Abstract
The question of how spatially organized activity in the visual cortex behaves during eyes‐closed, lysergic acid diethylamide (LSD)‐induced “psychedelic imagery” (e.g., visions of geometric patterns and more complex phenomena) has never been empirically addressed, although it has been proposed that under psychedelics, with eyes‐closed, the brain may function “as if” there is visual input when there is none. In this work, resting‐state functional connectivity (RSFC) data was analyzed from 10 healthy subjects under the influence of LSD and, separately, placebo. It was suspected that eyes‐closed psychedelic imagery might involve transient local retinotopic activation, of the sort typically associated with visual stimulation. To test this, it was hypothesized that, under LSD, patches of the visual cortex with congruent retinotopic representations would show greater RSFC than incongruent patches. Using a retinotopic localizer performed during a nondrug baseline condition, nonadjacent patches of V1 and V3 that represent the vertical or the horizontal meridians of the visual field were identified. Subsequently, RSFC between V1 and V3 was measured with respect to these a priori identified patches. Consistent with our prior hypothesis, the difference between RSFC of patches with congruent retinotopic specificity (horizontal–horizontal and vertical–vertical) and those with incongruent specificity (horizontal–vertical and vertical–horizontal) increased significantly under LSD relative to placebo, suggesting that activity within the visual cortex becomes more dependent on its intrinsic retinotopic organization in the drug condition. This result may indicate that under LSD, with eyes‐closed, the early visual system behaves as if it were seeing spatially localized visual inputs. Hum Brain Mapp 37:3031–3040, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: psychedelics, serotonin 5‐HT2 receptor agonists, LSD, visual cortex, fMRI, imagery, hallucinations
INTRODUCTION
Lysergic acid diethylamide (LSD) is a psychedelic drug and classic hallucinogen. Psychedelics have many interesting psychological effects; however, their potent hallucinogenic properties have long been a matter of scientific intrigue. It is assumed that LSD's principal “psychedelic” effects are mediated by activation of serotonin 2A receptor (5‐HT2AR) [Halberstadt, 2014]. The 5‐HT2AR is highly expressed in the visual cortex [Erritzoe et al., 2009; Ettrup et al., 2014; Pazos et al., 1987] and especially in the primary visual cortex (V1) [Zilles et al., 2002].
Studying mescaline in the mid‐1920s, Heinrich Klüver sought to document the peculiar geometric patterns that occur during intoxication with this particular compound [Klüver, 1942]. Later, Jack Cowan et al. proposed a mechanism by which such patterns could be perceived that was based on aberrant waves of excitation spreading across the approximately log‐polar map of primary visual cortex [Bressloff et al., 2002; Ermentrout and Cowan, 1979]. Although it has proved difficult to empirically test Cowan's model, the notion that hallucinogens induce aberrant dynamics within the visual cortex has indirect empirical support. For example, decreased alpha power has been observed in occipital areas with psilocybin [Kometer et al., 2013; Muthukumaraswamy et al., 2013], ayahuasca [Alonso et al., 2015; Riba et al., 2004; Schenberg et al., 2015] and LSD [Carhart‐Harris et al., 2016; Rodin and Luby, 1966], and in one early study, alpha suppression was predictive of the occurrence of complex psychedelic imagery [Shirahashi, 1960]. Furthermore, the relationship between decreased alpha power and psychedelic imagery has been found to be related to 5‐HT2AR activation [Valle et al., 2016]. Suppressed occipital alpha power is a hallmark of the eyes‐closed to eyes‐open transition, as is BOLD activation within the visual cortex [Goldman et al., 2002]. Elevated BOLD activation in the visual cortex during the induction of eyes‐closed visual imagery has been seen in studies with ayahuasca [de Araujo et al., 2012] and psilocybin [Carhart‐Harris et al., 2012] and recently we observed significant increases in resting‐state functional connectivity (RSFC) between visual networks and higher‐level associative networks with psilocybin [Roseman et al., 2014]. Furthermore, under LSD, increased RSFC between primary visual cortex and higher level associative areas and increased cerebral blood flow (CBF) in visual areas were correlated with subjective ratings of visual hallucinations and decreased occipital alpha power [Carhart‐Harris et al., 2016].
The visual field is retinotopically rerepresented several times in subregions of the occipital cortex [Hubel and Livingstone, 1987; Hubel and Wiesel, 1977; Zeki, 1978]. Retinotopic (or topological) organization means that nearby regions in the retina project to nearby cortical regions [Tootell et al., 1988]. By presenting videos of rotating wedges or expanding rings, it is possible to map brain areas that show activity that is dependent on the spatial location of the stimulus. This technique has helped identify the borders between neighboring visual regions (e.g., V1, V2, and V3) [Sereno et al., 1995]. Data obtained from retinotopic mapping has proved useful for understanding spontaneous (“resting‐state”) activity within the visual system [Kenet et al., 2003]. Moreover, given the involvement of retinotopically sensitive regions in the processing of spatial information, studying their spontaneous activity during resting‐state conditions, with and without LSD, may produce insights into the neural mechanisms underlying the emergence of psychedelic imagery.
