Abstract
The inhibitory gamma‐aminobutyric acid (GABA) system is involved in the etiology of most psychiatric disorders, including schizophrenia, autism spectrum disorder (ASD) and major depressive disorder (MDD). It is therefore not surprising that proton magnetic resonance spectroscopy (1H‐MRS) is increasingly used to investigate in vivo brain GABA levels. However, integration of the evidence for altered in vivo GABA levels across psychiatric disorders is lacking. We therefore systematically searched the clinical 1H‐MRS literature and performed a meta‐analysis. A total of 40 studies (N = 1,591) in seven different psychiatric disorders were included in the meta‐analysis: MDD (N = 437), schizophrenia (N = 517), ASD (N = 150), bipolar disorder (N = 129), panic disorder (N = 81), posttraumatic stress disorder (PTSD) (N = 104), and attention deficit/hyperactivity disorder (ADHD) (N = 173). Brain GABA levels were lower in ASD (standardized mean difference [SMD] = −0.74, P = 0.001) and in depressed MDD patients (SMD = −0.52, P = 0.005), but not in remitted MDD patients (SMD = −0.24, P = 0.310) compared with controls. In schizophrenia this finding did not reach statistical significance (SMD = −0.23, P = 0.089). No significant differences in GABA levels were found in bipolar disorder, panic disorder, PTSD, and ADHD compared with controls. In conclusion, this meta‐analysis provided evidence for lower brain GABA levels in ASD and in depressed (but not remitted) MDD patients compared with healthy controls. Findings in schizophrenia were more equivocal. Even though future 1H‐MRS studies could greatly benefit from a longitudinal design and consensus on the preferred analytical approach, it is apparent that 1H‐MRS studies have great potential in advancing our understanding of the role of the GABA system in the pathogenesis of psychiatric disorders. Hum Brain Mapp 37:3337–3352, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: 1H‐MRS, GABA, meta‐analysis, psychopathology, MDD, ASD
INTRODUCTION
There is ample evidence for involvement of the gamma‐aminobutyric acid (GABA) system in psychiatric disorders such as schizophrenia [Gonzalez‐Burgos et al., 2015; Lewis et al., 2005; Nakazawa et al., 2012], depression [Luscher et al., 2011], bipolar disorder [Brambilla et al., 2003], anxiety [Geuze et al., 2008; Kalueff and Nutt, 2007], autism [Marín, 2012], alcohol use disorder [Kumar et al., 2009], and attention deficit/hyperactivity disorder (ADHD) [Rivero et al., 2015]. A role for GABA neurotransmission across a wide spectrum of psychiatric disorders is not surprising since GABA is present at approximately one third of all synapses in the central nervous system and shapes neural network dynamics via GABAergic interneurons [Möhler, 2007]. As a result, GABA system functionality is pivotal for physiological processes that are often affected in psychiatric disorders, for example, neural plasticity, stress reactivity, sensory processing, memory formation, and attention [Mody and Pearce, 2004; Möhler, 2007; Vinkers et al., 2010].
A variety of approaches is applied to disentangle the role of the GABA system in the etiology of psychiatric disorders, e.g. involving (epi)genetics, post mortem studies and the measurement of GABA in plasma and cerebrospinal fluid [see, e.g., the review of Luscher et al., 2011]. Currently, the only methods to directly probe the GABA system in the living human brain are proton magnetic resonance spectroscopy (1H‐MRS), positron emission tomography (PET), and single photon emission computed tomography (SPECT). Of these methods, 1H‐MRS is the only one that does not require administration of radioactive tracers or drugs. Although GABA levels are relatively low in the human brain (±1 mmol/kg [Wijtenburg et al., 2015], compared with 5–15 mmol/kg for glutamate [Govindaraju et al., 2000] for example), recent advances in 1H‐MRS techniques and increased field strengths of MRI scanners have resulted in an improved GABA detection [Wijtenburg et al., 2015]. In light of the major overlapping signal for GABA with glutamine and glutamate in standard MRS sequences due to its chemical structure, it is vital to acknowledge the importance of GABA‐specific protocols reliably disentangling the GABA signal from the glutamate and glutamine signal. Moreover, editing techniques such as MEGA‐PRESS or MEGA‐sLASER [Andreychenko et al., 2012] allow for the quantification of brain GABA independent of overlapping spectral metabolites such as creatine [Mullins et al., 2014] and with reduced macromolecular contamination of the GABA signal [Arteaga De Castro et al., 2013].
These developments have resulted in a steady increase in 1H‐MRS studies examining GABA levels in psychiatric disorders ever since the first studies in 1999 [Behar et al., 1999; Sanacora et al., 1999]. However, it is currently unknown whether brain GABA levels are consistently altered across a range of psychiatric disorders. We also do not know whether GABA levels in these disorders are state‐dependent or represent a trait characteristic and whether brain GABA levels differ in developmental disorders (such as autism) from disorders with a stronger environmental component (such as MDD). In an attempt to clarify the potential relevance of brain GABA levels, we conducted a meta‐analysis of the existing 1H‐MRS GABA studies across psychiatric disorders. Moreover, to enhance the interpretation of our results and the implications for future 1H‐MRS studies, we provide a critical discussion on the challenges of GABA quantification that are associated with the use of proton magnetic resonance spectroscopy.
METHODS
Search Strategy and Selection
We conducted Pubmed and Embase searches for relevant 1H‐MRS studies comparing brain GABA levels between patients with a psychiatric disorder and healthy controls (Supporting Information Table 1, search performed August 21, 2015). Pre‐specified inclusion criteria were: (1) human in vivo 1H‐MRS studies; (2) psychiatric patients compared with healthy controls; (3) use of an editing technique or J‐resolved 1H‐MRS to measure GABA (to guarantee sufficient quality of distinct GABA signal); (4) original article; (5) article in English. Reference lists of retrieved articles were screened for additional relevant articles. Three studies per psychiatric disorder were minimally required for meta‐analysis.
Table 1.
Study characteristics: major depressive disorder, schizophrenia, and autism spectrum disorder
| Study | Diagnosis | Region(s)b | N (Pt/control) | Age (SD) | Female (%) | Meds (%, period) | Field strength (T) |
|---|---|---|---|---|---|---|---|
| MAJOR DEPRESSIVE DISORDER | |||||||
| Kugaya 2003 | MDD | OCC | 11 (6/5) | 34 (8) | 0 | 0 (10 days) | 2.1 |
| Epperson 2006 | MDD | OCC | 23 (9/14) | 31 (4) | 100 | 0 (9 mo)e | 2.1 |
| Bhagwagar 2008 | MDD‐R | ACC | 23 (12/11)c | 38 (4) | 52 | 0 (6 mo) | 3 |
| Walter 2009 | MDD | ACC (R) | 24 (11/13) | 37 (NA) | 67 | 0 (1 wk) | 3 |
| Hasler 2005 | MDD‐R |
dm/daPFC vmPFC |
31 (16/15) | 41 (12) | 77 | 0 (3 mo)f | 3 |
| Sanacora 1999 | MDD | OCC | 32 (14/18) | 40 (10) | 41 | 0 (2 wk)g | 2.1 |
| Bhagwagar 2007 | MDD‐R | OCC | 33 (15/18) | 40 (14) | 57 | 0 (3 mo) | 3 |
| Shaw 2013 | MDD‐R |
OCC PFC (L) Subcortical (L) |
34 (18/16) | 22 (2) | 100 | 0 (n.s.) | 3 |
| Hasler 2007 | MDD |
dm/daPFC vmPFC |
40 (20/20) | 34 (12) | 65 | 0 (1 mo) | 3 |
| Abdallah 2014 | MDD | OCC | 40 (23/17) | 43 (12) | 73 | 0 (4 wk) | 4 |
| Gabbay 2012 | MDD | ACC | 41 (20/21) | 16 (2) | 66 | 0 (3 mo)g | 3 |
| Price 2009 | MDD |
ACC OCC |
57 (33/24) | 40 (13) | 48 | 0 (2 wk) | 3 |
| Sanacora 2004 | MDD | OCC | 71 (33/38) | 39 (11) | 48 | 0 (2 wk)h | 2.1 |
| SCHIZOPHRENIA | |||||||
| Rowland Old 2013a | SZ |
ACC CSO |
20 (10/10) | 50 (4) | 30 | 100 | 3 |
| Rowland Young 2013a | SZ |
ACC CSO |
21 (11/10) | 32 (7) | 33 | 100 | 3 |
| Yoon 2010 | SZ | OCC | 26 (13/13) | 28 (9) | 15 | 62 | 3 |
| Marsman 2014 | SZ |
mPFC POC |
32 (13/19) 34 (15/19) |
28 (6)d | 28d | 100 | 7 |
| Stan 2015 | SZ | Hippocampus (L) | 34 (18/16) | 39 (10) | 32 | 61 | 3 |
| Goto 2009 | SZ |
FL Basal ganglia (L) POC |
36 (18/18) | 30 (11) | 50 | 100 | 4 |
| Ongur 2010 | SZ or SZAD |
POC ACC |
40 (21/19) | 38 (10) | 35 | 100 | 3 |
| Kelemen 2013 | SZ | OCC | 48 (28/20) | 25 (8) | 34 | 0 (naive) | 3 |
| Kegeles 2012 | SZ or SZAD |
dlPFC (L) mPFC |
54 (32/22) | 32 (10) | 33 | 50 (2 wk)i | 3 |
| Tayoshi 2010 | SZ |
Basal ganglia (L) ACC |
67 (38/29) | 34 (10) | 44 | 100 | 3 |
| Rowland Old 2015a | SZ or SZAD | mPFC | 68 (31/37) | 50 (6) | 35 | 90 | 3 |
| Rowland Young 2015a | SZ or SZAD | mPFC | 69 (29/40) | 26 (5) | 42 | 93 | 3 |
| AUTISM SPECTRUM DISORDER | |||||||
| Harada 2011 | ASD |
FL (L) Basal ganglia (L) |
22 (12/10) | 6 (3) | NA | NAj | 3 |
| Cochran 2015 | 5 autism, 6 Asperger's, 2 PDD‐NOS | ACC | 27 (13/14) | 15 (2) | 0 | 23 | 3 |
| Gaetz 2014 | ASD |
OCC Temp (L) PMC (L) |
18 (8/10) 24 (13/11) 32 (17/15) |
12 (3)d | 21d | 24d | 3 |
| Rojas 2014 | 9 autism, 7 Asperger's, 1 PDD‐NOS | Temp (L) | 34 (17/17) | 13 (5) | 35 | 29 | 3 |
| Brix 2015 | ASD | ACC (L) | 35 (14/21) | 10 (2) | 0 | 33 | 3 |
MDD(‐R), major depressive disorder (in remission); SZ, schizophrenia; SZAD, schizoaffective disorder; ASD, autism spectrum disorder; PDD‐NOS, pervasive developmental disorder, not otherwise specified; OCC, occipital cortex; ACC, anterior cingulate cortex; R, right; dm/daPFC, dorsomedial/dorsal anterolateral prefrontal cortex (region partly overlaps with vmPFC in the same study); (vm)PFC, (Ventromedial) prefrontal cortex; CSO, centrum semiovale; mPFC, medial prefrontal cortex; POC, parieto‐occipital cortex; L, left; FL, frontal lobe; dlPFC, dorsolateral prefrontal cortex; Temp, temporal lobe; PMC, primary motor cortex; Pt, patients; HC, healthy controls; SD, standard deviation; NA, not available; Meds, psychoactive medication use; mo, months; wk, weeks n.s., not specified; T, Tesla.
