Abstract
What is known as an odor object is an integrated representation constructed from physical features, and perceptual attributes mainly mediated by the olfactory and trigeminal systems. The aim of the present study was to comprehend how this multidimensional representation is organized, by deciphering how similarities in the physical, olfactory and trigeminal perceptual spaces of odors are represented in the human brain. To achieve this aim, we combined psychophysics, functional MRI and multivariate representational similarity analysis. Participants were asked to smell odors diffused by an fMRI‐compatible olfactometer and to rate each smell along olfactory dimensions (pleasantness, intensity, familiarity and edibility) and trigeminal dimensions (irritation, coolness, warmth and pain). An event‐related design was implemented, presenting different odorants. Results revealed that (i) pairwise odorant similarities in anterior piriform cortex (PC) activity correlated with pairwise odorant similarities in chemical properties (P < 0.005), (ii) similarities in posterior PC activity correlated with similarities in olfactory perceptual properties (P <0.01), and (iii) similarities in amygdala activity correlated with similarities in trigeminal perceptual properties (P < 0.01). These findings provide new evidence that extraction of physical, olfactory and trigeminal features is based on specific fine processing of similarities between odorous stimuli in a distributed manner in the olfactory system. Hum Brain Mapp 37:2161–2172, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: mental representation, similarity, smell, trigeminal, piriform, amygdala
Abbreviations
- LO
Lateral occipital complex,
- MDS
Multidimensional scaling,
- OFC
Orbito‐frontal cortex,
- PC
Piriform cortex,
- PCA
Principal component analysis,
- ROI
Region of interest,
- RSA
Representational similarity analysis,
- SPM
Statistical parametric mapping,
- TR
Time repetition
INTRODUCTION
An important issue in sensory and cognitive neuroscience is how the human brain mentally represents external stimuli. The physical and perceptual attributes of visual stimuli are represented in a distributed hierarchical manner [Nassi and Callaway, 2009; Van Essen et al., 1992] but, in the olfactory modality, it is still not clear how the chemical properties of odorant molecules and perceptual aspects of odors are represented along the human olfactory pathway.
In the visual system, several theories have suggested that discrimination between stimuli is related to their distance or similarity in brain representational spaces [Ashby and Lee, 1991; Edelman, 1998]. Functional MRI studies of the human visual system have demonstrated the relevance of the concept of similarity in understanding the representational spaces of the physical and perceptual components of visual input. For example, in a pairwise visual stimulus comparison, Haushofer, Livingstone and Kanwisher [Haushofer et al., 2008] showed that similarities in posterior lateral occipital complex (LO) patterns most closely matched similarities in physical shape, whereas similarities in anterior LO patterns most closely matched similarities in perceptual shape.
In human olfaction, whereas the concept of similarity has been largely studied at both physical and perceptual levels [Dravnieks et al., 1978; Gregson and Mitchell, 1974; Khan et al., 2007; Snitz et al., 2013], far less is known about similarity coding at the cerebral level. When an odorant molecule is sniffed, it reaches the nasal mucus and binds to one or several types of olfactory receptor. Beside this olfactory route, most odorant molecules are able to stimulate the intranasal trigeminal system that conveys sensations such are irritation or pain. This olfactory/trigeminal information is then transmitted to primary and secondary areas and a mental representation is thus generated from a combination of chemical and perceptual components. The chemical features of an odorant can be described by multiple molecular properties, including atom type, carbon chain length, type of bonds or functional groups etc., defining a multidimensional chemical space [Haddad et al., 2008]. Perceptual aspects notably include pleasantness, familiarity, intensity and edibility [Boesveldt et al., 2010; Small et al., 2005; Yeshurun and Sobel, 2010]. Moreover, the trigeminal attributes of smells consist in sensations such as irritation, pain, warmth and coolness [Hummel, 2000].
The present study firstly tested the hypothesis that similarities between the chemical features of an odorant and between its perceptual features are processed differently, along specific neural pathways. A candidate site likely to fulfill the function of this evaluation of distance is the piriform cortex (PC), since previous animal and human studies showed that the anterior PC encodes information about the chemical attributes of odorants, and the posterior PC encodes information about the perceptual quality of odors [Gottfried et al., 2006; Howard et al., 2009; Kadohisa and Wilson, 2006]. Secondly, the hypothesis that similarities in olfactory and in trigeminal perceptual spaces are represented in distinct areas was examined by considering additional regions known to be involved in both olfactory and intranasal trigeminal processing [Moessnang et al., 2013; Savic et al., 2002]: the orbito‐frontal cortex (OFC) and also the amygdala since it receives afferent projections from the trigeminal system [Hummel and Livermore, 2002]. Investigation combined psychophysics, functional MRI and representational similarity analysis (RSA), a multivariate statistical method used to characterize neural representations by distance functions within the response patterns evoked by different stimuli [Nili et al., 2014].
MATERIAL AND METHODS
Participants
Fifteen right‐handed volunteers were tested (seven males, eight females; mean age, 22.13 ± 4.41 years). Absence of olfactory deficit was assessed using the European Test of Olfactory Capabilities [Joussain et al., 2015], and detailed medical history combined with ENT examination ascertained that subjects were in good health. They received financial compensation for the time spent in the laboratory. The recording procedure was explained in great detail to the subjects, who provided written consent prior to participation. The study was conducted according to the Declaration of Helsinki and was approved by the Lyon Sud‐Est ethics committee.
Odorants
Odorants were delivered by an air‐dilution olfactometer, described in detail in Sezille et al. [Sezille et al., 2013]. Six odorants were used (CID and Odorant code): propanol (1031; PRO), isoamyl acetate (31276; ISO), benzaldehyde (240; BEN), citronellal (7794; CAL), citronellol (8842; COL) and trans‐2‐hexenyl acetate (17243; THA) (all from Sigma–Aldrich and diluted in mineral oil). To ensure iso‐intense perception, odorants were individually diluted to vol/vol concentrations of 22.5%, 15%, 0.6%, 75%, 1.5% and 1.5%, respectively for COL, CAL, ISO, BEN, PRO, and THA.
