such as birth weight and gestational duration, from prenatal and delivery medical records.
We examined potential confounding by other prenatal exposures for which we have reported associations with adverse neurodevelopment in CHAMACOS, including organophosphate pesticides (OPs) (Bouchard et al. 2011; Eskenazi et al. 2007; Marks et al. 2010), organochlorines (OCs) (Eskenazi et al. 2006; Gaspar et al. 2015), polybrominated diphenyl ether flame retardants (PBDEs) (Eskenazi et al. 2013; Sagiv et al. 2015), and manganese (Mn) (Mora et al. 2015). Detailed methods for prenatal biospecimen collection and quantification of these chemicals have been described previously (Bradman et al. 2005; Eskenazi et al. 2006; Eskenazi et al. 2013; Mora et al. 2015). Briefly, we quantified prenatal urinary dialkyl phosphate (DAP) metabolite concentrations as a measure of OP exposure in the same two maternal pregnancy urine samples we used to measure phthalate metabolites (collected at 13 and 26 wk gestation) (Bradman et al. 2005). We also measured OCs (Eskenazi et al. 2006) and PBDEs (Eskenazi et al. 2013) in pregnancy [] serum samples, or in maternal delivery samples for a subset of women () without pregnancy samples. We assessed prenatal exposure to Mn in children’s deciduous teeth (Mora et al. 2015).
Statistical Analysis
We averaged phthalate biomarker concentrations across the two urine samples to better estimate exposure throughout pregnancy (Hoppin et al. 2002) and to normalize the residuals and reduce the influence of the outliers. For concentrations below the LOD, we used the instrumental reading value, when available, and imputed nondetectable concentrations (without an instrumental reading) using maximum likelihood estimation following the log-normal distribution (Lubin et al. 2004).
We categorized phthalate metabolites based on their similarity in chemical structure and biological activity (Teitelbaum et al. 2008) as: a) molar sums of metabolites of LMW () phthalates (i.e., MEP, MBP, and MiBP) or of metabolites of HMW phthalates () (i.e., MBzP, MCPP, MCOP, and MCNP); and b) the molar sum of DEHP metabolites (; i.e., MEHP, MEHHP, MEOHP, and MECPP). We also looked at individual phthalate metabolites ().
We examined associations of average prenatal urinary phthalate biomarker concentrations (individual metabolites and summed concentrations) with neurodevelopmental outcomes assessed at a single time point (i.e., SRS, ENI, NEPSY-II, BASC-2 teacher-report, CADS teacher-report, and SRP internalizing scale) using multivariable linear regression and at multiple time points (i.e., WCST, BRIEF, WISC-IV, CADS, CPT-II, and other BASC-2 scales) using generalized estimating equations (GEE). If associations of phthalates with repeated measures of an outcome changed appreciably over time, we presented estimates from the linear regression analyses for each time point separately. To evaluate the presence of nonlinearity in exposure–outcome associations, we modeled phthalate concentrations categorized into tertiles.
We selected the following covariates a priori using a directed acyclic graph (See Figure S1): maternal age at enrollment (continuous), education (categorical: grade, 7th–12th grade, completed high school), country of birth (categorical: Mexico vs. other), years in the United States (continuous), and depression status at the time of assessment (dichotomous: vs. points in CES-D); child sex (dichotomous) and exact age at assessment (continuous); language of assessment or language of interview for parent-reported outcomes (dichotomous); HOME z-score at time of assessment (continuous); household income at the time of assessment (categorical: at or below poverty line vs. above poverty line); and psychometrician. Because the CPT-II and WCST were computerized tasks, we additionally adjusted these outcomes for children’s video game usage (average hours per week) at age 9 as reported by mothers. We imputed missing values for covariates (all missing) using data from the nearest available visit. If values for a missing covariate were not available from an earlier or later study visit, we randomly selected a value from the dataset ( participant missing HOME score and maternal depression values at the 14- and 16-y visits; participants missing maternal PPVT for sensitivity analyses).
We evaluated effect modification of the exposure–outcome associations by child sex using an augmented product term approach (Buckley et al. 2017) in which we specified a model including product terms between sex and each of the covariates, including sex. Interactions were assessed using sex-metabolite product terms from the same model and were considered statistically significant if .
We conducted sensitivity analyses to assess the robustness of our results. First, we fitted our regression models excluding preterm births (; defined as delivery ) and low birth weight (LBW) children (; defined as birthweight ) ( LBW and preterm). The number of children excluded varied by neurodevelopmental test because not all study participants completed each test. Second, we adjusted our regression models for additional potential confounders [i.e., breastfeeding duration ( vs. ) and maternal receptive vocabulary at the 9-y visit (PPVT score: , 75–99, ) by adding them individually to models. Last, we examined the confounding effects of prenatal exposure to other known neurotoxicants by adding prenatal PBDEs, DAPs, OCs, and Mn concentrations individually to our adjusted models. These additional neurotoxicants were modeled continuously and log-transformed (PBDEs, DAPs, and OCs transformed to the scale and Mn transformed to the scale). PBDEs were modeled as the lipid-adjusted sum of four congeners (, , , ), and OCs were modeled as the lipid-adjusted sum of dichlorodiphenyltrichloroethane (p,p′-DDT, o,p′-DDT), and dichlorodiphenyldichloroethylene (p,p′-DDE) from maternal serum collected at 26 wk gestation. Total DAP metabolite levels were calculated as the sum of six nonspecific urinary OP metabolites, averaged across two prenatal urine samples. All levels below the LOD were set at .
Results
Table 1 shows the sociodemographic characteristics of mother–child pairs from the initial cohort who delivered a live-born singleton () and participants with prenatal phthalate data who completed at least one neurodevelopmental test at age 7 y (). Table S1 shows the sociodemographic characteristics of participants with prenatal phthalate data who completed at least one neurodevelopmental test at age 9, 10.5, 12, 14, or 16 y. Among those with at least one neurodevelopmental assessment at age 7 y, most mothers were born in Mexico (86%), had not completed high school (78%), and had a family income below the U.S. poverty threshold (70%) (Table 1). Nearly 53% of CHAMACOS children were female, and 49% were breastfed for (Table 1). The standardized scores for each neurodevelopmental test are shown in Table S2.
Table 1.
Sociodemographic characteristics [n (%) or median (P25–P75)] of live-born singletons from the initial CHAMACOS mother–child cohort () and study participants with prenatal urinary phthalate metabolites who completed at least one of the neurobehavioral assessments at ages 7 ().a
| Characteristic | Live-born singletons (%) or Median (P25–P75) | Children with prenatal phthalates and at least one assessment at 7-y visit (%) or Median (P25–P75) |
|---|---|---|
| Maternal/household characteristics | ||
| Age at enrollment (y) | 25 (22–29) | 25.5 (22–29) |
| Education | ||
| grade | 228 (43.3) | 142 (44.1) |
| 7th–12th grade | 192 (36.4) | 110 (34.2) |
| Completed high school | 107 (20.3) | 70 (21.7) |
| Receptive vocabulary (PPVT score) at 9-y visit | 98 (80–108) | |
| Missing ()b | — | 23 |
| Country of birth | ||
| Mexico | 444 (84.3) | 277 (86.0) |
| Other | 83 (15.8) | 45 (14.0) |
| Years in U.S. | ||
| 270 (51.2) | 151 (46.9) | |
| 6–10 | 115 (21.8) | 84 (26.1) |
| 142 (27.0) | 88 (27.0) | |
| Parity | ||
| 0 | 180 (34.2) | 102 (31.7) |
| 347 (65.8) | 220 (68.3) | |
| Smoking during pregnancy | ||
| No | 496 (94.1) | 309 (96.0) |
| Yes | 31 (5.9) | 13 (4.0) |
| Maternal depression at 9-year visit ( CES-D score) | ||
| No | 225 (42.7) | 233 (72.4) |
| Yes | 78 (14.8) | 81 (25.1) |
| Missing ()b | 224 (42.5) | 8 (2.5) |
| Household income at 10.5-year visit | ||
| At or below poverty level | 242 (45.9) | 225 (69.9) |
| Above poverty level | 95 (18.0) | 97 (30.1) |
| Missing ()b | 190 (36.1) | 0 (0.0) |
| Child characteristics | ||
| Child’s sex | ||
| Boy | 263 (49.9) | 153 (47.5) |
| Girl | 264 (50.1) | 169 (52.5) |
| Birth weight (grams) | 3,440 (3,155–3,780) | 3,437.5 (3,170–3,785) |
| Gestational age at delivery (wk) | 39 (38–40) | 39 (38–40) |
| Breastfeeding duration (months) | ||
| 311 (59.0) | 163 (50.6) | |
| 205 (38.9) | 159 (49.4) | |
| Missing | 11 (2.1) | 0 (0.0) |
| Age at assessment (y) | ||
| 7-y assessment | — | 7.0 (7.0, 7.1) |
| Language of assessment | — | |
| English | — | 106 (33.8) |
| Spanish | — | 208 (66.2) |
| HOME Score Assessment | 0.1 () | |
| Missing ()b | — | — |
Note: —, no data; CES-D, Center for Epidemiologic Studies Depression Scale; , number of study participants; PPVT, Peabody Picture Vocabulary Test.
