Abstract
Using magnetoencephalography, we investigated the potential of perilesional and contralesional activity to support language recovery in patients with poststroke aphasia. In healthy young controls, left‐lateralized ventral frontotemporal regions responded to semantic anomalies during sentence comprehension and bilateral dorsal frontoparietal regions responded to syntactic anomalies. Older adults showed more extensive bilateral responses to the syntactic anomalies and less lateralized responses to the semantic anomalies, with decreased activation in the left occipital and parietal regions for both semantic and syntactic anomalies. In aphasic participants, we observed compensatory recruitment in the right hemisphere (RH), which varied depending on the type of linguistic information that was processed. For semantic anomalies, aphasic patients activated some preserved left hemisphere regions adjacent to the lesion, as well as homologous parietal and temporal RH areas. Patients also recruited right inferior and dorsolateral frontal cortex that was not activated in the healthy participants. Responses for syntactic anomalies did not reach significance in patients. Correlation analyses indicated that recruitment of homologous temporoparietal RH areas is associated with better semantic performance, whereas higher accuracy on the syntactic task was related to bilateral superior temporoparietal and right frontal activity. The results suggest that better recovery of semantic processing is associated with a shift to ventral brain regions in the RH. In contrast, preservation of syntactic processing is mediated by dorsal areas, bilaterally, although recovery of syntactic processing tends to be poorer than semantic. Hum Brain Mapp 37:2869–2893, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: : aphasia, stroke, sentence comprehension, language recovery, semantics, syntax, compensation, MEG, oscillations, beamforming, 8–30 Hz ERD
INTRODUCTION
Language comprehension requires rapid coordination of various types of information, including sounds, word meanings, and syntactic structure. Neuroimaging and lesion data indicate that the integration of these linguistic codes depends on dynamic interactions between frontal, temporal, and parietal brain regions [Hickok and Poeppel, 2004; Turken and Dronkers, 2011]. Damage to these regions, particularly in the left hemisphere (LH), is associated with disturbances in language comprehension or production, a disorder referred to as aphasia.
Aphasia is a common consequence of cerebrovascular stroke, and typically results from injury to structures perfused by the middle cerebral artery in the LH. Previous data indicate that considerable changes in the cortical representation of language processing can occur following stroke, and language recovery is assumed to occur as a result of cortical reorganization and neuroplastic changes that take place in structurally intact brain tissue [Angrilli and Spironelli, 2005; Saur et al., 2006; Thompson, 2000; Thompson and den Ouden, 2008]. Current evidence indicates that both LH and right hemisphere (RH) regions may support language recovery [Breier et al., 2007, 2009; Crinion and Leff, 2007; Fridriksson et al., 2006, 2007; Meinzer and Breitenstein, 2008; Meinzer et al., 2007]. However, the relative contributions of each hemisphere and neural mechanisms mediating recovery are not well understood. It is also not clear whether recovery of different linguistic components of language, such as syntax and semantics, proceeds at the same rate, or whether it engages distinct or overlapping neural processes and brain networks.
In functional neuroimaging studies, individuals with chronic aphasia caused by LH stroke often show increased neural activity in perilesional regions around the location of the stroke, and also in contralesional areas in the RH, including regions homologous to the LH language areas [Breier et al., 2007; Meinzer et al., 2007, Thompson, 2010; Vitali et al., 2007]. Recruitment of perilesional areas in the LH or reactivation of LH networks has been associated with the best clinical outcome [Cornelissen et al., 2003; Heiss et al., 1999; Heiss and Thiel, 2006; Léger et al., 2002; Vitali et al., 2007]. This conclusion is supported by recent TMS studies that have shown improved language performance after excitatory stimulation to the preserved LH cortex adjacent to the lesion [Baker et al., 2010; Fiori et al., 2011], or inhibitory stimulation to certain contralesional RH regions [Hamilton et al., 2011; Naeser et al., 2005, 2010; Winhuisen et al., 2007]. These findings are consistent with a critical role of perilesional tissue in recovery. Therefore, assessing the functionality of these areas is essential to tracking recovery and targeting interventions.
However, the role of the RH in language recovery after stroke has been more controversial. Some evidence suggests that RH activation represents adaptive plasticity or compensatory mechanisms, as new or homologous RH regions appear to take over functions of the damaged LH [Blasi et al., 2002; Meltzer et al., 2013; Musso et al., 1999; Thulborn et al., 1999]. Others instead suggest that activation of nondominant RH may actually be dysfunctional, caused by the loss of transcallosal inhibition from the damaged LH [Belin et al., 1996; Heiss and Thiel, 2006]. Based on this view, RH recruitment may interfere with language recovery by precluding reactivation of spared LH areas.
MEG Oscillatory Measures of Task‐Related Activation
Changes in task‐induced neural activity after stroke can be identified with high spatial resolution, and at the millisecond temporal scale using magnetoencephalography (MEG). This noninvasive technique may be a particularly useful tool in mapping the engagement of different brain areas for language processing in patients with poststroke aphasia. It detects magnetic fields at the surface of the head and can spatially localize postsynaptic currents generated in synchronously firing neuronal assemblies. More importantly, the scalp distribution of the magnetic fields is only minimally affected by the presence of the stroke, whereas the presence of lesions affects the EEG signal [Funke et al., 2011; Huang et al., 1990]. In addition, because the skull is almost transparent to magnetic fields, MEG provides better spatial resolution than EEG. MEG also measures electrical activity directly, offering superior temporal resolution than that of functional MRI, which is based on blood flow. Furthermore, with MEG measurements, we can access aspects of neuronal activity that are not reflected in the fMRI signal, such as oscillatory neural activity.
In recent years, oscillatory reactivity in MEG has been extensively studied using beamforming techniques for source analysis [Vrba, 2002; Vrba and Robinson, 2001]. This method estimates a virtual signal at a particular location in the brain while attenuating activity arising from other brain areas and extracranial sources, such as ocular artifacts [Cheyne et al., 2006; Robinson, 2004]. Several studies with neurologically unimpaired participants identified power decreases in the alpha and beta ranges as a reliable indicator of increased neural activity, with close correspondence to the BOLD responses in diverse parts of the cortex [Brookes et al., 2005; Hillebrand et al., 2005; Hanslmayr et al., 2012; Meltzer and Braun, 2011]. Changes in oscillatory power in these frequency bands have been induced in a wide range of cognitive paradigms, including those that target language processing [Singh et al., 2002; Kim and Chung, 2008; Meltzer and Braun, 2011].
In a recent study, we used MEG with beamforming to map the engagement of ventral and dorsal brain regions in the processing of different aspects of language [Kielar et al., 2015]. We measured brain activation with MEG while participants made acceptability judgements to sentences, some of which contained semantic and syntactic anomalies. We found that neural activation of specific language regions was detectable as an event‐related power decrease in the alpha and beta ranges (8–30 Hz). Processing of semantic anomalies was associated with 8–30 Hz event‐related desynchronization (ERD, i.e., 8–30 Hz power decreases) in a left‐lateralized set of ventral regions, whereas syntactic anomalies activated both dorsal and ventral cortex bilaterally. Power modulations in this frequency range have also been reported in other MEG studies examining oscillatory reactivity to semantically or syntactically anomalous words [Bastiaansen et al., 2009; Wang et al., 2012].
Relatively few studies have used this technique to map task‐related oscillatory activity in participants with stroke‐induced aphasia. In a recent study, Meltzer et al. [2013] mapped alpha and beta ERD in participants with chronic aphasia to reveal relationships between language comprehension, lesion characteristics, and compensatory neural activity. They found that in chronic stroke patients, 8–30 Hz power decreases in bilateral posterior temporal, right dorsal–parietal regions, and superior and middle frontal gyri were associated with better comprehension performance, suggesting the adoption of alternative cognitive strategies. These results suggest that mapping MEG oscillatory activity during cognitive tasks can reveal the engagement of specific brain regions during different aspects of sentence processing, such as semantics and syntax. The distinct contributions of dorsal and ventral brain pathways for language processing can be investigated, while at the same time, clarifying the supporting roles of RH regions and perilesional cortex in aphasia recovery.
Present Study
This study aims to build on our two recent findings: (1) that aphasic stroke patients show a correlation between MEG activation (indexed by 8–30 Hz ERD) in the RH and performance on a sentence comprehension task [Meltzer et al., 2013] and (2) that semantic and syntactic anomalies within a sentence comprehension task both elicit 8–30 Hz ERD [Kielar et al., 2014], but in dissociable sets of left‐lateralized or bilateral regions [Kielar et al., 2015]. In this study, we evaluate the neural response of older adults and stroke patients to semantic and syntactic anomalies, and seek to relate the patterns of activation to the degree of recovery and/or maintenance of linguistic processing present across the group of patients. The results illustrate the degree to which semantic and syntactic processing can reorganize to alternative brain regions in perilesional and contralesional cortex.
In this study, the choices of time windows, frequency bands, and conditions to compare were guided by our previous MEG study in young controls [Kielar et al., 2015]. In that study, ERD responses for semantic and syntactic anomalies peaked around 400 ms and ended about 1000 ms after critical word presentation. In both that study and a prior EEG study [Kielar et al., 2014], both semantic and syntactic anomalies induced ERD in a range of 8–30 Hz, with no discontinuity between the alpha and beta bands. Based on these findings, we elected to use a time window of 0.4–1 s in the 8–30 Hz band for this study.
Enhanced responses to semantic and syntactic anomalies reflect processing of specific linguistic content, not merely task engagement or sensory stimuli. Therefore, the presence of anomaly‐specific responses may provide a noninvasive measure of the functional adequacy of tissue to support language recovery. Additionally, the variable performance in detection of anomalies across aphasic participants allowed us to assess correlations between performance and functional activation. Areas exhibiting positive correlations are more likely to be involved in adaptive plasticity related to recovery, rather than being activated through a maladaptive process of transcallosal disinhibition.
The study design allowed us to investigate the adaptive role of activity in both perilesional and contralesional areas. Furthermore, it allowed us to investigate any possible differences in compensatory neural activity between semantic and syntactic processing. We were interested in determining the roles of dorsal and ventral brain regions in recovery from aphasia, specifically in relation to processing of semantic and syntactic information. The inclusion of both anomaly types illustrates differences in the brain's capacity for reorganization of semantic and syntactic functions. Finally, because we conducted the same paradigm with young and older adults, this study also demonstrates age‐related changes in oscillatory activity to semantic and syntactic processing, which must be considered in light of the fact that most normative studies are performed in participants considerably younger than the typical aphasic population.
METHODS
Participants
MEG data were acquired from three groups of participants: 19 patients with aphasia, 19 age‐matched healthy controls, and 21 young controls. Data from the 21 young controls was previously used in Kielar et al. [2015], but some analyses of that data are presented here for direct comparison with older controls. Two aphasic participants were essentially unable to read after their stroke and could not perform the MEG sentence comprehension task, resulting in 17 aphasic participants included in the language task analysis. This study was approved by the Research Ethics Board at Baycrest Hospital. All volunteers gave their written informed consent prior to the study and were compensated for their participation. Individual patient demographic and clinical characteristics are presented in Table 1.
Table 1.
Demographic, clinical, and lesion characteristics for stroke patients
| Patient | Age (years) | Education (years) | Handednessa | Time postonset | Aphasia type | Lesion volumeb | % of left cortex damaged | Lesion locationc |
|---|---|---|---|---|---|---|---|---|
| P1 | 47 | 18 | Right | 4 years 1 month | Nonfluent/agrammatic/(moderate Broca's) | 34,032 | 5.07 | L postcentral, BA47, insula, LSMG, LSTG, LMTG, LITG, L temporal pole |
| P2d | 67 | 21 | Right | 15 years 5 months | Nonfluent (severe Broca's) | 169,128 | 22.53 | Left postcentral, left precentral, LSFG, LMFG, BA44, BA45, BA 47, L insula, L STG, L temporal pole, L LMTG, LITG, FUS, LSPL, LSMG, LAG, LBG |
| P3 | 70 | 24 | Right | 1 year | Mild anomia | 4904 | Subcortical lesion | Basal ganglia |
| P4 | 75 | 15 | Right | 2 years 4 months | Conduction | 34,440 | 4.20 | Left precuneus, LSPL, LSMG, LAG, LSTG, LMTG, LITG |
| P5 | 79 | 10 | Right | 2 years 1 month | Mild nonfluent/expressive aphasia | 37,896 | 4.80 | Left postcentral, left precentral, BA44, insula, LSTG, LSPL, LSMG, LAG, precuneus |
| P6 | 46 | 15 | Right | 2 years 3 months | Mild anomia/conduction | 33,904 | 4.84 | BA 47, left insula, LSTG, LMTG, LITG, left temporal pole, LSMG, LAG, basal ganglia |
| P7 | 62 | 16 | Right | 1 year 2 months | Conduction/anomia | 53,456 | 6.92 | L insula, LSTG, LMTG, LITG, left temporal pole, LFUS, LSMG, LAG |
| P8 | 84 | 19 | Right | 10 years | Mild anomia | 3,176 | Subcortical lesion | Basal ganglia |
| P9 | 73 | 19 | Left | 5 years 8 months | Mild anomia | 27,440 | 4.09 | LSTG, LMTG, LITG, left temporal pole, LSPL, LSMG, LAG |
| P10 | 77 | 20 | Right | 7 months | Severe Wernicke's | 23,648 | 2.81 | LSTG, LMTG, LSMG, LAG |
| P11 | 66 | 20 | Right | 5 years 3 months | Conduction | 78,616 | 9.59 | Left postcentral, LSTG, LMTG, LITG, LSPL, LSMG, LAG, precuneus, basal ganglia |
| P12 | 58 | 14 | Right | 1 year 1 month | Nonfluent, expressive (severe Broca's) | 148,904 | 19.32 | Left postcentral, left precentral, LSFG, LMFG, BA44, BA45, BA47, LSTG, LMTG, left temporal pole, LIFG, L insula, LSMG, LAG, basal ganglia |
| P13 | 46 | 16 | Right | 4 years | Nonfluent (moderate Broca's) | 101,584 | 12.71 | Left postcentral, left precentral, LSFG, LMFG, BA44, BA45, BA47, L insula, left temporal pole, LSMG, basal ganglia |
| P14 | 57 | 12 | Right | 2 years | Nonfluent/expressive (severe Broca's) | 146,160 | 20.31 | Left postcentral, left precentral, LSFG, LMFG, BA44, BA45, BA47, L insula, LSPL, LSMG, LAG, LSTG, LMTG, left temporal pole |
| P15d | 65 | 20 | Right | 6 years 1 month | Nonfluent/expressive (severe Broca's) | 158,936 | 21.96 | Left postcentral, left precentral, LSFG, LMFG, BA44, BA45, BA47, L insula, LFUS, LSPL, LSTG, LMTG, LITG, left temporal pole, LSMG, LAG, left precuneus, basal ganglia |
| P16 | 68 | 13 | Right | 3 years 3 months | Mild anomia | 22,152 | 3.24 | Left postcentral, left precueneus, LSPL, LSMG, LAG |
| P17 | 60 | 14 | Right | 8 years 8 months | Mild conduction | 103,896 | 14.89 | Left precentral, LSFG, LMFG, BA44, BA45, LSMA, L insula, LSPL, LSMG, LAG, left precuneus, LSTG, LMTG |
| P18 | 69 | 15 | Right | 1 year | Moderate nonfluent, anomia | 9,104 | 0.0013 | Left basal ganglia |
| P19 | 68 | 14 | Right | 4 years 7 months | Mild anomia | 54,192 | 6.67 | Left precentral, left insula, LSPL, LSMG, LAG, LMTG |
| 65.11 | 16.58 | 4.26 | ||||||
| 10.86 | 3.56 | 3.89 |
Handedness assessed using Edinburgh Handedness Inventory.
