Abstract
Background:
Pregnancy is a sensitive condition during which adverse environmental exposures should be monitored thoroughly and minimized whenever possible. In particular, the hormone balance during gestation is delicate, and disturbance may cause acute or chronic long-term health effects. A potential endocrine disruption may be provoked by in utero exposure to xenoestrogens mimicking endogenous estrogens. The mycoestrogen zearalenone (ZEN), a toxic fungal secondary metabolite and mycotoxin found frequently in food and feed, constitutes a prominent example.
Objectives:
We performed a comprehensive assessment of the transfer as well as phase I and phase II metabolism of ZEN at the human placental barrier.
Methods:
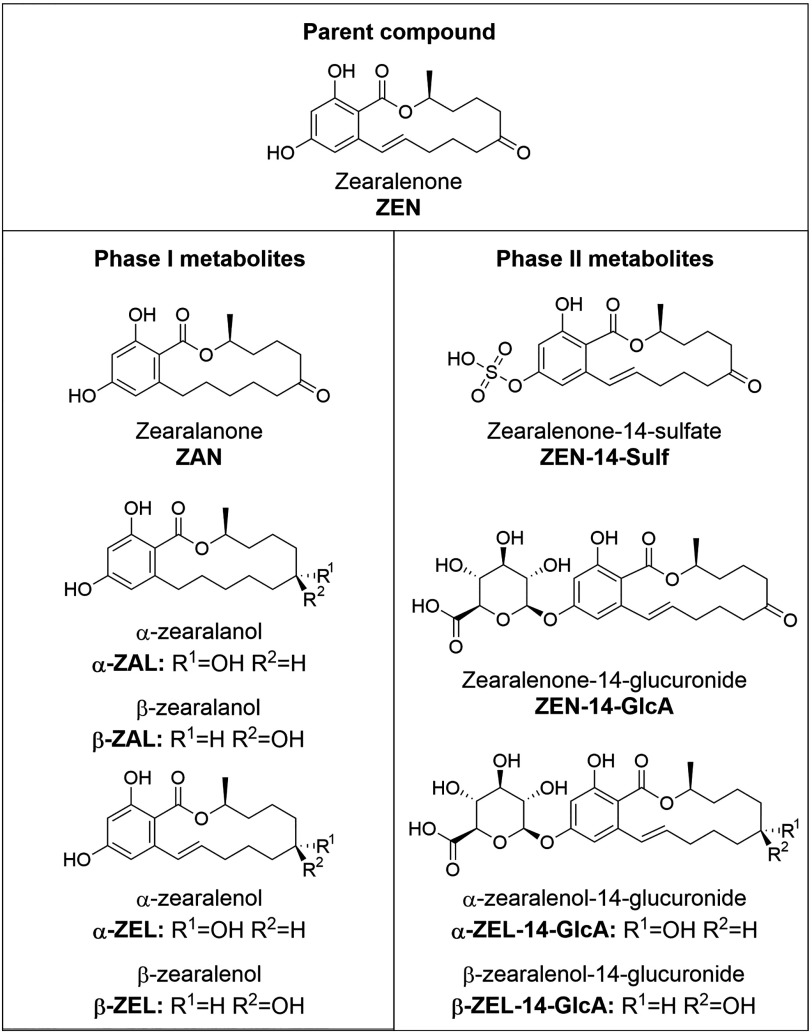
Human placentas were perfused with () ZEN for 6 h. Samples from the maternal and fetal compartment, placental tissue, and fetal plasma were analyzed by a highly sensitive UHPLC-MS/MS assay to detect ZEN as well as nine key metabolites (, , zearalanone, , , ZEN-14-glucuronide, , , ZEN-14-sulfate).
Results:
The model revealed a fast maternofetal transfer of ZEN across the human placental barrier. We also unraveled phase I and phase II metabolism of the parent toxin ZEN into the approximately 70-times more estrogenic and the less active ZEN-14-sulfate conjugate, which are effectively released into the maternal and fetal circulation in considerable amounts.
Conclusions:
Our findings suggest that exposure to ZEN (such as through consumption of ZEN-contaminated cereal-based products) during pregnancy may result in in utero exposure of the fetus, not only to ZEN but also some of its highly estrogenically active metabolites. In the light of the known affinity of ZEN and potentially co-occurring xenoestrogens to the estrogen receptor, and our results demonstrating placental transfer of ZEN and its metabolites in an ex vivo model, we recommend further research and more comprehensive assessment of gestational exposures in women. https://doi.org/10.1289/EHP4860
Introduction
Zearalenone (ZEN) is a fungal toxin (mycotoxin) produced by Fusarium species and is regularly found in cereal-based food and feed in various countries (Maragos 2010; EFSA 2011; Mally et al. 2016). Other sources, such as legume-based food and vegetable oils, were also reported (Schollenberger et al. 2007; Maragos 2010; EFSA 2011). Because this mycotoxin possesses potent estrogenic activity, it is often referred to as xeno- or mycoestrogen (Bennett and Klich 2003; Kowalska et al. 2016; Warth et al. 2018). Humans are frequently exposed to low doses, either directly by ingestion of contaminated food or indirectly by the consumption of livestock that were fed with contaminated chow (Kowalska et al. 2016).
The most prevalent phase I metabolites are and ( and ) and and ( and ) (Miles et al. 1996; Pfeiffer et al. 2011), whereas zearalanone (ZAN) is a metabolite of (Migdalof et al. 1983). The favorably formed metabolite is highly dependent on the species [reviewed in Zinedine et al. (2007)]. Detoxification occurs via phase II metabolism by sulfation and glucuronidation (Migdalof et al. 1983; Miles et al. 1996; Pfeiffer et al. 2011).
The human metabolism of ZEN has been characterized in cell models (Pfeiffer et al. 2010, 2011) as well as in vivo in human intervention studies (Mirocha et al. 1981; Warth et al. 2013). It is mainly metabolized via phase I metabolism to and to a minor extent to and is thereafter glucuronidated. ZEL metabolites were also shown in vitro using Caco-2 cells to conjugate to sulfate metabolites (Pfeiffer et al. 2011). In line with this, Huuskonen et al. (2015) unraveled human placental phase I metabolism of ZEN to and to a minor extent to in chorion carcinoma JEG-3 cells and human term placental subcellular fractions.
ZEN is often referred to as an endocrine-disrupting chemical (EDC) (Kowalska et al. 2016). According to the European Commission, EDCs are “substances that alter the functions of the hormonal system and consequently cause adverse effects” (EC 2018). ZEN and several of its metabolites can bind to estrogen receptors and resemble, therefore, with different potency, the estrogenic properties of endogenous estrogens. was demonstrated to be the most potent structure, being about 70 times more potent than the parent toxin ZEN (Frizzell et al. 2011), followed by and ZEN (Arukwe et al. 1999; Malekinejad et al. 2005; Frizzell et al. 2011; Tatay et al. 2017). Phase II metabolism (glucuronidation and sulfation) reduced estrogenicity in cell models (Jard et al. 2010; Frizzell et al. 2015).
Environmental exposure to molecules mimicking endogenous estrogens, so-called xenoestrogens, may result in various adverse effects in different human organs, including the reproductive tract and the nervous system (Singleton and Khan 2003). Such adverse effects have been reported frequently in animal studies [reviewed in Xu et al. (2017)] but human data are difficult to generate due to the complexity of the endocrine system and the diversity of xenoestrogens, which usually appear at low doses in various mixtures. Moreover, there is growing evidence that exposure to xenoestrogens at early developmental stages can be related to chronic diseases later in life, such as breast cancer and the development of other tumors (Palmlund 1996; Fernandez and Russo 2010; Fucic et al. 2012).
Consequently, the investigation of the in utero and early life exposure to xenoestrogens is of high priority. Different approaches have been developed to study the permeability of the human placental barrier [reviewed in Muoth et al. (2016)]. Among these, the dually perfused ex vivo placental perfusion model provides highly predictive, human-relevant data on the transfer and metabolism of compounds due to the use of intact human placental tissue and dynamic exposure conditions (Panigel et al. 1967; Hutson et al. 2011; Grafmüller et al. 2013; Mathiesen et al. 2014).
The unborn child is particularly responsive to endocrine disruption, and a perturbation of the fragile hormone balance may lead to a maldevelopment of the reproductive system (Palmlund 1996; Saunders et al. 1997; Welshons et al. 1999; Delbès et al. 2006; Reed and Fenton 2013). It has been described that the xenoestrogens bisphenol A (BPA), 4-nonylphenol, and genistein can cross the human placental barrier in ex vivo perfusion studies (Balakrishnan et al. 2010a, 2010b, 2011). Moreover, the detection of estrogenic compounds, such as parabens in umbilical cord plasma (Kolatorova et al. 2018) or nonylphenol and BPA in amniotic fluid (Shekhar et al. 2017) further supports that there is a transfer of these chemicals across the placenta in vivo. Therefore, an unborn child might be at immediate risk of chronic low-dose exposure to a mixture of xenoestrogens. The combination of different estrogenic compounds, even at very low levels, has been demonstrated to potentially result in synergistic adverse effects in cell models (Rajapakse et al. 2002; Vejdovszky et al. 2017).
