Abstract
The aim of this study was to investigate the age‐related changes in resting‐state neurometabolic connectivity from childhood to adulthood (6–50 years old). Fifty‐four healthy adult subjects and twenty‐three pseudo‐healthy children underwent [18F]‐fluorodeoxyglucose positron emission tomography at rest. Using statistical parametric mapping (SPM8), age and age squared were first used as covariate of interest to identify linear and non‐linear age effects on the regional distribution of glucose metabolism throughout the brain. Then, by selecting voxels of interest (VOI) within the regions showing significant age‐related metabolic changes, a psychophysiological interaction (PPI) analysis was used to search for age‐induced changes in the contribution of VOIs to the metabolic activity in other brain areas. Significant linear or non‐linear age‐related changes in regional glucose metabolism were found in prefrontal cortices (DMPFC/ACC), cerebellar lobules, and thalamo‐hippocampal areas bilaterally. Decreases were found in the contribution of thalamic, hippocampal, and cerebellar regions to DMPFC/ACC metabolic activity as well as in the contribution of hippocampi to preSMA and right IFG metabolic activities. Increases were found in the contribution of the right hippocampus to insular cortex and of the cerebellar lobule IX to superior parietal cortex metabolic activities. This study evidences significant linear or non‐linear age‐related changes in regional glucose metabolism of mesial prefrontal, thalamic, mesiotemporal, and cerebellar areas, associated with significant modifications in neurometabolic connectivity involving fronto‐thalamic, fronto‐hippocampal, and fronto‐cerebellar networks. These changes in functional brain integration likely represent a metabolic correlate of age‐dependent effects on sensory, motor, and high‐level cognitive functional networks. Hum Brain Mapp 37:3017–3030, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: brain development, physiologic aging, functional integration, [18F]‐fluorodeoxyglucose positron emission tomography (FDG‐PET)
INTRODUCTION
Human brain development and physiological aging are associated with major changes in the structural and the functional organization of neural systems that affect sensory perception, motor abilities, cognitive performance, and behavior [Reuter‐Lorenz and Park, 2010; Spreng, et al., 2010].
Development of the human nervous system starts in utero with progressive events like neural proliferation, neurite outgrowth, and synapse formation. The early occurrence of these constructive mechanisms represents a crucial contribution to the patterning of brain connectivity into functional neuronal networks [for reviews, see Low and Cheng, 2006; Vanderhaeghen and Cheng, 2010]. However, they result in an overabundance of neural connections, which must be subsequently reshaped to ensure an efficient communication flow within and between neural networks. The initial constructive events are therefore followed by regressive processes such as cell death, axon pruning, and synapse elimination, which occur after birth (during infancy, childhood, and adolescence) and gradually refine the connectivity patterns to a more precise and mature circuitry [Low and Cheng, 2006; Vanderhaeghen and Cheng, 2010]. These regressive processes occurring at the microscopic level translate into linear and non‐linear changes at the macroscopic level (i.e., regional changes in grey matter density, regional changes in diffusion tensor imaging [DTI] indices, etc.) as evidenced by structural neuroimaging investigations [see, e.g., Gogtay et al., 2004; Simmonds et al., 2014; Whitford et al., 2007]. In agreement with these observations, positron emission tomography studies using [18F]‐fluorodeoxyglucose positron emission tomography (FDG‐PET) have demonstrated that brain development is associated with changes in regional glucose metabolism in numerous cortical and subcortical structures from birth to adulthood [for a review, see Chugani, 1998]. Indeed, the measurement of absolute values of local cerebral metabolic rate for glucose (lCMRGlu) during development indicates that the cerebral cortex undergoes a dynamic course of metabolic maturation. This metabolic activity time‐course is characterized by a dramatic increase of lCMRGlu from birth in primary sensorimotor cortex, thalamus, cerebellar vermis and brainstem, followed by parietal, temporal and calcarine cortices, basal ganglia, and cerebellar hemisphere between 3 and 5 months, whereas frontal and occipital cortices started metabolic rise at 6–8 months [Chugani et al., 1987; Kinnala et al., 1996]. Then, lCMRGlu continues to increase in most brain structures up to about 4 years, when it plateaus until about 10 years when the children's brain consumes over twice the amount of glucose as the adults' brain does. Finally, lCMRGlu begins to decline and gradually reach adult values by 16–18 years [Chugani et al., 1987]. Also, from childhood to adulthood, regional metabolic changes predominate in the thalamus and the anterior cingulate cortex (ACC), but other cortical regions such as the insula, the postcentral gyrus, the prefrontal, and the posterior cingulate (PCC) cortices are also involved [Van Bogaert et al., 1998]. As expected, the metabolic pattern observed during brain development is closely associated with age‐related variations in regional cerebral blood flow (rCBF) [Chiron et al., 1992]. Indeed, high absolute rCBF values are found at birth in several cortical areas (temporal pole, visual, language, auditive, sensorimotor, parieto‐temporal, and frontal associative cortices), followed by rCBF increase in thalamic and cerebellar regions at 1 year of age. Values of rCBF reach a peak at 5 years of age, after which they progressively decrease to reach adult level at the end of the second decade of life [Chiron et al., 1992].
After the developmental phase—and partly superimposed to it—, physiological aging is characterized by a progressive decrease in synaptic density and neuronal loss in specific grey matter areas as well as a progressive demyelination of white matter tracts linking distant nodes of distributed neurocognitive networks [Madden et al., 2009; Moran et al., 2014; O'Sullivan et al., 2001; Resnick et al., 2003; Salat et al., 2005]. In line with these aging‐related microstructural changes, longitudinal structural MRI studies consistently demonstrated during adulthood an aging‐related pattern of progressive decrease in regional gray matter density predominating over associative cortices [Courchesne et al., 2000; Resnick et al., 2003]. Also, the progressive changes in DTI indices (e.g., decrease in fractional anisotropy) observed during physiological aging account for the progressive structural disconnection associated with (possibly activity dependent) demyelination [Bennett et al., 2010; Fields, 2015]. Accordingly, several FDG‐PET studies have shown significant decline in cerebral glucose consumption with normal aging (from 18 to 90 years) that mainly involves associative cortices [Pardo et al., 2007; Petit‐Taboue et al., 1998; Shen et al., 2012; Willis et al., 2002].
