Abstract
We investigated the effect of repeated delivery of anodal transcranial direct current stimulation (tDCS) on somatosensory performance and long‐term learning. Over the course of five days, tDCS was applied to the primary somatosensory cortex (S1) by means of neuronavigation employing magnetencephalography (MEG). Compared to its sham application, tDCS promoted tactile learning by reducing the two‐point discrimination threshold assessed by the grating orientation task (GOT) primarily by affecting intersessional changes in performance. These results were accompanied by alterations in the neurofunctional organization of the brain, as revealed by functional magnetic resonance imaging conducted prior to the study, at the fifth day of tDCS delivery and four weeks after the last application of tDCS. A decrease in activation at the primary site of anodal tDCS delivery in the left S1 along retention of superior tactile acuity was observed at follow‐up four weeks after the application of tDCS. Thus, we demonstrate long‐term effects that repeated tDCS imposes on somatosensory functioning. This is the first study to provide insight into the mode of operation of tDCS on the brain's response to long‐term perceptual learning, adding an important piece of evidence from the domain of non‐invasive brain stimulation to show that functional changes detectable by fMRI in primary sensory cortices participate in perceptual learning. Hum Brain Mapp 37:1277‐1295, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: longitudinal fMRI, neuronavigation, perceptual learning, somatosensory learning, (repeated) transcranial direct current stimulation
Abbreviations
- BDNF
Brain‐derived neutrotrophic factor
- FOV
Field of view
- GABA
Gamma‐aminobutyric acid
- GLM
General linear model approach
- GOT
Grating orientation task
- HRF
Hemodynamic response function
- LTP
Long‐term potentiation
- MEG
Magnetencephalography
- MNI
Montreal Neurological Institute
- MVPA
Multivoxel pattern analysis
- PKCγ
Protein kinase C γ
- RMANOVA
Repeated measure ANOVA
- ROI
Region‐of‐interest
- SPM
Statistical parametric map
- TE
Time to echo
- TR
Time to repeat
INTRODUCTION
Though everyday life requires use of perceptual skills, their eminent importance to daily functioning goes almost without notice. Yet perceptual learning takes time and requires extensive practice [Korman et al., 2003; Seitz and Dinse, 2007]. Likewise, regaining a lost skill is a laborious process [Conforto et al., 2010; Moseley and Wiech, 2009]. Thus, the idea of shortening the period of skill acquisition or refinement by auxiliary means is appealing, particularly in the domain of rehabilitation [Bolognini et al., 2009; Conforto et al., 2002, 2007]. One method that offers potential for promoting skill learning is transcranial direct stimulation (tDCS).tDCS is the non‐invasive application of a weak current (up to 2mA) through saline‐soaked sponges attached to the skull. It is thought to exert both small‐scale effects, for example on membrane potential [Bindman et al., 1965; Pupura and McMurtry, 1965] and on neurotransmitter release, especially gamma‐aminobutyric acid (GABA) [Clark et al., 2011; Stagg et al., 2009a], as well as large‐scale effects on task‐related brain metabolism [Antal et al., 2011; Jang et al., 2009; Kwon et al., 2008] and on resting‐state connectivity [Polania et al., 2011a, 2011b, 2012].
Skill learning can be categorised into online learning (i.e., learning within a session) and offline learning (i.e., learning between sessions) [Robertson et al., 2004; Karni and Sagi 1993]. A more recent distinction between performance, subsuming online and offline learning, and learning, referring to long‐term changes in performance, partially overlaps with but also complements the former conceptualization of skill learning [Söderström and Björk, 2015]. Importantly, tDCS affects both performance and learning.tDCS has become a popular tool for promoting short‐term performance, for example in the visual domain. tDCS is capable of influencing visual acuity [Kraft et al., 2010] and visual memory [Chi et al., 2010], as well as motion perception and visuo‐motor coordination [for a short review see Antal and Paulus, 2008]. Ragert et al. [2008] were the first to provide evidence of a short‐term effect of anodal tDCS on tactile acuity. The delivery of 20 minutes of anodal tDCS to the primary somatosensory cortex, with concomitant tactile testing during stimulation, was sufficient to cause a transient but profound improvement in two‐point discrimination performance—30% within 10 minutes of its onset. For the motor domain, it could be shown that the strength of effects that tDCS imposes on changes in motor performance are dependent on the relative timing of the onset of tDCS and the onset of the motor task [Stagg and Nitsche, 2011]. According to the Hebbian concept of synaptic plasticity, the largest effect was seen when the onset of tDCS and of the motor task were coupled and coincided. However, when anodal tDCS delivery was performed either before motor training [Amadi et al., 2015; Stagg and Nitsche, 2011] or after motor training [Reis et al., 2015], tDCS did not yield effects on short‐ or long‐term measures of motor learning. Unfortunately, no such study investigating the effect of tDCS‐dependent timing has been performed with regard to somatosensory functioning. All studies investigating and proving a beneficial effect of anodal tDCS on tactile acuity performed somatosensory testing during stimulation and therefore provided co‐stimulation [Fujimoto et al., 2014; Mori et al., 2013; Ragert et al., 2008]. However, co‐stimulation as implemented in these studies, was initiated with an offset of several minutes after the initiation of tDCS delivery. Thus, the studies provide evidence of an effect of tDCS from a hybrid model of timing.
Moreover, tDCS has been also been employed longitudinally to investigate and promote learning [Clark et al., 2009; Kadosh et al., 2010; Lindenberg et al., 2010; Reis et al., 2009], most recently with regard to somatosensory functioning. Mori et al. [2013] employed the repeated delivery of anodal tDCS to investigate tactile learning in patients suffering from multiple sclerosis, and observed a persistent increase in tactile acuity up to two weeks after the application of tDCS. However, Mori et al. [2013] did not investigate somatosensory learning with regard to the different stages of skill learning and thus did not investigate changes in performance. Yet their findings indicate that offline learning is the primary driver of tactile refinement. This parallels findings from the motor domain where longitudinal effects of tDCS delivery seem to be mediated by the enhancement of offline learning [Reis et al., 2009].
Despite growing evidence of an effect of anodal tDCS on skill learning [Clark et al., 2011; Kadosh et al., 2010; Lindenberg et al., 2010; Reis et al., 2009], the underlying functional and anatomical neural mechanisms of long‐term effects have rarely been investigated, and those few studies have produced inconsistent findings. Lindenberg et al. [2010] investigated the effects of longitudinally applied anodal tDCS on motor task‐related fMRI acitvation in stroke patients. They found increased activation in M1, the primary site of application. However, Lefebvre et al. [2014], who investigated the effects of repeated dual‐tDCS on stroke patients, reported a more effective recruitment of the motor network by more focalized activation after one week of intervention. As well, Kim et al. [2012] probed the effects of repeatedly applied anodal tDCS over M1 on motor function‐related fMRI activation in healthy subjects, observing a decrease in M1 ipsilateral to the side of stimulation. However, because Kim et al. [2012] did not provide a behavioural correlate of these neural changes and did not carry out an fMRI follow‐up session to evaluate its long‐term effects on neural functioning, the effects on learning are undetermined.
The aim of the present study, therefore, was to investigate the effects of repeatedly applied anodal tDCS on tactile skill learning in relation to online and offline learning (performance) and to skill retention (learning) as well as to identify their underlying neural correlate. Based on the available literature we hypothesise that (1) online learning will occur in response to anodal tDCS with the repeated assessment of GOT performance co‐occurring during stimulation. Repeated GOT‐testing, on its own, does not provide a stimulus strong enough to induce changes in performance or learning [Bleyenheuft and Thonnard 2007; Fujimoto et al., 2014; Mori et al., 2013; van Boven and Johnson, 1994]. Therefore, we do not expect learning in the sham condition where the delivery of tDCS is only simulated (2). Likewise online learning, we expect significant offline learning in the tDCSanodal condition, but no offline learning in the tDCSsham condition (3). We hypothesise a significant gain in tactile acuity in the tDCSanodal group after five days of repeated tDCS delivery with no change in tactile acuity in the tDCSsham group (4) resulting in superior GOT performance in the tDCSanodal group (5). At follow‐up, four weeks after the tDCS application, we expect that the tDCSanodal group will (continue to) exhibit superior performance in comparison to the tDCSsham group (6). Concerning brain activation, refined skill performance, also regarding the somatosensory domain [for a short review see Tommerdahl et al., 2010], is reflected by a set of neurons that is well‐defined and that, over time, is even more selective [Karni and Sagi 1993; Reed et al., 2011; Xu et al., 2009; Yang et al., 2009; Yotsumoto et al., 2009]. Based on the observations made by Kim et al. [2012] and Lefebvre et al. [2014], we propose that (7) longitudinal anodal tDCS will result in a more profound decrease in activation at its site of application (the primary somatosensory cortex) than in the sham condition.
METHODS
Participants
Altogether, thirty subjects were recruited to participate in the study [18 females; range 18–33 years, 24.1 ± 2.9 years (M ± SEM)] with no history of neurological or psychiatric disorders, trauma, or brain abnormalities. All subjects were right‐handed, as assessed by the high‐validity subset of the Edinburgh handedness inventory [Raczkowski et al., 1974]. All subjects gave informed written consent after explanation of the experimental procedure. The local ethics committee approved the study.
During the course of examinations we excluded five subjects from the analysis of behavioural and fMRI data due to brain abnormalities, intake of psychotropic drugs, technical problems at more than one testing session and GOT performance beyond measurability. Moreover, data from one subject was exclusively discarded from fMRI analysis as a result of excessive motion during scanning at two sessions. The final participant group, then, was 25 healthy subjects (16 females; range 18–33 years, 24.3 ± 2.9 years (M ± SEM)) with 12 subjects assigned to the tDCSanodal condition [eight females, 24.6 ± 3.7 years (M ± SEM)] and 13 subjects assigned to the tDCSsham condition [eight females, 24.0 ± 2.2 years (M ± SEM)].
Study Design
A pseudo‐randomized, double‐blind and sham‐controlled study design was employed to investigate the effects of repeatedly applied tDCS over the primary somatosensory cortex (S1). While subjects of one group received anodal tDCS, subjects of the other group were exposed to sham stimulation where tDCS delivery was only simulated.
