Abstract
Thermal taster status refers to the finding that, in some individuals, thermal stimulation of the tongue elicits a phantom taste. Little is known regarding the mechanism for this, it is hypothesised to be a result of cross‐wiring between gustatory and trigeminal nerves whose receptors co‐innervate papillae on the tongue. To address this, we use functional magnetic resonance imaging to perform the first study of whether the cortical response to gustatory‐trigeminal samples is altered with thermal taster status. We study the response to cold (6°C) gustatory (sweet) samples at varying levels of trigeminal stimulation elicited by CO2 (no CO2, low CO2, high CO2) in thermal taster (TT) and thermal non‐taster (TnT) groups, and evaluate associated behavioural measures. Behaviourally, the TT group perceived gustatory and trigeminal stimuli significantly more intense than TnTs, and were significantly more discriminating of CO2 level. fMRI data revealed elevated cortical activation to the no CO2 sample for the TT group compared to TnT group in taste, oral somatosensory and reward areas. In TnTs, a significant positive modulation in cortical response with increasing level of CO2 was found across taste, somatosensory and reward areas. In contrast, in TTs, a reduced positive modulation with increasing level of CO2 was found in somatosensory areas (SI, SII), whilst a significant negative modulation was found in taste (anterior insula) and reward (ACC) areas. This difference in cortical response to trigeminal stimuli supports cross‐modal integration in TTs, with gustatory and trigeminal nerves highly stimulated by cold gustatory samples due to their intertwined nature. Hum Brain Mapp 37:2263–2275, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: fMRI, BOLD, taste, thermal taster status, insula, oral somatosensory, trigeminal
INTRODUCTION
The perception of taste is known to vary widely across individuals. There are many factors that contribute to an individual's taste perception and subsequent food preferences, including the density of taste papillae on the tongue and genetic differences in taste receptors [Bajec and Pickering, 2010; Hayes and Keast, 2011]. Functional magnetic resonance imaging (fMRI) studies report that the primary taste cortex is located within the anterior insula/frontal operculum [Small et al., 1997, 1999; Veldhuizen et al., 2011] with secondary projections to the orbitofrontal cortex (OFC) [Francis et al., 1999], amygdala [O'Doherty et al., 2001], anterior cingulate cortex (ACC) [Small et al., 2003], ventral striatum [O'Doherty et al., 2003], and dorsolateral prefrontal cortex [Kringelbach et al., 2004]. However, few studies have investigated the impact of taste phenotype on the primary gustatory cortex and oral somatosensory areas. Eldeghaidy et al. [2011] showed a significant increase in the cortical BOLD response to oral fat in 6‐n‐propylthiouracil (PROP) tasters in key taste, texture and reward processing areas (super‐taster > taster > non‐taster).
A new taste phenotype known as “thermal taster status” has been described [Cruz and Green, 2000]. Thermal stimulation of small areas of the tongue has been shown to elicit a “phantom” taste in some individuals, ∼30–50% of the population [Bajec and Pickering, 2008; Cruz and Green, 2000; Green and George, 2004; Yang et al., 2014]. Since its discovery, behavioural differences have been reported between subjects who perceive a phantom taste, termed “thermal tasters” (TTs), and those who do not, termed “thermal non‐tasters” (TnTs), [Bajec and Pickering, 2008; Cruz and Green, 2000; Green and George, 2004; Green et al., 2005; Pickering et al., 2010a,b]. Thermal tasters have been shown to be more sensitive to pure taste stimuli at supra‐threshold levels [Bajec and Pickering, 2008; Green and George, 2004; Green et al., 2005], and both retro‐ and ortho‐nasal vanillin simulation [Green and George, 2004] compared with TnT, although an olfactory advantage was not found at detection threshold level in a more recent study [Yang et al., 2014]. However, there is conflicting evidence regarding the impact of thermal taster status on trigeminal stimuli; sensations induced by capsaicin and menthol (burning, stinging and prickling) were not rated differently between TTs and TnTs in a series of experiments by Green et al. [2005]. In contrast, the astringency of alum [Bajec and Pickering, 2008], the carbonation and fullness of beer [Pickering et al., 2010a], the astringency of red wine [Pickering et al., 2010b] and the temperature of warm and cold stimuli [Bajec and Pickering, 2008; Yang et al., 2014] have all been rated significantly higher in TTs than TnTs. Current evidence suggests that behavioural differences in TTs may be limited to the oral cavity, as no significant differences have been found for temperature intensity ratings at non‐gustatory sites (lip and hand) [Green and George, 2004]. The mechanism for this increase in sensitivity in TTs has been hypothesised to be due to a temperature sensitive chemosensory pathway [Cruz and Green, 2000]. This hypothesis is supported by the discovery that the TRPM5 cation channel, which responds to sweet, bitter and umami tastes is also heat activated and highly temperature sensitive [Talavera et al., 2008]. In TTs, the TRPM5 could depolarise the taste cells through thermal activation. However, the question remains as to whether the phenomenon of thermal taster status is limited to thermal‐taste activation, or whether a variety of trigeminal and gustatory stimuli can modulate a different cortical response in TTs compared to TnTs.
Here, the combination of behavioural sensory investigations and brain imaging allows the mechanism behind the thermal taster status phenomenon to be explored. We investigate whether the cortical response to gustatory‐trigeminal samples is altered with thermal taster status. Here, carbonation is chosen as a trigeminal stimulus to be modulated and combined with a sweet taste (dextrose) added at fixed levels. Few studies have investigated the effects of carbonation (CO2) as a somatosensory component of flavour perception and the pathways responsible for its perception in combination with taste stimuli are not fully understood.
MATERIALS AND METHODS
Participants and Screening
The study was approved by the University of Nottingham Medical School Research Ethics Committee. Recruitment questionnaires screened any volunteers with contraindications to MRI safety or those who had a known taste dysfunction. All subjects gave informed consent before enrolling in the study. 52 subjects (32 female/20 male, age 35 ± 7 years) underwent two separate screening sessions to determine their PROP and thermal taster status. PROP taster status was defined based on the intensity ratings of 0.32 mM PROP (Sigma Aldrich, UK) prepared in deionised water from a reverse osmosis unit, presented and classified according to a method described by Lim et al. [2008]. Intensity was rated on a general Labelled Magnitude Scale (gLMS) [Green et al., 1996], and training on how to use the scale was given prior to data collection in order to increase validity [Bartoshuk et al., 2002]. The gLMS is a category ratio scale used to measure intensity of sensation with categories of no sensation, barely detectable, weak, moderate, strong, very strong and strongest imaginable marked at distances of 0, 1.4, 17, 34.7, 52.5 and 100 mm along a continuous line. Only the verbal categories are given, and subjects are instructed to mark anywhere along the continuous line to register their sensation [Green et al., 1996]. All subjects were trained on the scale based on an approach by [Bartoshuk et al., 2002] which is described below. A reference sheet with a gLMS presented in exactly the same way as subsequent test sheets was given to each subject. Subjects received verbal and written instructions that the top of the scale corresponded to the strongest imaginable sensation of any kind and were asked to write down what this was at the top of their reference sheet. Subjects were asked to rate a list of 15 remembered or imagined sensations relative to their strongest imaginable sensation of any kind, Table 1.
