Abstract
Impairment in mental flexibility may be a key component contributing to cardinal cognitive symptoms among mood disorders patients, particularly thought control disorders. Impaired ability to switch from one thought to another might reflect difficulties in either generating new mental states, inhibiting previous states, or both. However, the neural underpinnings of impaired cognitive flexibility in mood disorders remain largely unresolved. We compared a group of mood disorders patients (n = 29) and a group of matched healthy subjects (n = 32) on a novel task‐switching paradigm involving happy and sad faces, that allowed us to separate generation of a new mental set (Switch Cost) and inhibition of the previous set during switching (Inhibition Cost), using fMRI. Behavioral data showed a larger Switch Cost in patients relative to controls, but the average Inhibition Cost did not differ between groups. At the neural level, a main effect of group was found with stronger activation of the subgenual cingulate cortex in patients. The larger Switch Cost in patients was reflected by a stronger recruitment of brain regions involved in attention and executive control, including the left intraparietal sulcus, precuneus, left inferior fontal gyrus, and right anterior cingulate. Critically, activity in the subgenual cingulate cortex was not downregulated by inhibition in patients relative to controls. In conclusion, mood disorder patients have exaggerated Switch Cost relative to controls, and this deficit in cognitive flexibility is associated with increased activation of the fronto‐parietal attention networks, combined with impaired modulation of the subgenual cingulate cortex when inhibition of previous mental states is needed. Hum Brain Mapp 37:1335‐1348, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: fMRI, mood disorders, task‐switch, inhibition, sgACC
INTRODUCTION
The nature and the specificity of impairments in cognitive functions among mood disorder patients is still debated [Gotlib and Joormann, 2010; McClintock et al., 2010; Sole et al., 2012]. One specific dimension frequently impaired is cognitive flexibility, in both unipolar and bipolar disorders, although results are inconsistent [Meiran et al., 2011; Sole et al., 2012] or heterogeneous [e.g., Arts et al., 2008]. One reason for discrepancies is that deficits in flexibility have been examined with various paradigms. However, particularly relevant to mood disorders is the type of cognitive flexibility underlying the control of thoughts and internal sources of interference. Thus, some aspects of ruminations, racing thoughts, or crowded thoughts observed in mood disorder patients might at least partly reflect disturbances in cognitive switching processes [Piguet et al., 2010], which subserve the ability to alternate between different cognitive states, and imply not only generating a new mental set but also inhibiting the previous mental set [Koch et al., 2010; Mayr and Keele, 2000; Monsell, 2003]. Our study specifically aimed at testing these switching components and their neural underpinning in mood disorder patients. Further, our task design also allowed us to examine whether these switching components are differentially modulated when processing positive or negative emotional information [Hare et al., 2008; Piguet et al., 2013; Sagaspe et al., 2011]. Because these deficits might represent a common and specific trait across the mood disorder spectrum, underlying cognitive dysfunction in these patients independent of mood anomalies, we took a dimensional approach combining patients with different clinical symptoms, as recently recommended in order to extract valid research domain criteria surpassing diagnostic boundaries (http://www.nimh.nih.gov/research-priorities/rdoc/nimh-research-domain-criteria-rdoc.shtml, Narrow and Kuhl, 2011; Keizer et al., 2013; Vieta and Valentí, 2013).
Deficits in cognitive control and inhibition represent an important vulnerability factor for depression [Joormann and D'Avanzato, 2010], especially when exposed to negative material [Joormann and Gotlib, 2010], which may exacerbate ruminations and crowded thoughts [Koster et al., 2011; Piguet et al., 2010; Whitmer and Gotlib, 2012]. Two recent studies suggested a direct relationship between ruminations and impaired cognitive control in tasks requiring mental flexibility. In healthy subjects, impaired shifting performance during an internal shift task was found to mediate an effect of stress on rumination [De Lissnyder et al., 2011]. In mood disorder patients, depressive symptoms correlated with performance on the same shifting task given one year before, and this relation was also mediated by rumination level [Demeyer et al., 2012]. In addition, the effect of internal shifting abilities on rumination tendency has been found to be specific for negative material in healthy subjects [Lo and Allen, 2011]. However, these studies used attention shifting tasks that also heavily relied on working memory capacities, rather than just switching processes.
Here, we probe cognitive control flexibility in mood disorder patients by using a task‐switching paradigm that provides separate measures for the generation and inhibition of current mental sets. To this aim, we adapted the switching task designed by Mayr and Keele [2000] for fMRI settings and included emotional stimuli in order to explore interactions between switching and emotional valence [Piguet et al., 2013]. As demonstrated in previous studies [De Lissnyder et al., 2010; Piguet et al., 2013; Whitmer and Gotlib, 2012], this paradigm provides a reliable way of measuring inhibition processes during cognitive switching [for a review, see Koch et al., 2010]. This is done by testing sequences of three alternating tasks (A, B, C) either in ABA order or in ABC order. Indeed, switching from A to B requires first inhibiting the ongoing mental processes engaged in A, so that returning to A shortly afterward will require stronger effort compared to switching to another task C, even though both sequences require two mental switches [Mayr and Keele, 2000]. This supplementary cognitive cost for ABA relative to ABC sequences represents a consequence of backward inhibition and leads to slower reaction time, in addition to the cost of switching itself. In contrast, a “pure” cost of switching can be estimated with a different trial sequence (BBA versus AAA), which only involves the interruption of the previous task‐set and the generation of a new, noninhibited task‐set. Behavioral studies using this kind of paradigm have found an impairment of inhibition with nonemotional stimuli in healthy participants showing ruminative tendencies [Whitmer and Banich, 2007] and depressed participants [Whitmer and Gotlib, 2012], whereas others reported an impairment of inhibition restricted to negative stimuli in healthy people with ruminative tendencies [De Lissnyder et al., 2010]. To our knowledge, no study has examined switching with a backward inhibition component using emotional material in mood disorders, and none probed for any distinctive pattern in brain activation in these patients.
Task switching paradigms have been extensively studied with neuroimaging in healthy volunteers and highlighted widespread activations in frontoparietal and basal ganglia networks [Brass and von Cramon, 2004; Dove et al., 2000; Dreher et al., 2002; Pollmann et al., 2000; Wager et al., 2004], but only few have sought to distinguish the specific neural correlates of inhibition and switching proper. In a study based on a variant of the current paradigm with healthy controls [Piguet et al., 2013], we found that switching activated the bilateral superior parietal lobules, intraparietal sulcus, and posterior cingulate cortex, whereas inhibition led to reduced activation in specific task‐related areas for inhibition, together with selective decreases in the anterior cingulate cortex (ACC) for the inhibition of emotional material. Another fMRI study in healthy participants also contrasted returning to a previously inhibited task versus shifting to a new task, and revealed increased activity in the right lateral prefrontal cortex, as well as left inferior temporal and occipital cortex, during inhibition [Dreher and Berman, 2002]. However, no comparison was made with a “pure” switching condition. Another study reported a correlation between behavioral inhibition cost and activations in basal ganglia and SMA/premotor area during a simple task switching paradigm [Whitmer and Banich, 2012].
Thus, the neural underpinnings of task reconfiguration and inhibition in task switching paradigm remain unclear, and their modulation by affective factors has not been explored yet in mood disorder patients. Moreover, despite abundant neuroimaging studies in mood disorders, little is known about brain systems responsible for thought disorders such as ruminations and other related cognitive symptoms. Hence the main goal of this study was to test for cognitive control abilities subserving mental flexibility and inhibition in mood disorder patients, and to examine any relation between these abilities and rumination across a range of mood states. We compared a group of patients with variable mood disorders in order to pinpoint common mechanisms across diagnostic categories [Almeida and Phillips, 2012], relative to a group of matched healthy controls, and employed a novel task‐switching paradigm [Piguet et al., 2013] that included an inhibition component and emotional material. We hypothesized that patients would show impaired cognitive flexibility in both the generation/reconfiguration and backward inhibition components of switching that are required during this task; and that this should correspond to not only different activation patterns in regions involved in executive control (such as parietal and prefrontal cortex) but also abnormal modulation by inhibition of regions implicated in the monitoring of current mental states and emotions (such as limbic areas and anterior cingulate cortex) [Piguet et al., 2010].