Rowland 2013; 2015 are two studies, both distinguishing a young and an old sample. Conform the original articles we kept this distinction in our analyses.
Region is midline unless otherwise specified.
All participants from the study of 2007 by the same group.
Mean of total sample, differs per region.
1 patient used lorazepam >2 weeks before imaging.
No antidepressants, other psychotropic medication not mentioned.
1 patient used lorazepam, 1 patient used thioridazine hydrochloride, 1 control and 1 patient received hormone replacement therapy.
Diphenhydramine hydrochloride use was accepted for insomnia.
Separate analysis with medication‐free patients.
10 autistic patients and 9 normal controls were sedated with triclofos sodium 20 min before imaging.
The initial search yielded 504 studies. All articles were screened on title and abstract. If uncertainty about aptness for inclusion remained, the full text article was read. This resulted in 49 relevant 1H‐MRS GABA studies (see Supporting Information Fig. S1 for a diagram according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analysis) [Moher et al., 2009]). Screening of reference lists yielded two additional articles. The pre‐specified minimum of three published studies was met for major depressive disorder (MDD) (N = 13) [Abdallah et al., 2014; Bhagwagar et al., 2007, 2008; Epperson et al., 2006; Gabbay et al., 2012; Hasler et al., 2005, 2007; Kugaya et al., 2003; Price et al., 2009; Sanacora et al., 1999, 2004; Shaw et al., 2013; Walter et al., 2009], schizophrenia (N = 10) [Goto et al., 2009; Kegeles et al., 2012; Kelemen et al., 2013; Marsman et al., 2014; Öngür et al., 2010; Rowland et al., 2013, 2015; Stan et al., 2015; Tayoshi et al., 2010; Yoon et al., 2010], autism spectrum disorder (ASD) (N = 5) [Brix et al., 2015; Cochran et al., 2015; Gaetz et al., 2014; Harada et al., 2011; Rojas et al., 2014], bipolar disorder (N = 4) [Bhagwagar et al., 2007; Brady et al., 2013; Kaufman et al., 2009; Wang et al., 2006], panic disorder (N = 3) [Goddard et al., 2001; Hasler et al., 2009; Long et al., 2013], posttraumatic stress disorder (PTSD) (N = 3) [Michels et al., 2014; Pennington et al., 2014; Rosso et al., 2014], and ADHD (N = 3) [Bollmann et al., 2015; Edden et al., 2012; Ende et al., 2015]. Less than three 1H‐MRS studies were available for alcohol dependence [Behar et al., 1999; Mason et al., 2006], premenstrual dysphoric disorder [Epperson et al., 2002; Liu et al., 2015], primary insomnia [Plante et al., 2012; Winkelman et al., 2008], borderline personality disorder [Ende et al., 2015], cocaine dependence [Ke et al., 2004], obsessive‐compulsive disorder [Simpson et al., 2012], nicotine dependence [Epperson et al., 2005], social anxiety disorder [Pollack et al., 2008], and Tourette syndrome [Tinaz et al., 2014].
Data Extraction
The following study characteristics were extracted:
Mean and standard deviations of GABA levels, selected brain region(s) and sample size. If means and/or standard deviations were not reported [Bhagwagar et al., 2008; Gaetz et al., 2014; Long et al., 2013; Rojas et al., 2014; Rowland et al., 2015; Yoon et al., 2010], freely available software was used (Window Ruler) to calculate these measures from the provided graphs. To ensure validity of this type of measurement, five studies were randomly chosen to calculate the correlation between factual and graphically acquired GABA levels, yielding a rho of 0.9998 (Supporting Information Fig. S2).
Clinical characteristics (i.e., gender, age, diagnosis, instruments used for diagnosis, use of psychotropic medication).
1H‐MRS methodology details, including: magnetic field strength, voxel size, specific editing technique or J‐resolved 1H‐MRS, GABA quantification using water or creatine as a reference, tissue composition correction and software used for metabolite quantification.
Statistical Analysis
If data were available, patients with symptoms and remitted patients were separately compared with healthy individuals where appropriate. For the primary analyses, GABA levels across multiple brain areas in the same individuals were interpreted to be independent, assuming that they are not homogenously distributed or comparably altered across brain regions. This approach is analogous to Aoki et al. [2012] and has the advantage that more data can be taken into account. An important disadvantage is that there is no correction for the fact that GABA data from different brain regions in the same individual may not be independent. Therefore, in secondary analyses, we calculated the weighted average and standard deviation of GABA levels across multiple brain regions in the same individuals analogous to Luykx et al. [2012a]. Moreover, we carried out analyses separately for frontal and occipital GABA levels in disorders for which sufficient studies were available (schizophrenia and MDD). Standardized mean differences (SMD) were calculated to compare effect sizes found in different studies. Heterogeneity was evaluated using Cochrane's Q‐test and the I 2 statistic [Higgins et al., 2003]. Funnel plots were constructed and Egger's test was used to establish possible publication bias [Egger et al., 1997]. All analyses were carried out using the Comprehensive Meta‐Analysis [Borenstein et al., 2005] software developed by Biostat. A random effects model was chosen since clinical and methodological heterogeneity was assumed to be present across studies. Moreover, we assumed a common/comparable among‐study variance component across subgroups (based on region or disorder state) and combined subgroups using a random effects model.
RESULTS
Study Characteristics
General
General study characteristics are shown in Table 1 for MDD, schizophrenia and ASD and in Table 2 for bipolar disorder, panic disorder, PTSD and ADHD. Additional information on diagnostic assessments and details of the applied 1H‐MRS methodology are included in the supplemental material (Supporting Information Tables S2 and S3).
Table 2.
Study characteristics: bipolar disorder, panic disorder, PTSD, and ADHD
| Study | Diagnosis | Region(s)a | N (Pt/control) | Age (SD) | Female (%) | Meds (%, period) | Field strength (T) |
|---|---|---|---|---|---|---|---|
| BIPOLAR DISORDER | |||||||
| Wang 2006 |
5 BD‐I, 9 BD‐II, 1 BD‐NOS (Eu: 8, D: 7) 9 BD‐I, 7 BD‐II (Eu: 10, D: 3, I/H: 3) |
mPFC OCC |
21 (15/6)b 22 (16/6)b |
37 (14) 34 (12) |
48 64 |
40 75 |
3 |
| Kaufman 2009 | BD (Eu: 10, D: 2, M: 1) |
Basal ganglia Whole brain |
24 (13/11) | 41 (13) | 38 | 100 | 4 |
| Brady 2013 | BD‐I (Eu: all) |
ACC POC |
28 (14/14) | 35 (12) | 36 | 86 | 4 |
| Bhagwagar 2007 | BD‐I (Eu: all) | OCC | 34 (16/18) | 37 (14) | 56 | 0 (3 mo) | 3 |
| PANIC DISORDER | |||||||
| Long 2013 | PD |
mPFC OCC |
19 (11/8) | 39 (12) | 47 | 0 (4 wk) | 3 |
| Goddard 2001 | PD | OCC | 28 (14/14) | 36 (8) | 57 | 0 (1 wk) | 2.1 |
| Hasler 2009 | PD |
dm/daPFC vmPFC |
34 (17/17) | 35 (11) | 69 | 0 (3 mo) | 3 |
| PTSD | |||||||
| Rosso 2014 | PTSD |
ACC Insula (R) |
26 (13/13) | 33 (12) | 46 | 8 | 4 |
| Michels 2014 | PTSD |
ACC dlPFC (L) |
29 (12/17c) | 40 (13) | 93 | 33 | 3 |
| Pennington 2014 | PTSD |
Temp ACC POC (R) |
40 (28/12)d 43 (31/12)d 49 (33/16)d |
36 (11)e | 0 | 0 (2 wk) | 4 |
| ADHD | |||||||
| Edden 2012 | ADHD: 10 C, 3 IA | PMC (L) | 32 (13/19) | 10 (NAf) | 28 | 0 (1 day) | 3 |
| Bollmann Children 2015 | ADHD | Basal ganglia (L) | 35 (16/19) | 11 (2) | 43 | 0 (3 days) | 3 |
| Ende 2015 | ADHD | ACC | 52 (22/30) | 29 (7) | 100 | 0 (2 wk) | 3 |
| Bollmann Adults 2015 | ADHD |
Basal ganglia (L) dlPFC (L) |
54 (16/38) | 34 (10) | 50 | 0 (3 days) | 3 |
BD(‐I/‐II/‐NOS), bipolar disorder type 1/type 2/not otherwise specified; Eu, euthymic; D, depressed; I/H, irritable/hypomanic; PD, panic disorder; M, manic; PTSD, posttraumatic stress disorder; ADHD, attention deficit/hyperactivity disorder; C, combined type; IA, predominantly inattentive type; (v)mPFC, (ventro)medial prefrontal cortex; OCC, occipital cortex; ACC, anterior cingulate cortex; POC, parieto‐occipital cortex; dm/daPFC, dorsomedial/dorsal anterolateral prefrontal cortex (region partly overlaps with vmPFC in the same study); R, right; dlPFC, dorsolateral prefrontal cortex; L, left; Temp, temporal cortex; PMC, primary motor cortex; Pt, patients; HC, healthy controls; SD, standard deviation; NA, not available; Meds, psychoactive medication use; mo, months; wk, weeks; T, Tesla.
