Abstract
Neural oscillations are essential for brain functions. Research has suggested that the frequency of neural oscillations is lower for more integrative and remote communications. In this vein, some resting‐state studies have suggested that large scale networks function in the very low frequency range (<1 Hz). However, it is difficult to determine the frequency characteristics of brain networks because both resting‐state studies and conventional frequency tagging approaches cannot simultaneously capture multiple large scale networks in controllable cognitive activities. In this preliminary study, we aimed to examine whether large scale networks can be modulated by task‐induced low frequency steady‐state brain responses (lfSSBRs) in a frequency‐specific pattern. In a revised attention network test, the lfSSBRs were evoked in the triple network system and sensory‐motor system, indicating that large scale networks can be modulated in a frequency tagging way. Furthermore, the inter‐ and intranetwork synchronizations as well as coherence were increased at the fundamental frequency and the first harmonic rather than at other frequency bands, indicating a frequency‐specific modulation of information communication. However, there was no difference among attention conditions, indicating that lfSSBRs modulate the general attention state much stronger than distinguishing attention conditions. This study provides insights into the advantage and mechanism of lfSSBRs. More importantly, it paves a new way to investigate frequency‐specific large scale brain activities. Hum Brain Mapp 37:381–394, 2016. © 2015 Wiley Periodicals, Inc.
Keywords: frequency tagging approach, low frequency oscillations, low frequency steady‐state brain responses, large scale networks, triple network system
INTRODUCTION
Neural oscillations are essential for establishing precise temporal relationships between neural responses that support various cognitive activities [Uhlhaas and Singer, 2010]. In other words, the particular pattern of neural oscillations corresponds to a specific cognitive state [Ward, 2003]. In the high frequency range (>1 Hz), some important rules of neural oscillations have been established. For instance, higher frequency (e.g., gamma band) oscillations are restricted to a small‐scale space, whereas long range networks are recruited during slow oscillations (e.g., delta band) [Buzsáki and Draguhn, 2004]. In addition, the intrinsic timescales are lengthened from sensory regions to areas engaged in higher order cognition [Murray et al., 2014]. Accordingly, large scale networks should work in a very low frequency range due to their long range communications and integrative roles in various brain functions. In fact, considerable evidence from resting‐state functional magnetic resonance imaging (fMRI) studies has shown that the strength of large scale networks is highest in lower frequencies (usually 0.01–0.1 Hz) and becomes weaker when the frequency increases [Gohel and Biswal, 2015; Li et al., 2015; Wu et al., 2008], supporting the low frequency dominance of large scale networks. However, the frequency characteristics of large scale networks have not been well understood.
Although the frequency characteristics of low frequency neural oscillations are primarily determined by blood oxygen level dependent (BOLD) signal, these characteristics are reported to be associated with neural activities. For instance, combined EEG‐fMRI studies indicated direct correlation between electrophysiological signal and BOLD measurement in the infraslow frequency range [Hiltunen et al., 2014; Pan et al., 2013; Thompson et al., 2014], suggesting that BOLD signal can reflect neural activities at the same frequency band. The BOLD signal is also related to band‐limited electrophysiological activities within high frequency range [Logothetis et al., 2001; Wang et al., 2012]. Furthermore, large scale networks defined by BOLD fluctuations not only have distinct frequency characteristics [Qian et al., 2015; Thompson and Fransson 2015; Wu et al., 2008], but also are associated with different high frequency electrophysiological activities [Mantini et al., 2007; Wang et al., 2012], indicating the relationship between BOLD signal and electrophysiological activities is frequency‐specific. Furthermore, the low frequency fluctuations (usually < 1 Hz) and high spatial resolutions make BOLD signal be appropriate to investigate frequency characteristics of large scale networks.
Although the BOLD signal can reflect neural activities, it is difficult for resting‐state studies to capture frequency characteristics of large scale networks in particular cognitive processes due to the mind wandering nature during resting state [Mason et al., 2007]. The frequency of neural oscillations in a specific brain region depends on what cognitive processes are performed in this area and which network is this region involved in Siegel et al. (2012). Therefore, a task‐based study is needed to reveal the cognitive significance of frequency characteristics of large scale networks.
In contrast to resting‐state study, the frequency tagging approach holds promise for revealing frequency characteristics of particular brain regions. Recently, several noninvasive stimulating methods have been extensively used to modulate rhythmic brain activities by means of frequency tagging, such as steady‐state sensory presentation, transcranial magnetic stimulation, transcranial direct current stimulation, and transcranial alternating current stimulation [Thut et al., 2011]. However, these techniques cannot modulate multiple large scale networks simultaneously. To overcome this limitation, we adopted a new frequency tagging method—the low frequency steady‐state brain responses (lfSSBRs)—to modulate large scale networks.
The lfSSBRs reflect entrained brain responses to a particular task operated in a constantly low frequency [Thut et al., 2012; Vialatte et al., 2010; Wang et al., 2014b]. These entrained responses are demonstrated to be phase‐locked to the onset of stimuli [Calderone et al., 2014; Lakatos et al., 2008]. According to the synchronized gating hypothesis [Florin and Baillet, 2015], the synchronization of low frequency oscillations opens the gate of information communication for high frequency oscillations. Because lfSSBRs can be evoked in many brain regions that are involved in different functional networks, we suggested that it can modulate both intra‐ and internetwork information communications via phase synchronization. In addition, the converging lines of evidence from the similarities between lfSSBRs and steady‐state evoked potentials (SSEPs), the survived lfSSBRs after hemodynamic response function (HRF) deconvolution, and the similar spatial distribution of lfSSBRs with regions defined by activation studies, indicate that lfSSBRs can reflect neural level activities. Therefore, we expected that lfSSBRs can modulate information communications among neural assemblies.
Furthermore, the lfSSBRs measure the variability of BOLD signal, and are different from the activation that measures the mean value of BOLD signal [Wang et al. 2015a, 2014b]. In prior studies, Garrett et al. [2013a], [2014] found that task‐related regions assessed by the BOLD signal variability are dispersive and distinctive from those measured by the mean BOLD signal. In contrast, we observed that lfSSBRs were evoked in brain regions defined by the activation [Wang et al., 2014b,]. Thus, the relationship between these two indices is under debate. In the current study, we expected to shed light on this important question by examining the spatial distribution of lfSSBRs.
The attention network test (ANT) [Fan et al., 2002] with a pure block design and nonorthogonal contrast [Wang et al., 2014a,2015b] was adopted to evoke lfSSBRs. The revised ANT included four task conditions with high similarity, providing reproducible evidence for the modulation of large scale networks. The 0.1 Hz was chosen as it is in the middle of the frequency range (0.05–0.1875 Hz) in which lfSSBRs have been successfully evoked [Wang et al., 2014b,2015c]. In the face of salient stimuli, the triple network system [the salience network (SN), central executive network (CEN), and default mode network (DMN)] would coordinate with each other to produce adaptive behavior [Menon, 2011; Wen et al., 2013]. The visual and sensorimotor networks were also expected to be modulated in the ANT because these networks are involved in all visual tasks that require motor responses, i.e., stimulus–response (S–R) tasks. Considering the sensorimotor bias of lfSSBRs [Wang et al., 2015a], the attention networks may be modulated more weakly than the sensory‐motor system and triple network system. Therefore, we hypothesized that the triple network system and the sensory‐motor system would be effectively modulated by lfSSBRs in a frequency‐specific pattern.
