Abstract
Our understanding of altered emotional processing in social anxiety disorder (SAD) is hampered by a heterogeneity of findings, which is probably due to the vastly different methods and materials used so far. This is why the present functional magnetic resonance imaging (fMRI) study investigated immediate disorder‐related threat processing in 30 SAD patients and 30 healthy controls (HC) with a novel, standardized set of highly ecologically valid, disorder‐related complex visual scenes. SAD patients rated disorder‐related as compared with neutral scenes as more unpleasant, arousing and anxiety‐inducing than HC. On the neural level, disorder‐related as compared with neutral scenes evoked differential responses in SAD patients in a widespread emotion processing network including (para‐)limbic structures (e.g. amygdala, insula, thalamus, globus pallidus) and cortical regions (e.g. dorsomedial prefrontal cortex (dmPFC), posterior cingulate cortex (PCC), and precuneus). Functional connectivity analysis yielded an altered interplay between PCC/precuneus and paralimbic (insula) as well as cortical regions (dmPFC, precuneus) in SAD patients, which emphasizes a central role for PCC/precuneus in disorder‐related scene processing. Hyperconnectivity of globus pallidus with amygdala, anterior cingulate cortex (ACC) and medial prefrontal cortex (mPFC) additionally underlines the relevance of this region in socially anxious threat processing. Our findings stress the importance of specific disorder‐related stimuli for the investigation of altered emotion processing in SAD. Disorder‐related threat processing in SAD reveals anomalies at multiple stages of emotion processing which may be linked to increased anxiety and to dysfunctionally elevated levels of self‐referential processing reported in previous studies. Hum Brain Mapp 37:1559‐1572, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: social phobia, psychophysiological interaction, default‐mode‐network, self‐referential processing, amygdala, globus pallidus, precuneus
INTRODUCTION
Social anxiety disorder (SAD) is defined by exaggerated fear in social and performance situations [DSM‐IV‐TR; American Psychiatric Association, 2000]. SAD patients are highly afraid of negative evaluation by others, especially when they are in the spotlight of attention, as, for example, when giving a speech, but also during small talk at parties and social gatherings [Stangier and Fydrich, 2002]. Models of SAD assume that self‐focus is increased, interoceptive information is prioritized, and external information is processed in a biased manner. Together, this leads to a biased self‐representation in social situations, thereby provoking and maintaining anxiety in such feared situations [Clark and Wells, 1995; Rapee and Heimberg, 1997].
A growing number of studies have investigated the neural correlates of SAD. (Para‐)limbic structures such as amygdala, insula, thalamus and globus pallidus, and cortical areas such as prefrontal cortex (PFC), anterior cingulate cortex (ACC), and superior temporal sulcus (STS) have been implied at different stages of threat processing in SAD [Brühl et al., 2014; Etkin and Wager, 2007; Freitas‐Ferrari et al., 2010; Gentili et al., 2015a, 2015b; Hattingh et al., 2013; Miskovic and Schmidt, 2012; Schulz et al., 2013]. In addition to deviations in this fear network, differential activation patterns have been observed in SAD in parieto‐occipital areas, such as posterior cingulate cortex (PCC), precuneus and cuneus [Brühl et al., 2014]. Medial PFC (mPFC), PCC and precuneus belong to the so‐called default mode network [DMN; Raichle et al., 2001] that is associated with self‐referential processing and emotion regulation.
Activation patterns reported in neuroimaging studies on affective processing in SAD are inconsistent and seem to depend on the paradigms and stimulus materials used. While most studies employed emotional faces as stimuli [Amir et al., 2005; Phan et al., 2006; Stein et al., 2002; Straube et al., 2004a], others used disorder‐related words [e.g. Schmidt et al., 2010], evaluative comments [e.g. Blair et al., 2008; Heitmann et al., 2014], voices [Quadflieg et al., 2008], or video clips showing social interaction situations [Boehme et al., 2014]. Some studies used anticipation of a public speech to provoke symptoms in SAD [Boehme et al., 2013; Lorberbaum et al., 2004]. With such diverse stimuli and designs, it is not surprising that results diverge between studies. Emotional faces seem to possess increased relevance for SAD [Schulz et al., 2013], but there is little evidence for specific fear of faces in SAD, since threat‐related rating data are lacking [Evans et al., 2008; Stein et al., 2002; Straube et al., 2004a; Straube et al., 2005]. Moreover, faces per se, in the absence of disorder‐relevant context, might not be sufficient to investigate emotion processing because processes such as identity recognition might interfere [Radua et al., 2014].
Studies applying more disorder‐related and anxiety‐inducing stimuli (e.g. words, evaluative comments, anticipation of speech) have neglected two aspects important for understanding disorder‐related threat processing in SAD. First, representativeness, naturalness und relevance as determinants of ecological validity [Schmuckler, 2001] is often rather limited for stimuli such as words. Second, neural responses to threatening stimuli in SAD may differ depending on the time window in which they occur. Studies investigating the immediate emotional response to ecologically valid, disorder‐related stimuli that provoke anxiety in SAD patients are as yet lacking. The only study, to our knowledge, was conducted by Nakao et al. [2011], with eight socially relevant complex scenes that were, unfortunately, low in ecological validity. For this reason, the present study validated and used stimuli specifically related to SAD with a high ecological validity.
Thus, due to heterogeneity of stimulus material and designs, several aspects of the neural basis of threat processing in SAD still remain to be elucidated. This particularly applies to interactions between brain structures relevant for affective processing in SAD. Recently, functional connectivity analysis has been employed to shed light onto functional brain networks in SAD [Ding et al., 2011; Liao et al., 2010; Prater et al., 2013]. Findings suggest altered functional connectivity between amygdala and prefrontal structures [Duval et al., 2015] and decoupling of posteromedial regions in SAD [Brühl et al., 2014]. Nonetheless, the number of functional connectivity studies in SAD is still very limited and conclusions remain tentative.
The aim of the present study was to investigate neural activation and connectivity patterns during emotional processing in SAD with tailor‐made stimuli. A novel, standardized stimulus set (Social Anxiety Picture Set Muenster, SAPS‐M) consisting of 50 disorder‐related and 50 neutral control scenes was used. Scene pictures were selected to be high in ecological validity and in relevance for SAD patients. Activity in brain regions assumed to be critical for affective processing in SAD [Brühl et al., 2014; Etkin and Wager, 2007; Freitas‐Ferrari et al., 2010; Miskovic and Schmidt, 2012; Schulz et al., 2013] as well as for general emotional scene processing [Sabatinelli et al., 2007; Vuilleumier and Driver, 2007] was analyzed. Moreover, functional connectivity among these structures was investigated with psychophysiological interaction (PPI) analysis. Regions of interest (ROIs) were amygdala, insula, thalamus, globus pallidus, mPFC, ACC, STS, PCC, precuneus, and cuneus. We expected to observe (1) hyperactivation in the above described ROIs for processing of disorder‐related versus neutral scenes in SAD patients, relative to healthy controls (HC). Next, (2), SAD patients as compared with HC should show altered functional connectivity between threat‐processing regions for disorder‐related as compared with neutral scenes.
MATERIALS AND METHODS
Subjects
Sixty‐seven participants took part in this study. SAD patients were recruited via public notices, local paper ads, and from collaborating psychotherapy institutes. HC were selected from a volunteer database at the Institute of Medical Psychology and Systems Neuroscience at the University of Münster, Germany. All participants had normal or corrected‐to‐normal vision, were right‐handed [Oldfield, 1971], met the general MRI requirements, had no history of neurological diseases or psychotic disorders, did not currently take psychotropic medication, and were screened by a psychologist using the standardized clinical interview [SCID; Wittchen et al., 1997]. SAD patients fulfilled the criteria for current generalized social anxiety disorder according to DSM‐IV as main diagnosis. HC were free of any diagnosis. All participants completed the German version of the Liebowitz‐Social‐Anxiety‐Scale [LSAS; German version; Stangier and Heidenreich, 2004], Social Phobia Scale [SPS; Stangier et al., 1999], Social Interaction Anxiety Scale [SIAS, Stangier et al., 1999], and the Beck Depression Inventory [BDI, Hautzinger et al., 1995]. Participants with a BDI score >30 (n = 2 SAD patients) and participants whose behavioral responses were not recorded properly due to technical problems (n = 2 SAD patients) were excluded from statistical analysis. Two HC with LSAS scores >30, which suggests a mild form of SAD [Mennin et al., 2002], were also excluded. The final sample comprised of 30 SAD patients (19 females, 11 males) and 30 HC (19 females, 11 males). The groups were matched according to gender, age, and educational attainment (see Table 1 for sample details). Comorbid diagnoses in SAD patients (n = 11, multiple entries possible) were current Major Depression Episode (n = 4), specific phobia (n = 7), Obsessive Compulsive Disorder (n = 1), and General Anxiety Disorder (n = 1). As expected, SAD patients scored higher than HC in social anxiety‐sensitive questionnaires (Table 1). BDI scores were also significantly increased in SAD patients, but are comparable with scores reported in other studies [e.g. Straube et al., 2004a].
