Abstract
Background:
Per- and polyfluoroalkyl substances (PFASs) are common industrial and consumer product chemicals with widespread human exposures that have been linked to adverse health effects. PFASs are commonly detected in foods and food-contact materials (FCMs), including fast food packaging and microwave popcorn bags.
Objectives:
Our goal was to investigate associations between serum PFASs and consumption of restaurant food and popcorn in a representative sample of Americans.
Methods:
We analyzed 2003–2014 serum PFAS and dietary recall data from the National Health and Nutrition Examination Survey (NHANES). We used multivariable linear regressions to investigate relationships between consumption of fast food, restaurant food, food eaten at home, and microwave popcorn and serum levels of perfluorooctanoic acid (PFOA), perfluorononanoic acid (PFNA), perfluorodecanoic acid (PFDA), perfluorohexanesulfonic acid (PFHxS), and perfluorooctanesulfonic acid (PFOS).
Results:
Calories of food eaten at home in the past 24 h had significant inverse associations with serum levels of all five PFASs; these associations were stronger in women. Consumption of meals from fast food/pizza restaurants and other restaurants was generally associated with higher serum PFAS concentrations, based on 24-h and 7-d recall, with limited statistical significance. Consumption of popcorn was associated with significantly higher serum levels of PFOA, PFNA, PFDA, and PFOS, based on 24-h and 12-month recall, up to a 63% (95% CI: 34, 99) increase in PFDA among those who ate popcorn daily over the last 12 months.
Conclusions:
Associations between serum PFAS and popcorn consumption may be a consequence of PFAS migration from microwave popcorn bags. Inverse associations between serum PFAS and food eaten at home—primarily from grocery stores—is consistent with less contact between home-prepared food and FCMs, some of which contain PFASs. The potential for FCMs to contribute to PFAS exposure, coupled with concerns about toxicity and persistence, support the use of alternatives to PFASs in FCMs. https://doi.org/10.1289/EHP4092
Introduction
Per- and polyfluoroalkyl substances (PFASs) are a diverse group of synthetic compounds with dual hydrophobic and hydrophilic properties and characteristic carbon–fluorine bonds that are extremely resistant to degradation even at high temperatures. They are widely used in nonstick, grease- and water-proof, and stain-resistant consumer products; firefighting foams; paints; and a range of industrial processes (U.S. EPA 2009). PFASs were first produced in the late 1940s (Lindstrom et al. 2011), and at least 4,700 PFASs are estimated to be on the global market (OECD 2018). Worldwide, PFASs have been detected in surface water, drinking water, and wildlife (Kelly et al. 2009; Weiss et al. 2015).
Human exposure in the general population can occur through diet, drinking water, air, dust, and direct contact with products (Trudel et al. 2008). Long-chain PFASs (carboxylates with perfluorinated carbon atoms, sulfonates with perfluorinated carbon atoms), particularly perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS), have been associated with cancer, immunotoxicity, weight gain, altered thyroid function, and reproductive and developmental toxicity (Wolf et al. 2007; Hines et al. 2009; Grandjean et al. 2012; Lopez-Espinosa et al. 2012; Barry et al. 2013). Concerns about persistence, bioaccumulation, and toxicity have led manufacturers to phase out production of long-chain PFASs and their precursors in North America and Europe, although production continues in other regions (Liu et al. 2017). As a way to prevent further exposures, the U.S. EPA issued Significant New Use Rules (SNURs) for a number of legacy PFASs, including PFOA and PFOS, requiring any new uses and imports of these chemicals to first be evaluated by the U.S. EPA (2019). Although alternative PFAS compounds, including short-chain PFASs, may be less bioaccumulative than long-chain compounds, they raise similar concerns in terms of persistence, mobility, and toxicity (Olsen et al. 2009; Butenhoff et al. 2012; Blaine et al. 2013; Rosenmai et al. 2016; Rushing et al. 2017).
Long-chain PFASs can persist in the human body for years. The human half-lives for PFOA, PFOS, and perfluorohexanesulfonic acid (PFHxS) in blood are approximately 3.5, 4.8, and 7.3 y, respectively (Olsen et al. 2007). The U.S. National Health and Nutrition Examination Survey (NHANES) detected these three PFASs, as well as perfluorononanoic acid (PFNA), in the blood of of Americans tested in 2003–2004 (Calafat et al. 2007), as well as in all children 3–11 y of age tested in 2013–2014 (Ye et al. 2018). Some short-chain replacement compounds, such as perfluorobutanesulfonic acid (PFBS) and perfluorohexanoic acid (PFHxA), have half-lives of about 1 month in blood (Olsen et al. 2009; Russell et al. 2013). Human half-lives for other short-chain compounds have not been evaluated, although they are also highly resistant to degradation (Danish Ministry of the Environment 2015).
Diet is considered a major route of PFAS exposure (Tittlemier et al. 2007; Trudel et al. 2008; Vestergren and Cousins 2009). PFASs have been found in many foods; a market basket study in Canada found PFASs in fish, meat, pizza, and microwave popcorn (Tittlemier et al. 2007). In particular, consumption of fish and shellfish has been positively associated with serum PFAS concentrations in studies in the United States, Europe, and Asia (Rylander et al. 2010; Bjermo et al. 2013; Manzano-Salgado et al. 2016; Christensen et al. 2017). An analysis of 2007–2014 NHANES data found positive associations between fish and shellfish consumption and serum PFAS concentrations (Christensen et al. 2017).
Migration of PFASs from food-contact materials (FCMs) into foods may also contribute to dietary exposure. PFASs have been used in FCMs for their grease- and water-resistant properties since the 1960s (Posner et al. 2013; Wang et al. 2017). PFASs that have been detected in fast food packaging, microwave popcorn bags, and other FCMs in North America, Europe, Asia, and Africa include PFOA and other perfluoroalkyl carboxylates (PFCAs), PFOS and other perfluoroalkyl sulfonates (PFSAs), fluorotelomer alcohols (FTOHs), and polyfluoroalkyl phosphate esters (PAPs) (Trier et al. 2011; Poothong et al. 2012; Gebbink et al. 2013; Shoeib et al. 2016; Yuan et al. 2016; Schaider et al. 2017). A 2017 survey of 400 FCMs collected from U.S. fast food restaurants (primarily large fast food chains with U.S. stores) found fluorinated chemicals in 46% of food-contact papers and 20% of paperboard samples, and a compound-specific analysis of a subset of samples detected 27 PFASs, including PFOA, PFHxA, and PFBS (Schaider et al. 2017). Compared with other types of FCMs, microwave popcorn bags have among the highest concentrations of PFASs, including PFCAs, PFOS, PAPs, fluorotelomer saturated carboxylic acids (FTCAs), and fluorotelomer unsaturated carboxylic acids (FTUCAs) (Poothong et al. 2012; Moreta and Tena 2014; Zabaleta et al. 2016, 2017).
PFASs in FCMs have been shown to migrate out of packaging and into food. PFAS migration from food-contact paper increases with higher temperatures, longer contact time, and the presence of emulsifiers (Begley et al. 2008). The extent of migration is compound-specific, and short-chain PFASs have higher migration efficiencies than longer-chain analogs (Yuan et al. 2016). A PAP surfactant was found in popcorn after heating in a microwave popcorn bag (Begley et al. 2008); however, another study did not find migration of four PFCAs from microwave popcorn bags into the popcorn after cooking (Moreta and Tena 2014). PFASs, especially volatile PFCA precursors such as FTOHs, have been found in the vapors from cooked microwave popcorn bags, indicating the potential for inhalation exposure (Sinclair et al. 2007). These findings indicate that migration of PFASs used in FCM formulations, such as PAPs and FTOHs, may occur at a higher rate than PFCAs, which are present in the additives as impurities (D’eon and Mabury 2011). PAPs and FTOHs are PFCA precursors and can contribute indirectly to PFCA exposure via in vivo biotransformation (Fasano et al. 2006; D’eon and Mabury 2007).
At a population level, the extent of PFAS exposure attributable to migration from FCMs is not well characterized. Several studies of the relationship between diet and PFAS blood concentrations have included popcorn and other foods likely to have been in contact with FCMs. Consumption of snacks (including popcorn) was positively associated with PFOS levels in the Danish National Birth Cohort (Halldorsson et al. 2008), and popcorn was among the foods associated with blood PFAS levels in four additional cohort studies (Halldorsson et al. 2008; Wu et al. 2015; Zong et al. 2018; Boronow et al. 2019). However, these studies did not more broadly investigate associations between the extent of food packaging, food sources (e.g., restaurants, grocery stores), and population PFAS exposures.
