Abstract
Introduction
Mild cognitive impairment (MCI) and visual hallucinations (VH) are common co‐morbidities and risk factors for dementia in Parkinson's disease (PD). The relative value of each of them in the progression to dementia is unknown. We investigated cognitive impairment and cerebral hypometabolism in PD‐MCI patients with VH (VH‐positive) and without (VH‐negative).
Methods
Twenty‐one PD‐MCI patients (12 VH‐negative, nine VH‐positive) and 19 controls were studied using a comprehensive neuropsychological battery and [18F]‐Fluorodeoxyglucose positron emission tomography (FDG‐PET). The neuropsychological assessment was repeated after 30 months. Regional FDG uptake was analyzed using statistical parametric mapping.
Results
VH‐positive patients had lower FDG uptake bilaterally in the occipital, and parietal cortex, right temporal lobe and in the left cingulum compared with VH‐negative patients. The two groups showed no significant differences in clinical characteristics and cognitive status at baseline. After 30 months of follow‐up, three (25%) and four (50%) of the VH‐negative and VH‐positive patients, respectively, had progressed to dementia.
Conclusion
Even in the absence of significant cognitive differences, PD‐MCI patients with VH exhibit more severe cerebral hypometabolism and had a higher rate of progression to dementia than VH‐negative patients, supporting the importance of VH and cerebral hypometabolism in establishing the risk of dementia in PD‐MCI. Hum Brain Mapp 37:968–977, 2016. © 2015 Wiley Periodicals, Inc.
Keywords: visual hallucinations, mild cognitive impairment, cerebral metabolism, Parkinson's disease, dementia, FDG‐PET
Abbreviations
- FDG‐PET
[18F]‐Fluorodeoxyglucose positron emission tomography
- IDDD
Interview for Daily Living Activities
- MCI
Mild cognitive impairment
- MDS
Movement Disorders Society
- MMSE
Mini mental state examination
- MNI
Montreal Neurological Institute
- PD
Parkinson's disease
- PDD
PD patients with dementia
- VH
Visual hallucinations
INTRODUCTION
Visual hallucinations (VH) [Aarsland et al., 2003; Fenelon et al., 2000; Goetz et al., 2001; Santangelo et al., 2007] and mild cognitive impairment (MCI) in patients with Parkinson's disease (PD) [Broeders et al., 2013; Gasca‐Salas et al., 2014; Janvin et al., 2006; Pedersen et al., 2013] are risk factors for dementia. In addition, [18F]‐Fluorodeoxyglucose positron emission tomography (FDG‐PET) studies have shown that both MCI [Garcia‐Garcia et al., 2012; Hosokai et al., 2009; Huang et al., 2008; Lyoo et al., 2010; Pappata et al., 2011] and VH [Boecker et al., 2007; Nagano‐Saito et al., 2004; Park et al., 2013] are associated with cerebral hypometabolism mainly in the temporoparietooccipital junction and the frontal cortex, a pattern which is more widespread in PD patients with dementia (PDD) [Bohnen et al., 2011; Garcia‐Garcia et al., 2012; Liepelt et al., 2009; Peppard et al., 1992; Yong et al., 2007]. However, MCI and VH are common co‐morbidities, with VH frequently associated with cognitive impairment in global function or in specific domains (executive [Barnes and Boubert, 2008; Factor et al., 2014; Hepp et al., 2013], attention, [Barnes and Boubert, 2008; Factor et al. 2014; Hepp et al., 2013], memory [Factor et al. 2014], language [Factor et al. 2014], visuospatial [Barnes and Boubert, 2008; Factor et al., 2014; Gallagher et al., 2011; Hepp et al., 2013; Llebaria et al., 2010]). Therefore, the specific value of MCI and VH in the risk of dementia and in the pattern of hypometabolism is not known. In this regard, not all PD‐MCI patients evolve towards dementia, and the features associated with the progression of cognitive decline in this population are not well known. We aimed to investigate if PD‐MCI patients with VH have a more severe degree of cognitive impairment and hypometabolism than PD‐MCI patients without VH, and how these features may influence the rate of progression to dementia.
METHODS
Subjects
Patients over the age 60 years with PD [Hughes et al., 1992] of at least 10 years' duration, who were on a stable medication regimen, and who had been diagnosed with MCI, were recruited from the Movement Disorder Unit of the University Clinic of Navarra. Patients were classified as MCI following the level II guidelines of the Movement Disorders Society (MDS) Criteria Task Force [Litvan et al., 2012] when the following criteria were accomplished: (i) cognitive decline with no functional impairment reported by either the patient or informant, or observed by the neurologist; (ii) the patient scored 1.5 standard deviations or more below the mean for age‐ and education‐matched healthy controls in at least two out of ten tests exploring five cognitive domains (two tests per domain). To determine test score deviations from control subjects, we calculated the Z‐score value according to the following formula: (test score of the patient—median test score from control sample)/test standard deviation from control sample); (iii) there was no evidence of abnormal activities of daily living. Dementia was diagnosed according to the MDS criteria [Emre et al., 2007], and was typified by the patient having a cognitive impairment in more than one cognitive domain that represented a decline from premorbid levels, and deficits that were severe enough to impair significantly functional independence. Functional independence was assessed with the Interview for Daily Living Activities (IDDD) [Teunisse and Derix, 1997].
VH were evaluated using item 2 in part I of the Unified Parkinson's Disease Rating Scale (UPDRS‐I). Patients were classified as: (i) VH‐negative (VH‐n) if they scored 0 and (ii) VH‐positive (VH‐p) for scores ≥ 1 [Goldman et al., 2014; Llebaria et al., 2010]. Patients with other neurological or psychiatric disorders, including severe depression (score higher than 20 in the Yesevage Geriatric Depression Rating Scale), MRI abnormalities, other neurological or medical causes for cognitive impairment, or who were on treatment with atypical neuroleptics, anticholinesterase or anticholinergic drugs, were excluded. Nineteen healthy controls were recruited from members of the Association of Blood Donors of Navarra (Spain) after excluding any history of major illness, psychiatric or neurological disease, memory complaints, neuropsychological assessment below normal scores, or MRI abnormalities. The local Medical Ethics Committee approved the study. Participants provided written informed consent.
