Abstract
Depression has been associated with various alterations in magnetic resonance imaging (MRI) derived resting‐state functional connectivity. Recently, homotopic connectivity, defined as functional connectivity between homotopic regions across hemispheres, has been reported to be reduced in patients with major depressive disorder (MDD). However, little is known about structural factors underlying alterations of homotopic connectivity, which would contribute to the understanding of the altered neurophysiological architecture in patients with MDD. We compared 368 patients with MDD and 461 never‐depressed controls regarding voxel‐mirrored homotopic connectivity (VMHC) and potential underlying mechanisms such as the structural connectivity of the corpus callosum, measured by DTI‐derived fractional anisotropy (FA), and left–right symmetries in homotopic gray matter volumes. Compared to controls, patients with MDD exhibited reduced VMHC in the cuneus, putamen, superior temporal gyrus, insula, and precuneus. Within these regions, no differences in left–right symmetries in homotopic gray matter volumes were evident across cohorts. FA of the corpus callosum correlated with VMHC in the entire sample. However, patients with MDD and controls did not differ with regard to callosal FA. The findings indicate that MDD is associated with a loss of interhemispheric synchrony in regions known to be implicated in self‐referential and reward processing. They also suggest that additional mechanisms are implicated in altered homotopic connectivity of patients with MDD, other than direct callosal fiber pathways or asymmetries in homotopic gray matter volumes. Hum Brain Mapp 37:1209–1217, 2016. © 2015 Wiley Periodicals, Inc.
Keywords: interhemispheric connectivity, functional connectivity, fMRI, resting‐state, structural connectivity
INTRODUCTION
Major depressive disorder (MDD) is a highly prevalent affective disorder [Richards, 2011] and a leading cause of the global burden of disease [Ferrari et al., 2013]. Previous research has established many different but complementary mechanisms and consequences associated with MDD from molecular, behavioral, and neuroimaging perspectives [Kupfer et al., 2012; Northoff, 2013]. Prominent features of MDD are structural as well as functional alterations of the brain as revealed by magnetic resonance imaging (MRI), such as gray matter volume reductions in the anterior cingulate cortex (ACC), hippocampus, and thalamus [Du et al., 2012] and increased blood oxygen level dependent (BOLD) signals in the amygdala while processing emotional stimuli with negative valence [Hall et al., 2014; Perlman et al., 2012].
A recent focus in research has been the investigation of alterations of resting‐state functional brain connectivity in patients with depression hereby measuring temporally correlated spontaneous fluctuations in low‐frequency (0.01–0.08 Hz) BOLD signals. Increased functional connectivity in default mode regions including the precuneus and ACC have been reported in patients with MDD and is thought to reflect autobiographical memory processing and rumination [Zhu et al., 2012]. Moreover, reduced functional connectivity has been revealed in frontoparietal networks related to attention and emotion regulation in patients with MDD [Kaiser et al., 2015]. Generally, the majority of previously identified resting‐state networks are distributed in homotopic regions across both hemispheres [Smith and Fox, 2009] and appear as an ubiquitous feature of the neurophysiological architecture of the brain [Salvador et al., 2005; Stark et al., 2008]. Such synchronized patterns of low‐frequency BOLD signals in homotopic regions across both hemispheres have been labeled as homotopic connectivity which can be examined by the method of voxel‐mirrored homotopic connectivity (VMHC) [Zuo et al., 2010]. Homotopic connectivity is altered in various psychiatric conditions [Anderson et al., 2011; Kelly et al., 2011]. In patients with first‐episode MDD, reduced homotopic connectivity of the medial prefrontal cortex and the precuneus have been reported [Guo et al., 2013a]. Moreover, reduced homotopic connectivity in the postcentral gyrus [Guo et al., 2013b], medial orbitofrontal gyrus, cuneus, and fusiform gyrus [Wang et al., 2013, 2015] were found in patients with MDD versus controls. Taken together, the pattern of reduced homotopic connectivity and the role of clinical variables are unclear in patients with MDD. Reasons might be that previous studies relied on relatively small sample sizes, focused on first‐episode depression or rather severe types of depression. Furthermore, underlying mechanisms of altered homotopic connectivity in patients with MDD are largely unknown. Possible structural changes underlying abnormal interhemispheric synchrony include left–right asymmetries regarding homotopic gray matter volumes as well as reduced structural connectivity of the corpus callosum. The corpus callosum is the main commissural fiber bundle mediating interhemispheric transfer [van der Knaap and van der Ham, 2011] and broad reductions of homotopic connectivity after dissection of the corpus callosum [Johnston et al., 2008] underscore the relevance of this structure for interhemispheric transfer. The corpus callosum has therefore been identified as a major pathway connecting several functionally linked resting‐state networks [Van Den Heuvel et al., 2009]. It is widely established that MDD is associated with diffuse reductions in gray matter [Bora et al., 2012; Du et al., 2012] volume which consequently appear to modify functional connectivity [Ma et al., 2012; van Tol et al., 2014]. Thus, left–right asymmetries in gray matter volume may represent a causal role in altered homotopic connectivity. Understanding the anatomical basis of altered homotopic connectivity would contribute to the understanding of the altered neurophysiological architecture in MDD.
