Abstract
In developed countries, obesity has become an epidemic resulting in enormous health care costs for society and serious medical complications for individuals. The homeostatic regulation of food intake is critically dependent on top‐down control of reward‐driven food craving. There is accumulating evidence from animal studies that the neuropeptide oxytocin (OXT) is involved in regulating hunger states and eating behavior, but whether OXT also contributes to cognitive control of food craving in humans is still unclear. We conducted a counter‐balanced, double‐blind, within‐subject, pharmacological magnetic resonance imaging experiment involving 31 healthy women who received 24 IU of intranasal OXT or placebo and were scanned twice while they were exposed to pictures of palatable food. The participants were instructed either to imagine the immediate consumption or to cognitively control the urge to eat the food. Our results show a trend that OXT specifically reduced food craving in the cognitive control condition. On the neural level, these findings were paralleled by an increase of activity in the middle and superior frontal gyrus, precuneus, and cingulate cortex under OXT. Interestingly, the behavioral OXT effect correlated with the OXT‐induced changes in the prefrontal cortex and precuneus. Collectively, the present study provides first evidence that OXT plays a key role in the cognitive regulation of food craving in women by strengthening activity in a broad neurocircuitry implicated in top‐down control and self‐referential processing. Hum Brain Mapp 37:4276–4285, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: craving, emotion regulation, food, functional magnetic resonance imaging, oxytocin, women
INTRODUCTION
Obesity and the associated serious medical complications belong to the largest public health and policy challenges in developed countries [Barness et al., 2007]. The worldwide prevalence of obesity has more than doubled in the last 35 years, with about 13% of the word's adult population fulfilling the criteria for obesity in 2014 [Webber et al., 2014]. Obesity‐related health problems are abundant, including diabetes mellitus type 2, stroke, cardiovascular disease, dyslipidemia, and increased risk for developing cancer [Flegal et al., 2012]. Current concepts of feeding regulation posit the existence of two parallel, but interacting, systems that modulate food intake. One system regulates food consumption to maintain energy balance at homeostatic levels (homeostatic feeding), while a reward‐based system mediates the consumption of palatable food independent of energy status (hedonic feeding) [Kenny, 2011; Volkow et al., 2013]. The ingestion of high‐calorie food as well as the observation of food cues induce activations in the hippocampus, insula, and in reward‐associated brain areas [Pelchat et al., 2004], whereas the ability to resist the temptation to eat past the point of satiety is primarily driven by prefrontal regions [Kenny, 2011]. In fact, reward‐related consumption can override homeostatic signals, resulting in food craving being associated with more snacking, less compliance with dietary restrictions, and a higher body mass index (BMI) [Delahanty et al., 2002]. Conditions with impaired regulatory capacities, such as binge eating disorder [Leehr et al., 2015], have been linked to an increased obesity risk. Modern treatment regimens for obesity combine diet, exercise, behavioral psychotherapy, and in severe cases, gastrointestinal surgery to reduce craving and resist food consumption [von Deneen and Liu, 2011]. Mindfulness‐based approaches and psychotherapy targeting the management of food craving have been effective in some studies, but there seems to be a large interindividual response variance [Potenza and Grilo, 2014]. The available pharmacological treatments for obesity are relatively ineffective and expansive, and have their drawbacks [Suplicy et al., 2014]. These problems highlight the need to better understand the psychological and neural determinants of craving to develop new, successful strategies for this growing epidemic [Goldman et al., 2013; Potenza and Grilo, 2014; Poulton et al., 2015].
The hypothalamic peptide oxytocin (OXT) not only plays a key role in attachment and bonding [Hurlemann and Scheele, 2015; Scheele et al., 2013] but is also critically involved in regulating hunger states [Atasoy et al., 2012]. Evidence from animal studies indicates that OXT suppresses food intake and especially the appetite for sugar, reduces visceral fat mass, and modulates energy expenditure and glucose homeostasis [Arletti et al., 1989; Maejima et al., 2011; Morton et al., 2012; Olson et al., 1991; Wu et al., 2012]. Hypothalamic OXT signaling is assumed to attenuate the effect of leptin, an adipokine that provides the brain with negative feedback on body fat stores and sensitizes caudal brainstem nuclei to satiety signals such as the release of cholecystokinin [Blevins et al., 2004]. Previous studies showed that mice with deficient OXT or OXT receptors develop late‐onset obesity [Camerino, 2009; Takayanagi et al., 2008]. Furthermore, OXT neuron loss‐of‐function is implicated in Prader–Willi syndrome, which is associated with excessive food craving and obesity [Swaab et al., 1995]. Surprisingly, however, the effects of intranasal OXT on ingestive behavior and the capacity to cognitively control food craving have largely been ignored in humans so far. Initial studies reported that the intranasal administration of OXT decreased body weight in obese patients [Zhang et al., 2013] and that OXT significantly decreased reward‐related snack consumption of chocolate cookies [Ott et al., 2013] and caloric intake in healthy men [Lawson et al., 2015].
Given this empirical background, oxytocinergic pathways seem a promising target for clinical interventions in obese patients, but it is still unknown whether OXT modulates food craving and if so which neural substrates are involved. In the light of demographic evidence indicating higher prevalence rates of eating disorders in women [Kessler et al., 2013], we tested the effects of intranasal OXT (24 IU) on behavioral and neural correlates of food craving in normal‐weight, healthy women. In accordance with the current literature, food craving was defined as a very strong desire to eat a specific food [Pelchat et al., 2004; Potenza and Grilo, 2014; Weingarten and Elston, 1990]. We used a counter‐balanced double‐blind, within‐subject, pharmaco‐functional magnetic resonance imaging (fMRI) approach in which we scanned 31 female volunteers twice while they were exposed to pictures of candy and dessert and indicated their craving for each of these food items on a visual analog scale. In half of the trials, participants were instructed to focus on the immediate consequences of consumption when rating their craving, while in the other half of the trials, participants were asked to consider the long‐term consequences. None of the subjects were not hungry during the experiment because hunger is not necessary for food craving [Small, 2002] and we wanted to exclude confounding effects of hunger [Pelchat et al., 2004]. In view of our previous finding that intranasal OXT can strengthen top‐down regulatory activity in the prefrontal cortex (PFC) [Eckstein et al., 2015], we hypothesized that OXT would augment the cognitive control of food craving, a behavioral effect paralleled by enhanced neural responses in PFC regions.