Table 1.
Mean age, mean educational attainment (years) and mean scores (±standard deviation) for social anxiety‐related questionnaires (LSAS, SPS, SIAS) and Beck Depression Inventory (BDI) for patients suffering from social anxiety disorder (SAD) and healthy controls (HC)
| SAD (M ± SD) | HC (M ± SD) | t‐value | P value (two‐tailed) | |
|---|---|---|---|---|
| Age | 27.5 ± 7.74 | 27.07 ± 5.35 | 0.252 | 0.802 |
| Education | 12.7 ± 1.32 | 13.27 ± 1.05 | −0.165 | 0.07 |
| LSAS | 67.2 ± 15.78 | 9 ± 6.44 | 18.7 | ≤0.001 |
| SPS | 32.27 ± 12.01 | 12.01 ± 3.28 | 13.18 | ≤0.001 |
| SIAS | 44.67 ± 12.85 | 9.03 ± 6.04 | 13.75 | ≤0.001 |
| BDI | 10.73 ± 7.57 | 1.17 ± 2.73 | 6.51 | ≤0.001 |
M = mean; SD = standard deviation. LSAS, Liebowitz social anxiety scale; SPS, social phobia scale; SIAS, social interaction anxiety scale; BDI, beck depression inventory.
The study conforms to the Declaration of Helsinki and was approved by the ethics committee of the University of Muenster, Germany. Written informed consent was obtained from each participant prior to the experiment. Participants received monetary compensation for participation.
Stimuli
The stimulus set (SAPS‐M) used here was developed in an extensive pilot study. Initially, 128 scene photographs thought to evoke social anxiety were either obtained from an internet‐based search using search terms such as “job interview”, “giving a speech” and “being the center of attention”, or were specifically created for the purpose. Clinical experts (n = 10) evaluated the suitability of these scenes to elicit fear in SAD patients. The 97 scenes deemed most suitable were then rated with regard to valence, arousal and anxiety‐induction by eight SAD patients who were currently under treatment and did not participate in the actual study, and by eight HC. For 72 scenes, anxiety ratings differed significantly between SAD patients and HC. From these 72 pictures, the 50 that discriminated best between SAD patients and HC were selected as final stimulus set of disorder‐related scenes. Scene photos depicted the following themes (multiple entries possible): giving a speech (10), job interview (14), round table/discussion (9), bullying (5), anxious body symptom (3), observation/evaluation (9), and request to speak (2).
Fifty neutral scenes were chosen for the control condition. Neutral scenes were selected based on ecological validity, taking into account representativeness, naturalness and relevance [Schmuckler, 2001], from the International Affective Picture System [IAPS; Lang et al., 2008], and the Emotional Picture Set [EmoPicS; Wessa et al., 2010]. Based on a small pilot study, in which four SAD patients who were not part of the final sample (but had already participated in the pilot study assessing disorder‐relatedness of the stimulus material) rated these pictures, 39 of these scenes (original IAPS/EmoPics ratings: mean valence: 5.004 ± 0.264; mean arousal: 3.211 ± 0.447) were confirmed as neutral also in SAD (mean ratings in pilot study: valence: 4.846 ± 0.508; mean arousal: 3.871 ± 0.845). In order to obtain a total of 50 neutral scenes, eleven other scene pictures, similar to the 39 in content and complexity, were added (obtained from IAPS, EmoPics, or internet search).
Importantly, disorder‐related and neutral scenes were matched with regard to properties such as central object (person or object), presence of facial expression, complexity, product‐entropy, luminance, and color (all P > 0.05). The number of illustrated persons and location depicted in the scene (indoor/outdoor) could not be matched. For task purposes, five additional scenes (originally EmoPics) were blurred with Adobe Photoshop CS6 (version 13.0.1, Adobe Sytems Inc., San Jose, CA). All in all, the final stimulus set consisted of 50 disorder‐related scenes (for SAD patients), 50 neutral scenes, and five blurred pictures.
Experimental Task
Hundred scene pictures, 50 disorder‐related and 50 neutral (with a resolution of 600 dpi), and five blurred pictures were each shown once in randomized order for 800 ms in the center of a black screen. Stimulus presentation was controlled by Presentation software (version 17.2, Neurobehavioral Systems, Albany, CA). Between stimulus presentations, a white fixation cross occurred for an average time period of 3,915 ms (jittered between 1,280 ms and 15,320 ms). To increase signal discriminability [Dale, 1999], the random stimulus sequence was optimized using the “optimal sequencing” (optseq) algorithm (http://www.surfer.nmr.mgh.harvard.edu/optseq/). Participants viewed scene pictures passively. To ensure attention to the scenes, they were instructed to press a button with the right index finger whenever they saw a blurred picture. No participant had more than one omission. Trials with blurred pictures were excluded from behavioral and fMRI analysis. Completion of the experimental task took approximately 9 min.
Recording and Analysis of Behavioral Data
Outside the scanner and after fMRI testing, participants rated the 50 disorder‐related and 50 neutral scenes. Nine‐point Likert scales were used to assess valence (1 = “very unpleasant” to 9 = “very pleasant”), arousal (1 = “not arousing” to 9 = “very arousing”), and anxiety levels subjects had experienced during picture presentation (1 = “not anxious” to 9 = “very anxious”). Rating was computer‐based, with each scene presented for 2 s, followed by the three rating scales, until participants responded.
Statistical analyses were performed using IBM SPSS Statistics 22 software (Armonk, New York). Valence, arousal and anxiety ratings were analyzed by means of repeated‐measures analyses of variance (ANOVAs) with emotion (disorder‐related or neutral) as within‐subjects factor and group (SAD or HC) as between‐subjects factor. For the ANOVAs, a probability level of P ≤ 0.05 was considered statistically significant. Post hoc t‐tests used to resolve interactions were Bonferroni‐corrected for multiple comparisons (corrected significance level P < 0.0125).
Functional MRI Data
Anatomical and functional MRI data were acquired with a 3T magnetic resonance scanner (“Magnetom PRISMA,” Siemens, Erlangen; GER) using a 20‐channel head‐neck coil. Functional data were measured using a T2*‐weighted echo‐planar sequence (TE = 30 ms, flip angle = 90°, matrix = 92 × 92 voxels, FOV = 208 mm2, TR = 2,080 ms). 255 volumes of 36 axial slices (thickness = 3 mm, 0.3 mm gap, in plane resolution = 2.26 mm × 2.26 mm) were acquired. To minimize susceptibility artifacts in inferior parts of anterior brain areas, the volumes were tilted approximately 20° from the AC/PC line. A shimming field was applied before functional imaging to reduce external magnetic‐field inhomogeneities. A high‐resolution T1‐weighted anatomical volume with 192 slices was also recorded.
Pre‐processing and analysis of functional data were performed using Brain Voyager QX software (version 2.4, Brain Innovation, Maastricht, NL). The first ten volumes were discarded from analysis to secure steady‐state tissue magnetization. Volumes were realigned to the first volume to minimize effects of head movements on data analysis. No participant showed excessive head movement (>1 voxel). Further data preprocessing comprised spatial (6 mm full‐width half‐maximum isotropic Gaussian kernel, FWHMK) as well as temporal (low pass filter: 2.8 s; high pass filter: 10 cycles in time course) smoothing. Anatomical and functional images were co‐registered and normalized to Talairach space [Talairach and Tournoux, 1988]. Finally, volumes were resampled to voxels of 2 × 2 × 2 mm, and slice time correction was applied.
