Abstract
The periaqueductal gray matter (PAG) is a key brain region of the descending pain modulation pathway. It is also involved in cardiovascular functions, anxiety, and fear; however, little is known about PAG subdivisions in humans. The aims of this study were to use resting‐state fMRI‐based functional connectivity (FC) to parcellate the human PAG and to determine FC of its subregions. To do this, we acquired resting‐state fMRI scans from 79 healthy subjects and (1) used a data‐driven method to parcellate the PAG, (2) used predefined seeds in PAG subregions to evaluate PAG FC to the whole brain, and (3) examined sex differences in PAG FC. We found that clustering of the left and right PAG yielded similar patterns of caudal, middle, and rostral subdivisions in the coronal plane, and dorsal and ventral subdivisions in the sagittal plane. FC analysis of predefined subregions revealed that the ventolateral(VL)‐PAG was supfunctionally connected to brain regions associated with descending pain modulation (anterior cingulate cortex (ACC), upper pons/medulla), whereas the lateral (L) and dorsolateral (DL) subregions were connected with brain regions implicated in executive functions (prefrontal cortex, striatum, hippocampus). We also found sex differences in FC including areas implicated in pain, salience, and analgesia including the ACC and the insula in women, and the MCC, parahippocampal gyrus, and the temporal pole in men. The organization of the human PAG thus provides a framework to understand the circuitry underlying the broad range of responses to pain and its modulation in men and women. Hum Brain Mapp 37:1514‐1530, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: fMRI, PAG, parcellation, resting‐state fMRI, pain, descending pain modulation
INTRODUCTION
The periaqueductal gray matter (PAG) is implicated in multiple functions, such as pain, analgesia, fear, anxiety, and cardiovascular responses and is believed to be a central hub for the integration of the pain experience. The PAG was first found to be involved in analgesia in the late 1960s when Reynolds observed significant analgesia following electrical stimulations of the PAG in animals [Mayer et al., 1971; Reynolds, 1969]. This effect was attributed to neuronal transmission through the dorsolateral funiculus [Basbaum and Fields, 1979], resulting in the inhibition of wide dynamic range neurons in the spinal dorsal horn [Zhang et al., 1991]. The pain reduction generated by PAG stimulation is now attributed to inhibitory effects on spinal nociceptive activity via monoaminergic projections from the rostroventral medulla (RVM) and the A7 noradrenergic nucleus of the medulla [Millan, 2002]. In humans, stimulation of the PAG, and its rostral extension, the periventricular gray, can produce analgesia but can also evoke unpleasant feelings [Hosobuchi, 1983], painful sensations [Nashold et al., 1969], and analgesia [Pereira et al., 2013b]. In addition to pain and analgesia, the PAG is also involved in fear, anxiety, vocalization, lordosis, and cardiovascular responses [Behbehani, 1995]. Thus, it is believed that information associated with stressful/painful events are integrated via the PAG to generate an appropriate coping response for survival in the face of a threat, also known as, “survival salience” [Linnman et al., 2012b].
The PAG does not show clear divisions based on cytoarchitecture but it has been subdivided into 4 columns in rats, cats, and primates [Carrive, 1993; Linnman et al., 2012b; Lumb, 2002; Mai and Paxinos, 2012]. The connectivity between PAG subregions and specific functions are thought to be modulated by a network, receiving inputs from the hypothalamus, autonomic system, and the prefrontal lobe, depending on the nature of an event [Behbehani, 1995]. Thus, the internal modulation network of the PAG, and its connectivity with these regions (through GABAergic inhibitory network and peptidergic afferents) provide a framework for responses to stressors and pain [Behbehani, 1995; Keay and Bandler, 2001; Lumb, 2004].
It has been proposed that different subregions of the PAG are involved in distinct stimulus‐evoked responses [Green et al., 2006; Linnman et al., 2012b; Pereira et al., 2010; Satpute et al., 2013]. For example, stimulation of the rostral dorsolateral region of the PAG in animals evokes defensive behavior, whereas stimulation of the caudal ventrolateral PAG results in hyporeactivity and quiescence [Carrive et al., 1988; Depaulis et al., 1994]. These subregions may also be involved in distinct coping strategies [Bandler et al., 2000b] and different types of analgesia (i.e., opioid vs nonopioid mediated) [Mai and Paxinos, 2012]. Coping strategies are often related to whether a stress is escapable or inescapable. An escapable threat can be considered temporary or spontaneous, such as a fight, predator, or natural disaster and typically activates the sympathetic nervous system to prepare the body for upcoming danger (e.g., tachycardia, hypertension, pupil dilatation, blood perfusion of the muscles, and increased vigilance). In contrast, inescapable stressors are generally longer term events, such as internal injuries, a chaotic work environment or bullying. This type of stressor induces disengagement or withdrawal from the environment, bradycardia, decreased reactivity, limitation of movement, and is associated with negative consequences such as anxiety and depression [Alloy et al., 1999; Samwel et al., 2006]. A painful stimulus can be classified as escapable or inescapable depending on the nature of the pain (i.e., intermittent cutaneous vs persistent cutaneous vs deep somatic pain) [Keay and Bandler, 2002]. Interestingly, it has been reported that there are differences in how men and women perceive pain [Mogil, 2012], and cope with stressful and painful situations [Keogh et al., 2000; Keogh and Herdenfeldt, 2002; Keogh and Mansoor, 2001], which could be associated with a dimorphic anatomy of the PAG [Kong et al., 2010; Linnman et al., 2012a; Loyd and Murphy, 2009].
rs‐fMRI can be used to identify FC based on task independent patterns of low‐frequency oscillations that are synchronous between brain regions [Biswal et al., 1995]. This technique is particularly useful to understand functionally complex regions, such as the PAG [Fox et al., 2006]. The study of PAG FC is of growing interest among pain scientists [Hemington and Coulombe, 2015], particularly in the field of chronic pain diseases; however, its subdivisions are not typically considered. Although some studies noted the existence of PAG subdivisions, they typically assume that the VL‐PAG represents the descending pain modulation system, disregarding the contribution of the DL‐PAG in nonopioid‐mediated analgesia. Despite our understanding of the PAG in animal studies, a recent review by Linnman et al [2012b] highlighted our poor understanding of the subdivisions and connectivity of the human PAG, particularly, related to pain functions. Therefore, the aims of this study were to use resting‐state fMRI (rs‐fMRI) functional connectivity (FC) to parcelate the PAG and to delineate the relationship of PAG subregions with the rest of the brain in humans. Additionally, based on previous studies demonstrating PAG dimorphism between males and females [Loyd and Murphy, 2009], we investigated sex differences in PAG FC. We hypothesized that FC can identify functional organization or patterns associated with different PAG subregions.
METHODS
Study Participants
A total of 80 right‐handed healthy subjects were recruited and provided informed written consent to procedures approved by the University Health Network Research Ethics Board. Portions of the study on this cohort have been previously published [Erpelding and Davis, 2013; Wang et al., 2014]. Subjects were excluded if they had any chronic illness including neurological/psychiatric disorders and recurrent or chronic pain, or if they were pregnant or breastfeeding.
This study consisted of rs‐fMRI data from 79 of the 80 right‐handed subjects (39 females, 40 males; 19–36 years old, age: mean ± SD, age 24.5 ± 5.0 years), as one subject was excluded due to incomplete data acquisition.
Data Acquisition and Preprocessing
Each participant underwent neuroimaging on a 3 T MRI system (GE Medical Systems, Milwaukee, WI) with an eight‐channel phased array head coil, including a high‐resolution T1‐weighted whole‐brain anatomical scan (180 axial slices; 256 × 256 matrix; 25.6 cm field of view; 1 × 1 × 1 mm voxels) using a T1‐weighted inversion recovery prepped, 3‐D fast spoiled gradient echo (IR‐FSGPR) sequence (flip angle = 15°; TE = 3 ms; TR = 7.8 ms; TI = 450 ms), and a 5 min rs‐fMRI T2*‐weighted EPI scan (40 slices; 64 × 64 matrix; 20 cm field of view; 3.125 × 3.125 × 4 mm voxels; TE = 30 ms; TR = 2000 ms; 150 volumes). Every subject was told to keep their eyes closed and to try to relax and think of nothing in particular [Erpelding and Davis, 2013].
Preprocessing was carried out using the FSL software (FMRIB's software library, http://www.fmrib.ox.ac.uk/fsl), Matlab customized scripts, and fMRISTAT toolbox. The brain was extracted using FSL's brain extraction tool, the first four volumes were deleted, and the data were corrected for head motion using six motion parameters (MCFLIRT). The scans were then linearly realigned to the first T1‐weighted image (FLIRT, 6 DOF) and spatially registered to the Montreal Neurological Institute (MNI) 152 2 mm standard space (FLIRT, 12 DOF). We used the aCompCor data processing method to correct for spurious noise sources and motion‐related artifacts, as described previously [Behzadi et al., 2007; Kucyi et al., 2014, 2013; Muschelli et al., 2014]. The aCompCor approach aims to identify patterns of structured noise in regions where there should not be signal from neuronal origin [Behzadi et al., 2007]. More precisely, the anatomical image was segmented into white matter (WM), grey matter (GM), and cerebrospinal fluid (CSF) using FMRIB's automated segmentation tool FAST to obtain masks of noise regions of interest (ROIs). Then, the segments were linearly registered to functional space, and the WM and CSF maps were eroded by thresholding to retain the top 198 cm3 and top 20 cm3 of voxels with highest probability of being WM and CSF [Chai et al., 2012; Kucyi et al., 2013]. Principal component analysis (PCA) was run on the time series of the eroded WM and CSF ROIs, and the top 5 WM and CSF components, respectively, and the six motion parameters previously obtained with MCFLIRT were regressed out of the whole‐brain data. Resulting images were then spatially smoothed with a 4 mm full‐width at half‐maximum (FWHM) kernel and temporally filtered using a 0.01–0.1 Hz bandpass filter.