Neuropsychological evaluation was undertaken with the mini mental state examination (MMSE) and with a comprehensive neuropsychological battery evaluating five cognitive domains (attention, memory, language and executive and visuospatial functions) as recommended by the MDS task force [Litvan et al., 2012] and as previously used by our team [Garcia‐Garcia et al., 2012; Gasca‐Salas et al., 2014; Gonzalez‐Redondo et al., 2014]. The global cognition Z‐score was calculated according to the following steps. First, individual neuropsychological test scores were transformed into Z‐scores (using the mean and standard deviation of the control sample). Second, Z‐score values for the different domains were calculated from the average of the Z‐scores of the tests assessing each domain. Finally, a global cognition Z composite score was obtained by the mean of the five cognitive domain Z‐scores. Motor assessment was undertaken with the Hoehn and Yahr scale and the motor section of the UPDRS (UPDRS‐III). Dopaminergic treatment as well as demographic and clinical variables were also recorded. Patients were evaluated at baseline and after 30 (range = 24–36) months in order to study the evolution of their cognitive state.
FDG‐PET
Image data acquisition
Subjects were scanned on a Siemens ECAT EXAT HR+ scanner (Siemens, Knoxville, TN) in the morning in a fasting state. Central nervous system depressant drugs (i.e., benzodiazepines or antidepressants) were withdrawn according to their pharmacological kinetics. Patients with PD were always scanned in the ‘on’ state. Before injection of the radiopharmaceutical agent, blood glucose was checked and confirmed to be <120 mg/dl in all cases. 18F‐FDG (370 MBq) was injected intravenously to all participants with eyes closed after a few minutes of rest in silence and with dimmed lighting. Subjects were required to rest for 40 min in the supine position in the PET scanner bed with their eyes closed. Seventy‐four planes (128x128 matrix) were acquired with a voxel size of 2.06x2.06x2.06 mm during a 20 min scan.
Data analysis
A FDG‐PET template in Montreal Neurological Institute (MNI) space was first created using FDG‐PET images and their corresponding MRI T1 from a control sample [Garcia‐Garcia et al., 2012]. All reconstructed FDG‐PET images were spatially normalized into the template standard stereotactic space and voxel values were normalized to pons activity.
Finally, images were smoothed using an 8 mm full width half‐maximum Gaussian filter. Differences in metabolism between the groups were analyzed using ANOVA, including age and educational level as covariates for comparisons between patients and controls, and age and global cognition Z‐score for comparisons between VH‐p and VH‐n patients.
The statistical threshold was P < 0.05 and corrected for multiple comparisons (FDR: false discovery rate) with a cluster size > 20 voxels. Correlation between the global cognition Z‐ score and FDG uptake was assessed using regression analysis with a cluster size of >20 voxels.
Data were analysed using SPM8 software (Wellcome Department of Neurology, London, UK) in MATLAB 7.13 (Mathworks Inc. Sherborn, MA).
We used the mni2tal program (http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach) to transform the coordinates of the voxel peaks into Talairach space and their anatomical locations were found using Talairach Daemon Client [Lancaster et al., 2000]
Statistical Analysis for Demographics and Neuropsychological Variables
ANOVA or Kruskall‐Wallis test followed by post‐hoc comparisons and Fisher's exact test were used for continuous or categorical variables, respectively. Educational level was used as a covariate in neuropsychological score comparisons. Statistical analyses were performed with SPSS 20. A P‐value <0.05 was considered to indicate statistical significance.
RESULTS
Twenty‐one PD‐MCI patients (9 VH‐p and 12 VH‐n) were studied. There were no differences between groups in general features (Table 1). Compared with VH‐p patients, VH‐n patients had a lower score in the Boston Naming Test, but they did not differ in any other neuropsychological variable (Table 2). After 29.1 ± 6.6 months of follow‐up, 3 out of 12 (25%) VH‐n and 4 out of 8 (50%) VH‐p patients had progressed to dementia. One patient with VH was lost to the follow‐up.
Table 1.
General features of Parkinson's disease patients and control subjects
| Controls (n = 19) | VH‐n (n = 12) | VH‐p (n = 9) | P value | P value | P value | |
|---|---|---|---|---|---|---|
| Controls vs. VH‐n | Controls vs. VH‐p | VH‐n vs. VH‐p | ||||
| Age (years) | 70.1 (3.1) | 70.8 (3.4) | 70.7 (3.9) | 0.1 | 0.2 | 0.91 |
| Male, n (%) | 10 (50) | 4 (33.3) | 6 (66.7) | 0.29 | 0.48 | 0.13 |
| Disease duration (years) | – | 14.3 (6.3) | 14.7 (5.4) | – | – | 0.87 |
| Most affected body side | – | Right: 6 (50%) | Right: 5 (55.5%) | |||
| Left: 6 (50%) | Left: 3 (33.3%) | – | – | 0.43 | ||
| Bilateral: 1 (11.1%) | ||||||
| UPDRS III OFF | – | 32.6 (12.9) | 33.2 (8.7) | – | – | 0.28 |
| UPDRS III ON | – | 17 (8.9) | 16.1 (7.3) | – | – | 0.12 |
| LEDD (mg/day) | – | 1187 (460) | 1102 (380) | – | – | 0.66 |
| DA LEDD (mg/day) | – | 343 (289) | 371 (314) | – | – | 0.84 |
| H&Y, n (%) | – | 1.5: 1 (8.3%) | 1.5: 1 (11.1%) | – | – | 0.78 |
| 2: 2 (16.7%) | 2: 1 (11.1%) | – | – | |||
| 3: 7 (58.3%) | 2.5: 1 (11.1%) | – | – | |||
| 4:2 (16.7%) | 3: 4 (44.4%) | – | – | |||
| 4: 2 (22.2%) | – | – | ||||
| Education (years) | 9.7 (3.1) | 9 (2.5) | 12 (4) | 1 | 0.22 | 0.07 |
Data are presented as mean (standard deviation) or number (frequency).
