Abstract
Expert performance constitutes the endpoint of skill acquisition and is accompanied by widespread neuroplastic changes. To reveal common mechanisms of reorganization associated with long‐term expertise in a cognitive domain (mental calculation, chess, language, memory, music without motor involvement), we used activation likelihood estimation meta‐analysis and compared brain activation of experts to nonexperts. Twenty‐six studies matched inclusion criteria, most of which reported an increase and not a decrease of activation foci in experts. Increased activation occurred in the left rolandic operculum (OP 4) and left primary auditory cortex and in bilateral premotor cortex in studies that used auditory stimulation. In studies with visual stimulation, experts showed enhanced activation in the right inferior parietal cortex (area PGp) and the right lingual gyrus. Experts' brain activation patterns seem to be characterized by enhanced or additional activity in domain‐specific primary, association, and motor structures, confirming that learning is localized and very specialized. Hum Brain Mapp 37:262–272, 2016. © 2015 Wiley Periodicals, Inc.
Keywords: activation likelihood estimation, expertise, prodigy, reorganization, skill development
INTRODUCTION
Expert performance is characterized by the consistent performance at a superior level in a cognitive, artistic, motor, or other domain such as chess, mathematics, music, and sports [Ericsson and Lehmann, 1996]. It constitutes the endpoint of skill acquisition, during which performance improves as a power function of practice and becomes faster and more accurate [Logan, 1992; Newell and Rosenbloom, 1981]. With long‐term practice, qualitative changes occur and skills develop from a controlled, effortful, attention demanding to a more automatic, rapid, and effortless mode of processing [Schneider and Shiffrin, 1977]. This process goes along with a redistribution of neural resources that may imply a decreased demand of control or attentional processes (reduced activation) as well as “true” reorganization (change in the location of activation) that is indicative of different tasks being performed at the beginning and end of training [Kelly and Garavan, 2005; Patel, et al., 2013]. Expertise‐related neuroplastic changes are widespread and concern multiple sensory, cognitive, motor, and affective networks of the brain [Harel, et al., 2013]. Generally, sensory and motor tasks that involve topographical cortical representation provoke different reorganizational mechanisms than higher cognitive tasks [Kelly and Garavan, 2005]. Further, reorganizational changes depend on the stage in the acquisition of a skill with a training of minutes or days resulting in a different outcome than a training of years. However, even after long‐lasting training, there is a considerable variability among people with regard to the level of skill they achieve, because high expertise does not naturally arise together with automaticity [Debarnot, et al., 2014; Ericsson, 2006]; for a review on the respective roles of practice versus ability for expertise, see special issue on “Acquiring expertise: ability, practice, and other influences, Intelligence, 45, 2014”). In order to clarify the neural mechanisms underlying high expertise, several functional imaging studies compared experts in different domains to a nonexpert control group [expert vs novice paradigm, Campitelli and Speelman, 2013]. Domains varied between music and sports as relatively frequent domains of expertise [for a qualitative review, see Chang, 2014], to more exclusive skills as mental arithmetic [e.g., Fehr, et al., 2010], or chess [e.g., Bilalic, et al., 2011b], to very particular skills related to special career groups, such as sommeliers [Castriota‐Scanderbeg, et al., 2005], perfumers [Plailly, et al., 2012], or architects [Kirk, et al., 2009]. Depending on the skill investigated, the number of available experts was limited and group sizes rather small. To date, there are no quantitative reviews that may summarize the neural effects of long‐lasting training in the cognitive domain and that compared experts to nonexperts. Thus the goal of the current activation likelihood estimation (ALE) meta‐analysis was to integrate studies investigating neuroplastic reorganization that underlies cognitive expertise based on many years of training. We aimed to consider expertise in cognitive domains to the exclusion of any motor or vocational expertise that may rely on very different reorganizational processes [see Kelly and Garavan, 2005; for a recent ALE meta‐analysis on motor learning, see Hardwick et al., 2013]. In this study, expert and nonexpert brain activation patterns were considered in contrast to each other, but not separately. By this, we intended to isolate long‐term expert‐specific plasticity processes across different domains. The following general reorganizational mechanisms were considered possible: (1) In concordance with the notion of redistribution of brain activity, there may be a decreased demand on attentional and control [“scaffolding,” Petersen, et al., 1998] processes, e.g., in the prefrontal cortex, the anterior cingulate, and posterior parietal cortex, and an increased demand on storage and processing in task‐specific areas in experts [Kelly and Garavan, 2005]. (2) Perceptual expertise that sometimes goes along with cognitive expertise, e.g., in chess [Gobet and Simon, 1996], has been shown to activate the fusiform face area on the lateral side of the fusiform gyrus [Bilalic, et al., 2011b; Gauthier, et al., 2000]. (3) Experts have been found to develop a special memory function that implies the setup of knowledge structures or templates in long‐term memory and their availability by retrieval cues in working memory [Ericsson and Kintsch, 1995; Gobet and Simon, 1996]. Activation of long‐term memory structures, such as the hippocampus and adjacent medial temporal areas, may be indicative of these processes. (4) Caudate nucleus activity has been demonstrated in implicit, automatic, and intuitive information processing, e.g., in Japanese chess professionals during quick, automatic generation of the next best move [Wan et al., 2011], in simultaneous interpreters [Hervais‐Adelman, et al., 2015], and in professional writers [Erhard et al., 2014]. Generally, increases in the subcortical striatum have been reported after training in motor and cognitive tasks (for a review, see Patel et al., 2013).
In sum, the objective of this study was to elucidate general reorganizational mechanisms of long‐term expertise across different cognitive domains. We hypothesized a decrease of attentional and control processing in the prefrontal cortex and an increase of activity in task‐specific areas, as well as a possible increase of activation in the fusiform face area, long‐term memory medial temporal structures, and the striatum.
METHODS
Literature Search and Study Selection
A systematic literature research was conducted to identify neuroimaging studies of expertise for inclusion in the meta‐analysis. Relevant papers were retrieved by searching Pubmed (http://www.ncbi.nlm.nih.gov/pubmed/) using the following terms: (fMRI OR “positron emission tomography”) AND (expertise). There were 745 results in Pubmed (May 2015). After screening these articles and applying the exclusion criteria as described below, we obtained the following categories for cognitive expertise: mental calculation, chess, language, memory, and music. We then continued searching within different domains by entering the keyword of the respective domain (e.g., (music) AND (expert OR expertise OR professional OR prodigy) AND (fMRI OR “positron emission tomography”). We obtained 10 results for mental calculation, 13 for chess, 148 for memory, 131 for language, and 99 for music. We again applied the exclusion criteria and checked the literature sections of the respective articles looking for additional publications.
