Abstract
Background:
The question of whether exposure to bisphenol A (BPA) contributes to the development of type 2 diabetes is still unresolved. Most epidemiological evidence on the association between BPA and diabetes is from cross-sectional studies or longitudinal studies with single urinary measurements. No prospective study has examined exposure to BPA analogs such as bisphenol S (BPS) in relation to incident type 2 diabetes.
Objectives:
We aimed to investigate whether exposure to BPA and BPS, assessed at up to two time points, was associated with the incidence of type 2 diabetes.
Methods:
We performed a case–cohort study on 755 participants without diabetes at baseline and followed-up over 9 y as part of the French prospective cohort Data from an Epidemiological Study on the Insulin Resistance Syndrome (D.E.S.I.R.). BPA-glucuronide (BPA-G) and BPS-glucuronide (BPS-G) were assessed in fasting spot urine samples collected during the health examinations at baseline and 3 y later. Associations with incident diabetes were examined using Prentice-weighted Cox regression models adjusted for potential confounders.
Results:
A total of 201 incident cases of type 2 diabetes were diagnosed over the follow-up, including 30 in the subcohort. Compared with participants with the lowest average BPA exposure (below the first quartile), participants in the second, third, and fourth quartile groups of exposure had a near doubling of the risk of type 2 diabetes, with a hazard ratio 2.56 (95% CI: 1.16, 5.65), 2.35 (95% CI: 1.07, 5.15), and 1.56 (95% CI: 0.68, 3.55), respectively. The detection of BPS-G in urine at one or both time points was associated with incident diabetes, with an 2.81 (95% CI: 1.74, 4.53).
Discussion:
This study shows positive associations between exposure to BPA and BPS and the incidence of type 2 diabetes, independent of traditional diabetes risk factors. Our results should be confirmed by recent, population-based observational studies in different populations and settings. Overall, these findings raise concerns about using BPS as a BPA substitute. Further research on BPA analogs is warranted. https://doi.org/10.1289/EHP5159
Introduction
The prevalence of type 2 diabetes has been rising steadily around the world (Chatterjee et al. 2017). Apart from traditional diabetes risk factors, a recent line of research has focused on the role of environmental toxicants and suggests that exposure to endocrine-disrupting chemicals may play a role in the incidence of diabetes (Neel and Sargis 2011). In particular, bisphenol A (BPA) has been suspected as a potential contributor to the etiology of metabolic diseases such as type 2 diabetes (Rancière et al. 2015). BPA is a chemical commonly used in the production of polycarbonate plastic and epoxy resins and is found in numerous consumer products such as food/beverage containers and thermal cash register receipts. BPA is structurally similar to the natural estrogen and, consequently, a known xenoestrogen. Experimental data from in vitro and in vivo studies have suggested plausible mechanisms implicating BPA in the development of type 2 diabetes through insulin resistance, pancreatic beta-cell dysfunction, adipogenesis, inflammation, oxidative stress, dysregulation of glucose metabolism, and disruption of thyroid hormone balance (Alonso-Magdalena et al. 2011).
The growing concern over BPA and its use restriction in some countries have prompted the replacement of BPA with alternative compounds, including BPA structural analogs such as bisphenol S (BPS), used in some BPA-free products such as thermal receipts and epoxy resins and in the coatings of food and drink cans (Chen et al. 2016). A study conducted in a convenience population sample in the United States between 2000 and 2014 suggested that exposure to BPS was increasing while exposure to BPA was declining, even though BPS concentrations measured in urine were still lower than those of BPA (Ye et al. 2015). Recently, a systematic review concluded that BPS has endocrine-disrupting effects similar to those of BPA (Rochester and Bolden 2015).
Evidence from cross-sectional epidemiological studies suggests that individuals with diabetes are more likely to have higher urinary bisphenols concentrations (Duan et al. 2018; Rancière et al. 2015). However, whether exposure to bisphenols is a risk factor for developing type 2 diabetes is still unclear due to the absence (for BPS) or lack (for BPA) of prospective data to ascertain the nature of the relationship. Existing longitudinal studies on BPA and diabetes reported no significant association (Shu et al. 2018) or they reported significant positive associations only in specific subgroups based on sex and age (Sun et al. 2014) or on a diabetes genetic risk score (Bi et al. 2016). Other limitations in the existing literature include self-reported diabetes diagnosis, lack of adjustment for important confounders such as dietary intake, as well as a single urinary BPA measurement per participant, which is a concern due to the short half-life of BPA in humans (Rancière et al. 2015).
The aim of the present study was to investigate the prospective association between repeated measures of urinary BPA and BPS and the risk of developing type 2 diabetes over 9 y in the French cohort study Data from an Epidemiological Study on the Insulin Resistance Syndrome (D.E.S.I.R.).
Methods
Source Population
D.E.S.I.R. is a prospective, population-based cohort study that aims to clarify the development of the insulin resistance syndrome and type 2 diabetes (Balkau et al. 2008). Between 1994 and 1996, this study included 5,212 men and women 30–65 years of age, enrolled from volunteers offered free periodic health examinations by the French Health Insurance System in 10 health examination centers in central-western France. Participants were clinically and biologically evaluated at inclusion and then at 3-, 6-, and 9-y visits. At each visit, urine and blood samples were collected in a standardized way, the morning after participants had fasted for at least 12 h. Detailed information about the follow-up has been previously published (Balkau et al. 2008). The study protocol was approved by French ethics committees [Comité Consultatif de Protection des Personnes dans la Recherche Biomédicale (CCPPRB) de Bicêtre, Hôpital de Bicêtre, Paris, France] and written informed consent was obtained from all participants.
