Abstract
Objective
To assess the structural correlates of cognitive and behavioral impairment in motor neuron diseases (MND) using multimodal MRI.
Methods
One hundred one patients with sporadic MND (56 classic amyotrophic lateral sclerosis, 31 upper motor neuron phenotype, and 14 lower motor neuron phenotype) and 51 controls were enrolled. Patients were classified into MND with a pure motor syndrome (MND‐motor) and with cognitive/behavioral symptoms (MND‐plus). Cortical thickness measures and diffusion tensor (DT) metrics of white matter (WM) tracts were assessed. A random forest approach was used to explore the independent role of cortical and WM abnormalities in explaining major cognitive and behavioral symptoms.
Results
There were 48 MND‐motor and 53 MND‐plus patients. Relative to controls, both patient groups showed a distributed cortical thinning of the bilateral precentral gyrus, insular and cingulate cortices, and frontotemporal regions. In all regions, there was a trend toward a more severe involvement in MND‐plus cases, particularly in the temporal lobes. Both patient groups showed damage to the motor callosal fibers, which was more severe in MND‐plus. MND‐plus patients also showed a more severe involvement of the extra‐motor WM tracts. The best predictors of executive and non‐executive deficits and behavioral symptoms in MND were diffusivity abnormalities of the corpus callosum and frontotemporal tracts, including the uncinate, cingulum, and superior longitudinal fasciculi.
Conclusions
Cortical thinning and WM degeneration are highly associated with neuropsychological and behavioral symptoms in patients with MND. DT MRI metrics seem to be the most sensitive markers of extra‐motor deficits within the MND spectrum. Hum Brain Mapp 37:1614‐1626, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: motor neuron disease, amyotrophic lateral sclerosis, cognitive and behavioral impairment, cortical thinning, diffusion tensor MRI
INTRODUCTION
There is increasing evidence that amyotrophic lateral sclerosis (ALS) is a clinically heterogeneous disease in terms of site of onset, degree of upper (UMN) and lower motor neurons (LMN) involvement, and rate of motor progression [Turner et al., 2013]. Several studies indicate that this heterogeneity also includes the presence and severity of cognitive and behavioral symptoms [Montuschi et al., 2015; Phukan et al., 2012]. Up to 50% of ALS patients have cognitive and/or behavioral changes, ranging from an overt frontotemporal dementia (FTD) to mild executive and/or non‐executive cognitive impairment and behavioral deficits [Montuschi et al., 2015; Phukan et al., 2012]. Cognitive and behavioral impairment, when evident early in the disease, is likely to be a predictor of a faster disease progression [Hu et al., 2013; Montuschi et al., 2015].
Findings from structural MRI studies have shown that frontotemporal and parietal cortical loss or thinning is more severe in patients with ALS with FTD and in those with mild cognitive and/or behavioral deficits than in patients with only motor ALS [Chang et al., 2005; Lillo et al., 2012; Schuster et al., 2014a]. More recently, diffusion tensor (DT) MRI studies suggested that ALS patients with cognitive and/or behavioral impairment had a more distributed white matter (WM) damage involving not only motor but also extra‐motor tracts, particularly in the frontotemporal regions, relative to patients with a pure motor syndrome [Canu et al., 2013; Kasper et al., 2014].
Some studies have directly investigated the relationship between brain structural abnormalities and cognitive and behavioral profiles in small samples of patients with ALS [Grossman et al., 2008; Kasper et al., 2014; Libon et al., 2012; Pettit et al., 2013; Sarro et al., 2011; Woolley et al., 2011]. In ALS, abnormalities of cognitive measures requiring action knowledge correlated with atrophy of the motor cortex [Grossman et al., 2008], while deficits in concept formation were related to cortical thinning of the left prefrontal‐parietal regions [Libon et al., 2012]. Performance in cognitive tests that assess executive functions was associated with DT MRI abnormalities of the corpus callosum, cingulum, corticospinal tract (CST), prefrontal and long association tracts [Kasper et al., 2014; Pettit et al., 2013], as we have previously shown in an independent patient population scanned at 1.5 T [Sarro et al., 2011]. A relationship between apathy and frontal cortical and WM alterations in patients with ALS has been suggested by a few studies [Kasper et al., 2014; Woolley et al., 2011]. Few studies have combined gray matter (GM) and WM assessments in motor neuron disease (MND) [Agosta et al., 2007; Filippini et al., 2010; Menke et al., 2014; Zhang et al., 2014] and only one, to date, has investigated their distinct roles in explaining the different aspects of the neuropsychological and behavioral profiles of patients with sporadic ALS [Menke et al., 2014]. Moreover, the structural correlates of extra‐motor manifestations in less frequent MND variants, such as UMN and LMN phenotypes, remain to be explored.
Aim of this multimodal MRI study was to assess the pattern of cortical thinning and WM tract damage in a large cohort of pure motor MND patients and MND patients with cognitive and/or behavioral impairment, including cases with classic ALS, and UMN and LMN phenotypes. In addition, a random forest approach [Breiman, 2001] was used to explore the independent role of cortical and WM abnormalities in explaining major cognitive deficits and behavioral symptoms in these patients. Random forest is able to take into account a high number of input features and can be used to select the most probable predictors of a given outcome at an individual patient level [Breiman, 2001].
MATERIALS AND METHODS
Subjects
One‐hundred and one right‐handed patients with sporadic MND (56 patients with classic ALS, 31 patients with a clinical UMN phenotype, and 14 patients with a clinical LMN phenotype) were consecutively recruited at four tertiary referral MND Clinics in Northern Italy (Table 1). Diagnosis of classic ALS was made according to the revised Escorial criteria [Brooks et al., 2000]. Patients with a clinical UMN phenotype did not have any LMN sign on clinical assessment or any evidence of active denervation on repeated electromyographical examinations [Chio et al., 2011]. Patients with a clinical LMN phenotype did not have signs of UMN involvement on clinical examination [Chio et al., 2011]. Patients underwent a comprehensive evaluation including neurological history, neurophysiological assessment, neuropsychological testing, and MRI. Blood samples were available for 81 patients. The presence of the GGGGCC hexanucleotide expansion in the first intron of C9Orf72 status was assessed using a repeat‐primed PCR assay [Renton et al., 2011]; a cut‐off of ≥30 repeats combined with a typical sawtooth pattern was considered pathological. In addition, the coding sequences and intron/exon boundaries of TARDBP and SOD1 genes were amplified by PCR using optimized protocols. Experienced neurologists blinded to the MRI results performed the clinical assessment. Site of disease onset and disease duration were recorded. Disease severity was assessed using the ALS Functional Rating Scale‐revised (ALSFRS‐r) [Cedarbaum et al., 1999] and clinical UMN involvement was graded using the UMN score [Turner et al., 2004]. The rate of disease progression was calculated as follows: (48: ALSFRS‐r score)/months from symptom onset. Fifty‐one right‐handed, age‐matched healthy controls were recruited among spouses of patients and by word of mouth (Table 1). Healthy controls were included if the neurological assessment was normal, the Mini‐Mental State Examination score was ≥28, and the Beck Depression Inventory score was < 9. In addition, subjects were excluded if they had: a family history of MND, dementia, or FTD‐related disorders; significant medical illnesses or substance abuse that could interfere with cognitive functioning; any other major systemic, psychiatric, or neurological illnesses; and other causes of focal or diffuse brain damage, including lacunae, and extensive cerebrovascular disorders at routine MRI. Approval of the institutional review boards and written informed consent were obtained from all participants (or their legal guardians).
Table 1.