In this paper, we report our investigation of whether consumption of microwave popcorn, fast food, and other food consumed outside and within the home is associated with population exposure to PFASs. We analyzed associations between serum PFAS concentrations and consumption of fast food, restaurant food, nonrestaurant food eaten at home, and microwave popcorn across multiple recall periods (24-h, 7- and 30-d, and 12-month) from six cycles of NHANES from 2003 to 2014. We included fish and shellfish consumption in our models because prior research found significant associations for these foods with serum PFASs (Christensen et al. 2017). Understanding sources of dietary exposures to PFASs can inform exposure assessment and identify intervention strategies to reduce exposure.
Methods
Serum PFAS Data
NHANES is an ongoing national survey that combines biomonitoring, physical examinations, and interviews to assess the health and nutrition of U.S. residents over time. The Centers for Disease Control and Prevention (CDC) has conducted the survey in biennial cycles beginning in 1999 and uses a complex survey design in which selected demographic subpopulations are oversampled and survey weights are used to construct a nationally representative sample. The study protocol was approved by the National Center for Health Statistics (NCHS) Research Ethics Review Board, and participants provided consent. For participants under 18, consent was provided by parents or guardians.
The NHANES biomonitoring program has measured PFAS serum concentrations since 1999. We compiled NHANES PFAS data from six cycles covering 2003–2014, including a subsample of 639 children (3–11 y of age) in the 2013–2014 cycle. We did not include data from the 1999–2000 cycle because PFAS data for that cycle were only available from surplus serum samples, which were treated differently than serum in later years, nor from the 2001–2002 cycle because only pooled data were available. Ten PFASs were analyzed in blood in all six cycles: perfluoroheptanoic acid (PFHpA), PFOA, PFNA, perfluorodecanoic acid (PFDA), perfluoroundecanoic acid (PFUnDA), perfluorododecanoic acid (PFDoDA), PFBS, PFHxS, PFOS, and 2-(N-methylperfluorooctane sulfonamido) acetic acid (Me-PFOSA-AcOH). Serum concentrations were reported in nanograms per milliliter. Details of analytical methods are described in CDC (2014) and include solid-phase extraction coupled to high-performance liquid chromatography–turbo ion spray ionization–tandem mass spectrometry. With the exception of PFBS and PFHpA, these compounds are all considered long-chain PFASs according to Buck et al. (2011). Table S1 provides additional information about each analyte.
Diet Recall Data
We compiled dietary information covering four timescales: 24-h diet recall, 7-d and 30-d meal recall, and 12-month food frequency questionnaires (FFQs). Table S2 shows available PFAS and dietary data for each NHANES cycle. Starting in 1999, 24-h dietary recall data were collected through an in-person interview at a Mobile Examination Center that was open to all ages (CDC 2009). Participants were asked to list every food or beverage item consumed, including portion size, in the previous day (midnight to midnight). Each item was later annotated by U.S. Department of Agriculture (USDA) analysts with nutritional information including caloric content, USDA food type code, and food source from 30 possible categories (including “Don’t Know”). For 24-h diet recall data, we assigned each meal to one of four categories: “Fast food and pizza restaurants,” “Other restaurants,” “Other sources—eaten at home,” and “Other sources—eaten outside the home.” For “fast food and pizza restaurants,” we used the NHANES definition of fast food as food from the “Restaurant fast food/pizza place” category. Lack of waiter/waitress service distinguishes fast food and pizza restaurants from other restaurants. Fast food does not include food from other similar categories such as “Vending machine,” “Bar/tavern/lounge,” and “Street vendor, vending truck.” This definition was used by Zota et al. (2016) in a previous study of chemical exposures and fast food consumption. “Other restaurants” included food from non-fast food restaurants, which we defined as any food items in the categories “Restaurant with waiter/waitress” and “Restaurant, no additional information.” “Other sources” included all other NHANES food source categories (including “Store grocery/supermarket,” “Cafeteria in a K-12 school,” and “Store—convenience type”). We further categorized meals from “Other sources” into those eaten at home and those eaten outside the home based on the field named “Did you eat this meal at home?” Any items without a food source specified were excluded.
We compiled information separately about several specific types of food that we hypothesized might contribute substantially to PFAS exposure. We classified microwave popcorn as any food item that had a USDA food code associated with microwave popcorn (see Table S3 for specific food codes). This excluded other types of popcorn, such as air popped popcorn. In addition, because a previous study using NHANES data found associations with serum PFAS and consumption of fish and shellfish, we also compiled data on consumption of fish and shellfish food items using USDA food codes for each food group (see Table S4).
Data from the 24-h diet recall were used to calculate caloric intake associated with certain food types and food sources. We calculated the total caloric intake over 24 h from microwave popcorn and from fish/shellfish for each participant by summing the total kilocalories (kcal) from each microwave popcorn or fish/shellfish food item in the recall data. Total caloric intake from fast food, restaurants, and other sources was calculated similarly, with each source category excluding any kilocalories from microwave popcorn or fish/shellfish.
From 2007 to 2014, NHANES included a consumer behavior module in the household interview that included questions on food consumption patterns over 7- and 30-d recall periods. Specifically, it asked the number of meals consumed in the last 7 d that were prepared outside the home (not including school breakfast or lunch, or from community programs) and how many of these meals were from fast food or pizza restaurants. The number of meals from other sources consumed in the past week was not gathered in the survey. These data were included in a separate analysis because they may be more representative of long-term fast food consumption than data from the 24-h diet recall (Willett 1998). The 30-d recall asked participants how many servings of various species of fish and shellfish they consumed over the past 30 d; we summed these to derive the total number of fish servings and total number of shellfish servings consumed.
A 12-month FFQ was included in the 2003–2004 and 2005–2006 NHANES cycles. Participants were mailed a questionnaire that asked how often they ate a number of different food types. The categorical responses were converted by NHANES, using the Diet*Calc software, to a continuous daily frequency measure that ranges from 0 (never) to 2 (two or more times per day). The factors for these conversions are listed in Table S5. We selected popcorn food frequency results (microwave popcorn was not differentiated in the questionnaire), and summed all seafood-related categories to form a combined “seafood” frequency measure. The data did not include frequency of fast food consumption over the last 12 months. We included these data as an indicator of long-term consumption habits to supplement the shorter-term recall data.
In addition, we compiled data for potential confounders that were previously included in related studies (Zota et al. 2016; Christensen et al. 2017), including age, gender, race/ethnicity, poverty–income ratio (PIR; ratio of family income to the U.S. Department of Health and Human Services poverty guideline; CDC 2015), and body mass index (BMI). Race/ethnicity was categorized as Hispanic (including both Mexican American and other Hispanic), non-Hispanic white, and non-Hispanic black. Age, PIR, and BMI were included as continuous variables.
Data Analysis
We combined data from six NHANES survey cycles covering 2003–2014, excluding the 2013–2014 child subsample, which was analyzed separately. The raw data set contained survey and laboratory results from 13,933 participants. We identified 10,578 qualifying participants by removing anyone who was missing PFAS serum concentration data (), missing race/ethnicity or reported as mixed race/other (), or missing household PIR data () or BMI data (). The 2013–2014 subsample of children 3–11 y of age was analyzed separately due to its use of special subsample survey weights that could not be combined with data from other subsamples. We identified 517 qualifying participants in the child subsample using the same criteria as the adolescent and adult data set.
We estimated associations between serum PFAS and dietary exposures that were derived using data from the four recall instruments (see Table S6). For participants of age, we estimated exposures based on: 24-h dietary recall from 10,106 NHANES participants during 2003–2014 who also had serum PFAS data; 7-d and 30-d meal recall data from NHANES consumer behavior modules for 5,261 participants during 2007–2014 (after excluding 1,407 participants missing data for weekly fast food consumption and fish/shellfish consumption); and 12-month FFQ data for 2,788 participants during 2003–2006 (after excluding 1,028 participants missing these data). We used separate models to estimate associations between serum PFAS and dietary exposures in children (3–11 y of age) based on 24-h recall data ( from the 2013–2014 NHANES cycle) and on 7- and 30-d recall data ( from the 2013–2014 NHANES cycle).
All analyses incorporated NHANES’ complex survey design and sample weights. We computed new sample weights according to NCHS guidelines for combining data from multiple cycles (Johnson et al. 2013). Concentrations below the lower limit of detection (LLOD) were substituted with a value of LLOD divided by the square root of 2. Analyses were only conducted on analytes with a high detection frequency () in order to avoid bias from substituted values.