At age 7, a total of 322 mothers and 314 youth completed at least one neurodevelopmental assessment.
Missing data for children followed-up were imputed with values collected on these variables at earlier or later time points for all analyses.
Phthalate metabolites were detected in 88%–100% of samples, depending on the metabolite (Table 2). Phthalate metabolite concentrations were generally similar to those measured in NHANES participants around the same time period (CDC 2018) (see Table S3 for uncorrected phthalate metabolite concentrations). Between-person variance exceeded within-person variance by a factor of two to eight (Table 2). Intraclass correlation coefficients (ICC) varied from 0.11 to 0.33 (Table 2). Spearman’s correlation coefficients among different phthalate metabolites ranged from 0.11 to 0.98 (see Table S4).
Table 2.
Distribution of phthalate biomarker concentrations (specific-gravity-corrected) measured in maternal urine samples collected at two time points during pregnancy, CHAMACOS ().
| Phthalate biomarkera | % | d | d | ICCd | Average of two measurements | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st measurementb | 2nd measurementc | GM (GSD) | Min | P25 | P50 | P75 | Max | ||||
| N/A | N/A | 0.94 | 2.14 | 0.31 | 1.5 (32.9) | 0.1 | 0.7 | 1.4 | 2.9 | 105.7 | |
| MEP | 100.0 | 99.7 | 1.26 | 2.59 | 0.33 | 241.7 (3.3) | 14.3 | 108.5 | 223.1 | 501.9 | 20,462.0 |
| MBP | 98.8 | 100.0 | 0.30 | 2.29 | 0.11 | 28.4 (2.4) | 1.3 | 16.2 | 26.8 | 47.6 | 529.8 |
| MiBP | 92.9 | 96.1 | 0.65 | 2.79 | 0.19 | 3.4 (2.7) | 0.1 | 1.8 | 3.3 | 6.7 | 138.0 |
| N/A | N/A | 0.34 | 1.27 | 0.21 | 0.3 (2.1) | 0.0 | 0.2 | 0.3 | 0.5 | 5.1 | |
| MBzP | 98.5 | 98.7 | 0.88 | 2.01 | 0.31 | 8.9 (2.6) | 0.5 | 4.9 | 9.0 | 17.7 | 137.0 |
| MCPP | 88.3 | 93.6 | 0.62 | 3.02 | 0.17 | 2.2 (2.3) | 0.0 | 1.4 | 2.4 | 3.8 | 28.5 |
| MCOP | 96.9 | 96.5 | 0.40 | 1.83 | 0.18 | 3.8 (2.1) | 0.2 | 2.4 | 3.8 | 5.6 | 93.2 |
| MCNP | 95.7 | 96.8 | 0.43 | 1.46 | 0.23 | 2.3 (2.0) | 0.2 | 1.5 | 2.3 | 3.4 | 42.7 |
| N/A | N/A | 0.38 | 1.43 | 0.21 | 0.2 (2.2) | 0.0 | 0.1 | 0.2 | 0.4 | 5.0 | |
| MEHP | 88.6 | 92.0 | 0.59 | 2.80 | 0.17 | 4.5 (2.6) | 0.2 | 2.6 | 4.4 | 7.7 | 141.3 |
| MEHHP | 100.0 | 99.0 | 0.49 | 1.82 | 0.21 | 18.9 (2.4) | 0.9 | 11.3 | 17.9 | 32.6 | 458.4 |
| MECPP | 100.0 | 100.0 | 0.36 | 1.29 | 0.22 | 32.4 (2.2) | 4.2 | 20.9 | 31.4 | 49.6 | 631.8 |
| MEOHP | 98.2 | 98.7 | 0.52 | 2.10 | 0.20 | 13.8 (2.4) | 0.6 | 8.1 | 14.0 | 22.4 | 335.0 |
Note: ICC, intraclass correlation coefficient; LOD, limit of detection; MBP, mono-n-butyl phthalate; MBzP, monobenzyl phthalate; MCPP, mono(3-carboxypropyl) phthalate; MCOP, mono(carboxyoctyl) phthalate; MCNP, mono(carboxynonyl) phthalate; MECCP, mono(2-ethyl-5-carboxypentyl) phthalate; MEHP, mono-2-ethylhexyl phthalate; MEHHP, mono(2-ethyl-5-hydroxyhexyl) phthalate; MEOHP, mono(2-ethyl-5-oxohexyl) phthalate; MEP, monoethyl phthalate; MiBP, mono-isobutyl phthalate; , sum of di-2-ethylhexyl phthalate metabolites; , sum of high-molecular-weight phthalate metabolites; , sum of low-molecular-weight phthalate metabolites.
Units are ng/mL for all individual metabolites and nmol/mL for the sums of metabolites (, , ).
Median (range) gestational age at (5.3–28.5) weeks gestation.
Median (range) gestational age at (21.8–39.5) weeks gestation.
Variances between- and within-woman and ICC were calculated and reported for specific-gravity adjusted urinary phthalate concentrations (nonaveraged).
Our analysis of tertiles provided evidence of nonlinearity for some associations, such as for and with WCST executive function outcomes (see Table S5). However, given that most exposure–outcome associations were linear (see Tables S5–S11), we included phthalate biomarker concentrations parameterized as continuous variables in all models, with point estimates representing the change in outcome for each 2-fold increase in biomarker concentrations. In our main analyses, we present results for the phthalate biomarkers , , and . Results for individual phthalate metabolites are included in Supplementary Material.
Executive Function
Most associations between prenatal concentrations of , , and with executive function outcomes were null in both combined (Table 3) and sex-stratified (see Table S12) analyses. When stratified by sex, we found that higher prenatal concentrations were associated with better scores for perseverative errors among boys (, 95% CI: 0.3, 2.5), but not among girls (, 95% CI: , 1.1; ; see Table S12).
Table 3.
Adjusted associations [ (95% CI)] of prenatal urinary phthalate biomarker concentrations (nmol/mL; and specific gravity-adjusted)a with executive function and cognition outcomes at 7, 9, 10.5, and/or 12 y using GEE models, CHAMACOS.
