Abstract
Resting‐state connectivity has become an increasingly important measure in characterizing the functional integrity of brain circuits in neuro‐psychiatric conditions. One approach that has recently gained prominence in this regard—and which we use in this study—is to investigate how resting‐state connectivity depends on the integrity of certain neuromodulator systems. Here, we use a pharmacological challenge in combination with functional magnetic resonance imaging to investigate the impact of dopaminergic receptor blockade on whole brain functional connectivity in twenty healthy human subjects. Administration of the D2‐receptor antagonist haloperidol led to a profound change in functional integration in network nodes linked to the amygdala. Compared to placebo and baseline measurements, network‐based statistics and pairwise connectivity analyses revealed reduced connectivity and decreased link strength between the amygdala and the bilateral posterior cingulate cortex and other cortical areas. This was complemented by less extensive but very circumscribed enhanced connectivity between the amygdala and the right putamen during D2‐receptor blockade. It will be interesting to investigate whether these pharmacologically induced shifts in resting‐state connectivity will similarly be evident in clinical conditions that involve a dysfunction of the dopaminergic system. Our findings might also aid in interpreting alterations in more complex states, such as those seen psychiatric conditions and their treatment. Hum Brain Mapp 37:4148–4157, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: haloperidol, resting‐state, pharmacological connectomics, phMRI, connectome
INTRODUCTION
Functional magnetic resonance imaging (fMRI) studies have shown that the human brain displays a large amount of spontaneous hemodynamic fluctuations at rest [Biswal et al., 1995; Buckner et al., 2013; Fox and Raichle, 2007; Power et al., 2014]. These fluctuations in the blood‐oxygen‐level‐dependent (BOLD) signal are highly organized, as demonstrated by the existence of various reproducible resting‐state networks, such as the visual, auditory, sensorimotor, executive control, or default‐mode networks [Damoiseaux et al., 2006; Fox et al., 2005; Greicius et al., 2003]. Importantly, several studies have now established a neural basis for resting‐state BOLD fluctuations [Leopold and Maier, 2012], suggesting that they can indeed be used to probe the functional neural architecture of the human brain.
The organization of resting‐state fluctuations is not static, but is significantly modulated by various factors such as experience [Lewis et al., 2009], age [Salami et al., 2014], as well as disease states and corresponding medication [Fox and Greicius, 2010; Zhang and Raichle, 2010]. There is also growing evidence that resting‐state connectivity is dependent on endogenous factors, such as the state of neurotransmitter and neuromodulator systems: not only are glutamate and GABA levels related to functional connectivity, for example, within the default‐mode network [Kapogiannis et al., 2013], but more widespread effects of opioidergic, adrenergic and serotonergic challenges on resting‐state connectivity have also been observed [Metzger et al., 2015; Nasrallah et al., 2014; Patel et al., 2008; Schaefer et al., 2014].
In this study, we focus on alterations in networks dependent on dopaminergic neuromodulation, which is known to be involved in a number of neuro‐psychiatric conditions [Koob and Volkow, 2009; Laviolette, 2007; Wise, 2004]. Identification of basic processes in networks that show dopamine‐dependent functional connectivity might be a valuable approach to understand the neural mechanisms contributing to complex neuropsychiatric conditions and their treatment, within the framework of intermediate connectivity phenotypes [Buckholtz and Meyer‐Lindenberg, 2012; Cao et al., 2016b; Fornito et al., 2013, 2012; Tost et al., 2012].
In the healthy state, dopaminergic D1‐ and D2‐receptors modulate a finely tuned balance of functional integration, as evident for example in the control of striatal inputs to prefrontal and limbic regions in rodents [Goto and Grace, 2008, 2005; Groenewegen et al., 1999]. This balance can be disturbed however, for example, by D2‐receptor antagonism, which is known to reduce resting‐state functional connectivity between parts of the dopaminergic midbrain (substantia nigra) and medio‐cortical regions as well as limbic parts (hippocampus) of the rodent brain [Gass et al., 2012]. These decreases in connectivity within the dopaminergic nigrostriatal and mesolimbic pathways in rodents fit well with the effects of D2 blockade in humans. In the human brain, D2 blockade was found to decrease functional connectivity of the midbrain and striatal regions within large‐scale cortical resting‐state networks that were derived from an independent component analysis [Cole et al., 2012]. In comparison to placebo, the administration of haloperidol was also shown to have a predominantly negative effect on the functional connectivity pattern of both subcortical and cortical resting‐state networks, which is plausible given the neurobiological architecture of dopaminergic pathways [Cole et al., 2013]. Furthermore, such a D2 blockade—as well as depletion of dopamine precursors—distorts the organizational principles of resting‐state networks [Achard and Bullmore, 2007; Carbonell et al., 2014]. D2 blockade was found to reduce the economical properties (e.g., efficiency) of connectivity within whole‐brain networks, and these organizational principles were found to be altered in patients with schizophrenia as well [Alexander‐Bloch et al., 2010; Vértes et al., 2012]. Together these studies suggest that D2 blockade impairs functional connectivity in the resting‐state. It is, however, important to investigate connectivity changes after D2 blockade in a regionally unbiased way both on the network as a whole as well as on its constituent elements—something which has not been done yet and which we aim to do here. This should aid to understand basic processes in the pathophysiology and treatment of complex neuropsychiatric conditions that affect the whole neuro‐architecture.
Here, we thus set out to explore dopamine D2‐receptor dependent changes in resting‐state connectivity of cortical and subcortical regions throughout the whole brain. To this end, we manipulated D2‐receptor functioning by the administration of the D2‐receptor antagonist haloperidol in a randomized placebo‐controlled double‐blind crossover study, using analysis approaches that focussed on both network comparisons and their constituting individual connections. In analogy to the aforementioned reports on dopaminergic challenges, we expected to observe a decrease in connectivity caused by the dopaminergic challenge, both in global connectivity measures and in the constituting pair‐wise connectivity between nodes.