Taken together, these data support the association of brain development and physiological aging with major changes in brain micro‐ and macrostructural organization that affect brain connectivity and functional network patterns. Surprisingly, no PET study has yet investigated the connectivity changes associated with the age‐related modifications in regional brain glucose metabolism observed from childhood to adulthood. Such study would provide novel insights into how age‐dependent regional cerebral metabolic changes influence the large‐scale brain architecture across the life span and, in particular, the functional integration between cortical and subcortical structures. In this voxel‐based analysis study, we first identified the brain areas showing linear or quadratic metabolic changes from 6 to 50 years of age. This approach was motivated by a previous FDG‐PET study from our group, which has shown that the non‐linear age‐related regional cerebral glucose metabolism changes observed in some associative cortical and subcortical brain regions followed a quadratic evolution [Van Bogaert et al., 1998]. We then performed psychophysiological interaction (PPI) analyses between these areas and the rest of the brain in order to characterize the age‐related modifications in effective connectivity associated with the evolution of regional brain glucose metabolism from childhood to adulthood. In other words, we tested for an effect of age on the linear coupling between two regions as indexed by their metabolism. We expected that the age‐related metabolic changes observed in some cortical (e.g., medial prefrontal cortices) and subcortical (e.g., thalamus) brain areas would translate into a significant age‐dependent change in the contribution of activity in one region when explaining the activity of another. Such finding would provide a novel metabolic correlate of the effects of age on large‐scale brain functional networks involved in high‐level cognitive functions such as, for example, executive or working memory processes.
METHODS
Subjects
A group of 77 subjects aged from 6 to 50 years (42 females, 35 males, mean age: 26 years) was included in this study. This group was composed of 23 children (15 girls, 8 boys, mean age: 10.9, age range: 6–17 years) with cryptogenic focal epilepsy and 54 healthy adults (27 females, 27 males, mean age: 32.8, age range: 18–50 years).
FDG‐PET data of 29 of the 54 healthy adults were acquired at the PET/Biomedical Cyclotron Unit of the ULB‐Hôpital Erasme, Brussels, Belgium and those of the 25 others were acquired at the Nuclear Medicine division of the UZ Leuven, Leuven, Belgium. All subjects had no prior neurologic or psychiatric history.
The group of 23 epileptic children was selected from a pediatric population investigated with FDG‐PET at the Service Hospitalier F. Joliot (Orsay, France) and validated in a previous work as representative of the normal pediatric population [Archambaud et al., 2013]. This population was composed of 24 children with focal cryptogenic epilepsy, normal structural MRI, and normal FDG‐PET (visual and statistical parametric assessments). One 5‐year‐old child was excluded from this study to avoid the normalization artifact issues associated with the use of an adult template for children aged less than 6 years [Muzik et al., 2000].
In order to assess the effect of epilepsy versus maturational processes on the age‐related changes in regional cerebral metabolism disclosed in this study, a second set of pediatric FDG‐PET data acquired at the PET/Biomedical Cyclotron Unit of the ULB‐Hôpital Erasme was retrospectively selected among children affected by various types of focal neurological disease or by a systemic disease but with normal cerebral FDG‐PET. No children ever suffered from epilepsy. This group was composed of 13 children (6 girls and 7 boys, mean age: 12.1, age range: 6–17 years) with cerebellar (4 patients) or brainstem (3 patients) tumors, post‐catatonic state (2 patients), systemic inflammatory disease (2 patients), nystagmus of unknown origin (1 patient), and post‐herpes zoster vasculitis (1 patient). FDG‐PET showed minor metabolic abnormalities in 5 patients with infratentorial lesions (hypometabolic brainstem in 3 patients and focal hypermetabolism in 2 patients with cerebellar tumors) whereas it was normal in the 8 other children. FDG‐PET data of these non‐epileptic children were entered in a conjunction analysis in order to isolate metabolic features common to the two pediatric populations [Price and Friston, 1997; Price et al., 1997] (see below). They were not included in the main voxel‐based analyses that were conducted only using the homogeneous and validated group of 23 epileptic children (see below).
All FDG‐PET data were acquired on the same type of PET camera using the same data acquisition and reconstruction procedures (see below).
The institutional Ethics Committees gave approval for these FDG‐PET investigations from which control data were obtained and for which written informed consent was obtained from all subjects (or their parents in case of children). The ULB‐Hôpital Erasme Ethics Committee waived the requirement for obtaining parents' (on the behalf of children participants) informed consent (oral and written) in the context of this retrospective study. The ULB‐Hôpital Erasme Ethics Committee gave approval for conducting the present study.
FDG‐PET Data Acquisition
FDG‐PET data were acquired using an ECAT 962 Exact HR+ camera (CTI‐Siemens, Knoxville, TN), the characteristics of which have been previously described [Brix et al., 1997].
All adult subjects and non‐epileptic children fasted for at least 4 hours, were awake in an eye‐closed rest and received an intravenous bolus injection of 2–5 mCi (74–185 MBq) of FDG before a 20‐minute PET data acquisition in three‐dimensional mode. One emission frame composed of 63 transaxial slices was obtained and realigned to the canthomeatal line. Images were corrected for photon attenuation with a post‐injection 10‐minute transmission scan obtained using 68Ge line sources. Each PET image was reconstructed using filtered back projection (Hanning filter applied) and displayed in a 128 × 128 × 63 voxel format, with a slice thickness of 2.4 mm.
Epileptic children were also investigated in a fasting and resting state, in a quiet, dimly light environment. The last seizure had occurred more than 6 h before PET examination except for 3 patients (between 1 and 6 h). All patients received an intravenous bolus injection of 3.7 MBq/kg (maximum 180 MBq) of FDG while lying in the scanner. Patients were not sedated for PET data acquisition, which started 30 minute after FDG injection.
FDG‐PET Data Preprocessing
FDG‐PET data were analyzed using the voxel‐based Statistical Parametric Mapping method (SPM8, http://www.fil.ion.ucl.ac.uk/spm/, Wellcome Trust Centre for Neuroimaging, London, UK). The PET images were first spatially normalized into the Montreal Neurologic Institute template (Montreal Neurologic Institute, Quebec, Canada) and then smoothed using a 12‐mm full‐width at half‐maximum Gaussian isotropic kernel. Global activity normalization was performed by proportional scaling [Van Bogaert et al., 2000].
Age‐Related Changes in Regional Cerebral Glucose Metabolism
A group‐level SPM analysis was performed to investigate the effects of age on regional cerebral glucose metabolism. For this analysis, we constructed a general linear model of the preprocessed FDG‐PET data of adult subjects and epileptic children, including a total of 77 scans. As age‐related changes in regional cerebral glucose metabolism were previously shown to evolve quadratically during development [Van Bogaert et al., 1998], we considered the subjects' age and age squared as covariates of interest. A F‐test was first used to search for brain areas showing statistically significant linear or non‐linear age dependent effects. Then, another F‐test was used to identify the prevalence of second‐order age effects by testing only the second‐order polynomial parameter or coefficient of our general linear model. Possible confounding effects were regressed out, by adding the subjects' gender and gray matter volume (GMV) as covariates of no‐interest in the design matrix. As structural MRI data were not available in the healthy subjects, GMV was calculated by applying the segmentation tool in SPM8 to the subjects' FDG‐PET images. GMV was added as a covariate of no‐interest in the design matrix to take into account the partial volume effect (PVE) associated with PET imaging that could bias the study of age‐related changes in metabolism [Curiati et al., 2011; Meltzer et al., 2000].
Finally, age‐dependent effects were further characterized by computing the second‐order polynomial regression plots for the brain areas showing statistically significant metabolic changes using Matlab 7.6 R2008a (Mathworks Inc., Sherborn, MA).