Study Population
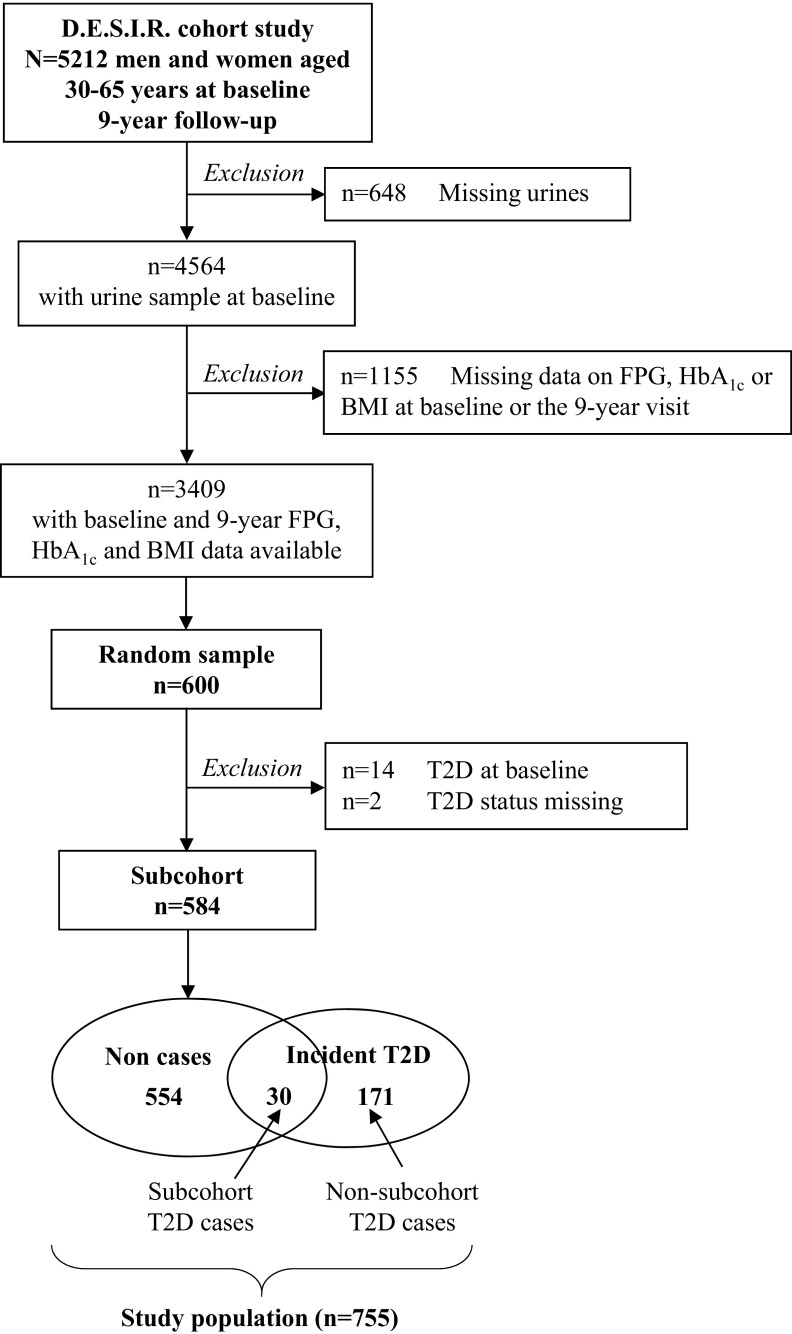
We conducted a case–cohort study nested in the D.E.S.I.R. cohort. All participants with a baseline urine sample and blood glucose and body mass index (BMI) data at baseline and 9-y visits were eligible for this study (). We randomly selected 600 participants from all the eligible participants and which we refer to as the subcohort. This group corresponds to a subcohort sampling fraction of 11.5%, which is in line with other case–cohort studies (Sharp et al. 2014). Incident diabetes was defined by treatment with glucose-lowering agents or a fasting plasma glucose of or glycated hemoglobin ( at any of the three 3-yearly health examinations after inclusion. After excluding participants with prevalent diabetes or uncertain status at baseline, the final study population included 584 subcohort members and 201 incident cases of diabetes, 30 of whom were in the subcohort. Among the 201 incident cases, 82 were diagnosed with diabetes between baseline and year 3, 50 between year 3 and year 6, and 69 between year 6 and year 9. In total, 47 participants were diagnosed with type 2 diabetes based on treatment, and 154 were diagnosed based on fasting plasma glucose or testing. The selection of participants is described in detail in Figure 1.
Figure 1.
Flow chart of the case–cohort study nested in the French prospective cohort study D.E.S.I.R. BMI, body mass index; D.E.S.I.R., Data from an Epidemiological Study on the Insulin Resistance Syndrome; FPG, fasting plasma glucose; , glycated hemoglobin; T2D, type 2 diabetes.
Assessment of Urinary BPA-Glucuronide and BPS-Glucuronide
We measured BPA-glucuronide (BPA-G) and BPS-glucuronide (BPS-G), major BPA and BPS urinary metabolites, in spot urine samples obtained at baseline and at the second (3-y) study examination [median 3.0 y after baseline, interquartile range (IQR): 2.8, 3.1 y]. All samples were stored at . BPA and BPS exposure can be assayed from stored frozen urine (Nepomnaschy et al. 2009). Samples () were analyzed as dansyl chloride derivatives using labeled BPA-G and BPS-G as internal standards. Liquid chromatography–mass spectrometry analyses were carried out on an Acquity U-HPLC device [Waters Cortecs C18 U-HPLC column (; ) with an acidified water/acetonitrile gradient elution (, 40°C)] coupled to a Xevo-TQ mass spectrometer (Waters) using positive electrospray ionization and multiple reaction monitoring (MRM) mode. The method was validated according to guidelines of the FDA (2013), from 0.5 to , using a linear model weighted by (). The limits of detection (LOD) for both BPA-G and BPS-G were validated at .
Within- and between-day precisions were calculated on six replicates of quality control points (QC) at three concentration levels (1.5, 7.5, and ) on 3 d. Within- and between-day precisions [expressed by coefficient of variation (CV)] were lower than 6% and 14% for BPA-G and 16% and 17% for BPS-G, respectively. The accuracy varied from 92% to 101% for BPA-G and from 85% to 91% for BPS-G. Recoveries, evaluated with three human urine samples spiked with BPA-G and BPS-G at 1.5 and , were 88% and 99% for BPA-G and BPS-G, respectively. The limits of quantification (LOQs) were validated at with a precision less than 11% and 13% and accuracy of 94% and 105% for BPA-G and BPS-G, respectively. Stability of the dansyl chloride labeling was evaluated with the spiked human urine samples over 24 h. Labeled bisphenol-glucuronides were stable over 12 h with a variation between nominal and observed concentration less than 14%.
Health Outcomes and Covariates
Blood samples were analyzed for metabolic markers including glucose and , and urine samples were analyzed for creatinine, using techniques previously described (Balkau et al. 2008). Weight and height were measured in lightly clad participants, by trained personnel following a standardized protocol. Blood pressure was measured in duplicate using a mercury sphygmomanometer with the participant in a supine position, and mean values were used for analyses. Information on familial and personal history of diabetes and treatment for diabetes and hypertension was collected during an interview with a physician. A self-administered questionnaire provided data on education level, employment, smoking status, diet, and degree of physical activity. Participants were asked if they currently smoked or have been regular smokers in their lifetime and were categorized as nonsmokers, former smokers, or current smokers. Physical activity was categorized as sedentary, moderately active, or active based on the frequency of participation in active sporting activities (never, time a week, 1–2 times a week, times a week) and the intensity of usual physical activity at home and at work (low, moderate, important, intensive). The dietary intake (in kilocalories per day) was calculated using the New Alimentary Self Questionnaire (NAQA) food consumption questionnaire (Lasfargues et al. 1990) describing usual food consumption, based on the composition of breakfast and the frequency of consumption of meat, fish, fried foods, butter, cheese, dairy products, bread, sugary desserts, sugar, soft drinks, and alcoholic beverages (wine, beer, cider, aperitif, digestive).
