Abstract
Generalized anxiety disorder (GAD) and panic disorder (PD) are most common anxiety disorders with high lifetime prevalence while the pathophysiology and disease‐specific alterations still remain largely unclear. Few studies have taken a whole‐brain perspective in the functional connectivity (FC) analysis of these two disorders in resting state. It limits the ability to identify regionally and psychopathologically specific network abnormalities with their subsequent use as diagnostic marker and novel treatment strategy. The whole brain FC using a novel FC metric was compared, that is, scaled correlation, which they demonstrated to be a reliable FC statistics, but have higher statistical power in two‐sample t‐test of whole brain FC analysis. About 21 GAD and 18 PD patients were compared with 22 matched control subjects during resting‐state, respectively. It was found that GAD patients demonstrated increased FC between hippocampus/parahippocampus and fusiform gyrus among the most significantly changed FC, while PD was mainly associated with greater FC between somatosensory cortex and thalamus. Besides such regional specificity, it was observed that psychopathological specificity in that the disrupted FC pattern in PD and GAD correlated with their respective symptom severity. The findings suggested that the increased FC between hippocampus/parahippocampus and fusiform gyrus in GAD were mainly associated with a fear generalization related neural circuit, while the greater FC between somatosensory cortex and thalamus in PD were more likely linked to interoceptive processing. Due to the observed regional and psychopathological specificity, their findings bear important clinical implications for the potential treatment strategy. Hum Brain Mapp 37:1459‐1473, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: anxiety disorders, functional magnetic resonance imaging (fMRI), fear, generalization, interoception; whole brain functional connectivity analysis
INTRODUCTION
Anxiety disorders are highly prevalent [Kessler et al., 1994, 2005a,b] and affect many aspects of daily life. Patients with anxiety disorders exhibit reduced quality of life, impaired social functioning, and higher morbidity and mortality [Rodriguez et al., 2005; Wittchen and Fehm, 2001]. Generalized anxiety disorder (GAD) and panic disorder (PD) are two common subtypes of anxiety disorders. GAD has the central defining feature of chronic, excessive anxiety and worrying, as described in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM‐5). Previous research has linked various fear‐learning processes to GAD, including fear generalization [Cha et al., 2014a, 2014b; Greenberg et al., 2013; Lissek et al., 2014; Mineka and Zinbarg, 2006]. Fear generalization is the transfer of conditioned fear to perceptually similar stimuli [Greenberg et al., 2013] and may contribute to an increase in the number of cues/events capable of triggering worry [Borkovec et al., 1991]. PD is characterized by recurrent unexpected panic attacks [American Psychiatric Association, 2013] and considerable studies have reported somatic abnormalities in PD, such as increased heart rate, higher skin conductance and increased respiration value [Fleet et al., 1996; Friedman and Thayer, 1998; Hoehn‐Saric et al., 2004; Stein and Asmundson, 1994]. Studies suggested that these somatic symptoms in PD reflect abnormal interoceptive processing including visceroception (from inner organs) as well as proprioception (from musculoskeletal organs) [Domschke et al., 2010; Ehlers and Breuer, 1992; Qiang et al., 2012; Van der Does et al., 1997; Willem Van der Does et al., 2000].
Imaging studies in GAD showed structural abnormalities in various regions including cingulate cortex, precentral gyrus, precuneus, temporal, and frontal gyrus [De Bellis et al., 2002; Strawn et al., 2013; Zhang et al., 2013]. Task‐related activity and connectivity changes were reported in parietal cortices (in an affective Stroop task) [Blair et al., 2012], Broca's area and the occipitotemporal area (during a verbal fluency task) [Kalk et al., 2012], the putamen (during anticipation) [Guyer et al., 2012], the bed nucleus of the stria terminalis (BNST) (during uncertainty) [Yassa et al., 2012], the amygdala, the prefrontal cortex (PFC) and the insula, respectively, related to the specific paradigms used [Etkin et al., 2009, 2010; Etkin and Schatzberg, 2011; McClure et al., 2007; Roy et al., 2013; Strawn et al., 2012]. Resting state studies mainly concentrate in amygdala circuits involved in emotion processing [Etkin et al., 2009; Liu et al., 2015; Roy et al., 2013]. It remains unclear though whether these changes are specific for GAD in particular or are rather related to anxiety in general. Accordingly, comparison of resting state FC between different subgroups of anxiety disorders like GAD and PD is warranted.
Structure neuroimaging data in PD showed changes in amygdale [Hayano et al., 2009; Massana et al., 2003b] and other subcortical structures, such as parahippocampus gyrus, caudate nucleus, basal ganglion, insula [Lai 2011; Massana et al., 2003a; Uchida et al., 2008], and cortical areas like anterior cingulated cortex (ACC), frontal and temporal areas [Asami et al., 2008; Fontaine et al., 1990; Han et al., 2008; Sobanski et al., 2010; Uchida et al., 2003]. Recent task‐related functional magnetic resonance imaging (fMRI) studies suggested that increased activation in fear system structures like amygdala, insula, and hippocampus during agoraphobia‐specific stimuli [Wittmann et al., 2011], and increased activation in the right inferior frontal area, the cingulate cortex during panic anticipation and imagery exposure [Bystritsky et al., 2001]. Investigations about resting‐state in PD suggested abnormal FC within the salience network [Pannekoek et al., 2013] and default mode network (DMN) [Shin et al., 2013]. However, whether these resting state FC are regionally and psychopathologically specific to PD as distinguished from for instance GAD remains unclear at this point.
To address both regional and psychopathological specificity, we here investigated and respectively compared resting‐state FC between GAD and healthy controls (HC), and between PD and HC. The resting‐state functional connectional analysis is a sound approach in the neuropsychiatric disorders [Chen et al., 2015; Duncan et al., 2014; Luo et al., 2011; Northoff 2013]. Based on prior studies, we hypothesized that the FC among GAD and PD were associated with different neural circuits. Specifically, we expected to find altered FC in network involved in fear and generalization among GAD. For PD network we expected an abnormal perception of bodily signals like areas involved in somatosensory processing. In addition, there might be correlations between abnormal FC and disease severity. In order to avoid the confounding effects of medication, we only included drug‐free (or drug‐naïve) patients in our study.
Unlike previous studies [Blair et al., 2012; Bystritsky et al., 2001; Etkin et al., 2009, 2010; Etkin and Schatzberg, 2011; Guyer et al., 2012; Kalk et al., 2012; McClure et al., 2007; Pannekoek et al., 2013; Roy et al., 2013; Strawn et al., 2012; Wittmann et al., 2011] that mostly focused on ROIs defined as a priori, we relied on a whole‐brain approach, that is, Brain‐wide Association Study (BWAS) [Tao et al., 2013] to identify those networks that are specifically associated with GAD as distinguished from HC and with PD as distinguished from HC from the whole brain FC. BWAS is totally data‐driven therefore avoids the shortcomings of ROI‐based approaches. However, with whole brain analysis the traditional FC metric such as Pearson correlation may not provide enough statistical power required for multiple comparison, especially when the number of subjects enrolled is not large, like the current study. To obtain statistically significant results in datasets with relatively small sample size, we proposed to use a novel FC metric, that is, scaled correlation [Folias et al., 2013; Nikolic et al., 2012] in this article, which we demonstrate to have higher statistical power in two sample t‐test of whole brain analysis than traditional Pearson correlation. This is because scaled‐correlation can reduce the variance of FC distribution, with the mean of FC distribution unchanged compared with that from Pearson correlation. Therefore, the estimate of P value in two sample t‐test becomes more significant than that from Pearson correlation. Our analysis was finally complemented by correlating psychopathological scores of GAD and PD with the respective FC changes.
METHOD
Subjects
Sixty‐one right‐handed subjects were included in the study: 21 subjects with GAD, 18 subjects with PD, and 22 HC. All patients were recruited from the psychological outpatient clinic at Shanghai Mental Health Center (SMHC), China. Matched HC were recruited from local communities and Shanghai Jiaotong University. One expert clinician (CBL) confirmed the diagnoses based on the DSM‐IV criteria for GAD and PD and the diagnoses are further checked by two research doctors (QH and LLZ) using Mini‐International Neuropsychiatric Interview (MINI), Chinese version [Si et al., 2009]. Inclusion criteria were: (i) aged 18–60 years; (ii) medication free for at least 2 weeks; (iii) the scores of Hamilton Anxiety Scale (HAMA) [Hamilton, 1959] ≥14, and the scores of Hamilton Depression Scale (HAMD) [Hamilton, 1960] ≤14; (iv) have completed at least 6 years of primary education. Exclusion criteria were: (i) head trauma leading to loss of consciousness; (ii) severe somatic diseases, for example, cancer, heart failure, pneumonia; (iii) alcohol or substances abuse; (iv) neurological illnesses such as stroke and dementia; (v) contraindication to magnetic resonance (MR) scanning. HC were included in this study if they were 18–60 years of age and had at least 6 years of education. HC were also assessed using the MINI, and did not qualify for any psychiatric diagnoses. The same exclusion criteria were applied to HC. All subjects performed HAMA, HAMD, Spielberger State‐Trait Anxiety Inventory (STAI) [Spielberger et al., 1970], Body Perception Questionnaire (BPQ) [Porges, 1993] before the fMRI scan. HAMA is a clinician‐administered scale to assess anxiety symptoms which have two subscales, the psychic anxiety subscale and the somatic anxiety subscale. STAI is a self‐administered assessment scale for the evaluation of severity of anxiety, containing trait anxiety and state anxiety. BPQ scale aims to capture body perception such as various bodily symptoms and sensations, which includes five factors: awareness, stress response, autonomic nervous system reactivity (ANSR), stress style, and disease history. Demographic and neuropsychological characteristics are listed in Table 1.