To date, the ability of mycotoxins to cross the placental barrier in the ex vivo placental perfusion model has been investigated only for the carcinogenic aflatoxin (Partanen et al. 2010), the nephrotoxic ochratoxin A (Woo et al. 2012), and the type-B trichothecene deoxynivalenol (Nielsen et al. 2011), which inhibits protein synthesis. Although aflatoxin and deoxynivalenol can pass through the human placental barrier, ochratoxin A could not in the described experiments. In a rat model, ZEN and its phase I metabolite were shown to be transferred to the fetus (Bernhoft et al. 2001) and cause adverse effects (Gao et al. 2017), but human data is still lacking to date. In addition, ZEN conversion to all of its potential metabolites has not yet been systematically investigated in an advanced human placenta model. Verification of ZEN translocation and metabolism in a predictive human placenta model is urgently needed, considering the significant species-specific differences in placental development, structure, function, and pathology (Enders and Blankenship 1999; Malassine et al. 2003; Schmidt et al. 2015). Important considerations are that the mouse and rat placenta does not produce estrogens (aromatase-negative), and the level of circulating estrogens is much lower than in human pregnancy, resulting in different pharmacokinetics and metabolic profiles (Witorsch 2002; Al-Bader 2006). Therefore, the primary aims of this study were to a) determine the permeability of the human placental barrier in respect to ZEN and b) to investigate the human placental metabolism of ZEN that might lead to biotransformation products exhibiting enhanced estrogenic activity.
Material and Methods
Chemicals and Reagents
Liquid chromatography–mass spectrometry (LC-MS)-grade acetonitrile (ACN) and methanol (MeOH) were obtained from Sigma-Aldrich, and water (, LC-MS grade) was purchased from VWR International GmbH. Ammonium fluoride (LC-MS grade) was obtained from Honeywell Fluka. ZEN, , , , , and were obtained from either Romerlabs or Sigma-Aldrich, whereas ZEN-14-GlcA, , and were synthesized at the Technical University of Vienna and kindly provided by Dr. Mikula (Mikula et al. 2012, 2013). ZEN-14-Sulf was a kind gift from Prof. Berthiller from the University of Natural Resources and Life Sciences, Vienna (Vendl et al. 2010). Antipyrine was obtained from Sigma-Aldrich. All reference standards were dissolved in ACN. ZEN and its metabolites were combined manually to a multianalyte calibration solution (each analyte at a concentration of ). All single and combined analytes were stored at . ZEN for transfusion experiments was obtained from Toronto Research Chemicals (purity 98.9%).
Ex Vivo Placental Perfusion
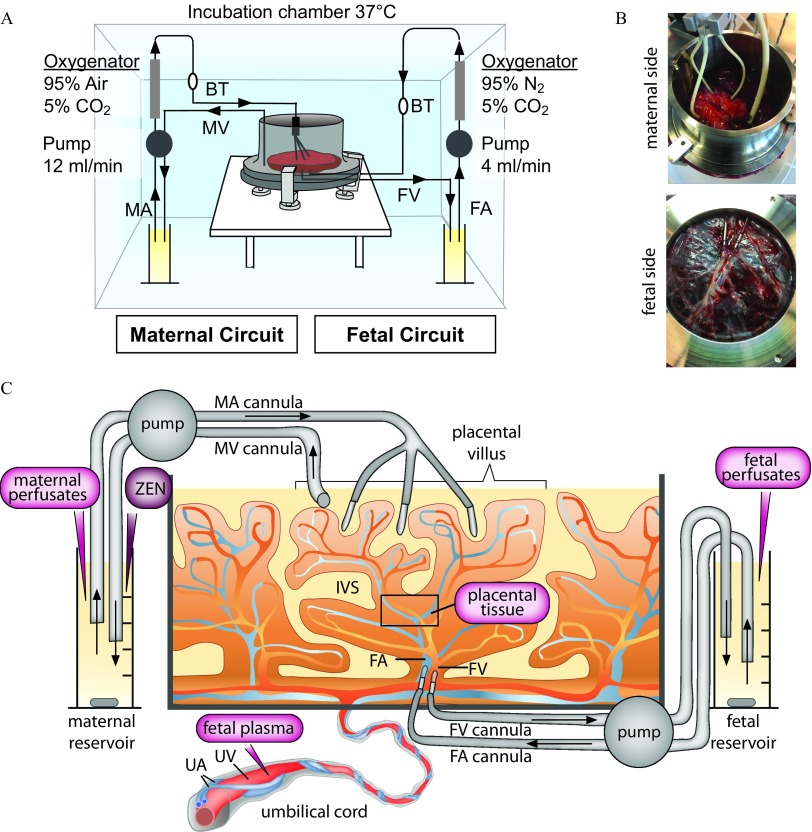
In total, six placentas were obtained from uncomplicated pregnancies after caesarean section from the Kantonsspital St. Gallen and Hirslanden Klinik Stephanshorn St. Gallen with written informed consent from the expecting mothers. The study was approved by the local ethics committee (EKOS 10/078; PB-2018-00,069) and performed according to the principles of the Declaration of Helsinki. The recirculating dually perfused ex vivo placental perfusion model was performed as previously described with some modifications (Figure 1) (Grafmüller et al. 2013; Grafmueller et al. 2015). Briefly, a fetal artery–vein pair of an intact cotyledon was cannulated, and the placenta was placed into a perfusion chamber with the maternal side up. To connect the maternal side, three blunt cannulas were gently inserted in the intervillous space, and a venous drain was introduced to return the fluid to the maternal circulation. Perfusion was achieved with two peristaltic pumps at for fetal flow and for maternal flow. Oxygenators were applied to maintain gas compositions of 95% /5% at the fetal side and 95% synthetic air/5% at the maternal side. All components of the perfusion system were kept in perfusion chamber at 37°C. To flush out the blood and allow recovery of the tissue from the ischemic period after the delivery, the cotyledon was perfused for 20 min with perfusion medium. The perfusion medium was M199 tissue culture medium, which was diluted with Earl’s buffer (1:2) and further supplemented with glucose (), bovine serum albumin (BSA; ), dextran 40 (), sodium heparin [], amoxicillin (), and sodium bicarbonate (; medium and all supplements were obtained from Sigma-Aldrich). After the recovery phase, the medium was replaced by fresh medium in the presence or absence of ZEN. In total, six placentas from six different women were perfused. Three placentas were perfused with perfusion medium without addition of ZEN to obtain baseline data. In the other three perfusions, ZEN was added to the maternal circulation at a final concentration of , corresponding to . The concentration was chosen to be in a nontoxic range [nontoxic on human choriocarcinoma cells (BeWo)] up to (Prouillac et al. 2009, 2012) but high enough to enable quantitative measurements also of minor biotransformation products. Concentrations of ZEN in maternal blood during pregnancy are not reported to date to the best of our knowledge. However, based on available exposure data and current legislation [in the EU EC (2007); EFSA (2011)] we expect chronic low-dose exposure to be frequent.
Figure 1.
Ex vivo dually perfused human placenta perfusion model. (A) Scheme of the perfusion system. (B) Photograph showing cannulation from the maternal and fetal side, respectively. (C) Scheme with details of maternal and fetal side cannulation of an intact cotyledon and sampling sites (magenta). Fetal plasma is isolated from the umbilical vein blood before perfusion. ZEN is introduced to the maternal reservoir at the start of perfusion. Maternal and fetal perfusates are sampled from the corresponding reservoirs at different time points during perfusion. Placental tissue (black quadrant) is taken from the intervillous region of a perfused cotyledon at the end of perfusion. Note: BT, bubble trap; FA, fetal artery; FV, fetal vein; IVS, intervillous space (maternal blood space); MA, maternal artery; MV, maternal vein; UA, umbilical artery; UV, umbilical vein.
Criteria for a successful perfusion were: a) the pre-perfusion of the tissue showed no leakage; b) the leakage (fetal to maternal) was less than during the translocation experiment; and c) the pH remained constant during the experiment (7.2–7.4). Moreover, the passive diffusion reference markers antipyrine and creatinine were added to all perfusions (final concentration of in maternal reservoir) to ensure sufficient overlap between maternal and fetal circulation.
Samples () from the maternal and fetal circulations (referred to as maternal and fetal perfusates) were collected at 0, 0.25, 0.5, 1, 2, 3, 4, 5, and 6 h of perfusion and analyzed immediately for pH, creatinine, glucose, and lactate using a blood gas analyzer (epoc® Blood Analysis system with BGEM test cards; Epocal Inc.). One of the control perfusions (medium without addition of ZEN) showed a slight leakage after 5 h of perfusion and was therefore stopped at that time point. Afterwards, supernatants were stored at for further UHPLC-MS/MS analysis. Placental tissue samples from the intervillous region were taken before (from a nonperfused neighboring cotyledon) and after perfusion and stored at for UHPLC-MS/MS analysis. Fetal plasma was isolated from umbilical vein blood by centrifugation [, 10 min, in S-Monovette with Li-Heparin (Sarstedt)] and stored at for later UHPLC-MS/MS analysis.
Sample Preparation
A volume of of maternal and fetal perfusates and fetal plasma, respectively, was spiked with of internal standard (IS) solution (), vortexed, mixed with MeOH/ACN (1/1), and thereafter sonicated for 10 min in an ice bath. To assist protein precipitation, the samples were stored at for 1 h and subsequently centrifuged at at for 15 min. The supernatant was evaporated at with a CentriVap Vacuum concentrator (Labconco) and resolved with (1/9). The samples were centrifuged for 5 min (, ), and the supernatant was transferred into a glass vial containing an insert. All steps were performed on ice.