Clustering
A mask of the PAG was drawn, guided by the Duvernoy's atlas, on the MNI 152 1 mm standard space (Fig. 1) [Naidich et al., 2009]. The mask was linearly transformed to 4 mm standard space and binarized. We used a script publicly available via http://fcon_1000.projects.nitrc.org [Kelly et al., 2012] that classifies voxels into natural subsets according to their whole brain correlation map (data‐driven). The time series of each voxel of the PAG mask was extracted and a correlation map was computed using Pearson correlation between the time series of each voxel of the PAG mask and every voxel of the brain. Then, the eta2 statistic (as described by Kelly et al. [2012] and Cohen et al. [2008]) was used to evaluate the similarity between all pairs of PAG voxel correlation maps. The eta2 statistic computation provided a similarity matrix which was used in a subsequent spectral clustering step. This step used the spectral clustering Meila‐Shi algorithm [Meila and Shi, 2001] performed with Matlab (http://www.stat.washington.edu/spectral/). This step resulted in a generalized eigen‐decomposition of the eta2 matrix. Then, a k‐means clustering algorithm was used to classify the data into clusters, i.e. group of contiguous voxels. Since a clustering solution was derived for every subject, we evaluated the most stable clustering results using a consensus clustering approach across subjects as described previously [Kelly et al., 2012]. This final step provides a heightened robustness of the results for K = 2 to K = 7, ensuring stability between subjects [Kelly et al., 2012]. Detailed description of the scripts and in‐depth explanations about this method were described in Kelly et al. [2012]. In the following sections, those clusters will be referred as “parcellation cluster” (i.e., group of contiguous voxel obtain through a parcellation algorithm).
Figure 1.
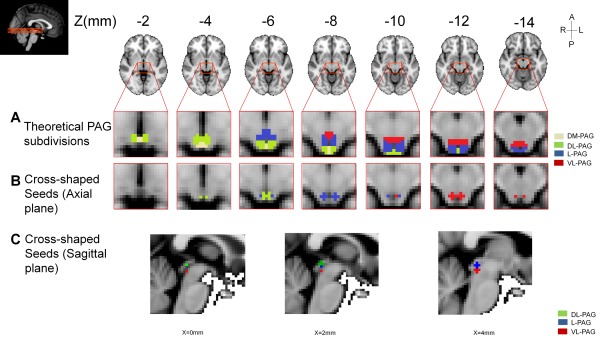
Theoretical subdivision of the PAG (A) and seeds used in the seed‐to‐whole brain analysis (B and C) shown in 2 mm3 standard space. Seeds were designed as 2‐mm‐radius spherical seeds in 1 mm standard space (cross‐shaped once transformed to 2 mm3 standard space and binarized). 1 mm standard space MNI coordinates: VL‐PAG (red) [±3, −32, −12], L‐PAG (blue) [±4, −31, −8], DL‐PAG (green) [±2, −32, −5]. R: right; L: left; A: anterior; P: posterior. The theoretical subdivision of the PAG is provided to illustrate the uneven distribution of the DL‐, L‐, and VL‐PAG in the inferior part of the PAG compared to rostral part of the PAG, for example, the superior part of the PAG is composed mainly of dorsal PAG (medial and lateral) and the inferior part of the PAG is composed mainly of lateral and ventrolateral PAG, with a smaller portion for the dorsal PAG. Seeds shown on sagittal plane are presented in Supporting Information (Fig. S1). For a complete schematic representation of the PAG, see Bandler and Shipley [1994] and Linnman et al. [2012b].
Resting‐State Connectivity Analysis
We next used spherical 2‐mm‐radius seeds originally drawn in 1 mm MNI space (coordinates: VL‐PAG [±3, −32, −12], L‐PAG [±4, −31, −8], DL‐PAG [±2, −32, −5]). The location of these seeds were based on previous studies [Linnman et al., 2012b] and each were registered to 2 mm3 MNI space using trilinear interpolation and binarized (Fig. 1B). [Note 1: the relationship between these seeds and parcellation cluster are showed as Supporting Information, Fig. S2; Note 2: Limitation and experimental design are further discussed in the limitation section.] The mean fMRI time series across all seed voxels was then extracted from the preprocessed functional data of every subject. The time series of each seed was correlated with the time series of every voxel of the brain. The analysis of single subject was done using FMRIB's FMRI Expert Analysis Tool (FEAT) with FILM general linear model. The functional data were prewhitened and each seed was used as an explanatory variable (using multiple regressions) so that the effect of the seeds’ FC on one another would be controlled for. The analyses used in this GLM were (1) VL‐PAG positive correlation, (2) VL‐PAG negative correlation, (3) L‐PAG positive correlation, (4) L‐PAG negative correlation, (5) DL‐PAG positive correlation, (6) DL‐PAG negative correlation, (7) VL‐PAG > L‐PAG, (8) L‐PAG > VL‐PAG, (9) VL‐PAG > DL‐PAG, (10) DL‐PAG > VL‐PAG, (11) L‐PAG > DL‐PAG, and (12) DL‐PAG > L‐PAG. The higher level analysis used the 12 resulting contrast of parameter estimate (COPE) images in a FMRIB's mixed effects (FLAME 1 + 2) thresholded at Z > 2.3 and a cluster‐based P < 0.05. To investigate potential sex difference, we only used COPEs 1, 3, 5 in the mixed effects analysis (thresholded at Z > 2.3 and a cluster‐based P < 0.05) in the following group contrasts: (1) female positive correlation, (2) female negative correlation, (3) male positive correlation, (4) male negative correlation, (5) female > male, and (6) male > female. [Note: The FLAME 1 + 2 analysis results in a group of contiguous voxels (cluster) with correlated functional connectivity. Each voxel has a probability of being part of the cluster (thresholded at Z > 2.3 and a cluster‐based P < 0.05. Every voxels of the brain were used in the analysis; therefore, a significant group of voxels (cluster) can contain voxels of distinct anatomic brain regions. A group of significant voxels can contain more than one “peak region,” and these peak regions can be located in a same or a different brain region. These “peak regions” are those described in the tables. In the following sections, the group of voxels obtained through FMRIB's mixed effects analysis will be referred as a “correlation cluster.”
The higher level analysis used FMRIB's mixed effects thresholded at Z = 2.3 and a cluster‐based P = 0.05 (Flame 1 + 2).
RESULTS
PAG Parcellation Clusters
Parcellation clusters are shown in Figure 2 (Fig. 2 is showing the results on axial planes; Supporting Information, Fig. S1 showing the results on sagittal planes). The parcellation of the right and the left PAG yielded very similar, symmetrical results. There were some minor differences that were observed in the K = 2 (axial slice Z = −8 mm) and in the K = 5 (axial slice Z = −12 mm). The most rostral parcellation cluster was identical for K = 2 to K = 5. A comparison to a theoretical PAG subdivision and K = 3–4 (transformed to 2 mm3 standard space, thresholded between 0.5 and 1) is presented in Supporting Information, as well as the position of small seeds in relation with those subdivisions (Supporting Information, Fig. S2). For the K = 2 parcellations clusters, there is a subdivision into distinct rostral and caudal parcellation clusters; the middle section (Z = −8 mm) of the left PAG is equally divided into parcellation cluster 1 (green) and 2 (purple) (Fig. 2). In K = 3, there was a division in rostral, middle, and caudal parcellation clusters with a similar overlap of parcellation cluster 1 (green) and 2 (pink) in the middle section of the PAG (Z = −8 mm). In K = 4, this overlap between parcellation cluster was observed across the middle and inferior slices. These overlapping parcellation clusters were column shaped, and interestingly, the ventrodorsal division was observed in many clustering solutions (for example, see K = 2: Z = −8 mm, K = 3: Z = −8 mm, K = 4: Z = −8 and −12, and K = 5: Z = −8 and −12 mm) and appear very similar to the ones previously described in animal studies [Behbehani, 1995].
Figure 2.
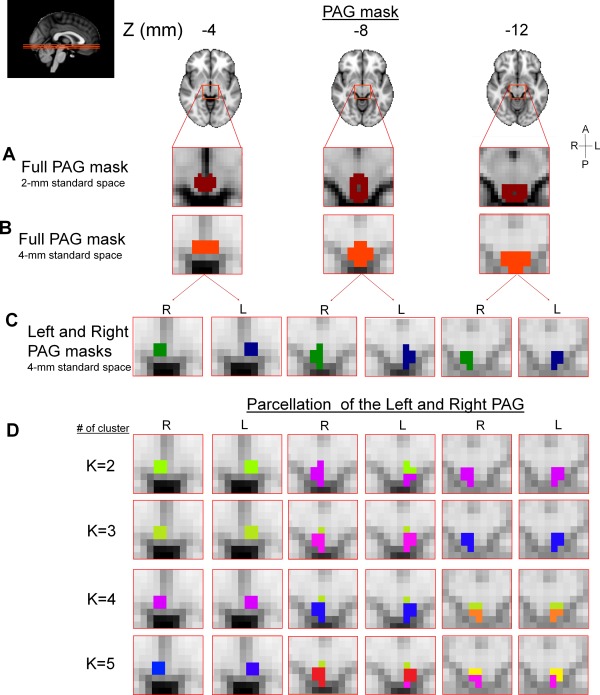
(A) Full PAG mask as drawn in 2 mm3 MNI space. (B) Full PAG mask transformed to 4 mm3 MNI space. (C) 4 mm3 standard space masks used for the PAG parcellation (right and left, 15 4 × 4 × 4 voxels each). (D) Parcellation of the right and left PAG into 2–5 clusters (using K = 2–5) projected onto on 3 axial slices (left panel, MNI152, Z = −12, −8, and −4). Different colors represent the different clusters. R: right; L: left; A: anterior; P: posterior.
PAG Subregions Connectivity with the Brain
The general pattern of PAG subdivisions obtained from the parcellation was similar to the one previously observed in animal studies [Bandler and Shipley, 1994; Behbehani, 1995; Lumb, 2002]. However, the parcellation clusters obtained from this study consisted of large 4 × 4 × 4 mm voxels. This is problematic because of the close proximity to the aqueduct. Thus, to increase precision in PAG subregions targeted, we conducted seed‐to‐whole brain analyses using small seeds based on the theoretical subdivisions of the PAG in order to understand their functions. [Note: Supplementary material shows similar results using previously obtained parcellation cluster as seed, Supporting Information, Figs. S6 and S7.] Our choice of methodology will be discussion in the limitation section. Seeds were selected using both our parcellation and general knowledge of the PAG. They were distributed both rostrocaudally and taking into account the ventral (VL), lateral (L), and dorsal (DL) localization of theoretical subdivisions of the PAG (Fig. 1). The correlation/anticorrelation maps are described below. These maps are composed of clusters of voxels significantly (anti‐)correlated with the seed. Each correlation cluster, as given using FSL, contains one or many peak values (i.e., coordinates with the highest Z‐score) (Figs. 3 and 4 and Tables 1 and 2).