Table 1.
Demographic and neuropsychological characteristics
| Variables | GAD | PD | HC | Groups comparisons | |||
|---|---|---|---|---|---|---|---|
| n = 21 | n = 18 | n = 22 | GAD vs. HC | PD vs. HC | GAD vs. PD | ||
| Mean (SD) | Mean (SD) | Mean (SD) | P value | P value | P value | ||
| Ages in years | 39.95 (12.24) | 37.17 (11.31) | 38.05 (10.32) | 0.593 | 0.801 | 0.473 | |
| Education in years | 11.19 (3.31) | 10.89 (2.68) | 12.50 (2.59) | 0.142 | 0.084 | 0.746 | |
| Sex (male/female) | 13/7 | 12/6 | 14/8 | 0.927 | 0.842 | 0.914 | |
| HAMA‐psychic anxiety | 13.60 (8.78) | 10.33 (4.01) | 0.81 (1.44) | 0 | 0 | 0.182 | |
| HAMA‐somatic anxiety | 8.83 (3.26) | 13.33 (11.89) | 0.14 (0.36) | 0 | 0 | 0.084 | |
| STAI‐trait | 50.50 (12.0) | 54.25 (8.00) | 39.35 (8.33) | 0.002 | 0 | 0.298 | |
| STAI‐state | 49.67 (15.30) | 50.00 (10.78) | 35.17 (9.09) | 0.001 | 0 | 0.943 | |
| BPQ‐awareness | 99.44 (22.75) | 110.06 (24.97) | 75.30 (20.40) | 0.001 | 0 | 0.197 | |
| BPQ‐stress response | 28.11 (9.76) | 30.94 (8.60) | 16.60 (5.77) | 0 | 0 | 0.37 | |
| BPQ‐ANSR | 51.39 (14.38) | 53.59 (13.92) | 38.40 (8.01) | 0.001 | 0 | 0.649 | |
| BPQ‐stress style1 | 25.56 (3.97) | 24.47 (4.12) | 20.6 (4.42) | 0.001 | 0.01 | 0.434 | |
| BPQ‐stress style2 | 8.44 (2.53) | 8.82 (2.63) | 5.65 (2.16) | 0.001 | 0 | 0.349 | |
| BPQ‐diseases history | 39.83 (12.61) | 36.12 (7.02) | 29.65 (3.50) | 0.001 | 0.001 | 0.862 | |
SD, standard deviation; HAMA, Hamilton Anxiety Scale; STAI, Spielberger State‐Trait Anxiety Inventory; BPQ, Body Perception Questionnaire; ANSR, autonomic nervous system reactivity; GAD, generalized anxiety disorder; PD, panic disorder; HC, healthy control.
Our study was approved by the Research Ethics Committee at the SMHC of Shanghai Jiaotong University, China. All participants gave written informed consent before participating in this study.
Image Acquisition
All images were acquired on a 3.0‐T SIMENS MAGNETOM TrioTim syngo MR B17 scanner equipped with a 12‐channel head coil at the Shanghai Key Laboratory of Magnetic Resonance of East China Normal University, Shanghai, China. Foam paddings were used to reduce head motion and earplugs were used to reduce scanner noise. Prior to the scan, the subjects were instructed to lie still with their eyes closed, not to fall asleep, to relax and to move as little as possible during scanning. A questionnaire after the MRI indicated that whether or not the subjects had fallen asleep and open their eyes during the scanning. The data with abnormal head move would be discarded. High‐resolution T1‐weighted anatomical images were acquired for registration in the sagittal orientation using a magnetization‐prepared rapid gradient‐echo sequence (repetition time = 1900 ms, echo time = 2.46 ms, flip angle = 9°, 32 transverse slices, field of view = 240 × 240 mm, matrix = 256 × 256, slice thickness = 1 mm). Resting‐state functional MRI data were acquired using a single‐shot, gradient‐recalled echo planar imaging sequence (repetition time = 2000 ms, echo time = 25 ms, flip angle = 90°). About 32 transverse slices (field of view = 240 × 240 mm, matrix = 64 × 64, slice thickness = 5 mm) resulting in a total of 157 volumes and a scan time of 314 s. No abnormalities were found upon inspection of the subjects’ structural images by a radiologist (JQL).
fMRI Data Preprocessing
Data quality control include: (i) any data affected by head motion (maximal motion between volumes in each direction, and rotation about each axis) of greater than 3 mm or rotation of greater than 3° was excluded; (ii) we discard subjects with greater than 10% displaced frames from the analysis as it is likely that such high‐level of movement would have had an influence on several volumes.
For the three datasets prior to preprocessing, the first 10 volumes were discarded to allow for scanner stabilization and the subjects’ adaptation to the environment. The fMRI data preprocessing was then conducted by SPM8 (http://www.fil.ion.ucl.ac.uk/spm) and a Data Processing Assistant for Resting‐State fMRI (DPARSF). The remaining functional scans were first corrected for within‐scan acquisition time differences between slices and then realigned to the middle volume to correct for inter‐scan head motions. Subsequently, the functional scans were spatially normalized to a standard template (Montreal Neurological Institute) and resampled to 3 × 3 × 3 mm3. After normalization, BOLD signal of each voxel was firstly detrended to abandon linear trend and then passed through a band‐pass filter (0.01–0.08 Hz) to reduce low‐frequency drift and high‐frequency physiological noise. Finally, nuisance covariates including head motion parameters, global mean signals, white matter signals and cerebrospinal signals were regressed out from the BOLD signals. A volume scrubbing movement correction [Liu et al., 2015] is carried out so that head‐motion artifacts are not influencing observed effects. The mean framewise displacement (FD) was computed with FD threshold for displacement being 0.5. In addition to the frame corresponding to the displaced time point, one preceding and two succeeding time points were also deleted to reduce the “spill‐over” effect from head movements. After data preprocessing, the time series were extracted in each ROI by averaging the signals of all voxels within that region.
In preprocessing we removed the global signal. Currently there is no consensus on the removal of global signal when computing FC. It has been shown that global signal removal can affect between‐group analyses in schizophrenia but not in bi‐polar disorder [Yang et al., 2014]. The global signal removal has been shown to reduce physiological noise, especially the variance due to movement related effects, thus improving the reliability of resting fMRI [Yan et al., 2013]. It also remove specific confounds from the data to facilitate the evaluation of neurophysiological relationships [Fox et al., 2009]. Currently there is no research on the global signal effect on anxiety disorders. The major argument against the removal of global signal is the introduction of spurious correlations. In our study the main interest is the difference between two groups in terms of the FC strength, irrespective of the sign of the FC, it is therefore important that both groups are treated identically. Note that the global signal in both groups are removed using identical approach, thus reducing the possibility of introducing spurious group differences. Moreover, though global signal removal can increase the frequency of pairwise negative correlation coefficients [Saad et al., 2012], we consider negative correlation only on relative terms rather than anticorrelations, which is consistent with Murphy et al. [2009]. It will be our future work to investigate the influence of global signal in whole‐brain FC analysis.
For all three datasets the automated anatomical labeling atlas (AAL) was used to partition the brain into 90 regions of interest (ROIs) (45 per hemisphere). The names of the ROIs and their corresponding abbreviations are listed in Supporting Information Table S1.
Scaled Correlation Coefficient as a Reliable FC Metric and Its High Statistical Power
Pearson correlation, or cross‐correlation, has been extensively used as a FC metric [Biswal et al., 1995; Greicius et al., 2003]. In this article we used another version of cross‐correlation, that is, scaled correlation coefficient [Nikolic et al., 2012] as a FC metric, which evaluates correlation coefficient at certain frequency ranges of the signal. Scaled correlation has been used to investigate synchronization hubs in the visual cortex [Folias et al., 2013]. In the following we first introduce the definition of Scaled correlation. We then describe the procedure needed to verify that scaled correlation has more statistical power in two sample t‐test than Pearson correlation.
Scaled correlation between two time series is defined as the average correlation coefficient computed across short segments (or windows, with length s) of the two signals. Each signal is divided into K non‐overlapped segments K = round(T/s), where T and s are the length of the entire signal and segment, respectively. Scaled correlation (r s) across the entire signals then is: , where is the cross‐correlation of the Kth segments.