Volume of lesioned voxels in microliters.
ROIs defined using the macroanatomical cortical parcellation of Tzourio‐Mazoyer et al. [2002], implemented in AFNI as the Macrolabel atlas.
Participants P2 and P15 lost reading ability after stroke and could not perform the MEG sentence comprehension task.
Participants with aphasia suffered a single LH stroke at least 6 months prior to the study. They were recruited from several sources in Toronto, Ontario and surrounding areas. These included the stroke clinics at Baycrest and Sunnybrook Health Sciences Centres, the Aphasia Institute (http://www.aphasia.ca), and the March of Dimes York‐Durham Aphasia Centre (http://www.marchofdimes.ca/EN/programs/ydac). Patients ranged in age from 46 to 84 years (mean = 65.1, SE = 2.49), and had 10–24 years of education (mean = 16.58, SE = 0.82). All aphasic participants but one were right handed as measured by Edinburgh Handedness Inventory [Oldfield, 1971; Williams, 2010]. They were native speakers of English, and had normal hearing and normal or corrected to normal vision. All patients retained sufficient capacity of language comprehension to consent for the study and follow task instructions. Exclusion criteria were earlier neurological diseases, language disorders, head traumas or brain surgery, epilepsy, severe psychiatric disorders, and unstable or poor health. Participants were diagnosed with aphasia prior to the study by a speech language pathologist and/or board‐certified neurologist. Aphasia diagnosis was based on the basis of the convergence of the clinical presentation, narrative speech samples, and the results of standardized tests.
Aphasic participants were matched with a group of healthy older controls for gender, age (t (36) = 0.155, p > 0.05), and education (t (36) = 1.02, p > 0.05). All healthy volunteers were recruited from the greater Toronto area by REB‐approved advertisements from the University of Toronto community and from the Baycrest Health Sciences subject pool. Both groups of neurologically unimpaired participants were native speakers of English. All young (10 females; age: mean = 24.55 years, SE = 0.63; education: mean = 16.45 years, SE = 0.46) and older, age‐matched controls (3 females, age range: 45–80 years old, mean = 65.63, SE = 2.31; education range: 12–21 years, mean = 17.57, SE = 0.53) were right handed and reported normal hearing and normal or corrected‐to‐normal vision. Participants had no history of neurological, psychiatric, speech, language, or learning disorders and none were taking neuroleptic or mood altering medications at the time of the study. Age‐matched controls participated in all behavioral and neuroimaging assessments completed by the stroke patients, whereas the younger controls only completed the neuroimaging components. All older control participants tested within normal limits on all cognitive and linguistic tests.
Cognitive and Language Assessment
Prior to participation in the MEG experiment, patients and age‐matched controls completed an extensive neuropsychological battery to assess several domains of cognitive and language functioning. PALPA tests were used to assess reading ability in patients, and to make sure that they could perform the MEG task. Table 2 lists all the cognitive and language tests that were administered. The selected language test scores for each patient, as well as means for older controls, are presented in Table 3.
Table 2.
List of language and cognitive tests
| Cognitive domain | Test | References |
|---|---|---|
| General | Geriatric Depression Scale | Sheikh and Yesavage [1986] |
| Montreal Cognitive Assessment | Nasreddine et al. [2005] | |
| Episodic memory | Word Lists (KBNA) | Leach et al. [2000] |
| Logical Memory (WMS‐IV) | Wechsler [2009] | |
| Facial Recognition Test | Warrington [1984] | |
| Visuospatial abilities | Complex Figure Drawing (KBNA) | Leach et al. [2000] |
| Symbol Cancelation (KBNA) | Leach et al. [2000] | |
| Benton's Judgment of Line Orientations (Short) | Benton et al. [1983]; Calami et al. [2011] | |
| Executive function | Trail Making Test (KBNA, Trails A and B) | Leach et al. [2000] |
| Digit Span (WAIS‐IV) | Wechsler [2008] | |
| Language | Letter Fluency (D‐KEFS) | Delis et al. [2001] |
| Category Fluency (Cambridge semantic battery) | Hodges et al. [1990] | |
| Boston Naming Test | Kaplan et al. [2001] | |
| Northwestern Assessment of Verbs and Sentences | Thompson [2011]; Cho‐Reyes and Thompson [2012] | |
| Northwestern Anagram Test | Thompson et al. [2011]; Weintraub et al. [2009] | |
| Cinderella Story telling (AphasiaBank) | MacWhinney et al. [2011] | |
| Picture descriptions (AphasiaBank) | MacWhinney et al. [2011] | |
| Semantics | Camel and Cactus Test (Cambridge semantic battery) | Adlam et al. [2010] |
| Repeat and Point | Hodges et al. [2008] | |
| Peabody Picture Vocabulary Test | Dunn and Dunn [2007] | |
| Reading and repetition | PALPA 8: nonword reading | Kay et al. [1992] |
| PALPA 9: word repetition | Kay et al. [1992] | |
| PALPA 35:word reading | Kay et al. [1992] | |
| PALPA 44: spelling | Kay et al. [1992] | |
| Children's Nonword Repetition | Gathercole et al. [1991] | |
| Sentence repetition (AphasiaBank) | MacWhinney et al. [2011] | |
| Discourse task (AphasiaBank) | MacWhinney et al. [2011] | |
| Aphasia severity | Western Aphasia Battery | Kertesz [1982, 2007] |
| Motor‐speech exam | Dabul [1979] |
Table 3.
Language test scores for individual patients and control group means
| NAVS_VNT | NAVS_SCT | Western Aphasia Battery (WAB) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BNT | Total | Can | Non‐Can | Total All | Flu | Comp | Rep | Naming | BAS | BLS | |
| P1 | 56 | 90.91 | 93.33 | 93.33 | 93.33 | 5 | 8 | 7.5 | 9.5 | 78.3 | 75 |
| P2a | NT | 13.64 | 73.33 | 40.00 | 56.67 | NT | NT | NT | NT | NT | NT |
| P3 | 46 | 86.36 | 100.00 | 100.00 | 100 | 10 | 10 | 10 | 10 | 96.7 | 95 |
| P4 | 41 | 81.82 | 100.00 | 73.33 | 86.67 | 8 | 10 | 7.5 | 7.5 | 80 | 77.5 |
| P5 | 59 | 90.91 | 100.00 | 100.00 | 100 | 8 | 10 | 9.5 | 10 | 95.8 | 91.9 |
| P6 | 49 | 95.45 | 100.00 | 73.33 | 86.67 | 8 | 9 | 7.5 | 10 | 90.83 | 86.87 |
| P7 | 42 | 59.09 | 80.00 | 40.00 | 60 | 6 | 8 | 4 | 8 | 62 | 62.5 |
| P8 | 45 | 95.45 | 100.00 | 100.00 | 100 | 9 | 9 | 10 | 9.5 | 94.17 | 89.37 |
| P9 | 54 | 95.45 | 80.00 | 66.67 | 73.33 | 9 | 10 | 10 | 10 | 98.3 | 97.5 |
| P10 | 26 | 63.63 | 60.00 | 53.33 | 56.67 | 7 | 9 | 6 | 6 | 62 | 51 |
| P11 | 56 | 90.91 | 66.67 | 73.33 | 70 | 9 | 10 | 9 | 10 | 97 | 96 |
| P12 | 7 | 9.091 | 80.00 | 86.67 | 83.33 | 2 | 10 | 5 | 2 | 45 | 38.75 |
| P13 | 48 | 86.36 | 100.00 | 100.00 | 100 | 6 | 10 | 9.5 | 10 | 52.5 | 70.5 |
| P14 | 25 | 45.45 | 80.00 | 46.67 | 63.33 | 2 | 8 | 6 | 7 | 58.3 | 48.75 |
| P15a | 8 | 22.73 | 53.33 | 80.00 | 66.67 | 0 | 7 | 3.5 | 0.5 | 25 | 22.5 |
| P16 | 57 | 95.45 | 100.00 | 60.00 | 80 | 9 | 9 | 10 | 10 | 95 | 96.25 |
| P17 | 41 | 95.45 | 100.00 | 60.00 | 80 | 8 | 9 | 7 | 10 | 86.7 | 85 |
| P18 | 42 | 81.82 | 86.67 | 86.67 | 86.67 | 4 | 9 | 9 | 9 | 75 | 66.88 |
| P19 | 55 | 100 | 93.33 | 100.00 | 96.67 | 8 | 10 | 8.5 | 10 | 90.8 | 86.9 |
| Mean (SD) | 42 (15.8) | 73.68 (29.71) | 86.67 (15.07) | 75.44 (21.09) | 81.05 (15.40) | 6.56 (15.39) | 9.17 (0.92) | 7.75 (2.12) | 8.28 (2.85) | 76.86 (21.46) | 74.34 (22.03) |
| Control mean (SD) | 56.63 (3.2) | 96.65 (5.21) | 100 | 100 | 100 | N/A | N/A | N/A | N/A | N/A | N/A |
Abbreviations: BNT: Boston Naming Test (score out of 60); NAVS_VNT: Northwestern Assessment of Verbs and Sentences Verb Naming Test (Total score averaged across all verb types); NAVS_SCT: Northwestern Assessment of Verbs and Sentences‐Sentence Comprehension Test; Can: Total score on all canonical sentences; Non‐Can: total score on all noncanonical sentences; total: overall score on all sentence types; Western Aphasia Battery: Bedside Version, Flu: spontaneous speech fluency; Comp: auditory verbal comprehension; Rep: repetition; BAS: bedside aphasia score; BLS: bedside language score.
NT: test scores not available.
Bedside Language Score (WAB_BLS) was determined by summing the speech content, fluency, auditory verbal comprehension, sequential commands, repetition, object naming, reading, and writing scores, dividing the sum by 8 and multiplying the result by 10.
Bedside Aphasia Score (WAB_ BAS) was determined by summing the speech content, fluency, auditory verbal comprehension, sequential commands, repetition, and object naming scores, dividing the sum by 6 and then multiplying the result by 10.
Participants P2 and P15 could not perform the MEG sentence comprehension task.
Sentence Comprehension Task
All participants completed a visual sentence‐judgement task during MEG data acquisition. More detailed description of the sentence materials can be found in our previous paper reporting results from young healthy controls [Kielar et al., 2015]. A brief description of the materials and paradigm is given below. Examples of experimental sentences are presented in Table 4.
Table 4.
Example sentences used in the experiment
| Code | Condition | Example sentences |
|---|---|---|
| COR | Correct | She will go to the bakery for a loaf of bread |
| SEM | Semantic anomaly | She will go to the bakery for a loaf of books |
| SYN | Syntactic anomaly | She will going to the bakery for a loaf of bread |
The experimental materials consisted of 400 sentence triplets. The sentences in each triplet were identical except for the critical words that were either anomalous or correct. The sentences were selected from a set of normed materials by Block and Baldwin [2010], for which participants were asked to provide the most likely completion of the sentence. Each triplet consisted of sentences in three conditions. The correct condition (COR) consisted of grammatically and semantically correct English sentences, taken directly from the normed materials, ranging 6–12 words in length. In the correct condition, the final word of the sentence was the one most frequently provided by the subjects in Block and Baldwin [2010] based on the cloze completion procedure. The sentences met criteria for high cloze probability with proportions ranging from 0.67 to 0.99. To create sentences with semantic anomaly conditions (SEM), the final words of the sentences were shuffled randomly creating unexpected completions, with the constraint that the final word should be the same part of speech as the original word. After the random shuffle, the placement of the words was adjusted manually to avoid SEM sentences that were judged insufficiently anomalous by the authors. The syntactic anomaly (SYN) was introduced at the sentence's main verb and took the form of an anomaly of either tense or agreement. For analyses of semantic anomalies, the anomalous final word was compared directly with correct final words, whereas for syntactic anomalies, the anomalous main verb was compared with correct main verbs. Although it may seem desirable to place both kinds of anomalies in the same sentence position, we elected not to do so for two reasons. In English, the syntactic anomalies that elicit the P600 are mainly associated with verbs, and due to English word order, they would normally go in the middle of the sentence. Although some studies have demonstrated N400 responses to semantically anomalous English verbs in sentence‐middle position [Osterhout and Nicol, 1999; Moreno et al., 2010], the N400 responses obtained in these studies are relatively small compared to the more traditional paradigm of sentence‐final elicitation (e.g., Kutas and Hillyard [1980]). Furthermore, most semantic anomalies in midsentence verbs involve animacy violations (e.g., “The cats won't bake…”), which have also been shown to elicit P600 responses, (see Kuperberg [2007] for a review). To maximize the separability of the two responses, we chose to place each anomaly in the optimal sentence positions that have been most commonly used in studies examining semantic and syntactic anomalies in English.