The paradigm is demonstrated in Figure 1. tDCS was applied on five consecutive days. Behavioural effects induced by tDCS were quantified by the grating orientation task (GOT) described below. The daily testing procedure began with baseline assessment of the right index finger (IF) by three GOT measurements. Each GOT measurement lasted nine to ten minutes, which is comparable to the duration of GOT testing reported previously [Fujimoto et al., 2014]. After baseline assessment, a questionnaire was administered to capture level of attention, perception of fatigue, and amount of discomfort (range 1–10; 1=no attention/fatigue/discomfort, 10=maximum attention/fatigue/discomfort; Ragert et al., 2008). Thereafter, tDCSanodal/sham was applied for 20 minutes. To assess effects of stimulation on tactile performance, the GOT was conducted 10, 30, 60 and 90 minutes after the onset of the tDCSanodal/sham stimulation. Therefore, the first GOT measurement and the delivery of anodal tDCS overlapped for nine to ten minutes, resulting in a co‐stimulation protocol as was implemented in previous studies [Fujimoto et al., 2014; Ragert et al., 2008]. After the final GOT measurement, participants again rated their levels of attention, fatigue, and discomfort, as well as their sensations associated with the application of tDCS (range 1–10; 1=no sensation, 10=burning pain) and the duration of its application (range 1–5; 1=only initially, 10=throughout; Gandiga et al., 2004; Ragert et al., 2011; Reis et al., 2009). Moreover, when the experimental design was disclosed on the last day of testing, subjects of the tDCSsham condition were asked whether they had suspected feigned tDCS delivery. On each day testing was conducted in the forenoon between 8:00 and 13:00; the overall experimental procedure lasted three hours on average.
Figure 1.
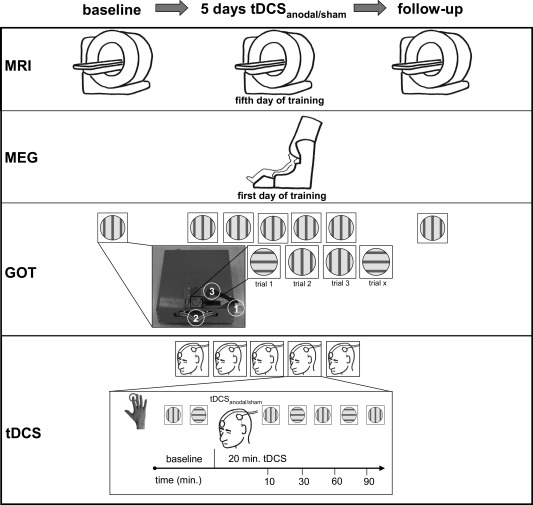
Schematic representation of the experimental design. tDCS was applied on five consecutive days, using a GOT to quantify behavioural effects. Each testing session began with baseline assessment of the right index finger (IF). Thereafter, tDCSanodal/sham was applied for 20 minutes. To measure effects on tactile performance, the GOT was conducted at intervals 10, 30, 60, and 90 minutes after the onset of the tDCSanodal/sham delivery. fMRI sessions were conducted one week prior to the first tDCS application, on the last day of tDCS application and about four weeks after the second fMRI session. On the first day of testing, MEG and a neuronavigational system were used for tDCS electrode placement. A front view of the device used for GOT testing is depicted in small image in Figure 1. GOT‐pens were presented by releasing a lever on side of the device (1) and were changed by another control shifter on its front (2, traced by white lines).
The effect of repeatedly applied tDCS on S1 was assessed, as well, by means of longitudinal fMRI. A first fMRI session was conducted about 7–21 days (mean: 8.3 days) prior to the first tDCS application. A second fMRI measurement was scheduled on the last day of tDCS application, with an offset of 4–6 hours after the final application of tDCSanodal/sham. A third fMRI session was conducted 24–28 days (mean: 25.3 days) after the second fMRI session. Three subjects missed a follow‐up measurement on one occasion.
GOT
The GOT [Van Boven and Johnson, 1994] was used to determine tactile acuity (Fig. 1). Subjects were asked to verbally report the orientation of a haptic pattern consisting of ridges and grooves that were either lengthwise or crosswise with regard to the long‐axis of the IF. Haptic patterns were applied to the tip of the IF by pens, each pen with a surface of different ridge and groove width (0.25, 0.5, 0.7, 1.0, 1.2 1.5, 2.0, 3.0 mm). A custom‐made device was used for reliable assessment of GOT thresholds. Pens were applied to the tip of the IF by manually releasing a lever on the side of the device. Pens were always applied with the same force (about 0.75 Newton). The IF was immobilized by hook‐and‐loop tape to render the task free of confounds introduced by movement. Moreover, pens were always applied at the same location on the finger tip by fixating the finger. A red circle was drawn on the tip of the right index finger to ensure a constant position of the examined part of the IF between testing sessions and days.
Testing was conducted in a stepwise block procedure. Each pen was presented 20 times; thereby, each orientation (lengthwise, crosswise) was presented 10 times in a pseudo‐randomized sequence. Six different sequences for each pen were chosen pseudo‐randomly to prevent implicit learning. Testing was conducted with the next lower grating until tactile acuity dropped below the 75% threshold [Ragert et al., 2008; Sathian and Zangaladze, 1997; van Boven and Johnson, 1994; Sens et al., 2012; Weiss et al., 2011]. Thus, a single measurement lasted nine to ten minutes. The tactile threshold was quantified by the following formula that allows interpolation of tactile acuity as assessed by the GOT:
Threshold G75 = the estimated threshold for the grating spacing on which the subject scored 25% correct responses; G = the grating spacing; P = trials correct/number of trials; below = the grating spacing or probability of correct response on the highest grating spacing to which the subject responded correctly less than 75% of the time; above = grating spacing or the probability of a correct response on the lowest grating spacing to which the subject responded correctly more than 75% of the time [Ragert et al., 2008].
On the first day of testing the pen with the largest grating was chosen for all subjects. Subsequent testing was adapted to individual performance. Therefore, testing started two grating levels above the level at which subjects failed to meet the above defined criterion of 75% correct responses.
TDCS Application
Direct current was generated by a battery‐driven electric stimulator (NeuroConn, Ilmenau, Germany) and delivered via a pair of square electrodes (5 × 5 cm). The stimulation electrodes were made of conductive rubber and were enclosed in saline‐soaked sponges. Electrodes were fixed to the head by a custom‐made headband.
Anodal tDCS (tDCSanodal) was applied over the left S1 with a total current of 2 mA and a local current density of 0.08 mA/cm2 using a fade in and fade out time of 10 seconds. To apply tDCS above S1, the primary somatosensory source in BA 3b was located using a whole head MEG‐system (306 channels, Elekta/Neuromag, Finland/USA). For this purpose, the median nerve was stimulated at the wrist of the right hand, according to the IFCN recommendations [Cruccu et al., 2008], and cortical responses were recorded at a sampling rate of 2000 Hz. The recorded responses were filtered using the signal space separation method [Taulu and Simola, 2005], averaged (n ≈ 256, trials containing artifacts were excluded from averaging) and bandpass filtered 0.3 to 150 Hz. The DANA software (release 3.3, Elekta Neuromag Oy) was used for source localization. The spatial alignment to the head‐based coordinate system was performed with the help of cardinal landmarks (preauricular points, nasion) and refined using a large number of head surface points. The best fitting dipole (goodness of fit > 85%, confidence volume < 110 mm) during the N20m response was selected to individually fit the spherical volume conductor derived from structural MRI. Prior to the first tDCS examination, the source location was spherically projected onto the head surface with the help of a ZEBRIS 3D neuronavigation system (CMS20‐USB) in order to place the stimulation electrode (anode) over the hand representation of BA 3b. The other electrode (cathode) was placed on the forehead above the contralateral (right) orbit [Mori et al., 2013; Ragert et al., 2008]. Both electrode positions were marked with a water‐insoluble red pen, and were renewed upon fading. Thus the positioning of the electrodes remained the same throughout the study.
In total, tDCS was delivered for 20 minutes in the tDCSanodal condition. In the sham condition, tDCS was applied for 30 seconds altogether, which has been shown to effectively stimulate the application of tDCS [Gandiga et al., 2006, Mori et al., 2013], while having no effect on the neural tissue underneath the electrodes [Nitsche et al., 2008].
MRI Pretesting
Two GOT test sessions were conducted outside the scanner to prepare the subsequent fMRI assessment of tactile acuity. A modified version of the GOT was employed, requiring subjects to indicate the orientation of a stimulus pair. Testing was conducted in a stepwise block procedure, beginning with the pen having the largest “resolution” (3 mm). Within each block, 20 pseudo‐randomized stimulus pairs (five for each of the four possible combinations of lengthwise and crosswise presentations) were applied to the length axis of the right IF while subjects verbally indicated the orientation of the stimulus pair. Subjects also rated their level of confidence in the decisions about the orientation of stimulus pairs, using the descriptors “guess”, “little certain”, “moderately certain” and “certain” [Petrusic and Baranski, 2003]. Note that, in contrast to the pretesting stage outside the scanner, responses on the orientation of GOT stimuli as well as confidence ratings inside the scanner were provided via a keypad with five buttons.
For subsequent fMRI testing, we selected two stimuli, individualised for each subject, one grating for which the orientation was easily identified [“easy pen”, P(correct) = 75–100%], as well as another grating for which the orientation was more difficult to identify (“difficult pen”, P(correct) = 30–60%). Two fMRI practice sessions inside the scanner were carried out to refine the choice of pens for the main fMRI experiment and to acquaint subjects with the MRI setting.
For each fMRI session (prior to the delivery of tDCS, at the fifth day of tDCS delivery, and after another four weeks; see below), GOT pens were adapted according to the above‐mentioned procedure. Thus, we tried to account for effects of learning (and forgetting) between testing sessions.
Figure 2 provides a schematic representation of the fMRI design. At each practice session, 30 trials of the modified GOT were presented. A pneumatically driven, MRI‐compatible stimulator was used for stimulus presentation. The sequence and duration of stimulus presentation were controlled by use of the software “Presentation” (Neurobehavioral Systems, Albany, CA).
Figure 2.
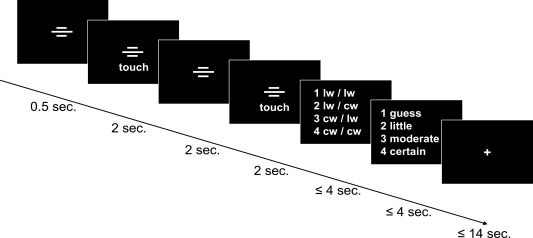
Schematic representation of the experimental fMRI design. Trials were announced by three transverse white bars that were presented for 0.5 seconds. Subsequently, GOT‐pens were presented twice, for 2 seconds, with a pause of 2 seconds between. Subjects rated the orientation (lw = lengthwise; cw =crosswise) of a stimulus pair, and immediately thereafter were given 4 seconds to rate their level of confidence associated with the preceding decision (guess; little=little certain; moderate=moderately certain; certain) within 4 seconds. A fixation cross was shown between trials. The long interstimulus intervals were required for technical reasons. Text and symbols are enlarged here for illustration clarity. Reprinted from Neuroimage, 99, Hilgenstock R, Weiss T, Witte OW, you'd better think twice: post‐decision perceptual confidence, 323‐331, Copyright 2014, with permission from Elsevier.