Multiple linear regression of the signal time course at each voxel was calculated with adjustment for autocorrelation following a global AR(2) model. The expected blood oxygenation level‐dependent (BOLD) signal change for each predictor was modeled by a canonical double‐gamma hemodynamic response function (HRF). The two predictors of interest were disorder‐related scene and neutral scene. First, voxel‐wise statistical maps were generated and predictor estimates (beta weights) were computed for each individual. Second, a random‐effects group analysis of the individual contrasts was calculated. Analyses were conducted for specific regions of interest (ROIs), defined a priori according to Automated Anatomical Labeling atlas [Maldjian et al., 2003; Tzourio‐Mazoyer et al., 2002] and transformed into Talairach space according to Lancaster et al. [2007] using ICBM2TAL in Matlab (version 8.2, The MathWorks Inc, Natick, MA). ROIs were insula (dilated 1 mm in radius), thalamus, globus pallidus, ACC, mPFC, STS, PCC, precuneus, and cuneus. The analysis of activation patterns in the amygdala was conducted in accordance with Boll et al. [2013]: ROIs for centromedial, basolateral and superficial amygdala were taken from the Anatomy Toolbox [Amunts et al., 2005], and the centromedial and superficial nuclear group will be referred to as corticomedial amygdala. The anatomical assignment of the observed activation patterns to an amygdala subregion was also verified using the anatomical atlas by Mai et al. [2004]. In addition, we conducted an exploratory whole‐brain analysis to investigate reliable task‐related activations outside the ROIs. Psychophysiological interaction (PPI) analysis was performed to explore differences between the groups' emotion‐dependent connectivity patterns within ROIs for the disorder‐related > neutral contrast. Significant activation clusters within ROIs for the contrast disorder‐related > neutral that differentiated between SAD and HC were defined as seed regions. PPI analysis was conducted with an interaction regressor that is the product of the HRF‐convolved task regressor (psychological factor) and the seed region time course (physiological factor).
Statistical parametric maps resulting from voxel‐wise analyses were considered significant for clusters that survived cluster‐based correction for multiple comparisons. For ROI‐ and PPI‐analyses, voxel‐level threshold was initially set to P ≤ 0.005 (uncorrected); for exploratory whole brain analysis, it was set to P ≤ 0.001 (uncorrected). Using a cluster‐level statistical threshold plugin in Brain Voyager [Goebel et al., 2006], thresholded maps were then submitted to a ROI‐specific correction criterion for the ROI‐analysis, or to a correction criterion based on the whole brain for exploratory analysis. For analysis of amygdala activations, correction criterions specific to the subregions were calculated to avoid missing activations in these small subregions. Correction criteria were always based on the estimate of the maps' spatial smoothness and on an iterative procedure (Monte Carlo simulation) used to estimate cluster‐level false‐positive rates [Forman et al., 1995]. After 1,000 iterations, the minimum cluster size threshold that yielded a cluster‐level false‐positive rate of 5% was applied to the statistical maps. For PPI‐analyses, a Bonferroni‐corrected threshold was used (P < 0.00625) due to multiple testing.
To account for dimensional effects between brain activation and social anxiety, correlational analysis between self‐reported social anxiety measures (LSAS, SPS‐, and SIAS‐scores) and extracted mean beta values for all participants within the ROI analysis‐based clusters showing differential neural effects were calculated (Bonferroni‐corrected significance level P < 0.00625).
Furthermore, to control for effects of depression in SAD patients, mean beta values of significant activation patterns yielded by ROI‐analyses in SAD patients were correlated with BDI‐scores (Bonferroni‐corrected significance level P < 0.00625).
RESULTS
Behavioral Data: Valence, Arousal, and Anxiety Ratings
Mean ratings of valence, arousal and perceived anxiety for SAD patients and HC according to emotion (disorder‐related versus neutral) are provided in Figure 1. Rating data revealed significant main effects of emotion (valence: F (1,58) = 177.871, P ≤ 0.001; arousal: F (1,58) = 193.585, P ≤ 0.001; anxiety: F (1,58) = 118.441, P ≤ 0.001) and group (valence: F (1,58) = 57.068, P ≤ 0.001; arousal: F (1,58) = 35.938, P ≤ 0.001; anxiety: F (1,58) = 66.805, P ≤ 0.001). Moreover, significant emotion × group interaction effects emerged, indicating that SAD patients rated disorder‐related as compared with neutral scenes as more unpleasant (F (1,58) = 33.247, P ≤ 0.001), more arousing (F (1,58) = 66.736, P ≤ 0.001) and more anxiety‐inducing (F (1,58) = 75.819, P ≤ 0.001) than HC.
Figure 1.
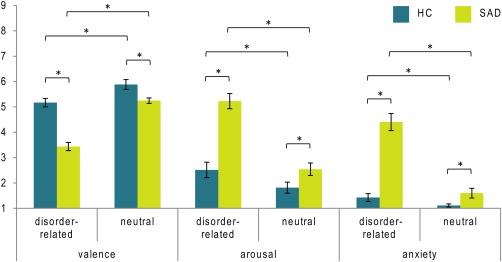
Mean valence, arousal and anxiety ratings for disorder‐related and neutral scenes in patients suffering from social anxiety disorder (SAD) and healthy controls (HC). Asterisks mark significant differences (P ≤ 0.0125). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
fMRI Data: Interaction Emotion by Group
ROI analyses for the contrast disorder‐related versus neutral scenes showed increased brain activation in SAD patients as compared with HC (see Fig. 2) in right corticomedial amygdala (peak voxel Talairach coordinates: x = 17, y = −5, z = −6, size: 104 mm3, average t‐value: 3.065, maximal t‐value: 3.901, P < 0.005 uncorrected, P < 0.05 corrected), left insula (peak voxel Talairach coordinates: x = −31, y = 11, z = −8, size: 608 mm3, average t‐value: 3.112, maximal t‐value: 4.109, P < 0.005 uncorrected, P < 0.05 corrected), left thalamus (peak voxel Talairach coordinates: x = −6, y = −11, z = 0, size: 304 mm3, average t‐value: 3.977, maximal t‐value: 6.601, P < 0.005 uncorrected, P < 0.05 corrected), left globus pallidus (peak voxel Talairach coordinates: x = −21, y = −1, z = 2, size: 1,056 mm3, average t‐value: 3.543, maximal t‐value: 4.829, P < 0.005 uncorrected, P < 0.05 corrected), left dorsal mPFC (dmPFC; peak voxel Talairach coordinates: x = −5, y = 41, z = 33, size: 984 mm3, average t‐value: 3.164, maximal t‐value: 4.213, P < 0.005 uncorrected, P < 0.05 corrected), bilateral precuneus (left: peak voxel Talairach coordinates: x = −12, y = −55, z = 48, size: 1,912 mm3, average t‐value: 3.284, maximal t‐value: 4.387, P < 0.005 uncorrected, P < 0.05 corrected; right: peak voxel Talairach coordinates: x = 12, y = −55, z = 23, size: 336 mm3, average t‐value: 2.949, maximal t‐value: 3.411, P < 0.005 uncorrected, P < 0.05 corrected) and left PCC/precuneus (peak voxel Talairach coordinates: x = −11, y = −53, z = 26, size: 2872 mm3, average t‐value: 3.17, maximal t‐value: 4.402, P < 0.005 uncorrected, P < 0.05 corrected). Note that the PCC/precuneus cluster extended into left cuneus. For SAD patients, there were no significant correlations with BDI‐scores (all effects failed to reach the Bonferroni‐corrected significance level of P < 0.00625).
Figure 2.