Eight counterbalanced lists of experimental materials were created: four for visual and four for auditory presentation (not reported here) to ensure that each participant was presented with only one sentence from each experimental triplet. Each list consisted of 75 COR, 50 SEM, and 50 SYN sentences. The experimental lists were pseudorandomized, such that no more than three consecutive trials appeared of the same anomaly condition (although sequences of control trials of any length were allowed). To allow subjects to have rest periods, the experimental lists were split into 5 runs of 35 trials each, consisting of 15 COR, 10 SEM, and 10 SYN. Each participant completed a total of 8 runs: 5 visual and 3 auditory. Participants completed all runs of one modality before switching to the other modality. Participants performed a sentence acceptability judgement task in both visual and auditory modalities. In this article, we report results of the visual presentation, which elicited more robust responses in young healthy controls [Kielar et al., 2015] and was also somewhat easier for most aphasic patients to perform.
Each trial started with a 500 ms fixation cross, followed by word‐by‐word presentation of the sentence. The words were presented in white font on a black background in the center of the screen. Each word appeared for 350 ms, followed by a blank screen for 400 ms. The last word of the sentence was followed by a blank screen of 2500 ms, after which a response prompt (a question mark) was presented. At this point, participants performed a button‐press judgement on whether the sentence was correct (i.e., free of semantic and syntactic errors), or “unacceptable.” One button was used for correct sentences and another for incorrect sentences. The buttons were pressed by the same hand, and the same hand was used across participants.
Subjects were instructed to withhold their button‐press judgment until the response cue appeared. Visual stimuli were displayed on a screen approximately 0.5 m from the participant's face, projected via mirrors from an LCD projector placed outside the magnetically shielded room to avoid interference.
Behavioral data analysis
The accuracy and reaction time for one stroke participant were not recorded because this patient had difficulty pressing the buttons. The reaction time for another participant could not be recorded due to a response button malfunction. In this MEG study, participants were required to press button A when the sentence was correct and button B when the sentence contained an anomaly. Because the overall error rate on the sentence comprehension task could be influenced by participants' response bias, we used a signal detection method to estimate individual participant's ability to discriminate targets (anomalous sentences) from nontargets (correct sentences). Using each participant's proportion of hits (pressing button B after the presentation of an anomalous sentence) and proportion of false alarms (pressing button B after the presentation of a correct control sentence), we calculated d′ values separately for semantic and syntactic anomalies. This procedure provides an estimate of performance on each sentence type corrected for response bias. Higher d′ values reflect greater discrimination sensitivity to anomalies and, thus, a better ability to discriminate semantic and syntactic anomalies from correct sentences. We used d′ values for behavioral analyses, to perform the voxel‐based lesion symptom mapping (VLSM) analyses, and to correlate MEG task performance with MEG activation (reflected by 8–30 Hz ERD).
MRI Scans Acquisition and Processing
MRI data were always acquired after the MEG session, either on the same day or up to 2 weeks later. MRI scans were acquired on a 3 T scanner (Siemens TIM Trio) located at Baycrest. Anatomical scans used for MEG source localization and lesion tracing included T1‐weighted MPRAGE (1 mm isotropic voxels) and T2 FLAIR. While in the scanner, participants also completed resting‐state fMRI, arterial spin labelling, and diffusion tensor imaging scans (to be reported elsewhere). The MPRAGE image was used to construct a head model for MEG source modeling. MR‐visible markers were placed at the fiducial points for accurate registration, aided by digital photographs from the MEG session. T1 images were skull stripped by applying a stripping procedure implemented in AFNI.
Lesion borders were delineated in a semiautomated approach. First, T1 MRI images were segmented using FSL, and voxels assigned a non‐zero probability of being CSF were identified. Using region of interest (ROI) drawing tools in AFNI, we drew a very liberal mask around the lesion as identified visually. The intersection of the liberal mask and the segmentation results was used as the initial draft of the lesion tracing. Finally, the lesion masks were adjusted manually using the AFNI ROI tools. Manual adjustments included excluding enlarged ventricles and CSF outside the cortical mantle from the lesion mask, and also adding in areas of white matter gliosis, as identified on the basis of the hyperintense signal seen in a coregistered T2‐FLAIR image. Both T1 images and lesion masks were warped into MNI space for group analysis of lesion characteristics and to overlay source‐localized MEG images.
For display of MEG activation maps derived from patient data, a composite lesion mask was constructed to identify regions that were damaged in the patient group. In addition, the spatially normalized T1‐weighted anatomical images of all patients were averaged together. Next, the lesion mask was overlaid on this image by subtracting a percentage of the signal proportional to the number of patients with lesions in that voxel. This procedure provides a visual approximation of the “average” pattern of lesion extent across individuals, and provides a suitable anatomical underlay on which to display group‐averaged functional imaging data. Selected slices from T1 images of individual aphasic participants showing lesion sites are shown in Figure 1A. A lesion overlap map showing the distribution of lesions in the LH is presented in Figure 1B.
Figure 1.
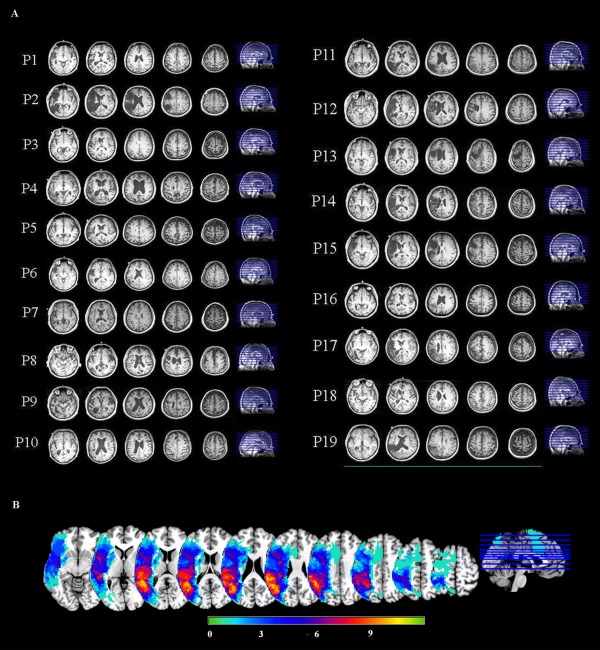
(A) Selected slices from T1 images of individual aphasic participants showing lesion sites. (B) An overlay of stroke patients' lesion distributions displayed on a template brain in MNI space. Colors represent the number of patients with a lesion in each voxel. Warmer colors indicate areas of greater lesion overlap.
Voxel‐Wise Lesion Symptom Analysis
Because our stroke participants showed considerable variability in performance on anomaly detection and lesion extent, we performed a quantitative assessment of the relationship between these variables using VLSM [Bates et al., 2003] implemented in the MRIcron software [Rorden et al., 2007]. The relationship between behavioral performance and lesion location was tested on a voxel‐wise basis using nonparametric mapping, which has been used previously with small sample sizes [Wu et al., 2007]. At each voxel, the behavioral scores were compared between the group of patients with a lesion at that voxel and those without a lesion. Statistical significance was determined by a nonparametric permutation test, with permutations generated by randomly permuting the mapping of lesion score to the behavioral score. Using this method, a maximum statistic across the whole brain is calculated for each permutation, and significance thresholds calculated from the 95th percentile of this distribution. This procedure ensures a family‐wise false‐positive rate of 0.05. Multiple comparisons are corrected by using the maximum statistic, such that a t value that exceeds the threshold derived using this procedure would be expected to occur by chance anywhere in the brain only 5% of the time. Permutation testing does not require parametric assumptions and allows calculating the t‐value corresponding to a specified alpha level [Nichols & Holmes, 2001]. We limited inference to voxels that were lesioned in at least 3 out of 17 patients.
To characterize the lesion location in each patient, we also performed an ROI‐based analysis of lesion extent. Using the macroanatomical cortical parcelation of Tzourio‐Mazoyer et al. [2002], implemented in AFNI as the macrolabel atlas, we defined 23 ROIs in the LH as binary masks. For each region, we computed the percentage of overlap between each patient's binary lesion mask (warped to MNI space) and the atlas‐derived ROI. This quantity reflected the percentage of the ROI covered by the lesion. The lesion locations identifying each anatomical region involved for each patient are presented in Table 1.
MEG Acquisition and Processing
MEG signals were recorded with a 151‐channel whole‐head system with axial gradiometers (VSMMedTech, Coquitlam, Canada). MEG was recorded continuously at a sampling rate of 625 Hz, and acquired with online synthetic third‐order gradient noise reduction [Vrba and Robinson, 2001]. Continuous signals were cut into epochs surrounding the critical word presentation times. Head position with respect to the MEG helmet was monitored using three coils placed at anatomical landmarks of the head (nasion, left, and right preauricular points). The head position was measured before and after each run, and averaged across runs for source analysis.
To construct head models for MEG analysis, the MR‐visible markers were manually identified on the T1 image and used to mark locations of the fiducial points in AFNI software [Cox, 1996]. The T1‐weighted MRI was spatially transformed into the coordinate space of the MEG data. The skull was stripped using Brain Extraction Tool, and a 3‐D convex hull approximating the inner surface of the skull was constructed using the software package Brainhull (http://kurage.nimh.nih.gov/meglab/Meg/Brainhull). Taking into account the position of the head relative to the sensors, a multisphere model [Huang et al., 1999] was computed for each MEG session. To normalize MEG source estimates into MNI space, we computed a nonlinear warp of each subject's brain to a single‐subject template, the “colin27” brain, using the software package ANTS [Avants et al., 2011]. This warp was then used to transform single‐subject MEG activity maps into MNI space.
MEG data analysis
Raw MEG sensor signals were screened for artifacts, and trials containing obvious signal disruptions were rejected (<1% of all trials). All further signal analysis was conducted in source space using synthetic aperture magnetometry (SAM) beamforming. Analysis of “virtual channel” signals in source space has two advantages (beyond localization) compared to analysis of sensor data: (1) the beamforming procedure attenuates extracranial artifacts such as blinks, eye movements, and muscle activity [Cheyne et al., 2007; Vrba, 2002]; and (2) source‐space analysis compensates for differences in head shape and head position across participants, which strongly affect the propagation of electromagnetic activity from the brain to the sensors, which are fixed in the MEG helmet. Note that we did not reject trials based on blinks because the beamforming procedure effectively removes them from the virtual signals estimated for intracranial locations, with the possible exception of orbitofrontal cortex adjacent to the eye orbits [Bardouille et al., 2006]. The remaining artifacts were caused by disturbances arising from environmental noise and subject motion.
Task‐Related MEG Analysis
To test for statistical significance of power changes throughout the brain in specified frequency ranges (8–30 Hz), we generated whole‐brain maps using SAM. For each subject, at a regular grid of locations spaced 7 mm apart throughout the brain, we computed the pseudo‐T value, which is a normalized measure of the difference in signal power between two time windows [Vrba and Robinson, 2001]. To ensure that equal amounts of data were used in both conditions, a random selection of 50 control trials (out of 75) were used to compare with the 50 trials in each anomaly condition. Due to this “dual‐state” analysis approach, multisubject statistical maps were derived from subtractive contrast images computed on the single‐subject level, not from individual conditions. Beamformer weights for this analysis were computed from data within specific time (0.4–1 s) and frequency windows (8–30 Hz), providing greater spatial resolution than nonspecific weights derived from broadband data [Brookes et al., 2008]. The analysis window was measured with respect to the critical word onset. Our selection of 8–30 Hz was motivated by the presence of continuous ERD across this entire range, both in our MEG study with young controls [Kielar et al., 2015] and in our previous EEG study that employed statistical cluster analysis of time–frequency responses [Kielar et al., 2014]. The same time and frequency windows were used to compare violation trials with control trials. Maps of pseudo t‐values throughout the brain were spatially normalized to MNI space by applying the nonlinear transforms computed by ANTS (by warping the T1‐weighted MRI to an MNI template), enabling random‐effects analysis at the group level.
Group statistics on SAM results were computed in a similar fashion as is customary in fMRI studies. For each experimental comparison, the spatially normalized whole‐brain map of pseudo t‐values was submitted to a voxel‐wise one‐sample t‐test across subjects. All statistical tests were two‐tailed. To test whether comprehension performance was related to differences in MEG activation (reflected by 8–30 Hz ERD), we performed voxel‐wise rank‐order correlations (Spearman's Rho) across patients between the accuracy scores (quantified using d′) and MEG activation (event‐related power in 8–30 Hz range). We used d′ values for this analysis because they provide an estimate of participants' task accuracy adjusted for response bias. These correlations were tested separately for semantic and syntactic anomalies.
To correct for multiple comparisons across the whole brain, resulting statistical maps were subjected to voxel‐wise thresholding and a minimum cluster‐size criterion of 90 voxels, resulting in a cluster‐wise corrected family‐wise error rate of p < 0.05. The cluster size criterion was determined by Monte Carlo simulations conducted in the AFNI program Alphasim, with a voxel‐wise threshold of p < 0.01, which was the most lenient threshold used in this study. For comparisons with stronger effects (e.g., syntactic anomaly‐control), we used a stricter threshold of p = 0.001. The simulations in Alphasim also require an estimate of the smoothness (FWHM: full‐width at half‐maximum) of the data in the absence of a true effect. For this, we computed “null” SAM maps by comparing the prestimulus intervals for two different conditions, which should not differ. Two null maps were computed for each young control subject for each frequency band. Smoothness estimates of these maps were highly consistent (FWHM range: 17.1–18.5), so the mean value of 18 mm was used in the simulations.
