Abstract
Background:
Long-term exposure to particulate matter (PM) in ambient air has been associated with cardiovascular mortality, but few studies have considered incident disease in relation to PM from different sources.
Objectives:
We aimed to study associations between long-term exposure to different types of PM and sources, and incident ischemic heart disease (IHD) and stroke in three Swedish cities.
Methods:
Based on detailed emission databases, monitoring data, and high-resolution dispersion models, we calculated source contributions to PM with aerodynamic diameter (), PM with aerodynamic diameter (), and black carbon (BC) from road wear, traffic exhaust, residential heating, and other sources in Gothenburg, Stockholm, and Umeå. Registry data for participants from four cohorts were used to obtain incidence of IHD and stroke for first hospitalization or death. We constructed time windows of exposure for same-year, 1- to 5-y, and 6- to 10-y averages preceding incidence from annual averages at residential addresses. Risk estimates were based on random effects meta-analyses of cohort-specific Cox proportional hazard models.
Results:
We observed 5,166 and 3,119 incident IHD and stroke cases, respectively, in 114,758 participants. Overall, few consistent associations were observed between the different air pollution measures and IHD or stroke incidence. However, same-year levels of ambient locally emitted BC (range: ) were associated with a 4.0% higher risk of incident stroke per interquartile range (IQR), [95% confidence interval (CI): 0.04, 7.8]. This association was primarily related to BC from traffic exhaust. (range: ) and (range: ) were not associated with stroke. Associations with incident IHD were observed only for exposure from residential heating.
Discussion:
Few consistent associations were observed between different particulate components and IHD or stroke. However, long-term residential exposure to locally emitted BC from traffic exhaust was associated with stroke incidence. The comparatively low exposure levels may have contributed to the paucity of associations. https://doi.org/10.1289/EHP4757
Introduction
Long-term particulate air pollution exposure has been associated with all-cause mortality in several studies, and the strongest associations have been repeatedly reported for cardiovascular causes (Hoek et al. 2013). Cardiovascular disease is the leading cause of death in a majority of countries and represents a substantial public health threat. Consequently, health effects of particulate air pollution on cardiovascular disease are of particular value for health risk assessment. Ischemic heart disease (IHD) and stroke constitute the major diagnoses contributing to the cardiovascular disease burden and contribute to both increased morbidity and mortality. Long-term exposure to particulate matter with aerodynamic diameter () has previously been associated with progression in atherosclerosis (Kaufman et al. 2016), the pathophysiological process behind IHD and a large share of strokes. Efforts to concomitantly investigate strengths of associations between different types of particulate air pollution and incidence in ischemic heart disease and stroke are warranted and are so far relatively limited (Hime et al. 2018). In addition, research is needed to identify which source emissions are especially important to monitor and regulate as a basis for adequate prevention.
Available studies of long-term exposure to PM with aerodynamic diameter of () or and associations with IHD and stroke incidence have employed different methods of exposure assessment with variable spatial resolution. Associations have been reported between and and incident IHD (Miller et al. 2007; Tonne et al. 2009; Madrigano et al. 2013; Cesaroni et al. 2014), although other large-cohort studies have not confirmed associations (Puett et al. 2009; Gan et al. 2011; Lipsett et al. 2011; Hoffmann et al. 2015). Only one study of long-term black carbon (BC) exposure reported associations with IHD but considered few individual-level covariates (Gan et al. 2011). Exposure metrics highly related to BC (Jeong et al. 2004), such as elemental carbon, PM absorbance, black smoke, etc., may also convey similar risk, and some studies have investigated PM absorbance and incident IHD with associations indicating increased risk but were statistically nonsignificant (Puett et al. 2011; Cesaroni et al. 2014; Hoffmann et al. 2015). In relation to stroke incidence, there are no previous studies investigating long-term BC exposure, and studies of PM absorbance have not consistently demonstrated associations with stroke incidence (Stafoggia et al. 2014; Hoffmann et al. 2015). A meta-analysis of 20 studies, some of them on incident stroke, indicated positive and stronger associations for than (Scheers et al. 2015). Importantly, few studies of long-term air pollution exposure and incident IHD or stroke have attempted to address sources of PM exposure or relevant time periods of exposure. This is despite that studies focusing on IHD mortality suggest differences in associations depending on PM source. Stronger associations were demonstrated between long-term levels of fossil fuel–related than overall mass concentrations, indicating greater health effects from combustion sources, particularly from coal burning (Thurston et al. 2016). There are also studies indicating stronger associations in low-level exposure settings such as Sweden (Stafoggia et al. 2014). Previous studies in Stockholm and Gothenburg have demonstrated associations between PM exposure and stroke and coronary events (Korek et al. 2015; Stockfelt et al. 2017), including source-specific analyses in Gothenburg that were inconclusive possibly due to sample size. In summary, the current sparsity of long-term studies with detailed source-specific PM exposure investigating incident IHD and stroke warrants further attention. Differences in associations between particulate air pollution and incident stroke and IHD based on particle size fraction and source emissions may have important policy-relevant implications even in low-level pollution environments such as in Swedish cities.
We aimed to use detailed high-resolution dispersion modeling, specifically designed to identify PM source contributions, to study associations between , , BC, and their sources with incident IHD and stroke, leveraging a meta-analysis of large cohorts from Gothenburg, Stockholm, and Umeå. We hypothesized that traffic exhaust sources would demonstrate the strongest associations with incident IHD and stroke.
Materials and Methods
Study Cohorts
Our study period ranged from 1 January 1990 to 31 December 2011 and included individuals in two cohorts from Gothenburg, four pooled cohorts from Stockholm, and one cohort from Umeå. Cohorts were selected based on study designs incorporating data collection of cardiovascular risk factors at recruitment from questionnaires and examinations.
The study population in Gothenburg has been described previously in more detail (Stockfelt et al. 2017). Briefly, it consisted of two general population cohorts: the Primary Prevention Study (PPS) cohort and the GOT-MONICA (Multinational Monitoring of Trends and Determinants in Cardiovascular Diseases) cohort. The PPS cohort consists of a random third of all men in Gothenburg born between 1915 and 1925 screened in 1970–1973 to study predictors of cardiovascular disease (Wilhelmsen et al. 1986). At baseline, the participants filled out questionnaires on background data and cardiovascular risk factors and were examined by health care professionals. The GOT-MONICA cohort is part of the MONICA project, an international multicohort study of risk factors for cardiovascular diseases (WHO MONICA Project Principal Investigators 1988). Recruitment and characteristics of the cohort have been described previously (Wilhelmsen et al. 1997). Briefly, participants recruited via random selection from all residents in Gothenburg aged 25–64 y at the time of inclusion, at baseline in 1985, 1990, or 1995, filled out questionnaires on background data and cardiovascular risk factors and were examined by health care professionals.
Four subcohorts from Stockholm County were pooled to form the Cardiovascular Effects of Air pollution and Noise Study (CEANS) cohort (Korek et al. 2015). One of these was a population-based prospective cohort, Stockholm Diabetes Prevention Program (SDPP), that recruited 3,128 men between 1992 and 1994 and 4,821 women between 1996 and 1998 in five municipalities in Stockholm County. Participants were 35–56 y of age and free of diabetes at recruitment. Approximately half (53%) had a first- or second-degree relative with a history of diabetes, and the remaining recruits were matched on age and sex. The second subcohort, the Cohort of 60-y-olds (60YO), included a population-based random sample of 60-y-olds constituting about one-third () of all men and women who turned 60 and were living within Stockholm County between August 1997 and March 1999. In the third subcohort, the Stockholm Screening Across the Lifespan Twin study and TwinGene (SALT), twins born before 1958 across Sweden were recruited between 1998 and 2002 and screened for complex diseases, particularly cardiovascular diseases. Only SALT participants residing in Stockholm County at recruitment were included (). The fourth subcohort included the Swedish National Study on Aging and Care in Kungsholmen (SNAC-K) with randomly sampled participants among prespecified age cohorts from 60–100 y old between March 2001 and June 2004 and living in a central area in Stockholm city (). Descriptive data comparing the four subcohorts in CEANS are provided in Table S1.
The Västerbotten Intervention Programme (VIP) was launched in 1985 in Västerbotten County in northern Sweden, including the city of Umeå. Recruitment and follow-up have been described in detail (Norberg et al. 2010). Briefly, all inhabitants were invited to their local primary health care center for health examination and counseling during their 40th, 50th, and 60th y of age with a gradual scale-up within the county to include primary health centers in all of the municipalities by 1992 and totaling 117,710 individuals, of which 81,821 residents of the Umeå municipality were included for the present study.
