Abstract
Background
Mounting evidence suggests that autism is a network disorder, characterized by atypical brain connectivity, especially in the context of high level cognitive processes such as working memory (WM). Accordingly, atypical WM processes have been related to the social and cognitive deficits observed in children with autism spectrum disorder (ASD).
Methods
We used magnetoencephalography (MEG) to investigate connectivity differences during a high memory load (2‐back) WM task between 17 children with ASD and 20 age‐, sex‐, and IQ‐matched controls.
Results
We identified reduced inter‐regional alpha‐band (9‐15 Hz) phase synchronization in children with ASD during the WM task. Reduced WM‐related brain synchronization encompassed fronto‐temporal networks (ps < 0.04 corrected) previously associated with challenging high‐level conditions (i.e. the left insula and the anterior cingulate cortex (ACC)) and memory encoding and/or recognition (i.e. the right middle temporal gyrus and the right fusiform gyrus). Additionally, we found that reduced connectivity processes related to the right fusiform were correlated with the severity of symptoms in children with ASD, suggesting that such atypicalities could be directly related to the behavioural deficits observed.
Discussion
This study provides new evidence of atypical long‐range synchronization in children with ASD in fronto‐temporal areas that crucially contribute to challenging WM tasks, but also emotion regulation and social cognition processes. Thus, these results support the network disorder hypothesis of ASD and argue for a specific pathophysiological contribution of brain processes related to working memory and executive functions on the symptomatology of autism. Hum Brain Mapp 37:153–164, 2016. © 2015 Wiley Periodicals, Inc.
Keywords: alpha oscillations, phase‐locking values, executive functions, MEG, children
Abbreviations
- ACC
anterior cingulate cortex
- ASD
autism spectrum disorder
- fMRI
functional magnetic resonance imaging
- IFG
inferior frontal gyrus
- IPS
intraparietal sulcus
- MEG
magnetoencephalography
- PLV
phase locking value
- WM
working memory
INTRODUCTION
Autism is a neurodevelopmental disorder characterized by social deficits but also restricted and repetitive behavior [American Psychiatric Association, 2013]. Mounting evidence suggests that several behavioral symptoms of autism spectrum disorder (ASD) are the consequence of a “network disorder” [Frith, 2004; Geschwind and Levitt, 2007] that may itself result from an atypical differentiation of complex networks underlying high level cognitive tasks such as language, mental flexibility, or working memory [Anagnostou and Taylor, 2011; Barendse et al., 2013; Belmonte et al., 2004; Geschwind and Levitt, 2007; Minshew and Williams, 2007; Misic et al., 2014; O'Hearn et al., 2008; Vissers et al., 2012; Ye et al., 2014].
Functional magnetic resonance imaging (fMRI) studies have shown reduced long‐distance connectivity (i.e. cortical underconnectivity) in ASD compared with control groups across several brain regions including the fronto‐parietal [Just et al. 2007; Kana et al., 2006), fronto‐fusiform [Koshino et al. 2008], or fronto‐striatal [Silk et al., 2006] networks. While some results show local over‐connectivity in the frontal and temporal areas, long‐range disconnection between frontal and posterior areas are predominant findings in ASD [see Just et al., 2012; Maximo et al., 2014 for a recent review].
To our knowledge only two studies have investigated both structural and functional connectivity simultaneously in ASD. Ray et al. [2014] showed that patients with ASD exhibited higher connectivity in structural and functional networks within but not between the “rich‐club” (i.e. hub) networks, while Verly et al. [2014] found that a reduction of both functional and structural connectivity processes was predictive of language difficulties in autism. These results suggest a direct relation between structural and functional abnormalities in ASD. Accordingly, it has been hypothesized that the structural abnormalities, but also the associated compensatory brain processes (unusual activation of alternative brain regions) observed in ASD, may lead to inefficient functional connectivity processes among brain regions to achieve task performance [Just et al., 2007; Maximo et al., 2014]. In particular, high level cognitive functions such as language, mental flexibility, or working memory that are impaired in ASD rely on optimal inter‐regional connectivity (i.e. the transfer of information) of complex networks that synchronize fronto‐posterior specialized areas through the white matter tracts [Just et al., 2012; O'Hearn et al., 2008]. Accordingly, structural MRI studies have shown some atypicalities in ASD such as abnormal white matter volume or white matter microstructure [Herbert et al., 2004; Shukla et al., 2011] as well as altered long‐range white matter connections that might be related to socio‐cognitive symptoms of ASD [see Ameis and Catani, 2015 for a recent review]. In addition, an abnormal neurodevelopmental trajectory of white matter maturation processes and of several cortical, subcortical, and cerebellar brain volumes have been identified in children with ASD [Sussman et al., 2015; Weinstein et al., 2011; Wolff et al., 2012].
In the present study, we investigated the brain connectivity mechanisms that underlie working memory (WM) processes in ASD as it represents an essential feature of social cognition and executive processes that could explain many behavioural symptoms of ASD [Barendse et al., 2013].
The development of WM functions that allows one to transiently store and manipulate “online” information [Baddeley, 2012], relies mainly on the functional specialization and integration (communication) of three brain regions; (1) the prefrontal cortex that allows the manipulation and maintenance of information in WM [Curtis and D'Esposito, 2003; D'Esposito et al., 1999], (2) the inferior parietal lobe which acts as an information buffer [Koshino et al., 2005] and (3) the temporal lobe which allows the encoding storage and retrieval of information in WM [Bergmann et al., 2012].
Impaired WM processes, have been frequently reported in autism, with WM deficits reported most often in the visuo‐spatial domain in ASD [Landa and Goldberg, 2005; Steele et al., 2007; Williams et al., 2014] and when the task imposes a heavier load on WM [de Vries and Geurts, 2014; Landa and Goldberg, 2005; McGonigle‐Chalmers et al., 2008; Williams et al., 2006]. However, behavioral results are inconsistent as a number of studies also found normal WM performance in this population [Geurts et al., 2004; Happe et al., 2006; Ozonoff and Strayer, 2001; Sinzig et al., 2008].
One possible explanation for these discrepant results may be related to the atypical strategies that some children with ASD use to perform complex tasks [Rump et al., 2009; Salter et al., 2008]. We recently identified using magnetoencephalography (MEG), important qualitative differences in the timing and localisation of brain activations underlying WM processes in children with ASD. WM‐related brain processes were associated with the activation of the left insula from 225 to 275 ms and of the left intraparietal sulcus (IPS) from 325 to 375 ms in typically developing children. However, although no between‐group difference was observed at the behavioral level, the ASD group recruited the left angular gyrus and the left precuneus in this time window, suggesting that WM processes rely on different brain networks in ASD [Urbain et al., 2015].
Functional connectivity studies showed that, although the specific areas involved may differ according to the task specificities, WM involves the long‐range communication of fronto‐posterior brain areas [Koshino et al., 2005; Koshino et al., 2008]. In their first study, Koshino et al. [2005] found that during an n‐back WM task with letters, brain activity within bilateral frontal regions was correlated with the left parietal regions in control adults but with the right parietal regions in ASD participants. In their second study, Koshino et al. [2008] found, using an n‐back task that relied on face stimuli, reduced connectivity between the left frontal regions (IFG and MFG) and the temporal (fusiform) areas in the adult ASD group.
