Abstract
This study aimed to explore structural and functional reorganization of the brain in the early stages of spinal cord injury (SCI) and identify brain areas that contribute to motor recovery. We studied 25 patients with SCI, including 10 with good motor recovery and 15 with poor motor recovery, along with 25 matched healthy controls. The mean period post‐SCI was 9.2 ± 3.5 weeks in good recoverers and 8.8 ± 2.6 weeks in poor recoverers. All participants underwent structural and functional MRI on a 3‐T magnetic resonance system. We evaluated differences in cross‐sectional spinal cord area at the C2/C3 level, brain cortical thickness, white matter microstructure, and functional connectivity during the resting state among the three groups. We also evaluated associations between structural and functional reorganization and the rate of motor recovery. After SCI, compared with good recoverers, poor recoverers had a significantly decreased cross‐sectional spinal cord area, cortical thickness in the right supplementary motor area and premotor cortex, and fractional anisotropy (FA) in the right primary motor cortex and posterior limb of the internal capsule. Meanwhile, poor recoverers showed decreased functional connectivity between the primary motor cortex and higher order motor areas (supplementary motor area and premotor cortex), while good recoverers showed increased functional connectivity among these regions. The structural and functional reorganization of the spine and brain was associated with motor recovery rate in all SCI patients. In conclusion, structural and functional reorganization of the spine and brain directly affected the motor recovery of SCI. Less structural atrophy and enhanced functional connectivity are associated with good motor recovery in patients with SCI. Multimodal imaging has the potential to predict motor recovery in the early stage of SCI. Hum Brain Mapp 37:2195–2209, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: spinal cord injury, atrophy, cortical reorganization, magnetic resonance imaging, motor recovery
Abbreviations
- ANCOVA
Analysis of co‐variance
- ASIA
American Spinal Injury Association
- FLAIR
Fluid‐attenuated inversion recovery
- fMRI
Functional MRI
- MP‐RAGE
Magnetization‐prepared rapid gradient‐echo sequence
- SCI
Spinal cord injury
- SMA
Supplementary motor area
INTRODUCTION
Spinal cord injury (SCI) is a common cause of disability, which significantly decreases quality of life [Ma et al., 2014]. During the past 20 years, although various therapies have been developed for restoring motor function in SCI, to date, there are no efficient and reliable clinical treatments available for SCI patients [Dietz and Curt, 2006]. One potential reason holding back improvements in SCI therapy is that current strategies, such as physical therapy, drug therapy, tissue engineering, and cell‐based therapy generally assume intact brain motor system function for driving limb movements. Most of these studies have focused on local changes in the spinal injury site and neglect the intimate interconnection with the brain [Cramer et al., 2005].
In recent years, there has been increasing evidence that loss of sensory and motor function following SCI results in extensive functional reorganization of the sensorimotor cortex in humans and animals [Moxon et al., 2014; Nardone et al., 2013a]. Several studies have shown that SCI contributes to structural reorganization of the spine and brain [Freund et al., 2011; Jurkiewicz et al., 2006; Lundell et al., 2011]. Henderson et al. [2011] showed that brain functional reorganization following long‐standing SCI is associated with a significant change in brain structure. To date, spinal cord atrophy [Freund et al., 2011; Lundell et al., 2011], brain white matter and grey matter atrophy [Freund et al., 2012; Jurkiewicz et al., 2006; Wrigley et al., 2009], and cortical functional reorganization [Henderson et al., 2011; Jurkiewicz et al., 2007] in the sensorimotor areas have been demonstrated in the later stages of SCI. Although it is well established that brain reorganization occurs in chronic SCI, the onset and role of these changes are still unknown in the acute phase of injury. Given that motor recovery of SCI occurs mainly in the early stages of disease (within 1 year after injury), studies of structural and functional reorganization in acute patients appear more meaningful than studies of chronic patients [Steeves et al., 2011].
Aguilar et al. [2010] investigated the acute neurophysiological changes occurring in the primary somatosensory cortex of anesthetized rats after spinal cord transection. They demonstrated that the complete thoracic transection of the spinal cord could immediately (within 1 h) change the state of large cortical networks, and that these changes play a critical role in the early brain functional reorganization after SCI. Similarly, our studies have also shown that SCI causes significant functional and structural changes of the sensorimotor system in the early stage of disease (within 8–10 weeks) [Hou et al., 2014a, 2014b]. However, it remains unclear whether this structural and functional reorganization contributes to the motor recovery of SCI. To the best of our knowledge, only one prospective longitudinal study has been performed in patients with acute traumatic SCI. That study showed extensive corticospinal tract and sensorimotor cortex atrophy at 40 days after SCI, with faster degenerative changes relating to poorer recovery [Freund et al., 2013]. These early studies of SCI are important but are not sufficient to draw conclusions on the role of brain reorganization in motor recovery, especially whether the functional reorganization translates into functional gain or further impairment. This question is important because it could further our understanding of the mechanism of spontaneous functional recovery following SCI. It also has the potential to provide additional information for predicting clinical outcomes, as well as leading to new therapeutic approaches to enhance beneficial or reduce maladaptive reorganization.
To address this question in the present study, we used multimodal MRI to perform a systemic investigation of the cross‐sectional cord area proximal to the site of injury (C2/C3 level) and cortical structural/functional changes after SCI and define their effects on motor recovery. We hypothesized that patients with different outcomes of motor function would show different structural and functional reorganization patterns following SCI. Furthermore, we hypothesized that reorganization of the spine and brain would directly affect the motor recovery of SCI.
MATERIAL AND METHODS
Study Participants
This study was approved by the Medical Ethics Committee of the Third Military Medical University (Chongqing, China), and written informed consent was obtained from all participants before enrollment. Patients with acute SCI were recruited consecutively from the Department of Rehabilitation at the Southwest Hospital (Chongqing, China) from September 2012 to December 2013.
SCI was confirmed by routine clinical X ray computed tomography images, electromyography, and neurological assessment. No patient had a psychiatric disorder or a history of traumatic brain injury. Neurological assessment was performed using the American Spinal Injury Association (ASIA) Standard Neurologic Classification of SCI (Committee et al., 2012). The ASIA impairment scale (AIS) and ASIA motor score were assessed at admission (baseline) and 6 months later. The evaluators who conducted the ASIA assessments were blinded to the study hypothesis. MRI was conducted on the day of the patient's arrival and took place prior to any treatment.
Disease duration was calculated from the time of injury to MRI scan. The interval between injury and MRI scanning ranged from 2 to 12 weeks (mean 9 weeks). Secondary injury including ischemic/apoptotic cascade, edema, and inflammation usually occur within the first 2 weeks after SCI [Park et al., 2004], therefore, these potentially confounding factors may have had little influence on brain structural and functional reorganization. All the patients with SCI received 3 h of therapy per day, 6 or 7 days per week. The length of stay in the acute rehabilitation unit depended on the patient's condition and progress and varied from one to several weeks (average length of stay was 40 days).
After discharge, all of the patients continued to receive care at the rehabilitation clinic and/or as outpatients. Patients with SCI were split into two groups (good recoverers and poor recoverers) according to the clinical outcome of motor recovery at 6 months follow‐up. Good recoverers achieved an increase of at least one AIS grade from baseline to 6 months follow‐up. Poor recoverers had no significant upward conversion of AIS grade at 6 months follow‐up. We also calculated the recovery rate of motor function in all patients with SCI, which was defined as follows: (motor score at 6 months follow‐up − motor score on admission) ÷ (100 − motor score on admission) ×100% [Waters et al., 1996].
Twenty‐five right‐handed healthy volunteers with no history of neurological or psychiatric diseases were recruited as controls, and were well matched for age and gender with the patients.