For the four cohorts, we obtained residential address history, updated yearly by linking personal identification numbers with mandatory records of residential addresses at Statistics Sweden or the Swedish Taxation Authority. Residential addresses were geocoded by automatically matching against the Swedish Mapping Cadastral and Land Registration Authority databases. Addresses were then manually checked and corrected for inconsistencies and assigned geographical coordinates.
Health Outcomes
Hospitalization and cause-specific mortality for stroke and IHD were determined by linkage of national personal identification numbers to the well-validated in-patient and death registries of the Swedish National Board of Health and Welfare (https://www.socialstyrelsen.se/en/statistics-and-data/registers/) (Ludvigsson et al. 2011). We used the International Classification of Diseases, Ninth Revision (ICD-9) codes 410–414 and ICD-10 I20-25 codes to define IHD and ICD-9 codes 431–436 and ICD-10 codes I61–I65 to define stroke (WHO 2015). We excluded prevalent cases at baseline identified by linkage to registries 5 y prior to recruitment in the current study. For stroke, in addition to the codes above, we also excluded individuals with diagnosis of late effects of previous stroke (ICD-9 code 438; ICD-10 codes I69.1–I69.8) at baseline.
Exposure Assessment
Details of exposure assessment have been published elsewhere (Segersson et al. 2017). In brief, high-resolution dispersion modeling of locally generated , , and BC was performed based on local emission inventories for the years 1990, 2000, and 2011 in Gothenburg and Umeå and the years 1990, 1995, 2000, 2005, and 2011 in Stockholm. For Gothenburg and Umeå, annual mean levels of the simulated contribution from local sources were linearly interpolated between 1990, 2000, and 2011 and corrected with a year-specific ventilation index calculated from air dispersion modeling over the two cities for each year from 1990 to 2011. For Stockholm, the annual levels were obtained from linear interpolation over the 4 y between each model simulations 1990, 1995, 2000, 2005, and 2011.
Most of the long-range transported consists of , and comparisons of measurements from several regional background sites in Sweden have shown small spatial variations on the regional scale (Forsberg et al. 2005). Thus, we assumed geographically homogeneous levels of transported and for each city. Annual averaged long-range contributions were taken from regional background stations outside the cities for Stockholm and Umeå, whereas in Gothenburg, due to lack of time series of measured concentrations of rural background air pollution, we indirectly calculated the long-range transport contribution by subtracting, from monitored urban background stations, the simulated contributions from local sources at the same location.
Due to the low availability of monitored BC, and noting low year-to-year variability in existing data of the related metric black smoke, the long-range transport contributions of BC were assumed constant over the period 1990–2011.
Emission factors for traffic exhaust were calculated for particles using geographically assigned inventory data on different vehicle types, speeds, and driving conditions based on Handbook on Emission Factors for Road Traffic version 3.1 (Hausberger et al. 2009) and for BC based on TRANSPHORM (Ilias et al. 2013). Nonexhaust emissions took into account road wear, and some contributions from brake and tire wear and contributions relative to exhaust emissions were estimated by methods previously described in detail (Omstedt et al. 2005; Denby et al. 2013a,b). Small-scale residential heating emissions in Gothenburg and Stockholm were determined using household energy consumption data from Statistics Sweden gridded to according to proxy data such as number of stoves or boilers per municipality, living space of small houses per , population density per , and availability of district heating. In Umeå, estimates for particle emissions from residential heating used a detailed inventory of individual stoves and boilers with data from chimney sweepers and interviews about amount of wood burning (Segersson et al. 2017). Large industrial sources and energy production facilities were included as point sources, based on information from the Swedish national emission inventory (Andersson et al. 2015), or using information from environmental permits and emission reports. Shipping emissions were described using a methodology described elsewhere (Jalkanen et al. 2012). Other emission sources, e.g., off-road machinery and diffuse emissions related to agriculture, were also based on the national Swedish inventory (Andersson et al. 2015).
The dispersion models ultimately provided impact of locally emitted pollutants with spatiotemporal contrasts (, annual averages) for the three study areas, either collapsed into one variable (local , local , and local BC) or as separate contributions for (traffic road wear, traffic exhaust, residential heating, shipping, industry, or a combined group of other sources) and BC (traffic exhaust, residential heating, or a combined group of other sources). Although PM contributions to traffic exhaust and residential heating are almost exclusively contained within (consequently, ), other source contributions may contain some fraction of larger particles, and thus, we homogeneously broke down the sources for for simplicity. Validation of estimated total concentrations against measured levels showed values of 0.87, 0.65, and 0.93 for , , and BC, respectively (Segersson et al. 2017).
In a second step, we indirectly estimated the contribution of long-range transport pollutants for , , and BC by the difference between total concentrations measured at monitoring stations and modeled local particle concentrations at the same location, taking into account hourly meteorological data. The city-specific annual averages of the long-range contributions were then added to the dispersion model estimates of local emissions to obtain total , total , and total BC at every grid point, whereby each estimate had a spatiotemporal contrast for the local component and a purely temporal contrast for the long-range transport component.
In a final step, we assigned the modeled annual averages for total and local , , and BC, as well as source-specific averages, to each study participant’s home address. Each participant’s average exposure was weighted according to time living at each address registered during the study period; thus, we accounted for any changes in residential address history in our exposure assessment.
Statistical methods.
We employed Cox proportional hazard models to estimate hazard ratios (HRs) in cohort-specific analyses after covariate adjustment and, in the case of the Stockholm CEANS study, used a stratified Cox regression. The stratified Cox regression in CEANS allowed us to estimate hazard functions adjusted for subcohort in CEANS and reduced the risk of violating the proportional hazards assumption across the geographically and temporally somewhat differently sampled subcohorts in Stockholm County. We expected age to be a more important confounder than calendar time, and thus, age was used as the time scale, consistent with previous studies (Thiébaut and Bénichou 2004; Beelen et al. 2014). Covariates were collected at recruitment, and exposure was modeled yearly. We separately modeled incident IHD and stroke based on first hospitalization or cause-specific death, whichever came first. We censured individuals at death for other causes at the end of study period or at time of permanent emigration from the study areas. Cohort-specific models were adjusted for potential confounders based on their expected association with place of residence and studied outcome. When covariate definitions varied slightly across cohorts we strived to use as similar definitions as possible for comparability. Final cohort-specific models included: sex, calendar year, subcohort (in Stockholm), smoking status (current, former, never smoker), alcohol consumption in Stockholm and Umeå (daily, weekly, seldom, never), physical activity (sedentary, moderate, intermediate, or vigorous in Gothenburg and Umeå and once a month or less/ per week, about once a month/1 h per week, 3 times a week or more/ per week in Stockholm), marital status (single, married or living with partner, no answer), socioeconomic index by occupation (blue-collar, low and intermediate white-collar and self-employed, high-level white-collar and self-employed, professionals with academic degrees, no answer), education level (primary school or less, up to secondary school or equivalent, university degree or more, no answer), occupation status (gainfully employed, unemployed/not gainfully employed, retired, no answer), and mean neighborhood individual income in persons of working age by Small Areas for Market Statistics (SAMS) provided by Statistics Sweden for years 1994 in Gothenburg and Stockholm and 2012 in Umeå. We used a later year for SAMS data in Umeå so that the average socioeconomic levels better reflected the growth of the city during the study period. Research regarding the pathophysiological mechanisms for cardiovascular effects of long-term PM exposure have indicated pathways linked to inflammation, atherosclerosis, and metabolic changes (Brook et al. 2010). Therefore, cardiovascular risk factors such as body mass index (BMI), diabetes, lipids, and hypertension were considered potential mediators on the causal pathway and were thus not adjusted for.
To explore the relevant induction period, we used three different time windows of exposure: exposure during the year of event, mean exposure during 1–5 calendar years preceding the event, and, finally, mean exposure 6–10 y preceding the event. We required individuals to have at least 80% available exposure data for the time windows. We expressed hazard ratios and 95% confidence intervals (CIs) per fixed increment of air pollution based on the mean of the interquartile ranges (IQRs) across the three study areas.
A meta-analysis of cohort-specific estimates was performed assuming random effects (DerSimonian and Laird 1986). We assessed presence of heterogeneity across estimates by applying the test from Cochran’s Q statistic and then quantifying the statistic (Higgins and Thompson 2002). Cohort-specific effect estimates were considered significantly heterogeneous if either the was greater than 50% or the p-value of the test was less than 0.05.
We performed analyses with total , total , and total BC for each outcome and time window. Models of total pollutants demonstrating positive associations were further explored in models restricted to local emissions. Finally, we employed two-pollutant models of specific sources relevant to the associations seen in the main models and not hampered by high collinearity.
