Abstract
Introduction
Cerebral small vessel disease is one of the most important risk factors for dementia, and has been related to hippocampal atrophy, which is among the first observed changes on conventional MRI in patients with dementia. However, these volumetric changes might be preceded by loss of microstructural integrity of the hippocampus for which conventional MRI is not sensitive enough. Therefore, we investigated the relation between the hippocampal diffusion parameters and the risk of incident dementia, using diffusion tensor imaging, independent of hippocampal volume.
Methods
The RUNDMC study is a prospective study among 503 elderly with small vessel disease, without dementia, with 5 years follow‐up in 2012 (99.6% response‐rate). Cox regression analysis was performed to calculate hazard ratios for dementia, of fractional anisotropy and mean diffusivity within the hippocampus, adjusted for demographics, hippocampal volume, and white matter. This was repeated in participants without evident hippocampal volume loss, because in these participants the visible damage might not yet have already started, whereas damage might have started on a microstructural level.
Results
43 participants developed dementia (8.6%), resulting in a 5.5‐year cumulative risk of 11.1% (95%CI 7.7–14.6). Higher mean diffusivity was associated with an increased 5‐year risk of dementia. In the subgroup of participants with the upper half hippocampal volume, higher hippocampal mean diffusivity, more than doubled the 5‐year risk of dementia.
Conclusion
This is the first prospective study showing a relation between a higher baseline hippocampal mean diffusivity and the risk of incident dementia in elderly with small vessel disease at 5‐year follow‐up, independent of hippocampal volume and white matter volume. Hum Brain Mapp 37:327–337, 2016. © 2015 Wiley Periodicals, Inc.
Keywords: dementia, elderly, cerebral small vessel disease, hippocampus, imaging, magnetic resonance imaging, diffusion tensor imaging
INTRODUCTION
The spectrum of cerebral small vessel disease (SVD) includes white matter hyperintensities (WMH) and lacunes of presumed vascular origin, microbleeds, and subcortical atrophy [Wardlaw et al., 2013]. SVD increases the risk of cognitive decline, including memory loss, ultimately leading to dementia in some [Vermeer et al., 2003; Wiesmann et al., 2013]. The presence and progression of SVD has previously been related to the progression of hippocampal atrophy [de Leeuw et al., 2004, 2006], which is thought to be a major cause of the profound deficit in memory consolidation in Alzheimer's dementia (AD) [Squire et al., 2004].
However, this hippocampal atrophy, visible on conventional MRI, occurs at a relatively late stage of dementia when the cognitive disturbances have already become apparent. Earlier detection of hippocampal pathology may provide insights in the etiology and course of dementia and offer opportunities for early treatment strategies, before irreversible degenerative damage of the hippocampus has occurred. Conceptually, there may be changes in the microstructural integrity of the hippocampus before macroscopic loss of volume occurs.
As conventional MRI is not sensitive to loss of microstructural integrity of the hippocampus, diffusion tensor imaging (DTI) might be of use to provide an early marker for dementia, using the diffusion properties of unbound water molecules [Basser et al., 1994; Pierpaoli et al., 1996]. Two DTI parameters are of special interest: mean diffusivity (MD) a measure water diffusion averaged in all spatial directions, and fractional anisotropy (FA), which provides information about the directionality of water diffusion. A low FA and high MD are believed to be an indication for lower microstructural integrity [Jones et al., 1999]. Cross‐sectionally we showed that hippocampal diffusion parameters (especially high MD) were related to verbal memory performance, in participants with a volumetric intact appearing hippocampus [van Norden et al., 2012a]. One prospective study in 13 MCI patients showed higher left hippocampal MD at baseline in those who converted to AD after a follow up of 1.5 years, compared to those who did not convert [Fellgiebel et al., 2006]. To the best of our knowledge there is a lack of large longitudinal studies, which predict dementia using diffusion data from the hippocampus, independent of hippocampal, and gray matter (GM) volume.
We therefore aimed to investigate the role of the diffusion parameters FA and MD within the hippocampus in the development of incident dementia, independent of hippocampal volume, GM volume, and SVD. Additionally, we investigated the role of hippocampal diffusion parameters as an early marker for dementia in elderly participants without evident hippocampal volume loss, because in those patients the irreversible degenerative damage might not have started yet. The study was part of the RUNDMC study, a prospective cohort study of 503 individuals, with SVD yet without dementia at the baseline assessment in 2006, with a subsequent follow‐up assessment in 2012.
METHODS
Study Population
The Radboud University Nijmegen Diffusion Tensor and Magnetic resonance Cohort (RUN DMC) study prospectively investigates risk factors and clinical consequences of brain changes as assessed by MRI among 503, 50–85 year old, nondemented elderly with cerebral SVD. On the basis of established research criteria, SVD was defined as the presence of lacunes and/or WMH on neuroimaging [Erkinjuntti, 2002]. Symptoms of SVD include acute symptoms, such as Transient Ischemic Attacks or lacunar syndromes, or subacute manifestations such as cognitive, motor disturbances and/or depressive symptoms [Erkinjuntti, 2002]. The baseline data collection was performed in 2006. Inclusion criteria were age between 50 and 85 years and cerebral SVD on neuroimaging (WMH and/or lacunes). Main exclusion criteria were dementia, (psychiatric) disease interfering with cognitive testing or follow‐up; WMH or SVD mimics (e.g., MS) and MRI contraindications or known claustrophobia [van Norden et al., 2011].
Follow‐up was completed in 2012. Of 503 baseline participants, 2 were lost to follow‐up (but not deceased according to the Dutch Municipal Personal Records database) and 49 had died. In person follow‐up was performed in 398 participants (Fig. 1), while 54 refused in person follow‐up, but clinical endpoints were available for this group.
Figure 1.