Table 1.
Remembered or imagined sensations used in gLMS training
| Remembered or imagined sensation |
|---|
|
1 The brightness of a dimly lit restaurant 2 The brightness of a well‐lit room 3 Staring at the sun 4 The loudness of a whisper 5 The loudness of a conversation 6 Hearing a nearby jet‐plane take off 7 Warmth of freshly baked bread in your mouth 8 The coldness experienced sucking on an ice‐cube 9 The smell of a rose 10 The strongest smell ever experienced 11 The sweetness of candyfloss 12 The bitterness of grapefruit 13 The strongest taste ever experienced 14 The strongest oral burn experienced 15 The strongest oral pain ever experienced |
Thermal taster status was assessed using a Medoc Pathway with intra‐oral ATS (advanced thermal stimulator) thermode (Medoc, Israel) on one tongue location only. Subjects were asked with the guidance of researcher to place the intra‐oral thermode (6 mm diameter round surface) on the anterior tongue tip, the area which is the most responsive to thermal taste [Cruz and Green, 2000] and where fungiform papillae are most densely innervated [Shahbake et al., 2005]. Subjects were instructed to hold the thermode firmly in place during all temperature trials. Two warming (from 15°C to 40°C) and two cooling (from 35°C to 5°C) trials were carried out following the procedure of Bajec and Pickering [2008]. The warming trial started at 35°C, cooled to 15°C and rewarmed to 40°C and held for 1 s. The cooling trial started at 35°C was cooled to 5°C and held for 10 s before rising to baseline (35°C), as illustrated in Figure 1a,b respectively. Warming trials always preceded cooling trials to avoid possible adaptation from the intense, sustained cold stimulation [Green and George 2004]. Subjects were told to wait until tongue temperature and sensation had returned to normal before proceeding onto the next trial, with a minimum of two minutes break. If a thermally induced taste was perceived, subjects were asked to state the taste quality perceived from a selected list (“sweet,” “salty,” “bitter,” “sour,” “umami” and “other please specify”), and rate its intensity for each trial on a gLMS. Thermal tasters were classified as those who perceived a taste, above weak during both replicates of either the warming or cooling trial. Thermal non‐tasters were classified as those who did not perceive a taste on any replicate of any trial.
Figure 1.
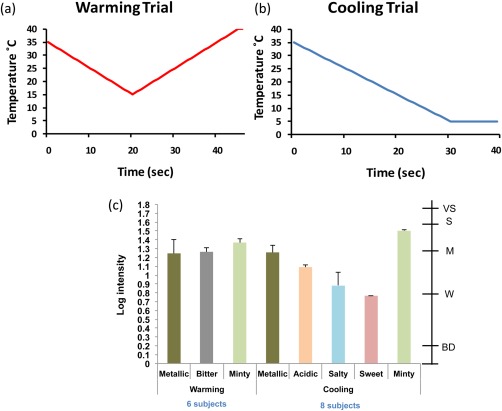
Thermal taster screening protocol. Graphical representation of (a) warming trial: cooling to 15°C before warming to 40°C, (b) cooling trial: cooling to 5°C where temperature is held for 10 s. (c) Taste quality and intensity experienced by thermal tasters to warming (six subjects) and cooling trials (eight subjects). Note: two subjects were both warming and cooling tasters. Secondary scale indicates labels on the gLMS: BD = barely detectable, W = weak, M = moderate, S = strong, VS = very strong. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Oral Responsiveness Assessments
Subjects were invited to another session on a separate day to rate the intensity of suprathreshold taste and temperature (warming and cooling) stimuli using the gLMS and to check for taste dysfunction. Taste dysfunction was classified by the authors as any subject who rated the stimuli barely detectable or below on the gLMS. Taste samples included 0.32 M sucrose (Tate and Lyle, UK), 0.56 M sodium chloride (NaCl) (Sainsbury, UK), 56 mM citric acid (Sigma Aldrich, UK), 1 mM quinine hydrochloride (QHCl) (Sigma Aldrich, UK), 0.32 mM PROP which were all prepared using deionised water and presented to subjects in a random order according to the method and concentrations defined by Lim et al. [2008]. The ATS was used to deliver temperature stimuli as described for thermal taster screening. For taste response assessment, each subject was instructed to rinse their mouth 3 times with deionised water before applying the taste. All stimuli were applied to the tongue by rolling a saturated cotton swab across the tip of the tongue for approximately 3 s. The subjects were instructed to actively taste the stimulus between the tongue and the hard palate using a gentle “smacking” motion and rate the perceived intensity of the taste once it had reached its maximum using the gLMS provided. Separate gLMS were provided for each stimulus. Subjects were presented with their own gLMS reference sheet from the training session and were encouraged to refer to it for guidance on where to rate the intensity of the taste. The four taste stimuli were presented first in a randomised order, PROP was presented last to avoid any cross over effects in PROP sensitive individuals. Subjects were given a 1 min interstimulus‐interval (lSI) and instructed to take longer if needed. During the lSI, subjects cleansed their palate with the deionised water and unsalted crackers (Rakusen's, Leeds, UK) provided. After a 5 min break, the procedure was repeated to collect duplicate ratings of each stimulus.
Samples and Subject's Preference
Three sweet samples of differing CO2 level were prepared for the fMRI scan session: (i) a gustatory (sweet + no CO2) sample “no CO2” and two gustatory‐trigeminal samples, (ii) a sweet + low CO2 “low CO2” and (iii) a sweet + high CO2 “high CO2” sample. Samples were based on a model beverage system following [Clark et al., 2011]. Samples were prepared by dissolving 70 g/L of polydextrose (Litesse® Ultra powder, Danisco, New Century, KS) and 30 g/L of dextrose (MyProtein, Manchester, UK) into still mineral water (Danone, Paris, France) and mixed on a roller bed for 6 h to ensure full dispersion. Samples were refrigerated until they reached 5 ± 1ºC. Polydextrose was added to give “body” whilst not contributing a taste quality (subthreshold), dextrose was added to impart suprathreshold sweetness. Samples to be carbonated were aliquoted into 100 mL Schott bottles (Fisher Scientific, Loughborough, UK) fitted with modified (Medical Engineering Unit, University of Nottingham, UK) Schott bottle caps (Fisher Scientific, Loughborough, UK) to allow a one‐way flow of food grade CO2 (BOC, Guildford, UK) directly into the vessel ensuring accurate carbonation levels. Once disconnected, the samples maintained pressure and therefore CO2 level. The low CO2 samples were carbonated to 1 volume and the high CO2 samples to 2 volumes. One volume equates to 1 litre of CO2 in 1 L of liquid. Two volumes represent a carbonation level similar to most standard beers. Samples were stored at 5 ± 1°C until required.