Scaled correlation with segment length s generally removes the correlation between frequency components (in two signals) lower than 1/s. The useful frequency range of BOLD signal is 0.01–0.08 Hz, which roughly corresponds to a time window of s = 100 seconds (0.01 Hz) and 12 seconds (0.08 Hz). Considering that the BOLD signal is sampled every 2 seconds, 100 and 12 seconds thus correspond to 50 and 6 sampled points, respectively. Therefore we compute scaled correlation with the segment length s varying from 6 to 50 sampled points, that is, s = 6, 7, 8, 9, …, 50.
The reason why we perform scaled correlation analysis at each possible segment length is that: (1) We want to cover the 0.01–0.08 Hz frequency range that is shown to carry useful information in fMRI data. (2) To avoid arbitrary choice of segment length s. In practice, we used segment length varying from 6 to 30 (rather than 50) sampled points as the length of entire signal is relatively short and segment length larger than 30 sampled points will lead to too few segments to reliably estimate the scaled correlation. By adopting scaled correlation coefficient at each specific segment length s, we are able to pick out subtle FC changes in specific frequency bands that may not be identified under the scrutiny of traditional Pearson correlation.
Now we describe how to validate scaled correlation as a reliable FC metric by showing that it contains similar information to the traditional Pearson correlation, but has a high statistical power in t‐test (i.e., more significant P value than that of Pearson correlation). We use a typical segment length (30 s) for demonstration. We perform t‐test between scaled‐ and Pearson correlation for both the control and patient group for a given FC, and examine if scaled‐ and Pearson correlation differs significantly. We further more perform a correlation analysis between scaled‐ and Pearson correlation to see if they are correlated. Our hypothesis is that the mean of scaled correlation and Pearson correlation would not differ significantly in control and patient group. Furthermore, we check variance of scaled correlation in both control and patient group and compare to those obtained by Pearson correlation. If scaled correlation has smaller sample variance, then this could increase the statistical power in two‐sample t‐test.
Statistical Analysis
For demographic and clinical characteristics, t‐tests were analyzed using the Statistical Package for the Social Science version 19.0 (SPSS Inc., Chicago). These data were compared using t‐tests for continuous variables, and chi‐square test for categorical variables.
For FC analysis using scaled correlation, we perform two sample t‐test for all connectivity of the brain network and identify those that show significant difference between patient and control groups (FDR, q = 0.05 and 0.1). Since scaled correlation is calculated with a given segment length s, to avoid arbitrary choice of segment length, we performed whole brain FC analysis using scaled correlation for each segment length s (ranging from 6 to 30 sampled‐points, as was explained above). We then average the result obtained under each segment length, that is, we count the average number of significantly changed FC (denoted by m), and pick top m FC that appears most frequently in the whole ensemble of identified FC under each segment length. This way, we are expected to obtain more stable FC changes. To evaluate the association between altered FC and symptom severity, we applied cross‐correlation analysis (P < 0.05).
RESULTS
Demographic and Clinical Characteristics
As is shown in Table 1, both patients’ groups and HC group did not differ in age, gender and years of education. Both patients’ groups rated higher than HC on the two items (psychic anxiety and somatic anxiety) of the HAMA, the state and trait scales of the STAI, and the six subscales within BPQ (Table 1). There was no statistically significant difference between GAD and PD subjects on all those scales mentioned above. On the HAMA‐somatic anxiety subscale there was a trend for higher score in PD patients than in GAD, with the relatively small P value 0.084.
FC Alterations in GAD and PD
As can be seen in Figure 1a,b and Table 2, totally 20 abnormal FC were found for GAD, the most significant abnormality in FC is seen between hippocampus/parahippocampus and fusiform gyrus (increased positive connectivity), compared with HC. Besides, disrupted FC also involve connectivities from precentral gyrus to visual areas such as lingual, calcarine and cuneus (decreased connectivity when compared with HC), as well as to inferior parietal (increased connectivity when compared with HC). FC from postcentral gyrus to cuneus and lingual gyrus are decreased and the connectivities from thalamus to posterior cingulate gyrus (PCC)/ACC are also decreased in GAD. Moreover, FC between middle frontal gyrus and angular gyrus is decreased and FC between supramarginal gyrus and precuneus is increased, in GAD compared with HC.
Figure 1.
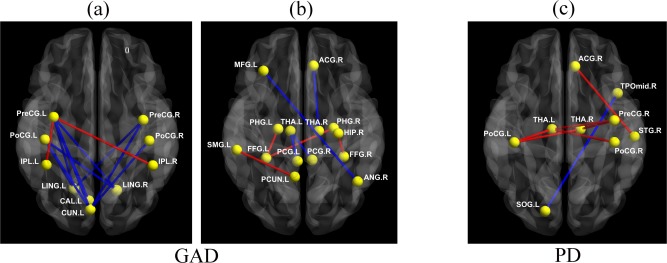
Altered functional connectivity of GAD (a, b) and PD (c). Abnormal FC in GAD were divided into two graphs (a, b) in order to display more clearly. Red line indicates increased FC in patient group with respective to matched controls, and blue line indicates the opposite. (Note that names and abbreviations of brain regions can be referred to Table S1, Supporting Information).
Table 2.
Significantly altered functional connectivity by comparing GAD patients with matched controls (FDR q = 0.1; “*” represent the FC that also survive FDR q = 0.05 correction)
| Functional connectivity | Increased↑ | GAD | Control | P value |
|---|---|---|---|---|
| Decreased↓ | (mean) | (mean) | ||
| *Precentral gyrus (L)—Inferior parietal (R) | ↑ | 0.1 | −0.215 | 0.00009 |
| ParaHippocampal gyrus (L)—Fusiform gyrus (L) | ↑ | 0.422 | 0.171 | 0.00022 |
| *Posterior cingulate gyrus (L)—Thalamus (L) | ↓ | 0.067 | 0.27 | 0.00025 |
| Hippocampus (R)—Fusiform gyrus (R) | ↑ | 0.345 | 0.083 | 0.00028 |
| *Precentral gyrus (L)—Lingual gyrus (L) | ↓ | −0.212 | 0.094 | 0.00028 |
| *ParaHippocampal gyrus (R)—Fusiform gyrus (L) | ↑ | 0.408 | 0.199 | 0.0003 |
| Middle frontal gyrus (L)—Angular gyrus (R) | ↓ | 0.187 | 0.431 | 0.00034 |
| Precentral gyrus (R)—Cuneus (L) | ↓ | −0.15 | 0.128 | 0.00041 |
| *Posterior cingulate gyrus (R)—Thalamus (L) | ↓ | 0.09 | 0.321 | 0.00043 |
| Cuneus (L)—Postcentral gyrus (R) | ↓ | −0.12 | 0.176 | 0.00048 |
| Precentral gyrus (L)—Calcarine cortex (L) | ↓ | −0.222 | 0.07 | 0.00055 |
| SupraMarginal gyrus (L)—Precuneus (L) | ↑ | −0.003 | −0.222 | 0.00056 |
| Precentral gyrus (L)—Lingual gyrus (R) | ↓ | −0.206 | 0.048 | 0.00071 |
| Precentral gyrus (L)—Cuneus (L) | ↓ | −0.266 | −0.013 | 0.00071 |
| Precentral gyrus (L)—Inferior parietal (L) | ↑ | 0.397 | 0.132 | 0.00072 |
| Precentral gyrus (R)—Lingual gyrus (L) | ↓ | −0.044 | 0.219 | 0.00075 |
| Cuneus (L)—Postcentral gyrus (L) | ↓ | −0.16 | 0.148 | 0.00079 |
| Anterior cingulate gyrus (R)—Thalamus (R) | ↓ | 0.092 | 0.357 | 0.00086 |
| Lingual gyrus (R)—Postcentral gyrus (L) | ↓ | −0.137 | 0.16 | 0.00092 |
| Lingual gyrus (L)—Postcentral gyrus (L) | ↓ | −0.112 | 0.2 | 0.00093 |
Upward arrow indicates increased FC in patient group with respective to matched controls, and downward arrow indicates the opposite.
R, right. L, left. Names and abbreviations of brain regions can be referred to Supporting Information Table S1.
For PD, the FC of postcentral gyrus with thalamus are the most significant, which are increased in patients when compared with HC. Moreover, increased FC also involves the right ACC and the superior temporal gyrus, the right precentral gyrus and the thalamus. The FC from the left superior occipital gyrus to the medial temporal lobe is decreased in PD patients (see Fig. 1c and Table 3).
Table 3.