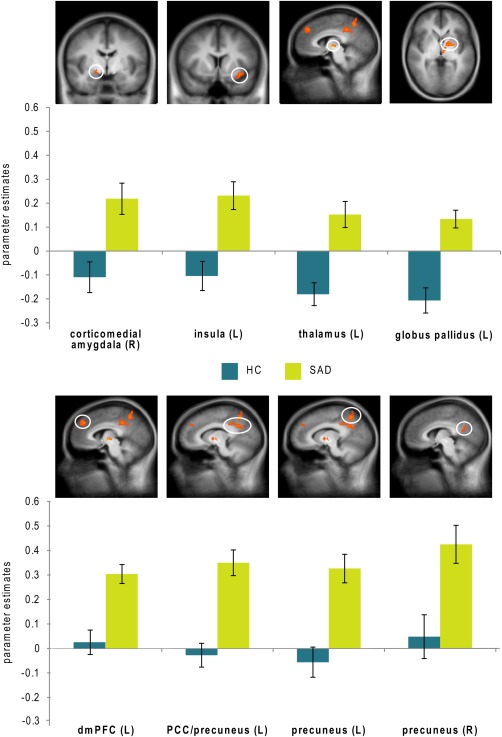
Differential brain activations during disorder‐related versus neutral scene processing in patients suffering from social anxiety disorder (SAD) as compared with healthy controls (HC). Statistical parametric maps are overlaid on a T1 scan (P < 0.005 uncorrected, P < 0.05 corrected; radiological convention: left (L) = right (R)). SAD patients display enhanced activation in corticomedial amygdala (y = 5), insula (y = 9), thalamus (x = −5), globus pallidus (z = 2), dorsomedial prefrontal cortex (dmPFC) (x = 5), posterior cingulate cortex (PCC)/precuneus (x = −10), left precuneus (x = −10) and right precuneus (x = 6). Diagrams show contrasts of parameter estimates (disorder‐related versus neutral; mean ± SE). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
The extracted mean beta values of these clusters for all participants showed highly significant correlations with self‐reported social anxiety measures: with all regions for the LSAS‐score (r = 0.354–0.527, P = 0.000015–0.005563), and with all clusters except right precuneus for the SPS‐score (r = 0.371–0.58, P = 0.000001–0.003507) and the SIAS‐score (r = 0.378–0.568, all P = 0.00002–0.00288).
Results of the exploratory whole‐brain analysis are provided in Table 2. Activation patterns revealed by whole brain analysis were largely consistent with activation clusters yielded by ROI‐analysis. Whole brain analysis did yield additional activations in angular gyrus, cerebellum, lateral PFC (lPFC), middle temporal gyrus, precentral gyrus and superior parietal lobule (SPL).
Table 2.
Significant hyperactivations for disorder‐related versus neutral scenes in patients suffering from social anxiety disorder (SAD) relative to healthy controls (HC) as revealed by exploratory whole brain analysis (P ≤ 0.001 uncorrected, and P ≤ 0.05 corrected)
| Region | Lateralization | Talairach coordinates of peak voxel | Cluster size (mm3) | t‐value average | t‐value maximum | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| angular gyrus | L | −44 | −75 | 24 | 360 | 3.568 | 4.171 |
| cerebellum lobule V | L | −11 | −42 | −13 | 464 | 3.714 | 4.929 |
| dmPFC | L | −6 | 40 | 33 | 392 | 3.56 | 4.213 |
| lPFC | L | −26 | 55 | 3 | 256 | 3.536 | 4.075 |
| middle temporal gyrus | L | −55 | −39 | −2 | 248 | 3.597 | 4.159 |
| PCC | L | −3 | −41 | 35 | 416 | 3.702 | 4.389 |
| PCC | L | −11 | −54 | 25 | 584 | 3.571 | 4.402 |
| precentral gyrus | L | −40 | 4 | 35 | 744 | 3.637 | 4.629 |
| precuneus | L | −11 | −55 | 48 | 816 | 3.726 | 4.387 |
| globus pallidus/putamen | L | −22 | −1 | 2 | 728 | 3.814 | 4.829 |
| SPL | R | 15 | −53 | 58 | 216 | 3.529 | 4.165 |
| thalamus | L | −5 | −12 | −1 | 632 | 4.19 | 6.601 |
dmPFC = dorsomedial prefrontal cortex; lPFC = lateral prefrontal cortex; PCC = posterior cingulate cortex; SPL = superior parietal lobule; L = left; R = right.
Overall, processing of disorder‐related versus neutral scenes in SAD patients recruited a distributed network of brain regions previously implied in emotional processing, particularly in threat processing (e.g. amygdala, insula, dmPFC, thalamus), alongside with several parietal, prefrontal and temporal regions.
fMRI Data: PPI Analysis
PPI analyses investigated functional connectivity between ROI‐Clusters differentiating between SAD patients and HC for the contrast disorder‐related > neutral scenes and other ROIs. Table 3 provides connectivity patterns for which significant differences between SAD patients and HC were found. In SAD patients relative to HC, hyperconnectivities were found for PCC/precuneus with insula and precuneus, and for globus pallidus with mPFC, ACC, and amygdala (stretching from basolateral to medial areas). Moreover, hypoconnectivity was found between left dmPFC and left PCC/precuneus (see Table 3, see Figs. 3 and 4).
Table 3.
PPI analysis: Significant differences between patients suffering from social anxiety disorder (SAD) and healthy controls (HC) in connectivity patterns for the contrast disorder‐related versus neutral scenes (P ≤ 0.005 uncorrected, and P ≤ 0.00625 corrected)
| Seed region | Finding region | Lateralization | Talairach coordinates of peak voxel | Cluster size (mm3) | t‐value average | t‐value maximum | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| SAD > HC | ||||||||
| PCC/precuneus | insula | R | 28 | −23 | 5 | 512 | 3.008 | 3.633 |
| precuneus | R | 11 | −59 | 36 | 1168 | 3.067 | 4.222 | |
| precuneus | L | −1 | −75 | 34 | 1872 | 3.155 | 4.289 | |
| globus pallidus | mPFC | R | 15 | 45 | 8 | 688 | 2.95 | 3.718 |
| ACC | R | 7 | 39 | 0 | 600 | 3.007 | 4.169 | |
| amygdala (BL) | R | 13 | −1 | −12 | 200 | 2.901 | 3.454 | |
| HC > SAD | ||||||||
| dmPFC | PCC/precuneus | L | −5 | −47 | 38 | 464 | 3.092 | 4.021 |
PCC = posterior cingulate cortex; mPFC = medial prefrontal cortex; ACC = anterior cingulate cortex; BL = basolateral; L = left; R = right.
Figure 3.
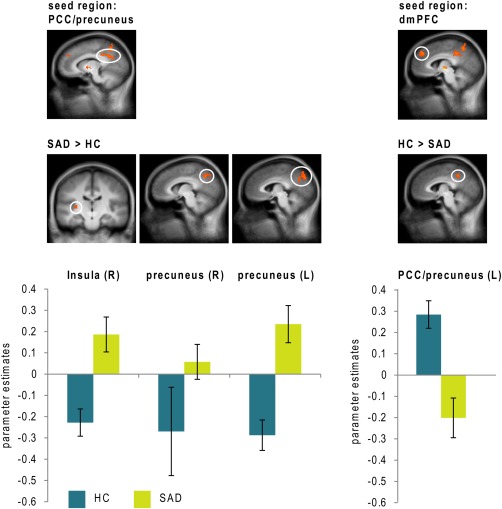
Differential psychophysiological interactions in patients suffering from social anxiety disorder (SAD) and healthy controls (HC) seeded from posterior cingulate cortex (PCC)/precuneus with findings (all SAD > HC) in insula (y = −18), left precuneus (x = −4) and right precuneus (x = 7), as well as seeded from dorsomedial prefrontal cortex (dmPFC) with a finding (HC > SAD) in PCC/precuneus (x = −4). Statistical parametric maps are overlaid on a T1 scan (P < 0.005 uncorrected, P < 0.05 corrected; radiological convention: left (L) = right (R)). Diagrams show contrasts of parameter estimates (disorder‐related versus neutral; mean ± SE). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 4.