MATERIALS AND METHODS
Participants
Data from 20 healthy male right‐handed participants (mean age: 28.9 years, range: 21–40 years) were analyzed in this study; 23 participants were originally recruited, but one participant had to be excluded due to intake of alcohol before one session and two other participants had to be excluded because they were unavailable at later test days. General exclusion criteria were any known current or prior neurological or psychiatric disorders (self‐report by a structured medical screening questionnaire), non‐removable metal parts in the body, use of prescription drugs within the past two months or use of nonprescription drugs during the last 2 weeks preceding the first experimental session as well as the use of illegal drugs; for further information, please see the section “Additional information on participants” in the Supporting Information. The Ethics Committee of the Medical Board in Hamburg, Germany, approved the study (PV3660) and all participants gave written informed consent.
Experimental Design
Each participant was scanned on three days—with an interval between scan‐days of at least two weeks—as part of a large pharmacological study. In this manuscript, we only report data from two days (placebo and haloperidol administration); data from a third day (naloxone administration) will be reported separately. Each day consisted of four resting‐state fMRI sessions (each with 175 images resulting in a duration of about 7 min per session): one before oral administration of either 2 mg haloperidol or placebo (i.e., baseline session) and three sessions afterwards (i.e., two intermediate sessions and one peak session), scheduled with 108 min intervals (on average) between each session. This resulted in 5:12 h:min on average between haloperidol/placebo administration and the start of the peak session. Here, we concentrate on the comparisons of baseline and peak sessions (Fig. 1); data from intermediate sessions will not be reported here, as these were carried out solely to allow comparisons with data from the day of naloxone administration.
Figure 1.
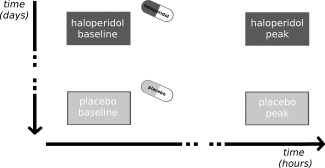
Experimental design. In a within‐subject design, each participant either received placebo or 2 mg haloperidol on one of the days, with the assignment of haloperidol/placebo carried out in a randomized double‐blind manner. Resting‐state functional connectivity was assessed preceding pharmacological intervention (baseline sessions) and at the approximate peak level of plasma concentration of haloperidol (peak sessions).
We expected to find haloperidol‐induced differences in the last session (peak session) when all participants were expected to have reached peak plasma concentration [Cheng et al., 1987, Froemming et al., 1989], but not in the first session (baseline session; which occurred before haloperidol/placebo administration); The last session in the placebo condition was used as the “placebo peak session” to control for unspecific factors such as the timing of data acquisition, habituation to the MR‐environment, and so forth (within‐day comparisons of placebo or haloperidol peak and baseline sessions are included in the Supporting Information together with other control analyses; see below). Each participant received placebo or haloperidol on one of the days, with the assignment of haloperidol/placebo carried out in a randomized double‐blind manner (Fig. 1).
Data Acquisition
Resting‐state fMRI data were acquired on a 3 Tesla system (Magnetom Trio, Siemens) equipped with a 32‐channel head coil. Thirty‐five transversal slices (3‐mm thick, 1‐mm gap) were acquired using a T2*‐sensitive gradient‐echo echo‐planar imaging (EPI) sequence (repetition time: 2.29 s; echo time: 30 ms; flip angle: 80°; field of view: 222 × 222 mm, in‐plane resolution: 3 × 3 mm, 74 × 74 matrix). High‐resolution T1‐weighted images (1 × 1 × 1 mm) were acquired after the last session. To allow for retrospective physiological noise correction of fMRI data, we also acquired pulse and respiration data, using the vendor‐supplied pulse sensor and respiratory bellows.
Data Processing
Data pre‐processing was carried out using statistical parametric mapping (SPM8, http://www.fil.ion.ucl.ac.uk/spm) and consisted of realignment with unwarping, coregistration (between EPI images and the skull‐stripped T1 image), spatial normalization using the DARTEL toolbox [Ashburner, 2007], and smoothing with a 6 mm FWHM isotropic Gaussian kernel. Pre‐processed data from both the baseline and the peak sessions were then prepared for functional connectivity analysis using CONN, a Matlab‐based toolbox (http://www.nitrc.org/projects/conn, [Whitfield‐Gabrieli and Nieto‐Castanon, 2012]). In a first step, we removed possible confounds, including motion parameters, white‐matter signal, cerebrospinal fluid signal, nonphysiological artefactual signals (spikes) and physiological influences of cardiac and respiratory nature (which we modelled using the method developed by [Deckers et al., 2006]; this method is a model‐free alternative to RETROICOR [Glover et al., 2000] and performs at least as well). In a second step (after the above‐described nuisance regression [Hallquist et al., 2013],) we band‐pass filtered the data between 0.008 to 0.09 Hz, as recommended in the CONN toolbox. In a third step we used an anatomical parcellation into a set of 90 discrete regions (based on regions defined in the Automated Anatomical Labelling software [Tzourio‐Mazoyer et al., 2002]), as provided by the CONN toolbox; this last step included 45 regions in each hemisphere, excluding the cerebellum. Finally, pair‐wise correlations were computed between the average (confound‐corrected and band‐pass filtered) time‐course of each region and the time‐courses of all other regions using Pearson's correlation coefficient. The results of these procedures were four 90 × 90 connectivity matrices for each participant (baseline and peak session under placebo and baseline and peak session under haloperidol), containing Fisher‐z transformed r‐values of all between‐region pair‐wise correlations.