Odor descriptions provided by the Arctander atlas [Arctander, 1994] and The Good Scents Company (http://www.thegoodscentscompany.com) show that these odorants are qualified with the following terms: almond, apple, banana, citrus, floral, fruity, green, pear, rose, and woody (source: Arctander) and cherry, musty, spicy, waxy, alcoholic (source: The Good Scents Company). These terms (except alcoholic) are well spread in the olfactory perceptual space such as the one illustrated by Zarzo and Stanton [Zarzo and Stanton, 2006].
Experimental Procedure
An event‐related design was used, comprising the odorants (10 trials per stimulus, 20‐second inter‐stimulus interval, and 5‐second stimulus duration) and a non‐odorized clean air condition, all trials distributed randomly across 5 fMRI scans (sessions). During each trial, participants were instructed to breathe naturally, and odorants were diffused synchronously with the subject's nasal respiration: a 5‐second stimulus duration was chosen because the odor was released during exhalation and had to be maintained during at least the whole duration of the subsequent inhalation (∼2 sec). The recorded signals were: respiratory signal, odor valve opening and time repetition (TR) signal from the fMRI scanner, enabling event‐related statistical analysis. Subject's respiratory signal was acquired using an airflow sensor that was integrated on an amplifier interface. A microbridge mass airflow (AWM2100V, Honeywell, MN) allowed acquisition of both inhalation and exhalation phases. The airflow sensor was connected to a nasal cannula (Cardinal Health, OH; 2.8mm inner diameter tube) positioned in both nostrils. Sniffing was digitally recorded at 100 Hz and stored in a computer. Sniffs were preprocessed by removing baseline offsets, and aligned in time by setting the point where the sniff entered the inspiratory phase as time zero. Inspired volume was calculated for the first sniff of every trial and was used as a covariate in the fMRI contrast estimation.
At the end of the fMRI sessions, participants were asked to rate the odorants in terms of intensity, pleasantness, familiarity, edibility, warmth, coolness, irritation and pain, using a visual rating scale ranging from −2 (very unpleasant) to 2 (very pleasant), or from 0 (not at all intense, familiar, edible, warm, cool, painful (pungent) or irritating) to 4 (very intense, familiar, edible, warm, cool, painful (pungent) or irritating). Possible changes in the perception of intensity and irritation along the experiment have been tested with five participants (one woman, four men, aged 28.6 ± 7.5 years). The exact same protocol of odor presentation as described earlier was used (random presentation of 60 odorous and 12 clean air trials during five sessions), with 0–4 intensity and irritation rating after each trial. Because we were interested in distances between odors, we computed intensity and irritation distances between the six odorants (according to the procedure presented in the Data Analysis Section), averaged them by session and conducted a two‐way ANOVA with odor pairs and sessions as within‐subjects factors. We found no significant effect of the session (intensity: F (4,56) = 1.34, P = .297; irritation: F (4,56) = 1.10, P = .392), showing that distances between odorants were the same across sessions. Finally, a similar ANOVA with odors and sessions, followed by post‐hoc t‐tests corrected for repeated testing (Bonferroni), revealed that there was no consistent linear decrease or increase of raw intensity and irritation ratings across the sessions. The significant and nearly significant effects of the session were due to a significant difference between only two sessions that could not be predicted by sensitization or habituation effects (intensity: F (4,24) = 2.82, P = .060, session 1 > session 3; irritation: F (4,24) = 7.51, P < .01, session 2 > session 4).
fMRI Data
The experiment, which lasted approximately 60 min (from subject's arrival to departure), was performed on a 1.5 Tesla MR‐scanner (Siemens Magnetom). The fMRI data were collected in 142 volumes/session (interleaved, AC‐AP acquisition) with a 29 axial‐slice 2D EPI sequence (matrix: 64 × 64; TR: 2,500 ms; TE: 50 ms; FA: 90°; voxel size: 3.43 × 3.43 × 3.4mm; FOV: 220). In the 9 minutes immediately following the fMRI session, a high‐resolution T1‐weighted brain image (3D MPR sequence: TR = 1,970ms/TE = 3.93ms) was acquired. fMRI data were preprocessed using statistical parametric mapping (SPM). Preprocessing steps comprised realignment and coregistration with the anatomical T1. To keep the finest grained pattern of activity, no smoothing was performed. To obtain the estimated activity of each voxel for each condition, voxel responses were modeled using a design matrix built with standard linear hemodynamic response and experimental condition onsets. Nasal respiration and motion parameters were included in the model to remove potential confounding effects. A region of interest (ROI) approach was used in which patterns of neural activity in the anterior PC, posterior PC, amygdala and OFC (first taken as a single entity, and second by dissociating its medial, lateral, anterior and posterior parts) were extracted for each odorant condition and each participant (Fig. 1). ROI were dawn using MRIcro (http://www.mricro.com) with reference to the human brain atlas of Mai [Mai et al., 1997]. Each region was drawn individually for each participant from coronal and axial slices in both hemispheres.
Figure 1.
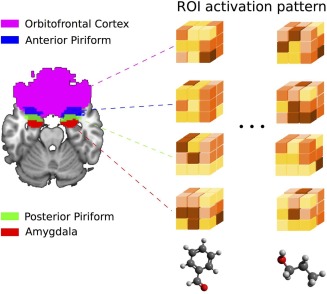
Illustration of activation pattern extraction in the different regions of interest (ROI). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Data Analysis
RSA was used to assess similarity between neural spaces on the one hand and chemical, olfactory and trigeminal spaces on the other hand.