Statistical Analysis
At both time points of exposure assessment, urinary BPA-G concentrations below the LOD were assigned the value of LOD divided by 2. BPA-G concentrations were categorized into four groups based on the 25th, 50th, and 75th percentiles of the distribution of pooled BPA-G concentrations at both time points in subcohort members. Due to a low detection rate of BPS-G, exposure to BPS was studied as a binary variable, as either detectable (BPS-G concentration ) or not.
As a way to reduce misclassification bias, we summarized exposure of participants by deriving BPA exposure and BPS detection from data from the two spot urine samples. BPA average exposure was defined as the mean of urinary BPA-G concentrations measured at baseline and year 3; BPS detection was defined as a binary variable, as either BPS-G detected at baseline and/or year 3 or BPS-G detected at none of the visits.
Within-person repeatability of BPA-G concentrations in spot urine samples collected 3 y apart was assessed using Spearman’s rank correlation coefficient () and creatinine-adjusted intraclass correlation coefficients (ICCs) estimated using one-way random-effect models, with BPA-G concentrations divided by the urinary creatinine concentration in the same sample to account for urine dilution. McNemar’s test and Cohen’s kappa () assessed the within-person stability of BPS detection categories over the two time points. At both time points, associations between detection of BPA-G and BPS-G were assessed using the chi-square test and Cohen’s kappa ().
First, we investigated the association between BPA and BPS exposure at baseline and diabetes incidence between baseline and year 9, after excluding prevalent cases at baseline. Second, we investigated the association between BPA and BPS exposure at year 3 and diabetes incidence between year 3 and year 9, after excluding prevalent cases at baseline and year 3 (see Figure S1). Last, we investigated BPA average exposure and BPS detection at least at one of the time points in relation with diabetes incidence between year 3 and year 9. We performed single-exposure models as well as models including both BPA and BPS exposures.
Multivariable Cox regression models adapted to the case–cohort design using the Prentice method (Barlow et al. 1999) were used to estimate adjusted hazard ratios (HRs) of incident diabetes with their 95% confidence intervals (CIs). Individual observations were censored on the approximate date of diagnosis for cases, defined as the midpoint between the last visit without diabetes and the first visit with diabetes.
The proportional-hazards assumption was checked using Schoenfeld residuals. We used Cox models stratified by smoking status (never, former, current) because this variable did not satisfy the proportional-hazards assumption (). Multivariable models with age as the timescale were adjusted for sex and the following variables from baseline (when examining diabetes incidence between baseline and year 9) or from year-3 (when examining diabetes incidence between year 3 and year 9): education level (, of education), employment (no, yes), marital status defined as married or living with a partner (no, yes), physical activity (sedentary, moderately active, active), caloric intake (in tertiles), family history of diabetes (no, yes), hypertension defined as systolic blood pressure or diastolic blood pressure or ongoing blood pressure-lowering treatment (no, yes), and BMI (continuous). All multivariable models were also adjusted for urinary creatinine levels, measured at the same time point as exposure assessment to account for urine dilution. Models estimating associations with average BPA concentration and BPS detection at least at one of the time points were adjusted for average urine creatinine. We included exposure variables (unadjusted for creatinine) in the analysis, with urinary creatinine concentration added as a separate independent variable so that urinary bisphenol concentration was appropriately adjusted for urinary creatinine and the statistical significance of other variables in the model was independent of the effects of creatinine concentration (Barr et al. 2005). Effect–measure modification by sex was investigated by testing multiplicative interaction terms. For BPA, we modeled three product interaction terms for the four categories of exposure, and the interaction p-values were derived using Wald tests.
In addition to the analyses described previously, and in order to better understand the shape of the relationship observed between groups of BPA exposure at year 3 and incidence of type 2 diabetes, we modeled the relationship between log-transformed BPA-G and the risk of type 2 diabetes using restricted cubic splines analysis after excluding participants with the highest 5% of BPA-G levels to minimize the potential impact of outliers. We performed the spline analysis with three knots placed at the 10th, 50th, and 90th percentiles of the log-transformed BPA-G concentrations distribution. The multivariable Cox regression model was adjusted for the same covariates as previously described. A test for nonlinearity was conducted by testing that the regression coefficient of the second spline variable equaled 0.
The analyses were performed in Stata/SE (version 13.1; StataCorp), using the STCASCOH procedure to build the data set suitable for case–cohort analysis and the MKSPLINE and XLBC procedures for the restricted cubic spline analysis. We followed the recommendations for reporting case–cohort studies as given by Sharp et al. (2014).
Results
Study Population
Baseline characteristics of subcohort members and incident diabetes cases are summarized in Table 1. The median age at inclusion was 47 (IQR: 39, 57) y in the subcohort, and 53% were women. Sociodemographic characteristics (sex, age, education level) of individuals from the subcohort did not substantially differ from those of other participants included in the D.E.S.I.R. cohort (see Table S1). The median age when diabetes was ascertained was 58 (IQR: 49, 65) y. The incidence rate of diabetes was 5.88 (95% CI: 4.11, 8.41) per 1,000 person-years in the subcohort (total number of ).
Table 1.
Baseline characteristics of participants in the case–cohort study nested in the D.E.S.I.R. cohort.
| Baseline characteristic | Subcohort | All incident type 2 diabetes cases |
|---|---|---|
| () | () | |
| Men | 273 (46.7) | 129 (64.2) |
| Age (y) | 47 (39, 57) | 53 (45, 60) |
| Education level (y) | ||
| 438 (75.6) | 158 (79.0) | |
| 141 (24.4) | 42 (21.0) | |
| Missing | 5 | 1 |
| Marital status | ||
| Married or living with a partner | 480 (82.3) | 160 (79.6) |
| Single, divorced, or widowed | 103 (17.7) | 41 (20.4) |
| Missing | 1 | 0 |
| Employment | ||
| No | 160 (27.4) | 87 (43.3) |
| Yes | 423 (72.6) | 114 (56.7) |
| Missing | 1 | 0 |
| Smoking status | ||
| Never | 313 (53.7) | 82 (40.8) |
| Former | 169 (29.0) | 61 (30.3) |
| Current | 101 (17.3) | 58 (28.9) |
| Missing | 1 | 0 |
| Physical activity | ||
| Sedentary | 135 (23.2) | 73 (36.3) |
| Moderately active | 312 (53.5) | 99 (49.3) |
| Active | 136 (23.3) | 29 (14.4) |
| Missing | 1 | 0 |
| Dietary intake (kcal/d) | 1,987 (1,685, 2,503) | 2,242 (1,754, 2,612) |
| Family history of diabetes | 94 (16.1) | 51 (25.4) |
| Hypertensiona | 200 (34.2) | 122 (60.7) |
| Body mass index () | ||
| 357 (61.1) | 57 (28.4) | |
| 25–29 | 188 (32.2) | 85 (42.3) |
| 39 (6.7) | 59 (29.3) | |
| Fasting blood glucose (mmol/L) | 5.25 (4.88, 5.63) | 5.92 (5.52, 6.37) |
Note: Data are (%) for categorical variables and median (P25, P75) for continuous variables. Percentages are based on nonmissing data. D.E.S.I.R., Data from an Epidemiological Study on the Insulin Resistance Syndrome; P, percentile.