We aimed to compare VMHC between a large sample of patients with a current episode of MDD and never‐depressed population controls. With respect to previous research, we expected patients with MDD to exhibit reduced homotopic connectivity, primarily in cortical midline structures. We also evaluated whether depression severity and illness duration were correlated with altered homotopic connectivity in patients with MDD to explore potential effects of current symptoms of MDD as well as cumulative long‐term consequences. This study also examined potential underlying structures of depression‐related VMHC changes by exploring group differences in the structural connectivity of the corpus callosum and in the left–right symmetry of gray matter volumes.
MATERIALS AND METHODS
Participants
The present work is based on data from the BiDirect study which is designed to prospectively investigate the bidirectional association between depression and subclinical arteriosclerosis. The BiDirect study comprises 2258 participants aged between 35 and 65 years including 999 in‐ and outpatients with an acute episode of depression, 912 population‐based controls, randomly invited by use of the population register of the city of Münster, and 347 patients with manifest cardiovascular disease. Details regarding the design and protocol of the BiDirect study have been described previously [Teismann et al., 2014]. In the present analysis, we included (1) patients from the cohort with depression who had been diagnosed with a current or recurrent unipolar major depressive episode (F32 or F33) according to ICD‐10 criteria and (2) population‐based controls without a history of depression and with a negative screening for current depressive symptoms by the Center for Epidemiologic Studies Depression Scale (CES‐D). A total score higher than 15 on the CES‐D was considered a positive screening for depression [Radloff, 1977]. We further only included participants who underwent resting‐state functional MRI (fMRI) and structural T1‐weighted or diffusion‐weighted imaging. Participants with brain lesions larger than 15 mm (maximum diameter) affecting the cortex or at least 3 mm displacement from the reference scan (first volume) in at least one single direction (translation/rotation) during resting‐state fMRI were excluded. Moreover, participants with known major neurological diseases—a history of anxiety disorders, psychotic features, substance abuse, or anorexia nervosa—were also excluded from the analysis. The final sample comprised 368 patients with depression and 461 never‐depressed controls. Data on sex, age education, and self‐reported handedness were collected. Education was assessed in four categories with each participant being assigned to the category reflecting her or his highest level of education. In patients with depression, the severity of current depressive symptoms on the CES‐D as well as illness duration, operationalized as years since first clinical diagnosis of MDD, were assessed. The BiDirect study was approved by the ethics committee of the University of Münster and the Westphalian Chamber of Physicians in Münster and was conducted according to the Declaration of Helsinki. Written informed consent for participation in the study was obtained from all participants.
MRI Acquisition
MRI data was collected on a scanner operating at 3 T (Intera, Philips, Best, NL). Participants underwent structural MRI and in most cases, a task‐related emotional faces paradigm [Dannlowski et al., 2009] prior to task‐free functional scans during the study session. Before the task‐free functional scan, participants were instructed to remain motionless, keep their eyes open, not to fall asleep, and not to think of anything in particular. After five dummy scans to allow for T1 equilibration functional data were obtained using T2*‐weighted fast field echo with echo‐planar imaging (2D FFE‐EPI) and the following parameters: repetition time (TR) = 3000 ms, echo time (TE) = 38 ms, flip angle = 90°, 72 volumes, matrix dimensions = 64 × 64, field of view (FOV) = 230 × 230 mm2, 36 axial slices, voxel‐size = 3.6 × 3.6 × 3.6 mm3. Structural 3D T1‐weighted turbo field echo images were recorded with the following parameters: TR = 7.26 ms, TE = 3.56 ms, 9° flip angle, matrix dimension 256 × 256, FOV = 256 × 256 mm2, 160 sagittal slices, 2 mm slice thickness (reconstructed to 1 mm) resulting in a voxel size of 1 × 1 × 1 mm3. Diffusion weighted images were acquired with single‐shot 2D spin echo EPI sequence, gradients were applied at 20 noncolinear directions (b = 1000 s/mm2) after collecting one image without diffusion weighting (b = 0 s/mm2). These images were acquired with the following specifications: TR = 5900 ms, TE = 95 ms, 90° flip angle, matrix dimensions = 128 × 128, FOV = 240 × 240 mm2, 36 axial slices, reconstructed to voxel size = 3.6 × 0.94 × 0.94 mm3.
MRI Preprocessing
We applied standardized methods for the preprocessing and analysis of resting‐state functional data implemented in the Data Processing Assistant for Resting‐State fMRI (DPARSF 2.3) [Chao‐Gan and Yu‐Feng, 2010], REST 1.8 [Song et al., 2011], and SPM8 (http://www.fil.ion.ucl.ac.uk/spm) running on Matlab R2010a (The MathWorks Inc., Natick, MA, USA). Volumes were head‐motion corrected, band‐pass filtered (0.01–0.08 Hz), spatially normalized to the Montreal Neurological Institute (MNI) echo‐planar imaging template, and resampled to a voxel size of 2 × 2 × 2 mm3. The resulting images were smoothed with a Gaussian kernel of 6 mm full width at half maximum (FWHM). Next, the images were linearly detrended. In order to minimize physiological artifacts, the six motion parameters as well as nuisance signals from white matter and cerebrospinal fluid were regressed out. A symmetric brain mask was applied. VMHC maps were computed for each participant by calculating Fisher z‐transformed Pearson correlations between each voxel and its mirrored counterpart in the opposite hemisphere. T1‐weighted structural images were preprocessed with the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm.html) and SPM8 running on Matlab. Native space images were segmented into gray and white matter in the first step and normalized to MNI standard space using DARTEL. Resulting images were smoothed with a Gaussian kernel of 6 mm FWHM. A gray matter mask for the analysis of homotopic connectivity was created. This was done by averaging all smoothed gray matter volumes. The resulting image was then resampled to a voxel‐size of 2 × 2 × 2 mm3, averaged with a left–right flipped version and thresholded at an intensity of 0.2 to produce a symmetrical gray matter mask for VMHC analysis. Diffusion‐weighted images were preprocessed and analyzed with FMRIB software library (http://www.fmrib.ox.ac.uk/fsl). Eddy current distortions and head motions were corrected with FMRIB's Diffusion Toolbox. Non‐brain tissue was removed with the Brain Extraction Tool. Subsequently, DTIFIT was used to fit the diffusion tensors and generate fractional anisotropy (FA) maps. These FA maps were nonlinearly registered to the FMRIB58_FA template in MNI space and subsequently smoothed with a Gaussian kernel of 6 mm FWHM. An anatomical mask of the corpus callosum was produced with Wake Forest University (WFU) pickatlas (http://fmri.wfubmc.edu/software/pickatlas) and FA within the corpus callosum was extracted in all participants.