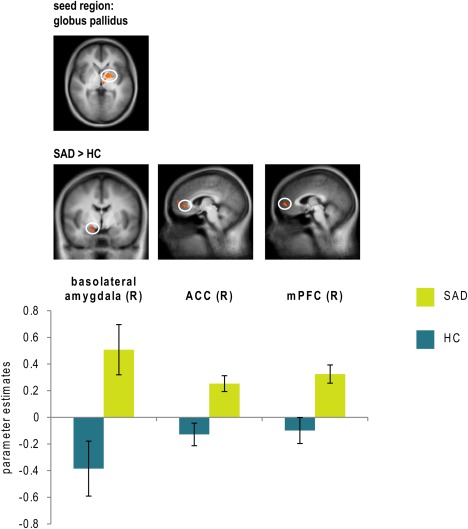
Differential psychophysiological interactions in patients suffering from social anxiety disorder (SAD) and healthy controls (HC) seeded from globus pallidus with findings (all SAD > HC) in basolateral amygdala (y = −4), anterior cingulate cortex (ACC; x = 5) and medial prefrontal cortex (mPFC; x = 3). Statistical parametric maps are overlaid on a T1 scan (P < 0.005 uncorrected, P < 0.05 corrected; radiological convention: left (L) = right (R)). Diagrams show contrasts of parameter estimates (disorder‐related versus neutral; mean ± SE). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
DISCUSSION
The aim of the present study was to investigate threat processing in SAD patients using a novel, standardized set of complex, ecologically valid disorder‐related visual scenes. Brain activation during scene presentation was examined using fMRI, and subjective ratings of perceived valence, arousal and anxiety were obtained. SAD patients as compared with HC rated disorder‐related versus neutral scenes as more unpleasant, more arousing and more anxiety‐inducing. In line with our first prediction, fMRI data yielded hyperactivations for disorder‐related versus neutral scenes in SAD patients1 in brain structures typically associated with different stages and functions of emotional processing: amygdala, insula, thalamus, globus pallidus, dmPFC, precuneus, and PCC. Supporting our second prediction, PPI analysis underlined the role of the PCC/precuneus region for disorder‐related threat processing in SAD, revealing altered functional connectivity of this region with insula, dmPFC and precuneus. In addition, globus pallidus showed hyperconnectivity in SAD patients as compared with HC with amygdala, mPFC and ACC. Overall, patterns of (hyper‐)activation and altered functional connectivity in SAD may be linked to increased anxiety and self‐referential processing. In the following sections, our results will be discussed in detail.
Rating results confirmed that disorder‐related scenes depicted situations SAD patients were indeed afraid of, thus corroborating their ecological validity. Differential activation patterns as revealed by fMRI data were suggestive of implicit emotional processing which emphasizes increased salience of disorder‐related scenes for SAD patients. Disorder‐related versus neutral scenes evoked hyperactivations in amygdala, insula, globus pallidus, thalamus, dmPFC, precuneus, and PCC in SAD patients. These regions have been discussed as crucial parts of the neural basis of SAD [e.g. Brühl et al., 2014; Gentili et al., 2015a,b].
Amygdala hyperactivation in response to threat is frequently observed in SAD [Blair et al., 2011; Schmidt et al., 2010; Stein et al., 2002; Straube et al., 2004a]. The recording and statistical analysis procedures of our study allowed more precise assessment of activity in amygdala subregions. In SAD patients, disorder‐related relative to neutral scenes evoked hyperactivation in corticomedial amygdala, more precisely in the central amygdaloid nucleus [cf. Mai et al., 2004]. Animal studies suggest that the central nucleus of the amygdala is involved in initiation of autonomic and behavioral responses via connections with subcortical regions [LeDoux, 2003; Pitkänen et al., 1997]. In humans, fewer studies have investigated this, but do report association with similar functions, for example attentional allocation towards or evaluation of significance of salient stimuli [Boll et al., 2011; Frühholz and Grandjean, 2013; Holland and Gallagher, 1999]. Thus, hyperactivation of the corticomedial amygdala complex in response to disorder‐related scenes in SAD may indicate increased attention and vigilance when perceiving potential threat.
In addition to amygdala hyperactivation, insula hyperactivation has also been observed in SAD patients in response to and also during anticipation of potential threat [Amir et al., 2005; Boehme et al., 2013; Gentili et al., 2009; Klumpp et al., 2010; Lorberbaum et al., 2004; Straube et al., 2004a, 2005]. The insula plays an important role in interoceptive processing [Craig, 2009; Critchley et al., 2004], more specifically for evaluating the emotional salience of interoceptive stimuli [Menon and Uddin, 2010; Reiman, 1997], and thereby for generating the subjective feeling of anxiety [Damasio et al., 2000]. Insula hyperactivation in our data may thus reflect perception of anxiety‐related bodily symptoms and enhanced self‐focus, both of which are core components of models of SAD [Clark and Wells, 1995; Rapee and Heimberg, 1997].
Complementary to amygdala and insula hyperactivations as indicators of anxious processing, hyperactivation in the thalamus may point to emotional impact at very early processing stages. The thalamus is an important structure for initial sensory integration and processing [Jones, 2003], and thalamus hyperactivation in SAD might be the correlate of an early fearful response in reaction to disorder‐related scenes [Brühl et al., 2011; Vuilleumier and Driver, 2007]. Moreover, hyperactivation in globus pallidus, putamen and prefrontal regions in concert with thalamic hyperactivation might reflect recruitment of cortico‐striatal‐thalamic‐cortical loops [e.g. Alexander and Crutcher, 1990] in threat processing in SAD.
In addition to its assumed involvement in the cortico‐striatal‐thalamic loop [Alexander and Crutcher, 1990], the globus pallidus is primarily associated with functions in voluntary movement [e.g. Sztainberg et al., 2011]. Interestingly, hyperactivation in this region during processing of potential threat in SAD has been described before [Binelli et al., 2014; Etkin and Wager, 2007; Gentili et al., 2015a,b; Hattingh et al., 2013]. These studies consider a possible role of the globus pallidus in modulation of motor aspects in response to salient emotional stimuli. Along these lines, globus pallidus hyperactivation in the present study might reflect modulation of emotion processing due to stimulus content in an additional domain of emotion processing, namely in brain structures associated with motor functions.
MPFC, PCC and precuneus as “cortical midline structures” [Schneider et al., 2008] are crucial components of the DMN which is involved in self‐referential processing and emotional regulation [Raichle et al., 2001]. In line with this, dmPFC has been directly linked to emotional awareness [Lane et al., 1997] and attentional allocation towards emotional stimuli and to their evaluation [Etkin et al., 2011; Ochsner and Gross, 2005; Phan et al., 2002]. Furthermore, dmPFC is associated with mentalizing of emotions in other persons [Frith and Frith, 2003; Olsson and Ochsner, 2008; Vogeley et al., 2001] and retrieval of self‐relevant autobiographic memories [Moran et al., 2009]. Complementing the alleged role of dmPFC in emotional processing, PCC and precuneus are assumed to be involved in processes of episodic memory retrieval [Maddock et al., 2002; Maddock and Buonocore, 1997] and self‐consciousness by maintaining internal representations [Cavanna and Trimble, 2006; Wolpert et al., 1998]. This is supported by altered activation patterns in these regions in anxious patients. Straube et al. [2004b] observed stronger PCC activation in spider phobics confronted with phobia‐related words, and Maddock et al. [2003] in panic patients in response to threat‐related words. These findings were interpreted as correlates of enhanced mnemonic processing. Gentili et al. [2009] described precuneus and PCC hyperactivations during performance of cognitive tasks in SAD and suggested that impairment of the DMN in social anxiety leads to increased self‐focused attention, thus contributing to the feeling of wariness of others' judgment. In an additional study, Gentili et al. [2015a,b] reported symptom severity of social anxiousness to predict the Hurst Exponent in the PCC/precuneus region, thus underlining the role of this region for SAD pathophysiology. In line with this, brain activations in PCC/precuneus correlated with LSAS‐scores as well as SPS‐ and SIAS score (except right precuneus) in the present study.