Figure 3.
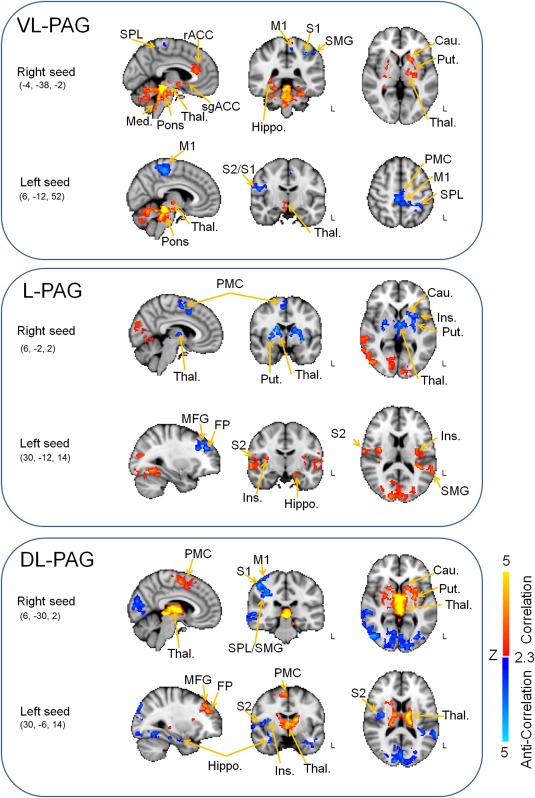
(Anti)‐correlation maps of the seed‐based analyses using left and right ventrolateral (VL)‐, lateral (L)‐, and dorsolateral (DL)‐PAG 2 mm spherical seeds. Red–yellow render shows correlation maps of the seeds, while blue–light blue render shows anticorrelation maps of the seeds. All clusters are thresholded at Z = 2.3–5, P < 0.05. rACC, rostral anterior cingulate cortex; sgACC, subgenual anterior cingulate cortex; Cau., caudate; FP, frontal pole; Hippo., hippocampus/parahippocampal gyrus; Ins., insular Cortex; M1, primary motor cortex; Med., medulla; MFG, middle frontal gyrus; PMC, premotor cortex; Put., putamen; S1, primary somatosensory cortex; S2, secondary somatosensory cortex; SMG, supramarginal gyrus; SPL, supraparietal lobule; Thal., Thalamus. Coordinates are in millimeters.
Figure 4.
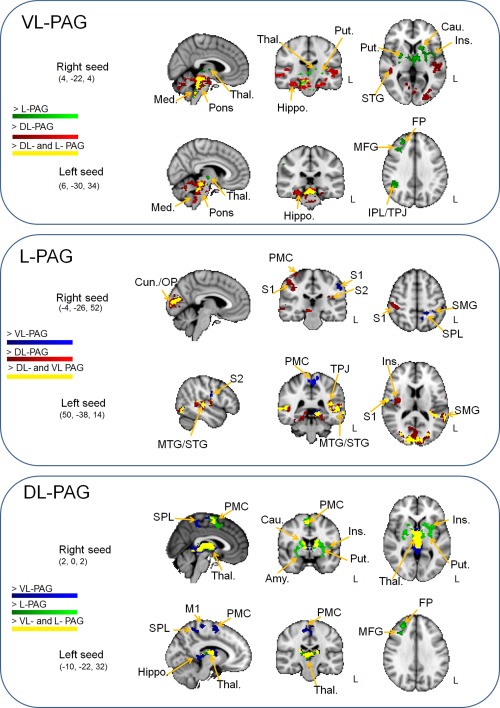
Contrast analyses between PAG subregions. All clusters are thresholded at Z = 2.3–5, P < 0.05. Amy., amygdala; Cau., caudate; FP, frontal pole; Hippo., hippocampus/parahippocampal gyrus; Ins., insular Cortex; IPL, intraparietal lobule; M1, primary motor cortex; Med., medulla; MFG, middle frontal gyrus; PMC, premotor cortex; Put., putamen; S1, primary somatosensory cortex; S2, secondary somatosensory cortex; SMG, supramarginal gyrus; SPL, supraparietal lobule; STG, superior temporal gyrus; Thal., Thalamus; TPJ, temporoparietal junction. Coordinates are in millimeters.
Table 1.
Peak MNI coordinates for brain regions positively correlated with ventrolateral‐, lateral‐, and dorsolateral‐PAG seeds
| Peak voxel, MNI coordinates | ||||
|---|---|---|---|---|
| Region | Z‐max | x | y | Z |
| Right ventrolateral PAG | ||||
| L/R PAG | 9.54 | 4 | −34 | −16 |
| R upper pons | 5.64 | 4 | −36 | −24 |
| R upper pons | 5.17 | 10 | −30 | −28 |
| R parahippocampal gyrus | 5.63 | 22 | −34 | −16 |
| L/R cerebellum | 5.11 | −10 | −58 | −20 |
| L rACC | 4.06 | −6 | 30 | 18 |
| L rACC/aMCC | 3.66 | −2 | 28 | 14 |
| R/L aMCC | 3.44 | −2 | 24 | 16 |
| R caudate/putamena | 3.77 | −16 | 14 | 2 |
| L putamena | 3.71 | 30 | −8 | −4 |
| L thalamusa | 3.57 | −10 | −16 | −2 |
| R/L putamena | 3.44 | 24 | 10 | −2 |
| R hippocampusa | 3.33 | 30 | −38 | 0 |
| L nucleus accumbensa | 2.94 | −12 | 16 | −4 |
| Left ventrolateral PAG | ||||
| L PAG | 10.8 | −2 | −32 | −12 |
| R upper pons | 5.14 | 6 | −36 | −26 |
| L/R cerebellum | 5.18 | 10 | −46 | −18 |
| R thalamusa | 3.33 | 8 | −14 | −2 |
| Right lateral PAG | ||||
| R lateral occipital cortex | 4.46 | 50 | −78 | 4 |
| L occipital pole/supracalcarine cortex | 4.05 | −4 | −90 | 10 |
| R occipital pole | 3.66 | 12 | −88 | 28 |
| R intracalcarine cortex/occipital pole | 3.65 | 10 | −86 | 12 |
| R middle temporal gyrus | 3.74 | 72 | −32 | −8 |
| R middle temporal gyrus | 3.71 | 72 | −42 | 4 |
| R occipital fusiform gyrus | 3.58 | 26 | −68 | −6 |
| Left lateral PAG | ||||
| L PAG | 8.66 | −6 | −32 | −10 |
| L medial geniculate body | 5.04 | −14 | −26 | −12 |
| R/L hippocampus/lingual gyrus | 4.89 | −14 | −36 | −4 |
| R occipital pole | 4.47 | 14 | −90 | 20 |
| R temporal occipital fusiform cortex | 4.28 | 28 | −56 | −10 |
| R/L superior temporal gyrus | 4.28 | −52 | −38 | 4 |
| L/R planum temporal | 4.06 | −40 | −40 | 12 |
| L/R middle temporal gyrus | 3.76 | −48 | −26 | −14 |
| L/R mid‐insulaa | 3.78 | 36 | −6 | 14 |
| L supramarginal gyrusa | 3.56 | −60 | −48 | 14 |
| R S1a | 3.08 | 60 | −4 | 22 |
| L/R S2a | 2.85 | 60 | −12 | 14 |
| Right dorsolateral PAG | ||||
| PAG | 10.7 | 0 | −32 | −4 |
| L/R thalamus | 7.39 | −2 | −16 | 8 |
| R premotor cortex BA6 (SMA) | 4.08 | 2 | −6 | 66 |
| Superior frontal gyrus BA8 | 3.32 | 0 | 34 | 50 |
| R paracingulate gyrus | 3.56 | 4 | 8 | 48 |
| L/R caudatea | 3.50 | −14 | 12 | 6 |
| L putamena | 4.83 | −22 | 6 | 8 |
| Left dorsolateral PAG | ||||
| PAG | 9.63 | 0 | −32 | −4 |
| L/R thalamus | 5.62 | −10 | −12 | 12 |
| R premotor cortex BA6 (SMA) | 4.11 | 10 | −6 | 62 |
| R frontal pole BA9 | 4.26 | 28 | 40 | 32 |
| R middle frontal gyrus BA8 | 4.26 | 34 | 32 | 42 |
| L cerebellum | 4.14 | −14 | −92 | −32 |
MNI coordinates (in mm) are reported at maximum peak and are corrected for multiple comparisons using family‐wise error correction at Z > 2.3 and P < 0.05. rACC, rostral anterior cingulate cortex; BA, Brodmann area; L, left; MCC, midcingulate cortex; PAG, periaqueductal gray; R, right; S1, primary somatosensory cortex; S2, secondary somatosensory cortex; SMA, supplementary motor area.
Subcluster peak coordinates.
Table 2.