Inclusion and Exclusion Criteria
To be included in the analysis, studies needed to have imaged and analyzed the whole head and reported coordinates in standard stereotaxic space. We did not include studies investigating structural brain differences or functional connectivity. As described in the introduction, we were interested in functional differences between experts with long‐term training and nonexperts. Thus only studies that investigated an adult nonclinical expert group in comparison to a nonexpert control group were included, and training studies that investigated longitudinal within‐group training effects as well as expert studies without control groups were discarded. Experts had to have many years of training to qualify as an expert in any domain.
Since we were interested in cognitive but not other types of expertise, all studies investigating motor and action expertise, motor imagery, or emotional processing in experts were excluded. Due to its motor involvement, studies implying singing during scanning were not included, as well as expert studies on meditation, acupuncture, and special career groups, such as architects, medical doctors, or sommeliers (no clearly defined cognitive training). Finally, since we intended to investigate aspects of convergent rather than divergent thinking, studies that examined creativity or musical improvisation were excluded. Finally, there were 26 studies included, 4 investigating expertise in mental arithmetic, 5 in chess, 1 in memory, 15 in music, and 1 in language and music (Table 1). The designation of a study as visual or auditory is indicated in Table 1. All the studies reported results for the contrast “expert > nonexpert”, but the reverse contrast “nonexpert > expert” did not yield significant effects, except in two studies [Krawczyk, et al., 2011; Schulze, et al., 2011b]. We chose contrasts in a way that the control condition was always subtracted from the mental task condition. Thus we do not report potential deactivations of brain areas.
Table 1.
Descriptive information of the studies included in the meta‐analysis
| Sample | |||||
|---|---|---|---|---|---|
| Authors | Area of expertise | No. experts | No. nonexperts | Experience (experts) | Task (presentation modality) |
| Fehr et al., 2010 | Mental calculation | 1 (m) | 11 (5 m) | 15 y | Simple and complex calculation (+, ×, *, /) tasks (v) |
| Hanakawa et al., 2003 | Mental calculation (abacus) | 6 (1 m) | 8 (1 m) | >17 y | Mental addition (single and multiple digit numbers) (v) |
| Pesenti et al., 2001 | Mental calculation | 1 (m) | 6 (all m) | 6 y | Simple and complex multiplication (v) |
| Tanaka et al. 2002 | Mental calculation (abacus) | 10 (all m) | 13 (7 m) | 8–16 y | Delayed match‐to‐sample working memory with digits (v) |
| Bartlett et al., 2013 | Chess | 11 (all m) | 11 (all m) |
Mean 16 y, Elo: >2353 |
One‐back task with chess boards (v) |
| Bilalic et al., 2010 | Chess | 8 (all m) | 15 (all m) | Elo: avg. 2108 | Visual search with chess stimuli (v) |
| Bilalic et al., 2011a | Chess | 8 (all m) | 8 (all m) | Elo: avg. 2130 | Object and pattern recognition with chess stimuli (v) |
| Bilalic et al., 2012 | Chess | 8 (all m) | 15 (all m) | Elo: avg. 2108 | Object and pattern recognition with chess stimuli (v) |
| Krawczyk et al., 2011 | Chess | 6 (all m) | 6 (all m) |
Mean 16 y, Elo: >2447 |
Recognition memory with chess patterns (v) |
| Maguire et al., 2003 | Memory | 10 (all m) | 10 (all m) | Attendance of world memory championship | Memory for order of digits (v) |
| Bangert et al., 2006 | Music | 8 (3 m) | 8 (3 m) | Mean 20 y | Listening to piano sequences (a) |
| Groussard et al., 2010 | Music | 20 (10 m) | 20 (10 m) | Mean 15 y | Listening to keyboard melodies and familiarity rating (a) |
| Habermeyer et al., 2009 | Music | 8 (7 m) | 8 (7 m) | Mean 37 y | Pattern deviance in melodies (a) |
| Harris and de Jong, 2014 | Music | 12 (all m) | 12 (all m) | Mean 25 y professional experience | Judging musical pieces (a) |
| Herdener et al., 2010 | Music | 7 (6 m) | 7 (6 m) | Mean 36 y | Acoustic temporal mismatch (a) |
| Herdener et al., 2014 | Music | 12 (all m) | 10 (all m) | >5 y professional experience | Listening to rhythm patterns (a) |
| Hoenig et al., 2011 | Music | 20 (14 m) | 20 (13 m) | Mean 27 y | Semantic match between word and picture of musical instruments (v) |
| Morrison et al., 2003 | Music | 6 (2 m) | 6 (2 m) | Professional level | Listening to culturally familiar and unfamiliar music (a) |
| Oechslin et al., 2010 | Music | 15 with absolute pitch (7 m) | 30 (14 m) | Mean 18 y | Listening to speech stimuli (a) |
| Oechslin et al., 2013 | Music | 20 (10 m) |
39 (20 m) (20 amateur musicians) |
Mean 18 y | Listening to musical pieces with (ir)regular endings (a) |
| Pallesen et al., 2010 | Music | 11 (2 m) | 10 (1 m) | Students or graduates of music academy | Working memory of musical sounds (a) |
| Pau et al., 2013 | Music | 14 pianists (8 m) | 15 (9 m) | Mean 11 y | Encoding of finger sequences (v) |
| Schulze et al., 2011a | Music | 16 (9 m) | 17 (9 m) | Mean 17 y | Working memory of tonal and atonal sequences (a) |
| Schulze et al., 2011b | Music | 16 (9 m) | 17 (9 m) | Students of music academy | Working memory of tonal and verbal stimuli (a) |
| Sluming et al., 2007 | Music | 10 (all m) | 10 (all m) | Members of philharmonic orchestra | Three‐dimensional mental rotation (v) |
| Dick et al., 2011 |
Music Language |
15 (no m) | 15 (no m) | > 16 y | Listening to violin and speech stimuli (a) |
m: males; y: years; (v): visual, (a); auditory; avg.: average; Elo: chess rating scale (mean: 1500, SD: 200).
ALE Meta‐Analysis
All meta‐analyses were carried out using the revised ALE algorithm for coordinate‐based meta‐analysis of neuroimaging results [Eickhoff, et al., 2009; Turkeltaub, et al., 2012]. This algorithm aims to identify areas with a convergence of reported coordinates across experiments that are higher than the expected from a random spatial association. Reported foci are treated as centers of 3D Gaussian probability distributions capturing the spatial uncertainty associated with each focus [Eickhoff, et al., 2009]. Here, the between‐subject variance is weighted by the number of participants per study since larger sample sizes should provide more reliable approximations of the “true” activation effect and should therefore be modeled by “narrower” Gaussian distributions.