VH‐n (VH‐negative): PD patients with mild cognitive impairment without visual hallucinations; VH‐p (VH‐positive): PD patients with mild cognitive impairment with visual hallucinations; H&Y: Hoehn and Yahr; UPDRS III: Unified Parkinson's disease Rating Scale motor section; LEDD: L‐dopa equivalent daily dose. LEDD was calculated using the following formula: levodopa 100 mg = levodopa retard 75 mg = pergolide 1mg = cabergoline 0.8 mg = ropinirole 4mg = rotigotine 4mg = pramipexole 1mg (Grosset, Needleman et al., 2004). DA LEDD: L‐dopa equivalent daily dose of dopamine agonist according to the same formula.
Table 2.
Neuropsychological test scores obtained by Parkinson's disease patients and control subjects
| Controls (n = 19) | VH‐n (n = 12) | VH‐p (n = 9) | P value | P value | P value | |
|---|---|---|---|---|---|---|
| Controls vs. VH‐n | Controls vs. VH‐p | VH‐n vs. VH‐p | ||||
| MMSE | 29.1 (1.2) | 25.9 (2.7) | 27 (1.7) | <0.0005 | 0.03 | 0.53 |
| IDDD | – | 36.6 (4) | 36.3 (4.1) | – | – | 0.52 |
| Attention domain | ||||||
| Digitsa | 11.8 (2.2) | 10.5 (2.6) | 11.5 (2.1) | 0.8 | 1 | 0.23 |
| TMTAa | 64.7 (27.1) | 91.5 (39.5) | 82.8 (39.6) | 0.15 | 0.66 | 1 |
| SW | 49.8 (6.7) | 33.8 (7.6) | 39.9 (9) | <0.00001 | 0.01 | 0.38 |
| SC | 43 (6.9) | 31.4 (5.6) | 35.4 (10) | 0.001 | 0.06 | 1 |
| Executive function domain | ||||||
| Phonemic fluency (60 seconds)a | 15 (5.3) | 7.8 (4.8) | 10.4 (4.1) | 0.001 | 0.09 | 1 |
| SWC | 46 (6.8) | 36.8 (9.3) | 38.9(8) | 0.014 | 0.12 | 1 |
| RPM | 27.4 (4.4) | 19 (3.8) | 22.5 (6.1) | <0.0005 | 0.06 | 0.51 |
| TMTBa | 139.2 (63.5) | 240 (65) | 234 (74.9) | 0.001 | 0.004 | 1 |
| Memory domain | ||||||
| Cerad Word delayed recalla | 5.7 (1.4) | 1.9 (2.1) | 2.1 (1.3) | <0.000005 | <0.00005 | 1 |
| Cerad Word Recognition | 9 (1.5) | 8.2 (1.4) | 7.1 (1.8) | 0.56 | <0.01 | 1 |
| FCSRT total recalla | 46.9 (1.3) | 46.2 (3.6) | 45.6 (2.1) | 1 | 0.41 | 1 |
| Recall of figurea | 6.5 (3.1) | 2.7 (3.2) | 4.5 (2.3) | 0.006 | 0.36 | 0.43 |
| Language domain | ||||||
| Bostona | 51 (5.4) | 39.4 (7.2) | 47.1 (5.9) | <0.0001 | 0.39 | 0.026 |
| Semantic fluency (60 seconds)a | 18.6 (4.6) | 10.9 (3.1) | 12.9 (5.6) | <0.0005 | 0.01 | 0.82 |
| Visuospatial function domain | ||||||
| Copy of figurea, b | 9.89 (0.5) | 9.7 (4) | 9.6 (0.9) | 1 | 1 | 1 |
| Copy of intersecting pentagonsa, c | 2: 19 (100%) | 2:7 (58.3%) | 2:5(55.6%) | 0.04 | 0.02 | 0.42 |
| 1:4(33.3%) | 1:1(11.1%) | |||||
| 0:1 (8.3%) | 0:2(22.2%) | |||||
Data are presented as mean (standard deviation) or number (frequency).
VH‐n (VH‐negative): Parkinson's disease patients with mild cognitive impairment without visual hallucinations; VH‐p (VH‐positive): Parkinson's disease patients with mild cognitive impairment with visual hallucinations.
MMSE: Mini‐Mental State Examination; IDDD: Interview for daily living activities; TMT‐A: Trail Making Test A; SW: Stroop Words; SC: Stroop Colors; SWC: Stroop Words‐Colors; RPM: Raven Progressive Matrices; TMT‐B: Trail Making Test B; FCSRT: Free and Cue Selective Reminding test; Boston: Boston Naming Test; ns: non‐significant.
Test used for the diagnosis of MCI following MDS level II guidelines.
Copy and delayed recall of the most complex figure from the two simple figures of Massachusetts General Hospital (Sala et al., 2008).
Copy of intersecting pentagon is represented by frequency of presentation of each score because of its short range. Scoring was performed using a 0–2 rating scale in which 2 points indicated that all 10 angles were present and the 2 pentagons were intersecting, 1 point indicated that the two intersecting figures were present and one of them had 5 angles, and 0 indicating a poorer performance than the first two cases (Ala et al., 2001).
Regional Differences in FDG‐PET
VH‐p patients showed lower FDG uptake bilaterally in the occipital and parietal cortices, and to a lesser extent in the right temporal and left cingulate than VH‐n patients (FDR P < 0.05). (Fig. 1C, Table 3)
Figure 1.
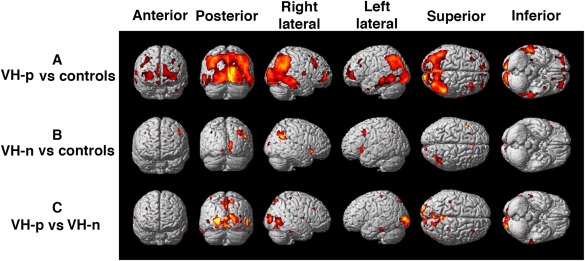
Brain regions with reduced metabolism in PD‐MCI patients with and without VH compared with controls. (A) PD‐MCI VH‐p < controls (P < 0.05 FDR corrected; age and educational level as covariates) (B) PD‐MCI VH‐n < controls (P < 0.001 uncorrected; age and educational level as covariates). (C) PD‐MCI VH‐p < PD‐MCI VH‐n (P < 0.05 FDR corrected; age and global cognition as covariates).
Table 3.