MATERIALS AND METHODS
Subjects
Thirty subjects took part in the experiment (15 females, ages ranged: 17–24 years, mean age: 21.20 ± 2.33). All of them were right‐handed (tested using the Chinese version of Edinburgh‐Handedness Questionnaire; coefficients >50) [Wang et al., 2013]). They were asked to have enough sleep and avoid any drugs and alcohol in 24 h before the experiment. All participants reported normal or correct‐to‐normal vision, without any medication, and neurological or psychiatric disorders. This study was approved by the research ethical committee of School of Life Science and Technology, University of Electronic Science and Technology of China. Participants were treated in compliance with the Declaration of Helsinki. Informed consents from participants were obtained before taking part in the study.
Stimuli Presentation and Task Procedure
The experiment was run on a Dell laptop computer. Pictures were projected to a screen via a projector and reflected to the eyes of subjects through a mirror. E‐Prime 2.0 software (http://www.pstnet.com; Psychology Software Tools) was used for programming, stimuli presentation, and timing control. Responses were collected through a response box. All stimuli were black figures presented at the center of a screen on a gray background. Fixation was a plus sign (0.22° visual angle). The visual angle subtended by the whole target (including five arrows and interarrow spaces, 0.53° visual angle for each arrow) was 3.1°. The peripheral target in the orienting condition was 2° away from the fixation.
All participants completed four blocks (alerting block, orienting block, executive control block, and baseline block) of the ANT. The order of four blocks was counterbalanced across subjects. Each block took 424 s, which contained buffer time of 4 s, two practice trials of 10 s and 40 experimental trials of 10 s. This design advanced our previous studies [Wang et al., 2014a,2015b] to provide common baseline (the baseline block) for all attention conditions. Participants were asked to focus on the fixation throughout the experiment and, during practice.
As shown in Figure 1, each trial began with a fixation or cue for 100 ms which was followed by 300 ms fixation. After that a target (congruent or incongruent, central, or spatial) appeared for 2,000 ms or until the participant pressed a key. Lastly, another fixation was presented to ensure the overall time of one trial was 10,000 ms (0.1 Hz). The subjects were asked to judge the direction of the third arrow (the central one) by pressing the left key if it points to the left and the right key if it points to the right. Before task blocks, there was a resting‐state block which had a comparable length to the task blocks. In the resting‐state block, subjects were asked to stay steady and awake, not to think of anything in particular.
Figure 1.
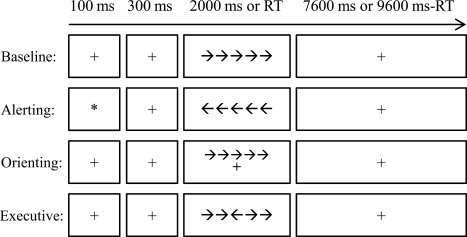
The procedure of attention network test with pure block design. The baseline, alerting, orienting, and executive control conditions were arranged in separated blocks. There was only one condition in each block. Each trial started with a cue (the alerting condition) or a fixation lasting for 100 ms. After a 300 ms interval, the target was presented for 2000 ms or disappeared after one key was pressed. After that a fixation was presented until the end of that trial. Each trial lasted for 10 s (0.1 Hz). RT: reaction time.
Behavioral Data Analysis
Instead of the conventional subtraction measure [Fan et al., 2009, 2002], we used ratio scores to define the efficiency of attention networks. The ratio scores could avoid the baseline difference and isolate the attention system from the overall reaction time (RT) [Westlye et al., 2011]. The attention network scores (ANSs) were computed by Eqs. (1), (2), (3):
| (1) |
| (2) |
| (3) |
We conducted one sample t tests to assess the effect of ANSs, paired‐sample t tests to test the difference between ANSs, Pearson's correlation to measure the dependence of ANSs, and split‐half reliability to evaluate the stability of ANSs. The split‐half reliability was estimated using Monte Carlo approach with 10,000 times of simulation [Wang et al., 2015b].
Image Acquisition
fMRI data were acquired using a 3.0T GE 750 scanner (General Electric, Fairfield, Connecticut) equipped with high‐speed gradients. An 8‐channel prototype quadrature birdcage head coil fitted with foam padding was applied to minimize head movement. Functional images were acquired using a gradient‐recalled echo‐planar imaging (EPI) sequence. The parameters were as follows: repetition time (TR) = 2,000 ms, echo time (TE) = 30 ms, bandwidth = 250 Hz/pixel, 90° flip angle, 43 axial slices (3.2 mm slice thickness without gap), 64 × 64 matrix, 22 cm field of view.
Image Preprocessing
Functional images were preprocessed using the Data Processing Assistant for Resting‐state fMRI (DPARSF 2.2, http://www.restfmri.net/forum/DPARSF). The preprocessing steps of task blocks included: the first 12 scans (including two trials) were discarded to allow evoked fluctuations to appear, signal to reach equilibrium and participants to adapt to the scanning noise; the remaining images were slice‐time corrected, spatially aligned and then spatially normalized to Montreal Neurological Institute (MNI) EPI template and resampled to 3 × 3 × 3 mm3 isotropic voxels. The normalized images were spatially smoothed with a 6 mm full width half maximum (FWHM) Gaussian kernel. The linear trend of time courses was then removed. The head motion parameter was further tested using the method proposed by Power et al. [2012]. There was no difference for the mean frame‐wise deviation among conditions: F (4, 116) = 0.73, P = 0.493, partial η 2 = 0.025. Then, six head motion parameters, white matter signal and cerebrospinal fluid signal were regressed out before power analysis. The global signal was not regressed out because this operation may remove a global neuronal signal that is induced by a widely distributed ascending input [Schölvinck et al., 2010] and reinforce the neuronal–hemodynamic correspondence [Keller et al., 2013]. After that the HRF was deconvoluted according to previous studies [Wang et al., 2014b; Wu et al., 2013] to eliminate different influences of neurovascular coupling on signals of lower (<0.1 Hz) and higher (>0.1 Hz) frequency bands [Robinson et al., 2006].
The Power Analysis
The SSEPs are defined by power increase at particular frequency after Fast Fourier Transform (FFT) [Herrmann, 2001]. In line with this, the FFT was used to uncover the lfSSBRs by converting the time course of each voxel to the frequency domain without bandpass filtering. The frequency resolution was 0.0025 Hz (sampling rate/sampled data: 0.5 Hz/200). The power spectrum of each block for each subject was obtained in the gray matter constrained by the gray matter probability template with a threshold of 0.2 [Liu et al., 2015b]. Both whole brain and regional power spectrums were measured. At the whole brain level, the power spectrum was defined as the average of power spectrum in all masked voxels. Regional lfSSBRs were defined as the power spectrum in each voxel. The effect of task was assessed using within‐subject analysis of variance (ANOVA) at each of the following frequency band: the fundamental frequency (0.097–0.103 Hz), the first harmonic (0.197–0.203 Hz), the remaining three frequency ranges (0.01–0.097, 0.103–0.197, and 0.203–0.25 Hz) as well as the full frequency band (0.01–0.25 Hz). These frequency bands were obtained by band pass filter with DPRSRF 2.2. The fundamental frequency and the first harmonic were selected via visual inspection (see Fig. 2) and according to previous studies [Wang et al., 2014b,2015b]. The remaining frequency bands were tested to demonstrate the frequency‐specific modulation of lfSSBRs to brain networks. The frequency interval of 0.0025–0.01 Hz was not included due to slow drift. Post hoc analysis was operated with paired‐sample t test using the SPM8 software (http://www.fil.ion.ucl.ac.uk/spm). All resulting statistic maps were corrected using the family wise error (FWE) method (P < 0.05) for multiple comparisons [Worsley et al., 1996].