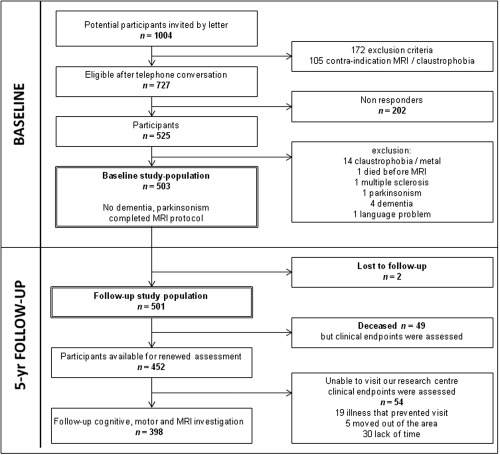
Flowchart study design baseline and follow‐up. Baseline and Follow‐up study population are indicated by double‐lined boxes. MRI: Magnetic Resonance Imaging
Dementia Case Finding
Dementia screening of participants was performed during a face‐to‐face follow‐up examination (n = 398) as follows. The Mini–Mental State Examination (MMSE) [Folstein et al., 1975] was used as a first screening tool. A score below 26 or a decline of 3 points or more from baseline was considered screen positive (n = 34). Of all screen‐positives, 20 were subsequently examined for the presence of dementia at the Radboud Alzheimer Center (7 were diagnosed with dementia and 13 were not). The remaining 14 refused additional analysis. For them, a consensus diagnosis of dementia was made by a panel, consisting of a neurologist, clinical neuropsychologist, and a geriatrician, all with expertise in dementia. They reviewed all available neuropsychological [van Norden et al., 2011] and imaging information, which included (i) the difference in neuropsychological performance between baseline and follow‐up, (ii) outcome of the Mini International Neuropsychiatric Interview MINI [Sheehan et al., 1998], (iii) the follow‐up MRI scan, or if not available, the baseline MRI‐scan (in 7 cases) for classification. (iv) For the interpretation of these tests, age and level of education were taken into account [Hochstenbach et al., 1998], next to interference with daily living, confirmed by family or caregivers. Of these 14 participants, 7 were diagnosed with dementia.
Medical records were reviewed from the participants who were not available for follow‐up assessment (49 deceased, from 54 follow‐up data were available, but did not visit the center). In addition, their general practitioners and medical specialists were contacted for information on their cognitive status. Dementia was suspected in 37 participants. After review by panel members, 29 of these were classified as having dementia (Fig. 2). In total, this resulted in 43 incident cases of dementia during a mean follow‐up period of 5.2 years (SD 0.7).
Figure 2.
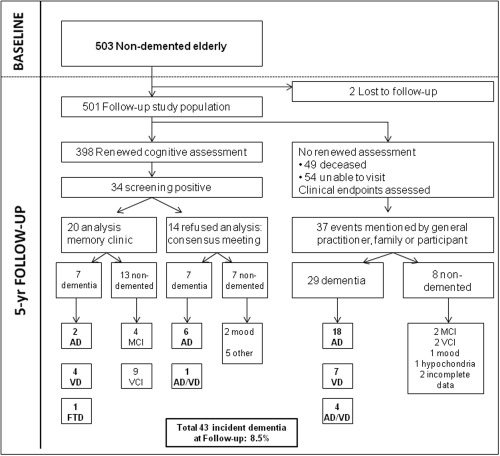
Flowchart diagnostic work‐up dementia. AD: Alzheimer's dementia; VD: vascular dementia; FTD: fronto‐temporal dementia; AD/VD: possible Alzheimer's Dementia with mixed etiology; MCI: mild cognitive impairment; VCI: vascular cognitive impairment.
The diagnosis of dementia was based on the Diagnostic and Statistical manual of mental disorders (IV) [American Psychiatric Association, 2000] criteria; probable Alzheimer's disease was based on the NIA‐AA criteria (n = 28) [McKhann et al., 2011], and vascular dementia (VaD) was based on NINDS‐AIREN criteria (n = 11) [Roman et al., 1993]. Individuals not fulfilling these criteria were classified as having possible AD with etiologically mixed presentation (n = 3) [McKhann et al., 2011] or frontotemporal dementia (n = 1). The onset of dementia was defined as the date on which the clinical symptoms allowed for the diagnosis [Vermeer et al., 2003]. When the date of diagnosis was not exactly known, we used the mid‐point between the baseline visit and the first date the diagnosis was confirmed [Ott et al., 1998], or the date the participant was admitted to a nursing home because of dementia.
MRI Resonance Scanning Protocol
MRI scans of all participants were acquired on a single 1.5‐Tesla MRI (Magnetom Sonata, Siemens Medical Solutions, Erlangen, Germany). The protocol included, the following whole brain scans: a T1‐weighted 3D magnetization‐prepared rapid gradient‐echo (MPRAGE) imaging (TR/TE/TI 2250/3.68/850 ms;flip angle15°; voxel size 1.0 × 1.0 × 1.0 mm); Fluid‐attenuated inversion recovery (FLAIR) pulse sequences TR/TE/TI 9000/84/2200 ms; voxel size 1.0 × 1.2 × 5.0 mm, with an interslice gap of 1 mm); a transversal T2*weighted gradient echo sequence (TR/TE 800/26 ms; voxel size 1.3 × 1.0 × 6.0 mm, with an interslice gap of 1 mm) and a Diffusion Tensor Imaging (DTI) sequence (TR/TE 10100/93 ms; voxel size 2.5 × 2.5 × 2.5 mm; 4 unweighted scans, 30 diffusion weighted scans with b‐value = 900 s mm−2) [van Norden et al., 2011].3
Conventional Imaging Analysis
WMH were manually segmented on FLAIR images and the total WMH volume was calculated by summing the segmented areas multiplied by slice thickness. The rating of lacunes, microbleeds and territorial infarcts were revised according to the recently published Standards for Reporting Vascular changes on neuroimaging, by trained raters blinded to all clinical data [Wardlaw et al., 2013]. There were good intra and inter‐rater variabilities with weighted kappa of 0.87 and 0.95 respectively for the presence of lacunes and 0.85 and 0.86 for the presence of microbleeds, calculated in 10% of the scans. Inter‐rater variability (assessed by intraclass correlation coefficient) for total WMH volume was 0.99.