Placental tissue samples were cut into pieces of approximately . In addition, 20 times the volume (4/4/2) was added as extraction solvent. Ceramic beads were added, and homogenization was performed with a Fast Prep Homogenizer (MP Biomedicals). Four cycles ( for 20 s; 120 s break) were conducted. After 10 min of sonication, the samples were centrifuged at at . of supernatant was spiked with of IS solution ( ), vortexed and put at for one hour. After 15 min of centrifugation at and , the supernatant was evaporated at with a CentriVap Vacuum concentrator and resolved in (1/9). The samples were centrifuged for 5 min (, ) and the supernatant transferred into a glass vial containing an insert. All steps were performed on ice.
LC-MS/MS Measurements
Measurements were performed utilizing a Dionex Ultimate 3000 UHPLC coupled to a TSQ Vantage triple quadrupole mass spectrometer (Thermo Scientific) as described in detail by Preindl et al. (2019). In brief, an Acquity HSS T3 column (, ) equipped with a VanGuard precolumn (; Waters Corporation) served for chromatographic separation. As mobile phases, water with ammonium fluoride as additive (A) and acetonitrile (B) were used at a flow rate of . Aqueous ammonium fluoride solutions have to be handled with care, because hydrogen fluoride may outgas. A stock solution of was prepared and stored at . The final concentration in the eluent was very low; however, contact with acid (e.g., in the waste bottle) was prevented for safety reasons. Separation was achieved by the following gradient: 0–1 min: 5% B; rise to 18% B until min 1.8; rise to 35% B until min 4.2; rise to 48% B until min 13; rise to 90% B until min 14; flush with 98% B from min 15.8 to min 17.6; re-equilibrate with 5% B from min 17.7 to min 20. The column compartment was operated at and the sample tray cooled to . The multiple reaction monitoring (MRM) experiments were performed in negative electrospray ionization (ESI) mode, using fast polarity switching. Electrospray settings were set as follows: spray voltage , vaporizer temperature , sheath gas pressure , ion sweep gas pressure , aux gas pressure and capillary temperature .
To account for matrix effects and potential retention time deviations in the different analyzed matrices, matrix-matched reference standards were prepared by resolving evaporated blank extractions of perfusion medium [pooled aliquots of the three control perfusions without ZEN (min 0, 120, and end of perfusion)], fetal plasma (pooled fetal plasma from six distinct experiments) and placental tissue (pooled tissue from six distinct placentas) with of standard level solutions (nine-point calibration, to in 1/9 ). ZEN was quantified using the IS. To determine extraction recoveries, blank extractions of perfusion medium [pooled aliquots of the three control perfusions without ZEN (min 0, 120, and end of perfusion)] and fetal plasma (pooled fetal plasma from six distinct experiments) were spiked in triplicate at before extraction and sample preparation. Placental tissue (pooled tissue from six distinct placentas) was spiked in triplicate at a concentration of post homogenization. For control measurements of antipyrine, a separate reference standard was dissolved in pure solvent at different concentrations for external calibration purpose (10 to in 1/9 ).
Data Processing and Statistics
Data evaluation and quantification was performed with the Xcalibur and TraceFinder software packages (version 4.1, Thermo Scientific). Limit of detection (LOD) and limit of quantification (LOQ) were estimated for each matrix in spiked blank matrix samples by a signal-to-noise ratio of three and ten, respectively. All results from medium-derived samples were corrected by the calculated analyte-specific recovery values.
Data regarding ZEN and its metabolites represent (SD) of three independent placentas, perfused with medium containing ZEN. Perfusion data comparing maternal and fetal perfusate concentrations were analyzed by unpaired Student’s t-test. Differences were considered statistically significant at . The percentages of ZEN metabolized to and ZEN-14-Sulf were calculated by the following formula: , where M and F is the amount of the metabolite ( or ZEN-14-Sulf) in the maternal and fetal circulation at the end of perfusion, T is the amount of metabolite in the placental tissue after perfusion, S is the amount of metabolites present in the samples being collected during perfusion, and is the amount of ZEN added to the maternal circulation at the beginning of perfusion. The percentages of ZEN released into the fetal or maternal circulation or present in placental tissue were calculated similarly as described above (% maternal: ; (% fetal: ; (% tissue: ).
Results
Analytical Performance
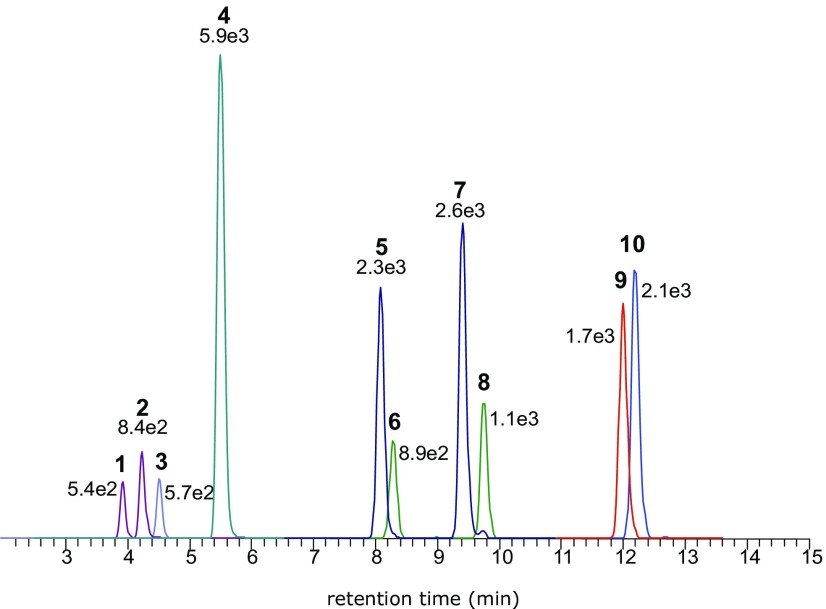
A highly sensitive and specific UHPLC-MS/MS multimethod for ZEN and nine of its key metabolites (chemical structures are depicted in Figure 2) was established for quantification. As demonstrated in Figure 3 and S1, we were able to achieve baseline chromatographic separations and desirable peak shapes despite the high structural similarity. MRM transitions and retention times of the measured analytes are summarized in Table 1. Estimated LOD values in perfusion medium ranged from 0.05 to , whereas in fetal plasma and placental tissue they ranged from 0.05 to and from 0.6 to , respectively (Table 1). Extraction recoveries as obtained from the spiking experiments were above 82% for placental tissue and plasma samples and 74% for the perfusion medium, respectively (Table S1).
Figure 2.
Chemical structures of ZEN and its metabolites investigated in this study.
Figure 3.
Multiple reaction monitoring (MRM) chromatograms of reference standards spiked into blank medium. () m/z (1); () m/z (2); zearalenone-14-glucuronide (ZEN-14-GlcA) m/z (3); zearalenone-14-sulfate (ZEN-14-Sulf) m/z (4); () m/z (5); () m/z (6); () m/z (7); () m/z (8); zearalanone (ZAN) m/z (9); zearalenone (ZEN) m/z (10); stated numbers represent the intensity arbitrary units (a.u.).
Table 1.
Chromatographic and mass spectrometric parameters of the assessed analytes.
| Analyte | Retention time (min) | Parent ion | Ion species | Quantifier ion | Qualifier ion | Ionization mode | Placental tissue (ng/g) | Fetal plasma () | Perfusion medium () | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LOD | LOQ | LOD | LOQ | LOD | LOQ | |||||||
| 3.82–4.03a | 495 | 319.3 | 175 | Negative | 2 | 6 | 5 | 15 | 1 | 1.5 | ||
| 4.10–4.35a | 495 | 319.3 | 175 | Negative | 6 | 6 | 5 | 5 | 0.5 | 1.5 | ||
| ZEN-14-GlcA | 4.39–4.64a | 493.2 | 317.2 | 175.1 | Negative | 6 | 20 | 5 | 15 | 1.5 | 5 | |
| ZEN-14-Sulf | 5.36–5.57a | 397.1 | 317.2 | 175.1 | Negative | 2 | 6 | 0.15 | 0.5 | 0.15 | 0.5 | |
| 8.08 | 321.2 | 277.2 | 303.2 | Negative | 2 | 2 | 0.15 | 0.5 | 0.5 | 1.5 | ||
| 8.32 | 319.2 | 275.2 | 160.1 | Negative | 2 | 2 | 0.5 | 1.5 | 0.5 | 1.5 | ||
| 9.40 | 321.2 | 277.2 | 303.2 | Negative | 2 | 2 | 0.15 | 0.5 | 0.5 | 0.5 | ||
| 9.74 | 319.2 | 275.2 | 160.1 | Negative | 2 | 6 | 0.5 | 1.5 | 0.5 | 1.5 | ||
| ZAN | 12.00 | 319.2 | 275.2 | 205.2 | Negative | 2 | 2 | 0.15 | 0.5 | 0.15 | 0.5 | |
| ZEN | 12.18 | 317 | 175.0 | 131.0 | Negative | 0.6 | 2 | 0.05 | 0.15 | 0.05 | 0.15 | |
| (IS) | 12.18 | 335 | 185.0 | — | Negative | |||||||
Note: —, indicate data not available, Multiple reaction monitoring (MRM) transitions, retention times and limit of detections (LOD), limit of quantifications (LOQ) of tested matrices. Zearalenone (ZEN), ZEN-14-glucuronide (ZEN-14-GlcA), ZEN-14-sulfate (ZEN-14-Sulf), (); (), (), zearalanone (ZAN); IS, Internal Standard.