RESULTS
Behavioral Results: Sentence Comprehension Performance
The behavioral results for both control groups and stroke patients are presented in Table 5. The behavioral analyses presented below were conducted using d′ values. The original accuracy scores prior to the adjustment are presented in Table 5.
Table 5.
Mean percent accuracy (standard error of the mean) and reaction time in milliseconds (standard error of the mean) on the sentence comprehension task for young controls (YC), age‐matched control group (AM), and stroke patients (STP)
| Group | Condition | Accuracy % (SE) | RT (SE) | d′ (SE) |
|---|---|---|---|---|
| YC | COR | 94 (0.01) | 512 (22.55) | |
| SEM | 97 (0.01) | 492 (24.60) | 3.51 (0.094) | |
| SYN | 94 (0.01) | 493 (23.94) | 3.26 (0.132) | |
| AM | COR | 93 (1.32) | 704 (44) | |
| SEM | 95 (1.02) | 649 (44) | 3.32 (0.156) | |
| SYN | 93 (1.76) | 624 (42) | 3.22 (0.152) | |
| STP | COR | 82 (2.88) | 818 (50.27) | |
| SEM | 84 (2.92) | 794 (44.29) | 2.1 (0.22) | |
| SYN | 42 (6.46) | 855 (54.76) | 0.79 (0.26) |
d′ statistic: accuracy corrected for response bias, calculated separately for semantic and syntactic anomalies. Higher value indicates better sensitivity to discriminate violations from correct sentences.
COR: correct sentences; SEM: semantic anomalies; SYN: syntactic anomalies.
Accuracy (d′ values) and reaction time (RT) data were entered into separate repeated measures analyses of variance (ANOVAs). For accuracy, d′ values for semantic and syntactic anomaly conditions were entered as a within‐subjects variable and participant group—stroke patient (STP), young control (YC), age‐matched (AM)—was entered as a between‐subjects variable. The analysis of accuracy data revealed a significant main effect of anomaly condition, F(1,53) = 45.28, p < 0.001, a significant main effect of group, F(2,53) = 47.55, p < 0.001, and a significant condition × group interaction, F(2,53) = 19.66, p < 0.001. To investigate the source of this interaction, a separate post hoc ANOVA with anomaly condition as a within‐subjects variable was performed for each participant group. For young controls, there was a significant main effect of anomaly condition, F(1,20) = 6.29, p = 0.021, indicating larger d′ prime (higher accuracy) for detecting semantic anomalies than for syntactic anomalies.
For stroke participants, the analysis of accuracy data revealed a significant main effect of anomaly condition, F(1,15) = 35.03, p < 0.001, indicating that patients were significantly less accurate in detecting syntactic anomalies than semantic anomalies. However, for older age‐matched controls, there was no significant main effect of anomaly condition, F(1,18) = 0.977, p = 0.336. The between‐group comparisons revealed that stroke participants were significantly less accurate on semantic and syntactic anomalies than both control groups (young vs stroke: semantic, t(35) = 5.944, p < 0.001, syntactic, t(35) = 8.368, p < 0.001 older vs stroke: semantic, t(33) = 4.571, p < 0.001, syntactic, t(33) = 7.954, p < 0.001. There were no significant differences in accuracy between young and older adults (semantic, t(38) = −1.001, p = 0.325; syntactic, t(38) = −0.225, p = 0.823.
We also assessed the reaction time pattern for the three participant groups and anomaly conditions (COR, SEM, and SYN). For the RT data, the ANOVA with anomaly condition as a within‐subjects variable and group as a between‐subjects variable revealed a significant main effect of anomaly condition, F(2,102) = 3.19, p = 0.045, a significant main effect of group, F(2,51) = 17.82, p < 0.001, and a significant group × condition interaction, F(4,102) = 3.51, p = 0.010.
The post hoc analysis of this interaction revealed that, for young controls, there was no significant difference in RT between the three condition types, F(2,40) = 0.659, p = 0.523. Similarly, there was no significant main effect of condition for stroke participants, F (2,26) = 2.04, p = 0.150. For the older control group, the analysis revealed a significant main effect of anomaly condition, F(2,36) = 8.57, p = 0.001, indicating significantly longer response latencies for correct sentences than for semantic and syntactic anomalies, which did not differ from each other (COR vs SEM, t(18) = 2.79, p = 0.012; COR vs SYN, t(18) = 4.19, p = 0.001; SEM vs SYN, t(18) =1.24, p = 0.232). The between‐group comparisons indicated that stroke patients were significantly slower than young controls on all sentence types (COR, t(33) = 5.176, p < 0.001, SEM, t(33) = 5.568, p < 0.001, SYN, t(33) = 5.635, p < 0.001). Also, stroke participants had longer RTs than older controls on semantic and syntactic anomalies, (SEM, t(31) = 2.202, p = 0.036; SYN, t(31) = 3.161, p = 0.004; COR, t(31) = 1.624, p = 0.116). In addition, older controls were significantly slower than young controls (COR, t(38) = 3.873, p = 0.001, SEM, t(38) = 3.101, p = 0.004, SYN, t(38) = 2.700, p = 0.011).
MEG Results
SAM localization of oscillatory responses
We applied SAM to localize brain responses to semantic and syntactic anomalies in stroke participants and the age‐matched control group. The choices of time windows, frequency bands, and conditions to compare were guided by our previous results in young controls [Kielar et al., 2015]. Informed by our previous results with the same paradigm, neural “activation” is indicated by power decrease, or ERD, in the frequency range of 8–30 Hz. Power decreases are mapped in a blue color scale on the surface of a standard reference brain in MNI space, while power increases (associated with reduced neural activity) are mapped in a yellow–red color scale. For stroke patients, the results were superimposed over an artificially darkened anatomical image representing the average lesion distribution across patients, with darker colors representing greater lesion overlap. To correct for multiple comparisons at a cluster‐wise level of p < 0.05, the statistical maps were thresholded at a voxelwise value of p = 0.01 or less and subjected to a minimum cluster size of 90 voxels (see methods).
Semantic responses: 8–30 Hz ERD
Figure 2A (adapted from Kielar et al. [2015]) shows the activation maps for semantic anomalies minus control words in young control participants. The comparison of semantic anomalies vs control words produced mostly left lateralized responses. Power decreases were present over most of the left and right occipital cortex. From the occipital areas, 8–30 Hz ERD extended into the left posterior superior temporal regions, and inferiorly into the fusiform gyrus, and included posterior parts of the left superior parietal lobule, supramarginal gyrus (SMG), and most of the left angular gyrus (AG). Power decreases were also observed in left frontal regions, including the left inferior frontal gyrus (IFG: BA 45, BA 44, BA 47) and the lateral surface of the middle frontal gyrus (BA 10, BA 46).
Figure 2.
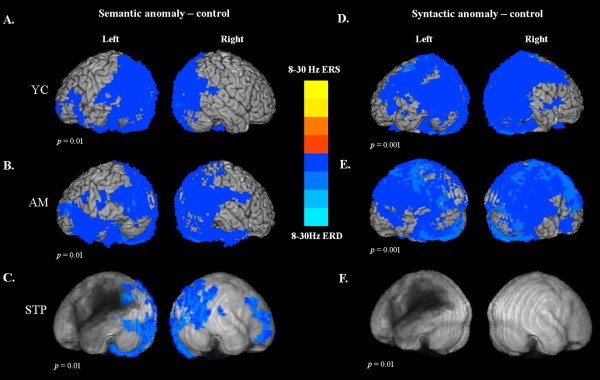
Synthetic aperture magnetometry (SAM) maps of power changes in the 8–30 Hz frequency range and 0.4–1 s time window after critical word onset for healthy controls and participants with poststroke aphasia. For comparisons with stroke patients, the results were overlaid on top of an artificially darkened anatomical image representing the lesion distribution across patients. Darker colors represent greater lesion overlap in these areas. The statistical maps were thresholded at a minimum cluster‐size criterion of 90 voxels and p < 0.01. Power changes for semantic anomalies vs control words for (A) healthy young controls, (B) older adults, and (C) stroke participants. Power changes for syntactic anomalies vs control words for (D) healthy young controls, (E) older adults, and (F) stroke patients.
The activation maps for semantic anomalies minus control words in older, age‐matched controls are shown in Figure 2B. The pattern of responses was similar to that observed for young controls, except that the power decreases had a more bilateral distribution for older adults. The comparison of semantic anomalies vs control words produced ERD in the occipital cortex bilaterally. From occipital areas, power decreases extended bilaterally to the posterior superior temporal and parietal regions (AG, SMG), and proceeded along the inferior temporal cortex into the left IFG. Power decreases were also observed along the lateral surface of the middle frontal gyrus (BA 10, BA 46), bilaterally.
Figure 2C presents the average activation maps for semantic anomalies minus control words in stroke participants. For aphasic patients, much less 8–30 Hz ERD was observed in the lesioned LH. Power decreases were present along the left occipital regions, fusiform gyrus, and small clusters in the posterior temporal and superior parietal cortex bordering the lesion zone, including left precuneus and the posterior part of the AG. In contrast, patients exhibited more activation in the RH, in occipital areas including precuneus, cuneus, and lingual gyrus. In the right parietal areas, power decreases were observed in AG and SMG, and extended into the right posterior superior and middle temporal areas. These RH regions were homologous to the LH areas activated in older control participants. In the frontal regions, semantic 8–30 Hz ERD was found in the right anterior, middle, and dorsolateral frontal cortex (BA 9, BA 10, and BA 46), and included right inferior frontal gyrus (BA 45/47), not activated in controls.
Syntactic Responses: 8–30 Hz ERD
For young controls, comparison of syntactic anomalies with control words produced widespread power decreases in both LH and RH (Fig. 2D). The 8–30 Hz ERD involved the entire occipital cortex, and included posterior superior temporal gyri, posterior portions of middle and inferior temporal cortices, and extended into the inferior and superior parietal lobules (most of the SMG and AG, precuneus). Power decreases were also observed along the precentral and postcentral gyri, including motor cortex, premotor and supplementary motor areas, and extended along middle frontal cortex into the posterior IFG in both hemispheres.
Older controls exhibited an extensive bilateral pattern of ERD in response to syntactic anomalies (Fig. 2E). The 8–30 Hz ERD involved the occipital cortex, posterior superior temporal gyri, middle and inferior temporal cortices, and the superior and inferior parietal lobules (most of the SMG, AG, and precuneus). Power decreases were also observed along the precentral and postcentral gyri—including motor cortex, premotor, and supplementary motor areas—and involved most of the inferior frontal gyrus, together with middle and superior frontal cortex.
There were no significant power decreases observed for stroke participants in response to syntactic anomalies (Fig. 2F).
Group comparisons: effects of stroke and aging
Comparison of semantic responses: 8–30 Hz ERD
Figure 3A displays the subtraction map for patients minus young controls for brain responses to semantic anomalies (semantic anomalies minus correct sentences). As ERD is a negative quantity, the activation decreases in patients are reflected by positive values in the subtraction map. These maps show reduced activation for patients in the left and right occipital regions, as well as in the left posterior temporal and parietal areas, including superior and inferior parietal lobules.
Figure 3.
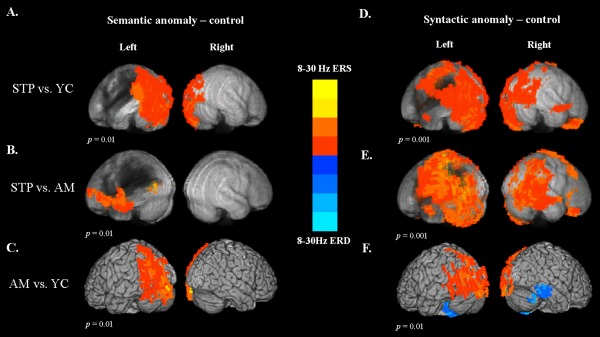
Between‐group voxel‐wise contrast maps of power changes in the 8–30 Hz frequency range and 0.4–1 s time window after critical word onset. The statistical maps were thresholded at a minimum cluster‐size criterion of 90 voxels and p < 0.01. (A) The subtraction map for stroke patients minus young controls on semantic anomalies (semantic anomalies vs correct sentences). (B) The subtraction map for patients minus older controls on semantic anomalies. (C) The subtraction map for older controls minus young controls on semantic anomalies. (D) The subtraction map for stroke patients minus young controls on syntactic anomalies (syntactic anomalies vs correct sentences). (E) The subtraction map for patients minus older controls on syntactic anomalies. (F) The subtraction map for older controls minus young controls on syntactic anomalies.
The comparison of patients with age matched older controls is presented in Figure 3B. The maps show less activation for patients in response to the semantic anomalies in the ventral frontotemporal regions, including inferior frontal gyrus (BA 44 and 45) and anterior temporal cortex. An additional cluster of decreased activation was found in the posterior temporal–occipital region bordering the lesion. There were no significant group differences in the RH.
Figure 3C shows the subtraction map comparing older controls with young controls. Older adults exhibited decreased activation in the left posterior and dorsal regions, including the left occipital cortex, and inferior and superior parietal regions. There were no significant differences in the RH.
Comparison of syntactic responses: 8–30 Hz ERD
The group comparison maps of brain responses to syntactic anomalies (syntactic anomalies minus correct sentences) for patients vs young controls are presented in Figure 3D, and for patients vs age‐matched older controls in Figure 3E. These maps show reduced activation for patients in extensive parts of the frontal, posterior temporal, and dorsal parietal regions that were activated in response to syntactic anomalies in healthy controls.
Figure 3F displays the subtraction map for older controls vs young controls. This comparison revealed decreased activation in older adults in the left and right occipital regions, left superior and inferior parietal regions, as well as in the left posterior temporal cortex. In addition, older adults exhibited increased activation in the right middle and inferior temporal gyrus, as well as bilateral fusiform gyrus and cerebellum, suggesting greater age‐related neural recruitment in these regions. In addition, compared to young controls, older adults exhibited significantly decreased ERD in left posterior brain regions for both semantic and syntactic anomalies although their activation was still statistically significant in these regions.