Although of interest, these results were obtained using fMRI which has a good spatial but a poor temporal resolution (> 1 s). This might have precluded a precise understanding of the timing of WM processes in the ASD population as an increasing number of studies in healthy adults suggest a crucial role of slow oscillations (i.e. < 15Hz; e.g. alpha, theta) during WM tasks [Brookes et al., 2011; Jensen et al., 2002; Jensen and Tesche, 2002; Palva et al., 2010a]. As MEG measures neuronal activity directly with millisecond (ms) time resolution and good spatial resolution, it has the ability to analyze interregional phase‐locking information [Engel et al., 2013]. In particular, the synchronization of theta (4–8 Hz) and/or alpha (9–15 Hz) frequency ranges help the communication between fronto‐posterior areas that serve the sub‐functions of WM processes [Klimesch et al., 2008; Sauseng et al., 2010]. For instance, connectivity analyses performed in adults showed that better memory performance (i.e., picture recognition) is associated with a short‐lasting event‐related increase in alpha and theta phase synchronization from 50 to 250 ms post‐stimulus onset [Klimesch et al., 2004]. Further evidence suggests that theta and alpha phase synchronization could reflect different processes of WM. Whereas anterior‐posterior alpha coherence seems to be involved in the manipulation of stored information [Palva et al., 2010b; Palva and Palva, 2011; Sauseng et al., 2005a], theta coherence may serve instead the central executive aspects of the WM task [Sauseng et al., 2006]. Moreover, looking at WM processes in a paediatric population using MEG, Doesburg et al. [2011], showed altered long‐range alpha‐band synchronization in children born very preterm.
Together, the above studies show that MEG enables the precise understanding of brain oscillatory correlates of WM constraints [Brookes et al., 2011; Doesburg et al., 2010; Doesburg et al., 2011; Jensen et al., 2002; Jensen and Tesche, 2002; Palva et al., 2010a] and may help us to better understand how these frequency‐specific mnemonic processes might be altered in atypical maturation conditions such as autism. To date, no studies have investigated the oscillatory and related brain connectivity mechanisms for WM in children with ASD.
The present study addresses this gap by investigating, with MEG, the inter‐regional brain synchronization elicited in a WM task in children with ASD compared to age‐, sex‐, IQ‐matched typically developing children. We hypothesize that although children with ASD may reach the same level of performance as typically developing children at the behavioral level, long‐range connectivity processes involving slow oscillatory mechanisms such as theta or alpha may differ between groups. Specifically, we predict that atypical WM processes may be associated with reduced brain synchronization of fronto‐posterior areas.
MATERIAL AND METHODS
Participants
Seventeen children with high functioning ASD and 20 age‐, sex‐, handedness, and IQ‐matched typically developing (TD) controls participated to this study. See Table 1 for demographic characteristics. These children were selected from a larger sample of 38 children with high functioning ASD and 26 TD control children [age range (year‐month): 7y 1mo–13y 11mo].
Table 1.
Demographic information
| Autism (n = 17) | Control (n =20) | ||||
|---|---|---|---|---|---|
| Age (years) | Mean ± SD | 11.17 ± 1.69 | 11.26 ± 1.64 | t (35) = 0.17 | P = 0.85 |
| IQ | Mean ± SD | 109.94 ± 13.92 | 115.95 ± 10.97 | t (35) = 1.46 | P= 0.15 |
| Sex | Male:female | 13:7 | 13:4 | χ 2(1)=0.57 | P= 0.44 |
| Handedness | Right:left | 17:3 | 16:1 | χ 2(1)=0.79 | P= 0.37 |
IQ, intellectual quotient; SD, standard deviation; t, value from Student's t‐distribution; χ 2, value from chi‐squared distribution.
Children with autism were not included in the study if they had an associated metabolic or genetic disorder, the presence of other neurological disorders, medical illnesses, any current significant Axis I psychiatric comorbidities, uncorrected vision, developmental delay, or learning disability as the primary diagnosis. TD children were not included if they reported a developmental delay, learning disability, any psychiatric, neurological, academic problem, or visual impairment. We arrived at our final sample of 37 children (20 TD and 17 ASD) after sex‐ and age‐matching and excluding children with inadequate task performance (e.g., performance below chance level) or excessive movement in the MEG or MRI scanners.
Clinical diagnoses of ASD were confirmed in all cases with a combination of the Autism Diagnostic Observation Schedule‐General (ADOS‐G; Lord et al., 2000) and expert clinical judgment. Nine children with ASD were on one psychotropic medication (Dexmethylphenidate, concerta, biphentin, fluoxetine, Prozac). Their MEG data were examined in comparison to children with ASD who were not taking medication, and the data did not differ between these subgroups (see results section).
Children with ASD were recruited through parent support groups, community support centres and hospital advertisements and TD children were recruited through brochures and flyers posted at the hospital and the surrounding community. MEG and MRI scanning, as well as cognitive and clinical testing, were performed at the Hospital for Sick Children in Toronto. All children gave informed assent and the parents provided informed written consent. Experimental procedures were approved by the Hospital's Research Ethics Board.
Experimental MEG Task and Procedure
Inter‐regional synchronization (connectivity) of neuronal oscillations associated with WM was investigated using a classical n‐back task (2‐back) that children performed in the MEG scanner. In the 2‐back task, where high level load is imposed on WM, children were instructed to press a key when they recognised the repetition of a complex multi‐coloured abstract image (target) presented two trials earlier (Fig. 1A).
Figure 1.
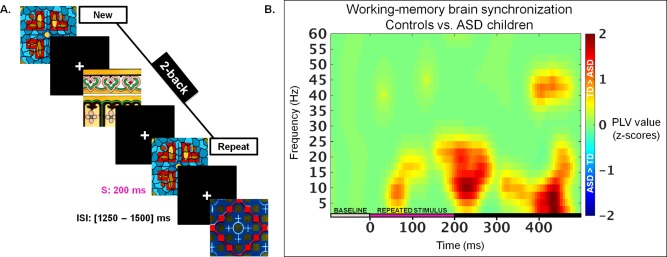
A: The N‐back task. Schematic of the 2–back condition where the child is required to recognize that an image (Repeat) is the same as two images before (New; first occurrence of a picture). Each image (stimulus, S) appeared for 200 ms and was followed by a fixation cross displayed with an ISI varying between 1250 and 1500 ms to reduce anticipation of the next trial. B: Differences in WM‐related brain synchronization processes between groups. Differences of averaged PLV values (inter‐regional brain synchrony; z‐scores; P < 0.05) between children with ASD and TD children across time and frequencies from which were selected the time windows and frequency bands of interest that were submitted to the nonparametric Network Based Statistic (NBS) toolbox.