Network‐Based Statistic
As a first approach we used the network‐based statistic (NBS; as implemented in a Matlab‐based toolbox: http://www.nitrc.org/projects/nbs, [Zalesky et al., 2010]), to investigate connectivity differences between the two pharmacological states (placebo and haloperidol). The NBS‐based investigation of changes in the whole network was chosen to identify regions of interest (i.e., the node displaying the largest change in connectivity between placebo and haloperidol), and proceeded as follows. First, each connection of a connectivity matrix is endowed with a statistical value, derived from the comparison of connectivity between the different sessions (please see below for the specific analyses). The resulting statistical maps/matrices are then thresholded (with a primary threshold) to receive a sparse set of regions in which the connectivity changes are likely to reject the null hypothesis. Of note, the evaluation of different primary thresholds is advised when using NBS to reduce effects of sparsity in the comparison of several graphs [Zalesky et al., 2010]. Here, we used primary thresholds of P < 0.01, P < 0.005, and P < 0.001. In a next step, these supra‐threshold connections are transformed into a connected graph component and its extent (number of connections between regions) is then tested for significance, using a family‐wise error (FWE) corrected threshold to correct for multiple comparisons. This is done by comparing the extent of the connected graph component against a null distribution of permuted graph components with randomized group assignment. In our analysis, we used 5,000 independent permutations with randomized interchange of group assignment within one person, to perform paired or repeated measures analyses [Suckling and Bullmore, 2004]. This method of permutation testing—also used in conventional cluster‐based thresholding of statistical parametric maps [Bullmore et al., 2009; Nichols and Holmes, 2002]—is valid to control for false‐positives in mass univariate testing scenarios as used here [Zalesky et al., 2010].
In a first analysis, we included all four connectivity matrices (baseline and peak sessions for haloperidol and placebo, Fig. 1) and used an undirected F‐test to test for any between‐session differences. In a second analysis—designed to test our hypothesis of decreases in connectivity after haloperidol administration as compared to placebo—we used a one‐sided paired t‐test to test for attenuation of connectivity under haloperidol as compared to placebo in the peak sessions. In a final set of analyses—that served as control analyses regarding the specificity and robustness of our findings and can be found in the Supporting Information (section “Network‐based statistic”)—we (a) tested the inverse contrast (haloperidol‐induced increases in connectivity during the peak sessions; Supporting Information Table 2), (b) tested for differences between baseline sessions (Supporting Information Tables 3 and 4), and (c) tested for differences between baseline and peak sessions (under placebo and haloperidol each; Supporting Information Tables 5‐9 and Supporting Information Figure 1).
Pair‐Wise Connectivity
We selected the region within the graph component that displayed the strongest changes in connectivity (highest node degree, i.e., number of connections, in both, the F‐test and the directed t‐test together) and explored changes of pair‐wise connectivity between this region and all other regions, and all other voxels, respectively. Detailed results using these approaches are provided in the Supporting Information (section “Pair‐wise connectivity”).
The pair‐wise region‐to‐region analysis (Supporting Information Table 10 and preceding text) supplements the NBS analysis by allowing inference on individual connections within a component (in NBS the null hypothesis can only be rejected at the level of the component as a whole, but not its constituent edges). The pair‐wise region‐to‐voxel analysis (Supporting Information Tables 11‐13 and Supporting Information Figures 3 and 4) also supplements the NBS analysis by allowing us to locate differences in connectivity without the constraints of ROI‐definition. As in preceding analyses, P‐values smaller than 0.05 were considered significant (after FWE correction at the cluster level); additional results using an uncorrected threshold (P < 0.001 with a 10‐voxel extent threshold) are provided in the Supporting Information.
RESULTS
Network‐Based Statistic
In a first exploratory analysis that was independent of our hypothesis (decreased connectivity under haloperidol), we tested for any between‐session differences (F‐Test across all four sessions depicted in Fig. 1) in connectivity of anatomically defined regions covering the whole cerebrum. At a primary threshold of P < 0.01, we found a significant (P = 0.0078, corrected) graph component consisting of 63 connections between 47 regions. The highest node degree within this graph was found in the right amygdala (11 connections), followed by the left paracentral gyrus (8 connections) and the left middle frontal gyrus (7 connections, see Supporting Information Figure 1 and Supporting Information Table 1 for details).
In a second analysis that was designed to test our main hypothesis, we specifically tested for a decrease in connectivity induced by haloperidol administration, that is, differences between peak sessions in haloperidol and placebo sessions. We found a graph component consisting of seven connections between eight regions (P = 0.048 at a primary threshold of P = 0.001 that were also present with P = 0.051 at a primary threshold of P = 0.01). This graph component (Fig. 2) consisted of connections (a) between the right amygdala and four regions (bilateral posterior cingulum, right precuneus and left middle temporal pole) and (b) between the right precuneus and three additional regions (bilateral hippocampus and left gyrus rectus). The graph component representing the undirected F‐test and the direct t‐test displayed a large overlap (see Supporting Information Figure 1). The right amygdala and right precuneus displayed the highest node degree (four connections) in the graph component representing the t‐test.
Figure 2.
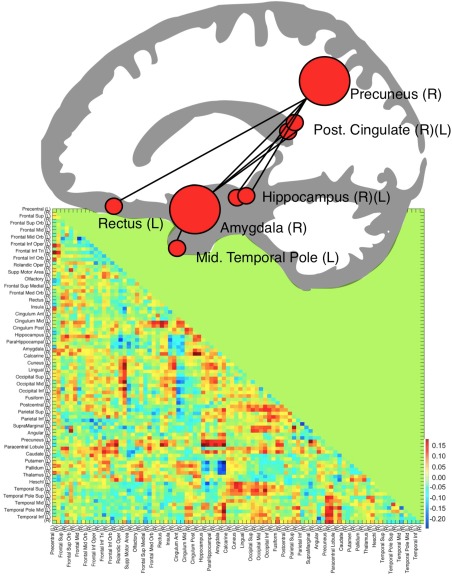
Illustration of the network showing reduced connectivity under haloperidol as compared to placebo. Each region is represented by a red circle, with the radius reflecting the relative node degree. ROIs were transformed onto the same x‐plane and superimposed on a vectorized average structural image. The correlation matrix depicts the difference in Fisher‐z transformed r‐values of the comparisons between haloperidol and placebo in all 90 ROIs; R = right, L = left. [Color figure can be viewed at http://wileyonlinelibrary.com.]