Statistical Analysis
First, sample characteristics of both cohorts including handedness and task‐sequence (prior presentation of the emotional faces paradigm) were compared. Hereby, t‐tests were applied for normally distributed data, Mann–Whitney U tests for data with skewed distributions, and χ 2 tests for categorial data. Additionally, we assessed individual mean framewise displacement during the acquisition of functional scans [Van Dijk et al., 2012] and compared framewise displacement across cohorts to minimize the impact of head micromovements on functional connectivity [Power et al., 2012; Satterthwaite et al., 2012]. Global thresholds for significance were set to p < 0.05, two‐tailed.
Two‐tailed voxel‐wise t‐tests were conducted on z‐transformed VMHC maps to compare patients with MDD and controls within the symmetrical gray matter mask, after adjustment for age, sex, education, task sequence, and framewise displacement. The threshold for significance was corrected for multiple comparisons at a global p < 0.05 (single voxel: p < 0.01; |T| > 2.58), requiring a cluster size of at least 97 voxel as defined by a Monte Carlo simulation conducted with AlphaSim in REST. The Monte Carlo simulation was conducted within a symmetrical, unilateral gray matter mask since each voxel is correlated with its counterpart in the opposite hemisphere. Masks were created for regions exhibiting significantly different VMHC in patients with MDD compared to controls and VMHC values within these regions were extracted. In order to explore possible effects of antidepressant medication in a nonindependent follow‐up analysis, extracted VMHC values were compared between patients with MDD using no antidepressant medication (n = 50) and controls by a multivariate analysis of covariance (MANCOVA), controlling for age, sex, education, task sequence, and framewise displacement. An additional fully adjusted MANCOVA was conducted to examine potential associations of CES‐D total score and illness duration with VMHC in patients with MDD.
To explore underlying structures of depression‐related differences in VMHC, left–right symmetries in homotopic gray matter volumes were compared between patients with MDD and controls in regions exhibiting significantly different VMHC between the two cohorts. Absolute left–right differences in gray matter volumes were computed for each subject and each homotopic cluster. These indices of asymmetry were compared across cohorts with a MANCOVA, adjusted for age, sex, and education. Moreover, callosal FA was compared between the cohorts using an analysis of covariance, adjusted for age, sex, and education.
In order to investigate the raw structure–function relationship between the structural connectivity of the corpus callosum and homotopic connectivity, correlations were computed between the respective measures of VMHC and FA of the corpus callosum in all subjects, while partialling out task sequence and framewise displacement. All analyses of demographic and extracted MRI data were conducted using SAS University Edition (Cary, NC, USA).
RESULTS
Demographic Characteristics
Comparisons of demographic characteristics between patients with depression and controls are presented in Table 1. Patients with depression were younger than controls and the proportion of women was slightly higher in the cohort of patients with depression. The two cohorts differed significantly by means of education, showing lower education to be more common in the cohort with depression. No significant difference in handedness was evident. The cohorts differed regarding the rate of prior presentation of the emotional faces paradigm. The magnitude of framewise displacement differed slightly but significantly across cohorts. As expected, patients with depression scored higher on the CES‐D.
Table 1.
Sample characteristics
| Patients with depression | Controls | ||
|---|---|---|---|
| Variable | n = 368 | n = 461 | p value |
| Age: mean (SD) | 48.84 (± 7.37) | 52.73 (± 8.00) | <0.01 |
| Women: n (%) | 211 (57.3%) | 224 (48.6%) | <0.01 |
| Prior presentation of the emotional faces paradigm: n (%) | 320 (87%) | 367 (79.6%) | <0.01 |
| Framewise displacement in mm: median (IQR) | 0.062 (0.04–0.10) | 0.056 (0.04–0.08) | <0.01 |
| Handedness: n (%) | 0.69 | ||
| Right | 334 (90.8%) | 425 (92.2%) | |
| Left | 27 (7.3%) | 27 (5.9%) | |
| Unspecified | 7 (1.9%) | 9 (2%) | |
| Education: n (%) | <0.01 | ||
| Primary or general secondary school | 95 (25.8%) | 82 (17.8%) | |
| Intermediate secondary school | 109 (29.6%) | 99 (21.5%) | |
| High school | 77 (20.9%) | 73 (15.8%) | |
| University graduates (>13 years) | 87 (23.6%) | 207 (44.9%) | |
| CES‐D total score: mean (SD) | 25.93 (± 12.15) | 6.33 (± 4.10) | <0.01 |
| Illness duration in years: median (IQR) | 2.00 (0–8.00) |
Abbreviations: SD, standard deviation; CES‐D, Center for Epidemiologic Studies Depression Scale; IQR, interquartile range.
