Abstract
The critical roles of frontostriatal circuits had been revealed in addiction. With regard to young smokers, the implication of frontostriatal circuits resting‐state functional connectivity (RSFC) in smoking behaviors and cognitive control deficits remains unclear. In this study, the volume of striatum subsets, i.e., caudate, putamen, and nucleus accumbens, and corresponding RSFC differences were investigated between young smokers (n 1 = 60) and nonsmokers (n 2 = 60), which were then correlated with cigarette smoking measures, such as pack_years‐cumulative effect of smoking, Fagerström Test for Nicotine Dependence (FTND)‐severity of nicotine addiction, Questionnaire on Smoking Urges (QSU)‐craving state, and Stroop task performances. Additionally, mediation analysis was carried out to test whether the frontostriatal RSFC mediates the relationship between striatum morphometry and cognitive control behaviors in young smokers when applicable. We revealed increased volume of right caudate and reduced RSFC between caudate and dorsolateral prefrontal cortex (DLPFC), orbitofrontal cortex in young smokers. Significant positive correlation between right caudate volume and QSU as well as negative correlation between anterior cingulate cortex‐right caudate RSFC and FTND were detected in young smokers. More importantly, DLPFC‐caudate RSFC strength mediated the relationship between caudate volume and incongruent errors during Stroop task in young smokers. Our results demonstrated that young smokers showed abnormal interactions within frontostriatal circuits, which were associated with smoking behaviors and cognitive control impairments. It is hoped that our study focusing on frontostriatal circuits could provide new insights into the neural correlates and potential novel therapeutic targets for treatment of young smokers. Hum Brain Mapp 37:2013–2026, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: young smokers, striatum, frontostriatal circuits, cognitive control, resting‐state functional connectivity
INTRODUCTION
According to the latest national survey of youth smoking by the Chinese Center for Disease Control and Prevention (announced in May, 2014), the smoking rate of junior high school students was 10.6% for males and 1.8% for females in China (http://www.chinacdc.cn/). In the United States of America, more than 80% of adult smokers begin smoking by 18 years of age, who are more likely to become life‐long smokers and are more susceptible to nicotine addiction than adults [White et al., 2009]. Animal study had demonstrated that nicotine use during adolescence caused long‐term disturbances in cognitive functioning and persistent behavioral changes that might contribute to subsequent psychopathologies [Counotte et al., 2009]. Besides, smoking‐related diseases, such as chronic obstructive pulmonary diseases [Caramori et al., 2015] and cardiovascular diseases [Japuntich et al., 2015], are primary cause of preventable early deaths [Bauer et al., 2014]. Therefore, it is of great significance to investigate the underlying neural mechanisms of young smokers.
Previous substance use disorder (SUD) studies had revealed the critical roles of frontostriatal circuits, which were mainly associated with reward (striatum) and cognitive control (prefrontal cortex) [Feil et al., 2010; Kober et al., 2010; Jin et al., 2015; Ma et al., 2010; Motzkin et al., 2014; Tomasi and Volkow, 2013; Yuan et al., 2015aa, 2015bb]. For smokers, modular dysfunction and structural deficits within frontostriatal circuits had also been detected [Jasinska et al., 2014; Li et al., 2015]. The striatum mainly regulated the rewarding effect of smoking [Barrett et al., 2004] and motivation to smoke [Le Foll et al., 2014]. The increased volume of striatum [Li et al., 2015] and the correlation with craving had been observed in young smokers [Das et al., 2012; Janes et al., 2015]. The prefrontal cortex (PFC) played important roles in cognitive control deficits in smokers [Feil et al., 2010; Feng et al., 2015; Yin et al., 2015]. The abnormalities in frontal brain regions had been detected in smokers, such as reduced gray matter and dysfunction of orbitofrontal cortex (OFC), anterior cingulate cortex (ACC), and dorsolateral prefrontal cortex (DLPFC) [Feil et al., 2010; Yu et al., 2011, 2013; Zhao et al., 2012]. It is worth to note that the striatum and the PFC are intermodulated via frontostriatal circuits modulated by dopamine (DA) [Volkow et al., 2011]. Striatum DA system dysfunction had been detected in smokers, such as decreased striatal DA transporter [Newberg et al., 2007] and increased DA activity [Salokangas et al., 2000]. The interactions between reward and cognitive control are especially important to investigate the underlying neural mechanisms of smoking [Kober et al., 2010]. Resting‐state functional connectivity (RSFC), which permits in vivo measurement of the degree of correlated activity (i.e., the strength of interaction) between macroscopic brain regions, offers a unique opportunity to examine these interactions in addiction [Bi et al., 2016; Feng et al., 2015; Ma et al., 2010; Yuan et al., 2010, 2015aa, 2015bb]. However, less is known about the frontostriatal circuits RSFC differences between young smokers and nonsmokers and how these differences relate to smoking behaviors (e.g., pack_years, craving).