Subsequently, probabilities of all foci reported of a given experiment were combined for each voxel, yielding a modeled activation (MA) map [Turkeltaub, et al., 2012]. Voxel‐ wise ALE scores (union across these MA maps) then quantified the convergence across experiments at each location in the brain. To distinguish “true” from random convergence, ALE scores were compared to an empirical null distribution reflecting a random spatial association among all MA maps. The resulting random‐effects inference focuses on the above‐chance convergence across studies rather than the clustering within a particular study [Eickhoff, et al., 2009]. This null hypothesis was derived by computing the distribution that would be obtained when sampling a voxel at random from each of the MA maps and taking the union of these values in the same manner as for the (spatially contingent) voxels in the original analysis [Eickhoff et al., 2012]. The P value of a “true” ALE score was then given by the proportion of equal or higher values obtained under the null distribution. The resulting nonparametric P values were then assessed at a familywise error (FWE) corrected threshold of P < 0.05 on cluster level (cluster‐forming threshold: P < 0.001 at voxel level, cf., [Eickhoff, et al., 2012]) and transformed into Z scores for display.
To reveal common mechanisms of reorganization after long‐term training in experts, an ALE meta‐analysis was conducted across all expertise studies. Further, to show possible modality‐specific effects of expertise, separate ALE analyses were calculated for studies that used visual and auditory stimulation. Since the ALE algorithm requires a minimum of nine studies, different areas of expertise could not be analyzed separately with the exception of music (16 studies including music and language). For the same reason, the ALE meta‐analysis of the contrast “nonexperts > experts” could not be calculated. Since musical expertise studies dominated the overall analysis of expertise studies, we conducted another ALE meta‐analysis across all expertise studies to the exclusion of music studies.
Anatomical Labelling
Resulting brain regions were macroanatomically labeled by reference to the probabilistic Harvard–Oxford atlas (Desikan, et al., 2006) included with FSLView v3.1 (http://www.fmrib.ox.ac.uk/fsl/fslview/index.html). For a more precise allocation, we made use of cytoarchitectonic maps of the human brain provided by the SPM Anatomy Toolbox [Eickhoff, et al., 2006b; Eickhoff, et al., 2007b; Eickhoff, et al., 2005]. Hereby, activations and deactivations were assigned to the most probable histologically defined area at the respective location. This probabilistic and histology‐based anatomical labeling is reported in Table 2; references to details of the cytoarchitecture are given in the results section.
Table 2.
Coordinates of peak activations, experts > nonexperts
| Cluster | Foci | Z‐value | Coordinates (MNI) | Probability for areas (%) | Anatomically assigned to | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| A: Main effect: auditory | |||||||
| I (148 voxel) | 1 | 4.68 | −58 | −8 | 12 | 60 | L. rolandic operculum, OP 4 |
| 2 | 3.68 | −58 | −8 | 6 | 30 | L. superior temporal gyrus, TE 1.2 | |
| II (124 voxel) | 1 | 4.24 | −52 | 2 | 44 | 50 | L. precentral gyrus, Area 6 |
| 2 | 3.80 | −52 | 6 | 36 | 10 | L. precentral gyrus, Area 6 | |
| III (95 voxel) | 1 | 3.82 | 56 | −6 | 46 | 80 | R. precentral gyrus, Area 6 |
| 2 | 3.67 | 48 | −2 | 56 | 30 | R. middle frontal gyrus, Area 6 | |
| 3 | 3.64 | 50 | −4 | 54 | 60 | R. middle frontal gyrus, Area 6 | |
| B: Main effect: visual | |||||||
| I (117 voxel) | 1 | 5.05 | 48 | −68 | 16 | 40 | R. inferior parietal cortex (PGp) |
| C: Main effect: expertise (auditory & visual) | |||||||
| I (170 voxel) | 1 | 4.80 | −52 | 4 | 40 | 30 | L. precentral gyrus, Area 6 |
| II (87 voxel) | 1 | 4.24 | −58 | −8 | 12 | 60 | L. rolandic operculum, OP 4 |
| 2 | 3.47 | −58 | −8 | 6 | 30 | L. superior temporal gyrus, TE 1.2 | |
| D: Main effect: music | |||||||
| I (71 voxel) | 1 | 4.89 | −52 | 6 | 40 | 20 | L. precentral gyrus, Area 6 |
| II (164 voxel) | 1 | 4.44 | −58 | −8 | 12 | 60 | L. rolandic operculum, OP 4 |
| 2 | 3.65 | −58 | −8 | 6 | 30 | L. superior temporal gyrus, TE 1.2 | |
| E: Main effect: expertise without music | |||||||
| I (141 voxel) | 1 | 5.49 | 48 | −68 | 16 | 40 | R. inferior parietal cortex (PGp) |
| II (89 voxel) | 1 | 4.56 | 14 | −52 | 6 | 20 | R. lingual gyrus, area 17 |
L.: left; R.: right.
RESULTS
The meta‐analysis of expertise studies that used auditory stimulation (13 studies, 365 participants) computed three activation clusters with altogether seven maxima (Table 2). Cluster I comprised the left rolandic operculum [OP 4, Eickhoff, et al., 2006a; Eickhoff, et al., 2007a] and the left superior temporal gyrus [TE 1.2, Morosan, et al., 2001]. Cluster II showed two maxima in the left precentral gyrus (Area 6, adjacent to Area 44). Cluster III comprised the right precentral gyrus (Area 6) extending to the right middle frontal gyrus [Area 6, Geyer, 2004] (Fig. 1).
Figure 1.
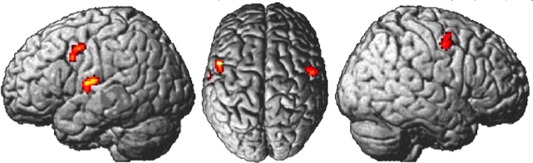
Convergent brain activation of expertise studies with auditory stimulation (colors represent z‐values between 3.1 (red) and 4.68 (yellow)). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
The meta‐analysis of investigations that used visual stimulation (13 studies, 261 participants) yielded a single activation cluster in the right inferior parietal cortex [PGp, Caspers, et al., 2006] (Fig. 2).
Figure 2.

Convergent brain activation of expertise studies with visual stimulation (colors represent z‐values between 3.1 (red) and 5.05 (yellow)). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
The meta‐analysis of all expertise studies computed two activation clusters with one maximum in the left precentral gyrus (Area 6) and two maxima in the left rolandic operculum and the left superior temporal gyrus (TE 1.2) (Fig. 3a).