MRI Acquisition
Images were obtained using a 3.0 T MRI system (TIM Trio, Siemens, Erlangen, Germany) with an eight‐channel phased‐array head coil. For each participant, conventional brain T1‐weighted, T2‐weighted and fluid‐attenuated inversion recovery (FLAIR) images were obtained to exclude organic disease and white matter hyperintensity lesions. All MR images were assessed by two experienced radiologists. Resting‐state functional MRI (fMRI), structural imaging and diffusion tensor imaging (DTI) were all performed on the same day. During MRI, all participants were instructed to relax with their eyes closed and lie still without moving. Resting‐state functional images were acquired using an echo‐planar‐imaging (EPI) sequence in contiguous axial planes with the following parameters: repetition time (TR) = 2000 ms, echo time (TE) = 30 ms, flip angle = 90°, number of slices = 36, slice thickness = 4 mm, field of view (FOV) = 256 × 256 mm2, and matrix = 64 × 64, isotropic size = 4 × 4 × 4 mm3. For each participant, fMRI lasted for 480 seconds, and 240 volumes were obtained. In addition, high‐resolution structural T1‐weighted anatomical images were acquired in a sagittal orientation using a 3D magnetization‐prepared rapid gradient‐echo sequence (MP‐RAGE) with the following parameters: TR = 1,900 ms, TE = 2.52 ms, flip angle = 15°, slice thickness = 1 mm, FOV = 256 × 256 mm2, matrix = 256 × 256, and isotropic voxel = 1 × 1 × 1 mm3. For each participant, MP‐RAGE scanning lasted for 272 seconds. DTI images were acquired using a single‐shot twice refocused spin‐EPI sequence in contiguous axial planes with the following parameters: TR = 10,000 ms, TE = 92 ms, flip angle = 90°, number of slices = 75, slice thickness = 2 mm, FOV = 256 × 248 mm2, matrix = 128 × 124, and isotropic size = 2 × 2 × 2 mm3. The diffusion‐sensitizing gradients were applied along 64 non‐collinear gradient directions with b = 1,000 s/mm2 and an acquisition without diffusion weighing with b = 0 s/mm2. For each participant, the DTI scanning lasted for 384 seconds.
Cross‐Sectional Spinal Cord Area Analysis
The methodology developed to measure cross‐sectional spinal cord area at cervical level C2/C3 was first used to quantify the clinical symptom severity of multiple sclerosis [Horsfield et al., 2010; Losseff et al., 1996], and was applied to SCI by Freund et al. [2010, 2011]. A transversal spinal cord mask was created on the 3D MP‐RAGE images with the C2 disc as a caudal landmark. The spinal cord area from the mask was automatically estimated in each slice and averaged using a semi‐automated segmentation method [Losseff et al., 1996]. To confirm the reliability of morphometric measurement of cross‐sectional spinal cord area, we repeated the measurement in all participants by two different investigators, and did not find any significant difference using a paired t test.
CORTICAL THICKNESS ANALYSIS
Cortical thickness measurements were performed using CIVET software (version 1.1.9; Montreal Neurological Institute at McGill University, Montreal, Quebec, Canada). The T1‐weighted MR structural images of each participant were corrected for non‐uniformity using the N3 algorithm [Sled et al., 1998] and linearly registered into an MNI152 standard space [Collins et al., 1994]. The corrected and registered images were automatically segmented into gray matter, white matter, cerebrospinal fluid, and background using the INSECT algorithm [Zijdenbos et al., 2002]. The Constrained Laplacian‐based Anatomic Segmentation with Proximity algorithm was applied to generate the inner and outer gray matter surfaces comprising 40,962 vertices for each hemisphere [Kim et al., 2005]. Finally, the cortical thickness was measured by taking the absolute distance between corresponding vertices on the extracted inner and outer surfaces [Lerch and Evans, 2005].
DTI Analysis
White matter image preprocessing and statistical analysis were performed using the DTI Studio software (version 2.4, Johns Hopkins Medical Institute, Laboratory of Brain Anatomical MRI; http://cmrm.med.jhmi.edu). After image acquisition, the echo planar distortions induced by eddy currents were corrected using an algorithm that determined the optimum affine transformation to be applied to each diffusion‐weighted image. Individual maps of FA were calculated from the DTI data on a pixel by pixel basis. Voxel‐based analysis was performed using SPM8 software (SPM8, Welcome Department of Imaging Neuroscience, Institute of Neurology, University of College London, UK; http://www.fil.ion.ucl.ac.uk/spm). After normalizing all b0 images to standard MNI space using the EPI template supplied by SPM8, the original voxel size was resampled to 3 × 3 × 3 mm. These derived parameters were applied to the FA maps to normalize them to the MNI space. Finally, the normalized FA maps were spatially smoothed using an isotropic Gaussian filter (8‐mm full width at half maximum) to improve the signal‐to‐noise ratio.
Functional Connectivity Analysis
Functional connectivity preprocessing and statistical analysis were also done with SPM8 software. To avoid manipulation error confounds and standardize the process, we used the batch‐processing tool data processing assistant for resting state fMRI (DPARSF; http://www.restfmri.net) [Chao‐Gan and Yu‐Feng, 2010]. For the resting state fMRI data of each participant, the first 10 volumes of functional images were discarded to allow for steady‐state magnetization and stabilization of participant status. EP images were slice‐timing corrected to the middle slice acquired in time, and realigned and resliced to correct for head motion with a mean volume created. Structural images were co‐registered with the mean volume of functional images and subsequently segmented by an inbuilt unified segmentation routine in SPM8. The parameter created by segmentation was applied to functional images, non‐linear normalization to the MNI template brain, and each voxel was resampled to isotropic 3 × 3 × 3 mm. As a final step, the resting state fMR images were smoothed using an 8‐8‐8 mm FWHM Gaussian kernel. The head motion of all participants during resting‐state fMRI acquisition was observed, and data were discarded if the translation exceeded 2 mm or if rotation exceeded 2°.
Functional connectivity was analyzed using the REST software package (http://www.restfmri.net) using a seed voxel correlation approach [Fox et al., 2005]. The right supplementary motor area (SMA) and right premotor cortex were selected as regions of interest (ROIs) for functional connectivity analysis on resting‐state fMRI data. Several possible spurious sources of variance, including the estimated head motion parameters, global brain average signals, and average signals from the cerebrospinal fluid and white matter, were removed from the data through linear regression. After band‐pass filtering (0.01–0.08 Hz) and linear trend removal, a reference time series for each seed was extracted by averaging the time series of voxels within each ROI. A correlation analysis was conducted between the seed ROI and the remaining voxels in the whole brain. The resulting r values were converted using Fisher's r‐to‐z transformation to improve the Gaussianity of their distribution.
To evaluate the reliability of the resting‐state fMRI findings, we conducted the following procedures. First, we randomly divided the healthy controls into two subgroups. Group I had 13 participants and Group II had 12. We then compared the functional connectivity differences between the groups using two‐sample t tests. The two control subgroups showed no significant differences in functional connectivity (P > 0.05, corrected for multiple comparisons), which suggested that resting state fMRI is a reliable method to assess brain functional abnormalities.
Statistical Analysis
Differences in age, sex, years of education, and cross‐sectional spinal cord area among the three groups were compared by conducting one‐way analysis of analysis of variance and the χ2 test using SPSS version 18.0. Statistical tests on the cortical thickness, white matter, and functional connectivity maps were performed using a voxel‐based, one‐way analysis of co‐variance (ANCOVA) with post hoc t tests controlling for age, sex and years of education. Statistical inferences were made with a voxel‐level threshold of P < 0.05, after family‐wise error correction for multiple comparisons. For clusters identified in the ANCOVA, we performed follow‐up between‐group, voxel‐wise t tests to characterize group differences using the same thresholds within a mask showing group differences from the ANCOVA. Finally, to determine the relationship between the structural and functional reorganization of the spine and brain and clinical characteristics, the mean values of all voxels in the abnormal areas revealed by ANCOVA were extracted separately using the volume of interest in SPM8. The mean values at the spinal level (cross‐sectional spinal cord area) and brain level (cortical thickness, fractional anisotropy value, and functional connectivity strength) were input into SPSS 18.0 to conduct Pearson correlation analysis with clinical motor recovery rate of all SCI patients.