MRI: Main Experiment
The main fMRI experiment consisted of 108 task trials (Fig. 2). In total, participants were presented with 48 pairs of “easy” tactile gratings and 60 pairs of “difficult” tactile gratings. The study design used a larger number of “difficult” trials in response to the phenomenon of underconfidence—corresponding to the observation that in “easy” choice sets people tend to display disproportionate levels of confidence (Baranski and Petrussic, 1994). In order to account for motor activation during task trials, a second trial type required subjects to press only one of two numbers (“3” or “4”) with either an index or middle finger, respectively [Zhang et al., 2005]. Overall, subjects were presented with 22 of these trials. Another 40 null trials, during which a fixation cross was shown for 13 seconds, were intermixed with task trials. These null trials were intended to prevent the formation of expectance based on trial length, as each trial's length had to be adapted to the number of rotations that were necessary for the disk to move to the next position for the presentation of a new stimulus pair (14 seconds at longest). Moreover, null trials were intended to establish baseline activity between trials. The overall fMRI paradigm for each subject took about 55 minutes to run.
MRI Data Acquisition
Images were acquired on a 3.0‐T MR whole body scanner (TRIO, Siemens, Erlangen, Germany) using a standard head coil. Approximately 1300 echo‐planar T2*‐weighted images (EPI) were recorded for each subject. Each functional volume consisted of 44 contiguous, ascending scanned axial slices of 3 mm thickness (voxel size 3 mm × 3 mm × 3 mm). Parallel imaging (iPAT) with an acceleration factor of two was used to record images, with a time to repeat (TR) of 2.53 seconds, a time to echo (TE) of 30 ms, and a field of view (FOV) of 192 mm.
High‐resolution anatomical images were acquired through use of a three‐dimensional MPRAGE consisting of 192 slices with a spatial resolution of 1 mm × 1 mm × 1 mm (TR 2300 ms, TE 3.03 ms and FOV 256 mm).
MRI Data Preprocessing
Data quality was checked by using ArtRepair (http://cibsr.stanford.edu/tools/human-brain-project/artrepair-software.htmland) and tsdiffana (http://imaging.mrc-cbu.cam.ac.uk/imaging/DataDiagnostics). Bad slices were fixed by the ArtRepair implementation of an interpolation‐algorithm that employs an adaptive threshold for each slice. For a discussion of applied data quality check methods, please refer to Mazaika et al. [2009].
Data analysis was performed using MATLAB (Mathworks, Natick, MA) and SPM8 (Wellcome Department of Cognitive Neurology, London, UK; http://www.fil.ion.ucl.ac.uk/spm/). The unwarp function as part of SPM8 was used to align all subjects’ images from the three (in three cases, two) scanning sessions to the first volume of the first scanning session by a fourth degree B‐spline interpolation. The high‐resolution anatomical image of each subject was coregistered to its mean (averaged over the images of all fMRI sessions) realigned EPI‐volume by a fourth degree B‐spline interpolation. Acquisition differences between slices were accounted for by slice time‐correction (with reference to the 22nd slice).
The “new segment” option was used for segmentation of the structural image from the first MRI measurement. Deformation fields were derived and subsequently used to normalise each subject's data to the Montreal Neurological Institute (MNI) standard space. The data was smoothed using an 8 mm, isotropic, three‐dimensional full‐width‐at‐half‐maximum Gaussian kernel.
MRI Design Coding
A general linear model approach (GLM) was used to obtain statistical maps. Ten regressors were specified by convolving SPM's canonical hemodynamic response function (HRF) with either a stick or a boxcar input for each condition that was modelled. Boxcar inputs were used to model higher order cognitive functions; stick functions were used to model all other events.
Four regressors coded the onset of the first stimulus of each stimulus pair. The first regressor was used to model the first stimulus onset of a combination of an “easy” trial that was answered correctly, the second was used to model an “easy–incorrect” combination, the third was used to model a “difficult–correct” combination, and a fourth regressor was used to model the onset of a “difficult–incorrect” combination regarding each stimulus pair. The onset of the decision on the stimulus pair's orientation was aligned with the presentation of the second stimulus, using a variable epoch approach. These regressors were specified according to the four regressors coding the first stimulus presentation. Hilgenstock et al. [2014] provide a more detailed explanation of the underlying reasoning for the coding of the second stimulus of the stimulus pair. The decision on the degree of certainty associated with the decision on the stimulus pair's orientation was modelled using a variable epoch approach and was coded by another regressor. An additional regressor was used to represent those trials in which subjects made a solely motor response, with no tactile stimulation. Altogether, ten regressors were included in the GLM.
MRI Model Estimation
The β‐weights for each regressor outlined above were estimated using a robust weighted least square regression (rWLS) approach that has been shown to successfully account for even severe “noise” in fMRI‐timeseries, independent of its source [Diedrichsen and Shadmehr 2005]. The rWLS employs a restricted maximum likelihood approach to estimate the variance of noise in each image of the specified time series. These estimates are then used to weight each observation by the inverse of its noise. High‐pass filtering, as part of the standard SPM model specification at 1/128 Hz, was performed to remove slowly varying trends.
Data Analysis: Questionnaire Data
Questionnaire data (on attention, fatigue and discomfort prior to and after delivery of tDCS) were collected on each day of testing, and were subjected to a repeated measure ANOVA (RMANOVA). Time (five levels, measurements on each day of testing) was modelled as a within‐subjects factor, and Group (two levels, anodal vs. sham) as a between‐subjects factor. Moreover, assessment of the perceived duration and intensity of tDCS (after the application of tDCS) was carried out via RMANOVAs. The Greenhouse‐Geiser correction was applied to non‐spherical data. A P value ≤ 0.05 was considered significant. Significant main effects and interactions were tested by post‐hoc contrasts with regard to the first point in time (baseline GOT performance), Bonferroni‐corrected for multiple comparisons. This criterion/procedure was applied to all analyses performed by RMANOVAs (please see below).
Data Analysis: Behavioural Data
Baseline performance of the right index finger prior to the first delivery of tDCSanodal/sham was compared between groups by a two‐sample test. A P value ≤ 0.05 was considered significant.
Online learning was defined as the difference between the baseline assessment of tactile performance on each day of testing and the GOT measurement 30 minutes after initiation of the tDCSanodal/sham application on the respective day of testing. The choice of this time schedule for online learning assessment was based on the observation that immediate effects of tDCS on the somatosensory system emerge within 10 minutes and cease within 40 minutes after the onset of stimulation [Ragert et al., 2008]. However, we defined three additional variables to test whether the repeated delivery of tDCS induced a prolonged or temporally shifted immediate online learning effect. These variables were computed by taking the difference between the GOT baseline assessment on each day of testing and the GOT performance at 10, 60 and 90 minutes (respectively) after the onset of tDCS delivery. Offline learning was defined as the difference between the GOT baseline measurement prior to the next tDCSanodal/sham application on a succeeding day of testing and the last recorded GOT performance on the preceding day of testing, in accordance with Reis et al. [2009]. Total learning was quantified by the absolute GOT baseline performance on the first day of testing, on the last day of testing and at follow‐up four weeks after the last application of tDCS. The assessment of baseline performance, also on day five of testing, was chosen to ensure comparability between measurements by excluding an immediate effect of tDCS delivery on GOT performance as well as by ruling out the influence of potential confounders (e.g., attention) on GOT performance.
Online learning, offline learning and total learning were assessed by RMANOVAs with the within‐subjects factor Time (online learning—five levels, learning on each day of testing; offline learning—four levels, difference variables of GOT performance on the preceding and succeeding day of testing; total learning ‐ three levels, performance prior to the study, on the fifth day and at follow‐up) and the between‐subjects factor Group (two levels, anodal vs. sham). To address specific hypotheses related to online learning, offline learning and total learning, we performed (where applicable) RMANOVAs separately for anodal and sham stimulation with the within‐subjects factor Time (for the number of levels, please see above). Another RMANOVA was employed to test for the temporal evolution of online learning effects in the tDCSanodal condition with the within‐subjects factors Time (five levels, learning on each day of testing) and Online Learning Measure (four levels, online learning 10, 30, 60 and 90 minutes after tDCS delivery). In relation to online learning and total learning hypotheses, we performed one‐sample t‐tests against zero and a two‐sample t‐test (with regard to total learning), Bonferroni‐corrected for multiple comparisons.
A RMANOVA was conducted to evaluate the comparability and stability of task difficulty for the fMRI implementation of the GOT both between and within groups. Therefore, Time (three levels—at baseline, at the fifth day of tDCS delivery, and at the four‐week follow‐up) and Difficulty (two levels, easy vs. difficult) were considered to be repeated factors, and Group (two levels, anodal vs. sham) was considered a between‐subjects factor.
Data were analysed with the SPSS software package (Version 13, SPSS Inc., Chicago) as well as MATLAB (Version 2009b, Mathworks, MA). Note that when specific hypotheses were tested, as in the example of total learning or fMRI changes [hypothesis (4) or hypothesis (7)], no correction for multiple comparisons was applied, in accord with Bortz et al. [2000] and Bortz [2009].
Data Analysis: fMRI—Magnitude of the BOLD Response
First level analysis was carried out on the regressor that coded the first stimulus presentation of those stimuli classified as “easy” and “correct” (the results section explains choice of analysis) against the regressor that coded those trials that required only a motor response. The same first‐level analysis was carried out for each of the three fMRI measurements, and the resulting first‐level contrast was taken to the second level by a one‐sample t‐test.
At the second level, a flexible factorial design was specified with a random subject, a Group factor (two levels, anodal vs. sham) and a Time factor (three levels, baseline, the fifth day of tDCS delivery, and follow‐up). Within the factorial design, we tested for differences between both groups at the three points in time, as well as for longitudinal changes of activational magnitude within groups.
Statistical parametric maps (SPM) were thresholded using the height as well as the spatial extent of activation. Thus, the SPM was initially thresholded at P ≤ 0.001 on the voxel level and subsequently thresholded at P ≤ 0.05 on a topological level, FWE‐corrected for multiple comparisons [Friston et al., 1994; Poline et al., 1997].