Neural spaces were constructed by extracting the activity pattern (vector of the voxel‐based contrast “odorant vs. air”) within individuals of each odorant for a particular ROI and the similarity in activity patterns between odors was represented by a matrix comprising the 6 experimental odorant conditions. In this matrix, each cell value represented the distance (or degree of dissimilarity) between the distributed brain activities generated by a pair of stimuli. The distance was measured as a Pearson distance (1 minus the Pearson correlation). These individual matrices were then averaged to form a group matrix for a particular neural space. These group matrices were then included in the statistical analyses.
To measure similarity in the olfactory perceptual space, a four‐dimensional space comprising intensity, pleasantness, familiarity and edibility ratings was constructed. Here, for each participant, the 4‐dimensional perceptual pattern was compared between odors (using a Euclidian distance metric), resulting, as for the neural spaces, in an olfactory similarity matrix or space between odors. The same analysis was conducted for the trigeminal similarity space, comprising four dimensions (warmth, coolness, irritation and pain). Again, participant's matrices were averaged to create group matrices for both the olfactory and the trigeminal spaces.
Similarity between odorants in the chemical space was measured using physicochemical descriptors generated by Dragon software (Talete ®). Each of the 6 odorants was defined by thousands of physicochemical descriptors projected into a principal component analysis (PCA), in which the 20 first components that covered 95% of the original variance were kept to form a 20‐dimensional chemical pattern. This pattern was compared between odorant stimuli. As for the neural spaces and the perceptual spaces, the chemical space was thus represented by a 6 × 6 matrix comprising all odorant conditions. Each cell value of the matrix represented the chemical similarity (Euclidian distance) between pairs of stimuli.
Regarding the choice of the metrics for similarity assessment, it must be noted that (i) Euclidian (rather than Pearson) distances were used for the perceptual and trigeminal spaces because of the limited number of data points in each vector (4), (ii) Pearson distances were preferred for neural space based on recent literature recommendations [Connolly et al., 2012; Kriegeskorte, 2008; Long Sha et al., 2015; Nili et al., 2014], and (iii) Euclidian distances metric was used for the chemical space for homogeneity purposes in the comparisons between neural and the other spaces.
RESULTS
Perceptual Differences Between Odorants
Figure 2 illustrates perceptual ratings for all six odorants. One‐way ANOVAs revealed that whereas no effect of odorants were seen for coolness [F(5,70) = 0.67, P > 0.05] and warmth [F(5,70) = 0.26, P > 0.05], the stimuli significantly differed in terms of irritation [F(5,70)=5.86, P < 0.0002], pain [F(5,70)=6.30, P < 0.0001], intensity [F(5,70)=3.13, P < 0.02], pleasantness [F(5,70)=6.09, P < 0.0001], familiarity [F(5,70)=8.03, P < 0.00001] and edibility [F(5,70)=7.85, P < 0.00001].
Figure 2.
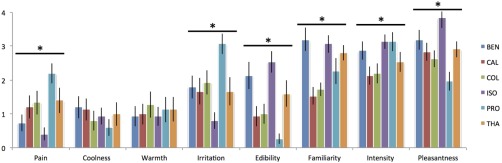
Perceptual ratings of the six chemosensory stimuli (PRO for propanol, ISO for isoamyl acetate, BEN for benzaldehyde, CAL for citronellal, COL for citronellol and THA for trans‐2‐hexenyl acetate). * means a significant effect of the odorant condition at the probability level of 5%. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Control for Perceptual Similarity
A control experiment was conducted to test whether odor similarity based on only four scales (intensity, pleasantness, familiarity, and edibility) was appropriate to represent olfactory perceptual proximity. Eleven participants (three women, nine men, aged 33.8 ± 9.7 years) who were independent from the main study were presented with each of the six odorants once, in the same conditions as during the fMRI study. They were asked to describe the odors, using a scale from 1 (not at all applicable) to 9 (very applicable), with 25 descriptors chosen as follows. We first localized the 15 terms typically used to describe the odorants (see Odorants section: sources Arctander atlas and The Good Scents Company website) in the olfactory perceptual space of Zarzo and Stanton [Zarzo and Stanton, 2006], among the many descriptors that are represented on the first two components of a PCA. Then, we chose 10 other descriptors so that the resulting 25 descriptors were well spread in the perceptual space. Consequently, animal, butter, cinnamon, coffee, ethereal, herbaceous, honey, medicinal, minty, and smoky completed the list. To analyze the participants' responses, an average 25‐dimensional perceptual space was constructed (based on each participant's distances between odors, see Data Analysis Section) and compared with the four‐dimensional perceptual space used in the main experiment. These two spaces were significantly and positively correlated (Spearman correlation rs = 0.56, P < .05), suggesting that the particular four‐dimensional space we used in the main study is adequate to represent odor perceptual similarities.
fMRI Activations and Representational Similarity Analysis
First, we determined the main effect of odors by contrasting the odorant conditions with the non‐odorized condition. This confirmatory group analysis revealed significant activations in PC, amygdala and OFC (Fig. 3).
Figure 3.
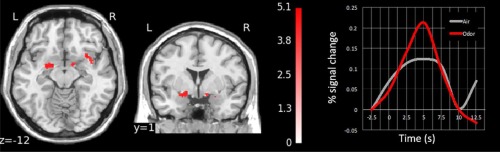
Odor‐induced activations in piriform cortex, amygdala and OFC in coronal, and axial views (P < 0.001 uncorrected). The right panel depicts peri‐stimulus plot activity in piriform cortex. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Second, the RSA method allowed us to test the extent to which the different spaces were similar. In this analysis, a Spearman distance (D S, corresponding to 1 minus the Spearman correlation) between each of the spaces was measured, and permutation tests for 10,000 permutations assessed the significance of the correlations. Since multiple testing was performed, P ‐values were adjusted using a FDR (false discovery rate) controlling procedure [Benjamini and Hochberg, 1995]. In total, three correlation analyses were performed between the neural spaces and (i) the chemical space, (ii) the olfactory perceptual space, and (iii) the trigeminal perceptual space.