Systolic blood pressure or diastolic blood pressure or ongoing blood pressure-lowering treatment.
Urinary BPA-G and BPS-G Levels
All of the 755 participants included in the study had at least one BPA-G estimate, and 723 (96%) had two estimates. Nearly all (98%) of the 755 participants had at least one BPS-G estimate, and 606 (80%) had two estimates.
In the subcohort, the detection rate and the distribution of urinary BPA-G and BPS-G concentrations at baseline and year 3 are shown in Table 2. Among the 562 subcohort members with BPA data at both time points, BPA-G was detected in 403 (72%) at both visits, in 146 (26%) at only one visit, and BPA-G was not detected at either visit in 13 (2%). Within-person variability of BPA-G was high relative to total variability (ICC for log-transformed creatinine-adjusted BPA-G concentrations between the two ) and rankings of concentrations between the two visits were weakly correlated ().
Table 2.
Urinary BPA-G, BPS-G, and creatinine concentrations at baseline and year 3 in the subcohort of the D.E.S.I.R. nested case–cohort study.
| Characteristic | BPA-G (ng/mL) | BPS-G (ng/mL) | Creatinine (mg/L) | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Year 3 | Pooled baseline-year 3 | Baseline | Year 3 | Pooled baseline-year 3 | Baseline | Year 3 | |
| Samples () | 584 | 562 | 1,146 | 511 | 516 | 1,027 | 584 | 562 |
| Detection rate [ (%)] | 448 (77) | 516 (92) | 964 (84) | 71 (14) | 45 (9) | 116 (11) | 584 (100) | 562 (100) |
| Minimum | 76 | 98 | ||||||
| Percentiles (P) | ||||||||
| P5 | 281 | 387 | ||||||
| P25 | 0.38 | 0.98 | 0.71 | 790 | 1,020 | |||
| P50 | 1.46 | 2.12 | 1.75 | 1,220 | 1,444 | |||
| P75 | 3.27 | 4.31 | 3.78 | 1,733 | 1,967 | |||
| P95 | 9.38 | 11.44 | 10.19 | 1.10 | 0.72 | 0.90 | 2,478 | 2,894 |
| Maximum | 75.77 | 67.93 | 75.77 | 84.45 | 439.28 | 439.28 | 6,168 | 4,700 |
Note: BPA-G, BPA-glucuronide; BPS-G, BPS-glucuronide; D.E.S.I.R., Data from an Epidemiological Study on the Insulin Resistance Syndrome; LOD, limit of detection ().
Among the 455 subcohort members with BPS-G data at both time points, BPS-G was detected in 5 participants (1%) at both visits, in 95 participants (21%) at one visit, and in 355 at none of the visits (78%). The within-person stability of categories of BPS-G detection over the two visits was null (p for ; ).
There was no difference in BPA-G concentrations according to BPS detection at the same time (p for and 0.47 at baseline and year 3, respectively). Moreover, there was no significant crude association between urinary detection of BPA-G and BPS-G among subcohort members, at baseline (p for ; ) or at year 3 (p for ; ). However, in the subcohort, the proportion of individuals with BPS-G detected at baseline and/or year 3 increased across average BPA exposure categories and was 15.8%, 19.1%, 28.0%, and 28.2% in the first, second, third, and fourth quartile groups, respectively (p for ).
Associations of BPA and BPS Exposure with Incident Diabetes
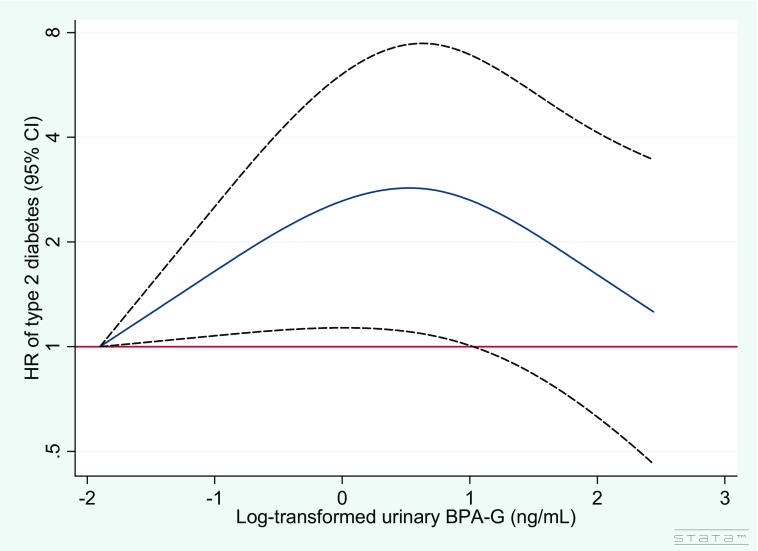
The associations between BPA and BPS exposure and the risk of developing diabetes are presented in Table 3. BPA-G concentrations at baseline were not associated with incident diabetes. However, positive associations of BPA-G concentrations at year 3 and BPA average exposure with diabetes risk were found. Compared with those in the first quartile group of average exposure, participants in the second, third, and fourth quartile groups had a near doubling of the risk of diabetes, although the association tended to be slightly reduced in the fourth quartile group: (95% CI: 1.16, 5.65), 2.35 (95% CI: 1.07, 5.15), and 1.56 (95% CI: 0.68, 3.55), respectively. Restricted cubic spline analysis confirmed that the risk for type 2 diabetes increased with increasing urinary BPA-G at year 3 () and that there was evidence for a nonlinear association (). The graphical representation of the relationship was consistent with a nonlinear association. Indeed, Figure 2 shows that the HRs of type 2 diabetes increased with urine BPA-G concentration until [ (95% CI: 1.10, 7.44)] and then began to decrease. In accordance with results obtained for groups of BPA exposure at baseline, we found no significant associations between urinary BPA-G at baseline and the incidence of type 2 diabetes using a restricted cubic spline model (, ), as shown in Figure S2.
Table 3.