The left and right hippocampus were manually segmented on the MPRAGE image, using the interactive software program “ITK‐SNAP” version 2.1 [Yushkevich et al., 2006] (http://www.itksnap.org). Segmentation was performed using a previously published protocol [Geuze et al., 2005] in which segmentation was performed from posterior to anterior. Inter‐rater studies showed an intraclass correlation coefficient of 0.73 and 0.79 for the left and right hippocampus respectively; intrarater showed an intraclass correlation coefficient for the left and right hippocampus of 0.97 and 0.96. All imaging analyses were performed by raters blinded to clinical information.
To obtain the GM (which was composed of the volume of the cortex, basal ganglia and thalamus), WM and cerebro spinal fluid (CSF) volume, the T1 MPRAGE images were segmented using Statistical Parametric Mapping 12 unified segmentation routines (SPM12; Wellcome Department of Cognitive Neurology, University College London, UK (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/). All images were visually checked for co‐registration errors and for motion and/or segmentation artefacts. The GM, WM, and CSF volumes were calculated by summing all voxels that had a P > 0.5 for belonging to that tissue class multiplied by the voxel volume in ml. The intracranial volume (ICV) was calculated by summing the volumes of GM, WM, and CSF. All volumes were normalized to total ICV [Colliot et al., 2008].
DTI‐Analysis
The raw diffusion weighted images of each patient were first denoised using the Local Principal Component Analyses filter [Manjon et al., 2013]. Diffusion data were then preprocessed using an in‐house developed algorithm named “patching artefacts from cardiac and head motion” [Zwiers, 2010]. In short, this iteratively reweighted‐least‐squares algorithm produces robust diffusion tensor estimates and provides weightings that are used to detect and correct head and cardiac motion artefacts in the diffusion‐weighted data. Next, affine misalignments from eddy currents and subject motion were corrected simultaneously which is based on the minimization of the normalized mutual information measure.
We then unwarped echo planar imaging (EPI) distortions by normalizing the EPI‐images to the T1‐image only in the phase‐encoding‐direction. DTI calculations were generated using DTIFIT from FSL's FDT toolbox. The skull‐stripped T1‐images were then nonlinearly registered to the FA map using FSL's nonlinear Image Registration Tool (FNIRT) with standard parameters. (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FNIRT) The transformation matrix derived from the nonlinear registration was then used to register the manually segmented hippocampus masks to the subject's DTI native space. The boundary voxels of these hippocampal masks were eroded to avoid potential partial volume effects. Mean FA and MD were then calculated within both hippocampi. All images were visually checked for motion artifacts and coregistration errors, especially for not including peri‐hippocampal CSF.
Other Measurements
Education was classified using 7 categories (1 being less than primary school, 7 reflecting academic degree) and then dichotomized in a group having only or less than primary school and a group having more than primary education [Hochstenbach et al., 1998]. Depressive symptoms were assessed with the Centre of Epidemiologic Studies Depression Scale (CES‐D); they were considered present in participants with CES‐D ≥ 16 and/or participants who currently used antidepressive medication, taken for depression [van Norden et al., 2011; van Uden et al., 2014].
Statistical Analysis
Person‐years at risk were calculated for each patient from date of the baseline assessment, until onset of dementia, death, or date of the follow‐up assessment. Patients who died or did not reach the endpoint were censored. WMH volume was log transformed because of the skewed distribution of the data. In view of the fact that etiology of Frontotemporal dementia is basically genetic, as opposed to the vascular etiology of VaD and dementia due to Alzheimer's disease, we excluded this participant from the analysis. Cumulative risk of incident dementia was estimated with Kaplan‐Meier analysis stratified by quartiles of total, left, and right MD and FA within the hippocampus. Subsequently, Kaplan‐Meier curves were compared between the subgroups using log‐rank test.
We used Cox regression analyses to calculate hazard ratios (HRs) with 95% confidence intervals (CI's) for baseline GM, HV, and the DTI parameters within the hippocampus, adjusted for age, sex, education, baseline MMSE and territorial infarcts (no.). Second, the HRs for the DTI parameters were calculated after additional adjustment for hippocampal and WM volume, because those were the only conventional MRI parameters independently related to the development of dementia, whereas WMH volume, GM volume, microbleeds, and lacunes were not. Finally, to identify diffusion parameters of the hippocampus as an early marker for incident dementia, we investigated the mean hippocampal FA and MD in patients with a “normal” hippocampal volume (median split; cut‐off of the normalized hippocampal volume 6.81 ml). In these participants the visible (volumetric) damage might not yet have started, but damage might possibly have started on a microstructural level. HRs for dementia were calculated for the DTI parameters within the hippocampus in that particular subgroup. After correction for age, sex, education, baseline MMSE and territorial infarcts (no.), these HRs were additionally adjusted for white matter volume. Two‐sided P values of <0.05 were considered to indicate statistical significance. Statistical analysis were performed using IBM SPSS Statistics version 20.
RESULTS
The final sample consisted of 501 participants from which clinical endpoints could be obtained (two were lost to follow up). One participant was additionally excluded because of baseline T1 and T2 artifacts. Baseline demographic and neuroimaging characteristics of 500 participants are shown in Table 1. Dementia developed in 43 participants during a mean follow‐up of 5.2 years (SD 0.7). Mean age at follow‐up was 70.8 years (SD 8.7) and 56.8% was male. Mean MMSE at baseline was 28.1 (SD 1.6). At baseline, median WMH volume was 7.2 ml (IQR 3.6; 18.4), mean WM volume was 464.5 ml (SD 44.4 ml), 26.8% had lacunes and 16.1% had microbleeds. Mean normalized hippocampal volume was 6.8 ml (SD 0.9). Mean FA in the hippocampus was 0.11 (SD 0.02), and mean MD in the hippocampus was 0.99 × 10−3 mm2/s (SD 0.06). The 5.5‐year cumulative risk of dementia was 11.1% (95%CI 7.7–14.6).
Table 1.