Immediately prior to the fMRI scan, subjects were familiarised with the three samples and presented with three 40 mL random 3 digit coded samples; no CO2, low CO2 and high CO2, in random order and were asked to evaluate them, using a palate cleanser before each sample (Danone, Paris, France) and place them in order of preference from most to least preferred. The number of subjects who most and least preferred each sample was determined in each group in order to identify any trends in the data. Statistical analysis was not carried out on preference data as the subject numbers were too low for such a behavioural test. After this task, subjects were told that the samples were no, low and high CO2 and that these same samples would be delivered during the fMRI scanning.
fMRI Paradigm Design
The samples were delivered to subjects using 60 mL syringes with Luer lock fittings to prevent loss of CO2 and control the flow of the sample. Thin plastic tubing, (68 cm long, 1.5 mm diameter) ran from the Luer stopcock to an individual subject’s bite bar created from dental putty to ensure consistent tube positioning. All samples and a water wash for cleansing between samples (Danone, Paris, France) were delivered at 6 ± 1°C.
Samples were delivered in a pseudo‐random order across fMRI cycles, with ten cycles of each sample delivered per fMRI scan. Three runs were acquired in each fMRI session, resulting in a total of 30 replicates of each sample for each subject. New samples were provided for each run in order to maintain sample temperature. The previous samples were drained from the tubing prior to new samples being connected. New samples were “washed” through the tubing before the next run commenced to ensure no air bubbles were blocking the flow. In each cycle, 2 mL of sample was manually delivered over a 2 s period (flow rate 1 mL/s). Manual delivery was found to be the most accurate method of delivering carbonated samples, due to the pressurised system and practice sessions prior to scanning showed that 2 mL could be consistently delivered over a 2 s period. The syringes were situated at a lower level than the subject's mouthpiece to ensure no residue sample was delivered to the subject during the ISI. Presentation software was used to deliver instructions to the researcher delivering the samples to ensure correct delivery to the subjects.
Following sample delivery, subjects were cued to swallow by a visual cue (Presentation Software, Neurobehavioral System, San Francisco) and surface electromyography (EMG) was acquired concurrently with the fMRI data acquisition [Eldeghaidy et al., 2011] to determine the exact time of swallow and to determine the duration each sample remained in the mouth. At 4 s after sample delivery, subjects were instructed to press a button to identify the level of carbonation in the sample received: 1 = no CO2, 2 = low CO2 and 3 = high CO2. The responses were collected and analysed to determine the subject's discrimination ability between sample CO2 level during fMRI scanning. At 12 s following the sample cessation, 1 mL still mineral water (Danone, Paris, France) wash was delivered over a 1 s period to clear the oral cavity of any lingering sample. A delay of 7.5 s was allowed before repeating the cycle. Each fMRI scan took ∼ 11 min to complete.
fMRI Data Acquisition
MRI data was acquired on a 3 T Philips Achieva scanner with a 32‐ch receive coil. fMRI data was collected using a double‐echo gradient‐echo, echo‐planar‐imaging (GE‐EPI) acquisition: TE = 25/40 ms, TR = 2,500 ms, flip angle (FA) 85°, 3 mm isotropic spatial resolution, 240 × 240 mm2 field of view (FOV), SENSE factor 2 in the right‐left (RL) direction and 34 slices aligned parallel with AC‐PC plane. Following fMRI acquisition, a T 1‐weighted MPRAGE image (1 mm isotropic resolution; TE/TR = 8.3/3.8 ms, FA = 8°, SENSE factor = 2, 160 slices, 256 × 256 matrix) was collected to aid registration of fMRI data to MNI space.
DATA ANALYSIS
Oral Responsiveness
Intensity ratings of taste and temperature samples were log10 transformed, with 0 ratings adjusted to 0.4 prior to transformation. A Multivariate ANOVA (MANOVA) was performed including all oral attributes as independent variables to enable the overall impact of thermal taster (TT) status on oral responsiveness to be determined. The effect of each individual variable was also determined from the MANOVA (α = 0.05). For those subjects classified as TTs, tastes perceived during screening were also log10 transformed, with 0 ratings adjusted to 0.4 prior to transformation, and the intensity of each taste perceived was averaged across the TT group.
Discrimination of CO2 Level
Discrimination of CO2 level, collected during the fMRI scan, was analysed by calculating the percentage of correctly identified samples for each subject and associated d’ value [Ennis, 1993], a measure of sensitivity representing probability of correct responses for that group. A d’ value above 1 indicates an ability to discriminate [Lawless and Heymann, 2010]. Significant differences (α = 0.05) between groups were evaluated using a student t‐test.
fMRI Data Analysis
fMRI data was processed using SPM5 (Statistical Parametric Mapping, Wellcome Department of Imaging Neuroscience; http://www.fil.ion.ucl.ac.uk/spm). T 2* maps were formed from the multi‐echo data set using a voxel‐by‐voxel, linear, weighted least squares fit and used in the weighted summation of the double‐echo fMRI data [Posse et al., 1999]. The weighted data was slice timing corrected and realigned. Individual realignment parameters were visually inspected to ensure no subject moved by more than one voxel during the fMRI scan. Data were then normalised to the MNI template, and spatially smoothed with 8 mm FWHM.
A first level GLM analysis was performed for each subject to generate contrasts for each sample (no CO2, low CO2 and high CO2), using the time each sample remained in the mouth calculated from the EMG trace convolved with a canonical hemodynamic response function (HRF), and temporally filtered with a 135 s high pass filter cut‐off. The water wash, button press and motion parameters were included as covariates of no interest. To identify areas of the brain which correlated with carbonation level, a linear (first order) parametric modulation with CO2 level was performed, and both positive and negative modulations were assessed.
Second level random effects (RFX) group analysis was then performed to determine brain areas active to each sample (no CO2, low CO2 and high CO2) for both the TT and TnT group, with maps threshold at a false discovery rate (FDR) corrected probability of P < 0.05. To assess the difference in brain activation between TTs and TnTs for each sample, a two‐sample t‐test was performed for each CO2 sample using a binary mask of “all” samples (P < 0.05 uncorrected) and assessed at a threshold level P < 0.005 uncorrected, k > 20 [Lieberman and Cunningham, 2009]. A second level RFX analysis of those areas displaying a linear parametric modulation with CO2 level was performed for both the TT and TnT group at a threshold level P < 0.005 uncorrected, k > 20. Finally, to determine whether subjective preference to the CO2 level of the sample could account for differences in taste activation, we performed a second level RFX analysis of CO2 level, and compared the inclusion/exclusion of subjective preference rating as a covariate of no interest to the response to CO2 level.