Results showed that pairwise stimulus similarities in anterior PC activity patterns closely correlated with pairwise stimulus similarities in the chemical space, whereas pairwise stimulus similarities in posterior PC activity patterns matched similarities in the olfactory perceptual space, and similarities in amygdala activity patterns correlated with similarities in the trigeminal perceptual space (Fig. 4a; highest level of correlation/similarity in blue). These correlation matrices were projected into a multidimensional scaling (MDS) representation (Fig. 4b) to illustrate the proximity between: (i) the olfactory perceptual space and the posterior PC activity pattern; (ii) the chemical space and the anterior PC activity pattern, and (iii) the trigeminal perceptual space and the amygdala activity pattern.
Figure 4.
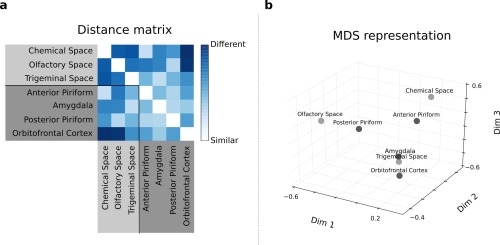
(a) Distance matrix: each square element of this matrix represents the distance (1 minus Spearman correlation) between pairs of spaces. (b) MDS representation of the distance matrix depicted in (a): each point represents a particular space, and the distance between two points represents the degree of similarity between the spaces (the smaller the distance, the greater the similarity). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
These correlations were statistically significant on permutation tests: D S = 0.2964, P < 0.005 for the correlation between chemical space and anterior PC activity, and D S = 0.3501, P < 0.01 for the correlations between olfactory space and posterior PC activity and D S = 0.3178, P < 0.01 between trigeminal space and amygdala activity (Figs. 5 and 6). By contrast, there was no significant correlation between OFC activity pattern and any of the chemical or perceptual similarity matrices. To examine whether the absence of significant relationship between OFC activity and the chemical and perceptual spaces may be due to the known heterogeneity of response within this area during olfactory processing [Anderson et al., 2003; de Araujo et al., 2005; Gottfried et al., 2002], we performed RSA in four OFC sub‐regions, namely anterior, medial, posterior and lateral OFC. As in the main analysis, results showed that pairwise stimulus similarities in activity patterns of the OFC sub‐regions did not correlate significantly with pairwise stimulus similarities in the chemical, olfactory or trigeminal spaces (Fig. 7; P > 0.05 in all cases).
Figure 5.
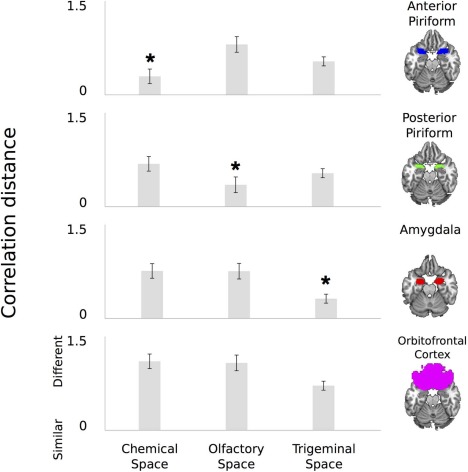
Correlation distances between neural spaces on the one hand, and chemical, olfactory and trigeminal spaces on the other hand. Error bars illustrate standard errors. * corresponds to a significant correlation at the corrected threshold of P < 0.05 (estimated by permutation tests). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 6.
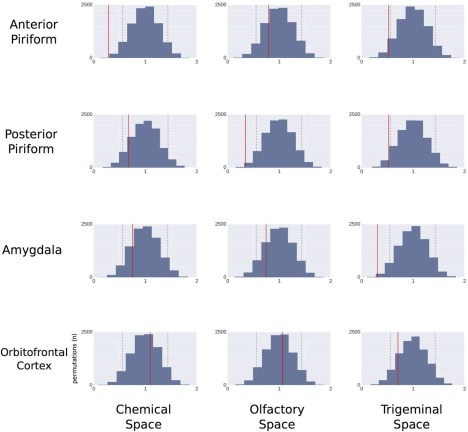
Permutations distribution of correlation distances between neural spaces (anterior piriform, posterior piriform, amygdala, OFC) on the one hand, and chemical, olfactory and trigeminal spaces on the other hand. On each graph, dotted gray lines represent the 5% confidence interval. Solid lines illustrate real correlation distance values. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 7.
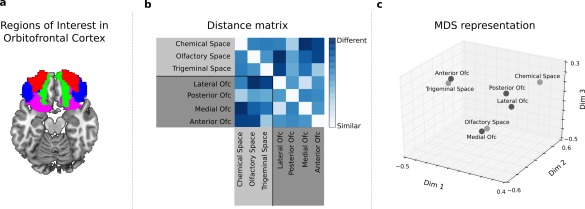
Representational Similarity Analysis in OFC sub‐regions. (a) Anatomical regions of interest in OFC (averaged across participants for visualization purposes; medial in green, posterior in purple, lateral in blue, anterior in red). (b) Distance matrix: each square element of this matrix represents the distance (1 minus Spearman correlation) between pairs of spaces. (c) MDS representation of the distance matrix depicted in (b): each point represents a particular space, and the distance between two points represents the degree of similarity between the spaces (the smaller the distance, the greater the similarity). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
In sum, these findings showed that different areas of the olfactory system, namely the anterior PC, posterior PC and amygdala, extract physical and perceptual (olfactory and trigeminal) similarities in a distributed manner.