The 2‐back task had a maximum of 330 trials including 221 ‘NEW’ and 109 ‘REPEAT’ stimuli. Practice series were given prior to entering the MEG to ensure that children understood the task. Stimuli used in the practice trials were not included in the experimental blocks. The 2‐back task was projected on a screen located 80 cm from the children's eyes; with a visual angle of approximately 4° of their visual field. Each complex picture (NEW and REPEAT) was shown for 200 ms followed by a fixation cross with an inter‐stimulus interval (ISI) varying between 1250 and 1500 ms – this jitter prevented anticipation of the next trial.
All children completed the Wechsler Abbreviated Scale of Intelligence (WASI) as well as the Forward and Backwards Digit Recall, Listening Recall, Mazes Memory and Block Recall subscales of the Working Memory Test Battery for Children (WMTB‐C) to supplement behavioral data collected during the MEG task.
MEG and MRI Data Acquisition
MEG data were recorded inside a magnetically‐shielded room on a CTF Omega 151 channel system (MISL Inc., Coquitlam, Canada) at 600 Hz. Throughout the run, head position was continuously recorded by three fiducial coils placed on the nasion, and left and right pre‐auricular points. After the MEG session, anatomical 3T MRI images were acquired (Magnetom 3T Tim Trio, Siemens AG, Erlangen, Germany), T1‐weighted magnetic resonance images using high resolution 3D MPRAGE sequences on a 12 channel head coil. MEG data were coregistered to the MRI structural images using the reference fiducial coil placements.
MEG and MRI Data Processing
Preprocessing, seed definition, and virtual electrode analyses
MEG analyses were performed only on trials associated with correctly recognized REPEAT stimuli. A third‐order spatial gradient environmental noise‐cancellation was applied to the MEG data that were band‐pass filtered offline at 1‐60 Hz. Data were then epoched from 200 ms prior to 1200 ms after stimulus (REPEAT picture) onset. Epochs contaminated by motion (>5 mm) were excluded from the analyses.
MEG data were coregistered with each participant's MRI image. Multisphere head models were constructed based on initial fiducial positions using each individual MRI scan [Lalancette et al., 2011]. MRIs were then normalized into standard MNI space using ANTS (http://picsl.upenn.edu/software/ants) with 5‐mm voxel‐grid of source power [Chau et al., 2004; Singh et al., 2003] viewable in AFNI software (http://afni.nimh.nih.gov/afni/). The coordinates of 90 seed locations representing the cortical and subcortical areas from the Automated Anatomical Labeling (AAL) atlas [Tzourio‐Mazoyer et al., 2002] were then unwarped from standard MNI space into each individual headspace.
An event‐related minimum variance vector beamformer was used to estimate the broadband time series for each source location and trial for each subject representing the activity of each of the 90 AAL sources. This beamformer technique allows the precise localization of cortical sources and deep brain structures as previously demonstrated by our group using the same n‐back task in a sample of healthy adults [Hung et al., 2013; Quraan et al., 2011]. Individual weight vectors were applied to each sensor measurement and summated to give an estimated source activity to each cortical or subcortical AAL seed location [Quraan and Cheyne, 2010].
Beamforming is a spatial filtering approach to MEG inverse source modeling. It relies on a minimization of total brain power, while being optimally sensitive to activity in a given brain location (i.e. each of the 90 seed locations), resulting in the suppression of background noise [Brookes et al., 2011]. Accordingly, beamformers are effective at suppressing ocular artefacts generated by eye movements, and non‐ocular artefacts, such as cardiac and muscle activity [Muthukumaraswamy, 2013].
Assessing functional connectivity: Inter‐regional phase‐locking analysis
We first extracted the instantaneous phase of each sample from a short‐time Fourier transform (sliding 200 ms time windows). Then, we performed a functional connectivity analysis between all pairwise combinations of the seeds. This was done by computing the degree of inter‐regional phase synchronization for every time (from −200 ms to 600 ms) and frequency (1‐60 Hz) point across trials using the phase locking value (PLV). Ranging between 0 and 1, PLV indexes reflect phase synchrony between two sources, which is understood to be a neurophysiological mechanism mediating communication among brain regions referred to as functional connectivity [Fries, 2005].
To study task‐dependent connectivity dynamics at the group level, PLVs were averaged across source pairs for each time point and frequency then subsequently averaged across individuals of each group. This produced time series representing source‐by‐source (90 × 90) adjacency matrices of the global network connectivity dynamics for each group (TD and ASD) at every time (from −200 ms to 600 ms) and frequency (1–60 Hz).
Statistical analysis
Averaged PLV values (mean inter‐regional brain synchrony; z‐scores) were compared between groups (TD vs. ASD) across time and frequency using a permutation test (3000 permutations; P < 0.05) to identify the time windows and frequency bands of interest subsequently submitted to the nonparametric Network Based Statistic (NBS) toolbox (see Supporting Information Fig. S1 for a schematic representation of the statistical analyses).
As illustrated on Figure 1B, we found stronger mean connectivity values (PLV, z‐scores C>ASD; P <0.05) in TD compared to ASD children from 0 to 150 ms and from 150 to 300 ms in the alpha band (9–15 Hz) and from 350–500 both in the theta (4–8 Hz) and the low gamma (40–50 Hz) bands. Theta (4–8 Hz), alpha, beta (16–30 Hz) and gamma (31–60 Hz) frequency ranges were selected as prior research has indicated they are critical for organizing communication among distributed brain areas in the context of working memory tasks [Brookes et al., 2011; Doesburg et al., 2011; Jensen et al., 2002; Jensen and Tesche, 2002; Palva et al., 2010a].
Adjacency matrices (90 by 90 ROIs) associated with the three time windows and frequency bands of interest [i.e. from 0 to 150 ms and from 150 to 300 ms in the alpha band (9–15 Hz) and from 350 to 500 both in the theta (4–8 Hz) and the gamma (30–60 Hz) bands, see above] were then submitted to a statistical between‐group comparison of inter‐regional connectivity differences using the nonparametric Network Based Statistic [NBS; Zalesky et al., 2012; Zalesky et al., 2010].
NBS initially performs multiple univariate tests on all analyzed edges [in this case each element in the adjacency matrix; see Maris and Oostenveld, 2007; Nichols and Holmes, 2002 for similar approaches). Using the NBS method, statistical significance is assigned at the level of the connectivity component as a whole, rather than at the level of the individual connections. As different stringencies for initial univariate threshold can yield differential sensitivities under various scenarios of differential connectivity (for example, small focal changes compared with weak diffuse changes) this threshold must be adapted to the data distribution under investigation [see Zalesky et al., 2012; Zalesky et al., 2010]. Accordingly and as recommended in previous studies [e.g. Bangel et al., 2014; Ye et al., 2014] the initial univariate test thresholds were set to t ≥ 3 for comparison of ASD participants with TD. This threshold corresponds to a P‐value of P = 0.005 (2–tailed) according to our sample size (df = 35; t = 3).