We performed sensitivity analyses, including modeling Cox regressions adjusting for BMI and BMI-squared, as well as excluding the Gothenburg PPS cohort, which included exclusively males and was recruited earlier than the other cohorts.
Finally, we performed exploratory interaction analyses for pollutants demonstrating associations in main analyses, including potential modifiers such as sex, smoking status (ever vs. never smokers), BMI (, 25–30, ), and education level (up to secondary school or equivalent vs. university degree or more, excluding the Gothenburg PPS cohort, for which we lacked this covariate). These analyses were first modeled as multiplicative interaction terms in cohort-specific analyses including the main effects of exposure and effect modifier as well as their product. We then meta-analyzed each stratum of the effect modifier across cohorts. A p-value to test the difference in st2ratum specific results of the effect modifier was derived by calculating the difference in betas (b-diff) across stratum and dividing it by the square root of the sum of the squared standard errors (SEs) for each level {}. This gave us a normal standard that was then checked against standard percentiles to arrive at a p-value. Age was not included since study start and recruitment age varied by cohort, making interpretation of results difficult in relation to time trends in exposures.
The study was reviewed and approved by the Ethical Review Boards of Gothenburg (references T800-08 and T547-13), Stockholm (reference 2018/2064-32), and Umeå (reference 2015/16-31Ö).
Results
Participant Characteristics
In total, 114,758 individuals were included from all study areas (Table 1). The total numbers of incident IHD and stroke were 5,166 and 3,119, respectively. The cohorts covered a wide range of age intervals, the youngest cohort being the Umeå VIP cohort and the eldest the Gothenburg PPS cohort. The Gothenburg PPS cohort was a male cohort by design, whereas the other cohorts had a slightly higher percentage of women compared with men. The proportion of current smokers varied from 21% in the Stockholm CEANS cohort to 39% in the Gothenburg PPS cohort. Physical activity was registered differently in the Stockholm CEANS cohort compared with the other cohorts, but overall, it all showed a majority of participants having low frequency of participation or intensity of physical activity. Alcohol consumption was only registered in Stockholm CEANS and Umeå VIP, with a greater frequency of alcohol consumption in CEANS. Less than one-third of participants had a university education in all cohorts, ranging from 20% in the Gothenburg MONICA cohort to 31% in the Stockholm CEANS cohort. Individuals in the PPS cohort were more likely to have blue-collar occupations compared with other cohort members.
Table 1.
Participant characteristics.
| Characteristic | Variable level | Gothenburg PPS | Gothenburg MONICA | Stockholm CEANS | Umeå VIP |
|---|---|---|---|---|---|
| Participants () | — | 5,850 | 4,500 | 22,314 | 81,821 |
| Baseline data collection (calendar years) | — | 1970–1977 | 1985, 1990, 1995 | 1992–2004 | 1990–2013 |
| Age in 1990 [median (range)] | — | 69 (64–75) | 46 (25–64) | 48 (31–92) | 39 (9–60) |
| Women (%) | — | 0 | 52 | 58 | 53 |
| Body mass index { [mean (SD)]} | — | 25.2 (3.0) | 24.9 (3.8) | 25.5 (4.0) | 25.4 (4.0) |
| Smoking status (%) | Current smoker | 39 | 29 | 21 | 22 |
| Former smoker | 33 | N/A | 36 | 29 | |
| Never smoker | 27 | 66a | 40 | 48 | |
| Leisure time physical activity (%) | Sedentary | 24 | 17 | 61b | 40 |
| Moderate | 58 | 63 | 26c | 43 | |
| Intermediate and vigorous | 17 | 18 | 8d | 15 | |
| Alcohol consumption (%) | Daily | N/A | N/A | 7 | 7 |
| Weekly | N/A | N/A | 55 | 20 | |
| Seldom | N/A | N/A | 31 | 50 | |
| Never | N/A | N/A | 6 | 5e | |
| Married/living with partner | Yes | 86 | 47 | 70 | 81 |
| Education level (%)g | Primary school or less | N/A | 13 | 30 | 43 |
| Up to secondary school or equivalent | N/A | 32f | 36 | 29 | |
| University degree and more | N/A | 20 | 31 | 27 | |
| Occupation | Gainfully employed | N/A | N/A | 66 | 84 |
| Unemployed/not gainfully employed | N/A | N/A | 6 | 6 | |
| Retired | N/A | N/A | 27 | 5 | |
| Socioeconomic index by occupation (%) | Blue-collar | 48 | N/A | 27 | N/A |
| Low and intermediate white-collar and self-employed | 41 | N/A | 51 | N/A | |
| High-level white-collar and self-employed professional with academic degrees | 11 | N/A | 18 | N/A | |
| Mean income by SAMS in 1994 (SEK) | — | 150,181 | 155,328 | 303,910 | 122,000 |
| Ischemic heart disease events () | — | 1,640 | 405 | 1,327 | 1,794 |
| Stroke events () | — | 1,016 | 238 | 864 | 1,001 |
Note: Missing information for variables not specified. —, no data; CEANS, Cardiovascular Effects of Air Pollution and Noise Study; MONICA, Multinational Monitoring of Trends and Determinants in Cardiovascular Diseases; N/A, not available; PPS, Primary Prevention Study; SAMS, Small Areas for Market Statistics; SEK, Swedish Krona; VIPS, Västerbotten Intervention Programme.
Did not distinguish between former and never smokers. Missing data for 15% of participants.
Once a month or less/ per week.
About once a month/ per week.
Three times a week or more/ per week.
Missing data for 19% of participants.
Includes technical training.
Missing data for 35% of Gothenburg Monica participants, since question was not included in the 1985 questionnaire.
Air Pollution Characteristics
Long-range transport particles accounted for 62–65, 75, and 81% of the average yearly exposure to ; 66–67, 78, and 77% of ; and 20–21, 45, and 54% of BC in Gothenburg, Stockholm, and Umeå, respectively. Distributions of total pollutants and local pollutants and sources for each participants’ residential histories are provided in Table 2. Traffic and road wear particles were the largest local contributor to in all cohorts except Umeå VIP, and ranged up to 67% in Stockholm CEANS (Figure S1). Combustion particles from residential heating were the second largest local contributor to in Gothenburg and Stockholm and the largest in the Umeå VIP cohort, where contributions from local sources were generally the lowest (). Traffic exhaust particles contributed to between 5–10% of local across cohorts, and mean person-year levels during follow-up ranged from in Umeå VIP to in the Gothenburg PPS cohort. Traffic exhaust was the largest local contributor to BC, constituting 64–66% in Gothenburg, 52% in Umeå VIP, and 81% of local BC in Stockholm CEANS (Figure S2). Residential heating contributed to 20–21%, 48%, and 16% of BC in Gothenburg, Umeå, and Stockholm, respectively.
Table 2.
