Abstract
Objectives
To validate the functional relevance of resting state networks (RSNs) by means of a comparison of resting state connectivity (RSC) between language regions elicited by direct cortical stimulation versus RSC between random regions; and to evaluate the accuracy of resting state fMRI in surgical planning by assessing the overlap between RSNs and intraoperative functional mapping results.
Methods
Sensorimotor and language eloquent sites were identified by direct electrical cortical stimulation in 98 patients with a diffuse low‐grade glioma. A seed to voxel analysis with inter‐language stimulation point connectivity versus inter‐random ROIs connectivity was performed (19 patients). An independant component analysis (ICA) was also applied to rsfMRI data. Language and sensorimotor components were selected over 20 independent components and compared to the corresponding stimulation points and resected cortex masks (31 and 90 patients, respectively).
Results
Mean connectivity value between language seeds was significantly higher than the one between random seeds (0.68 ± 0.39 and 0.12 ± 0.21 respectively, P < 10−10). 96 ± 11% of sensorimotor stimulation points were located within 10 mm from sensorimotor ICA maps versus 92 ± 21% for language. 3.1 and 15% of resected cortex overlapped sensorimotor and language networks, respectively. Mean sensorimotor stimulation points and resected cortex z‐scores were 2.0 ± 1.2 and −0.050 ± 0.60, respectively (P < 10−10). Mean language stimulation points and resected cortex z‐scores were 1.6 ± 1.9 and 0.68 ± 0.91, respectively, P < 0.005.
Conclusion
The significantly higher RSC between language seeds than between random seeds validated the functional relevance of RSC. ICA partly succeeded to distinguish eloquent versus surgically removable areas and may be possibly used as a complementary tool to intraoperative mapping. Hum Brain Mapp 37:3721–3732, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: functional magnetic resonance imaging, resting‐state fMRI, brain mapping, sensorimotor cortex, language, glioma, neurosurgery
INTRODUCTION
Task‐based fMRI is the leading technique used to map eloquent cortical sites before surgery [Bizzi et al., 2008; FitzGerald et al., 1997; Roux et al., 2003; Schneider et al., 2016]. Its accuracy compared to direct electrical cortical stimulations (DCS) is reported in some studies to be limited [Giussani et al., 2010], especially for language‐related functions [Kuchcinski et al., 2015], whereas other studies exhibited high sensitivity and specificity values of task‐based fMRI compared to DCS [Bizzi et al., 2008] and outlined its positive influence on presurgical decision making toward more aggressive approaches [Petrella et al., 2006]. rsfMRI is based on detection of low‐frequency fluctuations of BOLD signal (<0.1 Hz) at rest [Fransson, 2005] allowing neuroscientists to map the whole brain functional connectivity using a single scanning session, even on patients unable to perform tasks or presenting with altered states of consciousness [Boly et al., 2008]. rsfMRI is also a powerful tool to longitudinally assess neuroplasticity [Guerra‐Carrillo et al., 2014; Zhou et al., 2014], and to bring, consequently, valuable data on cortical functional reorganization after neurological insult.
Diffuse low‐grade glioma (LGG) is a slow growing neurological tumor (4–5 mm/year) that invades eloquent brain structures and induces important brain plasticity [Bonnetblanc et al., 2006; Desmurget et al., 2006; Ius et al., 2011]. Awake surgery with intraoperative cortical and subcortical mapping via direct electrostimulation is considered as the first‐line treatment option [Duffau, 2015]. Thanks to that surgical technique, both the extent of resection and the postoperative functional outcomes have substantially improved, leading to a prolonged survival and a better quality of life [De Witt Hamer et al., 2012].
RSNs are considered to reflect intrinsic functional networks [Biswal et al., 2010; Fox and Raichle, 2007; Smith et al., 2009], but the role of brain areas belonging to those networks identified at rest and their actual functional participation remains equivocal. Indeed, that assumption essentially results from the observation of similarities between those resting state networks (RSN) and cortical networks activated during task‐based fMRI [Biswal et al., 1995]. Thus comparison of rsfMRI and DCS could help to clarify the issue of the clinical relevance of RSNs. Moreover, previous studies outlined the challenge to map language function using rsfMRI [Mitchell et al., 2013; Tie et al., 2014]. A first step toward a better comprehension of temporal correlations of BOLD signal fluctuations would be to explore resting state connectivity (RSC) between reliably determined cortical areas involved in language processing to find evidences for an organized language network at rest.
Practical and theoretical attractive aspects of rsfMRI have resulted in several recent researches on its potential applications in surgical planning. Various approaches, either data driven [Mitchell et al., 2013], parcellation [Fox et al., 2016; Wang et al., 2015], or seed‐based approaches [Qiu et al., 2014; Zhang et al., 2009], have been used to map sensorimotor RSN and compare it to results from intraoperative stimulation mappings, but all of them used small and heterogeneous data sets.
Seed‐based approach is commonly used in rsfMRI [Biswal et al., 1995] to identify brain regions that are functionally connected. The seed identification is based on predetermined anatomical landmarks or on activated regions obtained during a separate task. However, anatomical boundaries may be difficult to determine, and the accuracy of task‐based fMRI to identify eloquent cortical areas is somewhat questionable [Giussani et al., 2010]. Seed‐based approach was used in diverse studies to compare motor cortex mapping using rsfMRI with a seed determined on anatomical landmarks versus intraoperative functional mapping [Qiu et al., 2014; Zhang et al., 2009]. This approach seems reasonable because of the low variability of the functional architecture of unimodal cortex across individuals [Mueller et al., 2013]. Hence, the use of seed to voxel approach is acceptable for motor cortex mapping but remains questionable for language mapping, because it relies on functionally variable heteromodal cortex.
Assessing rsfMRI accuracy in preoperative planning of critical cortical areas is mandatory before considering its use in daily clinical practice as a complementary tool. Sensorimotor and language networks are the most studied RSNs for surgical mapping because they are the most frequently assessed networks during awake surgery. They also represent different models to understand the relation between RSNs and functional networks [Langs et al., 2015; Mueller et al., 2013]. The necessity to use a data‐driven approach in clinical practice is constrained by the fact that canonical regions of interest are not reliable at the individual level [Vigneau et al., 2006], even more in a tumoral context because of the high tumor‐induced cortical plasticity [Duffau, 2005]. Independent component (IC) analysis is a data‐driven technique. It decomposes a two‐dimensional (time × voxels) data matrix into a set of time‐courses and associated spatial maps, which jointly describe the temporal and spatial characteristics of underlying hidden signals (components) [Beckmann et al., 2005]. Some studies successfully used IC analysis to compare rsfMRI cortical mapping with task‐based fMRI and/or direct electrical cortical mapping [Branco et al., 2016; Rosazza et al., 2014; Tie et al., 2014].