This study modified a previously used paradigm [Nir et al., 2006] to focus on activity in retinotopically sensitive patches of the lower level visual cortex, that is, discrete patches within V1 and V3 that are sensitive to visual stimuli presented along the horizontal or vertical meridians. Nir et al. [2006] showed that while observing a visual stimulus, the functional connectivity between different visual areas becomes related to their functional specialization (e.g., there is higher functional connectivity between faces–faces ROIs than faces–places ROIs). A similar organization of activity was observed when subjects freely viewed a movie [Hasson et al., 2004] but not during stimulus‐free resting‐state conditions [Nir et al., 2006]—thus, suggesting that visual stimulation drives this organized pattern of activity.
Broadly consistent with previously described effects of visual stimulation and imagery [Nir et al., 2006], we predicted that RSFC between retinotopically organized patches of V1 and V3 that possess congruent retinotopic specificity (i.e., V1 horizontal–V3 horizontal meridian or V1 vertical–V3 vertical meridian) would be greater than RSFC between incongruent patches (e.g., V1 horizontal–V3 vertical) under LSD, but not under placebo (see Raemaekers et al. [2014] for support for the assumption that polar retinotopic coordination should not be present during the resting state under placebo). That is, the impact of LSD on spontaneous activity within retinotopically organized patches of V1 and V3 would be consistent with what one would expect from visual stimulation [Nir et al., 2006].
MATERIALS AND METHODS
This is an extended analysis based on the data reported in Carhart‐Harris et al. [2016]. This study was approved by the National Research Ethics Service (NRES) committee London, West London and was conducted in accordance with the revised declaration of Helsinki (2000), the International Committee on Harmonisation Good Clinical Practice guidelines and National Health Service (NHS) Research Governance Framework. Imperial College London sponsored the research which was conducted under a Home Office license for research with schedule 1 drugs.
Participants
All participants were recruited via word of mouth and provided written informed consent to participate after study briefing and screening for physical and mental health. All the participants had experience with psychedelic drugs. The screening for physical health included electrocardiogram (ECG), routine blood tests, and urine test for recent drug use and pregnancy. A psychiatric interview was conducted and participants provided full disclosure of their drug use history. Key exclusion criteria included: <21 years of age, personal history of diagnosed psychiatric illness, immediate family history of a psychotic disorder, any psychedelic drug use within 6 weeks of the first scanning day, pregnancy, problematic alcohol use (i.e., >40 units consumed per week), or a medically significant condition rendering the volunteer unsuitable for the study.
Design
All study days were performed at Cardiff University Brain Research Imaging Centre (CUBRIC). Twenty healthy participants (4 females) attended two scanning days (LSD and placebo) at least 2 weeks apart in a balanced order, within‐subjects design. All participants received 75 µg of LSD, administered intravenously via a 10 ml solution, or placebo (10 ml saline) infused over a 2‐min period. The administration was followed by an acclimatization period of approximately 60 min, in which participants were encouraged to relax and lie with their eyes closed inside a mock MRI scanner. Participants reported noticing subjective drug effects between 5 and 15 min postdosing, and the effects approached peak intensity between 60 and 90 min postdosing. The duration of a subsequent plateau of drug effects varied among individuals but was generally maintained for approximately 4 h postdosing. MRI scanning started approximately 70 min postdosing, and lasted for approximately 60 min. Sessions included a structural scan, arterial spin labelling (ASL) fMRI, and BOLD fMRI. After the MRI scanning, there was a break of approximately 35 minutes, after which MEG scanning was performed. Once the subjective effects of LSD had sufficiently subsided, the study psychiatrist assessed the participant's suitability for discharge. For the analysis presented in this article, we used two eyes‐closed resting‐state BOLD scans totaling 14 min that were completed 125 min postinfusion (both placebo and LSD had two resting‐state scans within each session). The peak of the drug subjective experience occurred during these resting‐state scans and the effects were stable through the scanning period [Carhart‐Harris et al., 2016]. Immediately after the resting‐state scans, a BOLD retinotopic localizer scan was performed to localize subregions within the visual cortex. All subjects tolerated well the drug effects except for one subject that did not complete the study due to anxiety.
Subjective Ratings
Participants carried out VAS‐style ratings immediately after each scan using button press to rate the intensity of simple and complex visual hallucinations. They were phrased as follows: (1) “With eyes closed, I saw patterns and colors”; (2) “With eyes closed, I saw complex visual imagery”, with a bottom anchor of “no more than usual” and a top anchor of “much more than usual”. Furthermore, the 11 factor altered states of consciousness (ASC) questionnaire [Studerus et al., 2010] was completed at the end of each dosing day retrospectively referring to the peak drug effects. All participants reported eyes‐closed psychedelic imagery.
Anatomical Scans
Imaging was performed on a 3T GE HDx system. These were 3D magnetization prepared fast‐spoiled gradient echo scans in an axial orientation, with field of view = 256 × 256 × 192 and matrix = 256 × 256 × 192 to yield 1 mm isotropic voxel resolution. TR/TE = 7.9/3.0 ms; inversion time = 450 ms; flip angle = 20°.