Overall, the MEG task‐related results show that stoke patients show a better capacity to recruit LH perilesional areas and preserved RH areas for semantic than syntactic anomalies. The RH regions activated in patients were homologous to the LH regions that showed power decreases in healthy controls. In addition, patients recruited additional right inferior and dorsolateral frontal regions that were not observed in healthy participants, suggesting compensatory recruitment of alternative brain areas. Furthermore, comparison of stroke patients with the age‐matched control group confirmed that patients showed reduced task‐related activation along the left ventral frontotemporal regions during processing of semantic anomalies. However, the increased RH activity in patients did not reach statistical significance in direct comparison with the older control group. This may be due to the considerable variability in the patient group. The activation patterns observed at the group average level may not be representative of activity in the regions that correlate with performance across patients. This question is explored in the subsequent correlational analyses.
MEG 8–30 Hz ERD correlations with MEG sentence comprehension task performance
Although patients in our study were consistently impaired in detecting syntactic anomalies, they scored relatively well on semantic anomalies and control sentences. However, there was also considerable variability in performance (as shown in Table 5). >To examine the relationship between comprehension performance and differences in MEG activation (reflected by 8–30 Hz ERD), we performed voxelwise rank‐order correlations (Spearman's Rho) across patients between the accuracy scores (quantified using d′) and MEG activation, characterized by ERD in the 8–30 Hz range. Note that because ERD is a negative quantity, most of the observed correlations are negative, reflecting a greater degree of ERD corresponding to better performance.
Clusters of significant correlations were detected for both semantic and syntactic anomalies. The correlation maps for semantic anomalies in stroke patients are presented in Figure 4A. Activation in the right posterior superior and middle temporal gyrus (RSTG/RMTG), right inferior temporal gyrus (RITG), fusiform gyrus, inferior parietal regions (AG, SMG), and extending into middle occipital cortex was predictive of higher accuracy on the semantic anomalies.
Figure 4.
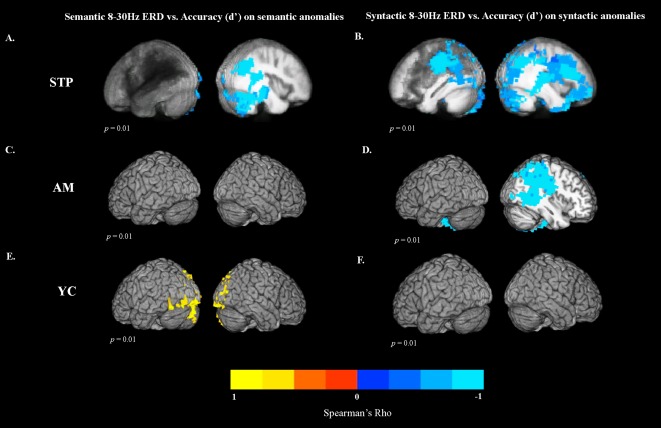
Correlations (Spearman's Rho) between MEG task activity and accuracy scores (quantified with d′). The statistical maps were thresholded at a minimum cluster‐size criterion of 90 voxels and p < 0.01. Correlations between 8–30 Hz ERD for semantic anomalies and d′ values for (A) stroke participants, (C) older controls, and (E) young controls. Correlations between 8 and 30 Hz ERD for syntactic anomalies and d′ values for (B) stroke participants, (D) older controls, and (F) young controls.
Figure 4B displays the correlation maps for syntactic anomalies in stroke patients. For syntactic anomalies, regions that correlated with better performance were found in the right cuneus and precuneus (BA7). They included right temporoparietal cortex (RTP), right superior temporal gyrus (RSTG), and extended into superior and dorsal occipital–parietal regions. Significant correlations were also observed along the right superior and middle frontal gyri, extending into medial frontal areas, precentral gyrus, and inferior frontal cortex. In the LH, higher accuracy correlated with activation in the posterior superior temporal cortex, and superior and inferior parietal cortex (LIPL), extending into the left precentral gyrus.
The same correlations were computed for both control groups. For older controls, there were no significant correlations between ERD and comprehension performance on semantic anomalies. For syntactic anomalies, correlations with performance were found in the right superior and inferior parietal lobule, extending into the superior temporal gyrus (Fig. 4D), indicating that better accuracy was associated with greater ERD in these regions.
For young controls, there was a significant positive correlation between event‐related power in the 8–30 Hz frequency range and comprehension performance on the semantic anomalies in the bilateral occipital regions (Fig. 4E). This positive correlation indicates that for young controls, reduced ERD (more power in 8–30 Hz range) for semantic anomalies relative to control words in these regions was associated with better performance. There were no significant correlations between ERD and comprehension performance for syntactic anomalies.
Accounting for lesion size on the relationship between MEG responses and performance
The correlational analyses between MEG task accuracy and MEG activation (reflected by 8–30 Hz ERD) revealed that for stroke patients, activation in RH regions was associated with better performance on the sentence comprehension task, indicating that RH recruitment may be compensatory. However, one caveat to this interpretation is that patients varied in the degree of damage to LH language regions, and this may account for some variability in their recruitment of RH cortex. For example, if RH activation is maladaptive and related to disinhibition from damaged LH cortex, one might expect larger lesions to predict greater RH activation. In such an account, larger lesions could drive poorer performance and more RH activation. This seems unlikely given that we found only correlations between better performance and RH activation. However, it is also possible that RH activation could be associated with smaller LH lesions, and thereby with performance, confounding our correlation results.
To estimate the contribution of LH lesion extent on both RH activation and performance accuracy, we employed hierarchical linear regression, as previously employed in a study examining LH lesions and RH compensatory changes [Xing et al., 2016]. The d′ values for semantic and syntactic anomalies were entered as dependent variables and age, education, lesion size, and event‐related power differences (8–30 Hz ERD, anomalies minus controls) in activated ROIs as independent variables. The ROIs used in the analysis were extracted from the correlation maps between ERD and task accuracy, separately for semantic and syntactic anomalies.
For semantic anomalies, the correlation between accuracy (d′ values) and ERD responses identified two significant clusters: one in the RSTG/RMTG and the other in the RITG. In the hierarchical regression, d′ values for semantic anomalies were entered as the dependent variable. Age and education were entered at step one, lesion size at step two, and ERD values extracted from the RH clusters in step three. The first hierarchical regression revealed that a model including only age and education was not predictive of accuracy (R 2 = 0.062, F(2,13) = 0.432, P = 0.658, R 2 change = 0.062, F(2,13) = 0.432, P = 0.658). When lesion size was added, the predictive value of the model increased (R 2 = 0.503, F(3,12) = 4.045, P = 0.034, R 2 change = 0.440, F(1,12) = 10.63, P = 0.007). The predictive value of the model improved further when the ERD values extracted from the RSTG/RMTG cluster were entered into the model (R 2 = 0.720, F(4,11) = 7.068, P = 0.005, R 2 change = 0.217, F(1,11) = 8.53, P = 0.014). The analysis showed that lesion size was a significant predictor of accuracy scores when ERD values were excluded from the model (beta = −0.0000156, t = (−3.26), P = 0.007). Larger lesions predicted poorer accuracy. However, when ERD values were added to the model, lesion size was no longer a significant predictor of accuracy (beta = −0.00000247, t = (−0.421), P = 0.682). Among the four variables entered in the analysis, only ERD in the RSTG/RMTG cluster was a significant independent predictor of accuracy on semantic violations (beta = −1.326, t(−2.920), P = 0.014), indicating that higher accuracy on semantic violations was associated with more activation in RSTG/RMTG independent of lesion size.
A similar pattern of results was obtained in the second hierarchical regression, where the ERD values extracted from the RITG cluster were entered into the model (R 2 = 0.697, F(4,11) = 6.334, P = 0.007, R 2 change = 0.194, F(1,11) = 7.067, P = 0.022). The analysis showed that among four variables entered in the analysis, only ERD in the RITG cluster was a significant independent predictor of accuracy on semantic violations, (beta = −1.322, t(−2.658), P = 0.022), indicating that higher accuracy on semantic violations was associated with greater activation (more 8–30 Hz ERD) in RITG after lesion size was accounted for.
For syntactic anomalies, higher task accuracy was associated with greater activation in four RH clusters and one LH cluster. These clusters included right temporoparietal cortex (RTP), right superior temporal gyrus (RSTG), right precuneus (RBA7), and middle frontal gyrus (RMF). The LH cluster was located in the inferior parietal cortex (LIPL). In each hierarchical regression analysis, d′ values for syntactic violations were entered as dependent variable. Age and education were entered at step one, lesion size at step two, and ERD values extracted from the activated clusters in step three.
The hierarchical regression revealed that a model including age and education was not predictive of accuracy on syntactic violations (R 2 = 0.122, F(2,13) = 0.904, P = 0.429, R 2 change = 0.122, F(2,13) = 0.904, P = 0.429). Addition of lesion size did not improve predictive value of the model (R 2 = 0.230, F(3,12) = 1.193, P = 0.354, R 2 change = 0.108, F(1,12) = 1.677, P = 0.220). The predictive value of the model improved when ERD values from the RH clusters and left IPL cluster were included in the model, (RTP: R 2 = 0.733, F(4,11) = 7.544, P = 0.004, R 2 change = 0.503, F(1,11) = 20.719, P = 0.001); RMF: R 2 = 0.544, F(4,11) = 3.286, P = 0.053, R 2 change = 0.315, F(1,11) = 7.599, P = 0.019; RSTG: R 2 = 0.764, F(4,11) = 8.886, P = 0.002, R 2 change = 0.534, F(1,11) = 24.853, P = 0.000; LIPL: R 2 = 0.755, F(4,11) = 8.464, P = 0.002, R 2 change = 0.525, F(1,11) = 23.553, P = 0.001). Adding ERD values from right BA7 ROI did not significantly improve predictive value of the model (RBA7: R 2 = 0.413, F(4,11) = 1.935, P = 0.175). Among the four variables entered in each hierarchical regression analysis, ERD values in the RH ROIs and LH ROI were significant independent predictors of accuracy on syntactic violations, indicating that higher accuracy on syntactic violations was associated with greater activation in the RH independent of lesion size (RTP: beta = −1.564, t(−4.552), P = 0.001; RMF: beta = −1.173, t(−2.757), P = 0.019; RSTG: beta = −1.234, t(−4.985), P = 0.000; LIPL: beta = −1.479, t(−4.853), P = 0.001).
Ratio of LH to RH ERD
The previous analyses showed that greater 8–30 Hz ERD in RH regions is predictive of better comprehension of semantic and syntactic anomalies in aphasic stroke patients, and that this relationship holds when taking into account lesion extent in the LH. These analyses support a compensatory role for RH engagement in aphasia. However, many studies have shown that preserved activation in perilesional tissue is associated with better outcomes. The approach taken in this study, examining voxel‐wise group correlations across subjects, may be less sensitive to perilesional activation because of the heterogeneity of lesion extent across patients. The variable presence of lesions in LH voxels may preclude the detection of a significant relationship between LH activation and performance, despite the common finding that preserved left‐dominant activation patterns are preferable in aphasia.
To supplement our previous analyses, therefore, we examined the ratio of LH to RH activation in ROIs associated with better performance. To limit the number of comparisons, analyses were only performed for ROIs (and their homologs in the opposite hemisphere) that showed significant correlations with performance in the whole‐brain analysis. For the semantic task, these regions were RSTG/RMTG and RITG, and their LH homologs. For the syntactic task, the regions were RTP, RMF, RBA7, RSTG, LIPL, and their homologs in the opposite hemisphere. The correlation coefficients and their corresponding significance levels are presented in Table 6. For semantic anomalies, there were significant negative correlations between ERD values and accuracy in the right STG/MTG and right ITG ROIs (as already shown in the analysis that identified these ROIs in the first place), but not in the LH homologs. There were no significant correlations between accuracy and the proportion of LH to RH ERD in these regions. These results indicate that for stroke patients, higher accuracy on semantic anomalies was associated with increased ERD in RH regions. Although there were associations between lesion extent and performance in the LH, there was no apparent benefit to having a left‐lateralized pattern of activation in these areas.
Table 6.
Pearson correlations between task accuracy and proportion of ERD in the left‐hemisphere regions relative to the RH regions in stroke patients
| Region | Correlation (r) | Significance (p) |
|---|---|---|
| d′ sem | ||
| RSTG/MTG | −0.832 | 0.000063b |
| RITG | −0.765 | 0.001b |
| LSTG/MTG | −0.378 | 0.149 |
| LITG | −0.374 | 0.153 |
| L/RMTG | −0.411 | 0.114 |
| L/RITG | −0.032 | 0.907 |
| d′ syn | ||
| RTP | −0.81 | 0.000141b |
| RMF | −0.69 | 0.003a |
| LIPL | −0.774 | 0.00043b |
| RBA7 | −0.559 | 0.024a |
| RSTG | −0.816 | 0.000115b |
| LTP | −0.724 | 0.002a |
| LMF | −0.616 | 0.011a |
| RIPL | −0.713 | 0.002a |
| LBA7 | −0.655 | 0.006a |
| LSTG | −0.527 | 0.036a |
| L/RTP | 0.083 | 0.761 |
| L/RMF | 0.082 | 0.763 |
| L/RIPL | 0.169 | 0.531 |
| L/RBA7 | −0.54 | 0.842 |
| L/RSTG | 0.098 | 0.718 |
The regions which showed significant correlations with performance in the whole brain analysis are shown in bold font. The significant correlations are marked in bold font (a: p < 0.05, uncorrected; b: significant with Bonferroni correction, d‐sem = p < 0.002, d‐syn = p < 0.0005).