We used a series of multivariable linear regression models to investigate the association between log-transformed PFAS serum concentrations and metrics of restaurant food and popcorn consumption in three data sets. All regressions included age, gender, race/ethnicity, cycle year, BMI, and PIR as covariates. Percent increase in the response variable was calculated from the regression coefficient and standard error (SE) by the formula , with a 95% confidence interval (CI) given by . A significance level of was set prior to analysis. Because gender was frequently a significant predictor and because of the potential for gender-specific differences in diet and physiological uptake of PFAS, we also conducted an additional set of analyses to investigate effects by gender (referred to below as gender effect modification models) in which we added interaction terms between gender and all other variables (including confounders), according to the augmented product term method described by Buckley et al. (2017). All analyses were conducted in R (version 3.4.0; R Development Core Team). Complex survey analyses were conducted using the survey R package (Lumley 2004). The R scripts used to conduct these analyses are provided in the R source zip file “FinalPaperCode” in the Supplemental Material.
Results
PFOA, PFNA, PFDA, PFHxS, and PFOS were detected in more than 70% of samples collected in 2003–2014 (see Table S7). All subsequent analyses are restricted to these five analytes. Median serum PFOS and PFOA concentrations decreased by a factor of 4 and 2, respectively, over the study period, whereas concentrations of PFNA, PFDA, and PFHxS were fairly constant or showed slight decreases.
As shown in Table 1, fast food consumption was common from 2003 to 2014, especially among adolescents: 35% of adults and 41% of adolescents (12–17 y of age) reported eating fast food in the last 24 h, and 70% of adults and 84% of adolescents ate at least one fast food meal in the past 7 d (2007–2014 data). Among participants who ate fast food in the last 24 h, median caloric intake from fast food was for adults and for adolescents (see Table S8). Microwave popcorn consumption was less common than fast food consumption in the 24-h recall; 5% of adults and 7% of adolescents reporting consuming microwave popcorn in the past 24 h, although 86% of participants reported eating popcorn of any kind in the past 12 months (2003–2006 data). Frequencies of microwave popcorn consumption in the 24-h recall were similar across demographic subgroups, ranging from 4% to 8%. Fish and shellfish consumption were common: 10% and 5% of participants reported fish and shellfish consumption, respectively, in the past 24 h (2003–2014), and 68% and 55% reported fish and shellfish consumption in the last month (2007–2014). The 12-month FFQ did not distinguish between fish and shellfish, but 89% of participants reported general seafood consumption in the past 12 months (2003–2006).
Table 1.
Consumption of fast food and pizza restaurant food and popcorn by demographic group in NHANES 2003–2014.
| Demographic group | 24-h (2003–2014) | 7-d (2007–2014) | 12-month (2003–2006) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fast food and pizza restaurant food | Food from other restaurant | Other food outlet, eaten at home | Other food outlet, not eaten at home | Microwave popcorn | Meals from fast food/pizza restaurant | Popcorn (any kind) | ||||
| Consumers (%) | Consumers (%) | Consumers (%) | Consumers (%) | Consumers (%) | Consumers (%) | Consumers (%) | ||||
| All | 10,106 | 36 | 23 | 95 | 56 | 5 | 5,261 | 71 | 2,788 | 86 |
| Female | 5,115 | 34 | 22 | 95 | 53 | 5 | 2,630 | 70 | 1,459 | 86 |
| Male | 4,991 | 38 | 24 | 95 | 59 | 5 | 2,631 | 72 | 1,329 | 86 |
| Adults ( y of age) | 8,419 | 35 | 24 | 95 | 55 | 5 | 4,551 | 70 | 2,183 | 86 |
| Non-Hispanic white adults | 4,309 | 34 | 25 | 95 | 55 | 5 | 2,385 | 68 | 1,233 | 87 |
| Non-Hispanic black adults | 1,884 | 43 | 15 | 92 | 53 | 5 | 998 | 80 | 460 | 81 |
| Hispanic adults | 2,226 | 37 | 22 | 94 | 58 | 4 | 1,168 | 72 | 490 | 78 |
| Adolescents (12–17 y of age) | 1,687 | 41 | 17 | 93 | 63 | 7 | 710 | 84 | 605 | 88 |
| Non-Hispanic white adolescents | 507 | 40 | 20 | 93 | 64 | 8 | 234 | 84 | 180 | 89 |
| Non-Hispanic black adolescents | 538 | 42 | 7 | 94 | 61 | 6 | 192 | 88 | 220 | 82 |
| Hispanic adolescents | 642 | 41 | 14 | 95 | 59 | 5 | 284 | 82 | 205 | 87 |
Note: NHANES, National Health and Nutrition Examination Survey.
Children (3–11 y of age) in 2013–2014 ate fast food at a similar frequency as adults and adolescents, with 39% reporting eating fast food in the last 24 h, and a median caloric intake from fast food of (see Table S9). A slightly higher proportion of children (11%) in 2013–2014 reported consuming microwave popcorn in the past 24 h, and a slightly lower proportion reported consuming fish and shellfish (5% and 2%, respectively, in the past 24 h).
The models included demographic and temporal variables as predictors to control for differences in exposure among subpopulations and over time. In the 24-h recall model, PFOS was lower in every cycle relative to the first cycle (2003–2004), and all five PFASs were lower in the last cycle (2013–2014) relative to the first cycle (see Table S10), reflecting the U.S. population’s generally declining exposures to long-chain PFASs. We found some evidence of different exposures among demographic subgroups. Both income and age were positively associated with all five PFASs and with their total concentration (). Females had lower levels of all five PFASs and compared with males. Compared with Non-Hispanic whites, Hispanics had lower levels of PFOA, PFHxS, and PFOS, whereas Non-Hispanic blacks were lower in PFOA and PFHxS and higher in the other three PFASs. Four of the five PFASs had statistically significant inverse relationships with BMI, with the exception of PFNA. Similar associations have been reported in prior analyses of NHANES data (Calafat et al. 2007; Christensen et al. 2017).
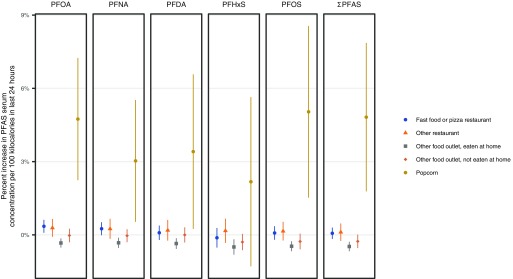
Consumption of microwave popcorn had significant positive associations with serum concentrations of PFOA, PFNA, and PFOS, as well as , in the 24-h recall model, and significant associations were also observed for consumption of popcorn (any type) with these PFASs, in addition to PFDA, in the 12-month recall model (Table 2 and Figure 1). Based on 24-h recall data, serum concentrations for these PFASs changed 3.0% (95% CI: 0.53, 5.5) for PFNA to 5.0% (95% CI: 1.5, 8.6) for PFOS per of microwave popcorn consumed daily, and the largest increases were observed for PFOS and PFOA (Table 2). For context, the median caloric intake from microwave popcorn among microwave popcorn consumers in the 24-h diet recall was (see Table S8). In the 12-month recall model, eating popcorn once a day was associated with 39% higher levels for PFNA (95% CI: 13, 73) up to 63% higher levels for PFDA (95% CI: 34, 99). Although we could not distinguish between microwave popcorn and other types of popcorn in the 12-month data, analysis of the 24-h recall data shows that microwave popcorn on average accounted for 85% (95% CI: 81, 89%) of overall popcorn consumption in the NHANES population. In our gender effect modification model, we did not find significant gender-specific differences in associations between popcorn consumption and serum PFAS levels (see Table S11 and Figure S1).
Table 2.
