Abstract
The use of top–down cognitive control mechanisms to regulate emotional responses as circumstances change is critical for mental and physical health. Several theoretical models of emotion regulation have been postulated; it remains unclear, however, in which brain regions emotion regulation goals (e.g., the downregulation of fear) are represented. Here, we examined the neural mechanisms of regulating emotion using fMRI and identified brain regions representing reappraisal goals. Using a multimethodological analysis approach, combining standard activation‐based and pattern‐information analyses, we identified a distributed network of lateral frontal, temporal, and parietal regions implicated in reappraisal and within it, a core system that represents reappraisal goals in an abstract, stimulus‐independent fashion. Within this core system, the neural pattern‐separability in a subset of regions including the left inferior frontal gyrus, middle temporal gyrus, and inferior parietal lobe was related to the success in emotion regulation. Those brain regions might link the prefrontal control regions with the subcortical affective regions. Given the strong association of this subsystem with inner speech functions and semantic memory, we conclude that those cognitive mechanisms may be used for orchestrating emotion regulation. Hum Brain Mapp 37:600–620, 2016. © 2015 Wiley Periodicals, Inc.
Keywords: reappraisal, inner speech, inferior frontal gyrus, fMRI, MVPA, decoding, neuroimaging, pattern classification
INTRODUCTION
The ability to use top–down cognitive control mechanisms, such as reappraisal, to regulate emotional responses as circumstances change is critical for mental and physical health [Eftekhari et al., 2009; Gross and John, 2003a; Gross and Muñoz, 1995]. Reappraisal refers to the cognitive reevaluation of a potentially emotionally arousing event, aimed at altering its emotional impact [Gross and Thompson, 2007]. Thus, reappraisal requires a goal to regulate either the magnitude or duration of an emotional response [Gross et al., 2011b] and could pursue two different goals, i.e., the downregulation (decrease) or upregulation (increase) of emotion [Gross, 2013; McRae et al., 2012; Ochsner et al., 2012]. Previous studies implemented different reappraisal goals depending on the valence of the emotional stimuli to either upregulate positive (e.g., Kim and Hamann, 2007) and negative emotions (e.g., Eippert et al., 2007; Kim and Hamann, 2007; Ochsner et al., 2004b; Urry et al., 2006), or to downregulate positive (e.g., Kanske et al., 2011; Kim and Hamann, 2007) and negative emotions (e.g., Eippert et al., 2007; Kanske et al., 2011; Kim and Hamann, 2007; Ochsner et al., 2004b; Urry et al., 2006). Reappraisal goals vary in their outcome, i.e., how affective responses change in their magnitude or duration, and can be achieved via different tactics, e.g., distancing or situation reinterpretation [Gross et al., 2011a; Gross, 2013; McRae et al., 2012; Ochsner et al., 2004b]. In this study, we aimed to investigate the up‐ and downregulation of emotion (often referred to as reappraisal goals by [Gross et al., 2011a; Gross, 2013; McRae et al., 2012; Ochsner et al., 2012; Wager et al., 2008]) and not the specific tactics to achieve these goals. Reappraisal represents a cognitively complex process, which has been proposed to involve several subprocesses important for generating, maintaining, and implementing a cognitive frame, as well as the tracking of changes in one's emotional states [Ochsner and Gross, 2008]. Here, we assumed that these subprocesses are equally instrumental for the up‐ and downregulation of emotional responses.
Research on emotion regulation has converged on a top–down model whereby neural responses to emotional stimuli in the amygdala and ventral striatum are downregulated by prefrontal regions [Johnstone et al., 2007; Kober et al., 2010; Phillips et al., 2008; Urry et al., 2006; Wager et al., 2008]. However, to date, it remains unclear which brain regions mediate the emotion regulation processes between the cortical control and the subcortical affective system, and which cognitive mechanisms underlie this mediation process. The recent model of cognitive control of emotion (MCCE) [Ochsner et al., 2012] posits that temporal brain regions (e.g., temporoparietal junction, middle temporal gyrus (MTG), superior temporal gyrus (STG)) play an intermediary role between DLPFC and amygdala during the emotion regulation process. In addition, medial prefrontal cortex (MPFC) regions and left inferior frontal gyrus (IFG) have also been suggested to link the cognitive control with the affect processing system [Ochsner et al., 2004a]. Numerous cognitive processes have been associated with these brain regions ranging from language, response selection and inhibition, attribution of mental states, emotional reflection, to suppression and distraction [Badre et al., 2005; Badre and Wagner, 2007; Binder and Desai, 2011; Buhle et al., 2013; Egner, 2011; Fletcher et al., 2000; Goldin et al., 2008; Jonides et al., 1998; Kanske et al., 2011; Kohn et al., 2014; Mitchell, 2009; Moss et al., 2005; Ochsner et al., 2004a, 2012; Olsson and Ochsner, 2008; Thompson‐Schill et al., 1997, 2005]. One less discussed cognitive process mediated by temporal regions as well as IFG is self‐directed inner speech [Geva et al., 2011b; Girbau, 2007; Jones and Fernyhough, 2007; Morin and Hamper, 2012; Shergill et al., 2003]. Inner speech might be involved in the evaluation of stimulus significance by reflecting on one's own internal state induced by the stimulus, through verbal labeling, categorizing, or separation of affect, and thus it could promote the reinterpretation of the meaning of the stimulus [Burns and Engdahl, 1998]. Indeed, verbal labeling of affect has previously been described as an incidental emotion regulation process [Lieberman et al., 2011].
The MCCE provides a framework for the study of emotion regulation and integrates different cognitive control processes involved in reappraisal, but it is limited in several important aspects. (1) Earlier studies have mainly focused on reappraisal in terms of decreasing negative emotions, and only few studies addressed the opposite goal of cognitive change, namely increasing emotions [Ochsner et al., 2012]. Whether or not the top–down model as described for downregulating emotion (e.g., Phillips et al., 2008) also holds for the upregulation of emotion is not clear yet. (2) Most studies to date used photos from the International Affective Picture System (IAPS) [Bradley and Lang, 2007] as stimulus material [Buhle et al., 2013; Frank et al., 2014; Kalisch, 2009; Kohn et al., 2014; Ochsner et al., 2012]. In a recent meta‐analysis, Ochsner et al. [Ochsner et al., 2012] found that 33 out of 43 reappraisal studies used IAPS pictures as stimuli. The number of neuroimaging studies that used more realistic material to induce emotions is much smaller. Some authors report having used affective film clips [Beauregard et al., 2001; Goldin et al., 2008; Lévesque et al., 2003], memories [Kross et al., 2009], photos of faces [McRae et al., 2012a; Ziv et al., 2013], or painful stimuli [Lorenz et al., 2003; Salomons et al., 2004, 2007; Wiech et al., 2006]. However, it remains untested to what extent stimulus material that is more similar to real‐world experiences overlaps with other stimuli such as photos when tested on the same sample (i.e., in a within subject design). (3) Previous studies primarily identified brain regions involved in emotion regulation by searching for average activation differences associated with reappraisal. This leaves the possibility that regions were overlooked in which reappraisal goals (i.e., up‐ and downregulating of emotions) are represented by distinct, fine‐grained neural patterns that do not necessarily lead to changes in the overall signal strength.
To answer the questions which brain regions mediate reappraisal processes and which cognitive mechanism underlies them, we aimed to identify those brain regions in which reappraisal is represented using functional magnetic resonance imaging (fMRI). We hypothesized that if the temporal regions, IFG and MPFC, took on the function of an intermediary role between the cortical control and the subcortical affective system [Ochsner et al., 2004a, 2012], then reappraisal goals should be represented in those regions to promote the fundamental cognitive processes important for successful emotion regulation. We predicted that reappraisal processes would recruit prefrontal regions generally implicated in the cognitive control of emotions independent of the reappraisal goal. Based on previous findings [Eippert et al., 2007; Ochsner et al., 2004b], we further hypothesized that increasing emotion would be associated with higher activity in the emotion regulation network compared to decreasing emotion. We sought to overcome some of the aforementioned methodological shortcomings. To address the issue of goal‐specificity and generalizability (limitation 1), we adapted an event‐related design with three experimental conditions (e.g., Kim and Hamann, 2007): Increase, Decrease, and Look. To overcome the issue of stimulus‐specific effects in emotion regulation (limitation 2), we used two sets of stimuli: aversive pictures from the IAPS and high‐arousing film clips of skydiving and BASE jumping that created a naturalistic setting for high emotional stress, strong hyperarousal, and anxiety. To attain high ecologic validity, we assured that the stimulus material was relevant to the subjects. Therefore, our sample represented a continuum of skydiving/BASE jumping experience and expertise by including novice and experienced sports participants likewise as well as nonsports participants. To capture the full extent of the emotion regulation network and determine in which regions reappraisal goals are represented, we implemented a multimethodological analysis approach moving beyond standard univariate fMRI analysis (limitation 3). First, we identified all brain regions involved in emotion regulation by contrasting reappraisal trials with control trials. We then identified regions that showed overall activation differences for up‐ and downregulation of emotions. Next, we applied multivariate voxel pattern analysis (MVPA) [Haynes and Rees, 2006; Norman et al., 2006] to all regions within the emotion regulation network that did not show activation differences between up‐ and downregulation of emotions to investigate whether fine‐grained activation patterns still contained regulation‐specific information. Finally, we explicitly tested the relationship between reappraisal success and neural activation patterns.
MATERIALS AND METHODS
Subjects
Sixty‐six right‐handed (Edinburgh‐Handedness Inventory; Oldfield, 1971), healthy subjects with normal or corrected to normal vision gave written, informed consent and participated in the study. Two subjects did not finish the experiment and five subjects' data had to be excluded due to head movement and technical problems with data acquisition. The final sample consisted of fifty‐nine subjects (mean age = 32.47 years, SD = 11.25; 20 female).
We presented participants with a scenario with high ecologic validity that also had a very high probability to be emotionally engaging for almost everyone, while allowing us to control for experience with emotion regulation. To capture a wide range of experience with these scenarios, our sample represented a continuum of skydiving/BASE jumping experience and expertise. We assumed that BASE jumpers and skydivers at different levels of expertise might possess a wide range of expertise in controlling their emotions. Thus, our sample included novice and experienced sports participants likewise (mean experience in years = 4.32 ± 7.53) as well as nonsports participants (i.e., not taking part in any extreme sports activities, and no previous experience in skydiving and/or BASE jumping). Indeed, our sample represented a wide range in emotion regulation skills (Fig. 1). Sports participants were also asked about how often they performed skydiving and BASE jumping in the past (number of jumps = 714.05, SD = 1798.68; range from 1 to 9,770 jumps). According to sports experience and expertise, our sample could be subdivided into three groups of subjects: nonsports participants (n = 20, mean age = 24.8 years, SD = 4.39), novice sports participants with an experience of less than 100 jumps (n = 19, mean age = 34.42 ± 12.26; mean number of jumps = 58 ± 29), and experienced sports participants with an experience of more than 100 jumps (n = 20, mean age = 38.30 ± 11.11; mean number of jumps = 1,337 ± 2,373).
Figure 1.
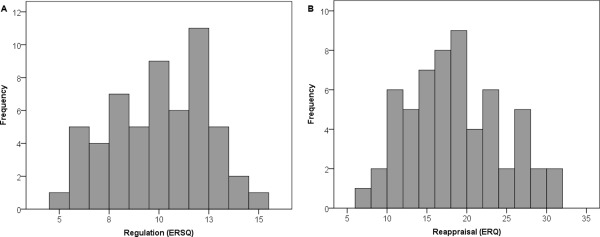
(A) Distribution of regulation scores on the Emotion Regulation Skills Questionnaire (ERSQ) across all participants. (B) Distribution of reappraisal scores on the Emotion Regulation Questionnaire (ERQ) across all participants.
This study was approved by the ethics committee of the German Psychological Society (DGPs).