Region is midline unless otherwise specified.
No overlap between the two samples.
Healthy controls were trauma‐exposed.
Also PTSD patients with comorbid alcohol abuse disorder.
Mean of total sample, differs per region.
Range 8.4–12.8 years old.
For MDD, nine studies investigated depressed patients and four studies examined remitted MDD patients (Table 1). For bipolar disorder, euthymic bipolar 1 disorder patients were generally included (Table 2).
Medication use
All MDD and panic disorder studies required patients to be medication‐free for at least 1 week (range: 1 week to 9 months). GABA data of unmedicated schizophrenia patients were only available in two studies [Kegeles et al., 2012; Kelemen et al., 2013] and no formal meta‐analysis was carried out. Around 25% of ASD patients used medication. In one study, the majority of subjects were sedated with triclofos sodium prior to the 1H‐MRS measurements [Harada et al., 2011]. The percentage of medicated bipolar disorder and PTSD patients per study ranged from zero [Bhagwagar et al., 2007; Pennington et al., 2014] to a hundred [Kaufman et al., 2009]. All ADHD patients were off medication for at least 1 day.
Diagnostic criteria
Three out of 10 1H‐MRS studies in schizophrenia also included patients with schizoaffective disorder (Table 1). Three out of five studies on ASD did not specify the Diagnostic and Statistical Manual of Mental Disorders (DSM) based diagnosis, while the other two included autism, Asperger's syndrome and pervasive developmental disorder not otherwise specified. The ADHD subtype (inattentive/hyperactive/combined) was only specified in one of the three 1H‐MRS studies.
1H‐MRS methodology
Methodological 1H‐MRS parameters varied widely across studies (Supporting Information Tables S2 and S3). In three studies from the same group, the two regions of interest partially overlapped but were treated as independent outcomes for this meta‐analysis [Hasler et al., 2005, 2007, 2009]. Voxel size ranged from 9 to 75 cm3 and MRI field strength varied from 2.1T to 7T. With regard to the editing technique, 21 studies used MEGA‐PRESS and 18 studies used an in‐house editing technique (J‐editing, JPRESS, MEGA‐sLASER or J‐resolved MRS). Creatine was used as a reference compound in 26 studies, water (H2O) in 13 studies and 1 study reported values for both [Brix et al., 2015]. Eight studies adjusted GABA levels for voxel tissue composition (gray/white matter and cerebrospinal fluid), 18 studies explored differences in tissue composition between groups and included gray or white matter proportion as a covariate in case of a significant difference, while 14 studies did not correct for or did not mention tissue composition correction. Software used for the quantification of GABA and other metabolites was LCModel in 16 studies, other generally available software in 11 studies (including Gannet, (j)MRUI, SAGE, MPFIT, and ProFit) and customized in‐house software in 12 studies.
Brain GABA Levels Across Psychiatric Disorders
MDD
MDD patients exhibited significantly lower GABA levels compared with healthy controls (SMD = −0.41, 95% confidence interval [CI]: −0.70 to −0.13, P = 0.005) (Fig. 1A). Separate analyses of depressed and remitted MDD individuals demonstrated that this was the result of significantly lower GABA levels in depressed (SMD = −0.52, 95% CI: −0.89 to −0.16, P = 0.005), but not in remitted MDD patients (SMD = −0.24, 95% CI: −0.70 to 0.22, P = 0.31) (Fig. 1A). Exclusion of the earliest 1H‐MRS study with the largest SMD (−2.24) [Sanacora et al., 1999] did not alter these results in depressed MDD patients (SMD = −0.39, 95% CI: −0.71 to −0.06, P = 0.019). GABA differences between depressed MDD patients and controls were larger in occipital (SMD = −0.60, 95% CI: −1.18 to −0.02, P = 0.043) than in prefrontal regions (SMD = −0.45, 95% CI: −1.05 to 0.16, P = 0.149) (Fig. 3; Supporting Information Fig. S3). Averaging GABA levels across multiple brain regions from the same study yielded similar results for the total sample (SMD = −0.45, 95% CI: −0.79 to −0.10, P = 0.012), for depressed patients (SMD = −0.52, 95% CI: −0.93 to −0.11, P = 0.014), and for remitted MDD patients (SMD = −0.27, 95% CI: −0.92 to 0.38, P = 0.416) (Supporting Information Fig. S4A).
Figure 1.
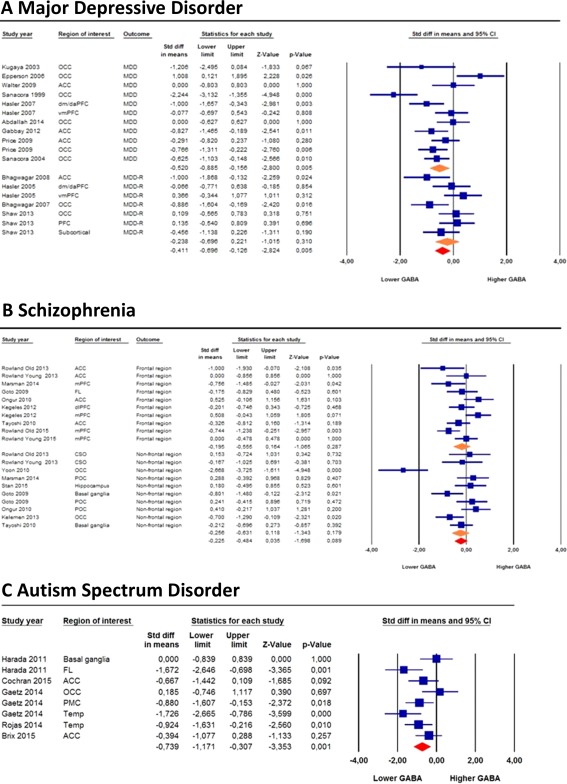
Forest plots of brain GABA levels in major depressive disorder, schizophrenia, and autism spectrum disorder. Diamond shaped orange symbols represent (from top to bottom) current MDD, remitted MDD, frontal regions in schizophrenia and non‐frontal regions in schizophrenia. Size of the blue squares is proportionate to the sample size used. OCC, occipital cortex; ACC, anterior cingulate cortex; dm/daPFC, dorsomedial dorsal anterolateral prefrontal (region partly overlaps with vmPFC in the same study); (vm/m)PFC, (Ventromedial/Medial) prefrontal cortex; MDD(‐R), major depressive disorder (remitted). FL, frontal lobe; dlPFC, dorsolateral prefrontal cortex; CSO, centrum semiovale; POC, parieto‐occipital cortex; Temp, temporal lobe; PMC, primary motor cortex. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 3.
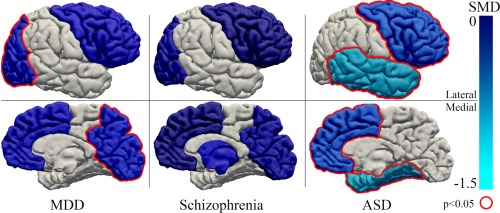
Schematic overview of SMDs in GABA per region of interest for MDD, schizophrenia and ASD (minimum of two studies per region). Frontal, temporal, parietal, occipital, and basal ganglia are color coded and the brain is shown from a lateral and medial perspective. Dark blue: SMD close to 0, light blue: SMD close to −1.5, gray: not reported. Red outline: significant difference between patients and controls (P < 0.05). The following SMDs were found in this meta‐analysis and used in this figure: MDD: occipital −0.597 (P = 0.043), frontal −0.445 (P = 0.149); schizophrenia: occipital −0.343 (P = 0.232), frontal −0.197 (P = 0.313), basal ganglia −0.483 (P = 0.259); ASD: frontal −0.831 (P = 0.001), temporal −1.252 (P = 0.001). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Schizophrenia
No statistically significant differences in GABA levels were found between schizophrenia patients and healthy individuals, even though GABA levels tended to be lower in schizophrenia patients (SMD = −0.23, 95% CI: −0.48 to 0.04, P = 0.089) (Fig. 1B). This trend level effect became statistically significant after averaging GABA levels across multiple brain regions from the same study (SMD = −0.29, 95% CI: −0.56 to −0.01, P = 0.039) (Supporting Information Fig. S4B). Exclusion of one study with the largest SMD (−2.67) [Yoon et al., 2010] rendered these results non‐significant (SMD = −0.18, 95% CI: −0.37 to 0.02, P = 0.078). A subanalysis in studies measuring GABA levels in frontal regions (medial [Kegeles et al., 2012; Marsman et al., 2014] and dorsolateral prefrontal cortex [Chen et al., 2014; Kegeles et al., 2012] and an unspecified region in the frontal lobe [Goto et al., 2009]) did not show significant differences (SMD = −0.20, 95% CI: −0.56 to 0.16, P = 0.287) (Fig. 3).