RESULTS
Demographic and Clinical Characteristics
We recruited 25 patients with SCI and 25 healthy controls. Ten patients showed an upward conversion of AIS grade at 6 months follow‐up (good recoverers) and 15 showed no significant upward conversion (poor recoverers). The demographic and clinical characteristics of all participants are given in Table 1. The three groups were well‐matched for age, sex and years of education. No significant differences were observed in disease duration between the two SCI subgroups (P > 0.05). However, the clinical baseline of injury severity between the two subgroups differed markedly: the good recovery group contained only one patient (AIS grade A), while the poor recovery group had seven patients. Significant differences were also observed in total ASIA motor score at baseline and at 6 months follow‐up (P < 0.05) between the two subgroups. As with motor functional recovery rate, good recoverers showed significantly greater motor recovery rates than poor recoverers showed (50.6% vs. 12.1%). The motor recovery rate was significantly negatively correlated with baseline neurological impairment (r = −0.33; P < 0.05) and disease duration since injury (r = −0.31; P = 0.005), while it did not correlate with the length of stay in acute unit, sex or age in the patients with SCI.
Table 1.
Demographic and clinical characteristics of healthy controls and patients with SCI
| Characteristics | Healthy controls (n = 25) | Good recoverers (n = 10) | Poor recoverers (n = 15) | P value |
|---|---|---|---|---|
| Mean age ± SD (years) | 36.5 ± 9.3 | 37.9 ± 13.9 | 35.8 ± 11.5 | 0.63a |
| Age range (years) | 20–45 | 19–56 | 19–52 | – |
| Gender (male/female) | 15/10 | 6/4 | 8/7 | 0.91c |
| Mean years of education (years) | 12.8 ± 3.9 | 12.1 ± 2.9 | 12.5 ± 3.3 | 0.71a |
| Disease duration (weeks) | 9.2 ± 3.5 | 8.8 ± 2.6 | 0.57b | |
| No. with cause of SCI | Fall (4), motor vehicle accident (6) | Fall (5), motor vehicle accident (10) | 0.73c | |
| Initial site of impairment (based on motor loss) | C6 (1), C8 (1), T5 (1), T6 (1), T7 (1), T8 (1), T9(1), T10 (2), T12 (1) | C5 (2), C6 (1), C8 (1), T5 (1), T6 (2), T7 (1), T8 (3), T9 (2), T11 (1), T12 (1) | 0.55c | |
| No. with AIS grades (baseline) | A(1), B(4), C(3), D(2) | A(7), B(3), C(3), D(2) | 0.35c | |
| No. with AIS grades conversion (6 months) | A to B (1), B to C (3), B to D (1), C to D (2), C to E (1), D to E (2) | No conversion | – | |
| Mean ASIA motor score (baseline) | 71.9 ± 16.3 | 58.5 ± 26.5 | 0.17b | |
| Mean ASIA motor score (6 months) | 85.5 ± 11.5 | 61.3 ± 26.4 | 0.01b | |
| Mean motor recovery rate (%) | 50.6 ± 14.4 | 12.1 ± 10.6 | <0.001b |
Abbreviation: ASIA, American Spinal Injury Association; AIS, ASIA impairment scale; SCI, spinal cord injury.
Indicated P value in one‐way analysis of variance among the three groups.
P value with independent samples t test.
P value with χ2 test.
Cross‐Sectional Spinal Cord Area Analysis
We measured cross‐sectional cord area at the C2/C3 level, which was above the site of injury. The C2/C3 cross‐sectional spinal cord area in healthy controls, good recoverers and poor recoverers was 83.2 ± 3.9, 80.5 ± 4.8 and 73.5 ± 5.2 mm2, respectively. Poor recoverers exhibited lower spinal cord area compared with healthy controls (P < 0.001) and good recoverers (P = 0.002). Although the spinal cord area was 2.7 mm2 lower in good recoverers than it was in healthy controls, this difference was not significant (P = 0.091) (Fig. 1).
Figure 1.
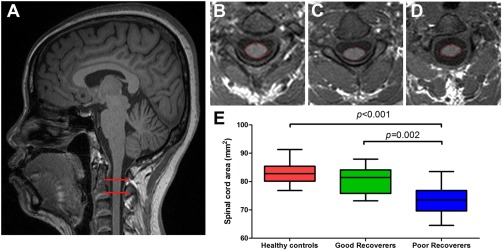
Cross‐sectional spinal cord area differences among three groups of participants. (A) T1‐weighted image showing the region of cross‐sectional cord area measurement (within red horizontal bars). Spinal cord area in a healthy control (B), good recoverer (C), and poor recoverer (D). (E) Box plots showing the atrophy of cord area in poor recoverers relative to both healthy controls and good recoverers. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Cortical Thickness Analysis
One‐way ANCOVA showed four brain regions that had significant differences in cortical thickness, namely the bilateral primary motor cortex, right SMA, and right premotor cortex. Compared to healthy controls, poor and good recoverers had significantly decreased cortical thickness in the bilateral primary motor cortex. In addition to reduced cortical thickness in the primary motor cortex, poor recoverers also showed decreased cortical thickness in the right SMA and premotor cortex when compared to healthy controls. A direct comparison between the two patient groups revealed significantly decreased cortical thickness in the right SMA and premotor cortex in the poor recoverers compared to the good recoverers (Fig. 2, Table 2).
Figure 2.
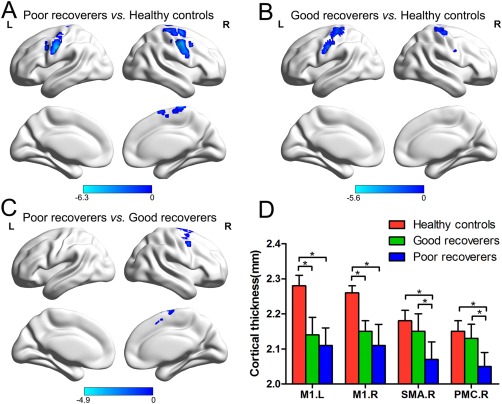
Cortical thickness atrophy in poor recoverers and good recoverers. (A) Smaller cortical thickness in the bilateral primary motor cortex (M1), right SMA and premotor cortex (PMC) in poor recoverers relative to healthy controls. (B) Smaller cortical thickness in the bilateral M1 in poor recoverers relative to healthy controls. (C) Smaller cortical thickness in the right SMA and PMC in poor recoverers relative to good recoverers. (D) Bar graph showing the cortical thickness values of the three groups at the peak of the abnormal regions. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 2.
Difference in cortical thickness among patients with SCI with good motor recovery, poor motor recovery and healthy controls
| MNI coordinate | Voxel | Peak | |||
|---|---|---|---|---|---|
| Anatomical region | x | y | z | size | t value |
| Poor recoverers < healthy controls | |||||
| Left M1 (BA4) | −49 | −10 | 39 | 85 | −6.28 |
| Right M1 (BA 4) | 49 | −7 | 45 | 125 | −5.89 |
| Right SMA (BA 6) | 3 | 6 | 72 | 50 | −4.76 |
| Right premotor cortex (BA 6) | 23 | −6 | 64 | 43 | −5.38 |
| Good recoverers < healthy controls | |||||
| Left M1 (BA 4) | −53 | −5 | 32 | 107 | −5.59 |
| Right M1 (BA 4) | 38 | −18 | 60 | 73 | −5.35 |
| Poor recoverers < good recoverers | |||||
| Right SMA | 5 | −27 | 70 | 29 | −4.17 |
| Right premotor cortex | 21 | −10 | 67 | 46 | −4.85 |
Abbreviation: SCI, spinal cord injury; BA, Broadmann area; M1, primary motor cortex; SMA, supplementary motor area; MNI, Montreal Neurological Institute. P < 0.05, corrected for multiple comparisons with family‐wise error correction.
White Matter Microstructural Analysis
The three groups differed in white matter fractional anisotropy (FA) mainly in the right internal capsule and bilateral primary motor cortex. Compared to the healthy controls, poor recoverers showed reduced FA in the right primary motor cortex and posterior limb of the internal capsule; good recoverers showed no significant difference in white matter microstructure. When comparing the two patients groups directly, the poor recoverers showed reduced FA in the bilateral primary motor cortex and right posterior limb of the internal capsule (Fig. 3, Table 3).
Figure 3.