We had reported the increased caudate volume in young smokers [Li et al., 2015]. Striatum (i.e., caudate) activation during cognitive control task (e.g., Stroop task) had also been detected [Kaufmann et al., 2005; Liu et al., 2004], which suggested a possible connection between striatum volume and cognitive control impairments in young smokers. Additionally, the increased caudate volume was associated with cognitive control deficits in previous addiction study (i.e., internet gaming disorder) [Cai et al., 2015]. However, these connections between striatum morphormetry and cognitive control in young smokers remain unknown.
Furthermore, previous SUD findings demonstrated that the impaired DA function in striatum (decreases in D2R, reduced DA release) were correlated with reduced baseline glucose metabolism in PFC in addicts [Tomasi and Volkow, 2013]. These results suggested that the improper regulation by DA of reward regions in addicted subjects probably modulate the function of prefrontal control [Volkow et al., 2011]. Our previous studies had also demonstrated that the striatum structural abnormalities were often accompanied with abnormal RSFC within frontostriatal circuits in brain diseases [Yuan et al., 2013, 2015bb]. Additionally, mounting evidence had revealed that frontostriatal circuits RSFC could modulate cognitive control [Liston et al., 2006; Vink et al., 2014; Yuan et al., 2015bb]. With regard to cigarette smoking, the altered reward‐related dorsal and ventral striatum activities had been revealed in smokers, such as decreased striatal DA transporter [Newberg et al., 2007] and increased DA activity [Salokangas et al., 2000]. Theoretically, the striatum might modulate the frontostriatal circuits to regulate addiction related behaviors. Taken together, we rationally hypothesized that frontostriatal circuits RSFC would mediate the relationship between striatum volume and cognitive control deficits in young smokers.
Therefore, the purposes of this study were to (1) assess the relationship between the volume of striatum subsets, i.e., caudate, putamen and nucleus accumbens (NAc), and smoking behaviors, and cognitive control deficits measured by Stroop task in young smokers; (2) detect the frontostriatal circuits RSFC differences between young smokers and nonsmokers and estimate their correlations with smoking behaviors and cognitive control deficits in young smokers; (3) test the possible mediator role of the frontostriatal circuits as the relationship between the striatum volume and cognitive control deficits in young smokers. It is hoped that our study relating circuit‐level interactions between brain regions (especially the striatum and PFC) to particular dimensions of behavioral dysfunction could shed new insights into the neural correlates of smoking behaviors in young smokers.
MATERIALS AND METHODS
Ethics Statement
This study was approved by the Medical Ethics Committee of the First Affiliated Hospital of the Medical College, Xi'an Jiaotong University. All participants and their parents gave the written informed consent after the experimental procedure was fully explained.
Participants
The young smokers were screened through advertisements in local universities with an interviewer‐administered assessment. Nicotine dependence severity was assessed with Fagerström Test for Nicotine Dependence (FTND) [Heatherton et al., 1991]. Craving state was assessed by the 10‐item brief Questionnaire on Smoking Urges (QSU) before scanning [Cox et al., 2001]. Pack_years of smoking were calculated by multiplying the average number of packs of cigarettes smoked per day by the number of years the participant smoked [Yu et al., 2015]. All young smokers had no attempt to quit or smoking abstinence in the past 6 months. The age‐, education‐, and gender‐matched healthy nonsmokers were also enrolled. None of nonsmokers had smoked more than 5 cigarettes in their lifetime.
