Abstract
Personality is known to be relatively stable throughout adulthood. Nevertheless, it has been shown that major life events with high personal significance, including experiences engendered by psychedelic drugs, can have an enduring impact on some core facets of personality. In the present, balanced‐order, placebo‐controlled study, we investigated biological predictors of post‐lysergic acid diethylamide (LSD) changes in personality. Nineteen healthy adults underwent resting state functional MRI scans under LSD (75µg, I.V.) and placebo (saline I.V.). The Revised NEO Personality Inventory (NEO‐PI‐R) was completed at screening and 2 weeks after LSD/placebo. Scanning sessions consisted of three 7.5‐min eyes‐closed resting‐state scans, one of which involved music listening. A standardized preprocessing pipeline was used to extract measures of sample entropy, which characterizes the predictability of an fMRI time‐series. Mixed‐effects models were used to evaluate drug‐induced shifts in brain entropy and their relationship with the observed increases in the personality trait openness at the 2‐week follow‐up. Overall, LSD had a pronounced global effect on brain entropy, increasing it in both sensory and hierarchically higher networks across multiple time scales. These shifts predicted enduring increases in trait openness. Moreover, the predictive power of the entropy increases was greatest for the music‐listening scans and when “ego‐dissolution” was reported during the acute experience. These results shed new light on how LSD‐induced shifts in brain dynamics and concomitant subjective experience can be predictive of lasting changes in personality. Hum Brain Mapp 37:3203–3213, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: LSD‐25, neuroplasticity, entropy, personality, transpersonal experience
INTRODUCTION
After several decades of effective prohibition, researchers have recently begun to re‐examine the therapeutic potential of psychedelic drugs, such as lysergic acid diethylamide (LSD). Small‐scale pilot studies have evaluated the safety and efficacy of psychedelics in the treatment of a number of psychiatric conditions with promising preliminary results [Bogenschutz et al., 2015; Gasser et al., 2015; Johnson et al., 2014; Osorio Fde et al., 2015; Thomas et al., 2013; Vollenweider and Kometer, 2010].
The dominant approach to psychedelic‐assisted therapy involves only one or a small number of high‐dose sessions that aim to evoke so‐called peak [Maslow, 1964] or mystical‐type experiences [Griffiths et al., 2006], characterized by dissolved ego boundaries and a concomitant sense of oneness or unity, the approach often referred as “psychedelic therapy” in the scientific literature [Chwelos et al., 1959; Grof et al., 2008]. Considerable emphasis in this framework is placed on “set” (i.e., beliefs, expectations and current psychological well‐being, as in “mind‐set”) and “setting” in psychedelic therapy, as it is recognized that psychological and environmental factors can be especially influential in shaping the nature of the drug experience [Faillace and Szara, 1968]. Consistent with this principle, psychedelic therapy sessions are typically accompanied by a carefully designed music playlist, which is intended to facilitate emotional release, personal insight and the occurrence of peak experiences [Bonny and Pahnke, 1972; Grof et al., 2008; Kaelen et al., 2015].
In healthy individuals, it has been shown that even a single session with the shorter‐acting LSD‐like psychedelic, psilocybin, can produce lasting increases in the personality trait openness [MacLean et al., 2011]. Openness is considered to be one of the major dimensions of personality and is linked to imagination, aesthetic appreciation, novelty‐seeking, non‐conformity, and creativity [Costa and McCrae, 1992]. Of relevance to psychedelic therapy, the observed increases in trait openness post‐psilocybin were greatest in those individuals who reported mystical‐type experiences in relation to their psilocybin session. Moreover, in some participants these changes persisted for more than one year after the single acute psychedelic experience [MacLean et al., 2011], which is remarkable given that personality is thought to be relatively fixed by adulthood [Costa and McCrae, 1988].
LSD has a rich pharmacology, with agonist properties at a range of different neurotransmitter receptors. However, its affinity for the serotonin 2A receptor (5‐HT2AR) is thought to largely determine its characteristic psychological and associated neurophysiological effects [Nichols and Sanders‐Bush, 2004; Passie et al., 2008]. Early human electrophysiological studies observed desynchronization of cortical activity during the acute LSD‐state and depth recordings detected increased amplitude of oscillations within limbic structures [Monroe and Heath, 1961; Schwarz et al., 1956]. More recent human imaging research has implicated disrupted activity and connectivity within the default mode and frontoparietal cortical regions under psychedelics [Carhart‐Harris et al., 2016, Carhart‐Harris et al., 2012; Kometer et al., 2015; Muthukumaraswamy et al., 2013], a wider repertoire of connectivity patterns [Roseman et al., 2014; Tagliazucchi et al., 2014], and a shift toward more random dynamics (increased entropy) within the higher‐order brain networks [Tagliazucchi et al., 2014]. This desegregating or globally “unifying” effect of psychedelics has been linked with the experience of disturbed ego‐boundaries [Tagliazucchi E, et al., 2016, Carhart‐Harris et al., 2013] and the so‐called “unitive experience” or “sense of oneness” that may be a definitive feature of mystical‐type experiences [Carhart‐Harris et al., 2014; Griffiths et al., 2006; MacLean et al., 2011; Stace, 1960]. These complex psychological phenomena have been described for millennia by certain spiritual movements and figureheads [The Rig Veda; Buddhaghosa, 430 CE, 2011] and are now capturing the interest of contemporary researchers working with psychedelics [Carhart‐Harris et al., 2014; Kometer et al., 2015; Lebedev et al., 2015].
Inspired by neuroimaging research with psychedelics, a testable hypothesis was recently formulated that proposed applying an information theory‐based measure of uncertainty or randomness (entropy) to the phenomenology and neurophysiology of altered states of consciousness [Carhart‐Harris et al., 2014]. The “entropic brain” hypothesis differentiates between the so‐called secondary consciousness of mature, healthy, and awake adult humans, in which brain activity is highly organized, and the primary consciousness of certain primitive and altered states, in which brain activity is characteristically disordered and therefore entropic or unpredictable. Phenomena such as ego‐dissolution and associated mystical‐type experiences are hypothesized to lie toward the primary consciousness end of this entropy continuum.
While there has been a concerted effort in recent years to map out the neural correlates of the acute psychedelic state [Carhart‐Harris et al., 2012; Carhart‐Harris et al., 2014; Kometer et al., 2015; Lebedev et al., 2015; Muthukumaraswamy et al., 2013], much less attention has been paid to how these acute effects of psychedelics relate to more enduring psychological and behavioral changes. The present study aimed to test the hypothesis that LSD increases the entropy of brain dynamics (reduced predictability of a time‐series) and that these effects have enduring consequences for personality. More specifically, we hypothesized that increased entropy within high‐level brain networks (e.g., the frontoparietal, salience and default‐mode networks) under LSD would positively predict subsequent increases in the personality trait openness. We also predicted that ego‐dissolution reported during the LSD‐state would be predictive of subsequent personality changes and that music‐listening would further enhance these relationships.