For syntactic anomalies, there were significant correlations between accuracy and ERD in the right temporoparietal region (RTP), and its LH homologue (LTP, uncorrected only). There were also significant correlations between accuracy and ERD values in the right STG, left IPL, and their homologs in the opposite hemisphere (uncorrected only). There were no significant correlations between accuracy and proportion of LH to RH ERD in these regions. These results suggest that better syntactic performance in patients was associated with recruitment of temporal and partial regions in both LH and RH, with no particular advantage for lateralized activation.
Relationship between MEG sentence comprehension task performance and lesion variability
To evaluate the relationship between lesion location and language impairment on the MEG sentence comprehension task, we used the VLSM technique implemented in MRIcron. The permutation analyses indicated that the t‐statistic threshold with an FWE significance level of p < 0.05 for semantic anomalies was 3.547, and for syntactic anomalies, it was 2.457. Figure 5 shows the VLSM results for MEG task performance accuracy on detecting semantic and syntactic anomalies, quantified using d′. For both semantic and syntactic anomalies, reduced accuracy was most strongly associated with lesions in the left superior temporal gyrus.
Figure 5.
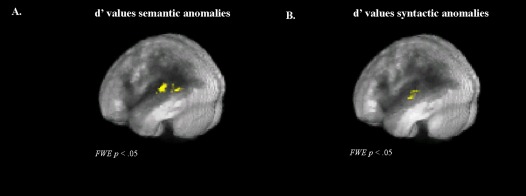
Voxel‐based lesion symptom mapping (VLSM) results for stroke patients. Positive values indicate that a lesion is correlated with reduced performance. Based on permutation tests, the maps were thresholded with an FWE significance level of p < 0.05. VLSM results on d′ values for (A) semantic anomalies and (B) syntactic anomalies.
DISCUSSION
Recovery of language function after stroke has been associated with changes in activation in different brain networks. This activation can take the form of restoration of function in the original language centers of the left, dominant hemisphere (if they are not destroyed), recruitment of new regions ipsilateral to the lesion, or a shift of language processing to the right, nondominant hemisphere. The RH recruitment may involve activation of regions homotopic to the damaged LH language centers, or recruitment of new regions not activated in controls during language tasks. Previous studies suggested that reactivation of preserved LH language regions leads to the best clinical outcome [Cornelissen et al., 2003; Heiss and Thiel, 2006; Léger et al., 2002, Tyler et al., 2010]. At the same time, many studies reported increased activity in the RH relative to healthy controls during language tasks [Blasi et al., 2002; Breier et al., 2007; Musso et al., 1999]. However, the significance of these findings in relation to aphasia recovery and language performance is not well understood, as RH activation has been associated with both improved performance and maladaptive processes.
In this study, we utilized MEG to understand the roles of perilesional and contralesional activity for processing semantic and syntactic information in patients with poststroke aphasia, and to explore the potential of RH activity to support recovery. The study design allowed us to directly assess the relationship between language‐related activation and behavioral task performance, as well as characterize the differences in activation to semantic and syntactic anomalies in older controls and aphasic patients. Comprehension performance in aphasia is likely dependent on both the extent of lesions and the presence of compensatory activation, so we considered both factors in our analyses.
In this study and in our previous study with young controls, we found a relatively broad pattern of ERD responses for semantic and syntactic anomalies. This widespread spatial distribution of responses in 8–30 Hz frequency range has been observed in other studies using linguistic stimuli [Meltzer and Braun, 2011; Meltzer et al., 2013]. Invasive electrophysiological studies indicate that ERD in this frequency range is frequently accompanied by gamma ERS and elevated neuronal firing rates, but has both a broader spatial distribution and a longer time course, making it more easily detectable with noninvasive methods like EEG and MEG [Crone et al., 2006]. Thus, 8–30 Hz ERD serves as a useful indicator of increased neural activity in noninvasive experiments, but may have a more limited spatial resolution compared to invasive measurements.
This study focused on 8–30 Hz ERD in response to anomalous words, which occurs over a relatively late and long‐lasting period (0.4–1.0 s). The time course of the effect suggests that alpha–beta power decreases in this task may index the protracted reprocessing of linguistic input after a semantic or syntactic violation is encountered, rather than an earlier and more automatic process. Consistent with this, it has been suggested that decreases in alpha and beta bands may be a general indicator of cortical information processing in short‐term and long‐term memory [Hanslmayr et al., 2012; Klimesch, 1999].
We found that, in young healthy controls, activation of left temporofrontal ventral brain regions was involved in processing of semantic anomalies, whereas syntactic anomalies recruited dorsal bilateral frontoparietal regions, as well as some of the ventral brain areas. For older controls, we observed more bilateral recruitment of the ventral regions for semantic anomalies, and extensive bilateral involvement of dorsal and ventral regions for syntactic anomalies. In stroke patients, linguistic processing recruited available regions in both hemispheres. However, the specific pattern of recruitment depended on the type of linguistic information that was processed. For semantic anomalies, patients showed decreased activation along the ventral left frontotemporal regions that were activated in controls. However, they recruited some of the preserved parietal and temporal cortex in the LH perilesional areas. In the RH, patients recruited inferior parietal and posterior temporal cortex that was also activated in controls. Correlations between behavioral performance and MEG activation (reflected by 8–30 Hz ERD) indicated that activation of the preserved right parietal and temporal regions is adaptive, as higher accuracy on the semantic task was associated with greater activity in these areas. In addition, patients showed activity in the right inferior frontal and anterior dorsolateral frontal cortex. However, this frontal activity was not correlated with task performance, nor was the difference in activation significant between patients and controls.
For syntactic anomalies, patients showed much less activity compared to controls. However, this lack of response to syntactic anomalies at the group level does not necessarily imply that patients completely failed to show differential brain activity to such stimuli. It suggests that the activation patterns were more variable for the syntactic task, leading to weak statistical significance at the voxel level. This is also consistent with the substantial behavioral variability for patients on the syntactic task. The lack of responses for syntactic anomalies may indicate that patients failed to detect syntactic errors. Consistent with this interpretation, a recent ERP study found that only violations that were detected evoked a later positivity [Batterink and Neville, 2013]. However, the correlations between brain activation and performance on the syntactic task revealed that higher accuracy for syntactic anomalies was associated with activity in the right and left dorsal parietal regions, including superior parietal cortex and occipital regions, as well as right superior middle frontal areas and the inferior frontal gyrus, which were also found to be activated in healthy controls.
These results suggest that recruitment of the RH facilitates semantic processing in aphasia, but less compensation occurs for syntactic processing. For semantic processing, patients recruited additional areas in the right dorsolateral frontal cortex (BAs 46/9) and right inferior frontal gyrus (BAs 45/47). The activation of these right dorsolateral frontal regions may indicate recruitment of alternative cognitive strategies for semantic processing in patients as it was not found in the healthy participants. However, this frontal activation for semantic anomalies was not significantly correlated with better performance, suggesting that it does not facilitate processing of semantic information directly. It appears that the shift of activation to right temporoparietal regions is more directly associated with successful semantic processing.
The recovery of semantic processing through recruitment of the RH cortex is most likely related to the ability of RH temporoparietal and frontal regions to support some aspects of semantic processing, even in the undamaged brain. The bilateral organization of semantic processing is postulated by the empirically based dual‐stream model of language processing [Hickok and Poeppel, 2004, 2007]. Previous studies found that involvement of RH homologs of the LH language regions increases as language comprehension demands become more complex [Bookheimer, 2002; Demonet et al., 2005]. Some evidence suggests that the LH performs fine‐gained coding of semantic information, whereas coding of semantic representation is more coarse and diffuse in the RH, making it particularly well suited for inferential processing [Beeman et al., 2000; Jung‐Beeman, 2005]. Some studies indicate that bilateral temporal regions support semantic integration, whereas inferior frontal areas are important for semantic selection or controlled retrieval from among competing alternatives [Thompson‐Schill et al., 1997; Wagner et al., 2001]. In particular, strong RH recruitment is observed for tasks which place greater demands on semantic integration and prediction processes [Beeman et al., 2000; Kircher et al., 2001; Meyer et al., 2000]. It is possible that for young healthy adults, processing semantic anomalies requires relatively little effort, engaging mostly dominant LH language regions. RH regions may still be active but at the subthreshold level. In patients with aphasia, it is possible that damage to the LH language regions prevents these areas from responding to language stimuli normally, unmasking RH activation. Responses in the right temporal–parietal regions may be inhibited in healthy participants by the dominant LH, but can be freed to activate after LH stroke. With time, these RH regions can become increasingly better adjusted to support language tasks that are more commonly supported by their LH counterparts.
The present results suggest that RH ventral regions are more strongly recruited for semantic processing in aphasia, whereas bilateral dorsal and right frontal areas are associated with more accurate syntactic performance. The RH frontal–parietal network connecting the right intraparietal sulcus to the right superior and middle prefrontal cortex has been also implicated in maintenance of sequence and serial order representations [Majerus, 2013]. The activation of these regions for syntax may reflect greater engagement of the preserved dorsal top–down attention system, executive control, and working memory when processing tense and agreement anomalies [Brownsett et al., 2014; Geranmayeh et al., 2014]. The present results are consistent with hypothesized roles of the left and right posterior superior temporal cortex in supporting syntactic comprehension [den Ouden et al., 2012; Friederici et al., 2009]. The observed activation in motor cortex and premotor regions has also been associated with complex syntactic computation and processing of incongruent or conflicting syntactic information [den Ouden et al., 2012; Christensen, 2010; Heim et al., 2009].
Results of Lesion Analysis
The VLSM results suggested that damage to the left superior temporal areas was associated with sentence comprehension impairment. The central role of these temporal regions in language comprehension has been suggested by previous studies investigating the relationship between lesion extent and language performance [Dronkers et al., 2004; Meltzer et al., 2013]. In addition, fiber tractography and functional connectivity studies indicate that the left‐middle temporal region is extensively connected with other temporal, parietal, and frontal regions [Turken and Dronkers, 2011]. Based on these findings, it could be predicted that the activity in the homologous right temporal areas will be associated with better performance on the sentence comprehension task across patients.
We found that MEG activity in the right posterior part of the superior temporal cortex correlated with higher accuracy on both semantic and syntactic tasks, suggesting a supportive role of this region in recovery of semantic and syntactic comprehension.
Vulnerability of Syntactic Processing
Although we did find correlations between activation in RH dorsal areas and successful detection of syntactic anomalies in aphasic patients, performance on syntactic anomaly detection was in general quite poor in that group (raw accuracy 42%). As a group, aphasic patients had no significant MEG activation in response to syntactic anomalies. This is striking as these anomalies elicited very large responses in healthy controls, considerably stronger in magnitude and greater in spatial extent than semantic anomalies (Fig. 2 and Figs. 1, 2, and 4 in Kielar et al. [2015]). This lack of sensitivity to syntactic anomalies in aphasics, compared to their relatively preserved semantic processing, points to the unique vulnerability of syntactic processing to perisylvian damage of any kind. Other studies have also found certain kinds of demanding syntactic processing to be disrupted in the majority of aphasic patients regardless of specific lesion site [Griffiths et al., 2013; Meltzer et al., 2013]. These results indicate that grammatical processing may be a highly vulnerable cognitive skill that is not easily regained after brain damage. Additionally, the uniformly poor performance on syntactic processing limits the interpretation of the VLSM results for syntactic anomaly detection. As patients are near floor level for that measure, there is less power to detect differences in lesion extent related to performance. Although the significant VLSM results indicate that intact tissue in the posterior superior temporal lobe does help support residual performance in aphasic patients, the overall reduced sensitivity in patients to syntactic anomalies suggests that syntactic processing is in general more vulnerable to disruption by brain damage than is semantic processing.
Effect of Aging
In addition to the effects of LH stoke on induced oscillatory activity, the present results revealed more subtle changes associated with aging. In comparison to the young controls, older adults showed more extensive bilateral responses to the syntactic anomalies and less lateralized responses to the semantic anomalies. The direct comparison between groups revealed that relative to younger controls, older participants exhibited decreased activation in the left occipital and parietal regions for both semantic and synaptic anomalies. For syntactic anomalies, older adults showed increased activation in the right middle and inferior temporal gyrus, as well as bilateral fusiform gyri and cerebellum. Similar changes in neural activation, including both increased and decreased activity, for older compared with younger participants have been observed in previous studies using a variety of memory and visual processing tasks (see Cabeza [2002] and Grady [2008] for reviews). Consistent with the present results, in these studies, older adults showed reduced activity in the occipital cortex and over‐recruitment of activity in other brain areas [Grady et al., 1994]. This pattern has been interpreted as compensation for age‐related decline in visual processing efficiency, by recruiting additional cognitive mechanisms [Grady, 2008].
Significantly stronger bilateral recruitment in older adults has also been found in language‐processing tasks [Kemmotsu et al., 2012; Manenti et al., 2013; Tyler et al., 2010]. Using MEG with a visual semantic judgment task, Kemmotsu et al. found stronger bilateral responses in older adults compared to the younger group. They also found an age‐related reduction in lateralization during language processing, which was accompanied by larger responses in the right temporal and inferior frontal regions. These age‐related changes in the perisylvian regions occurred in a time window from 250 to 450 ms that has been associated with linking word form to meaning and semantic processing [Halgren et al., 2002; Lau et al., 2013, 2008]. Consistent with the present findings, these results suggest that advanced age affects later stages of language processing, most likely associated with controlled retrieval and selection of lexical representations.