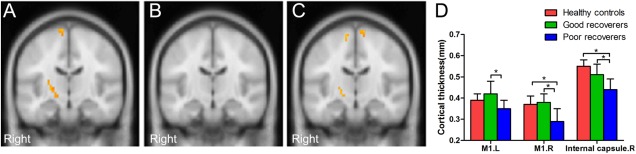
White matter fractional anisotropy differences among the three groups of participants. (A) Smaller fractional anisotropy in the right primary motor cortex (M1) and posterior limb of the internal capsule in poor recoverers relative to healthy controls. (B) No significant difference of fractional anisotropy in good recoverers relative to healthy controls. (C) Smaller fractional anisotropy in the bilateral M1 and posterior limb of the internal capsule in poor recoverers relative to good recoverers. (D) Bar graph showing the fractional anisotropy values of the three groups at the peak of the abnormal regions. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 3.
Difference in white matter fractional anisotropy among patients with SCI with good motor recovery, poor motor recovery and healthy controls
| MNI coordinate | Voxel | Peak | |||
|---|---|---|---|---|---|
| Anatomical region | x | y | z | size | t value |
| Poor recoverers < healthy controls | |||||
| Right M1 | −11 | −19 | 65 | 21 | −4.26 |
| Right internal capsule | −21 | −19 | 1 | 30 | −4.33 |
| Poor recoverers < good recoverers | |||||
| Right M1 | 5 | −27 | 70 | 29 | −4.37 |
| Left M1 | 28 | −19 | 61 | 25 | −4.28 |
| Right internal capsule | −21 | −18 | −3 | 19 | −4.15 |
Abbreviation: SCI, spinal cord injury; M1, primary motor cortex; MNI, Montreal Neurological Institute. P < 0.05, corrected for multiple comparisons with family‐wise error correction.
Resting‐State Functional Connectivity Analysis
Cortical thickness analysis showed significant anatomical differences between the two SCI subgroups, mainly in the right SMA and premotor cortex, therefore, we speculated that these two areas may play a vital role in the motor recovery following SCI. Previous studies have also shown that SMA and premotor cortex contribute to motor recovery of patients with stroke [Riecker et al., 2010; Wang et al., 2012]. Thus, we performed seed‐based resting‐state functional connectivity analysis to explore whether functional reorganization of these two areas plays an important role in motor functional recovery of SCI. We found that the two SCI groups showed distinct functional reorganization patterns in these two seed areas. Specifically, when the seed was located in the right SMA, poor recoverers showed decreased functional connectivity mainly in the right primary motor cortex and left SMA. On the contrary, the good recoverers showed increased functional connectivity in the bilateral primary motor cortex and left SMA. Similar results also were found in the seed of the right premotor cortex. Poor recoverers showed decreased functional connectivity in the right primary motor cortex; conversely, good recoverers showed increased functional connectivity. A direct comparison between the two patient groups showed significantly increased functional connectivity between the right SMA and right primary motor cortex and left SMA, as well as increased functional connectivity between the right premotor cortex and right primary motor cortex and right SMA in the good recoverers (Fig. 4, Table 4).
Figure 4.
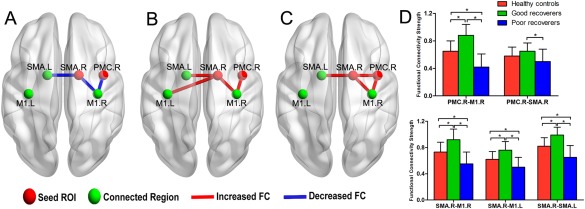
Resting state functional connectivity differences among the three groups of participants. (A) Decreased functional connectivity in poor recoverers relative to healthy controls. (B) Increased functional connectivity in good recoverers relative to healthy controls. (C) Increased functional connectivity in good recoverers relative to poor recoverers. (D) Bar graph showing the fractional strength of the three groups at the peak of the abnormal regions. Abbreviation: SMA, supplementary motor area; M1, primary motor cortex; PMC, premotor cortex; FC, functional connectivity. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 4.
Difference in functional connectivity among patients with SCI with good motor recovery, poor motor recovery and healthy controls
| Connected region | MNI coordinate | Voxel | Peak | |||
|---|---|---|---|---|---|---|
| Seed ROI | x | y | z | Size | t value | |
| Poor recoverers < healthy controls | ||||||
| Right SMA | Right M1 | 27 | −24 | 63 | 35 | −3.91 |
| Left SMA | −3 | 0 | 63 | 29 | −3.16 | |
| Right premotor cortex | Right M1 | 39 | −18 | 59 | 41 | −4.06 |
| Good recoverers > healthy controls | ||||||
| Right SMA | Right M1 | 36 | −12 | 57 | 32 | 4.12 |
| Left M1 | −21 | −13 | 62 | 23 | 3.25 | |
| Left SMA | −8 | −12 | 63 | 30 | 3.86 | |
| Right premotor cortex | Right M1 | 52 | −11 | 48 | 43 | 4.76 |
| Good recoverers > poor recoverers | ||||||
| Right SMA | Right M1 | 36 | −12 | 55 | 46 | 4.53 |
| Left SMA | −8 | −9 | 63 | 41 | 4.57 | |
| Right premotor cortex | Right M1 | 52 | −12 | 48 | 55 | 5.29 |
| Right SMA | 7 | −11 | 63 | 23 | 3.25 | |
Abbreviation: SCI, spinal cord injury; BA, Broadmann area; M1, primary motor cortex; SMA, supplementary motor area; MNI, Montreal Neurological Institute. P < 0.05, corrected for multiple comparisons with family‐wise error correction.
Correlations Between Structural/Functional Reorganization and Extent of Motor Recovery
At the spinal level, the cross‐sectional spinal cord area was positively correlated with the motor recovery rate in all patients with SCI (r = 0.59, P = 0.002). At the brain level, the FA values in the right primary motor cortex and the cortical thickness in the right SMA were positively correlated with the motor recovery rate in all the patients with SCI (r = 0.76, P < 0.001; r = 0.75, P < 0.001; respectively) (Fig. 5A). The functional connectivity strength between the right SMA and right primary motor cortex and left SMA were positively correlated with the motor recovery rate in all the patients with SCI (r = 0.78, P < 0.001; r = 0.75, P < 0.001; respectively). In addition, the functional connectivity strength between the right premotor cortex and right primary motor cortex was also positively correlated with the motor recovery rate in all the patients with SCI (r = 0.79, P < 0.001) (Fig. 5B).
Figure 5.
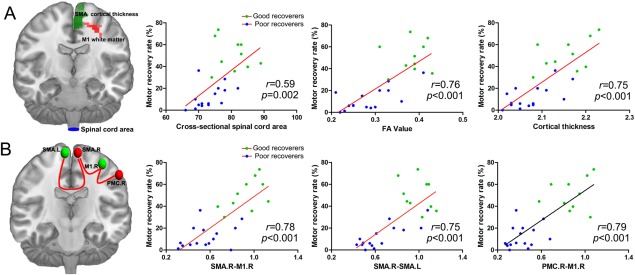
Correlations between structural and functional reorganization and the recovery rate of motor function in all patients with SCI. (A) Cross‐sectional spinal cord areas, fractional anisotropy (FA) values in the right primary motor cortex (M1) and the cortical thickness in the right SMA were positively correlated with the motor recovery rate. (B) The functional connectivity strength between the right SMA and the left SMA and right M1, and between the right premotor cortex (PMC) and the right M1 were positively correlated with the motor recovery rate in all patients with SCI. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Additional stepwise multiple regression analysis was conducted to test which spinal and supraspinal data were the superior predictors. The best set of parameters predicting the extent of motor recovery at 6 months included cortical thickness in the right SMA, functional connectivity strength between the right SMA and right primary motor cortex, and functional connectivity strength between the right premotor cortex and right primary motor cortex. The regression coefficient, standard error and t values are given in Table 5.
Table 5.