ASD
Patients with ASD showed significantly lower GABA levels compared with healthy controls (SMD = −0.74, 95% CI: −1.17 to −0.31, P = 0.001) (Fig. 1C). Averaging GABA levels across multiple brain regions from the same study yielded comparable results (SMD = −0.67, 95% CI: −0.94 to −0.39, P = 2.4 × 10 − 6) (Supporting Information Fig. S4C). The largest SMD (−1.25) was found in the two studies examining the temporal lobe (see Fig. 3 for a schematic overview of regional findings in ASD as well as in MDD and schizophrenia).
Bipolar disorder, panic disorder, PTSD, and ADHD
No significant differences in GABA levels were found for bipolar disorder (SMD = 0.23, 95% CI: −0.23 to 0.69, P = 0.326), panic disorder (SMD = −0.34, 95% CI: −0.90 to 0.22, P = 0.230), PTSD (SMD = 0.02, 95% CI: −0.56 to 0.59, P = 0.948), and ADHD (SMD=−0.22, 95% CI: −0.80 to 0.37, P = 0.472) compared with controls (Fig. 2). Averaging GABA levels across multiple brain regions from the same study did not change these non‐significant differences in GABA levels (bipolar: SMD = 0.099, 95% CI: −0.48 to 0.68, P = 0.734; panic disorder: SMD=−0.46, 95% CI: −1.14 to 0.23, P = 0.192; PTSD: SMD = 0.05, 95% CI: −0.76 to 0.86, P = 0.909; ADHD: SMD = −0.41, 95% CI: −1.00 to 0.18, P = 0.176) (Supporting Information Fig. S5).
Figure 2.
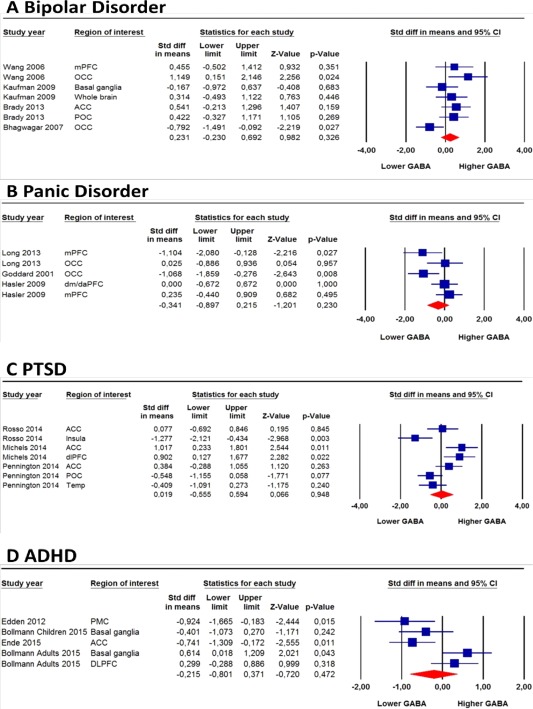
Forest plot showing brain GABA levels in bipolar disorder, panic disorder, PTSD, and ADHD. Size of the blue squares is proportionate to the sample size used. (v)mPFC, (ventro)medial prefrontal cortex; OCC, occipital cortex; ACC, anterior cingulate cortex; POC, parieto‐occipital cortex; dm/daPFC, dorsomedial/dorsal anterolateral prefrontal cortex (region partly overlaps with vmPFC in the same study); dlPFC, dorsolateral prefrontal cortex; Temp, temporal cortex; PMC, primary motor cortex. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Age
SMD size did not significantly depend on age in the meta‐analyses of at least five studies per diagnosis (MDD, schizophrenia and ASD; data not shown), even though the two studies examining GABA levels in a relatively older (∼50 years old) and a relatively younger sample (∼30 years old) [Rowland et al., 2013, 2015] only found significantly lower brain GABA levels in older schizophrenia patients compared with controls, even after adjusting for duration of the disorder [Rowland et al., 2015].
Publication bias
Funnel plots and Egger's tests showed no apparent publication bias and SMDs were more or less symmetrically distributed around the mean with greater dispersion of SMDs in studies that had higher standard errors (Supporting Information Figs. S6 and S7).
Heterogeneity
Significant heterogeneity was found for studies on MDD (P < 0.001, I2 = 68%), on current MDD (P < 0.001, I2 = 74%), but not on remitted MDD (P = 0.07, I2 = 49%). Exclusion of the study with the largest SMD [Sanacora et al., 1999] reduced heterogeneity but it remained significant (P = 0.003; I2 = 55%). A comparably large heterogeneity was found for schizophrenia studies (P < 0.001, I2 = 67%). Again, exclusion of the study with the largest SMD reduced heterogeneity, although it remained significant (P = 0.006, I2 = 51%) [Yoon et al., 2010]. Significant heterogeneity was found also for ASD (P = 0.026, I2 = 56%), bipolar disorder (P = 0.036, I2 = 55%), panic disorder (P = 0.043, I2 = 59%); PTSD (P < 0.001, I2 = 77%), and ADHD (P = 0.002, I2 = 77%). Heterogeneity remained significant after averaging GABA levels across multiple brain regions from the same study (data not shown), except for ASD (P = 0.775, I2 = 0%). Collectively, these findings indicate that heterogeneity may have influenced the results for all psychiatric disorders in this meta‐analysis.
DISCUSSION
The present study investigated whether brain GABA levels measured with 1H‐MRS are consistently altered across psychiatric disorders. Compared with healthy individuals, GABA levels were lower in depressed but not in remitted MDD patients. In addition, GABA levels were significantly lower in ASD patients compared with controls. For schizophrenia, the results were more equivocal: GABA levels were only significantly lower after averaging GABA levels across multiple brain regions from the same study. No significant differences in brain GABA levels were found in bipolar disorder, panic disorder, PTSD, and ADHD.
GABA in MDD
Our finding that brain GABA levels are lower in depressed MDD patient is in line with several studies showing that GABA deficits play a role in the etiology of MDD [Kalueff and Nutt, 2007; Luscher et al., 2011]. Compared with healthy individuals, there is evidence for lower GABA levels in plasma [Petty et al., 1992, 1995; Petty and Sherman, 1984] and cerebrospinal fluid [Gerner et al., 1984; Kasa et al., 1982], as well as a loss of GABAergic interneurons [Rajkowska et al., 2007] in MDD patients. We found that low brain GABA levels in MDD were state‐dependent, as there was no difference between remitted MDD patients and controls. Supporting state‐dependent GABA changes in MDD, longitudinal 1H‐MRS studies have shown normalization of brain GABA levels in MDD patients after electroconvulsive [Sanacora et al., 2003], cognitive behavioral therapy [Sanacora et al., 2006] and treatment with selective serotonin reuptake inhibitors[Sanacora et al., 2002]. Of note, some studies suggest that GABA levels in melancholic [Sanacora et al., 2004] and in treatment‐resistant MDD patients [Price et al., 2009] are lower compared with atypical or non‐treatment resistant MDD, respectively. Due to a lack of individual data, we could not distinguish between MDD subtypes in this meta‐analysis. Nevertheless, these findings underscore the potential utility of in vivo GABA levels for a diagnostic subdivision of MDD patients.
GABA in ASD
The finding that GABA levels were consistently lower in ASD fits a growing body of evidence that point to increased excitatory and reduced inhibitory neurotransmission in ASD [Hussman, 2001]. Both SPECT and PET studies have demonstrated a decrease in GABAA receptors in the frontal cortex [Mori et al., 2012] and of GABAA receptor alpha5 subunits in the nucleus accumbens and the amygdala in ASD patients [Mendez et al., 2013]. This evidence is further supported by postmortem studies that have shown decreased GABAA and GABAB receptor subunits in the superior frontal cortex [Fatemi et al., 2014] and reduced GAD65/67 levels in ASD [Fatemi et al., 2002; Yip et al., 2009]. However, evidence supporting the benefit of GABAergic drugs in ASD is limited and inconclusive [Brondino et al., 2015] and paradoxical response to treatment with conventional GABAergic agents has also been reported in ASD [Bruining et al., 2015]. Lower brain GABA levels in ASD could be the result of a loss of GABAergic interneurons [Barnes et al., 2015]. Alternatively, reduced GABA levels may be secondary and compensatory for the paradoxical excitatory effects of GABA described in some ASD patients [Bruining et al., 2015]. In contrast to the decreased central GABAergic transmission, most studies report higher peripheral GABA levels of ASD patients compared with controls [Dhossche et al., 2002; El‐Ansary et al., 2011; Russo, 2013], although conflicting evidence exists [Rolf et al., 1993]. A plausible explanation for this discrepancy of GABA findings in ASD is currently lacking, but underscores the relevance of measuring GABA indices in the brain.