Percentage difference in serum PFASs (95% CI) in association with self-reported consumption of food from fast food or pizza restaurants, other restaurants, or other food outlets, and of microwave popcorn, fish, and shellfish among NHANES participants of age.
| Recall period/food consumed | PFOA | PFNA | PFDA | PFHxS | PFOS | |
|---|---|---|---|---|---|---|
| 24-h recalla | ||||||
| Fast food or pizza restaurant | 0.35 (0.087, 0.62) |
0.25 (, 0.52) |
0.087 (, 0.38) |
(, 0.28) |
0.078 (, 0.35) |
0.066 (, 0.30) |
| Other restaurant | 0.29 (, 0.65) |
0.25 (, 0.66) |
0.19 (, 0.61) |
0.17 (, 0.66) |
0.15 (, 0.53) |
0.11 (, 0.47) |
| Other food outlet, eaten at home |
(, ) |
(, ) |
(, ) |
(, ) |
(, ) |
(, ) |
| Other food outlet, not eaten at home |
(, 0.25) |
(, 0.23) |
(, 0.31) |
(, 0.040) |
(, 0.051) |
(-0.54, 0.012) |
| Microwave popcorn | 4.7 (2.2, 7.2) |
3.0 (0.53, 5.5) |
3.4 (0.25, 6.6) |
2.2 (, 5.6) |
5.0 (1.5, 8.6) |
4.8 (1.8, 7.9) |
| Fish |
(, 1.3) |
1.3 (, 2.8) |
2.2 (0.65, 3.7) |
(, 2.2) |
0.44 (, 1.9) |
0.31 (, 1.7) |
| Shellfish | 0.70 (, 3.4) |
5.2 (2.1, 8.3) |
5.8 (2.9, 8.7) |
0.71 (, 5.2) |
2.7 (, 6.1) |
2.4 (, 5.4) |
| 7-d and 30-d recall | ||||||
| Fast food or pizza restaurantb | 0.91 (0.047, 1.8) |
0.63 (, 1.5) |
0.69 (, 1.7) |
0.042 (, 1.1) |
0.56 (, 1.4) |
0.49 (, 1.3) |
| Other restaurant or food outletb | 0.67 (0.071, 1.3) |
0.35 (, 0.97) |
0.94 (0.27, 1.6) |
1.1 (, 2.3) |
0.59 (, 1.3) |
0.51 (, 1.2) |
| Fishc |
(, 0.29) |
0.21 (, 0.97) |
1.0 (0.37, 1.7) |
(, 0.54) |
(, 0.76) |
(, 0.60) |
| Shellfishc | 1.1 (0.071, 2.2) |
2.9 (1.6, 4.1) |
3.3 (2.0, 4.6) |
0.66 (, 1.5) |
2.0 (0.81, 3.3) |
1.7 (0.69, 2.8) |
| 12-month FFQd | ||||||
| Popcorn | 43 (21, 71) |
39 (13, 73) |
63 (34, 99) |
36 (, 84) |
44 (19, 75) |
45 (26, 67) |
| Seafood | 15 (, 34) |
37 (12, 67) |
48 (24, 76) |
16 (, 39) |
19 (4.5, 36) |
19 (5.1, 36) |
Note: All estimates are from linear regression models that account for NHANES survey and sample weights and are adjusted for age, gender, race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic), NHANES cycle year, BMI (continuous), and PIR and are mutually adjusted for dietary intake variables. Models are limited to participants with complete data for PFAS, dietary variables, and model covariates. BMI, body mass index; CI, confidence interval; FFQ, food frequency questionnaire; NHANES, National Health and Nutrition Examination Survey; PFAS, per- and polyfluoroalkyl substances; PFDA, perfluorodecanoic acid; PFHxS, perfluorohexanesulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctanesulfonic acid; PIR, poverty–income ratio; , total concentration of PFASs.
Percentage difference in serum PFAS (95% CI) with each 100 kcal/d of food consumed at fast food or pizza restaurants, other types of restaurants, or other food outlets (e.g., grocery stores, convenience stores, cafeterias), excluding servings of microwave popcorn or seafood, or for each of microwave popcorn, fish, or shellfish (from any source), based on a 24-h dietary recall for the day before the NHANES study visit, NHANES 2003–2014, total .
Percentage difference in serum PFAS (95% CI) associated with one meal/week from a fast food or pizza restaurant, or one meal/week of food from other types of restaurants or food outlets, based on a 7-d dietary recall for the week before the NHANES study visit, NHANES 2007–2014, total . Estimates are also adjusted for the 30-d dietary variables.
Percentage difference in serum PFAS (95% CI) associated with one serving of fish or shellfish per 30 d (from any source) based on a 30-d dietary recall for the month before the NHANES study visit, NHANES 2007–2014, total . Estimates are also adjusted for the 7-d dietary variables.
Percentage difference in serum PFAS (95% CI) associated with a 1-unit increase in the average daily frequency of popcorn (of any type) or seafood consumption (from any source) during the previous year based on a FFQ, where a value of 1 indicates one serving/day, 2 indicates two servings/day, 0.14 indicates one serving/week, etc. (see Table S5 for a complete table of values), NHANES 2003–2006, total .
Figure 1.
Comparison of regression coefficients from the 24-h recall regression model, based on 2003–2014 data. The “Fast food or pizza restaurant,” “Other restaurant,” “Other food outlet, eaten at home,” and “Other food outlet, not eaten at home” categories exclude kilocalories from seafood and popcorn.
Among food sources, the strongest and most consistent associations we found were inverse associations between consumption of food from other sources (nonrestaurant) eaten at home and serum PFAS levels (Figure 1). According to the 24-h recall model, every of food per day eaten at home from nonrestaurant sources was associated with decreased concentrations of all five PFASs, from (95% CI: , ) for PFNA to (95% CI: , ) for PFHxS (Table 2). In our gender effect modification model, we found negative associations between serum PFAS and foods eaten at home among both men and women, and the coefficients for women were significantly more negative for PFOA, PFNA, and PFHxS (see Table S11 and Figure S1).
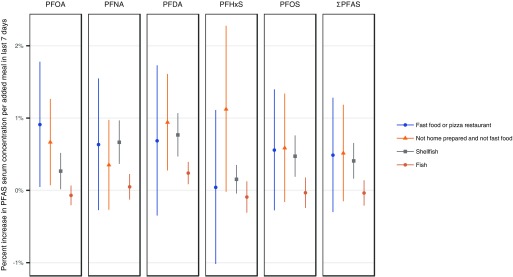
When we considered food from fast food/pizza restaurants and other restaurants in the 24-h and 7-d recall models, overall we found positive associations with serum PFAS levels. However, compared with associations with serum PFAS and nonrestaurant foods eaten at home, these associations were smaller in magnitude and few reached statistical significance. In the 24-h recall model, the only significant finding was that for every of fast food consumed in the past 24 h, serum concentrations of PFOA increased by 0.35% (95% CI: 0.087, 0.62). Similarly, results from the 7-d recall model also found nonsignificant positive associations between meals from fast food/pizza restaurants and serum PFAS, and the association with PFOA nearly reached significance (Figure 2). Calories of food eaten at other (non-fast food/pizza) restaurants in the past 24 h also had nonsignificant, positive associations with all five PFAS serum levels (Table 2 and Figure 1). In the 7-d recall model, the number of meals consumed from other restaurants in the last 7 d had positive associations with all five PFAS serum levels, with significant increases for PFOA and PFDA (Figure 2). In our gender effect modification model, we did not find gender-specific differences in associations between serum PFAS and consumption of food from fast food/pizza restaurants (see Table S11 and Figure S1).
Figure 2.
Comparison of regression coefficients from the 7-d/30-d recall model, based on 2007–2014 data.
Similar analyses of the subsample of children from 2013–2014 did not find significant relationships (after adjusting for multiple comparisons) between any variable and serum PFAS concentrations, which may be attributable to the small sample size relative to the models based on 2003–2014 data (see Tables S12 and S13).
Fish consumption was generally associated with various combinations of PFASs based on 24-h, 30-d, and 12-month recall. We found significant positive associations between shellfish consumption and serum levels of PFNA and PFDA according to 24-h and 30-d recall, and seafood consumption over the past 12 months was associated with higher serum levels of PFNA, PFDA, and PFOS (Table 2).
Discussion
To our knowledge, this study is the first to find associations between population-wide PFAS exposures and food consumption from various locations (e.g., restaurants, home). Overall, our analysis consistently found significant inverse associations between serum PFAS and calories from food eaten at home, whereas consumption of food from fast food/pizza restaurants and other restaurants generally showed weak positive associations with serum PFAS. We hypothesize that these associations may be related to the extent of contact that these foods have with food packaging, although we cannot rule out the possibility that differences in the types of food consumed in various locations may also contribute to these associations. We also observed significant positive associations with serum PFAS levels and consumption of popcorn, both in the 24-h and 12-month recall models, which are likely related to leaching of PFASs from microwave popcorn bags.
The consistent inverse associations between calories from nonrestaurant food eaten at home and serum PFAS concentrations may reflect a higher proportion of meals cooked at home and less contact with PFAS-containing FCMs than food prepared and consumed outside the home. In our analysis, the vast majority (90%) of the nonrestaurant meals reported to be eaten at home came from a grocery store (results not shown), although small proportions of these meals came from sources such as cafeterias and convenience stores. The consistency of these findings across different types of diet assessment methods is noteworthy because dietary recall is inexact and associations are likely to be biased heavily toward the null hypothesis.