MATERIALS AND METHODS
Participants
Thirty‐one healthy adult females (mean age ± SD: 25.35 ± 4.37 years; BMI: 22.26 ± 3.03) participated in the study after giving written, informed consent. The study was approved by the institutional review board of the Medical Faculty of the University of Bonn and was carried out in compliance with the latest revision of the Declaration of Helsinki. Subjects received monetary compensation for study participation. Screening of the subjects was conducted prior to the test sessions (see Supporting Information Table S1). During the screening session, all subjects filled out a German version of the Restraint Scale [Dinkel et al., 2005] measuring the extent to which subjects display a restrictive eating style. Subjects were free of current and past physical or psychiatric illness, as assessed by medical history and the Mini‐International Neuropsychiatric Interview (MINI) [Sheehan et al., 1998]. In addition, they were naive to prescription‐strength psychoactive medication, and had not taken any over‐the‐counter psychoactive medication in the past four weeks. Fifteen women used hormonal contraception (HC). The other 16 women without HC were tested in the follicular or luteal phase of their menstrual cycle as validated by blood assays obtained on the days of fMRI scanning (see Supporting Information Table S2). Furthermore, all subjects reported that they regularly eat candies and dessert, and both their baseline hunger ratings and blood glucose levels were comparable between the OXT and PLC sessions (see Supporting Information Table S2). To determine endogenous OXT concentrations, saliva samples were collected before the nasal spray administration and after the experiment using commercial sampling devices (Salivettes, Sarstedt). All subjects were in a romantic heterosexual relationship. The imaging paradigm was part of a larger study with 40 subjects [Scheele et al., 2016], but food craving data were available for only 31 participants.
EXPERIMENTAL DESIGN
We applied a randomized, placebo‐controlled, double‐blind, within‐group design. Subjects were randomly assigned to intranasal administration of either OXT (24IU; Syntocinon‐Spray, Sigma Tau; three puffs per nostril, each with 4IU OXT) or PLC (containing all ingredients except the peptide) 30 min before the start of an unrelated, nonaversive, fMRI task (reported elsewhere). The fMRI craving task started 45 min after the nasal spray administration. All subjects were tested after 12 pm. to minimize possible effects of circadian rhythms on cue reactivity. The minimum interval between the two fMRI sessions was 2 days. Details on the tasks, fMRI procedure, and analyses can be found in the Supporting Information section.
FMRI Paradigm
We adopted a modified version of a task previously developed to examine the regulation of craving [Kober et al., 2010]. Participants viewed pictures of candy and dessert (cf. Supporting Information) in two types of trials. In “NOW” trials, participants were instructed to consider the immediate consequence of consuming the pictured food, while “LATER” trials directed participants to think about the long‐term consequences. The instructional cue (NOW or LATER) was shown for 2 s, followed by a 6‐s presentation of the picture cue. After the food stimulus, a fixation cross was depicted in the center of the screen during the low‐level baseline period jittered on average 3 s (min: 2 s, max: 4 s). Next, the participants indicated their craving for the displayed food using a visual analog scale (0 not at all, 100 very much) that appeared on screen for 5 s. The order of instructional cues was randomized and trials were separated from each other by another low‐level baseline period jittered on average 5 s (min: 4 s, max: 6 s), during which a fixation cross was depicted in the center of the screen. In total, there were 30 NOW trials (picture set A, cf. Supporting Information) and 30 LATER trials (picture set B). Using Presentation 14 (Neurobehavioral Systems, Albany, CA), stimuli were presented on a 32‐inch MRI compatible TFT LCD monitor (NordicNeuroLab, Bergen, Norway) placed at the rear of the magnet bore. Lying inside the MRI scanner, subjects viewed the monitor via a first‐surface reflection mirror mounted on the head coil.
Acquisition of fMRI Data
A Siemens Trio MRI system (Siemens, Erlangen, Germany) operating at 3T was used to obtain T2*‐weighted echoplanar (EPI) images with blood‐oxygen‐level‐dependent contrast (TR = 2,500 ms, TE = 30 ms, pixel size: 2 × 2 × 3 mm, slice thickness= 3.0 mm, distance factor = 10%, FoV = 192 mm, flip angle = 90°, 37 axial slices). In addition, high‐resolution anatomical images were acquired on the same scanner using a T1‐weighted 3D MPRAGE sequence (imaging parameters: TR = 1,660 ms, TE = 2.54 ms, matrix size: 256 × 256, pixel size: 0.8 × 0.8 × 0.8 mm, slice thickness = 0.8 mm, FoV = 256 mm, flip angle = 9°, 208 sagittal slices).