Significantly altered functional connectivity by comparing PD patients with matched controls (FDR q = 0.1; “*” represent the FC that also survive FDR q = 0.05 correction)
| Functional connectivity | Increased↑ | PD | Control | P value | |
|---|---|---|---|---|---|
| Decreased↓ | (mean) | (mean) | |||
| *Postcentral gyrus (R)—Thalamus (L) | ↑ | −0.008 | −0.335 | 0.00007 | |
| *Anterior cingulate gyrus (R)—Temporal pole (superior) (R) | ↑ | 0.269 | −0.056 | 0.00009 | |
| *Superior occipital gyrus (L)—Temporal Pole (middle) (R) | ↓ | −0.309 | −0.009 | 0.0001 | |
| Postcentral gyrus (L)—Thalamus (R) | ↑ | 0.05 | −0.297 | 0.00015 | |
| Precentral gyrus (R)—Thalamus (L) | ↑ | 0.096 | −0.266 | 0.00018 | |
| Postcentral gyrus (L)—Thalamus (L) | ↑ | 0.079 | −0.27 | 0.00025 | |
| Precentral gyrus (R)—Thalamus (R) | ↑ | 0.105 | −0.244 | 0.00033 | |
Validation of Scaled Correlation as a More Powerful FC Metric
We choose the most significantly altered FC in GAD and PD (identified by scaled correlation, see Figs. 2 and 3) for demonstration. First we note that the mean FC obtained from scaled‐correlation does not differ significantly from that obtained from scaled‐correlation by Pearson‐correlation for both control and patient group (including GAD and PD), see Supporting Information Tables S2 and S4. The mean FC obtained from that Pearson‐correlation in fact show high level correlation (Supporting Information Tables S2 and S4). This indicates that scaled‐correlation and Pearson‐correlation bear similar information. However, the variance of the FC distribution from scaled‐correlation is much smaller than that of Pearson‐correlation in both control and patient group, see Supporting Information Tables S3 and S5. According to the definition of t‐value in two sample t‐test in identifying significant changes,
in which and and and are mean and standard deviation of two independent random variables and (i.e., the FC of control and patient group). The t value is proportional to the difference of the mean of and , but is inversely proportional to the sum of variance of and . As is shown in Supporting Information Tables S2–S5, the mean of scaled correlation as FC does not differ significantly with that of Pearson correlation, but the variance of scaled correlation is smaller, therefore the t value of t‐test obtained from scaled correlation is larger than that derived from Pearson correlation, and that the difference between two groups are more significant. This is why scaled correlation has higher statistical power and why we can identify significantly changed FC in GAD/PD group by scaled correlation, but not Pearson correlation.
Figure 2.
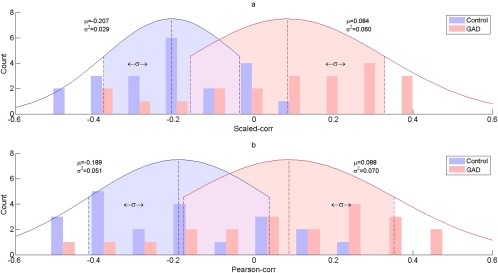
Distribution of scaled correlation (a) and Pearson correlation (b) for control and GAD group (for the first FC in Table II). As can be seen, the distribution of scaled correlation and Pearson correlation has similar mean (for both control and GAD group, with P > 0.05, see also Supporting Information Table S2), while the variance of the former is smaller than that of the latter (see Supporting Information Table S3).
Figure 3.
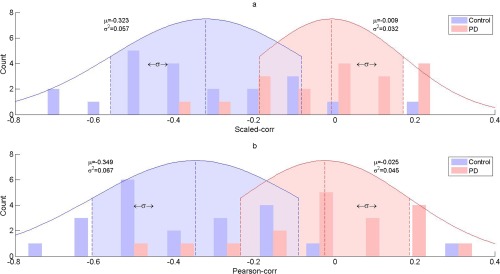
Distribution of scaled correlation (a) and Pearson correlation (b) for control and PD group (for the first FC in Table III). As can be seen, the distribution of scaled correlation and Pearson correlation has similar mean (for both control and PD group, with P > 0.05, see Supporting Information Table S4), while the variance of the former is smaller than that of the latter (see Table S5, Supporting Information).
In fact the top functional connectivities identified from two sample t‐test using Pearson correlation, though not significant (i.e., cannot survive correction), overlapped much with those obtained by scaled correlation, see Table 4. For GAD, the top 20 FC obtained by Pearson correlation has 10 that are overlapped with those from scaled correlation. For PD, the top 7 FC obtained by Pearson correlation are completely overlapped with those from scaled correlation. These results indicate the scaled correlation is a reliable but more powerful FC metric in two sample t‐test.
Table 4.
Top links identified by Pearson correlation for GAD (a, top 20 links) and PD (b, top 7 links) group
| Functional connectivity in GAD | HC (mean) | GAD (mean) | P (Pearson‐corr) |
|---|---|---|---|
| (a) GAD | |||
| *ParaHippocampal gyrus (L)—Fusiform gyrus (L) | 0.1835 | 0.4525 | 0.000016 |
| *Posterior cingulate gyrus (L)—Thalamus (L) | 0.2830 | 0.0839 | 0.000097 |
| Anterior cingulate gyrus (R)—Inferior parietal lobule (R) | 0.1506 | −0.1440 | 0.000126 |
| * Middle frontal gyrus (L)—Angular gyrus (R) | 0.4337 | 0.1795 | 0.000139 |
| * Cuneus (L)—Postcentral gyrus (R) | 0.1576 | −0.1399 | 0.000200 |
| *Anterior cingulate gyrus (R)—Thalamus (R) | 0.3830 | 0.1053 | 0.000207 |
| Anterior cingulate gyrus (L)— Inferior parietal lobule (R) | 0.0252 | −0.2545 | 0.000230 |
| Supplementary motor area (R)—Angular gyrus (L) | −0.3682 | −0.1777 | 0.000291 |
| Precentral gyrus (R)—Middle temporal gyrus (L) | 0.0681 | −0.1899 | 0.000312 |
| * Posterior cingulate gyrus (R)—Thalamus (L) | 0.3334 | 0.1117 | 0.000320 |
| Precentral gyrus (R)—Thalamus (R) | −0.2617 | 0.0170 | 0.000349 |
| *Precentral gyrus (L)— Inferior parietal lobule (R) | −0.1888 | 0.0879 | 0.000391 |
| *Precentral gyrus (R)—Cuneus (L) | 0.1002 | −0.1584 | 0.000432 |
| Superior frontal gyrus (R)—Hippocampus (L) | −0.0095 | −0.2302 | 0.000481 |
| Cuneus (R)—Postcentral gyrus (R) | 0.1955 | −0.0723 | 0.000495 |
| *Precentral gyrus (L)—Lingual gyrus (L) | 0.0750 | −0.1826 | 0.000579 |
| *Precentral gyrus (L)—Cuneus (L) | −0.0425 | −0.2610 | 0.000590 |
| Postcentral gyrus (L)—Thalamus (R) | −0.3219 | −0.0596 | 0.000621 |
| Hippocampus (L)—Fusiform gyrus (L) | 0.1438 | 0.3733 | 0.000652 |
| Supplementary motor area (L)—Calcarine cortex (R) | −0.0451 | −0.2558 | 0.000659 |
| (b) PD | |||
| *Superior occipital gyrus (L)—Temporal pole, middle (R) | −0.0058 | −0.3159 | 0.000020 |
| *Postcentral gyrus (L)—Thalamus (R) | −0.3219 | 0.0193 | 0.000039 |
| *Precentral gyrus (R)—Thalamus (L) | −0.2761 | 0.1013 | 0.000060 |
| *Postcentral gyrus (R)—Thalamus (L) | −0.3490 | −0.0253 | 0.000073 |
| *Postcentral gyrus (L)—Thalamus (L) | −0.2835 | 0.0552 | 0.000103 |
| *Precentral gyrus (R)—Thalamus (R) | −0.2617 | 0.0904 | 0.000154 |
| *Anterior cingulate gyrus (R)—Superior temporal gyrus (R) | −0.0564 | 0.2367 | 0.000208 |
Note that no significant FC changes were identified using Pearson correlation (FDR, q = 0.05) for both GAD and PD, and we listed the FC that changes most significantly in patient group (by their P value). The link marked with “*” are those identified by scaled correlation (see Table 2 for GAD and III for PD). As can be seen, for GAD, 10 of the top 20 links identified by Pearson correlation are found to change significantly using scaled correlation. For PD, the top 7 links by Pearson correlation are all identified by scaled correlation.
Correlation between FC Alteration and Symptom Scores
For GAD, the disrupted connectivity between hippocampus/parahippocampus and fusiform gyrus correlated to BPQ subscores (including subscores for awareness, stress response, autonomic nervous system reactivity and disease history) in positive way, while connectivity between ACC and thalamus correlated negatively with HAMA‐somatic/psychic anxiety and STAI‐trait. For PD, the altered connectivity between post/precentral and thalamus is found to be positively related to STAI‐state and BPQ subscores (subscales for awareness and stress response) (see Tables 5 and 6).
Table 5.