By contrast, consumption of food from locations outside the home showed either positive or no associations with serum PFAS levels. The 24-h and 7-d recall models showed positive associations with serum PFAS, although in the 24-h recall model, only the results for PFOA were significant. According to 24-h and 7-d recall models, meals consumed from other (not fast food/pizza) restaurants were also positively associated with serum PFAS, with significant associations for PFOA and PFDA in the 7-d recall model. One possible explanation for the association between consumption of fast food and restaurant meals and serum PFAS concentrations is that these meals may have higher PFAS concentrations on average compared with meals from other locations. We controlled for differences in consumption of fish and shellfish in our models because prior research found these were associated with PFAS levels in serum (Christensen et al. 2017), so differences in fish/shellfish consumption are unlikely to explain these associations. Associations with fast food consumption are a particular concern for adolescents: 84% reported eating a fast food/pizza restaurant meal in the past 7 d. Our results suggest that migration of PFASs from fast food and restaurant packaging may contribute to population-wide PFAS exposure. The five PFASs in our study have all been detected in fast food packaging, with results varying by type of packaging, location, and sampling time (Poothong et al. 2012; Zafeiraki et al. 2014; Schaider et al. 2017). The significant association between consumption of fast food/pizza and serum concentrations of PFOA in the 24-h recall model, and the nearly significant association in the 7-d recall model, may be explained by evidence that PFOA is more frequently detected than PFOS in fast food packaging (Schaider et al. 2017) and that PFOS production began to be phased out in the United States starting in 2000, so FCMs may play a larger role for PFOA than for PFOS.
The association we observed between microwave popcorn consumption and serum PFAS levels is likely explained by contamination from FCMs, given the consistent findings of PFASs in popcorn packaging (Poothong et al. 2012; Moreta and Tena 2014; Zabaleta et al. 2016, 2017). The four PFASs significantly associated with popcorn consumption (PFOA, PFNA, PFDA, and PFOS) have all been detected in microwave popcorn bags, as have PFCA-precursor compounds (Begley et al. 2005; Martinez-Moral and Tena 2012; Yuan et al. 2016). Recent analyses of PFOS in popcorn bags reflect the phase-out of PFOS production; two recent studies in 2016 and 2017 did not detect PFOS in microwave popcorn bags from the United States (Moreta and Tena 2014; Zabaleta et al. 2016, 2017). Thus, although the observed associations between microwave popcorn consumption and PFCA levels are more easily explained than those for PFOS, we did find a statistically significant PFOS association in both the 24-h and 12-month recall models. Other studies have found similar associations: Halldorsson et al. (2008) found consumption of snacks, including popcorn, was associated with PFOS serum levels, and Wu et al. (2015) found frequency of microwave popcorn consumption predictive of PFOS concentrations in serum from 128 adults.
In our gender effect modification model, we saw negative associations between consumption of nonrestaurant food at home and serum PFAS levels for women and men, with stronger associations for women. We also found that the point estimates for the associations with fast food/pizza restaurant food in the last 24 h were mostly slightly negative for women, whereas they were positive for men, with limited statistical significance for both genders (see Table S11). The differences between men and women are not likely to be explained entirely by differences in consumption patterns given that similar proportions of men and women consumed fast food/pizza restaurant food and food from other sources at home (Table 1) and that men and women had similar proportions of total caloric intake from foods from these sources (see Table S8). These differences are also unlikely to be entirely explained by differences in pharmacokinetics by gender given that we did not find gender-specific differences in the associations for popcorn and seafood (see Table S11). One possible explanation is that there may be differences in the types of food eaten, and the associated packaging, between men and women. Additional analysis of differences in dietary patterns between men and women, as well as additional information about PFAS levels in a wide range of foods, would help explain the different associations we observed between men and women.
A major strength of this study is that it comprehensively analyzed multiple types of dietary data over multiple timescales in a large representative sample of the U.S. population. Combining evidence from nationally representative exposure data sets with data from 24-h, 7- and 30-d, and 12-month diet and meal recall surveys and FFQs strengthens the evidence of associations between fast food, restaurant food, nonrestaurant food, and microwave popcorn and PFAS serum levels. The estimated relationships between popcorn consumption and serum PFAS concentrations are nearly consistent between 24-h recall and 12-month FFQ analyses; the same four PFASs are significantly associated with popcorn consumption in the 24-h and 12-month analyses. Furthermore, our results were robust to changes in how fish and shellfish consumption, previously identified as predictors of exposure, were incorporated into the analysis.
A potential limitation of our study is that NHANES questionnaires about recent behaviors may not represent long-term dietary habits that contribute to serum levels of long-chain PFASs with half-lives in human blood on the timescale of years. The ability of short-term diet recall to predict serum levels may be the most limited for PFASs with the longest human half-lives, notably PFHxS (Olsen et al. 2007), which had the fewest significant associations with dietary recall data in our analysis. It is also possible that food consumption patterns and sources are associated with one or more unidentified confounding factors or that self-reported diet recall may not accurately reflect food consumption. In addition, the cross-sectional design of the NHANES study limits our ability to investigate causal relationships between food consumption and PFAS exposures. Further, our study was not able to discern whether differences in associations between serum PFAS and different food outlets reflect differences in the types of foods consumed or how the foods were packaged or prepared.
The interpretation of our findings is complicated by changes in PFAS manufacturing during our study period. In 2000, 3M agreed to phase out production of perfluorooctane sulfonyl fluoride, the raw material used to produce PFOS, in the United States (U.S. EPA 2000; Buck et al. 2011), and U.S. EPA’s 2006 PFOA Stewardship Program included a commitment by eight global manufacturers to cut PFOA production in the United States by 95% by 2010 and its complete elimination by 2015 (U.S. EPA 2017). However, we still found associations between consumption of fast food and microwave popcorn and serum concentrations of PFOS and PFOA. One explanation for this finding is that recent consumption of fast food/pizza may reflect past behavior and that the association is still apparent because of the long biological half-lives of PFOA and PFOS. Ongoing exposure to long-chain PFASs may still occur in other parts of the world where production is still active or from imported products; an international study of microwave popcorn bags found predominantly short-chain PFASs in samples from Europe and the Americas and long-chain PFASs in samples from Asia (Zabaleta et al. 2017).
As the use of long-chain chemistries has declined, manufacturers have switched to alternative PFAS compounds, such as short-chain PFAS. Several short-chain compounds have been included in NHANES and other biomonitoring studies and have not been frequently detected in blood serum (Haug et al. 2009; Lee and Mabury 2011; Olsen et al. 2017). PFBS and PFHpA were the only short-chain PFASs included in the NHANES program, and both had relatively low detection frequencies across cycle years. Despite lower concentrations of short-chain PFASs than long-chain compounds in serum, there is evidence that concentrations are increasing in serum in the U.S. population (Glynn et al. 2012), and ongoing exposures are likely to increase because of growing production and use of short-chain PFASs (Wang et al. 2013). Twenty-two PFASs were approved for use in FCMs by the FDA as of 2016 (Schaider et al. 2017). In a study of serum, plasma, and whole blood from 61 participants, PFHxA was detected only in whole blood, suggesting that serum samples may not accurately reflect exposure to short-chain PFAS (Poothong et al. 2017). In addition, short-chain PFAs exhibit different patterns of accumulation in the body and in blood (Pérez et al. 2013; Burkemper et al. 2017; Poothong et al. 2017). Furthermore, although some studies have suggested that short-chain PFASs are less toxic based on administered PFAS dose, when differences in toxicokinetics are considered, some alternative PFASs may have similar or higher toxicity than the long-chain compounds they are meant to replace (Gomis et al. 2018). Other replacements have not yet been measured in population-wide biomonitoring (e.g., ammonium perfluoro(2-methyl-3-oxahexanoate) (GenX), ammonium 4,8-dioxa-3H-perfluorononanoate (ADONA)) (Wang et al. 2017), and there may be widespread exposures to other unidentified compounds. A biomonitoring study in China found that, depending on the city, known PFASs (analytes included 13 PFCAs and PFSAs) accounted for only 33–85% of total extractable organic fluorine in serum, with the balance consisting of unidentified organofluorine compounds (Yeung et al. 2008). Future research should evaluate the relationship between exposure to FCMs and body levels of a comprehensive set of PFAS compounds, including short-chain PFASs, to further elucidate the role of FCMs in population exposure. Given that our findings indicate FCMs are a source of exposure to PFASs, caution is warranted in replacing long-chain with short-chain compounds due to concerns about their toxicity and environmental mobility.