To assess the statistical reliability of differences in network connectivity, connectivity components are first identified, defined as groups on nodes which are contiguously connected by supra‐threshold edges/connections. The adjacency matrices are then shuffled between the to‐be compared groups and the largest connectivity component in the surrogated data is identified. This process is repeated 10,000 times to produce a null distribution. The sizes of connectivity components identified in the ‘real’ data are then compared with those from the surrogate data to assess the statistical reliability of connectivity differences. Since each component in the surrogate distribution is the largest difference in connectivity that could occur by chance considering each edge/connection in the entire 90 × 90 adjacency matrix, NBS effectively controls for false positives because of multiple comparisons. This statistical correction is effective for any initial univariate threshold since it is applied equally to obtain component extent for both the real and the surrogate data [Zalesky et al., 2012; Zalesky et al., 2010]. Time series of node strength and eigenvector centrality were calculated from the adjacency matrices using the Brain Connectivity Toolbox [Rubinov and Sporns, 2010] to index the network involvement of particular regions.
Results obtained using NBS and graph theoretical analysis for individual regions were plotted using the Brain Net Viewer toolbox [Xia et al., 2013]. Nodes and edges belonging to statistically significant components were plotted, and the size of each node represents connectivity strength for edges in the significant connectivity component.
Complementary correlation analyses were performed to investigate a priori associations between functional connectivity patterns and behavioural parameters. To do so, we computed Pearson correlations (P < 0.05 uncorrected) between the main significant connectivity hub difference (the right fusiform) and 3 measures of interest (1) task performance (2‐back accuracy), (2) a standardized visuo‐spatial WM measure (Block recall subtest of the Working Memory Test Battery for Children), and (3) the severity of the disorder (ADOS) which have shown negative relations between the various functional connectivity measures in different brain networks, such as fronto‐motor or fronto‐parietal networks [Just et al., 2007; Koshino et al., 2008] but also with hypoactivation of the fusiform area [Schultz, 2005].
Finally, non‐parametric analyses (Mann–Whitney U‐test for independent samples) examined whether the medication taken by some ASD children (9 with vs. 8 without medication) affected the connectivity results. To do so, the node strength values of the primary underconnected hubs (i.e. the left anterior insula (from 0–150 ms) and the right fusiform gyrus (from 150–300 ms); see the results section) were statistically compared between the two subgroups of ASD participants.
RESULTS
Working Memory Behavioral Performance
T‐test for independent samples analyses demonstrated only a trend for children with ASD to perform the task less accurately and more slowly than TD children (ps < 0.14; see Table 2), while variation coefficients (i.e. relative standard deviation) were similar between groups (P = 0.87).
Table 2.
Mean behavioral performance to the 2‐back task
| Autism (17) | Control (20) | |||
|---|---|---|---|---|
| Mean ± SD | Acc (%) | 59.34 ± 15 | 68.17 ± 16.66 | P = 0.10 |
| RTs (sec) | 0.66 ± 0.10 | 0.61 ± 0.09 | P = 0.13 | |
| V (%) | 0.39 ± 0.10 | 0.39 ± 0.06 | P = 0.87 |
Percentage of correct recognition associated with the repeated (target) picture, Accuracy [Acc], mean reaction times [RT], and RT coefficient of variation [CV] (calculated for each subject as the standard deviation of the mean RT divided by mean RT). SD, standard deviation.
No effect of Group (P > 0.34) and no interactions were found between the group factors and the standardized subscales scores of the Working Memory Test Battery for Children (subscales of the WMTB‐C X Group; P > 0.41). However, we found a main effect of subscales of the WMTB‐C (F(4,140) = 8.74, P < 0.00001) suggesting that regardless of the group of children, WM performance differed between subtests, with better performance for Digit Recall and Listening recall than for Backward Digit Recall, Block Recall and Mazes Memory (ps < 0.012) which otherwise did not differ from each other (ps > 0.19) as demonstrated by LSD Fisher post‐hoc analyses.
Brain Synchronization Differences During Working Memory Processing Between Children With ASD and TD Children
Statistical between‐groups comparisons revealed atypical alpha‐band brain synchronization processes in children with ASD (ps < 0.04). Atypical functional connectivity maps in axial and sagittal orientations are shown in Figure 2A,B, with the size of the node scaled to the magnitude of the significant connectivity difference between the groups; significant edges are also marked as interconnecting lines between AAL seed regions.
Figure 2.
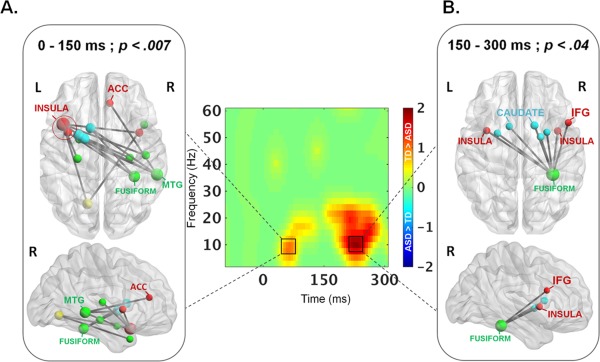
Reduced WM‐related fronto‐temporal alpha synchronization in ASD compared to TD children. Statistical between‐groups (TD>ASD) differences in the alpha band brain synchronization from (A) 0–150 ms and (B) from 150–300 ms in two fronto‐striato‐temporal networks. Nodes are scaled to the magnitude of the significant connectivity difference between the groups (TD>ASD); significant edges are also marked as interconnecting lines between AAL seed regions.
Abnormal oscillatory mechanisms found in ASD encompassed two fronto‐striato‐temporal networks from 0 to 150 ms and from 150 to 300 ms. Whereas the right fusiform, basal ganglia, and the bilateral insulae were consistently underconnected in children with ASD compared with controls within the first 300 ms, other connections differed more specifically during the first or the second 150 ms time window. In particular, the anterior cingulate cortex (ACC) but also the hippocampi and the left lingual gyrus were underconnected in ASD from 0 to 150 ms (P < 0.007) whereas the right inferior frontal gyrus was specifically underconnected from 150 to 300 ms in ASD compared to controls (P < 0.04).
Of note, the desynchronization pattern over the fronto‐temporal areas observed in ASD compared to TD children occurred in the absence of power differences between the groups (see Supporting Information Figs. S2 and S3), strengthening the finding of a lack of alpha synchronization in children with ASD during WM processes.
Graph theory analyses revealed that the left anterior insula (from 0 to 150 ms) and the right fusiform gyrus (from 150 to 300 ms) were the primary underconnected hubs in ASD compared to TD children according to eigenvector centrality values. Similarly, high‐strength node differences were noted in the left anterior insula and the right middle temporal gyrus from 0 to 150 ms and in the right fusiform gyrus from 0 to 300 ms after stimulus onset.
Complementary analyses investigated if medication taken by some ASD participants (9 with vs. 8 without medication) affected the connectivity results. Non‐parametric Mann–Whitney U‐test for independent samples did not reveal any differences of node strength values in either primary underconnected hub (i.e. the left anterior insula (from 0 to 150 ms; P = 1) or the right fusiform gyrus (from 150 to 300 ms; P = 0.63)) between the two subgroups of ASD participants.