Supplementing our focus on the comparisons of haloperidol and placebo peak sessions, we conducted a set of control analyses that are included in the Supporting Information. In short, they support the main finding of decreased connectivity under haloperidol and exclude unspecific baseline or session order effects. In more detail, we did not observe any significant graph component that described increasing connectivity under haloperidol in the peak sessions (i.e., the opposite of our hypothesis; Supporting Information Table 2) or differences between baseline sessions before administration of either haloperidol or placebo (Supporting Information Tables 3 and 4). Interestingly, we found a significant graph component that reflected decreasing connectivity from the baseline to the peak session in the haloperidol condition, including the amygdala, bilateral hippocampus and bilateral posterior cingulate (Supporting Information Tables 7 and 8)—this within‐day effect of decreased connectivity under haloperidol is thus in line with our between‐day effect reported above (Supporting Information Figure 1). Supporting the specificity of this finding, we did not observe any significant graph component that described an increase in connectivity from baseline to peak session after administration of haloperidol (Supporting Information Table 9) or differences between baseline and sessions peak in the placebo condition (Supporting Information Tables 5 and 6).
Pair‐Wise Region‐to‐Region Analysis
Due to the fact that NBS analyses only allow for inference on the graph component as a whole, but not on individual connections, we supplemented the NBS analyses with pair‐wise tests of region‐to‐region connectivity of the amygdala, as the major hub identified in the NBS analyses (highest node degree). These analyses revealed significantly decreased connectivity under haloperidol compared to placebo (peak sessions) between the amygdala and the bilateral posterior cingulate, right precuneus, and temporal pole (reported in detail in Supporting Information Table 10 and preceding text).
Pair‐Wise Region‐to‐Voxel Analysis
For a more fine‐grained analysis of the changes in the connectivity measures in the amygdala, we examined the correlation of the average time‐course between the bilateral amygdala and all other voxels in the brain. The amygdala was selected for its outstanding role in the NBS analysis (highest node degree). The pair‐wise region‐to‐voxel analysis revealed significantly reduced functional connectivity between the bilateral amygdala and a single cluster located in the right precuneus (Fig. 3). A post‐hoc analysis that examined the changes in connectivity between the bilateral precuneus (as the second major hub of the connected component in the NBS analyses) and all other voxels in the brain found reduced connectivity with the bilateral amygdala (Supporting Information Table 11; Supporting Information Figure 3).
Figure 3.
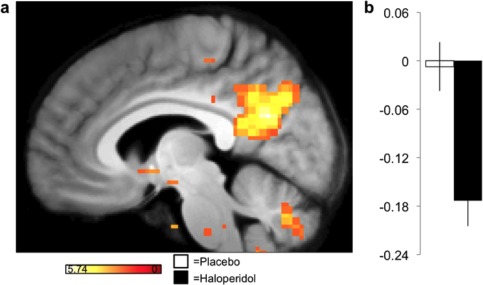
Functional connectivity from an amygdala seed is reduced in the haloperidol condition compared to placebo. Color‐coded t‐values are superimposed on the average structural image at a display‐threshold of P < 0.001 (uncorrected). The bar graph (b) represents averaged Fisher‐transformed correlation coefficient values between the amygdala seed ROI and the global maximum within the precuneus. Error bars indicate SEM. [Color figure can be viewed at http://wileyonlinelibrary.com.]
In analogy to the NBS analysis, we also explored whether the amygdala would show enhanced connectivity with other regions under D2 blockade and indeed observed enhanced connectivity in pair‐wise connectivity between the amygdala and the putamen (Supporting Information Table 13; Supporting Information Figure 4) that was found as well in the pair‐wise region‐to‐region connectivity (see the section “Pair‐wise connectivity” in the Supporting Information).
DISCUSSION
In this study, we were able to identify an amygdala‐centred network of functional connectivity changes as a result of blocking D2 type receptors in the resting state. Previous studies have linked variation of connectivity in amygdala‐related circuits with affective vulnerability in diseases such as schizophrenia [Fornito et al., 2013] and altered connectivity within graph components that include cortical (e.g., posterior cingulate) to limbic (e.g., amygdala and hippocampus) connections, have recently been identified as endo‐phenotypes in schizophrenic patients as well as first‐degree relatives [Cao et al., 2016a; Lo et al., 2015].
In more detail, in our study connectivity was reduced in a network of eight nodes comprising the right amygdala as the primarily connected hub. In this system, the amygdala is connected with the bilateral posterior cingulate cortex and the right temporal pole as well as the right precuneus that was further connected to the bilateral hippocampus and the left gyrus rectus. A finer‐grained region‐to‐voxel analysis from the amygdala seed region to all cerebral voxels contributes link strength and extent information for each node. The latter analysis confirmed reduced functional connectivity between the amygdala and the aforementioned posteromedial cortex network nodes in the haloperidol condition as compared to placebo.