BOLD fMRI Data Acquisition: Eyes‐Closed Resting State
Two sequences of BOLD‐weighted fMRI eyes‐closed resting‐state scans were acquired; for each condition, using a gradient‐echo echo‐planar imaging sequence (both the placebo and LSD sessions contained two resting‐state scans), TR/TE = 2000/35 ms, field‐of‐view = 220 mm, 64 × 64 acquisition matrix, parallel acceleration factor = 2, 90° flip angle. Thirty‐five oblique axial slices were acquired in an interleaved fashion, each 3.4 mm thick with zero slice gap (3.4 mm isotropic voxels). The precise length of each BOLD scan was 7 min 20 s.
BOLD Preprocessing
Three different but complementary imaging software packages were used to analyze the fMRI resting‐state data. Specifically, FMRIB Software Library (FSL) [Smith et al., 2004], AFNI [Cox, 1996] and Freesurfer [Dale et al., 1999] were used. One subject did not complete the resting‐state scans due to anxiety and an expressed desire to exit the scanner. Four others were discarded from the group analyses due to excessive head movement as measured using frame‐wise displacement (FD) [Power et al., 2014]. The criterion for exclusion was subjects with >15% scrubbed volumes when the scrubbing threshold is FD ≥ 0.5 mm. After discarding these subjects, we reduced the threshold to FD ≥ 0.4 mm. The mean between‐condition difference in mean FD for the 4 subjects that were discarded was 0.323 ± 0.254. Four more subjects were discarded due to imperfect identification of horizontal and vertical meridian retinotopic patches of V3 (see retinotopic localizer below). Another subject was identified as an outlier and was not included in the analysis (based on the retinotopic coordination result below and the Outlier Labelling Rule with g = 2.2 [Hoaglin et al., 1986]). For the 10 subjects (3 females) that were used in the analysis, the difference (LSD‐Placebo) in mean FD was 0.057 ± 0.029 (p = 0.0002). Therefore, many preprocessing stages were considered with respect to the difference in head motion and these are detailed below.
The following preprocessing stages were performed: (1) removal of the first three volumes; (2) despiking (3dDespike, AFNI); (3) slice time correction (3dTshift, AFNI); (4) motion correction (3dvolreg, AFNI) by registering each volume to the volume most similar, in the least squares sense, to all others (in‐house code); (5) brain extraction (BET, FSL); (6) rigid body registration to anatomical scans (nine subjects with FSL's BBR, one subject with Freesurfer's bbregister and one subject manually); (7) scrubbing (Power, et al., 2014), using an FD threshold of 0.4 (the mean percentage of volumes scrubbed for placebo and LSD was 0.4 ± 0.9% and 2.1 ± 2.6%, respectively). The maximum number of scrubbed volumes per scan was 7.1%. Scrubbed volumes were replaced with the mean of the surrounding volumes. Additional preprocessing steps included: (8) band‐pass filtering between 0.01 and 0.08 Hz (3dFourier, AFNI) (lowpass filter of 0.08 Hz is suggested to improve motion‐related artifacts [Satterthwaite et al., 2013]); (9) linear and quadratic detrending (3dDetrend, AFNI); (10) regressing out 9 nuisance regressors: out of these, 6 were motion‐related (3 translations and 3 rotations) and 3 were anatomically related (not smoothed). All nuisance regressors were band‐pass filtered with the same filter as in step 10. Specifically, the anatomical nuisance regressors were: (I) ventricles (Freesurfer, eroded in 2 mm space), (II) draining veins (DV) (FSL's CSF minus Freesurfer's Ventricles, eroded in 1 mm space), and (III) local white matter (WM) (FSL's WM minus Freesurfer's subcortical grey matter (GM) structures, eroded in 2 mm space). Regarding local WM regression, AFNI's 3dLocalstat was used to calculate the mean local WM time‐series for each voxel, using a 25‐mm‐radius sphere centered on each voxel. Local WM regression has been suggested to perform better than global WM regression [Jo et al., 2013] and is considered a promising approach to deal with motion‐related artifacts [Power et al., 2015].
Retinotopic Localizer
Subjects were presented with a 4 min 24 s video that alternated between vertical and horizontal polar angles (8 cycles, resolution = 1400 × 1050, visual angle = 23 × 23°, TR/TE = 2000/25 ms, 3 mm isotropic voxels). The wedge consisted of a colored checkerboard with superimposed moving black and white faces (from FERET database [Phillips et al., 1998]) and coherently moving white dots: new flow every 0.5 s. Fourier analysis with two distinct conditions was performed on the placebo data to identify activity corresponding to the vertical and horizontal polar angles [Sereno et al., 1995]. Borders between V1, V2, and V3 were identified manually for each subject (using an in‐house program). The middle of the vertical meridian activation was used to mark the border between V1 and V2 and the middle of the horizontal meridian to mark the border between V2 and V3. V1 and V3 were then subdivided using the vertical versus horizontal meridian activations to yield four patches (V1hor, V1ver, V3hor, and V3ver). The retinotopic localizer took place after the resting‐state scans to avoid a possible effect of the retinotopic localizer on the resting‐state scans [Albert et al., 2009; Hasson et al., 2009; Waites et al., 2005]. The retinotopic localizer took place for both placebo and LSD sessions. However, the BOLD data from the localizer of the LSD session was noisier due to head‐motion. Therefore, the retinotopic patches were identified solely on the placebo session.