Analysis of fMRI Data
fMRI data were preprocessed and analyzed using SPM8 software (Wellcome Trust Centre for Neuroimaging, London, UK; http://www.fil.ion.ucl.ac.uk/spm) implemented in MATLAB 7 (The MathWorks Inc., Natick, MA). On the first level, four conditions (“LATEROXT,” “NOWOXT,” “LATERPLC,” “NOWPLC”) were modeled by a boxcar function convolved with a hemodynamic response function. The movement parameters were included as confounds in the design matrix. Main effects of “treatment” (OXT, PLC) and “regulation” (LATER, NOW) were analyzed by comparing the conditions (OXT vs. PLC and LATER vs. NOW) relative to the low‐level baseline. Parameter estimates for each contrast were subjected to one‐sample t‐tests on the second level for the whole‐brain with a significance threshold of P < 0.05 corrected for multiple comparisons (family‐wise error [FWE]). To specifically examine the modulatory effects of OXT, a flexible factorial model was designed with the factors “treatment” (OXT, PLC) and “regulation” (LATER, NOW) to test the contrasts [(LATEROXT > NOWOXT) > (LATERPLC > NOWPLC)] and [(NOWOXT > LATEROXT) > (NOWPLC > LATERPLC)]. Based on our previous finding of an OXT effect in the middle frontal gyrus (Eckstein et al., 2015] and our current hypothesis, the Wake Forest University (WFU) Pickatlas (Version 3.0) was used to generate an anatomical region of interest (ROI) mask for the middle frontal gyrus. For the ROI analysis, the threshold for significance was set at P < 0.05, FWE‐corrected for multiple comparisons based on the size of the ROI.
RESULTS
Behavioral Ratings
A repeated measures analysis of variance (ANOVA) with the within‐subject factors “regulation” (NOW, LATER) and “treatment” (OXT, PLC) revealed a main effect of regulation (F (1, 30) = 24.34, P < 0.01, η2 = 0.45), but no further main or interaction effects (all Ps > 0.14). Food craving was significantly lower on LATER compared to NOW trials (PLC: 22.78% decrease, OXT: 25.76% decrease). Importantly, an exploratory analysis with paired t‐tests revealed a trend that OXT further reduced craving ratings on LATER trials (t (30) = −1.71, P = 0.098, d = −0.21, cf. Fig. 1a), but not in the NOW condition (t (30) = −1.15, P = 0.26, d = −0.15). An additional correlational analysis revealed that participants who scored higher on a questionnaire measuring a restrictive eating style also displayed lower craving ratings in NOW trials under PLC (r = −0.37, P = 0.04, cf. Fig. 1b), but not in other conditions (all Ps > 0.13). Furthermore, baseline blood glucose levels as well as serum levels of estradiol, progesterone, follicle‐stimulating, and luteinizing hormones (cf. Supporting Information Table S2) were comparable between the OXT and PLC sessions and OXT had no effect on mood and anxiety ratings (cf. Supporting Information Table S3). In the PLC session, OXT concentrations did not significantly change in the course of the experiment and there were no significant associations between OXT levels, BMI, or food craving ratings (cf. Supporting Information).
Figure 1.
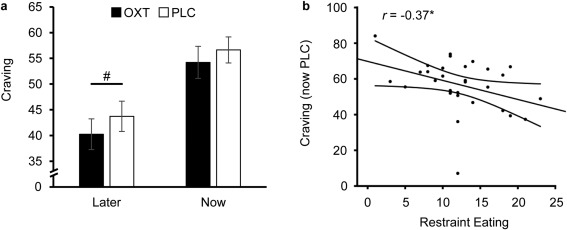
Oxytocin (OXT) effects on food craving ratings. Reported food craving was significantly reduced if subjects were instructed to consider the long‐term consequences of repeatedly consuming candy and desserts (LATER trials) compared to NOW trials in which the participants were instructed to consider the immediate consequences of consuming the food. The intranasal administration of OXT showed a trend to decreased craving in LATER trials (a), indicating an improved cognitive regulation of craving. Furthermore, subjects who scored higher on a questionnaire measuring a restrictive eating style displayed lower food craving ratings under PLC in the NOW trials (b). Error bars indicate the standard error of the mean (SEM). The curves next to the regression lines indicate 95% confidence intervals. Abbreviations: OXT, oxytocin; PLC, placebo; # P < 0.10.
Imaging Results