Baseline characteristics of participants with and without incident dementia
| Total, n = 500 | Dementia, n = 42 | Nondement, n = 458 | |
|---|---|---|---|
| Demographics | |||
| Age at baseline (SD) | 65.6 ± 8.8 | 74.6 ± 6.5 | 64.8 ± 8.5 |
| Men, n (%) | 284 (56.8) | 24 (57.1) | 260 (56.8) |
| Only primary education, n (%) | 49 (9.8) | 8 (19.0) | 41 (9.0) |
| MMSE baseline (SD) | 28.1 ± 1.6 | 27.1 ± 1.7 | 28.2 ± 1.6 |
| Depressive symptoms baseline, n (%) | 166 (33.2) | 21 (50.0) | 145 (31.7) |
| Baseline neuroimaging characteristics | |||
| ICV (ml; SD) | 1456.8 ± 137.4 | 1438.6 ± 142.4 | 1458.4 ± 137.0 |
| White matter volume (ml; SD) | 464.5 ± 44.4 | 439.8 ± 40.4 | 466.7 ± 44.1 |
| WMH volume (ml)a | 7.2 (3.5;17.9)a | 15.3 (7.4;37.9)a | 6.8 (3.3;17.4)a |
| NAWM volume (ml; SD) | 450.4 ± 49.10 | 413.8 ± 45.1 | 453.8 ± 48.1 |
| Lacunes, presence, n (%) | 134 (26.8) | 15 (35.7) | 119 (26.0) |
| Microbleeds, presence, n (%)b | 80 (16.1) | 9 (21.4) | 73 (15.9) |
| Territorial infarcts, presence, n (%) | 56 (11.2) | 7 (16.7) | 47 (10.3) |
| GM volume (ml; SD) | 616.0 ± 50.8 | 576.7 ± 42.2 | 619.7 ± 50.0 |
| Hippocampal volume (ml; SD)c | 6.8 ± 0.9 | 6.2 ± 1.0 | 6.9 ± 0.9 |
| Baseline DTI‐parameters | n = 497 | n = 42 | n = 455 |
| Hippocampus, mean FA (SD) | 0.11 ± 0.02 | 0.10 ± 0.02 | 0.11 ± 0.02 |
| Left hippocampus, mean FA (SD) | 0.11 ± 0.02 | 0.10 ± 0.02 | 0.11 ± 0.02 |
| Right hippocampus, mean FA (SD) | 0.10 ± 0.02 | 0.10 ± 0.02 | 0.11 ± 0.02 |
| Hippocampus, mean MD (SD) | 0.99 ± 0.06 | 1.04 ± 0.06 | 0.98 ± 0.06 |
| Left hippocampus, mean MD SD) | 1.00 ± 0.07 | 1.06 ± 0.07 | 0.99 ± 0.07 |
| Right hippocampus, mean MD (SD) | 0.98 ± 0.07 | 1.01 ± 0.07 | 0.97 ± 0.06 |
MMSE: Minimental State Examination; ml: milliliters; SD: standard deviation; FU: Follow‐up; WMH: White Matter Hyperintensities; NAWM: Normal Appearing White Matter; FA: Fractional Anisotropy; MD: Mean Diffusivity (10−3 mm2/s). Brain volumes represent normalized brain volumes to the total ICV.
Data shown are unadjusted values, and represent numbers (%), mean (SD), or median (interquartile range).
Three were excluded because of missing values of microbleeds.
Four were excluded because of missing values of hippocampal volume.
Three were additionally excluded for the DTI analysis because of baseline DTI‐scan artifacts.
This risk of dementia was higher in participants with the lowest quartile hippocampal FA at baseline compared with participants with the highest quartile FA (lowest 19.1% vs. highest 10.0%, Log‐Rank P < 0.022). The risk of dementia also was higher in participants with the highest quartile hippocampal MD at baseline compared with those with the lowest quartile MD (highest 24.7% vs. lowest 0.8%, Log‐Rank P < 0.001 (Fig. 3). Baseline hippocampal diffusion parameters were a significant predictor of the risk of dementia, corrected for age, sex, education, baseline MMSE, and territorial infarcts (no.) (Table 2). After additional adjustment for baseline WM and hippocampal volume, FA values of the hippocampus no longer were significant predictors. The mean MD of the hippocampus (left and right combined) (HR 1.44; 95%CI 1.10–1.88 per SD increase) remained a significant predictor of incident dementia at follow‐up, as did the mean MD in the left hippocampus (HR 1.44; 95%CI 1.10–1.90 per SD increase). The analysis was rerun with an interaction term for hippocampal volume and mean hippocampal MD, which was however not significant. Furthermore, investigating whole brain FA and MD values as a predictor of dementia we found no significant relation with dementia (data not shown).
Figure 3.
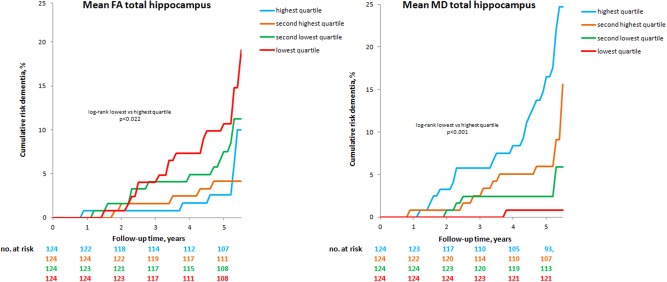
Cumulative risk for dementia, stratified by quartiles structural integrity of the hippocampus. Cumulative risk of dementia in elderly with cerebral Small vessel disease, stratified by quartiles structural integrity of the hippocampus. P‐values were obtained by log‐rank test, which was used to compare the curves of the highest and lowest quartile structural integrity. FA: Fractional Anisotropy, MD: Mean Diffusivity. The ranges of the Quartiles FA: 0.060–0.093 for the first quartile; 0.093–0.104 for the second quartile; 0.105–0.115 for the third quartile and 0.115–0.165 for the fourth quartile. The ranges of the Quartiles MD are (10−4 mm2/s): 8.28–9.46 for the first quartile; 9.47–9.79 for the second quartile; 9.80–10.22 for the third quartile and 10.22–12.5 for the last quartile.