MATERIAL AND METHODS
Participants
Patients were recruited in the department of adult psychiatry at the Geneva University Hospital, as well as through advertising on classified advertisements websites. Healthy participants (HP) were selected from a local database or through web advertising. All subjects gave informed written consent before inclusion in the study that was approved by the ethical committee of the Geneva University Hospital. Inclusion criteria for patients were a diagnostic of Major Depressive Disorder or Bipolar Disorder, aged between 18 and 60, under stable medication for 4 weeks, with no contraindication for MRI. Exclusion criteria for HP were past or present history of any neurological or psychiatric problem, use of medication, and contraindication for MRI. The Mini International Neuropsychiatric Interview [Sheehan et al., 1998] and the Structured Clinical Interview for the DSM‐IV, Mood Disorders section [First et al., 1997] were administered to patients and healthy subjects for evaluation of current axis‐I diagnostic (during a separate visit for the patients). HP were carefully matched with patients for age, gender, laterality, and level of education.
In total, 32 patients were included in the study but not all patients were able to complete the task‐switching paradigm, leaving 29 patients and 32 controls for the subsequent analyses.
Besides meeting criteria for MDD (N = 9), BD‐I (N = 6), BD‐II (N = 11), or BD‐others (N = 3) disorder (lifetime) according to DSM‐IV, some patients also met clinical criteria for anxiety disorder (N = 14), borderline personality disorder (N = 9), and ADHD (N = 2), reflecting the high prevalence of comorbid anxiety and personality disorder in the general mood disorder population as found in other studies [Friborg et al., 2014; Kauer‐Sant'Anna et al., 2009]. The severity of the disease was assessed by calculating the number of episodes (manic, hypomanic, and depressive) and the mean duration of disease in years as accurately as possible (patient recall and medical records, see supplementary Table).
During the medical interview, the Montgomery‐Asberg Depression Rating Scale [Montgomery and Asberg, 1979; French translation: Pellet et al., 1980], Young Mania Rating scale [French translation: Favre et al., 2003; Young et al., 1978], Ruminative Response Scale [RRS, Nolen‐Hoeksema and Morrow, 1991], and Hamilton anxiety scale [Hamilton, 1959; French translation: Pichot et al., 1981] were filled by both the patients and HP. Laterality was measured by the Edinburgh handedness inventory [Oldfield, 1971]. The ability to control thoughts, in particular the experience of intrusive thoughts, was also assessed with two questionnaires, the Thought Control Ability Questionnaire (TCAQ) [French translation: Gay et al., 2008; Luciano et al., 2005] and the White Bear Suppression Inventory (WBSI) [French translation: Schmidt et al., 2009; Wegner and Zanakos, 1994].
Apparatus and Stimuli
The task‐switching paradigm consisted of 3 possible tasks (color, gender, and emotion) made on face pictures. Each trial displayed three different faces arranged in a triangle. These faces were men or women with or without an emotional expression (happy, sad, and neutral) and shown in either red or green color (Fig. 1). Participants had to decide which of the three faces was different from the two others, according to one particular dimension (color, gender, or emotion) that was indicated on each trial by an instruction cue written in the middle of the screen. This cue appeared for 150 ms before the faces and stayed with the stimulus for the whole trial duration (1200 ms). The faces were followed by a fixation cross of 650 ms.
Figure 1.
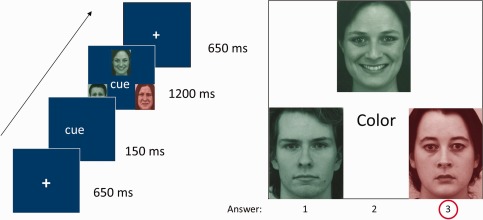
Emotional task‐switching paradigm. Illustration of stimuli and trial sequence.
Participants had to press the left button if the face differing from the others was on the left of the screen, the middle button if it was in the upper middle location, and the right button if it was on the right (using the index, middle, or annular fingers). This direct response‐mapping set avoids any working memory load and thus separates the executive control processes associated with switching from other working memory and retrieval processes. Extensive piloting prior to the study ensured that this task provided reliable measures of both inhibition and switching costs [Piguet et al., 2013]. There was no feedback about task performance, but the average accuracy score was presented to the subject at the end of each block.
The paradigm was implemented using E‐Prime 2.0 software (Psychology Software Tools Inc., USA) on a standard office PC (Optiplex 755, Dell S.A., Switzerland) running the Windows XP SP3 operating system. Responses were recorded with a response button box (HH‐1 × 4‐CR, Current Designs Inc., USA).
Design
The order of trials determined the condition in which any given trial was assigned [Piguet et al., 2013]. For example, for a sequence of trials requiring judgments of gender–gender–color, the color task performance reflects pure switching (= BBA); whereas for the sequence color–gender–color, the second color task additionally reflects the effect of previous inhibition (= ABA) taking place on the N‐2 trial [Piguet et al., 2013]. To optimize our fMRI paradigm, following extensive piloting, we constructed a blocked design protocol that included blocks with only repeat trials (10 consecutive trials with the same task, e.g., AAAA…) for the repetition condition, and task‐weighted blocks for each of the other experimental conditions. Thus, switch blocks contained 5 switch trials (repetition of the same task followed by a switch, e.g., BBA), 4 repeat trials (AA), plus the first (nonassigned) trial of the block. The inhibition blocks contained 4 inhibition trials (N‐1 different and N‐2 same, e.g., ABA), 3 switch trials, 2 repeat trials, plus the first nonassigned trial. The double‐switch blocks served as a control to the inhibition condition, by involving two successive switches but no recent task inhibition: they contained 4 double‐switch trials (N‐1 and N‐2 different, e.g., CBA), 3 switch trials, 2 repeat trials, plus the first nonassigned trial. Thus, each block type contained a predominance of a given task sequence, but a similar proportion of repeat and simple switch trials. We chose this blocked procedure to ensure a reliable modulation of the BOLD signal in patients, after adaption from (and comparison with) an earlier paradigm in healthy subjects [Piguet et al., 2013] where we used a fully event‐related design and obtained the same results than with the current task version (in HP). The order of sequences was thoroughly counterbalanced and randomized. Thus, for every trial of the type ABA in an inhibition condition, there was also a combination BAB and ACA, such that each experimental condition was free of any differential influence due to the nature of a particular preceding task.
In addition, each block had either a positive emotional valence, with a mixture of happy and neutral faces, or a negative valence, with a mixture of sad and neutral faces. This overall produced a total of 8 experimental conditions (switch‐happy, switch‐sad, repeat‐happy, repeat‐sad, inhibition‐happy, inhibition‐sad, doubleswitch‐happy, and doubleswitch‐sad).
Each subject performed a total of 6 blocks for each condition. Blocks were separated by a 7 s interval with a white fixation cross. We created 4 different lists of 48 blocks (480 trials), divided in 2 runs of ∼10 min each, administered in a pseudorandom order counterbalanced between subjects. Since each trial lasted 2000 ms, each block of 10 trials had a duration of 20 s, suitable for optimal fMRI block‐design. The faces were randomly distributed across trials, but the identity, color, gender, and emotion were counterbalanced between lists. We made sure that they were no direct repetition of the same faces in successive trials. Each participant practiced one run before entering the scanner.
Data Acquisition
Data were acquired at the Brain and Behaviour Laboratory, University of Geneva, with a 3 T magnetic resonance (MR) scanner (TIM Trio, Siemens) using a gradient echo‐planar (EPI) sequence in a rapid event‐related model [36 transverse slices with 20% gap, voxel size: 3.2 × 3.2 × 3.2 mm, repetition time (TR): 2100 ms, echo time (TE): 30 ms, flip angle (FA): 80°, field of view (FOV): 192 mm]. Three‐hundred and sixteen images were acquired for each of the 2 runs of the task. A structural MR scan was acquired at the end of the fMRI session [T1‐weighted 3D MP‐RAGE sequence, TR: 1900 ms, TE: 2.32 ms, TI: 900 ms, FA: 9°, FOV: 230 mm, matrix size 256 × 256 × 192, voxel size: 0.898 × 0.898 × 0.9 mm]. Stimuli were displayed using an LCD projector (CP‐SX1350, Hitachi, Japan) on a screen positioned at the rear of the scanner, which the participants could comfortably see through a mirror mounted on the 32 channels head coil.
