Abstract
Obesity is associated with lowered brain's grey (GM) and white matter (WM) density as measured by voxel‐based morphometry (VBM). Nevertheless, it remains unknown whether obesity has a causal influence on cerebral atrophy. We recruited 47 morbidly obese subjects (mean BMI = 42.2, SD = 4.0, 42 females and five males) eligible for bariatric surgery and 29 non‐obese subjects (mean BMI = 23.2, SD = 2.8, 23 females and six males) served as controls. Baseline scans were acquired with T1‐weighted magnetic resonance imaging (MRI) at 1.5 Tesla; obese participants were scanned again six months after the surgery. Local GM and WM densities were quantified using VBM. Full‐volume analyses were used for comparing baseline between‐group differences as well as the effects of surgery‐induced weight loss in the morbidly obese. Metabolic variables were used in linear models to predict WM and GM densities. Obese subjects had initially lower GM densities in widespread cortical areas including frontal, parietal, and temporal regions as well as insulae. Lower WM densities were observed throughout the WM. Bariatric surgery and concomitant weight loss resulted in global increase in WM density. Grey matter increase was limited to occipital and inferior temporal regions. Metabolic variables were associated with brain densities. We conclude that weight loss results in global recovery of WM as well as local recovery of grey matter densities. These changes likely reflect improved brain tissue integrity. Hum Brain Mapp 37:3745–3756, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: structural MRI, voxel‐based morphometry, obesity, bariatric surgery, weight loss
Abbreviations
- DTI
Diffusion tensor imaging
- FWHM
Full width at half maximum
- GLM
General linear model
- GM
Grey matter
- IFG
Impaired fasting glucose
- IGT
Impaired glucose tolerance
- MRI
Magnetic resonance imaging
- OGTT
Oral glucose tolerance test
- SAT
Subcutaneous adipose tissue
- VAT
Visceral adipose tissue
- VBM
Voxel‐based morphometry
- WM
White matter
INTRODUCTION
Overeating and resulting obesity have adverse effects on the central nervous system, increasing its susceptibility to dysfunction and degeneration. Cross‐sectional studies in humans have shown that obesity is associated with brain volume reductions in both grey (GM) and white matter (WM) as measured by voxel‐based morphometry (VBM), and loss of structural integrity of the WM as measured by diffusion tensor imaging (DTI) [Karlsson et al., 2013; Raji et al., 2010; Stanek et al., 2011; Verstynen et al., 2012]. These findings parallel those showing brain volume decreases as a function of age especially in frontal areas, yet obesity further amplifies the observed volume reductions even in the elderly [Brooks et al., 2013]. These volumetric changes may be detrimental to cognitive performance, and obesity‐induced structural brain changes may translate to cognitive dysfunction later in life [Walther et al., 2010]. Interestingly, metabolic changes that accompany obese phenotype seem to mediate these changes.
Accumulating evidence suggests that increase in adipose tissue, elevated blood pressure, hyperglycemia, hyperlipidemia, and low‐grade systemic inflammation underlie obesity‐related loss of structural integrity of the brain [Cazettes et al., 2011; Friedman et al., 2014; Karlsson et al., 2013; Korf et al., 2007]. However, because focal volume reductions have been observed in the brain areas governing reward, inhibition, and appetite control, it has also been proposed that these abnormalities in brain structure may have preceded obesity and thus caused overeating [Diekhof et al., 2012; Mizuhiki et al., 2012; Pannacciulli et al., 2006; Raji et al., 2010; Small, 2010; Scharmüller et al., 2012; Walther et al., 2010]. Cross‐sectional studies cannot determine whether such cerebral changes are due to the obese phenotype or whether they reflect a risk factor for weight gain. Yet currently experimental studies on the effects of body weight loss or gain on human brain structure are scarce.
Bariatric surgery provides effective means for weight loss in the morbidly obese, inducing rapid weight loss and subsequent improvement of metabolic health [Chang et al., 2013; Gloy et al., 2013]. Moreover, it provides a powerful approach for studying the effects of weight loss on the brain. Here we used magnetic resonance imaging (MRI) and VBM to study the effects of bariatric surgery on morbidly obese patients' WM and GM densities at a baseline state and six months after bariatric surgery and concomitant weight loss. First, we predicted that GM and WM densities would be initially lower in the morbidly obese subjects in comparison with healthy controls. Second, we hypothesized that the rapid weight loss after bariatric surgery would recover at least some of the obesity‐related volume reductions.
METHODS
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethical Committee of the Hospital District of South‐Western Finland (Sleevepass NCT00793143 and SleevePET2 NCT01373892, http://www.clinicaltrials.gov). All participants signed ethical committee‐approved, informed consent form prior to study. The study population encompasses some subjects from our previous study [Karlsson et al., 2013].
PARTICIPANTS
We initially scanned 30 non‐obese participants and 51 morbidly obese participants. One non‐obese and one obese subject were excluded from the study due to microvascular lesions in the WM, as revealed by neuroradiological examinations. Three further obese participants were excluded due to motion artifacts in the T1 images. The final sample included 42 morbidly obese females and five males (mean BMI = 42.2, SD = 4.0) about to undergo bariatric surgery and 23 non‐obese females and six males (mean BMI = 23.2, SD = 2.8) (Table 1). Subject groups were matched for age and height. Exclusion criteria for all subjects included binge‐eating disorders, neurological or mental disorders, as well as substance abuse and excessive alcohol consumption determined by clinical interviews, medical history, and blood tests. The screening procedure entailed routine doctor checkup and taking of blood samples for assessing metabolic health. Weight and conductance‐derived fat percentages were measured with Omron BF 400‐E (Omron Healthcare Europe, Netherlands) scale. The patients did not use their medications at the day of the screening measurements.
Table 1.