| Outcomes | Orientationb | k | ||||
|---|---|---|---|---|---|---|
| Executive function | ||||||
| WCST (T-score) (9, 12 y) | ||||||
| Errors | () | 593 | 318 | 0.2 (, 0.8) | 0.5 (, 1.4) | 0.5 (, 1.3) |
| Perseverative Errors | () | 593 | 318 | 0.7 (, 1.4) | 0.7 (, 1.9) | 0.6 (, 1.8) |
| BRIEF—parent report (T-score) (7, 9, 12 y) | ||||||
| Behavioral Regulation Index | () | 923 | 331 | 0.1 (, 0.6) | 0.1 (, 1.0) | 0.0 (, 0.8) |
| Metacognition Index | () | 922 | 330 | (, 0.1) | (, 0.9) | (, 0.7) |
| Global Executive Composite | () | 923 | 331 | (, 0.3) | 0.0 (, 1.0) | (, 0.8) |
| NEPSY Tower (scaled score)c (9 y) | () | 313 | 313 | 0.1 (, 0.3) | 0.1 (, 0.4) | 0.1 (, 0.4) |
| Cognitiond | ||||||
| WISC-IV Full-Scale IQ (scaled scores) (7, 10.5 y) | () | 589 | 321 | (, 0.6) | 0.0 (, 1.4) | 0.1 (, 1.4) |
| Verbal comprehension IQ | () | 621 | 324 | (, 0.4) | (, 1.0) | (, 1.0) |
| Perceptual reasoning IQ | () | 621 | 324 | 0.2 (, 1.2) | 0.4 (, 1.9) | 0.3 (, 1.7) |
| Working memory IQ | () | 593 | 323 | (, 0.1) | (, 1.2) | (, 1.2) |
| Processing speed IQ | () | 593 | 323 | 0.2 (, 0.9) | 0.5 (, 1.7) | 0.5 (, 1.7) |
Note: Models were adjusted for maternal age at enrollment, education, country of birth, years in the U.S., depression at time of assessment; child sex, child age at time of assessment, language of assessment, HOME score, and household income at time of assessment. WCST models were also adjusted for children’s video game usage. BRIEF, Behavior Rating Inventory Executive Function; k, number of children with data for at least one time point; , number of observations from all time points; NEPSY, A Developmental Neuropsychological Assessment; , sum of metabolites of low-molecular-weight phthalates; , sum of metabolites of high-molecular-weight phthalates; , sum of di(2-ethylhexyl) phthalate metabolites; WCST, Wisconsin Card Sort Task-64: computer version 2—research edition; WISC-IV, Wechsler Intelligence Scale for Children 4th edition.
Separate models created for each sum of metabolites (, , ).
() Higher scores indicate poorer performance/more symptomatic behavior; () lower scores indicate poorer performance/more symptomatic behavior.
NEPSY tower measured at one time point and modeled using linear regression.
Data also presented in Figure 1.
Cognition
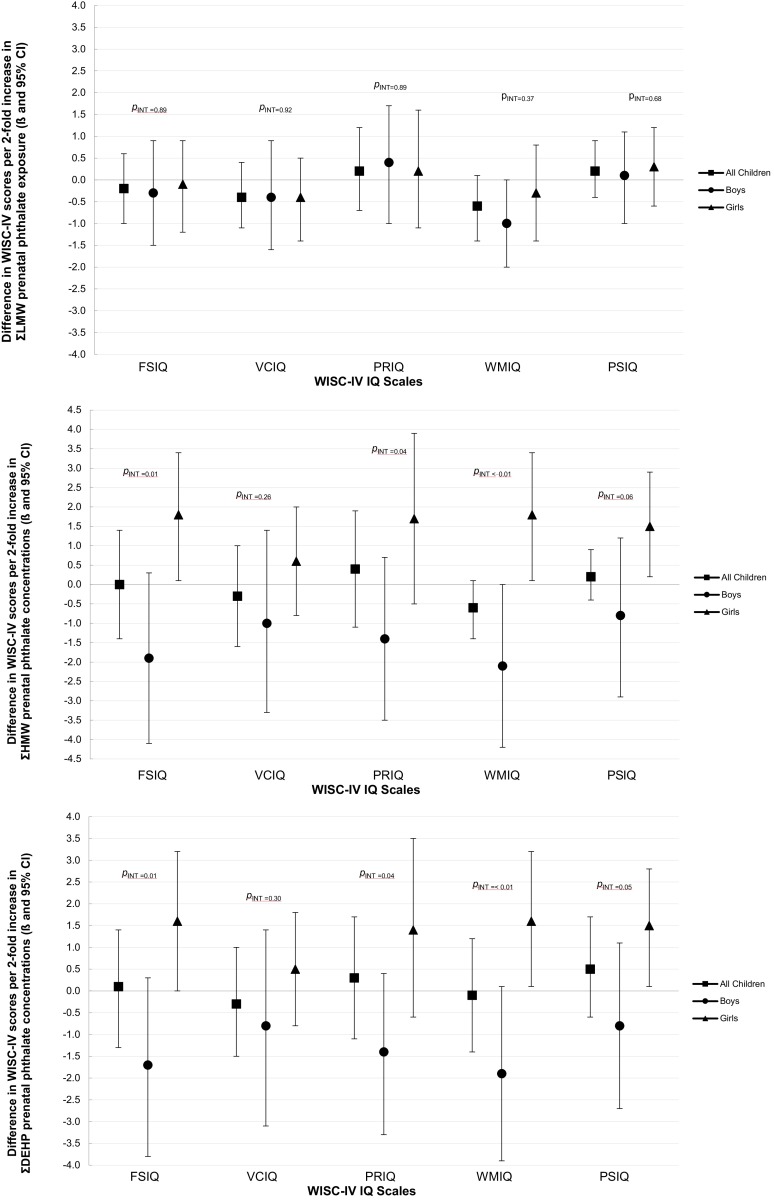
We found mostly null associations of prenatal , , and concentrations with IQ scores in analyses of boys and girls combined (Table 3). However, when we stratified by child’s sex, we observed that higher prenatal concentrations were associated with lower FSIQ (, 95% CI: , 0.3) and WMIQ (, 95% CI: , 0.0) scores among boys but higher FSIQ, WMIQ, and PSIQ scores among girls (, 95% CI: 0.1, 3.3; , 95% CI: 0.1, 3.4; and , 95% CI: 0.2, 2.9; respectively; all ; see Figure 1 and Table S12). Similarly, higher concentrations were associated with decreased WMIQ scores in boys (, 95% CI: , 0.1) and increased FSIQ, WMIQ, and PSIQ scores among girls (, 95% CI: 0.0, 3.2; , 95% CI: 0.1, 3.2; and , 95% CI: 0.1, 2.8; respectively; all ; see Figure 1 and Table S12). Among all children, MEP concentrations were associated with slightly decreased WMIQ scores (, 95% CI: , 0.1) and MiBP concentrations were associated with slightly increased PSIQ scores (, 95% CI: 0.0, 1.8; see Table S13). Effect estimates for VCIQ were null for all metabolites in both stratified and sex-stratified analyses (Table 3, Table S12, Table S13).
Figure 1.
Adjusted associations [ (95% CI)] for WISC-IV IQ scores per 2-fold increase in prenatal (A) , (B) , and (C) concentrations (nmol/mL; and specific gravity-adjusted) among all children and stratified by sex using GEE models. Models adjusted for maternal age at enrollment, education, country of birth, years in the United States, depression at time of assessment; child sex, child age at time of assessment, language of assessment, HOME score, and household income at time of assessment. Sex-specific effect estimates and p-int values obtained from models including cross-product terms between child sex and the exposure and child sex and each of the covariates. p-int represents interaction of exposure and sex obtained from these models. Data also shown in Tables 3 and S12.
Social Cognition
Most associations of prenatal phthalate concentrations with social cognition outcomes hovered at the null in both combined and sex-stratified analyses. Higher concentrations were associated with slightly better scores on the ENI assessment at 9 y among all children (, 95% CI: 0.0, 0.2; see Table 4) and among girls (, 95% CI: 0.0, 0.4; see Table S14).
Table 4.