VMHC in Patients With MDD Versus Controls
The comparison of both cohorts revealed significantly reduced VMHC in patients with MDD (Fig. 1). Lower VMHC was observed in the precuneus, cuneus, putamen, and in one cluster encompassing the superior temporal gyrus (STG) and insula, as presented in Table 2. In a nonindependent follow‐up analysis, VMHC values within these clusters were extracted and compared between depressed participants taking no antidepressant medication and controls. The omnibus test revealed a significant effect between the two cohorts (df = 4, F = 3.09, p = 0.02). Subsequent Bonferroni‐corrected univariate analyses confirmed significantly reduced VMHC within the precuneus (df = 1, F = 6.75, p = 0.04), the cluster encompassing STG and insula (df = 1, F = 7.79, p = 0.02) but not in the cuneus (df = 1, F = 2.63, p = 0.42) and putamen (df = 1, F = 1.66, p = 0.80) in patients with MDD.
Figure 1.
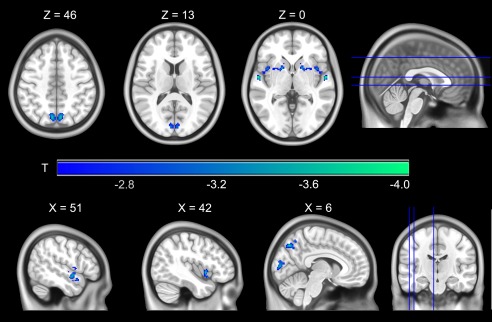
Regions exhibiting significantly reduced homotopic connectivity in patients with depression (n = 368) versus controls (n = 461) are presented as color overlays. No region showed increased homotopic connectivity in depressed participants. For visualization purposes, results are displayed on a symmetrical template provided by the Montreal Neurological Institute.
Table 2.
Regions with significantly lower VMHC in patients with depression
| Peak MNI coordinates | |||||
|---|---|---|---|---|---|
| Brain region (BA) | Cluster size (voxel) | X | Y | Z | T |
| STG (BA 38) and insula (BA 13) | 264 | ±56 | −6 | 0 | −4.06 |
| Putamen | 236 | ±26 | 12 | −14 | −3.60 |
| Precuneus (BA 7) | 173 | ±6 | −72 | 46 | −3.70 |
| Cuneus (BA 18) | 126 | ±4 | −88 | 10 | −3.77 |
Abbreviations: VMHC, voxel‐mirrored homotopic connectivity; BA, Brodmann area; MNI, Montreal Neurological Institute; STG, superior temporal gyrus.
Comparisons are adjusted for age, sex, education, framewise displacement, and task sequence.
Further analysis within patients with MDD revealed a nonsignificant omnibus test for CES‐D total score (df = 4, F = 0.65, p = 0.63) and a significant omnibus test for illness duration (df = 4, F = 2.45, p = 0.04). However, subsequent univariate tests for illness duration did not show significant effects in any respective region.
Homotopic Gray Matter Volume in Patients With MDD Versus Controls
The age, sex, and education‐adjusted MANCOVA omnibus test did not reveal differences in left–right gray matter symmetry in the four clusters across cohorts (df = 4, F = 0.30, p = 0.88).
VMHC and FA of the Corpus Callosum
Regarding the raw structure–function relationship in the entire study population, correlation analysis between FA of the corpus callosum and VMHC yielded significant associations in each respective region. The strongest association with FA was found in the cluster encompassing STG and insula (r = 0.21, p < 0.01) followed by the precuneus (r = 0.13, p < 0.01), the putamen (r = 0.11, p < 0.01), and the cuneus (r = 0.08, p = 0.02). However, the age‐, sex‐, and education‐adjusted ANCOVA did not reveal any differences with regard to callosal fractional anisotropy between the cohorts (df = 1, F = 1.62, p = 0.20).
DISCUSSION
This study aimed (1) to investigate potential alterations of homotopic connectivity in patients with depression and (2) to explore the anatomical basis of such alterations of homotopic connectivity, such as left–right asymmetries of homotopic gray matter volume and alterations in the structural connectivity of the corpus callosum.
Reduced Homotopic Connectivity in Patients with MDD
The results of this study revealed reduced homotopic connectivity in the precuneus, cuneus, putamen, and in one cluster encompassing the STG and insula associated with MDD. These structures appear to be affected by depression across various types of resting‐state fMRI‐derived measures [Sundermann et al., 2014].
Reduced homotopic connectivity in the cuneus, posterior cingulate cortex/precuneus, and STG has been previously reported in patients with MDD [Guo et al., 2013a, 2013b; Wang et al., 2013, 2015]. However, the pattern of VMHC found in MDD patients so far varied according to disease and treatment status. Whereas reduced homotopic connectivity in the precuneus was found in drug‐naïve patients with first‐episode MDD [Guo et al., 2013a], reduced homotopic connectivity in the STG has been reported in patients with treatment‐resistant MDD [Guo et al., 2013b]. Our finding of reduced homotopic connectivity in the insula and putamen is a new finding in MDD patients. On the other side, some previous findings such as reduced homotopic connectivity in the medial prefrontal cortex [Guo et al., 2013a] could not be replicated in this study. This is possibly due to different sample characteristics as previous studies focused on specific subgroups of MDD patients, such as first‐episoders [Guo et al., 2013a; Wang et al., 2013], treatment‐resistant MDD [Guo et al., 2013b], and severe depression [Wang et al., 2013, 2015] while our analyses was based on a heterogeneous sample.