Peak MNI coordinates for brain regions negatively correlated with ventrolateral‐, lateral‐, and dorsolateral‐PAG seeds
| Peak voxel, MNI coordinates | ||||
|---|---|---|---|---|
| Region | Z‐max | x | y | z |
| Right ventrolateral PAG | ||||
| L S1 BA2 | 4.18 | −32 | −38 | 50 |
| L IPL | 4.01 | −38 | −42 | 48 |
| L SPL 5M/Ci | 3.9 | −8 | −42 | 58 |
| L precuneus cortex | 3.54 | −10 | −54 | 56 |
| L M1a | 3.48 | −8 | −42 | 60 |
| L supramarginal gyrusa | 2.97 | −48 | −40 | 48 |
| Left ventrolateral PAG | ||||
| L SPL BA5‐7 | 4.38 | −38 | −44 | 48 |
| R S2 BA3 | 4.73 | 52 | −10 | 22 |
| R S1 BA3 | 3.48 | 46 | −8 | 28 |
| L/R M1 BA4 | 4.34 | 6 | −30 | 58 |
| L/R premotor cortex BA6a | 3.67 | −2 | −20 | 56 |
| Right lateral PAG | ||||
| PAG | 6.93 | 0 | −32 | −2 |
| L putamen | 4.83 | −20 | −4 | 10 |
| R caudate | 4.69 | 16 | 0 | 12 |
| L thalamus | 4.54 | −10 | −12 | 10 |
| R premotor cortex BA6/SMA | 3.65 | 6 | −4 | 56 |
| R paracingulate gyrus | 3.6 | 6 | 10 | 46 |
| L insulaa | 2.98 | −32 | 12 | 2 |
| Left lateral PAG | ||||
| R frontal pole BA10 | 4.44 | 28 | 40 | 32 |
| R middle prefrontal gyrus BA8/BA9 | 4.14 | 32 | 26 | 30 |
| Right dorsolateral PAG | ||||
| L occipital pole | 4.61 | −2 | −90 | 12 |
| R/L lingual gyrus | 4.6 | 14 | −60 | −4 |
| R/L occipital fusiform gyrus | 4.41 | 22 | −68 | −8 |
| R intracalcarine cortex | 4.2 | 10 | −84 | 12 |
| R lateral occipital cortex | 4.83 | 46 | −76 | 4 |
| R SPL (supramarginal gyrus) | 4.67 | 40 | −28 | 36 |
| R S1 BA1‐3 | 4.41 | 50 | −28 | 54 |
| R middle temporal gyrus | 3.84 | 64 | −24 | −6 |
| R supramarginal gyrus | 3.69 | 48 | −38 | 6 |
| R superior temporal gyrus | 3.65 | 52 | −34 | 4 |
| R M1a | 3.01 | 38 | −30 | 68 |
| Left dorsolateral PAG | ||||
| L PAG | 5.76 | −6 | −34 | −12 |
| R occipital pole | 4.63 | 16 | −92 | 22 |
| R lingual gyrus | 4.59 | 16 | −44 | −10 |
| R lateral occipital cortex | 4.42 | 44 | −80 | −8 |
| R/L middle temporal gyrus | 5.11 | 62 | −22 | −6 |
| R/L superior temporal gyrus | 4.09 | 54 | −22 | −2 |
| R/L middle temporal gyrus | 4.03 | 50 | −38 | 2 |
| R insula/S2 | 3.9 | 44 | −8 | 10 |
| L temporal pole | 3.93 | −34 | 14 | −34 |
| L temporal fusiform cortex, post. | 3.83 | −40 | −12 | −30 |
| L inferior temporal gyrus, ant. | 3.45 | −42 | −8 | −30 |
| L parahippocampal gyrus, ant. | 3.48 | −24 | −10 | −28 |
| L/R hippocampusa | 3.49 | 32 | −8 | −20 |
MNI coordinates (in mm) are reported at maximum peak and are corrected for multiple comparisons using family‐wise error correction at Z > 2.3 and P < 0.05. BA, Brodmann area; IPL, inferior parietal lobule; L, left; M1, primary motor cortex; PAG, periaqueductal gray; R, right; S1, primary somatosensory cortex; S2, secondary somatosensory cortex; SPL, superior parietal lobule.
Subcluster peak coordinates.
The analysis of the FC of each PAG seed region with voxels of the whole brain revealed different connectivity patterns among VL‐, L‐, and DL‐PAG subregions and the rest of the brain. The positive and negative correlation maps for each seed are described below.
FC of the VL‐PAG
The positive correlation map for the right VL‐PAG comprised one correlation cluster including areas within brainstem, cerebellum, and some subcortical regions (i.e., hippocampus, amygdala, caudate, and putamen) (Fig. 3 and Table 1). The second correlation cluster encompassed the anterior midcingulate cortex (aMCC) and the rostral anterior cingulate cortex (rACC). This second correlation cluster included the left and right anterior cingulate cortex (ACC), but the voxels in the aMCC were mainly in the right hemisphere, whereas the voxels located in the rACC were mainly in the left hemisphere. The left VL‐PAG showed positive correlation with voxels within the brainstem, the cerebellum, and some subcortical regions (i.e., hippocampus, thalamus).
Anticorrelations (i.e., negative FC) of the right VL‐PAG were also observed in the left parietal lobe including the primary somatosensory cortex (S1) and the superior parietal lobule (SPL) (Fig. 3 and Table 2). The anticorrelation map of the left VL‐PAG contained two distinct correlation clusters, including S1 and SPL, whereas the second correlation cluster included the primary motor cortex (M1).
Contrast analysis showed greater FC of the VL‐PAG with the brainstem (upper/lower pons, medulla) and the cerebellum compared to both L‐PAG and DL‐PAG regions (Fig. 4 and Table 3). Compared to L‐PAG, greater FC in the VL‐PAG compared to L‐PAG was observed in the dorsolateral prefrontal cortex (dlPFC), middle frontal gyrus, and frontal pole, as well as in many subcortical (thalamus, putamen, and caudate) and parietal regions (angular gyrus, supramarginal gyrus). Additionally, we found greater FC from the VL‐PAG to the parahippocampal gyrus/hippocampus, the temporal lobe (superior/middle temporal gyrus, planum polare), and the occipital lobe (occipital pole, lateral occipital cortex) compared to the DL‐PAG (Fig. 4 and Table 3).
Table 3.
Peak MNI coordinates for contrast comparisons analysis between PAG subregions
| Peak voxel, MNI coordinates | ||||
|---|---|---|---|---|
| Region | Z‐max | x | y | Z |
| Ventrolateral > lateral | ||||
| Right | ||||
| PAG | 8.12 | 4 | −34 | −16 |
| Hypothalamus/thalamus | 5.57 | 0 | −30 | −2 |
| L putamen | 4.58 | −26 | −4 | 8 |
| R caudate | 4.46 | 20 | −2 | 14 |
| L thalamus | 4.26 | −12 | −8 | 4 |
| Upper pons | 4.11 | 4 | −36 | −24 |
| Lower pons | 3.93 | −2 | −38 | −36 |
| Medulla | 3.92 | 4 | −44 | −54 |
| L insulaa | 3.03 | −40 | 16 | −2 |
| Left | ||||
| PAG/VTA/dorsal raphe | 7.89 | 0 | −32 | −16 |
| R frontal pole BA9 | 4.54 | 28 | 40 | 32 |
| R middle frontal gyrus BA9 | 4.2 | 32 | 26 | 30 |
| L cerebellum | 3.96 | −30 | −68 | −36 |
| R angular gyrus (TPJp) | 3.87 | 42 | −50 | 34 |
| R supramarginal gyrus (IPL) | 3.81 | 56 | −32 | 42 |
| R thalamus | 3.56 | 6 | −16 | −4 |
| R upper pons/central gray | 3.55 | 4 | −36 | −26 |
| R upper/mid pons | 3.54 | 4 | −38 | −30 |
| Lateral > ventrolateral | ||||
| Right | ||||
| L precuneus cortex | 4.35 | −10 | −56 | 56 |
| L SPL BA5/S1 BA2 | 4.18 | −20 | −44 | 50 |
| R/L occipital pole | 3.59 | −4 | −90 | 10 |
| L S1 | 3.53 | −32 | −40 | 50 |
| R/L intracalcarine cortex | 3.49 | 10 | −86 | 12 |
| L supramarginal gyrusa | 3.33 | −52 | −34 | 50 |
| Left | ||||
| R/L occipital pole | 4.38 | 14 | −90 | 20 |
| R/L superior temporal gyrus | 4.58 | −52 | −40 | 6 |
| R/L M1 BA4/SPL | 3.99 | −6 | −36 | 56 |
| R S1 BA3/S2 | 3.91 | 52 | −8 | 22 |
| R/L planum temporal (TPJa) | 3.78 | −40 | −40 | 14 |
| R/L superior temporal gyrus | 3.5 | −64 | −30 | 2 |
| R/L middle temporal gyrus | 3.42 | −54 | −56 | 4 |
| R lateral occipital cortex | 3.43 | 30 | −76 | 18 |
| R premotor cortex BA6 | 3.33 | 60 | 4 | 30 |
| R S2 | 3.19 | 56 | −10 | 14 |
| Ventrolateral > dorsolateral | ||||
| Right | ||||
| R PAG | 9.39 | 4 | −30 | −12 |
| R parahippocampal gyrus | 5.85 | 22 | −36 | −14 |
| R/L cerebellum | 5.59 | −12 | −50 | −22 |
| R superior temporal gyrus | 3.8 | 54 | −22 | −2 |
| R middle temporal gyrus | 3.65 | 62 | −26 | −6 |
| L occipital pole/lateral occipital cortex | 3.56 | −26 | −92 | −2 |
| L lingual gyrus | 3.36 | −20 | −84 | 0 |
| Left | ||||
| L PAG | 10 | −2 | −32 | −12 |
| R parahippocampal gyrus | 5.63 | 22 | −36 | −14 |
| R medulla | 4.72 | 2 | −42 | −54 |
| R upper pons | 4.55 | 6 | −34 | −24 |
| R mid pons | 4.51 | 4 | −38 | −34 |
| R temporal fusiform cortex/hippocampus | 4.3 | 34 | −28 | −20 |
| Dorsolateral > ventrolateral | ||||
| Right | ||||
| PAG | 11 | 0 | −32 | −4 |
| R/L thalamus | 6.41 | 0 | −28 | 6 |
| Premotor cortex BA6 (SMA) | 3.64 | 10 | 2 | 56 |
| Cingulate gyrus (pMCC) | 3.43 | −4 | −32 | 46 |
| R M1 BA4 | 3.28 | 4 | −36 | 58 |
| R SPL/M1a | 3.09 | 2 | −38 | 52 |
| Left | ||||
| PAG | 11.2 | 0 | −32 | −4 |
| R/L thalamus | 6.38 | −2 | −26 | 6 |
| R premotor cortex BA6 (SMA) | 4.05 | 10 | 0 | 58 |
| L superior frontal gyrus | 3.89 | −22 | 10 | 58 |
| R/L SPL/precuneous cortex | 3.71 | 12 | −20 | 58 |
| L hippocampusa | 3.40 | −10 | −40 | 2 |
| L M1a | 3.27 | −10 | −32 | 68 |
| Lateral > dorsolateral | ||||
| Right | ||||
| PAG | 6.86 | 8 | −34 | −6 |
| R/L occipital pole/intracalcarine cortex | 4.31 | −2 | −90 | 12 |
| R occipital fusiform gyrus/lingual gyrus | 4.1 | 22 | −68 | −8 |
| R lateral occipital cortex | 4.61 | 50 | −76 | 4 |
| R middle temporal gyrus | 3.81 | 70 | −32 | −10 |
| R superior temporal gyrus/lateral occipital cortex | 3.6 | 58 | −34 | 4 |
| S1 BA1‐3 | 4.22 | 50 | −28 | 54 |
| R/L supramarginal gyrus/SPL BA7 | 4 | 40 | −28 | 36 |
| L S2/insular gyrus | 4.32 | −42 | −30 | 20 |
| R premotor cortex/M1a | 3.31 | 34 | −28 | 70 |
| Left | ||||
| L PAG | 7.68 | −6 | −32 | −10 |