Correlation between altered functional connectivity and symptom scores for GAD patients
| Functional connectivity | Score type | P value | Corr. coef. |
|---|---|---|---|
| ParaHippocampal gyrus (L)—Fusiform gyrus (L) | BPQ‐awareness | 0.0081 | 0.487 |
| ParaHippocampal gyrus (L)—Fusiform gyrus (L) | BPQ‐stress response | 0.0022 | 0.5577 |
| ParaHippocampal gyrus (L)—Fusiform gyrus (L) | BPQ‐ANSR | 0.002 | 0.563 |
| ParaHippocampal gyrus (L)—Fusiform gyrus (L) | BPQ‐diseases history | 0.0103 | 0.472 |
| Hippocampus (R)—Fusiform gyrus (R) | BPQ‐diseases history | 0.0469 | 0.3671 |
| Cuneus (L)—Postcentral gyrus (R) | BPQ‐diseases history | 0.0314 | −0.3971 |
| Cuneus (L)—Postcentral gyrus (R) | STAI‐state | 0.0132 | −0.4536 |
| Precentral gyrus (L)—Calcarine cortex (L) | STAI‐trait | 0.0389 | −0.3792 |
| Anterior cingulate gyrus (R)—Thalamus (R) | STAI‐trait | 0.0093 | −0.6101 |
| Anterior cingulate gyrus (R)—Thalamus (R) | HAMA‐somatic anxiety | 0.0261 | −0.4227 |
| Anterior cingulate gyrus (R)—Thalamus (R) | HAMA‐psychic anxiety | 0.0399 | −0.5024 |
Table 6.
Correlation between altered functional connectivity and symptom scores for PD patients
| Functional connectivity | Score type | P value | Corr. coef. |
|---|---|---|---|
| Postcentral gyrus (L)—Thalamus (R) | STAI‐state | 0.046 | 0.3399 |
| Precentral gyrus (R)—Thalamus (L) | STAI‐state | 0.0366 | 0.4176 |
| Precentral gyrus (R)—Thalamus (R) | STAI‐state | 0.0366 | 0.4176 |
| *Superior occipital gyrus (L)—Temporal Pole (middle) (R) | HAMA‐psychic anxiety | 0.0412 | −0.515 |
| Postcentral gyrus (L)—Thalamus (L) | BPQ‐awareness | 0.076 | 0.3096 |
| Postcentral gyrus (L)—Thalamus (L) | BPQ‐stress response | 0.082 | 0.3076 |
DISCUSSION
To the best of our knowledge, our study is the first whole‐brain resting state FC analysis involving both GAD and PD. This allows the common and disease‐specific functional abnormalities to be systematically investigated. Our results, therefore, are expected to shed new lights onto the distinct neural mechanisms of these two major anxiety disorders. Note that no significant FC changes were identified using Pearson correlation for both GAD and PD group. Therefore, we described the two separate t‐test (GAD vs. HC, PD vs. HC) here.
Differences in Resting FC Between GAD and PD
As is shown in Tables II and III, much of the disrupted FC in GAD and PD shows regional and psychopathological specificity. For GAD, the aberrant connectivity of fusiform gyrus with hippocampus/parahippocampus are among the most significantly changed FC in GAD. Hippocampus and parahippocampus are parts of limbic system that are implicated in memory [Maren and Holt, 2000; Squire, 1992] and emotion [Alvarez et al., 2008; Chen and Etkin, 2013]. The reduction of hippocampus volume has been found in patients with GAD [Abdallah et al., 2013; Hettema et al., 2012; Moon et al., 2014] while the parahippocampus has also been implicated in anxiety disorders [Hattingh et al., 2012; Lorberbaum et al., 2004]. The fusiform gyrus is part of the temporal lobe and occipital lobe that has been suggested to play an important role in face, body, and word recognition [Grill‐Spector and Malach, 2004; Haxby et al., 2000, 2002; Kanwisher et al., 1997; Martin, 2007]. Abnormal activities in the fusiform gyrus in response to emotional faces have been reported in different anxiety disorders [Etkin and Wager, 2007; Gentili et al., 2008; Schultz et al., 2003; Syal et al., 2012]. The above findings of fusiform gyrus suggest that it might be a core brain region in visual and emotion processing in anxiety disorders. Both the visual cortex and hippocampus are involved in the processing of fear generalization [Lissek, 2012; Lissek et al., 2013a; Xu and Sudhof, 2013] which may contribute importantly to the psychopathology of GAD [Lissek et al., 2013b]. This is supported by our observation of the increased hippocampal‐fusiform gyrus FC correlating with BPQ subscores in GAD, since BPQ were used to measure multiple symptom areas [Stromback et al., 2015].
In addition, cuneus‐postcentral gyrus connection and precentral gyrus‐calcarine cortex connection are abnormal in GAD, and correlate with severity of symptoms, see Table 5. The cuneus is associated with contextual self‐descriptions [Chiao et al., 2009]. The postcentral gyrus and precentral gyrus have been associated with interoception processing [Critchley et al., 2004; Northoff, 2013; Pollatos et al., 2007]. Calcarine is reported to be associated with working memory in GAD [Moon and Jeong, 2015]. These abnormal connections may reflect a widely disturbed network in GAD.
Finally, three functional connections are associated with PCC/ACC‐thalamus pathway. PCC is the backmost part of the cingulated cortex and a main component of DMN which is associated with functions including emotional processing and social cognition [Broyd et al., 2009]. The abnormal FC within this area suggests that process relying on the DMN is affected in GAD. ACC is the frontal part of the cingulated cortex and participates in mediating visceromotor activity [Devinsky et al., 1995]. It is a main component of the interoceptive network [Khalsa et al., 2009]. The major role of the thalamus is to relay the information of sensation, motor signals, and spatial sense to the cerebral cortex [Sherman, 2007]. Given our observation that decreased functional connections between ACC and thalamus that was negatively correlated with HAMA in GAD patient therefore may cause some symptoms involve somatic disturbances like rapid heart rate, lower skin conductance, and difficulty breathing [Hoehn‐Saric et al., 2004]. Over‐generalization might be the main abnormal mechanism underlying GAD patients may answer why these patients experience less intense heartbeat perception than patients with PD.
For PD, the connectivity between postcentral gyrus and thalamus account for most of the significant alterations. Postcentral gyrus is known as the somatosensory cortex with function of receiving, integrating and interpreting most of the sensory information of the human body [Northoff, 2013]. Sensory information is carried to the brain by neural pathways to the thalamus, which project to the somatosensory cortex [Nelson and Chen, 2008]. Thalamus plays a crucial role in gating sensory responses [Saalmann and Kastner, 2011]. The increased FC from thalamus to somatosensory cortex found in PD patients therefore may cause abnormally high interoceptive sensitivity and somatosensory stimulus processing, which underlies the typical symptoms of PD, such as the extreme feeling of heartbeat. This finding is consistent with that the somatosensory cortex is the critical substrate for interoceptive awareness[Khalsa et al., 2009] and the thalamus has been shown to be strongly involved in interceptive processing [Cho et al., 2012; Craig, 2003; Craig and Zhang, 2006; Pollatos et al., 2007; Rieck et al., 2004]. Interestingly, thalamus does not only play a role in interoceptive processing, but may also be crucially involved in the fear circuit underlying the pathogenesis of PD [Gorman et al., 2000]. Considerable neuroimaging studies investigating functional and structural brain connectivity in PD show abnormalities in thalamus [Asami et al., 2009; Gorman et al., 2000; Lai and Wu, 2012, 2013; Ohrmann et al., 2010; Wintermann et al., 2013; Zhang et al., 2011]. Phobic patients also have increased activities in the thalamus during panic attacks [Caseras et al., 2010]. Therefore, the thalamus plays an important role in the pathogenesis of PD, possibly via the mediation of interoceptive processes. The postcentral gyrus showed increased resting‐state FC with left dorsal ACC for salience network in PD patients [de Carvalho et al., 2010; Pannekoek et al., 2013], with the finding possibly suggest that the processing of somatosensory information and self‐awareness is disturbed in PD. The postcentral gyrus also showed abnormal activities during a motor activation paradigm in female subjects with PD which suggest that the postcentral gyrus is implicated in the pathological mechanism of PD [Marchand et al., 2009]. In summary, combined with the positive correlation between strengthened connections and the severity of panic anxiety symptoms (Table 6), increased postcentral gyrus‐thalamus connectivity could be an early biomarker in PD patients.
Limitations
Our data have to be interpreted with caution due to several limitations. The main limitation of this study is the small number of sample subjects, which deteriorates the statistical power and makes it susceptible to type II error. However, we have used scaled correlation as FC, which we demonstrate to be a reliable (compared with Pearson correlation) but statistically more powerful metric. We also note that the identified FC in both GAD and PD group demonstrate symmetric patterns. In GAD, significant changes involve connectivity of left cuneus to bilateral pre/postcentral gyrus, bilateral lingual gyrus to bilateral precentral gyrus, and left thalamus to bilateral post‐cingulate. In PD, changes include FC of bilateral thalamus to bilateral postcentral gyrus. These symmetric FC changes add to the reliability of the results, as the probability for two symmetric connectivity to change significantly in a simultaneous manner would be low if they are true negatives. Secondly, the fMRI data was acquired using the parameters: TR = 2 s, slices = 30, bandpass filtering in the range 0.01 < f < 0.08 Hz. Under such condition of the acquisition, cardiac, and respiratory fluctuations may still reduce the specificity of low frequency fluctuations to functional connected regions [Lowe et al., 1998]. Finally, our resting‐state fMRI data were acquired after the acquisition of an anatomical scan and completion of four task‐related fMRI runs. The acquisition protocol could potentially have influence on resting‐state connectivity.