In this context, the aim of our study is (i) to validate the functional relevance of RSC, by comparing RSC between responsive areas for language versus RSC between random regions (distinct networks) and (ii) to evaluate the accuracy of resting state fMRI in surgical planning of sensorimotor and language networks using an IC analysis. More specifically we assess the overlap between responsive cortical sites and resected cortex with the corresponding sensorimotor and language RSNs.
METHOD
Patients
All patients were recruited from Montpellier University Medical Hospital from March 2012 to January 2015. Inclusion criteria were set as follows: an age superior or equal to 18, the completion of a preoperative rsfMRI and a neurosurgery performed under local anaesthesia with DCS. Only patients with a diffuse LGG as demonstrated by postoperative neuropathological examination were included in this study. Patients with missing clinical data (detailed description of clinical symptoms induced by DCS) or incomplete preoperative MR sequences (slices missing) were excluded at the outset. A total of 106 patients met the inclusion criteria. Among them, 8 patients were excluded because of incomplete or non‐exploitable preoperative MR. Hence, the data from 98 patients (55 males and 43 females) were consequently analyzed in our retrospective study. Nineteen patients were enrolled in the first part of the study (seed to voxel analysis using language seeds). Thirty‐one and ninety patients were included for language and sensorimotor networks comparison between RSNs and DCS mapping, respectively (see Fig. 1 for a complete description of patient assignment in the different analyses).
Figure 1.
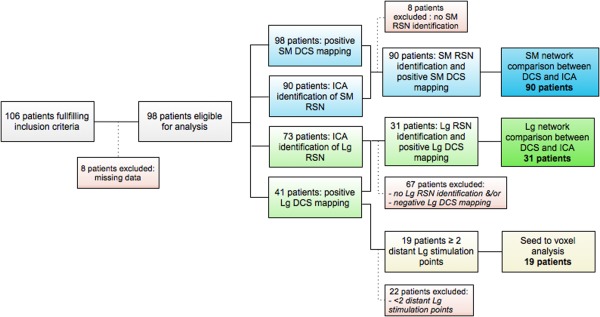
Flow chart of patients assignment to the different analyses. DCS = Direct Cortical Stimulation; ICA = Independent Component Analysis; Lg = Language; RSN = Resting State Network; SM = SensoriMotor. [Color figure can be viewed at http://wileyonlinelibrary.com.]
Standard Protocol Approvals, Registrations, and Patient Consents
The local institutional ethics review board approved the study. A written informed consent was obtained from all patients participating in the study.
MRI Data Acquisition
Structural and rsfMRI scans were performed within 48 h prior to surgery. Acquisitions were performed on 36 and 62 subjects on a 1.5T or 3T MR scanner, respectively, equipped with a 32‐channel receive‐only head coil (Avanto, Skyra Siemens Medical Systems, Erlangen, Germany). A field map was acquired with a gradient echo ‐ echo planar imaging (GE‐EPI) sequence for corrections of magnetic field distortions. Whole brain resting‐state fMRI data were acquired using a T2*‐weighted GE‐EPI sequence. Structural images were acquired for registration purposes using a 3D Magnetization‐Prepared, Rapid Acquisition Gradient Echo (MP‐RAGE) sequence. Parameters for the field map, rsfMRI, and 3D MP‐RAGE sequences acquisition are listed below.
Field Maps Acquisition
1.5T MR scanner: GE‐EPI sequence. Time repetition (TR) = 487 ms, time echo (TE)1 = 5.3 ms, TE2 = 10 ms, voxel size: 3.75 × 3.75 × 3 mm3, flip angle 60°.
3T MR scanner: GE‐EPI sequence. TR = 413 ms, TE1 = 4.9 ms, TE2 = 7.4 ms, voxel size: 3.44 × 3.44 × 3 mm3, flip angle 60°.
Resting State fMRI Acquisition
1.5T MR scanner: GE‐EPI sequence. TR = 2,320 ms, TE = 50 ms, 200 volumes, voxel size = 3 × 3 × 5.5 mm3, 28 interleaved slices, flip angle 90°, acquisition duration 8.07 min.
3T MR scanner: GE‐EPI sequence. TR = 2,400 ms, TE = 30 ms, 200 volumes, voxel size = 2.39 × 2.39 × 3 mm3, 39 interleaved slices, flip angle 90°, acquisition duration 8.07 min.
During the resting state acquisition, subjects were instructed to keep their eyes closed and not to think of anything in particular.
3DMP‐RAGE Sequence Acquisition
1.5T MR scanner: TR = 1,880 ms, TE = 2.5 ms, time inversion (TI) = 1,100 ms, flip angle 15°, voxel size: 1 × 1 × 1 mm3, with 176 slices.
3T MR scanner: TR = 1,700 ms, TE = 2.5 ms, TI = 922 ms, flip angle 9°, voxel size: 1 × 1 × 1 mm3, with 176 slices.
DCS Mapping
DCS was performed by the same well‐experienced neurosurgeon with 20 years of experience (H.D). Once the cortical surface was exposed, tumor and sulcal/gyral anatomy delineation with intraoperative ultrasound was performed. Stimulation of the cortical surface (between‐stimulus spacing of 5 mm) was performed using a bipolar electrode with 5 mm spacing (60 Hz, 1 ms pulse width, current amplitude 2–5 mA). Motor and sensory mapping was performed starting at 2 mA and increasing up to a maximum of 5 mA until reliable motor and/or sensory changes were elicited. Once the sensorimotor threshold was determined, that amplitude was used for the remainder of the cortical mapping. In our work, the tasks of interest included arm/leg movement, counting, object picture naming, and double‐task (picture naming in concert with contralateral arm movement) while systematically stimulating throughout the exposed cortical surface. Intraoperative evaluation of patient function was assessed by a licensed speech therapist (S.MG) and/or neuropsychologist (G.H) blinded to stimulation. Sites of stimulation that elicited errors in the aforementioned tasks were tested three times for reproducibility and marked with tags. Error characteristics were recorded (sensory, motor, or language impairment). Stimulation points eliciting semantic, phonologic and phonemic paraphasias, anomia, perseverations, and language switch were classified as language stimulation points whereas complete anarthria (speech arrest) was listed as motor rather than language impairment because it is caused by a dysfunction of the final common output for motor speech plans. Stimulations causing arrest of movement or involuntary movement were considered as positive motor sites. Stimulations causing transient paraesthesias were considered as positive sensory sites. Spatial cognition [de Schotten et al., 2005], semantic cognition [Duffau et al., 2013], theory of mind [Herbet et al., 2014], reading [Zemmoura et al., 2015], and visual field [Gras‐Combe et al., 2012] were also tested in some patients but results of those tests were not analysed in the context of study. After completion of cortical mapping, intraoperative images were taken and subsequently analysed offline. It is important to note that, once cortical resection was completed and before subcortical resection was started, no sensorimotor nor language deficit was observed.