Adjusted associations between exposure to BPA and BPS and the risk of type 2 diabetes in the D.E.S.I.R. cohort (single-pollutant models).
| Bisphenol exposure/detections | At baseline | At year 3 | Average exposure at baseline-year 3 | |||
|---|---|---|---|---|---|---|
| n/Na | aHR (95% CI)b | n/Na | aHR (95% CI)c | n/Na | aHR (95% CI)d | |
| BPA exposure | ||||||
| BPA-G concentration (ng/mL) | ||||||
| 62/233 | 1 | 11/94 | 1 | 10/75 | 1 | |
| 0.71–1.75 | 48/182 | 0.80 (0.53, 1.21) | 28/162 | 1.42 (0.66, 3.07) | 33/176 | 2.56 (1.16, 5.65) |
| 1.75–3.78 | 39/158 | 1.01 (0.65, 1.55) | 44/190 | 2.40 (1.16, 4.98) | 36/198 | 2.35 (1.07, 5.15) |
| 38/153 | 0.85 (0.54, 1.35) | 25/177 | 0.99 (0.44, 2.21) | 29/174 | 1.56 (0.68, 3.55) | |
| BPS detection | ||||||
| BPS-G concentration | ||||||
| No | 139/546 | 1 | 92/522 | 1 | 61/389 | 1 |
| Yes | 32/98 | 1.68 (1.09, 2.58) | 15/57 | 1.92 (1.02, 3.62) | 38/140 | 2.81 (1.74, 4.53) |
Note: Groups of BPA exposure were defined on the pooled baseline and year 3 BPA-G concentrations in subcohort members. aHR, adjusted hazard ratio; BMI, body mass index; BPA-G, BPA-glucuronide; BPS-G, BPS-glucuronide; CI, confidence interval; D.E.S.I.R., Data from an Epidemiological Study on the Insulin Resistance Syndrome; LOD, limit of detection ().
n/N indicates the numbers of type 2 diabetes cases relative to the total number of participants in each exposure category.
aHRs quantify the association between exposure to BPA/BPS and incidence of diabetes between baseline and year 9. Cox models with age as the timescale and stratified on smoking status were adjusted for sex and the following variables from baseline: urinary creatinine level, education level, employment, marital status, physical activity, caloric intake, family history of diabetes, hypertension, and BMI.
aHRs quantify the association between exposure to BPA/BPS and incidence of diabetes between year 3 and year 9. Cox models with age as the timescale and stratified on smoking status were adjusted for sex and the following variables from year 3: urinary creatinine level, education level, employment, marital status, physical activity, caloric intake, family history of diabetes, hypertension, and BMI.
aHRs quantify the association between exposure to BPA/BPS and incidence of diabetes between year 3 and year 9. Cox models with age as the timescale and stratified on smoking status were adjusted for sex, average urinary creatinine level, and the following variables from year 3: education level, employment, marital status, physical activity, caloric intake, family history of diabetes, hypertension, and BMI.
Figure 2.
Relationship between log-transformed BPA-G concentration at year 3 and adjusted hazard ratio (HR) of type 2 diabetes in the D.E.S.I.R. case–cohort study fitted with restricted cubic splines (three knots placed at the 10th, 50th, and 90th percentiles). Reference value for HRs is the minimum BPA-G concentration (); the solid line represents the HRs and the dashed lines the 95% confidence interval. , . Cox model with age as the timescale and stratified on smoking status was adjusted for urinary creatinine level, sex, education level, employment, marital status, physical activity, caloric intake, family history of diabetes, hypertension, and BMI (all variables from year 3). BMI, body mass index; BPA-G, bisphenol glucuronide; HR, hazard risk; D.E.S.I.R., Data from an Epidemiological Study on the Insulin Resistance Syndrome; LOD, limit of detection.
BPS-G detection in urine at baseline was positively associated with incident diabetes [ (95% CI: 1.09, 2.58)]. Considering BPS-G detection at year 3 led to similar results [ (95% CI: 1.02, 3.62)]. The association between BPS and diabetes risk was stronger for the detection of BPS-G at one or both time points versus at none of the visits [ (95% CI: 1.74, 4.53)]. In models including both exposures, the associations between BPS detection and diabetes risk remained of the same order of magnitude and significant after adjustment for BPA (see Table S2). The magnitude of the HRs for BPA exposure was slightly reduced when adjusted for BPS exposure.
Testing for effect–measure modification by sex revealed stronger associations between average BPS detection and diabetes risk in women [ (95% CI: 1.69, 10.63)] than in men [ (95% CI: 0.93, 3.33), ] (see Table S3). There was no interaction between average BPA exposure and sex regarding the risk of diabetes. However, stronger associations were observed in women than in men. Associations of BPS exposure (at baseline and/or year 3) with incident type 2 diabetes also appeared to be modified by age, BMI, and family history of diabetes (see Table S4). BPS exposure was significantly associated with increased risk of diabetes onset in participants who were overweight or obese at baseline, in participants of age at baseline, and in those with a family history of diabetes (all ). Lower and nonsignificant HR estimates were observed in normal weight participants and in participants of age. Significant but lower HR estimate was observed in those with no family history of diabetes. Because the numbers of cases became very small for these subgroup analyses, the 95% CIs were relatively wide.
Discussion
This study is one of the few prospective studies on the association between exposure to BPA and the subsequent development of type 2 diabetes and the first one on diabetes that relies on repeated urinary bisphenols measurements to assess exposure, which is crucial given the short half-life of BPA in the body. To the best of our knowledge, this is also the first study to investigate exposure to BPS in relation with the risk of developing diabetes. Our results suggest associations between diabetes risk and both BPA concentrations and BPS detection in urine, independent of traditional diabetes risk factors.
A major strength of the present study is the longitudinal design, in contrast to most of the previous epidemiological studies on BPA and diabetes, which were cross-sectional (Rancière et al. 2015). Human BPA exposure occurs primarily through the diet, and reverse causality cannot be excluded in cross-sectional studies showing associations between BPA and cardiometabolic disorders. Moreover, given the strong temporal within-person variability of urinary BPA concentrations (Ye et al. 2011), a spot urine sample at the time of diabetes diagnostic is likely to be a very poor proxy of exposure in the previous years in more toxicologically relevant exposure windows. The expected consequence, assuming a classical-type error structure, corresponds to a strong attenuation bias in dose–response functions (Perrier et al. 2016). Consequently, there is a need for results from prospective studies, in which BPA exposure has been assessed before the potential diabetes diagnosis, in order to better disentangle the nature of the relationship between BPA and diabetes. Our models were adjusted for total caloric intake, an important potential confounding factor rarely taken into account in previous studies (Rancière et al. 2015), even though adjustment for caloric intake does not fully represent dietary intake.