CONCLUSIONS
Taken together, our results suggest disease‐specific FC abnormalities responsible for GAD and PD patients. GAD is more associated with the fusiform gyrus and hippocampus/parahippocampus pathways suggesting the mechanism of fear generalization. PD is rather linked to inteoceptive pathways involving somatosensory cortex and thalamus. Future studies are needed to explore the effect of anti‐anxiety medications on resting state activity and its regionally‐ and psychopathologically specific changes in GAD and PD. This in turn may not only be relevant for diagnosis but also or selection of differential treatment strategies in GAD and PD.
Supporting information
Supporting Information
ACKNOWLEDGMENT
The authors wish to thank the subjects for their participation in the study.
REFERENCES
- Abdallah CG, Coplan JD, Jackowski A, Sato JR, Mao X, Shungu DC, Mathew SJ (2013): A pilot study of hippocampal volume and N‐acetylaspartate (NAA) as response biomarkers in riluzole‐treated patients with GAD. Eur Neuropsychopharmacol 23:276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez RP, Biggs A, Chen G, Pine DS, Grillon C (2008): Contextual fear conditioning in humans: Cortical‐hippocampal and amygdala contributions. J Neurosci 28:6211–6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (2013): Diagnostic and Statistical Manual of Mental Disorders, 5th ed. (DSM‐5). Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Asami T, Hayano F, Nakamura M, Yamasue H, Uehara K, Otsuka T, Roppongi T, Nihashi N, Inoue T, Hirayasu Y (2008): Anterior cingulate cortex volume reduction in patients with panic disorder. Psychiatry Clin Neurosci 62:322–330. [DOI] [PubMed] [Google Scholar]
- Asami T, Yamasue H, Hayano F, Nakamura M, Uehara K, Otsuka T, Roppongi T, Nihashi N, Inoue T, Hirayasu Y (2009): Sexually dimorphic gray matter volume reduction in patients with panic disorder. Psychiatry Res 173:128–134. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS (1995): Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magn Reson Med 34:537–541. [DOI] [PubMed] [Google Scholar]
- Blair KS, Geraci M, Smith BW, Hollon N, DeVido J, Otero M, Blair JR, Pine DS (2012): Reduced dorsal anterior cingulate cortical activity during emotional regulation and top‐down attentional control in generalized social phobia, generalized anxiety disorder, and comorbid generalized social phobia/generalized anxiety disorder. Biol Psychiatry 72:476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkovec TD, Shadick RN, Hopkins M (1991): The nature of normal and pathological worry In: Rapee RM, Barlow DH, editors. Chronic Anxiety: Generalized Anxiety Disorder and Mixed Anxiety‐Depression. New York, NY: Guilford Press; pp 29–51. [Google Scholar]
- Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga‐Barke EJ (2009): Default‐mode brain dysfunction in mental disorders: A systematic review. Neurosci Biobehav Rev 33:279–296. [DOI] [PubMed] [Google Scholar]
- Bystritsky A, Pontillo D, Powers M, Sabb FW, Craske MG, Bookheimer SY (2001): Functional MRI changes during panic anticipation and imagery exposure. Neuroreport 12:3953–3957. [DOI] [PubMed] [Google Scholar]
- Caseras X, Giampietro V, Lamas A, Brammer M, Vilarroya O, Carmona S, Rovira M, Torrubia R, Mataix‐Cols D (2010): The functional neuroanatomy of blood‐injection‐injury phobia: A comparison with spider phobics and healthy controls. Psychol Med 40:125–134. [DOI] [PubMed] [Google Scholar]
- Cha J, Carlson JM, Dedora DJ, Greenberg T, Proudfit GH, Mujica‐Parodi LR (2014a): Hyper‐reactive human ventral tegmental area and aberrant mesocorticolimbic connectivity in overgeneralization of fear in generalized anxiety disorder. J Neurosci 34:5855–5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha J, Greenberg T, Carlson JM, Dedora DJ, Hajcak G, Mujica‐Parodi LR (2014b): Circuit‐wide structural and functional measures predict ventromedial prefrontal cortex fear generalization: Implications for generalized anxiety disorder. J Neurosci 34:4043–4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AC, Etkin A (2013): Hippocampal network connectivity and activation differentiates post‐traumatic stress disorder from generalized anxiety disorder. Neuropsychopharmacology 38:1889–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Duan M, Xie Q, Lai Y, Dong L, Cao W, Yao D, Luo C (2015): Functional disconnection between the visual cortex and the sensorimotor cortex suggests a potential mechanism for self‐disorder in schizophrenia. Schizophr Res 166:151–157. [DOI] [PubMed] [Google Scholar]
- Chiao JY, Harada T, Komeda H, Li Z, Mano Y, Saito D, Parrish TB, Sadato N, Iidaka T (2009): Neural basis of individualistic and collectivistic views of self. Hum Brain Mapp 30:2813–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, YT , Fromm, S , Guyer, AE , Detloff, A , Pine, DS , Fudge, JL , Ernst, M (2012): Nucleus accumbens, thalamus and insula connectivity during incentive anticipation in typical adults and adolescents. NeuroImage 66C:508–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD (2003): Interoception: The sense of the physiological condition of the body. Curr Opin Neurobiol 13:500–505. [DOI] [PubMed] [Google Scholar]
- Craig AD, Zhang ET (2006): Retrograde analyses of spinothalamic projections in the macaque monkey: Input to posterolateral thalamus. J Comp Neurol 499:953–964. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ (2004): Neural systems supporting interoceptive awareness. Nat Neurosci 7:189–195. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Shifflett H, Iyengar S, Dahl RE, Axelson DA, Birmaher B, Hall J, Moritz G, Ryan ND (2002): Superior temporal gyrus volumes in pediatric generalized anxiety disorder. Biol Psychiatry 51:553–562. [DOI] [PubMed] [Google Scholar]
- de Carvalho MR, Dias GP, Cosci F, de‐Melo‐Neto VL, Bevilaqua MC, Gardino PF, Nardi AE (2010): Current findings of fMRI in panic disorder: Contributions for the fear neurocircuitry and CBT effects. Expert Rev Neurother 10:291–303. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA (1995): Contributions of anterior cingulate cortex to behaviour. Brain 118:279–306. [DOI] [PubMed] [Google Scholar]
- Domschke K, Stevens S, Pfleiderer B, Gerlach AL (2010): Interoceptive sensitivity in anxiety and anxiety disorders: An overview and integration of neurobiological findings. Clin Psychol Rev 30:1–11. [DOI] [PubMed] [Google Scholar]
- Duncan NW, Wiebking C, Northoff G (2014): Associations of regional GABA and glutamate with intrinsic and extrinsic neural activity in humans A review of multimodal imaging studies. Neurosci Biobehav Rev 47:36–52. [DOI] [PubMed] [Google Scholar]
- Ehlers A, Breuer P (1992): Increased cardiac awareness in panic disorder. J Abnorm Psychol 101:371–382. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD (2007): Functional neuroimaging of anxiety: A meta‐analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 164:1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Schatzberg AF (2011): Common abnormalities and disorder‐specific compensation during implicit regulation of emotional processing in generalized anxiety and major depressive disorders. Am J Psychiatry 168:968–978. [DOI] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD (2009): Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry 66:1361–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Hoeft F, Menon V, Schatzberg AF (2010): Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. Am J Psychiatry 167:545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleet RP, Dupuis G, Marchand A, Burelle D, Arsenault A, Beitman BD (1996): Panic disorder in emergency department chest pain patients: Prevalence, comorbidity, suicidal ideation, and physician recognition. Am J Med 101:371–380. [DOI] [PubMed] [Google Scholar]
- Folias SE, Yu S, Snyder A, Nikolic D, Rubin JE (2013): Synchronisation hubs in the visual cortex may arise from strong rhythmic inhibition during gamma oscillations. Eur J Neurosci 38:2864–2883. [DOI] [PubMed] [Google Scholar]
- Fontaine R, Breton G, Dery R, Fontaine S, Elie R (1990): Temporal lobe abnormalities in panic disorder: An MRI study. Biol Psychiatry 27:304–310. [DOI] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME (2009): The global signal and observed anticorrelated resting state brain networks. J Neurophysiol 101:3270–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman BH, Thayer JF (1998): Autonomic balance revisited: Panic anxiety and heart rate variability. J Psychosom Res 44:133–151. [DOI] [PubMed] [Google Scholar]
- Gentili C, Gobbini MI, Ricciardi E, Vanello N, Pietrini P, Haxby JV, Guazzelli M (2008): Differential modulation of neural activity throughout the distributed neural system for face perception in patients with Social Phobia and healthy subjects. Brain Res Bull 77:286–292. [DOI] [PubMed] [Google Scholar]