Demographic and clinical findings of healthy controls and patients with motor neuron disease
| Healthy controls | MND‐motor patients | MND‐plus patients | p: MND‐motor patients vs healthy controls | p: MND‐plus patients vs healthy controls | p: MND‐motor vs MND‐plus patients | |
|---|---|---|---|---|---|---|
| Number | 51 | 48 | 53 | — | — | — |
| Age (years) | 63 ± 8 | 59 ± 8 | 62 ± 10 | 0.06 | 0.70 | 0.12 |
| Sex (F/M) | 30/21 | 27/21 | 21/32 | 0.84 | 0.08 | 0.11 |
| Education (years) | 13 ± 5 | 11 ± 4 | 9 ± 5 | 0.01 | 0.001 | 0.21 |
| ALS/UMN variant/LMN variant | — | 24/15/9 | 32/16/5 | — | — | — |
| Disease duration (months) | — | 51 ± 64 | 43 ± 47 | — | — | 0.58 |
| Site of disease onset (limb/bulbar) | — | 39/9 | 45/8 | — | — | 0.61 |
| ALSFRS‐r | — | 37 ± 6 | 38 ± 7 | — | — | 0.96 |
| Rate of disease progression | — | 0.50 ± 0.56 | 0.61 ± 0.64 | — | — | 0.36 |
| UMN score | — | 10 ± 5 | 11 ± 5 | — | — | 0.70 |
Values are means ± standard deviations or number. P values refer to Fisher exact test or ANOVA models, followed by post‐hoc pairwise comparisons.
Abbreviations: ALS= amyotrophic lateral sclerosis; ALSFRS‐r= ALS functional rating scale‐revised; F= females; LMN= lower motor neuron; M= males; MND‐motor = patients with a pure motor syndrome; MND‐plus = patients with cognitive and/or behavioral symptoms; UMN= upper motor neuron.
Cognitive and Behavioral Assessment
Neuropsychological assessment was performed by a trained neuropsychologist unaware of the MRI and genetic results, and evaluated: global cognitive functioning with the Mini‐Mental State Examination; reasoning and executive functions with the Raven colored progressive matrices [Basso et al., 1987], phonemic and semantic fluency tests [Novelli et al., 1986], fluency indices (controlling for individual variations in motor disabilities) [Abrahams et al., 2000], digit span backward [Monaco et al., 2012], Cognitive Estimation Task [Della Sala et al., 2003], Wisconsin Card Sorting Test [Laiacona et al., 2000], and Weigl's Sorting test [Weigl, 1927]; verbal memory with the digit span forward [Orsini et al., 1987] and the Rey Auditory Verbal Learning Test immediate and delayed recall [Carlesimo et al., 1996]; and language with the oral noun confrontation naming subtest of BADA (Batteria per l'Analisi dei deficit Afasici) [Miceli et al., 1994]. Scores on neuropsychological tests were age‐, sex‐, and education‐corrected using corresponding normative values. Depression was assessed using the Hamilton Depression Rating Scale. The presence of behavioral disturbances was determined on the basis of the direct observation and patient's history, caregiver report, and the Frontal Behavioral Inventory (FBI) [Alberici et al., 2007] and the ALS‐FTD questionnaire [Raaphorst et al., 2012] administered to patients’ caregivers. Among the FBI items, “Personal Neglect” and “Logopenia” were excluded to minimize the effect of physical disability on behavioral performances.
According to cognitive and behavioral findings, patients were divided into two groups: MND with normal cognition (i.e., MND‐motor) and MND with cognitive and/or behavioral deficits (i.e., MND‐plus). MND‐plus patients included [Montuschi et al., 2015; Phukan et al., 2012]: MND with FTD (MND‐FTD), i.e., MND with a clinical diagnosis of the behavioral variant of FTD (bvFTD) or primary progressive aphasia; MND with executive cognitive impairment (MND‐ECI), i.e., MND patients who did not meet criteria for bvFTD but who had an impairment in at least two tests of executive functions; MND with non‐executive cognitive impairment (MND‐NECI), i.e., patients with impairment at two non‐executive domains and no executive impairment; MND with non‐classifiable impairment (MND‐NCCI), i.e., patients with impairment at one executive and/or one non‐executive test; and MND with behavioral impairment (MND‐bi), i.e., patients with behavioral disturbances associated with impairment in none or only one executive test and no impairment in non‐executive domains. The impairment at a cognitive test was defined as a score of two standard deviations (2.3rd percentile) below the mean score of independent normative control groups matched for age and education, representative of the Italian population when available (see the references of abovementioned tests for details) [Montuschi et al., 2015; Phukan et al., 2012]. Behavioral disturbances were defined according to the ALS‐FTD Consensus Criteria [Strong et al., 2009] as the presence of two behavioral abnormalities supported by at least two sources from among patient interview/observation, caregiver report, and structured questionnaires (FBI, ALS‐FTD questionnaire).
MRI Acquisition
Using a 3.0 T scanner (Intera, Philips Medical Systems, Best, the Netherlands), the following brain MRI sequences were obtained from all subjects: T2‐weighted spin echo (SE) (repetition time [TR] = 3,500 ms; echo time [TE] = 85 ms; echo train length = 15; flip angle = 90°; 22 contiguous, 5‐mm‐thick, axial slices; matrix size = 512 × 512; field of view [FOV] = 230 × 184 mm2); fluid‐attenuated inversion recovery (TR = 11 s; TE = 120 ms; flip angle = 90°; 22 contiguous, 5‐mm‐thick, axial slices; matrix size = 512 × 512; FOV = 230 mm2); 3D T1‐weighted fast field echo (FFE) (TR = 25 ms, TE = 4.6 ms, flip angle = 30°, 220 contiguous axial slices with voxel size = 0.89 × 0.89 × 0.8 mm, matrix size = 256 × 256, FOV = 230 × 182 mm2); and pulsed‐gradient SE echo planar with sensitivity encoding (acceleration factor = 2.5, TR = 8,986 ms, TE = 80 ms, 55 contiguous, 2.5 mm‐thick axial slices, number of acquisitions = 2; acquisition matrix 96 × 96, with an in‐plane pixel size of 0.94 × 0.94 mm and a FOV=240 × 240 mm2) and diffusion gradients applied in 32 noncollinear directions using a gradient scheme which is standard on this system (gradient over‐plus) and optimized to reduce echo time as much as possible. The b factor used was 1,000 s/mm2. Fat saturation was performed to avoid chemical shift artifacts. All slices were positioned to run parallel to a line that joins the most inferoanterior and inferoposterior parts of the corpus callosum.
MRI Analysis
Cortical thickness measurement
Cortical reconstruction and estimation of cortical thickness were performed on the 3D T1‐weighted FFE images using the FreeSurfer image analysis suite, version 5.0 (http://surfer.nmr.mgh.harvard.edu/) [Fischl and Dale, 2000], by a single observer unaware of the clinical and genetic results. After registration to Talairach space and intensity normalization, the process involved an automatic skull stripping, which removes extra‐cerebral structures, cerebellum, and brainstem, by using a hybrid method combining watershed algorithms and deformable surface models. Images were then carefully checked for skull stripping errors. After this step, images were segmented into GM, WM, and cerebrospinal fluid (CSF), cerebral hemispheres were separated, and subcortical structures divided from cortical components. The WM/GM boundary was tessellated and the surface was deformed following intensity gradients to optimally place WM/GM and GM/CSF borders, thus obtaining the WM and pial surfaces [Dale et al., 1999]. The results of this segmentation procedure were inspected visually, and if necessary, edited manually by adding control points. A maximum of 10 controls points were added to include areas that are obviously part of the brain but were not included in the first run; this step has been performed in 7 healthy controls, 8 MND‐motor, and 12 MND‐plus patients. Afterwards, surface inflation and registration to a spherical atlas were performed [Dale et al., 1999] and the cerebral cortex parcellated into 34 regions per hemisphere, based on gyral and sulcal structures, as described by Desikan et al. (2006). Finally, cortical thickness was estimated as the average shortest distance between the WM boundary and the pial surface.
DT MRI tractography
DT MRI analysis was performed using the FMRIB software library (FSL) tools (http://www.fmrib.ox.ac.uk/fsl/fdt/index.html) and the JIM6 software (Version 6.0, Xinapse Systems, Northants, UK, http://www.xinapse.com), by a single observer unaware of the clinical and genetic results. The diffusion‐weighted data were skull‐stripped using the Brain Extraction Tool implemented in FSL. Using FMRIB's Linear Image Registration Tool (FLIRT), the two diffusion‐weighted scans were coregistered by applying the rigid transformation needed to correct for position between the two b 0 images (T2‐weighted, but not diffusion‐weighted). The rotation component was also applied to diffusion‐weighted directions. Eddy currents correction was performed using the JIM6 software [Horsfield, 1999]. Then, the two acquisitions were concatenated. The DT was estimated on a voxel‐by‐voxel basis using DTIfit provided by the FMRIB Diffusion Toolbox. Maps of mean diffusivity (MD), fractional anisotropy (FA), axial diffusivity (axD), and radial diffusivity (radD) were obtained.