Summary of areas of hypometabolism obtained in the comparison between the different groups of PD‐MCI patients (VH‐positive, VH‐negative) and controls
| Cluster size | Coordinates | Hemisphere | Lobe | Gyrus | ||||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | T | |||||
| VH‐p vs. controls (FDR) | 51491 | 12 | −93 | −12 | 8,0 | Right | Occipital | Lingual Gyrus |
| 45 | −57 | 27 | 7,0 | Right | Temporal | Middle Temporal Gyrus | ||
| 51 | −52,5 | 31,5 | 7,0 | Right | Temporal | Supramarginal Gyrus | ||
| 15 | −94,5 | 15 | 6,0 | Right | Occipital | Cuneus | ||
| −7,5 | −97,5 | −3 | 6,0 | Left | Occipital | Lingual Gyrus | ||
| 31,5 | −69 | 45 | 6,0 | Right | Parietal | Precuneus | ||
| −42 | −57 | 49,5 | 5,0 | Left | Parietal | Inferior Parietal Lobule | ||
| 45 | −52,5 | 45 | 5,0 | Right | Parietal | Inferior Parietal Lobule | ||
| −42 | −61,5 | 22,5 | 5,6 | Left | Temporal | Middle Temporal Gyrus | ||
| −25,5 | −73,5 | 49,5 | 5,0 | Left | Parietal | Precuneus | ||
| 57 | −33 | −27 | 5,0 | Right | Temporal | Fusiform Gyrus | ||
| −46,5 | −57 | 36 | 5,0 | Left | Parietal | Angular Gyrus | ||
| −36 | −85,5 | 27 | 5,0 | Left | Occipital | Middle Occipital Gyrus | ||
| −4,5 | −85,5 | 12 | 4,0 | Left | Occipital | Cuneus | ||
| 245 | 66 | −3 | 9 | 5,0 | Right | Frontal | Precentral Gyrus | |
| 1336 | 27 | 52,5 | 0 | 5,0 | Right | Frontal | Superior Frontal Gyrus | |
| 48 | 55,5 | 6 | 4,0 | Right | Frontal | Middle Frontal Gyrus | ||
| 772 | 36 | 22,5 | 39 | 4,0 | Right | Frontal | Middle Frontal Gyrus | |
| 2900 | −60 | −57 | 0 | 4,0 | Left | Temporal | Middle Temporal Gyrus | |
| −46,5 | −79,5 | 0 | 3,0 | Left | Occipital | Middle Occipital Gyrus | ||
| −57 | −15 | −28,5 | 3,0 | Left | Temporal | Fusiform Gyrus | ||
| 2541 | −9 | 60 | 7,5 | 4,0 | Left | Frontal | Medial Frontal Gyrus | |
| −40,5 | 49,5 | 4,5 | 3,0 | Left | Frontal | Middle Frontal Gyrus | ||
| −13,5 | 57 | 12 | 4,0 | Left | Frontal | Superior Frontal Gyrus | ||
| 627 | 9 | 61,5 | 7,5 | 3,0 | Right | Frontal | Medial Frontal Gyrus | |
| 95 | −40,5 | 6 | −37,5 | 3,0 | Left | Temporal | Superior Temporal Gyrus | |
| 163 | 7,5 | 54 | −18 | 3,0 | Right | Frontal | Medial Frontal Gyrus | |
| 35 | 40,5 | 10,5 | 57 | 3,0 | Right | Frontal | Middle Frontal Gyrus | |
| 253 | −31,5 | 28,5 | 39 | 3,0 | Left | Frontal | Middle Frontal Gyrus | |
| 25 | 24 | 33 | 48 | 3,0 | Right | Frontal | Superior Frontal Gyrus | |
| VH−n vs. Controls (P < 0.001 uncorrected) | 620 | −12,2 | 4,5 | 12,6 | 4,6 | Left | Sub−lobar | Caudate |
| 1374 | 44,3 | −57,7 | 26,6 | 4,6 | Right | Temporal | Superior Temporal Gyrus | |
| 27,5 | −70,0 | 37,3 | 3,7 | Right | Parietal | Precuneus | ||
| 770 | 12,7 | 1,1 | 18,1 | 4,5 | Right | Sub‐lobar | Caudate | |
| 571 | 3,0 | −20,9 | 11,8 | 4,4 | Right | Sub‐lobar | Thalamus | |
| −2,6 | −19,4 | 11,9 | 3,9 | Left | Sub‐lobar | Thalamus | ||
| 243 | 42,2 | 19,8 | −1,3 | 4,3 | Right | Frontal | Inferior Frontal Gyrus | |
| 548 | 11,1 | −89,7 | 8,1 | 4,1 | Right | Occipital | Cuneus | |
| 10,0 | −89,0 | −13,4 | 4,1 | Right | Occipital | Lingual Gyrus | ||
| 107 | 8,3 | −21,3 | 30,8 | 4,0 | Right | Limbic | Cingulate Gyrus | |
| 242 | −45,9 | 4,9 | 39,1 | 4,0 | Left | Frontal | Middle Frontal Gyrus | |
| 67 | −8,1 | −90,6 | −9,9 | 3,8 | Left | Occipital | Lingual Gyrus | |
| 328 | −37,0 | 18,6 | −1,3 | 3,7 | Left | Sub‐lobar | Insula | |
| −41,3 | 16,5 | 6,5 | 3,7 | Left | Frontal | Precentral Gyrus | ||
| 206 | 3,0 | 16,1 | 23,4 | 3,7 | Right | Limbic | Anterior Cingulate | |
| 3,0 | 19,3 | 19,7 | 3,5 | Right | Limbic | Anterior Cingulate | ||
| 64 | 11,1 | −52,3 | 15,7 | 3,7 | Right | Limbic | Posterior Cingulate | |
| 26 | 33,3 | 14,1 | 42,7 | 3,7 | Right | Frontal | Middle Frontal Gyrus | |
| 21 | −36,4 | −65,2 | 34,0 | 3,4 | Left | Parietal | Angular Gyrus | |
| VH‐p vs. VH‐n(FDR) | 6160 | 21 | −88,5 | 7,5 | 6,0 | Right | Occipital | Lingual Gyrus |
| −7,5 | −66 | 4,5 | 5,0 | Left | Occipital | Lingual Gyrus | ||
| 7,5 | −96 | 13,5 | 5,0 | Right | Occipital | Cuneus | ||
| −1,5 | −96 | 9 | 5,0 | Left | Occipital | Cuneus | ||
| −24 | −99 | 16,5 | 3,0 | Left | Occipital | Middle Occipital Gyrus | ||
| 543 | 57 | −72 | 3 | 5,0 | Right | Occipital | Inferior Temporal Gyrus | |
| 54 | −75 | 7,5 | 4,0 | Right | Occipital | Middle Occipital Gyrus | ||
| 3063 | 16,5 | −72 | 57 | 5,0 | Right | Parietal | Precuneus | |
| −7,5 | −76,5 | 52,5 | 5,0 | Left | Parietal | Precuneus | ||
| 0 | −39 | 46,5 | 4,0 | Left | Limbic | Cingulate Gyrus | ||
| 114 | −39 | −31,5 | 64,5 | 4,0 | Left | Parietal | Postcentral Gyrus | |
| 69 | −49,5 | −79,5 | 4,5 | 4,0 | Left | Occipital | Middle Occipital Gyrus | |
| −43,5 | −87 | −1,5 | 3,0 | Left | Occipital | Inferior Occipital Gyrus | ||
| 54 | 58,5 | −31,5 | −27 | 4,0 | Right | Temporal | Inferior Temporal Gyrus | |
| 33 | 67,5 | −1,5 | 10,5 | 4,0 | Right | Frontal | Precentral Gyrus | |
| 34 | 34,5 | −81 | 48 | 4,0 | Right | Parietal | Precuneus | |
| 25 | 37,5 | −90 | 16,5 | 4,0 | Right | Occipital | Middle Occipital Gyrus | |
VH‐p (VH‐positive): Parkinson's disease patients with mild cognitive impairment with visual hallucinations; VH‐n (VH‐negative): Parkinson's disease patients with mild cognitive impairment without visual hallucinations. FDR: false discovery rate.