Adjusted associations [ (95% CI)] of prenatal urinary phthalate biomarker concentrations (nmol/mL; and specific gravity-adjusted)a with behavior and social cognition outcomes at 7, 9, 10.5, 12, 14, and/or 16 y using GEE models, CHAMACOS.
| Outcomes | Orientationb | k | ||||
|---|---|---|---|---|---|---|
| Social cognitionc | ||||||
| SRS Total Score (14 y) | () | 245 | 245 | 0.4 (, 0.9) | (, 0.5) | (, 0.5) |
| ENI (9 y) | () | 313 | 313 | 0.0 (, 0.1) | 0.1 (, 0.2) | 0.1 (0.0, 0.2) |
| NEPSY-II (12 y) | () | 310 | 310 | (, 0.2) | (, 0.1) | (, 0.2) |
| Behavior | ||||||
| BASC-2—parent-report (T-score) | ||||||
| Internalizing problems (7, 10.5, 14, 16 y) | () | 1,226 | 328 | 0.4 (, 1.0) | 0.5 (, 1.4) | 0.4 (,1.2) |
| Depression scalec (7 y) | () | 322 | 322 | 0.4 (, 1.0) | 0.4 (, 1.3) | 0.3 (, 1.1) |
| Anxiety scalec (7 y) | () | 313 | 313 | 0.3 (, 1.0) | 0.8 (, 1.9) | 0.6 (, 1.7) |
| Externalizing problems (7, 10.5, 14, 16 y) | () | 1,219 | 328 | 0.1 (, 0.5) | 0.2 (, 0.9) | 0.1 (, 0.7) |
| Hyperactivity scale (7, 10.5, 14, 16 y) | () | 1,226 | 328 | 0.0 (, 0.4) | (, 0.6) | (, 0.5) |
| Attention problems scale (7, 10.5, 14, 16 y) | () | 1,226 | 328 | 0.0 (, 0.5) | (, 0.7) | (, 0.7) |
| BASC-2—teacher-report (T-score)c | ||||||
| Internalizing problems (7 y) | () | 265 | 265 | (, 0.2) | (, 1.0) | (, 1.0) |
| Depression scale (7 y) | () | 265 | 265 | (, 0.1) | (, 0.9) | (, 0.9) |
| Anxiety scale (7 y) | () | 265 | 265 | (, 0.1) | (, 0.5) | (, 0.6) |
| Externalizing problems (7 y) | () | 265 | 265 | (, 0.6) | 0.0 (, 1.0) | 0.2 (, 1.1) |
| Hyperactivity scale (7 y) | () | 265 | 265 | 0.0 (, 0.7) | (, 0.9) | (, 0.9) |
| Attention problems scale (7 y) | () | 265 | 265 | 0.2 (, 0.4) | (, 0.4) | (, 0.5) |
| CADS—parent-report (T-score) | ||||||
| ADHD Index (7, 9, 12 y) | () | 920 | 332 | (, 0.1) | (, 0.5) | (, 0.5) |
| DSM-IV Total Scale (7, 9, 12 y) | () | 921 | 332 | (, 0.1) | (, 0.7) | (, 0.5) |
| Inattentive scale (7, 9, 12 y) | () | 920 | 332 | (, 0.1) | (, 0.5) | (, 0.4) |
| Hyperactive/impulsive scale (7, 9, 12 y) | () | 920 | 332 | (, 0.2) | 0.0 (, 0.9) | (, 0.7) |
| CADS—teacher-report (T-score)c | ||||||
| ADHD Index (7 y) | () | 261 | 261 | (, 0.5) | (, 0.9) | (, 0.9) |
| DSM-IV Total Scale (7 y) | () | 260 | 260 | (, 0.5) | (, 1.0) | (, 1.0) |
| Inattentive scale (7 y) | () | 264 | 264 | (, 0.5) | (, 0.9) | (, 0.9) |
| Hyperactive/impulsive scale (7 y) | () | 264 | 264 | (, 0.6) | 0.1 (, 1.3) | 0.2 (, 1.3) |
| CPT-II (T-score) | ||||||
| Errors of omission (9, 12 y) | () | 595 | 317 | 0.9 (0.0, 1.8) | (, 1.1) | (, 0.9) |
| Errors of commission (9, 12 y) | () | 595 | 317 | 0.3 (, 0.8) | (, 0.8) | (, 0.6) |
| ADHD confidence index (9, 12 y) | () | 596 | 317 | 0.7 (, 1.9) | (, 0.8) | (, 0.6) |
Note: Models were adjusted for maternal age at enrollment, education, country of birth, years in the U.S., depression at time of assessment; child sex, child age at time of assessment, language of assessment, HOME score, and household income at time of assessment. CPT-II models were also adjusted for children’s video game usage at time of assessment. ADHD, Attention Deficit Hyperactivity Disorder; BASC-2, Behavior Assessment System for Children, 2nd edition; CADS, Conners' ADHD/DSM-IV Scales; CPT-II, Continuous Performance Test, 2nd edition; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, 4th edition; ENI, Evaluación Neuropsicológica del Niño; k, number of children with data for at least one time point; , number of observations from all time points; NEPSY-II, A Developmental Neuropsychological Assessment, 2nd Edition; , sum of metabolites of low-molecular-weight phthalates; , sum of metabolites of high-molecular-weight phthalates; , sum of di(2-ethylhexyl) phthalate metabolites. SRS, Social Responsiveness Scale, version 2.
Separate models created for each sum of metabolites (, , ).
() Higher scores indicate poorer performance/more symptomatic behavior; () lower scores indicate poorer performance/more symptomatic behavior.
Measured at one time point and modeled using linear regression.
Behavior
Among all children, higher prenatal phthalate concentrations were associated with more errors of omission (higher T-scores) on the performance-based CPT-II (; 95% CI: 0.0, 1.8) measured at 9 and 12 y (Table 4) and poorer self-reported behaviors measured using the BASC-2 SRP scale, including more hyperactivity (; 95% CI: 0.1, 1.4), attention problems (; 95% CI: 0.7, 2.2), anxiety (; 95% CI: 0.0, 1.8), and overall internalizing problems on the composite scale (; 95% CI: 0.4, 1.9) measured at 16 y (Table 5). Similar, albeit weaker, trends were observed for parent-reported BASC-2 results modeled using GE; however, associations of phthalates with most teacher-reported behaviors were null or in the opposite direction of parent- and self-reported results (Table 4). We did not observe consistent sex-specific associations for behavior outcomes in GEE (Table S14) or linear regression models (Table S15). Prenatal MEP, MBzP, MCPP, and MCOP concentrations were associated with increased scores for parent-reported internalizing problems and/or errors of commission (Table S16). Similarly, prenatal concentrations of MEP and MCPP were associated with increased self-reported internalizing problems at age 16 (Table S17).
Table 5.
Adjusted associations [ (95% CI)] of prenatal urinary phthalate biomarker concentrations (nmol/mL; and specific gravity-adjusted)a with self-reported BASC-2 behavioral outcomes at 10.5, 14, and 16 y using linear regression models, CHAMACOS.
| Outcome | Orientationb | ||||
|---|---|---|---|---|---|
| 10.5-y assessment | |||||
| Internalizing problems | () | — | — | — | — |
| Depression scale | () | 289 | 0.5 (, 1.1) | 0.7 (, 1.9) | 0.6 (, 1.8) |
| Anxiety scale | () | 289 | 0.3 (, 1.0) | 0.5 (, 1.5) | 0.4 (, 1.4) |
| Externalizing problems | |||||
| Hyperactivity scale | () | 299 | 0.2 (, 0.8) | 0.5 (, 1.5) | 0.3 (, 1.3) |
| Attention problems scale | () | 290 | (, 0.7) | (, 0.8) | (, 0.7) |
| 14-y assessment | |||||
| Internalizing problems | () | — | — | — | — |
| Depression scale | () | 301 | 0.5 (, 1.2) | 0.6 (, 1.8) | 0.5 (, 1.6) |
| Anxiety scale | () | 300 | 0.4 (, 1.1) | (, 0.7) | (, 0.6) |
| Externalizing problems | |||||
| Hyperactivity scale | () | — | — | — | — |
| Attention problems scale | () | — | — | — | — |
| 16-y assessment | |||||
| Internalizing problems | () | 283 | 1.2 (0.4, 1.9) | 0.4 (, 1.4) | 0.4 (, 1.3) |
| Depression scale | () | 286 | 0.7 (, 1.5) | 0.2 (, 1.2) | 0.3 (, 1.2) |
| Anxiety scale | () | 286 | 0.9 (0.0, 1.8) | 0.7 (, 1.8) | 0.6 (, 1.7) |
| Externalizing problems | |||||
| Hyperactivity scale | () | 285 | 0.8 (0.1, 1.4) | 0.0 (, 1.0) | (, 0.8) |
| Attention problems scale | () | 286 | 1.5 (0.7, 2.2) | (, 0.8) | (, 0.6) |
Note: Models were adjusted for maternal age at enrollment, education, country of birth, years in the U.S., depression at time of assessment; —, no data; child sex, child age at time of assessment, language of assessment, HOME score, and household income at time of assessment. BASC-2, Behavior Assessment System for Children, 2nd edition; , number of children; , sum of metabolites of low-molecular-weight phthalates; , sum of metabolites of high-molecular-weight phthalates; , sum of di(2-ethylhexyl) phthalate metabolites.