- Gorman JM, Kent JM, Sullivan GM, Coplan JD (2000): Neuroanatomical hypothesis of panic disorder, revised. Am J Psychiatry 157:493–505. [DOI] [PubMed] [Google Scholar]
- Greenberg T, Carlson JM, Cha J, Hajcak G, Mujica‐Parodi LR (2013): Ventromedial prefrontal cortex reactivity is altered in generalized anxiety disorder during fear generalization. Depress Anxiety 30:242–250. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V (2003): Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A 100:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill‐Spector K, Malach R (2004): The human visual cortex. Annu Rev Neurosci 27:649–677. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Choate VR, Detloff A, Benson B, Nelson EE, Perez‐Edgar K, Fox NA, Pine DS, Ernst M (2012): Striatal functional alteration during incentive anticipation in pediatric anxiety disorders. Am J Psychiatry 169:205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M (1959): The assessment of anxiety states by rating. Br J Med Psychol 32:50–55. [DOI] [PubMed] [Google Scholar]
- Hamilton M (1960): A rating scale for depression. J Neurol Neurosurg Psychiatry 23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han DH, Renshaw PF, Dager SR, Chung A, Hwang J, Daniels MA, Lee YS, Lyoo IK (2008): Altered cingulate white matter connectivity in panic disorder patients. J Psychiatric Res 42:399–407. [DOI] [PubMed] [Google Scholar]
- Hattingh, CJ , Ipser, J , Tromp, SA , Syal, S , Lochner, C , Brooks, SJ , Stein, DJ (2012): Functional magnetic resonance imaging during emotion recognition in social anxiety disorder: An activation likelihood meta‐analysis. Front Human Neurosci 6:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI (2000): The distributed human neural system for face perception. Trends Cogn Sci 4:223–233. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI (2002): Human neural systems for face recognition and social communication. Biol Psychiatry 51:59–67. [DOI] [PubMed] [Google Scholar]
- Hayano F, Nakamura M, Asami T, Uehara K, Yoshida T, Roppongi T, Otsuka T, Inoue T, Hirayasu Y (2009): Smaller amygdala is associated with anxiety in patients with panic disorder. Psychiatry Clin Neurosci 63:266–276. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Kettenmann B, Ahluwalia V, McCarthy C, Kates WR, Schmitt JE, Silberg JL, Neale MC, Kendler KS, Fatouros P (2012): Pilot multimodal twin imaging study of generalized anxiety disorder. Depress Anxiety 29:202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn‐Saric R, McLeod DR, Funderburk F, Kowalski P (2004): Somatic symptoms and physiologic responses in generalized anxiety disorder and panic disorder: An ambulatory monitor study. Arch Gen Psychiatry 61:913–921. [DOI] [PubMed] [Google Scholar]
- Kalk NJ, Melichar J, Holmes RB, Taylor LG, Daglish MR, Hood S, Edwards T, Lennox‐Smith A, Lingford‐Hughes AR, Nutt DJ (2012): Central noradrenergic responsiveness to a clonidine challenge in generalized anxiety disorder: A Single photon emission computed tomography study. J Psychopharmacol (Oxford, England) 26:452–460. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM (1997): The fusiform face area: A module in human extrastriate cortex specialized for face perception. J Neurosci 17:4302–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS (1994): Lifetime and 12‐month prevalence of DSM‐III‐R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry 51:8–19. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE (2005a): Prevalence, severity, and comorbidity of 12‐month DSM‐IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry 62:617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Demler O, Frank RG, Olfson M, Pincus HA, Walters EE, Wang P, Wells KB, Zaslavsky AM (2005b): Prevalence and treatment of mental disorders, 1990 to 2003. N Engl J Med 352:2515–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa SS, Rudrauf D, Feinstein JS, Tranel D (2009): The pathways of interoceptive awareness. Nat Neurosci 12:1494–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CH (2011): Gray matter deficits in panic disorder: A pilot study of meta‐analysis. J Clin Psychopharmacol 31:287–293. [DOI] [PubMed] [Google Scholar]
- Lai CH, Wu YT (2012): Patterns of fractional amplitude of low‐frequency oscillations in occipito‐striato‐thalamic regions of first‐episode drug‐naive panic disorder. J Affect Disord 142:180–185. [DOI] [PubMed] [Google Scholar]
- Lai CH, Wu YT (2013): Changes in regional homogeneity of parieto‐temporal regions in panic disorder patients who achieved remission with antidepressant treatment. J Affect Disord 151:709–714. [DOI] [PubMed] [Google Scholar]
- Lissek S (2012): Toward an account of clinical anxiety predicated on basic, neurally mapped mechanisms of Pavlovian fear‐learning: the case for conditioned overgeneralization. Depress Anxiety 29:257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek, S , Bradford, DE , Alvarez, RP , Burton, P , Espensen‐Sturges, T , Reynolds, RC , Grillon, C (2013a): Neural substrates of classically conditioned fear‐generalization in humans: A parametric fMRI study. Social Cogn Affect Neurosci 9:1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek, S , Kaczkurkin, AN , Rabin, S , Geraci, M , Pine, DS , Grillon, C (2013b): Generalized anxiety disorder is associated with overgeneralization of classically conditioned fear. Biol Psychiatry 75:909–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Kaczkurkin AN, Rabin S, Geraci M, Pine DS, Grillon C (2014): Generalized anxiety disorder is associated with overgeneralization of classically conditioned fear. Biol Psychiatry 75:909–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WJ, Yin DZ, Cheng WH, Fan MX, You MN, Men WW, Zang LL, Shi DH, Zhang F (2015): Abnormal functional connectivity of the amygdala‐based network in resting‐state FMRI in adolescents with generalized anxiety disorder. Medical Science Monitor. Int Med J Exp Clin Res 21:459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorberbaum JP, Kose S, Johnson MR, Arana GW, Sullivan LK, Hamner MB, Ballenger JC, Lydiard RB, Brodrick PS, Bohning DE, George MS (2004): Neural correlates of speech anticipatory anxiety in generalized social phobia. Neuroreport 15:2701–2705. [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, Sorenson JA (1998): Functional connectivity in single and multislice echoplanar imaging using resting‐state fluctuations. NeuroImage 7:119–132. [DOI] [PubMed] [Google Scholar]
- Luo C, Qiu C, Guo Z, Fang J, Li Q, Lei X, Xia Y, Lai Y, Gong Q, Zhou D, Yao D (2011): Disrupted functional brain connectivity in partial epilepsy: A resting‐state fMRI study. PloS One 7:e28196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand WR, Lee JN, Healy L, Thatcher JW, Rashkin E, Starr J, Hsu E (2009): An fMRI motor activation paradigm demonstrates abnormalities of putamen activation in females with panic disorder. J Affect Disord 116:121–125. [DOI] [PubMed] [Google Scholar]
- Maren S, Holt W (2000): The hippocampus and contextual memory retrieval in Pavlovian conditioning. Behav Brain Res 110:97–108. [DOI] [PubMed] [Google Scholar]
- Martin A (2007): The representation of object concepts in the brain. Annu Rev Psychol 58:25–45. [DOI] [PubMed] [Google Scholar]
- Massana G, Serra‐Grabulosa JM, Salgado‐Pineda P, Gasto C, Junque C, Massana J, Mercader JM (2003a): Parahippocampal gray matter density in panic disorder: A voxel‐based morphometric study. Am J Psychiatry 160:566–568. [DOI] [PubMed] [Google Scholar]
- Massana G, Serra‐Grabulosa JM, Salgado‐Pineda P, Gasto C, Junque C, Massana J, Mercader JM, Gomez B, Tobena A, Salamero M (2003b): Amygdalar atrophy in panic disorder patients detected by volumetric magnetic resonance imaging. NeuroImage 19:80–90. [DOI] [PubMed] [Google Scholar]
- McClure EB, Monk CS, Nelson EE, Parrish JM, Adler A, Blair RJ, Fromm S, Charney DS, Leibenluft E, Ernst M, Pine DS (2007): Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Arch Gen Psychiatry 64:97–106. [DOI] [PubMed] [Google Scholar]
- Mineka S, Zinbarg R (2006): A contemporary learning theory perspective on the etiology of anxiety disorders: It's not what you thought it was. Am Psychol 61:10–26. [DOI] [PubMed] [Google Scholar]
- Moon CM, Jeong GW (2015): Functional neuroanatomy on the working memory under emotional distraction in patients with generalized anxiety disorder. Psychiatry Clin Neurosci 69:609–619. [DOI] [PubMed] [Google Scholar]
- Moon CM, Kim GW, Jeong GW (2014): Whole‐brain gray matter volume abnormalities in patients with generalized anxiety disorder: Voxel‐based morphometry. Neuroreport 25:184–189. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA (2009): The impact of global signal regression on resting state correlations: Are anti‐correlated networks introduced? NeuroImage 44:893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AJ, Chen R (2008): Digit somatotopy within cortical areas of the postcentral gyrus in humans. Cerebral Cortex (New York, N.Y.: 1991) 18:2341–2351. [DOI] [PubMed] [Google Scholar]