GABA in Schizophrenia
Notwithstanding previous evidence that GABA system functionality is associated with schizophrenia [Gonzalez‐Burgos et al., 2015; Lewis et al., 2005; Nakazawa et al., 2012] and the relatively large number of 1H‐MRS GABA studies, GABA levels were only significantly lower compared with controls after averaging GABA levels across multiple brain regions from the same study. In light of these equivocal findings, it is important to note that use of antipsychotics may have played a role. Only two studies included medication‐free patients [Kegeles et al., 2012; Kelemen et al., 2013], despite the fact that GABAergic transmission may be most prominently impaired in antipsychotic‐naïve patients [Frankle et al., 2015]. Other sources of clinical heterogeneity may have contributed to the inconclusive evidence such as a wide range of age (25–50 years old), gender (15%–50% female subjects), and duration of illness, which was reported in only five studies and varied from 5.6 months [Rowland et al., 2015] to 25.5 years [Rowland et al., 2013].
GABA in Bipolar Disorder, Panic Disorder, PTSD, and ADHD
Although there is some evidence for altered GABA system functionality in bipolar disorder [Brambilla et al., 2003], panic disorder [Kalueff and Nutt, 2007], PTSD [Geuze et al., 2008], and ADHD [Rivero et al., 2015], our meta‐analysis did not show significant differences in brain GABA levels between these patients and healthy controls. The limited number of published 1H‐MRS studies may partially account for these results.
Interpretation of the GABA MRS Signal
For a correct interpretation of this meta‐analysis, it is important to understand the background of the 1H‐MRS GABA signal. First, the signal originates from both intra‐ and extracellular GABA, even though the majority probably comes from within GABAergic interneurons [Petroff, 2002]. With regard to the biological significance of 1H‐MRS GABA signal, two not mutually exclusive hypotheses have been proposed [Hasler et al., 2007]: (1) lower GABA signal is indicative of a loss of GABAergic interneurons; (2) lower GABA signal quantifies GABAergic inhibition since intracellular GABA levels regulate extracellular GABA levels [Jackson et al., 2000]. If the 1H‐MRS GABA signal indeed reflects GABAergic inhibition, it is likely that GABA levels are dynamic and responsive to environmental challenges. In support, GABA levels are decreased in response to psychological stress [Hasler et al., 2010] and changes in GABA levels have been reported after gabapentin administration [Cai et al., 2012]. Nevertheless, the 1H‐MRS GABA signal in healthy individuals has been reported to be relatively stable; within‐session coefficients of variance (CV) range from 7% to 13% [Bogner et al., 2010; Near et al., 2013; O'Gorman et al., 2011], which is more or less consistent with CVs of measurements carried out up to 7 months apart (3.5%–21%) [Evans et al., 2010; Near et al., 2014; Stephenson et al., 2011; Wijtenburg et al., 2013]. Overall, we do not know to what degree the GABA signal varies as a result of normal physiological variation and whether absolute GABA levels and variability are specific for certain brain regions.
Methodological MRS Considerations
In addition to the interpretation of the 1H‐MRS GABA signal, there are several methodological issues that need to be considered. First, GABA levels probably differ across brain regions. For example, GABA levels differed two‐fold between brain regions measured in the same study [Kegeles et al., 2012; Tayoshi et al., 2010]. Therefore, a hypothesis‐driven regional approach is essential as long as whole‐brain approaches with sufficiently high spatial resolution of the 1H‐MRS signal are absent. Many studies have focused on the occipital cortex since the spectral resolution is higher compared with most other brain areas, as a result of a more homogeneous magnetic field [Puts and Edden, 2012]. However, this also implies that pragmatic reasons rather than hypothesis‐driven arguments (e.g., based on postmortem studies examining GAD67 mRNA levels or neuroimaging studies) may have been decisive in the selection of brain region. Fortunately, a hypothesis‐driven approach is increasingly common as illustrated by recent PTSD studies in this meta‐analysis which focused on prefrontal‐limbic structures that have been implicated in the etiology of this disorder [Koenigs and Grafman, 2009]. Moreover, some promising technical advances have been made to increase the 1H‐MRS signal‐to‐noise ratio [Boer et al., 2015] and it may eventually be possible to map GABA levels across the brain with a high spectral resolution.
Probably the greatest challenge in deriving a reliable GABA signal from 1H‐MRS measurements is the disentanglement of the GABA signal from the macromolecular signal [Mullins et al., 2014]. Editing techniques are essential for filtering out relatively large overlapping signals from both creatine and macromolecules [Rothman et al., 1993]. However, even editing techniques cannot cancel out all contamination: the proportion of macromolecules in the GABA signal after editing has been estimated at almost 50% using MEGA‐PRESS at 3T [Aufhaus et al., 2013]. It is also clear that the methods for reducing the macromolecular contamination of the GABA signal differ greatly across studies (for examples of strategies to deal with macromolecular contamination of the GABA signal, see Mullins et al. [2014]). As a result, the proportion of actual GABA in the signal differs across studies and its variance will also not be homogeneous. Moreover, it is unclear whether differences in macromolecule concentration exist between patients with specific psychiatric disorders and controls, although there is no reason to assume such difference. Unfortunately, there is currently no consensus on the method to minimize the macromolecular contribution to the GABA signal.
In addition to regional differences and macromolecule contamination, other methodological factors that may have affected quantification of the 1H‐MRS GABA signal in this meta‐analysis are: (i) tissue composition (i.e., the amount of gray matter, white matter and cerebrospinal fluid); (ii) whether GABA is reported as a ratio over creatine or water; (iii) the specific software used for GABA quantification; (iv) the scanner and head coil type; (v) pulse sequence acquisition parameters; and vi) the specific rules for quality control of the acquired spectra (e.g., using linewidth as an indicator for the quality of the shimming procedure) and of the fitting (e.g., evaluating the Cramér‐Rao lower bound). The importance of these methodological issues cannot be underestimated since they may result in an increased variability in GABA levels across 1H‐MRS studies. In summary, obtaining accurate in vivo GABA levels remains a challenge.
Future Directions
Although efforts have been made to provide guidelines for minimal best practice for MEGA‐PRESS at 3T regarding acquisition, processing and analysis framework [Mullins et al., 2014], overall consensus in the GABA 1H‐MRS field remains an important goal. In addition, evidence for disease‐related changes in GABA 1H‐MRS levels would greatly benefit from longitudinal studies that provide more information about disease course and stability of the GABA signal over time. Importantly, longitudinal studies are unaffected by many confounders such as genetically determined differences in GABA levels [Berrettini et al., 1982; Luykx et al., 2012b]. There is also a need for studies with larger and more detailed samples to obtain more robust results as well as to investigate possible confounders such as disease history and medication use. In this context, a more integrative approach taking neuroimmune, stress and epigenetic markers into account may be of particular interest. Finally, increased spatial resolution of the 1H‐MRS signal may specifically improve our understanding of how regional brain GABA levels relate to psychopathology. With new developments to suppress the lipid signal in the skull, whole brain MRSI (magnetic resonance spectroscopic imaging) is possible within much shorter acquisition times [Boer et al., 2015]. This would enable MRSI with GABA‐editing, given a homogeneous magnetic field throughout the brain.
CONCLUSIONS
The present 1H‐MRS meta‐analysis shows that in vivo GABA levels are lower in depressed MDD patients and in ASD patients compared with controls. These results substantiate the importance of GABA in the etiology of both developmental disorders and disorders with a greater environmental component. The evidence suggests that the GABA system remains a promising target for pharmacological interventions in MDD and ASD. However, future studies could benefit from increased fundamental knowledge of the physiological variation in GABA levels, longitudinal studies, and consensus on the preferred methodology and minimal standards of human 1H‐MRS studies. Beyond these improvements lies the promise that detailed and accurate brain GABA level measures may advance diagnostic precision and improve personalized medicine.
Supporting information
Supporting Information
Funders had no role in design and reporting of the study. All authors reported no biomedical financial interests or potential conflicts of interest.