Figure 2.
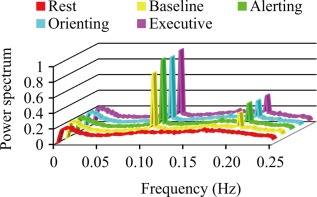
lfSSBRs at the whole brain level. Significant lfSSBRs were evoked by all four task conditions at the fundamental frequency and the first harmonic compared to the resting‐state.
The Functional Connectivity Analysis
To ensure the consistency of networks between power analysis and functional connectivity analysis, we constrained these networks within regions with significant main effect of power in the ANOVA at the fundamental frequency. The Anatomical Automatic Labeling (AAL) template with explicit labels was further used to restrict the boundary of networks [Tzourio‐Mazoyer et al., 2002]. As shown in Figures 3A and 4, the visual network (VN) includes the lateral and medial visual cortex; the sensorimotor network (SMN) includes the precentral gyrus, postcentral gyrus, supplementary motor area, and basal ganglia; the CEN includes the lateral frontal cortex and posterior parietal cortex; while the SN includes the anterior insula and dorsal anterior cingulate cortex. Because there was no significant main effect of power in the DMN, this network was constrained only by the AAL template, including the orbital part of medial frontal cortex and rectus (the ventral medial frontal cortex), posterior cingulate cortex/precuneus, and bilateral angular.
Figure 3.
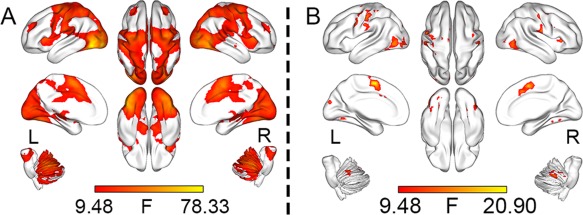
Main effects of regional lfSSBRs. At the fundamental frequency, significant lfSSBRs were evoked in the visual, sensorimotor, ventral attention, salience, and central executive networks (A). At the first harmonic, remarkable lfSSBRs were shown only in the visual and sensorimotor networks (B). All results were corrected with FWE method (P < 0.05) and visualized with the BrainNet Viewer [Xia et al., 2013].
Figure 4.
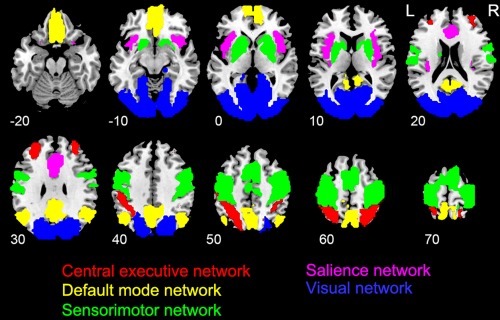
The schematic plot of the five networks.
The preprocessed imaging data was firstly band pass filtered at the aforementioned six frequency bands. Voxel‐wise functional connectivity was then calculated within each of these frequency bands. Both conventional time correlation and partial correlation analyses were conducted. The time correlation was computed between each pair of voxels within a network (intranetwork correlation) or between two networks (internetwork correlation). Noting that the partial correlation was not purely voxel‐wised for two reasons: first, the voxel‐wised partial correlation cannot be computed mathematically due to many more voxels than time points; second, the intranetwork partial correlation cannot be computed at network level with the mean signal of all voxels in one network. Therefore, the partial correlation estimated the linear conditional dependence between each pair of voxels within a network or between two networks, after regressing out the averaged time courses of remaining networks. Correlation coefficients were then normalized by Fisher's r‐to‐z transformation to facilitate group comparison [Liu et al., 2015a]. The correlation coefficient of each network was deemed as mean z values in this network. The ANOVA with task condition as within‐subject factor was performed for each intranetwork connection and each internetwork connection at each frequency band. Adjustments with Greenhouse–Geisser method were used wherever the sphericity assumption was violated [Wang et al., 2013]. Post hoc analysis was conducted with paired‐sample t test. Multiple comparisons were corrected using Bonferroni's method (P < 0.05).
Coherence Analysis
Besides traditional correlation in the time domain, the coherence is another index that measures functional connectivity in the frequency domain. Coherence, unlike the time correlation, captures temporal dependence even in the face of phase lags (Li et al., 2015). The voxel level coherence was calculated by employing the function ‘mscohere’ in MATLAB 8.4, the square root of the raw value was extracted and averaged across voxels within a network or between two networks. The mean coherence coefficient of one network or one pair of network was underwent ANOVA with task conditions as within subject factor. Post hoc analysis was conducted with paired‐sample t test. Multiple comparisons were corrected as we did in the functional connectivity analysis.
RESULTS
Behavioral Results
Table 1 summarizes the RT and accuracy of each task block. We then replaced RTs in erroneous trials (34 trials/4800 trials = 0.71%) and those larger than three standard deviations (56 trials/4800 trials = 1.17%) with the median of RT in that block for each subject. Then the median of each block was used to calculate ANSs according to Eqs. (1), (2), (3). As shown in Table 2, all effects of ANSs were significantly different from zero and from each other [all t (29) > 3.25, P < 0.003, Cohen's d > 0.705]. Furthermore, there were no (alerting vs. orienting, r = 0.03, P = 0.892; orienting vs. executive, r = 0.31, P = 0.098) or weak (alerting vs. executive, r = 0.40, P = 0.028) correlations between ANSs. All these correlations cannot survive Bonferroni's correction (P < 0.017 for triple comparisons). Finally, the ANSs are highly reliable (alerting: 0.736; orienting: 0.811; executive: 0.946) in our paradigm, indicating that the ANT with a pure block design and nonorthogonal contrast can get high reliability.
Table 1.
The reaction time and accuracy per condition (M ± SD)
| Condition | Reaction time (ms) | Accuracy (%) |
|---|---|---|
| Baseline | 490.18 ± 79.84 | 99.33 ± 1.12 |
| Alerting | 446.67 ± 66.83 | 99.17 ± 1.20 |
| Orienting | 634.41 ± 109.10 | 99.17 ± 1.20 |
| Executive | 606.18 ± 156.65 | 99.50 ± 1.02 |
M: mean; SD: standard deviation.
Table 2.
Statistical results of attention network scores
| Effect | Value (M ± SD.) | T (29) | P a |
|---|---|---|---|
| Alerting | −0.08 ± 0.07 | −6.74 | <0.0001 |
| Orienting | 0.30 ± 0.15 | 11.13 | <0.0001 |
| Executive | 0.24 ± 0.26 | 5.02 | <0.0001 |
Two‐tailed.
lfSSBRs at Different Frequency Bands
As shown in Figure 2, significant lfSSBRs were evoked by all four task conditions at the fundamental frequency and the first harmonic. ANOVA showed a remarkable main effect of condition at 0.01–0.097, 0.097–0.103, 0.197–0.203, and 0.01–0.25 Hz frequency bands (also see Table 3). The main effect of condition is not significant at 0.103–0.197 Hz [F (4,116) = 1.316, P = 0.268] and 0.203–0.25 Hz [F (4,116) = 2.174, P = 0.076] frequency bands. Post hoc analysis revealed that the power of task conditions is lower than that of the resting‐state at 0.01–0.097 Hz frequency band, whereas higher than that of the resting‐state at 0.097–0.103, 0.197–0.203, and 0.01–0.25 Hz frequency bands. There was no difference among four task conditions.