Group characteristics
| Normal weight subjects N = 29, 6 males | Morbidly obese subjects Preoperative, N = 47, 5 males | Morbidly obese subjects 6 months postoperative, N = 40, 3 males | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | p a | Mean | SD | Range | p b | |
| Age (years) | 45.9 | 11.8 | 19–65 | 44.9 | 9.0 | 24–60 | NS | ||||
| Weight (kg) | 66.8 | 11.3 | 49–93 | 117.3 | 13.8 | 91–149 | *** | 91.3 | 14.4 | 70–129 | *** |
| BMI (Body mass index) | 23.2 | 2.8 | 17.8–29.9 | 42.2 | 4.0 | 35–53 | *** | 32.7 | 4.1 | 27–44 | *** |
| Waist circumference (cm) | 75.1 | 8.4 | 63–92 | 120.8 | 11.0 | 110–159 | *** | 101.5 | 11.8 | 70–127 | *** |
| Fat percent | 30.25 | 6.7 | 16–42 | 49.4 | 5.6 | 33–55 | *** | 42.5 | 5.4 | 27–53 | *** |
| Subcutaneous fat mass (kg) | 4.2 | 1.3 | 2–7 | 19.2 | 5.1 | 9–35 | ** | 10.7 | 3.4 | 6.1–20.4 | *** |
| Visceral fat mass (kg) | 0.8 | 0.4 | 0.2–2.0 | 4.2 | 1.5 | 2–8 | ** | 1.8 | 1.0 | 0.5–4.2 | *** |
| Systolic blood pressure (mmHg) | 127.5 | 12.5 | 105–150 | 132.6 | 17.8 | 97–187 | NS | 125.8 | 11.3 | 109–155 | * |
| Diastolic blood pressure (mmHg) | 80.1 | 8.3 | 65–96 | 85.9 | 9.1 | 66–107 | ** | 80.0 | 9.5 | 63–101 | ** |
| HbA1c (%) | 5.6 | 0.3 | 5–6 | 5.9 | 0.7 | 5–8 | * | 5.6 | 0.6 | 4.3–7.5 | NS |
| Fasting glucose (mmol/l) | 5.4 | 0.4 | 4–6 | 6.2 | 1.2 | 5–12 | *** | 5.7 | 0.9 | 4.4–8.5 | NS |
| Triglycerides (mmol/l) | 0.7 | 0.3 | 0.3–1.0 | 1.3 | 0.5 | 1.0–3.0 | *** | 1.1 | 0.4 | 0.5–2.2 | NS |
| HDL (mmol/l) | 1.8 | 0.4 | 0.9–2.7 | 1.2 | 0.2 | 1.0–2.0 | *** | 1.4 | 0.3 | 0.9–2.0 | NS |
| LDL (mmol/l) | 2.5 | 0.9 | 1.1–4.0 | 2.5 | 0.7 | 1.0–4.0 | NS | 3.1 | 4.3 | 1.3–4.3 | NS |
| HDL/Kol ratio (%) | 40.4 | 8.9 | 27–56 | 29.4 | 7.1 | 15–47 | *** | 32.4 | 9.4 | 15–53 | NS |
| sensitive CRP (mg/l) | 0.73 | 1.0 | 0–4 | 4.4 | 3.8 | 0.2–19.0 | *** | 2.3 | 2.1 | 0.2–8.6 | NS |
| Thyroid stimulating hormone (mU/l) | 1.25 | 0.4 | 1–2 | 1.74 | 1.4 | 0–4 | * | 1.3 | 1.4 | 0.4–2.5 | NS |
| Beck depression inventory II | 3.7 | 4.4 | 0–15 | 4.4 | 4.4 | 0–19 | NS | 2.8 | 3.6 | 0–15 | NS |
| Educational backgroundc | Low | N = 14 | Low | N = 31 | 0.15d | ||||||
| High | N = 13 | High | N = 14 | *** | |||||||
*p < 0.05, **p < 0.01, ***p < 0.001, NS = non‐significant.
Independent samples t‐test.
Paired samples t‐test.
High refers to university or polytechnical school degree, low to all other backgrounds.
Mann‐Whitney U test.
Because depression might be associated with similar adverse brain changes as obesity [Cole et al., 2013], participants also completed the Beck Depression Inventory II as a part of the screening procedure. Some participants had mild depressive symptoms (Table 1), but they were deemed non‐depressive clinically prior to measurements. Some of the morbidly obese subjects had disturbed glucose and/or cholesterol metabolism, but they were currently medicated. Three obese individuals had diagnosed sleep apnea. Altogether, 14 subjects from the morbidly obese group had pre‐diabetes (IFG – impaired fasting glucose or IGT – impaired glucose tolerance) and 18 had diabetes as defined by ADA criteria in oral glucose tolerance test (OGTT). The non‐obese subjects had healthy glucose metabolism. 19 subjects underwent gastric bypass and 21 subjects underwent sleeve gastrectomy. 14 morbidly obese patients were light smokers (5‐20 cigarettes per day).
The screening checkups, interviews, and laboratory measurements were repeated 6 months postoperatively. Altogether seven obese subjects opted to drop out from the study prior to the postoperative scan or did not undergo the surgery. They did not differ from the participants that were included in the follow up in terms of metabolic parameters (Table 1).
IMAGE ACQUISITION
MR imaging was performed with Philips Gyroscan Intera 1.5 T CV Nova Dual scanner at Turku PET Centre. Anatomical images with 1 mm3 resolution were acquired using a T1‐weighted sequence (TR 25ms, TE 4.6 ms, flip angle 30°, scan time 376 s). To assess the volume of abdominal subcutaneous (SAT) and visceral (VAT) adipose tissue, axial T1‐weighted dual fast field echo images covering the abdominal area were acquired (TE 2.3 and 4.6 ms, TR 120 ms, slice thickness 10 mm without gaps). Abdominal subcutaneous and visceral fat was counted from top of liver until the top of femoral bone appeared on both sides were analyzed with the SliceOmatic software version 4.3 (http://www.tomovision.com/products/sliceomatic.htm). Sets of images were opened in SliceOmatic and the borders of adipose tissues were drawn manually (by TP).