Table 2.
Cox proportional hazards for dementia at follow‐up, derived from baseline hippocampal volume, GM volume, and DTI‐based metrics
| Baseline neuroimaging characteristics (n = 499) | HR + 95% CI | Significance | HR + 95% CI | Significance |
|---|---|---|---|---|
| Adjusted age, sex, education, baseline MMSE and territorial infarcts | Additionally adjusted for normalized WM volume | |||
| GM volume (mL; SD) | 0.75 (0.52–1.10) | P = 0.138 | 0.73 (0.49–1.07) | P = 0.102 |
| Hippocampal volume (ml; SD)a | 0.68 (0.49–0.96) | P = 0.028 | 0.69 (0.49–0.97) | P = 0.032 |
| Baseline DTI‐parametersb (n = 496) | Adjusted for age, sex, education baseline MMSE and territorial infarcts | Additionally adjusted for normalized HV and WM volume | ||
| Hippocampus, mean FA (SD) | 0.70 (0.49–0.99) | P = 0.044 | 0.72 (0.51–1.02) | P = 0.067 |
| Left hippocampus, mean FA (SD) | 0.78 (0.56–1.10) | P = 0.158 | 0.78 (0.55–1.11) | P = 0.163 |
| Right hippocampus, mean FA (SD) | 0.68 (0.48–0.96) | P = 0.029 | 0.73 (0.52–1.02) | P = 0.064 |
| Hippocampus, mean MD (SD) | 1.54 (1.20–1.99) | P = 0.001 | 1.44 (1.10–1.88) | P = 0.007 |
| Left hippocampus, mean MD (SD) | 1.60 (1.23–2.09) | P = 0.001 | 1.44 (1.10–1.90) | P = 0.009 |
| Right hippocampus, mean MD (SD) | 1.29 (1.02–1.64) | P = 0.034 | 1.26 (0.99–1.62) | P = 0.066 |
One was additionally excluded because of the diagnosis Frontotemporal Dementia.
Four participants were excluded because of missing values of hippocampal volume.
Three participants were additionally excluded for the DTI analysis because of baseline DTI‐scan artefacts.
SD: HR per standard deviation difference from the mean. Brain volumes, represent normalized brain volumes; normalized to the total ICV. WM: white matter; GM: gray matter; HV: hippocampus volume; FA: Fractional Anisotropy; MD: Mean Diffusivity (10−3 mm2/s).
The mean hippocampal MD (of both hippocampi combined and the left hippocampus separately), was a significant predictor of the risk of dementia, corrected for age, sex, education, baseline MMSE, and territorial infarcts in the subgroup of participants without evident hippocampal volume loss (Table 3). This association remained significant after additional correction for WM volume. This was not found for the mean hippocampal MD in participants with the lowest half of hippocampal volume (data not shown).
Table 3.
Cox proportional hazards; structural integrity of the volumetric intact hippocampi, and the risk of dementia at follow‐up
| Baseline DTI‐parameters (n = 246) | HR + 95% CI | Significance | HR + 95% CI | Significance |
|---|---|---|---|---|
| Adjusted age, sex, education, baseline MMSE and territorial infarcts | Additionally adjusted for normalized WM volume | |||
| Hippocampus, mean FA (SD) | 0.61 (0.28–1.30) | P = 0.198 | 0.63 (0.29–1.37) | P = 0.247 |
| Left hippocampus, mean FA (SD) | 0.75 (0.39–1.47) | P = 0.409 | 0.79 (0.40–1.56) | P = 0.501 |
| Right hippocampus, mean FA (SD) | 0.51 (0.22–1.20) | P = 0.123 | 0.53 (0.23–1.25) | P = 0.145 |
| Hippocampus, mean MD (SD) | 2.07 (1.11–3.88) | P = 0.022 | 2.34 (1.17–4.68) | P = 0.016 |
| Left hippocampus, mean MD (SD) | 2.44 (1.23–4.83) | P = 0.011 | 2.66 (1.30–5.44) | P = 0.007 |
| Right hippocampus, mean MD (SD) | 1.55 (0.82–2.92) | P = 0.176 | 1.64 (0.83–3.23) | P = 0.153 |
One was additionally excluded because of the diagnosis Frontotemporal Dementia. Eleven cases of incident dementia were found in participants with the highest half hippocampal volume. Four participants were excluded for the DTI analysis because of baseline DTI‐scan artefacts.
SD: HR per standard deviation difference from the mean. FA: Fractional Anisotropy, MD: Mean Diffusivity (10−3 mm2/s).
DISCUSSION
The present study in elderly participants with SVD initial free of dementia, demonstrated that higher hippocampal MD increased the risk of developing dementia after a 5‐year follow‐up. This was independent of demographics, WM and hippocampal volume and most pronounced in the left hippocampus, and the subgroup of elderly participants without evidence of hippocampal atrophy on conventional MRI. These findings are in line with the knowledge that the loss of function and structure of the hippocampus is a hallmark in the etiology of AD. [Alvarez et al., 1995; Zola‐Morgan et al., 1986]. Furthermore lower hippocampal microstructure, especially measured by MD in association to cognitive function, is consistent with previous cross‐sectional studies in both non‐demented elderly and elderly with MCI [Carlesimo et al., 2010; Cherubini et al., 2010; den Heijer et al., 2012; Fellgiebel et al., 2004].