In this study, we observed strongly bilateral responses in older adults to both semantic and syntactic anomalies, in the context of comparable levels of task accuracy for the two age groups. In addition, we found that compared to the younger group, older adults showed increased recruitment of right middle temporal cortex for the syntactic task. This neurobehavioral pattern is consistent with a compensatory process. Increased bilateral recruitment and increased activity in the RH frontotemporal regions has been found with fMRI during a syntactic processing task [Tyler et al., 2010], independent of performance. In that study, performance in older adults was preserved despite significant gray matter atrophy in the left frontotemporal brain regions that were activated during syntactic processing in the younger group. These results suggest that increased RH activity compensates for age‐related declines in LH function, resulting in a more bilateral language distribution and preserved task performance. In this study, the compensatory role of the RH is supported by the significant correlations between MEG activation (8–30 Hz ERD) and syntactic performance in the RH regions. Older adults who were more accurate on the syntactic task recruited right temporal–parietal regions, but this effect was not observed for the young controls. Together with previous studies, the present findings suggest that this bilateral pattern of activation may represent a compensatory recruitment of RH regions associated with healthy aging [Cabeza, 2001, 2002; Davis et al., 2012]. In our study, task accuracy did not differ between the older and young groups; however, older participants showed significantly longer reaction times. A similar pattern was observed by Kemmotsu et al. [2012], who found that prolonged RT was associated with a decreased N400 response in middle‐aged adults. This finding was interpreted as evidence for decreased efficiency in the controlled stages of the lexical‐semantic processing. Our results are consistent with this interpretation. Because participants responded after the complete sentences had been presented, the reaction time data most likely reflect the later stage of language processing associated with reanalysis and repair.
One surprising result was the positive correlation between semantic anomaly response and performance in young adults (Fig. 4E), in that better performance was associated with less ERD (more positive event‐related power values) in the occipital lobe. One possible interpretation of this is that the occipital ERD reflects extended visual processing in the later stages of word recognition, and that more skilled readers finish their processing faster, resulting in less ERD over the full time window. This possibility could be investigated in future studies examining oscillatory brain responses in healthy readers of various skill levels.
CONCLUSIONS
This study demonstrates that language comprehension in patients with poststroke aphasia involves reactivation of preserved perisylvian areas ipsilateral to the lesion and recruitment of preserved RH regions. Furthermore, the pattern of responses was different for semantics and syntax. Patients who were more accurate on the semantic task recruited preserved cortex in the right ventral temporal regions and inferior parietal lobe. In addition, patients activated part of the right dorsolateral frontal cortex that was not observed in controls. However, this right frontal activation was not associated with more accurate semantic performance, indicating that it may not contribute directly to semantic recovery. In contrast, preservation of syntactic processing seems to be mediated by left and right superior dorsal regions and right frontal cortex, although syntactic processing seems to show less capacity for recovery after damage to LH language centers.
These results have potential implications for the treatment of comprehension impairments in patients with aphasia. First, the pattern of activity associated with better performance is suggestive of a role for domain‐general processes in supporting language comprehension. These processes may themselves be the targets of fruitful interventions. It has been recently postulated that training focused on RH functions can stimulate cognitive reserve and provide additional cognitive mechanisms that help the brain adapt to age‐related changes and disease [Robertson, 2014]. The experimental evidence suggests that attention process training improves attention deficits in stroke patients [Baker‐Collo et al., 2009; Robertson, 2014], and executive function training that focuses on cognitive conflict resolution has a potential to improve syntactic ambiguity resolution [Hussey and Novick, 2012]. Such targeted interventions may stimulate recruitment of the intact domain‐general circuits to support recovery of language comprehension. Second, our findings add nuance to the ongoing debate about optimal treatment of aphasia with noninvasive brain stimulation. Several studies have achieved clinical improvements in picture naming [Chrysikou and Hamilton, 2011; Martin et al., 2009; Barwood et al., 2011] through the application of inhibitory stimulation to the RH homolog of Broca's area, supporting the maladaptive account of RH activity. The present results suggest that RH activity is in fact adaptive for receptive semantics, and thus inhibitory stimulation to the RH may not be optimal for the treatment of comprehension deficits.
REFERENCES
- Adlam AR, Patterson K, Bozeat S, Hodges JR (2010): The Cambridge semantic memory test battery: Detection of semantic deficits in semantic dementia and Alzheimer's disease. Neurocase 16:193–207. [DOI] [PubMed] [Google Scholar]
- Angrilli A, Spironelli C (2005): Cortical plasticity of language measured by EEG in case of anomic aphasia. Brain Lang 95:32–33. [Google Scholar]
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC (2011): A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 54:2033–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JM, Rorden C, Fridriksson J (2010): Using transcranial direct‐current stimulation to treat stroke patients with aphasia. Stroke 4:1229–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker‐Collo SL, Feigin VL, Lawes CM, Parag V, Senior H, Rodgers A (2009): Reducing attention deficits after stroke using attention process training a randomized controlled trial. Stroke 40:3293–3298. [DOI] [PubMed] [Google Scholar]
- Barwood CH, Murdoch BE, Whelan BM, Lloyd D, Riek S, JD OS, Coulthard A, Wong A (2011): Improved language performance subsequent to low‐frequency rTMS in patients with chronic non‐fluent aphasia post‐stroke. Eur J Neurol 18:935–943. [DOI] [PubMed] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, Dronkers NF (2003): Voxel‐based lesion‐symptom mapping. Nat Neurosci 6:448–450. [DOI] [PubMed] [Google Scholar]
- Batterink L, Neville HJ (2013): The human brain processes syntax in absence of consceious awareness. J Neurosci 33:85289–88533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardouille T, Picton TW, Ross B (2006): Correlates of eye blinking as determined by synthetic aperture magnetometry. Clin Neurophysiol 117:952–958. [DOI] [PubMed] [Google Scholar]
- Bastiaansen MC, Magyari L, Hagoort P (2009): Syntactic unification operations are reflected in oscillatory dynamics during on‐line sentence comprehension. J Cogn Neurosci 22:1333–1347. [DOI] [PubMed] [Google Scholar]
- Belin P, Van Eeckhort P, Zilbovicius M, Remy P, Francois C, Guillaume S, Chain F, Rancurel G, Samson Y (1996): Recovery from nonfluent aphasia after melodic intonation therapy: A PET study. Neurology 47:1504–1511. [DOI] [PubMed] [Google Scholar]
- Benton AL, Hamsher K, Varney NR, Spreen O (1983): Contributions to Neuropsychological Assessment: A Clinical Manual. New York: Oxford. [Google Scholar]
- Beeman MJ, Bowden EM, Gernsbacher MA (2000): Right and left hemisphere cooperation for drawing predictive and coherence inferences during normal story comprehension. Brain Lang 71:310–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi V, Young AC, Tansy AP, Petersen SE, Snyder AZ, Corbetta M (2002): Word retrieval learning modulates right frontal cortex in patients with left frontal damage. Neuron 36:159–170. [DOI] [PubMed] [Google Scholar]
- Block CK, Baldwin CL (2010): Cloze probability and completion norms for 498 sentences: Behavioral and neural validation using event‐related potentials. Behav Res Methods 42:665–670. [DOI] [PubMed] [Google Scholar]
- Bookheimer S (2002): Functional MRI of language: New approaches to understanding the cortical organization of semantic processing. Annu Rev Neurosci 25:151–188. [DOI] [PubMed] [Google Scholar]
- Breier JI, Juranek J, Maher LM, Schmadeke S, Men D, Papanicolaou AC (2007): Changes in language‐specific brain activation after therapy for aphasia using magnetoencephalography: A case study. Neurocase 13:169–177. [DOI] [PubMed] [Google Scholar]
- Breier JI, Juranek J, Maher LM, Schmadeke S, Men D, Papanicolaou AC (2009): Behavioral and neurophysiologic response to therapy for chronic aphasia. Arch Phys Med Rehabil 90:2026–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownsett SLE, Warren JE, Geranmayeh F, Woodhead Z, Leech R, Wise RJS (2014): Cognitive control and its impact on recovery from aphasiac stroke. Brain 137:242–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes MJ, Gibson AM, Hall SD, Furlong PL, Barnes GR, Hillebrand A, Singh KD, Holliday IE, Francis ST, Morris PG (2005): GLM‐beamformer method demonstrates stationary field, alpha ERD and gamma ERS co‐localisation with fMRI BOLD response in visual cortex. Neuroimage 26:302–308. [DOI] [PubMed] [Google Scholar]
- Brookes MJ, Vrba J, Robinson SE, Stevenson CM, Peters AM, Barnes GR, Hillebrand A, Morris PG (2008): Optimising experimental design for MEG beamformer imaging. Neuroimage 39:1788–1802. [DOI] [PubMed] [Google Scholar]
- Cabeza R (2001): Cognitive neuroscience of aging: Contributions of functional neuroimaging. Scand J Psychol 42:277–286. [DOI] [PubMed] [Google Scholar]
- Cabeza R (2002): Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychol Aging 17:85–100. [DOI] [PubMed] [Google Scholar]
- Calami M, Markon K, Denbur GNL, Tranel D (2011): Developing a short form of Benton's judgment of line orientation test: An item response theory approach. Clin Neuropsychol 25:670–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheyne D, Bakhtazad L, Gaetz W (2006): Spatiotemporal mapping of cortical activity accompanying voluntary movements using an event‐related beamforming approach. Hum Brain Mapp 27: 213–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheyne D, Bostan AC, Gaetz W, Pang EW (2007): Event‐related beamforming: A robust method for presurgical functional mapping using MEG. Clin Neurophysiol 118:1691–1704. [DOI] [PubMed] [Google Scholar]
- Cho‐Reyes S, Thompson CK (2012): Verb and sentence production and comprehension: Northwestern Assessment of Verbs and Sentences (NAVS). Aphasiology 26:1250–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen KR (2010): Syntactic reconstruction and reanalysis, semantic dead ends, and prefrontal cortex. Brain Cogn 73:41–50. [DOI] [PubMed] [Google Scholar]
- Chrysikou EG, Hamilton RH (2011): Noninvasive brain stimulation in the treatment of aphasia: Exploring interhemispheric relationships and their implications for neurorehabilitation. Restor Neurol Neurosci 29:375–394. [DOI] [PubMed] [Google Scholar]
- Cornelissen K, Laine M, Tarkiainen A, Järvensivu T, Martin N, Salmelin R (2003): Adult brain plasticity elicited by anomia treatment. J Cogn Neurosci 15:444–461. [DOI] [PubMed] [Google Scholar]
- Cox RW (1996): AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29:162–173. [DOI] [PubMed] [Google Scholar]
- Crinion JT, Leff AP (2007): Recovery and treatment of aphasia after stroke: Functional imaging studies. Curr Opin Neurol 20:667–673. [DOI] [PubMed] [Google Scholar]
- Crone NE, Sinai A, Korzeniewska A (2006): High‐frequency gamma oscillations and human brain mapping with electrocorticography. Progr Brain Res 159:275–295. [DOI] [PubMed] [Google Scholar]
- Dabul B (1979): Apraxia Battery for Adults. Tiugard, Oregon: CC Publications. [Google Scholar]
- Davis SW, Kragel JE, Madden DJ, Cabeza R (2012): The architecture of cross‐hemispheric communication in the aging brain: Linking behavior to functional and structural connectivity. Cereb Cortex 22:232–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH (2001): D‐KEFS: Delis–Kaplan Executive Function System Examiner's Manual. San Antonio, TX: Pearson, Inc. [Google Scholar]
- Demonet JF, Thierry G, Cardebat D (2005): Renewal of the neurophysiology of language: Functional neuroimaging. Physiol Rev 85:49–95. [DOI] [PubMed] [Google Scholar]
- den Ouden DB, Saur D, Mader W, Schelter B, Lukic S, Wali E, Timmer J, Thompson CK (2012): Network modulation during complex syntactic processing. Neuroimage 59:815–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dronkers NF, Wilkins DP, Van Valin RDJ, Redfern BB, Jaeger JJ (2004): Lesion analysis of the brain areas involved in language comprehension. Cognition 92:145–177. [DOI] [PubMed] [Google Scholar]
- Dunn LM, Dunn DM (2007): Peabody Picture Vocabulary Test. 4th ed. Wascana Limited Partnership. NCS Pearson, Inc. [Google Scholar]
- Fiori V, Coccia M, Marinelli CV, Vecchi V, Bonifazi S, Ceravolo MG, Provinciali L, Tomaiuolo F, Marangolo P (2011): Transcranial direct current stimulation improves word retrieval in healthy and nonfluent aphasic subjects. J Cogn Neurosci 23:2309–2323. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Makuuchi M, Bahlmann J (2009): The role of the posterior superior temporal cortex in sentence comprehnsion. NeuroReport 20:563–568. [DOI] [PubMed] [Google Scholar]
- Fridriksson J, Morrow‐Odom L, Moser D, Fridriksson A, Baylis G (2006): Neural recruitment associated with anomia treatment in aphasia. Neuroimage 32:1403–1412. [DOI] [PubMed] [Google Scholar]
- Fridriksson J, Moser D, Bonilha L, Morrow‐Odom L, Shaw H, Fridriksson A, Baylis G, Roden C (2007): Neural correlates of physiological and semantic based anomia treatment in aphasia. Neuropsychologia 45:1812–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funke ME, Moore K, Orrison JWW, Lewine JD (2011): The role of magnetoencephalography in “nonlesional” epilepsy. Epilepsia 52:10–14. [DOI] [PubMed] [Google Scholar]
- Gathercole SE, Willis C, Emslie H, Baddeley AD (1991): The influence of number of syllables and wordlikeness on children's repetition of nonwords. Appl Psycholinguist 12:349–367. [Google Scholar]
- Geranmayeh F, Brownsett SLE, Wise RJS (2014): Task‐induced brain activity in aphasic stroke patients: What is driving recovery? Brain 137:2632–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL (2008): Cognitive neuroscience of aging. Ann N Y Acad Sci 1124:127–144. [DOI] [PubMed] [Google Scholar]