Figure 3.
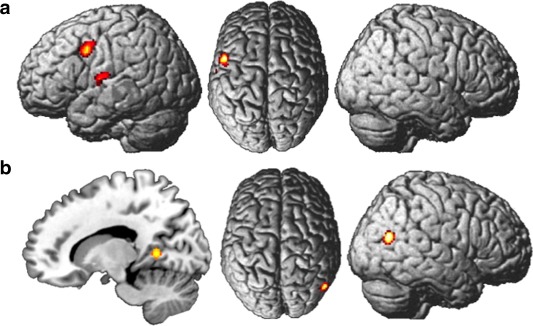
Convergent brain activation of expertise studies (a) including music studies (z‐values between 3.01 (red) and 4.8 (yellow)) and (b) excluding music studies (z‐values between 3.01 (red) and 5.49 (yellow)). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
The meta‐analysis of music studies calculated two activation clusters with one cluster in the left precentral gyrus (Area 6) and the second cluster with two maxima in the left rolandic operculum and the left superior temporal gyrus (TE 1.2).
The meta‐analysis of expertise excluding music studies (10 experiments, 172 participants) resulted in two clusters located in the right inferior parietal cortex (PGp), and the right lingual gyrus, [area 17, Amunts, et al., 2000] (Fig. 3b).
DISCUSSION
The current ALE meta‐analysis was conducted to highlight cerebral regions that show reorganization in response to long‐term training in a cognitive skill. Twenty‐six studies were identified that had investigated an expert group in comparison to a nonexpert control group in the domains of mental arithmetic, chess, language, memory, and music (without motor involvement). All studies found brain regions that showed larger activations in experts than in nonexperts, but only two provided results in the reverse direction. We may thus conclude that in the cognitive domain, experts' brains show larger activation magnitudes or activation in additional areas in contrast to the motor domain where brain activation patterns tend to be reduced and more focused in experts [Jäncke, et al., 2000; Lotze, et al., 2003; Picard, et al., 2013]. In detail, increased activation in experts occurred in the left rolandic operculum and left primary auditory cortex [TE 1.2, Morosan, et al., 2001] and in bilateral premotor cortex (area 6, left extending to area 44) in studies that used auditory stimulation. The same activation pattern was evident when separate ALE analyses were calculated across music studies and across all expertise studies combined, however, without the activation cluster in the right premotor cortex. In studies with visual stimulation, experts showed enhanced activation in the right inferior parietal cortex [area PGp, Caspers, et al., 2006]. When calculating a meta‐analysis of all studies excluding music, there was a comparable activation pattern as in studies with visual stimulation, however, with an additional cluster in the right lingual gyrus [Area 17, Amunts, et al., 2000]. Thus, across modalities and designs, brain activation patterns in experts were characterized by an enhanced activity in primary and association areas for the respective modality and motor associated regions.
Enhanced Activity in Experts in the Left Rolandic Operculum and Bilateral Premotor Cortex in Paradigms with Auditory Stimulation
Studies with auditory stimulation converged in an activation cluster in the left rolandic operculum (OP4) extending to the left primary auditory cortex [TE 1.2, Morosan, et al., 2001] and a co‐activation of the bilateral premotor cortex. The OP 4 of the human parietal operculum is a part of the secondary somatosensory cortex and represents the human analog of primate area PV [Eickhoff, et al., 2007a]. It has been associated with integrating functional coupling of primary sensory and motor regions [Eickhoff, et al., 2010]. In a recent functional network analysis of language areas, the OP 4 has been found a key structure connecting the primary auditory and motor regions responsible for language [Sepulcre, 2015]. In the context of music, the bilateral activity in the Rolandic operculum was interpreted as reflecting mechanisms of perception–execution matching during the perception of vocalizable auditory (e.g., musical) information [Koelsch, et al., 2006]. The same conclusion came from a meta‐analysis investigating language‐related networks that located the Rolandic operculum within a phonological network concerned with sensory‐motor coordination [Vigneau, et al., 2006]. Additionally, the Rolandic operculum includes the ventral part of the larynx motor cortex in the left hemisphere [Bouchard, et al., 2013; Conant, et al., 2014]. Together with the dorsal part that is located at the dorsolateral motor/premotor cortex [area 4p and 6, Brown and Martinez, 2007 ; Brown, et al., 2008; Loucks, et al., 2007], it regulates the production of pitch and voicing. Thus enhanced activation of experts in the left rolandic operculum together with the coactivation of the premotor cortex in this study may represent the two parts of the larynx motor cortex in the left hemisphere. Generally, premotor activation in the absence of any movement has been connected to the automatic access to a highly trained movement pattern [Rijntjes, et al., 1999] or movement imagery [Binkofski, et al., 2000]. The cluster of our meta‐analysis extended to Area 44 that has been described as belonging to an execution–observation matching system [mirror neuron system; Gallese et al., 1996; Rizzolatti et al., 1996] in the human cortex [Binkofski and Buccino, 2006]. Automatic cross‐modal transfer and coactivation of auditory and motor areas in musicians has been described in previous studies [Bangert, et al., 2006; Engel, et al., 2012; Lotze, et al., 2003] and is characteristic of expert behavior in the auditory, especially music domain. The results of this study may additionally suggest that musical expertise involves having greater activation in the pitch‐ producing centers of the brain. Subvocal articulatory activity has been reported in studies investigating auditory/musical imagery [Smith, et al., 1995] and notational audiation [the ability to “hear” music one is reading, Brodsky, et al., 2003; Brodsky, et al., 2008]. Subvocal repetition or imagery may be also present in musical experts across a diversity of tasks.