In the literature, BPA levels have mostly been measured in urine because blood levels of free BPA are often not detectable in the general population (Teeguarden et al. 2011). We studied the glucuronide metabolites of BPA and BPS as surrogates for BPA and BPS exposure. One of the advantages of studying these metabolites is to avoid contamination issues during sample collection or laboratory measurements (Vandentorren et al. 2011). In humans, after ingestion, BPA is rapidly metabolized and almost completely excreted in urine as the glucuronide conjugate because the free form of BPA is insoluble in water (Völkel et al. 2002). A study conducted in the United States showed among 31 volunteers that BPA-G was the dominant BPA form in human urine, representing 57% of the total BPA concentration, without significant differences by sex or ethnicity (Liao and Kannan 2012). We observed a large range of urinary BPA-G concentrations even after a 12-h fast, which is consistent with a study showing that urinary BPA levels did not decline rapidly with fasting time (Stahlhut et al. 2009). The urinary BPA-G concentrations that we observed in the D.E.S.I.R. subcohort ( and at baseline and year 3, corresponding to 0.82 and , respectively, BPA equivalent) are lower than those of the U.S. Nurses’ Health Study II (NHSII) participants (median of urinary BPA in ) who provided urine samples at about the same time period (1996–2001) (Sun et al. 2014). Only limited studies have reported the occurrence of BPS in human urine, all in samples collected in the 2000s (Wu et al. 2018). Compared with most of these studies, it is likely that the detection frequency of urinary BPS is lower in our study where urine samples were collected in the 1990s, before the use of BPS had increased in consumer products. In the literature, BPS was reported to be present in human urine for the first time in 2012 and was detected in 81% of the urine samples collected from the United States and seven Asian countries, with concentrations ranging from 0.02 to and a geometric (Liao et al. 2012). In comparison, we observed that 14% of urine samples from baseline and 9% from year 3 had detectable BPS-G among subcohort members ( corresponding to BPS equivalent).
Another major strength of our study is the assessment of BPA and BPS exposure based on up to two urine samples per participant. Although this is an improved assessment over the three other prospective studies that measured BPA in only one urine sample (Bi et al. 2016; Shu et al. 2018; Sun et al. 2014), the risk of exposure misclassification remains, given the high within-person temporal variability of biomarkers such as urinary BPA-G and BPS-G. The low ICC we found for BPA-G is consistent with the results by Reeves et al. (2014) and Townsend et al. (2013) as part of the Nurses’ Health Study (NHS) and NHSII cohorts where urinary spot samples were collected 1–3 y apart. This misclassification is expected to lead to strong loss of power and attenuation bias, by about 90% for an ICC of 0.1 (Perrier et al. 2016), assuming that this 3-y period corresponds to the toxicologically relevant time period. This could explain why our results are not consistent between the two time points for BPA. Inconsistent findings were also reported in the literature, for instance, in different cycles from the National Health and Nutrition Examination Survey (NHANES) (Casey and Neidell 2013) or in the prospective study among U.S. women from the NHS and the NHSII cohorts (Sun et al. 2014). In order to reduce misclassification bias, we derived average BPA exposure and BPS detection using data from the two visits; exposure averaging is expected to strongly decrease bias assuming that biospecimens were collected during the right time window (Perrier et al. 2016), and indeed, associations with both BPA and BPS were strengthened.
There is evidence from experimental studies that xenoestrogens, such as BPA and BPS, might have a role in the etiology of type 2 diabetes by affecting processes that are related to diabetes development, including dysfunction of pancreatic beta cells (Hectors et al. 2011). Experimental studies showed that BPA exposure can affect glucose metabolism at concentrations comparable with environmental levels measured in humans from the general population (Magliano and Lyons 2013). In humans, although several cross-sectional studies have reported positive associations between BPA and diabetes (Rancière et al. 2015), findings from the few prospective studies published so far are not entirely consistent. Among women from the NHS and the NHSII cohorts, a positive and significant association between urinary BPA and incident diabetes was observed among middle-aged women from the NHSII, but there was no association in older women from the NHS (Sun et al. 2014). Among middle-aged and elderly Chinese men and women, BPA exposure was not associated with incident diabetes, but positive associations were reported with a 4-y increase in fasting blood glucose in participants with a higher diabetes genetic risk score (Bi et al. 2016). More recently, a nested case–control study conducted in employed or retired university staff in China found no association between serum BPA levels and the 5-y incidence of type 2 diabetes (Shu et al. 2018).
The present study adds to the limited literature on the prospective relationship between BPA exposure and diabetes risk and contributes to the growing body of evidence that BPA exposure may be a risk factor for diabetes independent of traditional diabetes risk factors. Our results are in favor of a nonlinear relationship between exposure to BPA and diabetes risk. Even though nonmonotonic associations could arise by chance, this finding would be consistent with the nonlinear or nonmonotonic dose–response relationships commonly described in the BPA literature, possibly related to cytotoxicity, receptor selectivity, and receptor down-regulation or competition with endogenous hormones (Vandenberg 2013) as well as mitochondrial dysfunction (Lee et al. 2014).
Another important strength of this study is its investigation of exposure to BPS, a BPA analog. Because of health concerns and regulatory actions, the use of BPA is expected to decline while the use of BPA alternatives is expected to rise, which is supported by exposure data on BPA and three bisphenol analogs from NHANES between 2000 and 2014 (Ye et al. 2015). Evidence against BPS is growing from experimental studies highlighting similar endocrine-disrupting effects to those of BPA (Rochester and Bolden 2015). A case–control study conducted in China recently reported a cross-sectional association between urinary BPS concentrations and type 2 diabetes (Duan et al. 2018), whereas another case–control study from Saudi Arabia showed no evidence for an association between BPS and diabetes (Li et al. 2018). To our knowledge, no prospective studies have yet considered the association between BPS exposure and the incidence of diabetes. Our findings are in favor of “low-dose” effects of BPS on diabetes risk and suggest that BPS may not be a safe alternative to BPA. However, we cannot rule out residual confounding as an explanation for these observed associations. In particular, because humans are simultaneously exposed to various chemicals that can be correlated with BPA and BPS, we cannot exclude that the associations observed were partly due to other contaminants that were not measured in this study (Lee et al. 2014). Because our urine samples were collected in the 1990s when BPS use was limited, the prevalence of BPS exposure was low in our population, and we were not able to characterize a dose–response function. Thus, the strong effect size that we report needs to be replicated in more recent cohort studies. Epidemiological studies should be encouraged to document exposure to BPA alternative compounds in order to foster discussion on the current and crucial issue of chemicals used to replace BPA and to help public authorities make informed decisions in the fields of public health and environmental policies.
Our findings suggest that the relationship of BPS exposure with type 2 diabetes may be modified by sex, age, BMI, and family history of diabetes, even though the HR estimates for these subgroup analyses were imprecise. Although no sex or BMI differences were found for the associations of BPS with type 2 diabetes in the study by Duan et al. (2018), a few previous studies focusing on BPA exposure in relation to diabetes risk reported such modifying effects. For instance, our results are in line with those by Sun et al. (2014) who found BPA exposure associated with the risk of type 2 diabetes among middle-aged, but not older, women. We believe these sex and age differences are biologically plausible given that BPA and BPS are known xenoestrogens that can that can interfere with the endocrine system through binding and competing for the estrogen receptors ER-alpha (), ER-beta (), and estrogen-related receptor gamma (). The stronger associations we found among those who were overweight or obese are also consistent with the prospective study by Sun et al. (2014), where urinary BPA was positively associated with type 2 diabetes risk among women with , but no significant association was found for women with . Last, we also hypothesized that people with a family history of type 2 diabetes may be more susceptible to the deleterious effects of environmental exposures given the associations observed between BPA exposure and increased fasting blood glucose by Bi et al. (2016) in participants with a higher diabetes genetic risk score. For BPS, we detected effect modification by family history of diabetes, even though the limited statistical power led to extremely wide 95% CIs in the subgroup of individuals with family history. Overall, all the results from subgroup analyses should be interpreted with caution.