Seeds for tractography of the CST, corpus callosum, cingulum, superior longitudinal (SLF), inferior longitudinal (ILF), and uncinate fasciculi were defined in the Montreal Neurological Institute (MNI) space on the FA template provided by FSL, as previously described [Agosta et al., 2013, 2014]. Fiber tracking was performed in native DT MRI space using a probabilistic tractography algorithm implemented in FSL (probtrackx), which is based on Bayesian estimation of diffusion parameters (Bedpostx) [Behrens et al., 2007]. Fiber tracking was initiated from all voxels within the seed masks in the diffusion space to generate 5,000 streamline samples with a step length of 0.5 mm and a curvature threshold of 0.2. Using a “single‐seed” approach, the reconstructions of the CC and bilateral CST, SLF, and uncinate were obtained. For the ILF and cingulum, we used single seedmasks including three seeds each (anterior, middle, and posterior ILF; and anterior, isthmus, and parahippocampal cingulum). In addition, using a “seed to target” approach, the corpus callosum was segmented into three portions to identify the callosal fibers linking the primary motor cortices (CC‐PMC), lateral premotor cortices, and supplementary motor areas (CC‐SMA) [Agosta et al., 2014]. Tract maps were then normalized taking into consideration the number of voxels in the seed masks. To do so, the number of streamline samples present in the voxels of the tract maps was divided by the way‐total, which corresponds to the total number of streamline samples that were not rejected by the exclusion masks. The tract masks obtained were thresholded at a value equal to 40% of the 95th percentile of the distribution of the intensity values of the voxels included in the tract, as previously described [Galantucci et al., 2011]. This normalization procedure allowed us to correct for possible differences between tracts due to the different sizes of the starting seeds. In this way, we also excluded the background noise and avoided a too restrictive thresholding when the maximum intensity value was an outlier. Group probability maps of each thresholded tract were produced to visually check their anatomical consistency across study subjects. For each tract, the average MD, FA, axD, and radD were calculated in the native space.
Statistical Analysis
Demographic, clinical, and cognitive data
Normal distribution assumption was checked by means of Q‐Q plot and Shapiro‐Wilks and Kolmogorov–Smirnov tests. Group comparisons were performed using ANOVA models, followed by post hoc pairwise comparisons (SAS Release 9.3, SAS Institute, Cary, NC).
MRI data
The mean cortical thickness of 34 regions of interest per hemisphere and the mean DT MRI measures from WM tracts were compared between groups using ANOVA models, false‐discovery rate (FDR) corrected for multiple comparisons and adjusting for age. Patients vs. controls comparisons were also tested adjusting for years of education.
Random forest analysis
A random forest analysis [Breiman, 2001] was used to identify those MRI variables that best predict the MND cognitive and behavioral scores, providing information on variable importance (package ‘‘randomForest’’ version 4.5 implemented in R). The analysis was adjusted for physical disability (i.e., ALS Functional Rating Scale‐revised). To this end, 100,000 trees were built. The training set used to grow each tree was a 0.632+ bootstrap resample of the observations [Efron and Tibshirani, 1997]. The best split at each node was selected from a random subset of MRI metrics. The left‐out observations (i.e., “out of bag” observations) were then predicted to obtain the prediction error of the considered tree. The goodness of the fit of the random forest was assessed averaging the individual tree classification errors. The random forest framework allowed us to estimate the importance of each predictor by looking at how much the prediction error increased when out of bag data for that variable were permuted, while all others were left unchanged. The variables’ importance was ranked by assigning to each covariate a score based on the ability to predict correctly the outcome (i.e., patient cognitive and behavioral variables) according to the increase of prediction error when values of that covariate in a node were permuted randomly.
RESULTS
Demographic, Clinical, and Cognitive/Behavioral Findings
Forty‐eight MND patients (48%; 24 classic ALS, 15 UMN variant, and 9 LMN variant) were cognitively normal and 53 MND patients (53%; 32 classic ALS, 16 UMN variant, and 5 LMN variant) showed cognitive and/or behavioral disturbances (Table 1). The MND‐plus group included: 8 patients with MND‐FTD (all classic ALS), 5 patients with MND‐ECI (3 classic ALS, 1 UMN variant, 1 LMN variant), 9 patients with MND‐NECI (5 classic ALS, 4 UMN variant), 22 patients with MND‐NCCI (11 classic ALS, 7 UMN variant, 4 LMN variant), and 9 patients with MND‐bi (5 classic ALS, 4 UMN variant). All MND‐FTD patients presented with features consistent with a bvFTD. In MND‐bi patients, seven cases showed prominent negative symptoms (i.e., apathy, aspontaneity, indifference/emotional flatness, and disorganization), while two cases presented with predominant positive symptoms (i.e., impulsivity, irritability, and poor judgment). MND‐motor and MND‐plus patients had lower education relative to healthy controls, while no difference was found between patient groups in terms of demographic and clinical findings (Table 1). One MND‐motor, one MND‐FTD, and two MND‐ECI carried the C9Orf72 hexanucleotide repeat expansion. No TARDBP and SOD1 mutations were found in our patients. With the only exception of the Rey Auditory Verbal Learning Test (RAVLT) delayed recall performance, all cognitive/behavioral variables were different between MND‐motor and MND‐plus patients (Table 2). Two MND‐plus and one MND‐motor patients had an HDRS score between 17 and 23 indicating a moderate depression [Zimmerman et al., 2013], while a mild depression syndrome (HDRS score: 8–16) was found in 10 MND‐plus and 15 MND‐plus patients. No depression (HDRS ≤ 7) was observed in the remaining patients.
Table 2.
Neuropsychological and behavioral features of patients with motor neuron disease
| MND‐motor patients | MND‐plus patients | P | |
|---|---|---|---|
| General Cognition | |||
| MMSE (cut‐off 24) | 28.72 ± 1.16 | 27.11 ± 2.33 | <0.001 |
| Reasoning and executive functions | |||
| Raven's coloured progressive matrices (cut‐off 18) | 30.40 ± 3.66 | 25.98 ± 5.29 | <0.001 |
| Phonemic fluency (cut‐off 17) | 30.20 ± 9.11 | 23.66 ± 9.32 | <0.001 |
| Semantic fluency (cut‐off 25) | 40.72 ± 9.34 | 33.62 ± 10.05 | <0.001 |
| Phonemic fluency index | 6.32 ± 3.91 | 9.61 ± 8.43 | 0.03 |
| Semantic fluency index | 4.46 ± 1.40 | 5.40 ± 2.47 | 0.04 |
| Digit span backward (cut‐off 3.29) | 4.32 ± 0.99 | 3.57 ± 1.14 | 0.001 |
| CET (cut‐off 18) | 13.47 ± 2.75 | 15.09 ± 4.06 | 0.045 |
| WCST (cut‐off 90.5) | 52.50 ± 36.25 | 80.08 ± 43.20 | 0.002 |
| Weigl's Sorting test (cut‐off 4.50) | 12.66 ± 2.32 | 10.79 ± 3.66 | 0.01 |
| Verbal memory | |||
| Digit span forward (cut‐off 3.75) | 5.70 ± 1.17 | 5.17 ± 0.98 | 0.02 |
| RAVLT, immediate recall (cut‐off 28.53) | 43.67 ± 10.27 | 35.60 ± 12.36 | 0.001 |
| RAVLT, delayed recall (cut‐off 4.69) | 8.43 ± 2.91 | 7.12 ± 3.91 | 0.07 |
| Language | |||
| Oral noun confrontation naming subtest of BADA | 29.46 ± 0.71 | 27.84 ± 2.71 | <0.001 |
| Behavioral disturbances | |||
| FBI | 1.08 ± 2.19 | 6.68 ± 7.49 | <0.001 |
| ALS‐FTD questionnaire (cut‐off 22) | 7.33 ± 6.68 | 23.17 ± 14.03 | 0.03 |
| Depression | |||
| HDRS | 7.17 ± 4.90 | 6.08 ± 4.95 | 0.35 |
Values are means ± standard deviations. P values refer to ANOVA models. Abbreviations: CET=Cognitive Estimation Test; HC= healthy controls; HDRS=Hamilton Depression Rating Scale; FBI= Frontal behavioral inventory; MMSE= Mini Mental State Examination; MND‐motor= MND patients with a pure motor syndrome; MND‐plus= MND patients with cognitive and/or behavioral symptoms; RAVLT=Rey Auditory Verbal Learning Test; WCST=Wisconsin Card Sorting Test.