Neural activity in the amygdala is involved in basic motivational, emotional and social information processing [Adolphs, 2010; Balleine and Killcross, 2006; Davis and Whalen, 2001; Murray, 2007]. Together with cerebellum and thalamus the amygdala is a major [Tomasi and Volkow, 2011] and stable [Cao et al., 2014] subcortical node within the human connectome. Altered connectivity measures have been identified to be relevant as biological traits for neuropsychiatric disorders [Buckholtz and Meyer‐Lindenberg, 2012; Fornito et al., 2012] and are considered informative as intermediate phenotypes at the intersection of microscale synaptic processes and behavior [Buckholtz and Meyer‐Lindenberg, 2012; Cao et al., 2016b; Fornito et al., 2012; Tost et al., 2012]. To construct meaningful phenotypes, changes in connectivity have to be linked with genetic or pharmacological alteration at the synapse level. Importantly, the causality in our results can be attributed to the single factor of DA‐r blockade. Dopaminergic changes, however, are not only pathological but also a function of age. Our observation of connectomic changes in links to and from the amygdala is in line with the observation of reduced local cost‐efficiency in conntectomes including the amygdala in the elderly [Achard and Bullmore, 2007]. The authors of that study proposed the diminishing dopaminergic function with increasing age as a plausible factor for this effect, however they did not find comparably decreased connectivity after heuristic blockade of DA D2‐receptors (through sulpiride) in younger subjects. A recent study speaks in favor of their initial hypothesis revealing reduced connectivity measures through the depletion of dopamine precursors [Carbonell et al., 2014]. Our results support the hypothesis that the functional integration of an amygdala‐mediated network is affected by dopaminergic neurotransmission. Together with electrophysiological connectivity in rodents and functional connectivity measures in rodents and humans, the here presented “pharmacological connectomics” approach might be used to characterize effects of psychopharmacological agents on the organizational principles of large‐scale neural networks.
Furthermore, we identified altered connectivity of the posterior parietal cortices and the precuneus during DA‐r blockade. The precuneus constitutes yet another major hub in the human connectome [Tomasi and Volkow, 2011]. This important node in the default mode network (DMN, e.g., [Fransson and Marrelec, 2008; Utevsky et al., 2014]) is interconnected with a functional limbic network including ventromedial and dorsomedial regions of the prefrontal cortex as well as the parahippocampus, hippocampus and insula in primates including humans [Margulies et al., 2009]. Previous studies in rodents and humans found altered connectivity in medial parietal areas after dopaminergic receptor blockade especially with striatal seed‐regions [Cole et al., 2013, 2012; Gass et al., 2012; Kelly et al., 2009]. Similar to the age‐related changes in dopaminergic function in networks comprising the amygdala [Achard and Bullmore, 2007] decreased DMN activity in the elderly has been reported [Braskie et al., 2011]. Other catecholaminergic drugs such as atomoxetine, amphetamine or methylphenidate changed connectivity of the medial posterior cortex areas, as well [Farr et al., 2014; Lin and Gau, 2015; Mueller et al., 2014; Schrantee et al., 2016]. Interestingly, methylphenidate exerted also an effect on amygdala connectivity [Farr et al., 2014; Mueller et al., 2014]. In posterior cingulate cortex and precuneus, older adults showed diminished task‐induced deactivation in fMRI activity that was associated with lower dopamine synthesis capacity (FMT‐PET; [Braskie et al., 2011]). Thus, the original changes in amygdala‐mediated circuits may indirectly inflict an alternation upon areas of the DM network, which has been repeatedly associated with memory processes.
Complementary to NBS we also utilized pair‐wise connectivity to obtain information on link strength (region‐to‐region correlations) and node size/location (region‐to‐voxel correlations) within the identified network of altered connectivity. Here, the amygdala as the central hub (i.e., highest node degree) served as the region of interest. This analysis confirmed decreased connectivity with the posterior medial cortex (PMC) after dopaminergic receptor blockade, as compared to placebo. More precisely, the cluster‐location through anti‐correlation with the amygdala seed‐region resembled a subregion of the PMC that displayed limbic connectivity in the study by [Margulies et al., 2009]. This suggests that functional connectivity between ventral parts of the PMC and limbic regions are at least partly modulated by dopaminergic receptor function. This is of particular interest, given that the ventral PMC displays a high genetic heritability in its cost‐efficiency [Fornito et al., 2011]. Connectomics of the PMC might therefore be an interesting intermediate biological trait of neuropharmacological functioning that is for example associated with neuropsychiatric diagnosis [Arnold Anteraper et al., 2013], history of traumatic events [van der Werff et al., 2013] or neuropsychiatric traits [Aghajani et al., 2013]. Deducing from the importance of dopaminergic integrity in networks containing amygdala and precuneus in the elderly [Achard and Bullmore, 2007; Braskie et al., 2011; Salami et al., 2014], connectivity as a biological trait might be also of interest in light of successful aging, especially in regard to preserved emotional regulation and memory function [Brassen et al., 2012].
While our hypothesis for this study was a reduction of functional connectivity under haloperidol, we also conducted an exploratory analysis of the inverse effect, testing for enhanced connectivity after haloperidol administration. Interestingly, we identified enhanced pair‐wise connectivity after blockade of DA‐r between the amygdala and the putamen, as compared to placebo. This could resemble two functionalities of the same dopaminergic network. Given the previous results in different species that found altered connectivity between the striatum and parietal regions after dopaminergic challenges [Cole et al., 2013, 2012; Gass et al., 2012; Kelly et al., 2009], our results might point toward a role for the amygdala as the central connecting hub between these regions. Connectivity between the striatum and the amygdala is mediated indirectly through DA D2‐r, which are mainly affected by tonic dopaminergic activity [Floresco et al., 2003; Goto and Grace, 2005]. Acute DA D2‐r blockade then shifts the balance in dopaminergic receptor balance toward more phasic dopaminergic signalling via DA D1‐rs [Benoit‐Marand et al., 2011] which enhances limbic inputs to the striatum [Goto and Grace, 2008, 2005]. Even if this explanatory model may be far too specific to be proven here, there has been evidence for increasing blood perfusion in the striatum during rest after haloperidol administration [Lahti et al., 2003], particularly localized in the putamen [Bartlett et al., 1994; Handley et al., 2013]. The putamen is mutually connected with the amygdala, [Parent et al., 1983; Russchen et al., 1985; Smith and Parent, 1986] and amygdala seed‐regions display functional connectivity with striatal regions, including the putamen [Roy et al., 2009].