Separate models created for each sum of metabolites (, , ).
() Higher scores indicate poorer performance/more symptomatic behavior; () lower scores indicate poorer performance/more symptomatic behavior.
Sensitivity Analyses
In general, the point estimates did not change appreciably in sensitivity analyses after a) excluding preterm births and LBW children (see Tables S18–S20), and b) adjusting for additional potential confounders, including maternal receptive vocabulary (see Tables S21–S23) and breastfeeding duration (see Tables S24–S26). Associations of prenatal phthalate concentrations with self-reported hyperactivity, depression, and anxiety were attenuated in models adjusted for coexposure to PBDEs (Tables S27–S29), OP and OC pesticides (Tables S30–S35), and Mn (Tables S36-38), with all CI including the null.
Discussion
In this prospective cohort of low-income Mexican-American children, we observed primarily null associations of prenatal urinary phthalate biomarker concentrations with a wide range of measures of childhood and adolescent cognition and behavior. We did find suggestive associations of prenatal phthalates with more parent- and self-reported internalizing problems and poorer scores on performance-based behavioral assessments (i.e., CPT-II errors of omission). We also observed that prenatal and concentrations were associated with slightly higher IQ scores among girls and lower IQ scores among boys.
The previous literature from longitudinal birth cohort studies evaluating prenatal phthalate exposure and childhood neurodevelopment has produced mixed results. With respect to cognitive function, the Columbia Center for Children’s Environmental Health (CCCEH) study reported that prenatal concentrations of the LMW phthalate metabolites MBzP and MiBP were inversely associated with IQ scores among 328 children age 7 y (Factor-Litvak et al. 2014). Conversely, the Health Outcomes and Measures of the Environment (HOME) study found that higher prenatal concentrations of LMW phthalate metabolites, including MBzP and MBP, were associated with improved performance on the Virtual Morris Water Maze among 198 8-y-old children, particularly among boys (Braun et al. 2017). We also found evidence of better cognition in association with higher prenatal phthalate exposure in sex-stratified analyses in CHAMACOS; however, we observed that prenatal and concentrations were associated with slightly higher IQ scores among girls and lower IQ scores among boys.
Only one previous study, the Mount Sinai Children’s Environmental Healthy study, examined associations of prenatal phthalate exposure with executive function and found that higher metabolite concentrations from third-trimester maternal urine were associated with poorer BRIEF global executive composite index scores in 188 children ages 4–9 y (Engel et al. 2010). In contrast, we found null associations for phthalates and BRIEF behavioral regulation and global executive composite scores, and slightly better scores on the parent-reported BRIEF metacognition index.
Three previous studies have reported associations of prenatal phthalates with more externalizing behaviors, including aggression, hyperactivity, and conduct problems (Engel et al. 2010; Kobrosly et al. 2014; Lien et al. 2015). The Mount Sinai study reported associations of prenatal LMW phthalate concentrations with parent report of increased aggression, attention, and conduct problems on the BASC among children ages 4–9 y (Engel et al. 2010). A study of 122 mother–child pairs in Taiwan found that prenatal concentrations of LMW phthalate metabolites, as well as DEHP metabolites, were associated with parent report of more aggressive and delinquent behaviors on the Child Behavior Checklist (CBCL) at 8 y (Lien et al. 2015). In the multicenter Study for Future Families of 153 children ages 6 to 10 y in 4 U.S. states, prenatal MBP, a LMW phthalate, was associated with more parent-reported inattention, rule-breaking behavior, aggression, and conduct problems on the CBCL (Kobrosly et al. 2014). In addition, prenatal MBzP, a HMW phthalate, was associated with more parent-reported oppositional and conduct problems, but only among boys (Kobrosly et al. 2014). Only the Spanish INfancia y Medio Ambiente (INMA)-Sabadell birth cohort study () found essentially null associations for prenatal phthalate biomarkers and all behavioral outcomes, including ADHD-related behaviors, measured at 4 and 7 y (Gascon et al. 2015). In the CHAMACOS study, we observed modest associations of phthalates with attention problems, including poorer performance on the CPT-II at ages 9 and 12 and more symptomatic self-reported BASC-2 hyperactivity and attention problems scores at age 16. Associations with all other externalizing behaviors, including those from teacher-, parent-, and self-report, were essentially null.
Results from previous studies are less consistent with respect to internalizing behaviors. Although the Mount Sinai study reported associations of prenatal phthalate concentrations with more anxiety and depression among all children (Engel et al. 2010), the Study for Future Families found associations with less anxiety among girls (Kobrosly et al. 2014), and the Taiwanese study reported null associations for prenatal phthalates with all internalizing behaviors (Lien et al. 2015). Furthermore, the CCCEH study reported associations of prenatal MBP and MBzP concentrations (metabolites of LMW phthalates) with more internalizing behaviors among all children, with stronger associations observed for MBzP among girls (Whyatt et al. 2012). Associations of prenatal phthalates and BASC self-report of internalizing behaviors at age 16 were among the strongest in the CHAMACOS study, though associations with BASC parent report were considerably weaker, and BASC teacher-report associations were in the opposite direction. Although these results may seem paradoxical, they are largely in line with previous studies that have reported poor multiple informant agreement for parent-, teacher-, and child-reported behaviors, with worse concordance among participants with internalizing disorders (Miller et al. 2014; Verhulst and Akkerhuis 1989). Although externalizing problems may be more easily observed by others, a child can choose not to disclose feelings of anxiety or depression to teachers or caregivers (Salbach-Andrae et al. 2009). Previous studies indicate that teacher–youth agreement may be less than parent–youth agreement (Youngstrom et al. 2000), particularly for internalizing disorders (Salbach-Andrae et al. 2009), which is largely consistent with findings from our study. We observed the strongest findings for self-reported behavior at age 16, and evidence suggests that discrepancies in parent- and self-report and the reliability of self-reported behaviors increase with age (Edelbrock et al. 1985; Verhulst and Akkerhuis 1989). Although reports from multiple informants provide a more comprehensive picture of behaviors of children and adolescents throughout time, adolescents may be a more reliable source about their own internalizing behavior.
Consistent with the results from a study of 201 children age 3 y from the Markers of Autism Risk in Babies—Learning Early Signs (MARBLES) cohort (Shin et al. 2018), a study of 175 children ages 4–5 y from the HOME cohort (Braun et al. 2014), and the Spanish INMA-Sabadell birth cohort (Gascon et al. 2015), we found null associations of prenatal phthalate exposure with social cognition outcomes. In contrast, a study of 137 children ages 7–9 y from the Mount Sinai cohort found that higher prenatal phthalate concentrations were associated with poorer SRS scores (Miodovnik et al. 2011). To our knowledge, ours is the first study to investigate phthalate-related associations with tests of affect recognition (i.e., ENI and NEPSY-II), which may provide more comprehensive and objective data than parent-report alone.
Potential reasons for the heterogeneity in our findings and those from previous studies include differences in a) sample size, b) timing and number of prenatal exposure measurements, c) specific phthalate metabolites measured, d) neurodevelopmental tests administered, e) age of child at neurodevelopmental assessments, and f) sociodemographic characteristics that may influence phthalate exposure. For example, we assessed a larger diversity of neurodevelopmental outcomes than many previous studies assessed, and ours is the only study to examine associations of prenatal phthalates exposure with behavior and executive function using computer-based assessments. Additionally, we had a larger sample size than those in some previous investigations and assessed phthalate exposure from two prenatal urine samples, whereas many previous studies relied on one measurement. Notably, some of our strongest findings were for self-reported behavioral outcomes at age 16, and to our knowledge, ours is the first study to examine associations of prenatal phthalate exposure and neurodevelopment past age 10 y.