Cortical thickness
Relative to controls, both MND‐motor and MND‐plus patients revealed a distributed pattern of cortical thinning including the precentral gyrus, superior, middle, and inferior frontal gyri, orbitofrontal cortex, insular cortex, fusiform, superior and inferior temporal gyri, superior and inferior parietal gyri, supramarginal gyrus, lingual gyrus, lateral occipital cortex, and cingulate cortex bilaterally, left entorhinal cortex, temporal pole, right parahippocampal, middle temporal, postcentral gyri, posterior cingulate, and transverse temporal cortex (Fig. 1; Supporting Information Table 1). Compared with controls, MND‐plus patients also showed cortical thinning of the left middle temporal and postcentral gyrus, precuneus, and right entorhinal cortex (Fig. 2; Supporting Information Table 1). Adjusting for years of education, findings in patients vs. controls did not change (data not shown). Comparing the two MND groups, MND‐plus patients had a more severe cortical thinning of the middle temporal gyrus bilaterally, left orbitofrontal cortex, and right superior frontal gyrus, insula, fusiform, superior temporal, inferior temporal gyri, transverse temporal cortex, and rostral anterior cingulate cortex (Fig. 3; Supporting Information Table 1). When patients with MND‐FTD were excluded from the MND‐plus group, the results of the direct comparison between patient groups did not change, with exception of the left orbitofrontal cortex and right superior frontal gyrus which did not show significant thickness differences. Excluding the three MND‐plus patients carrying the C9Orf72 hexanucleotide expansion, results did not change (data not shown).
Figure 1.
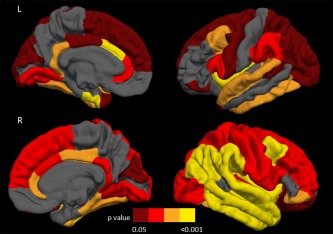
Distribution of the cortical thinning on the pial surface in motor neuron disease patients with a pure motor syndrome (MND‐motor) compared with healthy controls. Results are false‐discovery rate corrected for multiple comparisons and adjusted for subject's age. Colors represent p values: yellow = P < 0.001; orange = 0.001≥ P <0.01; red= 0.01≥ P ≤0.02; dark red= 0.02> P <0.05. L= left; R= right.
Figure 2.
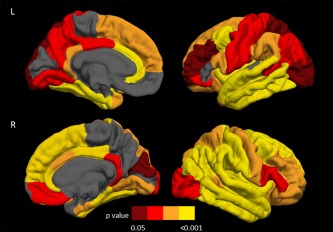
Distribution of the cortical thinning on the pial surface in motor neuron disease patients with cognitive and/or behavioral symptoms (MND‐plus) compared with healthy controls. Results are false‐discovery rate corrected for multiple comparisons and adjusted for subject's age. Colors represent P values: yellow= P < 0.001; orange= 0.001≥ P <0.01; red= 0.01≥ P ≤0.02; dark red= 0.02> P <0.05. L= left; R= right.
Figure 3.
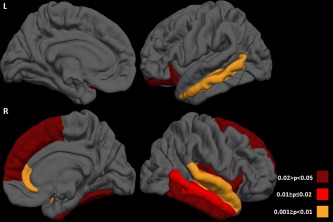
Distribution of the cortical thinning on the pial surface in motor neuron disease (MND) patients with cognitive and/or behavioral symptoms (MND‐plus) compared with those with a pure motor syndrome (MND‐motor). Results are false‐discovery rate corrected for multiple comparisons and adjusted for subject's age. Colors represent P values: orange= 0.001≥ P <0.01; red= 0.01≥ P ≤0.02; dark red= 0.02 > P < 0.05. L= left; R= right.
DT MRI tractography
Compared with healthy controls, MND‐motor patients showed a decreased FA and an increased radD of the callosal fibers linking the primary motor cortices (CC‐PMC) and supplementary motor area (CC‐SMA) (Supporting Information Tables 2 and 3). MND‐plus patients showed a severe involvement of the majority of WM tracts relative to healthy controls, including the whole corpus callosum, CC‐PMC, CC‐SMA, CST, and SLF bilaterally, and left uncinate and cingulum fasciculi (Supporting Information Tables 2 and 3). Adjusting for years of education, findings in patients vs. controls did not change (data not shown). A similar distributed pattern of WM damage was observed when MND‐plus patients were compared with MND‐motor cases (Supporting Information Tables 2 and 3). When patients with MND‐FTD were excluded from the MND‐plus group, the pattern of differences between patient groups was less distributed including the CC‐PMC, CC‐SMA, CST bilaterally, left uncinate, and right SLF (Supporting Information Tables 2 and 3). Excluding the three MND‐plus patients carrying the C9Orf72 hexanucleotide expansion, results did not change (data not shown).
Association Between Cognitive/Behavioral Deficits and MRI Findings
Table 3 shows the random forest analysis results in MND patients. For each cognitive or behavioral score, the first three MRI metrics in terms of importance in identifying impairment in the given scale/test are provided. Behavioral symptoms were predicted by damage to the left uncinate and callosal fibers (including the CC‐SMA). DT MRI metrics of the CC‐SMA and left SLF, cingulum, and CST were the best predictors of fluency deficits, while right temporal and left frontal thinning contributed together with left SLF and cingulum damage to categorization and abstract reasoning impairments. Left cingulum and uncinate damage and middle temporal gyrus thinning were the best predictors of verbal memory deficits.
Table 3.
Random forest analysis results in patients with motor neuron diseases
| Cognitive or behavioral tests | MRI variables | Normalized variable importance |
|---|---|---|
| Frontal Behavioral Inventory | Left uncinate MD | 100.00 |
| CC‐SMA FA | 64.01 | |
| CC FA | 56.60 | |
| Phonemic fluency | CC‐SMA MD | 100.00 |
| Left cingulum MD | 77.71 | |
| Left CST MD | 70.38 | |
| Semantic fluency | Left SLF MD | 100.00 |
| CC‐precentral MD | 39.65 | |
| Left SLF FA | 32.84 | |
| Wisconsin Card Sorting Test | Right transverse temporal cortex thickness | 100.00 |
| Right superior temporal gyrus thickness | 73.29 | |
| Left SLF FA | 73.22 | |
| Weigl's Sorting test | Left SLF FA | 100.00 |
| Left cingulum MD | 81.46 | |
| Left inferior frontal gyrus (pars opercularis) thickness | 60.17 | |
| Rey's List delayed recall | Left cingulum MD | 100.00 |
| Left middle temporal gyrus thickness | 39.04 | |
| Left uncinate MD | 28.62 |
Abbreviations: CC=corpus callosum; CC‐premotor=callosal fibers linking the premotor cortices; CC‐SMA=callosal fibers linking the supplementary motor areas; CST= corticospinal tract; FA=fractional anisotropy; MD= mean diffusivity; SLF= superior longitudinal fasciculus.
DISCUSSION
To date, this is the largest study assessing the structural correlates of cognitive and behavioral impairment in patients with different MND phenotypes. A distributed cortical thinning of the bilateral precentral, insular, cingulate, and frontotemporal cortices was observed in MND patients, regardless of the presence of cognitive and behavioral dysfunctions. In addition, in all regions, there was a trend toward a more severe involvement in MND‐plus cases, particularly in the temporal lobes. Both MND‐motor and MND‐plus patients showed damage to the motor callosal fibers, which was more severe in MND‐plus. MND‐plus cases also showed a more severe involvement of the extra‐motor WM tracts. Finally, the best predictors of executive and non‐executive deficits and behavioral symptoms in MND were diffusivity abnormalities of the corpus callosum and frontotemporal tracts. These findings suggest that cortical thinning and WM degeneration are highly associated with neuropsychological and behavioral symptoms in patients with MND. In addition, they showed that DT MRI measures of WM involvement are the most sensitive markers of extra‐motor deficits in these patients. Such markers may refine the diagnostic pathway and the prognostic stratification of patients, as well as provide an objective assessment of changes in disease activity in response to future therapeutic interventions.