VBM METHODS
Prior to analysis, the image quality was checked visually and the origo of each T1 image was set to anterior commissure. Structural images were analyzed with Matlab 2012a (The MathWorks Inc., Natick, MA) using the SPM8 (http://www.fil.ion.ucl.ac.uk/spm/) software and vbm8 toolbox (http://dbm.neuro.uni-jena.de/vbm/download/), which enables automated spatial normalization, tissue classification and radio‐frequency bias correction to be combined with the segmentation step. Cut‐off of spatial normalization was 25 mm and medium affine regularization 0.01 was used. Following normalization and segmentation into GM and WM, a modulation step was incorporated to take into account volume changes caused by spatial normalization. Importantly, the modulation step corrects for the differences in total brain size across subjects. Finally, the segmented, normalized, and modulated GM and WM images were smoothed using a Gaussian kernel of 8 mm full width at half maximum (FWHM).
STATISTICAL ANALYSIS
The smoothed images were analyzed using general linear model [GLM; Friston et al., 1994] in SPM8. First, a between‐subjects model (one‐way ANOVA) was used to compare GM and WM densities between controls and obese patients, in the baseline state before surgery. Second, to answer the main hypothesis of the study, a within‐subjects model (one‐way within‐subjects ANOVA) was used to estimate GM and WM changes following bariatric surgery by comparing patients' postoperative and preoperative scans with each other. An absolute threshold mask in the 2nd level designs was set at 0.2 for all comparisons to avoid possible edge effects around the border between GM and WM. Subjects' ages in years were entered into the between groups comparison models as a regressor of no interest to account for age‐related changes across subjects. Primary statistical threshold was set at whole brain FDR corrected p < 0.05. Additionally, more lenient threshold (p < 0.05, FDR corrected at the cluster level) was used to explore possible weaker effects. To determine which metabolic variables best predict initial brain densities and surgery‐induced changes in brain structure, we extracted GM and WM densities from the clusters resulting from the full‐volume analyses using Marsbar (http://marsbar.sourceforge.net). Cortical regions were identified from MNI coordinates of the SPM output with AAL atlas.
We also conducted complementary SPM analysis to reveal possible confounding effects of diabetic status and type of surgical technique on GM and WM atrophy. First, we used one‐way ANOVA to reveal possible differences between diabetic groups using one‐way ANOVA (non‐diabetic vs. IFG/IGT/diabetic; obese subjects only). Second, we also tested, whether there are linear associations between preoperative brain densities and preoperative BMI as well as preoperative brain densities and BMI change after surgery (preoperative minus postoperative) within the obese group that completed the follow up (N = 40). These two cross sectional comparisons were corrected for age. Third, we analyzed the effects of the surgical technique to changes in brain using a 2 × 2 mixed ANOVA (gastric bypass – sleeve gastrectomy × preop ‐ postop).
All region‐of‐interest (ROI) based between groups comparisons and correlational statistical analysis were carried out in IBM SPSS Statistics version 22. Additionally, we evaluated the possible confounding effects of smoking and depressive symptoms in the ROI analysis by correlating local GM and WM densities with smoking status and BDI scores. Finally, the metabolic characteristics of the morbidly obese that dropped out from the study were compared to those participating to all scans.
RESULTS
Cross‐Sectional Differences Between Non‐obese and Morbidly Obese Subjects
Preoperatively, the morbidly obese subjects had widespread decreases GM density in bilateral inferior orbitofrontal and frontal regions and bilateral insula (Fig. 1). We also found lower densities in temporal, cerebellar, and occipital regions (Fig. 1 and Table 2). WM reductions were most prominent beneath bilateral orbitofrontal gyri and midbrain/medulla (Fig. 2, Table 2).
Figure 1.
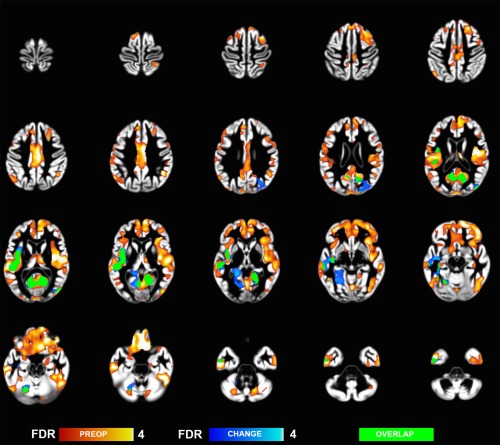
Grey matter regions where morbidly obese subjects had decreased brain densities as compared to non‐obese controls (hot colors), and regions showing postoperative density changes (cool colors) (whole brain FDR corrected p < 0.05).
Table 2.