| R lingual gyrus | 4.61 | 16 | −44 | −10 |
| R/L occipital pole | 4.54 | 14 | −90 | 22 |
| L substantia nigra | 4.5 | −14 | −26 | −12 |
| R temporal occipital fusiform cortex | 4.37 | 28 | −56 | −10 |
| R/L middle temporal gyrus | 4.05 | −48 | −26 | −14 |
| R/L superior temporal gyrus | 4.05 | −50 | 0 | −20 |
| L planum temporal | 3.88 | −40 | −40 | 12 |
| L mid‐insulaa | 3.90 | 36 | −8 | 14 |
| L supramarginal gyrusa | 3.58 | −60 | −48 | 14 |
| Dorsolateral > lateral | ||||
| Right | ||||
| PAG | 8.65 | 0 | −32 | −2 |
| R/L thalamus | 5.78 | −4 | −16 | 8 |
| R premotor cortex BA6 (SMA) | 3.85 | 6 | −4 | 56 |
| R paracingulate gyrus | 3.56 | 4 | 8 | 48 |
| Left | ||||
| R PAG | 7.24 | 0 | −34 | −2 |
| R/L thalamus | 5.08 | −10 | −12 | 12 |
| R frontal pole | 4.59 | 28 | 40 | 32 |
| R middle frontal gyrus | 4.37 | 34 | 32 | 42 |
| R premotor cortex/SMA | 4.23 | 6 | −8 | 62 |
| L/R putamena | 4.88 | −24 | −6 | 10 |
| L/R caudatea | 4.33 | −16 | 0 | 18 |
| L insulaa | 3.50 | −34 | 6 | 6 |
| L/R amygdalaa | 2.91 | 26 | 0 | −12 |
MNI coordinates (in mm) are reported at maximum peak and are corrected for multiple comparisons using family‐wise error correction at Z > 2.3 and P < 0.05. BA, Brodmann area; IPL, inferior parietal lobule; L, left; M1, primary motor cortex; PAG, periaqueductal gray; R, right; S1, primary somatosensory cortex; S2, secondary somatosensory cortex; SMA, supplementary motor area; SPL, superior parietal lobule; TPJa, temporoparietal junction, anterior division; TPJp, temporoparietal junction, posterior division.
Subcluster peak coordinates.
FC of the L‐PAG
The positive correlation map of the right L‐PAG seed comprised a first correlation cluster located in the occipital lobe (lateral occipital cortex, occipital pole) and a second one localized in the right temporal lobe (middle temporal gyrus) (Fig. 3 and Table 1). The left L‐PAG showed correlation with peri‐PAG regions, some subcortical regions (thalamus and hippocampus), and the occipital lobe (lateral occipital cortex and occipital pole). The second and third correlation clusters were located in the left and the right temporal lobe, respectively. Both these correlation clusters contained the insular cortex, planum polare, middle and superior temporal gyrus, and secondary somatosensory cortex (S2), and on the right side, also S1.
The anticorrelation map of the right L‐PAG FC contained the rostral PAG and the subcortical regions including the caudate, putamen, pallidum, and thalamus, and a second correlation cluster located in the premotor cortex (Fig. 3 and Table 2). The anticorrelation map associated with the left L‐PAG was composed of a correlation cluster in the right frontal lobe (frontal pole and middle frontal gyrus).
Compared to both VL‐ and DL‐PAG, contrast analysis showed greater FC of L‐PAG with the S1, S2, parietal lobe (precuneus, supramarginal gyrus, SPL), temporal lobe (superior/middle frontal gyrus, planum polare/temporal), and occipital lobe (occipital pole, lateral occipital cortex, intracalcarine cortex) (Fig. 4 and Table 3). The insula, the premotor cortex, and M1 showed greater FC with L‐PAG compared to VL‐PAG (Fig. 4 and Table 3).
FC of the DL‐PAG
The positive correlation map of the right DL‐PAG seed contained two correlation clusters. The first correlation cluster was located within the PAG and subcortical regions (thalamus, putamen, caudate, amygdala) (Fig. 3 and Table 2). The second correlation cluster included mainly the premotor cortex (from the precentral gyrus to the superior frontal gyrus) (Fig. 3 and Table 1). The correlation map of the left DL‐PAG seed had four distinct correlation clusters. The first correlation cluster was composed of the PAG and subcortical regions (thalamus, caudate, putamen, and hippocampus) (Fig. 3 and Table 1). The second correlation cluster was formed by the right premotor cortex (supplementary motor area and superior frontal gyrus). The third correlation cluster was located in the right frontal lobe (frontal pole and middle frontal gyrus), and the fourth correlation cluster was located in the cerebellum (Fig. 3 and Table 1).
The anticorrelation map of the right DL‐PAG seed contained the occipital lobe, including the occipital pole, lingual gyrus, right S1, right SPL, right middle temporal gyrus, superior temporal gyrus, and supramarginal gyrus (Fig. 3 and Table 2). The anticorrelation map of the left DL‐PAG seed was composed of the right occipital lobe (occipital pole, lateral occipital cortex, lingual gyrus), right temporal lobe (middle temporal gyrus, superior temporal gyrus, insula, S2, temporal fusiform cortex, inferior temporal gyrus, and parahippocampal gyrus), and two correlation clusters located in the left temporal pole (Fig. 3 and Table 2).
Compared with both VL‐ and L‐PAG, the contrast analysis showed greater FC of DL‐PAG with the thalamus, cingulate/paracingulate gyrus and premotor cortex (Fig. 4 and Table 3). The DL‐PAG also had greater FC with the superior frontal gyrus, M1, and SPL/precuneus compared to VL‐PAG. Finally, DL‐PAG showed greater FC in the dlPFC (middle frontal gyrus, frontal lobe) compared to L‐PAG (Fig. 4 and Table 3).
Sex Differences in FC of PAG Subregions
FC of the VL‐PAG in men and women
The VL‐PAG correlation maps showed sex differences (particularly in the cingulate cortex) with some minor overlap between the FC for men and women (Supporting Information, Figs. S4 and S5 and Tables S1 and S2). In women, both right and left VL‐PAG were functionally connected to the cerebellum, the pons, and the ACC (including aMCC, pACC and sgACC) (Supporting Information, Fig. S4 and Table S1). The left VL‐PAG was connected to the temporal lobe and temporal pole, as well as the left insula, whereas the right VL‐PAG was connected with the putamen. In men, the left and right VL‐PAG are functionally connected to the pons and the cerebellum (Supporting Information, Fig. S4 and Table S2). The left VL‐PAG also had FC with the MCC, while the right VL‐PAG had FC with the temporal lobe and the parahippocampal gyrus. Compared to men, women had greater FC with the left insula, left inferior frontal gyrus, left orbitofrontal cortex, left temporal pole, left cerebellum, and left and right aMCC and premotor cortex (Supporting Information, Fig. S5 and Table S3).
FC of the L‐PAG in men and women
The FC of the L‐PAG in women was mainly observed within regions of the occipital lobe (i.e, occipital pole, intracalcarine cortex, lateral occipital cortex, temporal fusiform cortex, and occipital fusiform gyrus) (Supporting Information, Fig. S4 and Table S1). Men exhibited FC of the L‐PAG with the occipital lobe (i.e., occipital pole, lateral occipital cortex, and the cuneus), the temporal lobe (i.e., temporal pole, superior and middle temporal gyrus), as well as the inferior frontal gyrus and the frontal pole (Supporting Information, Fig. S4 and Table S2). Compared to women, men had significantly greater L‐PAG FC to the superior and middle temporal gyrus, the vlPFC, the orbitofrontal cortex, and the inferior frontal gyrus (Supporting Information, Fig. S5 and Table S3).
FC of the DL‐PAG in men and women
In both men and women, the left DL‐PAG was functionally connected with the thalamus and the premotor cortex, whereas the right DL‐PAG was functionally connected with the thalamus only (Supporting Information, Fig. S4 and Tables S1 and S2). In women, the right DL‐PAG was connected to the primary motor cortex and the midcingulate cortex (MCC), and the left DL‐PAG was connected with the caudate nucleus (Supporting Information, Fig. S4 and Table S1). In men, the right DL‐PAG was also connected with the cerebellum (Supporting Information, Fig. S4 and Table S2). Compared to women, men had greater FC between the left DL‐PAG and the lateral occipital cortex and the cuneus (Supporting Information, Fig. S5 and Table S3); the right DL‐PAG did not yield any significance FC between men and women.
DISCUSSION
In this study, we investigated the intrinsic FC of PAG subregions using rs‐fMRI. We hypothesized that the PAG in healthy volunteers subdivides into columns similar to those seen in animal studies. We also expected different FC between subregions, particularly between VL‐ and DL‐PAG. The clusters formed by our parcellation of the PAG voxels had some similarities with the theoretical subdivision of the PAG previously observed in animal studies [Bandler and Shipley, 1994; Keay et al., 2001; Keay et al., 1994]. Furthermore, we observed distinct connectivity patterns among PAG subregions using spherical seeds located in specific regions of the PAG. Finally, we have identified sex differences in the FC that included the cingulate and insula cortices.