Table 3.
Difference of power at six frequency bands
| Frequencya | 0.01–0.097 | 0.097–0.103 | 0.197–0.203 | 0.01–0.25 | ||||
|---|---|---|---|---|---|---|---|---|
| ANOVA | F b | P | F | P | F | P | F | P |
| 24.995 | <0.0001 | 71.311 | <0.0001 | 26.784 | <0.0001 | 12.694 | <0.0001 | |
| t test | t c | P | t | p | t | P | t | P |
| A_R | −6.699 | <0.0001 | 10.467 | <0.0001 | 7.436 | <0.0001 | 6.066 | <0.0001 |
| B_R | −6.083 | <0.0001 | 9.567 | <0.0001 | 5.753 | <0.0001 | 3.595 | 0.001 |
| E_R | −8.234 | <0.0001 | 11.287 | <0.0001 | 6.667 | <0.0001 | 4.867 | <0.0001 |
| O_R | −8.703 | <0.0001 | 12.333 | <0.0001 | 6.450 | <0.0001 | 4.375 | 0.0001 |
Hz.
Degree of freedom (df): 4, 116.
df: 29.
A: alerting; B: baseline; E: executive; O: orienting; R: resting‐state; there was no significant power effect at 0.103–0.197 Hz and 0.203–0.25 Hz frequency bands.
For the fundamental frequency (Fig. 3A), voxel‐wise power analysis revealed significant effects of lfSSBRs in the VN, SMN, CEN, SN, cerebellum network (CN; most parts of the cerebellum), and ventral attention network (VAN; the right temporal–parietal junction). These results are consistent with previous findings [Wang et al., 2014b] that lfSSBRs modulate not only task‐general networks (e.g., the triple network system) but also task‐specific networks such as the VAN.
For the first harmonic (Fig. 3B), remarkable lfSSBRs were only observed in the VN and SMN. These results replicated previous findings that only the sensory‐motor system was strikingly modulated at relative high frequency bands [Wang et al., 2014b], indicating that sensory‐motor system involves a wider frequency range than the more integrative networks do. The power analysis in other frequency bands showed no significant main effect of power. This suggests that the power within these frequency bands is not significantly modulated by the task.
Post hoc analysis for both the fundamental frequency and the first harmonic showed that the power was different between task conditions and the resting‐state rather than among task conditions, indicating that the modulation of brain states by lfSSBRs overwhelms the modulation of different attention conditions. Meanwhile, it is consistent with previous results of sensorimotor bias [Wang et al., 2015a, 2014b]. That is to say, the lfSSBRs modulate sensorimotor network much more that task‐related regions.
Intra‐ and Internetwork Time Correlations
For the time correlation, Table 4 shows the main effect of condition at six frequency bands. Most effects of inter‐ and intranetwork relationships were significant at the fundamental frequency, the first harmonic, and the full frequency band. Only a small part of main effects were significant at other frequency bands. Therefore, the significant effect at full frequency band was mainly contributed by the fundamental frequency and the first harmonic. Strong effects at the fundamental frequency and the first harmonic along with weak effects at other frequency intervals indicated that lfSSBRs modulate the activity of large scale networks in a frequency‐specific means.
Table 4.
Intra‐ and internetwork correlations modulated by tasks
| Frequencya | 0.01–0.097 | 0.097–0.103 | 0.103–0.197 | 0.197–0.203 | 0.203–0.25 | 0.01–0.25 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Comparison | F b | P | F | P | F | P | F | P | F | P | F | P |
| CEN | 1.572 | 0.186 | 51.983 | <0.001 | 7.599 | <0.001 | 15.474 | <0.001 | 4.914 | 0.001 | 23.071 | <0.0001 |
| DMN | 0.470 | 0.757 | 2.150 | 0.079 | 1.092 | 0.364 | 2.814 | 0.028 | 1.452 | 0.221 | 0.320 | 0.864 |
| SMN | 1.102 | 0.359 | 58.857 | <0.001 | 2.365 | 0.084 | 24.207 | <0.001 | 2.769 | 0.048 | 17.871 | <0.0001 |
| SN | 3.789 | 0.014 | 33.504 | <0.001 | 0.702 | 0.517 | 12.951 | <0.001 | 0.251 | 0.909 | 1.377 | 0.246 |
| VN | 0.327 | 0.860 | 33.248 | <0.001 | 5.102 | 0.004 | 11.819 | <0.001 | 3.478 | 0.021 | 13.690 | <0.0001 |
| CEN_DMN | 0.101 | 0.982 | 5.807 | <0.001 | 3.028 | 0.020 | 3.915 | 0.005 | 2.113 | 0.111 | 4.627 | 0.002 |
| CEN_SMN | 1.095 | 0.363 | 55.708 | <0.001 | 4.231 | 0.009 | 18.372 | <0.001 | 4.879 | 0.005 | 25.425 | <0.0001 |
| CEN_SN | 0.529 | 0.715 | 51.685 | <0.001 | 1.433 | 0.228 | 16.869 | <0.001 | 1.247 | 0.298 | 17.813 | <0.0001 |
| CEN_VN | 1.762 | 0.141 | 39.333 | <0.001 | 4.809 | 0.001 | 15.224 | <0.001 | 2.346 | 0.081 | 21.617 | <0.0001 |
| DMN_SMN | 0.131 | 0.971 | 5.994 | <0.001 | 1.867 | 0.121 | 1.218 | 0.307 | 2.601 | 0.066 | 3.128 | 0.017 |
| DMN_SN | 0.218 | 0.928 | 3.485 | 0.01 | 1.229 | 0.303 | 1.484 | 0.212 | 0.557 | 0.694 | 2.979 | 0.022 |
| DMN_VN | 0.453 | 0.770 | 5.383 | <0.001 | 2.806 | 0.029 | 3.604 | 0.008 | 2.912 | 0.024 | 4.451 | 0.002 |
| SMN_SN | 0.665 | 0.617 | 56.145 | <0.001 | 0.363 | 0.772 | 19.320 | <0.001 | 0.907 | 0.446 | 15.253 | <0.0001 |
| SMN_VN | 2.508 | 0.046 | 38.316 | <0.001 | 3.781 | 0.006 | 12.591 | <0.001 | 3.959 | 0.005 | 24.967 | <0.0001 |
| SN_VN | 0.491 | 0.742 | 34.435 | <0.001 | 1.851 | 0.124 | 11.619 | <0.001 | 1.739 | 0.146 | 15.450 | <0.0001 |
Hz.
df: 4, 116.
The bold values indicate significant correlation with q < 0.05.
Post hoc analysis revealed that most of the differences were between task conditions and resting‐state (see Fig. 5 for intranetwork correlation and Fig. 6 for internetwork correlation) which is consistent with the results of power analysis.
Figure 5.