Age‐Related Changes in Functional Integration
For these analyses, the peak voxels within the clusters showing significant age‐related changes in relative glucose metabolism identified in the previous analyses were considered as voxels of interest (VOIs) for PPI analyses. These analyses specifically assessed the occurrence of any change with age in the contribution of the brain areas showing significant (linear or non‐linear) age‐related changes in glucose metabolism to the level of metabolic activity in other brain areas in the group of 77 subjects. In other words, these PPI analyses aimed at identifying brain regions in which metabolic activity depends on an interaction between age and the metabolic activity of the brain areas showing significant age‐related changes in glucose metabolism. As such, this analysis is an adaptation of the previously developed psycho‐ or physio‐physiological interaction analyses [Friston et al., 1997] to a specific experimental factor, that is, the subjects' age. In practice, for each VOI, the [VOI metabolic activity × age] values were used as a covariate of interest (centered around their mean). Separate t‐contrast analyses then searched, throughout the brain, for regions showing significant age‐related change (i.e., increase or decrease) in the contribution of the considered VOI to their metabolism. For these analyses, metabolic activity and age vectors constituted covariates of no‐interest. Finally, to account for the effects of PVE on connectivity or contribution changes, the PPI analyses were performed with GMV as covariate of no‐interest.
Effect of Epilepsy Versus Maturational Processes on Age‐Related Metabolic Changes
To assess the effect of epilepsy versus maturational processes on age‐related metabolic changes in our pediatric population, a general linear model of the FDG‐PET data of epileptic and non‐epileptic children as well as adult subjects taken as separate groups was constructed. Using this design matrix, separate t‐contrasts first identified brain regions where glucose metabolism was significantly lower or higher in (1) epileptic children versus adult controls, and (2) non‐epileptic children versus adult controls. Subsequently, a conjunction analysis between epileptic children versus adult subjects t‐contrasts and non‐epileptic children versus adult subjects t‐contrasts was performed to identify the metabolic changes that were common to both pediatric populations compared with adults. For these analyses, gender was used as covariate of no interest to regress out the potential confounding effects of this variable. This analysis provided evidence for consistent age‐related changes in metabolism between the epileptic and the non‐epileptic children. Furthermore, it serves as a test of reproducibility when splitting our (younger) cohort into two groups.
Results of all SPM analyses performed in this study were considered significant at P < 0.05 corrected for multiple comparisons over the entire brain volume (Family Wise Error, FWE). In addition, SPM analyses investigating the effects of age on regional cerebral glucose metabolism were considered significant when the cluster size k was greater than 1,000 voxels.
RESULTS
Variation of Cerebral Grey Matter Volume with Age
Figure 1 illustrates the evolution of cerebral GMV with age.
Figure 1.

Linear decrease of cerebral grey matter volume with age.
Significant linear decrease of cerebral GMV was observed with age in the population of 77 subjects (epileptic children and adult controls; Pearson's correlation coefficient r = −0.35, P value = 0.002).
Age‐Related Changes in Regional Cerebral Glucose Metabolism
Figure 2 illustrates the brain areas showing significant linear or non‐linear age‐related changes in regional cerebral glucose metabolism as well as the associated polynomial regressions characterizing the corresponding evolution of the metabolism with age. Table 1 details the brain areas showing significant linear or non‐linear age‐related changes with age.
Figure 2.

Brain areas showing significant linear or non‐linear age‐related changes in regional glucose metabolism. Top. Significant changes were found in dorsomedial prefrontal/anterior cingulate cortices (DMPFC/ACC), thalamic (thal) and hippocampal (hipp) regions bilaterally as well as in the cerebellum (lobules VIIIb and IX). R, right; L, left. Images are thresholded at P < 0.05 corrected for multiple comparisons over the entire brain volume (Family Wise Error, FWE). The color scale on the right of the figure represents the F‐statistic of the significant voxels. Bottom. Regression plots illustrating the evolution of the metabolic activity with age for these brain areas.
Table 1.
Brain areas showing significant age‐related changes with age
| MNI coordinates (mm) | |||
|---|---|---|---|
| Region | x | y | z |
| R ACC | 4 | 30 | 30 |
| L hippocampus | −28 | −8 | −30 |
| R hippocampus | 24 | −10 | −28 |
| L thalamus | −16 | −26 | 4 |
| R thalamus | 8 | −16 | 2 |
| L lobule VIIIb | −4 | −60 | −38 |
| R lobule IX | 4 | −54 | −50 |
R, right; L, left; ACC, anterior cingulate cortex.
Significant linear or non‐linear age‐related metabolic changes were found in a large bilateral mesiofrontal cluster—covering the dorsomedial prefrontal cortex (DMPFC) and the anterior cingulate cortex (ACC) where the peak voxel of this DMPFC/ACC cluster was found—, the thalamic and the hippocampal regions bilaterally, as well as the cerebellum (lobule IX and lobule VIIIb).
Regression plots showed that the relative metabolic activity in ACC and thalami increased from childhood to reach its maximal values at 30–35 years, after which it tends to decrease through adulthood. By contrast, hippocampal and cerebellar regions followed a different trend, slowly increasing through the considered life span.
Figure 3 illustrates the brain areas showing a significant non‐linear evolution of regional cerebral glucose metabolism with age. Second‐order age‐dependent effects were observed in the DMPFC/ACC cluster and thalamic regions bilaterally as suggested by polynomial regression plots.
Figure 3.

Brain areas showing significant prevalence of second‐order age effects in regional glucose metabolism. Significant non‐linear changes were found in dorsomedial prefrontal/anterior cingulate cortices and thalamic regions bilaterally. For viewing purpose, images are thresholded at P < 0.001 uncorrected. The color scale on the right of the figure represents the F‐statistic of the significant voxels.
Based on these results, peak voxels in the ACC (MNI coordinates: [4 30 30] mm), the hippocampi (left: [−28 −8 −30] mm, right: [24 −10 −28] mm), the thalami (left: [−16 −26 4] mm, right: [8 −16 2] mm) and the cerebellar lobules (VIIIb: [−4 −60 −38] mm, IX: [4 −54 −50] mm) were considered as VOI for further functional integration analyses.
Age‐Related Changes in Functional Integration
Figures 4 and 5 illustrate the brain areas showing age‐related changes in PPI with the brain regions characterized by significant (linear or non‐linear) changes in regional glucose metabolism with age. Tables 2 and 3 detail the results of PPI analyses.
Figure 4.

Results of PPI analyses showing significant age‐related decreases in seed contribution. Top, Left. Coronal slices showing the seed voxels (white dot = VOI) used for PPI analyses. Middle. Coronal or sagittal slices illustrating the brain regions showing significant age‐related decrease in the contribution of the seed voxels to their metabolic activity. For viewing purpose, images are thresholded at P < 0.001 uncorrected. Right. Color scale representing the T‐statistic of the significant voxels. Bottom. Regression plots illustrating the age‐related decrease in the contribution of seed voxels to the level of the metabolic activity in other brain areas. These regression plots were obtained by dividing subjects into young (6–31 years) and old (32–50 years) subjects. They are given for illustrative purpose only and do not reflect underlying parametric effects of age on the inter‐regional contribution.