Our study has several strengths and limitations. The longitudinal design of CHAMACOS and collection of rich data, including biological specimens, a wide array of neurodevelopmental measures, behavioral measures from multiple reporters (e.g., parents, teachers, and participants), and covariates, allowed for a very thorough and nuanced examination of the potential impacts of in utero phthalate exposure throughout childhood and adolescence. Ours is the first study to our knowledge to investigate the impacts of prenatal phthalate exposure and neurocognitive outcomes during adolescence, allowing us to examine the persistence of effects identified in cohorts of younger children. In addition, although previous studies have relied solely on parent-report of executive function, behavior, and social cognition, we directly assessed participants with respect to executive function (WCST and NEPSY Tower), attention and impulse control (CPT-II), and social cognition (ENI and NEPSY Affect Recognition). These arguably more objective, performance-based tests contribute to the richness of our neurodevelopmental assessments. Furthermore, to our knowledge, we are the first longitudinal study to examine the effects of prenatal phthalate exposure on adolescent self-reported behavior, which may provide more nuanced information on internalizing behaviors such as anxiety and depression.
Although the collection of rich neurodevelopmental data across various domains is a primary strength of our study, it also makes interpretation of our results more difficult, as demonstrated by inconsistency in our findings across these multiple outcomes. More important, we conducted a large number of analyses with multiple comparisons, which could produce spurious associations by chance alone. Therefore, we were careful to report patterns in our results rather than highlighting isolated findings. Overall, the richness and diversity of our outcomes allow us to conclude with some confidence that prenatal exposure to select phthalates is not strongly associated with neurodevelopment in our cohort.
The prenatal period has been identified as the most vulnerable window of neurotoxicity (Lioy et al. 2015); however, emerging evidence indicates that childhood phthalate exposure may be associated with adverse cognitive outcomes among children ages 2 to 12 y (Cho et al. 2010; Factor-Litvak et al. 2014; Huang et al. 2015; Kim et al. 2017; Li et al. 2019). We did not measure childhood phthalate concentrations in our cohort, and it is possible that postnatal exposures could affect neurodevelopment in this population, particularly during puberty, a period during which the brain is developing rapidly and may be particularly susceptible to EDCs (Wang et al. 2016). Previous research has shown sex-specific changes in internalizing and externalizing behaviors, executive function, and social cognition during puberty (Blakemore et al. 2010; Gur and Gur 2016; Spear 2013), and a toxicology study found that DEHP exposure during puberty was associated with increased anxiety among female mice (Wang et al. 2016). In addition to prenatal exposures, future studies should consider also assessing phthalate concentrations during childhood and adolescence to identify critical periods of susceptibility.
Our study and others have shown relatively low correlation between phthalate measurements collected at multiple times during pregnancy (Gascon et al. 2015) and poor reproducibility between measurements (Johns et al. 2015). Other studies have also suggested that phthalate metabolite concentrations can vary depending on the time of day at which the urine sample was collected (Silva et al. 2004) and that exposure biomarker patterns may vary, depending on the primary source of phthalate exposure (Johns et al. 2015; Preau et al. 2010). In addition to introducing nondifferential exposure misclassification that may have biased our results toward the null, the nonpersistent nature of phthalates makes it difficult to identify windows of susceptibility based on the timing of prenatal exposure (Braun et al. 2014). Future research investigating these exposure–outcome relationships should include a more critical evaluation of the most robust sampling strategy (Shin et al. 2019) (i.e., spot, first morning void, 24-h collection) to reduce exposure measurement error for the phthalate metabolite of interest.
Overall, we found mostly null associations of prenatal phthalate exposure with child and adolescent neurodevelopmental outcomes in the CHAMACOS cohort, though we observed some suggestive associations of prenatal LMW phthalate biomarker concentrations with more internalizing and externalizing behaviors, particularly from self-reported and performance-based assessments. These findings add to a growing literature addressing the potential developmental neurotoxicity of phthalate exposure.
Supplementary Material
Acknowledgments
We gratefully acknowledge the CHAMACOS laboratory and field staff, students, community partners, and participants and families.
This work was funded by research grant numbers R03 ES027139, P01 ES009605, R01 ES017054, and R01 ES021369 from the National Institute of Environmental Health Sciences (NIEHS); R82670901, RD83171001, and RD83451301 from the U.S. Environmental Protection Agency (EPA); and R01 DA035300 from the National Institute on Drug Abuse (NIDA).
Footnotes
Supplemental Material is available online (https://doi.org/10.1289/EHP5165).
The authors declare they have no actual or potential competing financial interests.
The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Note to readers with disabilities: EHP strives to ensure that all journal content is accessible to all readers. However, some figures and Supplemental Material published in EHP articles may not conform to 508 standards due to the complexity of the information being presented. If you need assistance accessing journal content, please contact ehponline@niehs.nih.gov. Our staff will work with you to assess and meet your accessibility needs within 3 working days.
References
- Adibi JJ, Perera FP, Jedrychowski W, Camann DE, Barr D, Jacek R, et al. 2003. Prenatal exposures to phthalates among women in New York City and Krakow, Poland. Environ Health Perspect 111(14):1719–1722, PMID: 14594621, 10.1289/ehp.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry). 1995. Toxicological Profile for diethyl phthalate (DEP). Atlanta, GA: Agency for Toxic Substances and Disease Registry. [PubMed] [Google Scholar]
- ATSDR. 2001. Toxicological Profile for di-n-butyl phthalate (DBP). Atlanta, GA: Agency for Toxic Substances and Disease Registry. [PubMed] [Google Scholar]
- ATSDR. 2002. Toxicological Profile for di(2-ethylhexyl)phthalate (DEHP). Atlanta, GA: Agency for Toxic Substances and Disease Registry. [PubMed] [Google Scholar]
- Bellinger DC. 2013. Prenatal exposures to environmental chemicals and children's neurodevelopment: an update. Saf Health Work 4(1):1–11, PMID: 23515885, 10.5491/SHAW.2013.4.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S-J, Burnett S, Dahl RE. 2010. The role of puberty in the developing adolescent brain. Hum Brain Mapp 31(6):926–933, PMID: 20496383, 10.1002/hbm.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boas M, Frederiksen H, Feldt-Rasmussen U, Skakkebæk NE, Hegedüs L, Hilsted L, et al. 2010. Childhood exposure to phthalates: associations with thyroid function, insulin-like growth factor I, and growth. Environ Health Perspect 118(10):1458–1464, PMID: 20621847, 10.1289/ehp.0901331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borch J, Metzdorff SB, Vinggaard AM, Brokken L, Dalgaard M. 2006. Mechanisms underlying the anti-androgenic effects of diethylhexyl phthalate in fetal rat testis. Toxicology 223(1–2):144–155, PMID: 16690193, 10.1016/j.tox.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Bouchard MF, Chevrier J, Harley KG, Kogut K, Vedar M, Calderon N, et al. 2011. Prenatal exposure to organophosphate pesticides and IQ in 7-year-old children. Environ Health Perspect 119(8):1189–1195, PMID: 21507776, 10.1289/ehp.1003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradman A, Eskenazi B, Barr DB, Bravo R, Castorina R, Chevrier J, et al. 2005. Organophosphate urinary metabolite levels during pregnancy and after delivery in women living in an agricultural community. Environ Health Perspect 113(12):1802–1807, PMID: 16330368, 10.1289/ehp.7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Bellinger DC, Hauser R, Wright RO, Chen A, Calafat AM, et al. 2017. Prenatal phthalate, triclosan, and bisphenol A exposures and child visual-spatial abilities. Neurotoxicology 58:75–83, PMID: 27888119, 10.1016/j.neuro.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalkbrenner AE, Just AC, Yolton K, Calafat AM, Sjödin A, et al. 2014. Gestational exposure to endocrine-disrupting chemicals and reciprocal social, repetitive, and stereotypic behaviors in 4- and 5-year-old children: the HOME study. Environ Health Perspect 122(5):513–520, PMID: 24622245, 10.1289/ehp.1307261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Sathyanarayana S, Hauser R. 2013. Phthalate exposure and children's health. Curr Opin Pediatr 25(2):247–254, PMID: 23429708, 10.1097/MOP.0b013e32835e1eb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley JP, Doherty BT, Keil AP, Engel SM. 2017. Statistical approaches for estimating sex-specific effects in endocrine disruptors research. Environ Health Perspect 125(6):067013, PMID: 28665274, 10.1289/EHP334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell B, Bradley R. 1984. Home Observation for Measurement of the Environment. Little Rock, AR: University of Arkansas. [Google Scholar]