Minor deviations in different matrices.
ZEN and Metabolite Levels in Placental Tissue and Fetal Plasma
To understand potential background levels of ZEN and its metabolites present in the placental tissue or introduced during the perfusion process, we determined their concentrations in three control perfusion experiments (placentas perfused with perfusion medium without addition of ZEN) from maternal and fetal perfusate as well as placental tissue samples taken before and after 6 h of perfusion (Table S1). In each experiment (all six perfused placentas), placental tissues and perfusates were assayed for ZEN and nine metabolites before the experimental addition of ZEN. In all these measurements, ZEN and the nine metabolites were not detected. Moreover, these compounds were also absent in fetal vein plasma from any of the six placentas investigated.
Human Placental Metabolism of ZEN
The human placenta expresses a wealth of enzymatic machinery responsible for both phase I and phase II reactions (Prouillac and Lecoeur 2010). Therefore, we explored the biotransformation of ZEN into previously described metabolites in the human ex vivo placental perfusion model as well as the bi-directional release of the metabolites into the maternal and fetal circulation.
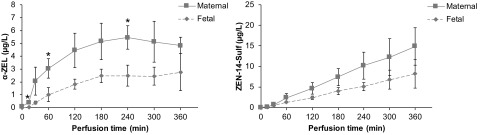
After 6 h of perfusion with ZEN, only the parent compound ZEN and the metabolites and ZEN-14-Sulf were detected in the placental tissue at concentrations of tissue, tissue, and tissue, respectively. Although ZEN standard material of highest quality was purchased (98.9% purity), our highly sensitive UHPLC-MS/MS method revealed that this material contained trace amounts of the metabolites ZAN and (each about 1% of the applied ZEN concentration) (Table S2). Although placental tissue concentrations of ZAN were below LOQ, a small amount of tissue was detected after 6 h of perfusion with ZEN. Release of the newly formed metabolites and ZEN-14-Sulf into the maternal and fetal circulation was observed already within the first 15 min of perfusion (Figure 4, Table S1). For , a plateau was reached after 3 h of perfusion with approximately double the concentrations in the maternal in comparison with the fetal compartment ( and at 6 h of perfusion, respectively) (Figure 4). In contrast, ZEN-14-Sulf concentrations showed a linear increase to in the maternal and in the fetal circulation at 6 h of perfusion (Figure 4, Table S1). Overall, ∼4 and 8% respectively of the introduced ZEN was metabolized to and ZEN-14-Sulf and released into the maternal and fetal circulation at the end of the perfusion studies, whereas no significant metabolism to ZAN, , , ZEN-14-GlcA, or was observed.
Figure 4.
Perfusion profiles of () and zearalenone-14-sulfate (ZEN-14-Sulf). Release of and ZEN-14-Sulf to the maternal and fetal circulation during human placental perfusion with zearalenone (ZEN) over 6 h. Data represent of three independent placentas perfused with medium containing ZEN. Note: is considered statistically significant (* denotes differences between maternal and fetal concentrations in perfusions). Perfusion data comparing maternal and fetal concentrations were analyzed by unpaired Student's t-test.
Human Placental Transfer of ZEN
To investigate if ZEN can cross the human placental barrier and reach the fetal circulation, we applied the dually perfused ex vivo placental perfusion model that is extensively used to assess the transplacental transfer of compounds and delivers highly predictive results (Hutson et al. 2011).
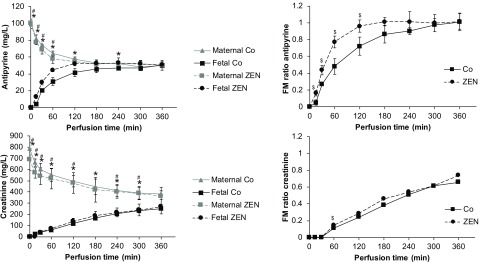
In all placental perfusion experiments (three control perfusions without addition of ZEN and three perfusions with addition of ZEN), two reference compounds with fast or slow passive diffusion kinetics, namely antipyrine and creatinine, were added to ensure sufficient overlap between the maternal and fetal circulation and to normalize the translocation data. Antipyrine showed a rapid translocation to the fetal circulation similar to those reported in previous publications (Wick et al. 2010; Nielsen et al. 2011; Woo et al. 2012; Grafmueller et al. 2015) (Figure 5). In contrast, transplacental transfer of creatinine was slower and did not reach an equilibrium within 6 h of perfusion (Figure 5). Addition of ZEN did not alter the transplacental transfer kinetics of creatinine but slightly increased the transfer of antipyrine (Figure 5).
Figure 5.
Perfusion profiles and fetal–maternal (FM) ratio of the reference compounds antipyrine and creatinine. Antipyrine () and creatinine () were added to the maternal circulation in six independently perfused placentas from six different mothers (three placentas were perfused with medium without zearalenone to obtain baseline data (Co) and three placentas were perfused with medium containing ZEN). The concentrations of both reference compounds were measured from the maternal and fetal perfusates by UHPLC-MS/MS at several time points during 6 h of perfusion. Data represent of three independently perfused placentas [three control perfusions without ZEN (Co) and three perfusions with addition of ZEN (ZEN)]. is considered statistically significant (* and # denote differences between maternal and fetal concentrations in Co and ZEN perfusions, respectively; $ denotes differences in FM ratio between Co and ZEN). Perfusion data comparing maternal and fetal concentrations were analyzed by unpaired Student's t-test.
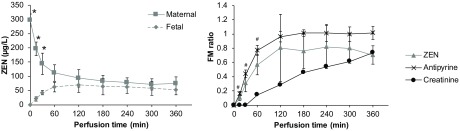
After addition of ZEN () to the maternal circulation, its levels rapidly decreased in the maternal compartment and increased in the fetal compartment to a concentration of after 6 h of perfusion (Figure 6, Table S1). Translocation of ZEN across the placental barrier was only slightly slower than the reference compound antipyrine but substantially faster than creatinine as indicated by the fetal/maternal (FM) ratios (Figure 6). At the end of perfusion, of the initially added ZEN was present in the fetal circulation, in the maternal circulation, and in the cotyledon. The rest of ZEN was metabolized to and ZEN-14-Sulf, and usually a small amount of the perfused substance will diffuse into the tissue surrounding the perfused cotyledon (Frederiksen et al. 2010) or bind to the perfusion system (Mose et al. 2008; Aengenheister et al. 2018).
Figure 6.
Perfusion profile and fetal–maternal (FM) ratio of zearalenone (ZEN). ZEN () was added to the maternal circulation and its concentration was measured from the maternal and fetal perfusates by LC-MS/MS at several time points during 6 h of perfusion. FM ratios were calculated for each time point and FM ratios of antipyrine and creatinine were added for comparison. Data represent of three independent placentas, perfused with medium containing ZEN. is considered statistically significant (* denotes differences between maternal and fetal concentrations in ZEN perfusions; # denotes differences in FM ratio between ZEN and creatinine). Perfusion data comparing maternal and fetal concentrations were analyzed by unpaired Student's t-test.
Due to the above-mentioned minimal contamination of ZEN with ZAN and , we could additionally obtain placental transfer data of these two compounds. Transfer of ZAN and across the placental barrier was observed, but due to the low initial amount in the maternal circulation (), the deviations of the individual measurements were relatively large (Figure S2).
Discussion
To the best of our knowledge, we report the first examination of the human placental transfer and metabolism of ZEN in an ex vivo human placental perfusion model and determined a placental transfer of ZEN at only a slightly slower pace than the highly diffusible reference compound antipyrine. Additionally, we showed that the human placenta is capable of phase I and II metabolism of ZEN to and ZEN-14-Sulf. The metabolites were quickly released into the fetal and maternal circulation with a preferential release toward the maternal circulation. Our findings are in good agreement with previous results from pregnant rats fed with ZEN in mid and end gestation (Bernhoft et al. 2001). In contrast, no placental transfer of ZEN was observed upon exposure of mice in early pregnancy (Appelgren et al. 1982). Therefore, placental metabolism and transfer of ZEN may be different in early human pregnancy as well, when the placental barrier is much thicker to protect the developing fetus. However, this possibility is difficult to investigate because human placental perfusion studies are limited to term placentas.