V1–V3 Retinotopic Coordination
After horizontal and vertical retinotopic patches of V1 and V3 were identified using the retinotopic localizer described above, the mean time‐series for V1hor, V1ver, V3hor, and V3ver (hor = horizontal orientation, ver = vertical orientation) were derived from each LSD and placebo resting‐state scans in native space. The results below were averaged across the two resting‐state scans (both placebo and LSD had two resting‐state scans within each session). Parameter estimates (β) of linear regression were calculated between V1hor –V3hor (β hor_hor), V1hor–V3ver (βhor_ver), V1ver–V3hor (βver_hor), V1ver–V3ver (β ver_ver). These β values represent RSFC between two retinotopic patches. Regression was used rather than correlation coefficients because differences between Pearson's correlations could be a result of either signal or noise differences; therefore, it is preferable to perform regression and look for drug‐placebo differences on the β values [Friston, 2011]. Retinotopic coordination (Fig. 3A) was calculated as follows:
Figure 3.
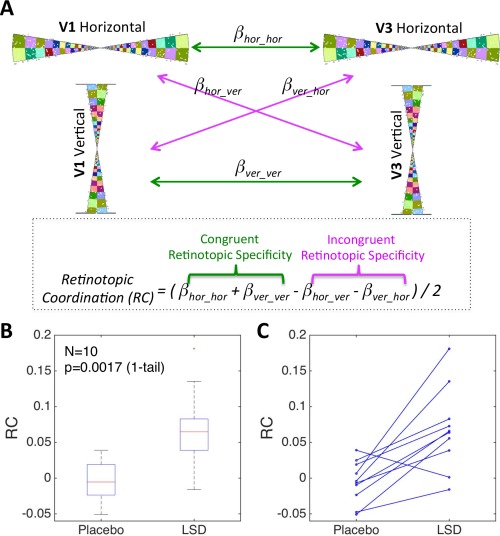
Increased retinotopic coordination (RC) between V1 and V3 under LSD relative to Placebo. (A) Calculating RC for each subject for each condition. Horizontal and vertical patches of V1 and V3 were identified using a retinotopic localizer (the wedges in this figure are taken from the video). Four regressions between patches of V1 and V3 produced four regression coefficients (β values) that represent the strength of RSFC. RC was calculated by adding the RSFC (β) values of patches with the congruent retinotopic specificity and then subtracting the RSFC β values of patches with incongruent retinotopic specificity. (B) Boxplot of RC for Placebo and LSD. (C) RC for all 10 subjects for Placebo and LSD. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
RESULTS
Subjective Ratings
The mean between‐condition difference in within‐scanner VAS ratings of visual hallucinations/imagery were 9.73 ± 6.43 and 7.37 ± 6.87 for simple and complex hallucinations, respectively (range = 0–20). The mean difference in the retrospective ratings of visual hallucinations/imagery were 65 ± 34 and 42 ± 28 for elementary and complex imagery, respectively (range = 0–100), p < 0.05 (Bonferonni corrected) for all the above changes in subjective ratings.
Retinotopic Localizer
Retinotopic patches (V1hor, V1ver, V3hor, and V3ver) of the 10 subjects and their surface areas (mm2) are presented in Figure 1. For illustration purposes, the surface average of the retinotopic activation is presented in Figure 2. The average was calculated by sampling each subject's morphed sphere onto the canonical icosahedral sphere surface (1 smoothing step; nearest neighbor, forward, and reverse). The averaged sphere was sampled onto Freesurfer's average surface.
Figure 1.
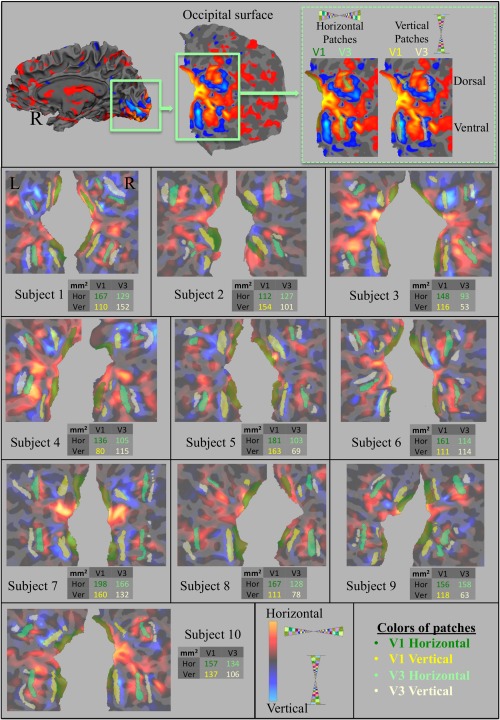
Identifying retinotopic patches for all subjects. Activity based on retinotopic localizer is projected onto the occipital surface of each subject. Hot and cold colors represent horizontal and vertical meridian activations, respectively. The top panel is the right hemisphere of an illustrative subject. Below, horizontal (green) and vertical (yellow) patches were identified for each subject in both V1 and V3. For each subject, the surface areas (mm2) of these patches are displayed in a table. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 2.