DCS Mapping Data Registration on Volumetric MRI
Positive stimulation sites were manually recorded on systematic intraoperative photographs and individually registered on each patient's 3DT1 MP‐RAGE preoperative imaging according to anatomical landmarks (sulci, gyri, and cortical veins) and detailed operative reports using MRIcron software (http://www.mccauslandcenter.sc.edu/mricro/mricron/)—resulting into motor, language, and sensory regions of interest (ROIs). To ensure the accuracy of our work, registration of stimulation sites was carried out independently by two investigators (the neurosurgeon and the lead author). The resulting cortical maps were then compared and (in the event of disparities) modified by consensus.
Functional response corresponding to each stimulation point was also recorded (sensorimotor or language response). Stimulation points were all considered independently. Resected cortex was manually registered on patient's individual anatomical imaging according to intraoperative photographs (at the end of the resection) and post‐operative MRI. It was represented by a 5‐mm depth mask starting from the surface of the resected cortex. Five millimeter is the estimated depth of electrical current diffusion into the brain. The cortical surface exposed by the bone flap was manually registered on anatomical imaging thanks to preoperative/postoperative 3DT1 MP‐RAGE images coregistration and delineation of bone flap on postoperative imaging (See Fig. 2).
Figure 2.
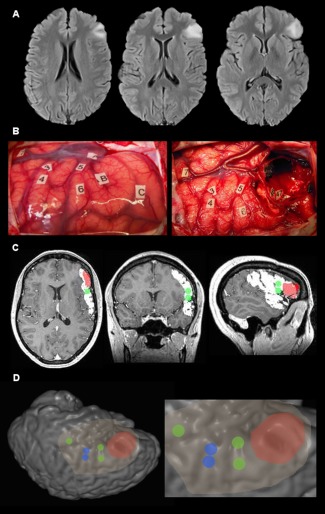
Illustrative case of stimulation points and resected cortex registration on patient's anatomical imaging. (A) FLAIR axial slices of a patient's left fronto‐opercular glioma. (B) Intraoperative photograph of the cortical surface before (left) and afer (right) resection. Number tags represent cortical eloquent sites elicited by DCS whereas letter tags represent tumor boundaries defined with intraoperative ultrasound imaging. (C) Multiplanar reconstruction of preoperative 3DT1 MP‐RAGE imaging. White delineation = exposed cortical surface. Red delineation = resected cortex. Green dots = language stimulation points reprensented with a 5 mm radius sphere. (NB: motor stimulation points are not visible here). (D) Surface rendering (operative view) of the patient's brain. Stimulation points are represented with 5 mm radius spheres (blue for motor and green for language). Highlighted cortex = exposed cortical surface. Red cortex = resected cortex. [Color figure can be viewed at http://wileyonlinelibrary.com.]
rsfMRI pre‐processing
Matlab (The Mathworks; MA) and Statistical Parametric Mapping (Ashburner, 2012) (SPM8; Wellcome Department of Imaging Neuroscience, London, UK, http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) were used for resting state image pre‐processing. The processing included removal of the first five volumes to ensure that steady resting state was achieved, corrections of magnetic field distortions, slice‐timing correction, head motion correction, and gray/white matter/CSF segmentation. Finally, the EPI images were coregistered to the individual anatomical images. A spatial smoothing using a Gaussian filter with a full width at half maximum of 8 mm was then performed.
Seed to voxel connectivity maps generation. We selected patients with at least two positive left‐sided language stimulation points located in distinct gyri to assess BOLD signal timecourse similarities between distant nodes of the same functional network (language network). Seed‐based correlation analyses were performed using the Functional Connectivity (CONN) toolbox [Whitfield‐Gabrieli and Nieto‐Castanon, 2012] (http://web.mit.edu/swg/software.htm) implemented in SPM 8. This toolbox performs seed‐based analysis by computing the temporal correlation (bivariate correlation) between the mean BOLD signals from a given ROI to all other voxels in the brain. To remove possible sources of confounds present in BOLD signal data, all functional MRI time‐series underwent signal compensation from the ventricles, deep white matter and head motion followed by temporal filtering (0.009 to 0.08 Hz) on unsmoothed volumes. Seed to voxel connectivity maps were generated using language ROIs as seeds but also with random ROIs selected from gray matter voxels in the exposed cortical surfaced for comparison purpose. Random ROIs were generated for each patient. Fifty‐nine connectivity maps were generated from 59 language stimulation sites in the 19 patients. The number of random and language ROIs was identical. Two types of random ROIs were computed: purely random ROIs and randomly assigned ROIs located 1 cm from language ROIs. All ROIs were defined as 5 mm radius spheres masked with gray matter.
ICA Connectivity Maps Generation
To identify functional network maps across patients, the NEDICA (for Network Detection using IC Analysis; Perlbarg et al., 2007; Perlbarg and Marrelec, 2008) (https://sites.google.com/site/netbrainwork/, Laboratoire d'Imagerie Fonctionnelle, Paris, France) [Perlbarg et al., 2008, 2007] software was used. We used an ICA to extract 20 ICs for each subject according to the literature to examine large‐scale brain networks [Ray et al., 2013]. Then connectivity values of IC maps were converted into z‐scores for further analysis.
Sensorimotor and Language ICs Selection
A three‐step process was performed for ICs selection. We performed (i) an automatic selection process, (ii) a manual selection process and, (iii) in case of disagreement between the previous methods, we re‐examined the different ICs and chose the one matching the best sensorimotor/language networks. We used sensorimotor (available online: http://findlab.stanford.edu/functional_ROIs.html, methodology described in [Shirer et al., 2012]) and language [Tie et al., 2014] validated templates originating from resting state data to extract sensorimotor and language ICs respectively. An automatic similarity detection method was used to outline the best matching ICs to the templates by calculating a goodness‐of‐fit (GOF) score. First, templates were normalized using SPM8 from the MNI space to the subject space to yield subject specific templates.