For our studies, we applied a high but noncytotoxic concentration of ZEN (; ) based on previous in vitro studies showing that ZEN as well as and were not cytotoxic up to a concentration of to BeWo cells after 48 h of exposure (Prouillac et al. 2009, 2012). However, ZEN and its metabolites might induce subtoxic effects to the placental tissue. For instance, ZEN () has been shown to induce trophoblast differentiation and alter ABC transporter gene expression in BeWo trophoblast cells (Prouillac et al. 2009). In contrast, its reduced metabolites were not able to induce trophoblast differentiation but did again modulate ABC transporter gene expression (Prouillac et al. 2012). Additionally, ABC transporters were altered in placentas of pregnant rats exposed to ZEN (Gao et al. 2017). An interesting aspect is that we also observed a potential impact of ZEN on human placental permeability in our ex vivo placental perfusion experiments. Translocation of the passive transcellular diffusion marker antipyrine was increased in the presence of ZEN, suggesting that ZEN may interfere with placental transfer of essential endogenous compounds or decrease barrier function toward xenobiotic substances. However, ZEN did not affect paracellular transport because it did not alter the translocation of the passive paracellular diffusion marker creatinine. Another explanation for how small amounts of xenoestrogens could affect fetal health is by disturbance of endocrine signaling pathways. In pregnancy, endogenous estrogen levels are very high in the maternal circulation as a consequence of placental estrogen production and preferential release toward the maternal circulation [reviewed in Kaludjerovic and Ward (2012)]. However, the high maternal estrogen plasma levels appear to be shielded from the embryo by the placenta as shown in rhesus monkeys (Slikker et al. 1982) and through fetal sequestering by alpha-fetoprotein, protecting the developing embryo from the bulk of maternal circulating estrogens [reviewed in Bondesson et al. (2015)]. Therefore, in light of the lower fetal estrogen levels, maternofetal translocation of xenoestrogens may significantly contribute to fetal estrogen levels and interfere with the fetomaternal endocrine system that regulates estrogen production during development. Evidence exists that a decrease in placental estrogen production during sensitive stages of development may have the potential to alter gene transcription and DNA methylation patterns of cells, suggesting that environmental estrogens may be able to induce epigenetic changes in the developing fetus [reviewed in Kaludjerovic and Ward (2012)].
In humans, ZEN has been shown to be predominately metabolized into (major phase I metabolite) and to a minor extent also to and thereafter glucuronidated. These forms are then, together with free and glucuronidated ZEN, excreted via urine (Mirocha et al. 1981; Warth et al. 2013). Until now, to the best of our knowledge, human sulfation pathways have been reported only in epithelial colorectal adenocarcinoma cells (Caco-2) (Pfeiffer et al. 2011).
Researchers have proposed that hydroxylation of ZEN is mediated through 3 and 3 hydroxysteroid dehydrogenases (HSD) (Olsen et al. 1981; Malekinejad et al. 2005). The enzyme 3 has been shown to be highly expressed in the human placenta (Mason et al. 1993). Previous studies in cell models revealed that the human placenta is likely capable of metabolizing ZEN and ZAN via phase I metabolism into their reduced forms (Huuskonen et al. 2015). We could partly confirm this data in the ex vivo perfusion model. Although ∼4% of the introduced ZEN was reduced to after 360 min of perfusion, a significant metabolism to was not observed. The placental metabolism of ZEN into is of particular relevance due to its far higher relative estrogenic potency in comparison with the parent compound (Arukwe et al. 1999; Frizzell et al. 2011; Tatay et al. 2017).
We further found phase II metabolism of ZEN to ZEN-14-Sulf, to the best of our knowledge for the first time in human placenta; ∼8% of ZEN introduced to the test system was converted to this metabolite after 360 min of perfusion. ZEN-14-Sulf has been described as fungal ZEN conjugate (Binder et al. 2017); however, as mentioned before, it can also be produced by human epithelial colorectal adenocarcinoma cells (Pfeiffer et al. 2011) in vitro. Because , , ZAN, , and carry the same 14-hydroxyl group as ZEN, we also expected possible sulfation of these compounds (in our system especially sulfation of ) but could not verify this hypothesis due to a lack of appropriate standard material. The placenta is known to require sulfate for sulfation of endogenous estrogens and thyroid hormones. The sulfate supply for the fetus is also mediated through the placenta. Reduced availability of sulfate due to detoxification processes may therefore result in a placental phenotype and disturbed fetal development (Dawson et al. 2017).
Human glucuronidation of ZEN and its reduced metabolites is mediated by UDP-glucuronosyltransferases (UGTs), mainly by UGT1A1, 1A3, and 1A8 in the liver, intestine, and eventually also in other organs. These UGTs preferably glucuronidate the unhindered 14-hydroxyl moiety of ZEN and its phase I metabolites (Pfeiffer et al. 2010). It has been shown before, that the human placenta expresses UGT2B isoforms (Collier et al. 2002). For placental UGT1A isoform expression conflicting results are reported, but evidence is growing that UGT1A1, 1A4, and 1A6 are expressed in healthy placentas (Collier et al. 2002; Reimers et al. 2011; Collier et al. 2015). From the principal potential of the placenta to express UGT1A1, it can be assumed that ZEN and its metabolites might be glucuronidated also in our placental perfusion model. However, this assumption was not confirmed in this study. A possible explanation could be that the glucuronidation potential of the placenta may be far lower than its sulfation potential, and consequently, the glucuronides are produced in such a low concentration that detection by means of the applied analytical method was not feasible.
ZEN and its nine analyzed metabolites were detected neither in placental tissue previous to the perfusion nor in fetal plasma obtained from a fetal placental vein, indicating that ZEN did not accumulate in the fetal circulation during the pregnancies of these mothers. However, we point out that a relatively low number of placentas and corresponding fetal plasma samples () were examined, and the mothers were probably fasting due to planned caesarian sections. Therefore, previously ingested ZEN might have been cleared already because ZEN elimination has been shown to be nearly completed within 3 d in various mammals (Ueno et al. 1977; Mirocha et al. 1981).
Despite some obvious strengths of the ex vivo perfusion model, such as the use of a complex and highly functional human tissue in dynamic conditions and its accessibility for mechanistic studies, there are certain limitations to this approach. One limitation is the restriction of the perfusion duration to approximately 6 h. However, this duration was sufficient to detect placental metabolism of ZEN to and ZEN-14-sulfate as well as maternofetal transfer of ZEN with a plateau already at 3 h of perfusion. Another drawback is the use of term placenta, which does not provide insights on placental transfer and metabolism at earlier stages of pregnancy. Moreover, the throughput of this method is limited, yet we obtained highly consistent results from three independent perfusion studies. Finally, due to the absence of maternal and fetal tissues, kinetic and metabolic data reflect those of the isolated human placental tissue only and may not be fully representative of the physiological situation in pregnant women. An analytical limitation of the study is the targeted approach applied for the identification of ZEN metabolites which takes only known biotransformation products into account. Future work may exploit an isotope-based metabolomic workflow (Bueschl et al. 2017) to understand the full extent of ZEN metabolism in an untargeted manner. Finally, researchers need to confirm whether similar findings can be achieved with lower ZEN doses, although this will likely result in a lower number of identified metabolites due to limitations in sensitivity even when applying highly sensitive tandem mass-spectrometry–based assays.
A nonbalanced diet among pregnant women may increase the chance that the fetus is exposed to higher levels of these mycoestrogens, and more emphasis should be placed on accurate exposure assessment via human biomonitoring of ZEN and its key metabolites throughout pregnancy.
ZEN and some of its metabolites have been frequently detected in human urine from various countries at low doses. For example, ZEN, , and were found in urine samples from Germany (Ali and Degen 2018), southern Italy (Solfrizzo et al. 2014), and Nigeria (Šarkanj et al. 2018) at median concentrations of 0.04, 0.056, and (ZEN), 0.23, 0.074, and (), 0.03, 0.088, and (), respectively. A study on New Jersey girls revealed urinary ZEN (median ), (median ), (median ), and (median ) (Bandera et al. 2011). Further urinary biomonitoring studies were summarized by Mally et al. (2016). The few biomonitoring studies investigating blood revealed ZEN levels typically below (Massart et al. 2008; Sun et al. 2019).
In addition, these natural contaminants likely contribute significantly to the overall xenoestrogen exposure that unborn fetuses may face. Elevated in utero exposure to endogenous estrogens or xenoestrogens has been shown to disturb the development of the reproductive system of the fetus in human and animals (Palmlund 1996; Saunders et al. 1997; Welshons et al. 1999; Delbès et al. 2006). In pigs, a decreased fetus and uterus weight (Etienne and Jemmali 1982) as well as a decreased number of fetuses were observed upon maternal ingestion of zearalenone (Young et al. 1990). In mice, exposure to ZEN in early pregnancy caused delayed implantation, loss of conceptuses, and a decreased growth of the fetuses (Kunishige et al. 2017). In addition, environmental exposure to xenoestrogens like BPA may be related to the carcinogenesis of breast cancer and various other tumors (Fernandez and Russo 2010; Fucic et al. 2012).
Conclusions
We revealed in an ex vivo human placental perfusion model the fast transfer of ZEN across the human placental barrier as well as phase I and phase II metabolism of the parent toxin ZEN into the highly estrogenic and the less active ZEN-14-sulfate. Our study will help to further evaluate the risks of an in utero exposure to xenoestrogens and highlights the need for a more comprehensive assessment of exposure and combinatory toxicological effects in the context of the exposome paradigm (Dennis et al. 2017; Warth et al. 2017).
Supplementary Material
Acknowledgments
The authors want to acknowledge all participants of the study. Moreover, we would like to extend our gratitude towards D. Braun (University of Vienna) for skillful technical support and critical discussions. We further thank the staff of the Mass Spectrometry Centre of the University of Vienna, where LC-MS/MS measurements have been performed and all collaborators who kindly contributed reference standards. This work was financed by the University of Vienna and Empa.