Regional maxima for brain regions identified in preoperative comparison (non‐obese > morbidly obese)
| MNI coordinates | ||||
|---|---|---|---|---|
| Region | x | y | z | T value |
| Grey matter | ||||
| Left middle temporal lobe | −52 | −30 | 4 | 6.9 |
| 3 | 36 | −30 | 6.1 | |
| 9 | 21 | −30 | 5.9 | |
| Right thalamus | 10 | −16 | 16 | 5.1 |
| 14 | −6 | 15 | 2.7 | |
| −8 | 10 | 15 | 2.5 | |
| Left calcarine gyrus | −6 | −99 | 12 | 4.5 |
| −6 | −96 | 24 | 4.0 | |
| 16 | −94 | 21 | 4.0 | |
| Right angular gyrus | 44 | −49 | 33 | 4.4 |
| Left inferior occipital lobe | −52 | −69 | −17 | 4.3 |
| −48 | −55 | −9 | 4.1 | |
| −36 | −52 | −11 | 3.8 | |
| Right inferior temporal lobe | 60 | −8 | −35 | 3.9 |
| 57 | 0 | −38 | 3.7 | |
| 34 | −4 | −44 | 3.3 | |
| Right cerebellum | 16 | −66 | −32 | 3.9 |
| 39 | −63 | −35 | 3.1 | |
| 10 | −85 | −21 | 3.0 | |
| Left middle occipital gyrus | −22 | −97 | 12 | 3.5 |
| −32 | −93 | 12 | 2.9 | |
| −40 | −88 | 15 | 2.8 | |
| Right postcentral gyrus | 33 | −46 | 66 | 3.4 |
| 32 | −48 | 57 | 3.0 | |
| Left cerebellum | −27 | −72 | −32 | 3.4 |
| −22 | −66 | −17 | 3.1 | |
| Left inferior temporal lobe | −38 | −27 | −15 | 3.2 |
| −34 | −42 | −21 | 3.1 | |
| Right fusiform gyrus | 38 | −25 | −17 | 3.2 |
| Right parahippocampal gyrus | 20 | −16 | −24 | 2.7 |
| 22 | −7 | −20 | 2.6 | |
| White matter | ||||
| Left inferior frontal | −33 | 54 | −11 | 6.36 |
| −42 | 42 | −8 | 5.7 | |
| 8 | −4 | 34 | 5.16 | |
| Left medial frontal | −8 | 8 | 39 | 3.4 |
| Right inferior occipital | 38 | −87 | −9 | 3.39 |
| Right medial frontal | 8 | 44 | 28 | 2.95 |
| 20 | 38 | 37 | 2.92 | |
| 18 | 47 | 31 | 2.91 | |
| Left cerebellum | −38 | −66 | −38 | 2.59 |
| −33 | −60 | −35 | 2.39 | |
The data are thresholded at p < 0.05 (FDR corrected).
Figure 2.
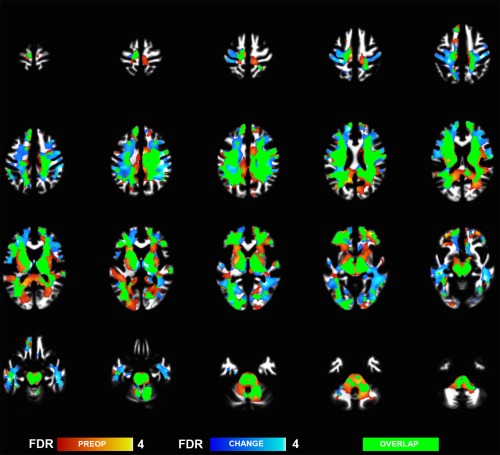
White matter regions where morbidly obese subjects had decreased brain densities as compared to non‐obese controls (hot colors), and regions showing postoperative density changes (cool colors) (whole brain FDR corrected p < 0.05).
In general, multiple metabolic variables linked with obesity (BMI, waist circumference, fat percent, abdominal and visceral fat volumes, systolic blood pressure, fasting glucose and plasma lipids) were negatively associated with GM and WM densities (Tables III and IV). Plasma HDL cholesterol levels were positively associated with both GM and WM in most brain regions in (Tables 3, 4 and Fig. 3).
Table 3.
Spearman correlations between grey matter densities and metabolic variables in normal weight and morbidly obese participants (preoperative)
| Left Cere bellum | Right Cere bellum | Left Calcarine Gyrus | Right Fusiform Gyrus | Right Angular Gyrus | Left Middle Occipital Gyrus | Left Inferior Occipital Lobe | Right Thalamus | Right Inferior Temporal Lobe | Left Inferior Temporal Lobe | Left Middle Temporal Lobe | Right Para hippo campal Gyrus | Right Post central Gyrus | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | −.287* | −.240* | −.251* | −.416** | −.259* | ||||||||
| BMI | −.469** | −.397*** | −.271* | −.411*** | −.294* | −.352** | −.399*** | −.297** | −.472*** | −.484*** | −.291* | −.259* | −.273* |
| Waist Circumference (cm) | −.358** | −.295* | −.332** | −.305* | −.408** | −.267* | −.267* | ||||||
| Fat percent | −.335** | −.237* | −.297* | −.307** | −.388** | −.318** | −.363** | −.280* | |||||
| Subcutaneous fat mass (kg) | −.420** | −.366** | −.289* | −.405** | −.295* | −.363** | −.429** | −.382** | |||||
| Visceral fat mass (kg) | −.417** | −.289* | −.472** | −.280* | −.334* | −.392** | |||||||
| Systolic blood pressure | −.251* | −.318** | |||||||||||
| diastolic blood pressure | −.253* | ||||||||||||
| HbA1c(%) | −.271* | −.417** | |||||||||||
| Fasting glucose (mmol/l) | −.294* | −.461*** | |||||||||||
| Triglycerides (mmol/l) | −.358** | −.283* | −.313* | −.273* | −.263* | −.288* | −.262* | −.537*** | |||||
| HDL (mmol/l) | .368** | .355** | .311** | .288* | |||||||||
| LDL (mmol/l) | |||||||||||||
| HDL/Kol ratio (%) | .376** | .379** | .391** | .351** | .274* | ||||||||
| sensitive CRP (mg/l) | −.322* | −.265* | |||||||||||
| Thyroid stimulating hormone (mU/l) | |||||||||||||
| Beck depression inventory II | −.273* |
Only statistically significant correlations (p < 0.05) are shown. * p < 0.05, **p < 0.01, *** p < 0.001.
Table 4.
Spearman correlations between white matter densities and metabolic variables in normal weight and morbidly obese participants (preoperative)
| Left Cerebellum | Right inferior Occipital | Left Inferior Frontal | Left Medial Frontal | Right Medial Frontal | |
|---|---|---|---|---|---|
| Age (years) | .256* | ||||
| BMI | −.251* | −.268* | −.322** | −.439*** | −.320** |
| Waist Circumference (cm) | −.280* | ||||
| Fat percent | −.300** | −.307** | −.294* | −.467*** | −.324** |
| Systolic blood pressure | −.242* | ||||
| Subcutaneous fat mass (kg) | −.299* | −.366** | −.284* | ||
| Visceral fat mass (kg) | −.384** | −.462** | −.317* | ||
| diastolic blood pressure | |||||
| HbA1c (%) | −.325* | ||||
| Fasting glucose (mmol/l) | −.276* | −.321* | −.326** | ||
| Triglycerides (mmol/l) | −.293* | −.297* | |||
| HDL (mmol/l) | .270* | .318** | |||
| LDL (mmol/l) | |||||
| HDL/Kol ratio (%) | |||||
| sensitive CRP (mg/l) | −.264* | −.313* | −.345** | ||
| Thyroid stimulating hormone (mU/l) | |||||
| Beck depression inventory II | |||||
Only statistically significant correlations (p < 0.05) are shown. * p < 0.05, **p < 0.01, *** p < 0.001.