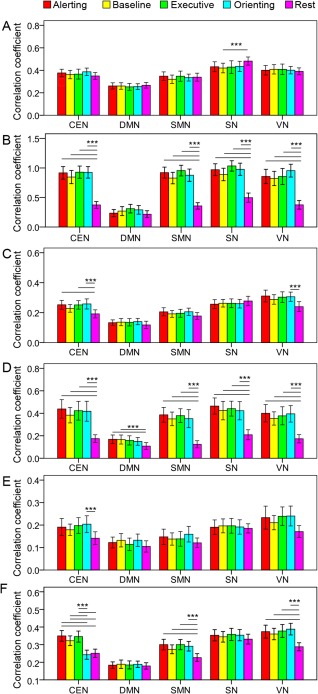
The intranetwork synchronization. Systematic modulation of the five networks is shown at the fundamental frequency (B), the first harmonic (D), and full frequency band (F) rather than other frequency bands (A: 0.01–0.097 Hz; C: 0.103–0.197 Hz; E: 0.203–0.25 Hz). *** P < 0.005. Error bar shows the 95% confidence interval.
Figure 6.
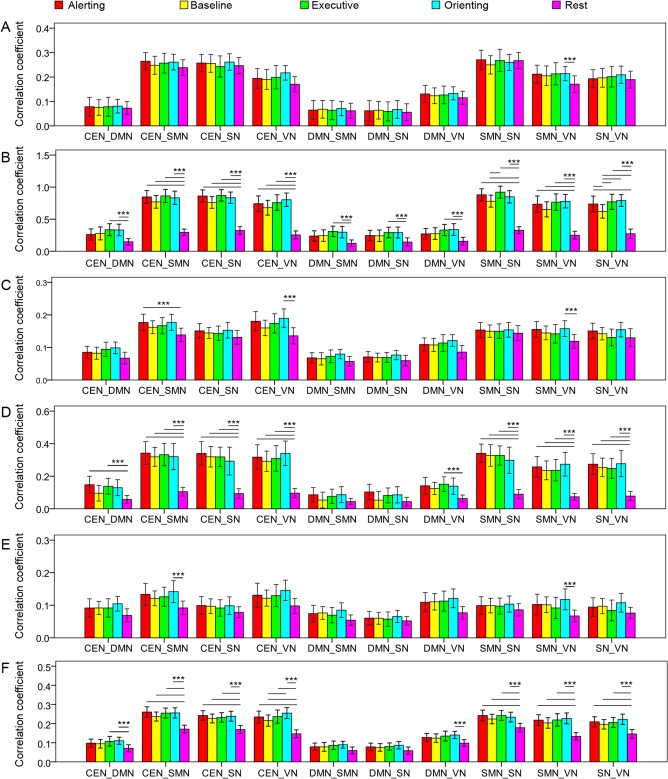
The internetwork synchronization. Similar to intranetwork correlation, systematic modulation of internetwork correlation is shown at the fundamental frequency (B), the first harmonic (D), and full frequency band (F) rather than other frequency bands (A: 0.01–0.097 Hz; C: 0.103–0.197 Hz; E: 0.203–0.25 Hz). ***P < 0.005. Error bar shows the 95% confidence interval.
For the intranetwork correlation (Fig. 5), only the DMN was not modulated at the fundamental frequency. All networks were modulated at the first harmonic. The DMN and SN were not modulated at the full frequency band. In addition, the CEN, SN, and VN were modulated by a few task conditions at other frequency intervals.
For the internetwork correlation (Fig. 6), all networks were modulated by task conditions at the fundamental frequency. All relationships except those of DMN‐SMN and SMN‐SN were modulated at the first harmonic. The results at full frequency band were much similar to those at the first harmonic. Furthermore, the VN, SMN, and CEN were modulated by a few task conditions at other frequency bands.
These results suggest that both the triple network system and sensory‐motor system and internetwork relationships are modulated by the task conditions. The modulation effect is mainly at the fundamental frequency and the first harmonic, indicating frequency‐specific modulation for large scale networks. However, there was no condition effect at any frequency band in the partial correlation analysis, indicating mutually influences of intra‐ and internetwork information communications.
Inter‐ and Intranetwork Coherences
As shown in Figure 7, the intra‐ and internetwork coherences were modulated around the fundamental frequency and the first harmonic. The DMN was not significantly modulated by the task, while coherence between the DMN and other networks was modulated at only the fundamental frequency. Post hoc analysis showed that the effect of condition was principally explained by the difference between task and resting conditions rather than among task conditions. These results were much similar to those in power analysis and time correlation analysis, indicating that lfSSBRs modulate intra‐ and internetwork information communications in a frequency‐specific way.
Figure 7.
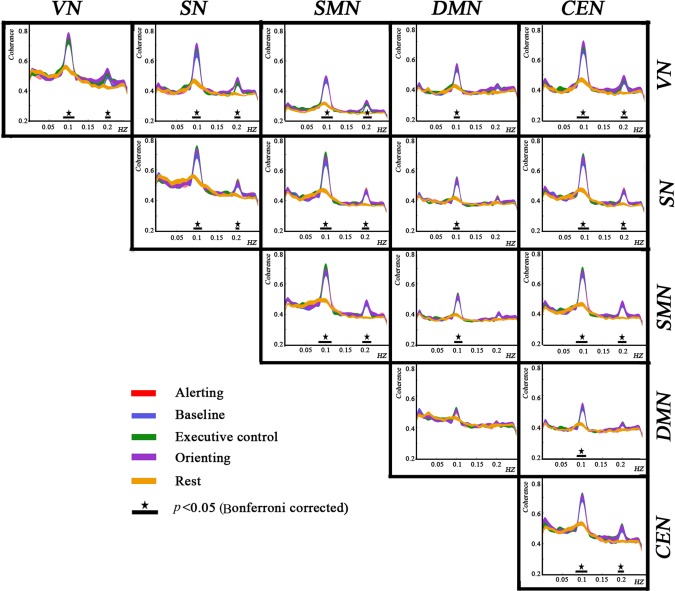
Intra‐ and internetwork coherences. lfSSBRs modulate coherence around the fundamental frequency and the first harmonic. The modulation was resulted from the difference between task and resting conditions rather than among different task conditions. Noting that coherence in the DMN was not modulated, while coherence between the DMN and other networks was modulated at the fundamental frequency.
DISCUSSION
Clarifying frequency characteristics of large scale brain networks in the low frequency range is of importance for understanding how the brain implements various cognitive activities. To the best of our knowledge, this is the first study that simultaneously modulates multiple large scale networks with lfSSBRs. Although this study cannot tell whether the entrainment mechanism [Thut et al., 2012] or the linear superposition mechanism [Capilla et al., 2011] or both of them are responsible for lfSSBRs, our evidence suggests that lfSSBRs can modulate information communications of large scale networks. Given the essential roles of large scale networks in normal brain functions [Krishnadas et al., 2014; Vahdat et al., 2014], this study opens a new window to study large scale brain activity in a frequency‐specific means.
lfSSBRs Modulate Large Scale Networks
The lfSSBRs enhance the power as well as the information communication of large scale networks. First, we observed lfSSBRs in large scale networks indexed by power increase. At the fundamental frequency, the triple network system and sensory‐motor system are universally modulated by different attention conditions. The sensory‐motor system is also modulated at the first harmonic. This resonant phenomenon is similar to that of SSEPs and that in our previous studies [Wang et al., 2014b, 2015c]. These results suggest different frequency characteristics of the triple network system and sensory‐motor system, and different mechanisms of lfSSBRs and SSEPs. Specifically, the advantage of lfSSBRs compared to SSEPs lie in that they can simultaneously modulate multiple large scale networks.