Footnotes
Supplemental Material is available online (https://doi.org/10.1289/EHP4860).
Co-first authorship.
The authors declare they have no actual or potential competing financial interests.
Note to readers with disabilities: EHP strives to ensure that all journal content is accessible to all readers. However, some figures and Supplemental Material published in EHP articles may not conform to 508 standards due to the complexity of the information being presented. If you need assistance accessing journal content, please contact ehponline@niehs.nih.gov. Our staff will work with you to assess and meet your accessibility needs within 3 working days.
References
- Aengenheister L, Dietrich D, Sadeghpour A, Manser P, Diener L, Wichser A, Karst U, Wick P, Buerki-Thurnherr T. 2018. Gold nanoparticle distribution in advanced in vitro and ex vivo human placental barrier models. J Nanobiotechnology 16(1):79, PMID: 30309365, 10.1186/s12951-018-0406-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Bader MD. 2006. Estrogen receptors alpha and beta in rat placenta: detection by RT-PCR, real time PCR and Western blotting. Reprod Biol Endocrinol 4:13, 10.1186/1477-7827-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali N, Degen GH. 2018. Urinary biomarkers of exposure to the mycoestrogen zearalenone and its modified forms in German adults. Arch Toxicol 92(8):2691–2700, PMID: 29980802, 10.1007/s00204-018-2261-5. [DOI] [PubMed] [Google Scholar]
- Appelgren LE, Arora RG, Larsson P. 1982. Autoradiographic studies of [3H]zearalenone in mice. Toxicology 25(2–3):243–253, PMID: 6218655, 10.1016/0300-483x(82)90033-6. [DOI] [PubMed] [Google Scholar]
- Arukwe A, Grotmol T, Haugen TB, Knudsen FR, Goksøyr A. 1999. Fish model for assessing the in vivo estrogenic potency of the mycotoxin zearalenone and its metabolites. Science of The Total Environment 236(1–3):153–161, PMID: 10535150, 10.1016/S0048-9697(99)00275-2. [DOI] [PubMed] [Google Scholar]
- Bennett JW, Klich M. 2003. Mycotoxins. Clinical Microbiology Reviews 16(3):497–516, 10.1128/CMR.16.3.497-516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan B, Henare K, Thorstensen EB, Ponnampalam AP, Mitchell MD. 2010a. Transfer of bisphenol A across the human placenta. Am J Obstet Gynecol 202(4):e391–397. 393, PMID: 20350650. [DOI] [PubMed] [Google Scholar]
- Balakrishnan B, Thorstensen EB, Ponnampalam AP, Mitchell MD. 2010b. Transplacental transfer and biotransformation of genistein in human placenta. Placenta 31(6):506–511, PMID: 20413155, 10.1016/j.placenta.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Balakrishnan B, Thorstensen E, Ponnampalam A, Mitchell MD. 2011. Passage of 4-nonylphenol across the human placenta. Placenta 32(10):788–792, PMID: 21821285, 10.1016/j.placenta.2011.07.014. [DOI] [PubMed] [Google Scholar]
- Bandera EV, Chandran U, Buckley B, Lin Y, Isukapalli S, Marshall I, King M, Zarbl H. 2011. Urinary mycoestrogens, body size and breast development in New Jersey girls. Sci Total Environ 409(24):5221–5227, PMID: 21975003, 10.1016/j.scitotenv.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhoft A, Behrens GH, Ingebrigtsen K, Langseth W, Berndt S, Haugen TB, et al. 2001. Placental transfer of the estrogenic mycotoxin zearalenone in rats. Reprod Toxicol 15(5):545–550, PMID: 11780962, 10.1016/S0890-6238(01)00159-9. [DOI] [PubMed] [Google Scholar]
- Binder SB, Schwartz-Zimmermann HE, Varga E, Bichl G, Michlmayr H, Adam G, et al. 2017. Metabolism of zearalenone and its major modified forms in pigs. Toxins 9(2):56, PMID: 28208710, 10.3390/toxins9020056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondesson M, Hao R, Lin C-Y, Williams C, Gustafsson J-Å. 2015. Estrogen receptor signaling during vertebrate development. Biochim Biophys Acta 1849(2):142–151, PMID: 24954179, 10.1016/j.bbagrm.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueschl C, Kluger B, Neumann NKN, Doppler M, Maschietto V, Thallinger GG, et al. 2017. MetExtract II: a software suite for stable isotope-assisted untargeted metabolomics. Anal Chem 89(17):9518–9526, PMID: 28787149, 10.1021/acs.analchem.7b02518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier AC, Ganley NA, Tingle MD, Blumenstein M, Marvin KW, Paxton JW, et al. 2002. UDP-glucuronosyltransferase activity, expression and cellular localization in human placenta at term. Biochem Pharmacol 63(3):409–419, PMID: 11853692, 10.1016/S0006-2952(01)00890-5. [DOI] [PubMed] [Google Scholar]
- Collier AC, Thévenon AD, Goh W, Hiraoka M, Kendal-Wright CE. 2015. Placental profiling of UGT1A enzyme expression and activity and interactions with preeclampsia at term. Eur J Drug Metab Pharmacokinet 40(4):471–480, PMID: 25465229, 10.1007/s13318-014-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson PA, Richard K, Perkins A, Zhang Z, Simmons DG. 2017. Review: Nutrient sulfate supply from mother to fetus: placental adaptive responses during human and animal gestation. Placenta 54:45–51, PMID: 28089504, 10.1016/j.placenta.2017.01.001. [DOI] [PubMed] [Google Scholar]
- Delbès G, Levacher C, Habert R. 2006. Estrogen effects on fetal and neonatal testicular development. Reproduction 132(4):527–538, PMID: 17008464, 10.1530/rep.1.01231. [DOI] [PubMed] [Google Scholar]
- Dennis KK, Marder E, Balshaw DM, Cui Y, Lynes MA, Patti GJ, et al. 2017. Biomonitoring in the era of the exposome. Environ Health Perspect 125(4):502–510, PMID: 27385067, 10.1289/EHP474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EC (European Commission). 2007. Commission Regulation (EC) No 1126/2007 of 28 September 2007 amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards Fusarium toxins in maize and maize products. Official Journal of the European Union L 255/14(29.9.2007): 14–17. [Google Scholar]
- EC. 2018. Towards a more comprehensive EU framework on endocrine disruptors. Brussels, Belgium: European Commission; 2018. [Google Scholar]
- EFSA (European Food Safety Authority). 2011. Scientific opinion on the risks for public health related to the presence of zearalenone in food. EFSA Journal 9(6):2197. [Google Scholar]
- Enders AC, Blankenship TN. 1999. Comparative placental structure. Adv Drug Deliv Rev 38(1):3–15, PMID: 10837743, 10.1016/S0169-409X(99)00003-4. [DOI] [PubMed] [Google Scholar]
- Etienne M, Jemmali M. 1982. Effects of zearalenone (F2) on estrous activity and reproduction in gilts. J Anim Sci 55(1):1–10, PMID: 6214538, 10.2527/jas1982.5511. [DOI] [PubMed] [Google Scholar]
- Fernandez SV, Russo J. 2010. Estrogen and xenoestrogens in breast cancer. Toxicol Pathol 38(1):110–122, PMID: 19933552, 10.1177/0192623309354108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen M, Vorkamp K, Mathiesen L, Mose T, Knudsen LE. 2010. Placental transfer of the polybrominated diphenyl ethers BDE-47, BDE-99 and BDE-209 in a human placenta perfusion system: an experimental study. Environ Health 9(1):32. PMID: 20598165, 10.1186/1476-069X-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frizzell C, Ndossi D, Verhaegen S, Dahl E, Eriksen G, Sørlie M, et al. 2011. Endocrine disrupting effects of zearalenone, alpha- and beta-zearalenol at the level of nuclear receptor binding and steroidogenesis. Toxicol Lett 206(2):210–217, PMID: 21803136, 10.1016/j.toxlet.2011.07.015. [DOI] [PubMed] [Google Scholar]
- Frizzell C, Uhlig S, Miles CO, Verhaegen S, Elliott CT, Eriksen GS, et al. 2015. Biotransformation of zearalenone and zearalenols to their major glucuronide metabolites reduces estrogenic activity. Toxicol In Vitro 29(3):575–581, PMID: 25645597, 10.1016/j.tiv.2015.01.006. [DOI] [PubMed] [Google Scholar]