A region of interest (ROI) analysis based on a priori areas was performed on right and left hemispheres for each individual subject's first level maps. The insula was subdivided into anterior (40, 10, −2) and posterior (44, −32, 12) parts, defined as an 8 mm sphere centred at the peak active voxel, as reported in [Eldeghaidy et al. 2011]. Thalamus, amygdala and SII (BA 43) were anatomically defined by the PickAtlas, and SI as an 8 mm sphere centred at (60, −6, 20). In addition, lateral (26, 32, −10) and medial OFC (−6, 44, −2) ROIs were defined as reported by de Araujo and Rolls [2004], and dorsolateral prefrontal cortex (DLPFC; 44, 32, 12) as reported by [Kringelbach et al., 2004]. The ROIs contained a large number of voxels (>250) which encompassed all activated areas of interest, allowing for variability in the location of the activation peak within cortical regions across all subjects. The ROI analysis was performed for (1) TT and TnT contrast maps at each CO2 level and (2) the linear parametric modulation of CO2 maps. For each sample, the mean of the top 5% parameter estimate (β‐value) was calculated for each ROI [Fernandez et al., 2003; Mitsis et al., 2008]. Since all ROIs were first defined to comprise a large number of voxels, this analysis approach ensured the assessment of the activity in each functional area with a high signal‐to‐noise ratio, while accounting for any between‐subject functional variability (e.g., arising due to differences in cortical folding patterns).
A two‐factor ANOVA (group and sample) to assess any significant differences (P < 0.05) at a global level was performed. Tukey's HSD post hoc multiple comparison tests determined significant differences between groups for each sample and ROI. For the parametric modulations, significant differences between groups were calculated using Student t‐test.
RESULTS
Screening
No subjects were excluded due to taste dysfunction. Of the 52 subjects screened, 12 were classified as TTs (23%) and 40 (77%) as TnTs. Twenty four subjects were invited to take part in the fMRI scanning, twelve thermal tasters (TT) (8 females, 4 males, 30 ± 7 years) and 12 thermal non‐tasters (TnT; 7 females, 5 males, 32 ± 5 years). Both TT and TnT groups were matched for PROP taster status (4 PROP non‐tasters (pNTs), 6 PROP medium‐tasters (pMTs) 2 PROP super‐tasters (pSTs) in each group). The 12 TnTs were randomly selected from the group of 24, taking into account availability, for each PROP taster status sub group. During thermal stimulation of the tongue, the intensity of tastes reported by each thermal taster during each replicate was between weak and strong on the gLMS, with an average intensity rating across all tastes and subjects just below moderate. TTs reported perceiving tastes during warming trials, cooling trials or both, with bitter reported as the taste most often perceived during warming trials and metallic most commonly perceived during cooling trials Figure 1c.
Oral Responsiveness
The MANOVA revealed a significant effect of TT group according to the Wilks’ Lambda test (P = 0.041), with TTs rating oral responsiveness significantly more intense than TnTs. Although this trend was observed across attributes (except Quinine and Cooling) (Fig. 2a), it only approached significance for sucrose (P = 0.054) and for warming (P = 0.056; Table 2), thus these latter two responses drove the overall significance observed.
Figure 2.
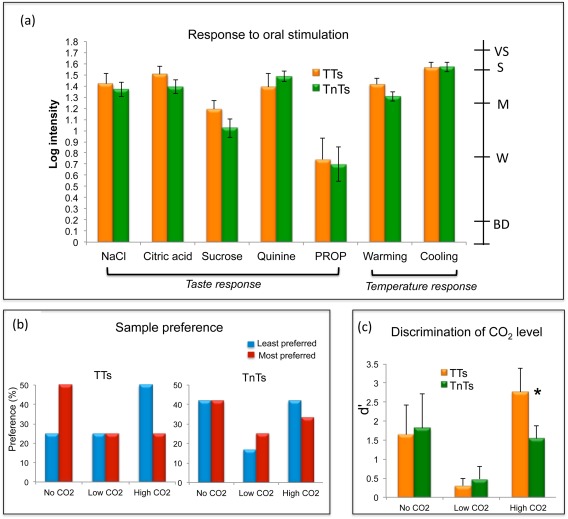
Behavioural response: (a) Intensity of oral responses perceived by the thermal taster (TT) and thermal non‐taster (TnT) group. Secondary scale indicates labels on the gLMS: BD = barely detectable, W = weak, M = moderate, S = strong, VS = very strong. (b) Percentage of subject's preference by each group. (c) The discrimination ability of subjects to correctly identify the CO2 level delivered during the fMRI scan session, with the d’ value of the TT and TnT group provided. Asterisks indicate a significant difference between groups at P < 0.05. Error bars show standard error. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 2.
Mean value of oral responsiveness to individual attributes for thermal tasters (TTs) and thermal‐non tasters (TnTs), F‐values and associated P‐values from the MANOVAa are provided
| Stimulus | Group | Mean | F‐value | P‐value | |
|---|---|---|---|---|---|
| Taste | NaCl | TnTs | 1.369 | 0.35 | 0.558 |
| TTs | 1.420 | ||||
| CitricAcid | TnTs | 1.392 | 1.90 | 0.175 | |
| TTs | 1.504 | ||||
| Sucrose | TnTs | 1.023 | 3.91 | 0.054b | |
| TTs | 1.193 | ||||
| Quinine | TnTs | 1.487 | 0.93 | 0.340 | |
| TTs | 1.387 | ||||
| PROP | TnTs | 0.698 | 0.59 | 0.810 | |
| TTs | 0.741 | ||||
| Temperature | Warming | TnTs | 1.305 | 3.84 | 0.056b |
| TTs | 1.416 | ||||
| Cooling | TnTs | 1.569 | 0.01 | 0.935 | |
| TTs | 1.565 |
Wilks’ Lambda test indicated a significant group effect for overall responsiveness (P = 0.041).
Approaching significance (P = 0.05).
Sample Preference
Figure 2b shows the preference of each CO2 sample in percentage values for TTs and TnTs respectively. There is a clear difference in the pattern of response between the TT and TnT group. The TT group most preferred the no CO2 sample and least preferred the high CO2 sample. In contrast, TnTs did not show a clear preference for any sample. The “no CO2” sample was both most preferred and least preferred by the same number of subjects in the TnT group. For both the TT and TnT group a Spearman rank correlation was performed between the rank of CO2 level and preference. For both groups a non‐significant correlation coefficient was found (TT: ρ = −0.25, P = 0.14; TnT: ρ =−0.042, P = 0.81).