Distributions of air pollutants at residential addresses for each cohort. All units in
| Exposure | Gothenburg PPS | Gothenburg MONICA | Stockholm CEANS | Umeå VIP | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Range | IQR | Mean | Range | IQR | Mean | Range | IQR | Mean | Range | IQR | Meana | IQRb | |
| Total | ||||||||||||||
| 13.2 | 4.8–33 | 3.9 | 13.1 | 4.4–37 | 4.5 | 12.3 | 7.4–42 | 2.7 | 9.9 | 6.9–52 | 2 | 12.1 | 3.3 | |
| 9.2 | 2.9–17 | 2.4 | 8.6 | 2.9–16 | 2.7 | 7.3 | 4.7–19 | 1.5 | 5.8 | 3.6–22 | 1.12 | 7.7 | 1.9 | |
| BC | 1.0 | 0.3–4.6 | 0.39 | 0.9 | 0.2–4.3 | 0.40 | 0.67 | 0.31–4.9 | 0.3 | 0.46 | 0.2–7.8 | 0.13 | 0.76 | 0.3 |
| Local | ||||||||||||||
| 5.1 | 0.4–22 | 2.2 | 4.6 | 0.4–30 | 2.4 | 3.0 | 0.09–32 | 2.6 | 1.9 | 0.2–38 | 0.9 | 3.7 | 2.0 | |
| 3.1 | 0.2–9.6 | 1.3 | 2.8 | 0.2–9.3 | 1.5 | 1.6 | 0.07–12.7 | 1.2 | 1.3 | 0.08–15 | 0.6 | 2.2 | 1.1 | |
| BC | 0.8 | 0.05–4.3 | 0.39 | 0.7 | 0.04–4.1 | 0.4 | 0.37 | 0.007–4.6 | 0.3 | 0.21 | 0.01–4 | 0.1 | 0.52 | 0.3 |
| Residential heating | 1.4 | 0.09–6.7 | 0.6 | 1.3 | 0.07–4.3 | 0.7 | 0.71 | 0.04–12.6 | 0.4 | 0.8 | 0.09–6.7 | 0.3 | 1.1 | 0.5 |
| Wear | 2.4 | 0.1–16 | 1.4 | 2.2 | 0.2–25 | 1.5 | 2.0 | 0.01–28 | 2.2 | 0.6 | 0.07–30 | 0.5 | 1.8 | 1.4 |
| Traffic exhaust | 0.5 | 0.04–3.6 | 0.3 | 0.4 | 0.03–3.2 | 0.3 | 0.24 | 0.0008–3.8 | 0.2 | 0.1 | 0.01–7 | 0.08 | 0.32 | 0.2 |
| Industry | 0.4 | 0.01–5.2 | 0.3 | 0.2 | 0.01–5.2 | 0.2 | — | — | — | 0.1 | 0.01–3 | 0.05 | 0.23 | 0.1 |
| Shipping | 0.04 | 0–0.5 | 0.03 | 0.04 | 0–0.5 | 0.03 | 0.01 | 0.0003–0.12 | 0.01 | 0.01 | 0–0.4 | 0 | 0.03 | 0.02 |
| Other | 0.4 | 0.02–0.8 | 0.19 | 0.3 | 0.01–0.8 | 0.23 | 0.05 | 0.002–0.53 | 0.04 | 0.3 | 0–0.6 | 0.22 | 0.26 | 0.2 |
| BC | ||||||||||||||
| Traffic exhaust | 0.53 | 0.03–4.0 | 0.32 | 0.45 | 0.02–3.7 | 0.32 | 0.3 | 0.001–4.5 | 0.3 | 0.11 | 0.01–7.4 | 0.08 | 0.35 | 0.3 |
| Residential heating | 0.16 | 0.01–1.05 | 0.07 | 0.15 | 0.01–0.7 | 0.07 | 0.06 | 0.003–1.1 | 0.04 | 0.1 | 0.01–0.85 | 0.04 | 0.12 | 0.05 |
Note: —, no data; BC, black carbon; CEANS, Cardiovascular Effects of Air Pollution and Noise Study; IQR, interquartile range; MONICA, Multinational Monitoring of Trends and Determinants in Cardiovascular Diseases; , particulate matter with aerodynamic diameter ; , PM with aerodynamic diameter ; PPS, Primary Prevention Study; VIPS, Västerbotten Intervention Programme.
Mean across study mean values.
Mean of IQRs across cities.
contributions from road wear and from traffic exhaust were highly correlated (Pearson coefficients ranging from 0.95 to 0.97 across cohorts), prohibiting two-pollutant models including both sources (Table S2). However, contributions from traffic exhaust and residential heating were weakly correlated (Pearson’s coefficients: across cohorts), and contributions to BC from traffic exhaust sources and from residential heating were similarly weakly correlated (r: ), permitting two-pollutant, source-specific models.
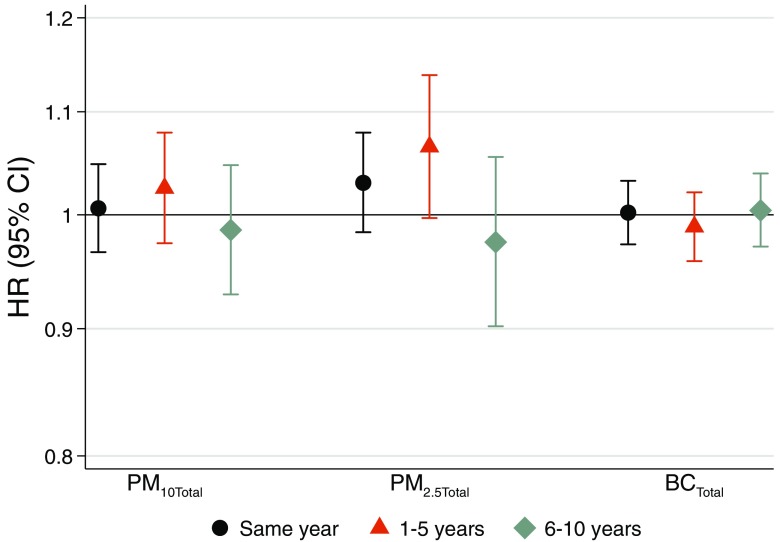
Associations with Ischemic Heart Disease
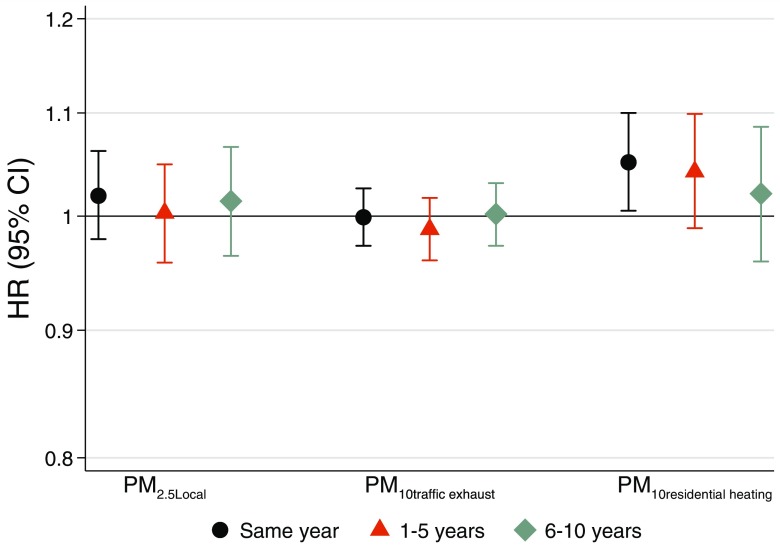
An IQR () of total at residency in the preceding 1- to 5-y time window was associated with a 6.5% (95% CI: , 14) higher risk of incident IHD (Figure 1). Other time windows, as well as total and total BC, did not demonstrate associations with IHD incidence. City-specific results are provided in Figures S3–S5. The Gothenburg PPS cohort was the main driver of the overall meta-analyses estimate for the association for the 1- to 5-y exposure time window, but this was not consistently the case for 0-y and 6- to 10-y time windows, and the statistics and p-values of the overall meta-analysis estimates did not indicate significant heterogeneity (Figure S4). Results from analysis of total for 1- to 5-y averages were not corroborated in models of locally emitted , which demonstrated no associations for any time window (Figure 2). In two-pollutant models, we observed no associations for traffic exhaust particles but a 5.1% (95% CI: 0.5, 10) higher risk of incident IHD per IQR increase () of same-year averaged PM emitted from residential heating (Figure 2). City-specific estimates of same-year levels of PM emitted from residential heating and incident IHD indicated no clear heterogeneity between the cities (Figure S6).
Figure 1.
Long-term exposure to total levels of particulate matter with aerodynamic diameter (), PM with aerodynamic diameter (), and black carbon (BC) and incident ischemic heart disease based on meta-analyses of four cohorts from Gothenburg, Stockholm, and Umeå. Hazard ratios (HRs) and 95% confidence intervals (CIs) for same year, 1–5 y, and 6–10 y preceding hospitalization or mortality for ischemic heart disease per interquartile range of (), (), and BC ().
Figure 2.
Long-term exposure to local particulate matter (PM) emissions and incident ischemic heart disease based on meta-analyses of four cohorts from Gothenburg, Stockholm, and Umeå. Estimates for PM with aerodynamic diameter () include all local sources in a single-pollutant model, and estimates for components of local sources [PM with aerodynamic diameter () traffic exhaust and residential heating] are derived from two-pollutant models. Although the components of local PM are derived from , the particle sizes of traffic exhaust and residential heating are almost exclusively in diameter. Hazard ratios (HRs) and 95% confidence intervals (CIs) for same year, 1–5 y, and 6–10 y preceding hospitalization or mortality for ischemic heart disease per interquartile range of local (), traffic exhaust (), and residential heating ().