Second, time correlation and coherence analyses show further evidence that intra‐ and internetwork synchronizations are considerably modulated by lfSSBRs. Strong modulations can be detected at the fundamental frequency and the first harmonic rather than at other frequency bands. These results indicate that information communications are enhanced by lfSSBRs and this enhancement is frequency specific. The frequency‐specific modulation indicates that lfSSBRs can specifically influence brain activities without affecting activities at nontarget frequency bands. The similarity among results of multiple analytical approaches may help to elucidate a hot issue in recent literature: the relationship between local activities and network architectures [Baria et al., 2013; Di et al., 2013]. According to a popular perspective of functional connectivity [Guerra‐Carrillo et al., 2014], large scale networks originate from long term co‐activation between brain regions. Therefore, the lfSSBRs may be an effective means to link local brain activities with inter‐regional communications and shed light on the development of large scale networks.
The partial correlation analysis provides further evidence for the simultaneous modulation of lfSSBRs to both intra‐ and internetwork synchronizations. The disappearance of condition effect in the partial correlation is consistent with the entrainment hypothesis of SSBRs [Calderone et al., 2014; Lakatos et al., 2008] and the synchronized gating hypothesis of information communication [Florin and Baillet, 2015]. Accordingly, the lfSSBRs reset the phase of low frequency fluctuations in multiple networks, opening the gate of intra‐ and internetwork information communications. Under this condition, the information flows among all these networks are enhanced and mutually dependent.
Furthermore, the power increase reflects the enhanced BOLD variability. We observed lfSSBRs in the task‐related regions primarily defined by previous studies measuring brain activation, indicating similar mechanisms of BOLD variability and BOLD activation. However, the spatial distributions revealed by these two indices are largely nonoverlapping in previous studies [Garrett et al., 2011, 2013b]. This distinction may be resulted from that Garrett et al. did not focus on specific frequencies whereas both neural oscillations and BOLD fluctuations are frequency‐specific [Buzsáki and Draguhn, 2004; Garrett et al., 2013b; Li et al., 2015]. Distinctive analytical approaches in their studies [Garrett et al., 2010, 2011, 2014] and ours [Wang et al., 2014b,] may also lead to different results. Combining steady‐state and unsteady‐state experimental designs may help to clarify the relationship between BOLD variability and BOLD activation in frequency‐dependent and frequency‐independent means.
It is worth noting that the DMN and its relationship with other networks were seldom modulated by lfSSBRs. These results may be explained by two reasons: first, although the DMN has been suggested to be involved in attention processing [Raichle, 2015], it is mainly responsible for self‐reference processing and autobiographical memory [Andrews‐Hanna et al., 2010b]. Its activities are principally internal and spontaneous rather than being stimulus‐locked [Andrews‐Hanna, 2012; Andrews‐Hanna et al., 2010a]. Therefore, top‐down or spontaneous cognitive tasks may modulate activities in the DMN; alternatively, previous studies have revealed that the dominant frequency of DMN is 0.06 Hz or between 0.01 and 0.04 Hz [Li et al., 2015; Wu et al., 2008], indicating that a greater resonance may be occurred at lower frequency rather than at 0.1 Hz [Rosanova et al., 2009]. Both functional and frequency‐specific reasons should be tested in future studies.
Possible Mechanism of lfSSBRs
The entrainment and the linear superposition of transient activity are two dominating theories in deciphering SSEPs [Capilla et al., 2011; Thut et al., 2012, 2011]. The entrainment theory proposes that brain activities can be entrained by regularly repeated exogenous stimulus [Thut et al., 2012], whereas the linear superposition hypothesis insists that SSEPs are the results of linear superposition of transient activity [Capilla et al., 2011]. Both perspectives can explain the phenomenon of lfSSBRs, considering similar waveforms of lfSSBRs and SSEPs and similar spatial distributions of lfSSBRs and activation. Moreover, it is not known whether lfSSBRs are driven by other mechanisms. Therefore, the mechanism of lfSSBRs warrants future research.
The significance of this study lies in (1) the modulation of large scale networks is limited in predefined frequency band, providing a controllable approach to investigate large scale brain activity; (2) it may uncover frequency characteristics of large scale networks such as those rules established in higher frequency ranges. For instance, the sensorimotor bias at the first harmonic may indicate that the sensory‐motor system has higher natural frequency than the more integrative triple network system [Wang et al., 2014b]; and (3) it provides a new means to study brain functions in different states. It is suggested that the HRF is different in task‐ and resting‐state [Chen and Glover, 2015], while the lfSSBRs are independent of HRF [Wang et al., 2014b,2015b]. Therefore, lfSSBRs provide a new method using power and correlation analyses to expound different mechanisms of cognitive activities at different states.
Implications for Attention Network Test
We obtained highly reliable ANSs by combining a pure block design and nonorthogonal contrast, replicating previous findings [McConnell and Shore, 2011; Wang et al., 2014a, 2015b]. High reliability is suggested to benefit the estimation of true internetwork correlation [MacLeod et al., 2010]. However, there is no strong internetwork correlation in the present study, indicating relatively independent ANSs. Furthermore, we cannot evaluate how these three attention networks cooperate with each other due to the pure block design. What we can conclude is the independence of ANSs which may be cause by personal specific profile of ANSs [Wang et al., 2015b].
The lack of distinctive BOLD effects for the three attention networks may be due to their weak effects in this task. Although some regions are overlapped with attention networks (e.g., the lateral frontal cortex, temporoparietal junction), the lack of difference among three attention networks indicates that these regions are associated with exogenous attention or brain state switch rather than with distinctive attention states. Several factors may be responsible for this phenomenon. On the one hand, although we have demonstrated the high reliability of ANSs by nonorthogonal method and block design [Wang et al., 2014a,2015b], the validity of ANSs may be not so high. For instance, the long intertrial interval may be involved in dynamic tonic/phasic alertness and voluntary temporal preparation [Matthias et al., 2010]. On the other hand, the three attention networks have been suggested to share common attention sources [Fan et al., 2009; Wang et al., 2015b]. For example, the norepinephrine and dopamine which support alerting and executive control functions may have similar neural effects [Aston‐Jones and Cohen, 2005; Bromberg‐Martin et al., 2010; Snyder, 2011]. In fact, the functions of three attention networks are largely complementary and overlapped on the one hand [Callejas et al., 2004; Fan et al., 2009; Wang et al., 2014a,2015b], enabling them to dynamically adapt the ever changing environment; on the other hand, the profile of ANSs is different across subjects, resulting in weak or no correlation between ANSs [Wang et al., 2015b]. In the present study, all three attention tasks as well as the baseline task require the subject to focus attention on exogenous stimuli. This endows them more similarity than difference. However, this does not mean they have the same neural mechanism because the measurement of their similarity and difference depends on lots of factors such as the reliability, validity, and the state of subject at that moment. Therefore, we suggest that the lack of differences among attention conditions results from that the swing of focusing attention on inward or outward environments covers the effects of different attention conditions.
Limitations and Future Directions
There are some unanswered questions in this preliminary study. First, the comprehensive frequency characteristics of the five networks cannot be determined because only one type of cognitive task and one task frequency were used here. The comprehensive frequency characteristics of large scale networks are an important issue for understanding the mechanism of low frequency oscillations and should be investigated in multiple cognitive activities and frequencies by simultaneous EEG‐fMRI recording. Second, there is no detected correlation between lfSSBRs and behavioral performance at the network level. In contrast, behavioral performance may be more related to the effects of regional activities or information communication [Burzynska et al., 2013; Wang et al., 2015a]. How the brain–behavior relationship is established by lfSSBRs warrants future study. Third, the neurovascular coupling contributes more for signals in the infraslow frequency range (<0.1 Hz) than those in the slow frequency range (0.1–1 Hz) [Chen and Glover, 2015; Robinson et al., 2006]. Whether lfSSBRs in these two frequency ranges are distinctively impacted by the neurovascular coupling is unknown. Setting task frequency within these ranges may help to solve this problem. Furthermore, the validity of ANSs should be emphasized. Different versions of ANT may measure different components of attention due to the complexity of attention system [Ishigami and Klein, 2010; Petersen and Posner, 2012]. A clear definition of attention components is needed in future research.