Procrustes Analysis
Finally, to examine how odorants are close in the two spaces of interest, a complementary Procrustes analysis was performed comparing the alignment of the same set of odorants in two different spaces. This analysis uses a standard linear transformation algorithm that consists in scaling, rotating, and translating the objects (odorants) in the different spaces (neural on the one hand, and chemical, olfactory, trigeminal on the other hand). The goodness of this alignment was based on the sum of the square errors between the two spaces. The statistical significance of this alignment criterion was evaluated by permutation procedures: the neural matrix was randomized using label permutation (since the study included 6 odorants (labels), we generated 720 possible permutations). The analysis of the similarities between the chemical space and anterior piriform activity showed that the distances between odorants were well preserved across spaces. The same findings were observed when considering the correlation between the olfactory perceptual space and posterior piriform activity, and between the trigeminal perceptual space and amygdala activity; here again, a close correspondence of pairwise distances between odorants (relative alignment) in each of the two spaces (perceptual vs. neural spaces) was observed (Fig. 8a–c; P < 0.009 in all cases).
Figure 8.
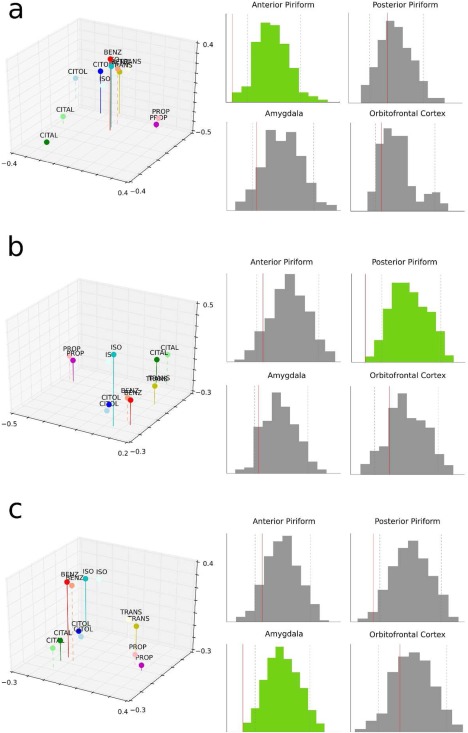
Procrustes analysis comparing odorant alignments in: (a) anterior piriform space vs. the chemical space, (b) posterior piriform space vs. the olfactory space, and (c) amygdala space vs. the trigeminal space. The histograms on the right side of the panels correspond to the permutation test distributions between neural spaces (anterior piriform, posterior piriform, amygdala, OFC) on the one hand, and chemical, olfactory and trigeminal spaces on the other hand. On each graph, dotted gray lines represents the 5% confidence interval. Solid lines illustrate real procruste distances values. Significant alignments after FDR corrections are depicted in lighter color (green in the online version). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
DISCUSSION
Philosophical theories have proposed several definitions of mental representations. While empiricism gives a central role to the senses, rationalism assumes the pre‐existence of reason and the need to take into account mental abilities and reasoning as sources of cognition. From this last perspective, cognitive activity transforms physical and sensory matter into knowledge [Gallina, 2006]; thus, any mental representation requires physical, sensory and cognitive experience to be built and organized. Psychological and biological investigations provide evidence of the existence of such multiple levels of representation of the “objects” of the perceived world, showing that “traces” of environmental objects are extracted and processed by different neural subsystems. This question was formulated and discussed mainly in the visual and auditory areas whereby similarity was introduced as a central concept in mental representation theories [Ashby and Lee, 1991; Edelman, 1998]. As regards olfactory representations, we know far less about the coding of similarity. The main aim of our study was to decipher how similarities in the chemical, olfactory and trigeminal perceptual spaces of odors are represented in the human brain.
A first result of interest was that whereas similarity in the chemical space of odorants was correlated with similarity in anterior PC activity, similarity in the olfactory perceptual space was correlated with similarity in posterior PC. Past studies suggested that PC is more than a primary olfactory relay and plays an active role from sensory to more cognitive aspects of odor perception. The functional heterogeneity of the PC, in humans as in rodents [Kadohisa and Wilson, 2006; Litaudon et al., 1997; Wilson, 2003], was revealed by studies showing that neural activity in the anterior and in the posterior PC is tuned to different aspects of odor perception [Bensafi et al., 2007; Gottfried et al., 2002; Zelano et al., 2005]. For example, in a series of experiments, Gottfried et al. sought to determine whether the PC encoded information about odorant structure and/or quality [Gottfried et al., 2006; Howard et al., 2009] using odorants that varied in quality (lemon‐like and vegetable‐like) and in chemical structure (alcohol and aldehyde) in a cross‐adaptation paradigm. Their results revealed a dissociation whereby the anterior PC responded to variation in odorant structure whereas the posterior PC coded odor quality. The authors noted that such dual odor representation within different portions of the PC is in line with the functional anatomy of the olfactory system: the anterior PC (recipient of structure‐based code) should be viewed as a first relay from the olfactory bulb, while the posterior PC integrates odorant structure information into a qualitative representation (for more details about this theory, see a review by Gottfried [Gottfried, 2010]). Our findings not only strengthen this concept that the physicochemical and perceptual properties of smells are represented differently, but also and above all provide new evidence that extraction of such physical‐like and perceptual‐like features is based on fine processing of similarities between odorous stimuli in a distributed manner in the PC.