Correlations Between Functional Connectivity Measures and Behavioral Measures
Brain‐behavior analyses revealed a significant positive correlation between the connectivity index (i.e. node strength) of the right fusiform and behavioral performance (i.e. 2–back accuracy) in TD but not in children with ASD (average r = + 0. 49; P = 0.02 in TD children vs. r = + 0. 09; P = 0.73 in ASD children, see Fig. 3A). A similar correlation was observed between the right fusiform node strength and performance on the block recall subtest of the Working Memory Test Battery for Children (WMTB‐C) in TD but not in children with ASD (average r = + 0. 44; P = 0.04 in TD children vs. r = −0. 23; P = 0.38 in ASD children, see Fig. 3B). Finally, we observed a significant negative correlation between the right fusiform node strength during this 2‐back task and the severity of autistic symptoms assessed through the ADOS scores (average r = −0. 54; P = 0.02 in ASD children, see Fig. 3C). This last result indicates that the more severe the symptoms in children with ASD, the less right fusiform received connections during this working memory task.
Figure 3.
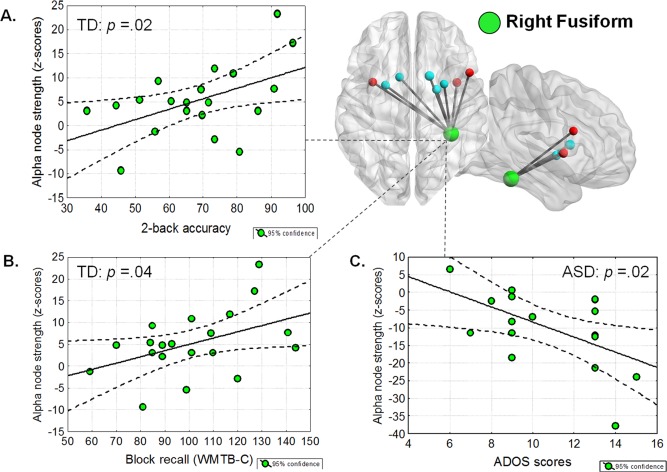
Correlation analyses. Significant correlation coefficients (all P < 0.05) between connectivity processes (alpha node strength; z‐scores) related to the right fusiform and WM performance (accuracy) in (A) the 2–back task (average r = + 0. 49; P = 0.02 in TD children vs. r = + 0. 09; P = 0.73 in ASD children and in (B) the block recall subtest of the Working Memory Test Battery for Children (WMTB‐C) (average r = + 0. 44; P =0.04 in TD children vs. r = −0. 23; P =0.38 in ASD children. C: Significant correlation coefficient (P < 0.05) between connectivity processes (alpha node strength; z‐scores) related to the right fusiform and symptoms severity in ASD.
DISCUSSION
Our study found abnormal alpha‐band brain synchronization in children with ASD compared to age‐, sex‐ and IQ‐matched controls during a 2‐back task. Although modulations of alpha amplitudes have been related to the inhibition of task irrelevant brain areas in the context of WM tasks, mounting evidence suggests that the synchronization of alpha rhythms, which reflect the time‐locked firing of inter‐regional neuronal processes [Varela et al., 2001], serve core WM processes [Freunberger et al., 2009; Klimesch et al., 2007; Sauseng et al., 2005a]. Hence, an atypical synchronization of alpha rhythms could reflect an inexact timing or dynamic of neural interconnectivity and, thus, result in less efficient WM‐related brain mechanisms. In line with this hypothesis, Freunberger et al. [2009] found that, in the context of a WM task, increased alpha phase‐locking synchronization processes were associated with the recognition of pictures that participants had to remember whereas pictures that had to be ignored (not‐to‐remember) were associated with an increase of alpha amplitude [Freunberger et al., 2009]. Thus, several studies have demonstrated a specific role of alpha synchronization in the top‐down control and binding of memory processes [Bauml et al., 2008; Freunberger et al., 2009; Sauseng et al., 2005b; von Stein et al., 2000].
In the present study, atypical alpha synchronization occurred in ASD within the first 300 ms after the repetition of the 2‐back trials (that were correctly recognized) in fronto‐temporal networks, in particular, between the left anterior insula and the right fusiform, that crucially contribute to WM processes. Long‐range fronto‐temporal alterations of brain structure and white matter tracts have been consistently reported in the ASD literature [see Ameis and Catani, 2015 for a review]. A recent meta‐analysis including voxel‐wise structural MRI studies shows a prevalence of alteration within the hippocampi, the fusiform gyri, the cingulate, and the insula in children and adolescents with ASD [Duerden et al., 2012]. In addition, an abnormal structure (i.e. enlargement, reduction or displacement) of the fusiform gyri has been identified in different studies in ASD [Koshino et al., 2008; van Kooten et al., 2008; Waiter et al., 2004], suggesting a possible overlap between structural and functional disruption of connectivity processes related to the fusiform, as well as some structural underpinnings to the atypical WM‐related connectivity processes identified in this study.
Graph theory analysis provided evidence of reduced connectivity processes in children with ASD among regions that are especially involved in challenging situations, such as the insula, the ACC and the inferior frontal gyrus (IFG).
These brain areas are involved in the executive control processes required in the context of a WM task [Bunge et al., 2002; Casey et al., 2002; Dove et al., 2000; Scherf et al., 2006] and challenging situations [Barch et al., 1997; Deng et al., 2015; Gehring and Knight, 2000] that involve decision making, response inhibition, error detection, conflict monitoring, problem solving, and performance monitoring [Casey et al., 2000; Huettel et al., 2005; Kim et al., 2014; Thielscher and Pessoa, 2007]. Moreover, the anterior insula is hypothesized to represent a network hub that is critical for switching among brain systems during task performance [Eckert et al., 2009; Sridharan et al., 2008] but also to provide a link between attention‐related problem solving and salience systems during the evaluation and the coordination of task performance [Eckert et al., 2009]. In parallel, hypoactivation in these brain regions has been related to the social and emotional difficulties of ASD [Di Martino et al., 2009; Leung et al., 2014], suggesting that the atypical connectivity processes found in these areas in our study might significantly affect the ability of ASD to process more complex WM situations such as real life interactions and, therefore, may contribute to some social deficits observed in autism.
This hypothesis is strengthened by the observation of a positive correlation between atypical connectivity (node strength) pattern in the right fusiform and the severity of symptoms in ASD. The right fusiform is known to facilitate the encoding and the recognition of visual objects [Haxby et al., 2000] and has been related to an object‐based encoding strategy in children [Scherf et al., 2006]. Moreover, increase of activity in the right fusiform is associated with both the manipulation and/or the maintenance of information in a WM task for faces but also is modulated by WM load [Druzgal and D'Esposito, 2001], suggesting its impact on core WM processes.
The central role of the right fusiform in WM processes in our study was further reinforced by the presence of a positive correlation between connectivity in this region and improved behavioural performance in the 2‐back task but also in the block recall subtest of the Working Memory Test Battery for Children (WMTB‐C) in TD children whereas a similar correlation was not present in children with ASD.
The fact that children with ASD achieved similar behavioral performance as TD controls in our study suggest that ASD performance may rely on a compensatory reorganization of function [Schafer et al., 2009]. However, we did not identify any obvious compensatory mechanisms as, across all frequency bands there was no evidence of any networks that were more synchronized in ASD compared to TD children. Thus, our results suggest that, although atypical WM‐related brain processes enable children with ASD to maintain a normal performance in the 2‐back task, the desynchronized networks found in our study may not support more challenging WM task conditions such as social interactions in this population.