The first goal of this study was to investigate the subdivision of the PAG in humans. The subdivisions of the PAG and its connectivity are well characterized in rats and monkeys [Bandler and Shipley, 1994; Keay et al., 2001; Keay et al., 1994], but not well described in humans. The parcellation analysis that we used in this study revealed that both left and right PAG can be subdivided into rostral and caudal as well as ventral and dorsal regions. The subdivision into rostral and caudal parcellation clusters is not typically what is expected of the PAG; however, this result corroborates previous findings and could be representative of the radial distribution of PAG columns. The radial distribution of PAG subregions is described in Supporting Information, Figure S2. By radial distribution, we mean that each column is not equally distributed rostrocaudally. More specifically, the dorsolateral subregion is more prominent in the rostral portion of the PAG, whereas the ventral subregion is predominant in the caudal portion of the PAG. Thus, the rostrocaudal pattern seen on lower clustering solution (K = 2–3) may represent the uneven distribution of PAG columns. Interestingly, this radial distribution was also seen by Ezra et al. [2015] who used probabilistic tractography to segment the PAG [Ezra et al., 2015]. In addition, our results are also in line with a study showing a rostrocaudal organization of the PAG as well as a radial pattern (from the caudal VL‐PAG, to the L‐PAG, and the rostral part of the DL‐PAG) during aversive‐image viewing [Satpute et al., 2013]. Finally, Van Oort et al. [2014] identified a rostrocaudal division of the PAG using instantaneous correlation parcellation. The observation of a rostrocaudal segmentation of intrinsic functional connectivity could also be associated with the somatotopy of the PAG, which is known to be rostrocaudally distributed [Pereira et al., 2013a].
In this parcellation analysis, we did not restrain the analysis to a specific cluster solution as did Ezra et al. [2015]. We evaluated many possibilities because we hypothesized that PAG subdivisions could be different in humans compared to animals. We investigated the parcellation of both the left and the right PAG (Fig. 2), but we also explored the parcellation of the PAG in men and women separately (Supporting Information, Figure S3). Parcellation of the left and right, as well as between males and females, yielded in similar results and therefore seem stable and consistent. It is very likely, as mentioned by Ezra et al. [2015], that the optimal clustering solution is K = 3 or K = 4. Indeed, the K = 5 clustering solution (Fig. 2) consisted of four parcellation clusters that were very similar to the K = 4 clustering solution, although, the fifth parcellation cluster is composed of a single voxel (Fig. 2, K = 5, the green cluster). The fact that the algorithm yielded a single voxel cluster suggests that the maximum of parcellation clusters was being approached. Therefore, to understand the function and the connectivity of these clusters, we investigated of the FC of these subdivisions using small and localized seeds. [Note: Seed‐to‐voxel analysis using parcellation cluster as seeds were obtained and showed as Supporting Information, Figs. S6 and S7, methodology and limitation are discussed later.]
In the context of pain, PAG subregions have been associated with different types of painful stimulations. However, it remains unknown whether the nature or the meaning of the pain is predominantly involved in the integration of the sensation of a noxious stimulus. Some studies showed the activation of L‐/DL‐PAG during intermittent noxious stimulations, whereas VL‐PAG was activated during continuous noxious stimulation [Keay and Bandler, 2001; Keay et al., 2002]. These stimuli are often described as escapable or inescapable. In animals, the VL‐PAG is often associated with opioid‐mediated analgesia, while DL‐PAG is associated with nonopioid analgesia [Behbehani, 1995; Behbehani et al., 1990; Linnman et al., 2012b]. However in humans, the subregion involved in opioid analgesia remains unknown. Many brain imaging pain studies of PAG connectivity have used a seed in the VL‐PAG because of its association with descending pain modulation and its relation with opioid‐mediated analgesia [Linnman et al., 2012b; Yu et al., 2014]. However, a study of local field potentials recorded in humans obtained from deep brain stimulation electrodes in the PAG and the periventricular gray (PAVG) showed that the DL‐PAVG may be related to pain relief through opioidergic mechanisms, while the VL‐PAVG may be associated with pain reduction involving vagal output and parasympathetic activity [Pereira et al., 2013b].
Additionally, the VL‐PAG has been implicated in passive coping [Behbehani, 1995; Linnman et al., 2012b]. In animals, the stimulation of this region triggers conservation/withdrawal behaviors and opioid‐mediated analgesia [Behbehani, 1995; Behbehani et al., 1990; Linnman et al., 2012b]. Our correlation map associated with this PAG subregion showed FC with the rACC/aMCC, cerebellum, pons, and medulla. These regions are known to be involved in descending pain modulation and opioid‐mediated analgesia [Bingel et al., 2006; Linnman et al., 2012b; Millan, 2002; Petrovic et al., 2002; Sprenger et al., 2011]. The FC with the medulla, as seen in Figure 4, seems specific to the VL‐PAG. In other words, it is the only region with which the VL‐PFC has greater FC than both L‐ and DL‐PAG. There was a negative correlation between VL‐PAG and a cluster extending over S1, SPL, and M1. These regions are involved in top–down attention during pain, spatial discrimination of painful stimulus, and self‐awareness [Bushnell et al., 2013; Kjaer et al., 2002; Villemure and Bushnell, 2009]. Also, negative correlations with basal ganglia (putamen, thalamus, caudate, and pallidum) were observed. It has been shown that some regions of the basal ganglia are directly correlated with S1 in situations of painful stimulation and may be associated with defensive behavior and motor responses [Bingel et al., 2004, 2002]. Further, dopamine release in the mesolimbic system is not only seen in reward situations but also in painful and aversive events [Brischoux et al., 2009; Scott et al., 2006]. Some animal studies have suggested that VL‐PAG receives projections from the orbitofrontal area [An et al., 1998]; however, we did not observe FC between these regions in our study. Kong et al. [2010] have measured the FC of the right VL‐PAG, using rs‐fMRI in 100 healthy subjects, and obtained similar FC of this subregion. Indeed, they showed FC between VL‐PAG and the cerebellum, RVM, rACC, aMCC, and hippocampus. In counterpart, we did not observe FC with the globus pallidus and the insula [Kong et al., 2010], but found FC with adjacent regions, such as the putamen, thalamus, and caudate. The negative correlations seen in our results are less similar to Kong et al. [2010], but could be due to the preprocessing protocol used in our study. It is known that aCompCor preprocessing technique has an impact on anticorrelation [Chai et al., 2012]. In summary, our results coincide with VL‐PAG functions found in animal and human behavioral studies [Keay and Bandler, 2001; Keay et al., 2002; Salomons et al., 2007]. VL‐PAG seems to be directly, and specifically, connected with brain regions involved in descending pain modulation (mainly RVM) and inversely correlated with brain regions involved in self‐awareness and defensive behaviors. As described by Kong et al. [2010], women showed greater FC between VL‐PAG and the ACC compared to men. This difference could be related to the fact that their analyses were statistically less stringent (P < 0.01 uncorrected). Our results are also similar to a previous study in our lab [Wang et al., 2014], which found greater FC between the sgACC and the descending pain modulation pathway (e.g., PAG) in women compared to men and greater FC between the sgACC and the salience network (namely the MCC) in men compared to women. This study did not subdivide the PAG but since the ACC is known to be connected mainly to the VL‐PAG, we can extrapolate from these results that the sex difference is driven by the caudal part of the PAG. Since the ACC is specialized in affective processing, this result is particularly interesting and could be responsible, at least in part, for sex differences in perceived pain perception and pain coping strategies [Hashmi and Davis, 2014].
The L‐ and DL‐PAG have been associated with active coping strategies [Bandler et al., 2000a; Keay and Bandler, 2001]. The stimulation of these regions induces defensive (rostral PAG) and escape (caudal PAG) behaviors in animals, as well as non‐opioid‐mediated analgesia [Behbehani, 1995; Linnman et al., 2012b]. The seeds located in the lateral columns of the PAG showed significant correlation with superior and medial temporal gyri involved in auditory and language functions, as well as with regions involved in the visual–auditory systems. Positive correlations were also observed with the insula, S2, and amygdala. These regions are known to serve the integration of an exogenous nociceptive stimulation, particularly sensory‐discriminative and motivo‐affective components of a painful stimulation [Apkarian et al., 2005; Davis and Moayedi, 2013; Tracey and Mantyh, 2007]. Regions of the occipital and temporal lobe could also be related to cross‐modal or multisensory processing of low‐level sensory cortex [Eckert et al., 2008]. Negative correlations were observed with the basal ganglia, premotor cortex, and PFC. These brain regions are associated with attention and right executive control networks [Shirer et al., 2012]. The functional connectivity with the occipital lobe is consistent with a previous study using tractography [Ezra et al., 2015]. Brain regions correlated with the DL‐PAG are also associated with attention and right executive control networks, including premotor cortex and PFC, but also with subcortical regions such as putamen, thalamus, insula, and amygdala. The amygdala is involved in emotional responses associated with fear and defensive behavior [LeDoux, 2003], thus FC between DL‐PAG and this region was not unexpected. Furthermore, the FC of DL‐PAG with the thalamus and the premotor cortex (Fig. 4) may be related to escape behavior [Doleys, 2014]. Consistent with animal studies, DL‐PAG had no FC with lower brainstem [Mai and Paxinos, 2012]. Accordingly, our findings are consistent with the notion of distinctive analgesia mechanisms between VL‐ and DL‐PAG [Behbehani, 1995; Behbehani et al., 1990; Chieng and Christie, 1994; Williams and Beitz, 1990]. In men, our findings showed greater FC connectivity between L‐PAG and DL‐PAG and temporal pole/inferior frontal gyrus, superior and middle temporal gyrus and to the lateral occipital pole. As noted above, the temporal gyri and occipital pole are involved in cross‐modal sensory processing, but are also activated during mind wandering away from pain [Kucyi et al., 2013]. There is no evidence for sex differences in attention to pain or mind wandering away from pain [Erpelding and Davis, 2013; Kucyi et al., 2013], but greater FC between L‐ and DL‐PAG and temporal gyri and occipital pole in men could be related to differences in coping strategies between men and women [Thompson et al., 2012, 2011].