Multivariate multiple regression analysis showing prognostic predictors of the extent of motor recovery at 6 months in patients with SCI
| Variables | Regression coefficient | SE | t | P |
|---|---|---|---|---|
| Cortical thickness in the right SMA | 2.63 | 1.32 | 1.92 | 0.001 |
| FC strength between the right SMA and right M1 | 1.08 | 0.75 | 1.24 | 0.03 |
| FC strength between the right premotor cortex and right M1 | 1.96 | 0.63 | 1.78 | 0.01 |
| Constant | −0.85 |
Abbreviation: SCI, spinal cord injury; M1, primary motor cortex; SMA, supplementary motor area; FC, functional connectivity; SE, standard error.
DISCUSSION
It is often seen in clinical settings that patients with acute SCI with similar AIS grades may have different outcomes in motor function; some patients show some recovery on motor function, whereas others show no obvious recovery. The present investigation addressed the question of whether different outcomes of motor function can be attributed to differences in structural and functional reorganization of the spine and brain. Our findings demonstrated that structural and functional reorganization patterns directly affect motor function recovery at 6 months after admission. Specifically, at the structural reorganization level, poor recoverers showed more serious and widespread structural damage of the spine and brain than the good recoverers showed. The extent of the structural damage was associated with the motor recovery rate in all the patients with SCI. At the functional reorganization level, poor recoverers had decreased functional connectivity between the primary motor cortex and higher order secondary motor areas (SMA and premotor cortex). Good recoverers mainly showed significantly increased functional connectivity among these regions, and functional reorganization in the brain was positively correlated with motor recovery rate in all patients with SCI. Our findings may shed some lights on the role of structural and functional reorganization in the early stage of SCI recovery, and further our understanding of the mechanisms underlying motor functional recovery after SCI.
Clinical Features
One of the main strengths of the present study was the early stage of SCI. Most previous structural and functional studies of SCI were in patients with chronic disease (range 1–30 years). Our present findings provide evidence that SCI causes significant structural and functional changes during the early stage of the disease, and may even be related to the extent of motor recovery at 6 months. Our structural findings are partly consistent with another recent study [Freund et al., 2013], which showed that extensive atrophic and microstructural changes of corticospinal axons and sensorimotor cortical areas occur as early as 40 days after SCI. These structural findings in the early stage of the disease may change the traditional view that degenerative changes in the brain motor systems occur slowly following SCI [Freund et al., 2007; Hains et al., 2003]. Meanwhile, our findings may provide a forceful imaging basis for the early clinical intervention.
In the study of the functional recovery in patients with SCI, one important question that remains largely unanswered is when one selects time points to study recovery and how long post‐injury is likely to optimize the ability to understand recovery curves. Previous animal studies have shown that complete thoracic transection of the spinal cord can immediately (within 1 h) change the functional state of large cortical networks [Aguilar et al., 2010]. Therefore, in theory at least, it is better to study reorganization of the brain as early as possible because it could lead to a better understanding of the pathophysiological mechanisms of long‐term cortical reorganization. However, the first few days of the acute stage following SCI are usually accompanied by spinal shock, in which the person's reflexes do not work [Boland et al., 2011]. During this stage (within 1 week), it is difficult to determine an exact illness evaluation and prognosis, because some function beyond what is currently being seen may occur later. At this acute stage, doctors may need to operate to remove the fractured fragments that are compressing the spine and stabilize the spine [Dvorak et al., 2015]. Once the acute phase is over and the person has been stabilized (∼2 weeks after injury), patients enters the rehabilitation stage of treatment. Treatment during this phase has the goal of returning as much function as possible to the person. Although neurological improvement following SCI may continue to occur beyond 1 year after injury [Kirshblum et al., 2004], most neurological recovery is thought to occur within the first 3 months after injury [Fawcett et al., 2007; Steeves et al., 2011]. Pollard and Apple [2003] have noted that >70% of neurological recovery occurs before discharge from rehabilitation. Therefore, the time points selected by the current study (range: 2–12 weeks, median: 4 weeks) seem to be a good choice to study the motor recovery in patients with SCI. Future longitudinal studies that enroll patients from 2 weeks after SCI might be appropriate, and provide further insights into the mechanisms of brain structural and functional reorganization and its effect on the extent of motor recovery.
Our study suggests that in patients with SCI, the extent of motor recovery in the 6 months following study enrolment is significantly negatively correlated with the time elapsed since injury. This finding is in line with a recent clinical study [Dvorak et al., 2015] and further supports the need to start rehabilitation in patients with SCI as soon as possible. In the current study, the clinical baseline of injury severity between the two subgroups differed markedly. Most patients in the good recovery group showed incomplete SCI, while most patients in the poor recovery group showed complete SCI. This finding is consistent with the subsequent finding that the extent of motor recovery is significantly negatively correlated with baseline neurological impairment. Previous clinical studies also showed that the baseline neurological impairment could be considered as a good prognostic indicator in the acute stage of SCI [Coleman and Geisler, 2004]. The age of the patient, considered to be a good prognostic indicator in the acute stage of SCI [Gwak et al., 2004; Wilson et al., 2014], was not associated with improvement of the motor deficit in our patients, which is in agreement with other studies [Furlan et al., 2010; New and Epi, 2007]. The previous studies suggested that medication and depression have an influence on motor recovery after central nervous system injury [Flaster et al., 2013]. However, in this study, we did not observe these potential confounding factors, given that there was no conclusive evidence in clinical studies to show the exact relationship between these potential confounding factors and outcome of SCI. Future clinical studies using large samples should demonstrate the potential relationship of medication and depression with functional recovery.
Morphological Findings
There is evidence in patients with chronic SCI that cortical gray matter and white matter undergo atrophic changes in the sensorimotor system [Freund et al., 2011; Freund et al., 2012; Wrigley et al., 2009]. One recent study reported that significant structural atrophy of the spine and brain occurs in the first few months after SCI, and structural reorganization progresses with a specific spatial and temporal pattern [Freund et al., 2013]. In line with this early stage study, we also showed that patients with SCI had spinal cord atrophy, cortical atrophy and white matter microstructural abnormalities in the early stage of the disease. Furthermore, our study showed that poor recoverers had more serious and widespread structural damage of the spine and brain than good recoverers had. Thus, it seems that structural integrity of the spine and brain directly affects motor recovery in the early stage of SCI. Although the exact mechanisms for structural impairment of the brain motor systems following SCI remain unclear, it is possible that retrograde degeneration accounts for this finding [Guleria et al., 2008].
One of the most interesting outcomes of our study was that significantly decreased cortical thickness was found in the right SMA and premotor cortex in the poor recoverers more than that in the good recoverers. In particular, the cortical thickness in the right SMA was positively correlated with the motor recovery rate in all patients. To the best of our knowledge, no previous structural MRI study has directly located the structural atrophy of these two regions during the early stage of SCI, however, it was captured in our study for the first time. These findings highlight the importance of the SMA and premotor cortex in the motor recovery of SCI. The importance of the SMA in motor recovery is supported by studies showing its role in the motor system, through efferent nerves to the primary motor cortex [Strick, 1988] and directly to the spinal cord [Biber et al., 1978]. The premotor cortex projects directly to the spinal cord and plays a role in planning movement, in the spatial and sensory guidance of movement, and in using abstract rules to perform specific tasks [Busan et al., 2009; Pastor‐Bernier et al., 2012; Weinrich et al., 1984]. In other central nervous system injury, such as stroke, the SMA and premotor cortex also play an important role in the motor recovery of patients [Pantano et al., 1996; Riecker et al., 2010].
Functional Findings
There is increasing recent evidence in humans that the adult brain is capable of extensive functional reorganization following central nervous system injury [Agosta et al., 2011; Chen et al., 2002; Fridman et al., 2004]. One of the main functional reorganizations in patients with stroke and traumatic brain injury is recruitment of additional motor areas (SMA and premotor cortex) to compensate for reduced capacity of the primary sensorimotor cortex to generate sufficient motor output [Lotze et al., 2006; Wang et al., 2010]. These functional reorganizations are believed to play an important role in motor recovery and may be a hallmark of recovery from brain trauma (Grefkes and Fink, 2011. Our findings showed that patients with good and poor motor recovery had distinct functional reorganization patterns in these two areas. Increased functional connectivity of the primary motor cortex with the SMA and premotor cortex occurred in patients with good motor recovery, but not in those with poor motor recovery. Overall, it may reflect a compensatory role of the over‐recruited neural resources for the recovery of SCI. These findings are consistent with a previous positron emission tomography study, which showed that the premotor cortex was the prominent contributor to motor recovery at 3–4 months post‐SCI [Nishimura et al., 2007].