In the PLC session, we replicated the previous finding that cognitive down‐regulation of craving increases activity in prefrontal control systems involving the dorsolateral and dorsomedial PFC and inferior frontal gyrus (cf. Supporting Information Table S4). Cognitive regulation (i.e., LATER compared to NOW trials) also produced a decrease in activations in a broad reward‐associated neurocircuit including the caudate nucleus, putamen, and midbrain regions (cf. Supporting Information Table S5). For LATER trials, OXT enhanced neural responses in several large clusters entailing the precuneus (peak MNI x, y, z: 32, −16, 30, t (30) = 5.20, peak MNI x, y, z: 34, −34, 14, t (30) = 4.31, and peak MNI x, y, z: 14, −38, 4, t (30) = 4.30, k = 2,954, P FWE < 0.01, cf. Fig. 2), cingulate cortex and precentral gyrus (peak MNI x, y, z: −14, −30, 34, t (30) = 5.01, peak MNI x, y, z: −38, −6, 30, t (30) = 4.81, k = 2,517, P FWE < 0.01; peak MNI x, y, z: 12, −38, 38, t (30) = 4.41, peak MNI x, y, z: −6, −38, 28, t (30) = 3.91, k = 360, P FWE = 0.02), and superior temporal gyrus (peak MNI x, y, z: 50, −10, 2, t (30) = 4.57, peak MNI x, y, z: 62, −12, 8, t (30) = 3,75, and peak MNI x, y, z: 56, −18, 6, t (30) = 3.26, k = 296, P FWE = 0.046). Importantly, a ROI‐based analysis revealed that OXT also elicited stronger activations in the middle frontal gyrus (peak MNI x, y, z: 24, 34, 44, t (30) = 3.96, peak MNI x, y, z: 30, 50, −2, t (30) = 3.74, and peak MNI x, y, z: 34, −2, 64, t (30) = 3.59, k = 2,191, P FWE < 0.01), very close to the coordinates where OXT has been previously found to augment responses in a fear extinction paradigm [Eckstein et al., 2015]. Interestingly, OXT was also accompanied by increased activations in NOW trials in a cluster ranging from the hippocampus to the precuneus (peak MNI x, y, z: 12, −38, 8, t (30) = 4.96, peak MNI x, y, z: 8, −32, 16, t (30) = 4.79, and peak MNI x, y, z: 20, −44, 12, t (30) = 4.33, k = 521, P FWE < 0.01). Moreover, OXT did not diminish neural activations for either LATER or NOW trials. To determine whether OXT modulated regulation‐specific responses, we computed the contrast [(LATEROXT > NOWOXT) > (LATERPLC > NOWPLC)]. For this specific contrast, OXT enhanced activations in a cluster spanning from the precuneus to the cingulate gyrus (peak MNI x, y, z: −2, −62, 46, t (90) = 3.86, peak MNI x, y, z: −10, −54, 54, t (90) = 3.73, and peak MNI x, y, z: 20, −52, 26, t (30) = 3.52, k = 565, P FWE < 0.01, cf. Fig. 3a). There was no significant OXT effect for the reversed contrast [(NOWOXT > LATEROXT) > (NOWPLC > LATERPLC)]. To further elucidate possible brain‐behavior‐associations, we also calculated a differential behavioral score [(LATEROXT > NOWOXT) > (LATERPLC > NOWPLC)] and submitted it as a covariate in the second‐level fMRI analysis. A stronger behavioral OXT effect correlated with a stronger recruitment of the precuneus and inferior parietal lobule (peak MNI x, y, z: 22, −52, 16, t (29) = 4.07, peak MNI x, y, z: 38, −66, 36, t (29) = 3.86, and peak MNI x, y, z: 48, −44, 50, t (29) = 3.83, k = 436, P FWE < 0.01, cf. Fig. 3b) as well as the superior frontal gyrus (peak MNI x, y, z: −16, 34, 52, t (29) = 4.98, peak MNI x, y, z: −8, 30, 52, t (29) = 4.16, and peak MNI x, y, z: 2, 28, 52, t (29) = 3.98, k = 361, P FWE < 0.01). Separate analyses for women using or not using HC yielded a comparable pattern of results in both groups.
Figure 2.
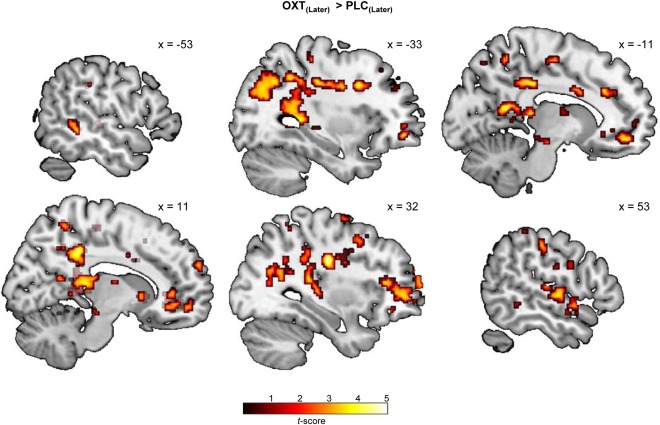
Oxytocin (OXT) effects on neural responses in LATER trials. Intranasal OXT enhanced neural activations in a broad neurocircuitry including the precuneus, cingulate gyrus, and superior temporal gyrus in LATER trials in which participants were instructed to consider the long‐term consequences of repeatedly consuming candy and desserts (for visualization purposes an uncorrected threshold of P < 0.005 was chosen). Abbreviations: OXT, oxytocin; PLC, placebo.
Figure 3.
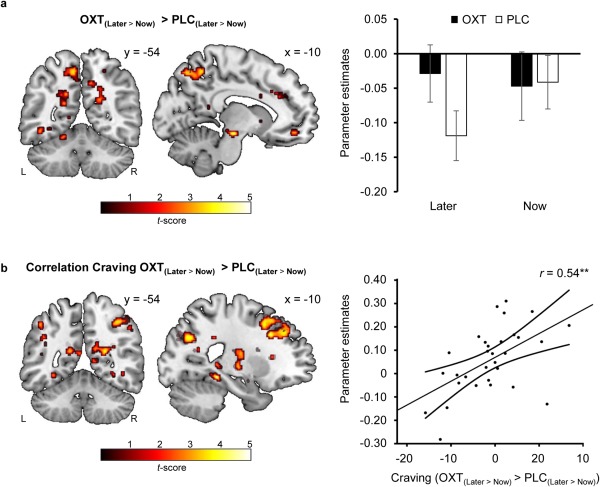
Oxytocin (OXT) effects on neural responses in LATER vs. NOW trials. Intranasal OXT increased neural activations in the precuneus in LATER relative to NOW trials (i.e., when participants used a cognitive strategy to reduce their craving) (a). Intriguingly, the differential behavioral score [(LATEROXT > NOWOXT) > (LATERPLC > NOWPLC)] positively correlated with enhanced activations in the precuneus and superior frontal gyrus (b). Thus, an OXT‐induced strengthening of cognitive craving regulation seems to depend on the recruitment of these areas. Error bars indicate the SEM. The curves next to the regression lines indicate 95% confidence intervals. For visualization purposes an uncorrected threshold of P < 0.005 was chosen. Abbreviations: L, left hemisphere; OXT, oxytocin; PLC, placebo; R, right hemisphere.