When compared to control subjects (FDR P < 0.05), VH‐p patients exhibited extensive hypometabolic areas involving mainly the bilateral parieto‐occipital and temporal cortex, and to a lesser extent the bilateral frontal cortex (Fig. 1A, Table 3) No differences were observed between VH‐n patients and control subjects in a stringently corrected analysis (FDR corrected). Using a less‐stringent statistical analysis (P < 0.001 uncorrected), VH‐n patients had small regions of reduced FDG uptake in the right superior temporal gyrus, left insula, parietal lobe (right precuneus and left angular gyrus), occipital lobe (right cuneus and bilateral lingual gyrus), frontal gyrus (right inferior and left middle), right cingulate gyrus, bilateral thalamus and right caudate (Fig. 1B, Table 3).
In relation to the progression to dementia in each group of patients, VH‐p patients who progressed (converters) and who did not progress (non‐converters) to dementia, in comparison with healthy controls (FDR corrected), exhibited hypometabolism with a similar distribution to that observed in the whole group of VH‐p patients (Fig. 2A,B, Supporting Information Table). In the comparison between the two subgroups (Fig. 2C, Supporting Information Table), VH‐p converters had more extended hypometabolism than VH‐p non‐converters in the left parahippocampal gyrus, left uncus, right temporal lobe and bilateral caudate (P < 0.001 uncorrected) (Fig. 2C, Supporting Information Table). VH‐n patients who developed dementia had hypometabolism in both temporal lobes, right cuneus and smaller areas in areas in right precentral and angular gyrus compared with controls (Fig. 2D, Supporting Information Table), whereas VH‐n patients who did not develop dementia did not show any difference with respect to control subjects (P < 0.001 uncorrected). No differences were observed between VH‐n patients who progressed or not to dementia.
Figure 2.

Comparison of brain regions with reduced metabolism in PD‐MCI VH‐p converters and non‐converters to dementia and controls. (A) PD‐MCI VH‐p converters < controls (P < 0.05 FDR corrected; age and educational level as covariates). (B) PD‐MCI VH‐p non‐converters < controls (P < 0.05 FDR corrected; age and educational level as covariates). (C) PD‐MCI VH‐p converters < PD‐MCI VH‐p non‐converters (P < 0.001 uncorrected; age and global cognition as covariates). (D) PD‐MCI VH‐n converters < controls (P < 0.001 uncorrected; age and educational level as covariates)
There was a positive correlation between global cognition and FDG uptake in the left inferior parietal lobe (49 voxels, T = 5.1; X = −29, Y = −71, Z = 57) for the whole sample of PD‐MCI patients and in VH‐p patients. These findings disappeared after FDR correction. No correlation was observed in the group of VH‐n patients.
DISCUSSION
We have undertaken a study in PD‐MCI patients with and without VH, and shown that, even when the severity of cognitive impairment was not different, the presence of VH in such patients was associated with more severe hypometabolism in extended areas of the occipital‐parietal and inferior temporal cortices. Interestingly, this pattern of hypometabolism seems to exceed that encountered in studies undertaken in PD‐MCI patients [Garcia‐Garcia et al., 2012; Hosokai et al., 2009; Huang et al., 2008; Lyoo et al., 2010; Pappata et al., 2011] not controlled by the presence of VH, but resembles the pattern associated with PDD [Bohnen et al., 2011; Garcia‐Garcia et al., 2012; Liepelt et al., 2009; Peppard et al., 1992; Yong et al., 2007]. Indeed, 50% of patients with VH had developed dementia in contrast to 25% in the group of MCI patients without VH at 30 months follow‐up. In addition, compared with healthy subjects, only VH‐p patients had reduced FDG uptake in the occipital, parietal and temporal cortices. Interestingly, as the number of VH‐n patients was low, when applying a less‐stringent statistical analysis, VH‐n patients had small areas of hypometabolism mainly in the superior temporal, precuneus, cuneus and lingual gyri and in subcortical structures (caudate nucleus and thalamus). These results suggest that the pattern of hypometabolism currently associated with PD‐MCI [Garcia‐Garcia et al., 2012; Hosokai et al., 2009; Huang et al., 2008; Lyoo et al., 2010; Pappata et al., 2011] is not only due to cognitive decline but also to the presence of VH. Thus, MCI could be associated with small cortical areas of reduced FDG in the frontal lobe, superior temporal, precuneus, cuneus and lingual gyrus that expand at the time that new areas of hypometabolism are added to the occipitoparietotemporal junction, occipital regions (lingual, fusiform, medial and inferior lobules) and cuneus when VH are present. In this sense, an association between global cognitive function and FDG uptake was encountered only in a reduced area of the parietal cortex both in the whole group of patients with MCI and in VH‐p patients, suggesting that most of the hypometabolism observed was related to VH. In addition, despite thalamic and caudate hypometabolism is also being observed in MCI‐VH‐n patients (uncorrected analysis), suggesting this deficit might be more related to cognitive decline than to VH, we did not encounter any association between global cognitive function and FDG uptake in these regions. This last result is apparently at odds with previous studies showing hypometabolism in the caudate of PDD patients [Garcia‐Garcia et al., 2012], and in the caudate and thalamus of non‐demented PD patients who develop dementia after two years of follow‐up [Bohnen et al., 2011]. This discrepancy might be explained by the fact that, in contrast to these studies where all patients were demented at the time of the study or two years later, we have studied a much more heterogeneous group of PD‐MCI patients. In this sense, we have observed that VH‐p patients that progressed to dementia had lower FDG uptake in the caudate bilaterally, and also in the left parahippocampal gyrus, left uncus, and right middle temporal lobe than VH‐p patients who did not progress to dementia. This result coincides with the finding in VH‐n patients who developed dementia; these patients also showed hypometabolism mainly in both temporal lobes and right cuneus, indicating that subcortical, temporal and hippocampal hypometabolism might be related purely to cognition and not to VH.