Figure 3.
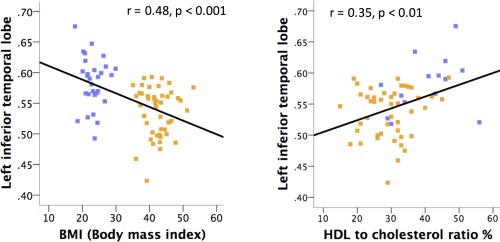
Linear relationship between grey matter densities, BMI and total cholesterol‐to‐HDL ratio (in the scatter plot, non‐obese subjects appear in dark blue and obese subjects in orange). See Tables 3, 4 and 6 for full report on the correlations between brain tissue densities and metabolic variables. [Color figure can be viewed at http://wileyonlinelibrary.com.]
The results remained essentially unchanged when including only female participants, and leaving smokers out of the analysis did not change the overall pattern of results. BDI scores did not correlate systematically with brain densities in ROI analysis (Tables III and IV). Also, no differences between smokers and non‐smokers in metabolic variables or brain densities were found. No differences were found in GM densities between morbidly obese with and without disturbances in glucose metabolism. However, brain stem WM densities were lower in morbidly obese subjects with versus without disturbed glucose metabolism (data not shown). Finally, BMI did not have linear associations to brain densities within the obese group preoperatively.
Effects of Bariatric Surgery and Weight Loss
Bariatric surgery led to significant weight loss (M = 26 kg, SD = 14) and decrease in fat percentage (M = 6.9%, SD = 5.4) (Table 1). Nine subjects with initial diabetes and four with IGT were in remission postoperatively. At brain level we found extensive WM volume recovery, spanning throughout the WM (Fig. 2 and Table 5). GM recovery was observed only by using more lenient statistical threshold (p < 0.05 FDR corrected at cluster level) and spanned occipital and temporal cortical regions (Fig. 1 and Table 5). These effects partially overlapped with initial volume differences observed in the non‐obese versus obese comparison in the preoperative state (see Figs. 1 and 2).
Table 5.
Regional maxima for brain regions identified in follow up comparison of the morbidly obese (postop. > preop.)
| MNI coordinates | ||||
|---|---|---|---|---|
| Region | x | y | z | T value |
| Grey Matter | ||||
| Right lingual gyrus | 21 | −63 | 0 | 4.3 |
| −20 | −45 | −2 | 3.8 | |
| −42 | −15 | −14 | 3.8 | |
| White matter | ||||
| Left inferior temporal | −42 | −28 | −18 | 5.8 |
| −8 | −10 | −12 | 5.2 | |
| 10 | −12 | 1 | 5.1 | |
| Left inferior temporal | −28 | −13 | −30 | 3.9 |
| Left superior temporal | −52 | −4 | −9 | 3.3 |
| −54 | −13 | −3 | 3.1 | |
| Left cerebellum | −30 | −45 | −35 | 2.9 |
| −32 | −58 | −39 | 2.6 | |
The data are thresholded at p < 0.05, FDR corrected.
The postoperative GM and WM changes were similar between the two surgical procedures (sleeve gastrectomy and gastric bypass). The dropped‐out obese participants did not differ from the ones that concluded the study with respect to demographic and metabolic variables. We found no significant correlations between preoperative GM or WM brain densities and surgery‐induced BMI changes in the ROI analysis. Correlation analysis between the postoperative increases in brain densities and changes in metabolic variables showed only limited correlations with each other and the changes did not appear directly proportional to BMI change (Table 6). Decreases in plasma triglycerides, LDL cholesterol, and long term glucose balance marker HbA1c correlated positively with the density increases (Table 6).
Table 6.
Pearson correlations between changes of brain densities and metabolic metrics after surgical weight loss
| Right Lingual Gyrus | Left Inferior Temporal white matter | Left Cerebellar white matter | Left Inferior Temporal white matter | Left Superior Temporal white matter | |
|---|---|---|---|---|---|
| BMI | |||||
| Subcutaneous fat mass (kg) | −.459* | .396* | |||
| Triglycerides (mmol/l) | .433** | ||||
| LDL cholesterol (mmol/l) | .330* | ||||
| HbA1c (%) | .402* |
Brain density increase is correlated with decreases in metabolic metrics.
Only statistically significant correlations (p < 0.05) are shown. * p < 0.05, **p < 0.01, *** p < 0.001.
Finally, we used complementary full‐volume GLM to test whether preoperative regional GM and WM densities would be associated with surgery‐induced weight loss. Significant positive associations with GM density were found in right‐hemispheric frontotemporal and insular cortices, right thalamus, and bilateral cerebellum. In WM positive associations with weight loss were found beneath bilateral posterior temporal cortex, and precentral and superior frontal cortex. Negative associations with weight change and GM or WM density were not found (data not shown).
DISCUSSION
Morbidly obese subjects had initially decreased GM and WM densities yet bariatric surgery and the following weight loss resulted in global WM recovery six months after the surgery. GM recovery was also observed but it was not as prominent. Some—but not all—of these changes occurred at sites where atrophy was observed in the preoperative comparison to control subjects (Figs. 1 and 2). At the preoperative state, increased adiposity and brain densities were associated negatively (Tables III and IV). Furthermore, decreases in plasma triglycerides, LDL cholesterol, and HbA1c after weight loss were associated positively with increased brain densities (Table 6). Altogether these findings suggest that weight loss has a causal influence on brain tissue densities.