GOF‐score was first published by Tie et al. [2014] and consist in the following equation:
where Zin is the average z‐score of the voxels within the template, Zout is the average z‐score of the voxels outside the template, and the difference between them is scaled by the maximum z‐score in the component map (Zmax). Then, GOF‐score were converted onto z‐scores and sorted in descending order with deletion of ICs with GOF z‐score < 2. Manual inspection of ICs relied on the same clusters as those used in sensorimotor and language templates. We systematically selected components that matched manual selection and the highest GOF z‐score.
Statistical Analysis
Statistical tests were performed using Matlab R2014a (The Mathworks; MA).
Seed to voxel connectivity versus DCS mapping
We performed two different analyses. For the first one, we compared the median connectivity values between language seeds and the median connectivity values between purely random ROIs. The second analyses compared median connectivity values between language seeds and between randomly assigned ROIs 1 cm from language seeds. Seed to voxel analysis principle is illustrated in Figure 3. Difference between median connectivity values was assessed with the non‐parametric Mann‐Whitney U test Bonferroni‐corrected for multiple comparisons.
Figure 3.
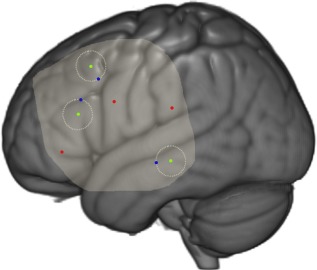
Seed to voxel connectivity analysis illustration. Left lateral view of a 3D rendered MNI brain. Green dots = language stimulation points with a 1 cm circle (dotted line) around. Randomly assigned ROIs 1 cm from language stimulation points in blue. Random ROIs in red. Exposed cortical surface is highlighted. [Color figure can be viewed at http://wileyonlinelibrary.com.]
ICA networks versus DCS mapping
Based on previous studies [Meier et al., 2013], individual language and sensorimotor IC masks were extracted after thresholding IC maps to save voxels with a z‐score > 1.96 (cluster extent > 40 contiguous voxels), corresponding to 5% of the voxels with the highest connectivity value. Stimulation points and resected cortex ROIs were compared to RSNs in terms of spatial distribution on the basis of these thresholded IC maps. We considered that a 1 cm margin between RSNs and positive stimulation points was reasonable to consider a stimulation point as accurately identified by rsfMRI—taking into account current diffusion and large size of rsfMRI voxels. Conversely, a 5 mm margin was considered more relevant in a clinical perspective. This is the reason why we used these two different cut‐off. A second comparison was made on the mean z‐score of stimulation points and resected cortex using a paired one‐sided t‐test.
Volumes of sensorimotor and language IC masks overlap with resected cortex mask were compared using a Mann‐Whitney U test.
RESULTS
Patients' and DCS' Characteristics
A complete overview of sociodemographic and clinical data is provided in Table 1. Sensori‐motor positive sites were elicited in every case whereas DCS caused language impairment in 41 (42%) cases, 37 (90%) on the left hemisphere and 4 (10%) on the right hemisphere. Among the later 4 patients, 3 were right‐handed and 1 was left‐handed.
Table 1.
Characteristics of the study population, tumor, and stimulation points
| Patients (n) | 98 |
| Age (years) | 40.5 ± 10.8 |
| Male: Female ratio | 1.3 |
| Handedness (%) | |
| Right | 84 |
| Left | 11 |
| Ambidextrous | 5 |
| Tumor side (%) | |
| Right | 43 |
| Left | 57 |
| Tumor location (n) | |
| FTI | 27 |
| F | 23 |
| T | 12 |
| P | 9 |
| FI | 9 |
| TI | 7 |
| R | 4 |
| I | 3 |
| TO | 2 |
| C | 2 |
| O | 0 |
| Tumor volume (ml) (mean ± SD; [range]) | 58.0 ± 42.8; [0.8–180] |
| Stimulation current intensity (mA) (mean ± SD; [range]) | 2.23 ± 0.524; [1.1–5.0] |
| Sensori‐motor positive mapping (%) | 100 |
| Language positive mapping (%) | 42 |
| Stimulation points (n, mean ± SD; [range]) | n = 462, 4.7 ± 2.5; [1–11] |
| Sensori‐motor stimulation points (n, mean ± SD; [range]) | n = 383, 3.9 ± 2.2; [1–11] |
| Language stimulation points (n, mean ± SD; [range]) | n = 79, 0.8 ± 1.2; [0–6] |
Legend: F = frontal; T = temporal; I = insular; P = parietal; R = rolandic; O = occipital; C = cingular.
Seed to Voxel Analysis Results
Nineteen patients were included for this analysis. Fifty‐nine connectivity maps were generated (59 language stimulation points among 19 patients, range: 2–6) from language seeds. Language stimulation points were located as follows: 25 in the inferior frontal gyrus, 18 in the superior temporal gyrus, 7 in the middle temporal gyrus, 6 in the middle frontal gyrus, 2 in the rolandic operculum, and 1 in the supramarginal gyrus. The same number of connectivity maps was generated from random ROIs and from randomly assigned ROIs 1 cm from language seeds. Mean connectivity value between language seeds was significantly higher than mean connectivity value between both random seeds and randomly assigned 1 cm from language seeds ROIS: 0.68 (SD = 0.39) versus 0.12 (SD = 0.21) and 0.16 (SD = 0.39), respectively, (P < 10−10 in both cases). See Figure 4A for boxplot representation of seed to voxel analysis results.
Figure 4.
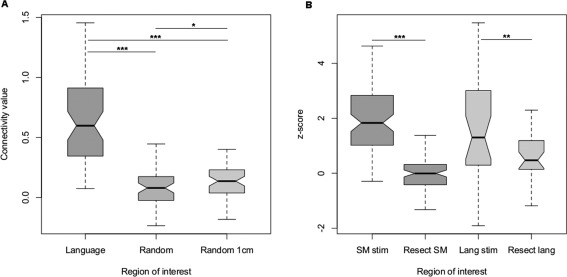
Boxplot representation of connectivity analysis results. (A) Seed to voxel analysis results. Random 1 cm = randomly assigned ROIs 1 cm from language seeds. (B) Independant component analysis results. SM stim = sensorimotor stimulation points, Lang stim = language stimulation points, Resect = resected cortex. ***= P < 0.001; **= P < 0.005.
ICA Results
Our semi‐automatic IC detection method succeeded to find sensori‐motor and language networks in 90 (92%) and 73 (75%) cases respectively (χ2 = 10.5, P = 0.001). Accordance between manual and automatic detection (IC with the highest GOF score) was achieved in 58 (64%) and 54 (74%) cases for sensorimotor and language networks respectively (χ2 = 1.70, P = 0.19). However, manually selected ICs always fitted one of the three highest GOF score ICs.