While the cuneus plays a role in vision‐related networks [Smith and Fox, 2009; Wang et al., 2008], the precuneus is an important structure of the default mode network [Raichle et al., 2001] and is shown to exhibit altered patterns of functional connectivity in depression [Bluhm et al., 2009]. However, recent evidence also suggests that the precuneus not only is a hub of the default mode network but also interacts with frontoparietal network nodes, indicating that the precuneus is functionally flexible [Utevsky et al., 2014], or that regions revealed to exhibit reduced VMHC in this study participate in other prototypical networks [Buckner et al., 2008]. Evidence from task‐related fMRI suggests that the precuneus is implicated in visuo‐spatial imagery, autobiographical memory, and self‐processing [Cabanis et al., 2013; Cavanna and Trimble, 2006]. Especially, the engagement of the precuneus in autobiographical memory and self‐processing appears relevant for depression: compared to controls, patients with depression were found to more often evaluate negative emotional stimuli as self‐related and, importantly, this self‐attribution of negative emotional stimuli was accompanied by diminished BOLD signals in the precuneus [Grimm et al., 2009]. The STG and insula have been associated with reward processing in general and also with depression‐specific alterations in reward processing and anhedonia [Keedwell et al., 2005; Paulus et al., 2005; Zhang et al., 2013]. These structures are also implicated in other functional and behavioral domains relevant for depression. For instance, altered activity of the insula was observed during tasks evaluating emotional salience, empathy, and also self‐attribution [Cabanis et al., 2013; Lamm and Singer, 2010]. Interestingly, the insula and putamen are part of the so‐called “hate circuit” [Zeki and Romaya, 2008], which can also be regarded as a network implicated in emotion regulation [Ochsner et al., 2012] that has been reported as aberrant in patients with MDD [Tao et al., 2013]. The clinical utility of these resting‐state fMRI‐derived measures, apart from functional connectivity, remains to be established. However, VMHC represents a straight forward and relatively easy method allowing to investigate interhemispheric synchrony in detail, showing distinct effects in first‐episode [Guo et al., 2013a] and treatment‐resistant depression [Guo et al., 2013b]. This study is the first one investigating interhemispheric synchrony in a large sample with heterogeneous symptom severity also assessing possible anatomical mechanisms. Although more research regarding the association between clinical variables and functional connectivity is required, locations revealed to exhibit reduced interhemispheric synchrony could for instance serve as reference regions regarding attempts to classify depression via resting‐state fMRI [Sundermann et al., 2014].
We did not observe an association between homotopic connectivity and the severity of depressive symptoms as measured by the CES‐D or illness duration, suggesting that reduced VMHC in patients with MDD might be state independent. Presumably, reduced homotopic connectivity is a trait characteristic of MDD, probably related to features such as deficits in emotion regulation and self‐attributive processing as described above. The effects of antidepressant medication on homotopic connectivity are still far from clear but the results of our exploratory analysis suggest that interhemispheric synchrony may depend on medication intake in certain regions. This corresponds with previous studies showing that antidepressant medication as well as electroconvulsive therapy can potentially alter measures of functional connectivity [McCabe and Mishor, 2011; Wei et al., 2014]. However, the subsample of antidepressant‐free patients with MDD is relatively small (n = 50) and does not allow a final conclusion regarding the effects of antidepressant medication on homotopic connectivity.
In summary, the current results corroborate and also extend previous findings from task‐free fMRI showing a depression‐related distortion in synchronous brain activity at rest in regions involved in self‐referential processing and emotion regulation that appear as a state‐marker of MDD and are to some extent independent of antidepressant medication.
Homotopic Differences in Gray Matter Volume
The underlying mechanism of homotopic connectivity is not clear yet. A previous study investigated alterations of gray matter as underlying cause of altered homotopic connectivity in depression but did not find any evidence for gray matter reductions in regions exhibiting reduced homotopic connectivity in patients with MDD [Wang et al., 2013]. The results of this study add that left–right asymmetries in homotopic gray matter volumes did not differ across cohorts and therefore cannot explain reduced homotopic connectivity observed in patients with MDD.
Structural Connectivity of the Corpus Callosum and Homotopic Connectivity
The corpus callosum is an important structure for interhemispheric information transfer [van der Knaap and van der Ham, 2011] and has thus been a candidate for the underlying mechanism of homotopic connectivity. Moderate correlations between VMHC and FA of the corpus callosum have been reported in patients with migraine and multiple sclerosis [Yuan et al., 2012; Zhou et al., 2013]. The results of this study also support a general association between callosal white matter structure and homotopic functional connectivity. However, the two cohorts did not differ with regard to FA in the corpus callosum. Since measures of FA cannot be directly attributed to specific white matter microstructure properties such as fiber density, myelination, or membrane permeability [Jones et al., 2013], more sophisticated approaches measuring specific properties of white matter microstructure may shed additional light on the etiology of homotopic connectivity. The corpus callosum has been identified as a major pathway for interhemispheric transfer in functional networks [Van Den Heuvel et al., 2009; Johnston et al., 2008], but there is also evidence that other pathways contribute to interhemispheric synchronization. For instance, it has also been reported that participants with agenesis of the corpus callosum exhibited similar functional networks as controls [Tyszka et al., 2011] although showing reduced homotopic connectivity in the precuneus. Together with these previous findings, the results of this study suggest underlying mechanisms of synchronization beyond direct callosal connections, e.g., via indirect callosal, interthalamic, corticocerebellar, or other commissural pathways.