Obesity Is Associated With Fronto‐Insular Atrophy
At the baseline state, obesity was associated with lowered GM and WM densities. GM atrophy was observed in frontal and orbitofrontal cortices and insulae, and tissue densities were lower throughout the WM. This accords with previous results suggesting that brain volume reductions are a common feature of obesity [Karlsson et al., 2013], even though opposite findings have also been reported [Haltia et al., 2007; Taki et al., 2008]. The insular cortex is involved in homeostatic and motivational control and has also recently been found to be critical in maintenance of addictions [Mizuhiki et al., 2012; Naqvi and Bechara, 2009; Small, 2010], whereas the orbitofrontal cortex links rewards to hedonic experience [Kringelbach, 2005]. Accordingly, alterations in these circuits may make the obese individuals prone to eat regardless of internal state of hunger or satiety [Diekhof et al., 2012; Karlsson et al., 2013; Scharmüller et al., 2012], but the changes may also reflect the consequences of obesity‐related adverse metabolic health profile.
Brain Integrity Is Associated With Metabolic Factors
Preoperatively, GM and WM atrophy was associated with multiple metabolic parameters including fat percentage, systolic blood pressure, fasting glucose, and triglycerides. However, clear tissue type specific trends were not revealed in the analyses and none of the used parameters appeared prominent as compared to others (Tables III and IV). Yet, preoperative regional GM and WM densities were not associated with subsequent weight loss in the ROI analysis. Complementary full‐volume analysis, however, revealed that fronto‐temporal, insular, thalamic, and cerebellar GM densities, as well as frontotemporal WM densities were positively associated with the magnitude of weight loss.
Based on previous studies, adverse cellular effects in the brain could follow vascular damage caused by hypertension [Breteler et al.,1994; Korf et al., 2007] and elevated plasma lipids [Cohen et al., 2011], which are features of obesity [Cazettes et al., 2011; Hotamisligil, 2006; Lumeng and Saltiel, 2011; Pannaciulli et al., 2007; Vachharajani and Granger, 2009]. On the other hand, brain volume alterations among the obese may also result from chronic hyperglycemia and glucose neurotoxicity through increased amount of oxygen radicals [Tomlinson and Gardiner, 2008; Tuulari et al., 2013], as weight gain and peripheral insulin resistance are generally thought to cause adverse structural and functional brain changes [Craft, 2006; Messier, 2005; Williamson et al., 2012]. In the current study, plasma HDL cholesterol levels were positively associated with GM and WM densities, which highlights possible positive effects of healthy lipid profile on brain health (Tables III and IV). Thus, the associations between metabolic variables and brain tissue integrity accord with some of the well‐known risk and protecting factors for atherosclerosis, vascular diseases [Friedman et al., 2014] and cognitive decline with increasing age [Jagust et al., 2005; Pannaciulli et al., 2007, Walther et al., 2010], yet their specific and individual contributions to cerebral atrophy cannot be resolved on the basis of the present data, in part because the study lacks longitudinal follow up prior to surgery.
Possible Causal Links Between Weight Gain, Weight Loss, and Brain Tissue
Given that obesity is associated with brain tissue volume reductions, it could be expected that losing weight would reverse these changes. This was the case in the current study, where we observed widespread recovery of WM and less profound recovery of GM densities (Figs. 1 and 2). WM may be the most vulnerable part of the brain under the multifaceted metabolic stress caused by obesity. Furthermore, WM may have greater capacity for regeneration, here probably through recovery in myelination [Bhatt et al, 2014]. It is generally thought that neurons within the cortical GM are not able to recover after cellular insult. Should more extensive recovery within the GM take place, in terms of what can be quantified with VBM, it may be that brain gray matter needs more time than six months to recuperate.
The obesity‐related GM atrophy might reflect an obese‐prone endophenotype rather than consequence of obesity [Karlsson et al., 2013]. Supporting this view, we observed GM atrophy in components of the homeostatic and reward circuitry, whose atrophy has been shown to predict future weight gain in prior prospective studies [Smucny et al., 2012; Yokum and Stice, 2012]. GM in these regions did not exhibit as strong recovery as in WM regions after bariatric surgery. It is thus possible that lowered brain densities in these regions involved in appetite and eating control may predispose an individual to overeating and obesity, whereas the WM changes might be caused by obese phenotype.
Although our results imply that the extreme obese phenotype is associated with decreased tissue integrity, a recent large epidemiological study highlights that obesity might even protect against the onset of dementia [Qizilbash et al., 2015]. This implies that the association between adiposity and cognitive performance is not as straightforward as has previously been thought. Overall, the metabolic variables explained a rather small amount of the tissue changes after bariatric surgery. It is thus imperative to assess the effects of additional factors such as changes of non‐adipose body composition, exercise habits [Coen et al., 2015], sleep quality [Peromaa‐Haavisto et al., 2015] and gastrointestinal hormone secretion [McIntyre et al., 2013; Meek et al., 2015] to brain tissue after the bariatric surgery changes in future studies.
LIMITATIONS
The age range of our obese subjects was fairly broad (Mean age of the groups was ca. 45 years; range 19‐65). It is thus possible that some changes (both structural and functional) may unfold only after decades of hyperglycemia, hyperlipidemia, and low‐grade inflammation ‐ or may manifest only with more extreme degrees or prolonged length of these states. The obese subjects diagnosed for type 2 diabetes, hypertension, and hypercholesterolemia were using oral medication, which may have resulted in flattened associations to brain volumes. Further, our sample comprised mainly of females thus the present findings may not directly generalize to males [Mueller et al., 2011]. Although grey reductions may be associated with decreased functional capability, VBM does not address brain function per se and that cellular changes corresponding to volumetric changes as observed here are not yet fully understood or characterized. Finally, although our study establishes a causal link between weight loss and brain tissue recovery, it remains unresolved whether weight gain actually leads to the atrophy observed in the morbidly obese subjects at the preoperative stage.