Exclusion criteria for both groups were (1) any physical illness such as a brain tumor, obstructive lung disease, hepatitis, or epilepsy as assessed according to clinical evaluations and medical records; (2) any Axis I psychiatric disorder, including current drug abuse (except nicotine for the smokers) by the structured clinical interview for DSM‐IV; (3) urine test demonstrating current substance use other than nicotine dependence; (4) alcohol use disorders measured by Alcohol Use Disorders Identification Test (AUDIT); (5) any medications currently that may affect cognitive functioning; (6) pregnancy or menstrual period in women; (7) IQ score < 90 (measured by Wechsler intelligence Scale); and (8) claustrophobia. All the participants were right‐handed as measured by the Edinburgh Handedness Inventory [Oldfield, 1971].
At last, 60 young smokers (53 males, aged 16–24 years, mean age, 20 ± 1.7 years) and 60 matched healthy nonsmokers (52 males, aged 17–23 years, mean age, 19.95 ± 1.8 years) were recruited in our study. Expiratory carbon monoxide (CO) levels of all participants were measured using the Smokerlyzer System (Bedfont Scientific, Ltd, Rochester, UK). CO level in expired air was verified as ≥10 ppm in smokers and ≤3 ppm in nonsmokers. Participants were asked to refrain from smoking during the 60 min immediately preceding the scan (average duration of abstinence before scan: 40.8 ± 15.8 min). The clinical and demographic characteristics of participants were shown in Table 1.
Table 1.
Demographic characteristics of young smokers and nonsmokers in this study
| Items | Smokers (n = 60) | Nonsmokers (n = 60) | t/χ 2 | p‐value |
|---|---|---|---|---|
| Age (years) | 20.0 ± 1.7 | 19.95 ± 1.8 | −0.51 | 0.96 |
| Sex, male (n%)a | 53 (88.3%) | 52 (86.7%) | 0.139 | 0.71 |
| IQ | 101.9 ± 6.6 | 101.2 ± 5.9 | 0.596 | 0.13 |
|
Levels of education (years) Cigarettes per day (CPD) |
13.8 ± 0.7 16.9 ± 5.4 |
13.6 ± 0.9 ‐ |
−1.295 ‐ |
0.20 ‐ |
| Age at start of smoking | 15.4 ± 1.5 | ‐ | ‐ | ‐ |
| Years of smoking | 4.4 ± 1.6 | ‐ | ‐ | ‐ |
| Pack‐yearsIAT | 3.6 ± 1.7 | ‐ | ‐ | ‐ |
| FTND | 6.0 ± 0.8 | ‐ | ‐ | ‐ |
| QSU | 30.7 ± 8.3 | ‐ | ‐ | ‐ |
Values are mean ± SD unless otherwise indicated. Pack‐years: smoking years × daily consumption/20. FTND: Fagerström Test for Nicotine Dependence. QSU: questionnaire on smoking urges. *p < 0.05.
The p value for gender distribution in the two groups was obtained by chi‐square test.
Cognitive Control Measurements
Cognitive control deficits in young smokers were measured by Stroop color‐word test as described in our previous studies [Cai et al., 2015; Feng et al., 2015; Xing et al., 2014; Yuan et al., 2015aa, 2015bb]. The task employed a block design with three conditions: congruent, incongruent, and rest. Three words—Red, Blue, and Green—were displayed in three colors (red, blue, and green) as the congruent and incongruent stimuli. During the rest phase, a cross was displayed at the center of the screen, and subjects were required to fix their eyes on this cross without responding. All stimuli were programmed into two runs with different sequences of congruent and incongruent blocks and counter‐balanced in order across participants. Each run consisted of four congruent, four incongruent, and nine rest blocks. There were seven trials in each task block, and each stimulus was presented for 1 s with an interstimulus interval of 2 s. All rest blocks lasted 17 s, except for the first one, which lasted 19 s. Before each task block, the instruction “Identify the Color” was presented, and before each rest block, the instruction “Rest” was presented. All instructions were presented for 2 s. The entire run lasted 355 s. Participants were instructed to respond to the displayed color as fast as possible by pressing a button on a Serial Response Box™ with their right hand. Button presses by the index, middle, and ring finger corresponded to red, blue, and green color, respectively. All the participants had normal vision without color blindness. The behavioral task was tested individually in a quiet room when the participants were in a calm state of mind. The participants were not permitted to enter the Stroop task until they all indicated clear understanding of the task, which was supported by the 90% correction rate in the congruent condition in practice runs. Through the practice, all participants finished the task without missing even one trial in this study. During the task, we collected five parameters for the cognitive control measurements, i.e., reaction time (RT) and errors in congruent condition, RT and errors in incongruent condition, reaction delay (RD) measured by reaction time during the incongruent condition minus congruent condition.