Data Analysis
Statistical analyses of the behavioral data were carried‐out using SPSS software (IBM) version 19. Performance was compared using ANOVAs with task conditions (Switch, Repeat, Inhibition, DoubleSwitch) and emotions (Happy, Sad) as within‐subject factor, and groups (patients vs HP) as between‐subject factor. Our behavioral analysis excluded trials with response time shorter than 300 ms, plus the first trial of each block to avoid starting costs after a pause.
Functional MRI data were preprocessed and analyzed with SPM8 (http://www.fil.ion.ucl.ac.uk) implemented in Matlab (R2007b Mathworks). Functional scans were first realigned using iterative rigid body transformations that minimize the residual sum of square between the first and subsequent images, and corrected for differences in acquisition time between slices. They were then normalized to the MNI EPI template (2D spline, voxel size: 3 × 3 × 3 mm) and finally spatially smoothed with a Gaussian kernel with full‐width at half maximum (FWHM) of 8 mm. High‐resolution structural image was co‐registered and normalized with the mean image of the EPI series.
Data were processed using a two‐step analysis, taking into account the intraindividual and interindividual variance. For each participant, brain responses were modeled at each voxel, using a general linear model (GLM), for each of the 8 conditions, with 20 s blocks starting at the presentation of the first image of the block. The ensuing vector was convolved with the canonical hemodynamic response function (HRF) and used as a regressor in the individual design matrix. Movement parameters estimated during realignment and a session constant vector were also included as a variable of no interest. A high‐pass filter was implemented using a cut‐off period of 128 s to remove low‐frequency drifts from the time‐series. Serial autocorrelations were estimated with a restricted maximum likelihood algorithm using an autoregressive model of order 1.
The main effect of switching was estimated by linear contrasts between “Switch” blocks versus “Repeat” blocks, whereas the effect of inhibition was obtained by contrasting “Inhibition” blocks versus “DoubleSwitch” blocks. Note that the inverse of the latter contrast (DoubleSwitch versus Inhibition) is also of particular interest as it represents regions that would be deactivated after task inhibition [Piguet et al., 2013]. The individual statistical images of each contrast of interest were used in a second‐level group analysis, corresponding to random‐effect statistics, using two‐sample t‐tests for the main effects of group and conditions. To identify significant differences between groups, we masked exclusively the activation map from one condition in one group (one‐sample t‐test) by the same contrast from the other group (using a threshold of p < 0.05 for the exclusive mask). This procedure reveals activations that are unique to one group relative to the other. Group differences were also assessed by formal interaction contrasts at the whole brain level. We report activations thresholded at p < 0.001 at the voxel level and surviving correction for multiple comparisons at p < 0.05 FWE, using small volume correction (SVC) based on regions activated in previous studies (Tables 1 and 2), unless mentioned otherwise. For activations related to switching and inhibition processes, we defined regions of interest (ROIs) based on coordinates from our previous study [Piguet et al., 2013] using a version of the same task in healthy volunteers (including left and right medial superior parietal cortex, left intraparietal sulcus, posterior parietal cortex (PCC), left and right inferior frontal gyrus, left and right anterior cingulate cortex (ACC). For some areas, several coordinate peaks were extracted from a single larger cluster (not detailed in the previous paper). For other regions not observed in healthy volunteers (subgenual ACC (sgACC) and medial prefrontal cortex (PFC)), we used ROIs based on relevant studies on mood disorder patients [Chai et al., 2011; Davey et al., 2012], as noted in Tables 1 and 2. All ROIs were 10 mm spheres centered on the selected coordinates. Additional exploratory analyses were also performed with parametric tests at the whole‐brain level to examine whether specific contrasts of interest were modulated by clinically relevant factors (such as rumination scale, duration of disease, etc.). These parametric analyses used random‐effect group statistics in SPM similar to the above, with the addition of behavioral scores or clinical measures as linear regressors.
Table 1.
MNI coordinates of main contrasts for switching and masking (p < 0.001 uncorrected)
| x | y | z | nbr vox | Z‐score | p FWE‐corrected (SVC)* | |
|---|---|---|---|---|---|---|
| Patients > HP, all conditions | ||||||
| Left subgenual cingulate cortex | −3 | 26 | −8 | 22 | 4.07 | p = 0.009 |
| HP>Patient, all conditions | ||||||
| No significant activations | ||||||
| Switch>Repeat, all subjects | ||||||
| Left precuneus/medial sup parietal | −9 | −64 | 31 | 3699 | 6.82 | p < 0.001 |
| Left intraparietal sulcus | −33 | −58 | 40 | above | 5.45 | p < 0.001 |
| Right precuneus/medial sup parietal | 15 | −64 | 31 | above | 5.17 | p < 0.001 |
| Posterior cingulate cortex | −6 | −31 | 31 | 492 | 5.42 | p < 0.001 |
| Left inferior frontal gyrus | −30 | 53 | 10 | 1065 | 5.13 | p < 0.001 |
| Right anterior cingulate cortex | 12 | 35 | 13 | 28 | 3.76 | p = 0.009 |
| Left anterior cingulate cortex | −9 | 29 | 31 | 14 | 3.61 | p = 0.009 |
| Switch>Repeat, Patients masked by HP exclusively | ||||||
| Left precuneus | −18 | −58 | 31 | 636 | 4.64 | p = 0.023 |
| Right posterior cingulate cortex | 9 | −34 | 28 | above | 4.47 | p = 0.018 |
| Left intraparietal sulcus | −36 | −64 | 37 | above | 4.31 | p = 0.024 |
| Left inferior frontal gyrus (3 peaks) | −51 | 23 | 31 | 244 | 4.46 | p = 0.021 |
| Right anterior cingulate cortex | 12 | 35 | 16 | 19 | 3.77 | n.s. (p < 0.001 unc) |
| Repeat>Switch, all subjects | ||||||
| Right inferior frontal gyrus | 51 | 32 | −8 | 31 | 4 | p = 0.006 |
SVC: small volume correction. * based on coordinates from Piguet et al. [2013].
Table 2.
MNI coordinates of inhibition effect and masking (p < 0.001 uncorrected)
| x | y | z | nbr vox | Z‐score | p FWE‐corrected (SVC)* | |
|---|---|---|---|---|---|---|
| Inhibition > DoubleSwitch, all subjects | ||||||
| No significant activation | ||||||
| DoubleSwitch > Inhibition, all subjects | ||||||
| Medial prefrontal cortex | −15 | 53 | 1 | 19 | 3.79 | p = 0.024 |
| DoubleSwitch> Inhibition, HP masked by Patients, exclusively | ||||||
| Medial prefrontal cortex | −12 | 59 | 1 | 74 | 3.87 | p = 0.004 |
| Right subgenual cortex | 9 | 32 | −8 | 8 | 3.37 | p = 0.017 |
RESULTS
Participants
The two groups did not differ significantly regarding gender, age, laterality, and level of education (Table 3). Patients were included regardless of their current mood state, allowing us to measure switching abilities in a dimensional manner across mood and diagnostic boundaries [Krystal and State, 2014; Narrow and Kuhl, 2011; Vieta and Valentí, 2013] (see also http://www.nimh.nih.gov/research-priorities/rdoc/nimh-research-domain-criteria-rdoc.shtml). Our final sample included 13 euthymic patients, 10 depressed, 1 hypomanic, and 5 in subdepressive/mixed state not meeting DSM‐IV‐TR criteria. We report levels of depression, mania, anxiety, ruminations and thought control as assessed by self‐report questionnaires in Table 3. Medication was taken by 24 out of 29 patients as detailed in Supplementary Table. Medication effects were considered in complementary analyses (see below) but did not appear to influence the results. Additional clinical information such as comorbidities, severity of the disease, and history of suicidal behavior are reported in Supplementary Table as well. Effects of other clinical parameters on behavioral and fMRI results are reported below when present; however, most effects of interest reported here were independent of particular diagnostic features of the patients, in agreement with the validity of a dimensional approach comparing patients to controls regardless of subgroups.
Table 3.