Figure 5.
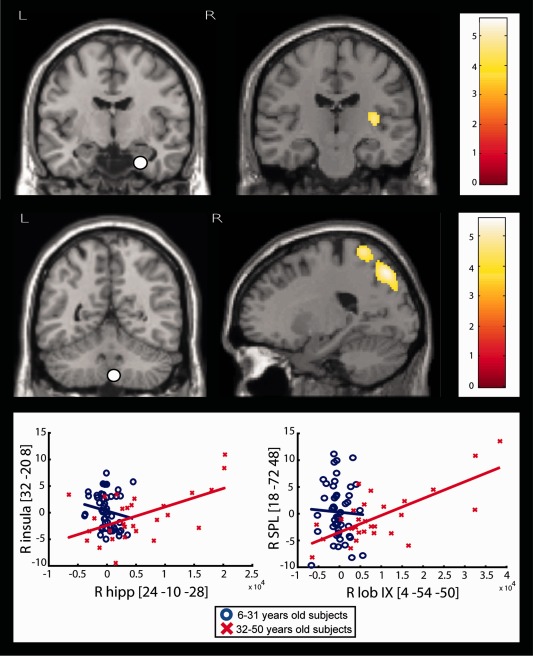
Results of PPI analyses showing significant age‐related increases in seed contribution. Top, Left. Coronal slices showing the seed voxels (white dot = VOI) used for PPI analyses. Middle. Coronal or sagittal slices illustrating the brain regions showing significant age‐related increase in the contribution of the seed voxels to their metabolic activity. For viewing purpose, images are thresholded at P < 0.001 uncorrected. Right. Color scale representing the T‐statistic of the significant voxels. Bottom. Regression plots illustrating the age‐related increase in the contribution of seed voxels to the level of the metabolic activity in other brain areas. These regression plots were obtained by dividing subjects into young (6–31 years) and old (32–50 years) subjects. They are given for illustrative purpose only and do not reflect underlying parametric effects of age on the inter‐regional contribution.
Table 2.
Results of PPI analyses: significant age‐related decreases in seed contribution
| Seed voxel | Voxels showing significant PPI | P valuesa | ||||||
|---|---|---|---|---|---|---|---|---|
| Region | MNI coordinates (mm) | Region | MNI coordinates (mm) | |||||
| x | y | z | x | y | z | |||
| L hipp | −28 | −8 | −30 | L DMPFC/ACC | −6 | 38 | 20 | 0.009 |
| R hipp | 24 | −10 | −28 | R IFG | 44 | 16 | 4 | 0.006 |
| R DMPFC/ACC | 4 | 48 | 4 | 0.026 | ||||
| L preSMA | −10 | 14 | 70 | 0.027 | ||||
| R thal | 8 | −16 | 2 | L DMPFC/ACC | −10 | 34 | 32 | 0.021 |
| L lob VIIIb | −4 | −60 | −38 | L DMPFC/ACC | −2 | 46 | 14 | 0.006 |
| R lob IX | 4 | −54 | −50 | L DMPFC/ACC | −6 | 50 | 6 | 0.003 |
R, right; L, left; hipp, hippocampus; thal, thalamus; lob, lobule; DMPFC/ACC, dorsomedial prefrontal cortex/anterior cingulate cortex; IFG, inferior frontal gyrus; preSMA, presupplementary motor area.
a P corr.
Table 3.
Results of PPI analyses: significant age‐related increases in seed contribution
| Seed voxel | Voxels showing significant PPI | P valuesa | ||||||
|---|---|---|---|---|---|---|---|---|
| Region | MNI coordinates (mm) | Region | MNI coordinates (mm) | |||||
| x | y | z | x | y | z | |||
| R hipp | 24 | −10 | −28 | R post insula | 32 | −20 | 8 | 0.035 |
| R lob IX | 4 | −54 | −50 | R SPL | 18 | −72 | 48 | 0.016 |
R, right; hipp, hippocampus; post, posterior; SPL, superior parietal lobule.
a P corr.
PPI analyses first demonstrated that the changes in regional glucose metabolism described above were associated with significant decreases with age in the level of the contribution of bilateral hippocampi to the metabolism in DMPFC/ACC, of the left hippocampus to the metabolism in right inferior frontal gyrus (IFG), of the right hippocampus to the metabolism in presupplementary motor area (preSMA), of the right thalamus to the metabolism in DMPFC/ACC as well as of both cerebellar lobules to the metabolism in DMPFC/ACC.
They also showed that these age‐related changes were associated with significant increases with age in the level of the contribution of the right hippocampus to the metabolism in the right posterior insula as well as of the lobule IX to the metabolism in the right superior parietal lobule (SPL).
Effect of Epilepsy Versus Maturational Processes on Age‐Related Metabolic Changes
Figure 6 illustrates the metabolic changes characterizing both pediatric populations compared with adults.
Figure 6.

Effect of maturational processes on regional cerebral glucose metabolism. The conjunction analysis revealed common significant hypometabolism in various cortical, subcortical, and cerebellar areas in children compared with adults. Images are thresholded at P < 0.05 corrected for multiple comparisons over the entire brain volume (Family Wise Error, FWE). The color scale on the right of the figure represents the T‐statistics of the significant voxels.
The conjunction analysis revealed the existence of highly significant hypometabolism in both populations of children compared with adults bilaterally in the ACC, the hippocampi, the thalami, and the cerebellum as well as in the basal ganglia (caudate nuclei, putamen), and lateral temporal neocortex. No hypermetabolic changes were common to both pediatric populations compared with adults.
DISCUSSION
Using a voxel‐based approach, this study demonstrates that the age‐related changes in regional glucose metabolism occurring in mesial prefrontal, thalamic, hippocampal, and cerebellar areas are associated with significant age‐related changes in PPI between these regions and other brain structures from childhood to adulthood. The contribution of these regions to the metabolic activity of other brain structures present significant increases or decreases with age, depending on the region considered. These results provide a metabolic correlate of the age‐dependent effects on multiple large‐scale neuronal networks involved in major sensory, motor, and high‐level cognitive functions.
Methodological Considerations
Like in previous FDG‐PET studies designed to identify age‐related cerebral metabolic changes [Chugani et al., 1987; Kinnala et al., 1996; Van Bogaert et al., 1998], for obvious ethical reasons, we had no access to PET data from healthy children. We rather used a group of epileptic children with normal (so called negative) structural MRI and FDG‐PET data that have been assessed as valid pseudo‐controls in a previous work [Archambaud et al., 2013]. We tested the assumption that epilepsy was not a confounding variable, by searching for common age‐related changes in regional brain glucose metabolism in our pseudo‐control pediatric group and in a group of non‐epileptic children with various focal brain or systemic disorders. When compared with the adult control group, the conjunction analysis of those two pediatric populations showed common significant hypometabolism in various subcortical (caudate nuclei, putamen, thalami), cortical, and cerebellar areas. This is in agreement with our previous FDG‐PET study that assessed the age‐related changes in regional brain glucose metabolism [Van Bogaert et al., 1998]. It therefore confirms our hypothesis that the age‐related changes in regional cerebral glucose metabolism disclosed in this study are indeed related to physiological brain maturational processes rather than to epilepsy‐induced changes in regional brain metabolism.