METHODS
Participants
Twenty healthy subjects (aged 30.9 ± 7.8 years, 15 males) recruited via word‐of‐mouth were included in the study. Informed consent was obtained from all subjects prior to enrolment. Standardized physical and psychiatric examinations, electrophysiological, blood and urine tests for drugs of abuse, and pregnancy were also carried out at screening.
Inclusion criteria were: age 21+ years, no personal or immediate family history of major psychiatric disorders, no cardiovascular disease, and no history of a significant adverse response to psychedelics and negative pregnancy and drug tests. All of the subjects had used classic psychedelics at least once before but not within 2 weeks of their first study day (Supporting Information Table S1).
The MRI part of the study was not completed by one subject who requested the scanning session to be stopped prematurely due to transient but nevertheless significant anxiety. His behavioral data, however, was collected and the pattern of personality change was consistent with what was observed at the group level. Thus, the personality‐related effects reported below include this subject.
Study Design and Procedures
Two scanning sessions with either 75 µg of LSD or placebo (each given intravenously over two minutes) were scheduled for each participant, in a counter‐balanced order, with an interval of at least two weeks between each session (Supporting Information Figure S1). For each session, three 7.5‐min BOLD resting‐state fMRI scans were acquired (lasting 25 min, in total): resting state 1 (no music), resting state 2 (music), and resting state 3 (no music). For the first and third states, the subjects were instructed to rest quietly and keep their eyes closed. Instructions were the same for the second state, except that the subjects were informed that they would listen to ambient music played through headphones. Two tracks from the album “Yearning” by Robert Rich and Lisa Moskow were selected in an independent sample to be balanced for emotional potency and were presented in a counter‐balanced order. The infusion (drug/placebo) was administered over 2 min and occurred 115 min before the resting‐state scans were initiated. Imaging data were acquired on a 3‐Tesla MRI scanner (GE Signa HDx) with a standardized protocol described in Supporting Information (Note 1).
Personality Assessment and In‐Scanner Subjective Measures
The standard 240‐item revised five‐factor personality inventory (NEO‐PI‐R) [McCrae and Costa, 2010] was administered at baseline and 2 weeks after the LSD/placebo session to evaluate changes in personality (Supporting Information Figure S1). A visual analogue scale assessing the intensity of ego‐dissolution (“I experienced a disintegration of my sense of self or ego”. Lower anchor = “not at all”; highest anchor = “most imaginable”) was completed inside the scanner immediately after each scan. This measure was strongly correlated with scores on the 11‐factor altered states of consciousness questionnaire [Studerus et al., 2010] related to mystical quality of the experiences (unity, spiritual experience, blissful state, and changed meaning of percepts) administered after the scanning session had been completed (Supporting Information Note 2).
Ethics
This study was approved by the National Research Ethics Service Committee London–West London and was conducted in accordance with the revised declaration of Helsinki [2000], the International Committee on Harmonization Good Clinical Practice guidelines and National Health Service Research Governance Framework. Imperial College London sponsored the research, which was conducted under a Home Office license for research with schedule 1 drugs.
Image Preprocessing
A standardized pipeline based on “SPM12” (Wellcome Trust Center for Neuroimaging, UCL) was used using the Data Processing Assistant for Resting‐State fMRI: Advanced Edition (DPARSFA, version 3.2) [Chao‐Gan and Yu‐Feng, 2010], installed in the MATLAB R2013a environment.
For each subject, echo‐planar images subsequently underwent steps for slice‐timing correction, spatial realignment, and registration to standardized MNI space carried out with a population template generated from the T1 structural images using the Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra algorithm [Ashburner, 2007]. Spurious variance was reduced by regressing‐out signal from white matter, cerebrospinal fluid, and by voxel‐specific motion correction [Satterthwaite et al., 2013]. Next, the images were detrended and band‐pass filtered (0.01–0.1 Hz) to eliminate biologically non‐relevant signals and the resulting output was used to calculate sample entropy [Liu et al., 2013; Richman and Moorman, 2000] at the voxel‐level, as implemented in the “complexity” toolbox (LOFT Lab).
Sample entropy is formally defined as the negative logarithm of the conditional probability that if two sets of simultaneous data points (vector pairs) with length m meet similarity criterion (having distance < r) then vector pairs with length m + 1 will also have distance < r. Practically, it measures the complexity of a signal and is negatively related to the predictability of a time‐series, that is, the greater a signal's sample entropy, the lower is its predictability [Richman and Moorman, 2000]. See Supporting Information for illustration of the measure (Supporting Information Note 3, Figure S2). The entropy maps were parcellated with Yeo's 17‐networks functional scheme [Yeo et al., 2011] and used in the subsequent statistical analyses. For the voxel‐based part, entropy maps were smoothed with a Gaussian kernel, full width at half maximum of 6 mm.
In order to evaluate robustness of the effects, we used a multi‐scale version of sample entropy [Costa et al., 2002], calculating the measure across time scales from 1 to 5 [Yang et al., 2015]. This procedure is equivalent to splitting a time‐series into non‐overlapping time‐windows (with the length of a scale from 1 to 5) and averaging the data points for each of them followed by SamEn calculation.
Statistical Analysis
Statistical analyses were carried out using R programming language, version 3.2.2 [R Development Core Team, 2015] with “nlme” package used for mixed‐effect modeling [Pinheiro et al., 2016]. Voxel‐wise contrast estimations were accomplished after the ROI‐based analyses using SPM12. For the voxel‐wise part, a family‐wise correction for multiple comparisons was carried out with a cluster‐wise method of Monte Carlo as implemented in the AlphaSim algorithm [Ward, 2000] with 5,000 synthesized Gaussian noise simulations (initial cluster‐forming threshold of P < 0.005).
A direct comparison of brain dynamics under LSD and placebo was then performed using mixed‐effects modeling (random effect: subjects). Additional covariates included motion and state‐by‐drug interaction (both linear and quadratic terms). There were minor sound problems for four subjects, specifically a disconnection of a headphone contact on one side during music scan. Therefore, an additional two‐level nuisance factor was introduced to the models, specifying whether a subject experienced any sound problems during the session. Post hoc evaluation revealed that this nuisance factor only slightly improved the model fit but generally had little impact on the results.
A second block of analyses addressed LSD‐induced entropy increases relative to placebo as predictors of personality trait openness evaluated 2 weeks after the LSD session relative to screening scores. This was done using mixed‐effects modeling of these relationships (ΔEntropy − ΔPersonality) and a state‐by‐change interaction with music listening (ΔEntropy − ΔPersonality × State).