DISCUSSION
The rationale of the present study was to examine the modulatory effect of OXT on the cognitive control of food craving in normal‐weight, healthy women. As hypothesized, we found a trend that OXT reduced food craving when participants thought about the long‐term consequences associated with eating high‐calorie food. On the neural level, these findings were paralleled by an OXT‐induced increase of activity in the middle and superior frontal gyrus, precuneus, and cingulate cortex during the cognitive control condition. Intriguingly, a stronger behavioral OXT effect correlated with a stronger recruitment of the precuneus, the inferior parietal lobe, and the superior frontal gyrus. In accordance with previous findings, the cognitive strategy to regulate food craving by focusing on the long‐term consequences of consuming palatable foods elicited activation of prefrontal regions and concomitantly caused a down‐regulation of reward‐associated neurocircuitry including the caudate nucleus, putamen, and midbrain regions [Hollmann et al., 2012; Kober et al., 2010; Pelchat et al., 2004]. The negative correlations between a restrictive eating style and food craving ratings under PLC support the ecological validity of the paradigm.
Importantly, the observed pattern of results indicate that intranasal OXT strengthens the regulation of food craving by enhancing both inhibitory control mediated by prefrontal regions and a self‐referential processing bias driven by activation in the precuneus and cingulate cortex. In the frontal cortex, OXT receptors are expressed in a high density [Boccia et al., 2013; Gould and Zingg, 2003] and an OXT‐mediated augmentation of prefrontal top‐down control has been previously documented in the context of fear extinction [Eckstein et al., 2015]. Furthermore, in patients with alcohol use disorder, enhanced prefrontal activation after neuro‐feedback‐training was associated with reduced craving [Karch et al., 2015]. Interestingly, excitatory stimulation of the PFC with transcranial direct current stimulation [Kekic et al., 2014] and transcranial magnetic stimulation [Uher et al., 2005] also reduced food craving and improved the self‐reported ability to resist food in healthy women. Interoceptive awareness and self‐reflection have been previously identified as key processes during active regulation of desire for food [Hollmann et al., 2012], and the notion that OXT induces a self‐referential processing bias is consistent with previous observations of context‐dependent OXT effects [Hurlemann and Scheele, 2015; Scheele et al., 2014a]. When the participants were instructed to think about the long‐term consequences of unhealthy food, OXT may have enhanced the perception and awareness of their own negative feelings which in turn contributed to diminished food craving. Notably, OXT effects on activations in the cingulate cortex, precuneus, and superior temporal gyrus resonate well with previous studies showing that the regional cerebral blood flow in the anterior cingulate cortex inversely correlated with the desirability of chocolate [Small et al., 2001] and that cingulate cortex activation is associated with cue‐induced cocaine craving [Childress et al., 1999; Garavan et al., 2000; Maas et al., 1998]. Furthermore, Brody et al. [2007] reported that resisting craving during cigarette cue exposure involves activation of the cingulum and precuneus in cigarette smokers. Likewise, Hartwell and colleagues [2011] found increased activation in the superior temporal pole when dependent smokers resisted nicotine craving.
At first glance, the observation of increased neural responses in the precuneus and hippocampus in NOW trials under OXT seems at odds with the finding that OXT inhibits reward‐driven eating in healthy men [Ott et al., 2013]. Mechanistically, the anorexigenic effect of OXT on food consumption may be mediated by its modulatory impact on leptin, which provides the brain with negative feedback on body fat stores and sensitizes caudal brainstem nuclei to satiety signals such as the release of cholecystokinin [Blevins et al., 2004]. However, we did not measure leptin levels and previous studies detected only weak or nonsignificant effects of OXT on leptin levels in humans [Lawson et al., 2015, Ott et al., 2013]. Importantly, we examined the modulatory effect of OXT on food cravings, which may differ from food consumption. Moreover, the lack of a significant OXT effect on food craving ratings in NOW trials together with its effect for the specific contrast “LATER > NOW” speaks against a mere reduction of hunger feelings. Instead, the neural profile suggests that OXT influenced self‐control and the cognitive evaluation of food stimuli. From an evolutionary perspective, the anorectic properties of OXT may be important during states of elevated endogenous OXT concentrations (e.g., lactation) to ensure that the mother nurses the offspring despite higher energy demands [Sabatier et al., 2013]. In contrast to rodents, hyperosmolarity does not stimulate OXT secretion in humans [Williams et al., 1986] and therefore the OXT‐mediated suppression of food craving may be particularly driven by cognitive control. In the present study, we found significant effects of OXT on activity in the superior temporal gyrus, precentral gyrus, and inferior parietal lobule, which constitute core regions of the mirror neuron system [Caspers et al., 2010; Iacoboni and Dapretto, 2006]. Evidence for the impact of OXT on mirror neuron activity comes from studies showing that OXT has a general suppressive effect on lower alpha/mu and on beta electroencephalography rhythms during perception of biological motion [Perry et al., 2010] and that OXT leads to a decrease of control over automatic imitative behavior [De Coster et al., 2014]. While the tendency to mimic the behavior of others has been linked to food consumption [Cohen, 2008], it is unclear why the modulatory effect of OXT on mirror neuron activity should be restricted to the cognitive control condition (LATER trials) in the present study.
Given the substantial commonalities between the neural underpinnings of obesity and drug addiction [Volkow et al., 2013], these data provide further supporting evidence for the concept of OXT as a pharmacological augmentation of psychotherapy in patients with eating disorders or addiction. Interestingly, effects of intranasal OXT in previous studies were particularly pronounced in social conditions. For instance, intranasal OXT selectively facilitated the attribution of animacy to social stimuli in women [Scheele et al., 2015] and enhanced adaptation in social contexts in men as evidenced by neural interactions of OXT treatment and social content during fear conditioning [Eckstein et al., 2016]. As such, one could speculate that the OXT effects on cognitive regulation strategies would be even stronger in psychotherapy sessions with intimate social interactions.