Ninety patients fulfilled the association of motor IC identification and sensori‐motor response elicited by DCS whereas the same condition was fulfilled by 31 patients for language, mainly because of the lower number of patients with peroperative language network identification compared to sensorimotor network (41 versus 98 patients respectively). Hence, our analysis focused on 90 and 31 patients for sensorimotor and language functions, respectively.
A mean of 96 ± 11% and 84 ± 24% of sensorimotor stimulation points were located within 10 and 5 mm from sensorimotor IC maps respectively. A mean of 92 ± 21% and 70 ± 41% of language stimulation points were located within 10 and 5 mm from language IC maps respectively. Mean sensorimotor network and resected cortex overlapping was significantly superior to the mean language network and resected cortex overlapping, 3.1 ± 5.8% and 15 ± 17% respectively, P = 0.001. Mean sensorimotor positive stimulation points z‐score was significantly superior to mean resected cortex z‐score for sensorimotor network, 2.0 ± 1.2 and −0.050 ± 0.60, respectively, (t = 14.3, P < 10−10). Mean language positive stimulation points z‐score was significantly superior to mean resected cortex z‐score for language network, 1.6 ± 1.9 and 0.68 ± 0.91, respectively, (t = 2.8, P < 0.005). See Figure 4B for boxplot representation of ICA z‐score results. Figure 5 illustrates one case of ICA result.
Figure 5.
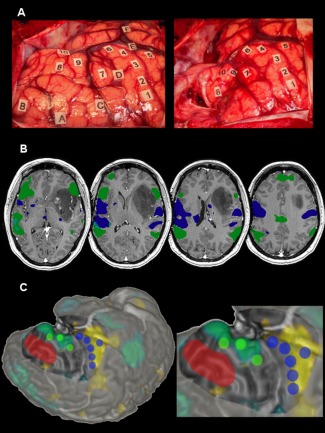
Illustrative case of stimulation points and ICA connectivity maps overlapping. (A) Intraoperative photograph of a 40‐year old left‐handed female's brain before (left) and after (right) resection. Lettered tags represent tumor boundaries defined with intraoperative ultrasound imaging. Numbered tags represent positive stimulation sites. 1:6 = motor response; 7:9 = language response; 10 = theory of mind response. (B) Axial multislice view of 3DT1 MP‐RAGE preoperative imaging with z‐score > 1.96 thresholded language (green) and sensorimotor (blue) selected independent components overlaid. (C) Surface rendering (operative view) of the patient's brain with language (green) and sensorimotor (blue) stimulation points represented with 5 mm radius spheres. Resected cortex (red) and exposed cortical surface are represented. The z > 1.96 thresholded language IC mask is overlaid in a blue/green color whereas the sensorimotor mask is represented in yellow. [Color figure can be viewed at http://wileyonlinelibrary.com.]
DISCUSSION
In this study, we report the largest series comparing preoperative resting state fMRI to intraoperative cortical electrical stimulation in a homogeneous set of diffuse LGG patients. Our study presents several strengths. First, the technique we used during awake surgery differs from the one used in other studies. Indeed we used very low stimulation intensities (mean intensity = 2.23 mA, SD = 0.524) hence eloquent cortical areas were more accurately mapped thanks to a lower current diffusion. Second, we used two well‐applied rsfMRI analysis techniques, namely a seed to voxel analysis and a data‐driven analysis (ICA), enabling us to benefit from concurrent approaches to validate rsfMRI.
Prior to consider rsfMRI as a potential tool for the preoperative mapping of eloquent cortical areas, we needed to validate the clinical relevance of RSN. Seed to voxel analysis confirmed for the first time to our knowledge the significant correlation between resting‐state low frequency fluctuations of BOLD signal similarities and the functional unity of distinct cortical areas confirmed by DCS. This is in accordance with numerous studies suggesting that low frequency fluctuations of BOLD signal are not due to physiological noise but rather arises from an intrinsically organized functional architecture of the brain at rest [Biswal et al., 1995; Damoiseaux et al., 2006; Fox et al., 2005; Fox and Raichle, 2007; Mennes et al., 2010; Smith et al., 2012]. In this study, we chose to focus on the language network because of the larger spatial distribution of cortical positive sites than sensorimotor positive sites that were centered on the central sulcus. Our aim was to explore the functional connectivity between distant regions of the brain located in different lobes or at least in different gyri to control confound effects due to spatial proximity. We took into account in our analysis the variable distance between stimulation points by generating random ROIs that were located close to the stimulation points (1 cm). Seed based connectivity analyses usually rely on canonical ROIs [Muller and Meyer, 2014; Tomasi and Volkow, 2012], namely Broca's and Wernicke's areas for language. In the study of Muller and Meyer, it is reported high correlation values between Broca and Wernicke's areas ranging from 0.3 to 0.4 while in the study of Tomasi and Volkow it is showed a significant and reproducible connectivity pattern between Broca and Wernicke's areas when applying a correlation value threshold superior to 0.64. Although these results are interesting and in some extent comparable to the connectivity values reported in the present study, they rely on canonical ROIs without addressing the actual function of the outlined networks. Indeed, several studies using DCS [Tate et al., 2014] or task‐based fMRI [Vigneau et al., 2006] have shown the highly variable location of critical cortical regions for language. Thanks to awake surgery and DCS we obtained for each patient a precise location of critical cortical areas of language. Seed to voxel analysis based on each patient's language stimulation points could help to define a subject‐level language RSN.
For the second part of the study, we decided not to report negative stimulation points because they were not reported on intraoperative photographs. In a perspective of preoperative functional mapping, the most important issue is to identify the peritumoral eloquent cortex and the resectable noneloquent cortex. Our results showed that ICA was to some extent effective to predict eloquent sensorimotor areas because 84 ± 4% of sensorimotor stimulation points were in a 5 mm range from sensorimotor z‐score > 1.96 thresholded connectivity map and resected cortex and sensorimotor connectivity maps overlap was 3.1 ± 5.8%. This low overlap along with a significant z‐score difference between stimulation points and resected cortex suggests that ICA could give reliable negative predictive data for sensorimotor function. Our results are consistent with previous studies comparing rsfMRI and DCS for sensorimotor cortex mapping using different approaches [Fox et al., 2016; Mitchell et al., 2013; Qiu et al., 2014; Zhang et al., 2009]. Our approach differed from the one used by Mitchell et al. [Mitchell et al., 2013] who used a data driven approach based on a trained classifier on a subset of healthy controls. ICA of rsfMRI was less effective to predict positive and negative language areas. These results are consistent with previous task‐based and resting‐state fMRI studies [Böttger et al., 2011; Giussani et al., 2010; Mitchell et al., 2013; Kuchcinski et al., 2015], which suggest that language mapping with fMRI presents several unsolved pitfalls.