LIMITATIONS
The PAG connectivity has been studied in animals using retrograde and anterograde tract tracing. This technique allows visualization of axonal projections. In our study, we measured the ultralow frequency oscillation of the BOLD signal at rest (rs‐fMRI). Significant correlations between the frequency oscillations from two brain regions can be associated with direct or indirect connections, as well as mono‐ and polysynaptic connections, between these brain regions, or can correspond to common inputs [Biswal et al., 1995; Davis and Moayedi, 2013]. Accordingly, the information obtained in this study only allows visualization of brain regions with synchronized BOLD oscillations as fMRI cannot differentiate between afferent and efferent connections.
Furthermore, brainstem imaging poses a challenge due to motion artifacts created by the constant CSF flow and the presence of major arteries [Beissner et al., 2014]. Recently, Beissner et al. [2014] showed that neither low‐pass filter nor a physiological noise model was sufficient to give good independent component analysis results on rs‐fMRI data. In our study, we used a state‐of‐the‐art preprocessing technique, aCompCor, which has shown to be particularly effective in decreasing motion artifacts produced by autonomic function, as well as increasing the discrepancy between noise and GM [Behzadi et al., 2007; Chai et al., 2012]. This technique—similar to most processes aiming at decreasing artifacts in the brainstem—is not perfect and was not specifically designed for the brainstem. Because of the voxel size in relation to the size and location of the aqueduct, it is possible that our results may include some noise artifacts.
We also note a study limitation due to the size of voxels within the PAG masks used in the parcellation analysis. As shown in Figure 2B, larger voxels precludes filtering out the aqueduct, and so decreases specificity of the analysis. To image the decrease in specificity and its comparison to our results, the three parcellation clusters of the clustering solution K = 3 result were used as seeds and showed as Supporting Information (Figs. S6 and S7). These results showed that there is, indeed, more noise associated with the correlation map of these seeds (Supporting Information, Figs. S6 and S7, showed with asterisk). However, even considering the noise, results are very similar, showing that there is still a greater correlation of the inferior PAG seed (similar localization as VL‐PAG seed) with the brainstem and a greater correlation of the superior PAG seed (similar localization as DL‐PAG) with the thalamus. Whereas, most of the FC observed using small seeds was located in GM brain regions and we did not observe any obvious artifacts. A limitation regarding the small seeds result is the transformation of the seeds from standard space to native space (3 × 3 × 4 voxels). This necessary transformation limits the precision of our seeds, increasing overlapping sections between seeds.
Finally, it is important to mention that PAG subregions oscillations were highly correlated with one another, whereas our multiple regression analyses aimed specifically at identifying unique features of PAG subregions (see Fig. 4 for subregion connectivity shown in yellow). Thus, it is possible that we were unable to clearly identify certain PAG functions.
CONCLUSION
We have identified distinct rs‐fMRI FC patterns of PAG subregions. The VL‐PAG was functionally connected with brain regions associated with descending pain modulation (i.e., rACC/aMCC, pons/medulla), whereas the L‐ and DL‐PAG were connected to brain regions involved in executive functions (i.e., prefrontal cortex, striatum, hippocampus). These results provide new insights into PAG subregions and their possible roles in pain and coping. Our findings are also important in the context of understanding chronic pain and its associated coping behavior related to inescapable stressors, such as avoidance of movement/social interactions and negative expectations that trigger worrying and catastrophizing [Samwel et al., 2006]. Therefore, important future studies should examine how the connectivity of these regions is modulated in chronic pain pathologies and contribution of individual factors and pain perception.
Supporting information
Supporting Information Figure S1‐S7
Supporting Information Tables S1‐S3
ACKNOWLEDGMENTS
The authors thank Kasey Hemington and Joshua Cheng for insights and assistance with data analysis, and Eugen Hlasny and Keith Ta for expert technical assistance. This work was performed at Toronto Western Research Institute, University Health Network. The authors have no conflicts of interest to disclose.
REFERENCES
- Alloy LB, Abramson LY, Whitehouse WG, Hogan ME, Tashman NA, Steinberg DL, Rose DT, Donovan P (1999): Depressogenic cognitive styles: Predictive validity, information processing and personality characteristics, and developmental origins. Behav Res Therapy 37:503–531. [DOI] [PubMed] [Google Scholar]
- An X, Bandler R, Ongur D, Price JL (1998): Prefrontal cortical projections to longitudinal columns in the midbrain periaqueductal gray in macaque monkeys. J Comparat Neurol 401:455–479. [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK (2005): Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 9:463–484. [DOI] [PubMed] [Google Scholar]
- Bandler R, Keay KA, Floyd N, Price J (2000a): Central circuits mediating patterned autonomic activity during active vs. passive emotional coping. Brain Res Bull 53:95–104. [DOI] [PubMed] [Google Scholar]
- Bandler R, Price JL, Keay KA (2000b): Brain mediation of active and passive emotional coping. Progr Brain Res 122:333–349. [DOI] [PubMed] [Google Scholar]
- Bandler R, Shipley MT (1994): Columnar organization in the midbrain periaqueductal gray: Modules for emotional expression? Trends Neurosci 17:379–389. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL (1979): The origin of descending pathways in the dorsolateral funiculus of the spinal cord of the cat and rat: Further studies on the anatomy of pain modulation. J Comparat Neurol 187:513–531. [DOI] [PubMed] [Google Scholar]
- Behbehani MM (1995): Functional characteristics of the midbrain periaqueductal gray. Progr Neurobiol 46:575–605. [DOI] [PubMed] [Google Scholar]
- Behbehani MM, Jiang M, Chandler SD (1990): The effect of [Met]enkephalin on the periaqueductal gray neurons of the rat: An in vitro study. Neuroscience 38:373–380. [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT (2007): A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage 37:90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beissner F, Schumann A, Brunn F, Eisentrager D, Bar KJ (2014): Advances in functional magnetic resonance imaging of the human brainstem. NeuroImage 86:91–98. [DOI] [PubMed] [Google Scholar]
- Bingel U, Glascher J, Weiller C, Buchel C (2004): Somatotopic representation of nociceptive information in the putamen: An event‐related fMRI study. Cereb Cortex 14:1340–1345. [DOI] [PubMed] [Google Scholar]
- Bingel U, Lorenz J, Schoell E, Weiller C, Buchel C (2006): Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain 120:8–15. [DOI] [PubMed] [Google Scholar]
- Bingel U, Quante M, Knab R, Bromm B, Weiller C, Buchel C (2002): Subcortical structures involved in pain processing: Evidence from single‐trial fMRI. Pain 99:313–321. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS (1995): Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magn Reson Med 34:537–541. [DOI] [PubMed] [Google Scholar]
- Brischoux F, Chakraborty S, Brierley DI, Ungless MA (2009): Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci USA 106:4894–4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell MC, Ceko M, Low LA (2013): Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci 14:502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrive P (1993): The periaqueductal gray and defensive behavior: Functional representation and neuronal organization. Behav Brain Res 58:27–47. [DOI] [PubMed] [Google Scholar]
- Carrive P, Bandler R, Dampney RA (1988): Anatomical evidence that hypertension associated with the defence reaction in the cat is mediated by a direct projection from a restricted portion of the midbrain periaqueductal grey to the subretrofacial nucleus of the medulla. Brain Res 460:339–345. [DOI] [PubMed] [Google Scholar]
- Chai XJ, Castanon AN, Ongur D, Whitfield‐Gabrieli S (2012): Anticorrelations in resting state networks without global signal regression. NeuroImage 59:1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chieng B, Christie MJ (1994): Inhibition by opioids acting on mu‐receptors of GABAergic and glutamatergic postsynaptic potentials in single rat periaqueductal gray neurones in vitro. Br J Pharmacol 113:303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AL Cohen, DA Fair, NU Dosenbach, FM Miezin, D Dierker, DC Van Essen, BL Schlaggar, SE Petersen (2008): Defining functional areas in individual human brains using resting functional connectivity MRI. Neuroimage 41:45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KD, Moayedi M (2013): Central mechanisms of pain revealed through functional and structural MRI. J Neuroimmune Pharmacol 8:518–534. [DOI] [PubMed] [Google Scholar]
- Depaulis A, Keay KA, Bandler R (1994): Quiescence and hyporeactivity evoked by activation of cell bodies in the ventrolateral midbrain periaqueductal gray of the rat. Exp Brain Res 99:75–83. [DOI] [PubMed] [Google Scholar]
- Doleys DM. 2014. Pain: Dynamics and Complexities: Oxford University Press. 304 p.