In the next set of analyses, we introduced the in‐scanner measures of ego‐dissolution as an additional variable and estimated a full four‐way interaction effect: ΔEntropy − ΔPersonality × State × Ego‐dissolution. This enabled us to address the questions of whether increases in openness were larger in those who reported greater ego‐dissolution and also showed more prominent increases in entropy, and whether such effects interacted with music‐listening.
An additional set of analyses, evaluated stability of the results after taking into account effects of drug administration order and the impact of previous psychedelic experience on the main contrasts. For the latter part, we calculated two composite scores, defined as the first principal components extracted from total number of experiences with different psychedelics (LSD, Psilocybin, DMT, Salvia Divinorum, Ketamine, and MDMA) and days passed since last intake of these drugs.
Prior to the analyses, normality of distributions was confirmed for all continuous measures (except for the voxel‐wise entropy measures) using Shapiro‐Wilk tests. For the direct effects of the drug on brain dynamics, we set a family‐wise error‐corrected (FWE) threshold for significance at P FWE < 0.05, and for all analyses of the behavioral data at P uncorrected < 0.05.
Results visualization was carried out using “MRIcroGL” software (McCausland Center for Brain Imaging).
RESULTS
Direct Effects of LSD on Sample Entropy
Overall, LSD's effect on brain entropy was well spatially distributed (global cortical effect: T = 4.34, P < 0.001), significantly affecting 11 out of 17 functional systems, including primary and secondary sensory and associative networks as well as hierarchically higher divisions (Fig. 1 and Table 1). Voxel‐wise analysis yielded consistent results with FWE‐corrected clusters of entropy increases located in the frontoparietal, medial occipital, posterior and dorsal cingulate regions (Fig. 2).
Figure 1.
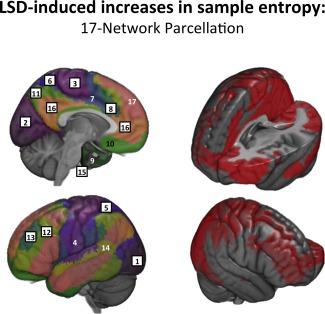
LSD‐induced entropy increases (17‐Network parcellation: LSD vs. Placebo). LEFT: Black frames with numbers represent networks with significant sample entropy increases seen under LSD. RIGHT: Networks with significant entropy increases are colored in red. Networks: 1 ‐ Secondary Visual; 2 ‐ Primary Visual; 3 ‐ Superior Sensorimotor; 4 ‐ Inferior Sensorimotor; 5 ‐ Superior Parietal; 6 ‐ Posterior Sensorimotor; 7 ‐ Posterior Salience; 8 ‐ Anterior Salience; 9 ‐ Anterior MTL; 10 ‐ Orbitofrontal; 11 ‐ Precuneus; 12 ‐ Inferior Frontoparietal; 13 ‐ Superior Frontoparietal; 14 ‐ Auditory; 15 ‐ Hippocampal; 16 ‐ Default Mode; 17 ‐ Frontotemporal. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 1.
Direct effects of LSD on network‐level sample entropy (mixed‐effects model)
| Network | Stats | ||||||
|---|---|---|---|---|---|---|---|
| N | Description |
T Drug |
P Drug |
T Drug × state:L |
P Drug × state:L |
T Drug × state:Q |
P Drug × state:Q |
| 1 | Secondary Visual | 4.698 | <0.001*** | −0.536 | 0.594 | 1.647 | 0.103 |
| 2 | Primary Visual | 6.019 | <0.001*** | −0.034 | 0.973 | 1.567 | 0.121 |
| 3 | Superior Sensorimotor | 6.209 | <0.001*** | 0.050 | 0.961 | 0.387 | 0.699 |
| 4 | Inferior Sensorimotor | 1.238 | 0.219 | 0.111 | 0.912 | 0.124 | 0.902 |
| 5 | Superior Parietal | 4.961 | <0.001*** | −0.206 | 0.837 | 0.486 | 0.628 |
| 6 | Posterior Sensorimotor | 5.971 | <0.001*** | −0.320 | 0.750 | 0.187 | 0.852 |
| 7 | Posterior Salience | 1.651 | 0.102 | 0.304 | 0.762 | −0.097 | 0.923 |
| 8 | Anterior Salience | 4.020 | <0.001*** | 0.582 | 0.562 | 0.017 | 0.986 |
| 9 | Anterior MTL | −0.710 | 0.479 | 0.258 | 0.797 | 0.169 | 0.866 |
| 10 | Orbitofrontal | 0.651 | 0.517 | −0.114 | 0.910 | 0.387 | 0.700 |
| 11 | Precuneus | 6.068 | <0.001*** | 0.556 | 0.580 | −0.119 | 0.905 |
| 12 | Inferior Frontoparietal | 6.401 | <0.001*** | −0.264 | 0.792 | 0.786 | 0.434 |
| 13 | Superior Frontoparietal | 5.171 | <0.001*** | 0.288 | 0.774 | 0.638 | 0.525 |
| 14 | Auditory | 0.511 | 0.610 | 0.139 | 0.889 | 0.020 | 0.984 |
| 15 | Hippocampal | 2.531 | 0.013* | 0.914 | 0.363 | 0.088 | 0.930 |
| 16 | Default Mode | 3.594 | 0.001** | 0.643 | 0.522 | 0.258 | 0.797 |
| 17 | Frontotemporal | 1.560 | 0.122 | 0.217 | 0.828 | 0.271 | 0.787 |
Global (cortical) effect of LSD on brain entropy: T = 4.34 (P < 0.001).
Caption:
Networks showing significant effects of the drug are highlighted in bold.
T = t‐value; P = P‐value;
Drug × state:L = drug‐by‐state interaction: linear term.
Drug × state:Q = drug‐by‐state interaction: quadratic term.
***P<0.001
**P<0.01
*P<0.05
Figure 2.
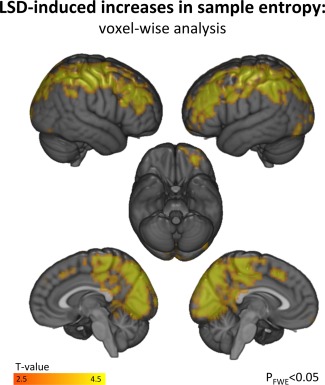
LSD‐induced entropy increases (Voxel‐wise analysis: LSD vs. Placebo). P FWE < 0.05: Family‐wise correction for multiple comparisons by 5,000 Monte Carlo simulations with initial cluster‐forming threshold of P < 0.005. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Further analysis of multi‐scale entropy revealed that the effects are present mostly within the first three scales and are not significant at scales 4 and 5 (Fig. 3). Post hoc exploration revealed some network‐specific effects of LSD on sample entropy at higher time scales, which, however, were beyond the scope of the present article (For details, see Supporting Information Figure S3, Note 4).