Strengths of our study include its longitudinal design in a population that covers the whole spectrum of cerebral SVD and the large sample size. In addition, the assessment of endpoints in 99.6% of our participants is a major strength. Collection of our data in a single centre allowed us to assemble baseline and follow‐up data according to identical procedures, reducing the risk of procedural bias. Furthermore, we manually segmented the WMH and hippocampal volumes without prior knowledge of the clinical data. We performed a nonlinear algorithm for the registration between the DTI and T1‐image to deal with possible differential susceptibility effects. Furthermore, the boundary voxels of the manually segmented hippocampal masks were eroded, to deal with potential partial volume effects. Finally, the relation between hippocampal diffusion parameters and dementia was investigated with adjustment for demographics, baseline global cognitive status and other brain imaging characteristics including WM and hippocampal volume, reducing confounds. Adjusting the associations between hippocampal diffusion parameters and dementia‐risk for hippocampal volume, this remained a significant predictor for dementia, suggesting that this association was not accounted for by concomitant small hippocampal volumes. Due to the relatively small number of incident dementia cases in our analyses (n = 41 in Table 2 and n = 11 Table 3), the analyses could not be adjusted for all possible confounders (WMH, presence of lacunes, microbleeds and GM volume, on top of age, sex, education, baseline MMSE, and territorial infarcts (no.), WM and Hippocampal volume), to avoid over‐fitting. However, investigating this association between the hippocampal diffusion parameters and the risk of dementia for all above mentioned confounders, the results did not change.
Several methodological issues need to be addressed. First, the nosological dementia diagnosis in our study was a clinical diagnosis supported by MR imaging at the moment of diagnosis, and if not available, baseline MR imaging. In some cases, especially in the elderly, a distinction between AD and VaD is hard to make, because neurodegeneration and vascular diseases often cooccur [Breteler, 2000; Launer, 2002; van Norden et al., 2012b; Viswanathan et al., 2009]. For this reason, we investigated “overall dementia” as outcome measure. Second, it is possible that some patients with incident dementia were missed, because the cut‐off point of 26 in the MMSE, although widely used, might not be sensitive enough, especially for cases of dementia in early stage of the disease, VaD, or dementia in participants with higher education levels. We think that if misclassification has occurred, it may have led to an underestimation of the effect. Third, we were not informed on the APOE status of our participants, which prevented us from further increasing the predictive value of our analysis. Fourth, we measured WMH using the FLAIR images with a non‐isotropic voxel size. For this reason some over or under‐classification could not be excluded.
We think that our study has a high generalizability to patients between 50 and 85 years presenting with SVD on neuroimaging in a general neurology clinic. At baseline we included independent living, participants, with a mean MMSE of 28.1, corresponding with estimates in the general population [Au et al., 2006; de Groot et al., 2000]. All had some degree of SVD on neuroimaging, which has a prevalence of over 90% in the elderly population over 60 years of age [de Leeuw et al., 2001]. However, the median of WMH in our study is higher than found in population based studies. For this reason, it is not surprising that our overall incidence rate of 16.4 per 1000 person years is higher than the overall incidence rate of 10.7 per 1000 person years found in a large population based study [Ott et al., 1998]. Comparing our cases of incident dementia with another study in participants with cerebral small vessel disease [Verdelho et al., 2013] we had less cases of incident dementia (43/501 after 5 years of follow up vs. 90/588 after 3 years of follow up). This could be due to the fact that our population had a mean age which was ∼10 years younger and has less severe WMH at baseline; both factors are known to be related with the incidence of dementia [Fratiglioni et al., 1999; Ott et al., 1998].
We found that the relation between the FA of the hippocampus and dementia was less prominent than for MD. This may be because FA mostly reflects the dominant directionality of the water diffusion. Multiple fibers present on the same location may all have different directions (crossing‐fibers), influencing FA. It might be that because of this, low FA may not necessarily reflect a lower underlying structural integrity in the hippocampus [Pierpaoli et al., 1996]. In contrast, MD is less affected by fiber‐crossing because it reflects the magnitude of water diffusion, which is not influenced by direction [Pierpaoli et al., 1996]. A recent meta‐analysis comparing DTI parameters and volume measures in MCI and AD, indicated that MD values had more discriminative power than FA values [Clerx et al., 2012], which is in line with our findings.
It could be that the discriminative power of the DTI parameters is higher in the group of elderly classified in the upper half of hippocampal volume, because the low microstructural integrity of the hippocampus may reflect changes that have not yet resulted in visible hippocampal volume loss. Possibly, once the hippocampal atrophy has started, this atrophy is of more predictive value for the development of dementia, than the diffusion changes, lowering the discriminative power of the DTI parameters. Furthermore, DTI parameters are less sensitive in a smaller hippocampus than in a volumetric intact one, which also might contribute to a stronger effect for the DTI parameters in our participants with the upper half of hippocampal volume. We did not find a significant relation between whole brain DTI metrics and dementia, which might indicate that hippocampal DTI parameters are more specific predictors for the development of dementia. In contrast, a recent longitudinal study [Jokinen et al., 2013] showed that microstructural changes in the WM predict a faster decline in several cognitive domains, but dementia has never been included as an outcome measure.
The underlying mechanisms of higher hippocampal MD (and lower FA), indicating a lower microstructural integrity still remains to be elucidated. Several mechanisms, whether or not related to SVD, have been proposed. Possibly severe WMH lead to axonal loss by anterograde or Wallerian degeneration in the areas that are connected by these WM structures [Dziedzic et al., 2010; Seo et al., 2010], such as the hippocampus, and as such lead to higher MD. Alternatively the accumulation of intraneuronal tau in the hippocampus [Braak and Braak, 1997] may lead to axonal damage and lower microstrucural integrity of the hippocampus, and as a consequence to loss of function.
Ideally, a surrogate marker for dementia identifies those at higher risk during the pre‐clinical period, because identification of this disease at an earlier stage (that is, before the irreversible damage has been done), might lead to early intervention with for instance vascular risk factors as a target. In turn, individuals identified as having a lower risk of developing dementia, can possibly be comforted. DTI of the hippocampus might identify the persons at risk in an earlier stage than possible with conventional MRI and may be a promising a surrogate marker of disease progression for use in therapeutic trials. However to answer the question whether dementia can really be prevented by treating or targeting these vascular risk factors we need randomized clinical trials with large sample size and sufficient long follow‐up [Richard et al., 2012].
In conclusion, results of our study show that hippocampal diffusion parameters at baseline are associated with an increased risk of developing dementia five years later, even when macroscopical loss of hippocampal volume is not apparent. These results might suggest that diffusion changes in the hippocampus precede volumetric changes as seen in dementia. DTI may offer an earlier insight in the development of dementia, and therefore provide an earlier window for possible preventive strategies.