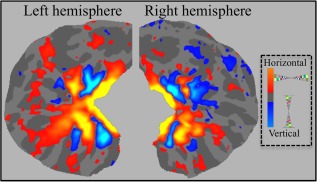
Average of retinotopic activation across subjects. Average retinotopic activation of 10 subjects is projected onto the Freesurfer average occipital surface. Hot and cold colors represent horizontal and vertical meridian activations, respectively. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
V1–V3 Retinotopic Coordination
Mean values across subjects for retinotopic coordination (i.e., the difference in RSFC between retinotopically congruent patches and incongruent patches) were significantly greater (t = 3.93, p = 0.0018, 1‐tail, paired t‐test, Cohen's d = 1.6) under LSD (0.068 ± 0.058) than under placebo (−0.005 ± 0.02) (boxplot is presented in Fig. 3B). Retinotopic coordination for each subject and for each condition is shown in Figure 3C (9 of 10 subjects showed a change in the predicted direction). This result is based on the mean of the two resting‐state scans (both the placebo and the LSD session contained two resting‐state scans) but it was also significant for each of the resting‐state scans alone (p = 0.0288 and p = 0.0057). Furthermore, the results showed the same trend for the right and left hemispheres separately (p = 0.022 and p = 0.078, respectively) and the result was also significant when using Pearson's correlation coefficient instead of regression to calculate RSFC (p = 0.033). Importantly, the increased retinotopic coordination across subjects did not correlate with increased head motion under LSD compared with placebo (r = −0.83, p = 0.999, 1‐tail), nor did it correlate with rating scales of psychedelic imagery (e.g., Elementary & Complex Imagery factors of ASC, or VAS ratings of Simple & Complex Hallucinations).
DISCUSSION
This study found that LSD modulated RSFC within the visual cortex reflects the intrinsic retinotopic architecture; i.e., RSFC between patches of V1 and V3 that possess a congruent retinotopic representation was stronger than RSFC between patches possessing incongruent retinotopic representations. Consistent with previous studies [Carhart‐Harris et al., 2016; de Araujo et al., 2012], these findings suggest that the primary visual cortex is involved in the processing of eyes‐closed psychedelic imagery.
Interpretation of the present results regarding psychedelic imagery may be informed by more general research on visual imagery [Kosslyn et al., 2006; Pylyshyn, 2002]. A key question in this research area is whether lower‐level stages in the visual system (e.g., the primary visual cortex) contribute to the representation of complex mental images [de Gelder et al., 2014; Pearson and Kosslyn, 2015]. Another related debate is whether primary visual cortex is engaged [Hong et al., 2009; Miyauchi et al., 2009] or disengaged [Braun et al., 1998] during REM sleep. This study addresses a third related question: the role of lower level visual areas in eyes‐closed psychedelic imagery. Our findings imply that low‐level components of the visual system (i.e., retinotopically mapped regions in V1 and V3) are indeed modified under LSD. Moreover, our results suggest that under LSD, the early visual system behaves “as if” it were receiving spatially localized visual information.
Early electrophysiological studies involving psychedelics (chiefly LSD) reported altered activity in the retina (Apter and Pfeiffer, 1956; Mouriz‐Garcia et al., 1969), LGN (Morgane and Stern, 1972; Phillis et al., 1967; Walter et al., 1971) and visual cortex (Evarts et al., 1955) under these drugs. However, the nature of altered neural activity in the visual cortex appeared to be strongly dose‐dependent (Dray et al., 1980) and these studies said little about the functional implications of altered activity. This study directly addresses how activity within low‐level aspects of the visual system is altered under a psychedelic (i.e., that V1 and V3 show increased retinotopic coordination under LSD).
This study found that the increased retinotopic coordination between V1 and V3 under LSD did not correlate with ratings of visual hallucinations. One possible explanation for this is that increased retinotopic coordination reflects a specific alteration in the spatial properties of psychedelic imagery and not its general intensity. For example, while the overall intensity of the hallucinatory experience may increase (e.g., with a higher dose of LSD) the psychedelic imagery may lose some of its spatial properties and this might be expressed as a decrease rather than an increase in retinotopic coordination. This matter could be addressed by including a different measure of the hallucinatory experience that enquires specifically about the spatial acuity of the psychedelic imagery, as well as its location in space. We would predict that psychedelic visions that are especially sharp or vivid and clearly located in space would relate to an increase in retinotopic coordination. Another possible explanation for the lack of correlation between the subjective intensity of the psychedelic imagery and the reported RSFC results is that higher levels of motion interfered with accurate measurements of retinotopic coordination: indeed, subjects that had higher differences (LSD‐placebo) in head motion had a lower difference in retinotopic coordination, i.e., head motion “diluted” the main drug effect on retinotopic coordination.
Another possible explanation of our main finding is that the retinotopic coordination observed under LSD was an epiphenomenon of a more general increase in “activity” within the relevant brain regions. Other results from the same LSD dataset do suggest that there are changes in activity within visual areas that might be construed as consistent with increased “activity”: e.g., increased CBF and decreased MEG alpha power [Carhart‐Harris et al., 2016]. However, these effects did not correlate with the increased retinotopic coordination observed here (p = 0.98, p = 0.97, 1‐tail, respectively) and therefore cannot be used to explain this study's main findings.