CONCLUSIONS
We show that obesity is associated with cerebral atrophy, yet particularly the WM (and to some extent GM) atrophy is recoverable by weight loss. This suggests a causal link between weight loss and brain tissue integrity, which might reflect improved brain health after surgical weight loss. Finally, obesity‐related accumulation of cardiovascular risk factors best explains the degree of atrophy and recovery to some extent, suggesting that efficient vascular risk factor control in clinical setting is also beneficial to brain integrity.
ACKNOWLEDGMENTS
The authors thank the staff of the Turku PET Centre for performing the PET imaging together with the researchers. Jarna C. Hannukainen and Minna Soinio are acknowledged for their valuable contribution to data collection. Special thanks goes to research nurse Mia Koutu for data collection together with researchers. The study was conducted within the Finnish Center of Excellence in Molecular Imaging in Cardiovascular and Metabolic Research. Jetro J. Tuulari is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- Bhatt A, Fan L, Pang Y (2014): Strategies for myelin regeneration: Lessons learned from development. Neural Regen Res 9:1347–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breteler MM, van Swieten JC, Bots ML, Grobbee DE, Claus JJ, van den Hout JH, van Harskamp F, Tanghe HL, de Jong PT, van Gijn J (1994): Cerebral white matter lesions, vascular risk factors, and cognitive function in a population‐based study: The Rotterdam Study. Neurology 44:1246–1252. [DOI] [PubMed] [Google Scholar]
- Brooks SJ, Benedict C, Burgos J, Kempton MJ, Kullberg J, Nordenskjöld R, Kilander L, Nylander R, Larsson E‐M, Johansson L, Ahlström H, Lind L, Schiöth HB (2013): Late‐life obesity is associated with smaller global and regional gray matter volumes: A voxel‐based morphometric study. Int J Obes 37:230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazettes F, Cohen J, Yau P, Talbot H, Convit A (2011): Obesity‐mediated inflammation may damage the brain circuit that regulates food intake. Brain Res 1373:101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft S (2006): Insulin resistance syndrome and Alzheimer disease: Pathophysiologic mechanisms and therapeutic implications. Alzheimer Dis Assoc Disord 20:298–301. [DOI] [PubMed] [Google Scholar]
- Chang, SH , Stoll C, Song J, Varela JE, Eagon C, Colditz G (2013): The effectiveness and risks of bariatric surgery: An updated systematic review and meta‐analysis, 2003‐2012. JAMA Surg 149:275–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, Cazettes F, Convit A (2011): Abnormal cholesterol is associated with prefrontal white matter abnormalities among obese adults, a diffusion tensor imaging study. Neuroradiol J 1:989–997. [PMC free article] [PubMed] [Google Scholar]
- Cole JH, Boyle CP, Simmons AC, Woods S, Rivera M, McGuffin P, Thompson PM, Fu CHY (2013): Body mass index, but not FTO genotype or major depressive disorder, influences brain structure. Neuroscience 252:109–117. [DOI] [PubMed] [Google Scholar]
- Coen PM, Menshikova EV, Giovanna DG, Zheng D, Charles J, Tanner CJ, Standley RA, Helbling NL, Dubis GS, Ritov VB, Xie H, Desimone ME, Smith SR, Stefanovic‐Racic M, Toledo FGS, Houmard Goodpaster BH (2015): Exercise and weight loss improve muscle mitochondrial respiration, lipid partitioning, and insulin sensitivity after gastric bypass surgery. Diabetes 64:3737–3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof E, Kaps L, Falkai P, Gruber O (2012): The role of the human ventral striatum and the medial orbitofrontal cortex in the representation of reward magnitude ‐ an activation likelihood estimation meta‐analysis of neuroimaging studies of passive reward expectancy and outcome processing. Neuropsychologia 50:1252–1266. [DOI] [PubMed] [Google Scholar]
- Friedman JI, Tang CY, de Haas HJ, Changchien L, Goliasch G, Dabas P, Wang V, Fayad ZA, Fuster V, Narula J (2014): Brain imaging changes associated with risk factors for cardiovascular and cerebrovascular disease in asymptomatic patients. JACC Cardiovasc Imaging 7:1039–1053. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J‐, Frith CD, Frackowiak RSJ (1994): Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp 2:189–210. [Google Scholar]
- Gloy V, Briel M, Bhatt D, Kashyap S, Schauer P, Mingrone G, Bucher HC, Nordmann AJ (2013): Bariatric surgery versus non‐surgical treatment for obesity: A systematic review and meta‐analysis of randomised controlled trials. BMJ.Br Med J 347:f5934–f5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haltia L, Viljanen A, Parkkola R, Kemppainen N, Rinne J, Nuutila P, Kaasinen V (2007): Brain white matter expansion in human obesity and the recovering effect of dieting. J Clin Endocrinol Metab 92:3278–3284. [DOI] [PubMed] [Google Scholar]
- Hotamisligil G (2006): Inflammation and metabolic disorders. Nature 444:860–867. [DOI] [PubMed] [Google Scholar]
- Jagust W, Harvey D, Mungas D, Haan M (2005): Central obesity and the aging brain. Arch Neurol 62:1545–1548. [DOI] [PubMed] [Google Scholar]
- Karlsson HK, Tuulari JJ, Hirvonen J, Lepomäki V, Parkkola R, Hiltunen J, Hannukainen JC, Soinio M, Pham T, Salminen P, Nuutila P, Nummenmaa L (2013): Obesity is associated with white matter atrophy: A combined diffusion tensor imaging and voxel‐based morphometric study. Obesity 21:2530–2537. [DOI] [PubMed] [Google Scholar]