Figure 3.
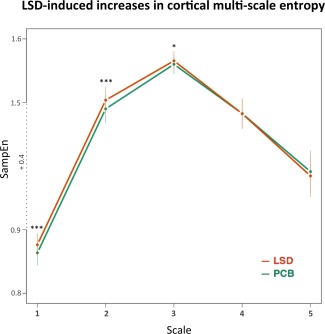
LSD‐induced increases in sample entropy across multiple time scales: global (cortical) effect. The plot shows effects for all 3 states combined. The bars represent standard deviations. X‐axis represents scaling factor used to yield coarse‐grained time‐series prior to SamEn estimation. SampEn – sample entropy (m = 2, r = 0.3, scale: 1–5). *** – P < 0.001; * – P < 0.05; A segment (+0.4) of Y‐axis was omitted for visualization purposes (due to large overall increases in sample entropy values when moving from scale 1 to 2). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Personality Changes
A comprehensive description of the psychological data acquired in this study is reported in a separate paper [Carhart‐Harris et al., 2016]. Among all evaluated traits, we focused on changes in trait openness after LSD, which were significant in the present study (T = 1.95[19], P = 0.03, Cohen's d = 0.16) and previously found to be affected by psychedelics [MacLean et al., 2011]. No significant changes in openness were observed post‐placebo. The assessment of additional traits was performed post hoc as an exploratory part of the study and can be found in Supporting Information (Supporting Information Notes 5‐6, Tables S3‐5).
Acute Shifts in Brain Dynamics as a Predictor of Changes in Openness
The LSD‐induced increases in brain entropy showed correlations with subsequent increases in trait openness. Global entropy increases predicted changes in openness (T = 2.3, P = 0.035) with the change‐by‐state interaction term also being significant (T = 3.83, P = 0.0005), meaning that music enhanced the predictive value of the relationship between increased entropy and subsequent increases in openness (Fig. 4). At the network level, both direct effects of the drug on entropy (i.e., independent of “state”) and change‐by‐state interactions (i.e., mediated by music) were predictive of subsequent increases in openness with both sensory and higher cognitive networks being involved in these effects (Table 2 and Supporting Information Figure S4).
Figure 4.
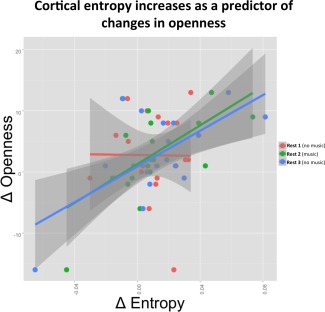
Global (cortical) increases in entropy during psychedelic state predict changes in trait openness from baseline to two weeks after the study sessions. X‐axis: global sample entropy changes under LSD relative to placebo. Y‐axis: changes in personality trait openness two weeks after the LSD session relative to screening values. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 2.
Increases in entropy under LSD and changes in trait openness.
| Network | Stats | ||||||
|---|---|---|---|---|---|---|---|
| N | Description |
T ΔO |
P ΔO |
P FDR |
T ΔO × state |
P ΔO × state |
P FDR |
| 1 | Secondary Visual a | 1.409 | 0.178 | 0.228 | 4.025 | <0.001*** | 0.003 |
| 2 | Primary Visual a | 1.240 | 0.233 | 0.264 | 2.797 | 0.009** | 0.015 |
| 3 | Superior Sensorimotor b | 2.562 | 0.021* | 0.183 | 3.672 | <0.001*** | 0.004 |
| 4 | Inferior Sensorimotor a | 1.544 | 0.142 | 0.228 | 3.965 | <0.001*** | 0.003 |
| 5 | Superior Parietal b | 2.493 | 0.024* | 0.183 | 3.056 | 0.004** | 0.009 |
| 6 | Posterior Sensorimotor b | 2.312 | 0.034* | 0.183 | 2.787 | 0.009** | 0.015 |
| 7 | Posterior Salience a | 1.658 | 0.117 | 0.228 | 3.226 | 0.003** | 0.008 |
| 8 | Anterior Salience a | 1.516 | 0.149 | 0.228 | 3.531 | 0.001** | 0.004 |
| 9 | Anterior MTL | 1.408 | 0.178 | 0.228 | 1.801 | 0.081 | 0.081 |
| 10 | Orbitofrontal a | 0.375 | 0.712 | 0.712 | 2.674 | 0.012* | 0.016 |
| 11 | Precuneus | 1.939 | 0.070 | 0.183 | 1.918 | 0.064 | 0.068 |
| 12 | Inferior Frontoparietal a | 1.917 | 0.073 | 0.183 | 2.421 | 0.021* | 0.028 |
| 13 | Superior Frontoparietal | 1.902 | 0.075 | 0.183 | 1.973 | 0.057 | 0.064 |
| 14 | Auditory a | 1.097 | 0.289 | 0.307 | 3.880 | <0.001*** | 0.003 |
| 15 | Hippocampal a | 2.102 | 0.052 | 0.183 | 2.674 | 0.012* | 0.016 |
| 16 | Default Mode | 1.380 | 0.187 | 0.228 | 2.015 | 0.052 | 0.063 |
| 17 | Frontotemporal a | 1.377 | 0.187 | 0.228 | 3.188 | 0.003** | 0.008 |
Global (cortical) effect: ΔO: T = 2.3 (P = 0.035); ΔO × state: T = 3.83 (P = 0.0005).
Caption:
Networks showing significant effects are highlighted in bold.
T = t‐value; P = P‐value; P FDR = P‐value adjusted for multiple tests (false discovery rate);
All results are adjusted for mean frame‐wise displacement (in‐scanner motion) used as a covariate;
ΔO = statistics for relations between acute entropy shifts [LSD vs. Placebo] and changes in Openness [2 weeks follow‐up vs. Screening] (Do acute LSD‐induced shifts in brain entropy predict changes in Openness?);
ΔO × state = Interactions with state (Does music affect predictive power of brain entropy shifts?);
Only interaction with state (ΔO ×state) is significant.
Both effects (ΔO and ΔO × state) are significant.
***P<0.001
**P<0.01
*P<0.05
N.B. A suspected outlier was identified during the analysis of openness, that is, one subject showed an anomalous decrease in openness post‐LSD. The reported results were therefore re‐evaluated using non‐parametric tests (Spearman rank correlation) and these yielded consistent results. See Supporting Information, Table S2 for details.