In conclusion, this study on pharmacological connectomics of DA‐r blockade revealed a shift of functional integration in an amygdala‐mediated network. The amygdala was reduced in its functional connectivity with nodes in the PMC and other brain areas on one hand and suggested enhanced connectivity with the putamen on the other hand. These connectomic measures add valuable information for our understanding of basic neuropsychopharmacological processes in amygdala‐centred circuits that might contribute to alterations in neural networks in complex conditions, as psychiatric disease and its treatment. Additionally, they also increase our knowledge about intervention‐independent but dopamine‐dependent changes in amygdala mediated networks as they occur, for example, over the life span.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The authors would like to thank Katrin Müller, Kathrin Wendt, and Timo Krämer for their support with acquiring the MRI data and Jürgen Finsterbusch for setting up the fMRI protocol. The authors declare no competing financial interests. MMM thanks the Zonta Club Hamburg‐Alster for support.
REFERENCES
- Achard S, Bullmore E (2007): Efficiency and cost of economical brain functional networks. PLoS Comput Biol 3:e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R (2010): What does the amygdala contribute to social cognition? Ann N Y Acad Sci 1191:42–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajani M, Veer IM, van Tol MJ, Aleman A, van Buchem MA, Veltman DJ, Rombouts SARB, van der Wee NJ (2013): Neuroticism and extraversion are associated with amygdala resting‐state functional connectivity. Cogn Affect Behav Neurosci 14:836–848. [DOI] [PubMed] [Google Scholar]
- Alexander‐Bloch AF, Gogtay N, Meunier D, Birn R, Clasen L, Lalonde F, Lenroot R, Giedd J, Bullmore ET (2010): Disrupted modularity and local connectivity of brain functional networks in childhood‐onset schizophrenia. Front Syst Neurosci 4:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold Anteraper S, Triantafyllou C, Sawyer AT, Hofmann SG, Gabrieli JD, Whitfield‐Gabrieli S (2013): Hyper‐connectivity of subcortical resting state networks in social anxiety disorder. Brain Connect 4:81–90. [DOI] [PubMed] [Google Scholar]
- Ashburner J (2007): A fast diffeomorphic image registration algorithm. Neuroimage 38:95–113. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Killcross S (2006): Parallel incentive processing: An integrated view of amygdala function. Trends Neurosci 29:272–279. [DOI] [PubMed] [Google Scholar]
- Bartlett EJ, Brodie JD, Simkowitz P, Dewey SL, Rusinek H, Wolf AP, Fowler JS, Volkow ND, Smith G, Wolkin A (1994): Effects of haloperidol challenge on regional cerebral glucose utilization in normal human subjects. Am J Psychiatry 151:681–686. [DOI] [PubMed] [Google Scholar]
- Benoit‐Marand M, Ballion B, Borrelli E, Boraud T, Gonon F (2011): Inhibition of dopamine uptake by D2 antagonists: An in vivo study. J Neurochem 116:449–458. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS (1995): Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magn Reson Med 34:537–541. [DOI] [PubMed] [Google Scholar]
- Braskie MN, Landau SM, Wilcox CE, Taylor SD, O'Neil JP, Baker SL, Madison CM, Jagust WJ (2011): Correlations of striatal dopamine synthesis with default network deactivations during working memory in younger adults. Hum Brain Mapp 32:947–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brassen S, Gamer M, Peters J, Gluth S, Büchel C (2012): Don't look back in anger! Responsiveness to missed chances in successful and nonsuccessful aging. Science 336:612–614. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Meyer‐Lindenberg A (2012): Psychopathology and the human connectome: Toward a transdiagnostic model of risk for mental illness. Neuron 74:990–1004. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Yeo BT (2013): Opportunities and limitations of intrinsic functional connectivity MRI. Nat Neurosci 16:832–837. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Barnes A, Bassett DS, Fornito A, Kitzbichler M, Meunier D, Suckling J (2009): Generic aspects of complexity in brain imaging data and other biological systems. Neuroimage 47:1125–1134. [DOI] [PubMed] [Google Scholar]
- Cao H, Bertolino A, Walter H, Schneider M, Schäfer A, Taurisano P, Blasi G, Haddad L, Grimm O, Otto K, Dixson L, Erk S, Mohnke S, Heinz A, Romanczuk‐Seiferth N, Mühleisen TW, Mattheisen M, Witt SH, Cichon S, Noethen M, Rietschel M, Tost H, Meyer‐Lindenberg A (2016a): Altered functional subnetwork during emotional face processing: A potential intermediate phenotype for Schizophrenia. JAMA Psychiatry 73:598–605. [DOI] [PubMed] [Google Scholar]
- Cao H, Dixson L, Meyer‐Lindenberg A, Tost H (2016b): Functional connectivity measures as schizophrenia intermediate phenotypes: Advances, limitations, and future directions. Curr Opin Neurobiol 36:7–14. [DOI] [PubMed] [Google Scholar]
- Cao H, Plichta MM, Schäfer A, Haddad L, Grimm O, Schneider M, Esslinger C, Kirsch P, Meyer‐Lindenberg A, Tost H (2014): Test‐retest reliability of fMRI‐based graph theoretical properties during working memory, emotion processing, and resting state. Neuroimage 84:888–900. [DOI] [PubMed] [Google Scholar]
- Carbonell F, Nagano‐Saito A, Leyton M, Cisek P, Benkelfat C, He Y, Dagher A (2014): Dopamine precursor depletion impairs structure and efficiency of resting state brain functional networks. Neuropharmacology 84:90–100. [DOI] [PubMed] [Google Scholar]
- Cheng YF, Paalzow LK, Bondesson U, Ekblom B, Eriksson K, Eriksson SO, Lindberg A, Lindström L (1987): Pharmacokinetics of haloperidol in psychotic patients. Psychopharmacology 91:410–414. [DOI] [PubMed] [Google Scholar]