Questionnaires
After the fMRI session, subjects completed several questionnaires. Because neural responses to threat‐related stimuli can differ with respect to state anxiety levels [Bishop et al., 2004] subjects rated their state anxiety before and after the experiment using the STAI [Laux et al., 1981; Spielberger et al., 1970] to assure that our sample did not differ from the population average. Alexithymia, the inability to describe and regulate one's emotions, was assessed using the TAS‐20 [Bach et al., 1996]. Personality characteristics were measured using the NEO‐Five Factory Inventory [Borkenau and Ostendorf, 1999] and the Sensation Seeking Scale (SSS‐V) [Beauducel et al., 2003]. To assess individual differences in general emotion regulation strategies (suppression and reappraisal), we obtained self‐ratings of emotion experience and expression using the emotion regulation questionnaire (ERQ) [Abler, 2009; Gross and John, 2003b] and the Emotion Regulation Skills Questionnaire (ERSQ) [Berking and Znoj, 2008]. In addition, subjects rated their ability to regulate their emotions for all experimental conditions in a separate general questionnaire on a seven‐point scale (1: “not successful at all” to 7: “very successful”). In two open questions, subjects were given the opportunity to provide additional comments on their emotion regulation strategies: (1) “Which strategy did you use to regulate your emotions during the Increase condition?” and (2) “Which strategy did you use to regulate your emotions during the Decrease condition?” Both questions were followed by “Please describe it in a few words.”
Electrodermal Activity
Electrodermal activity (EDA) can serve as an index of affective value of stimuli and is affected by emotional arousal and emotion regulation [Urry et al., 2009]. We recorded EDA using two cup electrodes with an internal impedance of 15 kΩ (7 mm) filled with isotonic paste and attached to the proximal phalanges of the index and middle fingers on the left hand. EDA was acquired at a sampling rate of 5,000 Hz using an MR‐compatible amplifier system (BrainAmp GSR‐module, Brain Products, Gliching, Germany) and constant voltage electrode excitation. The data were down‐sampled offline to 10 Hz and averaged across trials within each condition applying an 8 s time window using Ledalab Version 3.3.1 [Benedek and Kaernbach, 2010a]. Continuous decomposition analysis was performed to decompose skin‐conductance data into continuous tonic and phasic activity [Benedek and Kaernbach, 2010b]. Skin‐conductance responses (SCRs) were defined as a deflection of at least 0.01 muS occurring 1–8 s after stimulus onset. Only runs including more than 10% SCRs exceeding the above criterion were used for analysis. Values for phasic SCRs were extracted as the difference between a local minimum and the succeeding local maximum within the response window. EDA data from 12 subjects had to be excluded due to technical problems at recording.
Stimuli
Stimuli consisted of a set of (a) film clips as well as (b) pictures from the International Affective Picture System (IAPS) [Bradley and Lang, 2007]. One hundred and eighty film clips were evaluated previously by a different group of seventeen subjects (mean age = 26.82 ± 6.91 years; 13 female). (a) The film clips consisted of 120 highly arousing extreme sports film clips (Skydiving, BASE Jumping, Downhill‐Skateboarding, Freeride Snowboarding, Big Wave Surfing, Kite Surfing, Whitewater Kayaking, Snow Paragliding) and 60 neutral control film clips (Landscape, Airplane Flights, Helicopter Flights). The skydiving/BASE jumping film clips were obtained from a skydiving school (http://www.gojump.de), and all other film clips were obtained from a professional videographer (http://www.danny-strasser.de) as well as Red Bull Media House GmbH (http://www.redbull.com). Note that no participant had previously seen any of the film clips, which have not been published or used for any commercial purposes. The film clips were rated on valence and arousal using a nine‐point Likert scale (1: very negative/calm to 9: very positive/high arousing). On the basis of these ratings, 62 high‐arousing film clips of skydiving and BASE Jumping (using a cutoff value of >5 for arousal) were selected for the fMRI study. An additional 21 neutral film clips (using a cutoff value of <2 for arousal and >7 for valence) were selected to provide a baseline for post‐hoc ratings.
Skydiving and BASE jumping are considered to be extreme sports, which carry a high risk of severe physical injury or even death [Brymer, 2005]. Skydiving involves willfully stepping through the open door of an airplane, while jumping off a rock or a building with a parachute is part of BASE jumping. The latter is ranked as being among the most dangerous sports in the world [Pedersen, 1997]. To induce strong feelings of anxiety and high arousal, only extreme sports film clips from the first person perspective (helmet camera) were used (e.g., depicting the actual jump of an airplane or off a rock). Emotionally neutral film clips were matched to the sports clips with respect to the perspective (i.e., bird's eye view of landscapes taken from an airplane or helicopter), but did not involve dangerous scenarios. The final selection showed significantly greater negative emotion experience (sports: mean = 4.81, SD = 1.83, neutral: mean = 6.55 ± 1.10, t(16) = 3.84; p < 0.001) and higher arousal (sports: mean = 6.81 ± 1.07, neutral: mean = 3.57 ± 1.61, t(16) = 7.91, p < 0.001) for sports versus neutral film clips. (b) The pictures set consisted of 90 IAPS pictures that were highly aversive according to the existing norms (nine‐point rating scale, with 1 = negative/calm and 9 = positive/arousing) [Bradley and Lang, 2007] (mean valence = 2.54, SD = 0.67; mean arousal = 6.04, SD = 0.53), as well as 21 neutral pictures (mean valence = 5.19, SD = 0.32; mean arousal = 3.16, SD = 0.29). These served as a control condition for nonmotion stimuli. The content of the IAPS pictures was not related to the content of the film clips and included e.g., car accidents, mutilation, attacks, aimed guns, war, animals, and burned victims. After scanning, subjects of the fMRI experiment rated all stimuli on valence and arousal on a nine‐point Likert scale. Note that for the actual fMRI experiment, only sports film clips and negative pictures were used and neutral control stimuli were only included in the postratings.
During the fMRI experiment, film clips and images were presented in the middle of the screen with an 800 × 600 pixel display subtending 32° × 24° visual angle on dual display goggles (VisuaStim, MR Research, USA) using the stimulation software Presentation (Version 14.1, Neurobehavioral Systems, USA). Film clips subtended a 32° × 21° visual angle and IAPS pictures, a 24° × 18° visual angle, presented against a black background.
Experimental Design and Procedure
Before the scanning session, subjects received an introduction to skydiving and BASE jumping explaining the sport and watched a short movie for illustration.
Task design has been adapted from previous studies on emotion regulation (e.g., Kim and Hamann, 2007). Three task conditions were implemented in the experiment (Fig. 2). (1) In the Look condition, subjects were presented with film clips or pictures and were asked to view the stimuli attentively and allow themselves to experience/feel any emotional responses, which these might elicit without trying to manipulate them. (2) In the Increase condition, subjects were asked to engage themselves with the depicted situation and to increase their sense of subjective closeness to the displayed events by imaging a close friend/family member in the situation depicted in the picture (IAPS) or by imagining themselves as the skydiver or BASE jumper (film clips) [Eippert et al., 2007; Ochsner et al., 2004b; Urry et al., 2009]. This active engagement with the material generated an amplification of their sense of personal and subjective experience as well as the intensity of their negative emotions as the actions unfolded in the film clip. Subjects were explicitly trained before the scanning session to imagine a negative outcome of the depicted situation, e.g., accident and parachute not opening. (3) Conversely, in the Decrease condition, subjects were instructed to reduce the intensity of the negative emotion by distancing themselves from the image (IAPS) or by imagining a positive outcome of the situation by interpreting it as a fun, safe, and joyful event (film clips) [Eippert et al., 2007; Ochsner et al., 2004b; Urry et al., 2009]. Subjects then received a training session to practice the reappraisal strategies before scanning.
Figure 2.
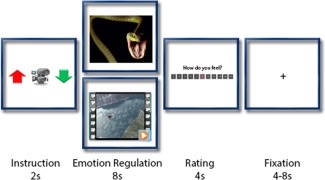
Task design. Each trial started with an instruction screen of 2s, displaying a cue for each experimental condition: Red arrow pointing upward symbolized Increase, video camera symbolized Look and green arrow pointing downwards indicated Decrease. The instruction was followed by an emotion regulation phase in which either an IAPS picture or a film clip was presented, depending on the run. During this 8s phase, participants were asked to either upregulate (Increase) or downregulate (Decrease) their emotions or not modulate their emotions at all (Look). In the next display, participants were asked to rate their current emotional state on a scale from −5 (very negative) to +5 (very positive), followed by a jittered fixation phase of 4–8s. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
An event‐related design was used implementing the three task conditions in pseudorandomized order. Each trial started with an instruction screen (2 s) indicating the experimental condition using a cue (camera symbol: Look; red arrow pointing upwards: Increase; green arrow pointing downwards: Decrease). A film clip or image was presented subsequently for 8 s during which the neutral strategy or the emotion regulation strategy had to be applied. Next, subjects were asked to rate their current emotional state on a scale from −5 to +5 (extremely negative to extremely positive) by pressing a button on a two button fiber optic response pad (fORP, Cambridge Research Systems Ltd., England), providing a measure of trial‐by‐trial emotion regulation success. The extremes of the emotional state ratings were not labelled during the fMRI experiment, only the scale from −5 to +5 was presented. Finally, a fixation cross presented in the middle of the screen for 4, 6, or 8 s concluded the trial.
Subjects performed 10 runs of the experiment, five of which used film clips and five used pictures in alternating order, starting with a film clip run. Each run consisted of 18 trials (6 trials per condition). The presentation of the film clips and images was randomized within one run and between subjects. One trial lasted 20s on average, one run lasted about 6 min, thus one scanning session with 10 runs resulted in about 1 h of scanning.
fMRI Data Acquisition
Whole brain functional and anatomical images were acquired using a 3.0 T Magnetom TrioTim MRI scanner (Siemens, Erlangen, Germany) and a 12‐channel head coil. A high‐resolution 3D T1‐weighted dataset was acquired for each subject (176 sagittal sections, 1 × 1 × 1 mm³; 256 × 256 data acquisition matrix). Functional images were acquired using a T2*‐weighted, gradient‐echo echo planar imaging (EPI) pulse sequence recording 37 sections oriented parallel to the anterior and posterior commissure at an in‐plane resolution of 3 × 3 × 3 mm³ (interslice gap = 0; TE = 30 ms; TR = 2s; FA = 90°; FoV = 192 × 192 mm2; 64 × 64 data acquisition matrix). For each experimental run, 185 whole‐brain volumes were recorded.
Data Analyses
Behavioral data
As we were interested in successful emotion regulation, we calculated reappraisal success scores based on the affect ratings acquired after each trial. Reappraisal success was defined as either a decrease or increase in reported emotion when applying a cognitive reappraisal strategy relative to the mean affect ratings of the Look condition representing the “natural” emotional response to the stimuli. On this basis each reappraisal trial (Increase or Decrease) was categorized as either successful or unsuccessful by subtracting the affect rating from the mean baseline (Look) [Wager et al., 2008]. Hence, negative values during Increase represent successful trials (subject reported stronger negative affect) while positive values represent unsuccessful trials (subject reported weaker negative affect) and vice versa for Decrease. Reappraisal success scores were calculated as the total number of successful reappraisal trials for each subject for both reappraisal conditions separately.
fMRI data
Functional imaging data analysis was performed using SPM8 (Statistical Parametric Mapping, Wellcome Institute for Cognitive Neurology, London, UK) and Matlab 8.0.0 (MathWorks, Natick, MA, USA). For all univariate analyses, preprocessing of fMRI data included slice time correction, realignment to the mean image, coregistration to the individual T1‐weighted anatomical images as well as spatial normalization to the standard EPI template, reslicing to 3 × 3 × 3 mm voxels (Montreal Neurological Institute, MNI template, as implemented in SPM8). Spatial smoothing was performed using an 8mm full‐width at half‐maximum (FWHM) isotopic Gaussian kernel. For the multivariate analysis, data were slice time corrected and realigned to the mean image, but no normalization and smoothing was performed. Regions of interest (see below) were defined at group level and transformed back into individual space for analysis.
The fMRI analysis proceeded in four steps. (1) First, we determined regions showing significant reappraisal‐related activation (Increase>Look, Decrease>Look), which was the basis for the identification of the General Emotion Regulation Network (GERN). (2) Then, we determined regional‐average activation differences between the reappraisal goals (Increase>Decrease, Decrease>Increase) within the GERN (obtained from Step 1). Based on this contrast, the regions of the GERN were subdivided into regions with and without corresponding differences in average local activation. (3) In the next step, we investigated the representational content of those regions (obtained from Step 2) in which regional‐average activation differences between reappraisal goals were absent by applying pattern information analysis. (4) Finally, we examined the relation between patterns of neural activity and behavioral performance in terms of reappraisal success.