Discrimination of CO2 Level
During the fMRI scan session, both the TT and TnT groups had a good level of discrimination ability when the sample was un‐carbonated “no CO2.” This discrimination ability was reduced for the “low CO2” sample and was similar between TT and TnT groups. However, there was a significant difference between groups for the discrimination of the high CO2 sample. TTs could correctly identify the high CO2 sample significantly more than the TnTs (P < 0.05), Figure 2c. It should be noted that the high CO2 sample was least preferred by the TT group, Figure 2b.
fMRI Results
The activation maps for TT and TnT revealed brain areas activated in response to each sample including primary taste areas (anterior insula and frontal operculum), oral somatosensory areas (mid and posterior insula, somatosensory cortices (SI and SII) and rolandic operculum), reward areas (including ACC and amygdala), dorsolateral prefrontal cortex (DLPFC) and the thalamus.
The parameter estimates (β‐values) in each ROI were first assessed for each sample in right and left hemispheres. A trend of higher activation in the left hemisphere for both TT and TnT was observed, with a significant increase in left thalamus for “no CO2” and “high CO2” samples in TT, whereas the left anterior insula was significantly higher in TnT for the “high CO2” sample. We then assessed each ROI combined across hemispheres for both groups, Figure 3a. A two‐factor ANOVA (group and sample) across all ROI's revealed a significant main effect at a global level for group (P < 0.05) of higher cortical activation across all ROI's in TTs, but not for sample (P > 0.05). Analysis across each ROI revealed significantly higher activation in the secondary somatosensory cortex (SII) for TTs compared to TnTs (P < 0.05), with a trend for higher activation in the posterior insula for TTs (P = 0.067). The activation maps for a two‐sample t‐test between TT and TnT groups for the no CO2 sample revealed significantly greater BOLD response for the TT group in SII, DLPFC and ACC, as shown in the differential activation maps in Figure 3b. Table 3 gives a summary for those brain areas. When assessing the effect of sample across both groups, a trend of higher activation to the high CO2 sample compared to the no CO2 sample was found in the ACC (P = 0.068).
Figure 3.
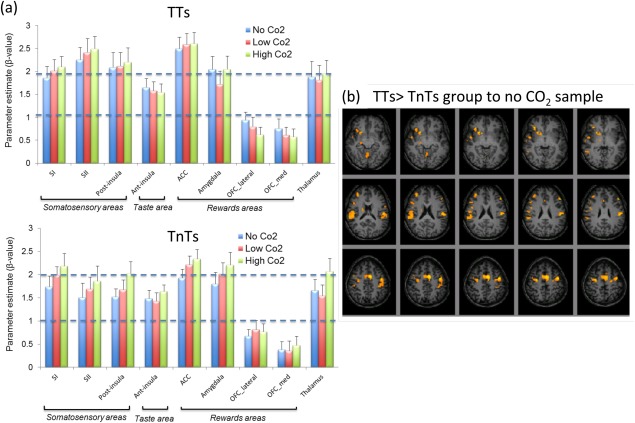
(a) Parameter estimate (β‐value) for TT and TnT groups highlighting the response in a priori cortical areas (error bars indicate the standard error). (b) Random effects group analysis map showing contrast of (TT > TnT group) to the no CO2 sample (sweet taste alone). Maps overlaid on T 1‐weighted images, assessed at threshold P < 0.005. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 3.
Brain areas showing higher response to thermal tasters (TTs) compared with thermal non‐tasters (TnTs) for gustatory sample “no CO2”
| Area | Side | MNIa | Z‐score | P‐value | Cluster sizeb, k |
|---|---|---|---|---|---|
| ACC* | L | −6, 2, 48 | 3.20 | 0.001 | 202 |
| Rolanic operculum* | L | −44, −4, 52 | 3.32 | <0.001 | 106 |
| Secondary somatosensory cortex (SII)* | L | −54, −30, 32 | 2.99 | 0.001 | 132 |
| Precentral gyrus* | R | 30, −8, 56 | 2.93 | 0.002 | 59 |
| Middle frontal gyrus/DLPFC | R | 36, 42, 24 | 2.73 | 0.003 | 22 |
R, right hemisphere; L, left hemisphere; MNI, Montreal Neurological Institute.
Peak voxel coordinates given in MNI space (x,y,z).
Reported clusters threshold at P < 0.005, uncorrected for multiple comparisons, with a cluster extent threshold k > 20 voxels, and “sub” indicates sub‐cluster level. Asterisks indicate areas activated with FDR corrected P < 0.05.
Group ROI analysis on the CO2 level parametric modulation β‐values showed a positive modulation of cortical activation with CO2 level in all brain areas in the TnT group including somatosensory, taste and reward areas and a negative correlation in the DLPFC. Combining data across hemispheres in the TT group, a significant negative modulation of cortical activation with CO2 level was found in the anterior insula, DLPFC, lateral and medial OFC and a trend in the ACC. A positive modulation in the SI, SII and a trend in the posterior insula (Fig. 4a) was also found. A significant difference in the linear parametric modulation with CO2 level was found between TT and TnT groups in the anterior insula, the DLPFC and the ACC (P < 0.05), with a trend in the lateral OFC (P = 0.069). Of note, the DLPFC showed an increase in the left hemisphere compared to the right hemisphere for both TTs and TnTs, whereas the response in left SII was significantly higher than right in TnTs.
Figure 4.
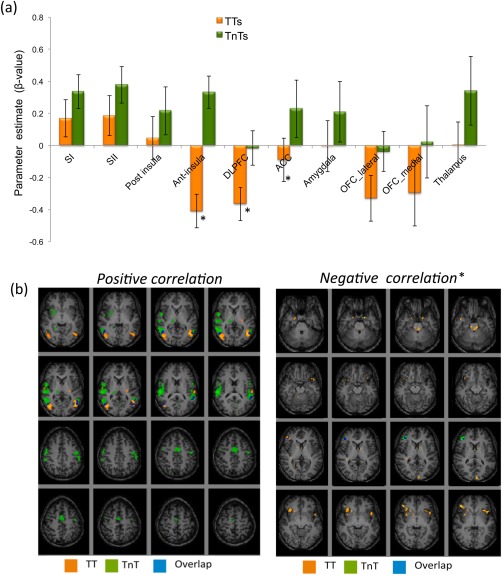
(a) Positive and negative parameter estimate (β‐value) for TT and TnT groups in a priori cortical areas (error bars indicate the standard error). Asterisks indicate a significant difference between groups at P < 0.05. (b) Cortical areas showing a positive and negative correlation with CO2 level. Maps displayed with P < 0.005, * P < 0.05 uncorrected. In each figure the TT group is shown in orange, the TnT group in green, and the overlap of these groups in blue. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Activation maps for the positive modulation of cortical activation with CO2 level are shown for the TT and TnT groups in Figure 4b, and Table 4. The RFX maps of the negative modulation with the CO2 level was found in the DLPFC [(46, 34, 14), z = 3.04, P = 0.001] in the TnT group maps, whereas the TT showed a negative modulation with the CO2 level in left anterior insula; [(−34, 24, 0), z= 2.63, P = 0.004] and left amygdala [(−18, 2, −26), z= 2.62, P = 0.004], as shown in Figure 4b.