MRI Data Acquisition
Imaging acquisition was carried out at the First Affiliated Hospital of the Medical College, Xi'an Jiaotong University, China. First, individual high‐resolution T1‐weighted image was acquired via a 3‐T MRI system (EXCITE, General Electric, Milwaukee, Wisconsin), with a volumetric three‐dimensional spoiled gradient recall sequence with the parameters as TR = 8.5 ms, TE = 3.4 ms, flip angle = 12°, slice thickness = 1 mm, 140 slices in axial plane, data matrix = 240 × 240, FOV = 240 × 240 mm. Subsequently, the resting‐state functional images were acquired with an echo‐planar imaging (EPI) with the following parameters: 35 contiguous slices with a slice thickness = 4 mm, TR = 2,000 ms, TE = 30 ms, flip angle = 90°, FOV = 240 × 240 mm, data matrix = 64 × 64, and total volume = 185. During the 6 min 10 s functional scan, participants were instructed to keep their eyes closed but stay awake, and not to think about anything. After the data acquisition, subjects were asked whether or not they remained awake during the whole procedure.
Structural MRI: Subcortical Volume Segmentation
Subcortical volumetric segmentation was performed by FreeSurfer 5.0 (http://surfer.nmr.mgh.harvard.edu) as described in our previous studies [Cai et al., 2015; Li et al., 2015; Yuan et al., 2013]. The main process included (1) removal of nonbrain tissue, (2) automated Talairach transformation, (3) segmentation of the subcortical white matter and deep gray matter volumetric structures, (4) intensity normalization, (5) tessellation of the gray matter/white matter boundary, (6) automated topology correction, (7) surface deformation, and (8) registration of the subjects' brains to a common spherical atlas.
Resting‐State Functional Connectivity of Striatum
The fMRI resting‐state images were processed using AFNI/SUMA (http://afni.nimh.nih.gov/) and FSL (http://fsl.fmrib.ox.ac.uk/fsl/) software. As described in our previous study [Yuan et al., 2015bb], the preprocessing was divided into two sections, i.e., core image processing and denoising. Core image processing consists of the following steps: (1) slice timing correction; (2) rigid‐body head motion correction (3 mm displacements and 3° rotations); (3) obliquity transform to the structural image; (4) affine coregistration to the skull‐stripped structural image; (5) standard spatial transform to the MNI152 template; (6) spatial smoothing (6 mm full‐width at half‐maximum); and (7) intensity normalization to a whole‐brain median of 1000. Previous studies had pointed out that nuisance regression and bandpass filtering alone are often insufficient to control head movement induced noise [Patel et al., 2014]. Therefore, wavelet despiking was used in this study for the functional connectivity [Patel et al., 2014]. Denoising steps included (8) time series despiking (wavelet domain), (9) nuisance signal regression including the 6 motion parameters estimated in (2), their first‐order temporal derivatives, white matter, and ventricular cerebrospinal fluid (CSF) signal (14‐parameters regression), and (10) a temporal Fourier filter. The striatum subsets (caudate, putamen and NAc) were chosen as our seeds according to Harvard‐subcortical structural atlas (http://www.cma.mgh.harvard.edu/). The regional resting‐state fMRI time series was extracted for each ROI (bilateral caudate, putamen, and NAc) by averaging all of the voxels within each region at each time point in the preprocessed data. Pearson correlation was employed to investigate the RSFC strength between striatum subsets and the whole‐brain regions, and then a Fisher's r‐to‐z transform was applied.