Univariate Analyses
The first‐level fixed effects model consisted of a set of five regressors (Instruction, Increase, Decrease, Look, Rating) convolved with the hemodynamic response function and six regressors describing head motion. In a second‐level random effects group analysis effects of reappraisal condition and stimulus type were compared using a flexible factorial design (3 × 2 design with the factors condition (Increase, Decrease, and Look) and stimulus type (IAPS, film clips)). T‐statistics for each voxel were thresholded at p < 0.05 corrected for multiple comparisons across whole brain with family wise error rate (FWE) using a cluster size of 100 voxels. This cluster size was chosen to assist in the identification of the major hubs implicated in emotion regulation. All trials were included in the univariate analysis regardless whether it was a successful or unsuccessful trial.
First, we tested for stimulus‐dependent differences in emotion regulation. We identified regions, which were involved in emotion regulation independent of the reappraisal goal used [(Increase > Look)OR(Decrease > Look)], but depending on stimulus type (film clips, IAPS). This analysis showed a large regional overlap between the activation patterns induced by the film clips and the IAPS and enhanced activity for film clips compared to IAPS in general. Regions involved in both contrasts were subsumed and labeled the general emotion regulation network (GERN) (Fig. 4A). To validate the identification of the GERN, a control analysis was conducted using a conjunction analysis [(Increase > Look)AND(Decrease > Look)], which included all regions involved in both reappraisal goals but exhibited no differential activity between them, again separately for both stimulus types. Thus, the GERN involved all regions responding to the Increase or the Decrease condition and all regions that demonstrated differential activity between those conditions, revealing the maximal extent of the network. In contrast, the network revealed by the conjunction contrast would be restricted to conjointly activated regions during reappraisal, and probably best described as the minimal network.
Figure 4.
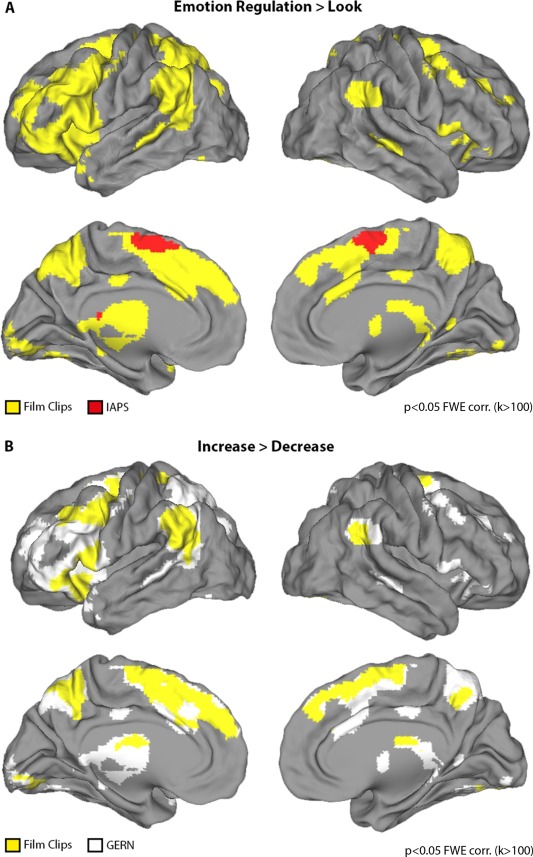
(A) Effects of reappraisal. Contrast [(Increase>Look) OR (Decrease>Look)] for film clips (yellow) and IAPS (red). (B) Contrast between Increase > Decrease for film clips (yellow) overlaid on the General Emotion Regulation Network (GERN) indicated in white. The same contrast for IAPS did not reveal any significant clusters at this threshold.
Second, within the GERN, which was used as a mask, we determined differences between the reappraisal conditions by contrasting Increase versus Decrease: (Decrease > Increase) and (Increase > Decrease), separately for each stimulus type. This analysis investigated activation differences between reappraisal conditions independent of the difference in activation of each reappraisal condition from baseline (Look). The identified regions represented the part of the GERN in which goal‐specific activation differences could be observed.
On the basis of those two analysis steps, we also identified a set of ROIs within the GERN in which no activation differences between Increase and Decrease were found. This was achieved by subtracting the goal‐specific ROIs from the GERN and obtaining remaining clusters (with a minimal voxel size of 100) (Fig. 5A). The ROIs were defined using the toolbox MarsBaR (http://marsbar.sourceforge. net/; [Brett et al., 2002]). We then asked whether these regions, which were found to be part of the GERN but show no differential effect in favor of one of the reappraisal goals, were truly goal‐unspecific (i.e., involved in both reappraisal goals) or still encoded information about the applied reappraisal goal. For this, we performed a subsequent pattern classification analyses on the second set of ROIs (obtained on a group level) (Table 1), which were transferred back into individual subject space and used as masks.
Figure 5.
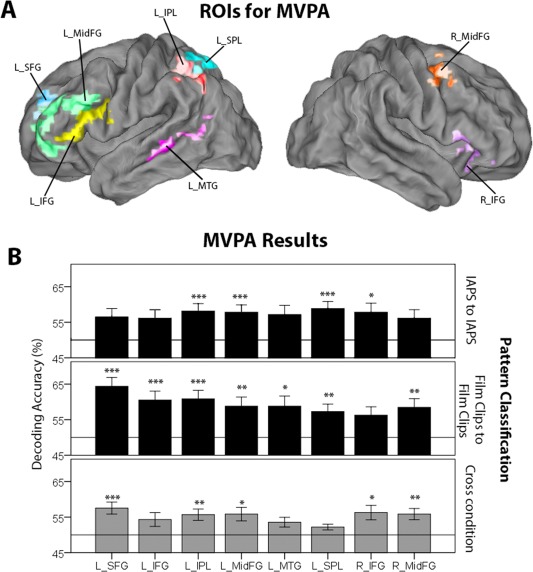
(A) Subtracting regions that showed goal‐specific activation differences (Increase>Decrease; Decrease>Increase) from the General Emotion Regulation Network (GERN) resulted in regions of interests (ROIs) for the multivoxel pattern analysis (MVPA): Left inferior drontal gyrus (L_IFG, yellow), left superior frontal gyrus (L_SFG, blue), left middle frontal gyrus (L_MidFG, green), left intraparietal lobe (L_IPL, red), left superior parietal lobe (L_SPL, turquoise), left middle temporal gyrus (L_MTG, pink), right middle frontal gyrus (R_MidFG, orange), and right inferior frontal gyrus (R_IFG, violet). (B) Pattern classification results for reappraisal goals. The upper panel shows the results of the pattern classification based only on IAPS. The middle panel displays the results of the pattern classification based on the film clips. The lower panel illustrates the results of the cross‐condition decoding. Error bars represent standard errors. ***Indicates significance from chance level (50%) at p < 0.001, ** at p < 0.01, and * at p < 0.05 (Bonferroni corrected).
Table 1.
Regions of Interest for MVPA
| Coordinates | ||||
|---|---|---|---|---|
| Region | x | y | z | size |
| Left Superior Frontal Gyrus (L_SFG) | −23 | 50 | 26 | 126 |
| Left Inferior Frontal Gyrus (L_IFG) | −49 | 20 | 16 | 176 |
| Left Intraparietal Lobe (L_IPL) | −41 | −52 | 48 | 185 |
| Left Middle Frontal Gyrus (L_MidFG) | −37 | 40 | 17 | 337 |
| Left Middle Temporal Gyrus (L_MTG) | −50 | −44 | 2 | 109 |
| Left Superior Parietal Lobe (L_SPL) | −25 | −60 | 56 | 147 |
| Right Inferior Frontal Gyrus (R_IFG) | 45 | 24 | −1 | 149 |
| Right Middle Frontal Gyrus (R_MidFG) | 38 | 3 | 52 | 175 |
Coordinates refer to MNI coordinate system.
Size is given in mm³.
Multivariate Analyses
The multivariate analysis comprised two different pattern classification techniques [Haynes and Rees, 2006; Norman et al., 2006]. In the first step, we applied a multivariate pattern classification algorithm to the predefined ROIs, while in the second step, we performed a control analysis using a searchlight approach throughout the whole brain [Kriegeskorte et al., 2006]. All trials were included in the multivariate analysis regardless whether it was a successful or unsuccessful trial.
ROI approach
The GLM parameter estimates were extracted for each voxel within the ROIs that showed no goal‐specific activation effect. This was done separately for each stimulus category (IAPS, film clips) and each reappraisal goal (Decrease and Increase), and separately for each run and each subject. First, for each subject, the parameter estimates from each region and each run were transformed into pattern vectors, representing the spatial activation patterns associated with each reappraisal goal in the respective region. These pattern vectors from each ROI were used as input for separate pattern classification analyses.
Whole‐brain approach
This analysis included the whole brain excluding all regions showing goal‐dependent activation effects and all ROIs. For a given position in the brain, a searchlight cluster was defined as a sphere of N voxels (c1… N), with radius of 3 voxels, constructed around the central voxel νi and transformed in an N‐dimensional pattern vector (see Bode et al., 2013, 2012). The GLM parameter estimates were extracted for these searchlight voxels for each stimulus category (IAPS, film clips) and each reappraisal goal (Decrease and Increase), and separately for each run and each subject. Again, parameter estimates from each searchlight cluster were transformed into pattern vectors and used to separate the pattern classification analyses.
Multivariate pattern classification algorithm
A multivariate pattern classification algorithm was applied to decode the reappraisal goal from the pattern vectors of each ROI cluster and searchlight cluster. We used a linear support vector machine (SVM) (LIBSVM toolbox, http://www.csie.ntu.edu.tw/~cjlin/libsvm) with a fixed regularization parameter C = 1 [Chang and Lin, 2011]. For data from each ROI, the classifier was trained on pattern vectors (Increase vs Decrease) from all runs but one to establish a decision boundary to distinguish between spatial activation patterns associated with the two reappraisal goals on a single subject level. This is referred to as “training data set.” The trained classifier was then tested on data from the independent left‐out run, i.e., the “test data set.” A fivefold cross‐validation was performed by repeating the classification process independently with the pattern vectors of each run as the “test data set” while training the classifier on data from the remaining runs. The average accuracy of all cross‐validation steps finally reflected how well the patterns of activation within the particular region allowed classifying the reappraisal goal, or in other words, whether this region encoded information about the reappraisal goals. Statistical significance was assessed by testing decoding accuracy values across subjects and runs for each ROI or searchlight cluster (chance level was 50% for two goals) using Bonferroni correction. Note that using independent cross‐validation as well as a selection of ROIs that was not based on a difference contrast between the reappraisal goals circumvented circular inference at any stage of the analyses [Mur et al., 2009]. The use of the same number of exemplars for decoding for each condition and each run further guaranteed that both reappraisal conditions were always equally represented in the training and test data.
Cross‐condition classification
Separate analyses were conducted for each stimulus type (IAPS, film clips) for (A) the ROIs and (B) the searchlight clusters. For the Within‐GERN ROIs, in a second step, we also performed a cross‐condition classification analysis [Bode et al., 2013]. For this, the pattern vectors from all runs of one stimulus category (either IAPS or film clips) were used to train the classifier to distinguish between activation patterns associated with Increase and Decrease. Instead of predicting the reappraisal goals from data from the left‐out run of the same stimulus condition as before, this time we predicted the reappraisal goal for each run of the other stimulus category (i.e., film clips for training on IAPS, IAPS for training on film clips). We then repeated the analysis with switched training and testing categories and averaged across both cross‐condition analyses. Finally, we tested whether the obtained cross‐condition classification accuracies were significant.
Correlation with emotion regulation success
Decoding accuracies of all ROIs (average accuracy obtained for each participant for each ROI) were correlated with the reappraisal success score for each stimulus category separately. We used a partial correlation controlling for age and total number of jumps. We correlated the accuracy of the classifier (predicting the emotion regulation goal: Increase vs Decrease) of each stimulus category (IAPS/film clips) with the reappraisal success (mean % successful trials in Increase and Decrease). Thus, a positive correlation of reappraisal success and decoding accuracy during emotion regulation reflected increased prediction of the emotion regulation goal with higher success scores. Such a relationship would imply that the better the classifier could differentiate between Increase and Decrease, the better participants could regulate their emotions.