Demographic and clinical characteristics
| Patients | Controls | Independent t‐test | ||
|---|---|---|---|---|
| Characteristics | M (SD) | M (SD) | p value | |
| N (males) | 29 (14) | 32 (14) | ||
| Age | 39.4 (8.5) | 39.8 (8.6) | n.s. | |
| Level of education | 13.3 (3.1) | 13.9 (2.9) | n.s. | |
| Laterality (not right‐handed) | 21 (8) | 25 (7) | ||
| MADRS | 14.1 (9.7) | 1.94 (1.8) | <0.001 | |
| Young | 1.97 (2.5) | 0.5 (0.8) | <0.001 | |
| Hamilton Anxiety | 12.8 (8.1) | 3.3 (2.3) | <0.001 | |
| RRS | 55.7 (11.1) | 34.2 (9.9) | <0.001 | |
| RRS‐brooding subscore | 12.7 (3.2) | 8.1 (2.4) | <0.001 | |
| RRS‐clinical subscore* | 25.2 (5.4) | 16.7 (5.2) | <0.001 | |
| TCAQ | 58.7 (13.3) | 79.3 (15.9) | <0.001 | |
| WBSI | 53.6 (11) | 38.3 (11.3) | <0.001 |
M: mean; SD: standard deviation; MADRS: Montgomery Asberg Depression rating Scale; RRS: Ruminative Response Scale; WBSI: White Bear Supression Inventory; TCAQ: Thought Control Ability Questionnaire; * Whitmer and Gotlib [2011].
Behavioral Data
Performance of patients and HP in the switching task differed in terms of accuracy but not response times (RTs). The mean percentage of correct response was 61.06% for patients (min. 28.75%–max. 90%, standard deviation [std] 16.14), versus 73.65% for HP (min. 63.1%–max. 91%, std 8.7; independent t‐test, p < 0.001). Accuracy did not correlate with level of depression or age, but with level of education (p = 0.04). Mean RTs were not different between patients (mean= 1014.5 ms, std 113.3) and HP (mean= 985.6 ms, std 104.8; independent t‐test, p = 0.304). RTs correlated with age (p = 0.002), not with level of depression or education.
To compare RTs in the different task conditions, we performed a 4 (Switch, Repeat, Inhibition, DoubleSwitch) × 2 (Happy, Sad) mixed repeated‐measure ANOVA with the between‐subject factor of group (Patients, HP). Results yielded no main effect of group or emotion, but a main effect of condition (F(3,57) = 40.52, p < 0.001) and an interaction of condition × group (F(3,57) = 5.625, p = 0.002). There was also a marginal triple interaction of condition × group × emotion (p = 0.060). Pairwise comparisons showed a significant effect for Switch versus Repeat (p < 0.001) and for Inhibition versus DoubleSwitch (p = 0.032), corresponding to the predicted “Switch cost” and “Inhibition cost”, respectively (Fig. 2 and Table 4). Post‐hoc t‐tests also showed that the difference between Switch and Repeat (Switch cost) was significantly larger for patients than HP (p = 0.006), which was not the case for the Inhibition cost (Table 4). The former difference was mainly due to the Switch cost in the happy condition, which was smaller in HP than patients (p < 0.001) and accounted for the triple interaction trend above.
Figure 2.
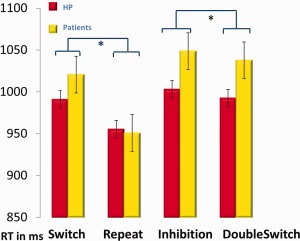
Behavioral data. Reaction time plotted for the 4 task conditions in ms (*significant, Switch > Repeat, p < 0.001, Inhibition > DoubleSwitch, p = 0.032).
Table 4.
Behavioral results: average reaction times in each condition and comparisons of Switch cost and Inhibition cost between patients and controls
| Switch | Repeat | Inhibition | DoubleSwitch | |||||
|---|---|---|---|---|---|---|---|---|
| Happy | Sad | Happy | Sad | Happy | Sad | Happy | Sad | |
| HP | 988.7 | 994.0 | 962.0 | 946.9 | 1005.8 | 1000.2 | 989.0 | 995.6 |
| Patients | 1022.2 | 1015.5 | 941.6 | 960.1 | 1051.2 | 1045.9 | 1036.0 | 1035.9 |
| t‐test | Switch > Repeat: p < 0.001 | Switch cost | Inhibition cost | |||||
| Inhibition > DoubleSwitch: p = 0.032 | HP: 35.6 (37.8) | 10.4 (37.4) | ||||||
| Happy > Sad: p = 0.921 | P: 69.8 (54.8) | 11 (46.4) | ||||||
| Patients > HP: | p = 0.006 | p = 0.96 | ||||||
Inspection of clinical factors indicated that the Switch cost was smaller (p = 0.038) for patients with a low severity of disease (number of episodes 1–4) than those with high severity (number of episodes > 10). However, the Switch cost did not differ between patients who took antipsychotics and those who did not (p = 0.734). These costs did not differ either between MDD and BD‐I, MDD and BD‐II, BD‐I and BD‐II, or between depressed and euthymic patients (independent sample t‐test). They were not correlated with rumination scores (RRS and subscores) or depression score (MADRS) but Switch cost did correlate positively with WBSI (r(61) = 0.289, p = 0.044): the more intrusive the thoughts, the longer it takes to switch from one mental set to the other.
Overall, these data confirm that our paradigm could successfully separate the recruitment of distinct switching and inhibition components in cognitive control during the fMRI task, and reveal significant differences in switching abilities in patients relative to controls. Behaviorally, these RT differences mainly concerned switching to a new set while the magnitude of inhibition costs was not significantly larger in patients.
Brain Imaging Data
The whole‐brain analysis of the main effect of group, comparing patients versus HP regardless of conditions, revealed selective increases in the left subgenual ACC (sgACC) and right supramarginal gyrus (Table 1). The main effect of emotion showed only increased activity in visual areas for blocks with Happy vs Sad faces (for both groups together), but no activity for the reverse contrast or for the interactions Group × Emotion.
The main effect of condition, Switch (S) vs Repeat (R) (for both groups together), showed significant activation in fronto‐parietal areas, bilaterally in the left precuneus/medial parietal cortex, intraparietal sulcus (IPS), and ACC, plus the left PCC and left inferior frontal gyrus (IFG; cf. Table 1). To identify regions more activated for patients than HP during switching, we then masked exclusively the effect of Switch versus Repeat in patients by the same contrast in HP (see methods). This procedure revealed activations present for patients, but not HP, in the left precuneus, right PCC, left IPS, left IFG, and right ACC (Table 1 and Fig. 3). These effects were further confirmed by a formal whole‐brain interaction test between patient versus HP for the same contrast (S vs R > Patients vs HP), which revealed activation peaks in the dorsal parietal cortex. There was no significant effect for the reverse interaction (S vs R > HP vs Patients) or with the reverse masking procedure, testing for regions more activated in HP than patients during switching.
Figure 3.
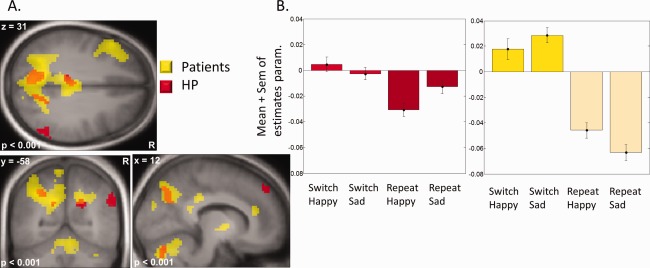
Effects of switching. (A) Main contrast Switch versus Repeat, for patients and HP. Patients show a greater network of activations. (B) Parameters estimates of left precuneus/medial superior parietal cortex (x, y, z = −18, −58, 31) showing lower recruitment for HP compared to patients, and no effect of emotion.
The parameters estimates of activity extracted from the whole‐brain analysis peak in the left precuneus/medial parietal cortex identified above are plotted in Fig. 3 to illustrate the difference between groups and to compare the two emotion conditions. These areas (left IPS, precuneus) showed no significant modulation by the emotional expression of face stimuli, as statistically verified by a 2 groups × 2 conditions (Switch, Repeat) × 2 emotions (Happy, Sad) repeated‐measure mixed ANOVA on these data.