- Fucic A, Gamulin M, Ferencic Z, Katic J, Krayer von Krauss M, Bartonova A, et al. 2012. Environmental exposure to xenoestrogens and oestrogen related cancers: reproductive system, breast, lung, kidney, pancreas, and brain. Environ Health 11(Suppl 1):S8, PMID: 22759508, 10.1186/1476-069X-11-S1-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Sun L, Zhang N, Li C, Zhang J, Xiao Z, Qi D. 2017. Gestational zearalenone exposure causes reproductive and developmental toxicity in pregnant rats and female offspring. Toxins (Basel) 9(1):21, PMID: 28067781, 10.3390/toxins9010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafmueller S, Manser P, Diener L, Diener PA, Maeder-Althaus X, Maurizi L, et al. 2015. Bidirectional transfer study of polystyrene nanoparticles across the placental barrier in an ex vivo human placental perfusion model. Environ Health Perspect 123(12):1280–1286, PMID: 25956008, 10.1289/ehp.1409271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafmüller S, Manser P, Krug HF, Wick P, von Mandach U. 2013. Determination of the transport rate of xenobiotics and nanomaterials across the placenta using the ex vivo human placental perfusion model. JoVE (76):50401, PMID: 23851364, 10.3791/50401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson JR, Garcia-Bournissen F, Davis A, Koren G. 2011. The human placental perfusion model: a systematic review and development of a model to predict in vivo transfer of therapeutic drugs. Clin Pharmacol Ther 90(1):67–76, PMID: 21562489, 10.1038/clpt.2011.66. [DOI] [PubMed] [Google Scholar]
- Huuskonen P, Auriola S, Pasanen M. 2015. Zearalenone metabolism in human placental subcellular organelles, JEG-3 cells, and recombinant CYP19A1. Placenta 36(9):1052–1055, PMID: 26188906, 10.1016/j.placenta.2015.06.014. [DOI] [PubMed] [Google Scholar]
- Jard G, Liboz T, Mathieu F, Guyonvarc'h A, André F, Delaforge M, et al. 2010. Transformation of zearalenone to zearalenone-sulfate by Aspergillus spp. World Mycotoxin J 3(2):183–191, 10.3920/WMJ2009.1184. [DOI] [Google Scholar]
- Kaludjerovic J, Ward WE. 2012. The interplay between estrogen and fetal adrenal cortex. J Nutr Metab 2012:837901–837901, PMID: 22536492, 10.1155/2012/837901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolatorova L, Vitku J, Hampl R, Adamcova K, Skodova T, Simkova M, et al. 2018. Exposure to bisphenols and parabens during pregnancy and relations to steroid changes. Environ Res 163:115–122, PMID: 29433019, 10.1016/j.envres.2018.01.031. [DOI] [PubMed] [Google Scholar]
- Kowalska K, Habrowska-Górczyńska DE, Piastowska-Ciesielska AW. 2016. Zearalenone as an endocrine disruptor in humans. Environ Toxicol Pharmacol 48:141–149, PMID: 27771507, 10.1016/j.etap.2016.10.015. [DOI] [PubMed] [Google Scholar]
- Kunishige K, Kawate N, Inaba T, Tamada H. 2017. Exposure to zearalenone during early pregnancy causes estrogenic multitoxic effects in mice. Reprod Sci 24(3):421–427, PMID: 27485361, 10.1177/1933719116657194. [DOI] [PubMed] [Google Scholar]
- Malassine A, Frendo JL, Evain-Brion D. 2003. A comparison of placental development and endocrine functions between the human and mouse model. Hum Reprod Update 9(6):531–539, PMID: 14714590, 10.1093/humupd/dmg043. [DOI] [PubMed] [Google Scholar]
- Malekinejad H, Maas-Bakker RF, Fink-Gremmels J. 2005. Bioactivation of zearalenone by porcine hepatic biotransformation. Vet Res 36(5–6):799–810, PMID: 16120254, 10.1051/vetres:2005034. [DOI] [PubMed] [Google Scholar]
- Mally A, Solfrizzo M, Degen GH. 2016. Biomonitoring of the mycotoxin zearalenone: current state-of-the art and application to human exposure assessment. Arch Toxicol 90(6):1281–1292, PMID: 27034246, 10.1007/s00204-016-1704-0. [DOI] [PubMed] [Google Scholar]
- Maragos C. 2010. Zearalenone occurrence and human exposure. World Mycotoxin J 3(4):369–383, 10.3920/WMJ2010.1240. [DOI] [Google Scholar]
- Mason JI, Ushijima K, Doody KM, Nagai K, Naville D, Head JR, et al. 1993. Regulation of expression of the 3 beta-hydroxysteroid dehydrogenases of human placenta and fetal adrenal. J Steroid Biochem Mol Biol 47(1–6):151–159, PMID: 8274430, 10.1016/0960-0760(93)90069-9. [DOI] [PubMed] [Google Scholar]
- Massart F, Meucci V, Saggese G, Soldani G. 2008. High growth rate of girls with precocious puberty exposed to estrogenic mycotoxins. J Pediatr 152(5):690–695, PMID: 18410776, 10.1016/j.jpeds.2007.10.020. [DOI] [PubMed] [Google Scholar]
- Mathiesen L, Mørck TA, Zuri G, Andersen MH, Pehrson C, Frederiksen M, et al. 2014. Modelling of human transplacental transport as performed in Copenhagen, Denmark. Basic Clin Pharmacol Toxicol 115(1):93–100, PMID: 24646015, 10.1111/bcpt.12228. [DOI] [PubMed] [Google Scholar]
- Migdalof BH, Dugger HA, Heider JG, Coombs RA, Terry MK. 1983. Biotransformation of zeranol: disposition and metabolism in the female rat, rabbit, dog, monkey and man. Xenobiotica 13(4):209–221, PMID: 6624136, 10.3109/00498258309052257. [DOI] [PubMed] [Google Scholar]
- Mikula H, Hametner C, Berthiller F, Warth B, Krska R, Adam G, et al. 2012. Fast and reproducible chemical synthesis of zearalenone-14-β,D-glucuronide. World Mycotoxin J 5(3):289–296, 10.3920/WMJ2012.1404. [DOI] [Google Scholar]
- Mikula H, Weber J, Lexmüller S, Bichl G, Schwartz H, Varga E, et al. 2013. Simultaneous preparation of alpha/beta-zearalenol glucosides and glucuronides. Carbohydr Res 373:59–63, PMID: 23584236, 10.1016/j.carres.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Miles CO, Erasmuson AF, Wilkins AL, Towers NR, Smith BL, Garthwaite I, et al. 1996. Ovine metabolism of zearalenone to α-zearalanol (zeranol). J Agric Food Chem 44(10):3244–3250, 10.1021/jf9601325. [DOI] [Google Scholar]
- Mirocha CJ, Pathre SV, Robison TS. 1981. Comparative metabolism of zearalenone and transmission into bovine milk. Food Cosmet Toxicol 19:25–30, PMID: 6455340, 10.1016/0015-6264(81)90299-6. [DOI] [PubMed] [Google Scholar]
- Mose T, Kjaerstad MB, Mathiesen L, Nielsen JB, Edelfors S, Knudsen LE. 2008. Placental passage of benzoic acid, caffeine, and glyphosate in an ex vivo human perfusion system. J Toxicol Environ Health A 71(15):984–991, PMID: 18569607, 10.1080/01932690801934513. [DOI] [PubMed] [Google Scholar]
- Muoth C, Aengenheister L, Kucki M, Wick P, Buerki-Thurnherr T. 2016. Nanoparticle transport across the placental barrier: pushing the field forward! Nanomedicine (Lond) 11(8):941–957, PMID: 26979802, 10.2217/nnm-2015-0012. [DOI] [PubMed] [Google Scholar]
- Nielsen JK, Vikström AC, Turner P, Knudsen LE. 2011. Deoxynivalenol transport across the human placental barrier. Food Chem Toxicol 49(9):2046–2052, PMID: 21620924, 10.1016/j.fct.2011.05.016. [DOI] [PubMed] [Google Scholar]
- Olsen M, Pettersson H, Kiessling KH. 1981. Reduction of zearalenone to zearalenol in female rat liver by 3 alpha-hydroxysteroid dehydrogenase. Acta Pharmacol Toxicol (Copenh) 48(2):157–161, PMID: 6455043, 10.1111/j.1600-0773.1981.tb01602.x. [DOI] [PubMed] [Google Scholar]
- Palmlund I. 1996. Exposure to a xenoestrogen before birth: the diethylstilbestrol experience. J Psychosom Obstet Gynaecol 17(2):71–84, PMID: 8819018, 10.3109/01674829609025667. [DOI] [PubMed] [Google Scholar]
- Panigel M, Pascaud M, Brun JL. 1967. [Radioangiographic study of circulation in the villi and intervillous space of isolated human placental cotyledon kept viable by perfusion] [in French]. J Physiol (Paris) 59(1 Suppl):277. [PubMed] [Google Scholar]
- Partanen HA, El-Nezami HS, Leppänen JM, Myllynen PK, Woodhouse HJ, Vähäkangas KH. 2010. Aflatoxin B1 transfer and metabolism in human placenta. Toxicol Sci 113(1):216–225, PMID: 19875679, 10.1093/toxsci/kfp257. [DOI] [PubMed] [Google Scholar]
- Pfeiffer E, Hildebrand A, Mikula H, Metzler M. 2010. Glucuronidation of zearalenone, zeranol and four metabolites in vitro: formation of glucuronides by various microsomes and human UDP-glucuronosyltransferase isoforms. Mol Nutr Food Res 54(10):1468–1476, PMID: 20397195, 10.1002/mnfr.200900524. [DOI] [PubMed] [Google Scholar]
- Pfeiffer E, Kommer A, Dempe JS, Hildebrand AA, Metzler M. 2011. Absorption and metabolism of the mycotoxin zearalenone and the growth promotor zeranol in Caco-2 cells in vitro. Mol Nutr Food Res 55(4):560–567, PMID: 21462323, 10.1002/mnfr.201000381. [DOI] [PubMed] [Google Scholar]