RESULTS
Individual Differences
Several questionnaires were administered to ensure that our sample did not differ from the reported population averages on relevant scales (less than one standard deviation (SD) from the mean). Our sample did not differ from the reported population averages on state anxiety, alexithymia, sensation seeking, and reappraisal (Table 2).
Table 2.
Questionnaires
| female (n = 20) | Male (n = 39) | Total (n = 59) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Questionnaire | M | SD | PA | PA SD | M | SD | PA | PA SD | M | SD | PA | PA SD |
| STAI (pre‐experiment) | 35.50 | 5.8 | 35.76 | 10.5 | 35.67 | 9.1 | 36 | 10 | ||||
| STAI (postexperiment) | 37.36 | 6.0 | 36.02 | 7.48 | 36.58 | 7.0 | ||||||
| TAS‐20 | 43.00 | 7.5 | 43.45 | 9.8 | 44.89 | 9.9 | 47.19 | 10.3 | 44.25 | 9.16 | ||
| Reappraisal (ERQ) | 3.16 | 1.07 | 4.24 | 0.9 | 2.92 | 0.9 | 4.07 | 1.2 | 3.00 | 0.9 | ||
| Suppression (ERQ) | 5.23 | 1.13 | 2.92 | 0.93 | 4.39 | 1.20 | 3.45 | 1.12 | 4.68 | 1.23 | ||
| ERSQ | 3.44 | 1.15 | 3.38 | 1.04 | 3.40 | 1.06 | 2.71 | 0.52 | ||||
| SSS | 18.85 | 3.5 | 16.9 | 5.5 | 19.53 | 3.0 | 19.9 | 6.6 | 19.30 | 3.16 | ||
STAI: State Trait Anxiety Inventory; TAS‐20: Toronto Alexithymia Scale‐20; ERQ: Emotion Regulation Questionnaire; SSS: Sensation Seeking Questionnaire.
M, mean; SD, standard deviation; PA, population average; PA SD, standard deviation of population average.
To assess whether subjects differed in the habitual use of emotion regulation strategies (reappraisal and suppression) and emotion regulation skills due to the participation in extreme sports, we tested for differences on the ERQ and ERSQ between the three subject groups. There were no significant differences between non‐sports participants and novice skydivers (ERQ: reappraisal: t(37) = 0.96, p = 0.34; suppression: t(37) = −0.26, p = 0.79; ERSQ: t(34) = −1.32, p = 0.19), between nonsports participants and experienced skydivers/BASE jumpers (ERQ: reappraisal: t(38) = 0.57, p = 0.56; suppression: t(38) = −0.87, p = 0.39; ERSQ: t(35) = −0.15, p = 0.87), and between novice and experienced sports participants (ERQ: reappraisal: t(37) = −0.36, p = 0.71; suppression: t(37) = −0.67, p = 0.51; ERSQ: t(37) = 1.22, p = 0.23).
Furthermore, we tested whether the three groups of subjects differed in terms of emotion regulation success. For this, we performed a repeated measures 3 (groups: Control, Novice, and Experts) by 2 (tasks: Successful Increase and Successful Decrease) by 2 (stimulus category: IAPS and film clips) ANOVA. The emotional state ratings inside the scanner did not reveal any significant differences between groups in respect to successful emotion regulation (interaction effects: task*group: F(2,174) = 0.25, p = 0.78; stimulus category*group: F(2,174) = 1.58, p = 0.22; task*stimulus category*group: F(2,178) = 2.58, p = 0.09), but only a main effect of task (F(1,18) = 15.79, p < 0.001).
The absence of group differences at the behavioral level does not necessarily indicate the use of equal emotion regulation strategies, which most likely would play out more strongly in real‐world situations. However, it indicates similar performance in emotion regulation in the task in the scanner.
Behavioral Results
Emotion induction
After scanning, subjects rated their ability to regulate their emotions during the study (1 = very bad to 7 = very good) as above the mean of the rating scale for Increase (IAPS: M = 4.49, SD = 1.31, t(58) = 5.77, p < 0.001; film clips: M = 4.62, SD = 1.25, t(58) = 6.88, p < 0.001) and Decrease (IAPS: M = 4.23, SD = 1.22, t(58) = 4.63, p < 0.001; film clips: M = 4.31, SD = 1.28, t(58) = 4.84, p < 0.001), and for their ability to vividly put themselves into the depicted situation (IAPS: M = 4.99, SD = 1.07, t(58) = 10.64, p < 0.001; film clips: M = 5.33, SD = 1.19, t(58) = 11.79, p < 0.001).
To assess whether the stimuli induced the desired emotion, subjects rated all previously seen film clips on valence and arousal in a post‐scan rating session on a nine‐point Likert scale. Applying a 2 (stimulus category: IAPS, film clips) × 2 (emotional category: negative/sports film clips, neutral) repeated measures ANOVA, we observed a significant main effect for stimulus category (F(1,58) = 303.0, p < 0.001) and emotional category (F(1,58) = 194.55, p < 0.001), as well as a significant interaction effect between both factors (F(1,58) = 88.81, p < 0.001). Post‐hoc t‐test showed that negative IAPS pictures were rated more negatively than extreme sports film clips (t(58) = −16.75, p < 0.001). Neutral film clips were rated less negatively than neutral IAPS pictures (t(58) = 12.90, p < 0.001). Negative IAPS pictures were rated more negative than neutral ones (t(58) = −20.41, p < 0.001) and extreme sports film clips were perceived more negative than neutral film clips (t(58) = −4.21, p < 0.001). Similar effects were observed for arousal ratings. The ANOVA revealed significant main effects for stimulus category (F(1,58) = 28.43, p < 0.001) and emotional category (F(1,58) = 332.67, p < 0.001) as well as an interaction effect between both factors (F(1,58) = 10.60, p < 0.01). Negative IAPS pictures were rated less arousing than extreme sports film clips (t(58) = −3.21, p < 0.01). Neutral IAPS pictures were rated less arousing than neutral film clips (t(58) = −5.49, p < 0.001). Extreme sports film clips and negative IAPS pictures were rated more arousing than their neutral counterparts (extreme sports film clips: t(58) = 14.63, p < 0.001; IAPS: t(58) = 16.64, p < 0.001). As expected, negative IAPS pictures were rated as being more negative than film clips, but film clips were rated as being more arousing, indicating that the experimental manipulation was successful.
Skin conductance measures were acquired during scanning and provided additional evidence that the emotion induction was successful. A 3 (task: Increase, Decrease, Look) × 2 (stimulus category: IAPS, film clips) repeated measures ANOVA showed main effects of task (F(1,45) = 3.81, p < 0.05) and stimulus category (F(1,46) = 27.45, p < 0.001), but no significant interaction effect (Fig. 3A). The skin conductance response amplitudes were significantly greater for extreme sports film clips compared to IAPS pictures (t(46) = 5.23, p < 0.001), and within the film clips condition a significant difference between Increase and Decrease was observed (t = 46) = 2.15, p < 0.05), confirming the rating results. A significant difference between Increase and Look within the IAPS condition was also observed (t = 46) = 2.36, p < 0.05).
Figure 3.
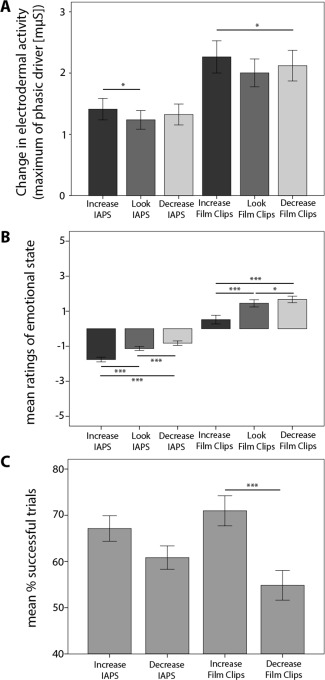
(A) Skin conductance responses during scanning. Mean changes in electrodermal activity as a function of experimental condition and stimulus category. (B) Emotional state ratings as a function of task condition and stimulus category. After each emotion regulation phase, subjects rated their current emotional state on a scale from −5 (very negative) to +5 (very positive). (C) Effective emotion regulation during scanning. Mean success scores as a function of task condition and stimulus category. Error bars represent standard errors. ***Indicates significant difference between conditions at p < 0.001 and * at p < 0.05.
Emotion regulation
In addition to the postscan measures, participants rated their emotional state (on a scale from −5 to +5) after each trial in the scanner. A significant main effect was found for task condition (Increase, Decrease, Look; F(1,58) = 47.53, p < 0.001) and stimulus category (IAPS, film clips; F(1,58) = 118.83, p < 0.001), as well as an interaction effect between task and stimulus category, bordering significance (F(1,58) = 2.95, p = 0.05) (Fig. 3B). Significantly greater negative affect was reported for the IAPS pictures compared to the film clips for all task conditions (Look: t(58) = −10.78, p < 0.001; Increase: t(58) = −9.21, p < 0.001; Decrease: t(58) = −11.20, p < 0.001). These ratings also allowed us to determine whether emotion regulation was successful. Subjects reported significantly more negative emotions when intentionally upregulating compared to when downregulating emotional responses for both stimulus categories (film clips: t(58) = −6.14, IAPS: t(58) = −7.73, p < 0.001), and the reappraisal conditions significantly differed from Look in the expected direction (Increase > Look: film clips: t(58) = −5.86, p < 0.001; IAPS: t(58) = −8.01, p < 0.001; Look > Decrease: film clips: t(58) = 2.21, p < 0.05; IAPS: t(58) = 3.52, p = 0.001), confirming the successful increase or decrease of emotional responses. Participants were more effective in emotion regulation for the Increase condition compared to the Decrease condition, as can be seen when ratings were converted into Effectiveness scores (t(58) = 3.57, p < 0.001) (see Experimental Procedures). A 2 (task: Increase, Decrease) × 2 (stimulus category: IAPS, film clips) ANOVA revealed a significant main effect for task (F = (1,58) = 12.89, p < 0.001) (Fig. 3C). The difference in effectiveness for Increase and Decrease was more pronounced for film clips (t(58) = 3.86, p < 0.001).
As the sample consisted of novice and experienced skydivers/BASE jumpers as well as participants with no experience in skydiving/BASE jumping, we investigated the association of reappraisal success and sports experience represented in the total number of jumps. A partial correlation (controlling for age) between reappraisal success and number of jumps did not reveal any significant results (IAPS: r = 0.05, p = 0.74; Film Clips: r = 0.19, p = 0.25).
fMRI Results
Identification of the general emotion regulation network (GERN) using mean activation‐based analysis
Results from the first contrast between both regulation goals (Decrease, Increase) against the control trials (Look) are shown in Figure 4 A and Table 3. This analysis was conducted separately for film clips and IAPS pictures (see Data Analysis for details) and included all regions, which were activated during either reappraisal condition. Consistent with previous work we found enhanced activity in a number of prefrontal regions in response to the reappraisal goals in dorsal and ventral PFC, including bilateral IFG, left middle frontal gyrus (MidFG), and left superior frontal gyrus (SFG). MPFC regions included anterior cingulate cortex (ACC), supplementary motor area (SMA), and DMPFC. Posterior cortical regions included the left inferior parietal lobe (IPL), bilateral angular gyrus, bilateral supramarginal gyrus, bilateral precuneus, and bilateral middle temporal gyrus (MTG). Note that the activated network for reappraisal was more widespread for film clips than for IAPS pictures.
Table 3.