Enhanced Activity in Experts in the Right Inferior Parietal Cortex (Area PGp, Caspers, et al., 2006) in Paradigms with Visual Stimulation
Across visual paradigms including primarily mental arithmetic and chess studies, there was one significant activation cluster in the right inferior parietal cortex. In the context of chess expertise, the right parieto‐occipito‐temporal junction was connected to superiority of experts in chess specific object recognition [Bartlett, et al., 2013; Bilalic, et al., 2010; Bilalic, et al., 2012] and was discussed in terms of a qualitatively different way of processing with the additional recruitment of right homologue areas in experts [Bilalic, et al., 2010]. This activation pattern was also observed in abacus experts during calculation [Hanakawa, et al., 2003] and a mental calculation prodigy [Pesenti, et al., 2001] in line with the notion of true functional reorganization [Kelly and Garavan, 2005]. Our cluster extended to region PGp of the right angular gyrus that has been linked to mathematical cognition [Wu, et al., 2009], however, as a deactivation correlated with poorer accuracy in the calculation task. Deactivation of the right angular gyrus, however in the more anterior region PGa, was also evident in chess grandmasters during a Chinese chess solving task [Duan, et al., 2012]. In contrast right area PGp was active in Bilalic et al. (2011a) (named posterior occipito‐temporal junction), with deactivation occurring only in control persons during a chess piece identity task. These inconsistent findings may be reconciled taking into account that deactivation in that area has been shown to go along with increasing task difficulty [Greicius and Menon, 2004; Wu, et al., 2009]. Thus in study paradigms that were quite demanding [e.g., Duan, et al., 2012], the right angular gyrus may show more deactivation than in easy tasks presented to chess experts in Bilalic et al. (2011a).
Enhanced Activation of Primary Visual and Auditory Cortices Across Different Domains of Expertise
Taking into account music studies, the ALE meta‐analysis across all studies pointed to the left primary auditory cortex (in addition to motor‐related areas) as differentiating expert from non‐experts. Without music studies, there was a cluster in the medial part of the right lingual gyrus, assigned to area 17 that has been related to pattern recognition processes [Bilalic, et al., 2012] going along with enhanced parafoveal vision in chess experts [Bilalic, et al., 2010]. In the learning literature it has been frequently described, that perceptual learning occurs at the location where a specific aspect of perception is processed, i.e. all along the processing stream [Karni, 1996]. Providing that, it is not surprising that expertise also develops in brain areas connected to the earlier information processing of stimuli in question. Furthermore, the primary auditory cortex is not confined to the analysis of sound, but is also involved in auditory learning, memory and even problem solving [for a review see Weinberger, 2012]. Likewise, the right lingual gyrus has been shown to participate in cognitive tasks, such as working memory [possibly due to visual strategies applied; Gerton, et al., 2004], visual imagery [Kosslyn, et al., 1999], and perceptual learning [Maertens and Pollmann, 2005]. Early low‐level information processing has been suggested to contribute to outstanding skills observed in savants [Snyder and Mitchell, 1999] as well as other prodigies [Birbaumer, 1999]. Thus expertise, even if widely distributed in the brain [Harel, et al., 2013], may start with alterations in primary sensory areas.
Apart from bottom‐up mechanisms, top‐ down modulation may also explain enhanced primary sensory cortex activation in experts. Experts show enhanced engagement with their area of expertise and pay more attention to expertise‐related objects [Golan, et al., 2014; Hershler and Hochstein, 2009], what is reflected by an enhanced activity in early visual areas [Harel, et al., 2010]. Likewise, top‐down modulatory effects can be observed in audition [Tervaniemi, et al., 2009].
Methodological Constraints
In the current meta‐analysis, we included 26 studies investigating very different areas of cognitive expertise. Although the number of studies included is within the normal range for meta‐analyses, common mechanisms across these studies may be difficult to capture. In a recent ALE analysis about within‐group training effects across a range of cognitive and motor tasks, the authors found domain‐general effects regardless of the different paradigms included (Patel, et al., 2013). One reason why we did not find these effects may be the generally lower power in between‐subject designs. Another explanation may be that in most studies, experts conducted so‐called “contrived” tasks [Chi, 2006], i.e. tasks that were constructed in a way that both experts and non‐experts can complete them. There are several advantages of contrived tasks, however, one disadvantage is that they may lack ecological validity. Thus in the current meta‐analysis we may have missed some expert‐specific mechanisms (e.g. long‐term working memory), because the tasks did not require to engage them.
Summary and Conclusions
In the current meta‐analysis of cognitive expertise, brain activation patterns in experts (in comparison to nonexperts) were characterized by an enhanced or additional activity in primary and association areas for the respective modality, and motor‐associated regions. With regard to our hypotheses specified in the introduction, there were no domain‐general mechanisms evident, such as a reduction of activity in the control network as observed in short‐term practice studies [Kelly and Garavan, 2005; Patel, et al., 2013], nor expert‐specific information processing in long‐term working memory structures, the fusiform gyrus, or the striatum. Instead, there was an enhanced activity in domain‐specific structures related to the tasks performed (hypothesis 1b), confirming that learning is localized and very specialized [Hill and Schneider, 2006]. Indeed, there is a considerable behavioral evidence that superior performance of experts is due to their extensive experience with domain‐related patterns and configurations, e.g., in chess [Reingold, et al., 2001; Reingold and Sheridan, 2011]. We were not able to confirm large networks of expertise, as suggested by Harel et al. (2013), what may be due to the variety of expertise studies included. Most of the studies reported an increase and not a decrease of activation foci in contrast to the nonexpert group. Thus true functional reorganization, possibly connected to enhanced tissue in cortical areas devoted to the task, can be observed after long periods of training.
Conflict of interest: none.