Results from the present functional connectivity analysis further support a pivotal role of the PCC/precuneus region in disorder‐related scene processing in SAD, thus corroborating neurobiological models of SAD [Brühl et al., 2014]. PPI analysis revealed altered interplay between PCC/precuneus and paralimbic (insula) as well as cortical regions (dmPFC, precuneus) in SAD patients relative to HC: functional connectivity of PCC/precuneus with insula and precuneus was increased, while hypoconnectivity was observed between PCC/precuneus and dmPFC. Brühl et al. [2014] described decoupling of the medio‐parietal region which was assumed to lead to deficits in bottom‐up activation regulation. This is in line with the present findings of hypoconnectivity between dmPFC and PCC/precuneus, while contrasting with the observed hyperconnectivity between PCC/precuneus and insula and precuneus. Due to extensive cortical and subcortical connectivity, the PCC/precuneus region is regarded as a central transfer point for information in the brain [Cavanna and Trimble, 2006; Tomasi and Volkow, 2011]. Thus, stronger functional connections between PCC/precuneus and other brain regions in SAD patients during disorder‐related scene processing possibly reflect more intense processing of these significant stimuli. This assumption is in line with Dennis et al. [2011] who suggested anxiety to lead to increased functional connectivity between DMN and insula.
Furthermore, connectivity analysis with globus pallidus as seed region yielded functional hyperconnectivities with amygdala, mPFC and ACC. Note that the hyperconnectivity originating from globus pallidus to amygdala refers to basolateral and extending into medial parts of the amygdala. To date and to our knowledge, abnormal globus pallidus activation in SAD patients during emotion processing has only been reported in meta‐analytic approaches [Binelli et al., 2014; Etkin and Wager, 2007; Gentili et al., 2015a,b; Hattingh et al., 2013]. Thus, the present results, that is, the differential effect between SAD patients and HC as well as the increased functional connectivity of globus pallidus with well‐known areas in emotion processing (amygdala, mPFC, ACC) in SAD patients, reinforce the relevance of this brain structure in social anxious threat processing.
The results of the exploratory analysis, i.e. hyperactivations of SAD patients in dorsolateral PFC (dlPFC), superior parietal lobule (SPL), middle temporal and angular gyrus, may demonstrate modulation of different stages of emotional processing such as attentional allocation, mentalizing and emotion regulation by stimulus content (i.e. disorder‐relatedness) in SAD [Diekhof et al., 2011; Etkin et al., 2011; Kohn et al., 2014; Saxe and Kanwisher, 2003]. Disorder‐related scenes thus provoke altered activity and connectivity in and between brain areas specifically involved in self‐referential processing, and in cognitive functions such as interoceptive awareness, self‐consciousness, maintenance of internal representations, evaluation of emotional states, and autobiographic memory retrieval. These processes are crucial for the formation of a maladaptive mental self‐representation in a potentially threatening situation in SAD. Maladaptive self‐representation is considered to be a critical point for the emergence and maintenance of anxiety in SAD patients [Clark and Wells, 1995; Rapee and Heimberg, 1997]. This is supported by findings of increased mPFC activation during autobiographic memory retrieval in a novel photo paradigm [Cabeza et al., 2004]. Higher dmPFC, PCC and thalamus activation was also observed in response to emotional pictures, with activation depending on self‐relatedness of the stimuli [Schneider et al., 2008].
Activation patterns for disorder‐related scene processing in SAD in the present study suggest involvement of a large network of distributed brain regions that have previously been implicated in threat processing in SAD. Notably, only few studies to date have succeeded in painting such a comprehensive picture. For instance, PCC and precuneus hyperactivation has rarely been reported in studies using emotional faces, although meta‐analytic approaches and reviews have stressed the importance of this region [Brühl et al., 2014; Gentili et al., 2015a,b]. It seems plausible that high ecological validity of the stimulus material may allow for a more thorough investigation of different aspects of the fear response in SAD.
However, it has to be pointed out that we did not compare results for our new stimulus set with results from other disorder‐related stimuli for SAD, such as emotional faces. Although we suggest that our stimuli are associated with high ecological validity, future studies should include additional behavioral measures to corroborate this statement, and compare results of different classes of stimuli.
A further limitation is that 11 patients with SAD as main diagnosis also fulfilled the criteria for other psychological disorders (mainly other anxiety disorders, but also depression). While comorbid diagnoses may present an important limitation of the present study, including patients with comorbid diagnoses does increase representativeness of the patient sample as well as statistical power because fewer individuals need to be excluded and sample sizes of n = 30 can realistically be reached. Furthermore, activation patterns in SAD patients did not correlate with BDI‐scores, even though approx. 13% of patients presented with comorbid MDD.
In sum, we obtained evidence for hyperactivation in designated areas and for altered connectivity in SAD patients when confronted with threatening, disorder‐specific materials. Hyperactivations in a widespread emotion‐processing network including (para‐)limbic structures (e.g. amygdala, insula, thalamus, globus pallidus) and cortical regions (e.g. dmPFC, PCC, precuneus) were observed when SAD patients processed disorder‐related scenes. Analysis of amygdala subregions revealed hyperactivation specifically in corticomedial regions, and more specifically, in the central nucleus. PPI analysis revealed altered interplay between PCC/precuneus and paralimbic (insula) as well as cortical regions (dmPFC, precuneus) in SAD patients. Hyperconnectivity between globus palidus and limbic (amygdala) and cortical areas (ACC, mPFC) was also observed. These results underline a pivotal role of the PCC/precuneus region and emphasize the relevance of globus pallidus for threat processing in SAD. Overall, our findings appear to indicate that stimulus content, that is, specific relevance for the disorder, modulates multiple stages of emotion processing in SAD, which may be linked to enhanced vigilance and interoceptive awareness. This may affect higher‐order cognitive processes such as building of internal (self‐)representations, perspective taking and episodic memory retrieval. Generally, disorder‐related threat processing in SAD seems to be characterized by increased anxiety as well as dysfunctionally elevated levels of self‐referential processing.
Footnotes
When referring to hyper‐ or hypoactivation or ‐connectivity in SAD patients, this is in relation to HC, unless stated otherwise.