REFERENCES
- Abdallah C, Jiang L, De Feyter H, Fasula M, Krystal JH, Rothman DL, Mason GF, Sanacora G (2014): Glutamate metabolism in major depressive disorder. Am J Psychiatry 171:1320–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreychenko A, Boer VO, Arteaga De Castro CS, Luijten PR, Klomp DWJ (2012): Efficient spectral editing at 7 T: GABA detection with MEGA‐sLASER. Magn Reson Med 68:1018–1025. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Kasai K, Yamasue H (2012): Age‐related change in brain metabolite abnormalities in autism: A meta‐analysis of proton magnetic resonance spectroscopy studies. Transl Psychiatry 2:e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arteaga De Castro C, Boer V, Andreychenko A, Wijnen J, Van der Heide U, Luijten P, Klomp D (2013): Improved efficiency on editing MRS of lactate and gamma‐aminobutyric acid by inclusion of frequency offset corrected inversion pulses at high fields. NMR Biomed 26:1213–1219. [DOI] [PubMed] [Google Scholar]
- Aufhaus E, Weber‐Fahr W, Sack M, Tunc‐Skarka N, Oberthuer G, Hoerst M, Meyer‐Lindenberg A, Boettcher U, Ende G (2013): Absence of changes in GABA concentrations with age and gender in the human anterior cingulate cortex: A MEGA‐PRESS study with symmetric editing pulse frequencies for macromolecule suppression. Magn Reson Med 69:317–320. [DOI] [PubMed] [Google Scholar]
- Barnes SA, Kappe A, Zembrzycki A, Metzler A, Mukamel EA, Lucero J, Wang X, Sejnowski TJ (2015): Disruption of mGluR5 in parvalbumin‐positive interneurons induces core features of neurodevelopmental disorders. Mol Psychiatry 20:1161–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar KL, Rothman DL, Petersen KF, Hooten M, Delaney R, Petroff OAC, Shulman GI, Navarro V, Petrakis IL, Charney DS, Krystal JH (1999): Preliminary evidence of low cortical GABA levels in localized 1H‐MR spectra of alcohol‐dependent and hepatic encephalopathy patients. Am J Psychiatry 156:952–954. [DOI] [PubMed] [Google Scholar]
- Berrettini WH, Nurnberger JI, Hare T, Gershon ES, Post RM (1982): Plasma and CSF GABA in affective illness. Br J Psychiatry 141:483–487. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z, Wylezinska M, Jezzard P, Evans J, Ashworth F, Sule A, Matthews PM, Cowen PJ (2007): Reduction in occipital cortex gamma‐aminobutyric acid concentrations in medication‐free recovered unipolar depressed and bipolar subjects. Biol Psychiatry 61:806–812. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z, Wylezinska M, Jezzard P, Evans J, Boorman E, M Matthews P, J Cowen P (2008): Low GABA concentrations in occipital cortex and anterior cingulate cortex in medication‐free, recovered depressed patients. Int J Neuropsychopharmacol 11:255–260. [DOI] [PubMed] [Google Scholar]
- Boer VO, Van de Lindt T, Luijten PR, Klomp DWJ (2015): Lipid suppression for brain MRI and MRSI by means of a dedicated crusher coil. Magn Reson Med 73:2062–2068. [DOI] [PubMed] [Google Scholar]
- Bogner W, Gruber S, Doelken M, Stadlbauer A, Ganslandt O, Boettcher U, Trattnig S, Doerfler A, Stefan H, Hammen T (2010): In vivo quantification of intracerebral GABA by single‐voxel 1H‐MRS‐How reproducible are the results? Eur J Radiol 73:526–531. [DOI] [PubMed] [Google Scholar]
- Bollmann S, Ghisleni C, Poil S, Martin E, Ball J, Eich‐Höchli D, Edden R, Klaver P, Michels L, Brandeis D, O'Gorman RL (2015): Developmental changes in gamma‐aminobutyric acid levels in attention‐deficit/hyperactivity disorder. Transl Psychiatry 5:e589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenstein M, Hedges L, Higgins J, Rothstein H (2005): Comprehensive Meta‐Analysis Version 2. Engelwood, NJ: Biostat. [Google Scholar]
- Brady RO, Mccarthy JM, Prescot AP, Jensen JE, Cooper AJ, Cohen BM, Renshaw PF, Öngür D (2013): Brain gamma‐aminobutyric acid (GABA) abnormalities in bipolar disorder. Bipolar Disord 15:434–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla P, Perez J, Barale F, Schettini G, Soares JC (2003): GABAergic dysfunction in mood disorders. Mol Psychiatry 8:721–715. [DOI] [PubMed] [Google Scholar]
- Brix MK, Ersland L, Hugdahl K, Grüner R, Posserud M, Hammar Å, Craven AR, Noeske R (2015): “Brain MR spectroscopy in autism spectrum disorder — the GABA excitatory/inhibitory imbalance theory revisited.”. Front Hum Neurosci 9:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brondino N, Fusar‐Poli L, Panisi C, Damiani S, Barale F, Politi P (2015): Pharmacological modulation of gaba function in autism spectrum disorders: A systematic review of human studies. J Autism Dev Disord 46:825–839. [DOI] [PubMed] [Google Scholar]
- Bruining H, Passtoors L, Goriounova N, Jansen F, Hakvoort B, De Jonge M, Poil S (2015): Paradoxical benzodiazepine response: A rationale for bumetanide in neurodevelopmental disorders? Pediatrics 136:e539–e543. [DOI] [PubMed] [Google Scholar]
- Cai K, Nanga RP, Lamprou L, Schinstine C, Elliott M, Hariharan H, Reddy R, Epperson CN (2012): The impact of gabapentin administration on brain gaba and glutamate concentrations: A 7t 1h‐mrs study. Neuropsychopharmacology 37:2764–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CMA, Stanford AD, Mao X, Abi‐Dargham A, Shungu DC, Lisanby SH, Schroeder CE, Kegeles LS (2014): GABA level, gamma oscillation, and working memory performance in schizophrenia. NeuroImage Clin 4:531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran DM, Sikoglu EM, Hodge SM, Edden RAE, Foley A, Kennedy DN, Moore CM, Frazier JA (2015): Relationship among glutamine, gamma‐aminobutyric acid, and social cognition in autism spectrum disorders. J Child Adolesc Psychopharmacol 25:314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhossche D, Applegate H, Abraham A, Maertens P, Bland L, Bencsath A, Martinez J (2002): Elevated plasma gamma‐aminobutyric acid (GABA) levels in autistic youngsters: Stimulus for a GABA hypothesis of autism. Med Sci Monit 8:PR1–PR6. [PubMed] [Google Scholar]
- Edden R, Crocetti D, Zhu H, Gilbert D, Mostofsky S (2012): Reduced GABA concentration in attention‐deficit/hyperactivity disorder. Arch Gen Psychiatry 69:750–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C (1997): Bias in meta‐analysis detected by a simple, graphical test. BMJ 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Ansary AK, Bacha AB, Al‐Ayahdi LY (2011): Relationship between chronic lead toxicity and plasma neurotransmitters in autistic patients from Saudi Arabia. Clin Biochem 44:1116–1120. [DOI] [PubMed] [Google Scholar]
- Ende G, Cackowski S, Van Eijk J, Sack M, Demirakca T, Kleindienst N, Bohus M, Sobanski E, Krause‐Utz A, Schmahl C (2015): Impulsivity and aggression in female bpd and adhd patients: Association with acc glutamate and gaba concentrations. Neuropsychopharmacology 41:410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperson CN, Haga K, Mason GF, Sellers E, Gueorguieva R, Zhang W, Weiss E, Rothman D, Krystal JH (2002): Cortical gamma‐aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: A proton magnetic resonance spectroscopy study. Arch Gen Psychiatry 59:851–858. [DOI] [PubMed] [Google Scholar]
- Epperson CN, Malley SO, Czarkowski KA, Gueorguieva R, Sanacora G, Rothman DL, Krystal JH, Mason GF (2005): Sex, GABA and nicotine: The impact of smoking on cortical GABA levels across the menstrual cycle as measured with proton magnetic resonance spectroscopy. Biol Psychiatry 57:44–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperson CN, Gueorguieva R, Czarkowski KA, Stiklus S, Sellers E, Krystal JH, Rothman DL, Mason GF (2006): Preliminary evidence of reduced occipital GABA concentrations in puerperal women: A 1H‐MRS study. Psychopharmacology (Berl) 186:425–433. [DOI] [PubMed] [Google Scholar]
- Evans CJ, McGonigle DJ, Edden RAE (2010): Diurnal stability of γ‐aminobutyric acid concentration in visual and sensorimotor cortex. J Magn Reson Imaging 31:204–209. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Halt AR, Stary JM, Kanodia R, Schulz SC, Realmuto GR (2002): Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in autistic parietal and cerebellar cortices. Biol Psychiatry 52:805–810. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD, Rustan OG, Rooney RJ, Thuras PD (2014): Downregulation of GABAA receptor protein subunits alpha6, beta2, delta, eta, gamma2, theta, and rho2 in superior frontal cortex of subjects with autism. J Autism Dev Disord 44:1833–1845. [DOI] [PubMed] [Google Scholar]
- Frankle WG, Cho RY, Prasad KM, Mason NS, Paris J, Himes M, Walker C, Lewis D, Narendran R (2015): In vivo measurement of gaba transmission in healthy subjects and schizophrenia patients. Am J Psychiatry 172:1148–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Mao X, Klein R, Ely B, Babb J, Panzer A, Alonso C, Shungu D (2012): Anterior cingulate cortex gamma‐aminobutyric acid in depressed adolescents: Relationship to anhedonia. Arch Gen Psychiatry 69:139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetz W, Bloy L, Wang D, Port R, Blaskey L, Levy S, ROberts T (2014): GABA estimation in the brains of children on the autism spectrum: Measurement precision and regional cortical variation. Neuroimage 86:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerner R, Fairbanks L, Anderson G, Young J, Scheinin M, Linnoila M, Hare T, Shaywitz B, Cohen D (1984): CSF neurochemistry in depressed, manic, and schizophrenic patients compared with that of normal controls. Am J Psychiatry 141:1533–1540. [DOI] [PubMed] [Google Scholar]
- Geuze E, van Berckel BNM, Lammertsma AA, Boellaard R, de Kloet CS, Vermetten E, Westenberg HGM (2008): Reduced GABAA benzodiazepine receptor binding in veterans with post‐traumatic stress disorder. Mol Psychiatry 13:74–83. [DOI] [PubMed] [Google Scholar]