Data Analysis: fMRI—Spatial Extent of the BOLD Response
An established method to test for effects of tactile training on neural processing is the analysis of change in activational extent [Dinse et al., 2003; Pleger et al., 2001, 2003; Tegenthoff et al., 2005]. This analysis used the same second‐level data as outlined above, and the same thresholding procedure was employed. To assess the size of activation, we used a region‐of‐interest (ROI) analysis at all three points in time, separately for each group. A pool of potential ROIs was determined from literature that investigated the processing of haptic information by tactile gratings [Van Boven et al., 2005, Zhang et al., 2005]. Those regions involved in tactile information processing identified at the first fMRI session were chosen for subsequent analyses from the pool of ROIs (Table 1). Therefore, we applied the ROI masks of these regions provided by the Anatomy toolbox [Eickhoff et al., 2005] to the analyses outlined in the preceding methods section (fMRI ‐ magnitude of the BOLD response), and counted the number of significantly activated voxels within each region using the two‐stage thresholding procedure outlined above by custom‐written MATLAB‐code. Moreover, we visually verified activation of the hand area in left S1 as the primary target of fMRI testing and of anodal tDCS delivery for very subject as well as at the group level of analysis based on the morphological criteria of the hand knob as defined by Yousry et al. [1997].
Table 1.
Anatomical regions of interest (ROI)
| Region name | Abbreviation | Localization |
|---|---|---|
| Primary somatosensory cortex | S1 | Inferior parietal |
| Secondary somatsensory cortex (= OP 1/4) | S2 | Inferior parietal |
| BA 4 | Operculum | |
| BA 6 | SMA | Postcentral |
| BA 39 (IPC (PGa, PGp)) | Precentral | |
| BA 40 [IPC (PFt, PF, PFm)] | Precentral |
According to Caspers et al. [2006], regions of the inferior parietal cortex (IPC) correspond to Brodmann areas 39 and 40. The secondary somatosensory cortex is located in subdivisions of the parietal operculum (OP) [Eickhoff et al., 2006, 2010]. ROIs are alphabetically ordered by their localization.
Longitudinal changes in activational size within the predefined ROIs were investigated by a RMANOVA with the within‐subjects factor Time (three levels, prior to the study, on its fifth day and at follow‐up) and the between‐subjects factor Group (two levels, anodal vs. sham). The Greenhouse‐Geiser correction was applied to non‐spherical data. A P value ≤ .05 was considered significant. Significant main effects and interactions where tested by post‐hoc contrasts with regard to the first point in time (baseline GOT performance), Bonferroni‐corrected for multiple comparisons. RMANOVAs were conducted separately for each Group (two levels, anodal vs. sham) with the within‐subject factor Time (three level, prior to the study, the fifth day of the study, follow‐up).
To identify brain regions exhibiting changes specifically attributable to the repeated delivery of tDCS, we defined four criteria to be fulfilled by a single brain region: (1) a significant longitudinal change in at least one stimulation group, (2) a resulting group difference at least at one point in time, (3) a significant interaction of the extent of fMRI activation and the stimulation group and (4) a correlation between the extent of activation and the amount of improvement in tactile acuity (see below). These criteria (or subsets of the criteria) have also been used in other studies investigating the relationship between tDCS delivery and (changes in) fMRI activation [Antal et al., 2011; Stagg et al., 2009a,b, 2011].
Data analysis: fMRI—correlation analysis
The size of activation of each ROI at the second fMRI session (fifth day of the study) and at the third fMRI session (four weeks after the last delivery of tDCS) was correlated with the improvement in tactile acuity at the fifth day of the study (with regard to baseline performance) (1) and at follow‐up (with regard to baseline performance) (2) by computing the Pearson product‐moment correlation coefficient (P ≤ 0.05, uncorrected for multiple comparisons).
RESULTS
Outliers and Baseline
In the tDCSanodal group, one subject of the experimental group maxed out the upper measurement range of the GOT at the baseline assessment on the first day of testing, and was consequently excluded from subsequent analyses. Therefore, data from 11 subjects of the tDCSanodal and 13 subjects of the tDCSsham condition were subjected to further analyses.
There was no significant difference in GOT baseline performance between the tDCSanodal and tDCSsham right index finger [tDCSanodal: M = 1.32, SD = 0.42; tDCSsham: M = 1.58, SD = 0.40; t(23) = 1,543, P = 0.137].
Questionnaire Data
On each day of testing, participants rated their levels of attention, fatigue and discomfort prior to and after the delivery of tDCSanodal/sham. RMANOVAs showed no significant difference between the tDCSanodal and tDCSsham condition on any measure [attention: F(1, 23) = 0.502, P = 0.486; fatigue: F(1, 23) = 0.422, P = 0.522; discomfort: F(1, 23) = 0.563, P = 0.461]. Moreover, there was no difference between groups for the indicated duration or intensity of stimulation measured [intensity: F(1, 23) = 0.137, P = 0.715; duration: F(1) = 2.203, P = 0.151].
Offline learning: A RMANOVA on online learning revealed a significant main effect of Time [five levels, each day of testing; F(2.562, 58.936) = 3.721, P = 0.021] but not Group (two levels, anodal vs. sham; F(1, 23) = 0.074, P = 0.788), and a significant interaction of Time and Group [F(2.562, 58.936) = 4.566, P = 0.009]. Moreover, performing a RMANOVA separately for the anodal and sham conditions revealed that there was no online learning in the tDCSsham condition [F(1, 12) = 2.190, P = 0.165] but there was significant online learning in the tDCSanodal condition [F(1, 11) = 5.352, P = 0.041]. Using t‐tests against zero to analyze the time course of online learning in the tDCSanodal condition in more detail disclosed swiftly saturating online learning, as can been seen from Figure 3. Only on the first day of testing was there significant online learning [t(11) = 3.357, P = 0.005], even when Bonferroni‐corrected for multiple comparisons. Moreover, only on the first day of testing was there a significant difference in the amount of online learning between the tDCSanodal and the tDCSsham condition [t(23) = 2.575, P = 0.017].
Figure 3.
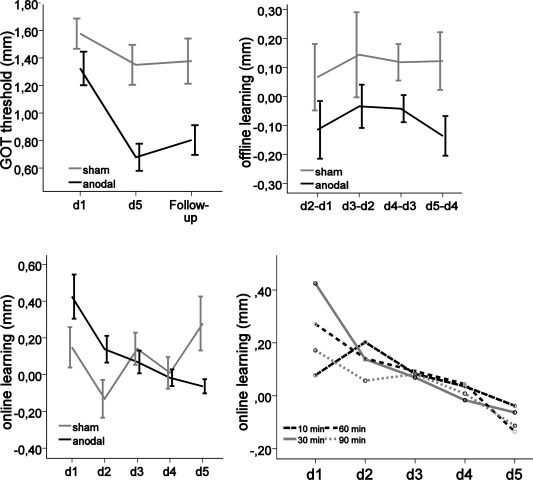
Online learning, offline learning and the GOT threshold in mm (M ± SEM), displayed separately for tDCSanodal (black) and tDCSsham (light gray) conditions over the course of the study. Online learning is displayed separately, timed at 10, 30, 60, and 90 minutes after the onset of tDCS, with regard to baseline performance. Negative values with regard to offline learning correspond to an improvement in tactile acuity. D = day, Dx‐Dy = difference between baseline on day x and final measurement on day y. Follow‐up = assessment of tactile acuity four weeks after the last delivery of tDCSanodal/sham.
We performed additional analyses to determine the temporal evolution of tDCS effects and a potential shift or extension in the duration of its immediate effects on tactile acuity. As can been seen from Figure 3, a very similar pattern of online learning effects emerged at 10, 30, 60 and 90 minutes after tDCS delivery. Thus, there was no significant difference between the four different types of online learning measures F(3, 132) = 1.198, P = 0.326).
Offline Learning
Results indicated that there was neither a significant effect of Time [F(2.219, 48.968) = 0.338, P = 0.728] nor a significant interaction of Time and Group [F(2.219, 48.968) = 0.115, P = 0.902]. As seen in Figure 3, there was constant offline learning in the tDCSanodal group, peaking between day 2 and day 3, as well as between day 4 and day 5. In the tDCSsham group there was a persistent loss of GOT performance between testing days, resulting in a significant main effect of Group [F(1, 23) = 6.190, P = 0.021]. As well, subjecting offline learning data to a RMANOVA (separately for both groups) showed that there was significant offline learning in the tDCSanodal condition [F(1, 11) = 6.376, P = 0.028] but no offline learning (and also no “warm‐up decrement”) in the tDCSsham condition [F(1, 12) = 2.683, P = 0.127].
Total Learning
Using a RMANOVA to compare the absolute GOT threshold of the right IF between both groups of stimulation at the baseline, at the fifth day of tDCS delivery and at follow‐up resulted in a significant main effect of Time [F(2, 44) = 14.301, P = < 0.001] and Group [F(1, 22) = 9.607, P = 0.005], and a significant interaction of Time and Group [F(2, 44) = 4.827, P = 0.013], picturing the improvement of GOT performance over the course of the study with a superior performance of subjects in the tDCSanodal condition. Post‐hoc analyses revealed that in comparison to baseline performance on day one of testing, there was a significant difference between the GOT performance in the tDCSanodal and tDCSsham conditions at day five of testing [Manodal = 0.68, SD = 0.341; Msham = 1.35, SD = 0.524; F(1, 22) = 8.037, P = 0.010; Fig. 3]. However, at follow‐up we only observed a trend of a significant difference in GOT thresholds [tDCSanodal: M = 0.80, SD = 0.373; tDCSsham: M = 1.38, SD = 0.569; F(1, 22) = 4.264, P = 0.051, η2 = 0.162], but with a large effect size [Cohen, 1988]. Using a two‐sample t‐test to compare the absolute GOT threshold of the tDCSanodal and tDCSsham conditions, we found a significant and Bonferroni‐corrected group difference in two‐point discrimination at follow‐up [t(22) = 2.917, P = 0.008]. Despite a pooled RMANOVA main effect of Time as reported above, however, a separate RMANOVA conducted for the tDCSsham condition revealed no significant change and therefore no learning in tDCSsham condition over the course of the study [F(2, 22) = 1.355, P = 0.279].
fMRI Data: Behavioural fMRI‐Data
A RMANOVA proved that difficulty did not change over time [F(2, 51.66) = 0.092, P = 0.912], and did not differ between groups at any point in time [F(1, 25.14) = 0.047, P = 0.829]. As expected, stimuli classified as “easy” were significantly different from stimuli categorized as “difficult” [F(1, 77.24) = 30.551, P < 0.001]. However, as Table 2 shows, stimuli that were supposed to be “difficult” exceeded the upper category boundary (50 percent correct trials) set for “difficult” stimuli at all three points in time, as well as in both groups.
Table 2.