The present study has some limitations. We have included only healthy female volunteers since women are more likely to experience food craving than men [Weingarten and Elston, 1991] and because they have an elevated lifetime risk of eating disorders [Kessler et al., 2013]. However, there is accumulating evidence that OXT can have sexual‐dimorphic effects in some social domains [Preckel et al., 2014; Scheele et al., 2012, 2014b] and thus we cannot extrapolate our findings to men. Furthermore, food craving in the LATER condition was reduced after OXT treatment, but the OXT effect was only marginally significant. The moderate effect sizes may result from our relatively low baseline food craving scores and it is conceivable that the effects of OXT are more pronounced during acute hunger or when craving is experimentally induced. In our sample of normal‐weight women, we did not detect an association between endogenous OXT concentrations and BMI, but there are reports of lower than normal OXT levels in obese individuals [Qian et al., 2014]. Along these lines, future studies are warranted to translate our findings to clinical contexts and to elucidate the OXT effect in combination with cognitive psychotherapy of obesity targeting the management of food craving. While intranasal OXT ameliorates cravings in marijuana‐dependent individuals [McRae‐Clark et al., 2013] and blocks withdrawal in alcohol‐dependent patients [Pedersen et al., 2013], two recent studies also report divergent effects of intranasal OXT in opioid‐dependent individuals [Woolley et al., 2016] and subjects with alcohol use disorder [Mitchell et al., 2016], with OXT reducing only cue‐induced alcohol cravings. Thus, the findings of the present study should be cautiously interpreted and may not extend to severe craving in all addiction disorders.
Taken together, the present study provides first evidence that OXT may play a key role in the cognitive regulation of food craving in women by strengthening activity in a broad neurocircuitry implicated in top‐down control and self‐referential processing. Against this background, intranasal OXT could be a viable option as an adjunct therapy in patients with binge eating disorder or metabolic syndrome and for relapse preventions in patients with addictive disorders.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The authors wish to thank Jesscia Plota for help with the data acquisition, Paul Jung for programming assistance, and Alexandra Patin for proofreading the manuscript. Financial Disclosures: The authors report no competing biomedical financial interests or personal affiliations in connection with the content of this manuscript. Author Contributions: R.H. and D.S. designed the experiments; F.S. and D.S. conducted the experiments; N.S., F.S., R.H. and D.S. analyzed the data; N.S., F.S., B.S., W.M., R.H. and D.S. wrote the paper.
REFERENCES
- Arletti R, Benelli A, Bertolini A (1989): Influence of oxytocin on feeding behavior in the rat. Peptides 10:89–93. [DOI] [PubMed] [Google Scholar]
- Atasoy D, Betley JN, Su HH, Sternson SM (2012): Deconstruction of a neural circuit for hunger. Nature 488:172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barness LA, Opitz JM, Gilbert‐Barness E (2007): Obesity: Genetic, molecular, and environmental aspects. Am J Med Genet A 143A:3016–3034. [DOI] [PubMed] [Google Scholar]
- Blevins JE, Schwartz MW, Baskin DG (2004): Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size. Am J Physiol Regul Integr Comp Physiol 287:R87–R96. [DOI] [PubMed] [Google Scholar]
- Boccia ML, Petrusz P, Suzuki K, Marson L, Pedersen CA (2013): Immunohistochemical localization of oxytocin receptors in human brain. Neuroscience 253:155–164. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Jou J, Tiongson E, Allen V, Scheibal D, London ED, Monterosso JR, Tiffany ST, Korb A, Gan JJ, Cohen MS (2007): Neural substrates of resisting craving during cigarette cue exposure. Biol Psychiatry 62:642–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camerino C (2009): Low sympathetic tone and obese phenotype in oxytocin‐deficient mice. Obesity (Silver Spring) 17:980–984. [DOI] [PubMed] [Google Scholar]
- Caspers S, Zilles K, Laird AR, Eickhoff SB (2010): ALE meta‐analysis of action observation and imitation in the human brain. Neuroimage 50:1148–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP (1999): Limbic activation during cue‐induced cocaine craving. Am J Psychiatry 156:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen DA (2008): Neurophysiological pathways to obesity: Below awareness and beyond individual control. Diabetes 57:1768–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Coster L, Mueller SC, T'Sjoen G, De Saedeleer L, Brass M (2014): The influence of oxytocin on automatic motor simulation. Psychoneuroendocrinology 50:220–226. [DOI] [PubMed] [Google Scholar]
- Delahanty LM, Meigs JB, Hayden D, Williamson DA, Nathan DM (2002): Psychological and behavioral correlates of baseline BMI in the diabetes prevention program (DPP). Diabetes Care 25:1992–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkel A, Berth H, Exner C, Rief W, Balck F (2005): German version of the restraint scale for the assessment of restrained eating. Diagnostica 51:67–74. [Google Scholar]
- Eckstein M, Becker B, Scheele D, Scholz C, Preckel K, Schlaepfer TE, Grinevich V, Kendrick KM, Maier W, Hurlemann R (2015): Oxytocin facilitates the extinction of conditioned fear in humans. Biol Psychiatry 78:194–202. [DOI] [PubMed] [Google Scholar]