The frequency of cognitive impairment and behavioral abnormalities in our series of classic ALS patients was similar to those described in prior population‐based studies [Montuschi et al., 2015; Phukan et al., 2012]. As previously suggested [Montuschi et al., 2015; Phukan et al., 2012], our study showed that cognitive impairment in this condition is more heterogeneous than expected earlier [Strong et al., 2009], and involves cognitive domains other than executive functions, such as memory and language. The slightly higher representation of NECI and NCCI patients in our sample relative to previous reports [Montuschi et al., 2015; Phukan et al., 2012] is likely due to the fact that we included also patients with the UMN and LMN variants [Chio et al., 2011]. A few studies so far have investigated the cognitive/behavioral symptoms of these MND phenotypes. In our series, 39% of patients with clinical UMN phenotype showed variable patterns of cognitive impairment, including not only executive but also memory and language deficits, in line with previous smaller reports [Piquard et al., 2006; Zago et al., 2008]. We also identified a group of UMN patients (13%) with an isolated behavioral impairment. In patients with the LMN phenotype, we found the lowest percentage of cases with cognitive impairment (36%) and no subject showing behavioral symptoms. In addition, the majority of LMN patients with cognitive deficits had a MND‐NCCI profile, i.e., impairment at one executive and/or non‐executive test who did not fulfill the criteria for other cognitive groups, and only one case showed an overt executive dysfunction. Non‐motor clinical manifestations are disputed in the LMN variant, as a few previous studies found executive and memory impairments [Raaphorst et al., 2011; van der Graaff et al., 2011] but others did not [Wicks et al., 2006]. In keeping with previous studies [Montuschi et al., 2015; Phukan et al., 2012], our findings highlight that the use of standardized and comprehensive methodologies for the evaluation of cognitive and behavioral impairment is essential to capture the multifaceted picture of cognitive and behavioral manifestations in the MND spectrum.
In addition to the cortical thinning of the precentral gyrus, cortical thickness measurements were found to be altered in multiple brain areas encompassing (predominantly right‐sided) frontal, insular, temporal, parietal, and occipital regions in both MND‐motor and MND‐plus patients. The evidence of a distributed involvement of extra‐motor areas in MND confirms recent pathological data [Brettschneider et al., 2013] and previous neuroimaging studies [Agosta et al., 2012; d'Ambrosio et al., 2014; Mezzapesa et al., 2013; Schuster et al., 2014b; Thorns et al., 2013; Verstraete et al., 2011]. In all cortical regions, we found a trend toward a more severe thinning in MND‐plus relative to MND‐motor patients underscoring the morphological continuum within the MND spectrum. Moreover, MND‐plus patients showed a more severe cortical thinning of the anterior cingulate cortex, insula, temporal pole, and lateral temporal cortices, particularly prominent in the right hemisphere, relative to MND‐motor. A more severe involvement of extra‐motor frontotemporal cortical areas has been shown in ALS patients carrying C9orf72 repeat expansion, which is a high pathogenic mutation for the development of both ALS and FTD, than in negative patients [Bede et al., 2013; Walhout et al., 2015a]. Together with previous studies [Chang et al., 2005; Lillo et al., 2012; Montuschi et al., 2015; Phukan et al., 2012; Schuster et al., 2014a], our findings provide further support for the overlapping brain morphology between FTD and MND, also in sporadic cases. Interestingly, cortical thinning in the temporal regions had a higher level of significance compared to healthy controls than the precentral gyrus, even in MND‐motor patients. Although we cannot exclude that this finding is partly related to the inclusion of patients with varying disease phenotypes (including LMN variants), it is also worth noting that the involvement of temporal gyri was previously associated with a more rapid clinical deterioration in ALS patients [Verstraete et al., 2011; Walhout et al., 2015b]. Furthermore, a recent study in asymptomatic C9orf72 repeat expansion carriers showed an effect in temporal, parietal, and occipital cortical regions but not in the primary motor cortex relative to asymptomatic noncarriers [Walhout et al., 2015a]. Longitudinal investigations are warranted to clarify the temporal dynamics of motor and extra‐motor cortical changes in sporadic MND cases.
MND‐motor patients showed a severe damage to the motor callosal fibers relative to controls. The involvement of motor fibers of the body of the CC has been consistently proposed as a possible hallmark of UMN degeneration in MND, and seems to be more pronounced in cases with a clinical UMN phenotype [Agosta et al., 2014], who are highly represented in our sample. The lack of significant abnormalities of the CST in MND‐motor patients is likely to be related to the fact that DT MRI tractography measurements are derived by averaging values over all voxels within the tract, which could limit the ability to detect discrete areas of difference between groups. Indeed, previous voxel‐wise reports revealed that CST damage in MND is more severe at the level of the posterior limb of the internal capsule and rostral portions of the CST underneath the primary motor cortices [Agosta et al., 2014; Menke et al., 2012]. MND‐plus group relative to MND‐motor cases showed a prominent reduction of WM integrity of the frontal, temporal, and parietal long‐range tracts. These findings support the hypothesis that WM involvement underlies both cognitive and behavioral changes in MND patients [Grossman et al., 2008; Kasper et al., 2014; Libon et al., 2012; Pettit et al., 2013; Sarro et al., 2011; Woolley et al., 2011].
When effects of cortical and WM damage on cognitive and behavioral deficits were examined at an individual patient level adjusting for clinical disease severity, we found that the relative contribution of WM tract abnormalities was greater compared to that of cortical thinning. Unlike classic correlation analyses that often provide a large number of statistically significant results, some of which are likely to be false discoveries that may not be confirmed by future studies, the random forest approach is an established and highly accurate classifier, which can handle a large number of input variables, even when the number of observations is small [Breiman, 2001]. Furthermore, introducing an appropriate level of randomness, random forest allows to model interactions between predictors identifying which, among them, is the best one [Breiman, 2001]. The best predictor of behavioral disturbances of MND patients was the damage to the uncinate fasciculus. The uncinate fasciculus connects the ventral anterior temporal lobe, which subtends social and emotional memories, with the lateral orbitofrontal cortex, which subtends the reward‐based decision making [Von Der Heide et al., 2013]. Although the role of the uncinate fasciculus is still unclear, it has been suggested that it contributes to social behavior by transmitting the mnemonic associations between objects, people, and emotions stored in the anterior temporal lobe to the orbitofrontal cortex for making choices [Von Der Heide et al., 2013]. This bidirectional interaction seems to be instrumental in assigning value (rewards/punishments) to the stored representations. In bvFTD patients, the involvement of this tract was found to correlate with measures of disinhibition and apathy [Hornberger et al., 2011]. Furthermore, we observed that the damage to the long associative WM tracts linking frontal and parietal regions, such as the SLF and cingulum, are the best predictors of the severity of executive function impairment in MND patients investigated with verbal fluency (involving speed selection of specific material), Wisconsin Card Sorting Test (WCST) (involving response selection, inhibition and management of conflicting task) and Weigl's Sorting test (involving abstract reasoning). A number of previous studies in healthy populations have demonstrated that the success in executive functioning tasks, due to their complexity, needs to be supported by the integrity of a widely distributed network of fronto‐parietal WM connections [Gold et al., 2010]. Performances at the WCST were also predicted by temporal cortical thickness, probably due to the strong contribution of conceptual knowledge representation to this task [Barcelo et al., 1997]. Phonemic fluency was associated with altered metrics of the callosal fibers linking SMA and CST, confirming that such a test offers a sensitive measure of disease severity in MND [Abrahams et al., 2000; Pettit et al., 2013]. Finally, the best predictors of the RAVLT delayed recall performance were the metrics of the cingulum, middle temporal thickness, and uncinate, confirming that the recall process, unlike the recognition, need not only the integrity of memory components, but also of the executive searching strategies [Ricci et al., 2012].