In terms of percept, what is actually known as “smell” is in fact constituted by diverse chemosensory attributes mainly mediated by both the olfactory system and the trigeminal system [Hummel and Livermore, 2002]. The two systems contribute to construct a whole sensory experience, and have complementary functions. Olfactory perceptual attributes enable recognizing environmental sources such as food or flowers. The trigeminal system plays also a fundamental role in shaping chemosensory representation. It provides relevant information regarding sensations like pain, irritation, warmth or coolness produced by almost all odorant molecules [Hummel, 2000]. These sensations enable everyone to detect irritant or poisonous chemicals in order to avoid them. On the other hand, the trigeminal system can also be involved in detection and recognition of appetitive environmental sources (e.g., through the freshness of a minty candy). A second result of interest in our study was that similarity between trigeminal attributes was correlated with similarity in amygdala activity pattern. This finding has anatomical supports. Indeed, cell bodies of trigeminal afferents lie in the gasserian ganglion. Their axons project to the spinal nucleus, and trigeminal information is then relayed to the amygdala from the trigeminal sensory nuclei via the lateral parabranchial complex [Brand, 2006]. The amygdala is a heterogeneous structure of the medial temporal lobe involved in emotional processing of sensory information. A prominent role of this region has been demonstrated during the processing of fearful and aversive stimuli [Morris et al., 1996; Zald and Pardo, 1997], but also during the treatment of pleasant environmental stimuli [Hamann et al., 2002]. In the olfactory domain, increased amygdala activity was observed in relation to increase in odor intensity [Anderson et al., 2003] or odor emotional salience [Winston et al., 2005]. In the trigeminal system, sensations conveyed by the fifth cranial nerve (pain, irritation, warmth, coolness) are “features” of high biological relevance since they often enable us to avoid harmful sources for our biological balance. Our findings suggest that these features, alone or in conjunction with others, are detected and processed by the amygdala. In sum, combined with the above, our findings confirmed that the human amygdala is involved in detecting relevant emotional stimuli, and to the best of our knowledge, they generalize this concept for the first time to the trigeminal system.
While the present study provides new evidence that chemical and olfactory/trigeminal perceptual dimensions of smells are treated in a distributed manner, some of our methodological choices and findings require discussion. First, using more odorants and more odor repetitions would likely increase the statistical power of our analyses, and therefore enable better discrimination between subtle perceptual features.
Second, whereas one would expect a significant relationship between odorant physicochemical and perceptual properties [Amoore et al., 1964; Haddad et al., 2010; Joussain et al., 2011; Kermen et al., 2011; Khan et al., 2007; Mandairon et al., 2009; Poncelet et al., 2010; Shiffman, 1974; Zarzo, 2011], we did not observe such correlation in the current study. Different factors may explain this lack of relationship including the small number of odors and perceptual descriptors used. Another factor could be the number of physicochemical properties considered in the analysis. Indeed, whereas we chose to analyze the chemical similarity in a space containing more components and explaining 95% of the total variance, previous studies linked the perceptual space (often its pleasantness dimension) with a reduced number of physicochemical components (often the main components explaining between 30 and 50% of the total variance). To check whether such a link could be observed in our dataset when we only consider the 3 first principal components (PC1, PC2, PC3) of the physicochemical space, we performed correlation analyses between these PCs on the one hand and perceptual and trigeminal dimensions on the other hand. We replicated the findings of previous studies by finding a significant link: (i) between similarities on PC2 and pleasantness dimension (r = 0.68, P = 0.005) and (ii) between similarities on PC3 and trigeminal space (r = 0.65, P = 0.01).
Third, in our experiment, similarities between odorant molecules in chemical and perceptual spaces did not match similarities in any OFC sub‐region activity patterns. The involvement of the OFC in odor processing has been shown by a large number of brain imaging studies: OFC activity in response to smells is modulated by cognitive experimental tasks [Zatorre et al., 2000] and reflects assignment of hedonic value [Anderson et al., 2003; Rolls et al., 2003]. OFC is most likely involved in the construction of mental representation of odors but its activation pattern may reflect something else than chemical similarity or perceptual similarity. It may reflect identification or proximity in terms of semantic category, two dimensions that were not tested in the current study. Interestingly, its modulation by verbal labeling has been shown in odor processing [Bensafi et al., 2014; de Araujo et al., 2005].
One question that may be raised from the current findings is how relevant they are in the light of the processing of novel odorant stimuli. Odor identification under varying contextual conditions is one of the challenges the human olfactory system has to face. To accurately estimate the familiarity of an odor object, the system may consider the degree of similarity with previously collected odor objects, representations of which were stored in the course of experience. Once the representation most similar to the new odorant object is activated, it is then useful to define the category (“edible fruit,” “pleasant flower,” “toxic,” “irritant”) and identity (“orange,” “rose,” “spoiled food”) of the stimulus in order to decide and act. Of course, olfactory areas alone cannot achieve this decoding of chemical reality into a multidimensional representational space in which the odorous object can be categorized and perhaps identified, and the systems involved in memory storage of knowledge and of decisions in the temporal [Olofsson et al., 2013] and frontal lobes [Grabenhorst and Rolls, 2009] are needed.
To conclude, another question raised by our findings concerns how they relate to current theories of mental representation of smells. Recent literature suggests that a given odorant produces a neural signature that the brain processes as a whole and recognizes as an odor object [Stevenson and Wilson, 2007]. These odor objects include chemical molecular components and perceptual components [Yeshurun and Sobel, 2010] and can be inferred from odor‐evoked PC activity patterns [Gottfried, 2010]. This complex picture is significantly enhanced by the present findings that the odor objects of the perceived world are composed of multiple traces, from PC to amygdala. The use of RSA in particular constituted a novel way of investigating odor processing in the brain, notably revealing how differences and similarities between odor characteristics are finely and distinctively reflected in the various olfactory areas. The physical and olfactory/trigeminal perceptual “traces” of odors were shown to be extracted and sustained by different neural subsystems in the olfactory cortex; an odor object may then result from the interaction between these subsystems, each generating a partial neuronal representation of this multidimensional representation.
Correction added on 24 June 2016, after first online publication.