Table 4.
Brain areas showing positive modulation in BOLD amplitude with CO2 level in thermal tasters (TTs) and thermal non tasters (TnTs)
| Area | Side | MNIa | Z‐score | P‐value | Cluster sizeb, k |
|---|---|---|---|---|---|
| Thermal taster (TT) | |||||
| SII | |||||
| L | −62, −28,16 | 5.41 | <0.001 | 51 | |
| R | 64, −14, 8 | 3.34 | 0.003 | 26 | |
| Thermal non‐ taster (TnT) | |||||
| Primary Somatosensory Cortex (SI)* | R |
62, −12, 42 54, −16, 22 58, −20, 26 |
3.59 3.25 3.23 |
<0.001 <0.001 0.001 |
797 |
| L | −52, −14, 46 | 3.52 | <0.001 | sub | |
| Secondary somatosensory cortex (SII)* | R |
62, −26, 18 68, −28, 8 |
4.55 3.89 |
<0.001 <0.001 |
sub |
| Rolandic operculum* | R | 56, 0, 6 | 4.55 | <0.001 | sub |
| L | −56, 2, 14 | 3.54 | <0.0001 | 44 | |
| ACC* | R | 2, 0,50 | 3.31 | <0.0001 | 312 |
| L | −6, −10, 48 | 3.34 | <0.0001 | sub | |
| Precentral gyrus* | R | 60, −2, 42 | 3.58 | <0.0001 | sub |
R, right hemisphere; L, left hemisphere; MNI, Montreal Neurological Institute.
Peak voxel coordinates given in MNI space (x,y,z).
Reported clusters threshold at P < 0.005, uncorrected for multiple comparisons, with a cluster extent threshold k > 20 voxels, and “sub” indicates sub‐cluster level, Asterisks indicate areas activated with FDR corrected P < 0.05.
We assessed whether differences in preference rating could explain the observed differences in taste activation to CO2 level, but found no difference in the statistically thresholded activation maps when including preference rating as a covariate of no interest compared to when preference was not included as a covariate. Thus we conclude that the observed differences in taste activation patterns are related to CO2 level alone and not preference.
DISCUSSION
In this study, thermal tasters perceived a phantom taste during thermal stimulation which was of a similar intensity to the oral response to taste samples themselves, Figures 1c and 2a. Basic tastes reported during thermal stimulation were bitter, sweet, salty and sour/acidic. The “other” category was selected by six subjects who self‐reported metallic or minty tastes. Metallic is purported to have a taste component as well as trigeminal and aroma elements for some divalent salts [Epke et al., 2009; Lawless et al., 2005; Lim and Lawless, 2005]. We concluded that minty sensation was important as it may result from the subject experiencing a phantom sweet taste, shown in literature to be an important component of mintiness, [Davidson et al., 1999], in conjunction with the trigeminal temperature stimulation. This may explain why none of the TTs in this study reported sweetness during the warming trial when other studies have reported sweetness on warming [Cruz and Green 2000; Yang et al., 2014]. The incidence of tastes reported by thermal tasters has been reported in one other study [Yang et al., 2014] and the most frequent taste reported was metallic. It would be interesting to compare the cortical response in TTs who report basic tastes with those who report other taste sensations in order to understand this further. The ability of thermal stimulation to elicit such a clear taste response in thermal tasters is intriguing. Furthermore, behaviourally, TTs perceived the intensity of oral response (taste and temperature) higher than TnTs (Fig. 2a). This suggests that TTs could have a perceptual advantage for some gustatory and trigeminal stimuli when presented in isolation, as previously reported [Bajec and Pickering, 2008; Cruz and Green, 2000]. During the fMRI scan session, TTs were significantly more able to discriminate the high CO2 sample compared to TnTs, and, the high CO2 sample was clearly the least preferred sample for TTs, supporting a perceptual advantage in this group, Figure 2b,c. Perceptually increasing levels of CO2 may have reduced perceived sweetness due to cross‐modal interactions [Clark et al., 2011] which could account for the preference trend towards the no CO2 sample as it may have been perceived sweeter by TTs.
Taste and somatosensory stimuli are usually simultaneously present during food intake. Function convergence between these two modalities has been documented [Cerf‐Ducastel et al., 2001; Guest et al., 2007; Rudenga et al., 2010]. Cerf‐Ducastel et al. [2001] showed an overlap in taste and lingual somatosensory representation in the insula, rolandic, frontal and temporal operculum, with superior and inferior parts of the insula being more discriminating to gustatory only samples (sweet, salt, sour and umami) compared with somato‐gustatory samples (pungent sour and astringent sweet). In this study, the samples developed for use in the fMRI protocol were designed to elicit a gustatory‐trigeminal response (sweet + low CO2; sweet + high CO2). Cortical activation to the sweet + no CO2 sample was significantly higher in the TT group compared to the TnT group in, oral somatosensory (SII, rolandic operculum) and reward areas (ACC), in addition to the DLPFC, an area linked to cognitive evaluation processes, such as evaluation of rewarding taste stimulation [Kringelbach et al., 2004], suggesting that the increase in intensity perception measured behaviourally by the TT group is a result of elevated cortical activation across areas associated with taste perception. This may be due to an elevated perception of sweetness intensity or a modified oral perception due to the sample delivery temperature in TTs compared to TnTs.
When the trigeminal component (CO2 level) of the stimulus increased, the pattern of cortical activation between TT and TnT groups was significantly different. A significant increase in cortical response with increasing CO2 level (P < 0.05, Fig. 4) was seen across all a priori ROIs (taste, somatosensory and reward areas) in the TnT group. In contrast, only the somatosensory areas (SI, SII) showed a significant positive modulation (P < 0.05) with increasing CO2 level in the TT group. Interestingly, TTs showed a significant negative parametric modulation (P < 0.05) with CO2 level in primary taste (anterior insula) and reward (ACC) areas, in addition to a negative modulation for the DLPFC in both TT and TnT groups. Previous studies have reported DLFPC activation to food‐related studies [Small et al., 2001; Tataranni et al., 1999], and Kringelbach et al. [2004] showed DLFPC activation to unimodal taste and multimodal flavour stimuli in the human brain.