Associations with Stroke
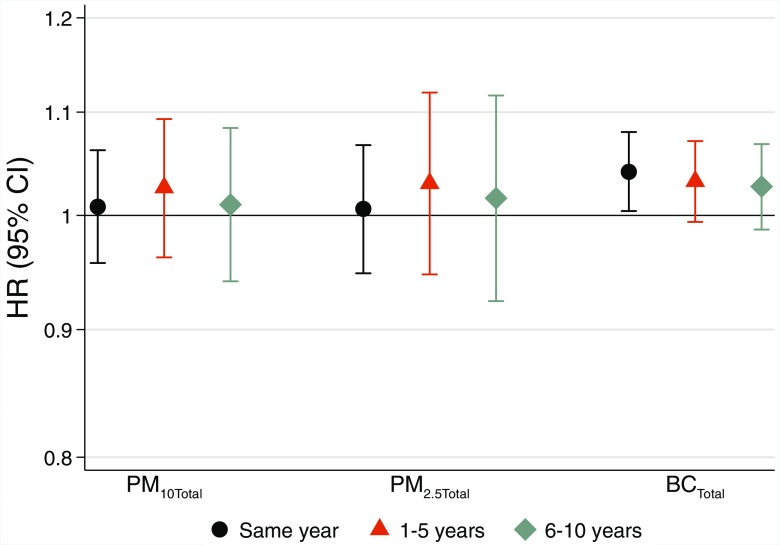
Total BC exposure in the concurrent calendar year was associated with a 4.1% per (95% CI: 0.4, 8) higher risk of incident stroke (Figure 3). Similar point estimates were observed for 1- to 5-y and 6- to 10-y time windows. Residential and levels were not associated with incident stroke, irrespective of exposure time window. City-specific estimates indicated no clear heterogeneity (Figures S7–S9).
Figure 3.
Long-term exposure to total levels of particulate matter with aerodynamic diameter (), PM with aerodynamic diameter (), and black carbon (BC) and incident stroke based on meta-analyses of four cohorts from Gothenburg, Stockholm, and Umeå. Hazard ratios (HRs) and 95% confidence intervals (CIs) for same year, 1–5 y, and 6–10 y preceding hospitalization or mortality for stroke per interquartile range of (), (), and BC ().
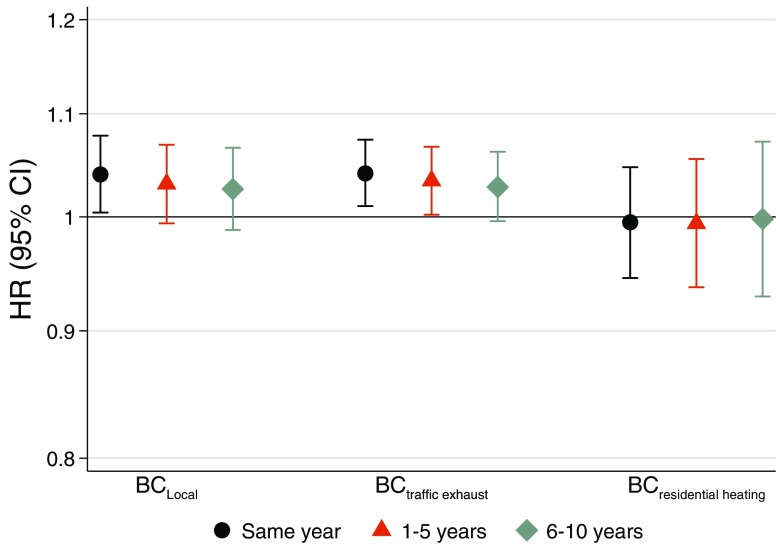
In source-specific analyses of BC exposure during the same year as stroke incidence, we included BC from traffic exhaust and BC from residential heating in a two-pollutant model. We observed stronger and more precise estimates for BC from traffic sources (4.1% per ; 95% CI: 1.0, 7.4) and no associations for residential heating sources (Figure 4). City-specific estimates for residential levels of BC from traffic sources during the same year as incident stroke indicated no heterogeneity (Figure S10).
Figure 4.
Long-term exposure to local black carbon (BC) emissions and incident stroke based on meta-analyses of four cohorts from Gothenburg, Stockholm, and Umeå. Estimates for BC include all local sources in single-pollutant models, and estimates for components of local sources (BC traffic exhaust and BC residential heating) are derived from two-pollutant models. Hazard ratios (HRs) and 95% confidence intervals (CIs) for same year, 1–5 y, and 6–10 y preceding hospitalization or mortality for stroke per interquartile range of pollutant (BC local: , BC traffic exhaust: , and BC residential heating: ).
In sensitivity analyses, results from statistical models including BMI and were very similar, with a significant association observed for 1- to 5-y averages of and incident IHD (Tables S3 and S4). Meta-analyses, excluding the Gothenburg PPS cohort, demonstrated wider CIs but similar results as the main analyses (Table S5 and S6). We did not observe differences in associations according to sex, smoking status, BMI, and education level in stratified analyses of BC and incident stroke (Table S7).
Discussion
In a meta-analysis of long-term particulate air pollution exposure and incidence of IHD and stroke in four Swedish cohorts from Gothenburg, Stockholm, and Umeå, we observed no associations between total levels of air pollutants and incident IHD but positive associations between BC exposure and incidence of stroke. Associations between BC and stroke incidence were most apparent for exposure averaged over the same year as disease incidence and from local traffic sources. Intriguingly, source-specific analyses of PM suggested positive associations between residential heating sources and same-year incident IHD.
Air Pollution and Ischemic Heart Disease
We observed results suggestive of a positive association between 1- to 5-y averages of and incident IHD; however, CIs spanned the null. The effect seemed driven by one cohort in Gothenburg where an increased risk of coronary heart disease has previously been reported among local transit and taxi drivers (Rosengren et al. 1991). We did not observe significant heterogeneity of cohort-specific results, but given the low observation counts (four cohorts), this test has low power. In addition, locally emitted was not associated with incident IHD, providing further reason for cautious interpretation. The positive association observed for total might be argued to be a result of long-range transport particles; however, our study was not designed for investigating spatial contrasts of long-range transport, using meta-analyses of city-specific estimates, and only included temporal contrasts for long-range transport contributions.
In more exploratory analyses, we observed an association between same-year averaged PM from residential heating and incident IHD. Up to 98% of the emissions from residential heating in our study were from combustion of solid fuels from wood logs or pellets. However, the uncertainty of these emissions was much higher than for traffic exhaust, especially in Stockholm and Gothenburg, where we lacked the detailed inventory of stoves and boilers from chimney sweeps obtained in Umeå (Segersson et al. 2017). The relative uncertainty of the underlying data for residential heating sources warrants cautious interpretation of observed associations from these sources. Interestingly though, the observed associations between PM from residential heating sources and incident IHD were apparent in the Umeå cohort, with the most reliable exposure assessment, and in the Gothenburg PPS cohort, and showed consistency over several time windows of exposure. PM from residential heating accounted for about a third of the local emissions and 10% or less of the total levels, possibly representing a risk for IHD independent of traffic and long-range sources of .
In previous literature, long-term exposure to was associated with higher risk of myocardial infarction incidence in two analyses of the Worcester Heart Attack Study in Massachusetts, United States, modeling exposure both by use of satellite-derived data or a latent variable regression of rotating-site monitoring data collection to construct 18-month averaged exposure (Tonne et al. 2009, Madrigano et al. 2013). The latent variable regression captured traffic particle contrasts on a spatial scale of kilometers and did not include temporal variability, similar to the limitation of land-use regression models. Both of these studies reported stronger associations for regional compared with local traffic particles. This lack of association between local and myocardial infarction is consistent with the lack of positive associations between local emissions and incident IHD in our study. Other large studies of exposure and IHD incidence did not demonstrate significant associations, such as the Nurses’ Health Study (Puett et al. 2009), the California Teachers Study (Lipsett et al. 2011), the population-based Vancouver study (Gan et al. 2011), and the Heinz Nixdorf Recall Study (Hoffmann et al. 2015). However, The Women’s Health Initiative study used a cruder exposure assessment than ours, based on residential distance to nearest monitors, and used the annual average of a single year (2000) to represent spatial contrast in exposure, disregarding any temporal component. Nevertheless, they reported a 21% increased risk of coronary heart disease per overall and stronger associations for contrasts within rather than between cities (HR: 1.21; 95% CI: 1.04, 1.42) (Miller et al. 2007). Similar nonsignificant associations were reported in a meta-analysis of 11 European cohorts demonstrating a 13% higher risk of incident IHD per (95% CI: , 30) but this primarily used land-use regression models to assess annual exposure at recruitment and consequently ignored any change in exposure between recruitment and disease incidence (Cesaroni et al. 2014).
Cesaroni et al. 2014 also observed associations between and incidence of acute coronary events in the meta-analysis of 11 European cohorts [European Study of Cohorts for Air Pollution Effects (ESCAPE)] [12% (95% CI: 1, 25) increased risk per residential average at study recruitment]. However, several other studies of and incidence of IHD are more in line with our findings and have not affirmatively demonstrated associations (Puett et al. 2008; Lipsett et al. 2011; Atkinson et al. 2013; Katsoulis et al. 2014; Hoffmann et al. 2015).