Experimental part of this study was performed on the imaging facilities of CERMEP ‐ imagerie du vivant, Bron, F‐69677, France. We wish to thank all team members from the CERMEP and especially Danielle Ibarrola, Dominique Sappey‐Marinier and Jamila Lagha, for their help in preparing, designing and running the experiments with us
REFERENCES
- Amoore JE, Johnston JW Jr, Rubin M (1964): The stereochemical theory of odor. Sci Am 210:42–9. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Christoff K, Stappen I, Panitz D, Ghahremani DG, Glover G, Gabrieli JDE, Sobel N (2003): Dissociated neural representations of intensity and valence in human olfaction. Nat Neurosci 6:196–202. [DOI] [PubMed] [Google Scholar]
- Arctander S (1994): Perfume and flavor materials of natural origin (Allured Publishing, Carol Stream (Illinois), 1994).
- Ashby FG, Lee WW (1991): Predicting similarity and categorization from identification. J Exp Psychol Gen 120:150. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y (1995): Controlling the false discovery rate: A practical and powerful approach to multiple testing. J Roy Stat Soc Ser B (Methodological) 57:289–300. [Google Scholar]
- Bensafi M, Croy I, Phillips N, Rouby C, Sezille C, Gerber J, Small DM, Hummel T (2014): The effect of verbal context on olfactory neural responses. Hum Brain Mapp 35:810–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensafi M, Sobel N, Khan RM (2007): Hedonic‐specific activity in piriform cortex during odor imagery mimics that during odor perception. J Neurophysiol 98:3254–3262. [DOI] [PubMed] [Google Scholar]
- Boesveldt S, Frasnelli J, Gordon AR, Lundström JN (2010): The fish is bad: Negative food odors elicit faster and more accurate reactions than other odors. Biol Psychol 84:313–317. [DOI] [PubMed] [Google Scholar]
- Brand G (2006): Olfactory/trigeminal interactions in nasal chemoreception. Neurosci Biobehav Rev 30:908–917. [DOI] [PubMed] [Google Scholar]
- Connolly AC, Guntupalli JS, Gors J, Hanke M, Halchenko YO, Wu Y‐C, Abdi H, Haxby JV (2012): The representation of biological classes in the human brain. J Neurosci 32:2608–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araujo IE, Rolls ET, Velazco MI, Margot C, Cayeux I (2005): Cognitive modulation of olfactory processing. Neuron 46:671–679. [DOI] [PubMed] [Google Scholar]
- Dravnieks A, Bock FC, Powers JJ, Tibbetts M, Ford M (1978): Comparison of odors directly and through profiling. Chem Senses 3:191–225. [Google Scholar]
- Edelman S (1998): Representation is representation of similarities. Behav Brain Sci 21:449–467. [DOI] [PubMed] [Google Scholar]
- Gallina J‐M (2006): Les représentations mentales. Dunod.
- Gottfried JA (2010): Central mechanisms of odour object perception. Nat Rev Neurosci 11:628–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA, Deichmann R, Winston JS, Dolan RJ (2002): Functional heterogeneity in human olfactory cortex: An event‐related functional magnetic resonance imaging study. J Neurosci 22:10819–10828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA, Winston JS, Dolan RJ (2006): Dissociable codes of odor quality and odorant structure in human piriform cortex. Neuron 49:467–479. [DOI] [PubMed] [Google Scholar]
- Grabenhorst F, Rolls ET (2009): Different representations of relative and absolute subjective value in the human brain. NeuroImage 48:258–268. [DOI] [PubMed] [Google Scholar]
- Gregson RAM, Mitchell MJ (1974): Odor quality similarity scaling and odor‐word profile matching. Chem Senses 1:95–101. [Google Scholar]
- Haddad R, Khan R, Takahashi YK, Mori K, Harel D, Sobel N (2008): A metric for odorant comparison. Nat Meth 5:425–429. [DOI] [PubMed] [Google Scholar]
- Haddad R, Medhanie A, Roth Y, Harel D, Sobel N (2010): Predicting odor pleasantness with an electronic nose. Graham Lyle J., editor. PLoS Computational Biology 6:e1000740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann SB, Ely TD, Hoffman JM, Kilts CD (2002): Ecstasy and agony: Activation of the human amygdala in positive and negative emotion. Psychol Sci 13:135–141. [DOI] [PubMed] [Google Scholar]
- Haushofer J, Livingstone MS, Kanwisher N (2008): Multivariate patterns in object‐selective cortex dissociate perceptual and physical shape similarity. PLoS Biol 6:e187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard JD, Plailly J, Grueschow M, Haynes J‐D, Gottfried JA (2009): Odor quality coding and categorization in human posterior piriform cortex. Nat Neurosci 12:932–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel T (2000): Assessment of intranasal trigeminal function. Int J Psychophysiol 36:147–155. [DOI] [PubMed] [Google Scholar]
- Hummel T, Livermore A (2002): Intranasal chemosensory function of the trigeminal nerve and aspects of its relation to olfaction. Int Arch Occup Environ Health 75:305–313. [DOI] [PubMed] [Google Scholar]
- Joussain P, Chakirian A, Kermen F, Rouby C, Bensafi M (2011): Physicochemical influence on odor hedonics: where does it occur first? Commun Integr Biol 4:563–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joussain P, Bessy M, Faure F, Bellil D, Landis BN, Hugentobler M, Tuorila H, Mustonen S, Vento SI, Delphin‐Combe F, Krolak‐Salmon P, Rouby C, Bensafi M (2015): Application of the European test of olfactory capabilities in patients with olfactory impairment. Eur Arch Otorhinolaryngol 1–10. [DOI] [PubMed] [Google Scholar]