- Eckstein M, Scheele D, Patin A, Preckel K, Becker B, Walther A, Domschke K, Grinevich V, Maier W, Hurlemann R (2016): Oxytocin facilitates Pavlovian fear learning in males. Neuropsychopharmacology 41:932–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Kit BK, Ogden CL (2012): Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999‐2010. JAMA 307:491–497. [DOI] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA (2000): Cue‐induced cocaine craving: Neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry 157:1789–1798. [DOI] [PubMed] [Google Scholar]
- Goldman RL, Canterberry M, Borckardt JJ, Madan A, Byrne TK, George MS, O'Neil PM, Hanlon CA (2013): Executive control circuitry differentiates degree of success in weight loss following gastric‐bypass surgery. Obesity (Silver Spring) 21:2189–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould BR, Zingg HH (2003): Mapping oxytocin receptor gene expression in the mouse brain and mammary gland using an oxytocin receptor‐LacZ reporter mouse. Neuroscience 122:155–167. [DOI] [PubMed] [Google Scholar]
- Hartwell KJ, Johnson KA, Li X, Myrick H, LeMatty T, George MS, Brady KT (2011): Neural correlates of craving and resisting craving for tobacco in nicotine dependent smokers. Addict Biol 16:654–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann M, Hellrung L, Pleger B, Schlogl H, Kabisch S, Stumvoll M, Villringer A, Horstmann A (2012): Neural correlates of the volitional regulation of the desire for food. Int J Obes (Lond) 36:648–655. [DOI] [PubMed] [Google Scholar]
- Hurlemann R, Scheele D (2015): Dissecting the role of oxytocin in the formation and loss of social relationships. Biol Psychiatry 79:185–193. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Dapretto M (2006): The mirror neuron system and the consequences of its dysfunction. Nat Rev Neurosci 7:942–951. [DOI] [PubMed] [Google Scholar]
- Karch S, Keeser D, Hummer S, Paolini M, Kirsch V, Karali T, Kupka M, Rauchmann BS, Chrobok A, Blautzik J, and others (2015): Modulation of craving related brain responses using real‐time fMRI in patients with alcohol use disorder. PLoS One 10:e0133034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kekic M, McClelland J, Campbell I, Nestler S, Rubia K, David AS, Schmidt U (2014): The effects of prefrontal cortex transcranial direct current stimulation (tDCS) on food craving and temporal discounting in women with frequent food cravings. Appetite 78:55–62. [DOI] [PubMed] [Google Scholar]
- Kenny PJ (2011): Reward mechanisms in obesity: New insights and future directions. Neuron 69:664–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund PA, Chiu WT, Deitz AC, Hudson JI, Shahly V, Aguilar‐Gaxiola S, Alonso J, Angermeyer MC, Benjet C, Bruffaerts R, de Girolamo G, de Graaf R, Maria Haro J, Kovess‐Masfety V, O'Neill S, Posada‐Villa J, Sasu C, Scott K, Viana MC, Xavier M (2013): The prevalence and correlates of binge eating disorder in the World Health Organization World Mental Health Surveys. Biol Psychiatry 73:904–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Mende‐Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, Ochsner KN (2010): Prefrontal‐striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci USA 107:14811–14816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson EA, Marengi DA, DeSanti RL, Holmes TM, Schoenfeld DA, Tolley CJ (2015): Oxytocin reduces caloric intake in men. Obesity (Silver Spring) 23:950–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leehr EJ, Krohmer K, Schag K, Dresler T, Zipfel S, Giel KE (2015): Emotion regulation model in binge eating disorder and obesity–a systematic review. Neurosci Biobehav Rev 49:125–134. [DOI] [PubMed] [Google Scholar]
- Maas LC, Lukas SE, Kaufman MJ, Weiss RD, Daniels SL, Rogers VW, Kukes TJ, Renshaw PF (1998): Functional magnetic resonance imaging of human brain activation during cue‐induced cocaine craving. Am J Psychiatry 155:124–126. [DOI] [PubMed] [Google Scholar]
- Maejima Y, Iwasaki Y, Yamahara Y, Kodaira M, Sedbazar U, Yada T (2011): Peripheral oxytocin treatment ameliorates obesity by reducing food intake and visceral fat mass. Aging (Albany NY) 3:1169–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae‐Clark AL, Baker NL, Maria MM, Brady KT (2013): Effect of oxytocin on craving and stress response in marijuana‐dependent individuals: A pilot study. Psychopharmacology (Berl) 228:623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JM, Arcuni PA, Weinstein D, Woolley JD (2016): Intranasal oxytocin selectively modulates social perception, craving, and approach behavior in subjects with alcohol use disorder. J Addict Med 10:182–189. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Thatcher BS, Reidelberger RD, Ogimoto K, Wolden‐Hanson T, Baskin DG, Schwartz MW, Blevins JE (2012): Peripheral oxytocin suppresses food intake and causes weight loss in diet‐induced obese rats. Am J Physiol Endocrinol Metab 302:E134–E44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson BR, Drutarosky MD, Chow MS, Hruby VJ, Stricker EM, Verbalis JG (1991): Oxytocin and an oxytocin agonist administered centrally decrease food intake in rats. Peptides 12:113–118. [DOI] [PubMed] [Google Scholar]
- Ott V, Finlayson G, Lehnert H, Heitmann B, Heinrichs M, Born J, Hallschmid M (2013): Oxytocin reduces reward‐driven food intake in humans. Diabetes 62:3418–3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CA, Smedley KL, Leserman J, Jarskog LF, Rau SW, Kampov‐Polevoi A, Casey RL, Fender T, Garbutt JC (2013): Intranasal oxytocin blocks alcohol withdrawal in human subjects. Alcohol Clin Exp Res 37:484–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelchat ML, Johnson A, Chan R, Valdez J, Ragland JD (2004): Images of desire: Food‐craving activation during fMRI. Neuroimage 23:1486–1493. [DOI] [PubMed] [Google Scholar]
- Perry A, Bentin S, Shalev I, Israel S, Uzefovsky F, Bar‐On D, Ebstein RP (2010): Intranasal oxytocin modulates EEG mu/alpha and beta rhythms during perception of biological motion. Psychoneuroendocrinology 35:1446–1453. [DOI] [PubMed] [Google Scholar]