In order to assess if the non‐linear changes in regional metabolism observed with age within specific brain areas were associated with any change in functional integration with other brain structures, we adapted the previously developed PPI analysis [Friston et al., 1997] to a specific experimental factor, that is, the subjects' age. Initially, PPI analysis was developed to determine which brain voxels modulate (increase or decrease) their relationship with a seed region of interest in a given context, such as during a particular behavioral/cognitive task [Friston et al., 1997; O'Reilly et al., 2012]. In other words, these analyses were used to identify brain regions whose activity depends on an interaction between psychological (e.g., task, behavioral performance) and physiological (the metabolic activity or the time course of a region of interest) factors [O'Reilly et al., 2012]. In the present study, we simply adapted this popular technique of effective connectivity analysis to our question of interest by replacing the psychological factor by a particular experimental factor (i.e., age), leading to PPI. Finally, these PPI analyses are statistically identical to PPI analysis performed in PET or fMRI time‐series studies.
Age‐Related Changes in Regional Glucose Metabolism
We found significant linear or non‐linear age‐related changes in regional glucose metabolism in various brain structures such as DMPFC/ACC, thalamic and hippocampal regions bilaterally as well as cerebellar lobules VIIIb and IX. Interestingly, these metabolic changes were more widespread than expected based on a previous study performed by our group [Van Bogaert et al., 1998] as they also involved the hippocampi and the cerebellum. This is probably due to the use of a more recent PET camera, with a wider field of view (FOV), and the use of a larger set of subjects covering a more extended age range (6–50 years), hence more statistical power.
Furthermore, our analyses disclosed differences in the evolution of relative glucose metabolism with age in mesial frontal structures (including the ACC) and thalamus compared with hippocampal and cerebellar regions. Indeed, relative metabolic activity in those mesial prefrontal and thalamic areas evolved quadratically with age and increased from childhood to reach maximal values at 30–35 years, after which it tended to decrease through adulthood. DMPFC/ACC plays a pivotal role in evaluating the outcomes of actions, conflict monitoring as well as error processing, and it is also known to become metabolically more active at a later age than other cortical and cerebellar areas [Botvinick et al., 2004; Gogtay et al., 2004]. The continued maturation of this brain region during childhood and adolescence supports the protracted development of inhibitory control over adolescence [Ordaz et al., 2013]. Also, this brain region is considered to subtend higher‐order cognitive functions, such as executive control processes or episodic memory, which may pursue their maturation during adulthood [Bastin et al., 2012; Shen et al., 2012; Willis et al., 2002]. The later phase of the inverse‐U‐shaped metabolic evolution in DMPFC/ACC and thalamic regions is in agreement with previous FDG‐PET studies on healthy subjects from adulthood to senescence, showing a significant decrease with age in glucose uptake consumption in mesial frontal regions and dorsomedial thalamus [Martin et al., 1991; Pardo et al., 2007; Petit‐Taboue et al., 1998]. Furthermore, in one study, the metabolic decline was modeled as a linear trend over the whole 18–90 years life span (significant r of Pearson's correlation coefficient) and interpreted as a correlate of the age‐associated cognitive decline observed in normal aging [Pardo et al., 2007]. Our non‐linear analysis over a more definite period covering early adulthood provides a more precise view about the initiation of the metabolic decline that appears to occur around 30–35 years of age.
In our study, the relative glucose metabolism in hippocampal and cerebellar regions increased linearly with age until around 40–50 years when it tended to slightly plateau. This finding could be related to the fact that the period of life considered in this study (<50 years) only captures the early phase of the age‐related metabolic changes involving these structures. Indeed, previous FDG‐PET studies performed in healthy subjects showed a profile of increased metabolic activity in hippocampal and cerebellar areas until the 5th–6th decades of life, after which it tends to decrease until late senescence [Loessner et al., 1995; Moeller et al., 1996; Petit‐Taboue et al., 1998; Willis et al., 2002]. Consistent with this literature, our data bring further support to the developmental theory postulating that the first regions to emerge phylogenetically and ontogenetically are the most resistant to age effects, whereas the last ones are the most vulnerable [Chugani et al., 1987; Kinnala et al., 1996]. Moreover, several FDG‐PET studies performed in healthy populations aged greater than 50 years suggested that the progressive metabolic increase in mesiotemporal regions, occurring when the metabolic decline has already started in mesiofrontal and thalamic areas, might correspond to a compensation mechanism to cope with the impairment observed in higher‐order cognitive functions [Kalpouzos et al., 2009; Kantarci et al., 2010; Mosconi et al., 2008]. Indeed, this compensatory mechanism could take place until late adulthood to preserve a certain level of performance in brain functions that are more affected by the age‐related physiologic changes, bringing further support to the cognitive reserve model [Steffener et al., 2011].
Age‐Related Changes in Functional Integration
PPI analyses revealed a significant age‐related decrease in the contribution of hippocampal, thalamic as well as inferoposterior cerebellar areas to the metabolic activity of DMPFC/ACC. This finding practically means that the age‐related changes in glucose metabolism observed within these brain areas are associated with a progressive decrease of their functional integration with the DMPFC/ACC. These age‐related changes in PPI might be related to the physiologic decline of performances in higher‐level cognition functions, like working memory and executive control. Indeed, the executive control network [Habas et al., 2009; Seeley et al., 2007] is responsible for the achievement of executive‐function tasks demanding attentional, working memory, and cognitive function resources. This network is most commonly activated by tasks involving manipulation and rearrangement of items in working memory as well as flexible allocation of attention according to the source of stimulus [for a review, see Wager and Smith, 2003]. Our results suggest that the early occurrence of age‐related metabolic decrease in mesial prefrontal cortex might be causally related to a significant decrease in connectivity affecting the fronto‐thalamic, fronto‐hippocampal, and fronto‐cerebellar connections, which are pivotal to the performances in higher‐level cognitive functions and the functioning of the corresponding functional networks. Moreover, the observed changes in PPI involving mesial prefrontal regions bring support to the frontal lobe hypothesis of aging [West, 1996], which posits that cognitive functions supported by the prefrontal cortex should decline at an earlier age than those supported by other associative cortices [Rabbitt and Lowe, 2000]. In particular, the age‐related cognitive deficits involve many component processes of executive control, including working memory, attention switching, sustained attention, inhibition, and goal maintenance [Andrews‐Hanna et al., 2007; Hogan, 2004]. As the hippocampal‐thalamic complex is associated to specific cognitive abilities in healthy subjects, playing a central role in the modulation of memory performances probably because of its connections with prefrontal cortical areas, the deterioration of fronto‐thalamic and fronto‐hippocampal connectivity may represent a functional correlate of the age‐related memory impairments observed in healthy subjects and neurodegenerative conditions [Tekin and Cummings, 2002]. Besides, changes in fronto‐cerebellar connectivity might influence feedback and feedforward control loops for integrating multiple internal representations with external stimuli and self‐generated responses [Schmahmann, 1996]. This connectivity decay may be involved in the age‐related changes in processing speed, intra‐individual variability, automaticity and higher‐level cognitive functions [Andrews‐Hanna et al., 2007; Hogan, 2004].