REFERENCES
- Amunts K, Malikovic A, Mohlberg H, Schormann T, Zilles K (2000): Brodmann's areas 17 and 18 brought into stereotaxic space‐where and how variable? NeuroImage 11:66–84. [DOI] [PubMed] [Google Scholar]
- Bangert M, Peschel T, Schlaug G, Rotte M, Drescher D, Hinrichs H, Heinze HJ, Altenmüller E (2006): Shared networks for auditory and motor processing in professional pianists: Evidence from fMRI conjunction. NeuroImage 30:917–926. [DOI] [PubMed] [Google Scholar]
- Bartlett JC, Boggan AL, Krawczyk DC (2013): Expertise and processing distorted structure in chess. Front Hum Neurosci 7:825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilalic M, Kiesel A, Pohl C, Erb M, Grodd W (2011a): It takes two‐skilled recognition of objects engages lateral areas in both hemispheres. PloS One 6:e16202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilalic M, Langner R, Erb M, Grodd W (2010): Mechanisms and neural basis of object and pattern recognition: A study with chess experts. J Exp Psychol Gen 139:728–742. [DOI] [PubMed] [Google Scholar]
- Bilalic M, Langner R, Ulrich R, Grodd W (2011b): Many faces of expertise: Fusiform face area in chess experts and novices. J Neurosci 31:10206–10214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilalic M, Turella L, Campitelli G, Erb M, Grodd W (2012): Expertise modulates the neural basis of context dependent recognition of objects and their relations. Hum Brain Mapp 33:2728–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binkofski F, Amunts K, Stephan KM, Posse S, Schormann T, Freund HJ, Zilles K, Seitz RJ (2000): Broca's region subserves imagery of motion: A combined cytoarchitectonic and fMRI study. Hum Brain Mapp 11:273–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binkofski F, Buccino G (2006): The role of ventral premotor cortex in action execution and action understanding. J Phys Paris 99:396–405. [DOI] [PubMed] [Google Scholar]
- Birbaumer N (1999): Neurobiology. Rain Man's revelations. Nature 399:211–212. [DOI] [PubMed] [Google Scholar]
- Bouchard KE, Mesgarani N, Johnson K, Chang EF (2013): Functional organization of human sensorimotor cortex for speech articulation. Nature 495:327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky W, Henik A, Rubinstein BS, Zorman M (2003): Auditory imagery from musical notation in expert musicians. Percep Psychophys 65:602–612. [DOI] [PubMed] [Google Scholar]
- Brodsky W, Kessler Y, Rubinstein BS, Ginsborg J, Henik A (2008): The mental representation of music notation: Notational audiation. J Exp Psychol Hum Percept Perform 34:427–445. [DOI] [PubMed] [Google Scholar]
- Brown S, Martinez MJ (2007): Activation of premotor vocal areas during musical discrimination. Brain Cognit 63:59–69. [DOI] [PubMed] [Google Scholar]
- Brown S, Ngan E, Liotti M (2008): A larynx area in the human motor cortex. Cereb Cortex 18:837–845. [DOI] [PubMed] [Google Scholar]
- Campitelli G, Speelman C (2013): Expertise paradigms for investigating the neural substrates of stable memories. Front Hum Neurosci 7:740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers S, Geyer S, Schleicher A, Mohlberg H, Amunts K, Zilles K (2006): The human inferior parietal cortex: Cytoarchitectonic parcellation and interindividual variability. NeuroImage 33:430–448. [DOI] [PubMed] [Google Scholar]
- Castriota‐Scanderbeg A, Hagberg GE, Cerasa A, Committeri G, Galati G, Patria F, Pitzalis S, Caltagirone C, Frackowiak R (2005): The appreciation of wine by sommeliers: A functional magnetic resonance study of sensory integration. NeuroImage 25:570–578. [DOI] [PubMed] [Google Scholar]
- Chang Y (2014): Reorganization and plastic changes of the human brain associated with skill learning and expertise. Front Hum Neurosci 8:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi MT (2006): Laboratory methods for assessing experts' and novices' knowledge In: Ericsson KA, Charness N, Feltovich PJ, Hoffman RR, editors. The Cambridge Handbook of Expertise and Expert Performance. Cambridge, New York: Cambridge University Press; pp 167–184. [Google Scholar]
- Conant D, Bouchard KE, Chang EF (2014): Speech map in the human ventral sensory‐motor cortex. Curr Opin Neurobiol 24:63–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debarnot U, Sperduti M, Di Rienzo F, Guillot A (2014): Experts bodies, experts minds: How physical and mental training shape the brain. Front Hum Neurosci 8:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ (2006): An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 31:968–980. [DOI] [PubMed] [Google Scholar]
- Dick F, Lee HL, Nusbaum H, Price CJ (2011): Auditory‐motor expertise alters “speech selectivity” in professional musicians and actors. Cereb Cortex 21:938–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X, Liao W, Liang D, Qiu L, Gao Q, Liu C, Gong Q, Chen H (2012): Large‐scale brain networks in board game experts: Insights from a domain‐related task and task‐free resting state. PloS One 7:e32532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Amunts K, Mohlberg H, Zilles K (2006a): The human parietal operculum. II. Stereotaxic maps and correlation with functional imaging results. Cereb Cortex 16:268–279. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT (2012): Activation likelihood estimation meta‐analysis revisited. NeuroImage 59:2349–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Grefkes C, Zilles K, Fink GR (2007a): The somatotopic organization of cytoarchitectonic areas on the human parietal operculum. Cereb Cortex 17:1800–1811. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Heim S, Zilles K, Amunts K (2006b): Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. NeuroImage 32:570–582. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Jbabdi S, Caspers S, Laird AR, Fox PT, Zilles K, Behrens TE (2010): Anatomical and functional connectivity of cytoarchitectonic areas within the human parietal operculum. J Neurosci 30:6409–6421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT (2009): Coordinate‐based activation likelihood estimation meta‐analysis of neuroimaging data: A random‐effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp 30:2907–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras MH, Evans AC, Zilles K, Amunts K (2007b): Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. NeuroImage 36:511–521. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K (2005): A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage 25:1325–1335. [DOI] [PubMed] [Google Scholar]
- Engel A, Bangert M, Horbank D, Hijmans BS, Wilkens K, Keller PE, Keysers C (2012): Learning piano melodies in visuo‐motor or audio‐motor training conditions and the neural correlates of their cross‐modal transfer. NeuroImage 63:966–978. [DOI] [PubMed] [Google Scholar]
- Erhard K, Kessler F, Neumann N, Ortheil HJ, Lotze M (2014): Professional training in creative writing is associated with enhanced fronto‐striatal activity in a literary text continuation task. NeuroImage 100:15–23. [DOI] [PubMed] [Google Scholar]