The heterogeneous interindividual accuracy of rsfMRI is a matter of concern. The influence of tumor location on RSC could play a role in that interindividual variability. How does infiltration of functional hubs and/or white matter pathways disturb RSC? Some tumors infiltrate networks connectivity and functional epicenters more than others. That might cause disturbances in RSC [Maesawa et al., 2015] resulting in difficulties to identify RSN using an ICA approach. One can hypothesize that IC analysis failed to identify the functional networks of interest in patients whom tumor induced significant RSC impairment.
Resting‐state fMRI offers the opportunity to preoperatively adapt the surgical strategy according to the cortical functional anatomy. As depicted in previous task‐based fMRI studies [Petrella et al., 2006], rsfMRI may adress the expected extent of resection according to functional boundaries and help to decide through which transcortical corridor the surgery should be performed. Combining preoperative rsfMRI and diffusion tensor imaging informations would offer the possibility to assemble functional and structural connectivity data at the patient's level allowing the neurosurgeon to virtually perform tumor resection before confronting those data to electrical cortico‐sub‐cortical stimulation. Additionally, informations provided by rsfMRI could be of prime interest especially in cases of repeated surgeries to follow the longitudinal remapping of functional networks [Duffau, 2013] and decide for the optimal surgeries timing.
Limitations
Several study's limitations can explain the difference between sensorimotor and language mapping with ICA. The analysis focused on 31 patients for language versus 90 patients for sensorimotor function. Recent findings suggest that interindividual variability in functional connectivity is not uniformly distributed across the cortex. Functional connectivity within associative regions, including the main nodes of language, executive, and attention networks, are likely to be more variable than those within unimodal regions, such as visual and sensorimotor structures [Langs et al., 2015; Mueller et al., 2013]. That might explain why mapping of unimodal cortex (i.e., sensorimotor cortex) via ICA seems more robust than mapping of associative cortex subserving distributed networks such as the language or other higher order cognitive network. Moreover, tumor growth causes important cortical plasticity [Duffau, 2013]. Long range functional connectivity within language network in glioma patients has been studied and it appears that gliomas induce important functional connectivity decrease within language labelled regions compared to healthy controls [Briganti et al., 2012]. These factors and the lower number of patients with positive language mapping might explain the better results for sensorimotor function and the difference in ICs detection rate between sensorimotor and language networks (92 and 75% respectively, P = 0.001).
The number of ICs (20) was limited and we chose to identify only one component by manual inspection and spatial similarity detection [Tie et al., 2014]. However, the language network might be subdivided into several subcomponents [Cordes et al., 2000; Hampson et al., 2002] we might have missed by selecting only one subcomponent for language.
Negative stimulation points unavailability prevented from providing sensitivity and specificity values of ICA maps compared to DCS functional mapping maps.
Another limitation is the inability of cortical stimulations to map the cortex buried into the sulci. This can account for false positive outcomes. However, after cortical resection, no neurological deficit was encountered, emphasizing the reliability of DCS for eloquent cortex sparing. A possible supplementary limitation is the different signal to noise ratio between rsfMRI acquisition performed at 1.5 versus 3.0 Tesla.
CONCLUSION AND PERSPECTIVES
Our study succeeded to establish a correlation between networks identified at rest and eloquent cortical areas identified by DCS with a seed‐based approach. It supports the assumption that rsfMRI identifies networks involved in task performance.
Despite important interindividual variability in the spatial location of cortical functional epicenters, especially in glioma patients [Tate et al., 2014], ICA succeeded in some extent to distinguish eloquent versus resectable sensorimotor and language areas. However, even if the results are promising, they reveal a high interindividual variability of mapping accuracy and a rate around 80% in the detection of eloquent cortical sites is clearly insufficient to use rsfMRI on its own for preoperative cortical functional mapping. Task‐based fMRI remains the technique of choice to preoperatively map eloquent cortex. Further validation studies are needed to increase rsfMRI reliability for surgical planning, using in particular network automatic identification and/or subnetwork analysis.
To conclude, rsfMRI is a very promising technique that could be used in a large range of clinical conditions in which task‐based fMRI cannot be used. For example, it can be applied to patients with severe neurological deficits or altered states of consciousness, and multiple functional networks are explored in a single short session providing a complete overview of the whole cortical functional organization.
ACKNOWLEDGMENT
The study was not industry‐sponsored.
Hugues Duffau and Nicolas Menjot de Champfleur contributed equally to the manuscript.