CONCLUSIONS
We demonstrated by power analysis, time correlation and coherence analyses that lfSSBRs modulate large scale networks in specific frequency bands. Almost the same pattern is revealed by multiple analytical approaches that the triple network system and the sensory‐motor system can be modulated in a frequency tagging means. This opens a new window to investigate large scale brain activities and their frequency characteristics.
Disclosure: The authors declare no competing financial interests.
REFERENCES
- Andrews‐Hanna JR (2012): The brain's default network and its adaptive role in internal mentation. Neuroscientist 18:251–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews‐Hanna JR, Reidler JS, Huang C, Buckner RL (2010a): Evidence for the default network's role in spontaneous cognition. J Neurophysiol 104:322–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews‐Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL (2010b): Functional‐anatomic fractionation of the brain's default network. Neuron 65:550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston‐Jones G, Cohen JD (2005): An integrative theory of locus coeruleus–norepinephrine function: Adaptive gain and optimal performance. Ann Rev Neurosci 28:403–450. [DOI] [PubMed] [Google Scholar]
- Baria A, Mansour A, Huang L, Baliki M, Cecchi G, Mesulam M, Apkarian A (2013): Linking human brain local activity fluctuations to structural and functional network architectures. NeuroImage 73:144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg‐Martin ES, Matsumoto M, Hikosaka O (2010): Dopamine in motivational control: Rewarding, aversive, and alerting. Neuron 68:815–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzynska AZ, Garrett DD, Preuschhof C, Nagel IE, Li SC, Backman L, Heekeren HR, Lindenberger U (2013): A scaffold for efficiency in the human brain. J Neurosci 33:17150–17159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Draguhn A (2004): Neuronal oscillations in cortical networks. Science 304:1926–1929. [DOI] [PubMed] [Google Scholar]
- Calderone DJ, Lakatos P, Butler PD, Castellanos FX (2014): Entrainment of neural oscillations as a modifiable substrate of attention. Trends Cogn Sci 18:300–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callejas A, Lupiáñez J, Tudela P (2004): The three attentional networks: On their independence and interactions. Brain Cogn 54:225–227. [DOI] [PubMed] [Google Scholar]
- Capilla A, Pazo‐Alvarez P, Darriba A, Campo P, Gross J (2011): Steady‐state visual evoked potentials can be explained by temporal superposition of transient event‐related responses. PLoS One 6:e14543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JE, Glover GH (2015): BOLD fractional contribution to resting‐state functional connectivity above 0.1 Hz. NeuroImage 107:207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di X, Kim EH, Huang CC, Tsai SJ, Lin CP, Biswal BB (2013): The influence of the amplitude of low‐frequency fluctuations on resting‐state functional connectivity. Front Human Neurosci 7:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Gu X, Guise KG, Liu X, Fossella J, Wang H, Posner MI (2009): Testing the behavioral interaction and integration of attentional networks. Brain Cogn 70:209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI (2002): Testing the efficiency and independence of attentional networks. J Cogn Neurosci 14:340–347. [DOI] [PubMed] [Google Scholar]
- Florin E, Baillet S (2015): The brain's resting‐state activity is shaped by synchronized cross‐frequency coupling of neural oscillations. NeuroImage 111:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett DD, Kovacevic N, McIntosh AR, Grady CL (2010): Blood oxygen level‐dependent signal variability is more than just noise. J Neurosci 30:4914–4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett DD, Kovacevic N, McIntosh AR, Grady CL (2011): The importance of being variable. J Neurosci 31:4496–4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett DD, Kovacevic N, McIntosh AR, Grady CL (2013a): The modulation of BOLD variability between cognitive states varies by age and processing speed. Cereb Cortex 23:684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett DD, McIntosh AR, Grady CL (2014): Brain signal variability is parametrically modifiable. Cereb Cortex 24:2931–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett DD, Samanez‐Larkin GR, MacDonald SW, Lindenberger U, McIntosh AR, Grady CL (2013b): Moment‐to‐moment brain signal variability: A next frontier in human brain mapping? Neurosci Biobehav Rev 37:610–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohel SR, Biswal BB (2015): Functional integration between brain regions at rest occurs in multiple‐frequency bands. Brain Connect 5:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra‐Carrillo B, Mackey AP, Bunge SA (2014): Resting‐state fMRI A window into human brain plasticity. Neuroscientist 20:522–533. [DOI] [PubMed] [Google Scholar]
- Herrmann CS (2001): Human EEG responses to 1–100 Hz flicker: Resonance phenomena in visual cortex and their potential correlation to cognitive phenomena. Exp Brain Res 137:346–353. [DOI] [PubMed] [Google Scholar]
- Hiltunen T, Kantola J, Elseoud AA, Lepola P, Suominen K, Starck T, Nikkinen J, Remes J, Tervonen O, Palva S (2014): Infra‐slow EEG fluctuations are correlated with resting‐state network dynamics in fMRI. J Neurosci 34:356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishigami Y, Klein RM (2010): Repeated measurement of the components of attention using two versions of the Attention Network Test (ANT): Stability, isolability, robustness, and reliability. J Neurosci Methods 190:117–128. [DOI] [PubMed] [Google Scholar]
- Keller CJ, Bickel S, Honey CJ, Groppe DM, Entz L, Craddock RC, Lado FA, Kelly C, Milham M, Mehta AD (2013): Neurophysiological investigation of spontaneous correlated and anticorrelated fluctuations of the BOLD signal. J Neurosci 33:6333–6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnadas R, Ryali S, Chen T, Uddin L, Supekar K, Palaniyappan L, Menon V (2014): Resting state functional hyperconnectivity within a triple network model in paranoid schizophrenia. Lancet 383:S65. [Google Scholar]
- Lakatos P, Karmos G, Mehta AD, Ulbert I, Schroeder CE (2008): Entrainment of neuronal oscillations as a mechanism of attentional selection. Science 320:110–113. [DOI] [PubMed] [Google Scholar]
- Li JM, Bentley WJ, Snyder AZ, Raichle ME, Snyder LH (2015): Functional connectivity arises from a slow rhythmic mechanism. Proc Natl Acad Sci 112:E2527–E2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Xie B, Wang Y, Guo W, Fouche JP, Long Z, Wang W, Chen H, Li M, Duan X, Zhang J, Qiu M, Chen H (2015a): Characterization of post‐traumatic stress disorder using resting‐state fMRI with a multi‐level parametric classification approach. Brain Topogr 28:221–237. [DOI] [PubMed] [Google Scholar]
- Liu F, Zhu C, Wang Y, Guo W, Li M, Wang W, Long Z, Meng Y, Cui Q, Zeng L, Chen H (2015b): Disrupted cortical hubs in functional brain networks in social anxiety disorder. Clin Neurophysiol 22:102–115. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A (2001): Neurophysiological investigation of the basis of the fMRI signal. Nature 412:150–157. [DOI] [PubMed] [Google Scholar]
- MacLeod JW, Lawrence MA, McConnell MM, Eskes GA, Klein RM, Shore DI (2010): Appraising the ANT: Psychometric and theoretical considerations of the attention network test. Neuropsychology 24:637–651. [DOI] [PubMed] [Google Scholar]