Statistical Analyses
The volumes of the striatum structures (i.e., bilateral caudate, putamen, NAc) and intracranial volume (ICV) were extracted and imported into the SPSS 20.0 (SPSS Statistics, IBM, Armonk, NY). A univariate analysis of variance for each of the segmented volumes was performed separately using SPSS 20.0 to assess the differences between young smokers and nonsmokers while accounting for ICV. To correct for multiple comparisons, the Bonferroni procedure was applied. All tests were two‐tailed, and the level of significance was P < 0.0083 (0.05/6). Then, after adjusting by the number of ROIs used (i.e., 6), two‐sample t‐tests were used to compare z value maps between smokers and nonsmokers (FWE corrected at P < 0.0083 (0.05/6)). Furthermore, correlation analysis was carried out to assess the relationship between the neuroimaging findings (i.e., the absolute volumes of the striatum subsets and abnormal RSFC within frontostriatal circuits) and behavioral data in smokers (i.e., the reduced cognitive control task performances and smoking variables (FTND, QSU, age of onset, pack_years)). The correction results were corrected by the Bonferroni correction (i.e., the number of corrections) for multiple testing.
Finally, mediation analyses test whether the relationship between any two correlated variables (striatum volume abnormalities and Stroop task performance) can be explained by the values from a third variable (frontostriatal RSFC) [Kober et al., 2010]. If frontostriatal RSFC is a full mediator of the relationship between striatum volume abnormalities and cognitive control impairment, then this relationship will become insignificant when frontostriatal RSFC is controlled for in the model. According to standard convention [Kober et al., 2010], “a” refers to the striatum volume abnormalities—frontostriatal RSFC effect, “b” refers to the frontal‐striatum RSFC—cognitive control impairment, and “c” refers to the direct striatum volume abnormalities and cognitive control impairment, controlling for the mediator frontostriatal RSFC. The product “a × b” tests the significance of the direct mediator. As is customary, we used a bootstrapping test for the statistical significance of the product “a × b”. All the mediation analysis processing was finished according to the SPSS macros downloaded from the Psychonomic Society's Web archive at http://www.psychonomic.org/archive/(Preacher and Hayes, 2004].
RESULTS
Cognitive Control Impairments in Young Smokers
Detailed demographical analysis showed no significant differences in the distributions of age, gender, years of education, or IQ between the two groups. According to the self‐reports, the number of cigarettes per day was 16.9 ± 5.4 in young smokers; the mean FTND was 6.0 ± 0.8 (Table 1). During the Stroop task, both groups showed a significant Stroop effect (Fig. 1), where the RT was longer during incongruent than congruent condition (smokers: 633.3 ± 73.0 ms vs 548.7 ± 58.8 ms, T = 6.99, P < 0.005; nonsmokers: 651.8 ± 77.6 ms vs 542.7 ± 61.3 ms, T = 8.54, P < 0.005). The young smokers committed more errors than the nonsmokers during the incongruent condition (6.6 ± 3.8 vs 5.3 ± 2.4, T = 2.25, P = 0.027). Additionally, RD measured by RT during the incongruent condition minus congruent condition was shorter in young smokers than nonsmokers (84.6 ± 41.4 ms vs 109.1 ± 51.4 ms, T = −2.87, P = 0.005). The pack_years were positively correlated with the incongruent errors in young smokers (r = 0.416, P = 0.001) (Fig. 1).
Figure 1.