Conflicts of interest: No conflicts of interest.
REFERENCES
- Alvarez P, Zola‐Morgan S, Squire LR (1995): Damage limited to the hippocampal region produces long‐lasting memory impairment in monkeys. J Neurosci 15:3796–3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (2000): Diagnostic and statistical manual of mental disorders, 4th ed. Washington DC. [Google Scholar]
- Au R, Massaro JM, Wolf PA, Young ME, Beiser A, Seshadri S, D'Agostino RB, DeCarli C (2006): Association of white matter hyperintensity volume with decreased cognitive functioning: The Framingham Heart Study. Arch Neurol 63:246–250. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D (1994): Estimation of the effective self‐diffusion tensor from the NMR spin echo. J Magn Reson Ser B 103:247–254. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E (1997): Diagnostic criteria for neuropathologic assessment of Alzheimer's disease. Neurobiol Aging 18:S85–S88. [DOI] [PubMed] [Google Scholar]
- Breteler MM (2000): Vascular risk factors for Alzheimer's disease: An epidemiologic perspective. Neurobiol Aging 21:153–160. [DOI] [PubMed] [Google Scholar]
- Carlesimo GA, Cherubini A, Caltagirone C, Spalletta G (2010): Hippocampal mean diffusivity and memory in healthy elderly individuals: A cross‐sectional study. Neurology 74:194–200. [DOI] [PubMed] [Google Scholar]
- Cherubini A, Peran P, Spoletini I, Di Paola M, Di Iulio F, Hagberg GE, Sancesario G, Gianni W, Bossu P, Caltagirone C, Sabatini U, Spalletta G (2010): Combined volumetry and DTI in subcortical structures of mild cognitive impairment and Alzheimer's disease patients. J Alzheimer's Dis 19:1273–1282. [DOI] [PubMed] [Google Scholar]
- Clerx L, Visser PJ, Verhey F, Aalten P (2012): New MRI markers for Alzheimer's disease: A meta‐analysis of diffusion tensor imaging and a comparison with medial temporal lobe measurements. J Alzheimer's Dis 29:405–429. [DOI] [PubMed] [Google Scholar]
- Colliot O, Chetelat G, Chupin M, Desgranges B, Magnin B, Benali H, Dubois B, Garnero L, Eustache F, Lehericy S (2008): Discrimination between Alzheimer disease, mild cognitive impairment, and normal aging by using automated segmentation of the hippocampus. Radiology 248:194–201. [DOI] [PubMed] [Google Scholar]
- de Groot JC, de Leeuw FE, Oudkerk M, van Gijn J, Hofman A, Jolles J, Breteler MM (2000): Cerebral white matter lesions and cognitive function: The Rotterdam Scan Study. Ann Neurol 47:145–151. [DOI] [PubMed] [Google Scholar]
- de Leeuw FE, de Groot JC, Achten E, Oudkerk M, Ramos LM, Heijboer R, Hofman A, Jolles J, van Gijn J, Breteler MM (2001): Prevalence of cerebral white matter lesions in elderly people: A population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry 70:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leeuw FE, Barkhof F, Scheltens P (2004): White matter lesions and hippocampal atrophy in Alzheimer's disease. Neurology 62:310–312. [DOI] [PubMed] [Google Scholar]
- de Leeuw FE, Korf E, Barkhof F, Scheltens P (2006): White matter lesions are associated with progression of medial temporal lobe atrophy in Alzheimer disease. Stroke 37:2248–2252. [DOI] [PubMed] [Google Scholar]
- den Heijer T, der Lijn F, Vernooij MW, de Groot M, Koudstaal PJ, der Lugt A, Krestin GP, Hofman A, Niessen WJ, Breteler MM (2012): Structural and diffusion MRI measures of the hippocampus and memory performance. Neuroimage 63:1782–1789. [DOI] [PubMed] [Google Scholar]
- Dziedzic T, Metz I, Dallenga T, Konig FB, Muller S, Stadelmann C, Bruck W (2010): Wallerian degeneration: A major component of early axonal pathology in multiple sclerosis. Brain Pathol 20:976–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkinjuntti T (2002): Subcortical vascular dementia. Cerebrovasc Dis 13 Suppl 2:58–60. [DOI] [PubMed] [Google Scholar]
- Fellgiebel A, Wille P, Muller MJ, Winterer G, Scheurich A, Vucurevic G, Schmidt LG, Stoeter P (2004): Ultrastructural hippocampal and white matter alterations in mild cognitive impairment: A diffusion tensor imaging study. Dement Geriatr Cogn Disord 18:101–108. [DOI] [PubMed] [Google Scholar]
- Fellgiebel A, Dellani PR, Greverus D, Scheurich A, Stoeter P, Muller MJ (2006): Predicting conversion to dementia in mild cognitive impairment by volumetric and diffusivity measurements of the hippocampus. Psychiatry Res 146:283–287. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR (1975): “Mini‐mental state”. A practical method for grading the cognitive state of Patients for the Clinician. J Psychiatric Res 12:189–198. [DOI] [PubMed] [Google Scholar]
- Fratiglioni L, De Ronchi D, Aguero‐Torres H (1999): Worldwide prevalence and incidence of dementia. Drugs Aging, 15:365–375. [DOI] [PubMed] [Google Scholar]
- Geuze E, Vermetten E, Bremner JD (2005): MR‐based in vivo hippocampal volumetrics: 2. Findings in neuropsychiatric disorders. Mol Psychiatry 10:160–184. [DOI] [PubMed] [Google Scholar]
- Hochstenbach J, Mulder T, van Limbeek J, Donders R, Schoonderwaldt H (1998): Cognitive decline following stroke: A comprehensive study of cognitive decline following stroke. J Clin Exp Neuropsychol 20:503–517. [DOI] [PubMed] [Google Scholar]
- Jokinen H, Schmidt R, Ropele S, Fazekas F, Gouw AA, Barkhof F, Scheltens P, Madureira S, Verdelho A, Ferro JM, Wallin A, Poggesi A, Inzitari D, Pantoni L, Erkinjuntti T, Group LS (2013): Diffusion changes predict cognitive and functional outcome: the LADIS study. Ann Neurol 73:576–583. [DOI] [PubMed] [Google Scholar]