Another potential limitation is the possibility that there was a difference in the level of arousal between the two conditions. Decreased arousal can increase BOLD signal variance (Fukunaga et al., 2006; Larson‐Prior et al., 2009; Wong et al., 2013) and thus, differential levels of arousal in the drug vs placebo condition may have caused a difference in the global signal that impacted on the retinotopic coordination outcomes reported here. We did notice decreased variance under LSD in medial visual network [Carhart‐Harris et al., 2016]; however, crucially, it did not correlate significantly with increased retinotopic coordination (p = 0.35, 1‐tail), and therefore this explanation for the present data seems unlikely. Nevertheless, future studies could incorporate simple measures of subjective (e.g., VAS ratings) and/or objective arousal (e.g., of heart rate) to test for differences between conditions. These measures could then be included in the regression analyses to test whether they have significant explanatory value.
A further limitation of this study is the sample size (N = 10). Unfortunately, psychedelic neuroimaging studies are sensitive to data loss issues, mainly related to high levels of head motion associated with the drug condition. Future studies should take this into account and collect more data than would ordinarily be needed to compensate for potential data loss. Even after correcting for motion, however, we had a very clear prior hypothesis that proved correct in 9 out of the 10 subjects and post‐hoc analyses indicated that head motion had a deleterious rather than a contributory influence on this predicted effect. Ideally, to show that retinotopic coordination is related to visual processing, we could have acquired fMRI scans while our subjects watched a movie and then measured retinotopic coordination in this condition.
CONCLUSION
This study suggests that under the influence of LSD, the visual cortex acts as if it is processing spatially localized visual information. However, further work is required to investigate the specific regional source/s of eyes‐closed psychedelic imagery; for example, does it arise purely from changes localized to the early visual cortex, or are upstream or downstream regions also implicated? More work is also required to identify associations between the subjective quality of psychedelic imagery and underlying changes in brain activity. These investigations should improve our understanding of the function of the visual system during normal conditions and how this can go awry in certain abnormal states.
CONFLICT OF INTEREST
The authors declare no competing financial interests.
ACKNOWLEDGMENTS
The researchers would like to thank supporters of the http://Walacea.com crowd‐funding campaign for helping to secure the funds required to complete the study. This report presents an independent research carried out at the NIHR/Wellcome Trust Imperial Clinical Research Facility.
REFERENCES
- Albert NB, Robertson EM, Miall RC (2009): The resting human brain and motor learning. Curr Biol 19:1023–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso JF, Romero S, Mañanas MÀ, Riba J (2015): Serotonergic psychedelics temporarily modify information transfer in humans. Int J Neuropsychopharmacol pyv039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apter JT, Pfeiffer CC (1956): Effect of hallucinogenic drugs on the electroretinogram. Am J Ophthalmol 42:206–211. [DOI] [PubMed] [Google Scholar]
- Braun AR, Balkin TJ, Wesensten NJ, Gwadry F, Carson RE, Varga M, Baldwin P, Belenky G, Herscovitch P (1998): Dissociated pattern of activity in visual cortices and their projections during human rapid eye movement sleep. Science 279:91–95. [DOI] [PubMed] [Google Scholar]
- Bressloff PC, Cowan JD, Golubitsky M, Thomas PJ, Wiener MC (2002): What geometric visual hallucinations tell us about the visual cortex. Neural Comput 14:473–491. [DOI] [PubMed] [Google Scholar]
- Carhart‐Harris R, Muthukumaraswamy S, Roseman L, Kaelen M, Droog W, Murphy K, Tagliazucchi E, Schenberg EE, Nest T, Orban C, Leech R, Williams L, Williams TM, Bolstridge M, Sessa B, McGonigle J, Sereno MI, Nichols D, Hellyer PJ, Hobden P, Evans J, Singh KD, Wise RG, Curran HV, Feilding AJND (2016): Neural correlates of the LSD experience revealed by multimodal neuroimaging. Proc Natl Acad Sci USA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart‐Harris RL, Leech R, Williams TM, Erritzoe D, Abbasi N, Bargiotas T, Hobden P, Sharp DJ, Evans J, Feilding A, Wise RG, Nutt DJ (2012): Implications for psychedelic‐assisted psychotherapy: Functional magnetic resonance imaging study with psilocybin. Br J Psychiatry 200:238–244. [DOI] [PubMed] [Google Scholar]
- Cox RW (1996): AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29:162–173. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI (1999): Cortical surface‐based analysis: I. Segmentation and surface reconstruction. Neuroimage 9:179–194. [DOI] [PubMed] [Google Scholar]
- de Araujo DB, Ribeiro S, Cecchi GA, Carvalho FM, Sanchez TA, Pinto JP, De Martinis BS, Crippa JA, Hallak JE, Santos AC (2012): Seeing with the eyes shut: Neural basis of enhanced imagery following ayahuasca ingestion. Hum Brain Mapp 33:2550–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gelder B, Tamietto M, Pegna AJ, Van den Stock J (2014): Visual imagery influences brain responses to visual stimulation in bilateral cortical blindness. Cortex [DOI] [PubMed] [Google Scholar]