- Ericsson KA (2006): The influence of experience and deliberate practice of the development of superior expert performance In: Ericsson KA, Charness N, Feltovich PJ, Hoffman RR, editors. The Cambridge Handbook of Expertise and Expert Performance. Cambridge, New York: Cambridge University Press; pp 683–703. [Google Scholar]
- Ericsson KA, Kintsch W (1995): Long‐term working memory. Psychol Rev 102:211–245. [DOI] [PubMed] [Google Scholar]
- Ericsson KA, Lehmann AC (1996): Expert and exceptional performance: Evidence of maximal adaptation to task constraints. Ann Rev Psychol 47:273–305. [DOI] [PubMed] [Google Scholar]
- Fehr T, Weber J, Willmes K, Herrmann M (2010): Neural correlates in exceptional mental arithmetic–about the neural architecture of prodigious skills. Neuropsychologia 48:1407–1416. [DOI] [PubMed] [Google Scholar]
- Gallese, V. , Fadiga, L. , Fogassi, L. , Rizzolatti, G. (1996) Action recognition in the premotor cortex. Brain J Neurol 119 (Pt 2):593–609. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Skudlarski P, Gore JC, Anderson AW (2000): Expertise for cars and birds recruits brain areas involved in face recognition. Nat Neurosci 3:191–197. [DOI] [PubMed] [Google Scholar]
- Gerton BK, Brown TT, Meyer‐Lindenberg A, Kohn P, Holt JL, Olsen RK, Berman KF (2004): Shared and distinct neurophysiological components of the digits forward and backward tasks as revealed by functional neuroimaging. Neuropsychologia 42:1781–1787. [DOI] [PubMed] [Google Scholar]
- Geyer, S. (2004) The microstructural border between the motor and the cognitive domain in the human cerebral cortex. Adv Anat Embryol Cell Biol 174:I–VIII, 1–89. [DOI] [PubMed] [Google Scholar]
- Gobet F, Simon HA (1996): Templates in chess memory: A mechanism for recalling several boards. Cognit Psychol 31:1–40. [DOI] [PubMed] [Google Scholar]
- Golan T, Bentin S, DeGutis JM, Robertson LC, Harel A (2014): Association and dissociation between detection and discrimination of objects of expertise: Evidence from visual search. Attent Percept Psychophys 76:391–406. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Menon V (2004): Default‐mode activity during a passive sensory task: Uncoupled from deactivation but impacting activation. J Cognit Neurosci 16:1484–1492. [DOI] [PubMed] [Google Scholar]
- Groussard M, La Joie R, Rauchs G, Landeau B, Chetelat G, Viader F, Desgranges B, Eustache F, Platel H (2010): When music and long‐term memory interact: Effects of musical expertise on functional and structural plasticity in the hippocampus. PloS One 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermeyer B, Herdener M, Esposito F, Hilti CC, Klarhofer M, di Salle F, Wetzel S, Scheffler K, Cattapan‐Ludewig K, Seifritz E (2009): Neural correlates of pre‐attentive processing of pattern deviance in professional musicians. Hum Brain Mapp 30:3736–3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanakawa T, Honda M, Okada T, Fukuyama H, Shibasaki H (2003): Neural correlates underlying mental calculation in abacus experts: A functional magnetic resonance imaging study. NeuroImage 19:296–307. [DOI] [PubMed] [Google Scholar]
- Hardwick RM, Rottschy C, Miall RC, Eickhoff SB (2013): A quantitative meta‐analysis and review of motor learning in the human brain. NeuroImage 67:283–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel A, Gilaie‐Dotan S, Malach R, Bentin S (2010): Top‐down engagement modulates the neural expressions of visual expertise. Cereb Cortex 20:2304–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel A, Kravitz D, Baker CI (2013): Beyond perceptual expertise: Revisiting the neural substrates of expert object recognition. Front Hum Neurosci 7:885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R, de Jong BM (2014): Cerebral activations related to audition‐driven performance imagery in professional musicians. PloS One 9:e93681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdener M, Esposito F, di Salle F, Boller C, Hilti CC, Habermeyer B, Scheffler K, Wetzel S, Seifritz E, Cattapan‐Ludewig K (2010): Musical training induces functional plasticity in human hippocampus. J Neurosci 30:1377–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdener M, Humbel T, Esposito F, Habermeyer B, Cattapan‐Ludewig K, Seifritz E (2014): Jazz drummers recruit language‐specific areas for the processing of rhythmic structure. Cereb Cortex 24:836–843. [DOI] [PubMed] [Google Scholar]
- Hershler O, Hochstein S (2009): The importance of being expert: Top‐down attentional control in visual search with photographs. Attent Percept Psychophys 71:1478–1486. [DOI] [PubMed] [Google Scholar]
- Hervais‐Adelman A, Moser‐Mercer B, Golestani N (2015): Brain functional plasticity associated with the emergence of expertise in extreme language control. NeuroImage 114:264–274. [DOI] [PubMed] [Google Scholar]
- Hill NM, Schneider W (2006): Brain changes in the development of expertise: Neuroanatomical and neurophysiological evidence about skill‐based adaptations In: Ericsson KA, Charness N, Feltovich P, Hoffman RR, editors. The Cambridge Handbook of Expertise and Expert Performance. Cambridge, New York: Cambridge University Press; pp 653–682. [Google Scholar]
- Hoenig K, Muller C, Herrnberger B, Sim EJ, Spitzer M, Ehret G, Kiefer M (2011): Neuroplasticity of semantic representations for musical instruments in professional musicians. NeuroImage 56:1714–1725. [DOI] [PubMed] [Google Scholar]
- Jäncke L, Shah NJ, Peters M (2000): Cortical activations in primary and secondary motor areas for complex bimanual movements in professional pianists. Brain Res Cognit Brain Res 10:177–183. [DOI] [PubMed] [Google Scholar]
- Karni A (1996): The acquisition of perceptual and motor skills: A memory system in the adult human cortex. Brain Res Cognit Brain Res 5:39–48. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Garavan H (2005): Human functional neuroimaging of brain changes associated with practice. Cereb Cortex 15:1089–1102. [DOI] [PubMed] [Google Scholar]
- Kirk U, Skov M, Christensen MS, Nygaard N (2009): Brain correlates of aesthetic expertise: A parametric fMRI study. Brain Cognit 69:306–315. [DOI] [PubMed] [Google Scholar]
- Koelsch S, Fritz T, DY Muller VC, Friederici K, AD , (2006): Investigating emotion with music: An fMRI study. Hum Brain Mapp 27:239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosslyn SM, Pascual‐Leone A, Felician O, Camposano S, Keenan JP, Thompson WL, Ganis G, Sukel KE, Alpert NM (1999): The role of area 17 in visual imagery: Convergent evidence from PET and rTMS. Science 284:167–170. [DOI] [PubMed] [Google Scholar]
- Krawczyk DC, Boggan AL, McClelland MM, Bartlett JC (2011): The neural organization of perception in chess experts. Neurosci Lett 499:64–69. [DOI] [PubMed] [Google Scholar]
- Logan GD (1992): Shapes of reaction‐time distributions and shapes of learning curves: A test of the instance theory of automaticity. J Exp Psychol Learn Mem Cognit 18:883–914. [DOI] [PubMed] [Google Scholar]