Our study is not without limitations. First, it is a cross‐sectional study. The longitudinal assessment of our MND patients is ongoing and would likely identify MRI features that can have a predictive value in this population. Second, MND patients had lower education level compared with healthy controls, as shown in previous studies [Montuschi et al., 2015]; indeed, education was added as covariate in the patients vs. controls comparisons. It is worth noting, however, that years of education were similar between the two patients groups thus not affecting between patient comparisons and the random forest analysis findings. Third, although we recruited a large sample of MND patients, the numbers were necessarily lower when patients were divided in subgroups. Larger studies are warranted to investigate the underlying structure–function relationships in each cognitive/behavioral group, separately. Fourth, C9Orf72 genotyping was not available for 20% of our sample. Finally, our multiparametric study provides cortical and WM structural correlates of cognitive and behavioral manifestations in MND. Recent data indicates that hippocampal and basal ganglia impairment may be important contributors to extra‐motor deficits in MND [Barbagallo et al., 2014; Machts et al., 2015], with a gradient of incremental pathology across the ALS/ALS‐FTD spectrum [Machts et al., 2015]. In addition, evidence for involvement of the cerebellum in MND comes from several pathological reports, showing TDP‐43 inclusions in this structure, and imaging studies, which demonstrated cerebellar GM and WM abnormalities [Prell and Grosskreutz, 2013]. The structural damage to the cerebellum in MND may lead to an ineffective modulation of both motor and cognitive functions [Prell and Grosskreutz, 2013; Tan et al., 2014]. Future studies are needed to define the relative contributions of cortical, basal ganglia, WM, and cerebellar impairments in determining cognitive and behavioral dysfunctions in these patients.
Supporting information
Supporting Information
Supporting Information
Federica Agosta and Pilar M. Ferraro have equally contributed to the study and should both be considered as “first authors.”
Conflict of interest: F. Agosta serves on the editorial board of the Journal of Neurology; has received speaker honoraria from Biogen Idec and EXCEMED– Excellence in Medical Education; and receives research supports from the Italian Ministry of Health, and AriSLA (Fondazione Italiana di Ricerca per la SLA). P.M. Ferraro, N. Riva, E.G. Spinelli, E. Canu, P. Valsasina, C. Lunetta, S. Iannaccone, M. Copetti, E. Prudente, and A. Falini report no disclosures. A. Chiò has served on scientific advisory boards for Biogen Idec, Cytokinetcs, and Italfarmaco; and has received research support from the Italian Ministry of Health, Italian Ministry of Education University and Research, European Communities, Regione Piemonte, Compagnia di San Paolo, Agenzia Italiana per la Ricerca sulla SLA (ARISLA), Fondazione Vialli e Mauro Onlus, and Federazione Italiana Giuoco Calcio (FIGC). G. Comi has received compensation for consulting services and/or speaking activities from Novartis, Teva Pharmaceutical Ind., Sanofi, Genzyme, Merck Serono, Biogen, Bayer, Actelion, Serono Symposia International Foundation, Almirall, Chugai and Receptos. M. Filippi is Editor‐in‐Chief of the Journal of Neurology; serves on scientific advisory boards for Teva Pharmaceutical Industries; has received compensation for consulting services and/or speaking activities from Bayer Schering Pharma, Biogen Idec, Merck Serono, Novartis, and Teva Pharmaceutical Industries; and receives research support from Bayer Schering Pharma, Biogen Idec, Merck Serono, Teva Pharmaceutical Industries, Novartis, Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, Cure PSP, Alzheimer's Drug Discovery Foundation (ADDF), the Jacques and Gloria Gossweiler Foundation (Switzerland), and AriSLA (Fondazione Italiana di Ricerca per la SLA).
REFERENCES
- Abrahams S, Leigh PN, Harvey A, Vythelingum GN, Grise D, Goldstein LH (2000): Verbal fluency and executive dysfunction in amyotrophic lateral sclerosis (ALS). Neuropsychologia 38:734–747. [DOI] [PubMed] [Google Scholar]
- Agosta F, Pagani E, Rocca MA, Caputo D, Perini M, Salvi F, Prelle A, Filippi M (2007): Voxel‐based morphometry study of brain volumetry and diffusivity in amyotrophic lateral sclerosis patients with mild disability. Hum Brain Mapp 28:1430–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agosta F, Valsasina P, Riva N, Copetti M, Messina MJ, Prelle A, Comi G, Filippi M (2012): The cortical signature of amyotrophic lateral sclerosis. PLoS One 7:e42816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agosta F, Galantucci S, Canu E, Cappa SF, Magnani G, Franceschi M, Falini A, Comi G, Filippi M (2013): Disruption of structural connectivity along the dorsal and ventral language pathways in patients with nonfluent and semantic variant primary progressive aphasia: A DT MRI study and a literature review. Brain Lang 127:157–166. [DOI] [PubMed] [Google Scholar]
- Agosta F, Galantucci S, Riva N, Chio A, Messina S, Iannaccone S, Calvo A, Silani V, Copetti M, Falini A, Comi G, Filippi M (2014): Intrahemispheric and interhemispheric structural network abnormalities in PLS and ALS. Hum Brain Mapp 35:1710–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberici A, Geroldi C, Cotelli M, Adorni A, Calabria M, Rossi G, Borroni B, Padovani A, Zanetti O, Kertesz A (2007): The Frontal Behavioural Inventory (Italian version) differentiates frontotemporal lobar degeneration variants from Alzheimer's disease. Neurol Sci 28:80–86. [DOI] [PubMed] [Google Scholar]
- Barbagallo G, Nicoletti G, Cherubini A, Trotta M, Tallarico T, Chiriaco C, Nistico R, Salvino D, Bono F, Valentino P, Quattrone A (2014): Diffusion tensor MRI changes in gray structures of the frontal‐subcortical circuits in amyotrophic lateral sclerosis. Neurol Sci 35:911–918. [DOI] [PubMed] [Google Scholar]
- Barcelo F, Sanz M, Molina V, Rubia FJ (1997): The Wisconsin Card Sorting Test and the assessment of frontal function: A validation study with event‐related potentials. Neuropsychologia 35:399–408. [DOI] [PubMed] [Google Scholar]
- Basso A, Capitani E, Laiacona M (1987): Raven's coloured progressive matrices: Normative values on 305 adult normal controls. Funct Neurol 2:189–194. [PubMed] [Google Scholar]
- Bede P, Bokde AL, Byrne S, Elamin M, McLaughlin RL, Kenna K, Fagan AJ, Pender N, Bradley DG, Hardiman O (2013): Multiparametric MRI study of ALS stratified for the C9orf72 genotype. Neurology 81:361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW (2007): Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage 34:144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiman L (2001): Random forests. Mach Learn 45:5–32. [Google Scholar]
- Brettschneider J, Del Tredici K, Toledo JB, Robinson JL, Irwin DJ, Grossman M, Suh E, Van Deerlin VM, Wood EM, Baek Y, Kwong L, Lee EB, Elman L, McCluskey L, Fang L, Feldengut S, Ludolph AC, Lee VM, Braak H, Trojanowski JQ (2013): Stages of pTDP‐43 pathology in amyotrophic lateral sclerosis. Ann Neurol 74:20–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks BR, Miller RG, Swash M, Munsat TL (2000): El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 1:293–299. [DOI] [PubMed] [Google Scholar]