Conclusion
In this study, we conducted a comprehensive analysis of the association between PFAS exposure and consumption of food from fast food/pizza restaurants, other restaurants, and food eaten at home, as well as microwave popcorn, based on representative sampling of the U.S. population in 2003–2014, incorporating multiple data sets and recall periods. Microwave popcorn consumption was associated with increased PFAS serum concentrations; these results suggest that migration from fluorinated grease-proof coatings commonly applied to popcorn bags contributes to dietary PFAS exposures. We found inverse relationships between serum PFAS levels and consumption of food eaten at home, with stronger associations among women. By contrast, we generally found weak positive associations between serum PFAS concentrations and consumption of fast food and restaurant food. Taken together, these results may be attributed to PFAS migration from food packaging and other FCMs, to differences in the types of foods consumed in different locations, or to a combination of these two factors. FCMs are likely an ongoing source of exposure to long-chain PFASs in countries where they are still being produced; for example, long-chain PFASs remain common in Chinese FCMs (Yuan et al. 2016). In the United States, newer replacement PFASs are currently being used, but we have little information on the extent of exposure or health effects related to these newer compounds. Preliminary evidence suggests that short-chain PFASs migrate more readily from FCMs than long-chain compounds (Yuan et al. 2016) and may be associated with similar adverse effects (Rosenmai et al. 2016; Rushing et al. 2017). Schaider et al. (2017) found that approximately half of fast food paper wrappers and 80% of paperboard samples did not contain fluorinated chemicals, indicating that nonfluorinated options for grease-proof FCM are readily available in the United States. Concerns about persistence, mobility, and potential toxicity support a precautionary approach to protecting public and environmental health by avoiding the use of fluorinated chemicals in FCMs entirely.
Supplementary Material
Acknowledgments
We thank J. Brody for helpful discussion. This work was funded by charitable gifts to Silent Spring Institute and by the National Institute of Environmental Health Sciences of the National Institutes of Health (NIH; P42ES027706 and R01ES028311). All authors are or have been employed at Silent Spring Institute, a scientific research organization dedicated to studying environmental factors in women’s health. The institute is a 501(c)3 public charity funded by federal grants and contracts, foundation grants, and private donations, including from breast cancer organizations. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Supplemental Material is available online (https://doi.org/10.1289/EHP4092).
The authors declare they have no actual or potential competing financial interests.
Note to readers with disabilities: EHP strives to ensure that all journal content is accessible to all readers. However, some figures and Supplemental Material published in EHP articles may not conform to 508 standards due to the complexity of the information being presented. If you need assistance accessing journal content, please contact ehponline@niehs.nih.gov. Our staff will work with you to assess and meet your accessibility needs within 3 working days.
References
- Barry V, Winquist A, Steenland K. 2013. Perfluorooctanoic acid (PFOA) exposures and incident cancers among adults living near a chemical plant. Environ Health Perspect 121(11–12):1313–1318, PMID: 24007715, 10.1289/ehp.1306615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley TH, Hsu W, Noonan G, Diachenko G. 2008. Migration of fluorochemical paper additives from food-contact paper into foods and food simulants. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 25(3):384–390, PMID: 18311629, 10.1080/02652030701513784. [DOI] [PubMed] [Google Scholar]
- Begley TH, White K, Honigfort P, Twaroski ML, Neches R, Walker RA. 2005. Perfluorochemicals: potential sources of and migration from food packaging. Food Addit Contam 22(10):1023–1031, PMID: 16227186, 10.1080/02652030500183474. [DOI] [PubMed] [Google Scholar]
- Bjermo H, Darnerud PO, Pearson M, Barbieri HE, Lindroos AK, Nälsén C. 2013. Serum concentrations of perfluorinated alkyl acids and their associations with diet and personal characteristics among Swedish adults. Mol Nutr Food Res 57(12):2206–2215, PMID: 23934649, 10.1002/mnfr.201200845. [DOI] [PubMed] [Google Scholar]
- Blaine AC, Rich CD, Hundal LS, Lau C, Mills MA, Harris KM, et al. 2013. Uptake of perfluoroalkyl acids into edible crops via land applied biosolids: field and greenhouse studies. Environ Sci Technol 47(24):14062–14069, PMID: 24206563, 10.1021/es403094q. [DOI] [PubMed] [Google Scholar]
- Boronow KE, Brody JG, Schaider LA, Peaslee GF, Havas L, Cohn BA. 2019. Serum concentrations of PFASs and exposure-related behaviors in African American and non-Hispanic white women. J Expo Sci Environ Epidemiol. 29(2):206–217, PMID: 30622332, 10.1038/s41370-018-0109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, et al. 2011. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag 7(4):513–541, PMID: 21793199, 10.1002/ieam.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley JP, Doherty BT, Keil AP, Engel SM. 2017. Statistical approaches for estimating sex-specific effects in endocrine disruptors research. Environ Health Perspect 125(6):067013, PMID: 28665274, 10.1289/EHP334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkemper JL, Aweda TA, Rosenberg AJ, Lunderberg DM, Peaslee GF, Lapi SE. 2017. Radiosynthesis and biological distribution of 18F-labeled perfluorinated alkyl substances. Environ Sci Technol Lett 4(6):211–215. [Google Scholar]
- Butenhoff JL, Kennedy GL Jr, Chang SC, Olsen GW. 2012. Chronic dietary toxicity and carcinogenicity study with ammonium perfluorooctanoate in Sprague-Dawley rats. Toxicology 298(1–3):1–13, PMID: 22531602, 10.1016/j.tox.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, Needham LL. 2007. Polyfluoroalkyl chemicals in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000. Environ Health Perspect 115(11):1596–1602, PMID: 18007991, 10.1289/ehp.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention). 2009. National Health and Nutrition Examination (NHANES) MEC Interviewers Procedures Manual. Atlanta, GA: CDC; https://www.cdc.gov/nchs/data/nhanes/nhanes_09_10/mecinterviewers.pdf [accessed September 30, 2017]. [Google Scholar]
- CDC. 2014. Laboratory Procedure Manual, Perfluoroalkyl and Polyfluoroalkyl Substances, NHANES 2013–2014. Atlanta, GA: CDC; https://wwwn.cdc.gov/nchs/data/nhanes/2013-2014/labmethods/PFAS_H_MET.pdf [accessed September 30, 2017]. [Google Scholar]
- CDC. 2015. National Health and Nutrition Examination Survey, 2013–2014 Data Documentation, Codebook, and Frequencies. https://wwwn.cdc.gov/nchs/nhanes/2013-2014/demo_h.htm [accessed 25 August 2019].
- Christensen KY, Raymond M, Blackowicz M, Liu Y, Thompson BA, Anderson HA, et al. 2017. Perfluoroalkyl substances and fish consumption. Environ Res 154:145–151, PMID: 28073048, 10.1016/j.envres.2016.12.032. [DOI] [PubMed] [Google Scholar]
- D’eon JC, Mabury SA. 2007. Production of perfluorinated carboxylic acids (PFCAs) from the biotransformation of polyfluoroalkyl phosphate surfactants (PAPS): exploring routes of human contamination. Environ Sci Technol 41(13):4799–4805, PMID: 17695932, 10.1021/es070126x. [DOI] [PubMed] [Google Scholar]
- D’eon JC, Mabury SA. 2011. Is indirect exposure a significant contributor to the burden of perfluorinated acids observed in humans? Environ Sci Technol 45(19):7974–7984, PMID: 21630688, 10.1021/es200171y. [DOI] [PubMed] [Google Scholar]
- Danish Ministry of the Environment. 2015. Short-Chain Polyfluoroalkyl Substances (PFAS): A Literature Review of Information on Human Health Effects and Environmental Fate and Effect Aspects of Short-Chain PFAS. Environmental Project No. 1707. https://www2.mst.dk/Udgiv/publications/2015/05/978-87-93352-15-5.pdf [accessed 1 December 2017].