These results suggest that samples containing both gustatory and trigeminal stimulus input are processed differently by the TT and TnT groups. The significantly higher cortical response of TTs to the sweet + no CO2 sample compared with TnTs, and the limited change in activation in SI and SII with the addition of a trigeminal CO2 component (sweet + low/high CO2 samples), as well as behaviourally the higher intensity perception in TTs, supports the hypothesis that the gustatory and trigeminal nerves are intertwined at the periphery in TTs. These results support previous findings from Essick et al. [2003] that tactile and taste sensitivities covary. The close proximity of gustatory (chorda tympani nerve) and somatosensory (lingual nerve) afferents, particularly at the tongue tip, and small receptive fields at that location, supports coupling between the taste and somatosensory sensations [Whitehead et al., 1985]. This hypothesis supports the fact that thermal tasters can experience a phantom taste from temperature activating the gustatory nerve during thermal stimulation, as revealed behaviourally. Here, when gustatory and trigeminal stimuli are presented together (low and high CO2 level), activation in TTs remains unaltered, likely as both nerves are already highly stimulated, whilst in TnTs increased cortical activation results from the additional stimulation of the trigeminal nerve.
We hypothesise that the increase in cortical activation across taste and somatosensory ROIs for the TnT group in response to CO2 level, results in an increased intensity perception to CO2 level. However, for TTs cortical activation in taste (anterior insula), DLPFC and reward (lateral and medial OFC, ACC) areas is negatively modulated with CO2, suggesting that the sensory advantage of increased intensity perception of simple tastants by TT might be lost when another modality is added, with TTs further rating the high CO2 sample as least preferred. This could be due to a decrease in sweetness perception with increasing CO2 as found by others [Clark et al., 2011; Hewson et al., 2009] which is impacting the cortical activation patterns differently in each group. We hypothesise that this is due to cross‐wiring between gustatory and trigeminal receptors in TTs.
The differences in cortical response observed between TTs and TnTs contributes to understanding concerning differences in perception between these two groups. Such differences may impact food choice behaviour and the differences in response to carbonation here could impact on beverage choice and hence could impact on product design considerations in the beverage industry. Current research into the difference between TT and TnT groups for food and drink preferences is very limited. Liking of beer [Pickering et al., 2010a] and wine [Pickering et al., 2010b] was not found to be significantly different between groups. It is possible that the preference for uncarbonated samples in TTs found here might result in reduced preference for highly carbonated beers and other soft drinks, however, a fully controlled study with more complex beverage systems and a larger sample size is needed to confirm this. Differences between thermal taster groups have been found for food liking. TTs were found to like soft foods significantly less than TnTs, potentially indicating a difference between groups in their oral tactile sensitivity [Bajec and Pickering, 2010]. Analogous to our findings of thermal taster status, studies of lexical‐gustatory synaesthesia [Jones et al., 2011]—individuals who experience an automatic and highly consistent taste to spoken and written language—have demonstrated increased anterior insula activation related to viewing words that elicited tastes, and it has been shown that genes play a role in such a synaesthesia [Brang and Ramachandran, 2011; Simner and Ward, 2006]. Here, we show that TTs have different activation patterns compared with TnTs, and it is possible that genotype may also play a role here. Further research now needs to be conducted to understand the mechanism of thermal taster status and this cross‐modal gustatory and trigeminal interaction.
CONCLUSION
Few investigations of thermal taster status have been published. This work presents the first study to address changes in the cortical response in thermal tasters. We investigate the difference in cortical activation to trigeminal‐gustatory stimuli between thermal and non‐thermal taster groups. Behaviourally, thermal tasters respond to taste and temperature stimuli more intensely than TnTs. This is supported by this fMRI data which shows heightened cortical activation in taste, somatosensory and reward areas to gustatory stimuli in TTs compared to TnTs, and that the addition of a trigeminal CO2 component to stimuli leads to a limited change in cortical response in these areas in TTs. Evidence from this study supports a cross‐modal integration mechanism with interaction of stimulation to taste and trigeminal nerves in thermal tasters.
REFERENCES
- Bajec MR, Pickering GJ (2008): Thermal taste, PROP responsiveness, and perception of oral sensations. Physiol Behav 95:581–590. [DOI] [PubMed] [Google Scholar]
- Bajec MR, Pickering GJ (2010): Association of thermal taste and PROP responsiveness with food liking, neophobia, body mass index, and waist circumference. Food Qual Prefer 21:589–601. [Google Scholar]
- Bartoshuk LM, Duffy VB, Fast K, Green BG, Prutkin J, Snyder DJ (2002): Labeled scales (e.g., category, Likert, VAS) and invalid across‐group comparisons: What we have learned from genetic variation in taste. Food Qual Prefer 14:125–138. [Google Scholar]
- Brang D, Ramachandran V (2011): Survival of the Synesthesia Gene: Why Do People Hear Colors and Taste Words. PLoS Biology 9:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerf‐Ducastel B, Van de Moortele P, MacLeod P, Le Bihan D, Faurion A (2001): Interaction of gustatory and lingual somatosensory perceptions at the cortical level in the human: A functional magnetic resonance imaging study. Chem Senses 26:371–383. [DOI] [PubMed] [Google Scholar]
- Clark RA, Hewson L, Bealin‐Kelly F, Hort J (2011): The interactions of CO2, ethanol, hop acids and sweetener on flavour perception in a model beer. Chemosens Percept 4:42–54. [Google Scholar]
- Cruz A, Green BG (2000): Thermal stimulation of taste. Nature 403:889–892. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Rolls ET (2004): Representation in the human brain of food texture and oral fat. J. Neurosci 24:3086–3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JM Davidson, TA Hollowood, RST Linforth, AJ Taylor (1999): The effect of sucrose on the perceived flavour intensity of chewing gum. J. Agric. Food Chem 47:4336–4340. [DOI] [PubMed] [Google Scholar]
- Eldeghaidy S, Marciani L, McGlone F, Hollowood T, Hort J, Head K, Taylor AJ, Busch J, Spiller RC, Gowland PA, ST Francis (2011): The cortical response to the oral perception of fat emulsions and the effect of taster status. J Neurophysiol 105:2572–2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis DM (1993): The power of sensory discrimination methods. J Sens Stud 8:353–370. [Google Scholar]