In summary, this study is the first to demonstrate a desynchronization of alpha rhythms in fronto‐temporal networks in children with ASD during a working memory task. Poor brain connectivity encompassed fronto‐temporal areas previously associated with the processing of WM and challenging situations but also with social cognition and the regulation of emotion. Furthermore, atypical connectivity processes related to the right fusiform were associated with the severity of symptoms in ASD. Therefore, our results suggest that the atypical connectivity processes observed in the fronto‐temporal areas in children with ASD and, in particular, between the left anterior insula and the right fusiform, may contribute to the social cognitive deficits of autism. Finally, this study further strengthened the relevance of investigating neuronal synchronization processes in clinical populations to understand how these oscillatory codes are disrupted in pathophysiological conditions associated with neurodevelopmental disorders.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The authors thank Rachel Leung for administering the ADOS‐R. They sincerely thank their MEG and MRI technicians, Marc Lalancette, Ruth Weiss, and Tammy Rayner, for all their support in data acquisition. They also thank Daniel Cassel and Simeon Wong for their work and help in the MEG analyses. Lastly, they thank Crescent School in Toronto for their support and participation in this project.
REFERENCES
- Ameis SH, Catani M (2015): Altered white matter connectivity as a neural substrate for social impairment in autism spectrum disorder. Cortex 62:158–181. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . (2013): Diagnostic and Statistical Manual of Mental Disorders. (5th ed). Association AP, editor. Washington, DC: American Psychiatric Press. [Google Scholar]
- Anagnostou E, Taylor MJ. (2011): Review of neuroimaging in autism spectrum disorders: what have we learned and where we go from here. Mol Autism 2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A (2012): Working memory: theories, models, and controversies. Ann Rev Psychol 63:129. [DOI] [PubMed] [Google Scholar]
- Bangel KA, Batty M, Ye AX, Meaux E, Taylor MJ, Doesburg SM (2014): Reduced beta band connectivity during number estimation in autism. NeuroImage Clin 6:202–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Nystrom LE, Forman SD, Noll DC, Cohen JD (1997): Dissociating working memory from task difficulty in human prefrontal cortex. Neuropsychologia 35:1373–1380. [DOI] [PubMed] [Google Scholar]
- Barendse EM, Hendriks MP, Jansen JF, Backes WH, Hofman PA, Thoonen G, Kessels RP, Aldenkamp AP (2013): Working memory deficits in high‐functioning adolescents with autism spectrum disorders: neuropsychological and neuroimaging correlates. J Neurodev Disord 5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauml KH, Hanslmayr S, Pastotter B, Klimesch W (2008): Oscillatory correlates of intentional updating in episodic memory. Neuroimage 41:596–604. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Allen G, Beckel‐Mitchener A, Boulanger LM, Carper RA, Webb SJ (2004): Autism and abnormal development of brain connectivity. J Neurosci 24:9228–9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann HC, Rijpkema M, Fernandez G, Kessels RP (2012): Distinct neural correlates of associative working memory and long‐term memory encoding in the medial temporal lobe. Neuroimage 63:989–997. [DOI] [PubMed] [Google Scholar]
- Brookes MJ, Wood JR, Stevenson CM, Zumer JM, White TP, Liddle PF, Morris PG (2011): Changes in brain network activity during working memory tasks: a magnetoencephalography study. Neuroimage 55:1804–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JD (2002): Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron 33:301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Thomas KM, Davidson MC, Kunz K, Franzen PL (2002): Dissociating striatal and hippocampal function developmentally with a stimulus‐response compatibility task. J Neurosci 22:8647–8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Thomas KM, Welsh TF, Badgaiyan RD, Eccard CH, Jennings JR, Crone EA (2000): Dissociation of response conflict, attentional selection, and expectancy with functional magnetic resonance imaging. Proc Natl Acad Sci U S A 97:8728–8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau W, McIntosh AR, Robinson SE, Schulz M, Pantev C (2004): Improving permutation test power for group analysis of spatially filtered MEG data. Neuroimage 23:983–996. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D'Esposito M (2003): Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci 7:415–423. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Postle BR, Ballard D, Lease J (1999): Maintenance versus manipulation of information held in working memory: an event‐related fMRI study. Brain Cogn 41:66–86. [DOI] [PubMed] [Google Scholar]
- de Vries M, Geurts HM (2014): Beyond individual differences: are working memory and inhibition informative specifiers within ASD? J Neural Trans 121:1183–1198. [DOI] [PubMed] [Google Scholar]
- Deng Y, Wang Y, Ding X, Tang YY, Kim C, Gold JNF, BT (2015): Conflict adaptation in prefrontal cortex: now you see it, now you don't. Neuroreport 26:124–130. 25569792 [Google Scholar]
- Di Martino A, Ross K, Uddin LQ, Sklar AB, Castellanos FX, Milham MP (2009): Functional brain correlates of social and nonsocial processes in autism spectrum disorders: an activation likelihood estimation meta‐analysis. Biol Psychiatr 65:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doesburg SM, Herdman AT, Ribary U, Cheung T, Moiseev A, Weinberg H, Liotti M, Weeks D, Grunau RE (2010): Long‐range synchronization and local desynchronization of alpha oscillations during visual short‐term memory retention in children. Exp Brain Res 201:719–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doesburg SM, Ribary U, Herdman AT, Miller SP, Poskitt KJ, Moiseev A, Whitfield MF, Synnes A, Grunau RE (2011): Altered long‐range alpha‐band synchronization during visual short‐term memory retention in children born very preterm. Neuroimage 54:2330–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove A, Pollmann S, Schubert T, Wiggins CJ, von Cramon DY (2000): Prefrontal cortex activation in task switching: an event‐related fMRI study. Brain Res Cogn Brain Res 9:103–109. [DOI] [PubMed] [Google Scholar]
- Druzgal TJ, D'Esposito M (2001): Activity in fusiform face area modulated as a function of working memory load. Brain Res Cogn Brain Res 10:355–364. [DOI] [PubMed] [Google Scholar]
- Duerden EG, Mak‐Fan KM, Taylor MJ, Roberts SW (2012): Regional differences in grey and white matter in children and adults with autism spectrum disorders: an activation likelihood estimate (ALE) meta‐analysis. Autism Res 5:49–66. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Menon V, Walczak A, Ahlstrom J, Denslow S, Horwitz A, Dubno JR 2009. At the heart of the ventral attention system: the right anterior insula. Hum Brain Mapp 30 253041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AK, Gerloff C, Hilgetag CC, Nolte G (2013): Intrinsic coupling modes: multiscale interactions in ongoing brain activity. Neuron 80:867–886. [DOI] [PubMed] [Google Scholar]
- Freunberger R, Fellinger R, Sauseng P, Gruber W, Klimesch W (2009): Dissociation between phase‐locked and nonphase‐locked alpha oscillations in a working memory task. Hum Brain Mapp 30:3417–3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P (2005): A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci 9:474–480. [DOI] [PubMed] [Google Scholar]