- Grady CL, Maisog JM, Horwitz B, Ungerleider LG, Mentis MJ, Salerno JA, Pietrini P, Wagner E, Haxby JV (1994): Age‐related changes in cortical blood flow activation during visual processing of faces and location. J Neurosci 14:1450–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths JD, Marslen‐Wilson WD, Stamatakis EA, Tyler LK (2013): Functional organization of the neural language system: Dorsal and ventral pathways are critical for syntax. Cereb Cortex 23:139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halgren E, Dhond RP, Christiensen N, Van Petten C, Marinkovic K, Lewine DJ, Dale AM (2002): N400‐like magnetoencephalography responses modulated by semantic context, word frequency, and lexical class in sentences. NeuroImage 17:1101–1116. [DOI] [PubMed] [Google Scholar]
- Hamilton RH, Chrysikou EG, Coslett B (2011): Mechanism of aphasia recovery after stroke and the role of noninvasive brain stimulation. Brain Lang 118:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanslmayr S, Staudigl T, Fellner MC (2012): Oscillatory power decreases and long‐term memory: The information via desynchronization hypothesis. Front Hum Neurosci 6:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim S, Friederici AD, Schiller NO, Ruschemeyer SA, Amunts K (2009): The determiner congruency effect in language production investigated with functional MRI. Hum Brain Mapp 30:928–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss W‐D, Kessler J, Thiel A, Ghaemi M, Karbe H (1999): Differential capacity of left and right hemispheric areas for compensation of poststroke aphasia. Ann Neurol 45:430–438. [DOI] [PubMed] [Google Scholar]
- Heiss WD, Thiel A (2006): A proposed regional hierarchy in recovery of post‐stroke aphasia. Brain Lang 98:118–123. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D (2004): Dorsal and ventral streams: A framework for understanding aspects of the functional anatomy of language. Cognition 92:67–99. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D (2007): The cortical organization of speech processing. Nat Rev Neurosci 8:393–402. [DOI] [PubMed] [Google Scholar]
- Hillebrand A, Singh KD, Holliday IE, Furlong PL, Barnes G (2005): A new approach to neuroimaging with magnetoencephalography. Hum Brain Mapp 25:199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges JR, Martinos M, Woollams AM, Patterson K, Adlam ALR (2008): Repeat and point: Differentiating semantic dementia from progressive non‐fluent aphasia. Cortex 44:1265–1270. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Salmon DP, Butters N (1990): Differential impairment of semantic and episodic memory in Alzheimer's and Huntington's diseases: A controlled prospective study. J Neurol Neurosurg Psychiatry 53:1089–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M, Mosher JC, Leah R (1999): A sensor‐weighted overlapping‐sphere head model and exhaustive head model comparison for MEG. Phys Med Biol 44:423–440. [DOI] [PubMed] [Google Scholar]
- Huang JC, Nicholson C, Okada YC (1990): Distortions of magnetic evoked fields and surface potentials by conductivity differences at boundaries in brain tissue. Biophys J 57:1155–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussey EK, Novick JM (2012): The benefits of executive control training and the implications for language processing. Front Psychol 3: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung‐Beeman M (2005): Bilateral brain processes for comprehending natural language. Trends Cogn Sci 9:512–518. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S (2001): Boston Naming Test, 2nd ed Philadelphia: Lippincott Williams & Wilkins. [Google Scholar]
- Kay J, Coltheart M, Lesser R (1992): Psycholinguistic Assessments of Language Processing in Aphasia. Sussex, UK: Psychology Press. [Google Scholar]
- Kemmotsu N, Girard HM, Kucukboyaci NE, McEvoy LK, Hagler JDJ, Dale AM, Halgren E, McDonald CR (2012): Age‐related changes in the neurophysiology of language in adults: Relationship to regional cortical thinning and white matter microstructure. J Neurosci 32:12204–12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A (1982): Western Aphasia Battery. New York: Grune and Stratton. [Google Scholar]
- Kertesz A (2007): Western Aphasia Battery – Revised. San Antonio, TX: Pearson, Inc. [Google Scholar]
- Kielar A, Meltzer JA, Moreno S, Alain C, Bialystok E (2014): Oscillatory responses to semantic and syntactic violations in EEG. J Cogn Neurosci 26:2840–2862. [DOI] [PubMed] [Google Scholar]
- Kielar A, Panamsky L, Links KA, Meltzer JA (2015): Localization of electrophysiological responses to semantic and syntactic anomalies in language comprehension with MEG. Neuroimage 10:507–524. [DOI] [PubMed] [Google Scholar]
- Kim JS, Chung CK (2008): Language lateralization using MEG beta frequency desynchronization during auditory oddball stimulation with one‐syllable words. Neuroimage 42:1499–1507. [DOI] [PubMed] [Google Scholar]
- Kircher TTJ, Brammer M, Andreu NT, Williams SCR, McGuire PK (2001): Engagement of right temporal cortex during processing of linguistic context. Neuropsychologia 39:798–809. [DOI] [PubMed] [Google Scholar]
- Klimesch W (1999): EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res Rev 29:169–195. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR (2007): Neural mechanisms of language comprehension: Challenges to syntax. Brain Res 23–49. (1146): [DOI] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA (1980): Reading senseless sentences: Brain potentials reflect semantic incongruity. Science 207:203–205. [DOI] [PubMed] [Google Scholar]
- Lau EF, Gramfort A, Hämäläinen MS, Kuperberg GR (2013): Automatic semantic facilitation in anterior temporal cortex revealed through multimodal neuroimaging. J Neurosci 33:17174–17181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau EF, Phillips C, Poeppel D (2008): A cortical network for semantics: (De) Constructing the N400. Nat Rev Neurosci 9:920–933. [DOI] [PubMed] [Google Scholar]
- Leach L, Kaplan E, Rewilak D, Richards B, Proulx G‐B (2000): Kaplan Baycrest Neurocognitive Assessment Manual. United States: The Psychological Corporation. [Google Scholar]
- Léger A, Démonet J‐F, Ruff S, Aithamon B, Touyeras B, Puel M, Boulanouar K, Cardebat D (2002): Neural substrates of spoken language rehabilitation in an aphasic patient: An fMRI study. Neuroimage 17:174–183. [DOI] [PubMed] [Google Scholar]
- MacWhinney B, Fromm D, Forbes M, Holland A (2011): AphasiaBank: Methods for studying discourse. Aphasiology 25:1286–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerus S (2013): Language repetition and short‐term memory: An integrative framework. Front Hum Neurosci 7:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manenti R, Brambilla M, Petesi M, Miniussi C, Cotelli M. (2013): Compensatory networks to counteract the effects of ageing on language. Behav Brain Res 249:22–27. [DOI] [PubMed] [Google Scholar]
- Martin PI, Naeser MA, Ho M, Treglia E, Kaplan E, Baker EH, Pascual‐Leone A (2009): Research with transcranial magnetic stimulation in the treatment of aphasia. Curr Neurol Neurosci Rep 9:451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer M, Breitenstein C (2008): Functional imaging studies of treatment‐induced recovery in chronic stroke. Aphasiology 22:1251–1268. [Google Scholar]
- Meinzer M, Obleser J, Flaisch T, Eulitz C, Rockstroh B (2007): Recovery from aphasia as a function of language therapy on an early bilingual patient demonstrated by fMRI. Neuropsychologia 45:1247–1256. [DOI] [PubMed] [Google Scholar]
- Meltzer JA, Braun AR (2011): An EEG‐MEG dissociation between online syntactic comprehension and post hoc reanalysis. Front Hum Neurosci 5:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer JA, Wagage S, Ryder J, Solomon B, Braun AR (2013): Adaptive significance of right hemisphere activation in aphasic language comprehension. Neuropsychologia 51:1248–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M, Friederici AD, von Cramon DY (2000): Neurocognition of auditory sentence comprehension: Event‐related FMRI reveals sensitivity to syntactic violations and task demands. Cogn Brain Res 9:19–33. [DOI] [PubMed] [Google Scholar]
- Moreno S, Bialystok E, Wodniecka Z, Alain C (2010): Conflict resolution in sentence processing by bilinguals. J Neurolinguist 23:564–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso M, Weiller C, Kiebel S, Müller SP, Bülau P, Rijntes M (1999): Training‐induced brain plasticity in aphasia. Brain 122:1781–1790. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Martin PI, Nicholas M, Baker EH, Seekins H, Kobayashi M, Theoret H, Fregni F, Maria‐Tormos J, Kurland J, Doron KW, Pascual‐Leone A (2005): Improved picture naming in chronic aphasia after TMS to part of right Broca's area: An open‐protocol study. Brain Lang 93:95–105. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Martin PI, Treglia E, Ho M, Kaplan E, Bashir S, Hamilton R, Coslett HB, Pascual‐Leone A (2010): Research with rTMS in the treatment of aphasia. Restor Neurol Neurosci 28:511–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H (2005): The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc 63:695–699. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP (2001): Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum Brain Mapp 15:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Osterhout L, Nicol J (1999): On the distinctiveness, independence, and time course of the brain responses to syntactic and semantic anomalies. Lang Cogn Process 14:283–317. [Google Scholar]
- Robertson IH (2014): A right hemisphere role in cognitive reserve. Neurobiol Aging 35:1375–1385. [DOI] [PubMed] [Google Scholar]
- Robinson SE (2004): Localization of event‐related activity by SAM(erf). Neurol Clin Neurophysiol 109. [PubMed] [Google Scholar]
- Rorden C, Karnath HO, Bonilha L (2007): Improving lesion‐symptom mapping. J Cogn Neurosci 19:1081–1088. [DOI] [PubMed] [Google Scholar]
- Saur D, Lange R, Baumgaertner A, Schraknepper V, Willmes K, Rijntjes M, Weiller C (2006): Dynamics of language reorganization after stroke. Brain 129:1371–1384. [DOI] [PubMed] [Google Scholar]
- Sheikh JI, Yesavage JA (1986): Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clin Gerontologist 5:165–173. [Google Scholar]
- Singh KD, Barnes GR, Hillebrand A, Forde EME, Williams AL (2002): Task‐related changes in cortical synchronization are spatially coincident with the hemodynamic response. Neuroimage 16:103–114. [DOI] [PubMed] [Google Scholar]
- Thompson CK (2000): Neuroplasticity: Evidence from aphasia. J Commun Disord 33:357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CK (2010): Neuroplasticity and treatment‐induced recovery of sentence processing in agrammatism. Neuropsychologia 48:3211–3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CK (2011): Northwestern Assessment of Verbs and Sentences. Examiner's Manual. Northwestern University. [Google Scholar]
- Thompson CK, den Ouden D‐B (2008): Neuroimaging and recovery of language in aphasia. Curr Neurol Neurosci Rep 8:475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CK, Weintraub S, Mesulam MM (2011): Northwestern Anagram Test. Examiner's Manual. Northwestern University. [Google Scholar]
- Thompson‐Schill SL, D'Esposito M, Aguirre GK, Farah MJ (1997): Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A re‐evaluation. Proc Natl Acad Sci USA 94:14792–14797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thulborn KR, Carpenter PA, Just MA (1999): Plasticity of language‐related brain function during recovery from stroke. Stroke 30:749–754. [DOI] [PubMed] [Google Scholar]
- Turken AU, Dronkers NF (2011): The neural architecture of the language comprehension network: Converging evidence from lesion and connectivity analyses. Front Syst Neurosci 5:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler LK, Shafto MA, Randall B, Wright P, Marslen‐Wilson W, Stamatakis EA (2010): Preserving syntactic processing across the adult life span: The modulation of the frontotemporal language system in the context of age‐related atrophy. Cereb Cortex 20:352–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler LK, Wright P, Randall B, Marslen‐Wilson WD, Stamatakis EA (2010): Reorganization of syntactic processing following left‐hemisphere brain damage: Does right‐hemisphere activiyty preserve function. Brain 133:3396–3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15:273–289. [DOI] [PubMed] [Google Scholar]
- Vitali P, Abutalebi J, Tettamanti M, Danna M, Ansaldo A‐I, Perani D, Joanette Y, Cappa SF (2007): Training‐induced brain remapping in chronic aphasia: A pilot study. Neurorehabil Neural Repair 21:152–160. [DOI] [PubMed] [Google Scholar]
- Vrba J (2002): Magnetoencephalography: The art of finding a needle in a haystack. Physica C 368:1–9. [Google Scholar]
- Vrba J, Robinson SE (2001): Signal processing in magnetoencephalography. Methods 25:249–271. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Paré‐Blagoev EJ, Clark J, Poldrack RA (2001): Recovering meaning: Left prefrontal cortex guides controlled semantic retrieval. Neuron 31:329–338. [DOI] [PubMed] [Google Scholar]
- Wang L, Jensen O, van den Brink D, Weder N, Schoffelen JM, Magyari L, Hagoort P, Bastiaansen M (2012): Beta oscillations relate to the N400m during language comprehension. Hum Brain Mapp 33:2898–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington EK (1984): Recognition Memory Test. Los Angeles, CA: Western Psychological Sciences. [Google Scholar]
- Wechsler D (2008): Wechsler Adult Intelligence Scale – Fourth Edition: Canadian Manual. Toronto, ON: Pearson, Inc. [Google Scholar]
- Wechsler D (2009): Wechsler Memory Scale – Fourth Edition: Administration and Scoring Manual. San Antonio, TX: Pearson, Inc. [Google Scholar]
- Weintraub S, Mesulam MM, Wieneke C, Rademaker A, Rogalski EJ, Thompson CK (2009): The Northwestern anagram test: Measuring sentence production in primary progressive aphasia. Am J Alzheimers Dis Other Demen 24:408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S (2010): A major revision of the Edinburgh Handedness Inventory; Versions 1.1. Colchester, Essex, United Kingdom
- Winhuisen L, Thiel A, Schumacher B, Kessler J, Rudolf J, Haupt WF, Heiss WD (2007): The right inferior frontal gyrus and poststroke aphasia: A follow‐up investigation. Stroke 38:1286–1292. [DOI] [PubMed] [Google Scholar]
- Wu D, Waller S, Chatterjee A (2007): The functional neuroanatomy of thematic role and locative relational knowledge. J Cogn Neurosci 19:1542–1555. [DOI] [PubMed] [Google Scholar]
- Xing S, Lacey EH, Skipper‐Kallal LM, Jiang X, Harris‐Love M, Zeng J, Turkeltaub PE (2016): Right hemisphere gray matter structure and language outcome in chronic left hemisphere stroke. Brain 139:227–241. [DOI] [PMC free article] [PubMed] [Google Scholar]