- Dray A, Fox P, Hilmy M, Somjen G (1980): The effects of LSD and some analogues on the responses of single cortical neurons of the cat to optical stimulation. Brain Res 200:105–121. [DOI] [PubMed] [Google Scholar]
- Ermentrout GB, Cowan JD (1979): A mathematical theory of visual hallucination patterns. Biol Cybernet 34:137–150. [DOI] [PubMed] [Google Scholar]
- Erritzoe D, Frokjaer VG, Haugbol S, Marner L, Svarer C, Holst K, Baaré WF, Rasmussen PM, Madsen J, Paulson OB (2009): Brain serotonin 2A receptor binding: Relations to body mass index, tobacco and alcohol use. Neuroimage 46:23–30. [DOI] [PubMed] [Google Scholar]
- Ettrup A, da Cunha‐Bang S, McMahon B, Lehel S, Dyssegaard A, Skibsted AW, Jørgensen LM, Hansen M, Baandrup AO, Bache S (2014): Serotonin 2A receptor agonist binding in the human brain with [ 11C] Cimbi‐36. J Cereb Blood Flow Metab 34:1188–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evarts EV, Landau W, Freygang W, Marshall WH (1955): Some effects of lysergic acid diethylamide and bufotenine on electrical activity in the cat's visual system. Am J Physiol Legacy Content 182:594–598. [DOI] [PubMed] [Google Scholar]
- Friston KJ (2011): Functional and effective connectivity: A review. Brain Connect 1:13–36. [DOI] [PubMed] [Google Scholar]
- Fukunaga M, Horovitz SG, van Gelderen P, de Zwart JA, Jansma JM, Ikonomidou VN, Chu R, Deckers RH, Leopold DA, Duyn JH (2006): Large‐amplitude, spatially correlated fluctuations in BOLD fMRI signals during extended rest and early sleep stages. Magn Reson Imag 24:979–992. [DOI] [PubMed] [Google Scholar]
- Goldman RI, Stern JM, Engel J Jr, Cohen MS (2002): Simultaneous EEG and fMRI of the alpha rhythm. Neuroreport 13:2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL (2014): Recent advances in the neuropsychopharmacology of serotonergic hallucinogens. Behav Brain Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U, Nir Y, Levy I, Fuhrmann G, Malach R (2004): Intersubject synchronization of cortical activity during natural vision. Science 303:1634–1640. [DOI] [PubMed] [Google Scholar]
- Hasson U, Nusbaum HC, Small SL (2009): Task‐dependent organization of brain regions active during rest. Proc Natl Acad Sci 106:10841–10846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoaglin DC, Iglewicz B, Tukey JW (1986): Performance of some resistant rules for outlier labeling. J Am Stat Assoc 81:991–999. [Google Scholar]
- Hong CCH, Harris JC, Pearlson GD, Kim JS, Calhoun VD, Fallon JH, Golay X, Gillen JS, Simmonds DJ, van Zijl P (2009): fMRI evidence for multisensory recruitment associated with rapid eye movements during sleep. Hum Brain Mapp 30:1705–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Livingstone MS (1987): Segregation of form, color, and stereopsis in primate area 18. J Neurosci 7:3378–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN (1977): Ferrier lecture: Functional architecture of macaque monkey visual cortex. Proc R Soc Lond B Biol Sci 1–59. [DOI] [PubMed] [Google Scholar]
- Jo HJ, Gotts SJ, Reynolds RC, Bandettini PA, Martin A, Cox RW, Saad ZS (2013): Effective preprocessing procedures virtually eliminate distance‐dependent motion artifacts in resting state FMRI. J Appl Math 2013: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenet T, Bibitchkov D, Tsodyks M, Grinvald A, Arieli A (2003): Spontaneously emerging cortical representations of visual attributes. Nature 425:954–956. [DOI] [PubMed] [Google Scholar]
- Klüver H (1942): Mechanisms of Hallucinations. McGraw‐Hill. [Google Scholar]
- Kometer M, Schmidt A, Jäncke L, Vollenweider FX (2013): Activation of serotonin 2A receptors underlies the psilocybin‐induced effects on α oscillations, N170 visual‐evoked potentials, and visual hallucinations. J Neurosci 33:10544–10551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosslyn SM, Thompson WL, Ganis G (2006): The Case for Mental Imagery. Oxford University Press. [Google Scholar]
- Larson‐Prior LJ, Zempel JM, Nolan TS, Prior FW, Snyder AZ, Raichle ME (2009): Cortical network functional connectivity in the descent to sleep. Proc Natl Acad Sci 106:4489–4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyauchi S, Misaki M, Kan S, Fukunaga T, Koike T (2009): Human brain activity time‐locked to rapid eye movements during REM sleep. Exp Brain Res 192:657–667. [DOI] [PubMed] [Google Scholar]
- Morgane P, Stern W (1972): Relationship of sleep to neuroanatomical circuits, biochemistry, and behavior*. Ann N Y Acad Sci 193:95–111. [DOI] [PubMed] [Google Scholar]
- Mouriz‐Garcia A, Schmidt R, Arlazoroff A (1969): Effects of LSD on the spontaneous and evoked activity of retinal and geniculate ganglion cells. Psychopharmacology 15:382–391. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Carhart‐Harris RL, Moran RJ, Brookes MJ, Williams TM, Errtizoe D, Sessa B, Papadopoulos A, Bolstridge M, Singh KD (2013): Broadband cortical desynchronization underlies the human psychedelic state. J Neurosci 33:15171–15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir Y, Hasson U, Levy I, Yeshurun Y, Malach R (2006): Widespread functional connectivity and fMRI fluctuations in human visual cortex in the absence of visual stimulation. Neuroimage 30:1313–1324. [DOI] [PubMed] [Google Scholar]