REFERENCES
- J Ashburner (2012): SPM: A history. NeuroImage 62:791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM (2005): Investigations into resting‐state connectivity using independent component analysis. Philos Trans R Soc B Biol Sci 360:1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo X‐N, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski AM, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kötter R, Li SJ, Lin CP, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts SA, Rypma B, Schlaggar BL, Schmidt S, Seidler RD, Siegle GJ, Sorg C, Teng GJ, Veijola J, Villringer A, Walter M, Wang L, Weng XC, Whitfield‐Gabrieli S, Williamson P, Windischberger C, Zang YF, Zhang HY, Castellanos FX, Milham MP. (2010): Toward discovery science of human brain function. Proc Natl Acad Sci USA 107:4734–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Zerrin Yetkin F, Haughton VM, Hyde JS (1995): Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magn Reson Med 34:537–541. [DOI] [PubMed] [Google Scholar]
- Bizzi A, Blasi V, Falini A, Ferroli P, Cadioli M, Danesi U, Aquino D, Marras C, Caldiroli D, Broggi G (2008): Presurgical functional MR imaging of language and motor functions: Validation with intraoperative electrocortical mapping. Radiology 248:579–589. [DOI] [PubMed] [Google Scholar]
- Boetto J, Bertram L, Moulinié G, Herbet G, Moritz‐Gasser S, Duffau H (2015): Low rate of intraoperative seizures during awake craniotomy in a prospective cohort with 374 supratentorial brain lesions: Electrocorticography is not mandatory. World Neurosurg 84:1838–1844. [DOI] [PubMed] [Google Scholar]
- Boly M, Phillips C, Tshibanda L, Vanhaudenhuyse A, Schabus M, Dang‐Vu TT, Moonen G, Hustinx R, Maquet P, Laureys S (2008): Intrinsic brain activity in altered states of consciousness. Ann N Y Acad Sci 1129:119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnetblanc F, Desmurget M, Duffau H (2006): Gliomes de bas grade et plasticité cérébrale: Implications fondamentales et Cliniques. Médecine/Sciences 22:389–395. [DOI] [PubMed] [Google Scholar]
- Böttger J, Margulies DS, Horn P, Thomale UW, Podlipsky I, Shapira‐Lichter I, Chaudhry SJ, Szkudlarek C, Mueller K, Lohmann G, Hendler T, Bohner G, Fiebach JB, Villringer A, Vajkoczy P, Abbushi A (2011): A software tool for interactive exploration of intrinsic functional connectivity opens new perspectives for brain surgery. Acta Neurochir (Wien) 153:1561–1572. [DOI] [PubMed] [Google Scholar]
- Branco P, Seixas D, Deprez S, Kovacs S, Peeters R, Castro SL, Sunaert S (2016): Resting‐state functional magnetic resonance imaging for language preoperative planning. Front Hum Neurosci 10:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briganti C, Sestieri C, Mattei PA, Esposito R, Galzio RJ, Tartaro A, Romani GL, Caulo M (2012): Reorganization of functional connectivity of the language network in patients with brain gliomas. Am J Neuroradiol 33:1983–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Wendt GJ, Turski PA, Moritz CH, Quigley MA, Meyerand ME (2000): Mapping functionally related regions of brain with functional connectivity MR imaging. Am J Neuroradiol 21:1636–1644. [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts S, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF (2006): Consistent resting‐state networks across healthy subjects. Proc Natl Acad Sci USA 103:13848–13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Schotten MT (2005): Direct evidence for a parietal‐frontal pathway subserving spatial awareness in humans. Science 309:2226–2228. [DOI] [PubMed] [Google Scholar]
- De Witt Hamer PC, Robles SG, Zwinderman AH, Duffau H, Berger MS (2012): Impact of intraoperative stimulation brain mapping on glioma surgery outcome: A meta‐analysis. J Clin Oncol 30:2559–2565. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Bonnetblanc F, Duffau H (2006): Contrasting acute and slow‐growing lesions: A new door to brain plasticity. Brain 130:898–914. [DOI] [PubMed] [Google Scholar]
- Duffau H (2005): Lessons from brain mapping in surgery for low‐grade glioma: Insights into associations between tumour and brain plasticity. Lancet Neurol 4:476–486. [DOI] [PubMed] [Google Scholar]
- Duffau H (2013): The huge plastic potential of adult brain and the role of connectomics: New insights provided by serial mappings in glioma surgery. Cortex 58:325–337. [DOI] [PubMed] [Google Scholar]
- Duffau H, Herbet G, Moritz‐Gasser S (2013): Toward a pluri‐component, multimodal, and dynamic organization of the ventral semantic stream in humans: Lessons from stimulation mapping in awake patients. Front Syst Neurosci 7:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffau H (2015): Stimulation mapping of white matter tracts to study brain functional connectivity. Nat Rev Neurol 11:255–265. [DOI] [PubMed] [Google Scholar]
- FitzGerald DB, Cosgrove GR, Ronner S, Jiang H, Buchbinder BR, Belliveau JW, Rosen BR, Benson RR (1997): Location of language in the cortex: A comparison between functional MR imaging and electrocortical stimulation. Am J Neuroradiol 18:1529–1539. [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Qian T, Madsen JR, Wang D, Li M, Ge M, Zuo H, Groppe DM, Mehta AD, Hong B, Liu H (2016): Combining task‐evoked and spontaneous activity to improve pre‐operative brain mapping with fMRI. NeuroImage 124:714–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME (2007): Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8:700–711. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME (2005): The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P (2005): Spontaneous low‐frequency BOLD signal fluctuations: An fMRI investigation of the resting‐state default mode of brain function hypothesis. Hum Brain Mapp 26:15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giussani C, Roux F‐E, Ojemann J, Sganzerla EP, Pirillo D, Papagno C (2010): Is preoperative functional magnetic resonance imaging reliable for language areas mapping in brain tumor surgery? Review of language functional magnetic resonance imaging and direct cortical stimulation correlation studies. Neurosurgery 66:113–120. [DOI] [PubMed] [Google Scholar]
- Gras‐Combe G, Moritz‐Gasser S, Herbet G, Duffau H (2012): Intraoperative subcortical electrical mapping of optic radiations in awake surgery for glioma involving visual pathways: Clinical article. J Neurosurg 117:466–473. [DOI] [PubMed] [Google Scholar]
- Guerra‐Carrillo B, Mackey AP, Bunge SA (2014): Resting‐state fMRI: A window into human brain plasticity. Neuroscientist 20:522–533. [DOI] [PubMed] [Google Scholar]
- Hampson M, Peterson BS, Skudlarski P, Gatenby JC, Gore JC (2002): Detection of functional connectivity using temporal correlations in MR images. Hum Brain Mapp 15:247–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbet G, Lafargue G, Bonnetblanc F, Moritz‐Gasser S, Menjot de Champfleur N, Duffau H (2014): Inferring a dual‐stream model of mentalizing from associative white matter fibres disconnection. Brain 137:944–959. [DOI] [PubMed] [Google Scholar]
- Ius T, Angelini E, Thiebaut de Schotten M, Mandonnet E, Duffau H (2011): Evidence for potentials and limitations of brain plasticity using an atlas of functional resectability of WHO grade II gliomas: Towards a “minimal common brain.” NeuroImage 56:992–1000. [DOI] [PubMed] [Google Scholar]
- Kuchcinski G, Mellerio C, Pallud J, Dezamis E, Turc G, Rigaux‐Viodé O, Malherbe C, Roca P, Leclerc X, Varlet P, Chrétien F, Devaux B, Meder JF, Oppenheim C (2015): Three‐tesla functional MR language mapping comparison with direct cortical stimulation in gliomas. Neurology 84:560–568. [DOI] [PubMed] [Google Scholar]
- Langs G, Wang D, Golland P, Mueller S, Pan R, Sabuncu MR, Sun W, Li K, Liu H (2015): Identifying shared brain networks in individuals by decoupling functional and anatomical variability. Cereb Cortex:bhv189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maesawa S, Bagarinao E, Fujii M, Futamura M, Motomura K, Watanabe H, Mori D, Sobue G, Wakabayashi T (2015): Evaluation of resting state networks in patients with gliomas: Connectivity changes in the unaffected side and its relation to cognitive function. PloS One 10:e0118072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier MP, Ilmberger J, Fesl G, Ruge MI (2013): Validation of functional motor and language MRI with direct cortical stimulation. Acta Neurochir (Wien) 155:675–683. [DOI] [PubMed] [Google Scholar]
- Mennes M, Kelly C, Zuo X‐N, Di Martino A, Biswal BB, Castellanos FX, Milham MP (2010): Inter‐ individual differences in resting‐state functional connectivity predict task‐induced BOLD activity. NeuroImage 50:1690–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell TJ, Hacker CD, Breshears JD, Szrama NP, Sharma M, Bundy DT, Pahwa M, Corbetta M, Snyder AZ, Shimony JS, Leuthardt EC (2013): A novel data‐driven approach to preoperative mapping of functional cortex using resting‐state functional magnetic resonance imaging. Neurosurgery 73:969–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- S Mueller, D Wang, MD Fox, BTT Yeo, J Sepulcre, MR Sabuncu, R Shafee, J Lu, H Liu (2013): Individual Variability in Functional Connectivity Architecture of the Human Brain. Neuron 77:586–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller AM, Meyer M (2014): Language in the brain at rest: New insights from resting state data and graph theoretical analysis. Front Hum Neurosci 8:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlbarg V, Marrelec G, Doyon J, Pélégrini‐Issac M, Lehéricy S, Benali H (2008): NEDICA: Detection of group functional networks in fMRI using spatial independent component analysis. In: 5th IEEE International Symposium on Biomedical Imaging: From Nano to Macro, Paris. pp 1247–1250.