- Eckert MA, Kamdar NV, Chang CE, Beckmann CF, Greicius MD, Menon V (2008): A cross‐modal system linking primary auditory and visual cortices: Evidence from intrinsic fMRI connectivity analysis. Hum Brain Mapp 29:848–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erpelding N, Davis KD (2013): Neural underpinnings of behavioural strategies that prioritize either cognitive task performance or pain. Pain 154:2060–2071. [DOI] [PubMed] [Google Scholar]
- Ezra M, Faull OK, Jbabdi S, Shane Pattinson KT. (2015): Connectivity‐based segmentation of the periaqueductal gray matter in human with brainstem optimized diffusion MRI. Hum Brain Mapp [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME (2006): Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci USA 103:10046–10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AL, Wang S, Owen SL, Xie K, Bittar RG, Stein JF, Paterson DJ, Aziz TZ (2006): Stimulating the human midbrain to reveal the link between pain and blood pressure. Pain 124:349–359. [DOI] [PubMed] [Google Scholar]
- Hashmi JA, Davis KD (2014): Deconstructing sex differences in pain sensitivity. Pain 155:10–13. [DOI] [PubMed] [Google Scholar]
- Hemington KS, Coulombe MA (2015): The periaqueductal gray and descending pain modulation: Why should we study them and what role do they play in chronic pain? J Neurophysiol:Jn 00998 2014. [DOI] [PubMed] [Google Scholar]
- Hosobuchi Y (1983): Combined electrical stimulation of the periaqueductal gray matter and sensory thalamus. Appl Neurophysiol 46:112–115. [DOI] [PubMed] [Google Scholar]
- Keay KA, Bandler R (2001): Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosci Biobehav Rev 25:669–678. [DOI] [PubMed] [Google Scholar]
- Keay KA, Bandler R (2002): Distinct central representations of inescapable and escapable pain: Observations and speculation. Exp Physiol 87:275–279. [DOI] [PubMed] [Google Scholar]
- Keay KA, Clement CI, Depaulis A, Bandler R (2001): Different representations of inescapable noxious stimuli in the periaqueductal gray and upper cervical spinal cord of freely moving rats. Neurosci Lett 313:17–20. [DOI] [PubMed] [Google Scholar]
- Keay KA, Clement CI, Matar WM, Heslop DJ, Henderson LA, Bandler R (2002): Noxious activation of spinal or vagal afferents evokes distinct patterns of fos‐like immunoreactivity in the ventrolateral periaqueductal gray of unanaesthetised rats. Brain Research 948:122–130. [DOI] [PubMed] [Google Scholar]
- Keay KA, Clement CI, Owler B, Depaulis A, Bandler R (1994): Convergence of deep somatic and visceral nociceptive information onto a discrete ventrolateral midbrain periaqueductal gray region. Neuroscience 61:727–732. [DOI] [PubMed] [Google Scholar]
- Kelly C, Toro R, Di Martino A, Cox CL, Bellec P, Castellanos FX, Milham MP (2012): A convergent functional architecture of the insula emerges across imaging modalities. NeuroImage 61:1129–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keogh E, Hatton K, Ellery D (2000): Avoidance versus focused attention and the perception of pain: Differential effects for men and women. Pain 85:225–230. [DOI] [PubMed] [Google Scholar]
- Keogh E, Herdenfeldt M (2002): Gender, coping and the perception of pain. Pain 97:195–201. [DOI] [PubMed] [Google Scholar]
- Keogh E, Mansoor L (2001): Investigating the effects of anxiety sensitivity and coping on the perception of cold pressor pain in healthy women. Eur J Pain 5:11–22. [DOI] [PubMed] [Google Scholar]
- Kjaer TW, Nowak M, Lou HC (2002): Reflective self‐awareness and conscious states: PET evidence for a common midline parietofrontal core. NeuroImage 17:1080–1086. [PubMed] [Google Scholar]
- Kong J, Tu PC, Zyloney C, Su TP (2010): Intrinsic functional connectivity of the periaqueductal gray, a resting fMRI study. Behav Brain Res 211:215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucyi A, Moayedi M, Weissman‐Fogel I, Goldberg MB, Freeman BV, Tenenbaum HC, Davis KD (2014): Enhanced medial prefrontal‐default mode network functional connectivity in chronic pain and its association with pain rumination. J Neurosci 34:3969–3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucyi A, Salomons TV, Davis KD (2013): Mind wandering away from pain dynamically engages antinociceptive and default mode brain networks. Proc Natl Acad Sci USA 110:18692–18697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J (2003): The emotional brain, fear, and the amygdala. Cell Mol Neurobiol 23:727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnman C, Beucke JC, Jensen KB, Gollub RL, Kong J (2012a): Sex similarities and differences in pain‐related periaqueductal gray connectivity. Pain 153:444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnman C, Moulton EA, Barmettler G, Becerra L, Borsook D (2012b): Neuroimaging of the periaqueductal gray: State of the field. NeuroImage 60:505–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyd DR, Murphy AZ (2009): The role of the periaqueductal gray in the modulation of pain in males and females: Are the anatomy and physiology really that different? Neural Plasticity 2009:462879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumb BM (2002): Inescapable and escapable pain is represented in distinct hypothalamic‐midbrain circuits: Specific roles for Adelta‐ and C‐nociceptors. Exp Physiol 87:281–286. [DOI] [PubMed] [Google Scholar]
- Lumb BM (2004): Hypothalamic and midbrain circuitry that distinguishes between escapable and inescapable pain. News Physiol Sci 19:22–26. [DOI] [PubMed] [Google Scholar]
- Mai JK, Paxinos G. 2012. The Human Nervous System. Amsterdam; Boston: Elsevier Academic Press; xi, 1415 p. [Google Scholar]
- Mayer DJ, Wolfle TL, Akil H, Carder B, Liebeskind JC (1971): Analgesia from electrical stimulation in the brainstem of the rat. Science 174:1351–1354. [DOI] [PubMed] [Google Scholar]
- Meila M, Shi JB (2001): Learning segmentation by random walks. Adv Neural Inform Process Syst 13:873–879. [Google Scholar]
- Millan MJ (2002): Descending control of pain. Progr Neurobiol 66:355–474. [DOI] [PubMed] [Google Scholar]
- Mogil JS (2012): Sex differences in pain and pain inhibition: Multiple explanations of a controversial phenomenon. Nat Rev Neurosci 13:859–866. [DOI] [PubMed] [Google Scholar]
- Muschelli J, Nebel MB, Caffo BS, Barber AD, Pekar JJ, Mostofsky SH (2014): Reduction of motion‐related artifacts in resting state fMRI using aCompCor. NeuroImage 96C:22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidich TP, Duvernoy HM, Delman BN, Sorensen AG, Kollias SS, Haacke EM (2009): Duvernoy's Atlas of the Human Brain Stem and Cerebellum: High‐Field MRI, Surface Anatomy, Internal Structure, Vascularization and 3D Sectional Anatomy. Springer. [Google Scholar]
- Nashold BS, Jr , Wilson WP, Slaughter DG (1969): Sensations evoked by stimulation in the midbrain of man. J Neurosurg 30:14–24. [DOI] [PubMed] [Google Scholar]
- Pereira EA, Lu G, Wang S, Schweder PM, Hyam JA, Stein JF, Paterson DJ, Aziz TZ, Green AL (2010): Ventral periaqueductal grey stimulation alters heart rate variability in humans with chronic pain. Exp Neurol 223:574–581. [DOI] [PubMed] [Google Scholar]
- Pereira EA, Wang S, Owen SL, Aziz TZ, Green AL (2013a): Human periventricular grey somatosensory evoked potentials suggest rostrocaudally inverted somatotopy. Stereo Funct Neurosurg 91:290–297. [DOI] [PubMed] [Google Scholar]
- Pereira EA, Wang S, Peachey T, Lu G, Shlugman D, Stein JF, Aziz TZ, Green AL (2013b): Elevated gamma band power in humans receiving naloxone suggests dorsal periaqueductal and periventricular gray deep brain stimulation produced analgesia is opioid mediated. Exp Neurol 239:248–255. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Kalso E, Petersson KM, Ingvar M (2002): Placebo and opioid analgesia– imaging a shared neuronal network. Science 295:1737–1740. [DOI] [PubMed] [Google Scholar]
- Reynolds DV (1969): Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science 164:444–445. [DOI] [PubMed] [Google Scholar]
- Salomons TV, Johnstone T, Backonja MM, Shackman AJ, Davidson RJ (2007): Individual differences in the effects of perceived controllability on pain perception: Critical role of the prefrontal cortex. J Cogn Neurosci 19:993–1003. [DOI] [PubMed] [Google Scholar]
- Samwel HJ, Evers AW, Crul BJ Kraaimaat FW (2006): The role of helplessness, fear of pain, and passive pain‐coping in chronic pain patients. Clin J Pain 22:245–251. [DOI] [PubMed] [Google Scholar]
- Satpute AB, Wager TD, Cohen‐Adad J, Bianciardi M, Choi JK, Buhle JT, Wald LL, Barrett LF (2013): Identification of discrete functional subregions of the human periaqueductal gray. Proc Natl Acad Sci USA 110:17101–17106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DJ, Heitzeg MM, Koeppe RA, Stohler CS, Zubieta JK (2006): Variations in the human pain stress experience mediated by ventral and dorsal basal ganglia dopamine activity. J Neurosci 26:10789–10795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD (2012): Decoding subject‐driven cognitive states with whole‐brain connectivity patterns. Cereb Cortex 22:158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenger C, Bingel U, Buchel C (2011): Treating pain with pain: Supraspinal mechanisms of endogenous analgesia elicited by heterotopic noxious conditioning stimulation. Pain 152:428–439. [DOI] [PubMed] [Google Scholar]
- Thompson T, Keogh E, Chen MJ, French CC (2012): Emotion‐focused coping and distraction: Sex differences in the influence of anxiety sensitivity during noxious heat stimulation. Eur J Pain 16:410–420. [DOI] [PubMed] [Google Scholar]
- Thompson T, Keogh E, French CC (2011): Sensory focusing versus distraction and pain: Moderating effects of anxiety sensitivity in males and females. J Pain 12:849–858. [DOI] [PubMed] [Google Scholar]
- Tracey I, Mantyh PW (2007): The cerebral signature for pain perception and its modulation. Neuron 55:377–391. [DOI] [PubMed] [Google Scholar]
- Van Oort E, Mennes M, Beckmann C. Hierarchical functional atlas of human cortex using instantaneous correlation parcellations 2014; OHBM 2014 Annual Meeting, Hamburg, Germany.
- Villemure C, Bushnell MC (2009): Mood influences supraspinal pain processing separately from attention. J Neurosci 29:705–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Erpelding N, Davis KD (2014): Sex differences in connectivity of the subgenual anterior cingulate cortex. Pain 155:755–763. [DOI] [PubMed] [Google Scholar]
- Williams FG, Beitz AJ (1990): Ultrastructural morphometric analysis of enkephalin‐immunoreactive terminals in the ventrocaudal periaqueductal gray: Analysis of their relationship to periaqueductal gray‐raphe magnus projection neurons. Neuroscience 38:381–394. [DOI] [PubMed] [Google Scholar]
- Yu R, Gollub RL, Spaeth R, Napadow V, Wasan A, Kong J (2014): Disrupted functional connectivity of the periaqueductal gray in chronic low back pain. NeuroImage Clin 6:100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DX, Owens CM, Willis WD (1991): Two forms of inhibition of spinothalamic tract neurons produced by stimulation of the periaqueductal gray and the cerebral cortex. J Neurophysiol 65:1567–1579. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figure S1‐S7
Supporting Information Tables S1‐S3