Longitudinal GOT difficulty
| T1 | T2 | T3 | ||||
|---|---|---|---|---|---|---|
| Anodal | Sham | Anodal | Sham | Anodal | Sham | |
| Easy | 0.84 | 0.89 | 0.82 | 0.80 | 0.81 | 0.82 |
| Difficult | 0.70 | 0.64 | 0.73 | 0.71 | 0.68 | 0.65 |
Note: The table provides the percentage of correct trials separately for each group and stimulus category across the three fMRI measurements.
fMRI Data: Statistical Parametric Maps
Unfortunately, even though fMRI testing was individually adpated at each fRMI testing session, only “easy” stimuli could be held intraindividually constant across all three fMRI measurements. Only “easy” stimuli complied with their a priori category definition, while “difficult” stimuli proved to be too easy (Table 2). Therefore, the analysis of fMRI data was performed only on easy and correct trials.
With regard to activational magnitude, there was neither a difference between both groups of stimulation at any point in time nor a longitudinal change within the tDCSanodal or tDCSsham conditions. However, Figs. 4 and 5 reveal a significant interaction of Time and Group [F(2, 44) = 5.540, P = 0.007] with regard to the task‐related activational extent (number of voxels) of the left primary somatosensory cortex (S1), resulting in significantly less activation in the tDCSanodal condition. By the pattern of change in activational size, especially at follow‐up, we observed neither a main effect of Time [F(2, 44) = 0.676, P = 0.514] nor of Group [F(1, 22) = 0.0102, P = 0.920, Fig. 5] with regard to the activational extent of left S1. Yet post‐hoc tests showed that, in comparison to the activational extent at baseline, there was significantly less activation (number of voxels) in the tDCSanodal than in the tDCSsham condition at follow‐up [F(1, 22) = 11.101, P = 0.003]. An additional RMANOVA performed separately for the tDCSanodal condition revealed a significant longitudinal decrease in the number of activated voxels in the left S1 [F(2, 20) = 3.501, P = 0.048], with no such change in the tDCSsham condition [F(2, 24) = 2.649, P = 0.091]. As can ben seen from Figure 4B and Table 3 the decrease in activation in left S1 in the tDCSanodal condition is primarily located at the representation of the hand areas in S1 subjected to tactile training and targeted by anodal tDCS.
Figure 4.
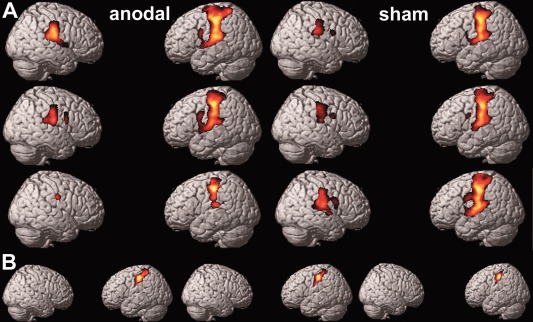
(A) Changes in activational size. Random effect group analysis of activations over the course of the study evoked by passive tactile stimulation of the right index finger in contrast to a button press. The upper row of brains corresponds to the baseline fMRI session, the middle row to the fMRI session after five days of anodal or sham tDCS delivery, and the lower row to the follow‐up fMRI session. (B) Change in the activational size of the hand area, selectively for the left S1 in the tDCSanodal condition, over the course of the study. An S1 mask was generated by the Anatomy toolbox. From left to right: baseline fMRI session, fMRI session after five days of anodal delivery, follow‐up fMRI session. Maps in (A) and (B) are thresholded at P ≤ .001. Data is projected on the single subject rendering template provided by SPM 8.
Figure 5.
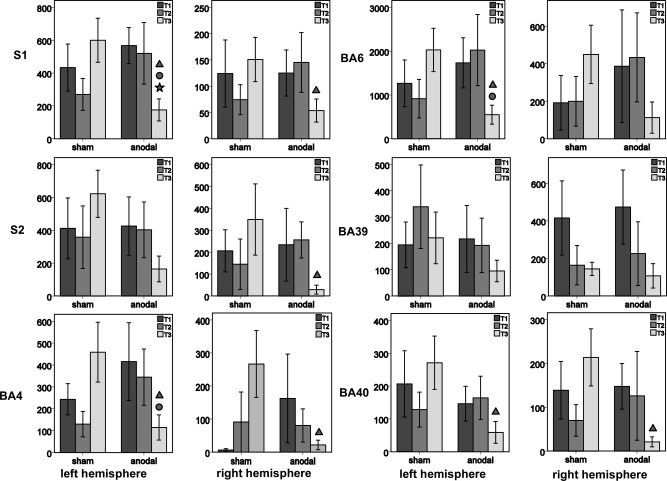
Bar charts represent the number of activated voxels (M ± SEM) within the investigated ROIs of the left and right hemisphere separately for the anodal and sham condition over the course of the study [dark gray—before tDCS (T1); mid‐gray—fifth day of tDCS (T2); light gray—follow‐up (T3)]. Triangles indicate a group difference at T3 with regard to activation at T2 (P = 0.05, uncorrected for multiple comparisons); dots indicate an interaction between both groups of stimulation over the course of the study (P = 0.05, uncorrected for multiple comparisons); stars indicate a longitudinal change (within one group of stimulation; P = 0.05, uncorrected for multiple comparisons). BA = Brodmann Area; S1 = primary somatosensory cortex; S2 = secondary somatosensory cortex. Images were resliced to 1.5 × 1.5 × 1.5 mm.
Table 3.
Maximum of activation of left S1 in MNI space
| Timepoint | Peak voxel MNI coordinates | Peak T‐statistic | P‐value | ||
|---|---|---|---|---|---|
| x | y | z | |||
| T1 | −54 | −21 | 51 | 12.13 | <0.001 |
| T2 | −56 | −21 | 51 | 14.33 | <0.001 |
| T3 | −57 | −19 | 46 | 7.83 | <0.001 |
First maximum of activation in left S1 separately for the tDCSanodal condition at baseline prior to the study (T1), at the fifth day of anodal tDCS delivery (T2) and at follow‐up four weeks after the last delivery of tDCS (T3) in the MNI space.
Figure 6.
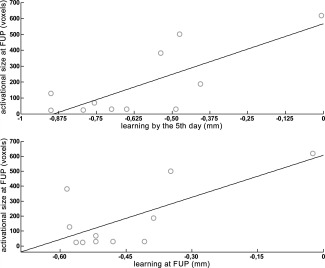
Correlation between the activational extent at follow‐up (FUP) four weeks after the last delivery of tDCS in voxels (voxel size 1.5 × 1.5 × 1.5 mm), and the GOT performance at the fifth day of study and at follow‐up, in relation to baseline GOT performance in mm in the tDCSanodal condition. Negative values with regard to GOT performance correspond to a reduction in GOT threshold, and thus to an improvement in tactile acuity.
Finally, two findings from the correlation analysis reveal (non‐causal) support for a relationship between the repeated delivery of anodal tDCS and changes in brain activation at the follow‐up fMRI sessions. First, there is a significant negative correlation between the amount of learning in the tDCSanodal condition at the fifth day of the study and the extent of brain activation at follow‐up [r(11) = −0.743, P = 0.009]. Put differently, the larger the gain in tactile acuity by the fifth day of the study, the more localized the activational fMRI extent was at the follow‐up fMRI session (Fig. 6). Second, we observed a significant negative correlation between the extent of brain activation at follow‐up and tactile acuity at follow‐up, with regard to baseline GOT performance [r(11) = −0.689, P = 0.019]. Thus, the left S1 satisfies all criteria defined in the Methods section to identify brain regions exhibiting a pattern of change attributable to the repeated delivery of tDCS. Moreover, no brain region other than the left S1 fulfilled these criteria. Indeed, two of four criteria, at most, could be fulfilled by any of the other investigated brain areas (Fig. 5).
Concomitant to a decrease in activation in the left S1, there was an overall, though not significant, trend towards a decrease in the activational size in response to tactile stimulation in both hemispheres in the tDCSanodal condition. A complementary (and similarly non‐significant) trend of more extensive activation at follow‐up was observed in the tDCSsham condition (Figs. 4 and 5).
DISCUSSION
The present study was intended to investigate effects of repeatedly applied anodal tDCS on tactile learning of the right index finger (IF), as well as to investigate concomitant change in brain activity that result from the application of anodal tDCS. We found that, in comparison to the sham application, anodal tDCS enhanced tactile learning primarily by effects on intersessional changes in performance within five days and at follow‐up four weeks after the last delivery of anodal tDCS. Alterations in the neurofunctional organization of the brain accompanied these behavioural changes. At follow‐up we observed a longitudinal decrease in the activational extent in the tDCSanodal condition, related to the change in tactile acuity and with significantly less activation in the tDCSsham group at the primary site of anodal tDCS delivery.
Online Learning
Importantly, all subsequently presented findings are not related to confounders such as differences in attention, fatigue, discomfort or the intensity or duration of stimulation, as there was no difference on any measure between groups. Moreover, no subject in the tDCSsham condition indicated suspicion of feigned tDCS delivery.
As hypothesised, we observed significant online learning the tDCSanodal group but not in the tDCSsham group (Fig. 3). We observed no shift in the onset of online learning effects, nor did we see any extension of the duration of online learning effects. Interestingly, and as previously reported in the Reis et al. [2009] study on the effects of repeated delivery of anodal tDCS on longitudinal motor skill learning, online learning was saturated early in the tDCSanodal group. This finding may be related to the observation of increased expression of the protein kinase C γ (PKC γ) in the cerebral cortex induced by tDCS [Islam et al., 1994]. The PKC γ is engaged in the phosphorylation of AMPA receptors [McDonald et al., 2001; Nanou and El‐Manira, 2010; Yan et al., 2011] that are related to the early stages of long‐term potentiation (LTP)—that is online learning [Derkach et al., 2007; Santos et al., 2007]. Thus, tDCS could induce larger gains in performance from a single session of application [Liebetanz et al., 2002; Nitsche et al., 2003, 2004; Ragert et al., 2008] by exerting a more profound effect on the phosphorylation of AMPA receptors. Moreover, changes in AMPA receptors may persist even for days [Clem and Huganir 2010] which may relate to the observation of faster saturation of online learning in the tDCSanodal condition.
Offline learning
Based on findings by Mori et al. [2013] with regard to the somatosensory system and by Reis et al. [2009] with regard to the motor system, we expected offline gains in tactile acuity in response to anodal tDCS, but not for its sham application. According to our hypotheses, we observed offline learning in the tDCSanodal group that was significantly greater than in the tDCSsham condition where no offline learning occurred (Fig. 3). Thus, we conclude that tDCS exerts the enhancement of skilled performance by affecting intersessional performance.