The age‐related disruption of fronto‐hippocampal connectivity is further evidenced by the decreased contribution of the hippocampal region to specific prefrontal areas metabolism (preSMA and right IFG) involved in mediating response inhibition, a hallmark of executive control [Sharp et al., 2010; Verbruggen and Logan, 2008]. PreSMA and right IFG appear to be pivotal for the ability to resolve competition between action contingencies and conflicting response tendencies, which is known to deteriorate with age [Hasher and Zacks, 1988; Lustig et al., 2001]. In particular, it has been suggested that age‐related inhibitory deficits stem from reduced frontal lobe integrity, especially involving its white matter tracts, and are associated with reduced processing speed, increased performance variability and general cognitive decline [Hedden and Gabrieli, 2004; Sullivan and Pfefferbaum, 2006]. Besides, hippocampus has been suggested to play a key role in representing contextual memory and utilizing contextual information for flexible response selection [Hirsh, 1974; Smith and Bulkin, 2014]. So, our results provide a neurophysiological substrate for the selective age‐related deficit in the ability to inhibit distracting task‐irrelevant information, which could potentially account for a wide range of age differences in memory and attention [Gazzaley et al., 2005; Hasher and Zacks, 1988; Lustig et al., 2001; May et al., 1999].
PPI analyses also disclosed significant increases with age in the contribution of the right hippocampus on posterior insula metabolism. This finding can be understood as a progressive increase in functional integration between the right hippocampus and posterior insula during the age‐related changes in right hippocampus metabolism. Functional imaging studies have shown that posterior insula contributes to sensorimotor integration in response to somatic, visual, and auditory stimuli [Ciccarelli et al., 2005; Dronkers, 1996; Johansen‐Berg and Matthews, 2002] as well as to the sensory control mechanisms of speech production [Ackermann and Riecker, 2004]. Interestingly, a recent fMRI study revealed specific co‐activation maps of the posterior region of the insula with right hippocampus, SMA and other cortical structures [Chang et al., 2012], associating this specific network with pain, sensorimotor and language‐related topics. Therefore, this age‐related change in functional integration between right hippocampal and posterior insular regions might represent a compensatory mechanism, mediated by the hippocampus, to sustain the physiologic decline of performances in some sensorimotor abilities.
Finally, we found significant age‐related increase in the contribution of the right inferoposterior cerebellar (lobule IX) to the metabolism in the SPL. This latter region heavily contributes to the cortico‐cerebellar system, responsible for the cognitive control of movement [Prevosto and Sommer, 2013]. Lobule IX of cerebellum has been implicated in various functional tasks including sensation [Hui et al., 2005], perception of change in stimulus timing [Liu et al., 2008], working memory [Desmond et al., 1997], and motor synchronization [Jantzen et al., 2004], whereas SPL is strongly involved in flexible allocation of resources for attention to sensory stimuli, sensorimotor integration, and spatial aspects of movement planning [Koenigs et al., 2009; Wenderoth et al., 2004]. Our finding is in agreement with fMRI studies showing that, besides typical motor areas, SPL and posterior cerebellum are additionally recruited in healthy elderly subjects and correlated positively with performance on sensorimotor coordination tasks [Debaere et al., 2004; Heuninckx et al., 2008]. This age‐related activation over a large‐scale network could be meaningful for preserving cognitive control and motor performance in the elderly, thus compensating for various neural or behavioral deficits related to neurodegeneration, and reduction in sensory function.
CONCLUSIONS
This study provides novel metabolic evidence supporting an effect of age on large‐scale brain functional network architectures. Indeed, it discloses significant linear or non‐linear age‐related changes in regional glucose metabolism of mesial prefrontal, mesiotemporal, thalamic, and cerebellar areas from childhood to adulthood, that were associated with significant changes in functional interactions between those brain areas and other associative cortical regions (i.e., frontal, insular, and parietal cortices). These age‐dependent connectivity changes probably account for some of the cognitive and behavioral changes occurring during brain development and physiologic aging.
ACKNOWLEDGMENTS
Koen Van Laere is Senior Clinical Researcher at the Fonds voor Wetenschappelijk Onderzoek (FWO, Belgium). Vincent Wens is Research Logistic Collaborator and Xavier De Tiège is Postdoctoral Clinical Master Specialist at the Fonds de la Recherche Scientifique (FRS‐FNRS, Belgium).
aIn Memoriam.
The authors have no conflicts of interests. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
REFERENCES
- Ackermann H, Riecker A (2004): The contribution of the insula to motor aspects of speech production: A review and a hypothesis. Brain Lang 89:320–328. [DOI] [PubMed] [Google Scholar]
- Andrews‐Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL (2007): Disruption of large‐scale brain systems in advanced aging. Neuron 56:924–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archambaud F, Bouilleret V, Hertz‐Pannier L, Chaumet‐Riffaud P, Rodrigo S, Dulac O, Chassoux F, Chiron C (2013): Optimizing statistical parametric mapping analysis of 18F‐ FDG PET in children. EJNMMI Res 3:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastin C, Yakushev I, Bahri MA, Fellgiebel A, Eustache F, Landeau B, Scheurich A, Feyers D, Collette F, Chetelat G, Salmon E (2012): Cognitive reserve impacts on inter‐ individual variability in resting‐state cerebral metabolism in normal aging. Neuroimage 63:713–722. [DOI] [PubMed] [Google Scholar]
- Bennett IJ, Madden DJ, Vaidya CJ, Howard DV, Howard JHJ (2010): Age‐related differences in multiple measures of white matter integrity: A diffusion tensor imaging study of healthy aging. Hum Brain Mapp 31:378–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS (2004): Conflict monitoring and anterior cingulate cortex: An update. Trends Cogn Sci 8:539–546. [DOI] [PubMed] [Google Scholar]
- Brix G, Zaers J, Adam LE, Bellemann ME, Ostertag H, Trojan H, Haberkorn U, Doll J, Oberdorfer F, Lorenz WJ (1997): Performance evaluation of a whole‐body PET scanner using the NEMA protocol. National Electrical Manufacturers Association. J Nucl Med 38:1614–1623. [PubMed] [Google Scholar]
- Chang LJ, Yarkoni T, Khaw MW, Sanfey AG (2012): Decoding the role of the insula in human cognition: Functional parcellation and large‐scale reverse inference. Cereb Cortex 23:739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiron C, Raynaud C, Maziere B, Zilbovicius M, Laflamme L, Masure MC, Dulac O, Bourguignon M, Syrota A (1992): Changes in regional cerebral blood flow during brain maturation in children and adolescents. J Nucl Med 33:696–703. [PubMed] [Google Scholar]