- Canu E, Agosta F, Galantucci S, Chio A, Riva N, Silani V, Falini A, Comi G, Filippi M (2013): Extramotor damage is associated with cognition in primary lateral sclerosis patients. PLoS One 8:e82017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlesimo GA, Caltagirone C, Gainotti G (1996): The Mental Deterioration Battery: Normative data, diagnostic reliability and qualitative analyses of cognitive impairment. The Group for the Standardization of the Mental Deterioration Battery. Eur Neurol 36:378–384. [DOI] [PubMed] [Google Scholar]
- Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, Nakanishi A (1999): The ALSFRS‐R: A revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci 169:13–21. [DOI] [PubMed] [Google Scholar]
- Chang JL, Lomen‐Hoerth C, Murphy J, Henry RG, Kramer JH, Miller BL, Gorno‐Tempini ML (2005): A voxel‐based morphometry study of patterns of brain atrophy in ALS and ALS/FTLD. Neurology 65:75–80. [DOI] [PubMed] [Google Scholar]
- Chio A, Calvo A, Moglia C, Mazzini L, Mora G (2011): Phenotypic heterogeneity of amyotrophic lateral sclerosis: A population based study. J Neurol Neurosurg Psychiatry 82:740–746. [DOI] [PubMed] [Google Scholar]
- d'Ambrosio A, Gallo A, Trojsi F, Corbo D, Esposito F, Cirillo M, Monsurro MR, Tedeschi G (2014): Frontotemporal cortical thinning in amyotrophic lateral sclerosis. AJNR Am J Neuroradiol 35:304–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI (1999): Cortical surface‐based analysis. I. Segmentation and surface reconstruction. Neuroimage 9:179–194. [DOI] [PubMed] [Google Scholar]
- Della Sala S, MacPherson SE, Phillips LH, Spinnler H (2003): How many camels are there in Italy? Cognitive estimates standardised on the Italian population. Neurol Sci 24:10–15. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ (2006): An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31:968–980. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani RJ (1997): Improvements on cross‐validation: The 632+ bootstrap method. J Am Statist Assoc 92:548–560. [Google Scholar]
- Filippini N, Douaud G, Mackay CE, Knight S, Talbot K, Turner MR (2010): Corpus callosum involvement is a consistent feature of amyotrophic lateral sclerosis. Neurology 75:1645–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM (2000): Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 97:11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galantucci S, Tartaglia MC, Wilson SM, Henry ML, Filippi M, Agosta F, Dronkers NF, Henry RG, Ogar JM, Miller BL, Gorno‐Tempini ML (2011): White matter damage in primary progressive aphasias: A diffusion tensor tractography study. Brain 134:3011–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold BT, Powell DK, Xuan L, Jicha GA, Smith CD (2010): Age‐related slowing of task switching is associated with decreased integrity of frontoparietal white matter. Neurobiol Aging 31:512–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, Anderson C, Khan A, Avants B, Elman L, McCluskey L (2008): Impaired action knowledge in amyotrophic lateral sclerosis. Neurology 71:1396–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornberger M, Geng J, Hodges JR (2011): Convergent grey and white matter evidence of orbitofrontal cortex changes related to disinhibition in behavioural variant frontotemporal dementia. Brain 134:2502–2512. [DOI] [PubMed] [Google Scholar]
- Horsfield MA (1999): Mapping eddy current induced fields for the correction of diffusion‐weighted echo planar images. Magn Reson Imaging 17:1335–1345. [DOI] [PubMed] [Google Scholar]
- Hu WT, Shelnutt M, Wilson A, Yarab N, Kelly C, Grossman M, Libon DJ, Khan J, Lah JJ, Levey AI, Glass J (2013): Behavior matters—Cognitive predictors of survival in amyotrophic lateral sclerosis. PLoS One 8:e57584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper E, Schuster C, Machts J, Kaufmann J, Bittner D, Vielhaber S, Benecke R, Teipel S, Prudlo J (2014): Microstructural white matter changes underlying cognitive and behavioural impairment in ALS—An in vivo study using DTI. PLoS One 9:e114543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laiacona M, Inzaghi MG, De Tanti A, Capitani E (2000): Wisconsin card sorting test: A new global score, with Italian norms, and its relationship with the Weigl sorting test. Neurol Sci 21:279–291. [DOI] [PubMed] [Google Scholar]
- Libon DJ, McMillan C, Avants B, Boller A, Morgan B, Burkholder L, Chandrasekaran K, Elman L, McCluskey L, Grossman M (2012): Deficits in concept formation in amyotrophic lateral sclerosis. Neuropsychology 26:422–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillo P, Mioshi E, Burrell JR, Kiernan MC, Hodges JR, Hornberger M (2012): Grey and white matter changes across the amyotrophic lateral sclerosis‐frontotemporal dementia continuum. PLoS One 7:e43993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machts J, Loewe K, Kaufmann J, Jakubiczka S, Abdulla S, Petri S, Dengler R, Heinze HJ, Vielhaber S, Schoenfeld MA, Bede P (2015): Basal ganglia pathology in ALS is associated with neuropsychological deficits. Neurology 85:1301–1309. [DOI] [PubMed] [Google Scholar]
- Menke RA, Abraham I, Thiel CS, Filippini N, Knight S, Talbot K, Turner MR (2012): Fractional anisotropy in the posterior limb of the internal capsule and prognosis in amyotrophic lateral sclerosis. Arch Neurol 69:1493–1499. [DOI] [PubMed] [Google Scholar]
- Menke RA, Korner S, Filippini N, Douaud G, Knight S, Talbot K, Turner MR (2014): Widespread grey matter pathology dominates the longitudinal cerebral MRI and clinical landscape of amyotrophic lateral sclerosis. Brain 137:2546–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzapesa DM, D'Errico E, Tortelli R, Distaso E, Cortese R, Tursi M, Federico F, Zoccolella S, Logroscino G, Dicuonzo F, Simone IL (2013): Cortical thinning and clinical heterogeneity in amyotrophic lateral sclerosis. PLoS One 8:e80748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miceli G, Laudanna A, Burani C, Capasso R. 1994. Batteria per l'Analisi del Deficit Afasico. B.A.D.A. [B.A.D.A. A Battery for the Assessment of Aphasic Disorders]. Roma: CEPSAG. [Google Scholar]
- Monaco M, Costa A, Caltagirone C, Carlesimo GA (2012): Forward and backward span for verbal and visuo‐spatial data: Standardization and normative data from an Italian adult population. Neurol Sci 34:749–754. [DOI] [PubMed] [Google Scholar]
- Montuschi A, Iazzolino B, Calvo A, Moglia C, Lopiano L, Restagno G, Brunetti M, Ossola I, Lo Presti A, Cammarosano S, Canosa A, Chio A (2015): Cognitive correlates in amyotrophic lateral sclerosis: A population‐based study in Italy. J Neurol Neurosurg Psychiatry 86:168–173. [DOI] [PubMed] [Google Scholar]
- Novelli G, Papagno C, Capitani E, Laiacona N, Vallar G, Cappa SF (1986): Tre test clinici di ricerca e produzione lessicale. Taratura su soggetti normali. Arch Psicol Neurol Psichiatr 47:477–506. [Google Scholar]
- Orsini A, Grossi D, Capitani E, Laiacona M, Papagno C, Vallar G (1987): Verbal and spatial immediate memory span: Normative data from 1355 adults and 1112 children. Ital J Neurol Sci 8:539–548. [DOI] [PubMed] [Google Scholar]
- Pettit LD, Bastin ME, Smith C, Bak TH, Gillingwater TH, Abrahams S (2013): Executive deficits, not processing speed relates to abnormalities in distinct prefrontal tracts in amyotrophic lateral sclerosis. Brain 136:3290–3304. [DOI] [PubMed] [Google Scholar]