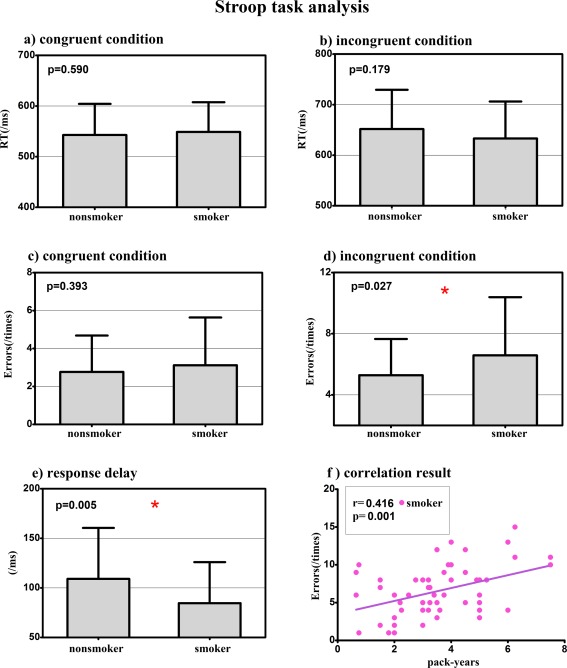
Cognitive control measurements in young smokers and nonsmokers. The young smokers committed more errors and shorter response delay (RD) than the nonsmokers during the incongruent condition. The pack_years, calculated by multiplying the average number of packs of cigarettes smoked per day with the number of years the participant smoked, were positively correlated with the incongruent errors in young smokers (r = 0. 164, P = 0.001). This result demonstrated that the cognitive control deficits measured by Stroop task was the behavioral marker of cumulative effect of long‐term smoking in young smokers. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Striatum Volumes Differences
No significant ICV difference (T = 0.638, P > 0.05) between the young smokers (1,094,979 ± 193,035 mm3) and nonsmokers (1,165,689 ± 195,399 mm3) was detected. The increased volumes of right caudate (F = 7.508, P =0.007) in young smokers were observed as compared with nonsmokers, controlling ICV as the covariates (Bonferroni corrected). Specifically, relative to nonsmokers, young smokers showed 5.3% increased volume of the right caudate (Fig. 2 and Table 2). No other significant differences were found between young smokers and nonsmokers when comparing the volume of other striatum subsets.
Figure 2.
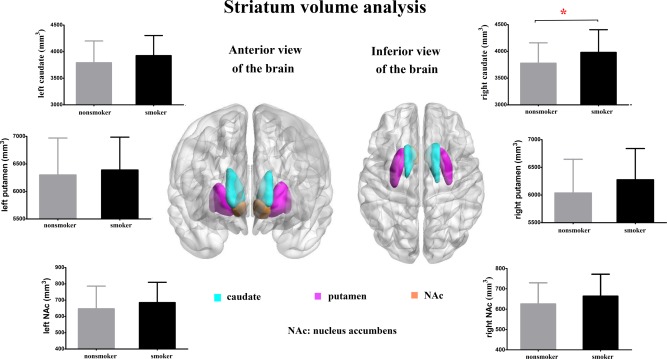
Striatum volume comparisons between young smokers and nonsmokers. The increased volumes of right caudate (F = 7.526, P =0.007) in young smokers were observed as compared with nonsmokers (Bonferroni corrected). No other significant differences were found between young smokers and nonsmokers when comparing the volume of the other subsets of the striatum. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 2.
Volume of striatum comparison between smokers and nonsmokers
| Region | Volume (mean ± SD mm3) | F value | Percent difference (%) | p‐value | |
|---|---|---|---|---|---|
| Smokers (N = 60) | Nonsmokers (N = 60) | ||||
| Left | |||||
| Caudate | 3925.5 ± 375.1 | 3792.2 ± 405.9 | 3.583 | 3.5 | 0.061 |
| Putamen | 6389.6 ± 591.9 | 6300.2 ± 664.6 | 0.287 | 1.4 | 0.593 |
| NAc | 685.1 ± 123.6 | 647.3 ± 137.8 | 1.374 | 5.8 | 0.243 |
| Right | |||||
| Caudate | 3981.4 ± 420.6 | 3779.4 ± 377.4 | 7.508 | 5.3 | 0.007* |
| Putamen | 6276.1 ± 559.5 | 6036.8 ± 604.6 | 3.671 | 4.0 | 0.058 |
| NAc | 673.8 ± 131.9 | 643.5 ± 109.0 | 1.516 | 4.7 | 0.221 |
Values are mean ± SD unless otherwise indicated. NAc: nucleus accumbens. ICV: intracranial volume. *p < 0.05/6.