- Kadohisa M, Wilson Da (2006): Separate encoding of identity and similarity of complex familiar odors in piriform cortex. PNAS 103:15206–15211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermen F, Chakirian A, Sezille C, Joussain P, Le Goff G, Ziessel A, Chastrette M, Mandairon N, Didier A, Rouby C, et al (2011): Molecular complexity determines the number of olfactory notes and the pleasantness of smells. Sci Rep 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan RM, Luk C‐H, Flinker A, Aggarwal A, Lapid H, Haddad R, Sobel N (2007): Predicting odor pleasantness from odorant structure: pleasantness as a reflection of the physical world. J Neurosci 27:10015–10023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N (2008): Representational similarity analysis—connecting the branches of systems neuroscience. Frontiers in Systems Neuroscience. Available at: http://journal.frontiersin.org/article/10.3389/neuro.06.004.2008/abstract. [DOI] [PMC free article] [PubMed]
- Litaudon P, Datiche F, Cattarelli M (1997): Optical recording of the rat piriform cortex activity. Prog Neurobiol 52:485–510. [DOI] [PubMed] [Google Scholar]
- Sha L, Haxby JV, Abdi H, Guntupalli JS, Oosterhof NN, Halchenko YO, Connolly AC (2015): The animacy continuum in the human ventral vision pathway. J Cogn Neurosci 27:665. [DOI] [PubMed] [Google Scholar]
- Mai JK, Assheuer J, Paxinos G (1997): Atlas of the Human Brain. San Diego: Academic Press. [Google Scholar]
- Mandairon N, Poncelet J, Bensafi M, Didier A (2009): Humans and mice express similar olfactory preferences. PLoS One 4:e4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moessnang C, Pauly K, Kellermann T, Krämer J, Finkelmeyer A, Hummel T, Siegel SJ, Schneider F, Habel U (2013): The scent of salience—Is there olfactory‐trigeminal conditioning in humans? NeuroImage 77:93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, Dolan RJ (1996): A differential neural response in the human amygdala to fearful and happy facial expressions. Nature 383:812–815. [DOI] [PubMed] [Google Scholar]
- Nassi JJ, Callaway EM (2009): Parallel processing strategies of the primate visual system. Nat Rev Neurosci 10:360–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nili H, Wingfield C, Walther A, Su L, Marslen‐Wilson W, Kriegeskorte N (2014): A toolbox for representational similarity analysis. PLoS Comput Biol 10:e1003553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson JK, Rogalski E, Harrison T, Mesulam M‐M, Gottfried JA (2013): A cortical pathway to olfactory naming: evidence from primary progressive aphasia Brain 136:1245–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poncelet J, Rinck F, Ziessel A, Joussain P, Thévenet M, Rouby C, Bensafi M (2010): Semantic knowledge influences prewired hedonic responses to odors. PLoS One 5:e13878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Kringelbach ML, De Araujo IET (2003): Different representations of pleasant and unpleasant odours in the human brain. Eur J Neurosci 18:695–703. [DOI] [PubMed] [Google Scholar]
- Savic I, Gulyás B, Berglund H (2002): Odorant differentiated pattern of cerebral activation: Comparison of acetone and vanillin. Hum Brain Mapp 17:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sezille C, Messaoudi B, Bertrand A, Joussain P, Thévenet M, Bensafi M (2013): A portable experimental apparatus for human olfactory fMRI experiments. J Neurosci Methods 218:29–38. [DOI] [PubMed] [Google Scholar]
- Shiffman SS (1974): Physicochemical correlates of olfactory quality. Science 185:112–117. [DOI] [PubMed] [Google Scholar]
- Small DM, Gerber JC, Mak YE, Hummel T (2005): Differential neural responses evoked by orthonasal versus retronasal odorant perception in humans. Neuron 47:593–605. [DOI] [PubMed] [Google Scholar]
- Snitz K, Yablonka A, Weiss T, Frumin I, Khan RM, Sobel N (2013): Predicting odor perceptual similarity from odor structure. PLoS Comput Biol 9:e1003184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson RJ, Wilson DA (2007): Odour perception: An object‐recognition approach. Perception 36:1821–1833. [DOI] [PubMed] [Google Scholar]
- Van Essen D, Anderson C, Felleman D (1992): Information processing in the primate visual system: an integrated systems perspective. Science 255:419–423. [DOI] [PubMed] [Google Scholar]
- Wilson DA (2003): Rapid, experience‐induced enhancement in odorant discrimination by anterior piriform cortex neurons. J Neurophysiol 90:65–72. [DOI] [PubMed] [Google Scholar]
- Winston JS, Gottfried JA, Kilner JM, Dolan RJ (2005): Integrated neural representations of odor intensity and affective valence in human amygdala. J Neurosci 25:8903–8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeshurun Y, Sobel N (2010): An odor is not worth a thousand words: From multidimensional odors to unidimensional odor objects. Ann Rev Psychol 61:219–241. [DOI] [PubMed] [Google Scholar]
- Zald Dh, Pardo Jv (1997): Emotion, olfaction, and the human amygdala: Amygdala activation during aversive olfactory stimulation. PNAS 94:4119–4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarzo M (2011): Hedonic judgments of chemical compounds are correlated with molecular size. Sensors 11:3667–3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarzo M, Stanton DT (2006): Identification of latent variables in a semantic odor profile database using principal component analysis. Chem Senses 31:713–724. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Jones‐Gotman M, Rouby C (2000): Neural mechanisms involved in odor pleasantness and intensity judgments. Neuroreport 11:2711–2716. [DOI] [PubMed] [Google Scholar]
- Zelano C, Bensafi M, Porter J, Mainland J, Johnson B, Bremner E, Telles C, Khan R, Sobel N (2005): Attentional modulation in human primary olfactory cortex. Nat Neurosci 8:114–120. [DOI] [PubMed] [Google Scholar]