GERN (Whole Brain Univariate Analysis)
| Coordinates | ||||||
|---|---|---|---|---|---|---|
| Region | Size | t‐value | p | x | y | z |
| Increase>Look (IAPS) | ||||||
| R Supp. Motor Area | 265 | 7.56 | 0.001 | 3 | 5 | 64 |
| L Hippocampus | 122 | 7.19 | 0.001 | −15 | −37 | 16 |
| R Hippocampus | 130 | 6.24 | 0.001 | 33 | −43 | 4 |
| R Cerebellum | 181 | 5.90 | 0.001 | 27 | −55 | −26 |
| Look>Increase (IAPS) | ||||||
| R Angular Gyrus | 139 | 6.51 | 0.001 | 39 | −61 | 52 |
| Decrease>Look (IAPS) | ||||||
| No sign. regions | ||||||
| Look>Decrease (IAPS) | ||||||
| No sign. regions | ||||||
| Increase>Look (Film Clips) | ||||||
| L Supp. Motor Area | 2587 | 11.78 | 0.001 | −6 | 5 | 61 |
| L Inferior Frontal Gyrus | 11.69 | 0.001 | −48 | 23 | −8 | |
| L Cerebellum | 780 | 11.60 | 0.001 | −42 | −61 | −29 |
| L Supramarginal Gyrus | 1112 | 11.57 | 0.001 | −54 | −52 | 31 |
| L Precuneus | 10.02 | 0.001 | −3 | −58 | 61 | |
| R Insula | 122 | 9.04 | 0.001 | 48 | 23 | −8 |
| R Inferior Frontal Gyrus | 8.40 | 0.001 | 54 | 20 | 1 | |
| L Middle Frontal Gyrus | 145 | 8.82 | 0.001 | −30 | 56 | 16 |
| L Thalamus | 114 | 8.09 | 0.001 | −1 | −13 | 16 |
| Look>Increase (Film Clips) | ||||||
| No sign. regions | ||||||
| Decrease>Look (Film Clips) | ||||||
| L Inferior Frontal Gyrus | 288 | 6.44 | 0.001 | −48 | 35 | −5 |
| L Supp. Motor Area | 158 | 6.29 | 0.001 | −6 | 8 | 61 |
| Look>Decrease (Film Clips) | ||||||
| No sign. regions | ||||||
L, left. R, right. Coordinates refer to MNI coordinate system.
p < 0.05 FWE corrected (k > 100).
Regional average activation differences between reappraisal goals within the GERN
The following analysis was restricted to the GERN identified in Step 1. The contrast between Increase versus Decrease revealed only differences in signal change during the film clips condition, as shown in Figure 4 B and reported in detail in Table 4. The reverse contrast, analyzing for more activation for Decrease versus Increase, did not show significant effects for any stimulus condition. Regional‐average activation differences between reappraisal goals were found in the dorsal and ventral PFC (IFG, SFG), MPFC (SMA, DMPFC), and posterior cortical regions (angular gyrus, supramarginal gyrus, precuneus, MTG). All other regions within the GERN were activated by both reappraisal goals similarly as no differences in local average activation were observed. These comprised a set of prefrontal regions (SFG, IFG, MidFG) and posterior cortical regions including IPL, superior parietal lobe (SPL), and MTG, which were subsequently used as ROIs for the pattern‐based classification analysis.
Table 4.
Contrast between Task Conditions (Masked with GERN)
| Coordinates | ||||||
|---|---|---|---|---|---|---|
| Region | size | t‐value | p | x | y | z |
| Increase>Decrease (IAPS) | ||||||
| No sign. regions | ||||||
| Decrease>Increase (IAPS) | ||||||
| No sign. regions | ||||||
| Increase>Decrease (Film Clips) | ||||||
| L Angular Gyrus | 476 | 8.54 | 0.001 | −51 | −52 | 31 |
| R Medial Superior Frontal Gyrus | 1803 | 7.54 | 0.001 | 3 | 32 | 52 |
| L Supplementary Motor Area | 7.03 | 0.001 | −9 | −1 | 64 | |
| L Insula | 498 | 6.70 | 0.001 | −30 | 26 | −8 |
| L Inferior Frontal Gyrus | 6.56 | 0.001 | −42 | 23 | −8 | |
| R Supramarginal Gyrus | 150 | 6.05 | 0.001 | 66 | −43 | 25 |
| R Middle Temporal Gyrus | 5.00 | 0.002 | 42 | −46 | 19 | |
| L Caudate | 239 | 5.74 | 0.001 | −15 | 11 | 13 |
| R Thalamus | 5.64 | 0.001 | 12 | −22 | 19 | |
| L Thalamus | 5.49 | 0.001 | −9 | −19 | 19 | |
| L Precuneus | 344 | 5.63 | 0.001 | −3 | −49 | 58 |
| R Fusiform Gyrus | 127 | 5.66 | 0.001 | 36 | −73 | −17 |
| L Cerebellum | 130 | 5.49 | 0.001 | −15 | −85 | −17 |
| Decrease>Increase (Film Clips) | ||||||
| No sign. regions | ||||||
L, left. R, right. Coordinates refer to MNI coordinate system.
p < 0.05 FWE corrected (k > 100).
Investigation of the representational content of the GERN regions using pattern‐information analysis
In this analysis step, we aimed to detect goal‐specific activation patterns within those GERN regions in which differences in regional‐average activation for both reappraisal goals were absent. No differences between activation patterns would point to a general involvement in emotion regulatory processes (for example, by general arousal up‐ or downregulation processes for both goals), while systematic, informative differences in patterns (i.e., the predictability of strategies from activation patterns) would point to a representation of reappraisal goals in these areas. To test whether regulation goals were represented in such an arousal‐independent manner, we applied MVPA to the eight ROIs obtained in Step 2: left SFG, bilateral IFG, bilateral MidFG, left IPL, left SPL and left MTG (Fig. 5A, details in Table 1). We trained a linear support vector machine classifier [Chang and Lin, 2011] on fine‐grained activation patterns in these ROIs, and then tested whether these allowed for the prediction of the reappraisal goals using independent data (see Data Analysis for details). MVPA was performed for both stimulus conditions separately.
For IAPS pictures, Increase and Decrease could successfully be decoded from left IPL, left MidFG, left SPL, and right IFG, shown in Figure 5B and Table 5 (upper panel). Left SFG, IFG, IPL, MTG, SPL, and bilateral MidFG significantly decoded the two reappraisal goals during the film clips runs, shown in Figure 5B (middle panel). These results demonstrate that in reappraisal regions that lack local average activation differences, robust spatial pattern differences were nevertheless present, and these regions contained representational information about the emotion regulation goals.
Table 5.
MVPA Results
| Accuracy | ||||||
|---|---|---|---|---|---|---|
| Pattern classification | Region | M | SE | t‐value | p‐value | Bonferroni |
| IAPS to IAPS | L_SFG | 56.44 | 2.42 | 2.65 | 0.01 | |
| L_IFG | 56.10 | 2.41 | 2.52 | 0.01 | ||
| L_IPL | 58.13 | 2.11 | 3.84 | 0.0001 | 0.001 | |
| L_MidFG | 57.79 | 2.08 | 3.74 | 0.0001 | 0.001 | |
| L_MTG | 57.11 | 2.63 | 4.35 | 0.009 | ||
| L_SPL | 58.81 | 2.02 | 2.69 | 0.0001 | 0.001 | |
| R_IFG | 57.79 | 2.58 | 3.02 | 0.004 | 0.05 | |
| R_MidFG | 56.10 | 2.44 | 2.49 | 0.01 | ||
| Film Clips to Film Clips | L_SFG | 64.40 | 2.45 | 5.86 | 0.0001 | 0.001 |
| L_IFG | 60.50 | 2.50 | 4.19 | 0.0001 | 0.001 | |
| L_IPL | 60.84 | 2.40 | 4.50 | 0.0001 | 0.001 | |
| L_MidFG | 58.81 | 2.54 | 3.45 | 0.001 | 0.01 | |
| L_MTG | 58.81 | 2.85 | 3.09 | 0.003 | 0.05 | |
| L_SPL | 57.28 | 2.09 | 3.48 | 0.001 | 0.01 | |
| R_IFG | 56.27 | 2.36 | 2.64 | 0.01 | ||
| R_MidFG | 58.47 | 2.41 | 3.50 | 0.001 | 0.01 | |
| Cross Prediction | L_SFG | 57.54 | 1.69 | 4.45 | 0.0001 | 0.001 |
| (IAPS to Fim Clips; | L_IFG | 54.32 | 1.94 | 2.22 | 0.03 | |
| Film Clips to IAPS) | L_IPL | 55.67 | 1.59 | 3.55 | 0.001 | 0.01 |
| L_MidFG | 55.84 | 1.90 | 3.07 | 0.003 | 0.05 | |
| L_MTG | 53.55 | 1.36 | 2.60 | 0.01 | ||
| L_SPL | 52.20 | 0.81 | 2.70 | 0.009 | ||
| R_IFG | 56.27 | 2.04 | 3.07 | 0.003 | 0.05 | |
| R_MidFG | 55.84 | 1.58 | 3.68 | 0.001 | 0.01 | |
One sample t‐tests against chance level (50%).
Bonferroni correction for 8 tests within each pattern classification.
We additionally performed a cross‐condition classification analysis (Bode et al., 2013), for which the classifiers were trained on IAPS and tested on film clips, and vice versa. This analysis allowed us to test for a stimulus‐independent coding of reappraisal goals in the ROIs. We found that goal‐associated activation patterns in the left SFG, left IPL, bilateral MidFG, and right IFG were highly similar for pictures and film clips as bidirectional cross‐decoding was successful, shown in Figure 5B (lower panel).
Relation between emotion regulation success and neural representations of reappraisal goals
We then sought to establish a relation between behavioral performance and the neural representations of reappraisal goals, as revealed by MVPA. Specifically, we tested the hypothesis that subjects' behavioral ability to up‐ or downregulate their emotions (reappraisal success score) depended on the distinctness of the underlying neural representations. The distinctness of the neural representations was measured in MVPA classification accuracy (i.e., the separability of the multivoxel spatial patterns for Increase and Decrease) and correlated with the individual subjects' reappraisal success scores. This analysis was conducted separately for film clips and IAPS pictures. Note that subjects were significantly more successful in up‐ compared to downregulation of emotion independent of stimulus category.
The results show that the separability of the spatial patterns during the film clip runs in left IFG, left IPL, and left MTG significantly predicted how well the subjects could regulate their emotions (IFG: r = 0.26 p < 0.05; IPL: r = 0.27, p < 0.05; MTG: r = 0.48, p < 0.001). As our sample consisted of participants with a wide range of experience in emotion regulation in relevant real‐life situations including novice and experienced extreme sports participants as well as participants with no extreme sports experience, we controlled for age and experience in sports in terms of total number of jumps. This was quantified using a partial correlation analysis, which confirmed the significant positive correlation after removing any effects of age and sports experience (IFG: r = 0.35, p < 0.05; IPL: r = 0.40, p < 0.05; MTG: r = 0.54, p < 0.001), shown in Figure 6. The more distinguishable the underlying neural representations of the reappraisal goals were for the classifier, the more successful participants were in regulating their emotions. For IAPS pictures all results were weaker in general and this association was not significant.
Figure 6.
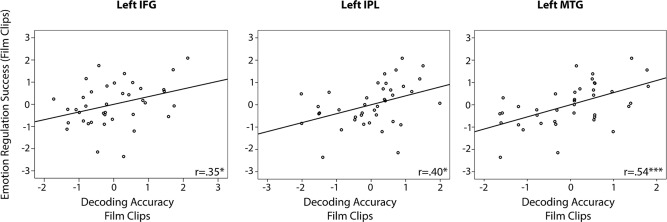
Partial correlations between emotion regulation success and decoding accuracies controlled for age and total number of jumps. Each data point represents a measurement from one participant (standardized residuals). The solid black lines indicate the linear regression for each panel. Correlation coefficients and statistical significance are denoted in the lower right corner of each panel. *Indicates significant a significant correlation at p < 0.05 and *** at p < 0.001.
Control analyses
First, we conducted a conjunction analysis searching for GERN regions that were activated conjointly by up‐ and downregulation dependent on stimulus type but exhibited no differential activity between them. This analysis showed that SMA, left IFG and left precuneus were jointly activated by both up‐ and downregulation (Table 6). Furthermore, we performed a broader, unrestricted conjunction analysis searching for regions that were activated conjointly by the up‐ as well as downregulation of emotion across stimulus type on a whole‐brain level. This analysis showed a more extended network of regions implicating the SMA, bilateral IFG, left angular gyrus, and left STG (Table 7); however, it did not allow us to separate out the joint network for both stimulus types. Both conjunction analyses confirmed that parts of the GERN were activated by reappraisal for both stimulus types.
Table 6.