Limitations
Certain limitations of this study have to be mentioned. First of all, the majority of patients with MDD was medicated which may have an influence on measures of functional connectivity [McCabe et al., 2011; McCabe and Mishor, 2011]. However, our findings are in good agreement with previous results [Guo et al., 2013a; Wang et al., 2013] and we validated our findings in a subsample of depressed participants using no antidepressant medication. Nevertheless, we had only information about current use or nonuse of antidepressant medication which does not exclude the possibility of usage during earlier stages of the disease. Furthermore, the results might have been confounded by the prior presentation of a task‐related paradigm, which can potentially impact on resting‐state fMRI derived measures [Pyka et al., 2013]. However, we included task‐sequence as a nuisance variable in all relevant models, making it unlikely that group differences were attributed to the emotional faces paradigm. The present findings are also inherently limited by the method of VMHC since brains are not exactly symmetric. However, to reduce the impact of the asymmetric nature of the brain, the data was smoothed and cluster based correction was employed. Also, it would be desirable to assess if reduced interhemispheric synchrony observed in patients with MDD is driven by brain activity in a single hemisphere. However, this is not feasible with this functional connectivity approach owing to the operational definition of VMHC analysis, which inheres that results are strictly symmetrical.
CONCLUSION
We found reduced homotopic connectivity in the cuneus, precuneus, putamen, and in one cluster encompassing the STG and insula in a well‐powered, real‐world sample of patients with a current episode of MDD compared to never‐depressed controls. Reduced homotopic connectivity in patients with MDD may rather reflect a pathophysiological trait marker than a state effect depending on acute depressive symptoms. Further research is required to investigate behavioral correlates of altered homotopic connectivity, which possibly involve autobiographical memory and self‐attributive processing. Homotopic connectivity was associated with structural connectivity of the corpus callosum across the entire sample. However, neither alterations in callosal fractional anisotropy nor differing left–right symmetries in homotopic gray matter volumes did explain the reduced interhemispheric synchrony in our cohort of patients with MDD, suggesting the importance of additional pathways for homotopic connectivity reductions in patients with MDD.
ACKNOWLEDGMENTS
The authors thank all participants and the whole team of the BiDirect study. The authors have no conflicts of interest to declare.
REFERENCES
- Anderson JS, Druzgal TJ, Froehlich A, DuBray MB, Lange N, Alexander AL, Abildskov T, Nielsen Ja, Cariello AN, Cooperrider JR, Bigler ED, Lainhart JE (2011): Decreased interhemispheric functional connectivity in autism. Cereb Cortex 21:1134–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluhm R, Williamson P, Lanius R, Théberge J, Densmore M, Bartha R, Neufeld R, Osuch E (2009): Resting state default‐mode network connectivity in early depression using a seed region‐of‐interest analysis: Decreased connectivity with caudate nucleus. Psychiatry Clin Neurosci 63:754–761. [DOI] [PubMed] [Google Scholar]
- Bora E, Fornito A, Pantelis C, Yücel M (2012): Gray matter abnormalities in major depressive disorder: A meta‐analysis of voxel based morphometry studies. J Affect Disord 138:9–18. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews‐Hanna JR, Schacter DL (2008): The brain's default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124:1–38. [DOI] [PubMed] [Google Scholar]
- Cabanis M, Pyka M, Mehl S, Müller BW, Loos‐Jankowiak S, Winterer G, Wölwer W, Musso F, Klingberg S, Rapp AM, Langohr K, Wiedemann G, Herrlich J, Walter H, Wagner M, Schnell K, Vogeley K, Kockler H, Shah NJ, Stöcker T, Thienel R, Pauly K, Krug A, Kircher T (2013): The precuneus and the insula in self‐attributional processes. Cogn Affect Behav Neurosci 13:330–345. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR (2006): The precuneus: A review of its functional anatomy and behavioural correlates. Brain 129:564–583. [DOI] [PubMed] [Google Scholar]
- Chao‐Gan Y, Yu‐Feng Z (2010): DPARSF: A MATLAB toolbox for “Pipeline” data analysis of resting‐state fMRI. Front Syst Neurosci 4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Ohrmann P, Konrad C, Domschke K, Bauer J, Kugel H, Hohoff C, Schöning S, Kersting A, Baune BT, Mortensen LS, Arolt V, Zwitserlood P, Deckert J, Heindel W, Suslow T (2009): Reduced amygdala‐prefrontal coupling in major depression: Association with MAOA genotype and illness severity. Int J Neuropsychopharmacol 12:11–22. [DOI] [PubMed] [Google Scholar]
- Van Dijk KRA, Sabuncu MR, Buckner RL (2012): The influence of head motion on intrinsic functional connectivity MRI. Neuroimage 59:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du M‐Y, Wu Q‐Z, Yue Q, Li J, Liao Y, Kuang W‐H, Huang X‐Q, Chan RCK, Mechelli A, Gong Q‐Y (2012): Voxelwise meta‐analysis of gray matter reduction in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 36:11–16. [DOI] [PubMed] [Google Scholar]
- Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJL, Vos T, Whiteford Ha (2013): Burden of depressive disorders by country, sex, age, and year: Findings from the global burden of disease study 2010. PLoS Med 10:e1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm S, Ernst J, Boesiger P, Schuepbach D, Hell D, Boeker H, Northoff G (2009): Increased self‐focus in major depressive disorder is related to neural abnormalities in subcortical‐cortical midline structures. Hum Brain Mapp 30:2617–2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Liu F, Dai Y, Jiang M, Zhang J, Yu L, Long L, Chen H, Gao Q, Xiao C (2013a): Decreased interhemispheric resting‐state functional connectivity in first‐episode, drug‐naive major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 41:24–29. [DOI] [PubMed] [Google Scholar]
- Guo W, Liu F, Xue Z, Gao K, Liu Z, Xiao C, Chen H, Zhao J (2013b): Decreased interhemispheric coordination in treatment‐resistant depression: A resting‐state fMRI study. PLoS One 8:e71368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall LMJ, Klimes‐Dougan B, Hunt HR, Thomas Houri MK, Noack AE, Mueller AB Lim OK, Cullen RK, (2014): An FMRI study of emotional face processing in adolescent major depression. J Affect Disord 168:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Heuvel MP, Mandl RCW, Kahn RS, Hulshoff Pol HE (2009): Functionally linked resting‐state networks reflect the underlying structural connectivity architecture of the human brain. Hum Brain Mapp 30:3127–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JM, Vaishnavi SN, Smyth MD, Zhang D, He BJ, Zempel JM, Shimony JS, Snyder AZ, Raichle ME (2008): Loss of resting interhemispheric functional connectivity after complete section of the corpus callosum. J Neurosci 28: 6453–6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Knösche TR, Turner R (2013): White matter integrity, fiber count, and other fallacies: The do's and don'ts of diffusion MRI. Neuroimage 73:239–254. [DOI] [PubMed] [Google Scholar]
- Kaiser RH, Andrews‐Hanna JR, Wager TD, Pizzagalli Da (2015): Large‐scale network dysfunction in major depressive disorder: A meta‐analysis of resting‐state functional connectivity. JAMA Psychiatry 72:603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML (2005): The neural correlates of anhedonia in major depressive disorder. Biol Psychiatry 58:843–853. [DOI] [PubMed] [Google Scholar]
- Kelly C, Zuo X‐N, Gotimer K, Cox CL, Lynch L, Brock D, Imperati D, Garavan H, Rotrosen J, Castellanos FX, Milham MP (2011): Reduced interhemispheric resting state functional connectivity in cocaine addiction. Biol Psychiatry 69:684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Knaap LJ, van der Ham IJM (2011): How does the corpus callosum mediate interhemispheric transfer? A review. Behav Brain Res 223:211–221. [DOI] [PubMed] [Google Scholar]
- Kupfer DJ, Frank E, Phillips ML (2012): Major depressive disorder: New clinical, neurobiological, and treatment perspectives. Lancet 379:1045–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Singer T (2010): The role of anterior insular cortex in social emotions. Brain Struct Funct 214:579–591. [DOI] [PubMed] [Google Scholar]
- Ma C, Ding J, Li J, Guo W, Long Z, Liu F, Gao Q, Zeng L, Zhao J, Chen H (2012): Resting‐state functional connectivity bias of middle temporal gyrus and caudate with altered gray matter volume in major depression. PLoS One 7:e45263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe C, Mishor Z, Filippini N, Cowen PJ, Taylor MJ, Harmer CJ (2011): SSRI administration reduces resting state functional connectivity in dorso‐medial prefrontal cortex. Mol Psychiatry 16:592–594. [DOI] [PubMed] [Google Scholar]
- McCabe C, Mishor Z (2011): Antidepressant medications reduce subcortical‐cortical resting‐state functional connectivity in healthy volunteers. Neuroimage 57:1317–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G (2013): Gene, brains, and environment‐genetic neuroimaging of depression. Curr Opin Neurobiol 23:133–142. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers Ja, Buhle JT (2012): Functional imaging studies of emotion regulation: A synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci 1251:E1–E24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Feinstein JS, Leland D, Simmons AN (2005): Superior temporal gyrus and insula provide response and outcome‐dependent information during assessment and action selection in a decision‐making situation. Neuroimage 25:607–615. [DOI] [PubMed] [Google Scholar]
- Perlman G, Simmons AN, Wu J, Hahn KS, Tapert SF, Max JE, Paulus MP, Brown GG, Frank GK, Campbell‐sills L, Yang TT (2012): Amygdala response and functional connectivity during emotion regulation: A study of 14 depressed adolescents. J Affect Disord 139:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes Ka, Snyder AZ, Schlaggar BL, Petersen SE (2012): Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyka M, Hahn T, Heider D, Krug A, Sommer J, Kircher T, Jansen A (2013): Baseline activity predicts working memory load of preceding task condition. Hum Brain Mapp 34:3010–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS (1977): The CES‐D scale: A self‐report depression scale for research in the general population. Appl Psychol Meas 1:385–401. [Google Scholar]