- Chugani HT (1998): A critical period of brain development: Studies of cerebral glucose utilization with PET. Prev Med 27:184–188. [DOI] [PubMed] [Google Scholar]
- Chugani HT, Phelps ME, Mazziotta JC (1987): Positron emission tomography study of human brain functional development. Ann Neurol 22:487–497. [DOI] [PubMed] [Google Scholar]
- Ciccarelli O, Toosy AT, Marsden JF, Wheeler‐Kingshott CM, Sahyoun C, Matthews PM, Miller DH, Thompson AJ (2005): Identifying brain regions for integrative sensorimotor processing with ankle movements. Exp Brain Res 166:31–42. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hinds S, Press GA (2000): Normal brain development and aging: Quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology 216:672–682. [DOI] [PubMed] [Google Scholar]
- Curiati PK, Tamashiro‐Duran JH, Duran FL, Buchpiguel CA, Squarzoni P, Romano DC, Vallada H, Menezes PR, Scazufca M, Busatto GF, Alves TC (2011): Age‐related metabolic profiles in cognitively healthy elders: Results from a voxel‐based [18F]fluorodeoxyglucose‐positron‐emission tomography study with partial volume effects correction. AJNR Am J Neuroradiol 32:560–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debaere F, Wenderoth N, Sunaert S, Van Hecke P, Swinnen SP (2004): Cerebellar and premotor function in bimanual coordination: Parametric neural responses to spatiotemporal complexity and cycling frequency. Neuroimage 21:1416–1427. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Gabrieli JD, Wagner AD, Ginier BL, Glover GH (1997): Lobular patterns of cerebellar activation in verbal working‐memory and finger‐tapping tasks as revealed by functional MRI. J Neurosci 17:9675–9685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dronkers NF (1996): A new brain region for coordinating speech articulation. Nature 384:159–161. [DOI] [PubMed] [Google Scholar]
- Fields RD (2015): A new mechanism of nervous system plasticity: Activity‐dependent myelination. Nat Rev Neurosci 16:756–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ (1997): Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 6:218–229. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, Rissman J, D'Esposito M (2005): Top‐down suppression deficit underlies working memory impairment in normal aging. Nat Neurosci 8:1298–1300. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF3, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM (2004): Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A 101:8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, Greicius MD (2009): Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci 29:8586–8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasher L, Zacks RT (1988) Working memory, comprehension, and aging: A review and a new view In: Bower GH, editor. The Psychology of Learning and Motivation: Advances in Research and Theory. San Diego, CA: Academic Press; pp 193–225. [Google Scholar]
- Hedden T, Gabrieli JD (2004): Insights into the ageing mind: A view from cognitive neuroscience. Nat Rev Neurosci 5:87–96. [DOI] [PubMed] [Google Scholar]
- Heuninckx S, Wenderoth N, Swinnen SP (2008): Systems neuroplasticity in the aging brain: Recruiting additional neural resources for successful motor performance in elderly persons. J Neurosci 28:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsh R (1974): The hippocampus and contextual retrieval of information from memory: A theory. Behav Biol 12:421–444. [DOI] [PubMed] [Google Scholar]
- Hogan JH (2004): The cerebellum in thought and action: A fronto‐cerebellar aging hypothesis. New Ideas Psychol 22:97–125. [Google Scholar]
- Hui KK, Liu J, Marina O, Napadow V, Haselgrove C, Kwong KK, Kennedy DN, Makris N (2005): The integrated response of the human cerebro‐cerebellar and limbic systems to acupuncture stimulation at ST 36 as evidenced by fMRI. Neuroimage 27:479–496. [DOI] [PubMed] [Google Scholar]
- Jantzen KJ, Steinberg FL, Kelso JA (2004): Brain networks underlying human timing behavior are influenced by prior context. Proc Natl Acad Sci U S A 101:6815–6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen‐Berg H, Matthews PM (2002): Attention to movement modulates activity in sensori‐ motor areas, including primary motor cortex. Exp Brain Res 142:13–24. [DOI] [PubMed] [Google Scholar]
- Kalpouzos G, Chetelat G, Baron JC, Landeau B, Mevel K, Godeau C, Barre L, Constans JM, Viader F, Eustache F, Desgranges B (2009): Voxel‐based mapping of brain gray matter volume and glucose metabolism profiles in normal aging. Neurobiol Aging 30:112–124. [DOI] [PubMed] [Google Scholar]
- Kantarci K, Senjem ML, Lowe VJ, Wiste HJ, Weigand SD, Kemp BJ, Frank AR, Shiung MM, Boeve BF, Knopman DS, Petersen RC, Jack CR, Jr (2010): Effects of age on the glucose metabolic changes in mild cognitive impairment. AJNR Am J Neuroradiol 31:1247–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnala A, Suhonen‐Polvi H, Aarimaa T, Kero P, Korvenranta H, Ruotsalainen U, Bergman J, Haaparanta M, Solin O, Nuutila P, Wegelius U (1996): Cerebral metabolic rate for glucose during the first six months of life: An FDG positron emission tomography study. Arch Dis Child 74:F153–F157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Barbey AK, Postle BR, Grafman J (2009): Superior parietal cortex is critical for the manipulation of information in working memory. J Neurosci 29:14980–14986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Xu D, Ashe J, Bushara K (2008): Specificity of inferior olive response to stimulus timing. J Neurophysiol 100:1557–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loessner A, Alavi A, Lewandrowski KU, Mozley D, Souder E, Gur RE (1995): Regional cerebral function determined by FDG‐PET in healthy volunteers: Normal patterns and changes with age. J Nucl Med 36:1141–1149. [PubMed] [Google Scholar]
- Low LK, Cheng HJ (2006): Axon pruning: An essential step underlying the developmental plasticity of neuronal connections. Philos Trans R Soc Lond Ser B, Biol Sci 361:1531–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig C, May CP, Hasher L (2001): Working memory span and the role of proactive interference. J Exp Psychol Gen 130:199–207. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Spaniol J, Costello MC, Bucur B, White LE, Cabeza R, Davis SW, Dennis NA, Provenzale JM, Huettel SA (2009): Cerebral white matter integrity mediates adult age differences in cognitive performance. J Cogn Neurosci 21:289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AJ, Friston KJ, Colebatch JG, Frackowiak RS (1991): Decreases in regional cerebral blood flow with normal aging. J Cereb Blood Flow Metab 11:684–689. [DOI] [PubMed] [Google Scholar]
- May CP, Hasher L, Kane MJ (1999): The role of interference in memory span. Mem Cognit 27:759–767. [DOI] [PubMed] [Google Scholar]
- Meltzer CC, Cantwell MN, Greer PJ, Ben‐Eliezer D, Smith G, Frank G, Kaye WH, Houck PR, Price JC (2000): Does cerebral blood flow decline in healthy aging? A PET study with partial‐volume correction. J Nucl Med 41:1842–1848. [PubMed] [Google Scholar]