These effects of anodal tDCS on offline learning are likely mediated by local changes in GABA concentration, driving plasticity as shown for the motor cortex [Amadi et al., 2015; Kim et al., 2014; Stagg and Nitsche, 2011]. GABAergic interneurons may be related to rapid remapping of cortical representations [Amadi et al., 2015] by affecting the receptive field of neurons. Moreover, they are intrinsically related to LTP [Amadi et al., 2015; Bachtiar and Stagg 2014; Stagg and Nitsche, 2011], driving lasting changes in synaptical architecture and neurotransmitter release [Pastalkova et al., 2006; Whitlock et al., 2006]. For example, applying anodal tDCS to the primary motor cortex (M1) reduces the local GABA concentration with a positive correlation between tDCS‐induced decreases in local GABA concentration and in the amount of motor learning [Stagg et al., 2009a,b, 2011]. Unfortunately, no study has investigated the relationship between anodal tDCS delivery, (changes in) local GABA concentration and (changes in) somatosensory performance. Yet the motor and somatosensory systems share, in principle, the same mechanism of plasticity [Abraham, 2003]. We therefore assume a central role for GABA in mediating superior offline learning in response to repeated anodal tDCS delivery.
As well, sleep may contribute to offline learning effects evoked by anodal tDCS. A recent study by Fritsch et al. [2010] revealed that the delivery of tDCS is related to brain‐derived neutrotrophic factor (BDNF) secretion. The later stage of LTP—that is offline learning ‐ is at least partially mediated by BDNF [Lu et al., 2008; Pang et al., 2004]. BDNF also appears to influence EEG power spectra in neonatal rats [Hairston et al., 2004], and its microinjection in awake adult rats has been shown to cause a focal increase in slow‐wave sleep [Faraguna et al., 2008]. Importantly, local changes in sleep have been related to learning and are dependent on prior novel experiences [Huber et al., 2004, 2007]. Thus, due to its effects on BDNF secretion, tDCS may promote learning by increasing local sleep, and consequently may promote greater offline learning gains. A more recent study by Reis et al. [2015], however, provides evidence that offline gains in motor skills occur in a time‐dependent but not a sleep‐dependent fashion.
Total Learning
On the fifth day of tDCS delivery, the GOT threshold (with regard to baseline performance at the first day of testing) in the tDCSanodal condition was significantly lower than in the tDCSsham condition (Fig. 3). Surprisingly, at follow‐up there was only the trend of a significant difference in two‐point discrimination with regard to baseline performance (P = 0.051). However, this finding came along with a large effect size [η2= 0.162; Cohen, 1988]. In addition, comparing the absolute GOT thresholds between groups at follow‐up, we observed a significant difference in tactile acuity between the tDCSanodal and tDCSsham conditions with superior performance in the tDCSanodal condition. As well, in the tDCSsham condition, no evidence of long‐term learning with regard to baseline performance was seen.
These results provide evidence of long‐term learning in the somatosensory domain in response to the repeated application of anodal tDCS. This finding, moreover, is in line with results from the somatosensory domain reported by Mori et al. [2013], but is also consistent with findings from the motor domain [Reis et al., 2009]. Therefore, we are led to conclude that improvement in perceptual performance induced by anodal tDCS does not seem to suffer from an accelerated decay. Rather, in contrast to its sham application, the repeated delivery of anodal tDCS seems to enhance tactile skills for at least a month.
fMRI Data
We found a longitudinal decrease in the task‐dependent activational extent of the hand area in left S1 in the tDCSanodal condition at follow‐up, causing significantly less activation at the primary site of repeated tDCS application (in contrast to the tDCSsham). Moreover, an overall trend towards a decrease of the activational size was observed in the tDCSanodal condition, primarily in the left hemispheres, while over the course of the study there was a complementary trend of more extensive activation in the tDCSsham condition. Only the left S1 satifies all conditions we predefined to identify brain regions exhibiting changes specifically attributable to the repeated delivery of tDCS. These are (1) a significant longitudinal change in at least one stimulation group, (2) a resulting group difference at (at least) one point in time, (3) a significant interaction of changes in the activational extent over the course of the study and the stimulation group and (4) a correlation between the extent of activation and the amount of improvement in tactile acuity. Indeed, we observed that the larger the gain in tactile acuity by the fifth day of the study and the larger the gain at follow‐up, the more focalized the activational fMRI extent in the left S1 at the follow‐up fMRI session would be. The analysis of the magnitude of activation did not result in a significant finding (Figs. 4 and 5).
Reports of short‐term effects of tDCS on the BOLD‐response in the motor domain are ambiguous. However, while there are also reports of a task‐related decrease in activation [Antal et al., 2011; Baudewig et al., 2001], the number of studies that report an increase in the BOLD‐response in motor‐related brain regions after the application of anodal tDCS seems to outweigh the reports showing decreases in activation [Jang et al., 2009; Kwon et al., 2008; Lang et al., 2005; Stagg et al., 2009b]. As with the short‐term findings, results regrading long‐term effects of tDCS are ambiguous. Lindenberg et al. [2010] investigated the effects of longitudinally applied tDCS and behavioural training on the brain's response to motor task‐related brain activation in stroke patients, and reported a longitudinal increase of the activational magnitude in M1, ipsilateral to the site of stimulation. Lefebvre et al. [2014], who employed a similar paradigm, observed more effective recruitment of the motor network by more focalized activation in motor areas in the damaged hemisphere. Kim et al. [2012] applied a similar paradigm but investigated effects of anodal tDCS in healthy subjects, observing a decrease in M1 as well as in a more widespread network of areas. Therefore, effects of the repeated application of anodal tDCS in healthy subjects as well as in stroke patients may be mediated primarily by a decrease in functional activation.
In addition, previous observations [Kim et al., 2012; Lefebvre et al., 2014] and the present finding of a decrease in the size of task‐dependent activation in S1 with regard to somatosensory processing are rather plausible against the background of the physiology of somatosensory information processing. Tactile information processing not only requires high resolution at the periphery, but also in the central nervous system [for a review see Tommerdahl et al., 2010]. Thus, extensive and widespread activation at a later stage of skilled performance is likely disadvantageous to performance. While the early stage of skill learning is characterized by recruitment of more extensive neural machinery to perform a task through selection of the most effective set of modules, skilled performance seems to be reflected by a smaller set of neurons and their connections [Hlustik et al., 2004; Lefebvre et al., 2014; Karni and Sagi 1993; Reed et al., 2011; Xu et al., 2009; Yang et al., 2009; Yotsumoto et al., 2009]. Along this line of reasoning and according to the present fMRI findings, anodal tDCS may promote tactile learning by boosting the transition from large‐scale recruitment to refined information processing.
Interestingly, and in contrast to the motor domain [Kim et al., 2012], we found no significant difference in the activational extent between the tDCSanodal and tDCSsham conditions at the fifth day of the study, while there was a significant difference in performance. Yet we observed a significant relationship between the gain in tactile acuity by the fifth day of the study and a reduction of the activational extent at the follow‐up fMRI session four weeks later. Thus, there could be a “silent” or “transitional” period in which neural changes detectable by fMRI are not yet present, but mature over a prolonged period in dependence of the (amount of) initial training and initially triggered changes. As no such “transitional” period has been observed with regard to the motor system, we speculate that differences in the cortical architecture of S1 and M1 may be related to the present finding. In comparison to the agranular motor cortex, there is a smaller number of stabile spines in S1, also in response to LTP [Castro‐Alamancos et al., 1995, 1996]. These spines, however, mediate local effects of neurotransmitter release and constitute an important neuroarchitectural feature of LTP [Pastalkova et al., 2006; Whitlock et al., 2006] on which long‐term effects of tDCS are dependent [Stagg and Nitsche, 2011]. Therefore, a longer period of time may have to pass, in comparison to the motor cortex, for the establishment of stable changes in the neurofunctional architecture of S1.
The fact that neurofunctional changes in response to the repeated delivery of tDCS are located at the primary side of its application provides an unprecedented piece of evidence with regard to the question of the neural level at which perceptual long‐term learning takes place. In contrast to the concept of top‐down perceptual learning [Ahissar et al., 2009]—that is, learning at higher levels of stimulus processing—broad and recent evidence [Bao et al., 2010; Hua et al., 2010] has argued that perceptual learning occurs at the level of primary sensory cortices [Adini et al., 2002, 2004; Sasaki et al., 2010]. The present study is the first to provide long‐term brain imaging evidence from the domain of non‐invasive brain stimulation to show that perceptual learning also targets the early level of stimulus processing in the somatomatosensory domain.
However, methodological issues related to the present fRMI design exist (not all trials could be evaluated as planned; a repetition suppression study may have been more sensitive) and data evaluation (a multivoxel pattern analysis (MVPA) could have detected more subtle changes) that primarily restrict the sensitivity of the present study to detect changes in activation at sites other than S1. Therefore, we conclude that the repeated delivery of anodal tDCS also affects the early level of somatosensory stimulus processing, but we do not exclude changes in distinct parts of the somatosensory network or changes at a network level in response to tDCS, as is suggested by the most recent studies investigating the effect of tDCS on, for example, resting state connectivity [Keeser et al., 2012; Polonia 2011a,b, 2012; Park et al., 2013].
CONCLUSION
Tactile learning, as investigated in the present study, is substantially modulated by the repeated application of anodal tDCS. Behaviourally, the repeated delivery of anodal tDCS boosts offline learning. Neuronally, anodal tDCS causes a long‐term decrease in the size of activation in response to passive tactile stimulation in the left S1, which is highly plausible based on the neuroanatomy of somatosensory information processing. Thus, the present study provides valuable insight into the mode of operation for anodal tDCS on the brain response to perceptual learning.