REFERENCES
- Alexander GE, Crutcher MD (1990): Functional architecture of basal ganglia circuits: Neural substrates of parallel processing. Trends Neurosci 13:266–271. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2000): Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition: DSM‐IV‐TR. Washington, DC: American Psychiatric Publications. [Google Scholar]
- Amir N, Klumpp H, Elias J, Bedwell JS, Yanasak N, Miller LS (2005): Increased activation of the anterior cingulate cortex during processing of disgust faces in individuals with social phobia. Biol Psychiatry 57:975–981. [DOI] [PubMed] [Google Scholar]
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, Zilles K (2005): Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: Intersubject variability and probability maps. Anat Embryol (Berl) 210:343–352. [DOI] [PubMed] [Google Scholar]
- Binelli C, Subirà S, Batalla A, Muñiz A, Sugranyés G, Crippa JA, Farré M, Pérez‐Jurado L, Martín‐Santos R (2014): Common and distinct neural correlates of facial emotion processing in social anxiety disorder and Williams syndrome: A systematic review and voxel‐based meta‐analysis of functional resonance imaging studies. Neuropsychologia 64:205–217. [DOI] [PubMed] [Google Scholar]
- Blair K, Geraci M, Devido J, McCaffrey D, Chen G, Vythilingam M, Ng P, Hollon N, Jones M, Blair RJR, Pine DS (2008): Neural response to self‐ and other referential praise and criticism in generalized social phobia. Arch Gen Psychiatry 65:1176–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair KS, Geraci M, Otero M, Majestic C, Odenheimer S, Jacobs M, Blair RJR, Pine DS (2011): Atypical modulation of medial prefrontal cortex to self‐referential comments in generalized social phobia. Psychiatry Res 193:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehme S, Ritter V, Tefikow S, Stangier U, Strauss B, Miltner WHR, Straube T (2013): Brain activation during anticipatory anxiety in social anxiety disorder. Soc Cogn Affect Neurosci nst129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehme S, Mohr A, Becker MP, Miltner WH, Straube T (2014): Area‐dependent time courses of brain activation during video‐induced symptom provocation in social anxiety disorder. Biol Mood Anxiety Disord 4:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boll S, Gamer M, Kalisch R, Büchel C (2011): Processing of facial expressions and their significance for the observer in subregions of the human amygda. Neuroimage 56:299–306. [DOI] [PubMed] [Google Scholar]
- Boll S, Gamer M, Gluth S, Finsterbusch J, Büchel C (2013): Separate amygdala subregions signal surprise and predictiveness during associative fear learning in humans. Eur J Neurosci 37:758–767. [DOI] [PubMed] [Google Scholar]
- Brühl AB, Rufer M, Delsignore A, Kaffenberger T, Jäncke L, Herwig U (2011): Neural correlates of altered general emotion processing in social anxiety disorder. Brain Res 1378:72–83. [DOI] [PubMed] [Google Scholar]
- Brühl AB, Delsignore A, Komossa K, Weidt S (2014): Neuroimaging in social anxiety disorder—A meta‐analytic review resulting in a new neurofunctional model. Neurosci Biobehav Rev 47:260–280. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Prince S, Daselaar S, Greenberg D, Budde M, Dolcos F, LaBar K, Rubin D (2004): Brain activity during episodic retrieval of autobiographical and laboratory events: An fMRI study using a novel photo paradigm. J Cogn Neurosci 16:1583–1594. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR (2006): The precuneus: A review of its functional anatomy and behavioural correlates. Brain J Neurol 129:564–583. [DOI] [PubMed] [Google Scholar]
- Clark DM, Wells A (1995): A cognitive model of social phobia In: Heimberg RG, Liebowitz MR, Hope DA, Schneier FR, editors. Social Phobia: Diagnosis, Assessment, and Treatment. New York: Guilford Press; pp 69–93. [Google Scholar]
- Craig ADB (2009): How do you feel–now? The anterior insula and human awareness. Nat Rev Neurosci 10:59–70. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Öhman A, Dolan RJ (2004): Neural systems supporting interoceptive awareness. Nat Neurosci 7:189–195. [DOI] [PubMed] [Google Scholar]
- Dale AM (1999): Optimal experimental design for event‐related fMRI. Hum Brain Mapp 8:109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LLB, Parvizi J, Hichwa RD (2000): Subcortical and cortical brain activity during the feeling of self‐generated emotions. Nat Neurosci 3:1049–1056. [DOI] [PubMed] [Google Scholar]
- Dennis EL, Gotlib IH, Thompson PM, Thomason ME (2011): Anxiety modulates insula recruitment in resting‐state functional magnetic resonance imaging in youth and adults. Brain Connect 1:245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof EK, Geier K, Falkai P, Gruber O (2011): Fear is only as deep as the mind allows: A coordinate‐based meta‐analysis of neuroimaging studies on the regulation of negative effect. Neuroimage 58:275–285. [DOI] [PubMed] [Google Scholar]
- Ding J, Chen H, Qiu C, Liao W, Warwick JM, Duan X, Zhang W, Gong Q (2011): Disrupted functional connectivity in social anxiety disorder: A resting‐state fMRI study. Magn Reson Imaging 29:701–711. [DOI] [PubMed] [Google Scholar]
- Duval ER, Javanbakht A, Liberzon I (2015): Neural circuits in anxiety and stress disorders: a focused review. Ther Clin Risk Manage 11:115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD (2007): Functional neuroimaging of anxiety: A meta‐analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 164:1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R (2011): Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci 15:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans KC, Wright CI, Wedig MM, Gold AL, Pollack MH, Rauch SL (2008): A functional MRI study of amygdala responses to angry schematic faces in social anxiety disorder. Depress Anxiety 25:496–505. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC (1995): Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster‐size threshold. Magn Reson Med 33:636–647. [DOI] [PubMed] [Google Scholar]
- Freitas‐Ferrari MC, Hallak JEC, Trzesniak C, Filho AS, Machado‐de‐Sousa JP, Chagas MHN, Nardi AE, Crippa JAS (2010): Neuroimaging in social anxiety disorder: A systematic review of the literature. Prog Neuropsychopharmacol Biol Psychiatry 34:565–580. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith CD (2003): Development and neurophysiology of mentalizing. Philos Trans R Soc Lond B Biol Sci 358:459–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frühholz S, Grandjean D (2013): Amygdala subregions differentially respond and rapidly adapt to threatening voices. Cortex 49:1394–1403. [DOI] [PubMed] [Google Scholar]
- Gentili C, Ricciardi E, Gobbini MI, Santarelli MF, Haxby JV, Pietrini P, Guazzelli M (2009): Beyond amygdala: Default mode network activity differs between patients with Social Phobia and healthy controls. Brain Res Bull 79:409–413. [DOI] [PubMed] [Google Scholar]
- Gentili C, Cristea IA, Angstadt M, Klumpp H, Tozzi L, Phan KL, Pietrini P (2015a): Beyond emotions: A meta‐analysis of neural response within face processing system in social anxiety. Exp Biol Med 0:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentili C, Vanello N, Cristea I, David D, Ricciardi E, Pietrini P (2015b): Proneness to social anxiety modulates neural complexity in the absence of exposure: A resting state fMRI study using Hurst exponent. Psychiatry Res 232:135–144. [DOI] [PubMed] [Google Scholar]
- Goebel R, Esposito F, Formisano E (2006): Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: From single‐subject to cortically aligned group general linear model analysis and self‐organizing group independent component analysis. Hum Brain Mapp 27:392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattingh CJ, Ipser J, Tromp SA, Syal S, Lochner C, Brooks SJ, Stein DJ (2013): Functional magnetic resonance imaging during emotion recognition in social anxiety disorder: an activation likelihood meta‐analysis. Front Hum Neurosci 6:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautzinger M, Bailer M, Worall H, Keller F (1995): Beck‐Depressions‐Inventar (BDI). Testhandbuch der deutschen Ausgabe. Bern: Huber. [Google Scholar]
- Heitmann CY, Peterburs J, Mothes‐Lasch M, Hallfarth MC, Böhme S, Miltner WHR, Straube T (2014): Neural correlates of anticipation and processing of performance feedback in social anxiety. Hum Brain Mapp 35:6023–6031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Gallagher M (1999): Amygdala circuitry in attentional and representational processes. Trends Cogn Sci 3:65–73. [DOI] [PubMed] [Google Scholar]
- Jones BE (2003): Arousal systems. Front Biosci J Virtual Libr 8:s438–s451. [DOI] [PubMed] [Google Scholar]
- Klumpp H, Angstadt M, Nathan PJ, Phan KL (2010): Amygdala reactivity to faces at varying intensities of threat in generalized social phobia: An event‐related functional MRI study. Psychiatry Res Neuroimaging 183:167–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn N, Eickhoff SB, Scheller M, Laird AR, Fox PT, Habel U (2014): Neural network of cognitive emotion regulation–An ALE meta‐analysis and MACM analysis. Neuroimage 87:345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas‐Gutiérrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT (2007): Bias between MNI and Talairach coordinates analyzed using the ICBM‐152 brain template. Hum Brain Mapp 28:1194–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RD, Fink GR, Chau PM, Dolan RJ (1997): Neural activation during selective attention to subjective emotional responses. Neuroreport 8:3969–3972. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN (2008): International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual. Technical Report A‐8. University of Florida, Gainesville, FL.