On the other hand, the hypometabolic areas found in VH‐p compared with VH‐n patients were located in the posterior cortex, and VH‐p patients, both converters and non‐converters to dementia, have a similar pattern of extensive, mainly posterior, cortical hypometabolism. These findings suggest a cortical substrate for VH in keeping with MRI studies of grey matter volume [Goldman et al., 2014] and clinico‐pathological studies showing that PD patients with VH exhibit a higher cortical density of Lewy bodies, mainly in the parietal and temporal lobes and in the amygdala [Harding et al. 2002, Papapetropoulos et al., 2006, Jacobson et al., 2014].
Taken together, the presence of VH in PD‐MCI patients should be considered as an indicator of higher cerebral dysfunction, closer to that observed in PDD, and therefore indicative of patients with a higher risk of evolution to dementia. Our results are also in keeping with the only previous study of VH controlled by cognitive status undertaken in PD patients, which showed that VH‐p patients had a greater reduction of grey matter volume in the cuneus, lingual and fusiform gyri, occipital and parietal lobule, and in the cingulate, paracentral and precentral gyri [Goldman et al., 2014]. The regions of hypometabolism we described in VH‐p patients, and which mostly coincide with the atrophied regions described by Goldman et al [Goldman et al., 2014], are involved in higher visual processing [Diederich et al., 2009]. This is in line with studies showing that visuospatial dysfunction [Gasca‐Salas et al., 2014; Pagonabarraga et al., 2008; Williams‐Gray et al., 2009] and posterior hypometabolism [Bohnen et al., 2011; Garcia‐Garcia et al., 2012] in PD are related to the development of dementia. The results also reinforce the importance of cerebral posterior dysfunction heralded by the presence of VH in PD‐MCI patients, who display a higher tendency to develop dementia. It must be noted that no significant differences in visuospatial function between VH‐p and VH‐n patients were encountered. This could be due to the fact that this domain was explored by two similar tests (drawing of figures) and that, similarly to previous studies [Boecker et al., 2007; Nagano‐Saito et al., 2004], the sample size was low, perhaps accounting for subtle differences that were not picked up. In addition, it should be noted that although the difference was not significant, VH‐p patients had more years of education, a parameter that could have influenced the cognitive performance in this group.
Our study is the first to assess cerebral metabolism associated with VH in PD‐MCI patients in whom clinical features (age, disease duration, motor and cognitive dysfunction) were similar to those of PD‐MCI patients without VH. Moreover, we have excluded patients with depression and under pharmacological treatment (antipsychotic, anticholinesterase, anticholinergic), which could have interfered with the expression of VH and affected cognitive and metabolic findings. Previous studies [Boecker et al., 2007; Nagano‐Saito et al., 2004] undertaken in VH‐p PD patients included non‐demented patients in whom no difference between MCI and normal cognition was distinguished, and where VH‐p had a longer disease duration [Nagano‐Saito et al., 2004] or where patients had poorer motor function [Boecker et al., 2007]. Only one study [Park et al. 2013] included a subgroup of VH‐p with cognitive impairment, but this sub‐group was compared with VH‐n patients with normal cognition, and cognitive impairment was not defined by the MCI diagnostic criteria.
Our findings have practical implications because, although MCI in PD patients is a well‐recognized risk factor for dementia [Broeders et al., 2013; Gasca‐Salas et al., 2014; Janvin et al., 2006; Pedersen et al., 2013], it is a heterogeneous entity and not all patients progress to dementia [Litvan et al., 2011]. Thus, the identification of subtypes of MCI or other clinical features that increase the risk of cognitive decline is relevant and may have practical applications in future therapeutic studies.
In summary, we have shown that even with a similar cognitive outcome, PD‐MCI patients with VH exhibited a more‐severe posterior cerebral hypometabolism than PD‐MCI patients without VH, indicating a higher cortical dysfunction closer to that observed in PDD patients and that actually the ratio of conversion to dementia was higher in this group of patients. The presence of hallucinations must be considered as a relevant indicator of poorer cognitive evolution in patients with PD‐MCI.
CONFLICTS OF INTEREST
Carmen Gasca‐Salas received a travel grant from the Movement Disorders Society. Pedro Clavero has received payment from UCB, Lundbeck and Abbvie for travel and accommodation to attend scientific meetings. David Garcia‐Garcia has no conflicts of interest. Prof Jose A Obeso: Received honorarium for lecturing in meetings organized by GSK, Lundbeck and UCB in Spain, Zambon, Italy and Boehringer Ingelheim Mexico No honorarium were received during 2015. Grants/Research: Funding from Spanish Science and Education Ministery and European Union (REPLACES). Maria C Rodriguez Oroz has received payment from UCB, Lundbeck, Abbvie and Boston Scientific for lectures, travel and accommodation to attend scientific meetings. She has received grants from CIBERNED, the Government of the Basque Country and Guipuzcoa, the Spanish Health Institute and Era‐net.