- Korf ESC, van Straaten ECW, de Leeuw F, van der Flier WM, Barkhof F, Pantoni L, Basile AM, Inzitari D, Erkinjuntti T, Wahlund LO, Rostrup E, Schmidt R, Fazekas F, Scheltens P, LADIS Study Group (2007): Diabetes mellitus, hypertension and medial temporal lobe atrophy: The LADIS study. Diabetic Med 24:166–171. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML (2005): The human orbitofrontal cortex: Linking reward to hedonic experience. Nat Rev Neurosci Sep 6:691–702. [DOI] [PubMed] [Google Scholar]
- López M, Soriano Mas L, Delgado Rico C, Rio Valle E, Verdejo J, García A (2012): Brain structural correlates of reward sensitivity and impulsivity in adolescents with normal and excess weight. PLoS One 7:e49185–e49185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng C, Saltiel A (2011): Inflammatory links between obesity and metabolic disease. J Clin Invest 121:2111–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre RS, Powell AM, Kaidanovich‐Beiline O, Soczynska JK, Alsuwaidanc M, Woldeyohannes HO, Kim AS, Gallaugher LA (2013): The neuroprotective effects of GLP‐1: Possible treatments for cognitive deficits in individuals with mood disorders. Behav Brain Res 237:164–171. [DOI] [PubMed] [Google Scholar]
- Meek CL, Lewis HB, Reimann F, Gribble FM, Park AJ (2016): The effect of bariatric surgery on gastrointestinal and pancreatic peptide hormones. Peptides 77:28–37. [DOI] [PubMed] [Google Scholar]
- Messier C (2005): Impact of impaired glucose tolerance and type 2 diabetes on cognitive aging. Neurobiol Aging 26 Suppl 1:26–30. [DOI] [PubMed] [Google Scholar]
- Mizuhiki T, Richmond B, Shidara M (2012): Encoding of reward expectation by monkey anterior insular neurons. J Neurophysiol 107:2996–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller K, Anwander A, Müller H, Horstmann A, Lepsien J, Busse F, Mohammadi S, Schroeter ML, Stumvoll M, Villringer A, Pleger B (2011): Sex‐dependent influences of obesity on cerebral white matter investigated by diffusion‐tensor imaging. PLoS One 6:e18544–e18544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A (2009): The hidden island of addiction: The insula. Trend Neurosci 32:56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannacciulli N, Del Parigi A, Chen K, Le, Duc Son NT, Reiman E, Tataranni P (2006): Brain abnormalities in human obesity: A voxel‐based morphometric study. Neuroimage 31:1419–1425. [DOI] [PubMed] [Google Scholar]
- Pannacciulli N, Le DSNT, Chen K, Reiman E, Krakoff J (2007): Relationships between plasma leptin concentrations and human brain structure: A voxel‐based morphometric study. Neurosci Lett 412:248–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peromaa‐Haavisto PH, Tuomilehto HJ, Kössi J, Virtanen JM, Luostarinen M, Pihlajamäki J, Käkelä P, Victorzon M (2015): Prevalence of obstructive sleep apnoea among patients admitted for bariatric surgery. A prospective multicentre trial. Obes Surg 1–7. [DOI] [PubMed] [Google Scholar]
- Qizilbash N, Gregson J, Johnson ME, Neil Pearce N, Douglas I, Wing K, Evans SJW, Pocock SJ (2015): BMI and risk of dementia in two million people over two decades: A retrospective cohort study. Lancet Diab Endocrinol 3:431–436. [DOI] [PubMed] [Google Scholar]
- Raji C, Ho A, Parikshak N, Becker J, Lopez O, Kuller L, et al. (2010): Brain structure and obesity. Hum Brain Mapp 31:353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharmüller W, Übel S, Ebner F, Schienle A (2012): Appetite regulation during food cue exposure: A comparison of normal‐weight and obese women. Neurosci Lett 518:106–110. [DOI] [PubMed] [Google Scholar]
- Small D (2010): Taste representation in the human insula. Brain Struct Funct 214:551–561. [DOI] [PubMed] [Google Scholar]
- Smucny J, Cornier MA, Lindsay E, Thomas E, Bechtell J, Tregellas J (2012): Brain structure predicts risk for obesity. Appetite 59:859–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanek K, Grieve S, Brickman A, Korgaonkar M, Paul R, Cohen R, Gunstad JJ (2011): Obesity is associated with reduced white matter integrity in otherwise healthy adults. Obesity 19:500–504. [DOI] [PubMed] [Google Scholar]
- Taki Y, Kinomura S, Sato K, Inoue K, Goto R, Okada K (2008): Relationship between body mass index and gray matter volume in 1,428 healthy individuals. Obesity 16:119–124. [DOI] [PubMed] [Google Scholar]
- Tomlinson D, Gardiner N (2008): Glucose neurotoxicity. Nat Rev Neurosci 9:36–45. [DOI] [PubMed] [Google Scholar]
- Tuulari J, Karlsson H, Hirvonen J, Hannukainen J, Bucci M, Helmiö M, Ovaska J, Soinio M, Salminen P, Savisto N, Nummenmaa L, Nuutila P (2013): Weight loss after bariatric surgery reverses insulin‐induced increases in brain glucose metabolism of the morbidly obese. Diabetes 62:2747–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vachharajani V, Granger DN (2009): Adipose tissue: A motor for the inflammation associated with obesity. IUBMB Life 61:424–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstynen T, Weinstein A, Schneider W, Jakicic J, Rofey D, Erickson K (2012): Increased body mass index is associated with a global and distributed decrease in white matter microstructural integrity. Psychosom Med 74:682–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther K, Birdsill A, Glisky E, Ryan L (2010): Structural brain differences and cognitive functioning related to body mass index in older females. Hum Brain Mapp 31:1052–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson R, McNeilly A, Sutherland C (2012): Insulin resistance in the brain: An old‐age or new‐age problem? Biochem Pharmacol 84:737–745. [DOI] [PubMed] [Google Scholar]
- Yokum S, Stice E (2012): Relation of regional gray and white matter volumes to current BMI and future increases in BMI: A prospective MRI study. Int J Obes 36:656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]