- CDC (Centers for Disease Control and Prevention). 2018. Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables, Volume One. Atlanta, GA: Centers for Disease Control and Prevention. [Google Scholar]
- Cho SC, Bhang SY, Hong YC, Shin MS, Kim BN, Kim JW, et al. 2010. Relationship between environmental phthalate exposure and the intelligence of school-age children. Environ Health Perspect 118(7):1027–1032, PMID: 20194078, 10.1289/ehp.0901376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra V, Harley K, Lahiff M, Eskenazi B. 2014. Association between phthalates and attention deficit disorder and learning disability in U.S. children, 6–15 years. Environ Res 128:64–69, PMID: 24267794, 10.1016/j.envres.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners CK. 2001. Conner's Rating Scales-Revised (CSR-R) Technical Manual (Includes Auxiliary Scales: CADS-P and CADS-T). North Tonawanda, NY: Multi-Health Systems Inc. [Google Scholar]
- Conners CK, MHS Staff. 2000. Conners' Continuous Performance Test (CPT II). North Towanda, NY: Multi-Health Systems Inc. [Google Scholar]
- Constantino JN, Gruber CP. 2012. Social Responsiveness Scale - Second Edition (SRS-2). Lutz, FL: Western Psychological Services. [Google Scholar]
- Dunn LM, Dunn D. 1981. Peabody Picture Vocabulary Test, Revised. Circle Pines, MN: American Guidance Service. [Google Scholar]
- Duty SM, Ackerman RM, Calafat AM, Hauser R. 2005. Personal care product use predicts urinary concentrations of some phthalate monoesters. Environ Health Perspect 113(11):1530–1535, PMID: 16263507, 10.1289/ehp.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelbrock C, Costello AJ, Dulcan MK, Kalas R, Conover NC. 1985. Age differences in the reliability of the psychiatric interview of the child. Child Dev 56(1):265–275, PMID: 3987406, 10.2307/1130193. [DOI] [PubMed] [Google Scholar]
- Ejaredar M, Nyanza EC, Ten Eycke K, Dewey D. 2015. Phthalate exposure and childrens neurodevelopment: a systematic review. Environ Res 142:51–60, PMID: 26101203, 10.1016/j.envres.2015.06.014. [DOI] [PubMed] [Google Scholar]
- Engel SM, Miodovnik A, Canfield RL, Zhu C, Silva MJ, Calafat AM, et al. 2010. Prenatal phthalate exposure is associated with childhood behavior and executive functioning. Environ Health Perspect 118(4):565–571, PMID: 20106747, 10.1289/ehp.0901470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Chevrier J, Rauch SA, Kogut K, Harley KG, Johnson C, et al. 2013. In utero and childhood polybrominated diphenyl ether (PBDE) exposures and neurodevelopment in the CHAMACOS study. Environ Health Perspect 121(2):257–262, PMID: 23154064, 10.1289/ehp.1205597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Harley K, Bradman A, Weltzien E, Jewell NP, Barr DB, et al. 2004. Association of in utero organophosphate pesticide exposure and fetal growth and length of gestation in an agricultural population. Environ Health Perspect 112(10):1116–1124, PMID: 15238287, 10.1289/ehp.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Marks AR, Bradman A, Fenster L, Johnson C, Barr DB, et al. 2006. In utero exposure to dichlorodiphenyltrichloroethane (DDT) and dichlorodiphenyldichloroethylene (DDE) and neurodevelopment among young Mexican American children. Pediatrics 118(1):233–241, PMID: 16818570, 10.1542/peds.2005-3117. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C, et al. 2007. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ Health Perspect 115(5):792–798, PMID: 17520070, 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Factor-Litvak P, Insel B, Calafat AM, Liu X, Perera F, Rauh VA, et al. 2014. Persistent associations between maternal prenatal exposure to phthalates on child IQ at age 7 years. PLoS One 9(12):e114003, PMID: 25493564, 10.1371/journal.pone.0114003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Wu W, Xu Y, Jin Z, Bao H, Zhu P, et al. 2017. Effects of prenatal phthalate exposure on thyroid hormone concentrations beginning at the embryonic stage. Sci Rep 7(1):13106, PMID: 29026179, 10.1038/s41598-017-13672-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascon M, Valvi D, Forns J, Casas M, Martínez D, Júlvez J, et al. 2015. Prenatal exposure to phthalates and neuropsychological development during childhood. Int J Hyg Environ Health 218(6):550–558, PMID: 26095249, 10.1016/j.ijheh.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Gaspar FW, Harley KG, Kogut K, Chevrier J, Mora AM, Sjödin A, et al. 2015. Prenatal DDT and DDE exposure and child IQ in the CHAMACOS cohort. Environ Int 85:206–212, PMID: 26414943, 10.1016/j.envint.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, Kenworthy L. 2000. Behavioral Rating Inventory of Executive Function (BRIEF). Lutz, FL: Psychological Assessment Resources, Inc. [Google Scholar]
- Gur RE, Gur RC. 2016. Sex differences in brain and behavior in adolescence: Findings from the Philadelphia Neurodevelopmental Cohort. Neurosci Biobehav Rev 70:159–170, PMID: 27498084, 10.1016/j.neubiorev.2016.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley KG, Berger K, Rauch S, Kogut K, Claus Henn B, Calafat AM, et al. 2017. Association of prenatal urinary phthalate metabolite concentrations and childhood BMI and obesity. Ped Res 82(3):405–415, PMID: 28426647, 10.1038/pr.2017.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, Calafat AM. 2005. Phthalates and human health. Occup Environ Med 62(11):806–818, PMID: 16234408, 10.1136/oem.2004.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK. 2000. WCST-64: Computer Version for Windows — Research Edition. Odessa, FL: Psychological Assessment Resources, Inc. [Google Scholar]
- Hoppin JA, Brock JW, Davis BJ, Baird DD. 2002. Reproducibility of urinary phthalate metabolites in first morning urine samples. Environ Health Perspect 110(5):515–518, PMID: 12003755, 10.1289/ehp.02110515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HB, Chen HY, Su PH, Huang PC, Sun CW, Wang CJ, et al. 2015. Fetal and childhood exposure to phthalate diesters and cognitive function in children up to 12 years of age: Taiwanese Maternal and Infant Cohort Study. PLoS One 10(6):e0131910, PMID: 26121592, 10.1371/journal.pone.0131910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns LE, Cooper GS, Galizia A, Meeker JD. 2015. Exposure assessment issues in epidemiology studies of phthalates. Environ Int 85:27–39, PMID: 26313703, 10.1016/j.envint.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JI, Hong YC, Shin CH, Lee YA, Lim YH, Kim BN. 2017. The effects of maternal and children phthalate exposure on the neurocognitive function of 6-year-old children. Environ Res 156:519–525, PMID: 28431379, 10.1016/j.envres.2017.04.003. [DOI] [PubMed] [Google Scholar]