Long-term exposure to coarse particles and absorbance, a metric similar to BC, has not been associated with IHD incidence in earlier studies (Puett et al. 2009; Cesaroni et al. 2014; Hoffmann et al. 2015). A large administrative cohort study from Vancouver lacking many individual-level confounders, but with a similarly low-pollution setting as ours, reported positive associations between BC exposure assessed by land-use regression and incident IHD, in contrast to our results (Gan et al. 2011).
In summary, long-term exposure to total levels of , , and BC in our study were not significantly associated with IHD incidence, and therefore, we were unable to confirm findings in some previous literature. We observed an interesting association for a component of PM from residential heating and IHD incidence, possibly implicating a role for combustion particles from solid fuels. Studies have used different exposure assessments and applied different time windows of exposure that may influence the discrepancies observed. Our results for IHD incidence are in contrast to studies of long-term exposure and IHD mortality, where many studies report positive associations with , , and BC (Puett et al. 2008, 2009; Lipsett et al. 2011; Thurston et al. 2016; Turner et al. 2016; Hvidtfeldt et al. 2019). However, several of these studies observed weaker or no associations with IHD incidence, possibly indicating that individuals with IHD who later die of the disease may be more susceptible to long-term air pollution preceding death when they are presumably older with greater disease severity and higher prevalence of comorbidities. This would indicate that PM exposure is a weak risk factor for IHD incidence but contributes more to IHD mortality.
Air Pollution and Stroke Incidence
We observed consistent associations between BC levels estimated at the home address and risk of stroke incidence, with the strongest association suggested for same-year exposure. BC was mostly from local sources where traffic was the dominant source. Consistently with this, we observed the strongest associations with BC from traffic sources. PM and BC from traffic exhaust assessed in our study used a high level of detail for measured traffic flows separated by vehicle type as well as modeled traffic flow in areas without measurements (Segersson et al. 2017). The emission factors were estimated by vehicle type, speed, and driving conditions. We did not, however, observe associations between BC from residential heating sources and risk of incident stroke, which, if true, possibly indicates that these combustion sources are less relevant to stroke risk. The contributions of BC from long-range transport were difficult to estimate, and therefore, a constant was used for all modeling years, leading to similar results for total and local BC.
Although other studies of long-term BC exposure and stroke incidence are lacking, previous studies of other metrics of long-term ambient particulate air pollution and incident stroke have been conducted using land-use regression (Puett et al. 2011; Katsoulis et al. 2014; Hoffmann et al. 2015), inverse density weighting of residential distance to monitors (Johnson et al. 2010; Lipsett et al. 2011), satellite-derived models (Lin et al. 2017; Qiu et al. 2017), and dispersion models (Atkinson et al. 2013; Korek et al. 2015; Crichton et al. 2016; Stockfelt et al. 2017) to assess exposure to and . In contrast to our findings of no associations between or and stroke incidence, positive associations between and incident stroke have generally been reported in these studies but without demonstrated statistical significance. Compared with , studies that included long-term exposure to have, to a higher degree, reported statistically significant associations with incident stroke. A meta-analysis that included 20 studies of long-term exposure of and and incident stroke reported significant associations for both pollutants with stronger effects observed for [relative risk (RR): 1.06 per (95% CI: 1.02, 1.11)] than [RR: 1.06 per (95% CI: 1.02, 1.11)] (Scheers et al. 2015). Our results from long-term BC exposure and incident stroke fill an important knowledge gap and add to the evidence supporting the role of combustion particles from traffic exhaust in stroke incidence.
Other Considerations
Our study was designed to study associations between spatiotemporal contrasts in locally emitted particle pollution within cities and not between cities. Across our cities, there are known gradients of cardiovascular and cerebrovascular risk (Socialstyrelsen 2015), as well as in levels of pollutants (Segersson et al. 2017). In addition, for both air pollution and risk of IHD and stroke, there are concordant reductions over time (EEA 2013; Modig et al. 2013). In other words, there are independent temporal as well as spatial differences in both regional exposure and our outcomes of interest that need to be controlled for in order to analyze associations specific to long-range transported particle pollution. Since we only had citywide temporal data for long-range transport particles without geographical variation and a staggered recruitment of cohorts, as a consequence, we were not able to assess the contribution to risks of IHD and stroke incidence from long-range transport particles. Some of the inconsistencies across studies of long-term exposure to particle pollution and incident IHD or stroke may also be due to different toxicities of source components to total levels.
The literature concerning long-term air pollution exposure from particle fractions and incidence in IHD and stroke is more limited and heterogeneous than for mortality (Hoek et al. 2013). Our study, like others, only considered associations between pollutant exposure and first hospitalization or death, whichever came first. Compared with mortality studies, this also includes outcomes in healthier individuals. It is possible that air pollution exposures have stronger effects in populations who already have overt disease, which may partly explain differences between mortality and incidence studies of air pollution.
Limitations
Our study grew out of an ambitious effort to more closely understand how long-term exposure to components and sources of particulate air pollution might affect incidence in IHD and stroke, yet several limitations should be acknowledged. We aimed to disentangle associations by specific PM sources, including road wear and traffic exhaust, but we were limited in this study aim by the high correlation in their spatial dispersion due to their common generation from vehicular traffic. We could not include the effect of having a wood stove indoors, which may be important according a study from Umeå (Oudin et al. 2018). In addition, the contributions of other sources to local emissions from, for example, industry and shipping, were too small to meaningfully address in analyses. While we were able to validate the total levels of pollutants against monitoring data, we were unable to conduct validations of source components, which we recognize as a potential limitation that may have contributed to our varying estimates for source specific analyses. However, encouragingly, we did see a good agreement between modeled total levels and measured levels at monitoring stations in close proximity to traffic where emission sources are clearly dominated by traffic (Segersson et al. 2017). Although we adjusted for calendar year in our statistical models, we cannot exclude the possibility that expected time trends in PM air pollution during the study period may have affected our results. We included several cohorts in our study with a fair amount of heterogeneity regarding when they were recruited, what covariate data was collected, and distributions of characteristics such as age and sex. Some covariate data was missing entirely for some cohorts such as alcohol consumption, education, occupation, and socioeconomic index by occupation. This potentially increased the possibility for different levels of residual confounding by cohort, but the alternative approach, settling for the same set of confounders, would, as whole, most likely have introduced more confounding in the meta-analytical estimates. We created cohort-specific models to adjust for confounding, and although we tried to harmonize these to the best of our ability and used a meta-analyses methodology with random effects for cities, we cannot exclude that cohort differences may have influenced our results. We tested for heterogeneity in city-specific estimates using both the statistic and the p-value of the chi-squared test, but their power to detect heterogeneity across the four cohorts was relatively low. While we characterized incident IHD and stroke according to the first hospitalization event or death, whichever came first in our high-coverage registries, we recognize that, in some cases, the clinical debut may have occurred at an earlier date. This is expected to contribute to some nondifferential misclassification of outcome but not likely bias results substantially, since this misclassification would be expected to be largely independent of air pollution. Finally, we did not consider possible differences in associations across subtypes of stroke due to the reduced power to detect such differences and the concerns in registry data of diagnostic specificity of stroke etiology.
Several strengths should also be mentioned. We had uniquely detailed exposure data including source and components of PM and BC. We were able to acquire a large number of participants by combining several cohorts and had detailed data concerning their address history, including being able to account for any relocation during the study period. We used high-quality and comprehensive national patient and death registries, minimizing loss to follow-up for our outcomes of interest (Ludvigsson et al. 2011). We also conducted our study in a low-pollutant environment with annual averages ranging from 5.8 to , well within the current World Health Organization (WHO) air quality guideline of and considerably lower than current European Union standard of . Although we did not observe associations with total levels of and , we did observe associations for BC and stroke, shedding some doubt on the efficacy of these standards.
Conclusions
In a low-pollution-level setting, long-term exposure to BC, more specifically from local traffic exhaust sources, was associated with increased risk of incident stroke. However, we did not observe associations between total levels of , , and IHD or stroke incidence. There was some evidence of an association between specifically from local emissions of residential heating and incidence of IHD that warrant further investigation. BC may be an important air pollution metric to further guide air quality control policy and likely has broader implications than traffic particles in other environments with contributions from combustion sources such as oil or coal.