- Fasano WJ, Carpenter SC, Gannon SA, Snow TA, Stadler JC, Kennedy GL, et al. 2006. Absorption, distribution, metabolism, and elimination of 8-2 fluorotelomer alcohol in the rat. Toxicol Sci 91(2):341–355, PMID: 16543293, 10.1093/toxsci/kfj160. [DOI] [PubMed] [Google Scholar]
- Gebbink WA, Ullah S, Sandblom O, Berger U. 2013. Polyfluoroalkyl phosphate esters and perfluoroalkyl carboxylic acids in target food samples and packaging—method development and screening. Environ Sci Pollut Res Int 20(11):7949–7958, PMID: 23494682, 10.1007/s11356-013-1596-y. [DOI] [PubMed] [Google Scholar]
- Glynn A, Berger U, Bignert A, Ullah S, Aune M, Lignell S, et al. 2012. Perfluorinated alkyl acids in blood serum from primiparous women in Sweden: serial sampling during pregnancy and nursing, and temporal trends 1996–2010. Environ Sci Technol 46(16):9071–9079, PMID: 22770559, 10.1021/es301168c. [DOI] [PubMed] [Google Scholar]
- Gomis MI, Vestergren R, Borg D, Cousins IT. 2018. Comparing the toxic potency in vivo of long-chain perfluoroalkyl acids and fluorinated alternatives. Environ Int 113:1–9, PMID: 29421396, 10.1016/j.envint.2018.01.011. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Andersen EW, Budtz-Jørgensen E, Nielsen F, Mølbak K, Weihe P, et al. 2012. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA 307(4):391–397, PMID: 22274686, 10.1001/jama.2011.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halldorsson TI, Fei C, Olsen J, Lipworth L, McLaughlin JK, Olsen SF. 2008. Dietary predictors of perfluorinated chemicals: a study from the Danish National Birth Cohort. Environ Sci Technol 42(23):8971–8977, PMID: 19192827, 10.1021/es801907r. [DOI] [PubMed] [Google Scholar]
- Haug LS, Thomsen C, Becher G. 2009. Time trends and the influence of age and gender on serum concentrations of perfluorinated compounds in archived human samples. Environ Sci Technol 43(6):2131–2136, PMID: 19368225, 10.1021/es802827u. [DOI] [PubMed] [Google Scholar]
- Hines EP, White SS, Stanko JP, Gibbs-Flournoy EA, Lau C, Fenton SE. 2009. Phenotypic dichotomy following developmental exposure to perfluorooctanoic acid (PFOA) in female CD-1 mice: low doses induce elevated serum leptin and insulin, and overweight in mid-life. Mol Cell Endocrinol 304(1–2):97–105, PMID: 19433254, 10.1016/j.mce.2009.02.021. [DOI] [PubMed] [Google Scholar]
- Johnson CL, Paulose-Ram R, Ogden CL, Carroll MD, Kruszon-Moran D, Dohrmann SM, et al. 2013. National Health and Nutrition Examination Survey: analytic guidelines, 1999–2010. Vital Health Stat 2(161):1–24, PMID: 25090154. [PubMed] [Google Scholar]
- Kelly BC, Ikonomou MG, Blair JD, Surridge B, Hoover D, Grace R, et al. 2009. Perfluoroalkyl contaminants in an Arctic marine food web: trophic magnification and wildlife exposure. Environ Sci Technol 43(11):4037–4043, PMID: 19569327, 10.1021/es9003894. [DOI] [PubMed] [Google Scholar]
- Lee H, Mabury SA. 2011. A pilot survey of legacy and current commercial fluorinated chemicals in human sera from United States donors in 2009. Environ Sci Technol 45(19):8067–8074, PMID: 21486041, 10.1021/es200167q. [DOI] [PubMed] [Google Scholar]
- Lindstrom AB, Strynar MJ, Libelo EL. 2011. Polyfluorinated compounds: past, present, and future. Environ Sci Technol 45(19):7954–7961, PMID: 21866930, 10.1021/es2011622. [DOI] [PubMed] [Google Scholar]
- Liu ZY, Lu YL, Wang P, Wang TY, Liu SJ, Johnson AC, et al. 2017. Pollution pathways and release estimation of perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) in central and eastern China. Sci Total Environ 580:1247–1256, PMID: 28040212, 10.1016/j.scitotenv.2016.12.085. [DOI] [PubMed] [Google Scholar]
- Lopez-Espinosa M-J, Mondal D, Armstrong B, Bloom MS, Fletcher T. 2012. Thyroid function and perfluoroalkyl acids in children living near a chemical plant. Environ Health Perspect 120(7):1036–1041, PMID: 22453676, 10.1289/ehp.1104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley T. 2004. Analysis of complex survey samples. J Stat Softw 9(8):1–19, 10.18637/jss.v009.i08. [DOI] [Google Scholar]
- Manzano-Salgado CB, Casas M, Lopez-Espinosa MJ, Ballester F, Martinez D, Ibarluzea J, et al. 2016. Variability of perfluoroalkyl substance concentrations in pregnant women by socio-demographic and dietary factors in a Spanish birth cohort. Environ Int 92–93:357–365, PMID: 27132161, 10.1016/j.envint.2016.04.004. [DOI] [PubMed] [Google Scholar]
- Martinez-Moral MP, Tena MT. 2012. Determination of perfluorocompounds in popcorn packaging by pressurised liquid extraction and ultra-performance liquid chromatography–tandem mass spectrometry. Talanta 101:104–109, PMID: 23158298, 10.1016/j.talanta.2012.09.007. [DOI] [PubMed] [Google Scholar]
- Moreta C, Tena MT. 2014. Determination of perfluorinated alkyl acids in corn, popcorn and popcorn bags before and after cooking by focused ultrasound solid–liquid extraction, liquid chromatography and quadrupole-time of flight mass spectrometry. J Chromatogr A 1355:211–218, PMID: 24986069, 10.1016/j.chroma.2014.06.018. [DOI] [PubMed] [Google Scholar]
- OECD (Organization for Economic Cooperation and Development). 2018. Toward a New Comprehensive Global Database of Per- and Polyfluoroalkyl Substances (PFASs): Summary Report on Updating the OECD 2007 List of Per- and Polyfluoroalkyl Substances (PFASs). ENV/JM/MONO(2018)7. Paris, France: OECD Environment Directorate, Environment, Health and Safety Division; http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV-JM-MONO(2018)7&doclanguage=en [accessed 25 August 2019]. [Google Scholar]
- Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, et al. 2007. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect 115(9):1298–1305, PMID: 17805419, 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW, Chang SC, Noker PE, Gorman GS, Ehresman DJ, Lieder PH, et al. 2009. A comparison of the pharmacokinetics of perfluorobutanesulfonate (PFBS) in rats, monkeys, and humans. Toxicology 256(1–2):65–74, PMID: 19059455, 10.1016/j.tox.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Mair DC, Lange CC, Harrington LM, Church TR, Goldberg CL, et al. 2017. Per- and polyfluoroalkyl substances (PFAS) in American Red Cross adult blood donors, 2000–2015. Environ Res 157:87–95, PMID: 28528142, 10.1016/j.envres.2017.05.013. [DOI] [PubMed] [Google Scholar]
- Pérez F, Nadal M, Navarro-Ortega A, Fàbrega F, Domingo JL, Barceló D, et al. 2013. Accumulation of perfluoroalkyl substances in human tissues. Environ Int 59:354–362, PMID: 23892228, 10.1016/j.envint.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Poothong S, Boontanon SK, Boontanon N. 2012. Determination of perfluorooctane sulfonate and perfluorooctanoic acid in food packaging using liquid chromatography coupled with tandem mass spectrometry. J Hazard Mater 205–206:139–143, PMID: 22265653, 10.1016/j.jhazmat.2011.12.050. [DOI] [PubMed] [Google Scholar]
- Poothong S, Thomsen C, Padilla-Sanchez JA, Papadopoulou E, Haug LS. 2017. Distribution of novel and well-known poly-and perfluoroalkyl substances (PFASs) in human serum, plasma, and whole blood. Environ Sci Technol 51(22):13388–13396, PMID: 29056041, 10.1021/acs.est.7b03299. [DOI] [PubMed] [Google Scholar]
- Posner S, Roos S, Poulsen P, Jörundsdottir H, Gunnlaugsdóttir H, Trier X, et al. 2013. Per- and Polyfluorinated Substances in the Nordic Countries: Use, Occurence and Toxicology. Copenhagen, Denmark: Nordic Council of Ministers; https://www.diva-portal.org/smash/get/diva2:1128873/FULLTEXT01.pdf [accessed September 30, 2017]. [Google Scholar]
- Rosenmai AK, Taxvig C, Svingen T, Trier X, van Vugt-Lussenburg BMA, Pedersen M, et al. 2016. Fluorinated alkyl substances and technical mixtures used in food paper-packaging exhibit endocrine-related activity in vitro. Andrology 4(4):662–672, PMID: 27152447, 10.1111/andr.12190. [DOI] [PubMed] [Google Scholar]