- Pazos A, Probst A, Palacios J (1987): Serotonin receptors in the human brain—IV. Autoradiographic mapping of serotonin‐2 receptors. Neuroscience 21:123–139. [DOI] [PubMed] [Google Scholar]
- Pearson J, Kosslyn SM (2015): The heterogeneity of mental representation: Ending the imagery debate. Proc Natl Acad Sci 112:10089–10092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips PJ, Wechsler H, Huang J, Rauss PJ (1998): The FERET database and evaluation procedure for face‐recognition algorithms. Image Vision Comput 16:295–306. [Google Scholar]
- Phillis J, Tebēcis A, York D (1967): The inhibitory action of monoamines on lateral geniculate neurones. J Physiol 190:563–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE (2014): Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage 84:320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Schlaggar BL, Petersen SE (2015): Recent progress and outstanding issues in motion correction in resting state fMRI. Neuroimage 105:536–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pylyshyn ZW (2002): Mental imagery: In search of a theory. Behav Brain Sci 25:157–182. [DOI] [PubMed] [Google Scholar]
- Raemaekers M, Schellekens W, van Wezel RJ, Petridou N, Kristo G, Ramsey NF (2014): Patterns of resting state connectivity in human primary visual cortical areas: A 7T fMRI study. Neuroimage 84:911–921. [DOI] [PubMed] [Google Scholar]
- Riba J, Anderer P, Jané F, Saletu B, Barbanoj MJ (2004): Effects of the South American psychoactive beverage ayahuasca on regional brain electrical activity in humans: A functional neuroimaging study using low‐resolution electromagnetic tomography. Neuropsychobiology 50:89–101. [DOI] [PubMed] [Google Scholar]
- Rodin E, Luby E (1966): Effects of LSD‐25 on the EEG and photic evoked responses. Arch Gen Psychiatry 14:435–441. [DOI] [PubMed] [Google Scholar]
- Roseman L, Leech R, Nutt DJ, Feilding A, Carhart‐Harris RL (2014): The effects of psilocybin and MDMA on between‐network resting state functional connectivity in healthy volunteers. Front Hum Neurosci 8:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, Eickhoff SB, Hakonarson H, Gur RC, Gur RE (2013): An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting‐state functional connectivity data. Neuroimage 64:240–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenberg EE, Alexandre JFM, Filev R, Cravo AM, Sato JR, Muthukumaraswamy SD, Yonamine M, Waguespack M, Lomnicka I, Barker SA (2015): Acute biphasic effects of ayahuasca. PLoS One 10:e0137202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno MI, Dale A, Reppas J, Kwong K, Belliveau J, Brady T, Rosen B, Tootell R (1995): Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science 268:889–893. [DOI] [PubMed] [Google Scholar]
- Shirahashi K (1960): Electroencephalographic study of mental disturbances experimentally induced by LSD25. Psychiatry Clin Neurosci 14:140–155. [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen‐Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE (2004): Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23:S208–S219. [DOI] [PubMed] [Google Scholar]
- Studerus E, Gamma A, Vollenweider FX (2010): Psychometric evaluation of the altered states of consciousness rating scale (OAV). PLoS One 5:e12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootell RB, Switkes E, Silverman MS, Hamilton SL (1988): Functional anatomy of macaque striate cortex. II. Retinotopic organization. J Neurosci 8:1531–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle M, Maqueda A, Rabella M, Rodríguez‐Pujadas A, Antonijoan R, Romero S, Alonso J, Mañanas M, Friedlander P, Feilding A, Riba J (2016): Inhibition of alpha oscillations through serotonin 2A receptor activation underlies the visual effects of ayahuasca in humans. Eur Neuropsychopharmacol [DOI] [PubMed] [Google Scholar]
- Waites AB, Stanislavsky A, Abbott DF, Jackson GD (2005): Effect of prior cognitive state on resting state networks measured with functional connectivity. Hum Brain Mapp 24:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter S, Balzano E, Vuillon‐Cacciuttolo G, Naquet R (1971): Behavioural and electrographic effects of d‐lysergic acid diethylamide (LSD 25) on the photosensitive Papio papio. Electroencephalogr Clin Neurophysiol 30:294–305. [DOI] [PubMed] [Google Scholar]
- Wong CW, Olafsson V, Tal O, Liu TT (2013): The amplitude of the resting‐state fMRI global signal is related to EEG vigilance measures. Neuroimage 83:983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki S (1978): Uniformity and diversity of structure and function in rhesus monkey prestriate visual cortex. J Physiol 277:273–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilles K, Palomero‐Gallagher N, Grefkes C, Scheperjans F, Boy C, Amunts K, Schleicher A (2002): Architectonics of the human cerebral cortex and transmitter receptor fingerprints: Reconciling functional neuroanatomy and neurochemistry. Eur Neuropsychopharmacol 12:587–599. [DOI] [PubMed] [Google Scholar]