- Phukan J, Elamin M, Bede P, Jordan N, Gallagher L, Byrne S, Lynch C, Pender N, Hardiman O (2012): The syndrome of cognitive impairment in amyotrophic lateral sclerosis: A population‐based study. J Neurol Neurosurg Psychiatry 83:102–108. [DOI] [PubMed] [Google Scholar]
- Piquard A, Le Forestier N, Baudoin‐Madec V, Delgadillo D, Salachas F, Pradat PF, Derouesne C, Meininger V, Lacomblez L (2006): Neuropsychological changes in patients with primary lateral sclerosis. Amyotroph Lateral Scler 7:150–160. [DOI] [PubMed] [Google Scholar]
- Prell T, Grosskreutz J (2013): The involvement of the cerebellum in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener 14:507–515. [DOI] [PubMed] [Google Scholar]
- Raaphorst J, de Visser M, van Tol MJ, Linssen WH, van der Kooi AJ, de Haan RJ, van den Berg LH, Schmand B (2011): Cognitive dysfunction in lower motor neuron disease: Executive and memory deficits in progressive muscular atrophy. J Neurol Neurosurg Psychiatry 82:170–175. [DOI] [PubMed] [Google Scholar]
- Raaphorst J, Beeldman E, Schmand B, Berkhout J, Linssen WH, van den Berg LH, Pijnenburg YA, Grupstra HF, Weikamp JG, Schelhaas HJ, Papma JM, van Swieten JC, de Visser M, de Haan RJ (2012): The ALS‐FTD‐Q: A new screening tool for behavioral disturbances in ALS. Neurology 79:1377–1383. [DOI] [PubMed] [Google Scholar]
- Renton AE, Majounie E, Waite A, Simon‐Sanchez J, Rollinson S, Gibbs JR, Schymick JC, Laaksovirta H, van Swieten JC, Myllykangas L, Kalimo H, Paetau A, Abramzon Y, Remes AM, Kaganovich A, Scholz SW, Duckworth J, Ding J, Harmer DW, Hernandez DG, Johnson JO, Mok K, Ryten M, Trabzuni D, Guerreiro RJ, Orrell RW, Neal J, Murray A, Pearson J, Jansen IE, Sondervan D, Seelaar H, Blake D, Young K, Halliwell N, Callister JB, Toulson G, Richardson A, Gerhard A, Snowden J, Mann D, Neary D, Nalls MA, Peuralinna T, Jansson L, Isoviita VM, Kaivorinne AL, Holtta‐Vuori M, Ikonen E, Sulkava R, Benatar M, Wuu J, Chio A, Restagno G, Borghero G, Sabatelli M, Heckerman D, Rogaeva E, Zinman L, Rothstein JD, Sendtner M, Drepper C, Eichler EE, Alkan C, Abdullaev Z, Pack SD, Dutra A, Pak E, Hardy J, Singleton A, Williams NM, Heutink P, Pickering‐Brown S, Morris HR, Tienari PJ, Traynor BJ (2011): A Hexanucleotide Repeat Expansion in C9ORF72 Is the Cause of Chromosome 9p21‐Linked ALS‐FTD. Neuron 72:257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci M, Graef S, Blundo C, Miller LA (2012): Using the Rey Auditory Verbal Learning Test (RAVLT) to differentiate alzheimer's dementia and behavioural variant fronto‐temporal dementia. Clin Neuropsychol 26:926–941. [DOI] [PubMed] [Google Scholar]
- Sarro L, Agosta F, Canu E, Riva N, Prelle A, Copetti M, Riccitelli G, Comi G, Filippi M (2011): Cognitive functions and white matter tract damage in amyotrophic lateral sclerosis: A diffusion tensor tractography study. AJNR Am J Neuroradiol 32:1866–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster C, Kasper E, Dyrba M, Machts J, Bittner D, Kaufmann J, Mitchell AJ, Benecke R, Teipel S, Vielhaber S, Prudlo J (2014a): Cortical thinning and its relation to cognition in amyotrophic lateral sclerosis. Neurobiol Aging 35:240–246. [DOI] [PubMed] [Google Scholar]
- Schuster C, Kasper E, Machts J, Bittner D, Kaufmann J, Benecke R, Teipel S, Vielhaber S, Prudlo J (2014b): Longitudinal course of cortical thickness decline in amyotrophic lateral sclerosis. J Neurol 261:1871–1880. [DOI] [PubMed] [Google Scholar]
- Strong MJ, Grace GM, Freedman M, Lomen‐Hoerth C, Woolley S, Goldstein LH, Murphy J, Shoesmith C, Rosenfeld J, Leigh PN, Bruijn L, Ince P, Figlewicz D (2009): Consensus criteria for the diagnosis of frontotemporal cognitive and behavioural syndromes in amyotrophic lateral sclerosis. Amyotroph Lateral Scler 10:131–146. [DOI] [PubMed] [Google Scholar]
- Tan RH, Devenney E, Dobson‐Stone C, Kwok JB, Hodges JR, Kiernan MC, Halliday GM, Hornberger M (2014): Cerebellar integrity in the amyotrophic lateral sclerosis‐frontotemporal dementia continuum. PLoS One 9:e105632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorns J, Jansma H, Peschel T, Grosskreutz J, Mohammadi B, Dengler R, Munte TF (2013): Extent of cortical involvement in amyotrophic lateral sclerosis—An analysis based on cortical thickness. BMC Neurol 13:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner MR, Cagnin A, Turkheimer FE, Miller CC, Shaw CE, Brooks DJ, Leigh PN, Banati RB (2004): Evidence of widespread cerebral microglial activation in amyotrophic lateral sclerosis: An [11C](R)‐PK11195 positron emission tomography study. Neurobiol Dis 15:601–609. [DOI] [PubMed] [Google Scholar]
- Turner MR, Hardiman O, Benatar M, Brooks BR, Chio A, de Carvalho M, Ince PG, Lin C, Miller RG, Mitsumoto H, Nicholson G, Ravits J, Shaw PJ, Swash M, Talbot K, Traynor BJ, Van den Berg LH, Veldink JH, Vucic S, Kiernan MC (2013): Controversies and priorities in amyotrophic lateral sclerosis. Lancet Neurol 12:310–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Graaff MM, Sage CA, Caan MW, Akkerman EM, Lavini C, Majoie CB, Nederveen AJ, Zwinderman AH, Vos F, Brugman F, van den Berg LH, de Rijk MC, van Doorn PA, Van Hecke W, Peeters RR, Robberecht W, Sunaert S, de Visser M (2011): Upper and extra‐motoneuron involvement in early motoneuron disease: A diffusion tensor imaging study. Brain 134:1211–1228. [DOI] [PubMed] [Google Scholar]
- Verstraete E, Veldink JH, Hendrikse J, Schelhaas HJ, van den Heuvel MP, van den Berg LH (2011): Structural MRI reveals cortical thinning in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 83:383–388. [DOI] [PubMed] [Google Scholar]
- Von Der Heide RJ, Skipper LM, Klobusicky E, Olson IR (2013): Dissecting the uncinate fasciculus: Disorders, controversies and a hypothesis. Brain 136:1692–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhout R, Schmidt R, Westeneng HJ, Verstraete E, Seelen M, van Rheenen W, de Reus MA, van Es MA, Hendrikse J, Veldink JH, van den Heuvel MP, van den Berg LH (2015a): Brain morphologic changes in asymptomatic C9orf72 repeat expansion carriers. Neurology 85:1780–1788. [DOI] [PubMed] [Google Scholar]
- Walhout R, Westeneng HJ, Verstraete E, Hendrikse J, Veldink JH, van den Heuvel MP, van den Berg LH (2015b): Cortical thickness in ALS: Towards a marker for upper motor neuron involvement. J Neurol Neurosurg Psychiatry 86:288–294. [DOI] [PubMed] [Google Scholar]
- Weigl E (1927): On the psychology of so‐called processes of abstraction. Zeitschrift Für Psychologie 103:245–300. [Google Scholar]
- Wicks P, Abrahams S, Leigh PN, Williams T, Goldstein LH (2006): Absence of cognitive, behavioral, or emotional dysfunction in progressive muscular atrophy. Neurology 67:1718–1719. [DOI] [PubMed] [Google Scholar]
- Woolley SC, Zhang Y, Schuff N, Weiner MW, Katz JS (2011): Neuroanatomical correlates of apathy in ALS using 4 Tesla diffusion tensor MRI. Amyotroph Lateral Scler 12:52–58. [DOI] [PubMed] [Google Scholar]
- Zago S, Poletti B, Corbo M, Adobbati L, Silani V (2008): Dysgraphia in patients with primary lateral sclerosis: A speech‐based rehearsal deficit? Behav Neurol 19:169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Yin X, Zhao L, Evans AC, Song L, Xie B, Li H, Luo C, Wang J (2014): Regional alterations in cortical thickness and white matter integrity in amyotrophic lateral sclerosis. J Neurol 261:412–421. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Martinez JH, Young D, Chelminski I, Dalrymple K (2013): Severity classification on the Hamilton Depression Rating Scale. J Affect Disord 150:384–388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