Supplementary Material
Acknowledgments
This study was funded by the Swedish Environmental Protection Agency as part of the Swedish Clean Air and Climate Research Program (grant NV-06576-13). The GOT-MONICA and PPS cohorts were supported by the Swedish Research Council, 2013-5187 (SIMSAM). The SDPP cohort was funded by the Stockholm County Council, the Swedish Research Council, the Swedish Diabetes Association, the Novo Nordisk Scandinavia, and GlaxoSmithKline. The 60YO cohort was funded by the Stockholm County Council and the Swedish Research Council. The Swedish Twin Registry is managed by Karolinska Institutet and receives funding through the Swedish Research Council under the grant no. 2017-00641. Data collection in SALT was supported by a grant from the National Institutes of Health (NIH), grant no. 1R01 AG08724. The VIP-Umeå cohort was funded by the Västerbotten County Council. P.L.L. was supported by funding from the Swedish Research Council for Health, Working Life and Welfare (FORTE) 2015-00917 and Karolinska Institute’s Strategic Research Area in Epidemiology (SFO-EPI). L.B. was financed by grants from the Swedish state under the agreement between the Swedish government and the county councils: the ALF-agreement (74580).
Footnotes
Supplemental Material is available online (https://doi.org/10.1289/EHP4757).
The authors declare they have no actual or potential competing financial interests.
Note to readers with disabilities: EHP strives to ensure that all journal content is accessible to all readers. However, some figures and Supplemental Material published in EHP articles may not conform to 508 standards due to the complexity of the information being presented. If you need assistance accessing journal content, please contact ehponline@niehs.nih.gov. Our staff will work with you to assess and meet your accessibility needs within 3 working days.
References
- Andersson S, Arvelius J, Gerner A, Danielsson H, Ortiz C, Svanström S. 2015. Description of Methods and Quality of Spatially Distributed Emissions to Air during 2015; Swedish EPA, Contract No. 309 1235; Swedish EPA: Stockholm, Sweden, 2015. [Google Scholar]
- Atkinson RW, Carey IM, Kent AJ, van Staa TP, Anderson HR, Cook DG. 2013. Long-term exposure to outdoor air pollution and incidence of cardiovascular diseases. Epidemiology 24(1):44–53, PMID: 23222514, 10.1097/EDE.0b013e318276ccb8. [DOI] [PubMed] [Google Scholar]
- Beelen R, Raaschou-Nielsen O, Stafoggia M, Andersen ZJ, Weinmayr G, Hoffmann B, et al. 2014. Effects of long-term exposure to air pollution on natural-cause mortality: an analysis of 22 European cohorts within the multicentre ESCAPE project. Lancet 383(9919):785–795, PMID: 24332274, 10.1016/S0140-6736(13)62158-3. [DOI] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AF, et al. 2010. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation 121(21):2331–2378, PMID: 20458016, 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Cesaroni G, Forastiere F, Stafoggia M, Andersen ZJ, Badaloni C, Beelen R, et al. 2014. Long term exposure to ambient air pollution and incidence of acute coronary events: prospective cohort study and meta-analysis in 11 European cohorts from the ESCAPE Project. BMJ 348:f7412, PMID: 24452269, 10.1136/bmj.f7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crichton S, Barratt B, Spiridou A, Hoang U, Liang SF, Kovalchuk Y, et al. 2016. Associations between exhaust and non-exhaust particulate matter and stroke incidence by stroke subtype in South London. Sci Total Environ 568:278–284, PMID: 27295599, 10.1016/j.scitotenv.2016.06.009. [DOI] [PubMed] [Google Scholar]
- Denby BR, Sundvor I, Johansson C, Pirjola L, Ketzel M, Norman M, et al. 2013a. A coupled road dust and surface moisture model to predict non-exhaust road traffic induced particle emissions (NORTRIP). Part 1: road dust loading and suspension modelling. Atmos Environ 77:283–300, 10.1016/j.atmosenv.2013.04.069. [DOI] [Google Scholar]
- Denby BR, Sundvor I, Johansson C, Pirjola L, Ketzel M, Norman M, et al. 2013b. A coupled road dust and surface moisture model to predict non-exhaust road traffic induced particle emissions (NORTRIP). Part 2: surface moisture and salt impact modelling. Atmos Environ 81:485–503, 10.1016/j.atmosenv.2013.09.003. [DOI] [Google Scholar]
- DerSimonian R, Laird N. 1986. Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188, PMID: 3802833, 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- EEA (European Environment Agency). 2013. Air Pollution Fact Sheet 2013. Sweden. Copenhagen, Denmark: EEA. [Google Scholar]
- Forsberg B, Hansson HC, Johansson C, Areskoug H, Persson K, Jarvholm B. 2005. Comparative health impact assessment of local and regional particulate air pollutants in Scandinavia. Ambio 34(1):11–19, PMID: 15789513, 10.1639/0044-7447(2005)034[0011:CHIAOL]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Gan WQ, Koehoorn M, Davies HW, Demers PA, Tamburic L, Brauer M. 2011. Long-term exposure to traffic-related air pollution and the risk of coronary heart disease hospitalization and mortality. Environ Health Perspect 119(4):501–507, PMID: 21081301, 10.1289/ehp.1002511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausberger S, Rexeis M, Zallinger M. 2009. Emission Factors from the Model PHEM for the HBEFA Version 3. Report Nr. I-20/2009 Haus-Em 33/08/679 from 07.12.2009. Graz, Austria: Institute for Internal Combustion Engines and Thermodynamics, Graz University of Technology. [Google Scholar]
- Higgins JP, Thompson SG. 2002. Quantifying heterogeneity in a meta-analysis. Stat Med 21(11):1539–1558, PMID: 12111919, 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Hime NJ, Marks GB, Cowie CT. 2018. A comparison of the health effects of ambient particulate matter air pollution from five emission sources. Int J Environ Res Public Health 15(6):E1206, PMID: 29890638, 10.3390/ijerph15061206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek G, Krishnan RM, Beelen R, Peters A, Ostro B, Brunekreef B, et al. 2013. Long-term air pollution exposure and cardio- respiratory mortality: a review. Environ Health 12(1):43, PMID: 23714370, 10.1186/1476-069X-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann B, Weinmayr G, Hennig F, Fuks K, Moebus S, Weimar C, et al. 2015. Air quality, stroke, and coronary events: results of the Heinz Nixdorf Recall Study from the Ruhr Region. Dtsch Arztebl Int 112(12):195–201, PMID: 25838021, 10.3238/arztebl.2015.0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hvidtfeldt UA, Sorensen M, Geels C, Ketzel M, Khan J, Tjonneland A, et al. 2019. Long-term residential exposure to PM2.5, PM10, black carbon, NO2, and ozone and mortality in a Danish cohort. Environ Int 123:265–272, PMID: 30551059, 10.1016/j.envint.2018.12.010. [DOI] [PubMed] [Google Scholar]
- Ilias V, Leonidas N, Zissis S. 2013. Methodology for the Quantification of Road Transport PM-Emissions, Using Emission Factors or Profiles. Deliverable D1.1.2, Updated February 2013 TRANSPHORM, http://www.transphorm.eu/Portals/51/Documents/Deliverables/New%20Deliverables/D1.1.2_updated.pdf [accessed 26 October 2018]. [Google Scholar]
- Jalkanen JP, Johansson L, Kukkonen J, Brink A, Kalli J, Stipa T. 2012. Extension of an assessment model of ship traffic exhaust emissions for particulate matter and carbon monoxide. Atmos Chem Phys 12(5):2641–2659, 10.5194/acp-12-2641-2012. [DOI] [Google Scholar]
- Jeong CH, Hopke PK, Kim E, Lee DW. 2004. The comparison between thermal-optical transmittance elemental carbon and Aethalometer black carbon measured at multiple monitoring sites. Atmos Environ 38(31):5193–5204, 10.1016/j.atmosenv.2004.02.065. [DOI] [Google Scholar]
- Johnson JY, Rowe BH, Villeneuve PJ. 2010. Ecological analysis of long-term exposure to ambient air pollution and the incidence of stroke in Edmonton, Alberta, Canada. Stroke 41(7):1319–1325, PMID: 20538697, 10.1161/STROKEAHA.110.580571. [DOI] [PubMed] [Google Scholar]
- Katsoulis M, Dimakopoulou K, Pedeli X, Trichopoulos D, Gryparis A, Trichopoulou A, et al. 2014. Long-term exposure to traffic-related air pollution and cardiovascular health in a Greek cohort study. Sci Total Environ 490:934–940, PMID: 24908651, 10.1016/j.scitotenv.2014.05.058. [DOI] [PubMed] [Google Scholar]