REFERENCES
- Abraham WC (2003): How long will long‐term potentiation last? Philos T Roy Soc B 358:735–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adini Y, Sagi D, Tsodyks M (2002): Context‐enabled learning in the human visual system. Nature 415:790–793. [DOI] [PubMed] [Google Scholar]
- Adini Y, Wilkonsky A, Haspel R, Tsodyks M, Sagi D (2004): Perceptual learning in contrast discrimination: The effect of contrast uncertainty. J Vision 4:993–1005. [DOI] [PubMed] [Google Scholar]
- Ahissar M, Nahum M, Nelken I, Hochstein S (2009): Reverse hierarchies and sensory learning. Philos Trans R Soc Lond B 364:285–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amadi U, Allman C, Johansen‐Berg H, Stagg CJ (2015): The homeostatic interaction between anodal transcranial direct current stimulation and motor learning in humans is related to GABA A activity. Brain Stimul 8:898–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal A, Paulus W (2008): Transcranial direct current stimulation and visual perception. Perception 37:367–374. [DOI] [PubMed] [Google Scholar]
- Antal A, Polania R, Schmidt‐Samoa C, Dechent P, Paulus W (2011): Transcranial direct current stimulation over the primary motor cortex during fMRI. Neuroimage 55:590–596. [DOI] [PubMed] [Google Scholar]
- Bachtiar V, Stagg CJ (2014): The role of inhibition in human motor cortical plasticity. Neurosci 278:93–104. [DOI] [PubMed] [Google Scholar]
- Bao M, Yang L, Rios C, He B, Engel SA (2010): Perceptual learning increases the strength of the earliest signals in visual cortex. J Neurosci 30:15080–15084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudewig J, Nitsche MA, Paulus W, Frahm J (2001): Regional modulation of BOLD MRI responses to human sensorimotor activation by transcranial direct current stimulation. Magn Reson Med 45:196–201. [DOI] [PubMed] [Google Scholar]
- Bindman LJ (1965): Long‐lasting changes in firing frequency of neurones in rat cerebral cortex and radial potential gradients. J Physiol London 179:369–382. [Google Scholar]
- Bleyenheuft Y, Thonnard JL (2007): Tactile spatial resolution measured manually: A validation study. Somatosens Mot Res 24:111–114. [DOI] [PubMed] [Google Scholar]
- Bolognini N, Pascual‐Leone A, Fregni F (2009): Using non‐invasive brain stimulation to augment motor training‐induced plasticity. J Neuroeng Rehab 6:103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortz J (2009). Statistik für Human‐ und Sozialwissenschaftler. Berlin: Springer. [Google Scholar]
- Bortz J, Lienert GA, Boehnke K (2000): Verteilungsfreie Methoden in der Biostatistik. Berlin: Springer. [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB (2002): Region of interest analysis using an SPM toolbox. In: 8th International Conference on Functional Mapping of the Human Brain, Sendai, Japan.
- Caspers S, Geyer S, Schleicher A, Mohlberg H, Amunts K, Zilles K (2006): The human inferior parietal cortex: Cytoarchitectonic parcellation and interindividual variability. Neuroimage 33:430–448. [DOI] [PubMed] [Google Scholar]
- Castro‐Alamancos MA, Donoghue JP, Connors BW (1995): Different forms of synaptic plasticity in somatosensory and motor areas of the neocortex. J Neurosci 15:5324–5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro‐Alamancos MA, Connors BW (1996): Short‐term synaptic enhancement and long‐term potentiation in neocortex. Pnas 93:1335–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi RP, Fregni F, Snyder AW (2010): Visual memory improved by noninvasive brain stimulation. Brain Res 1353:168–175. [DOI] [PubMed] [Google Scholar]
- Clark VP, Coffman BA, Mayer AR, Weisend MP, Lane TD, Calhoun VD (2010): TDCS guided using fMRI significantly accelerates learning to identify concealed objects. Neuroimage 59:117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark VP, Coffman BA, Trumbo MC, Gasparovic C (2011): Transcranial direct current stimulation (tDCS) produces localized and specific alterations in neurochemistry: A (1)H magnetic resonance spectroscopy study. Neurosci Lett 500:67–71. [DOI] [PubMed] [Google Scholar]
- Clem RL, Huganir RL (2010): Calcium‐permeable AMPA receptor dynamics mediate fear memory erasure. Science 330:1108–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J ( 1988): Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Erlbaum. [Google Scholar]
- Conforto AB, Kaelin‐Lang A, Cohen LG (2002): Increase in hand muscle strength of stroke patients after somatosensory stimulation. Ann Neurol 51:122–125. [DOI] [PubMed] [Google Scholar]
- Conforto AB, Cohen LG, dos Santos RL, Scaff M, Marie SKN (2007): Effects of somatosensory stimulation on motor function in chronic corticosubcortical strokes. J Neurol 254:333–339. [DOI] [PubMed] [Google Scholar]
- Conforto AB, Ferreiro KN, Tomasi C, dos Santos RL, Moreira VL, Marie SKN, Cohen LG (2010): Effects of somatosensory stimulation on motor function after subacute stroke. Neurorehabil Neural Repair 24:263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruccu G, Aminoff MJ, Curio G, Guerit JM, Kakigi R, Mauguiere F, Garcia‐Larrea L (2008): Recommendations for the clinical use of somatosensory‐evoked potentials. Clin Neurophysiol 119:1705–1719. [DOI] [PubMed] [Google Scholar]
- Derkach VA, Oh MC, Guire ES, Soderling TR (2007): Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nature Rev Neurosci 8:101–113. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Shadmehr R (2005): Detecting and adjusting for artifacts in fMRI time series data. Neuroimage 27:624–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinse HR, Ragert P, Pleger B, Schwenkreis P, Tegenthoff M (2003): Pharmacological modulation of perceptual learning and associated cortical reorganization. Science 301:91–94. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K (2005): A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25:1325–1335. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Amunts K, Zilles K (2006): Incorporating cytoarchitectonic probability maps into the analysis of fMRI data. J Psychophysiol 20:320–320. [Google Scholar]
- Eickhoff SB, Jbabdi S, Caspers S, Laird AR, Fox PT, Zilles K, Behrens TEJ (2010): Anatomical and functional connectivity of cytoarchitectonic areas within the human parietal operculum. J Neurosci 30:6409–6421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraguna U, Vyazovskiy VV, Nelson AB, Tononi G, Cirelli C (2008): A causal role for brain‐derived neurotrophic factor in the homeostatic regulation of sleep. J Neurosci 28:4088–4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC (1994): Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp 1:210–220. [DOI] [PubMed] [Google Scholar]
- Fritsch B, Reis J, Martinowich K, Schambra HM, Ji YY, Cohen LG, Lu B (2010): Direct current stimulation promotes BDNF‐dependent synaptic plasticity: Potential implications for motor learning. Neuron 66:198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto S, Yamaguchi T, Otaka Y, Kondo K, Tanaka S (2014): Dual‐hemisphere transcranial direct current stimulation improves performance in a tactile spatial discrimination task. Clin Neurophysiol 125:1669–1674. [DOI] [PubMed] [Google Scholar]
- Gandiga PC, Hummel FC, Cohen LG (2006): Transcranial DC stimulation: A tool for double‐blind sham‐controlled clinical studies in brain stimulation. Clin Neurophysiol 117:845–850. [DOI] [PubMed] [Google Scholar]
- Hairston IS, Peyron C, Denning DP, Ruby NF, Flores J, Sapolsky RM, O'Hara BF (2004): Sleep deprivation effects on growth factor expression in neonatal rats: A potential role for BDNF in the mediation of delta power. J Neurophysiol 91:1586–1595. [DOI] [PubMed] [Google Scholar]
- Hilgenstock R, Weiss T, Witte OW (2014): You'd better think twice: Post‐decision perceptual confidence. NeuroImage 99:323–331. [DOI] [PubMed] [Google Scholar]
- Hlustik P, Solodkin A, Noll DC, Small SL (2004): Cortical plasticity during three‐week motor skill learning. J Clin Neurophysiol 21:180–191. [DOI] [PubMed] [Google Scholar]
- Hua TM, Bao PL, Huang CB, Wang ZH, Xu JW, Zhou YF, Lu ZL (2010): Perceptual learning improves contrast sensitivity of V1 neurons in cats. Curr Biol 20:887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Tononi G (2004): Local sleep and learning. Nature 430:78–81. [DOI] [PubMed] [Google Scholar]
- Huber R, Esser SK, Ferrarelli F, Massimini M, Peterson MJ, Tononi G (2007): TMS‐induced cortical potentiation during wakefulness locally increases slow wave activity during sleep. PloS One 2:e276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam N, Moriwaki A, Hattori Y, Hori Y (1994): Anodal polarization induces protein Kinase‐C‐Gamma (PKC‐gamma)‐like immunoreactivity in the rat cerebral‐cortex. Neurosci Res 21:169–172. [DOI] [PubMed] [Google Scholar]
- Jang SH, Ahn SH, Byun WM, Kim CS, Lee MY, Kwon YH (2009): The effect of transcranial direct current stimulation on the cortical activation by motor task in the human brain: An fMRI study. Neurosci Lett 460:117–120. [DOI] [PubMed] [Google Scholar]
- Kadosh RC, Soskic S, Iuculano T, Kanai R, Walsh V (2010): Modulating neuronal activity produces specific and long‐lasting changes in numerical competence. Curr Biol 20:2016–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni A, Sagi D (1993): The time‐course of learning a visual skill. Nature 365:250–252. [DOI] [PubMed] [Google Scholar]
- Keeser D, Meindl T, Bor J, Palm U, Pogarell O, Mulert C, Padberg F (2011): Prefrontal transcranial direct current stimulation changes connectivity of resting‐state networks during fMRI. J Neurosci 31:15284–15293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CR, Kim DY, Kim LS, Chun MH, Kim SJ, Park CH (2012): Modulation of cortical activity after anodal transcranial direct current stimulation of the lower limb motor cortex: A functional MRI study. Brain Stimul 5:462–467. [DOI] [PubMed] [Google Scholar]
- Kim S, Stephenson MC, Morris PG, Jackson SR (2014): tDCS‐induced alterations in GABA concentration within primary motor cortex predict motor learning and motor memory: A 7T magnetic resonance spectroscopy study. NeuroImage 99:237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korman M, Raz N, Flash T, Karni A (2003): Multiple shifts in the representation of a motor sequence during the acquisition of skilled performance. Proc Natl Acad Sci USA 100:12492–12497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft A, Roehmel J, Olma MC, Schmidt S, Irlbacher K, Brandt SA (2010): Transcranial direct current stimulation affects visual perception measured by threshold perimetry. Exp Brain Res 207(3‐4): 283–290. [DOI] [PubMed] [Google Scholar]
- Kwon YH, Ko MH, Ahn SH, Kim YH, Song JC, Lee CH, Jang SH (2008): Primary motor cortex activation by transcranial direct current stimulation in the human brain. Neurosci Lett 435:56–59. [DOI] [PubMed] [Google Scholar]