To explore further the compensatory role of brain functional reorganization, we investigated the relationships between motor recovery rate and functional connectivity measures. Positive correlations were found between the rate of motor recovery and functional connectivity between the primary motor cortex and SMA and premotor cortex. This finding emphasizes that increased functional connectivity between the primary motor cortex and higher order secondary motor areas (SMA and premotor cortex) plays a vital role in the motor recovery of SCI. It is, therefore, suggested that methods that enhance cortical functional connectivity with the higher order motor areas might be useful for further promoting recovery after SCI.
Clinical Implications
Our work provides useful information on brain reorganization associated with motor recovery in the early stage of SCI. The most striking finding of our study was that the extent of structural damage to the spine and brain was associated with the motor recovery rate in patients with SCI. Notably, this finding indicated that structural atrophy of the spinal cord and brain motor cortex was predictive of slow or poor recovery, whereas the better the structural integrity of the spinal cord and brain, the faster and better the motor recovery. Thus, preserved structural integrity of the brain motor system above the injury site appears to be critical for good functional outcome after SCI, and could therefore be a primary target for therapeutic repair strategies.
Our findings may also explain why current therapeutic approaches for SCI have limited effectiveness and are far from satisfactory. Over the past decade, a variety of experimental therapies has emerged to promote axon regeneration at the lesion site in the hope of promoting functional recovery [Thuret et al., 2006], including the application of neurotrophic factors [Kwon et al., 2002], drug therapy [Baptiste and Fehlings, 2006], and cell‐based therapies [Guest et al., 2005]. These therapeutic approaches to reduce disability following SCI generally assume intact brain motor system function, that is, the cell bodies of damaged axons remain alive. However, the current findings showed that SCI could cause significant structural damage in the brain motor systems, which may indicate damage to the cell bodies in the brain motor system. If the cell body is damaged, the axon will die as well [Carlson et al., 2000] and therapeutic interventions to promote axon regeneration would be futile. In general, loss of cortical projection neurons may contribute to the lack of functional recovery and limit the gains that can be achieved with exogenous treatment. That is the reason why current therapeutic approaches promoting regeneration of the injured spinal cord have limited effectiveness. Thus, future interventional studies should target both the injured spinal cord and brain [Nardone et al., 2013b]. It is also reasonable to speculate that therapeutic repair strategies aimed at modulating or reversing structural reorganization of the spine and brain may have therapeutic potential [Choe et al., 2013; Dobkin et al., 2007]. Given that structural changes occurred early, as revealed by the current study, these interventions should ideally be used in the early stages after SCI.
From a clinical point of view, establishing whether measurable early alterations in structural or functional reorganization affect final functional outcome would be of importance for early diagnosis, prediction and treatment selection. A previous study using positron emission tomography has shown that motor recovery is associated with metabolic improvement in the primary motor cortex and premotor cortex in macaque monkeys in the first 3 months of SCI [Nishimura et al., 2007]. Structural atrophy of the corticospinal axons and sensorimotor cortical areas remote from the site of SCI also could be considered as neuroimaging biomarkers for interventional studies [Freund et al., 2013]. Similarly, we observed a significant correlation between motor recovery at 6 months after admission and structural and functional reorganization of the spine and brain. The best predictors included the cortical thickness in the right SMA, and functional connectivity strength among the primary motor cortex, SMA and premotor cortex. Our findings could be meaningful for the prognostic evaluation of SCI. If regression of SMA atrophy or increased functional connectivity between the primary motor cortex and higher order secondary motor areas observed in the early stage of SCI, either spontaneous or therapy‐induced, the motor recovery is potentially reversible as well. These findings might be valuable for optimizing the therapeutic strategies by stratification of patients for selection of drug or rehabilitation strategies and to assess treatment effects by monitoring the dynamic brain structural and functional changes during the course of treatment [Endo et al., 2009; Freund et al., 2013].
Limitations
The current study had some limitations. First, because this was a preliminary study, our results were limited to a small sample of patients with SCI with a heterogeneous level and degree of injury. Future studies that focus on different levels and degrees of SCI may help to further our understanding of SCI‐related structural and functional reorganization. Second, imaging data at 6 months follow‐up were not acquired because of budget limitations. We could not fully assess the dynamic changes in the patterns of structural and functional reorganization following SCI. Third, this study focused on motor function recovery in patients with SCI. Whether the cortical reorganization after SCI contributed to maladaptive changes such as neuropathic pain and mood disorders [Mole et al., 2014; Nicotra et al., 2006] remains to be determined. Theoretically, cortical reorganization following SCI is neither good nor bad [Moxon et al., 2014]. The good side of cortical reorganization can contribute to functional recovery, just as the current study found, but its bad side can be maladaptive and lead to phantom sensation and neuropathic pain [Gustin et al., 2010; Makin et al., 2013]. It is therefore critical for future studies to explore the phenomenology and mechanisms of cortical reorganization to develop and optimize cost‐effective therapies to maximize functional recovery while minimizing neuropathic pain [Engineer et al., 2011]. Finally, given that this was a preliminary study to explore the role of brain structural and functional reorganization in motor recovery, we only used conventional voxel‐based analysis to explore abnormalities in the brain. Future studies using the tract‐based spatial statistics and tractographic approach will be necessary to gain further insight into the microstructural abnormalities in the white matter of patients with SCI.
CONCLUSIONS
To the best of our knowledge, this is the first study combining structural and functional imaging protocols to explore the relationship between recovery of motor function and reorganization of the spine and brain. Our study demonstrated that patients with good and poor motor recovery showed distinct functional reorganization patterns. Structural atrophy of the spinal cord and brain motor cortex was predictive of slow or poor recovery, whereas the better the structural integrity of the spinal cord and brain, the faster and better the motor recovery. Furthermore, increased functional connectivity between the primary motor cortex and higher order secondary motor areas play a vital role in the motor recovery of SCI. Consequently, our results indicate that structural and functional reorganization of the spine and brain directly affects motor recovery in the early stage of SCI, and future novel interventions should target both the injured spinal cord and brain. Meanwhile, the strong correlations between the structural and functional changes and the rate of motor recovery indicate that multimodal imaging of the spine and brain has the potential to predict motor recovery in the early stage of SCI. Future studies using a large study population to differentiate the level and degree of SCI, and performing MRI for a longer time (6 or 12 months) will be important to further our understanding of the patterns of SCI‐related structural and functional reorganization.
ACKNOWLEDGMENT
The authors thank the patients who participated in this study and gave generously of their time.