Interaction with In‐Scanner Measures of Ego‐Dissolution
A four‐way interaction for openness (ΔEntropy − ΔOpenness × State × Ego‐dissolution; random effect: “subjects”) was significant for the orbitofrontal (N10; T = 2.07[27], P = 0.048) and superior frontoparietal (N13; T = 2.36[27], P = 0.026) networks. This means that those who had the highest enhancement of ego‐dissolution during and after music‐listening and the most prominent increases in brain entropy in those compartments were also those who demonstrated the greatest increases in openness. This was a network‐specific effect, however, as it did not reach significance at the global level: T = 1.71[27], P = 0.099.
Effects of Drug Order and Previous Psychedelic Experience
Introducing drug order (i.e., whether participants received LSD in their first or second scanning session) into the models did not eliminate the above‐described effects on openness. This was also true for both composite scores of previous psychedelic experience See Supporting Information for related post hoc analyses (Supporting Information Notes 6‐7).
DISCUSSION
In line with the entropic brain hypothesis [Carhart‐Harris et al., 2014], exposure to LSD was associated with prominent increases in brain entropy affecting both sensory and higher‐order networks. These effects were observed mainly within short time scales, possibly indicating shifts in complexity toward randomness [Yang et al., 2015]. These changes in brain dynamics were predictive of subsequent increases in trait openness measured 2 weeks later, and this relationship was especially strong for the music and post‐music scans. This is one of the first fMRI studies of LSD and the first to identify a biological predictor of subsequent changes in personality [Griffiths et al., 2006, 2008; MacLean et al., 2011].
Based on the present study's findings and those that have preceded it, there are reasons to believe that classic psychedelics alter brain dynamics in a way that promotes lasting psychological changes. For example, LSD and other psychedelics have been found to enhance associative learning [Harvey, 2003; Romano et al., 2010], cognitive performance [Jensen et al., 2013], extinction of conditioned fear in rodents [Catlow et al., 2013], and creativity [Frecska et al., 2012] in humans. Furthermore, psychedelic drugs are currently showing promise in the treatment of drug addiction [Bogenschutz et al., 2015; Johnson et al., 2014; Thomas et al., 2013], depression [Osorio Fde et al., 2015; Vollenweider and Kometer, 2010], and anxiety associated with life‐threatening illnesses [Gasser et al., 2015]. Further work is clearly required to help delineate not just whether but also how these drugs can be useful in these disorders.
A key question for future studies to address is: are there any lasting changes in brain function and/or anatomy post‐treatment with psychedelics that can explain their putative therapeutic effects? Since previous research has demonstrated a link between peak mystical experiences and persisting changes in personality [Griffiths et al., 2008; Grof et al., 2008; MacLean et al., 2011; Majic et al., 2015; Smart and Storm, 1964], one might also wish to focus on psychological causation. In other words, the profound, transformative nature of the acute psychological experience may drive subsequent personality change. While this may well be true, it would be naive to assume that such processes do not have biological counterparts. Clearly, investigating the neurobiological underpinnings of the apparent long‐term effects of psychedelics on outlook and behavior is an important area for future research.
Personality traits reach maturity by adulthood and thereafter remain relatively stable until old age. However, it has been shown that major life events can have a significant impact on personality [Jeronimus et al., 2013; Lai et al., 2007; Tedeschi and Calhoun, 2004; Updegraff and Taylor, 2000]. Interestingly, in a study administering a single high‐dose of psilocybin in a sample of psychedelic‐naïve individuals, 67% of the subjects considered their psychedelic session to be either the single most personally meaningful or among the top five most meaningful experiences of their life, comparing it, for example, to the birth of their first child [Griffiths et al., 2006]. Such sessions, especially those accompanied by peak mystical experiences, have been shown to promote sustained positive changes in attitudes and behavior [Griffiths et al., 2008; Griffiths et al., 2006], and to have a lasting impact on trait openness [MacLean et al., 2011]. Consistent effects were observed in the present study and here we extend on current knowledge by highlighting increased brain entropy as a potential cause of the sustained psychological changes.
It is well known that highly profound psychological experiences, whatever their cause, can lead individuals to question prior assumptions and change their behavior and outlook, sometimes in a fundamental and lasting way [Griffiths et al., 2008; Jeronimus et al., 2013; Lai et al., 2007; MacLean et al., 2011; Updegraff and Taylor, 2000]. In a similar way, psychedelics may serve as a kind of “existential shock” therapy [Yalom, 1980], confronting individuals with the illusory nature of their self or ego and its attachments. If handled with appropriate care, such experiences may have a unique role to play in psychotherapy, promoting insights and self‐actualization, as detailed in certain schools of psychology [Jung, 1951] and religious/spiritual philosophy [The Rig Veda; Buddhaghosa, 430 CE, 2011]. It is incumbent on modern science to study and understand these important matters.
Most, if not all of the so‐called “classic” psychedelic drugs have agonist properties at the serotonin 2A receptor and it is thought that activation of this particular receptor sub‐type is necessary for the occurrence of the above‐described profound psychological experiences. This leads one to enquire about the functional and evolutionary significance of the serotonin 2A receptor and the physiological and psychological phenomena that can follow from its stimulation. One possibility is that 5‐HT2AR stimulation initiates the kind of processes in the brain that are required for major behavioral and psychological change. One can easily conceive of conditions where such change may be evolutionarily advantageous; for example, in situations of significant stress and/or threat to life. In line with this reasoning, serotonin release is known to be greatly elevated during conditions of arousal and stress [Rueter et al., 1997]. Stress‐induced serotonin release and subsequent 5‐HT2AR stimulation may explain associations between major life stress and putatively spontaneous changes in behavior and outlook. It would be interesting to probe further the hypothesis that 5‐HT2AR signaling plays a role in psychological changes observed in relation to trauma, psychosis and/or putatively non‐pathological existential crises. The involvement of 5‐HT2AR signaling in major psychological/behavioral change is supported by findings of 5‐HT2AR‐mediated enhancements in neural plasticity [Nichols and Sanders‐Bush, 2002; Reissig et al., 2008; Vaidya et al., 1997], associative learning [Harvey, 2003; Romano et al., 2010], and cognitive performance in animals [Jensen et al., 2013], as well as previous [Griffiths et al., 2006, 2008; MacLean et al., 2011] and the present findings of major personality change in association with psychedelic drugs.
It may also be relevant that 5‐HT2AR density is highest during critical periods of development [Sheline et al., 2002], suggesting its possible involvement in the mediation of normal and abnormal developmental processes. Further work is required to elaborate this potentially very important area of enquiry, especially given the current interest in 5‐HT2AR antagonism as a purely pharmacotherapeutic strategy for managing certain psychiatric disorders [Carpenter et al., 2002; Marek et al., 2003]—which would be at loggerheads with the drug‐assisted psychotherapeutic model touted here and elsewhere [Bogenschutz et al., 2015; Gasser et al., 2015; Grob et al., 2011; Osorio Fde et al., 2015; Thomas et al., 2013; Vollenweider and Kometer, 2010] for psychedelics. Specifically, it would be interesting to further investigate brain structural and functional changes related to the so‐called psychedelic afterglow [Majic et al., 2015], a period sometimes lasting several weeks after a high‐dose psychedelic experiences that is typically associated with improved mood, liberation from emotional burdens and renewed resilience. It has previously been commented that psychotherapy may be especially effective during this period [Majic et al., 2015], suggesting the involvement of enhanced psychological and neurobiological plasticity.