- Cole DM, Beckmann CF, Oei NYL, Both S, van Gerven JMA, Rombouts SARB (2013): Differential and distributed effects of dopamine neuromodulations on resting‐state network connectivity. NeuroImage 78:59–67. [DOI] [PubMed] [Google Scholar]
- Cole DM, Oei NYL, Soeter RP, Both S, van Gerven JMA, Rombouts SARB, Beckmann CF (2012): Dopamine‐dependent architecture of cortico‐subcortical network connectivity. Cereb Cortex 23:1509–1516. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts S, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF (2006): Consistent resting‐state networks across healthy subjects. Proc Natl Acad Sci USA 103:13848–13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Whalen PJ (2001): The amygdala: Vigilance and emotion. Mol Psychiatry 6:13–34. [DOI] [PubMed] [Google Scholar]
- Deckers RHR, van Gelderen P, Ries M, Barret O, Duyn JH, Ikonomidou VN, Fukunaga M, Glover GH, de Zwart JA (2006): An adaptive filter for suppression of cardiac and respiratory noise in MRI time series data. Neuroimage 33:1072–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr OM, Zhang S, Hu S, Matuskey D, Abdelghany O, Malison RT, Li CSR (2014): The effects of methylphenidate on resting‐state striatal, thalamic and global functional connectivity in healthy adults. Int J Neuropsychopharmacol 17:1177–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA (2003): Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci 6:968–973. [DOI] [PubMed] [Google Scholar]
- Fornito A, Harrison BJ, Goodby E, Dean A, Ooi C, Nathan PJ, Lennox BR, Jones PB, Suckling J, Bullmore ET (2013): Functional dysconnectivity of corticostriatal circuitry as a risk phenotype for psychosis. JAMA Psychiatry 70:1143–1151. [DOI] [PubMed] [Google Scholar]
- Fornito A, Zalesky A, Bassett DS, Meunier D, Ellison‐Wright I, Yücel M, Wood SJ, Shaw K, O'Connor J, Nertney D, Mowry BJ, Pantelis C, Bullmore ET (2011): Genetic influences on cost‐efficient organization of human cortical functional networks. J Neurosci 31:3261–3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A, Zalesky A, Pantelis C, Bullmore ET (2012): Schizophrenia, neuroimaging and connectomics. Neuroimage 62:2296–2314. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME (2007): Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8:700–711. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME (2005): The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Greicius M (2010): Clinical applications of resting state functional connectivity. Frontiers in systems neuroscience 4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P, Marrelec G (2008): The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. NeuroImage 42:1178–1184. [DOI] [PubMed] [Google Scholar]
- Froemming JS, Lam YW, Jann MW, Davis CM (1989): Pharmacokinetics of haloperidol. Clin Pharmacokinet 17:396–423. [DOI] [PubMed] [Google Scholar]
- Gass N, Schwarz AJ, Sartorius A, Cleppien D, Zheng L, Schenker E, Risterucci C, Meyer‐Lindenberg A, Weber‐Fahr W (2012): Haloperidol modulates midbrain‐prefrontal functional connectivity in the rat brain. Eur Neuropsychopharmacol 23:1310–1319. [DOI] [PubMed] [Google Scholar]
- Glover GH, Li T-Q, Ress D (2000): Image‐based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med 44:162–167. [DOI] [PubMed] [Google Scholar]
- Goto Y, Grace AA (2008): Limbic and cortical information processing in the nucleus accumbens. Trends Neurosci 31:552–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Grace AA (2005): Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal‐directed behavior. Nat Neurosci 8:805–812. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V (2003): Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 100:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AV, Voorn P (1999): Convergence and segregation of ventral striatal inputs and outputs. Ann N Y Acad Sci 877:49–63. [DOI] [PubMed] [Google Scholar]
- Hallquist MN, Hwang K, Luna B (2013): The nuisance of nuisance regression: Spectral misspecification in a common approach to resting‐state fMRI preprocessing reintroduces noise and obscures functional connectivity. Neuroimage 82:208–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley R, Zelaya FO, Reinders AATS, Marques TR, Mehta MA, O'Gorman R, Alsop DC, Taylor H, Johnston A, Williams S, McGuire P, Pariante CM, Kapur S, Dazzan P (2013): Acute effects of single‐dose aripiprazole and haloperidol on resting cerebral blood flow (rCBF) in the human brain. Hum Brain Mapp 34:272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapogiannis D, Reiter DA, Willette AA, Mattson MP (2013): Posteromedial cortex glutamate and GABA predict intrinsic functional connectivity of the default mode network. Neuroimage 64:112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C, Zubicaray G, de Martino AD, Copland DA, Reiss PT, Klein DF, Castellanos FX, Milham MP McMahon K (2009): l‐Dopa modulates functional connectivity in striatal cognitive and motor networks: A double‐blind placebo‐controlled study. J Neurosci 29:7364–7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND (2009): Neurocircuitry of addiction. Neuropsychopharmacology 35:217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahti AC, Holcomb HH, Weiler MA, Medoff DR, Tamminga CA (2003): Functional effects of antipsychotic drugs: Comparing clozapine with haloperidol. Biol Psychiatry 53:601–608. [DOI] [PubMed] [Google Scholar]