- Frith C (2004): Is autism a disconnection disorder? Lancet 3:577. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Knight RT (2000): Prefrontal‐cingulate interactions in action monitoring. Nat Neurosci 3:516–520. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, Levitt P (2007): Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol 17:103–111. [DOI] [PubMed] [Google Scholar]
- Geurts HM, Verte S, Oosterlaan J, Roeyers H, Sergeant JA (2004): How specific are executive functioning deficits in attention deficit hyperactivity disorder and autism? J Child Psychol Psychiatry 45:836–854. [DOI] [PubMed] [Google Scholar]
- Happe F, Booth R, Charlton R, Hughes C (2006): Executive function deficits in autism spectrum disorders and attention‐deficit/hyperactivity disorder: examining profiles across domains and ages. Brain Cogn 61:25–39. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Petit L, Ungerleider LG, Courtney SM (2000): Distinguishing the functional roles of multiple regions in distributed neural systems for visual working memory. Neuroimage 11:145–156. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Makris N, Filipek PA, Kemper TL, Normandin JJ, Sanders HA, Kennedy DN, Caviness VS Jr. ( 2004): Localization of white matter volume increase in autism and developmental language disorder. Ann Neurol 55:530–540. [DOI] [PubMed] [Google Scholar]
- Huettel SA, Song AW, McCarthy G (2005): Decisions under uncertainty: probabilistic context influences activation of prefrontal and parietal cortices. J Neurosci 25:3304–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung Y, Smith ML, Taylor MJ (2013): Functional dissociations in prefrontal‐hippocampal working memory systems. Cortex 49:961–967. [DOI] [PubMed] [Google Scholar]
- Jensen O, Gelfand J, Kounios J, Lisman JE, Jensen O, Tesche CD (2002): Oscillations in the alpha band (9‐12 Hz) increase with memory load during retention in a short‐term memory task. Cereb Cortex 12:877–882. [DOI] [PubMed] [Google Scholar]
- Jensen O, Tesche CD (2002): Frontal theta activity in humans increases with memory load in a working memory task. Eur J Neurosci 15:1395–1359. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ (2007): Functional and anatomical cortical underconnectivity in autism: evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cereb Cortex 17:951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Keller TA, Malave VL, Kana RK, Varma S (2012): Autism as a neural systems disorder: a theory of frontal‐posterior underconnectivity. Neurosci Biobehav Rev 36:1292–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, NJ Minshew, MA Just (2006): Sentence comprehension in autism: thinking in pictures with decreased functional connectivity. Brain: a journal of neurology 129:2484–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Johnson NF, Gold BT (2014): Conflict adaptation in prefrontal cortex: now you see it, now you don't. Cortex 50:76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W, Freunberger R, Sauseng P, Gruber W (2008): A short review of slow phase synchronization and memory: evidence for control processes in different memory systems? Brain Res 1235:31–44. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S (2007): EEG alpha oscillations: the inhibition‐timing hypothesis. Brain Res Rev 53:63–88. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Schack B, Schabus M, Doppelmayr M, Gruber W, Sauseng P (2004): Phase‐locked alpha and theta oscillations generate the P1‐N1 complex and are related to memory performance. Brain Res Cogn Brain Res 19:302–316. [DOI] [PubMed] [Google Scholar]
- Koshino H, Carpenter PA, Minshew NJ, Cherkassky VL, Keller TA, Just MA (2005): Functional connectivity in an fMRI working memory task in high‐functioning autism. Neuroimage 24:810–821. [DOI] [PubMed] [Google Scholar]
- Koshino H, Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA (2008): fMRI investigation of working memory for faces in autism: visual coding and underconnectivity with frontal areas. Cereb Cortex 18:289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalancette M, Quraan M, Cheyne D (2011): Evaluation of multiple‐sphere head models for MEG source localization. Phys Med Biol 56:5621–5635. [DOI] [PubMed] [Google Scholar]
- Landa RJ, Goldberg MC (2005): Language, social, and executive functions in high functioning autism: a continuum of performance. J Autism Dev Disord 35:557–573. [DOI] [PubMed] [Google Scholar]
- Leung RC, Ye AX, Wong SM, Taylor MJ, Doesburg SM 2014. Reduced beta connectivity during emotional face processing in adolescents with autism. Mol Autism 5:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr., Leventhal BL, PC DiLavore, A Pickles, M Rutter (2000): The autism diagnostic observation schedule‐generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 30:205–223. [PubMed] [Google Scholar]
- Maris E, Oostenveld R (2007): Nonparametric statistical testing of EEG‐ and MEG‐data. J Neurosci Methods 164:177–190. [DOI] [PubMed] [Google Scholar]
- Maximo JO, Cadena EJ, Kana RK (2014):The implications of brain connectivity in the neuropsychology of autism. Neuropsychol Rev 24:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGonigle‐Chalmers M, Bodner K, Fox‐Pitt A, Nicholson L (2008): Size sequencing as a window on executive control in children with autism and Asperger's syndrome. J Autism Dev Disord 38:1382–1390. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Williams DL (2007): The new neurobiology of autism: cortex, connectivity, and neuronal organization. Arch Neurol 64:945:950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misic B, Doesburg SM, Fatima Z, Vidal J, Vakorin VA, Taylor MJ, McIntosh AR (2014):coordinated information generation and mental flexibility: large‐scale network disruption in children with autism. Cereb Cortex 25:2815–2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy SD 2013. High‐frequency brain activity and muscle artifacts in MEG/EEG: a review and recommendations. Front Hum Neurosci 7:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP (2002): Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 15:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hearn K, Asato M, Ordaz S, Luna B (2008): Neurodevelopment and executive function in autism. Dev Psychopathol 20:1103–1132. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Strayer DL (2001): Further evidence of intact working memory in autism. J Autism Dev Disord 31:257–263. [DOI] [PubMed] [Google Scholar]
- Palva JM, Monto S, Kulashekhar S, Palva S (2010a): Neuronal synchrony reveals working memory networks and predicts individual memory capacity. Proc Natl Acad Sci U S A 107:7580–7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva S, Monto S, Palva JM (2010b): Graph properties of synchronized cortical networks during visual working memory maintenance. Neuroimage 49:3257–3268. [DOI] [PubMed] [Google Scholar]
- Palva S, Palva JM (2011): Functional roles of alpha‐band phase synchronization in local and large‐scale cortical networks. Front Psychol 2:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quraan MA, Cheyne D (2010): Reconstruction of correlated brain activity with adaptive spatial filters in MEG. Neuroimage 49:2387–2400. [DOI] [PubMed] [Google Scholar]