Conjunction Analysis: Emotion Regulation versus Look (Whole Brain Univariate Analysis)
| Coordinates | ||||||||
|---|---|---|---|---|---|---|---|---|
| Contrast | Region | L/R | Size | t‐value | p (FWE‐corr.) | x | y | z |
| IAPS: (Increase>Look)+(Decrease>Look) | Supplementary Motor Area | L | 131 | 5.69 | 0.001 | −6 | 11 | 64 |
| Film Clips: (Increase>Look)+(Decrease>Look) | Cerebellum | R | 259 | 5.32 | 0.001 | 6 | −82 | −26 |
| Inferior Frontal Gyrus | L | 492 | 5.04 | 0.001 | −48 | 35 | −5 | |
| Precuneus | L | 199 | 4.97 | 0.001 | −9 | −64 | 64 | |
| Supplementary Motor Area | L | 233 | 4.90 | 0.001 | −6 | 8 | 61 | |
| Hippocampus | L | 313 | 4.86 | 0.001 | −27 | −43 | 1 | |
L, left. R, right. Coordinates refer to MNI coordinate system. p < 0.05 FWE corrected (k > 100).
Table 7.
Conjunction Analysis: Emotion Regulation versus Look across Stimulus Material (Whole Brain Univariate Analysis)
| Coordinates | ||||||||
|---|---|---|---|---|---|---|---|---|
| Contrast | Region | L/R | Size | t‐value | p (FWE‐corr.) | x | y | z |
| (Increase>Look)+(Decrease>Look) | Supplementary Motor Area | L | 875 | 13.34 | 0.001 | −3 | 11 | 64 |
| Inferior Frontal Gyrus | R | 550 | 9.82 | 0.001 | −45 | 14 | −2 | |
| Cerebellum | L | 611 | 9.34 | 0.001 | 42 | −64 | −29 | |
| Superior Temporal Gyrus | L | 513 | 8.25 | 0.001 | −51 | −34 | −2 | |
| Angular Gyrus | L | 183 | 8.02 | 0.001 | −45 | −52 | 25 | |
| Inferior Frontal Gyrus | L | 157 | 7.87 | 0.001 | 51 | 14 | 1 | |
L, left. R, right. Coordinates refer to MNI coordinate system. p < 0.05 FWE corrected (k > 100).
Second, to identify regions in which the differential local average activation for the reappraisal goals was modulated by stimulus material, we directly contrasted Increase versus Decrease as function of stimulus type. This analysis showed that the Increase > Decrease activation difference was greater for film clips compared to IAPS pictures in prefrontal regions including medial SFG, MidFG, IFG, and posterior cortical regions including angular gyrus (Table 8). We also conducted a whole‐brain exploratory search for differential average signal changes in response to both reappraisal goals to determine regions that may have been missed in our more constrained masked analysis, but no significant effects were found for this contrast outside the GERN.
Table 8.
Contrast between Task Conditions in Respect to Stimulus Type (Whole Brain Univariate Analysis)
| Coordinates | ||||||||
|---|---|---|---|---|---|---|---|---|
| Contrast | Region | L/R | Size | t‐value | p (FWE‐corr.) | x | y | z |
| (Increase>Decrease)Film Clips > (Increase>Decrease)IAPS | Medial Superior Frontal Gyrus | R | 440 | 7.87 | <0.001 | 6 | 35 | 52 |
| Angular Gyrus | R | 209 | 7.14 | <0.001 | 57 | −52 | 34 | |
| Middle Frontal Gyrus | R | 175 | 6.77 | <0.001 | 39 | 20 | 46 | |
| Inferior Frontal Gyrus | L | 121 | 5.77 | <0.001 | −48 | 29 | −5 | |
| (Decrease>Increase)Film Clips > (Decrease>Increase)IAPS | No sign. regions | |||||||
L, left. R, right. Coordinates refer to MNI coordinate system. p < 0.05 FWE corrected (k > 100).
Third, to locate regions that might have been missed in our constrained masked analysis, we applied the same pattern classification analysis to all other brain regions, sparing the GERN, using a searchlight approach (for details see Methods). This approach controlled for the possibility that other regions, which did not show an overall increase in signal change in response to reappraisal, could still be involved in emotion regulation. Crucially, however, no regions carrying representational information about reappraisal goals were found outside the GERN.
Fourth, we tested for group differences according to sports experience. We performed a whole‐brain analysis using a threshold of FWE p < 0.05 (k > 100) for all relevant contrasts (Increase > Look, Decrease > Look, Increase > Decrease, Decrease > Increase) between groups (Control, Novice, Experts) and for each stimulus category (IAPS, film clips). Only one contrast revealed a significant result, [(Increase film‐clips> Look film‐clips)novice > (Increase film‐clips > Look film‐clips)controls], in the SMA (x = 6, y = 29, z = 52; t = 6.69, k = 111).
DISCUSSION
Several models for the neural mechanisms underlying emotion regulation have been put forth, all of them based on the notion that prefrontal regions modulate amygdala activity [Ochsner et al., 2012; Ochsner and Gross, 2005; Phillips et al., 2008; Quirk and Beer, 2006; Wager et al., 2008]. Two important questions remain unanswered, however: First, which brain regions exactly build the core within the emotion regulation network, linking cortical control and subcortical affective systems? Second, which brain regions contain reappraisal goal‐specific information?
This study used a combination of mean activation‐based and information‐based analyses to identify the general emotion regulation network (GERN) and regions that encoded information of reappraisal goals. We found that the different reappraisal goals were indeed represented in distinct spatial neuronal patterns within the GERN, and that the distinctness of those spatial patterns of brain activation further predicted individual differences in participants' ability to successfully regulate their emotions.
The basic characterization of the GERN extends previous findings in two main aspects regarding stimulus material and emotion regulation abilities. We included film clips as stimulus material that created a more realistic experience of highly salient emotional situations, and by doing so we demonstrated the involvement of a broad network of brain regions in emotion regulation. Furthermore, our sample of participants had a wide range of experience in emotion regulation in real‐life situations similar to the scenes depicted in the film clips, as we deliberately included novice and experienced extreme sports athletes as well as nonsports participants. Our findings can therefore be considered a robust demonstration of the GERN across levels of experience and different stimulus categories, extending previous studies (e.g., Morawetz et al., 2015; Drabant et al., 2009; Eippert et al., 2007; Kim and Hamann, 2007; McRae et al., 2010; Ochsner et al., 2002, 2004a; Urry et al., 2009; Wager et al., 2008). Our results align well with findings of prior studies on reappraisal that consistently reported the recruitment of broad areas of the PFC, including bilateral dorsolateral and ventrolateral PFC (more left‐lateralized), and regions of dorsal ACC and MPFC [Buhle et al., 2013; Morawetz et al., 2015; Ochsner et al., 2012; Ray and Zald, 2012]. Dorsolateral and MPFC regions have been implicated in cognitive control, strategy selection, implementation, monitoring, selective attention, and working memory [Ochsner et al., 2012; Ochsner and Gross, 2005]. Those PFC regions in conjunction with left ventrolateral PFC, appear to modulate the neural processing of intensity and salience of emotional responses in limbic brain regions [Goldin et al., 2008; Ochsner et al., 2004a, 2012] via temporal and inferior parietal lobe regions, which are involved in linguistic processing [Geva et al., 2011a; Jones and Fernyhough, 2007], e.g., labeling affective stimuli [Iacoboni and Wilson, 2006].
Only very few studies so far examined different goals in reappraisal [Ochsner et al., 2012]. Two studies directly contrasted Increase versus Decrease [Eippert et al., 2007; Ochsner et al., 2004b] and showed incoherent results. Ochsner et al. [2004b] found enhanced responses in left rostral MPFC and the posterior cingulate cortex during Increase, whereas right DLPFC and lateral orbital PFC were associated with greater signal changes during decreasing emotions. In contrast, Eippert et al. [2007] reported only significant activation for the Increase versus Decrease contrast in the amygdala, right DLPFC, ACC and right orbitofrontal cortex. Our findings are partly in accord with both studies: a greater recruitment of left frontal regions was found during upregulation [Ochsner et al., 2004b], while there was no significant activation difference for Decrease versus Increase [Eippert et al., 2007]. It has to be noted, however, that we did not use the exact same contrast as previous studies, which used a masking procedure (e.g., Increase > Decrease was masked inclusively with Increase > Look). Furthermore, the differences in task conditions in our study were only observed in the film clips condition, while the other two studies used IAPS pictures. Our study demonstrates that when realistic, highly engaging stimulus material is used, pronounced differences in activation between up‐ and downregulation of emotion can be observed throughout the entire GERN. This is supported by the observation that the response to film clips compared to the pictures was not only stronger at the neural level, but also at the level of subjective experience, as expressed in higher arousal ratings and higher physical arousal, as reflected in enhanced skin conductance responses [Eippert et al., 2007; Urry et al., 2009].
Most regions involved in emotion regulation in our study were activated in a goal‐specific way. Importantly, however, some brain regions within the GERN were likely to be engaged in reappraisal processes in general, as they showed an overall increase in activation, but no differences between reappraisal goals in average activation. These regions, including several frontal (SFG, MidFG/DLPFC, IFG), temporal (MTG), and parietal (IPL, SPL) regions, nonetheless encoded information about the reappraisal goal, which can be interpreted as reflecting systematic differences in underlying neuronal population activity [Mur et al., 2009]. Fine‐grained neural activation patterns for reappraisal goals in all these regions were further highly stable and allowed accurately predicting reappraisal goals across stimulus material, as demonstrated by our cross‐condition classification analysis (Bode et al., 2013). This implies that reappraisal goals were encoded in an abstract fashion, independent of the coding of specific stimulus categories. These are coding principles that could be expected from higher level control regions that might modulate the use of emotion regulation strategies.
A remaining possibility is that decoding in these regions might reflect other correlated aspects of the task in addition to the reappraisal goals that the mass‐univariate analysis was not sensitive for Todd et al. [2013]. However, such potential confounds would be the same for the mass‐univariate analysis and all reported comparisons between these goals. Reappraisal goals are complex and cannot be fully decomposed based on any of our analyses alone. However, meaningful differences in the neural patterns underlying the representation of the up‐ and downregulation of emotion that are not linked to strong differences in overall activation, as demonstrated here, are likely to reflect more abstract intrinsic properties of reappraisal [Woolgar et al., 2014]. It has to be kept in mind that the up‐ and downregulation of emotion investigated in this study are complex processes and naturally involve different subprocesses, e.g., different mental imagery. This means, we cannot rule out that the representation of up‐ and downregulation of emotion might be driven by these different components rather than reflecting an abstract code. However, with the current methodology, these subprocesses appear inseparable from the specific strategies and can be regarded as an integral feature of their representation [Woolgar et al., 2014].
We further found that the same neural patterns in some of these regions were predictive of participants' reappraisal success. Specifically, a direct link between strategy encoding and reappraisal success was found for left IFG, IPL and MTG: The more separable participants' neural representations for Increase and Decrease were, the better their ability to up‐ or downregulate emotions. Such a direct link between the distinctness of neural spatial activation patterns and individual differences in behavior so far has been restricted to perceptual discrimination [Raizada et al., 2010] and has not been demonstrated for emotion regulation before. This provides additional strong evidence that left IFG, IPL, and MTG are involved in processes functionally highly important for emotion regulation and that they form the core regions of the brain's emotion regulation network.
Conceptually, our findings align well with the recently revised MCCE [Ochsner et al., 2012], which comprises three neural systems involved in reappraisal: the first system implicated in the cognitive modulation of emotional responses based upon prefrontal and parietal areas; the second system implicated in generating emotion represented by ventral striatum, amygdala, ventromedial PFC, and insula; the third system implicated in representing the perceptual and semantic properties of stimuli and playing an intermediary role between both aforementioned systems based on temporal regions [Ochsner et al., 2012]. We identified regions within the first system (SFG, MidFG/DLPFC, IFG, IPL, SPL) and the third system (MTG) that contained representational information about both reappraisal goals, supporting the idea that emotion regulation processes are instantiated in those regions. Moreover, we demonstrated that behavioral success in emotion regulation depends upon the properties of neural representations in a subset of these regions, namely the left IFG, MTG, and IPL. One likely account for these results might be the involvement of inner speech [Geva et al., 2011b; Girbau, 2007; Shergill et al., 2003] and semantic memory [Badre and Wagner, 2007; Binder and Desai, 2011; Thompson‐Schill, 2003], which have been associated with activity in all three regions.