- Raichle ME, MacLeod aM, Snyder aZ, Powers WJ, Gusnard Da, Shulman GL (2001): A default mode of brain function. Proc Natl Acad Sci USA 98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards D (2011): Prevalence and clinical course of depression: A review. Clin Psychol Rev 31:1117–1125. [DOI] [PubMed] [Google Scholar]
- Salvador R, Suckling J, Coleman MR, Pickard JD, Menon D, Bullmore E (2005): Neurophysiological architecture of functional magnetic resonance images of human brain. Cereb Cortex 15:1332–1342. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Loughead J, Ruparel K, Elliott MA, Hakonarson H, Gur RC, Gur RE (2012): Impact of in‐scanner head motion on multiple measures of functional connectivity: Relevance for studies of neurodevelopment in youth. Neuroimage 60:623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S, Fox P (2009): Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci USA 106:13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X‐W, Dong Z‐Y, Long X‐Y, Li S‐F, Zuo X‐N, Zhu C‐Z, He Y, Yan C‐G, Zang Y‐F (2011): REST: A toolkit for resting‐state functional magnetic resonance imaging data processing. PLoS One 6:e25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark DE, Margulies DS, Shehzad ZE, Reiss P, Kelly aMC, Uddin LQ, Gee DG, Roy AK, Banich MT, Castellanos FX, Milham MP (2008): Regional variation in interhemispheric coordination of intrinsic hemodynamic fluctuations. J Neurosci 28:13754–13764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundermann B, Olde lütke Beverborg M, Pfleiderer B (2014): Toward literature‐based feature selection for diagnostic classification: A meta‐analysis of resting‐state fMRI in depression. Front Hum Neurosci 8:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao H, Guo S, Ge T, Kendrick KM, Xue Z, Liu Z, Feng J (2013): Depression uncouples brain hate circuit. Mol Psychiatry 18:101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teismann H, Wersching H, Nagel M, Arolt V, Heindel W, Baune BT, Wellmann J, Hense H‐W, Berger K (2014): Establishing the bidirectional relationship between depression and subclinical arteriosclerosis ‐ rationale, design, and characteristics of the BiDirect Study. BMC Psychiatry 14:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tol M‐J, Li M, Metzger CD, Hailla N, Horn DI, Li W, Heinze HJ, Bogerts B, Steiner J, He H, Walter M (2014): Local cortical thinning links to resting‐state disconnectivity in major depressive disorder. Psychol Med 44:2053–2065. [DOI] [PubMed] [Google Scholar]
- Tyszka JM, Kennedy DP, Adolphs R, Paul LK (2011): Intact bilateral resting‐state networks in the absence of the corpus callosum. J Neurosci 31:15154–15162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utevsky AV, Smith DV, Huettel Sa (2014): Precuneus is a functional core of the default‐mode network. J Neurosci 34:932–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Jiang T, Yu C, Tian L, Li J, Liu Y, Zhou Y, Xu L, Song M, Li K (2008): Spontaneous activity associated with primary visual cortex: A resting‐state fMRI study. Cereb Cortex 18:697–704. [DOI] [PubMed] [Google Scholar]
- Wang L, Li K, Zhang Q‐E, Zeng Y‐W, Jin Z, Dai W‐J, Su Y‐A, Wang G, Tan Y‐L, Yu X, Si T‐M (2013): Interhemispheric functional connectivity and its relationships with clinical characteristics in major depressive disorder: A resting state fMRI study. PLoS One 8:e60191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhong S, Jia Y, Zhou Z, Wang B, Pan J, Huang L (2015): Interhemispheric resting state functional connectivity abnormalities in unipolar depression and bipolar depression. Bipolar Disord 17:486–495. [DOI] [PubMed] [Google Scholar]
- Wei Q, Tian Y, Yu Y, Zhang F, Hu X, Dong Y, Chen Y, Hu P, Hu X, Wang K (2014): Modulation of interhemispheric functional coordination in electroconvulsive therapy for depression. Transl Psychiatry 4:e453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan K, Qin W, Liu P, Zhao L, Yu D, Zhao L, Dong M, Liu J, Yang X, von Deneen KM, Liang F, Tian J (2012): Reduced fractional anisotropy of corpus callosum modulates inter‐hemispheric resting state functional connectivity in migraine patients without aura. PLoS One 7:e45476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki S, Romaya JP (2008): Neural correlates of hate. PLoS One 3:e3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W‐N, Chang S‐H, Guo L‐Y, Zhang K‐L, Wang J (2013): The neural correlates of reward‐related processing in major depressive disorder: A meta‐analysis of functional magnetic resonance imaging studies. J Affect Disord 151:531–539. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Milham M, Zuo X‐N, Kelly C, Jaggi H, Herbert J, Grossman RI, Ge Y (2013): Functional homotopic changes in multiple sclerosis with resting‐state functional MR imaging. AJNR Am J Neuroradiol 34:1180–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Wang X, Xiao J, Liao J, Zhong M, Wang W, Yao S (2012): Evidence of a dissociation pattern in resting‐state default mode network connectivity in first‐episode, treatment‐naive major depression patients. Biol Psychiatry 71:611–617. [DOI] [PubMed] [Google Scholar]
- Zuo X‐N, Kelly C, Di Martino A, Mennes M, Margulies DS, Bangaru S, Grzadzinski R, Evans AC, Zang Y‐F, Castellanos FX, Milham MP (2010): Growing together and growing apart: Regional and sex differences in the lifespan developmental trajectories of functional homotopy. J Neurosci 30:15034–15043. [DOI] [PMC free article] [PubMed] [Google Scholar]