- Perlbarg V, Bellec P, Anton J‐L, Pélégrini‐Issac M, Doyon J, Benali H (2007): CORSICA: Correction of structured noise in fMRI by automatic identification of ICA components. Magn Reson Imaging 25:35–46. [DOI] [PubMed] [Google Scholar]
- Petrella JR, Shah LM, Harris KM, Friedman AH, George TM, Sampson JH, Pekala JS, Voyvodic JT (2006): Preoperative functional MR imaging localization of language and motor areas: Effect on therapeutic decision making in patients with potentially resectable brain tumors. Radiology 240:793–802. [DOI] [PubMed] [Google Scholar]
- Qiu T, Yan C, Tang W, Wu J, Zhuang D, Yao C, Lu J, Zhu F, Mao Y, Zhou L (2014): Localizing hand motor area using resting‐state fMRI: Validated with direct cortical stimulation. Acta Neurochir (Wien) 156:2295–2302. [DOI] [PubMed] [Google Scholar]
- Ray KL, McKay DR, Fox PM, Riedel MC, Uecker AM, Beckmann CF, Smith SM, Fox PT, Laird AR (2013): ICA model order selection of task co‐activation networks. Front Neurosci 7:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosazza C, Aquino D, D'Incerti L, Cordella R, Andronache A, Zacà D, Bruzzone MG, Tringali G, Minati L (2014): Preoperative mapping of the sensorimotor cortex: Comparative assessment of task‐based and resting‐state fMRI. Ed. Daniel Margulies. PLoS One 9:e98860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux F‐E, Boulanouar K, Lotterie J‐A, Mejdoubi M, LeSage JP, Berry I (2003): Language functional magnetic resonance imaging in preoperative assessment of language areas: Correlation with direct cortical stimulation. Neurosurgery 52:1335–1347. [DOI] [PubMed] [Google Scholar]
- Schneider FC, Pailler M, Faillenot I, Vassal F, Guyotat J, Barral F‐G, Boutet C (2016): Presurgical assessment of the sensorimotor cortex using resting‐state fMRI. Am J Neuroradiol 37:101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD (2012): Decoding subject‐driven cognitive states with whole‐brain connectivity patterns. Cereb Cortex 22:158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Miller KL, Moeller S, Xu J, Auerbach EJ, Woolrich MW, Beckmann CF, Jenkinson M, Andersson J, Glasser MF, Van Essen DC, Feinberg DA, Yacoub ES, Ugurbil K (2012): Temporally‐independent functional modes of spontaneous brain activity. Proc Natl Acad Sci USA 109:3131–3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R Laird AR, others (2009): Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci USA 106:13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate MC, Herbet G, Moritz‐Gasser S, Tate JE, Duffau H (2014): Probabilistic map of critical functional regions of the human cerebral cortex: Broca's area revisited. Brain J Neurol 137:2773–2782. [DOI] [PubMed] [Google Scholar]
- Tie Y, Rigolo L, Norton IH, Huang RY, Wu W, Orringer D, Mukundan S, Golby AJ (2014): Defining language networks from resting‐state fMRI for surgical planning‐a feasibility study: Resting‐state fMRI for language mapping. Hum Brain Mapp 35:1018–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND (2012): Resting functional connectivity of language networks: Characterization and reproducibility. Mol Psychiatry 17:841–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Hervé PY, Duffau H, Crivello F, Houdé O, Mazoyer B, Tzourio Mazoyer N (2006): Meta‐analyzing left hemisphere language areas: Phonology, semantics, and sentence processing. NeuroImage 30:1414–1432. [DOI] [PubMed] [Google Scholar]
- Wang D, Buckner RL, Fox MD, Holt DJ, Holmes AJ, Stoecklein S, Langs G, Pan R, Qian T, Li K, Baker JT, Stufflebeam SM, Wang K, Wang X, Hong B, Liu H (2015): Parcellating cortical functional networks in individuals. Nat Neurosci 18:1853–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield‐Gabrieli S, Nieto‐Castanon A (2012): Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2:125–141. [DOI] [PubMed] [Google Scholar]
- Zhang D, Johnston JM, Fox MD, Leuthardt EC, Grubb RL, Chicoine MR, Smyth MD, Snyder AZ, Raichle ME, Shimony JS (2009): Preoperative sensorimotor mapping in brain tumor patients using spontaneous fluctuations in neuronal activity imaged with functional magnetic resonance imaging: Initial experience. Neurosurgery 65:226–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemmoura I, Herbet G, Moritz‐Gasser S, Duffau H (2015): New insights into the neural network mediating reading processes provided by cortico‐subcortical electrical mapping: Neural basis of reading. Hum Brain Mapp 36:2215–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou IY, Liang Y‐X, Chan RW, Gao PP, Cheng JS, Hu Y, So K‐F, Wu EX (2014): Brain resting‐ state functional MRI connectivity: Morphological foundation and plasticity. NeuroImage 84:1–10. [DOI] [PubMed] [Google Scholar]