The ability of silent self‐directed inner speech appears to be tightly connected to emotion regulatory processes as reappraisal can be thought of as an intrinsically linguistic strategy [Bronowski, 1977, 1979; Luria, 1962; Vygotsky, 1962]: it comprises a set of cognitive processes which help to re‐evaluate the experienced emotion [Gyurak et al., 2011], which include the active reinterpretation of the meaning, cause, consequence, or personal significance of the emotion‐inducing stimulus by means of verbal labeling [Ochsner and Gross, 2008]. Left IFG, MTG, and IPL are implicated in the generation of inner speech [Geva et al., 2011b; Girbau, 2007; Jones and Fernyhough, 2007; Morin and Hamper, 2012; Shergill et al., 2003], especially during emotion tasks (e.g., evaluating one's emotional response to a stimulus) [Morin and Hamper, 2012; Morin and Michaud, 2007]. Inner speech as a central underlying mechanism for emotion regulation might serve not only the accurate identification of one's current emotions through verbal labeling [Morin, 2005; Ochsner et al., 2004a] but also the effective modulation of one's current emotions in terms of cognitive reappraisal. In line with our proposal of inner speech processes as underlying mechanism of emotion regulation, a recent meta‐analysis highlighted the role of IFG in language processing during emotion regulation [Kohn et al., 2014]. A meta‐analytic connectivity modeling approach showed that the functional role of IFG during emotion regulation is most likely associated with language and that IFG is primarily involved in tasks including word generation, reading, and episodic recall [Kohn et al., 2014]. In support of this account, the observed regions have also been implicated in semantic memory, that most likely represents a prerequisite for inner speech processes. While left IFG has been involved in the effortful selection of semantic information, IPL and MTG have been associated with the storage of the actual content of semantic knowledge [Binder and Desai, 2011]. In terms of emotion regulation this means that IFG might be implicated in the selection of the appropriate reappraisal content, while parietal and temporal regions could provide the semantic information retrieved from the emotional stimulus that is needed for the reinterpretation of the meaning of the stimulus. Thus, inner speech and related semantic memory processes may serve as potential basic mechanisms underlying successful emotion regulation.
Consequently, we suggest extending the MCCE by adding another subsystem that underlies inner speech and semantic memory, comprising left IFG, MTG, and IPL. Further support for the hypothesis of a sub‐system comprising these areas stems from diffusion tensor imaging studies (DTI) demonstrating a high interconnectivity between IFG, MTG, and IPL [Geva et al., 2011a]. Moreover, a DTI study demonstrated projections from left IFG to temporal and frontal regions via dorsal and ventral pathways, the latter encompassing the basal forebrain including the amygdala complex [Anwander et al., 2007]. The left IFG might therefore link the prefrontal control system to the affective appraisal system to orchestrate emotion regulatory processes. This refinement of the MCCE may shed light on how prefrontal cortex downregulates limbic regions during reappraisal processes.
Note that the goal of the study was not to investigate individual differences of particular subregions of the GERN, recruited for different regulation goals in people with different experiences. We deliberately included participants with a wide range of experience, as well as different materials (film clips and pictures) and different goals (up‐ and down regulation) to capture the maximal extent of the GERN rather than different core configurations. This, of course, also means that the network as revealed here cannot easily be generalized to be representative for every person in all possible scenarios. The inclusion of such a wide range of experience, however, allowed us to ensure that novice and professional “regulators” were equally represented and no group was overlooked, which would have led to an inadequate characterization of the GERN. The absence of behavioral differences in emotion regulation and in regulation success between the participant groups with different experience further points to the possibility that other factors than experience alone might be important for understanding individual differences in emotion regulation. These questions, however, were beyond the scope of this study.
It has to be noted that, as we did not obtain valence ratings during scanning, we cannot rule out that the film clips sometimes might have induced a positive instead of a negative valence, although SCRs indicate effects of increased arousal and ratings indicate changes in emotional state. If the film clips were not perceived as negative, then the subjects' emotional state ratings should be more positive during the Look and Decrease condition, an account which is contradicted by our behavioral results. A previous study [Kim and Hamann, 2007] investigated the up‐ and downregulation of emotions in response to positive and negative pictures and found that similar regions were activated independent of the valence of the stimuli. This indicates that the valence of the stimuli might not affect emotion regulation processes to a great extent. Furthermore, Ochsner et al. [2012] showed in a meta‐analysis that reappraisal of both negative and positive stimuli depends upon left‐hemispheric regions and that activation patterns are highly overlapping. Thus, even if the subjects perceived some of the stimuli as slightly positive, this does not invalidate our manipulation and also does not affect the interpretation of the results.
Furthermore, it is important to note that the IAPS pictures and the film clips differed in various aspects such as, e.g., the size of the stimuli, the motion component, content, valence, and arousal. However, both stimulus categories induce various kinds of emotions, ranging from anxiety, fear, disgust to thrill, allowing participants to regulate the negative aspect of these emotions in both cases. In consequence, the cognitive processes underlying the reappraisal of all these emotional stimuli can be assumed to be highly similar. In support, all regions that were found to be activated by emotion regulation for IAPS pictures were also activated when film clips were used. The definition of the core network of emotion regulation is based upon both stimuli sets and the stimulus‐independence of the GERN is further supported by the cross‐condition classification of the MVPA which showed that both reappraisal goals are represented in the identified ROIs independent of the stimulus set used. The identification of the core emotion regulation network of emotion regulation is consistent with previous literature [Buhle et al., 2013; Frank et al., 2014; Kohn et al., 2014; Ochsner et al., 2012] and extends previous process models [Kohn et al., 2014; Ochsner et al., 2012; Phillips et al., 2008]. It is important to note, however, that this network is restricted to the used stimulus sets. Importantly, however, as the core regions exhibited goal‐unspecific and stimulus‐independent activity, we assume the generalizability of this network to other kinds of emotional stimuli.
CONCLUSIONS
In summary, our study provides evidence for a distributed network of lateral frontal, temporal, and parietal regions implicated in reappraising emotional events. Further, we identified a core system of emotion regulation that represents strategies for up‐ and downregulation of emotion in an abstract, stimulus‐independent fashion. The neural pattern‐separability in a subset of these brain regions (left IFG, MTG, and IPL) was directly related to the success in emotion regulation. This direct association between the distinctness of neural spatial activation patterns and individual differences in behavior has only been demonstrated in the perceptual domain so far. Given their strong association with inner speech functions and semantic memory, we conclude that those cognitive mechanisms may be used for orchestrating emotion regulation. On a neural level, we suggest that those brain regions, in which reappraisal is represented, link the prefrontal regions involved in cognitive control with the subcortical regions involved in affective processes.
ACKNOWLEDGMENTS
We wish to thank Danny Strasser (http://www.danny-strasser.de), Juergen Brath (http://www.gojump.de), and Red Bull Media House (http://www.redbull.com) for providing the film clips, Anita Tusche for help on the data analyses, and Juergen Brath and Juergen Muehling (http://www.funjump.de) for helping with the recruitment of subjects.
Conflict of interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
REFERENCES
- Abler B, Kessler H (2009): Emotion regulation questionnaire – Eine deutschsprachige Fassung des ERQ von Gross und John. Diagnostica 55:144–152. [Google Scholar]
- Anwander A, Tittgemeyer M Cramon DY, Von (2007): Connectivity‐based parcellation of Broca' s area. Cereb Cortex 17:816–825. [DOI] [PubMed] [Google Scholar]
- Bach M, Bach D, Zwaan Serim DM, Bohmer MF (1996): Validierung der deutschen Version der 20‐Item Toronto Alxithymie Skala bei Normalpersonen und psychiatrischen Patienten. Psychother Psychosom Medizinische Psychol 46:23–28. http://psycnet.apa.org/psycinfo/1996-03240-003. [PubMed] [Google Scholar]
- Badre D, Poldrack RA, Paré‐Blagoev EJ, Insler RZ, Wagner AD (2005): Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron 47:907–918. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD (2007): Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia 45:2883–2901. [DOI] [PubMed] [Google Scholar]
- Beauducel A, Strobel A, Brocke B (2003): Psychometrische Eigenschaften und Normen einer deutschsprachigen Fassung der Sensation Seeking‐Skalen, Form V. Diagnostica 49:61–72. [Google Scholar]
- Beauregard M, Lévesque J, Bourgouin P (2001): Neural correlates of conscious self‐regulation of emotion. J Neurosci 21:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedek M, Kaernbach C (2010a): A continuous measure of phasic electrodermal activity. J Neurosci Methods 190:80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedek M, Kaernbach C (2010b): Decomposition of skin conductance data by means of nonnegative deconvolution. Psychophysiology 47:647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berking M, Znoj H (2008): Entwicklung und Validierung eines Fragebogens zur standardisierten Selbsteinschätzung emotionaler Kompetenzen (SEK‐27). Zeitschrift Für Psychiatrie Psychologie Und Psychotherapie 56:141–153. [Google Scholar]
- Binder JR, Desai RH (2011): The neurobiology of semantic memory. Trends Cogn Sci 15:527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SJ, Duncan J, Lawrence AD (2004): State anxiety modulation of the amygdala response to unattended threat‐related stimuli. J Neurosci 24:10364–10368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode S, Bogler C, Haynes J‐D (2013): Similar neural mechanisms for perceptual guesses and free decisions. Neuroimage 65:456–465. [DOI] [PubMed] [Google Scholar]
- Bode S, Bogler C, Soon CS, Haynes J‐D (2012): The neural encoding of guesses in the human brain. Neuroimage 59:1924–1931. [DOI] [PubMed] [Google Scholar]
- Borkenau P, Ostendorf F (1999): NEO‐Fünf‐Faktoren Inventar (NEO‐FFI) nach Costa und McCrae. Z Klin Psychol Psychother 28:145–146. [Google Scholar]
- Bradley MM, Lang PJ (2007): The International Affective Picture System (IAPS) in the study of emotion and attention In: Coan JA, Allen JJ, editors. Handbook of Emotion Elicitation and Assessment. Oxford University Press. Series in Affective Science pp 29–46. [Google Scholar]
- Brett M, Anton J, Valabregue R, Poline J (2002): Region of interest analysis using an SPM toolbox. Neuroimage 16: Abstract 497. [Google Scholar]
- Bronowski J (1977): Human and animal languages In: Bronowski J, editor. A Sense of the Future. Cambridge, MA: MIT Press; pp 104–131. [Google Scholar]
- Bronowski J (1979): The Origins of Knowledge and Imagination (The Mrs. Hepsa Ely Silliman Memorial Lectures Series). Yale University Press. [Google Scholar]
- Brymer GE (2005): Extreme dude! A phenomenological perspective on the extreme sport experience; University of Wollongong. Available at: http://ro.uow.edu.au/theses/379. Last acessed October 1, 2014.