- Goddard AW, Mason GF, Almai A, Rothman DL, Behar KL, Petroff OAC, Charney DS, Krystal JH (2001): Reductions in occipital cortex GABA levels in panic disorder detected with 1h‐magnetic resonance spectroscopy. Arch Gen Psychiatry 58:556–561. [DOI] [PubMed] [Google Scholar]
- Gonzalez‐Burgos G, Cho RY, Lewis DA (2015): Alterations in cortical network oscillations and parvalbumin neurons in schizophrenia. Biol Psychiatry 77:1031–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto N, Yoshimura R, Moriya J, Kakeda S, Ueda N, Ikenouchi‐Sugita A, Umene‐Nakano W, Hayashi K, Oonari N, Korogi Y, Nakamura J (2009): Reduction of brain gamma‐aminobutyric acid (GABA) concentrations in early‐stage schizophrenia patients: 3T Proton MRS study. Schizophr Res 112:192–193. [DOI] [PubMed] [Google Scholar]
- Govindaraju V, Young K, Maudsley A (2000): Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed 13:129–153. [DOI] [PubMed] [Google Scholar]
- Harada M, Taki MM, Nose A, Kubo H, Mori K, Nishitani H, Matsuda T (2011): Non‐invasive evaluation of the GABAergic/glutamatergic system in autistic patients observed by MEGA‐editing proton MR spectroscopy using a clinical 3 tesla instrument. J Autism Dev Disord 41:447–454. [DOI] [PubMed] [Google Scholar]
- Hasler G, Neumeister A, Van Der Veen JW, Tumonis T, Bain EE, Shen J, Drevets WC, Charney DS (2005): Normal prefrontal gamma‐aminobutyric acid levels in remitted depressed subjects determined by proton magnetic resonance spectroscopy. Biol Psychiatry 58:969–973. [DOI] [PubMed] [Google Scholar]
- Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC (2007): Reduced prefrontal glutamate/glutamine and gamma‐aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry 64:193–200. [DOI] [PubMed] [Google Scholar]
- Hasler G, Veen JW, Van Der Geraci M, Shen J, Pine D Drevets WC (2009): Prefrontal cortical gamma‐aminobutyric acid levels in panic disorder determined by proton magnetic resonance spectroscopy. Biol Psychiatry 65:273–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, van der Veen JW, Grillon C, Drevets WC, Shen J (2010): Effect of acute psychological stress on prefrontal GABA concentration determined by proton magnetic resonance spectroscopy. Am J Psychiatry 167:1226–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG (2003): Measuring inconsistency in meta‐analyses. BMJ Br Med J 327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussman JP (2001): Suppressed gabaergic inhibition as a common factor in suspected etiologies of autism. J Autism Dev Disord 31:247–248. [DOI] [PubMed] [Google Scholar]
- Jackson MF, Esplin B, Čapek R (2000): Reversal of the activity‐dependent suppression of GABA‐mediated inhibition in hippocampal slices from γ‐vinyl GABA (vigabatrin)‐pretreated rats. Neuropharmacology 39:65–74. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Nutt DJ (2007): Role of GABA in anxiety and depression. Depress Anxiety 24:495–517. [DOI] [PubMed] [Google Scholar]
- Kasa K, Otsuki S, Yamamoto M, Sato M, Kuroda H, Ogawa N (1982): Cerebrospinal fluid gamma‐aminobutyric acid and homovanillic acid in depressive disorders. Biol Psychiatry 17:877–883. [PubMed] [Google Scholar]
- Kaufman RE, Ostacher MJ, Marks EH, Simon NM, Sachs GS, Jensen JE, Renshaw PF, Pollack MH (2009): Brain GABA levels in patients with bipolar disorder. Prog Neuro‐Psychopharmacol Biol Psychiatry 33:427–434. [DOI] [PubMed] [Google Scholar]
- Ke Y, Streeter CC, Nassar LE, Sarid‐Segal O, Hennen J, Yurgelun‐Todd DA, Awad LA, Rendall MJ, Gruber SA, Nason A, Mudrick MJ, Blank SR, Meyer AA, Knapp C, Ciraulo DA, Renshaw PF (2004): Frontal lobe GABA levels in cocaine dependence: A two‐ dimensional, J‐resolved magnetic resonance spectroscopy study. Psychiatry Res 130:283–293. [DOI] [PubMed] [Google Scholar]
- Kegeles LS, Mao X, Stanford AD, Girgis R, Ojeil N, Xu X, Gil R, Slifstein M, Abi‐Dargham A, Lisanby SH, Shungu DC (2012): Elevated prefrontal cortex γ‐aminobutyric acid and glutamate‐glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch Gen Psychiatry 69:449–459. [DOI] [PubMed] [Google Scholar]
- Kelemen O, Kiss I, Benedek G, Kéri S (2013): Perceptual and cognitive effects of antipsychotics in first‐episode schizophrenia: The potential impact of GABA concentration in the visual cortex. Prog Neuro‐Psychopharmacol Biol Psychiatry 47:13–19. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Grafman J (2009): Posttraumatic stress disorder: The role of medial prefrontal cortex and amygdala. Neuroscientist 15:540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugaya A, Sanacora G, Verhoeff NPLG, Fujita M, Mason GF, Seneca NM, Bozkurt A, Khan SA, Anand A, Degen K, Charney DS, Zoghbi SS, Baldwin RM, Seibyl JP, Innis RB (2003): Cerebral benzodiazepine receptors in depressed patients measured with [123I]iomazenil SPECT. Biol Psychiatry 54:792–799. [DOI] [PubMed] [Google Scholar]
- Kumar S, Porcu P, Werner D, Matthews D, Diaz‐Granados J, Helfand R, Morrow A (2009): The role of GABAA receptors in the acute and chronic effects of ethanol: A decade of progress. Psychopharmacology 205:529–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW (2005): Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci 6:312–324. [DOI] [PubMed] [Google Scholar]
- Liu B, Wang G, Gao D, Gao F, Zhao B, Qiao M, Yang H, Yu Y, Ren F, Yang P, Chen W, Rae CD (2015): Alterations of GABA and glutamate‐glutamine levels in premenstrual dysphoric disorder: A 3T proton magnetic resonance spectroscopy study. Psychiatry Res Neuroimaging 231:64–70. [DOI] [PubMed] [Google Scholar]
- Long Z, Medlock C, Dzemidzic M, Shin YW, Goddard AW, Dydak U (2013): Decreased GABA levels in anterior cingulate cortex/medial prefrontal cortex in panic disorder. Prog Neuropsychopharmacol Biol Psychiatry 44:131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher B, Shen Q, Sahir N (2011): The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry 16:383–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luykx JJ, Laban KG, Van Den HMP, Boks MPM, Mandl RCW (2012a): Region and state specific glutamate downregulation in major depressive disorder: A meta‐analysis of 1 H‐MRS findings. Neurosci Biobehav Rev 36:198–205. [DOI] [PubMed] [Google Scholar]
- Luykx JJ, Vinkers CH, Bakker SC, Visser WF, van Boxmeer L, Strengman E, van Eijk KR, Lens JA, Borgdorff P, Keijzers P, Kappen TH, van Dongen EPA, Bruins P, Verhoeven NM, de Koning TJ, Kahn RS, Ophoff RA (2012b): A common variant in ERBB4 regulates GABA concentrations in human cerebrospinal fluid. Neuropsychopharmacology 37:2088–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín O (2012): Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci 13:107–120. [DOI] [PubMed] [Google Scholar]
- Marsman A, Mandl RCW, Klomp DWJ, Bohlken MM, Boer VO, Andreychenko A, Cahn W, Kahn RS, Luijten PR, Hulshoff Pol HE (2014): GABA and glutamate in schizophrenia: A 7 T 1H‐MRS study. NeuroImage Clin 6:398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason GF, Petrakis IL, Graaf RA, De Gueorguieva R, Guidone E, Coric V, Epperson CN, Rothman DL Krystal JH (2006): Cortical gamma‐aminobutyric acid levels and the recovery from ethanol dependence: preliminary evidence of modification by cigarette smoking. Biol Psychiatry 59:85–93. [DOI] [PubMed] [Google Scholar]
- Mendez MA, Horder J, Myers J, Coghlan S, Stokes P, Erritzoe D, Howes O, Lingford‐Hughes A, Murphy D, Nutt D (2013): The brain GABA‐benzodiazepine receptor alpha‐5 subtype in autism spectrum disorder: A pilot [11C]Ro15‐4513 positron emission tomography study. Neuropharmacology 68:195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels L, Schulte‐Vels T, Schick M, O'Gorman RL, Zef T, Hasler G, Mueller‐Pfeiffer C (2014): Prefrontal GABA and glutathione imbalance in posttraumatic stress disorder: Preliminary findings. Psychiatry Res Neuroimaging 224:288–295. [DOI] [PubMed] [Google Scholar]
- Mody I, Pearce RA (2004): Diversity of inhibitory neurotransmission through GABA A receptors. Trends Neurosci 27:569–575. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman D, PRISMA G. r (2009): Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. BMJ 339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möhler H (2007): Molecular regulation of cognitive functions and developmental plasticity: Impact of GABAA receptors. J Neurochem 102:1–12. [DOI] [PubMed] [Google Scholar]
- Mori T, Mori K, Fujii E, Toda Y, Miyazaki M, Harada M, Hashimoto T, Kagami S (2012): Evaluation of the GABAergic nervous system in autistic brain: 123I‐iomazenil SPECT study. Brain Dev 34:648–654. [DOI] [PubMed] [Google Scholar]
- Mullins PG, McGonigle DJ, O'Gorman RL, Puts NAJ, Vidyasagar R, Evans CJ, Edden RAE, Brookes MJ, Garcia A, Foerster BR, Petrou M, Price D, Solanky BS, Violante IR, Williams S, Wilson M (2014): Current practice in the use of MEGA‐PRESS spectroscopy for the detection of GABA. Neuroimage 86:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, Zsiros V, Jiang Z, Nakao K, Kolata S, Zhang S, Belforte JE (2012): GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacology 62:1574–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Near J, Evans C, Puts N, Barker P, Edden R (2013): J‐difference editing of gamma‐aminobutyric acid (GABA): Simulated and experimental multiplet patterns. Magn Reson Med 70:1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Near J, Ho YL, Sandberg K, Kumaragamage C, Blicher JU (2014): Long‐term reproducibility of GABA magnetic resonance spectroscopy. Neuroimage 99:191–196. [DOI] [PubMed] [Google Scholar]