- Moeller JR, Ishikawa T, Dhawan V, Spetsieris P, Mandel F, Alexander GE, Grady C, Pietrini P, Eidelberg D (1996): The metabolic topography of normal aging. J Cereb Blood Flow Metab 16:385–398. [DOI] [PubMed] [Google Scholar]
- Moran RJ, Symmonds M, Dolan RJ, Friston KJ (2014): The brain ages optimally to model its environment: Evidence from sensory learning over the adult lifespan. PLoS Comput Biol 10:e1003422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, De Santi S, Li J, Tsui WH, Li Y, Boppana M, Laska E, Rusinek H, de Leon MJ (2008): Hippocampal hypometabolism predicts cognitive decline from normal aging. Neurobiol Aging 29:676–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzik O, Chugani DC, Juhasz C, Shen C, Chugani HT (2000): Statistical parametric mapping: Assessment of application in children. Neuroimage 12:538–549. [DOI] [PubMed] [Google Scholar]
- O'Reilly JX, Woolrich MW, Behrens TE, Smith SM, Johansen‐Berg H (2012): Tools of the trade: Psychophysiological interactions and functional connectivity. Social Cogn Affect Neurosci 7:604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan M, Jones DK, Summers PE, Morris RG, Williams SC, Markus HS (2001): Evidence for cortical “disconnection” as a mechanism of age‐related cognitive decline. Neurology 57:632–638. [DOI] [PubMed] [Google Scholar]
- Ordaz SJ, Foran W, Velanova K, Luna B (2013): Longitudinal growth curves of brain function underlying inhibitory control through adolescence. J Neurosci 33:18109–18124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo JV, Lee JT, Sheikh SA, Surerus‐Johnson C, Shah H, Munch KR, Carlis JV, Lewis SM, Kuskowski MA, Dysken MW (2007): Where the brain grows old: Decline in anterior cingulate and medial prefrontal function with normal aging. Neuroimage 35:1231–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit‐Taboue MC, Landeau B, Desson JF, Desgranges B, Baron JC (1998): Effects of healthy aging on the regional cerebral metabolic rate of glucose assessed with statistical parametric mapping. Neuroimage 7:176–184. [DOI] [PubMed] [Google Scholar]
- Prevosto V, Sommer MA (2013): Cognitive control of movement via the cerebellar‐recipient thalamus. Front Syst Neurosci 7:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Friston KJ (1997): Cognitive conjunction: A new approach to brain activation experiments. Neuroimage 5:261–270. [DOI] [PubMed] [Google Scholar]
- Price CJ, Moore CJ, Friston KJ (1997): Subtractions, conjunctions, and interactions in experimental design of activation studies. Hum Brain Mapp 5:264–272. [DOI] [PubMed] [Google Scholar]
- Rabbitt P, Lowe C (2000): Patterns of cognitive ageing. Psychol Res 63:308–316. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C (2003): Longitudinal magnetic resonance imaging studies of older adults: A shrinking brain. J Neurosci 23:3295–3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter‐Lorenz PA, Park DC (2010): Human neuroscience and the aging mind: A new look at old problems. J Gerontol 65:405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Greve DN, van der Kouwe AJ, Hevelone ND, Zaleta AK, Rosen BR, Fischl B, Corkin S, Rosas HD, Dale AM (2005): Age‐related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging 26:1215–1227. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD (1996): From movement to thought: Anatomic substrates of the cerebellar contribution to cognitive processing. Hum Brain Mapp 4:174–198. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD (2007): Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp DJ, Bonnelle V, De Boissezon X, Beckmann CF, James SG, Patel MC, Mehta MA (2010): Distinct frontal systems for response inhibition, attentional capture, and error processing. Proc Natl Acad Sci U S A 107:6106–6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Liu H, Hu Z, Hu H, Shi P (2012): The relationship between cerebral glucose metabolism and age: Report of a large brain pet data set. PLoS One 7:e51517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds DJ, Hallquist MN, Asato M, Luna B (2014): Developmental stages and sex differences of white matter and behavioral development through adolescence: A longitudinal diffusion tensor imaging (DTI) study. Neuroimage 92:356–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DM, Bulkin DA (2014): The form and function of hippocampal context representations. Neurosci Biobehav Rev 40:52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Wojtowicz M, Grady CL (2010): Reliable differences in brain activity between young and old adults: A quantitative meta‐analysis across multiple cognitive domains. Neurosci Biobehav Rev 34:1178–1194. [DOI] [PubMed] [Google Scholar]
- Steffener J, Reuben A, Rakitin BC, Stern Y (2011): Supporting performance in the face of age‐related neural changes: Testing mechanistic roles of cognitive reserve. Brain Imaging Behav 5:212–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A (2006): Diffusion tensor imaging and aging. Neurosci Biobehav Rev 30:749–761. [DOI] [PubMed] [Google Scholar]
- Tekin S, Cummings JL (2002): Frontal‐subcortical neuronal circuits and clinical neuropsychiatry: An update. J Psychosom Res 53:647–654. [DOI] [PubMed] [Google Scholar]
- Van Bogaert P, Wikler D, Damhaut P, Szliwowski HB, Goldman S (1998): Regional changes in glucose metabolism during brain development from the age of 6 years. Neuroimage 8:62–68. [DOI] [PubMed] [Google Scholar]
- Van Bogaert P, Massager N, Tugendhaft P, Wikler D, Damhaut P, Levivier M, Brotchi J, Goldman S (2000): Statistical parametric mapping of regional glucose metabolism in mesial temporal lobe epilepsy. Neuroimage 12:129–138. [DOI] [PubMed] [Google Scholar]
- Vanderhaeghen P, Cheng HJ (2010): Guidance molecules in axon pruning and cell death. Cold Spring Harbor Perspect Biol 2:a001859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD (2008): Response inhibition in the stop‐signal paradigm. Trends Cogn Sci 12:418–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Smith EE (2003): Neuroimaging studies of working memory: A meta‐analysis. Cognit Affect Behav Neurosci 3:255–274. [DOI] [PubMed] [Google Scholar]
- Wenderoth N, Debaere F, Sunaert S, van Hecke P, Swinnen SP (2004): Parieto‐premotor areas mediate directional interference during bimanual movements. Cereb Cortex 14:1153–1163. [DOI] [PubMed] [Google Scholar]
- West RL (1996): An application of prefrontal cortex function theory to cognitive aging. Psychol Bull 120:272–292. [DOI] [PubMed] [Google Scholar]
- Whitford TJ, Rennie CJ, Grieve SM, Clark CR, Gordon E, Williams LM (2007): Brain maturation in adolescence: Concurrent changes in neuroanatomy and neurophysiology. Hum Brain Mapp 28:228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis MW, Ketter TA, Kimbrell TA, George MS, Herscovitch P, Danielson AL, Benson BE, Post RM (2002): Age, sex and laterality effects on cerebral glucose metabolism in healthy adults. Psychiatry Res 114:23–37. [DOI] [PubMed] [Google Scholar]