- Laviolette SR (2007): Dopamine modulation of emotional processing in cortical and subcortical neural circuits: Evidence for a final common pathway in schizophrenia? Schizophr. Bull 33:971–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold DA, Maier A (2012): Ongoing physiological processes in the cerebral cortex. Neuroimage 62:2190–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CM, Baldassarre A, Committeri G, Romani GL, Corbetta M (2009): Learning sculpts the spontaneous activity of the resting human brain. Proc Natl Acad Sci USA 106:17558–17563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HY, Gau SSF (2015): Atomoxetine treatment strengthens an anti‐correlated relationship between functional brain networks in medication‐naïve adults with attention‐deficit hyperactivity disorder: A randomized double‐blind placebo‐controlled clinical trial. Int J Neuropsychopharmacol 19:1461–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo CYZ, Su TW, Huang CC, Hung CC, Chen WL, Lan TH, Lin CP, Bullmore ET (2015): Randomization and resilience of brain functional networks as systems‐level endophenotypes of schizophrenia. Proc Natl Acad Sci USA 112:9123–9128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies DS, Vincent JL, Kelly C, Lohmann G, Uddin LQ, Biswal BB, Villringer A, Castellanos FX, Milham MP, Petrides M (2009): Precuneus shares intrinsic functional architecture in humans and monkeys. Proc Natl Acad Sci USA 106:20069–20074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger CD, Wiegers M, Walter M, Abler B, Graf H (2015): Local and global resting state activity in the noradrenergic and dopaminergic pathway modulated by reboxetine and amisulpride in healthy subjects. Int J Neuropsychopharmacol 19:pyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S, Costa A, Keeser D, Pogarell O, Berman A, Coates U, Reiser MF, Riedel M, Möller HJ, Ettinger U, Meindl T (2014): The effects of methylphenidate on whole brain intrinsic functional connectivity. Hum Brain Mapp 35:5379–5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA (2007): The amygdala, reward and emotion. Trends Cognit Sci 11:489–497. [DOI] [PubMed] [Google Scholar]
- Nasrallah FA, Lew SK, Low ASM, Chuang KH (2014): Neural correlate of resting‐state functional connectivity under α2 adrenergic receptor agonist, medetomidine. NeuroImage 84:27–34. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP (2002): Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum Brain Mapp 15:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent A, Mackey A, De Bellefeuille L (1983): The subcortical afferents to caudate nucleus and putamen in primate: A fluorescence retrograde double labeling study. Neuroscience 10:1137–1150. [DOI] [PubMed] [Google Scholar]
- Patel RS, Borsook D, Becerra L (2008): Modulation of resting state functional connectivity of the brain by naloxone infusion. Brain Imaging Behav 2:11–20. [Google Scholar]
- Power JD, Schlaggar BL, Petersen SE (2014): Studying brain organization via spontaneous fMRI signal. Neuron 84:681–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AM, Uddin LQ, Gotimer K, Biswal BB, Castellanos FX, Milham MP (2009): Functional connectivity of the human amygdala using resting state fMRI. Neuroimage 45:614–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russchen FT, Bakst I, Amaral DG, Price JL (1985): The amygdalostriatal projections in the monkey. An anterograde tracing study. Brain Res 329:241–257. [DOI] [PubMed] [Google Scholar]
- Salami A, Pudas S, Nyberg L (2014): Elevated hippocampal resting‐state connectivity underlies deficient neurocognitive function in aging. Proc Natl Acad Sci USA 111:17654–17659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer A, Burmann I, Regenthal R, Arélin K, Barth C, Pampel A, Villringer A, Margulies DS, Sacher J (2014): Serotonergic modulation of intrinsic functional connectivity. Curr Biol 24:2314–2318. [DOI] [PubMed] [Google Scholar]
- Schrantee A, Ferguson B, Stoffers D, Booij J, Rombouts S, Reneman L (2016): Effects of dexamphetamine‐induced dopamine release on resting‐state network connectivity in recreational amphetamine users and healthy controls. Brain Imaging Behav 10:548–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Parent A (1986): Differential connections of caudate nucleus and putamen in the squirrel monkey (Saimiri sciureus). Neuroscience 18:347–371. [DOI] [PubMed] [Google Scholar]
- Suckling J, Bullmore E (2004): Permutation tests for factorially designed neuroimaging experiments. Hum Brain Mapp 22:193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND (2011): Association between functional connectivity hubs and brain networks. Cereb Cortex 21:2003–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tost H, Bilek E, Meyer‐Lindenberg A (2012): Brain connectivity in psychiatric imaging genetics. Neuroimage 62:2250–2260. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15:273–289. [DOI] [PubMed] [Google Scholar]
- Utevsky AV, Smith DV, Huettel SA (2014): Precuneus is a functional core of the default‐mode network. J Neurosci 34:932–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Werff SJA, Pannekoek JN, Veer IM, van Tol MJ, Aleman A, Veltman DJ, Zitman FG, Rombouts SARB, Elzinga BM, van der Wee NJA (2013): Resting‐state functional connectivity in adults with childhood emotional maltreatment. Psychol Med 43:1825–1836. [DOI] [PubMed] [Google Scholar]
- Vértes PE, Alexander‐Bloch AF, Gogtay N, Giedd JN, Rapoport JL, Bullmore ET (2012): Simple models of human brain functional networks. Proc Natl Acad Sci USA 109:5868–5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield‐Gabrieli S, Nieto‐Castanon A (2012): Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2:125–141. [DOI] [PubMed] [Google Scholar]
- Wise RA (2004): Dopamine, learning and motivation. Nat Rev Neurosci 5:483–494. [DOI] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Bullmore ET (2010): Network‐based statistic: Identifying differences in brain networks. NeuroImage 53:1197–1207. [DOI] [PubMed] [Google Scholar]
- Zhang D, Raichle ME (2010): Disease and the brain's dark energy. Nat Rev Neurol 6:15–28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