In summary, these results indicate that switching recruited larger brain networks in patients than HP. This finding is further backed up by a whole‐brain parametric correlation analysis showing that the magnitude of the behavioral Switch cost (RTs) correlated positively with activity in the left IPS (x, y, z = −36, −70, 55), left middle frontal gyrus (x, y, z = −18, 29, 58), and ACC (x, y, z = −9, 41, 28) (p = 0.001 uncorrected, all subjects).
Next we investigated the effect of inhibition. The comparison of Inhibition vs DoubleSwitch yielded no significant activation; but the reverse contrast, DoubleSwitch vs Inhibition, showed a selective effect in the medial prefrontal cortex (mPFC, Fig. 4A and Table 2), for all subjects together, demonstrating that this region is generally less activated as a consequence of inhibition of the same task on a preceding trial. A masking procedure was again used to compare this effect between groups. The contrast DoubleSwitch vs Inhibition among HP, masked exclusively by the same contrast among patients, revealed stronger activation in both the right sgACC and the mPFC (Fig. 4B and Table 2). These regions are therefore deactivated by previous inhibition in HP, but not in patients. The formal interaction contrast across the whole brain (DoubleSwitch > Inhibition for HP > Patients) also confirmed a significant effect in the ventromedial PFC/sgACC (113 vox. cluster, peak x, y, z: −6 59 −2, Z‐score = 3.26).
Figure 4.
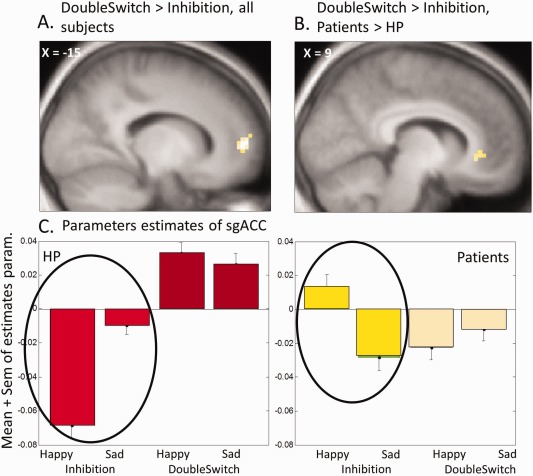
Effects of inhibition. (A) Main contrast DoubleSwitch > Inhibition, showing medial PFC deactivation after inhibition for all subjects. (B) Interaction DoubleSwitch > Inhibition * Patients > HP, showing activation of sgACC unique to patients. (C) Parameters estimates of sgACC (x, y, z = 9, 32, −8), showing an interaction of conditions with the emotional content of stimuli.
These group differences in the sgACC are illustrated by the parameters estimates of activity plotted in Fig. 4C, which additionally points to a modulation of inhibition effects by emotion. This modulation was confirmed by a repeated‐measure mixed ANOVA with the factors Group (HP, Patients) × Condition (DoubleSwitch, Inhibition) × Emotion (Happy, Sad), performed on the parameters estimates from the right sgACC. This analysis revealed a significant triple interaction (F(1,59) = 4.632, p = 0.035) driven by the fact that the deactivation of the sgACC during the inhibition condition was maximal in response to happy faces in HP (t = 3.49, p = 0.001), whereas this was not the case (or even reversed) in patients (t = −1.59, p = 0.124; Fig. 4).
The reverse masking procedure, testing for inhibition effects among patients not seen among HP, showed no significant activation.
Finally, we examined the relation between these brain responses and the behavioral measure of Inhibition cost (RTs in the Inhibition condition minus RT in the DoubleSwitch condition). When using this value from each individual as a covariate in a parametric regression analysis at the whole group level (patients and HP combined together), we found a positive correlation with activation in the sgACC for the contrast DoubleSwitch versus Inhibition (x, y, z = −3 26 −8, z = 2.52, p = 0.005 unc). Further inspection of the parameter estimates from this cluster for each group separately showed that this correlation with the behavioral Inhibition cost was actually driven by the effect in HP (r(32) = −0.458, p = 0.008): the bigger the Inhibition cost, the lower the activity in sgACC following inhibition. In other words, participants who are better at inhibiting a current mental set are also capable of better deactivating the sgACC when switching to new task goals. This correlation was not seen in patients (r(29) = 0.064, p = 0.743), due to the reduced modulation of sgACC by inhibition. Thus, their sgACC activity appeared globally constant and unaffected by task condition. Notably, however, activation in the sgACC did not correlate with depression scores on the MADRS, for all participants combined or each group taken separately (p ≥ 0.167).
Correlation with RRS
The tendency to ruminate as measured with the RRS did not correlate with behavioral measures of Switching cost or Inhibition cost. Nonetheless, given our hypothesis concerning the relation between cognitive flexibility and ruminations, we directly tested for a correlation of the RRS with brain activity in the two main fMRI contrasts. The parametric regression of individual RRS scores for the Switch > Repeat contrast across the whole brain yielded a negative correlation with activity in the right dorsal ACC (x, y, z = 9, 23, 34), right dorsolateral PFC (x, y, z = 57, 26, 28), and bilateral anterior insula (x, y, z = −39/30, 29, −5/1; all p ≤ 0.001 unc., all participants combined). Thus, the higher the tendency to ruminate, the lower the recruitment of these areas during task switching. This negative correlation was observed in both groups for the right ACC and the right insula, and only in HP for the dorsolateral PFC activity.
A similar parametric analysis showed no correlation of RRS scores with brain activity in the DoubleSwitch vs Inhibition contrast (either positive or negative). Additional analysis using the brooding subscore or the clinical subscore of the RRS instead of the total score showed the same pattern of results.
Relation with Other Clinical Factors
None of the mentioned questionnaires (MADRS, Hamilton Anxiety Scale, TCAQ, WBSI), when used as a regressor in our fMRI analysis, was found to explain the group differences reported above. For completeness, we performed auxiliary analyses to verify for any potential medication effects, even though none of the patients had the same treatment. At the behavioral level, patients with or without antipsychotic medication did not differ in any of our measures. Further, at the brain level, comparing patients with and without antipsychotic medication did not influence our results, as observed in other cognitive tasks in similar patients [Phillips et al., 2008; Piguet et al., 2014]. We also performed an additional analysis including as regressor the number of different classes of medication taken (0–4), which did not correlate with any brain activation. We also used two regressors in the statistical model for the severity of disease (number of episodes and duration of the disease) that did not impact our results. Finally, auxiliary analyses comparing subgroups of patients (unipolar vs bipolar type II, or depressed vs euthymic) did not show any modification of the general pattern of results described above (data not reported here).
DISCUSSION
We used a task‐switching paradigm to assess switching and inhibition processes in the control of mental sets, both behaviorally and in terms of brain activation, among mood disorders patients and HP. Behavioral results showed that the cost of switching from one task to another (Switch cost) is significantly larger for patients than for HP, irrespective of the DSM‐IV diagnostic, reflecting a cognitive impairment shared across different mood disorders. We did not find group differences for the cost of returning to a previously inhibited task (Inhibition cost), but this measure was more variable even in HP. In keeping with our behavioral data, fMRI revealed that switching produced greater activations in a wide fronto‐parieto‐cingulate network in patients than HP, with IPS correlating with the magnitude of the behavioral Switch cost. In addition, the sgACC showed not only a main effect of group, with significantly greater activation in patients overall, but more critically, this region also exhibited less deactivation in patients compared to HP as a consequence of task inhibition, suggesting a failure to downregulate this area during switching.
Increased Activations in Attention Network and Task Switching
Mood disorder patients have often been found to present hypoactivation in prefrontal “control” regions, particularly at rest [Savitz and Drevets, 2009]. Here, however, we found the opposite: a larger recruitment of regions involved in attention and switching, including the superior medial parietal cortex, IPS, PCC, and left IFG [Collette et al., 2005; Hampshire et al., 2010; Kim et al., 2012; Philipp et al., 2013], suggesting that patients make greater efforts when switching to a new mental set. These findings support the notion of a depletion of cognitive resources in these patients, who may need higher (or longer) activity than HP to achieve the same performance. Moreover, IPS activity was linearly correlated with the switch cost magnitude across participants, highlighting its direct functional relationship with task performance.