- Nikolic D, Muresan RC, Feng W, Singer W (2012): Scaled correlation analysis: A better way to compute a cross‐correlogram. Eur J Neurosci 35:742–762. [DOI] [PubMed] [Google Scholar]
- Northoff G. 2013. Unlocking the Brain: Coding Volume 1. New York: Oxford University Press. [Google Scholar]
- Ohrmann P, Pedersen A, Braun M, Bauer J, Kugel H, Kersting A, Domschke K, Deckert J, Suslow T (2010): Effect of gender on processing threat‐related stimuli in patients with panic disorder: Sex does matter. Depress Anxiety 27:1034–1043. [DOI] [PubMed] [Google Scholar]
- Pannekoek JN, Veer IM, van Tol MJ, van der Werff SJ, Demenescu LR, Aleman A, Veltman DJ, Zitman FG, Rombouts SA, van der Wee NJ (2013): Aberrant limbic and salience network resting‐state functional connectivity in panic disorder without comorbidity. J Affect Disord 145:29–35. [DOI] [PubMed] [Google Scholar]
- Pollatos O, Schandry R, Auer DP, Kaufmann C (2007): Brain structures mediating cardiovascular arousal and interoceptive awareness. Brain Res 1141:178–187. [DOI] [PubMed] [Google Scholar]
- Porges S. (1993): Body Perception Questionnaire. Laboratory of Developmental Assessment. Maryland: University of Maryland. [Google Scholar]
- Qiang H, LanLan Z, Hui L, YuMei W, Ting L, JiJun W, ChunBo L (2012): Heartbeat perception levels and related factors in patients with anxiety disorder. Chin Ment Health J 27:180–185. [Google Scholar]
- Rieck RW, Ansari MS, Whetsell WO, Jr. , Deutch AY, Kessler RM (2004): Distribution of dopamine D2‐like receptors in the human thalamus: Autoradiographic and PET studies. Neuropsychopharmacology 29:362–372. [DOI] [PubMed] [Google Scholar]
- Rodriguez BF, Bruce SE, Pagano ME, Keller MB (2005): Relationships among psychosocial functioning, diagnostic comorbidity, and the recurrence of generalized anxiety disorder, panic disorder, and major depression. J Anxiety Disord 19:752–766. [DOI] [PubMed] [Google Scholar]
- Roy AK, Fudge JL, Kelly C, Perry JS, Daniele T, Carlisi C, Benson B, Castellanos FX, Milham MP, Pine DS, Ernst M (2013): Intrinsic functional connectivity of amygdala‐based networks in adolescent generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry 52:290–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, Cox RW (2012): Trouble at rest: How correlation patterns and group differences become distorted after global signal regression. Brain Connect 2:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalmann YB, Kastner S (2011): Cognitive and perceptual functions of the visual thalamus. Neuron 71:209–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz RT, Grelotti DJ, Klin A, Kleinman J, Van der Gaag C, Marois R, Skudlarski P (2003): The role of the fusiform face area in social cognition: Implications for the pathobiology of autism. Philos Trans R Soc London Ser B Biol Sci 358:415–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM (2007): The thalamus is more than just a relay. Curr Opin Neurobiol 17:417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin YW, Dzemidzic M, Jo HJ, Long Z, Medlock C, Dydak U, Goddard AW (2013): Increased resting‐state functional connectivity between the anterior cingulate cortex and the precuneus in panic disorder: Resting‐state connectivity in panic disorder. J Affect Disord 150:1091–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si T, Shu L, Dang W, Su Y, Chen J, Dong W, Kong Q, Zhang W (2009): Evaluation of the reliability and validity of chinese version of the Mini. International Neuropsychiatric Interview in patients with mental disorders. Chin Ment Health J 23:493–497. [Google Scholar]
- Sobanski T, Wagner G, Peikert G, Gruhn U, Schluttig K, Sauer H, Schlosser R (2010): Temporal and right frontal lobe alterations in panic disorder: A quantitative volumetric and voxel‐based morphometric MRI study. Psychol Med 40:1879–1886. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE (1970): The State‐Trait Anxiety Inventory. Palo Alto, Calif: Consulting Psychologists Press Inc. [Google Scholar]
- Squire LR (1992): Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychol Rev 99:195–231. [DOI] [PubMed] [Google Scholar]
- Stein MB, Asmundson GJ (1994): Autonomic function in panic disorder: Cardiorespiratory and plasma catecholamine responsivity to multiple challenges of the autonomic nervous system. Biol Psychiatry 36:548–558. [DOI] [PubMed] [Google Scholar]
- Strawn JR, Bitter SM, Weber WA, Chu WJ, Whitsel RM, Adler C, Cerullo MA, Eliassen J, Strakowski SM, DelBello MP (2012): Neurocircuitry of generalized anxiety disorder in adolescents: A pilot functional neuroimaging and functional connectivity study. Depress Anxiety 29:939–947. [DOI] [PubMed] [Google Scholar]
- Strawn JR, Wehry AM, Chu WJ, Adler CM, Eliassen JC, Cerullo MA, Strakowski SM, Delbello MP (2013): Neuroanatomic abnormalities in adolescents with generalized anxiety disorder: A voxel‐based morphometry study. Depress Anxiety 30:842–848. [DOI] [PubMed] [Google Scholar]
- Stromback M, Wiklund M, Renberg ES, Malmgren‐Olsson EB (2015): Complex symptomatology among young women who present with stress‐related problems. Scand J Caring Sci 29:234–247. [DOI] [PubMed] [Google Scholar]
- Syal S, Hattingh CJ, Fouche JP, Spottiswoode B, Carey PD, Lochner C, Stein DJ (2012): Grey matter abnormalities in social anxiety disorder: A pilot study. Metab Brain Dis 27:299–309. [DOI] [PubMed] [Google Scholar]
- Tao H, Guo S, Ge T, Kendrick KM, Xue Z, Liu Z, Feng J (2013): Depression uncouples brain hate circuit. Mol Psychiatry 18:101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida RR, Del‐Ben CM, Santos AC, Araujo D, Crippa JA, Guimaraes FS, Graeff FG (2003): Decreased left temporal lobe volume of panic patients measured by magnetic resonance imaging. Braz J Med Biol Res 36:925–929. [DOI] [PubMed] [Google Scholar]
- Uchida RR, Del‐Ben CM, Busatto GF, Duran FL, Guimaraes FS, Crippa JA, Araujo D, Santos AC, Graeff FG (2008): Regional gray matter abnormalities in panic disorder: A voxel‐based morphometry study. Psychiatry Res 163:21–29. [DOI] [PubMed] [Google Scholar]
- Van der Does AJ, Van Dyck R, Spinhoven P (1997): Accurate heartbeat perception in panic disorder: Fact and artefact. J Affect Disord 43:121–130. [DOI] [PubMed] [Google Scholar]
- Willem Van der Does AJ, Antony MM, Ehlers A, Barsky AJ (2000): Heartbeat perception in panic disorder: A reanalysis. Behav Res Ther 38:47–62. [DOI] [PubMed] [Google Scholar]
- Wintermann GB, Donix M, Joraschky P, Gerber J, Petrowski K (2013): Altered olfactory processing of stress‐related body odors and artificial odors in patients with panic disorder. PloS One 8:e74655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen HU, Fehm L (2001): Epidemiology, patterns of comorbidity, and associated disabilities of social phobia. Psychiatr Clin N Am 24:617–641. [DOI] [PubMed] [Google Scholar]
- Wittmann A, Schlagenhauf F, John T, Guhn A, Rehbein H, Siegmund A, Stoy M, Held D, Schulz I, Fehm L, Fydrich T, Heinz A, Bruhn H, Strohle A (2011): A new paradigm (Westphal‐Paradigm) to study the neural correlates of panic disorder with agoraphobia. Eur Arch Psychiatry Clin Neurosci 261:185–194. [DOI] [PubMed] [Google Scholar]
- Xu W, Sudhof TC (2013): A neural circuit for memory specificity and generalization. Science 339:1290–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan CG, Cheung B, Kelly C, Colcombe S, Craddock RC, Di Martino A, Li Q, Zuo XN, Castellanos FX, Milham MP (2013): A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. NeuroImage 76:183–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang GJ, Murray JD, Repovs G, Cole MW, Savic A, Glasser MF, Pittenger C, Krystal JH, Wang XJ, Pearlson GD, Glahn DC, Anticevic A (2014): Altered global brain signal in schizophrenia. Proc Natl Acad Sci U S A 111:7438–7443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Hazlett RL, Stark CE, Hoehn‐Saric R (2012): Functional MRI of the amygdala and bed nucleus of the stria terminalis during conditions of uncertainty in generalized anxiety disorder. J Psychiatr Res 46:1045–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Duan L, Liao M, Yang F, Liu J, Shan B, Li L (2011): MRI for brain structure and function in patients with first‐episode panic disorder. Zhong Nan Da Xue Xue Bao Yi Xue Ban 36:1170–1175. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li L, Yu R, Liu J, Tang J, Tan L, Liao M, Yang F, Shan B (2013): White matter integrity alterations in first episode, treatment‐naive generalized anxiety disorder. J Affect Disord 148:196–201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