- O'Gorman RL, Michels L, Edden RA, James B, Martin E (2011): NIH Public Access. J Magn Reson Imaging 33:1262–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öngür D, Prescot AP, McCarthy J, Cohen BM, Renshaw PF (2010): Elevated gamma‐aminobutyric acid levels in chronic schizophrenia. Biol Psychiatry 68:667–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington DL, Abé C, Batki SL, Meyerhoff DJ (2014): A preliminary examination of cortical neurotransmitter levels associated with heavy drinking in posttraumatic stress disorder. Psychiatry Res Neuroimaging 224:281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroff OAC (2002): GABA and glutamate in the human brain. Neuroscientist 8:562–573. [DOI] [PubMed] [Google Scholar]
- Petty F, Sherman AD (1984): Plasma GABA levels in psychiatric illness. J Affect Disord 6:131–138. [DOI] [PubMed] [Google Scholar]
- Petty F, Kramer GL, Gullion CM, Rush AJ (1992): Low plasma gamma‐aminobutyric acid levels in male patients with depression. Biol Psychiatry 32:354–363. [DOI] [PubMed] [Google Scholar]
- Petty F, Kramer GL, Fulton M, Davis L, Rush AJ (1995): Stability of plasma GABA at four‐year follow‐up in patients with primary unipolar depression. Biol Psychiatry 37:806–810. [DOI] [PubMed] [Google Scholar]
- Plante DT, Jensen JE, Schoerning L, Winkelman JW (2012): Reduced gamma‐aminobutyric acid in occipital and anterior cingulate cortices in primary insomnia: A link to major depressive disorder? Neuropsychopharmacology 37:1548–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack MH, Jensen JE, Simon NM, Kaufman RE, Renshaw PF (2008): High‐field MRS study of GABA, glutamate and glutamine in social anxiety disorder: Response to treatment with levetiracetam. Prog Neuropsychopharmacol Biol Psychiatry 32:739–743. [DOI] [PubMed] [Google Scholar]
- Price RB, Shungu DC, Mao X, Nestadt P, Kelly C, Collins KA, Murrough JW, Charney DS, Mathew SJ (2009): Amino acid neurotransmitters assessed by proton magnetic resonance spectroscopy: Relationship to treatment resistance in major depressive disorder. Biol Psychiatry 65:792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puts N, Edden R (2012): In vivo magnetic spectroscopy of GABA: A methodological review. Prog Nucl Magn Spectrosc 60:1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, O'Dwyer G, Teleki Z, Stockmeier CA, Miguel‐Hidalgo JJ (2007): GABAergic neurons immunoreactive for calcium binding proteins are reduced in the prefrontal cortex in major depression. Neuropsychopharmacology 32:471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero O, Selten MM, Sich S, Popp S, Bacmeister L, Amendola E, Negwer M, Schubert D, Proft F, Kiser D, Schmitt AG, Gross C, Kolk S, Strekalova T, Van den Hove D, Resink T, Nadif Kasri N, Lesch K (2015): Cadherin‐13, a risk gene for ADHD and comorbid disorders, impacts GABAergic function in hippocampus and cognition. Transl Psychiatry 5:e655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas DC, Singel D, Steinmetz S, Hepburn S, Brown MS (2014): Decreased left perisylvian GABA concentration in children with autism and unaffected siblings. Neuroimage 86:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolf LH, Haarmann FY, Grotemeyer KH, Kehrer H (1993): Serotonin and amino acid content in platelets of autistic children. Acta Psychiatr Scand 87:312–316. [DOI] [PubMed] [Google Scholar]
- Rosso IM, Weiner MR, Crowley DJ, Silveri MM, Rauch SL, Jensen JE (2014): Insula and anterior cingulate GABA levels in post‐traumatic stress disorder: Preliminary findings using magnetic resonance spectroscopy. Depress Anxiety 31:115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman DL, Petroff OA, Behar KL, Mattson RH (1993): Localized 1H NMR measurements of gamma‐aminobutyric acid in human brain in vivo. Proc Natl Acad Sci U S A 90:5662–5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland LM, Kontson K, West J, Edden RA, Zhu H, Wijtenburg SA, Holcomb HH, Barker PB (2013): In vivo measurements of glutamate, GABA, and NAAG in schizophrenia. Schizophr Bull 39:1096–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland LM, Krause BW, Wijtenburg SA, Mcmahon RP, Chiappelli J, Nugent KL, Nisonger SJ, Korenic SA, Kochunov P (2015): Medial frontal GABA is lower in older schizophrenia: A MEGA‐PRESS with macromolecule suppression study. Mol Psychiatry 21:198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo AJ (2013): Correlation between hepatocyte growth factor (hgf) and gamma‐aminobutyric acid (gaba) plasma levels in autistic children. Biomark Insights 8:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Mason GF, Rothman DL, Behar KL, Hyder F, Petroff OA, Berman RM, Charney DS, Krystal JH (1999): Reduced cortical gamma‐aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry 56:1043–1047. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Mason GF, Rothman DL, Krystal JH (2002): Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am J Psychiatry 159:663–665. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Mason GF, Rothman DL, Hyder F, Ciarcia JJ, Ostroff RB, Berman RM, Krystal JH (2003): Increased cortical GABA concentrations in depressed patients receiving ECT. Am J Psychiatry 160:577–579. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Gueorguieva R, Epperson CN, Wu Y‐T, Appel M, Rothman DL, Krystal JH, Mason GF (2004): Subtype‐specific alterations of gamma‐aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry 61:705–713. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Fenton LR, Fasula MK, Rothman DL, Levin Y, Krystal JH, Mason GF (2006): Cortical gamma‐aminobutyric acid concentrations in depressed patients receiving cognitive behavioral therapy. Biol Psychiatry 59:284–286. [DOI] [PubMed] [Google Scholar]
- Shaw A, Brealy J, Richardson H, Muthukumaraswamy SD, Edden RA, John Evans C, Puts NAJ, Singh KD, Keedwell PA (2013): Marked reductions in visual evoked responses but not gamma‐aminobutyric acid concentrations or gamma‐band measures in remitted depression. Biol Psychiatry 73:691–698. [DOI] [PubMed] [Google Scholar]
- Simpson HB, Shungu DC, Bender JJ, Mao X, Xu X, Slifstein M, Kegeles LS (2012): Investigation of cortical glutamate‐glutamine and gamma‐aminobutyric acid in obsessive‐compulsive disorder by proton magnetic resonance spectroscopy. Neuropsychopharmacology 37:2684–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stan A, Ghose S, Zhao C, Hulsey K, Mihalakos P, Yanagi M, Morris S, Bartko J, Choi C, Tamminga C (2015): Magnetic resonance spectroscopy and tissue protein concentrations together suggest lower glutamate signaling in dentate gyrus in schizophrenia. Mol Psychiatry 20:433–439. [DOI] [PubMed] [Google Scholar]
- Stephenson MC, Gunner F, Napolitano A, Greenhaff PL, Macdonald IA, Saeed N, Vennart W, Francis ST, Morris PG, Stephenson MC, Francis ST, Morris PG (2011): Applications of multi‐nuclear magnetic resonance spectroscopy at 7T. World J Radiol 3:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayoshi S, Nakataki M, Sumitani S, Taniguchi K, Shibuya‐Tayoshi S, Numata S, Iga JI, Ueno SI, Harada M, Ohmori T (2010): GABA concentration in schizophrenia patients and the effects of antipsychotic medication: A proton magnetic resonance spectroscopy study. Schizophr Res 117:83–91. [DOI] [PubMed] [Google Scholar]
- Tinaz S, Belluscio BA, Malone P, Van der Veen JW, Hallett M, Horovitz SG (2014): Role of the sensorimotor cortex in Tourette syndrome using multimodal imaging. Hum Brain Mapp 35:5834–5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinkers CH, Mirza NR, Olivier B, Kahn RS (2010): The inhibitory GABA system as a therapeutic target for cognitive symptoms in schizophrenia: Investigational agents in the pipeline. Expert Opin Investig Drugs 19:1217–1233. [DOI] [PubMed] [Google Scholar]
- Walter M, Henning A, Grimm S, Schulte RF, Beck J, Dydak U, Schnepf B, Boeker H, Boesiger P, Northoff G (2009): The relationship between aberrant neuronal activation in the pregenual anterior cingulate, altered glutamatergic metabolism, and anhedonia in major depression. Arch Gen Psychiatry 66:478–486. [DOI] [PubMed] [Google Scholar]
- Wang P, Sailasuta N, Chandler R, Ketter T (2006): Magnetic resonance spectroscopic measure‐ ment of cerebral gamma‐aminobutyric acid concentrations in patients with bipolar disorders. Acta Neuropsychiatr 18:120–126. [DOI] [PubMed] [Google Scholar]
- Wijtenburg AS, Rowland LM, Edden RA, Barker PB, Phil D (2013): Reproducibility of brain spectroscopy at 7T using conventional localization and spectral editing techniques. J Magn Reson Imaging 38:460–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijtenburg SA, Yang S, Fischer BA, Rowland LM (2015): In vivo assessment of neurotransmitters and modulators with magnetic resonance spectroscopy: Application to schizophrenia. Neurosci Biobehav Rev 51:276–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelman JW, Buxton OM, Jensen JE, Benson KL, Connor SPO, Wang W, Renshaw PF (2008): Reduced brain GABA in primary insomnia: Preliminary data from 4T proton magnetic resonance spectroscopy (1H‐MRS). Sleep 31:1499–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip J, Soghomonian JJ, Blatt GJ (2009): Decreased GAD65 mRNA levels in select subpopulations of neurons in the cerebellar dentate nuclei in autism: An in situ hybridization study. Autism Res 2:50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Maddock RJ, Rokem A, Silver MA, Minzenberg MJ, Ragland JD, Carter CS (2010): GABA concentration is reduced in visual cortex in schizophrenia and correlates with orientation‐specific surround suppression. J Neurosci 30:3777–3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