- Potenza MN, Grilo CM (2014): How relevant is food craving to obesity and its treatment? Front Psychiatry 5:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulton AS, Hibbert EJ, Champion BL, Cook TL, Alais D, Coulshed DS (2015): Piloting a new approach to the treatment of obesity using dexamphetamine. Front Endocrinol (Lausanne) 6:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preckel K, Scheele D, Kendrick KM, Maier W, Hurlemann R (2014): Oxytocin facilitates social approach behavior in women. Front Behav Neurosci 8:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian W, Zhu T, Tang B, Yu S, Hu H, Sun W, Pan R, Wang J, Wang D, Yang L, Mao C, Zhou L, Yuan G (2014): Decreased circulating levels of oxytocin in obesity and newly diagnosed type 2 diabetic patients. J Clin Endocrinol Metab 99:4683–4689. [DOI] [PubMed] [Google Scholar]
- Sabatier N, Leng G, Menzies J (2013): Oxytocin, feeding, and satiety. Front Endocrinol (Lausanne) 4:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele D, Striepens N, Güntürkün O, Deutschlander S, Maier W, Kendrick KM, Hurlemann R (2012): Oxytocin modulates social distance between males and females. J Neurosci 32:16074–16079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele D, Wille A, Kendrick KM, Stoffel‐Wagner B, Becker B, Güntürkün O, Maier W, Hurlemann R (2013): Oxytocin enhances brain reward system responses in men viewing the face of their female partner. Proc Natl Acad Sci USA 110:20308–20313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele D, Kendrick KM, Khouri C, Kretzer E, Schlapfer TE, Stoffel‐Wagner B, Gunturkun O, Maier W, Hurlemann R (2014a): An oxytocin‐induced facilitation of neural and emotional responses to social touch correlates inversely with autism traits. Neuropsychopharmacology 39:2078–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele D, Striepens N, Kendrick KM, Schwering C, Noelle J, Wille A, Schlapfer TE, Maier W, Hurlemann R (2014b): Opposing effects of oxytocin on moral judgment in males and females. Hum Brain Mapp 35:6067–6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele D, Schwering C, Elison JT, Spunt R, Maier W, Hurlemann R (2015): A human tendency to anthropomorphize is enhanced by oxytocin. Eur Neuropsychopharmacol 25:1817–1823. [DOI] [PubMed] [Google Scholar]
- Scheele D, Plota J, Stoffel‐Wagner B, Maier W, Hurlemann R (2016): Hormonal contraceptives suppress oxytocin‐induced brain reward responses to the partner's face. Soc Cogn Affect Neurosci 11:767–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC (1998): The Mini‐International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. J Clin Psychiatry 59 Suppl 20:22–33. [PubMed] [Google Scholar]
- Small DM (2002): Toward an understanding of the brain substrates of reward in humans. Neuron 33:668–671. [DOI] [PubMed] [Google Scholar]
- Small DM, Zatorre RJ, Dagher A, Evans AC, Jones‐Gotman M (2001): Changes in brain activity related to eating chocolate: From pleasure to aversion. Brain 124:1720–1733. [DOI] [PubMed] [Google Scholar]
- Suplicy H, Boguszewski CL, dos Santos CM, do Desterro de Figueiredo M, Cunha DR, Radominski R (2014): A comparative study of five centrally acting drugs on the pharmacological treatment of obesity. Int J Obes (Lond) 38:1097–1103. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Purba JS, Hofman MA (1995): Alterations in the hypothalamic paraventricular nucleus and its oxytocin neurons (putative satiety cells) in Prader‐Willi syndrome: A study of five cases. J Clin Endocrinol Metab 80:573–579. [DOI] [PubMed] [Google Scholar]
- Takayanagi Y, Kasahara Y, Onaka T, Takahashi N, Kawada T, Nishimori K (2008): Oxytocin receptor‐deficient mice developed late‐onset obesity. Neuroreport 19:951–955. [DOI] [PubMed] [Google Scholar]
- Uher R, Yoganathan D, Mogg A, Eranti SV, Treasure J, Campbell IC, McLoughlin DM, Schmidt U (2005): Effect of left prefrontal repetitive transcranial magnetic stimulation on food craving. Biol Psychiatry 58:840–842. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Tomasi D, Baler RD (2013): The addictive dimensionality of obesity. Biol Psychiatry 73:811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Deneen KM, Liu Y (2011): Obesity as an addiction: Why do the obese eat more? Maturitas 68:342–345. [DOI] [PubMed] [Google Scholar]
- Webber L, Divajeva D, Marsh T, McPherson K, Brown M, Galea G, Breda J (2014): The future burden of obesity‐related diseases in the 53 WHO European‐Region countries and the impact of effective interventions: A modelling study. BMJ Open 4:e004787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten HP, Elston D (1990): The phenomenology of food cravings. Appetite 15:231–246. [DOI] [PubMed] [Google Scholar]
- Weingarten HP, Elston D (1991): Food cravings in a college population. Appetite 17:167–175. [DOI] [PubMed] [Google Scholar]
- Williams TD, Abel DC, King CM, Jelley RY, Lightman SL (1986): Vasopressin and oxytocin responses to acute and chronic osmotic stimuli in man. J Endocrinol 108:163–168. [DOI] [PubMed] [Google Scholar]
- Woolley JD, Arcuni PA, Stauffer CS, Fulford D, Carson DS, Batki S, Vinogradov S (2016): The effects of intranasal oxytocin in opioid‐dependent individuals and healthy control subjects: A pilot study. Psychopharmacology (Berl). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Xu Y, Zhu Y, Sutton AK, Zhao R, Lowell BB, Olson DP, Tong Q (2012): An obligate role of oxytocin neurons in diet induced energy expenditure. PLoS One 7:e45167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Wu C, Chen Q, Chen X, Xu Z, Wu J, Cai D (2013): Treatment of obesity and diabetes using oxytocin or analogs in patients and mouse models. PLoS One 8:e61477. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