- Buhle JT, Silvers J, a Wager TD, Lopez R, Onyemekwu C, Kober H, Weber J Ochsner KN (2013): Cognitive reappraisal of emotion: A meta‐analysis of human neuroimaging studies. Cereb Cortex 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns TR, Engdahl E (1998): The social construction of consciousness part 2: Individual selves, self‐awareness, and reflectivity. J Conscious Stud 2:166–184. [Google Scholar]
- Chang C‐C, Lin C‐J (2011): LIBSVM: a library for support vector machines. ACM Trans Intell Syst Technol 2:1–39. [Google Scholar]
- Drabant EM, McRae K, Manuck SB, Hariri AR, Gross JJ (2009): Individual differences in typical reappraisal use predict amygdala and prefrontal responses. Biol Psychiatry 65:367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eftekhari A, Zoellner LA, Vigil SA (2009): Patterns of emotion regulation and psychopathology. Anxiety Stress Coping 22:571–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egner T (2011): Right ventrolateral prefrontal cortex mediates individual differences in conflict‐driven cognitive control. J Cogn Neurosci 23:3903–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eippert F, Veit R, Weiskopf N, Erb M, Birbaumer N, Anders S (2007): Regulation of emotional responses elicited by threat‐related stimuli. Hum Brain Mapp 28:409–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC, Shallice T, Dolan RJ (2000): “Sculpting the response space”–an account of left prefrontal activation at encoding. Neuroimage 12:404–417. [DOI] [PubMed] [Google Scholar]
- Frank DW, Dewitt M, Hudgens‐Haney M, Schaeffer DJ, Ball BH, Schwarz NF, Hussein AA, Smart LM, Sabatinelli D (2014): Emotion regulation: Quantitative meta‐analysis of functional activation and deactivation. Neuroscience and Biobehavioral Reviews. Elsevier Ltd. [DOI] [PubMed] [Google Scholar]
- Geva S, Correia M, Warburton Ea (2011a): Diffusion tensor imaging in the study of language and aphasia. Aphasiology 25:543–558. [Google Scholar]
- Geva S, Jones P, Crinion J, Price C, Baron J, Warburton E (2011b): The neural correlates of inner speech defined by voxel‐based lesion‐symptom mapping. Brain 134:3071–3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girbau D (2007): A neurocognitive approach to the study of private speech. Span J Psychol 10:41–51. [DOI] [PubMed] [Google Scholar]
- Goldin PR, Mcrae K, Ramel W, Gross JJ (2008): The neural bases of emotion regulation: Reappraisal and suppression of negative emotion. Biol Psychiatry 63:577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ (2013): Emotion regulation: Taking stock and moving forward. Emotion 13:359–365. [DOI] [PubMed] [Google Scholar]
- Gross JJ, John OP (2003a): Individual differences in two emotion regulation processes: Implications for affect, relationships, and well‐being. J Pers Soc Psychol 85:348–362. [DOI] [PubMed] [Google Scholar]
- Gross JJ, John OP (2003b): ERQ emotion regulation questionnaire. Psychol Assess 2003–2003. [Google Scholar]
- Gross JJ, Muñoz RF (1995): Emotion regulation and mental health. Clin Psychol Sci Pract 2:151–164. [Google Scholar]
- Gross JJ, Sheppes G, Urry HL (2011a): Cognition and emotion lecture at the 2010 SPSP emotion preconference. Cognition 25:1–17. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Thompson RA (2007): Emotion regulation: Conceptual foundations In: Gross JJ, editor. Handbook of emotion regulation. New York: Guilford Press; pp 3–26. [Google Scholar]
- Gross JJ, Sheppes G, Urry HL (2011b): Emotion generation and emotion regulation: A distinction we should make (carefully). Cogn Emot 25:765–781. [DOI] [PubMed] [Google Scholar]
- Gyurak A, Gross JJ, Etkin A (2011): Explicit and implicit emotion regulation: A dual‐process framework. Cogn Emot 25:400–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes J‐D, Rees G (2006): Decoding mental states from brain activity in humans. Nat Rev Neurosci 7:523–534. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Wilson SM (2006): Beyond a single area: Motor control and language within a neural architecture encompassing Broca's area. Cortex 42:503–506. [DOI] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ (2007): Failure to regulate: counterproductive recruitment of top‐down prefrontal‐subcortical circuitry in major depression. J Neurosci 27:8877–8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Fernyhough C (2007): Neural correlates of inner speech and auditory verbal hallucinations: A critical review and theoretical integration. Clin Psychol Rev 27:140–154. [DOI] [PubMed] [Google Scholar]
- Jonides J, Smith EE, Marshuetz C, Koeppe Ra, Reuter‐Lorenz Pa (1998): Inhibition in verbal working memory revealed by brain activation. Proc Natl Acad Sci USA 95:8410–8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R (2009): The functional neuroanatomy of reappraisal: Time matters. Neurosci Biobehav Rev 33:1215–1226. [DOI] [PubMed] [Google Scholar]
- Kanske P, Heissler J, Schönfelder S, Bongers A, Wessa M (2011): How to regulate emotion? Neural networks for reappraisal and distraction. Cereb Cortex 21:1379–1388. [DOI] [PubMed] [Google Scholar]
- Kim SH, Hamann S (2007): Neural correlates of positive and negative emotion regulation. J Cogn Neurosci 19:776–798. [DOI] [PubMed] [Google Scholar]
- Kober H, Mende‐Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, Ochsner KN (2010): Prefrontal‐striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci USA 107:14811–14816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn N, Eickhoff SB, Scheller M, Laird aR, Fox PT, Habel U (2014): Neural network of cognitive emotion regulation ‐ An ALE meta‐analysis and MACM analysis. Neuroimage 87:345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Goebel R, Bandettini P (2006): Information‐based functional brain mapping. Proc Natl Acad Sci USA 103:3863–3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kross E, Davidson M, Weber J, Ochsner K (2009): Coping with emotions past: The neural bases of regulating affect associated with negative autobiographical memories. Biol Psychiatry 65:361–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laux L, Glanzmann P, Schaffner P, Spielberger CD (1981): Das State‐Trait‐Angstinventar (STAI). Weinheim Beltz. Weinheim, Germany: Beltz. [Google Scholar]
- Lévesque J, Eugène F, Joanette Y, Paquette V, Mensour B, Beaudoin G, Leroux J‐M, Bourgouin P, Beauregard M (2003): Neural circuitry underlying voluntary suppression of sadness. Biol Psychiatry 53:502–510. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Inagaki TK, Tabibnia G, Crockett MJ (2011): Subjective responses to emotional stimuli during labeling, reappraisal, and distraction. Emotion 11:468–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz J, Minoshima S, Casey KL (2003): Keeping pain out of mind: The role of the dorsolateral prefrontal cortex in pain modulation. Brain 126:1079–1091. [DOI] [PubMed] [Google Scholar]
- Luria AR (1962): The role of speech in the regulation of normal and abnormal behaviour. Proc R Soc Med 55:243 [Google Scholar]
- McRae K, Ciesielski B, Gross JJ (2012): Unpacking cognitive reappraisal: Goals, tactics, and outcomes. Emotion 12:250–255. [DOI] [PubMed] [Google Scholar]
- McRae K, Hughes B, Chopra S, Gabrieli JDE, Gross JJ, Ochsner KN (2010): The neural bases of distraction and reappraisal. J Cogn Neurosci 22:248–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP (2009): Inferences about mental states. Philos Trans R Soc Lond B Biol Sci 364:1309–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawetz C, Bode S, Baudewig J, Kirilina E, Heekeren HR (2015): Changes in effective connectivity between dorsal and ventral prefrontal regions moderate emotion regulation. Cereb Cortex. 2015 Jan 28. pii: bhv005. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Morin A (2005): Possible links between self‐awareness and inner speech: Theoretical background, underlying mechanisms, and empirical evidence. J Conscious Stud 4:115–134. [Google Scholar]
- Morin A, Hamper B (2012): Self‐reflection and the inner voice: Activation of the left inferior frontal gyrus during perceptual and conceptual self‐referential thinking. Open Neuroimag J 6:78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin A, Michaud J (2007): Self‐awareness and the left inferior frontal gyrus: Inner speech use during self‐related processing. Brain Res Bull 74:387–396. [DOI] [PubMed] [Google Scholar]
- Moss HE, Abdallah S, Fletcher P, Bright P, Pilgrim L, Acres K, Tyler LK (2005): Selecting among competing alternatives: Selection and retrieval in the left inferior frontal gyrus. Cereb Cortex 15:1723–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mur M, Bandettini Pa, Kriegeskorte N (2009): Revealing representational content with pattern‐information fMRI–an introductory guide. Soc Cogn Affect Neurosci 4:101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman Ka, Polyn SM, Detre GJ, Haxby JV (2006): Beyond mind‐reading: Multi‐voxel pattern analysis of fMRI data. Trends Cogn Sci 10:424–430. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE (2002): Rethinking feelings: An FMRI study of the cognitive regulation of emotion. J Cogn Neurosci 14:1215–1229. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ (2005): The cognitive control of emotion. Trends Cogn Sci 9:2429: [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ (2008): Cognitive emotion regulation: Insights from social cognitive and affective neuroscience. Curr Dir Psychol Sci 17:153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, Hanelin J, Ramachandran T, Glover G, Mackey SC (2004a): Reflecting upon feelings: An fMRI study of neural systems supporting the attribution of emotion to self and other. J Cogn Neurosci 16:1746–1772. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JDE, Gross JJ (2004b): For better or for worse: Neural systems supporting the cognitive down‐ and up‐regulation of negative emotion. Neuroimage 23:483–499. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers J, Buhle JT (2012): Functional imaging studies of emotion regulation: A synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci 1251:E1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Olsson A, Ochsner KN (2008): The role of social cognition in emotion. Trends in Cognitive Sciences 12:65–71. [DOI] [PubMed] [Google Scholar]
- JDA Parker, RM Bagby, GJ Taylor, NS Endler, P Schmitz (1993): Factorial validity of the 20‐item Toronto Alexithymia Scale. European Journal of Personality 7:221–232. [Google Scholar]
- Pedersen DM (1997): Perceptions of high risk sports. Percept Mot Skills 85:756–758. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC (2008): A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry 13:829, 833–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS (2006): Prefrontal involvement in the regulation of emotion: Convergence of rat and human studies. Curr Opin Neurobiol 16:723–727. [DOI] [PubMed] [Google Scholar]
- Raizada RDS, Tsao F‐M, Liu H‐M, Kuhl PK (2010): Quantifying the adequacy of neural representations for a cross‐language phonetic discrimination task: Prediction of individual differences. Cereb Cortex 20:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray RD, Zald DH (2012): Anatomical insights into the interaction of emotion and cognition in the prefrontal cortex. Neurosci Biobehav Rev 36:479–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomons TV, Johnstone T, Backonja M‐M, Davidson RJ (2004): Perceived controllability modulates the neural response to pain. J Neurosci 24:7199–7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomons TV, Johnstone T, Backonja M‐M, Shackman AJ, Davidson RJ (2007): Individual differences in the effects of perceived controllability on pain perception: Critical role of the prefrontal cortex. J Cogn Neurosci 19:993–1003. [DOI] [PubMed] [Google Scholar]
- Shergill SS, Brammer MJ, Fukuda R, Williams SCR, Murray RM, McGuire PK (2003): Engagement of brain areas implicated in processing inner speech in people with auditory hallucinations. Br J Psychiatry 182:525–531. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE (1970): The state‐trait anxiety inventory. Manual 1–23. [Google Scholar]
- Thompson‐Schill SL, D'Esposito M, Aguirre GK, Farah MJ (1997): Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A reevaluation. Proc Natl Acad Sci USA 94:14792–14797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson‐Schill SL (2003): Neuroimaging studies of semantic memory: Inferring “how” from “where”. Neuropsychologia 41:280–292. [DOI] [PubMed] [Google Scholar]
- Thompson‐Schill SL, Bedny M, Goldberg RF (2005): The frontal lobes and the regulation of mental activity. Curr Opin Neurobiol 15:219–224. [DOI] [PubMed] [Google Scholar]
- Todd MT, Nystrom LE, Cohen JD (2013): Confounds in multivariate pattern analysis: Theory and rule representation case study. Neuroimage 77:157–165. [23558095] [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Davidson RJ (2009): Individual differences in some (but not all) medial prefrontal regions reflect cognitive demand while regulating unpleasant emotion. Neuroimage 47:852–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL, Reekum V, Marije C, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, Davidson RJ (2006): Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci 26:4415–4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vygotsky LS (1962): Thought and Language. Ed. Eugenia Hanfmann, Gertrude Vakar. Annals of Dyslexia. MIT Press; Vol. 14 Available at: http://www.amazon.com/dp/0262720108. Last acessed October 1, 2014. [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN (2008): Prefrontal‐subcortical pathways mediating successful emotion regulation. Neuron 59:1037–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiech K, Kalisch R, Weiskopf N, Pleger B, Stephan KE, Dolan RJ (2006): Anterolateral prefrontal cortex mediates the analgesic effect of expected and perceived control over pain. J Neurosci 26:11501–11509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolgar A, Golland P, Bode S (2014): Coping with confounds in multivoxel pattern analysis: What should we do about reaction time differences? A comment on Todd, Nystrom & Cohen 2013. Neuroimage 98:506–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv M, Goldin PR, Jazaieri H, Hahn KS, Gross JJ (2013): Emotion regulation in social anxiety disorder: Behavioral and neural responses to three socio‐emotional tasks. Biol Mood Anxiety Disord 3:20. [DOI] [PMC free article] [PubMed] [Google Scholar]