- Rushing BR, Hu Q, Franklin JN, McMahen RL, Dagnino S, Higgins CP, et al. 2017. Evaluation of the immunomodulatory effects of 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)-propanoate in C57BL/6 mice. Toxicol Sci 156(1):179–189, PMID: 28115649, 10.1093/toxsci/kfw251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell MH, Nilsson H, Buck RC. 2013. Elimination kinetics of perfluorohexanoic acid in humans and comparison with mouse, rat and monkey. Chemosphere 93(10):2419–2425, PMID: 24050716, 10.1016/j.chemosphere.2013.08.060. [DOI] [PubMed] [Google Scholar]
- Rylander C, Sandanger TM, Frøyland L, Lund E. 2010. Dietary patterns and plasma concentrations of perfluorinated compounds in 315 Norwegian women: the NOWAC Postgenome Study. Environ Sci Technol 44(13):5225–5232, PMID: 20527765, 10.1021/es100224q. [DOI] [PubMed] [Google Scholar]
- Schaider LA, Balan SA, Blum A, Andrews DQ, Strynar MJ, Dickinson ME, et al. 2017. Fluorinated compounds in U.S. fast food packaging. Environ Sci Technol Lett 4(3):105–111, PMID: 30148183, 10.1021/acs.estlett.6b00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoeib T, Hassan Y, Rauert C, Harner T. 2016. Poly- and perfluoroalkyl substances (PFASs) in indoor dust and food packaging materials in Egypt: trends in developed and developing countries. Chemosphere 144:1573–1581, PMID: 26517384, 10.1016/j.chemosphere.2015.08.066. [DOI] [PubMed] [Google Scholar]
- Sinclair E, Kim SK, Akinleye HB, Kannan K. 2007. Quantitation of gas-phase perfluoroalkyl surfactants and fluorotelomer alcohols released from nonstick cookware and microwave popcorn bags. Environ Sci Technol 41(4):1180–1185, PMID: 17593716, 10.1021/es062377w. [DOI] [PubMed] [Google Scholar]
- Tittlemier SA, Pepper K, Seymour C, Moisey J, Bronson R, Cao XL, et al. 2007. Dietary exposure of Canadians to perfluorinated carboxylates and perfluorooctane sulfonate via consumption of meat, fish, fast foods, and food items prepared in their packaging. J Agric Food Chem 55(8):3203–3210, PMID: 17381114, 10.1021/jf0634045. [DOI] [PubMed] [Google Scholar]
- Trier X, Granby K, Christensen JH. 2011. Polyfluorinated surfactants (PFS) in paper and board coatings for food packaging. Environ Sci Pollut Res Int 18(7):1108–1120, PMID: 21327544, 10.1007/s11356-010-0439-3. [DOI] [PubMed] [Google Scholar]
- Trudel D, Horowitz L, Wormuth M, Scheringer M, Cousins IT, Hungerbühler K. 2008. Estimating consumer exposure to PFOS and PFOA. Risk Anal 28(2):251–269, PMID: 18419647, 10.1111/j.1539-6924.2008.01017.x. [DOI] [PubMed] [Google Scholar]
- U.S. EPA (Environmental Protection Agency). 2000. EPA and 3M announce phase out of PFOS. https://archive.epa.gov/epapages/newsroom_archive/newsreleases/33aa946e6cb11f35852568e1005246b4.html [accessed 11 October 2017].
- U.S. EPA. 2009. Long-chain perfluorinated chemicals (PFCs) action plan. https://www.epa.gov/sites/production/files/2016-01/documents/pfcs_action_plan1230_09.pdf [accessed 25 August 2019].
- U.S. EPA. 2017. PFOA Stewardship Program Baseline Year Summary Report. https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/pfoa-stewardship-program-baseline-year-summary-report [accessed 11/28/2017].
- U.S. EPA. 2019. Risk management for per- and polyfluoroalkyl substances (PFASs) under TSCA: access PFAS dockets. https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/risk-management-and-polyfluoroalkyl-substances-pfass [accessed April 22, 2019].
- Vestergren R, Cousins IT. 2009. Tracking the pathways of human exposure to perfluorocarboxylates. Environ Sci Technol 43(15):5565–5575, PMID: 19731646, 10.1021/es900228k. [DOI] [PubMed] [Google Scholar]
- Wang Z, Cousins IT, Scheringer M, Hungerbühler K. 2013. Fluorinated alternatives to long-chain perfluoroalkyl carboxylic acids (PFCAs), perfluoroalkane sulfonic acids (PFSAs) and their potential precursors. Environ Int 60:242–248, PMID: 24660230, 10.1016/j.envint.2013.08.021. [DOI] [PubMed] [Google Scholar]
- Wang ZY, DeWitt JC, Higgins CP, Cousins IT. 2017. A never-ending story of per- and polyfluoroalkyl substances (PFASs)? Environ Sci Technol 51(5):2508–2518, PMID: 28224793, 10.1021/acs.est.6b04806. [DOI] [PubMed] [Google Scholar]
- Weiss J, de Boer J, Berger U, Muir D, Ruan T, Torre A, et al. 2015. PFAS Analysis in Water for the Global Monitoring Plan of the Stockholm Convention: Set-Up and Guidelines for Monitoring. Geneva, Switzerland: United Nations Environment Programme, Chemicals Branch; https://www.unitar.org/cwm/sites/unitar.org.cwm/files/uploads/guide_pfas_water_unep_2015.pdf [accessed 25 August 2019]. [Google Scholar]
- Willett W. 1998. Nutritional Epidemiology. 2nd ed Cary, NC: Oxford University Press. [Google Scholar]
- Wolf CJ, Fenton SE, Schmid JE, Calafat AM, Kuklenyik Z, Bryant XA, et al. 2007. Developmental toxicity of perfluorooctanoic acid in the CD-1 mouse after cross-foster and restricted gestational exposures. Toxicol Sci 95(2):462–473, PMID: 17098816, 10.1093/toxsci/kfl159. [DOI] [PubMed] [Google Scholar]
- Wu XM, Bennett DH, Calafat AM, Kato K, Strynar M, Andersen E, et al. 2015. Serum concentrations of perfluorinated compounds (PFC) among selected populations of children and adults in California. Environ Res 136:264–273, PMID: 25460645, 10.1016/j.envres.2014.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Kato K, Wong LY, Jia T, Kalathil A, Latremouille J, et al. 2018. Per- and polyfluoroalkyl substances in sera from children 3 to 11 years of age participating in the National Health and Nutrition Examination Survey 2013–2014. Int J Hyg Environ Health 221(1):9–16, PMID: 28993126, 10.1016/j.ijheh.2017.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung LW, Miyake Y, Taniyasu S, Wang Y, Yu H, So MK, et al. 2008. Perfluorinated compounds and total and extractable organic fluorine in human blood samples from China. Environ Sci Technol 42(21):8140–8145, PMID: 19031915, 10.1021/es800631n. [DOI] [PubMed] [Google Scholar]
- Yuan G, Peng H, Huang C, Hu J. 2016. Ubiquitous occurrence of fluorotelomer alcohols in eco-friendly paper-made food-contact materials and their implication for human exposure. Environ Sci Technol 50(2):942–950, PMID: 26655429, 10.1021/acs.est.5b03806. [DOI] [PubMed] [Google Scholar]
- Zabaleta I, Bizkarguenaga E, Bilbao D, Etxebarria N, Prieto A, Zuloaga O. 2016. Fast and simple determination of perfluorinated compounds and their potential precursors in different packaging materials. Talanta 152:353–363, PMID: 26992531, 10.1016/j.talanta.2016.02.022. [DOI] [PubMed] [Google Scholar]
- Zabaleta I, Negreira N, Bizkarguenaga E, Prieto A, Covaci A, Zuloaga O. 2017. Screening and identification of per-and polyfluoroalkyl substances in microwave popcorn bags. Food Chem 230:497–506, PMID: 28407941, 10.1016/j.foodchem.2017.03.074. [DOI] [PubMed] [Google Scholar]
- Zafeiraki E, Costopoulou D, Vassiliadou I, Bakeas E, Leondiadis L. 2014. Determination of perfluorinated compounds (PFCs) in various foodstuff packaging materials used in the Greek market. Chemosphere 94:169–176, PMID: 24367824, 10.1016/j.chemosphere.2013.09.092. [DOI] [PubMed] [Google Scholar]
- Zong G, Valvi D, Coull B, Göen T, Hu FB, Nielsen F, et al. 2018. Persistent organic pollutants and risk of type 2 diabetes: a prospective investigation among middle-aged women in Nurses’ Health Study II. Environ Int 114:334–342, PMID: 29477570, 10.1016/j.envint.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zota AR, Phillips CA, Mitro SD. 2016. Recent fast food consumption and bisphenol A and phthalates exposures among the U.S. population in NHANES, 2003–2010. Environ Health Perspect 124(10):1521–1528, PMID: 27072648, 10.1289/ehp.1510803. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.