It is important to acknowledge some limitations of the present study. Our main interpretation of the observed effect of music on brain entropy and subsequent personality change is that it had a relaxing effect on the participants, possibly increasing the likelihood of entropic brain dynamics and associated psychological phenomena. The order of the music scan was not counter‐balanced, however, which precludes us from separating its influence from that of pharmacodynamics factors; although, the subjective intensity of LSD's effects was relatively stable across the three rest scans (See Supporting Information Note 9, Figure S5). Second, some of our participants had substantial previous experience with psychedelic drugs. The increases in openness observed in the present study were of a large magnitude however (i.e., the effects appeared not to be attenuated by past‐use), and consistent with those seen previously in a relatively large sample of psychedelic‐naïve participants administered psilocybin [MacLean et al., 2011]. Thus, the impact of LSD on personality in the present study was not significantly diminished by previous psychedelic use.
In summary, the present study discovered a significant relationship between acute LSD‐induced changes in brain activity and subsequent changes in personality. Individuals with more entropic brain activity under LSD showed larger increases in openness in the weeks following their experience. Moreover, this relationship was enhanced when participants listened to music and experienced ego‐dissolution.
These findings have implications for the development of psychedelic therapy, emphasizing the importance of music listening and the potential desirability of an “ego‐dissolution” experience. It is also noteworthy that the aforementioned relationships with personality change appeared to be driven by the music and post‐music scans, much more so than the pre‐music scan. One might infer from this this that music helped to establish the kind of (entropic) brain dynamics that are required for the occurrence of profound and potential transformative psychological experiences. Further work is required to explore and develop our understanding of the different factors (including music) that contribute to a positive therapeutic response to psychedelics and the present study is a start in this direction.
Our results also have important implications for the understanding and appreciation of the power of psychedelics to elicit major changes in outlook, well‐being and personality, and stimulate additional questions about what other psychological and/or behavioral traits might be sensitive to psychedelic‐induced change—for example, ones related to psychopathology [Frokjaer et al., 2010]. LSD and other psychedelics have notoriously paradoxical psychological effects [Carhart‐Harris et al., 2016]; they can induce psychosis‐like phenomena acutely, and yet appear to have more beneficial than detrimental effects on psychological well‐being in the long‐term [Hendricks et al., 2015], particularly if the experiences are mediated by therapeutic support [Bogenschutz et al., 2015; Gasser et al., 2015; Griffiths et al., 2006, 2008; Johnson et al., 2008; Krebs and Johansen, 2012; MacLean et al., 2011; Osorio Fde et al., 2015; Thomas et al., 2013; Vollenweider and Kometer, 2010]. The present results suggest that psychedelics' “entropic” effect on cortical activity may be responsible for these positive psychological effects, opening the mind up to change that can be profound and lasting in nature.
Supporting information
Supporting Information.
Conflicts of interest: The authors have no conflicts of interest, which may influence the results.
REFERENCES
- Ashburner J (2007): A fast diffeomorphic image registration algorithm. Neuroimage 38:95–113. [DOI] [PubMed] [Google Scholar]
- Bogenschutz MP, Forcehimes AA, Pommy JA, Wilcox CE, Barbosa PC, Strassman RJ (2015): Psilocybin‐assisted treatment for alcohol dependence: A proof‐of‐concept study. J Psychopharmacol 29:289–299. [DOI] [PubMed] [Google Scholar]
- Bonny HL, Pahnke WN (1972): The use of music in psychedelic (LSD) psychotherapy. J Music Ther 9:64–87. [Google Scholar]
- Buddhaghosa (430 CE) (2011) Visuddhimagga: The path of purification (english translation from Pali by Bhikkhu Nanamoli): Buddhist Publication Society.
- Carhart‐Harris RL, Erritzoe D, Williams T, Stone JM, Reed LJ, Colasanti A, Tyacke RJ, Leech R, Malizia AL, Murphy K, Hobden P, Evans J, Feilding A, Wise RG, Nutta DJ. (2012): Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc Natl Acad Sci USA 109:2138–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart‐Harris RL, Leech R, Erritzoe D, Williams TM, Stone JM, Evans J, Sharp DJ, Feilding A, Wise RG, Nutt DJ (2013): Functional connectivity measures after psilocybin inform a novel hypothesis of early psychosis. Schizophr Bull 39:1343–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart‐Harris RL, Leech R, Hellyer PJ, Shanahan M, Feilding A, Tagliazucchi E, Chialvo DR, Nutt D (2014): The entropic brain: A theory of conscious states informed by neuroimaging research with psychedelic drugs. Front Hum Neurosci 8:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart‐Harris RL, Kaelen M, Bolstridge M, Williams TM, Williams LT, Underwood R, Feilding A, Nutt DJ (2016): The paradoxical psychological effects of lysergic acid diethylamide (LSD). Psychol Med 46:1379–1390. [DOI] [PubMed] [Google Scholar]
- Carhart‐Harris RL, et al. (2016): Neural correlates of the LSD experience revealed by multimodal neuroimaging. Proc Natl Acad Sci U S A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Yasmin S, Price LH (2002): A double‐blind, placebo‐controlled study of antidepressant augmentation with mirtazapine. Biol Psychiatry 51:183–188. [DOI] [PubMed] [Google Scholar]
- Catlow BJ, Song S, Paredes DA, Kirstein CL, Sanchez‐Ramos J (2013): Effects of psilocybin on hippocampal neurogenesis and extinction of trace fear conditioning. Exp Brain Res 228:481–491. [DOI] [PubMed] [Google Scholar]
- Chao‐Gan Y, Yu‐Feng Z (2010): DPARSF: A MATLAB toolbox for “Pipeline” data analysis of resting‐state fMRI. Front Syst Neurosci 4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chwelos N, Blewett DB, Smith CM, Hoffer A (1959): Use of d‐lysergic acid diethylamide in the treatment of alcoholism. Q J Stud Alcohol 20:577–590. [PubMed] [Google Scholar]