- Lang N, Siebner HR, Ward NS, Lee L, Nitsche MA, Paulus W, Frackowiak S (2005): How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain? Eur J Neurosci. 22:495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre S, L Dricot, P Laloux, W Gradkowski, P Desfontaines, F Evrard, A Peeters, J Jamart, Y Vandermeeren (2015): Neural substrates underlying stimulation‐enhanced motor skill learning after stroke Brain 138:149–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebetanz D, Nitsche MA, Tergau F, Paulus W (2002): Pharmacological approach to the mechanisms of transcranial DC‐stimulation‐induced aftereffects of human motor cortex excitability. Brain 125:2238–2247. [DOI] [PubMed] [Google Scholar]
- Lindenberg R, Renga V, Zhu LL, Nair D, Schlaug G (2010): Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology 75:2176–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Christian K, Lu B (2008): BDNF: A key regulator for protein synthesis‐dependent LTP and long‐term memory? Neurobiol Learn Mem 89:312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald BJ, Chung HJ, Huganir RL (2001): Identification of protein kinase C phosphorylation sites within the AMPA receptor GluR2 subunit. Neuropharmacology 41:672–679. [DOI] [PubMed] [Google Scholar]
- Mazaika P, Hoeft F, Glover H, Reiss AL (2009): Methods and software for fMRI analysis for clinical subjects. Poster Session at the Meeting of Hum Brain Mapp, San Francisco, CA. [Google Scholar]
- Mori F, Nicoletti CG, Kusayanagi H, Foti C, Restivo DA, Marciani MG, Centonze D (2013): Transcranial direct current stimulation ameliorates tactile sensory deficit in multiple sclerosis. Brain Stimul 6:654–659. [DOI] [PubMed] [Google Scholar]
- Moseley GL, Wiech K (2009): The effect of tactile discrimination training is enhanced when patients watch the reflected image of their unaffected limb during training. Pain 144:314–319. [DOI] [PubMed] [Google Scholar]
- Nanou E, El Manira A (2010): Mechanisms of modulation of AMPA‐induced Na(+)‐activated K(+) current by mGluR1. J Neurophysiol 103:441–445. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, Paulus W (2003): Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol‐London 553:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Liebetanz D, Schlitterlau A, Henschke U, Fricke K, Frommann K, Tergau F (2004): GABAergic modulation of DC stimulation‐induced motor cortex excitability shifts in humans. Eur J Neurosci 19:2720–2726. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, Pascual‐Leone A (2008): Transcranial direct current stimulation: State of the art 2008. Brain Stim 1:206–223. [DOI] [PubMed] [Google Scholar]
- Pang PT, Teng HK, Zaitsev E, Woo NT, Sakata K, Zhen SH, Lu B (2004): Cleavage of proBDNF by tPA/plasmin is essential for long‐term hippocampal plasticity. Science 06:487–491. [DOI] [PubMed] [Google Scholar]
- Park CH, Chang WH, Park JY, Shin YI, Kim ST, Kim YH (2013): Transcranial direct current stimulation increases resting state interhemispheric connectivity. Neurosci Lett 539:7–10. [DOI] [PubMed] [Google Scholar]
- Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC (2006): Storage of spatial information by the maintenance mechanism of LTP. Science 313:1141–1144. [DOI] [PubMed] [Google Scholar]
- Petrusic WM, Baranski JV (2003): Judging confidence influences decision processing in comparative judgments. Psychon Bull Rev 10:177–183. [DOI] [PubMed] [Google Scholar]
- Pleger B, Dinse HR, Ragert P, Schwenkreis P, Malin JP, Tegenthoff M (2001): Shifts in cortical representations predict human discrimination improvement. Proc Natl Acad Sci USA 98:12255–12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleger B, Foerster AF, Ragert P, Dinse HR, Schwenkreis P, Malin JP, Tegenthoff M (2003): Functional imaging of perceptual learning in human primary and secondary somatosensory cortex. Neuron 40:643–653. [DOI] [PubMed] [Google Scholar]
- Polania R, Paulus W, Antal A, Nitsche MA (2011a): Introducing graph theory to track for neuroplastic alterations in the resting human brain: A transcranial direct current stimulation study. Neuroimage 54:2287–2296. [DOI] [PubMed] [Google Scholar]
- Polania R, Nitsche MA, Paulus W (2011b): Modulating functional connectivity patterns and topological functional organisation of the brain with direct current stimulation. Hum Brain Mapp 32:1236–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanía R, Paulus W, Nitsche MA (2012): Reorganizing the intrinsic functional architecture of the human primary motor cortex during rest with non‐invasive cortical stimulation. PloS One 7:e30971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poline JB, Worsley KJ, Evans AC, Friston KJ (1997): Combining spatial extent and peak intensity to test for activations in functional imaging. Neuroimag. 5:83–96. [DOI] [PubMed] [Google Scholar]
- Purpura DP, McMurtry JG (1965): Intracellular activities and evoked potential changes during polarization of motor cortex. J Neurophysiol 28:166–185. ( [DOI] [PubMed] [Google Scholar]
- Ragert P, Nierhaus T, Cohen LG, Villringer A (2011): Interhemispheric interactions between the human primary somatosensory cortices. PloS One 6:e16150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragert P, Vandermeeren Y, Camus M, Cohen LG (2008): Improvement of spatial tactile acuity by transcranial direct current stimulation. Clin. Neurophysiol 119:805–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raczkowski D, Kalat JW, Nebes R (1974): Reliability and validity of some handedness questionnaire items. Neuropsychologia 12:43–47. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Merzenich MM, Jenkins WM, Grajski KA, Dinse HR (1992): Topographic reorganization of the hand representation in cortical area 3b of owl monkeys trained in a frequency‐discrimination task. J Neurophysiol 67:1031–1056. [DOI] [PubMed] [Google Scholar]
- Reed A, Riley J, Carraway R, Carrasco A, Perez C, Jakkamsetti V, Kilgard MP (2011): Cortical map plasticity improves learning but is not necessary for improved performance. Neuron 70:121–131. [DOI] [PubMed] [Google Scholar]
- Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, Krakauer JW (2009): Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Pnas 106:1590–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis J, Fischer JT, Prichard G, Weiller C, Cohen LG, Fritsch B (2015): Time‐but not sleep‐dependent consolidation of tDCS‐enhanced visuomotor skills. Cereb Cortex 25:109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson EM (2004): Skill learning: Putting procedural consolidation in context. Curr Biol 14:1061–1063. [DOI] [PubMed] [Google Scholar]
- Santos SFA, Rebelo S, Derkach VA, Safronov BV (2007): Excitatory interneurons dominate sensory processing in the spinal substantia gelatinosa of rat. J Physiol London 581:241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Gold J, Watanabe T (2010): Perceptual learning: Cortical changes when cats learn a new trick. Curr Biol 20:R557–R558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathian K, Zangaladze A (1997): Tactile learning is task specific but transfers between fingers. Percept Psychophys 59:119–128. [DOI] [PubMed] [Google Scholar]
- Sathian K, Zangaladze A (1998): Perceptual learning in tactile hyperacuity: Complete intermanual transfer but limited retention. Exp Brain Res 118:131–134. [DOI] [PubMed] [Google Scholar]
- Seitz AR, Dinse HR (2007): A common framework for perceptual learning. Curr Opin Neurobiol 17:148–153. [DOI] [PubMed] [Google Scholar]
- Sens E, Teschner U, Meissner W, Preul C, Huonker R, Witte OW, Miltner WHR, Weiss T (2012): Effects of temporary functional deafferentation on the brain, sensation, and behavior of stroke patients. J Neurosci 32:11773–11779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderstrom NC, Bjork RA (2015): Learning versus performance: An integrative review. Perspect Psychol Sci 10:176–199. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Nitsche MA (2011): Physiological basis of transcranial direct current stimulation. Neuroscientist 17:37–53. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Best JG, Stephenson MC, O'Shea J, Wylezinska M, Kincses ZT, Johansen‐Berg H (2009a): Polarity‐sensitive modulation of cortical neurotransmitters by transcranial stimulation. J Neurosci 29:5202–5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, O'Shea J, Kincses ZT, Woolrich M, Matthews PM, Johansen‐Berg H (2009b): Modulation of movement‐associated cortical activation by transcranial direct current stimulation. Eur J Neurosci 30:1412–1423. [DOI] [PubMed] [Google Scholar]
- Taulu S, Simola J (2006): Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys Med Biol 51:1759–1768. [DOI] [PubMed] [Google Scholar]
- Tegenthoff M, Ragert P, Pleger B, Schwenkreis P, Forster AF, Nicolas V, Dinse HR (2005): Improvement of tactile discrimination performance and enlargement of cortical somatosensory maps after 5 Hz rTMS. Plos Biol 3:2031–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- M Tommerdahl, OV Favorov, BL Whitsel (2010): Dynamic representations of the somatosensory cortex. Neurosci Biobehav Rev 34:160–170. [DOI] [PubMed] [Google Scholar]
- Sommerdahl M, Favorov OV, Whitsel BL (2010): Dynamic representations of the somatosensory cortex. Neurosci Biobehav Rev 34:160–170. [DOI] [PubMed] [Google Scholar]
- Van Boven RW, Johnson KO (1994): The limit of tactile spatial resolution in humans: Grating orientation discrimination at the lip, tongue, and finger. Neurology 44:2361–2366. [DOI] [PubMed] [Google Scholar]
- Van Boven RW, Ingeholm JE, Beauchamp MS, Bikle PC, Ungerleider LG (2005): Tactile form and location processing in the human brain. Pnas 102:12601–12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss T, Sens E, Teschner U, Meissner W, Preul C, Witte OW, Miltner WH (2011): Deafferentation of the affected arm a method to improve rehabilitation? Stroke 42:1363–1370. [DOI] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF (2006): Learning induces long‐term potentiation in the hippocampus. Science 313:1093–1097. [DOI] [PubMed] [Google Scholar]
- Yan JZ, Xu Z, Ren SQ, Hu B, Yao W, Wang SH, Liu SY, Wei L (2011): Protein kinase C promotes N‐Methyl‐D‐aspartate (NMDA) receptor trafficking by indirectly triggering calcium/calmodulin‐dependent protein kinase II (CaMKII) autophosphorylation. J Biol Chem 286:25187–25200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Pan F, Gan WB (2009): Stably maintained dendritic spines are associated with lifelong memories. Nature 462:920–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotsumoto Y, Sasaki Y, Chan P, Vasios CE, Bonmassar G, Ito N, Watanabe T (2009): Location‐specific cortical activation changes during sleep after training for perceptual learning. Curr Biol 19:1278–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TA Yousry, UD Schmid, H Alkadhi, D Schmidt, A Peraud, A Buettner, P Winkler (1997): Localization of the motor hand area to a knob on the precentral gyrus. Brain 120:141–157. [DOI] [PubMed] [Google Scholar]
- Zhang MM, Mariola E, Stilla R, Stoesz M, Mao H, Hu XP, Sathian K (2005): Tactile discrimination of grating orientation: fMRI activation patterns. Hum Brain Mapp 25:370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]