- Lotze M, Scheler G, Tan HR, Braun C, Birbaumer N (2003): The musician's brain: Functional imaging of amateurs and professionals during performance and imagery. NeuroImage 20:1817–1829. [DOI] [PubMed] [Google Scholar]
- Loucks TM, Poletto CJ, Simonyan K, Reynolds CL, Ludlow CL (2007): Human brain activation during phonation and exhalation: Common volitional control for two upper airway functions. NeuroImage 36:131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maertens M, Pollmann S (2005): fMRI reveals a common neural substrate of illusory and real contours in V1 after perceptual learning. J Cognit Neurosci 17:1553–1564. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Valentine ER, Wilding JM, Kapur N (2003): Routes to remembering: The brains behind superior memory. Nat Neurosci 6:90–95. [DOI] [PubMed] [Google Scholar]
- Morosan P, Rademacher J, Schleicher A, Amunts K, Schormann T, Zilles K (2001): Human primary auditory cortex: Cytoarchitectonic subdivisions and mapping into a spatial reference system. NeuroImage 13:684–701. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Demorest SM, Aylward EH, Cramer SC, Maravilla KR (2003): FMRI investigation of cross‐cultural music comprehension. NeuroImage 20:378–384. [DOI] [PubMed] [Google Scholar]
- Newell A, Rosenbloom PS (1981): Mechanisms of skill acquisition and the law of practice In: Anderson J, editor. Cognitive Skills and Their Acquisition. Hillsdale, NJ: Erlbaum; pp 1–55. [Google Scholar]
- Oechslin MS, Meyer M, Jancke L (2010): Absolute pitch–functional evidence of speech‐relevant auditory acuity. Cereb Cortex 20:447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oechslin MS, Van De Ville D, Lazeyras F, Hauert CA, James CE (2013): Degree of musical expertise modulates higher order brain functioning. Cereb Cortex 23:2213–2224. [DOI] [PubMed] [Google Scholar]
- Pallesen KJ, Brattico E, Bailey CJ, Korvenoja A, Koivisto J, Gjedde A, Carlson S (2010): Cognitive control in auditory working memory is enhanced in musicians. PloS One 5:e11120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R, Spreng RN, Turner GR (2013): Functional brain changes following cognitive and motor skills training: A quantitative meta‐analysis. Neurorehabil Neural Repair 27:187–199. [DOI] [PubMed] [Google Scholar]
- Pau S, Jahn G, Sakreida K, Domin M, Lotze M (2013): Encoding and recall of finger sequences in experienced pianists compared with musically naive controls: A combined behavioral and functional imaging study. NeuroImage 64:379–387. [DOI] [PubMed] [Google Scholar]
- Pesenti M, Zago L, Crivello F, Mellet E, Samson D, Duroux B, Seron X, Mazoyer B, Tzourio‐Mazoyer N (2001): Mental calculation in a prodigy is sustained by right prefrontal and medial temporal areas. Nat Neurosci 4:103–107. [DOI] [PubMed] [Google Scholar]
- Petersen SE, van Mier H, Fiez JA, Raichle ME (1998): The effects of practice on the functional anatomy of task performance. Proc Natl Acad Sci USA 95:853–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard N, Matsuzaka Y, Strick PL (2013): Extended practice of a motor skill is associated with reduced metabolic activity in M1. Nat Neurosci 16:1340–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plailly J, Delon‐Martin C, Royet JP (2012): Experience induces functional reorganization in brain regions involved in odor imagery in perfumers. Hum Brain Mapp 33:224–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reingold EM, Charness N, Pomplun M, Stampe DM (2001): Visual span in expert chess players: Evidence from eye movements. Psychol Sci 12:48–55. [DOI] [PubMed] [Google Scholar]
- Reingold EM, Sheridan H (2011): Eye movements and visual expertise in chess and medicine In: Liversedge SP, Gilchrist ID, Everling S, editors. Oxford Handbook on Eye Movements. Oxford: Oxford University Press; pp 528–550. [Google Scholar]
- Rijntjes M, Dettmers C, Buchel C, Kiebel S, Frackowiak RS, Weiller C (1999): A blueprint for movement: Functional and anatomical representations in the human motor system. J Neurosci 19:8043–8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Gallese V, Fogassi L (1996): Premotor cortex and the recognition of motor actions. Brain Res Cognit Brain Res 3:131–141. [DOI] [PubMed] [Google Scholar]
- Schneider W, Shiffrin RM (1977): Controlled and automatic human information‐processing.1. Detection, search, and attention. Psychol Rev 84:1–66. [Google Scholar]
- Schulze K, Mueller K, Koelsch S (2011a): Neural correlates of strategy use during auditory working memory in musicians and non‐musicians. Eur J Neurosci 33:189–196. [DOI] [PubMed] [Google Scholar]
- Schulze K, Zysset S, Mueller K, Friederici AD, Koelsch S (2011b): Neuroarchitecture of verbal and tonal working memory in nonmusicians and musicians. Hum Brain Mapp 32:771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulcre J (2015): An OP4 functional stream in the language‐related neuroarchitecture. Cereb Cortex 25:658–666. [DOI] [PubMed] [Google Scholar]
- Sluming V, Brooks J, Howard M, Downes JJ, Roberts N (2007): Broca's area supports enhanced visuospatial cognition in orchestral musicians. J Neurosci 27:3799–3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD, Wilson M, Reisberg D (1995): The role of subvocalization in auditory imagery. Neuropsychologia 33:1433–1454. [DOI] [PubMed] [Google Scholar]
- Snyder AW, Mitchell DJ (1999): Is integer arithmetic fundamental to mental processing?: The mind's secret arithmetic. Proc Biol Sci R Soc 266:587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Michimata C, Kaminaga T, Honda M, Sadato N (2002): Superior digit memory of abacus experts: An event‐related functional MRI study. Neuroreport 13:2187–2191. [DOI] [PubMed] [Google Scholar]
- Tervaniemi M, Kruck S, De Baene W, Schroger E, Alter K, Friederici AD (2009): Top‐down modulation of auditory processing: Effects of sound context, musical expertise and attentional focus. Eur J Neurosci 30:1636–1642. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P (2012): Minimizing within‐experiment and within‐group effects in Activation Likelihood Estimation meta‐analyses. Hum Brain Mapp 33:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Herve PY, Duffau H, Crivello F, Houde O, Mazoyer B, Tzourio‐Mazoyer N (2006): Meta‐analyzing left hemisphere language areas: Phonology, semantics, and sentence processing. NeuroImage 30:1414–1432. [DOI] [PubMed] [Google Scholar]
- Wan X, Nakatani H, Ueno K, Asamizuya T, Cheng K, Tanaka K (2011): The neural basis of intuitive best next‐move generation in board game experts. Science 331:341–346. [DOI] [PubMed] [Google Scholar]
- Weinberger, N.M. (2012) Plasticity in the primary auditory cortex, not what you think it is: Implications for basic and clinical auditory neuroscience. Otolaryngology Suppl 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SS, Chang TT, Majid A, Caspers S, Eickhoff SB, Menon V (2009): Functional heterogeneity of inferior parietal cortex during mathematical cognition assessed with cytoarchitectonic probability maps. Cereb Cortex 19:2930–2945. [DOI] [PMC free article] [PubMed] [Google Scholar]