REFERENCES
- Agosta F, Valsasina P, Absinta M, Riva N, Sala S, Prelle A, Copetti M, Comola M, Comi G, Filippi M (2011): Sensorimotor functional connectivity changes in amyotrophic lateral sclerosis. Cereb Cortex 21:2291–2298. [DOI] [PubMed] [Google Scholar]
- Aguilar J, Humanes‐Valera D, Alonso‐Calvino E, Yague JG, Moxon KA, Oliviero A, Foffani G (2010): Spinal cord injury immediately changes the state of the brain. J Neurosci 30:7528–7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptiste DC, Fehlings MG (2006): Pharmacological approaches to repair the injured spinal cord. J Neurotrauma 23:318–334. [DOI] [PubMed] [Google Scholar]
- Biber MP, Kneisley LW, LaVail JH (1978): Cortical neurons projecting to the cervical and lumbar enlargements of the spinal cord in young and adult rhesus monkeys. Exp Neurol 59:492–508. [DOI] [PubMed] [Google Scholar]
- Boland RA, Lin CS, Engel S, Kiernan MC (2011): Adaptation of motor function after spinal cord injury: Novel insights into spinal shock. Brain 134:495–505. [DOI] [PubMed] [Google Scholar]
- Busan P, Barbera C, Semenic M, Monti F, Pizzolato G, Pelamatti G, Battaglini PP (2009): Effect of transcranial magnetic stimulation (TMS) on parietal and premotor cortex during planning of reaching movements. PLoS One 4:e4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson GD, Gorden CD, Nakazowa S, Wada E, Warden K, LaManna JC (2000): Perfusion‐limited recovery of evoked potential function after spinal cord injury. Spine (Phila Pa 1976) 25:1218–1226. [DOI] [PubMed] [Google Scholar]
- Chao‐Gan Y, Yu‐Feng Z (2010): DPARSF: A MATLAB Toolbox for “Pipeline” Data Analysis of Resting‐State fMRI. Front Syst Neurosci 4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Cohen LG, Hallett M (2002): Nervous system reorganization following injury. Neuroscience 111:761–773. [DOI] [PubMed] [Google Scholar]
- Choe AS, Belegu V, Yoshida S, Joel S, Sadowsky CL, Smith SA, van Zijl PC, Pekar JJ, McDonald JW (2013): Extensive neurological recovery from a complete spinal cord injury: A case report and hypothesis on the role of cortical plasticity. Front Hum Neurosci 7:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman WP, Geisler FH (2004): Injury severity as primary predictor of outcome in acute spinal cord injury: Retrospective results from a large multicenter clinical trial. Spine J 4:373–378. [DOI] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC (1994): Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr 18:192–205. [PubMed] [Google Scholar]
- Cramer SC, Lastra L, Lacourse MG, Cohen MJ (2005): Brain motor system function after chronic, complete spinal cord injury. Brain 128:2941–2950. [DOI] [PubMed] [Google Scholar]
- Dietz V, Curt A (2006): Neurological aspects of spinal‐cord repair: Promises and challenges. Lancet Neurol 5:688–694. [DOI] [PubMed] [Google Scholar]
- Dobkin B, Barbeau H, Deforge D, Ditunno J, Elashoff R, Apple D, Basso M, Behrman A, Harkema S, Saulino M and others. (2007): The evolution of walking‐related outcomes over the first 12 weeks of rehabilitation for incomplete traumatic spinal cord injury: The multicenter randomized Spinal Cord Injury Locomotor Trial. Neurorehabil Neural Repair 21:25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak MF, Noonan VK, Fallah N, Fisher CG, Finkelstein J, Kwon BK, Rivers CS, Ahn H, Paquet J, Tsai EC, et al. (2015): The influence of time from injury to surgery on motor recovery and length of hospital stay in acute traumatic spinal cord injury: An observational Canadian cohort study. J Neurotrauma 32:645–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo T, Tominaga T, Olson L (2009): Cortical changes following spinal cord injury with emphasis on the Nogo signaling system. Neuroscientist 15:291–299. [DOI] [PubMed] [Google Scholar]
- Engineer ND, Riley JR, Seale JD, Vrana WA, Shetake JA, Sudanagunta SP, Borland MS, Kilgard MP (2011): Reversing pathological neural activity using targeted plasticity. Nature 470:101–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett JW, Curt A, Steeves JD, Coleman WP, Tuszynski MH, Lammertse D, Bartlett PF, Blight AR, Dietz V, Ditunno J, et al. (2007): Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: Spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord 45:190–205. [DOI] [PubMed] [Google Scholar]
- Flaster M, Sharma A, Rao M (2013): Poststroke depression: A review emphasizing the role of prophylactic treatment and synergy with treatment for motor recovery. Top Stroke Rehabil 20:139–150. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME (2005): The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund P, Wannier T, Schmidlin E, Bloch J, Mir A, Schwab ME, Rouiller EM (2007): Anti‐Nogo‐A antibody treatment enhances sprouting of corticospinal axons rostral to a unilateral cervical spinal cord lesion in adult macaque monkey. J Comp Neurol 502:644–659. [DOI] [PubMed] [Google Scholar]
- Freund P, Dalton C, Wheeler‐Kingshott CA, Glensman J, Bradbury D, Thompson AJ, Weiskopf N (2010): Method for simultaneous voxel‐based morphometry of the brain and cervical spinal cord area measurements using 3D‐MDEFT. J Magn Reson Imaging 32:1242–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund P, Weiskopf N, Ward NS, Hutton C, Gall A, Ciccarelli O, Craggs M, Friston K, Thompson AJ (2011): Disability, atrophy and cortical reorganization following spinal cord injury. Brain 134:1610–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund P, Wheeler‐Kingshott CA, Nagy Z, Gorgoraptis N, Weiskopf N, Friston K, Thompson AJ, Hutton C (2012): Axonal integrity predicts cortical reorganisation following cervical injury. J Neurol Neurosurg Psychiatry 83:629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund P, Weiskopf N, Ashburner J, Wolf K, Sutter R, Altmann DR, Friston K, Thompson A, Curt A (2013): MRI investigation of the sensorimotor cortex and the corticospinal tract after acute spinal cord injury: A prospective longitudinal study. Lancet Neurol 12:873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman EA, Hanakawa T, Chung M, Hummel F, Leiguarda RC, Cohen LG (2004): Reorganization of the human ipsilesional premotor cortex after stroke. Brain 127:747–758. [DOI] [PubMed] [Google Scholar]
- Furlan JC, Bracken MB, Fehlings MG (2010): Is age a key determinant of mortality and neurological outcome after acute traumatic spinal cord injury? Neurobiol Aging 31:434–446. [DOI] [PubMed] [Google Scholar]
- Guest JD, Hiester ED, Bunge RP (2005): Demyelination and Schwann cell responses adjacent to injury epicenter cavities following chronic human spinal cord injury. Exp Neurol 192:384–393. [DOI] [PubMed] [Google Scholar]
- Guleria S, Gupta RK, Saksena S, Chandra A, Srivastava RN, Husain M, Rathore R, Narayana PA (2008): Retrograde Wallerian degeneration of cranial corticospinal tracts in cervical spinal cord injury patients using diffusion tensor imaging. J Neurosci Res 86:2271–2280. [DOI] [PubMed] [Google Scholar]
- Gustin SM, Wrigley PJ, Siddall PJ, Henderson LA (2010): Brain anatomy changes associated with persistent neuropathic pain following spinal cord injury. Cereb Cortex 20:1409–1419. [DOI] [PubMed] [Google Scholar]
- Gwak YS, Hains BC, Johnson KM, Hulsebosch CE (2004): Locomotor recovery and mechanical hyperalgesia following spinal cord injury depend on age at time of injury in rat. Neurosci Lett 362:232–235. [DOI] [PubMed] [Google Scholar]
- Hains BC, Black JA, Waxman SG (2003): Primary cortical motor neurons undergo apoptosis after axotomizing spinal cord injury. J Comp Neurol 462:328–341. [DOI] [PubMed] [Google Scholar]
- Henderson LA, Gustin SM, Macey PM, Wrigley PJ, Siddall PJ (2011): Functional reorganization of the brain in humans following spinal cord injury: Evidence for underlying changes in cortical anatomy. J Neurosci 31:2630–2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsfield MA, Sala S, Neema M, Absinta M, Bakshi A, Sormani MP, Rocca MA, Bakshi R, Filippi M (2010): Rapid semi‐automatic segmentation of the spinal cord from magnetic resonance images: Application in multiple sclerosis. Neuroimage 50:446–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou JM, Sun TS, Xiang ZM, Zhang JZ, Zhang ZC, Zhao M, Zhong JF, Liu J, Zhang H, Liu HL, et al. (2014a): Alterations of resting‐state regional and network‐level neural function after acute spinal cord injury. Neuroscience 277:446–454. [DOI] [PubMed] [Google Scholar]