- Preindl K, Braun D, Aichinger G, Sieri S, Fang M, Marko D, et al. 2019. A generic liquid chromatography−tandem mass spectrometry exposome method for the determination of xenoestrogens in biological matrices. Anal Chem 91(17):11334. PMID: 31398002, 10.1021/acs.analchem.9b02446. [DOI] [PubMed] [Google Scholar]
- Prouillac C, Koraichi F, Videmann B, Mazallon M, Rodriguez F, Baltas M, et al. 2012. In vitro toxicological effects of estrogenic mycotoxins on human placental cells: structure activity relationships. Toxicol Appl Pharmacol 259(3):366–375, PMID: 22310176, 10.1016/j.taap.2012.01.016. [DOI] [PubMed] [Google Scholar]
- Prouillac C, Lecoeur S. 2010. The role of the placenta in fetal exposure to xenobiotics: importance of membrane transporters and human models for transfer studies. Drug Metab Dispos 38(10):1623–1635, PMID: 20606001, 10.1124/dmd.110.033571. [DOI] [PubMed] [Google Scholar]
- Prouillac C, Videmann B, Mazallon M, Lecoeur S. 2009. Induction of cells differentiation and ABC transporters expression by a myco-estrogen, zearalenone, in human choriocarcinoma cell line (BeWo). Toxicology 263(2–3):100–107, PMID: 19580841, 10.1016/j.tox.2009.06.023. [DOI] [PubMed] [Google Scholar]
- Rajapakse N, Silva E, Kortenkamp A. 2002. Combining xenoestrogens at levels below individual no-observed-effect concentrations dramatically enhances steroid hormone action. Environ Health Perspect 110(9):917–921, PMID: 12204827, 10.1289/ehp.02110917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed CE, Fenton SE. 2013. Exposure to diethylstilbestrol during sensitive life stages: a legacy of heritable health effects. Birth Defects Res C Embryo Today 99(2):134–146, PMID: 23897597, 10.1002/bdrc.21035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimers A, Østby L, Stuen I, Sundby E. 2011. Expression of UDP-glucuronosyltransferase 1A4 in human placenta at term. Eur J Drug Metab Pharmacokinet 35(3–4):79–82, PMID: 21302032, 10.1007/s13318-010-0021-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šarkanj B, Ezekiel CN, Turner PC, Abia WA, Rychlik M, Krska R, et al. 2018. Ultra-sensitive, stable isotope assisted quantification of multiple urinary mycotoxin exposure biomarkers. Anal Chim Acta 1019:84–92, PMID: 29625687, 10.1016/j.aca.2018.02.036. [DOI] [PubMed] [Google Scholar]
- Saunders PT, Majdic G, Parte P, Millar MR, Fisher JS, Turner KJ, et al. 1997. Fetal and perinatal influence of xenoestrogens on testis gene expression. Adv Exp Med Biol 424:99–110, PMID: 9361775, 10.1007/978-1-4615-5913-9_19. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Morales-Prieto DM, Pastuschek J, Fröhlich K, Markert UR. 2015. Only humans have human placentas: molecular differences between mice and humans. J Reprod Immunol 108:65–71, PMID: 25817465, 10.1016/j.jri.2015.03.001. [DOI] [PubMed] [Google Scholar]
- Schollenberger M, Müller HM, Rüfle M, Terry-Jara H, Suchy S, Plank S, et al. 2007. Natural occurrence of Fusarium toxins in soy food marketed in Germany. Int J Food Microbiol 113(2):142–146, PMID: 16854487, 10.1016/j.ijfoodmicro.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Shekhar S, Sood S, Showkat S, Lite C, Chandrasekhar A, Vairamani M, et al. 2017. Detection of phenolic endocrine disrupting chemicals (EDCs) from maternal blood plasma and amniotic fluid in Indian population. Gen Comp Endocrinol 241:100–107, PMID: 27235644, 10.1016/j.ygcen.2016.05.025. [DOI] [PubMed] [Google Scholar]
- Singleton DW, Khan SA. 2003. Xenoestrogen exposure and mechanisms of endocrine disruption. Front Biosci 8:s110–s118, PMID: 12456297, 10.2741/1010. [DOI] [PubMed] [Google Scholar]
- Slikker W, Bailey JR, Newport D, Lipe GW, Hill DE. 1982. Placental transfer and metabolism of 17 alpha-ethynylestradiol-17 beta and estradiol-17 beta in the rhesus monkey. J Pharmacol Exp Ther 223(2):483–489, PMID: 7131302. [PubMed] [Google Scholar]
- Solfrizzo M, Gambacorta L, Visconti A. 2014. Assessment of multi-mycotoxin exposure in southern Italy by urinary multi-biomarker determination. Toxins (Basel) 6(2):523–538, PMID: 24476712, 10.3390/toxins6020523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Li C, Zhou S, Zhao Y, Gong YY, Gong Z, et al. 2019. Determination of trace zearalenone and its metabolites in human serum by a high-throughput UHPLC-MS/MS analysis. App Sci 9(4):741, 10.3390/app9040741. [DOI] [Google Scholar]
- Tatay E, Espín S, García-Fernández AJ, Ruiz MJ. 2017. Estrogenic activity of zearalenone, α-zearalenol and β-zearalenol assessed using the E-screen assay in MCF-7 cells. Toxicol Mech Meth 28(4):239–242, PMID: 26799652, 10.1080/15376516.2017.1395501. [DOI] [PubMed] [Google Scholar]
- Ueno Y, Ayaki S, Sato N, Ito T. 1977. Fate and mode of action of zearalenone. Ann Nutr Aliment 31(4–6):935–948, PMID: 613943. [PubMed] [Google Scholar]
- Vejdovszky K, Hahn K, Braun D, Warth B, Marko D. 2017. Synergistic estrogenic effects of Fusarium and Alternaria mycotoxins in vitro. Arch Toxicol 91(3):1447–1460, PMID: 27401186, 10.1007/s00204-016-1795-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendl O, Crews C, MacDonald S, Krska R, Berthiller F. 2010. Occurrence of free and conjugated Fusarium mycotoxins in cereal-based food. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 27(8):1148–1152, PMID: 20496251, 10.1080/19440041003801166. [DOI] [PubMed] [Google Scholar]
- Warth B, Raffeiner P, Granados A, Huan T, Fang M, Forsberg EM, Benton HP, Goetz L, Johnson CH, Siuzdak G. 2018. Metabolomics Reveals that Dietary Xenoestrogens Alter Cellular Metabolism Induced by Palbociclib/Letrozole Combination Cancer Therapy. Cell Chem Biol 25(3):291–300, PMID: 29337187, 10.1016/j.chembiol.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warth B, Spangler S, Fang M, Johnson CH, Forsberg EM, Granados A, et al. 2017. Exposome-Scale Investigations Guided by Global Metabolomics, Pathway Analysis, and Cognitive Computing. Anal Chem 89(21):11505–11513, PMID: 28945073, 10.1021/acs.analchem.7b02759. [DOI] [PubMed] [Google Scholar]
- Warth B, Sulyok M, Berthiller F, Schuhmacher R, Krska R. 2013. New insights into the human metabolism of the Fusarium mycotoxins deoxynivalenol and zearalenone. Toxicol Lett 220(1):88–94, PMID: 23623764, 10.1016/j.toxlet.2013.04.012. [DOI] [PubMed] [Google Scholar]
- Welshons WV, Nagel SC, Thayer KA, Judy BM, Vom Saal FS. 1999. Low-dose bioactivity of xenoestrogens in animals: fetal exposure to low doses of methoxychlor and other xenoestrogens increases adult prostate size in mice. Toxicol Ind Health 15(1–2):12–25, PMID: 10188188, 10.1177/074823379901500103. [DOI] [PubMed] [Google Scholar]
- Wick P, Malek A, Manser P, Meili D, Maeder-Althaus X, Diener L, et al. 2010. Barrier capacity of human placenta for nanosized materials. Environ Health Perspect 118(3):432–436, PMID: 20064770, 10.1289/ehp.0901200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witorsch RJ. 2002. Low-dose in utero effects of xenoestrogens in mice and their relevance to humans: an analytical review of the literature. Food Chem Toxicol 40(7):905–912, PMID: 12065211, 10.1016/S0278-6915(02)00069-8. [DOI] [PubMed] [Google Scholar]
- Woo CS, Partanen H, Myllynen P, Vähäkangas K, El-Nezami H. 2012. Fate of the teratogenic and carcinogenic ochratoxin A in human perfused placenta. Toxicol Lett 208(1):92–99, PMID: 22037670, 10.1016/j.toxlet.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Xu Z, Liu J, Wu X, Huang B, Pan X. 2017. Nonmonotonic responses to low doses of xenoestrogens: a review. Environ Res 155:199–207, PMID: 28231547, 10.1016/j.envres.2017.02.018. [DOI] [PubMed] [Google Scholar]
- Young LG, Ping H, King GJ. 1990. Effects of feeding zearalenone to sows on rebreeding and pregnancy. J Anim Sci 68(1):15–20, PMID: 2137439, 10.2527/1990.68115x. [DOI] [PubMed] [Google Scholar]
- Zinedine A, Soriano JM, Moltó JC, Mañes J. 2007. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: an oestrogenic mycotoxin. Food Chem Toxicol 45(1):1–18, PMID: 17045381, 10.1016/j.fct.2006.07.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.