- Costa PT Jr, McCrae RR (1988): Personality in adulthood: A six‐year longitudinal study of self‐reports and spouse ratings on the NEO Personality Inventory. J Pers Soc Psychol 54:853–863. [DOI] [PubMed] [Google Scholar]
- Costa PT, McCrae RR ( 1992): NEO PI‐R professional manual. Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Costa M, Goldberger AL, Peng CK (2002): Multiscale entropy analysis of complex physiologic time series. Phys Rev Lett 89:068102. [DOI] [PubMed] [Google Scholar]
- Faillace LA, Szara S (1968): Hallucinogenic drugs: Influence of mental set and setting. Dis Nervous Syst 29:124–126. [PubMed] [Google Scholar]
- Frecska E, More CE, Vargha A, Luna LE (2012): Enhancement of creative expression and entoptic phenomena as after‐effects of repeated ayahuasca ceremonies. J Psychoactive Drugs 44:191–199. [DOI] [PubMed] [Google Scholar]
- Frokjaer VG, Vinberg M, Erritzoe D, Baare W, Holst KK, Mortensen EL, Arfan H, Madsen J, Jernigan TL, Kessing LV, Knudsen GM (2010): Familial risk for mood disorder and the personality risk factor, neuroticism, interact in their association with frontolimbic serotonin 2A receptor binding. Neuropsychopharmacology 35:1129–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser P, Kirchner K, Passie T (2015): LSD‐assisted psychotherapy for anxiety associated with a life‐threatening disease: A qualitative study of acute and sustained subjective effects. J Psychopharmacol 29:57–68. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Richards WA, McCann U, Jesse R (2006): Psilocybin can occasion mystical‐type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology (Berl) 187:268–283. discussion 284‐92. [DOI] [PubMed] [Google Scholar]
- Griffiths R, Richards W, Johnson M, McCann U, Jesse R (2008): Mystical‐type experiences occasioned by psilocybin mediate the attribution of personal meaning and spiritual significance 14 months later. J Psychopharmacol 22:621–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grof S, Hofmann A, Weil A ( 2008): LSD Psychotherapy (The Healing Potential Potential of Psychedelic Medicine), Robinson Ave, Sarasota: MAPS.org, 4th ed. http://MAPS.org. [Google Scholar]
- Grob CS, Danforth AL, Chopra GS, Hagerty M, McKay CR, Halberstadt AL, Greer GR (2011): Pilot study of psilocybin treatment for anxiety in patients with advanced‐stage cancer. Arch Gen Psychiatry 68:71–78. [DOI] [PubMed] [Google Scholar]
- Harvey JA (2003): Role of the serotonin 5‐HT(2A) receptor in learning. Learn Mem 10:355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks PS, Thorne CB, Clark CB, Coombs DW, Johnson MW (2015): Classic psychedelic use is associated with reduced psychological distress and suicidality in the United States adult population. J Psychopharmacol 29:280–288. [DOI] [PubMed] [Google Scholar]
- Jensen AA, Plath N, Pedersen MH, Isberg V, Krall J, Wellendorph P, Stensbol TB, Gloriam DE, Krogsgaard‐Larsen P, Frolund B (2013): Design, synthesis, and pharmacological characterization of N‐ and O‐substituted 5,6,7,8‐tetrahydro‐4H‐isoxazolo[4,5‐d]azepin‐3‐ol analogues: Novel 5‐HT(2A)/5‐HT(2C) receptor agonists with pro‐cognitive properties. J Med Chem 56:1211–1227. [DOI] [PubMed] [Google Scholar]
- Jeronimus BF, Ormel J, Aleman A, Penninx BW, Riese H (2013): Negative and positive life events are associated with small but lasting change in neuroticism. Psychol Med 43:2403–2415. [DOI] [PubMed] [Google Scholar]
- Johnson M, Richards W, Griffiths R (2008): Human hallucinogen research: Guidelines for safety. J Psychopharmacol 22:603–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Garcia‐Romeu A, Cosimano MP, Griffiths RR (2014): Pilot study of the 5‐HT2AR agonist psilocybin in the treatment of tobacco addiction. J Psychopharmacol 28:983–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung CG ( 1951): Aion ‐ Researches Into the Phenomenology of the Self (Collected Works of C. G. Jung, Vol. 9). London: Routledge. [Google Scholar]
- Kaelen M, Barrett FS, Roseman L, Lorenz R, Family N, Bolstridge M, Curran HV, Feilding A, Nutt DJ, Carhart‐Harris RL (2015): LSD enhances the emotional response to music. Psychopharmacology (Berl) 232:3607–3614. [DOI] [PubMed] [Google Scholar]
- Kometer M, Pokorny T, Seifritz E, Volleinweider FX (2015): Psilocybin‐induced spiritual experiences and insightfulness are associated with synchronization of neuronal oscillations. Psychopharmacology (Berl) 232:3663–3676. [DOI] [PubMed] [Google Scholar]
- Krebs TS, Johansen PO (2012): Lysergic acid diethylamide (LSD) for alcoholism: Meta‐analysis of randomized controlled trials. J Psychopharmacol 26:994–1002. [DOI] [PubMed] [Google Scholar]
- Lai CF, Kao TW, Wu MS, Chiang SS, Chang CH, Lu CS, Yang CS, Yang CC, Chang HW, Lin SL, Chang CJ, Chen PY, Wu KD, Tsai TJ, Chen WY (2007): Impact of near‐death experiences on dialysis patients: A multicenter collaborative study. Am J Kidney Dis 50:124–132, 132.e1–2. [DOI] [PubMed] [Google Scholar]
- Lebedev AV, Lovden M, Rosenthal G, Feilding A, Nutt DJ, Carhart‐Harris RL (2015): Finding the self by losing the self: Neural correlates of ego‐dissolution under psilocybin. Hum Brain Mapp 36:3137–3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CY, Krishnan AP, Yan L, Smith RX, Kilroy E, Alger JR, Ringman JM, Wang DJ (2013): Complexity and synchronicity of resting state blood oxygenation level‐dependent (BOLD) functional MRI in normal aging and cognitive decline. J Magn Reson Imaging 38:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean KA, Johnson MW, Griffiths RR (2011): Mystical experiences occasioned by the hallucinogen psilocybin lead to increases in the personality domain of openness. J Psychopharmacol 25:1453–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majic T, Schmidt TT, Gallinat J (2015): Peak experiences and the afterglow phenomenon: When and how do therapeutic effects of hallucinogens depend on psychedelic experiences? J Psychopharmacol 29:241–253. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Carpenter LL, McDougle CJ, Price LH (2003): Synergistic action of 5‐HT2A antagonists and selective serotonin reuptake inhibitors in neuropsychiatric disorders. Neuropsychopharmacology 28:402–412. [DOI] [PubMed] [Google Scholar]
- Maslow AH (1964) Religions, Values, and Peak‐Experiences: Penguin Books, 1970, New York, USA.