- Hou JM, Yan RB, Xiang ZM, Zhang H, Liu J, Wu YT, Zhao M, Pan QY, Song LH, Zhang W et al. (2014b): Brain sensorimotor system atrophy during the early stage of spinal cord injury in humans. Neuroscience 266C:208–215. [DOI] [PubMed] [Google Scholar]
- Jurkiewicz MT, Crawley AP, Verrier MC, Fehlings MG, Mikulis DJ (2006): Somatosensory cortical atrophy after spinal cord injury: A voxel‐based morphometry study. Neurology 66:762–764. [DOI] [PubMed] [Google Scholar]
- Jurkiewicz MT, Mikulis DJ, McIlroy WE, Fehlings MG, Verrier MC (2007): Sensorimotor cortical plasticity during recovery following spinal cord injury: A longitudinal fMRI study. Neurorehabil Neural Repair 21:527–538. [DOI] [PubMed] [Google Scholar]
- Kim JS, Singh V, Lee JK, Lerch J, Ad‐Dab'bagh Y, MacDonald D, Lee JM, Kim SI, Evans AC (2005): Automated 3‐D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. Neuroimage 27:210–221. [DOI] [PubMed] [Google Scholar]
- Kirshblum S, Millis S, McKinley W, Tulsky D (2004): Late neurologic recovery after traumatic spinal cord injury. Arch Phys Med Rehabil 85:1811–1817. [DOI] [PubMed] [Google Scholar]
- Kwon BK, Liu J, Messerer C, Kobayashi NR, McGraw J, Oschipok L, Tetzlaff W (2002): Survival and regeneration of rubrospinal neurons 1 year after spinal cord injury. Proc Natl Acad Sci USA 99:3246–3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch JP, Evans AC (2005): Cortical thickness analysis examined through power analysis and a population simulation. Neuroimage 24:163–173. [DOI] [PubMed] [Google Scholar]
- Losseff NA, Webb SL, O'Riordan JI, Page R, Wang L, Barker GJ, Tofts PS, McDonald WI, Miller DH, Thompson AJ (1996): Spinal cord atrophy and disability in multiple sclerosis. A new reproducible and sensitive MRI method with potential to monitor disease progression. Brain 119:701–708. [DOI] [PubMed] [Google Scholar]
- Lotze M, Grodd W, Rodden FA, Gut E, Schonle PW, Kardatzki B, Cohen LG (2006): Neuroimaging patterns associated with motor control in traumatic brain injury. Neurorehabil Neural Repair 20:14–23. [DOI] [PubMed] [Google Scholar]
- Lundell H, Christensen MS, Barthelemy D, Willerslev‐Olsen M, Biering‐Sorensen F, Nielsen JB (2011): Cerebral activation is correlated to regional atrophy of the spinal cord and functional motor disability in spinal cord injured individuals. Neuroimage 54:1254–1261. [DOI] [PubMed] [Google Scholar]
- Ma VY, Chan L, Carruthers KJ (2014): Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: Stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pain. Arch Phys Med Rehabil 95:986–995 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makin TR, Scholz J, Filippini N, Henderson Slater D, Tracey I, Johansen‐Berg H (2013): Phantom pain is associated with preserved structure and function in the former hand area. Nat Commun 4:1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mole TB, MacIver K, Sluming V, Ridgway GR, Nurmikko TJ (2014): Specific brain morphometric changes in spinal cord injury with and without neuropathic pain. Neuroimage Clin 5:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moxon KA, Oliviero A, Aguilar J, Foffani G (2014): Cortical reorganization after spinal cord injury: Always for good? Neuroscience 283:78–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardone R, Holler Y, Brigo F, Seidl M, Christova M, Bergmann J, Golaszewski S, Trinka E (2013a): Functional brain reorganization after spinal cord injury: Systematic review of animal and human studies. Brain Res 1504:58–73. [DOI] [PubMed] [Google Scholar]
- Nardone R, Holler Y, Brigo F, Seidl M, Christova M, Bergmann J, Golaszewski S, Trinka E (2013b): Functional brain reorganization after spinal cord injury: Systematic review of animal and human studies. Brain Res 1504:58–73. [DOI] [PubMed] [Google Scholar]
- New PW, Epi MC (2007): Influence of age and gender on rehabilitation outcomes in nontraumatic spinal cord injury. J Spinal Cord Med 30:225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicotra A, Critchley HD, Mathias CJ, Dolan RJ (2006): Emotional and autonomic consequences of spinal cord injury explored using functional brain imaging. Brain 129:718–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y, Onoe H, Morichika Y, Perfiliev S, Tsukada H, Isa T (2007): Time‐dependent central compensatory mechanisms of finger dexterity after spinal cord injury. Science 318:1150–1155. [DOI] [PubMed] [Google Scholar]
- Pantano P, Formisano R, Ricci M, Di Piero V, Sabatini U, Di Pofi B, Rossi R, Bozzao L, Lenzi GL (1996): Motor recovery after stroke. Morphological and functional brain alterations. Brain 119:1849–1857. [DOI] [PubMed] [Google Scholar]
- Park E, Velumian AA, Fehlings MG (2004): The role of excitotoxicity in secondary mechanisms of spinal cord injury: A review with an emphasis on the implications for white matter degeneration. J Neurotrauma 21:754–774. [DOI] [PubMed] [Google Scholar]
- Pastor‐Bernier A, Tremblay E, Cisek P (2012): Dorsal premotor cortex is involved in switching motor plans. Front Neuroeng 5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard ME, Apple DF (2003): Factors associated with improved neurologic outcomes in patients with incomplete tetraplegia. Spine (Phila Pa 1976) 28:33–39. [DOI] [PubMed] [Google Scholar]
- Riecker A, Groschel K, Ackermann H, Schnaudigel S, Kassubek J, Kastrup A (2010): The role of the unaffected hemisphere in motor recovery after stroke. Hum Brain Mapp 31:1017–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC (1998): A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 17:87–97. [DOI] [PubMed] [Google Scholar]
- Steeves JD, Kramer JK, Fawcett JW, Cragg J, Lammertse DP, Blight AR, Marino RJ, Ditunno JF Jr, Coleman WP, Geisler FH, et al. (2011): Extent of spontaneous motor recovery after traumatic cervical sensorimotor complete spinal cord injury. Spinal Cord 49:257–265. [DOI] [PubMed] [Google Scholar]
- Strick PL (1988): Anatomical organization of multiple motor areas in the frontal lobe: Implications for recovery of function. Adv Neurol 47:293–312. [PubMed] [Google Scholar]
- Thuret S, Moon LD, Gage FH (2006): Therapeutic interventions after spinal cord injury. Nat Rev Neurosci 7:628–643. [DOI] [PubMed] [Google Scholar]
- Wang L, Yu C, Chen H, Qin W, He Y, Fan F, Zhang Y, Wang M, Li K, Zang Y, et al. (2010): Dynamic functional reorganization of the motor execution network after stroke. Brain 133(Pt 4):1224–1238. [DOI] [PubMed] [Google Scholar]
- Wang LE, Tittgemeyer M, Imperati D, Diekhoff S, Ameli M, Fink GR, Grefkes C (2012): Degeneration of corpus callosum and recovery of motor function after stroke: A multimodal magnetic resonance imaging study. Hum Brain Mapp 33:2941–2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters RL, Adkins RH, Sie IH, Yakura JS (1996): Motor recovery following spinal cord injury associated with cervical spondylosis: A collaborative study. Spinal Cord 34:711–715. [DOI] [PubMed] [Google Scholar]
- Weinrich M, Wise SP, Mauritz KH (1984): A neurophysiological study of the premotor cortex in the rhesus monkey. Brain 107:385–414. [DOI] [PubMed] [Google Scholar]
- Wilson JR, Davis AM, Kulkarni AV, Kiss A, Frankowski RF, Grossman RG, Fehlings MG (2014): Defining age‐related differences in outcome after traumatic spinal cord injury: Analysis of a combined, multicenter dataset. Spine J 14:1192–1198. [DOI] [PubMed] [Google Scholar]
- Wrigley PJ, Gustin SM, Macey PM, Nash PG, Gandevia SC, Macefield VG, Siddall PJ, Henderson LA (2009): Anatomical changes in human motor cortex and motor pathways following complete thoracic spinal cord injury. Cereb Cortex 19:224–232. [DOI] [PubMed] [Google Scholar]
- Zijdenbos AP, Forghani R, Evans AC (2002): Automatic “pipeline” analysis of 3‐D MRI data for clinical trials: Application to multiple sclerosis. IEEE Trans Med Imaging 21:1280–1291. [DOI] [PubMed] [Google Scholar]