- Kaufman JD, Adar SD, Barr RG, Budoff M, Burke GL, Curl CL, et al. 2016. Association between air pollution and coronary artery calcification within six metropolitan areas in the USA (the Multi-Ethnic Study of Atherosclerosis and Air Pollution): a longitudinal cohort study. Lancet 388(10045):696–704, PMID: 27233746, 10.1016/S0140-6736(16)00378-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korek MJ, Bellander TD, Lind T, Bottai M, Eneroth KM, Caracciolo B, et al. 2015. Traffic-related air pollution exposure and incidence of stroke in four cohorts from Stockholm. J Expo Sci Environ Epidemiol 25(5):517–523, PMID: 25827311, 10.1038/jes.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Guo Y, Di Q, Zheng Y, Kowal P, Xiao J, et al. 2017. Ambient PM2.5 and stroke: effect modifiers and population attributable risk in six low- and middle-income countries. Stroke 48(5):1191–1197, PMID: 28386038, 10.1161/STROKEAHA.116.015739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsett MJ, Ostro BD, Reynolds P, Goldberg D, Hertz A, Jerrett M, et al. 2011. Long-term exposure to air pollution and cardiorespiratory disease in the California teachers study cohort. Am J Respir Crit Care Med 184(7):828–835, PMID: 21700913, 10.1164/rccm.201012-2082OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, et al. 2011. External review and validation of the Swedish national inpatient register. BMC Public Health 11:450, PMID: 21658213, 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrigano J, Kloog I, Goldberg R, Coull BA, Mittleman MA, Schwartz J. 2013. Long-term exposure to PM2.5 and incidence of acute myocardial infarction. Environ Health Perspect 121(2):192–196, PMID: 23204289, 10.1289/ehp.1205284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL, et al. 2007. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med 356(5):447–458, PMID: 17267905, 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- Modig K, Andersson T, Drefahl S, Ahlbom A. 2013. Age-specific trends in morbidity, mortality and case-fatality from cardiovascular disease, myocardial infarction and stroke in advanced age: evaluation in the Swedish population. PLoS One 8(5):e64928, PMID: 23741426, 10.1371/journal.pone.0064928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norberg M, Wall S, Boman K, Weinehall L. 2010. The Vasterbotten Intervention Programme: background, design and implications. Glob Health Action 3(1):4643, PMID: 20339479, 10.3402/gha.v3i0.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omstedt G, Bringfelt B, Johansson C. 2005. A model for vehicle-induced non-tailpipe emissions of particles along Swedish roads. Atmos Environ 39(33):6088–6097, 10.1016/j.atmosenv.2005.06.037. [DOI] [Google Scholar]
- Oudin A, Segersson D, Adolfsson R, Forsberg B. 2018. Association between air pollution from residential wood burning and dementia incidence in a longitudinal study in Northern Sweden. PLoS One 13(6):e0198283, PMID: 29897947, 10.1371/journal.pone.0198283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puett RC, Hart JE, Suh H, Mittleman M, Laden F. 2011. Particulate matter exposures, mortality, and cardiovascular disease in the health professionals follow-up study. Environ Health Perspect 119(8):1130–1135, PMID: 21454146, 10.1289/ehp.1002921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puett RC, Hart JE, Yanosky JD, Paciorek C, Schwartz J, Suh H, et al. 2009. Chronic fine and coarse particulate exposure, mortality, and coronary heart disease in the Nurses’ Health Study. Environ Health Perspect 117(11):1697–1701, PMID: 20049120, 10.1289/ehp.0900572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puett RC, Schwartz J, Hart JE, Yanosky JD, Speizer FE, Suh H, et al. 2008. Chronic particulate exposure, mortality, and coronary heart disease in the nurses’ health study. Am J Epidemiol 168(10):1161–1168, PMID: 18835862, 10.1093/aje/kwn232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu H, Sun S, Tsang H, Wong CM, Lee RS, Schooling CM, et al. 2017. Fine particulate matter exposure and incidence of stroke: a cohort study in Hong Kong. Neurology 88(18):1709–1717, PMID: 28363975, 10.1212/WNL.0000000000003903. [DOI] [PubMed] [Google Scholar]
- Rosengren A, Anderson K, Wilhelmsen L. 1991. Risk of coronary heart disease in middle-aged male bus and tram drivers compared to men in other occupations: a prospective study. Int J Epidemiol 20(1):82–87, PMID: 2066248, 10.1093/ije/20.1.82. [DOI] [PubMed] [Google Scholar]
- Scheers H, Jacobs L, Casas L, Nemery B, Nawrot TS. 2015. Long-term exposure to particulate matter air pollution is a risk factor for stroke: meta-analytical evidence. Stroke 46(11):3058–3066, PMID: 26463695, 10.1161/STROKEAHA.115.009913. [DOI] [PubMed] [Google Scholar]
- Segersson D, Eneroth K, Gidhagen L, Johansson C, Omstedt G, Nylen AE, et al. 2017. Health impact of PM10, PM2.5 and black carbon exposure due to different source sectors in Stockholm, Gothenburg and Umea, Sweden. Int J Environ Res Public Health 14(7):E742, PMID: 28686215, 10.3390/ijerph14070742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socialstyrelsen. 2015. Regional comparisons 2014 public health. https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/artikelkatalog/oppna-jamforelser/2015-9-2.pdf [accessed 26 October 2018].
- Stafoggia M, Cesaroni G, Peters A, Andersen ZJ, Badaloni C, Beelen R, et al. 2014. Long-term exposure to ambient air pollution and incidence of cerebrovascular events: results from 11 European cohorts within the ESCAPE project. Environ Health Perspect 122(9):919–925, PMID: 24835336, 10.1289/ehp.1307301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockfelt L, Andersson EM, Molnár P, Gidhagen L, Segersson D, Rosengren A, et al. 2017. Long-term effects of total and source-specific particulate air pollution on incident cardiovascular disease in Gothenburg, Sweden. Environ Res 158:61–71, PMID: 28600978, 10.1016/j.envres.2017.05.036. [DOI] [PubMed] [Google Scholar]
- Swedish National Board of Health and Welfare (Socialstyrelsen). 1986. Klassifikation av sjukdomar 1987: Systematisk förteckning. Nordstedt Tryckeri, Stockholm. ISBN 91-38-09347-2. [Google Scholar]
- Thiébaut AC, Bénichou J. 2004. Choice of time-scale in Cox’s model analysis of epidemiologic cohort data: a simulation study. Stat Med 23(24):3803–3820, PMID: 15580597, 10.1002/sim.2098. [DOI] [PubMed] [Google Scholar]
- Thurston GD, Burnett RT, Turner MC, Shi Y, Krewski D, Lall R, et al. 2016. Ischemic heart disease mortality and long-term exposure to source-related components of U.S. fine particle air pollution. Environ Health Perspect 124(6):785–794, PMID: 26629599, 10.1289/ehp.1509777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonne C, Yanosky J, Gryparis A, Melly S, Mittleman M, Goldberg R, et al. 2009. Traffic particles and occurrence of acute myocardial infarction: a case-control analysis. Occup Environ Med 66(12):797–804, PMID: 19553228, 10.1136/oem.2008.045047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner MC, Jerrett M, Pope CA 3rd, Krewski D, Gapstur SM, Diver WR, et al. 2016. Long-term ozone exposure and mortality in a large prospective study. Am J Respir Crit Care Med 193(10):1134–1142, PMID: 26680605, 10.1164/rccm.201508-1633OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (World Health Organization). 2015. International Statistical Classification of Diseases and Related Health Problems, 10th Revision. http://apps.who.int/classifications/icd10/browse/2016/en [accessed 6 November 2015].
- WHO MONICA Project Principal Investigators. 1988. The World Health Organization MONICA Project (monitoring trends and determinants in cardiovascular disease): a major international collaboration. J Clin Epidemiol 41(2):105–114, PMID: 3335877, 10.1016/0895-4356(88)90084-4. [DOI] [PubMed] [Google Scholar]
- Wilhelmsen L, Berglund G, Elmfeldt D, Tibblin G, Wedel H, Pennert K, et al. 1986. The multifactor primary prevention trial in Göteborg, Sweden. Eur Heart J 7(4):279–288, PMID: 3720755, 10.1093/oxfordjournals.eurheartj.a062065. [DOI] [PubMed] [Google Scholar]
- Wilhelmsen L, Johansson S, Rosengren A, Wallin I, Dotevall A, Lappas G. 1997. Risk factors for cardiovascular disease during the period 1985–1995 in Goteborg, Sweden. The GOT-MONICA Project. J Intern Med 242(3):199–211, PMID: 9350164, 10.1046/j.1365-2796.1997.00163.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.