Supporting information
Supporting Information 1
Supporting Information 2
Correction added on 22 December 2015, after first online publication.
REFERENCES
- Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh‐Sorensen P (2003): Prevalence and characteristics of dementia in Parkinson disease: An 8‐year prospective study. Arch Neurol 60:387–392. [DOI] [PubMed] [Google Scholar]
- TA Ala, LF Hughes, GA Kyrouac, MW Ghobrial, RJ Elble (2001): Pentagon copying is more impaired in dementia with Lewy bodies than in Alzheimer's disease. J Neurol Neurosurg Psychiatry 70: 483–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes J, Boubert L (2008): Executive functions are impaired in patients with Parkinson's disease with visual hallucinations. J Neurol, Neurosurg, Psychiatry 79:190–192. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Koeppe RA, Minoshima S, Giordani B, Albin RL, Frey KA, Kuhl DE (2011): Cerebral glucose metabolic features of Parkinson disease and incident dementia: Longitudinal study. J Nuclear Med 52:848–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boecker H, Ceballos‐Baumann AO, Volk D, Conrad B, Forstl H, Haussermann P (2007): Metabolic alterations in patients with Parkinson disease and visual hallucinations. Arch Neurol 64:984–988. [DOI] [PubMed] [Google Scholar]
- Broeders M, de Bie RM, Velseboer DC, Speelman JD, Muslimovic D, Schmand B (2013): Evolution of mild cognitive impairment in Parkinson disease. Neurology 81:346–352. [DOI] [PubMed] [Google Scholar]
- Diederich NJ, Fenelon G, Stebbins G, Goetz CG (2009): Hallucinations in Parkinson disease. Nat Rev Neurol 5:331–342. [DOI] [PubMed] [Google Scholar]
- M Emre, D Aarsland, R Brown, DJ Burn, C Duyckaerts, Y Mizuno, GA Broe, J Cummings, DW Dickson, S Gauthier, J Goldman, C Goetz, A Korczyn, A Lees, R Levy, I Litvan, I McKeith, W Olanow, W Poewe, N Quinn, C Sampaio, E Tolosa, B Dubois (2007): Clinical diagnostic criteria for dementia associated with Parkinson's disease. Movement Disord 22: 1689–1707. [DOI] [PubMed] [Google Scholar]
- Factor SA, Scullin MK, Sollinger AB, Land JO, Wood‐Siverio C, Zanders L, Freeman A, Bliwise DL, McDonald WM, Goldstein FC (2014): Cognitive correlates of hallucinations and delusions in Parkinson's disease. J Neurol Sci 347:316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenelon G, Mahieux F, Huon R, Ziegler M (2000): Hallucinations in Parkinson's disease: Prevalence, phenomenology and risk factors. Brain 123:733–745. [DOI] [PubMed] [Google Scholar]
- Gallagher DA, Parkkinen L, O'Sullivan SS, Spratt A, Shah A, Davey CC, Bremmer FD, Revesz T, Williams DR, Lees AJ, Scrag A (2011): Testing an aetiological model of visual hallucinations in Parkinson's disease. Brain 134:3299–3309. [DOI] [PubMed] [Google Scholar]
- Garcia‐Garcia D, Clavero P, Gasca Salas C, Lamet I, Arbizu J, Gonzalez‐Redondo R, Obeso JA, Rodriguez‐Oroz MC (2012): Posterior parietooccipital hypometabolism may differentiate mild cognitive impairment from dementia in Parkinson's disease. Eur J Nucl Med Mol Imaging 39:1767–1777. [DOI] [PubMed] [Google Scholar]
- Gasca‐Salas C, Estanga A, Clavero P, Aguilar‐Palacio I, Gonzalez‐Redondo R, Obeso JA, Rodriguez‐Oroz MC (2014): Longitudinal assessment of the pattern of cognitive decline in non‐demented patients with advanced Parkinson's disease. J Parkinson Dis 4:677–686. [DOI] [PubMed] [Google Scholar]
- Goetz CG, Leurgans S, Pappert EJ, Raman R, Stemer AB (2001): Prospective longitudinal assessment of hallucinations in Parkinson's disease. Neurology 57:2078–2082. [DOI] [PubMed] [Google Scholar]
- Goldman JG, Stebbins GT, Dinh V, Bernard B, Merkitch D, deToledo‐Morrell L, Goetz CG (2014): Visuoperceptive region atrophy independent of cognitive status in patients with Parkinson's disease with hallucinations. Brain 137:849–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez‐Redondo R, Garcia‐Garcia D, Clavero P, Gasca‐Salas C, Garcia‐Eulate R, Zubieta JL, Arbizu J, Obeso JA, Rodriguez‐Oroz MC (2014): Grey matter hypometabolism and atrophy in Parkinson's disease with cognitive impairment: A two‐step process. Brain 137:2356–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- K Grosset, F Needleman, G Macphee, D Grosset (2004): Switching from ergot to nonergot dopamine agonists in Parkinson's disease: a clinical series and five‐drug dose conversion table. Mov Disord 19: 1370–1374. [DOI] [PubMed] [Google Scholar]
- Harding AJ, Broe GA, Halliday GM (2002): Visual hallucinations in Lewy body disease relate to Lewy bodies in the temporal lobe. Brain 125:391–403. [DOI] [PubMed] [Google Scholar]
- Hepp DH, da Hora CC, Koene T, Uitdehaag BM, van den Heuvel OA, Klein M, van de Berg MD, Berendse HW, Foncke EM (2013): Cognitive correlates of visual hallucinations in non‐demented Parkinson's disease patients. Parkinson Relat Dis 19:795–799. [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992): Accuracy of clinical diagnosis of idiopathic Parkinson's disease: A clinico‐pathological study of 100 cases. J Neurol, Neurosurg, Psychiatry 55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokai Y, Nishio Y, Hirayama K, Takeda A, Ishioka T, Sawada Y, Suzuki K, Itoyama Y, Takahashi S, Fukuda H, Mori E (2009): Distinct patterns of regional cerebral glucose metabolism in Parkinson's disease with and without mild cognitive impairment. Movement Disord 24:854–862. [DOI] [PubMed] [Google Scholar]