- The MathWorks (2013): MATLAB version 8.1. Natick, Massachusetts: The MathWorks Inc. [Google Scholar]
- McCrae RR, Costa PT (2010): NEO Inventories: Professional manual. Lutz, FL: Psychological Assessment Resources, Inc. [Google Scholar]
- Monroe RR, Heath RG (1961): Effects of lysergic acid and various derivatives on depth and cortical electrograms. J Neuropsychiatry 3:75–82. [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Carhart‐Harris RL, Moran RJ, Brookes MJ, Williams TM, Errtizoe D, Sessa B, Papadopoulos A, Bolstridge M, Singh KD, Feilding A, Friston KJ, Nutt DJ (2013): Broadband cortical desynchronization underlies the human psychedelic state. J Neurosci 33:15171–15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols CD, Sanders‐Bush E (2002): A single dose of lysergic acid diethylamide influences gene expression patterns within the mammalian brain. Neuropsychopharmacology 26:634–642. [DOI] [PubMed] [Google Scholar]
- Nichols CD, Sanders‐Bush E (2004): Molecular genetic responses to lysergic acid diethylamide include transcriptional activation of MAP kinase phosphatase‐1, C/EBP‐beta and ILAD‐1, a novel gene with homology to arrestins. J Neurochem 90:576–584. [DOI] [PubMed] [Google Scholar]
- Osorio Fde L, Sanches RF, Macedo LR, Santos RG, Maia‐de‐Oliveira JP, Wichert‐Ana L, Araujo DB, Riba J, Crippa JA, Hallak JE (2015): Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: A preliminary report. Rev Bras Psiquiatr 37:13–20. [DOI] [PubMed] [Google Scholar]
- Passie T, Halpern JH, Stichtenoth DO, Emrich HM, Hintzen A (2008): The pharmacology of lysergic acid diethylamide: A review. CNS Neurosci Ther 14:295–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, and R Core Team (2016): nlme: Linear and Nonlinear Mixed Effects Models. Springer, New York. Available at: https://cran.r-project.org/web/packages/nlme/citation.html.
- R Core Team (2015). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at: https://www.R-project.org/.
- Reissig CJ, Rabin RA, Winter JC, Dlugos CA (2008): d‐LSD‐induced c‐Fos expression occurs in a population of oligodendrocytes in rat prefrontal cortex. Eur J Pharmacol 583:40–47. [DOI] [PubMed] [Google Scholar]
- Richman JS, Moorman JR (2000): Physiological time‐series analysis using approximate entropy and sample entropy. Am J Physiol Heart Circ Physiol 278:H2039–H2049. [DOI] [PubMed] [Google Scholar]
- Romano AG, Quinn JL, Li L, Dave KD, Schindler EA, Aloyo VJ, Harvey JA (2010): Intrahippocampal LSD accelerates learning and desensitizes the 5‐HT(2A) receptor in the rabbit, Romano et al. Psychopharmacology (Berl) 212:441–448. [DOI] [PubMed] [Google Scholar]
- Roseman L, Leech R, Feilding A, Nutt DJ, Carhart‐Harris RL (2014): The effects of psilocybin and MDMA on between‐network resting state functional connectivity in healthy volunteers. Front Hum Neurosci 8:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueter LE, Fornal CA, Jacobs BL (1997): A critical review of 5‐HT brain microdialysis and behavior. Rev Neurosci 8:117–137. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, Eickhoff SB, Hakonarson H, Gur RC, Gur RE, Wolf DH (2013): An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting‐state functional connectivity data. Neuroimage 64:240–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz BE, Sem‐Jacobsen CW, Petersen MC (1956): Effects of mescaline, LSD‐25, and adrenochrome on depth electrograms in man. AMA Arch Neurol Psychiatry 75:579–587. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Mintun MA, Moerlein SM, Snyder AZ (2002): Greater loss of 5‐HT(2A) receptors in midlife than in late life. Am J Psychiatry 159:430–435. [DOI] [PubMed] [Google Scholar]
- Smart RG, Storm T (1964): The Efficacy of Lsd in the Treatment of Alcoholism. Q J Stud Alcohol 25:333–338. [PubMed] [Google Scholar]
- Stace WT ( 1960): Mysticism and Philosophy. New York: Jeremy P. Tarcher; First Paperback Edition (February 1, 1987).
- Studerus E, Gamma A, Vollenweider FX (2010): Psychometric evaluation of the altered states of consciousness rating scale (OAV). PLoS One 5:e12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliazucchi E, Carhart‐Harris R, Leech R, Nutt D, Chialvo DR (2014): Enhanced repertoire of brain dynamical states during the psychedelic experience. Hum Brain Mapp 35:5442–5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliazucchi E, et al (2016): Increased global functional connectivity correlates with LSD‐Induced ego dissolution. Current biology: CB. [DOI] [PubMed]
- Tedeschi RG, Calhoun LG (2004): Posttraumatic growth: Conceptual foundations and empirical evidence. Psychol Inq 15:1–18. [Google Scholar]
- The Rig Veda (translation by Griffith Ralph T.H.): London, England: Forgotten Books, p 760. (January 18, 2008).
- Thomas G, Lucas P, Capler NR, Tupper KW, Martin G (2013): Ayahuasca‐assisted therapy for addiction: Results from a preliminary observational study in Canada. Curr Drug Abuse Rev 6:30–42. [DOI] [PubMed] [Google Scholar]
- Updegraff JA, Taylor SE (2000): From vulnerability to growth: Positive and negative effects of stressful life events In: Harvey J, Miller E, editors. Loss and Trauma: General and Close Relationship Perspectives. Philadelphia: Brunner‐Routledge; pp 3–28. [Google Scholar]
- Vaidya VA, Marek GJ, Aghajanian GK, Duman RS (1997): 5‐HT2A receptor‐mediated regulation of brain‐derived neurotrophic factor mRNA in the hippocampus and the neocortex. J Neurosci 17:2785–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider FX, Kometer M (2010): The neurobiology of psychedelic drugs: Implications for the treatment of mood disorders. Nat Rev Neurosci 11:642–651. [DOI] [PubMed] [Google Scholar]
- Ward BD (2000): Simultaneous Inference for fMRI data. Biophysics Research Institute, Medical College of Wisconsin, Milwaukee, Wisconsin. Available at: http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf, accessed on March 3, 2016.
- Yalom ID (1980): Existential Psychotherapy: New York: Basic Books, 1st ed. 544 p.
- Yang AC, Hong CJ, Liou YJ, Huang KL, Huang CC, Liu ME, Lo MT, Huang NE, Peng CK, Lin CP, Tsai SJ. (2015): Decreased resting‐state brain activity complexity in schizophrenia characterized by both increased regularity and randomness. Hum Brain Mapp 36:2174–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L, Polimeni JR, Fischl B, Liu H, Buckner RL (2011): The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106:1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information.