Conclusions
Our findings suggest that exposure to BPA and BPS may be associated with an increased risk for type 2 diabetes and highlight the importance of assessing the potential health hazards associated with BPA substitutes. Further research in more recent prospective cohort studies, where the BPS exposure is likely to be higher, is needed to confirm these results. Overall, caution in interpretation is needed due to the limited ability to predict long-term bisphenol exposure from spot urine samples, even repeated, and to possible residual confounding.
Supplementary Material
Acknowledgments
The Data from an Epidemiological Study on the Insulin Resistance Syndrome (D.E.S.I.R.) Study Group: CESP, Inserm U1018: B. Balkau, P. Ducimetière, E. Eschwège; Université Paris Descartes: F. Rancière; Inserm U367: F. Alhenc-Gelas; Centre hospitalier universitaire (CHU) d’Angers: A. Girault; Bichat Hospital: F. Fumeron, M. Marre, R. Roussel; CHU de Rennes: F. Bonnet; CNRS UMR8090, Lille: A. Bonnefond, S. Cauchi, P. Froguel; Centres d’examens de santé de l’Assurance Maladie: Alençon, Angers, Blois, Caen, Chateauroux, Chartres, Cholet, Le Mans, Orléans, Tours; Institut de Recherche en Médecine Générale: J. Cogneau; General practitioners of the Region; IRSA: C. Born, E. Caces, M. Cailleau, O. Lantieri, J.G. Moreau, F. Rakotozafy, J. Tichet, and S. Vol.
The authors thank the D.E.S.I.R. participants and the D.E.S.I.R. team for their contributions. This study has been supported by the Francophone Diabetes Society (SFD) and the French Endocrine Disruptor Research Program (PNRPE) for the assessment of exposure to bisphenol A (BPA) and bisphenol S (BPS). F.R. was funded as a postdoctoral fellow by the Field of major interest of the Île-de-France Regional Council, Cardiovascular, Obesity, Kidney, Diabetes (CORDDIM).
The D.E.S.I.R. study has been supported by Inserm contracts with Caisse nationale de l’assurance maladie des travailleurs salariés (CNAMTS), Lilly, Novartis Pharma, and Sanofi-Aventis, by Inserm (Réseaux en Santé Publique, Interactions entre les déterminants de la santé), Cohortes Santé Très Grands Instruments de Recherche (TGIR), Association Diabète Risque Vasculaire, Fédération Française de Cardiologie, Fondation de France, Association de langue française pour l’étude du diabéte et des maladies métaboliques (ALFEDIAM), Office national interprofessionnel des vins (ONIVINS), Ardix Medical, Bayer Diagnostics, Becton Dickinson, Cardionics, Merck Santé, Novo Nordisk, Pierre Fabre, Roche, and Topcon.
Footnotes
Supplemental Material is available online (https://doi.org/10.1289/EHP5159).
The authors declare they have no actual or potential competing financial interests.
Note to readers with disabilities: EHP strives to ensure that all journal content is accessible to all readers. However, some figures and Supplemental Material published in EHP articles may not conform to 508 standards due to the complexity of the information being presented. If you need assistance accessing journal content, please contact ehponline@niehs.nih.gov. Our staff will work with you to assess and meet your accessibility needs within 3 working days.
References
- Alonso-Magdalena P, Quesada I, Nadal A. 2011. Endocrine disruptors in the etiology of type 2 diabetes mellitus. Nat Rev Endocrinol 7(6):346–353, PMID: 21467970, 10.1038/nrendo.2011.56. [DOI] [PubMed] [Google Scholar]
- Balkau B, Lange C, Fezeu L, Tichet J, de Lauzon-Guillain B, Czernichow S, et al. . 2008. Predicting diabetes: clinical, biological, and genetic approaches: Data from the Epidemiological Study on the Insulin Resistance Syndrome (DESIR). Diabetes Care 31(10):2056–2061, PMID: 18689695, 10.2337/dc08-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow WE, Ichikawa L, Rosner D, Izumi S. 1999. Analysis of case-cohort designs. J Clin Epidemiol 52(12):1165–1172, PMID: 10580779, 10.1016/s0895-4356(99)00102-x. [DOI] [PubMed] [Google Scholar]
- Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. 2005. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect 113(2):192–200, PMID: 15687057, 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi Y, Wang W, Xu M, Wang T, Lu J, Xu Y, et al. . 2016. Diabetes genetic risk score modifies effect of bisphenol A exposure on deterioration in glucose metabolism. J Clin Endocrinol Metab 101(1):143–150, PMID: 26523527, 10.1210/jc.2015-3039. [DOI] [PubMed] [Google Scholar]
- Casey MF, Neidell M. 2013. Disconcordance in statistical models of bisphenol A and chronic disease outcomes in NHANES 2003-08. PLoS One 8(11):e79944, PMID: 24223205, 10.1371/journal.pone.0079944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Khunti K, Davies MJ. 2017. Type 2 diabetes. Lancet 389(10085):2239–2251, PMID: 28190580, 10.1016/S0140-6736(17)30058-2. [DOI] [PubMed] [Google Scholar]
- Chen D, Kannan K, Tan H, Zheng Z, Feng YL, Wu Y, et al. . 2016. Bisphenol analogues other than BPA: environmental occurrence, human exposure, and toxicity—a review. Environ Sci Technol 50(11):5438–5453, PMID: 27143250, 10.1021/acs.est.5b05387. [DOI] [PubMed] [Google Scholar]
- Duan Y, Yao Y, Wang B, Han L, Wang L, Sun H, et al. . 2018. Association of urinary concentrations of bisphenols with type 2 diabetes mellitus: a case-control study. Environ Pollut 243(Pt B):1719–1726, PMID: 30408859, 10.1016/j.envpol.2018.09.093. [DOI] [PubMed] [Google Scholar]
- FDA (Food and Drug Administration). 2013. Guidance for Industry, Bioanalytical Method Validation Guidance. http://academy.gmp-compliance.org/guidemgr/files/UCM368107.PDF [accessed 13 April 2016].