This pattern is actually consistent with other studies reporting increased engagement of extended networks during various cognitive tasks among depressed patients [Fitzgerald et al., 2008], including the rostral ACC [Wagner et al., 2006], dorsal ACC, and dorsolateral prefrontal areas [Harvey et al., 2005]. This is also observed among the close healthy relatives of patients with depression [Mannie et al., 2010] or bipolar disorder [Fusar‐Poli et al., 2012], in whom increased frontal and insular recruitment during cognitive tasks is thought to represent a marker of “functional inefficiency” and compensatory mechanisms [Fusar‐Poli et al., 2012].
In conclusion, overactivation of brain regions implicated in switching processes corroborates the notion that mood disorder patients experience greater efforts to shift and less efficient engagement of attention in a new cognitive task, as directly demonstrated here by the larger behavioral switch cost in RTs and the corresponding increases in fronto‐parietal activity.
Subgenual Cingulate Cortex and Task Inhibition
Hyperactivation of the sgACC at rest is a well‐known marker of unipolar depression [Greicius et al., 2007; Mayberg et al., 1999; Mayberg, 2009, 2003]. A reduction in grey matter volume of this region is implicated in the pathophysiology of both unipolar and bipolar disorders, though without a clear understanding of its functional role [Drevets et al., 2008; Ellison‐Wright and Bullmore, 2010; Kempton et al., 2011]. The sgACC is also involved in emotional processing [Maddock et al., 2003; Mayberg et al., 1999]. Current views suggest that changes in sgACC in depression may reflect increased basal metabolism, leading to hyperactivity across different conditions, in a task‐independent fashion [Northoff, 2007]. This partly accords with what we observed here, with a significant main effect of group and lack of modulation by task demands for patients relative to HP. In our study, the sgACC was dynamically down‐regulated by the inhibition of a previous mental set in HP, and this modulation correlated with magnitude of the behavioral inhibition cost. By contrast, this region remained highly activated and unaffected by task inhibition in patients. Taken together, these data provide novel evidence for impaired cognitive flexibility in patients, leading to a reduced ability to suppress a current task set in order to switch to a new task, and reduced ability to downregulate the sgACC in response to the new task demands.
The sgACC is a key part of the default‐mode network (DMN), thought to be critically implicated in self‐referential processing as well as interoceptive monitoring [D'Argembeau et al., 2005; Qin and Northoff, 2011]. Activity in this region may contribute to the sense of self, but also influence rest–stimulus interactions [Qin and Northoff, 2011]. In accord with these views, our findings suggest that higher cognitive flexibility when responding to external tasks demands may require more efficient disengagement of current self‐referential processing, and that such ability might be reduced in mood disorder patients.
Depression is indeed known to be characterized by a failure to deactivate regions of the DMN, including ventromedial PFC, ACC, lateral parietal cortex, and lateral temporal cortex [Sheline et al., 2009], together with extra functional connectivity of sgACC and thalamus with the DMN [Greicius et al., 2007]. A study in MDD patients reported that during a self‐judgment task, ventro‐medial prefrontal regions were less deactivated for patients than healthy subjects, suggesting that MDD patients might fail to downregulate their high resting state activity in sgACC because they remain “stuck in their own self” [Grimm et al., 2009; Yoshimura et al., 2010]. These data therefore accord with the notion that higher sgACC activity may reflect increased self‐focus in depression. Here, however, our paradigm did not involve any explicit self‐related task, suggesting that such modulation of sgACC might reflect more automatic processes of self‐monitoring or interoceptive regulation associated with ongoing behavioral adjustments. Patients might be unable to inhibit self‐monitoring and affective regulation processes mediated by sgACC when required to focus on a new external task demand.
As proposed by other authors, the self is not a unitary construct but implicates several different levels, both implicit and explicit [Northoff, 2007]. Accordingly, activity in ventral mPFC, including sgACC, has been associated with both implicit and explicit self‐referential processing, contrarily to more specific explicit processing in dorsal mPFC [Rameson et al., 2010]. Moreover, tonic activation in ventral mPFC has been linked to automatic aspects of self‐focus [Lemogne et al., 2012].
Taken all together, these findings suggest that sgACC, known to be central in the pathophysiology of depression, is intimately associated with automatic self‐processing mechanisms that involve implicit emotion regulation and interoceptive signals, and shows a distinctive pattern of dysfunctional modulation by task demands in mood disorder patients. This is consistent with other findings that this region shows deactivation in attentional tasks only with much higher cognitive load in depressed patients as compared with healthy individuals [Desseilles et al., 2009]. More generally, this interpretation also accords with a recent theory of altered interoceptive state in MDD and anxiety, postulating the existence of noisily amplified self‐referential interoceptive belief states that may contribute to negative processing biases in these patients [Paulus and Stein, 2010].
In summary, our results add novel evidence for persistent hyperactivation of self‐related regions in mood disorder patients, selectively affecting subgenual cingulate areas implicated in automatic interoceptive and self‐monitoring processing. Critically, we show a striking failure of patients to deactivate this region by task set inhibition. This persistent “background” activity may contribute to deplete attentional resources and forces patients to recruit larger fronto‐attentional network to achieve the task.
We note, however, that neither neural changes in sgACC activity and behavioral measures of inhibition cost correlated with a measure of habitual rumination tendencies, as assessed by the RRS score. By contrast, more dorsal activation in cingulate cortex during switching correlated with lower rumination, together with dorsolateral PFC and insula. These areas may contribute to control thoughts and diminish subjective experience of ruminations by subserving more efficient generation of new task sets, in both controls and patients. However, rumination tendency measured by the RRS does not appear directly related to a deficit in the inhibition process associated with switching abilities.
Limitations: Heterogeneity of Patients and Diagnosis
Our study included patients with different diagnoses and different mood states. It is often assumed that bipolar disorders patients are more severely impaired in cognitive functioning than unipolar patients [Iverson et al., 2011]. However, our primary goal was to test for switching and inhibition abilities in mood disorders in a dimensional fashion [Krystal and State, 2014], regardless of diagnostic boundaries, and we therefore did not seek to separate patients in different groups. Moreover, when specifically comparing bipolar‐II and unipolar patients, we found no difference at either the behavioral or neural level concerning the cognitive switch processes investigated here. Likewise, we found that none of the reported effect could be explained by different patterns between depressed and euthymic patients, and none of the reported effects were modulated by the MADRS scale. The variability among our patients may have added some noise to our results, but at the same time also make our findings more robust and closer to typical populations encountered in clinical practice.
Similar concerns may also apply to the various treatments that our patients received. Because none of them was under the exact same drug combination or dosage, we could not fully control for medication effects. However, as described above, exploratory analyses both at the behavioral and brain levels did not point to any medication confound for our results. Altogether, these data accord with recent reviews in the literature showing no systematic effect of medication in similar patient group studies [Phillips et al., 2008].
Finally, future studies with larger patients samples would be valuable to allow more sophisticated mediational or multiple regression analyses to better assess the relation between changes in activity among different brain regions and the severity of clinical symptoms associated with thought control anomalies and mood disorders.
CONCLUSION
Our results reveal that normal cognitive flexibility is associated with the ability to deactivate the sgACC when attention must be engaged in a new task. This ability is impaired among mood disorders patients who show a persistent hyperactivation of sgACC, unaffected by task inhibition during switching. This deficit is associated with reduced efficiency in switching, enhanced recruitment of fronto‐parieto‐cingulate brain networks mediating attention shifts, and presumably greater efforts to change attention focus. This lack of mental flexibility among mood disorders patients, combined with a failure to deactivate sgACC, might parallel the difficulties of these patients to suppress self‐focused thoughts and affective concerns, a major symptom of depression.
Supporting information
Supporting Information Table 1.
ACKNOWLEDGMENT
None of the authors of this manuscript report biomedical financial interests or other potential conflicts of interest.