- Jones DK, Lythgoe D, Horsfield MA, Simmons A, Williams SC, Markus HS (1999): Characterization of white matter damage in ischemic leukoaraiosis with diffusion tensor MRI. Stroke 30:393–397. [DOI] [PubMed] [Google Scholar]
- Launer LJ (2002): Demonstrating the case that AD is a vascular disease: Epidemiologic evidence. Ageing Res Rev 1:61–77. [DOI] [PubMed] [Google Scholar]
- Manjon JV, Coupe P, Concha L, Buades A, Collins DL, Robles M (2013): Diffusion weighted image denoising using overcomplete local PCA. PloS One 8:e73021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr., Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH (2011): The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's Dement 7:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott A, Breteler MM, van Harskamp F, Stijnen T, Hofman A (1998): Incidence and risk of dementia. The Rotterdam Study. Am J Epidemiol 147:574–580. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G (1996): Diffusion tensor MR imaging of the human brain. Radiology 201:637–648. [DOI] [PubMed] [Google Scholar]
- Richard E, Moll van Charante EP, van Gool WA (2012): Vascular risk factors as treatment target to prevent cognitive decline. J Alzheimer's Dis 32:733–740. [DOI] [PubMed] [Google Scholar]
- Román GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, Amaducci L, Orgogozo J‐M, Brun A, Hofman A, Moody DM, O'Brien MD, Yamaguchi T, Grafman J, Drayer BP, Bennett DA, Fisher M, Ogata J, Kokmen E, Bermejo F, Wolf PA, Gorelick PB, Bick KL, Pajeau AK, Bell MA, DPhil, DeCarli C, Culebras A, Korczyn AD, Bogousslavsky J, Hartmann A, Scheinberg P (1993): Vascular dementia: Diagnostic criteria for research studies. Report of the NINDS‐AIREN International Workshop. Neurology 43:250–260. [DOI] [PubMed] [Google Scholar]
- Seo SW, Ahn J, Yoon U, Im K, Lee JM, Tae Kim S, Ahn HJ, Chin J, Jeong Y, Na DL (2010): Cortical thinning in vascular mild cognitive impairment and vascular dementia of subcortical type. J Neuroimaging 20:37–45. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC (1998) The Mini‐International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. J Clin Psychiatry 59 Suppl 20:22–33;quiz 34‐57. [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE (2004): The medial temporal lobe. Annu Rev Neurosci 27:279–306. [DOI] [PubMed] [Google Scholar]
- van Norden AG, de Laat KF, Gons RA, van Uden IW, van Dijk EJ, van Oudheusden LJ, Esselink RA, Bloem BR, van Engelen BG, Zwarts MJ, Tendolkar I, Olde‐Rikkert MG, van der Vlugt MJ, Zwiers MP, Norris DG, de Leeuw FE (2011): Causes and consequences of cerebral small vessel disease. The RUN DMC study: A prospective cohort study. Study rationale and protocol. BMC Neurol 11:29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Norden AG, de Laat KF, Fick I, van Uden IW, van Oudheusden LJ, Gons RA, Norris DG, Zwiers MP, Kessels RP, de Leeuw FE (2012a): Diffusion tensor imaging of the hippocampus and verbal memory performance: The RUN DMC study. Hum Brain Mapp 33:542–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Norden AG, van Dijk EJ, de Laat KF, Scheltens P, Olderikkert MG, de Leeuw FE (2012b): Dementia: Alzheimer pathology and vascular factors: from mutually exclusive to interaction. Biochim Biophys Acta 1822:340–349. [DOI] [PubMed] [Google Scholar]
- van Uden IW, Tuladhar AM, de Laat KF, van Norden AG, Norris DG, van Dijk EJ, Tendolkar I, de Leeuw FE (2014): White Matter Integrity and Depressive Symptoms in Cerebral Small Vessel Disease: The RUN DMC Study. Am J Geriatr Psychiatry. 23:525–535. doi: 10.1016/j.jagp.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Verdelho A, Madureira S, Moleiro C, Ferro JM, O'Brien JT, Poggesi A, Pantoni L, Fazekas F, Scheltens P, Waldemar G, Wallin A, Erkinjuntti T, Inzitari D, Study L (2013): Depressive symptoms predict cognitive decline and dementia in older people independently of cerebral white matter changes: the LADIS study. J Neurol Neurosurg Psychiatry 84:1250–1254. [DOI] [PubMed] [Google Scholar]
- Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM (2003): Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med 348:1215–1222. [DOI] [PubMed] [Google Scholar]
- Viswanathan A, Rocca WA, Tzourio C (2009): Vascular risk factors and dementia: How to move forward? Neurology 72:368–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O'Brien JT, Barkhof F, Benavente OR, Black SE, Brayne C, Breteler M, Chabriat H, Decarli C, de Leeuw FE, Doubal F, Duering M, Fox NC, Greenberg S, Hachinski V, Kilimann I, Mok V, Oostenbrugge R, Pantoni L, Speck O, Stephan BC, Teipel S, Viswanathan A, Werring D, Chen C, Smith C, van Buchem M, Norrving B, Gorelick PB, Dichgans M nEuroimaging STfRV, co (2013): Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 12:822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesmann M, Kiliaan AJ, Claassen JA (2013): Vascular aspects of cognitive impairment and dementia. J Cerebral Blood Flow Metabolism 33:1696–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G (2006): User‐guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 31:1116–1128. [DOI] [PubMed] [Google Scholar]
- Zola‐Morgan S, Squire LR, Amaral DG (1986): Human amnesia and the medial temporal region: Enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. J Neurosci 6:2950–2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwiers MP (2010): Patching cardiac and head motion artefacts in diffusion‐weighted images. Neuroimage 53:565–575. [DOI] [PubMed] [Google Scholar]