- Quraan MA, Moses SN, Hung Y, Mills T, Taylor MJ (2011): Detection and localization of hippocampal activity using beamformers with MEG: a detailed investigation using simulations and empirical data. Hum Brain Mapp 32:812–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Miller M, Karalunas S, Robertson C, Grayson DS, Cary RP, Hawkey E, Painter JG, Kriz D, Fombonne E, JT Nigg, DA Fair (2014): Structural and functional connectivity of the human brain in autism spectrum disorders and attention‐deficit/hyperactivity disorder: a rich club‐organization study. Hum Brain Mapp 35:6032–6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, Sporns O (2010): Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52:1059–1069. [DOI] [PubMed] [Google Scholar]
- Rump KM, Giovannelli JL, Minshew NJ, Strauss MS (2009): The development of emotion recognition in individuals with autism. Child Dev 80:1434–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter G, Seigal A, Claxton M, Lawrence K, Skuse D (2008): Can autistic children read the mind of an animated triangle? Autism 12:349–371. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Griesmayr B, Freunberger R, Klimesch W, Klimesch W, Freunberger R, Sauseng P (2010): Control mechanisms in working memory: a possible function of EEG theta oscillations. Neurosci Biobehav Rev 34:1015–1022. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Doppelmayr M, Pecherstorfer T, Freunberger R, Hanslmayr S (2005a): EEG alpha synchronization and functional coupling during top‐down processing in a working memory task. Hum Brain Mapp 26:148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Doppelmayr M, Pecherstorfer T, Freunberger R, Hanslmayr S (2005b): EEG alpha synchronization and functional coupling during top‐down processing in a working memory task. Hum Brain Mapp 26:148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Freunberger R, Pecherstorfer T, Hanslmayr S, Doppelmayr M (2006): Relevance of EEG alpha and theta oscillations during task switching. Exp Brain Res 170:295–301. [DOI] [PubMed] [Google Scholar]
- Schafer RJ, Lacadie C, Vohr B, Kesler SR, Katz KH, Schneider KC, Pugh KR, Makuch RW, Reiss AL, Constable RT, LR Ment (2009): Alterations in functional connectivity for language in prematurely born adolescents. Brain 132:661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf KS, Sweeney JA, Luna B (2006): Brain basis of developmental change in visuospatial working memory. J Cogn Neurosci 18:1045–1058. [DOI] [PubMed] [Google Scholar]
- Schultz RT (2005): Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. Int J Dev Neurosci 23:125–141. [DOI] [PubMed] [Google Scholar]
- Shukla DK, Keehn B, Muller RA (2011): Tract‐specific analyses of diffusion tensor imaging show widespread white matter compromise in autism spectrum disorder. J Child Psychol Psychiatry 52:286–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk TJ, Rinehart N, Bradshaw JL, B Tonge, G Egan, MW O'Boyle, R Cunnington (2006): Visuospatial processing and the function of prefrontal‐parietal networks in autism spectrum disorders: a functional MRI study. Am J Psychiatry 163:1440–1443. [DOI] [PubMed] [Google Scholar]
- Singh KD, Barnes GR, Hillebrand A (2003): Group imaging of task‐related changes in cortical synchronisation using nonparametric permutation testing. Neuroimage 19:1589–1601. [DOI] [PubMed] [Google Scholar]
- Sinzig J, Morsch D, Bruning N, Schmidt MH, Lehmkuhl G (2008): Inhibition, flexibility, working memory and planning in autism spectrum disorders with and without comorbid ADHD‐symptoms. Child Adolesc Psychiatry Ment Health 2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V (2008): A critical role for the right fronto‐insular cortex in switching between central‐executive and default‐mode networks. Proc Natl Acad Sci U S A 105:12569–12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele SD, Minshew NJ, Luna B, Sweeney JA (2007): Spatial working memory deficits in autism. J Autism Dev Disord 37:605–612. [DOI] [PubMed] [Google Scholar]
- Sussman D, Leung RC, Vogan VM, Lee W, Trelle S, Lin S, Cassel DB, Chakravarty MM, Lerch JP, Anagnostou E, MJ Taylor (2015): The autism puzzle: Diffuse but not pervasive neuroanatomical abnormalities in children with ASD. NeuroImage 8:1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thielscher A, Pessoa L (2007): Neural correlates of perceptual choice and decision making during fear‐disgust discrimination. J Neurosci 27:2908–2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15:273–289. [DOI] [PubMed] [Google Scholar]
- Urbain CM, Pang EW, Taylor MJ (2015): Atypical spatiotemporal signatures of working memory brain processes in autism. Transl Psychiatry 5:e617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kooten IA, Palmen SJ, von Cappeln P, Steinbusch HW, Korr H, Heinsen H, Hof PR, van Engeland H, Schmitz C (2008): Neurons in the fusiform gyrus are fewer and smaller in autism. Brain 131:987–999. [DOI] [PubMed] [Google Scholar]
- Varela F, Lachaux JP, Rodriguez E, Martinerie J (2001): The brainweb: phase synchronization and large‐scale integration. Nat Rev Neurosci 2:229–239. [DOI] [PubMed] [Google Scholar]
- Verly M, Verhoeven J, Zink I, Mantini D, Van Oudenhove L, Lagae L, Sunaert S, Rommel N (2014): Structural and functional underconnectivity as a negative predictor for language in autism. Hum Brain Mapp 35:3602–3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissers ME, Cohen MX, Geurts HM (2012): Brain connectivity and high functioning autism: a promising path of research that needs refined models, methodological convergence, and stronger behavioral links. Neurosci Biobehav Rev 36:604–625. [DOI] [PubMed] [Google Scholar]
- von Stein A, Chiang C, Konig P (2000): Top‐down processing mediated by interareal synchronization. Proc Natl Acad Sci U S A 97:14748–14753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waiter GD, Williams JH, Murray AD, Gilchrist A, Perrett DI, Whiten A (2004): A voxel‐based investigation of brain structure in male adolescents with autistic spectrum disorder. Neuroimage 22:619–625. [DOI] [PubMed] [Google Scholar]
- Weinstein M, Ben‐Sira L, Levy Y, Zachor DA, Ben Itzhak E, Artzi M, Tarrasch R, Eksteine PM, Hendler T, Ben Bashat D (2011): Abnormal white matter integrity in young children with autism. Hum Brain Mapp 32:534–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DL, Goldstein G, Minshew NJ (2006): The profile of memory function in children with autism. Neuropsychology 20:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DM, Jarrold C, Grainger C, Lind SE (2014): Diminished time‐based, but undiminished event‐based, prospective memory among intellectually high‐functioning adults with autism spectrum disorder: relation to working memory ability. Neuropsychology 28:30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JJ, Gu H, Gerig G, Elison JT, Styner M, Gouttard S, Botteron KN, Dager SR, Dawson G, Estes AM, AC Evans, HC Hazlett, P Kostopoulos, RC McKinstry, SJ Paterson, RT Schultz, L Zwaigenbaum, J Piven (2012): Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. Am J Psychiatry 169:589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M, Wang J, He Y (2013): BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS One 8:e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye AX, Leung RC, Schafer CB, Taylor MJ, Doesburg SM (2014): Atypical resting synchrony in autism spectrum disorder. Hum Brain Mapp 35:6049–6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A, Cocchi L, Fornito A, Murray MM, Bullmore E (2012): Connectivity differences in brain networks. Neuroimage 60:1055–1062. [DOI] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Bullmore ET (2010): Network‐based statistic: identifying differences in brain networks. Neuroimage 53:1197–1207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