REFERENCES
- Almeida JRCd, Phillips ML (2012): Distinguishing between unipolar depression and bipolar depression: Current and future clinical and neuroimaging perspectives. Biol Psychiatry. http://www.ncbi.nlm.nih.gov/pubmed/22784485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Argembeau A, Collette F, Van der Linden M, Laureys S, Del Fiore G, Degueldre C, Luxen A, Salmon E (2005): Self‐referential reflective activity and its relationship with rest: A PET study. Neuroimage 25:616–624. [DOI] [PubMed] [Google Scholar]
- Arts B, Jabben N, Krabbendam L, van Os J (2008): Meta‐analyses of cognitive functioning in euthymic bipolar patients and their first‐degree relatives. Psychol Med 38:771–785. [DOI] [PubMed] [Google Scholar]
- Brass M, von Cramon DY (2004): Decomposing components of task preparation with functional magnetic resonance imaging. J Cogn Neurosci 16:609–620. [DOI] [PubMed] [Google Scholar]
- Chai XJ, Whitfield‐Gabrieli S, Shinn AK, Gabrieli JDE, Nieto Castañón A, McCarthy JM, Cohen BM, Ongür D (2011): Abnormal medial prefrontal cortex resting‐state connectivity in bipolar disorder and schizophrenia. Neuropsychopharmacology 36:2009–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collette F, Van der Linden M, Laureys S, Delfiore G, Degueldre C, Luxen A, Salmon E (2005): Exploring the unity and diversity of the neural substrates of executive functioning. Hum Brain Mapp 25:409–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey CG, Harrison BJ, Yücel M, Allen NB (2012): Regionally specific alterations in functional connectivity of the anterior cingulate cortex in major depressive disorder. Psychol Med 42:2071–2081. [DOI] [PubMed] [Google Scholar]
- Demeyer I, De Lissnyder E, Koster EHW, De Raedt R (2012): Rumination mediates the relationship between impaired cognitive control for emotional information and depressive symptoms: A prospective study in remitted depressed adults. Behav Res Ther 50:292–297. [DOI] [PubMed] [Google Scholar]
- Desseilles M, Balteau E, Sterpenich V, Dang‐Vu TT, Darsaud A, Vandewalle G, Albouy G, Salmon E, Peters F, Schmidt C, Schabus M, Gais S, Degueldre C, Phillips C, Luxen A, Ansseau M, Maquet P, Schwartz S (2009): Abnormal neural filtering of irrelevant visual information in depression. J Neurosci 29:1395–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove A, Pollmann S, Schubert T, Wiggins CJ, von Cramon DY (2000): Prefrontal cortex activation in task switching: An event‐related fMRI study. Brain Res Cogn Brain Res 9:103–109. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Berman KF (2002): Fractionating the neural substrate of cognitive control processes. Proc Natl Acad Sci USA 99:14595–14600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher JC, Koechlin E, Ali SO, Grafman J (2002): The roles of timing and task order during task switching. Neuroimage 17:95–109. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M (2008): The subgenual anterior cingulate cortex in mood disorders. CNS Spectr 13:663–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison‐Wright I, Bullmore E (2010): Anatomy of bipolar disorder and schizophrenia: A meta‐analysis. Schizophr Res 117:1–12. [DOI] [PubMed] [Google Scholar]
- Favre S, Aubry J‐M, Gex‐Fabry M, Ragama‐Pardos E, McQuillan A, Bertschy G (2003): Translation and validation of a French version of the Young Mania Rating Scale (YMRS). Encephale 29:499–505. [PubMed] [Google Scholar]
- First, MB , Gibbon M., Spitzer RL, Williams, JBW , Benjamin LS (1997): Structured Clinical Interview for DSM‐IV Axis II Personality Disorders, (SCID‐II). Washington, DC: American Psychiatric Press, Inc.[TQ1] [Google Scholar]
- Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ (2008): A meta‐analytic study of changes in brain activation in depression. Hum Brain Mapp 29:683–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friborg O, Martinsen EW, Martinussen M, Kaiser S, Overgård KT, Rosenvinge JH (2014): Comorbidity of personality disorders in mood disorders: A meta‐analytic review of 122 studies from 1988 to 2010. J Affect Disord 152154:1–11. [DOI] [PubMed] [Google Scholar]
- Fusar‐Poli P, Howes O, Bechdolf A, Borgwardt S (2012): Mapping vulnerability to bipolar disorder: A systematic review and meta‐analysis of neuroimaging studies. J Psychiatry Neurosci 37:170–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay P, d'Acremont M, Schmidt RE, Van der Linden M (2008): Validation of a French adaptation of the thought control ability questionnaire. Eur J Psychol Assess 24:101–107. [Google Scholar]
- Gotlib IH, Joormann J (2010): Cognition and depression: Current status and future directions. Annu Rev Clin Psychol 6:285–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF (2007): Resting‐state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry 62:429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm S, Ernst J, Boesiger P, Schuepbach D, Hell D, Boeker H, Northoff G (2009): Increased self‐focus in major depressive disorder is related to neural abnormalities in subcortical‐cortical midline structures. Hum Brain Mapp 30:2617–2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton MM (1959): The assessment of anxiety states by rating. Br J Med Psychol 32:50–55. [DOI] [PubMed] [Google Scholar]
- Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM (2010): The role of the right inferior frontal gyrus: Inhibition and attentional control. Neuroimage 50:1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ (2008): Biological substrates of emotional reactivity and regulation in adolescence during an emotional go‐nogo task. Biol Psychiatry 63:927–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey P‐O, Fossati P, Pochon J‐B, Levy R, Lebastard G, Lehéricy S, Allilaire J‐F, Dubois B (2005): Cognitive control and brain resources in major depression: An fMRI study using the n‐back task. Neuroimage 26:860–869. [DOI] [PubMed] [Google Scholar]
- Iverson GL, Brooks BL, Langenecker SA, Young AH (2011): Identifying a cognitive impairment subgroup in adults with mood disorders. J Affect Disord 132:360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Gotlib IH (2010): Emotion regulation in depression: Relation to cognitive inhibition. Cogn Emot 24:281–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, D'Avanzato C (2010): Emotion regulation in depression: Examining the role of cognitive processes. Cogn Emot 24:913–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauer‐Sant'Anna M, Kapczinski F, Vieta E (2009): Epidemiology and management of anxiety in patients with bipolar disorder. CNS Drugs 23:953–964. [DOI] [PubMed] [Google Scholar]
- Keizer I, Piguet C, Favre S, Aubry J‐M, Dayer A, Gervasoni N, Gex‐Fabry M, Bertschy G (2013): Subjective experience of thought overactivation in mood disorders: Beyond racing and crowded thoughts. Psychopathology. zotero://attachment/4176/. [DOI] [PubMed] [Google Scholar]
- Kempton MJ, Salvador Z, Munafò MR, Geddes JR, Simmons A, Frangou S, Williams SCR (2011): Structural neuroimaging studies in major depressive disorder. Meta‐analysis and comparison with bipolar disorder. Arch Gen Psychiatry 68:675–690. [DOI] [PubMed] [Google Scholar]
- Kim C, Cilles SE, Johnson NF, Gold BT (2012): Domain general and domain preferential brain regions associated with different types of task switching: A meta‐analysis. Hum Brain Mapp 33:130–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch I, Gade M, Schuch S, Philipp AM (2010): The role of inhibition in task switching: A review. Psychon Bull Rev 17:1–14. [DOI] [PubMed] [Google Scholar]
- Koster EH, De Lissnyder E, Derakshan N, De Raedt R (2011): Understanding depressive rumination from a cognitive science perspective: The impaired disengagement hypothesis. Clin Psychol Rev 31:138–145. [DOI] [PubMed] [Google Scholar]
- Krystal JH, State MW (2014): Psychiatric disorders: Diagnosis to therapy. Cell 157:201–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemogne C, Delaveau P, Freton M, Guionnet S, Fossati P (2012): Medial prefrontal cortex and the self in major depression. J Affect Disord 136:e1–e11. [DOI] [PubMed] [Google Scholar]
- De Lissnyder E, Koster EH, Derakshan N, De Raedt R (2010): The association between depressive symptoms and executive control impairment in response to emotional and non‐emotional information. Cogn Emot 24:264–280. [Google Scholar]