- Mantini D, Perrucci MG, Del Gratta C, Romani GL, Corbetta M (2007): Electrophysiological signatures of resting state networks in the human brain. Proc Natl Acad Sci 104:13170–13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN (2007): Wandering minds: the default network and stimulus‐independent thought. Science 315:393–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthias E, Bublak P, Müller HJ, Schneider WX, Krummenacher J, Finke K (2010): The influence of alertness on spatial and nonspatial components of visual attention. J Exp Psychol Human Percept Perform 36:38–56. [DOI] [PubMed] [Google Scholar]
- McConnell MM, Shore DI (2011): Mixing measures: Testing an assumption of the attention network test. Attent Percept Psychophys 73:1096–1107. [DOI] [PubMed] [Google Scholar]
- Menon V (2011): Large‐scale brain networks and psychopathology: A unifying triple network model. Trends Cogn Sci 15:483–506. [DOI] [PubMed] [Google Scholar]
- Murray JD, Bernacchia A, Freedman DJ, Romo R, Wallis JD, Cai X, Padoa‐Schioppa C, Pasternak T, Seo H, Lee D (2014): A hierarchy of intrinsic timescales across primate cortex. Nat Neurosci 17:1661–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan WJ, Thompson GJ, Magnuson ME, Jaeger D, Keilholz S (2013): Infraslow LFP correlates to resting‐state fMRI BOLD signals. NeuroImage 74:288–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, Posner MI (2012): The attention system of the human brain: 20 years after. Ann Rev Neurosci 35:73–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012): Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage 59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian L, Zhang Y, Zheng L, Shang Y, Gao JH, Liu Y (2015): Frequency dependent topological patterns of resting‐state brain networks. PLoS One 10:e0124681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME (2015): The brain's default mode network. Ann Rev Neurosci 38:433–447. [DOI] [PubMed] [Google Scholar]
- Robinson P, Drysdale P, Van der Merwe H, Kyriakou E, Rigozzi M, Germanoska B, Rennie C (2006): BOLD responses to stimuli: dependence on frequency, stimulus form, amplitude, and repetition rate. NeuroImage 31:585–599. [DOI] [PubMed] [Google Scholar]
- Rosanova M, Casali A, Bellina V, Resta F, Mariotti M, Massimini M (2009): Natural frequencies of human corticothalamic circuits. J Neurosci 29:7679–7685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schölvinck ML, Maier A, Frank QY, Duyn JH, Leopold DA (2010): Neural basis of global resting‐state fMRI activity. Proc Natl Acad Sci 107:10238–10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel M, Donner TH, Engel AK (2012): Spectral fingerprints of large‐scale neuronal interactions. Nat Rev Neurosci 13:121–134. [DOI] [PubMed] [Google Scholar]
- Snyder SH (2011): What dopamine does in the brain. Proc Natl Acad Sci 108:18869–18871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson GJ, Pan WJ, Magnuson ME, Jaeger D, Keilholz SD (2014): Quasi‐periodic patterns (QPP): Large‐scale dynamics in resting state fMRI that correlate with local infraslow electrical activity. NeuroImage 84:1018–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson WH, Fransson P (2015): The frequency dimension of fMRI dynamic connectivity: Network connectivity, functional hubs and integration in the resting brain. NeuroImage 121:227–242. [DOI] [PubMed] [Google Scholar]
- Thut G, Miniussi C, Gross J (2012): The functional importance of rhythmic activity in the brain. Curr Biol 22:R658–R663. [DOI] [PubMed] [Google Scholar]
- Thut G, Schyns PG, Gross J (2011): Entrainment of perceptually relevant brain oscillations by non‐invasive rhythmic stimulation of the human brain. Front Psychol 2:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. NeuroImage 15:273–289. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W (2010): Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci 11:100–113. [DOI] [PubMed] [Google Scholar]
- Vahdat S, Darainy M, Ostry DJ (2014): Structure of plasticity in human sensory and motor networks due to perceptual learning. J Neurosci 34:2451–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialatte FB, Maurice M, Dauwels J, Cichocki A (2010): Steady‐state visually evoked potentials: Focus on essential paradigms and future perspectives. Prog Neurobiol 90:418–438. [DOI] [PubMed] [Google Scholar]
- Wang L, Saalmann YB, Pinsk MA, Arcaro MJ, Kastner S (2012): Electrophysiological low‐frequency coherence and cross‐frequency coupling contribute to BOLD connectivity. Neuron 76:1010–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YF, Cui Q, Liu F, Huo YJ, Lu FM, Chen H, Chen HF (2014a): A new method for computing attention network scores and relationships between attention networks. PLoS One 9:e89733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, YF , Dai, GS , Liu, F , Long, ZL , Yan, JH , Chen, HF (2015a): Steady‐state BOLD response to higher‐order cognition modulates low frequency neural oscillations. J Cogn Neurosci. [DOI] [PubMed] [Google Scholar]
- Wang YF, Liu F, Long ZL, Duan XJ, Cui Q, Yan JH, Chen HF (2014b): Steady‐state BOLD response modulates low frequency neural oscillations. Sci Rep 4:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Jing X, Liu F, Li M, Long Z, Yan J, Chen H (2015b): Reliable attention network scores and mutually inhibited inter‐network relationships revealed by mixed design and non‐orthogonal method. Sci Rep 5:10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Liu F, Jing X, Long Z, Chen H (2015c): Phase‐dependent alteration of functional connectivity density during face recognition in the infra‐slow frequency range In: Wang R, editor. The 5th International Conference on Cognitive Neurodynamics 2015. Sanya, China: Springer. [Google Scholar]
- Wang Y, Liu F, Li R, Yang Y, Liu T, Chen H (2013): Two‐stage processing in automatic detection of emotional intensity: A scalp event‐related potential study. Neuroreport 24:818–821. [DOI] [PubMed] [Google Scholar]
- Ward LM (2003): Synchronous neural oscillations and cognitive processes. Trends Cogn Sci 7:553–559. [DOI] [PubMed] [Google Scholar]
- Wen X, Liu Y, Yao L, Ding M (2013): Top‐down regulation of default mode activity in spatial visual attention. J Neurosci 33:6444–6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlye LT, Grydeland H, Walhovd KB, Fjell AM (2011): Associations between regional cortical thickness and attentional networks as measured by the attention network test. Cereb Cortex, 21:345–356. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC (1996): A unified statistical approach for determining significant signals in images of cerebral activation. Human Brain Mapp 4:58–73. [DOI] [PubMed] [Google Scholar]
- Wu CW, Gu H, Lu H, Stein EA, Chen JH, Yang Y (2008): Frequency specificity of functional connectivity in brain networks. NeuroImage 42:1047–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GR, Liao W, Stramaglia S, Ding JR, Chen H, Marinazzo D (2013): A blind deconvolution approach to recover effective connectivity brain networks from resting state fMRI data. Med Image Anal 17:365–374. [DOI] [PubMed] [Google Scholar]
- Xia M, Wang J, He Y (2013): BrainNet Viewer: A network visualization tool for human brain connectomics. PLoS One 8:e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]