- Huang C, Mattis P, Perrine K, Brown N, Dhawan V, Eidelberg D (2008): Metabolic abnormalities associated with mild cognitive impairment in Parkinson disease. Neurology 70:1470–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SAT, Morshed Dugger BN, Beach TG, Hentz JG, Adler CH, Shill HA, Sabbagh MN, Belden CM, Sue LI, Caviness JN, Hu C, Arizona Parkinson's Disease Consortium (2014): Plaques and tangles as well as Lewy‐type alpha synucleinopathy are associated with formed visual hallucinations. Parkinsonism Relat Disord 20:1009–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janvin CC, Larsen JP, Aarsland D, Hugdahl K (2006): Subtypes of mild cognitive impairment in Parkinson's disease: Progression to dementia. Movement Disord 21:1343–1349. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT (2000): Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp 10:120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepelt I, Reimold M, Maetzler W, Godau J, Reischl G, Gaenslen A, Herbst H, Berg D (2009): Cortical hypometabolism assessed by a metabolic ratio in Parkinson's disease primarily reflects cognitive deterioration‐[18F]FDG‐PET. Movement Disord 24:1504–1511. [DOI] [PubMed] [Google Scholar]
- Litvan I, Aarsland D, Adler CH, Goldman JG, Kulisevsky J, Mollenhauer B, Rodriguez‐Oroz MC, Troster A, Weintraub D (2011): MDS Task Force on mild cognitive impairment in Parkinson's disease: Critical review of PD‐MCI. Movement Disord 26:1814–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvan I, Goldman JG, Troster AI, Schmand BA, Weintraub D, Petersen RC, Mollenhauer B, Adler CH, Marder K, Williams‐Gray CH, Aarsland D, Kulisevsky J, Rodriguez‐Oroz MC, Burn DJ, Barker RA, Emre M (2012): Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. Movement Disord 27:349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llebaria G, Pagonabarraga J, Martinez‐Corral M, Garcia‐Sanchez C, Pascual‐Sedano B, Gironell A, Kulisevsky J (2010): Neuropsychological correlates of mild to severe hallucinations in Parkinson's disease. Movement Disord 25:2785–2791. [DOI] [PubMed] [Google Scholar]
- Lyoo CH, Jeong Y, Ryu YH, Rinne JO, Lee MS (2010): Cerebral glucose metabolism of Parkinson's disease patients with mild cognitive impairment. Eur Neurol 64:65–73. [DOI] [PubMed] [Google Scholar]
- Nagano‐Saito A, Washimi Y, Arahata Y, Iwai K, Kawatsu S, Ito K, Nakamura A, Abe Y, Yamada T, Kato T, Kachi T (2004): Visual hallucination in Parkinson's disease with FDG PET. Movement Disorders 19:801–806. [DOI] [PubMed] [Google Scholar]
- Pagonabarraga J, Kulisevsky J, Llebaria G, Garcia‐Sanchez C, Pascual‐Sedano B, Gironell A (2008): Parkinson's disease‐cognitive rating scale: A new cognitive scale specific for Parkinson's disease. Movement Disord 23:998–1005. [DOI] [PubMed] [Google Scholar]
- Papapetropoulos S, McCorquodale DS, Gonzalez J, Jean‐Gilles L, Mash DC (2006): Cortical and amygdalar Lewy body burden in Parkinson's disease patients with visual hallucinations. Parkinson Relat Disord 12:253–256. [DOI] [PubMed] [Google Scholar]
- Pappata S, Santangelo G, Aarsland D, Vicidomini C, Longo K, Bronnick K, Amboni M, Erro R, Vitale C, Pellecchia MT, Brunetti A, De Michele G, Salvatore M, Barone P (2011): Mild cognitive impairment in drug‐naive patients with PD is associated with cerebral hypometabolism. Neurology 77:1357–1362. [DOI] [PubMed] [Google Scholar]
- Park HK, Kim JS, Im KC, Kim MJ, Lee JH, Lee MC, Kim J, Chung SJ (2013): Visual hallucinations and cognitive impairment in Parkinson's disease. Can J Neurol Sci 40:657–662. [DOI] [PubMed] [Google Scholar]
- Pedersen KF, Larsen JP, Tysnes OB, Alves G (2013): Prognosis of mild cognitive impairment in early Parkinson disease: The Norwegian ParkWest study. JAMA Neurol 70:580–586. [DOI] [PubMed] [Google Scholar]
- Peppard RF, Martin WR, Carr GD, Grochowski E, Schulzer M, Guttman M, McGeer PL, Phillips AG, Tsui JK, Caine DB (1992): Cerebral glucose metabolism in Parkinson's disease with and without dementia. Arch Neurol 49:1262–1268. [DOI] [PubMed] [Google Scholar]
- I Sala, MB Sánchez‐Saudinós, L Molina‐Porcel, E Lázaro, I Gich, J Clarimón, F Blanco‐Vaca, R Blesa, T Gómez‐Isla, A Lleó (2008) Homocysteine and Cognitive Impairment Relation with Diagnosis and Neuropsychological Performance. Dement Geriatr Cogn Disord 26:506–512. [DOI] [PubMed] [Google Scholar]
- Santangelo G, Trojano L, Vitale C, Ianniciello M, Amboni M, Grossi D, Barone P (2007): A neuropsychological longitudinal study in Parkinson's patients with and without hallucinations. Movement Disord 22:2418–2425. [DOI] [PubMed] [Google Scholar]
- Teunisse S, Derix MM (1997): The interview for deterioration in daily living activities in dementia: Agreement between primary and secondary caregivers. Int Psychogeriatr 9:155–162. [DOI] [PubMed] [Google Scholar]
- Williams‐Gray CH, Evans JR, Goris A, Foltynie T, Ban M, Robbins TW, Brayne C, Kolachana BS, Weinberger DR, Sawcer SJ, Barker RA (2009): The distinct cognitive syndromes of Parkinson's disease: 5 year follow‐up of the CamPaIGN cohort. Brain 132:2958–2969. [DOI] [PubMed] [Google Scholar]
- Yong SW, Yoon JK, An YS, Lee PH (2007): A comparison of cerebral glucose metabolism in Parkinson's disease, Parkinson's disease dementia and dementia with Lewy bodies. Eur J Neurol 14:1357–1362. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information 1
Supporting Information 2