- Hectors TL, Vanparys C, van der Ven K, Martens GA, Jorens PG, Van Gaal LF, et al. . 2011. Environmental pollutants and type 2 diabetes: a review of mechanisms that can disrupt beta cell function. Diabetologia 54(6):1273–1290, PMID: 21442161, 10.1007/s00125-011-2109-5. [DOI] [PubMed] [Google Scholar]
- Lasfargues G, Vol S, Le Clésiau H, Bedouet M, Hagel L, Constans T, et al. . 1990. Validity of a short self-administered dietary questionnaire compared with a dietetic interview [in French]. Presse Med 19(20):953–957, PMID: 2141133. [PubMed] [Google Scholar]
- Lee DH, Porta M, Jacobs DR Jr, Vandenberg LN. 2014. Chlorinated persistent organic pollutants, obesity, and type 2 diabetes. Endocr Rev 35(4):557–601, PMID: 24483949, 10.1210/er.2013-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AJ, Xue J, Lin S, Al-Malki AL, Al-Ghamdi MA, Kumosani TA, et al. . 2018. Urinary concentrations of environmental phenols and their association with type 2 diabetes in a population in Jeddah, Saudi Arabia. Environ Res 166:544–552, PMID: 29960220, 10.1016/j.envres.2018.06.040. [DOI] [PubMed] [Google Scholar]
- Liao C, Kannan K. 2012. Determination of free and conjugated forms of bisphenol A in human urine and serum by liquid chromatography–tandem mass spectrometry. Environ Sci Technol 46(9):5003–5009, PMID: 22489688, 10.1021/es300115a. [DOI] [PubMed] [Google Scholar]
- Liao C, Liu F, Alomirah H, Loi VD, Mohd MA, Moon HB, et al. . 2012. Bisphenol S in urine from the United States and seven Asian countries: occurrence and human exposures. Environ Sci Technol 46(12):6860–6866, PMID: 22620267, 10.1021/es301334j. [DOI] [PubMed] [Google Scholar]
- Magliano DJ, Lyons JG. 2013. Bisphenol A and diabetes, insulin resistance, cardiovascular disease and obesity: controversy in a (plastic) cup? J Clin Endocrinol Metab 98(2):502–504, PMID: 23264398, 10.1210/jc.2012-3058. [DOI] [PubMed] [Google Scholar]
- Neel BA, Sargis RM. 2011. The paradox of progress: environmental disruption of metabolism and the diabetes epidemic. Diabetes 60(7):1838–1848, PMID: 21709279, 10.2337/db11-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepomnaschy PA, Baird DD, Weinberg CR, Hoppin JA, Longnecker MP, Wilcox AJ. 2009. Within-person variability in urinary bisphenol A concentrations: measurements from specimens after long-term frozen storage. Environ Res 109(6):734–737, PMID: 19463991, 10.1016/j.envres.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier F, Giorgis-Allemand L, Slama R, Philippat C. 2016. Within-subject pooling of biological samples to reduce exposure misclassification in biomarker-based studies. Epidemiology 27(3):378–388, PMID: 27035688, 10.1097/EDE.0000000000000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rancière F, Lyons JG, Loh VHY, Botton J, Galloway T, Wang T, et al. . 2015. Bisphenol A and the risk of cardiometabolic disorders: a systematic review with meta-analysis of the epidemiological evidence. Environ Health 14(1):46, PMID: 26026606, 10.1186/s12940-015-0036-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves KW, Luo J, Hankinson SE, Hendryx M, Margolis KL, Manson JE, et al. . 2014. Within-person variability of urinary bisphenol-A in postmenopausal women. Environ Res 135:285–288, PMID: 25462677, 10.1016/j.envres.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochester JR, Bolden AL. 2015. Bisphenol S and F: a systematic review and comparison of the hormonal activity of bisphenol A substitutes. Environ Health Perspect 123(7):643–650, PMID: 25775505, 10.1289/ehp.1408989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp SJ, Poulaliou M, Thompson SG, White IR, Wood AM. 2014. A review of published analyses of case-cohort studies and recommendations for future reporting. PLoS One 9(6):e101176, PMID: 24972092, 10.1371/journal.pone.0101176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu X, Tang S, Peng C, Gao R, Yang S, Luo T, et al. . 2018. Bisphenol A is not associated with a 5-year incidence of type 2 diabetes: a prospective nested case–control study. Acta Diabetol 55(4):369–375, PMID: 29387940, 10.1007/s00592-018-1104-4. [DOI] [PubMed] [Google Scholar]
- Stahlhut RW, Welshons WV, Swan SH. 2009. Bisphenol A data in NHANES suggest longer than expected half-life, substantial nonfood exposure, or both. Environ Health Perspect 117(5):784–789, PMID: 19479022, 10.1289/ehp.0800376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Cornelis MC, Townsend MK, Tobias DK, Eliassen AH, Franke AA, et al. . 2014. Association of urinary concentrations of bisphenol A and phthalate metabolites with risk of type 2 diabetes: a prospective investigation in the Nurses’ Health Study (NHS) and NHSII cohorts. Environ Health Perspect 122(6):616–623, PMID: 24633239, 10.1289/ehp.1307201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeguarden JG, Calafat AM, Ye X, Doerge DR, Churchwell MI, Gunawan R, et al. . 2011. Twenty-four hour human urine and serum profiles of bisphenol A during high-dietary exposure. Toxicol Sci 123(1):48–57, PMID: 21705716, 10.1093/toxsci/kfr160. [DOI] [PubMed] [Google Scholar]
- Townsend MK, Franke AA, Li X, Hu FB, Eliassen AH. 2013. Within-person reproducibility of urinary bisphenol A and phthalate metabolites over a 1 to 3 year period among women in the Nurses’ Health Studies: a prospective cohort study. Environ Health 12(1):80, PMID: 24034517, 10.1186/1476-069X-12-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN. 2013. Non-monotonic dose responses in studies of endocrine disrupting chemicals: bisphenol A as a case study. Dose Response 12(2):259–276, PMID: 24910584, 10.2203/dose-response.13-020.Vandenberg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandentorren S, Zeman F, Morin L, Sarter H, Bidondo ML, Oleko A, et al. . 2011. Bisphenol-A and phthalates contamination of urine samples by catheters in the Elfe pilot study: implications for large-scale biomonitoring studies. Environ Res 111(6):761–764, PMID: 21684541, 10.1016/j.envres.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Völkel W, Colnot T, Csanády GA, Filser JG, Dekant W. 2002. Metabolism and kinetics of bisphenol A in humans at low doses following oral administration. Chem Res Toxicol 15(10):1281–1287, PMID: 12387626, 10.1021/tx025548t. [DOI] [PubMed] [Google Scholar]
- Wu LH, Zhang XM, Wang F, Gao CJ, Chen D, Palumbo JR, et al. . 2018. Occurrence of bisphenol S in the environment and implications for human exposure: a short review. Sci Total Environ 615:87–98, PMID: 28963899, 10.1016/j.scitotenv.2017.09.194. [DOI] [PubMed] [Google Scholar]
- Ye X, Wong LY, Bishop AM, Calafat AM. 2011. Variability of urinary concentrations of bisphenol A in spot samples, first morning voids, and 24-hour collections. Environ Health Perspect 119(7):983–988, PMID: 21406337, 10.1289/ehp.1002701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Wong LY, Kramer J, Zhou X, Jia T, Calafat AM. 2015. Urinary concentrations of bisphenol A and three other bisphenols in convenience samples of U.S. adults during 2000–2014. Environ Sci Technol 49(19):11834–11839, PMID: 26360019, 10.1021/acs.est.5b02135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.