- Kobrosly RW, Evans S, Miodovnik A, Barrett ES, Thurston SW, Calafat AM, et al. 2014. Prenatal phthalate exposures and neurobehavioral development scores in boys and girls at 6–10 years of age. Environ Health Perspect 122(5):521–528, PMID: 24577876, 10.1289/ehp.1307063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkman M, Kirk U, Kemp S. 1998. NEPSY: A Developmental Neuropsychological Assessment. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Korkman M, Kirk U, Kemp S. 2007. NEPSY II: A Developmental Neuropsychological Assessment, Second Edition. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Li N, Papandonatos GD, Calafat AM, Yolton K, Lanphear BP, Chen A, et al. 2019. Identifying periods of susceptibility to the impact of phthalates on children's cognitive abilities. Environ Res 172:604–614, PMID: 30878731, 10.1016/j.envres.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien YJ, Ku HY, Su PH, Chen SJ, Chen HY, Liao PC, et al. 2015. Prenatal exposure to phthalate esters and behavioral syndromes in children at 8 years of age: Taiwan Maternal and Infant Cohort Study. Environ Health Perspect 123(1):95–100, PMID: 25280125, 10.1289/ehp.1307154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lioy PJ, Hauser R, Gennings C, Koch HM, Mirkes PE, Schwetz BA, et al. 2015. Assessment of phthalates/phthalate alternatives in children's toys and childcare articles: review of the report including conclusions and recommendation of the Chronic Hazard Advisory Panel of the Consumer Product Safety Commission. J Expo Sci Environ Epidemiol 25:343–353, PMID: 25944701, 10.1038/jes.2015.33. [DOI] [PubMed] [Google Scholar]
- Lubin JH, Colt JS, Camann D, Davis S, Cerhan JR, Severson RK, et al. 2004. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect 112(17):1691–1696, PMID: 15579415, 10.1289/ehp.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks AR, Harley K, Bradman A, Kogut K, Barr DB, Johnson C, et al. 2010. Organophosphate pesticide exposure and attention in young Mexican-American children: the CHAMACOS study. Environ Health Perspect 118(12):1768–1774, PMID: 21126939, 10.1289/ehp.1002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matute E, Rosselli M, Ardila A, Ostrosky-Solis F. 2007. Evaluación neuropsicológica infantil. Guadalajara, México: Manual Moderno. [Google Scholar]
- Meeker JD, Sathyanarayana S, Swan SH. 2009. Phthalates and other additives in plastics: human exposure and associated health outcomes. Philos Trans R Soc Lond B Biol Sci 364(1526):2097–2113, PMID: 19528058, 10.1098/rstb.2008.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LD, Martinez YJ, Shumka E, Baker H. 2014. Multiple informant agreement of child, parent, and teacher ratings of child anxiety within community samples. Can J Psychiatry 59(1):34–39, PMID: 24444322, 10.1177/070674371405900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miodovnik A, Engel SM, Zhu C, Ye X, Soorya LV, Silva MJ, et al. 2011. Endocrine disruptors and childhood social impairment. Neurotoxicology 32(2):261–267, PMID: 21182865, 10.1016/j.neuro.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora AM, Arora M, Harley KG, Kogut K, Parra K, Hernández-Bonilla D, et al. 2015. Prenatal and postnatal manganese teeth levels and neurodevelopment at 7, 9, and 10.5 years in the CHAMACOS cohort. Environ Int 84:39–54, PMID: 26209874, 10.1016/j.envint.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preau JL, Wong L-Y, Silva MJ, Needham LL, Calafat AM. 2010. Variability over 1 week in the urinary concentrations of metabolites of diethyl phthalate and di(2-ethylhexyl) phthalate among eight adults: an observational study. Environ Health Perspect 118(12):1748–1754, PMID: 20797930, 10.1289/ehp.1002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff L. 1977. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1(3):385–401, 10.1177/014662167700100306. [DOI] [Google Scholar]
- Reynolds CR, Kamphaus RW. 2004. BASC-2: Behavior Assessment System for Children, Second Edition Manual. Circle Pines, MN: AGS Publishing. [Google Scholar]
- Rudel RA, Camann DE, Spengler JD, Korn LR, Brody JG. 2003. Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers, and other endocrine-disrupting compounds in indoor air and dust. Environ Sci Technol 37(20):4543–4553, PMID: 14594359, 10.1021/es0264596. [DOI] [PubMed] [Google Scholar]
- Sagiv SK, Kogut K, Gaspar FW, Gunier RB, Harley KG, Parra K, et al. 2015. Prenatal and childhood polybrominated diphenyl ether (PBDE) exposure and attention and executive function at 9-12 years of age. Neurotoxicol Teratol 52:151, PMID: 26271888, 10.1016/j.ntt.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salbach-Andrae H, Lenz K, Lehmkuhl U. 2009. Patterns of agreement among parent, teacher and youth ratings in a referred sample. Eur Psychiatry 24(5):345–351, PMID: 18789656, 10.1016/j.eurpsy.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Shin HM, Bennett DH, Barkoski J, Ye X, Calafat AM, Tancredi D, et al. 2019. Variability of urinary concentrations of phthalate metabolites during pregnancy in first morning voids and pooled samples. Environ Int 122:222–230, PMID: 30477814, 10.1016/j.envint.2018.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H-M, Schmidt RJ, Tancredi D, Barkoski J, Ozonoff S, Bennett DH, et al. 2018. Prenatal exposure to phthalates and autism spectrum disorder in the MARBLES study. Environ Health 17(1):85, PMID: 30518373, 10.1186/s12940-018-0428-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M, Barr DB, Reidy JA, Malek NA, Hodge CC, Caudill SP, et al. 2004. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ Health Perspect 112(3):331–338, PMID: 14998749, 10.1289/ehp.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, Samandar E, Preau JL Jr, Reidy JA, Needham LL, Calafat AM. 2007. Quantification of 22 phthalate metabolites in human urine. J Chromatogr B Analyt Technol Biomed Life Sci 860(1):106–112, PMID: 17997365, 10.1016/j.jchromb.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Spear LP. 2013. Adolescent neurodevelopment. J Adolesc Health 52(2):S7–S13, PMID: 23332574, 10.1016/j.jadohealth.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanida T, Warita K, Ishihara K, Fukui S, Mitsuhashi T, Sugawara T, et al. 2009. Fetal and neonatal exposure to three typical environmental chemicals with different mechanisms of action: mixed exposure to phenol, phthalate, and dioxin cancels the effects of sole exposure on mouse midbrain dopaminergic nuclei. Toxicol Lett 189(1):40–47, PMID: 19481886, 10.1016/j.toxlet.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Teitelbaum SL, Britton JA, Calafat AM, Ye X, Silva MJ, Reidy JA, et al. 2008. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environ Res 106(2):257–269, PMID: 17976571, 10.1016/j.envres.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Verhulst FC, Akkerhuis GW. 1989. Agreement between parents' and teachers' ratings of behavioral/emotional problems of children aged 4-12. J Child Psychol Psychiatry 30(1):123–136, PMID: 2925818, 10.1111/j.1469-7610.1989.tb00772.x. [DOI] [PubMed] [Google Scholar]
- Wang R, Xu X, Zhu Q. 2016. Pubertal exposure to di-(2-ethylhexyl) phthalate influences social behavior and dopamine receptor D2 of adult female mice. Chemosphere 144:1771–1779, PMID: 26524146, 10.1016/j.chemosphere.2015.10.062. [DOI] [PubMed] [Google Scholar]
- Weiss B. 2012. The intersection of neurotoxicology and endocrine disruption. Neurotoxicology 33(6):1410–1419, PMID: 22659293, 10.1016/j.neuro.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weschler D. 2003. Weschler Intelligence Scale for Children - Fourth Edition (WISC-IV). Administration and Scoring Manual. San Antonio, TX: Harcourt Assessment Inc. [Google Scholar]
- Whyatt RM, Liu X, Rauh VA, Calafat AM, Just AC, Hoepner L, et al. 2012. Maternal prenatal urinary phthalate metabolite concentrations and child mental, psychomotor, and behavioral development at 3 years of age. Environ Health Perspect 120(2):290–295, PMID: 21893441, 10.1289/ehp.1103705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittassek M, Angerer J, Kolossa-Gehring M, Schäfer SD, Klockenbusch W, Dobler L, et al. 2009. Fetal exposure to phthalates–a pilot study. Int J Hyg Environ Health 212(5):492–498, PMID: 19423389, 10.1016/j.ijheh.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Youngstrom E, Loeber R, Stouthamer-Loeber M. 2000. Patterns and correlates of agreement between parent, teacher, and male adolescent ratings of externalizing and internalizing problems. J Consult Clin Psychol 68(6):1038–1050, PMID: 11142538, 10.1037//0022-006x.68.6.1038. [DOI] [PubMed] [Google Scholar]
- Zota AR, Calafat AM, Woodruff TJ. 2014. Temporal trends in phthalate exposures: findings from the National Health and Nutrition Examination Survey, 2001–2010. Environ Health Perspect 122(3):235–241, PMID: 24425099, 10.1289/ehp.1306681. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.