- Epke E, McClure S, Lawless H (2009): Effects of nasal occlusion and oral contact on perception of metallic taste from metal salts. Food Qual Prefer 20:133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essick GK, Chopra A, Guest S, McGlone F (2003): Lingual tactile acuity, taste perception, and the density and diameter of fungiform papillae in female subjects. Physiol Behav 80:289–302. [DOI] [PubMed] [Google Scholar]
- Fernandez G, Weis S, Stoffel‐Wagner B, Tendolkar I, Reuber M, Beyenburg S, Klaver P, Fell J, de Greiff A, Ruhlmann J and others. (2003): Menstrual cycle‐dependent neural plasticity in the adult human brain is hormone, task, and region specific. J Neurosci 23:3790–3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis S, Rolls ET, Bowtell R, McGlone F, O'Doherty J, Browning A, Clare S, Smith E (1999): The representation of pleasant touch in the brain and its relationship with taste and olfactory areas. Neuroreport 10:453–459. [DOI] [PubMed] [Google Scholar]
- Green B, George P (2004): Thermal taste' predicts higher responsiveness to chemical taste and flavor. Chem Senses 29:617–628. [DOI] [PubMed] [Google Scholar]
- Green BG, Dalton P, Cowart B, Shaffer G, Rankin K, Higgins J (1996): Evaluating the 'Labeled Magnitude Scale' for measuring sensations of taste and smell. Chem Senses 21:323–334. [DOI] [PubMed] [Google Scholar]
- Green BG, Alvarez‐Reeves M, George P, Akirav C (2005): Chemesthesis and taste: Evidence of independent processing of sensation intensity. Physiol Behav 86:526–537. [DOI] [PubMed] [Google Scholar]
- Guest S, Grabenhorst F, Essick G, Chen Y, Young M, McGlone F, de Araujo I, Rolls E (2007): Human cortical representation of oral temperature. Physiol Behav 92:975–984. [DOI] [PubMed] [Google Scholar]
- Hayes J, Keast S (2011): Two decades of supertasting: Where do we stand? Physiol Behav 104:1072–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewson L, Hollowood T, Chandra S, Hort J (2009): Gustatory, Olfactory and Trigeminal Interactions in a Model Carbonated Beverage. Chem Percept 2:94–107. [Google Scholar]
- C Jones, M Gray, L Minati, J Simner, HD Critchley, J Ward (2011): The neural basis of illusory gustatory sensations: Two rare cases of lexicalgustatory synaesthesia. J Neuropsychol 5:243–254. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, de Araujo IET, Rolls ET (2004): Taste‐related activity in the human dorsolateral prefrontal cortex. Neuroimage 21:781–788. [DOI] [PubMed] [Google Scholar]
- Lawless H, Stevens D, Chapman K, Kurtz A (2005): Metallic Taste from Electrical and Chemical Stimulation. Chem Senses 30:185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawless HT, Heymann H. 2010. Sensory Evaluation of Food. New York: Springer. [Google Scholar]
- Lieberman M, Cunningham W (2009): Type I and Type II error concerns in fMRI research: Re‐balancing the scale. Scan 4:423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Lawless H (2005): Oral sensations from iron and copper sulfate. Physiol Behav 85:308–313. [DOI] [PubMed] [Google Scholar]
- J Lim, L Urban, BG Green (2008): Measures of Individual Differences in Taste and Creaminess Perception. Chem Senses 33:493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsis G, Iannetti G, Smart T, Tracey I, Wise R (2008): Regions of interest analysis in pharmacological fMRI: How do the definition criteria influence the inferred result? NeuroImage 40:121–132. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F (2001): Representation of pleasant and aversive taste in the human brain. J. Neurophysiol 85:1315–1321. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP, Dayan P, Friston K, Critchley H, Dolan RJ (2003): Temporal difference models and reward‐related learning in the human brain. Neuron 38:329–337. [DOI] [PubMed] [Google Scholar]
- Pickering GJ, Bartolini JA, Bajec MR (2010a): Perception of beer flavour associates with thermal taster status. J Inst Brew 116:239–244. [Google Scholar]
- Pickering GJ, Moyes A, Bajec MR, Decourville N (2010b): Thermal taster status associates with oral sensations elicited by wine. Aust J Grape Wine Res 16:361–367. [Google Scholar]
- Posse S, Wiese S, Gembris D, Mathiak K, Kessler C, Grosse‐Ruyken ML, Elghahwagi B, Richards T, Dager SR, Kiselev VG (1999): Enhancement of BOLD‐contrast sensitivity by single‐shot multi‐ echo functional MR imaging. Magn Reson Med 42:87–97. [DOI] [PubMed] [Google Scholar]
- Rudenga K, Green B, Nachtigal D, Small D (2010): Evidence for an intgrated oral sensory module in the human ventral insula. Chem Senses 35:693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbake M, Hutchinson I, Laing DG, Jinks AL (2005): Rapid quantitative assessment of fungiforrn papillae density in the human tongue. Brain Res 1052:196–201. [DOI] [PubMed] [Google Scholar]
- J Simner, J Ward (2006): Synaesthesia: the taste of words on the tip of the tongue. Nature 444:438. [DOI] [PubMed] [Google Scholar]
- Small DM, Jones‐Gotman M, Zatorre RJ, Petrides M, Evans AC (1997): Flavor processing: More than the sum of its parts. Neuroreport 8:3913–3917. [DOI] [PubMed] [Google Scholar]
- Small DM, Zald DH, Jones‐Gotman M, Zatorre RJ, Pardo JV, Frey S, Petrides M (1999): Human cortical gustatory areas: A review of functional neuroimaging data. Neuroreport 10:7–13. [DOI] [PubMed] [Google Scholar]
- D Small, R Zatorre, A Dagher, A Evans, M Jones‐Gotman (2001): Changes in brain activity related to eating chocolate from pleasure to aversion. Brain 124:1720–1733. [DOI] [PubMed] [Google Scholar]
- Small DM, Gregory MD, Mak YE, Gitelman D, Mesulam MM, Parrish T (2003): Dissociation of neural representation of intensity and affective valuation in human gustation. Neuron 39:701–711. [DOI] [PubMed] [Google Scholar]
- Talavera K, Yasumatsu K, Yoshida R, Margolskee RF, Voets T, Ninomiya Y, Nilius B (2008): The taste transduction channel TRPM5 is a locus for bitter‐sweet taste interactions. FASEB J 22:1343–1355. [DOI] [PubMed] [Google Scholar]
- P Tataranni, J Gautier, K Chen, A Uecker, D Bandy, A Salbe, P RE, M Lawson, E Reiman, R E (1999): Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc. Natl Acad Sci 96:4569–4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuizen MG, Albrecht J, Zelano C, Boesveldt S, Breslin P, Lundström JN (2011): Identification of human gustatory cortex by activation likelihood estimation. Hum Brain Mapp 32:2256–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead M, Beeman C, Kinsella B (1985): Distribution of taste and general sensory nerve endings in fungiform papillae of the hamster. Am J Anat 173:185–201. [DOI] [PubMed] [Google Scholar]
- Yang Q, Hollowood T, Hort J (2014): Phenotypic variation in oronasal perception and the relative effects of PROP and Thermal Taster Status. Food Qual Prefer 38:83–91. [Google Scholar]