- De Lissnyder E, Koster EH, Goubert L, Onraedt T, Vanderhasselt MA, De Raedt R (2011): Cognitive control moderates the association between stress and rumination. J Behav Ther Exp Psychiatry 43:519–525. [DOI] [PubMed] [Google Scholar]
- Lo BCY, Allen NB (2011): Affective bias in internal attention shifting among depressed youth. Psychiatry Res 187:125–129. [DOI] [PubMed] [Google Scholar]
- Luciano JV, Algarabel S, Tomás JM, Martínez JL (2005): Development and validation of the thought control ability questionnaire. Pers Individ Diff 38:997–1008. [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH (2003): Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Hum Brain Mapp 18:30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannie ZN, Harmer CJ, Cowen PJ, Norbury R (2010): A functional magnetic resonance imaging study of verbal working memory in young people at increased familial risk of depression. Biol Psychiatry 67:471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS (2009): Targeted electrode‐based modulation of neural circuits for depression. J Clin Invest 119:717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS (2003): Modulating dysfunctional limbic‐cortical circuits in depression: Towards development of brain‐based algorithms for diagnosis and optimised treatment. Br Med Bull 65:193–207. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT (1999): Reciprocal limbic‐cortical function and negative mood: Converging PET findings in depression and normal sadness. Am J Psychiatry 156:675–682. [DOI] [PubMed] [Google Scholar]
- Mayr U, Keele SW (2000): Changing internal constraints on action: The role of backward inhibition. J Exp Psychol Gen 129:4–26. [DOI] [PubMed] [Google Scholar]
- McClintock SM, Husain MM, Greer TL, Cullum CM (2010): Association between depression severity and neurocognitive function in major depressive disorder: A review and synthesis. Neuropsychology 24:9–34. [DOI] [PubMed] [Google Scholar]
- Meiran N, Diamond GM, Toder D, Nemets B (2011): Cognitive rigidity in unipolar depression and obsessive compulsive disorder: Examination of task switching, Stroop, working memory updating and post‐conflict adaptation. Psychiatry Res 185:149–156. [DOI] [PubMed] [Google Scholar]
- Monsell S (2003): Task switching. Trends Cogn Sci 7:134–140. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M (1979): A new depression scale designed to be sensitive to change. Br J Psychiatry 134:382–389. [DOI] [PubMed] [Google Scholar]
- Narrow WE, Kuhl EA (2011): Dimensional approaches to psychiatric diagnosis in DSM‐5. J Ment Health Policy Econ 14:197–200. [PubMed] [Google Scholar]
- Nolen‐Hoeksema S, Morrow J (1991): A prospective study of depression and posttraumatic stress symptoms after a natural disaster: The 1989 Loma Prieta earthquake. J Pers Social Psychol 61:115–121. [DOI] [PubMed] [Google Scholar]
- Northoff G (2007): Psychopathology and pathophysiology of the self in depression ‐ Neuropsychiatric hypothesis. J Affect Disord 104:1–14. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB (2010): Interoception in anxiety and depression. Brain Struct Funct 214:451–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellet, J. , Bobon, D.P. , Mormont, I. , Lang, F. , Massardier, A. (1980): Etude Princeps de la Validation Française de la MADRS. Masson, Paris: Sous‐Echelle Dépression de la CPRS. [Google Scholar]
- Philipp AM, Weidner R, Koch I, Fink GR (2013): Differential roles of inferior frontal and inferior parietal cortex in task switching: Evidence from stimulus‐categorization switching and response‐modality switching. Hum Brain Mapp 34:1910–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Travis MJ, Fagiolini A, Kupfer DJ (2008): Medication effects in neuroimaging studies of bipolar disorder. Am J Psychiatry 165:313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichot, P , Pull, CB , von Frenckell, R , Pull, MC (1981): A factorial analysis of the Hamilton Anxiety Rating Scale. Psychiatrica Fennica 183. [Google Scholar]
- Piguet C, Dayer A, Kosel M, Desseilles M, Vuilleumier P, Bertschy G (2010): Phenomenology of racing and crowded thoughts in mood disorders: A theoretical reappraisal. J Affect Disord 121:189–198. [DOI] [PubMed] [Google Scholar]
- Piguet C, Desseilles M, Cojan Y, Sterpenich V, Dayer A, Bertschy G, Vuilleumier P (2014): Neural correlates of generation and inhibition of verbal association patterns in mood disorders. Soc Cogn Affect Neurosci 10:978–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet C, Sterpenich V, Desseilles M, Cojan Y, Bertschy G, Vuilleumier P (2013): Neural substrates of cognitive switching and inhibition in a face processing task. Neuroimage 82:489–499. [DOI] [PubMed] [Google Scholar]
- Pollmann S, Dove A, Yves von Cramon D, Wiggins CJ (2000): Event‐related fMRI: Comparison of conditions with varying BOLD overlap. Hum Brain Mapp 9:26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P, Northoff G (2011): How is our self related to midline regions and the default‐mode network? Neuroimage 57:1221–1233. [DOI] [PubMed] [Google Scholar]
- Rameson LT, Satpute AB, Lieberman MD (2010): The neural correlates of implicit and explicit self‐relevant processing. Neuroimage 50:701–708. [DOI] [PubMed] [Google Scholar]
- Sagaspe P, Schwartz S, Vuilleumier P (2011): Fear and stop: A role for the amygdala in motor inhibition by emotional signals. Neuroimage 55:1825–1835. [DOI] [PubMed] [Google Scholar]
- Savitz J, Drevets WC (2009): Bipolar and major depressive disorder: Neuroimaging the developmental‐degenerative divide. Neurosci Biobehav Rev 33:699–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RE, Gay P, Courvoisier D, Jermann F, Ceschi G, David M, Brinkmann K, Van der Linden M (2009): Anatomy of the White Bear Suppression Inventory (WBSI): A review of previous findings and a new approach. J Pers Assess 91:323–330. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC (1998): The Mini‐International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. J Clin Psychiatry 59:22–33. quiz 34–57. [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, Mintun MA, Wang S, Coalson RS, Raichle ME (2009): The default mode network and self‐referential processes in depression. Proc Natl Acad Sci USA 106:1942–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sole B, Bonnin CM, Torrent C, Martinez‐Aran A, Popovic D, Tabarés‐Seisdedos R, Vieta E (2012): Neurocognitive impairment across the bipolar spectrum. CNS Neurosci Ther 18:194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieta E, Valentí M (2013): Mixed states in DSM‐5: Implications for clinical care, education, and research. J Affect Disord 148:28–36. [DOI] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Reading S (2004): Neuroimaging studies of shifting attention: A meta‐analysis. Neuroimage 22:1679–1693. [DOI] [PubMed] [Google Scholar]
- Wagner G, Sinsel E, Sobanski T, Köhler S, Marinou V, Mentzel H‐J, Sauer H, Schlösser RGM (2006): Cortical inefficiency in patients with unipolar depression: An event‐related FMRI study with the Stroop task. Biol Psychiatry 59:958–965. [DOI] [PubMed] [Google Scholar]
- Wegner DM, Zanakos S (1994): Chronic thought suppression. J Pers 62:616–640. [DOI] [PubMed] [Google Scholar]
- Whitmer AJ, Banich MT (2007): Inhibition versus switching deficits in different forms of rumination. Psychol Sci 18:546–553. [DOI] [PubMed] [Google Scholar]
- Whitmer AJ, Gotlib IH (2012): Switching and backward inhibition in major depressive disorder: The role of rumination. J Abnorm Psychol. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=22468767. [DOI] [PubMed] [Google Scholar]
- Whitmer A, Gotlib IH (2011): Brooding and reflection reconsidered: A factor analytic examination of rumination in currently depressed, formerly depressed, and never depressed individuals. Cogn Therapy Res 35:99–107. [Google Scholar]
- Whitmer AJ, Banich MT (2012): Brain activity related to the ability to inhibit previous task sets: An fMRI study. Cogn Affect Behav Neurosci. http://www.ncbi.nlm.nih.gov/pubmed/22956332. [DOI] [PubMed] [Google Scholar]
- Yoshimura S, Okamoto Y, Onoda K, Matsunaga M, Ueda K Suzuki S, Shigetoyamawaki (2010): Rostral anterior cingulate cortex activity mediates the relationship between the depressive symptoms and the medial prefrontal cortex activity. J Affect Disord 122:76–85. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA (1978): A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 133:429–435. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Table 1.