- LeDoux JE (2003): The emotional brain, fear, and the amygdala. Cell Mol Neurobiol 23:727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W, Qiu C, Gentili C, Walter M, Pan Z, Ding J, Zhang W, Gong Q, Chen H (2010): Altered effective connectivity network of the amygdala in social anxiety disorder: A resting‐state fMRI study. PLoS One 5:e15238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorberbaum JP, Kose S, Johnson MR, Arana GW, Sullivan LK, Hamner MB, Ballenger JC, Lydiard RB, Brodrick PS, Bohning DE, George MS (2004): Neural correlates of speech anticipatory anxiety in generalized social phobia. Neuroreport 15:2701–2705. [PubMed] [Google Scholar]
- Maddock RJ, Buonocore MH (1997): Activation of left posterior cingulate gyrus by the auditory presentation ofthreat‐related words: An fMRI study. Psychiatry Res Neuroimaging 75:1–14. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH (2002): Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Hum Brain Mapp 18:30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock RJ, Buonocore MH, Kile SJ, Garrett AS (2003): Brain regions showing increased activation by threat‐related words in panic disorder. Neuroreport 14:325–328. [DOI] [PubMed] [Google Scholar]
- Mai JK, Assheuer J, Paxinos G (2004): Atlas of the Human Brain, 2nd Edition. Amsterdam: Elsevier Academic Press. [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH (2003): An automated method for neuroanatomic and cytoarchitectonic atlas‐based interrogation of fMRI data sets. Neuroimage 19:1233–1239. [DOI] [PubMed] [Google Scholar]
- Mennin DS, Fresco DM, Heimberg RG, Schneier FR, Davies SO, Liebowitz MR (2002): Screening for social anxiety disorder in the clinical setting: Using the Liebowitz social anxiety scale. J Anxiety Disord 16:661–673. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ (2010): Saliency, switching, attention and control: A network model of insula function. Brain Struct Funct 214:655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskovic V, Schmidt LA (2012): Social fearfulness in the human brain. Neurosci Biobehav Rev 36:459–478. [DOI] [PubMed] [Google Scholar]
- Moran JM, Heatherton TF, Kelley WM (2009): Modulation of cortical midline structures by implicit and explicit self‐relevance evaluation. Soc Neurosci 4:197–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao T, Sanematsu H, Yoshiura T, Togao O, Murayama K, Tomita M, Masuda Y, Kanba S (2011): fMRI of patients with social anxiety disorder during a social situation task. Neurosci Res 69:67–72. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ (2005): The cognitive control of emotion. Trends Cogn Sci 9:242–249. [DOI] [PubMed] [Google Scholar]
- Oldfield R (1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Olsson A, Ochsner KN (2008): The role of social cognition in emotion. Trends Cogn Sci 12:65–71. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I (2002): Functional neuroanatomy of emotion: A meta‐analysis of emotion activation studies in PET and fMRI. Neuroimage 16:331–348. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME (2006): Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biol Psychiatry 59:424–429. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Savander V, LeDoux JE (1997): Organization of intra‐amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci 20:517–523. [DOI] [PubMed] [Google Scholar]
- Prater KE, Hosanagar A, Klumpp H, Angstadt M, Phan KL (2013): Aberrant amygdala‐frontal cortex connectivity during perception of fearful faces and at rest in generalized social anxiety disorder. Depress Anxiety 30:234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadflieg S, Mohr A, Mentzel H‐J, Miltner WHR, Straube T (2008): Modulation of the neural network involved in the processing of anger prosody: The role of task‐relevance and social phobia. Biol Psychol 78:129–137. [DOI] [PubMed] [Google Scholar]
- Radua J, Sarró S, Vigo T, Alonso‐Lana S, Bonnín CM, Ortiz‐Gil J, Canales‐Rodríguez EJ, Maristany T, Vieta E, Mckenna PJ, Salvador R, Pomarol‐Clotet E (2014): Common and specific brain responses to scenic emotional stimuli. Brain Struct Funct 219:1463–1472. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001): A default mode of brain function. Proc Natl Acad Sci USA 98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapee RM, Heimberg RG (1997): A cognitive‐behavioral model of anxiety in social phobia. Beh Res Ther 35:741–756. [DOI] [PubMed] [Google Scholar]
- Reiman EM (1997): The application of positron emission tomography to the study of normal and pathologic emotions. J Clin Psychiatry 58:4–12. [PubMed] [Google Scholar]
- Sabatinelli D, Lang PJ, Keil A, Bradley MM (2007): Emotional perception: Correlation of functional MRI and event‐related potentials. Cereb Cortex 17:1085–1091. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N (2003): People thinking about thinking people. The role of the temporo‐parietal junction in “theory of mind.”. Neuroimage 19:1835–1842. [DOI] [PubMed] [Google Scholar]
- Schmidt S, Mohr A, Miltner WHR, Straube T (2010): Task‐dependent neural correlates of the processing of verbal threat‐related stimuli in social phobia. Biol Psychol 84:304–312. [DOI] [PubMed] [Google Scholar]
- Schmuckler MA (2001): What is ecological validity? A dimensional analysis. Infancy 2:419–436. [DOI] [PubMed] [Google Scholar]
- Schneider F, Bermpohl F, Heinzel A, Rotte M, Walter M, Tempelmann C, Wiebking C, Dobrowolny H, Heinze HJ, Northoff G (2008): The resting brain and our self: self‐relatedness modulates resting state neural activity in cortical midline structures. Neuroscience 157:120–131. [DOI] [PubMed] [Google Scholar]
- Schulz C, Mothes‐Lasch M, Straube T (2013): Automatic neural processing of disorder‐related stimuli in social anxiety disorder: faces and more. Front Psychol 4:282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stangier U, Heidenreich T, Berardi A, Golbs U, Hoyer J (1999): Die Erfassung sozialer Phobie durch die Social Interaction Anxiety Scale (SIAS) und die Social Phobia Scale (SPS). Z Für Klin Psychol Psychother 28:28–36. [Google Scholar]
- Stangier U, Fydrich T (2002): Soziale Phobie und Soziale Angststörung: Psychologische Grundlagen, Diagnostik und Therapie. Göttingen: Hogrefe Verlag. [Google Scholar]
- Stangier U, Heidenreich T (2004): Die Liebowitz Soziale Angst‐ Skala (LSAS) [Liebowitz Social Anxiety Scale]. Collegium Internationale Psychiatriae Scalarum (Ed.), Internationale Skalen für Psychiatrie [International Psychiatry Scales]. Weinheim: Beltz. German.
- Stein MB, Goldin PR, Sareen J, Zorrilla LTE, Brown GG (2002): Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch Gen Psychiatry 59:1027–1034. [DOI] [PubMed] [Google Scholar]
- Straube T, Kolassa I‐T, Glauer M, Mentzel H‐J, Miltner WHR (2004a): Effect of task conditions on brain responses to threatening faces in social phobics: An event‐related functional magnetic resonance imaging study. Biol Psychiatry 56:921–930. [DOI] [PubMed] [Google Scholar]
- Straube T, Mentzel HJ, Glauer M, Miltner WHR (2004b): Brain activation to phobia‐related words in phobic subjects. Neurosci Lett 372:204–208. [DOI] [PubMed] [Google Scholar]
- Straube T, Mentzel H‐J, Miltner WHR (2005): Common and distinct brain activation to threat and safety signals in social phobia. Neuropsychobiology 52:163–168. [DOI] [PubMed] [Google Scholar]
- Sztainberg Y, Kuperman Y, Justice N, Chen A (2011): An anxiolytic role for CRF receptor type 1 in the Globus pallidus . J Neurosci 31:17416–17424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988): Co‐Planar Stereotaxic Atlas of the Human Brain. 3‐Dimensional Proportional System: An Approach to Cerebral Imaging. New York: Thieme Medical Publishers. [Google Scholar]
- Tomasi D, Volkow ND (2011): Association between functional connectivity hubs and brain networks. Cereb Cortex 21:2003–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15:273–289. [DOI] [PubMed] [Google Scholar]
- Vogeley K, Bussfeld P, Newen A, Herrmann S, Happé F, Falkai P, Maier W, Shah NJ, Fink GR, Zilles K (2001): Mind reading: neural mechanisms of theory of mind and self‐perspective. Neuroimage 14:170–181. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Driver J (2007): Modulation of visual processing by attention and emotion: windows on causal interactions between human brain regions. Philos Trans R Soc Lond B Biol Sci 362:837–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessa M, Kanske P, Neumeister P, Bode K, Heissler J, Schönfelder S (2010): EmoPics: Subjektive und psychophysiologische Evaluation neuen Bildmaterials für die klinisch‐bio‐psychologische Forschung. Z Für Klin Psychol Psychother 39:77. [Google Scholar]
- Wittchen H‐U, Zaudig M, Fydrich T (1997): SKID Strukturiertes Klinisches Interview für DSM‐IV. Achse I und II. Göttingen: Hogrefe. Z Für Klin Psychol Psychother 28:68–70. [Google Scholar]
- Wolpert DM, Goodbody SJ, Husain M (1998): Maintaining internal representations: The role of the human superior parietal lobe. Nat Neurosci 1:529–533. [DOI] [PubMed] [Google Scholar]