Resting‐State Functional Connectivity Differences
Four young smokers and three nonsmokers were removed due to extra head motion (>3 mm and/or 3°). RSFC analysis generated similar caudate, putamen, and NAc networks in young smokers and nonsmokers, including the cortical regions (DLPFC, ACC, OFC, temporal, parietal, occipital, and limbic cortices), subcortical regions (thalamus, putamen, globus pallidus, caudate, midbrain, and pons), and cerebellum (Fig. 4 and Supporting information). However, further analysis revealed reduced RSFC between right caudate and several regions in young smokers (P < 0.05/6, FWE corrected), i.e., bilateral OFC, ACC, thalamus, angular gyrus, right DLPFC, and hippocampus (Table 3 and Fig. 5). In addition, the left caudate showed decreased RSFC with bilateral ACC, thalamus, and right superior frontal gyrus (P < 0.05/6, FWE corrected). No significant increased RSFC was observed within these two caudate networks in young smokers. With regard to the other striatal seeds (NAc and putamen), there were no significant group RSFC differences (P < 0.05/6, FWE corrected).
Figure 4.
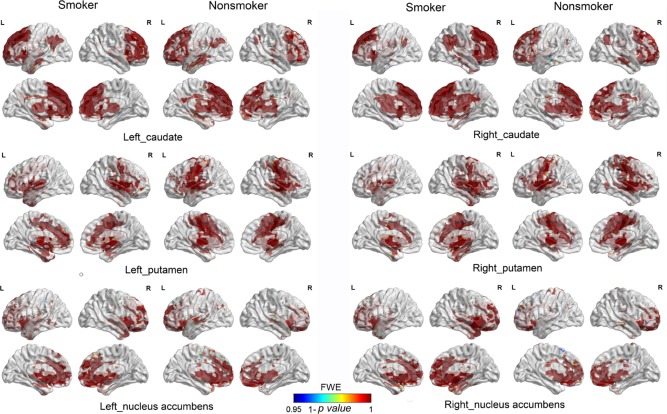
Striatum resting‐state functional connectivity (RSFC) networks. RSFC analysis generated similar caudate, putamen, and NAc networks in young smokers and nonsmokers, including the cortical regions (DLPFC, ACC, OFC, temporal, parietal, occipital, and limbic cortices), subcortical regions (thalamus, putamen, globus pallidus, caudate, midbrain, and pons), and cerebellum. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 3.
Between‐group differences of RSFC in young smokers and nonsmokers (p < 0.05/6, few‐corrected)
| Brain region | Side | BA | Voxels with maximum effect | Cluster size | ||||
|---|---|---|---|---|---|---|---|---|
| Talairach | t‐value | P‐value | ||||||
| x | y | z | ||||||
| Reduced RSFC of left caudate in young smokers compared with nonsmokers | ||||||||
| Anterior cingulate | L | 32 | −2 | 34 | 20 | 6.6726 | 3.1E‐05 | 35 |
| R | 32 | 14 | 34 | 20 | 5.5848 | 0.001021 | 20 | |
| Thalamus | L | −4 | −8 | 2 | 8.9414 | 9.69E‐09 | 443 | |
| R | 2 | −10 | 4 | 7.1727 | 5.55E‐06 | 64 | ||
| Superior frontal gyrus | R | 6/8 | 16 | 24 | 56 | 7.5321 | 1.57E‐06 | 231 |
| Reduced RSFC of right caudate in young smokers compared with nonsmokers | ||||||||
| Anterior cingulate | L | 24/32 | −6 | 19 | −6 | 6.7552 | 5.26E‐05 | 116 |
| R | 24/32 | 12 | 34 | 20 | 7.0943 | 1.67E‐05 | 199 | |
| Inferior frontal gyrus | L | 47 | −28 | 15 | −11 | 7.3278 | 7.46E‐06 | 136 |
| R | 47 | 22 | 23 | −15 | 7.4638 | 4.64E‐06 | 107 | |
| Thalamus | L | −20 | −19 | 8 | 7.0372 | 2.03E‐05 | 260 | |
| R | 4 | −10 | 4 | 6.9233 | 2.99E‐05 | 199 | ||
| Angular gyrus | L | 39 | −55 | −57 | 34 | 6.3809 | 0.00018 | 43 |
| R | 39 | 53 | −68 | 37 | 5.6617 | 0.00166 | 15 | |
| Middle frontal gyrus | R | 9/46 | 40 | 29 | 41 | 6.1036 | 0.000434 | 68 |
| Hippocampus | R | 32 | −22 | −9 | 6.3464 | 0.000201 | 38 | |
Figure 5.
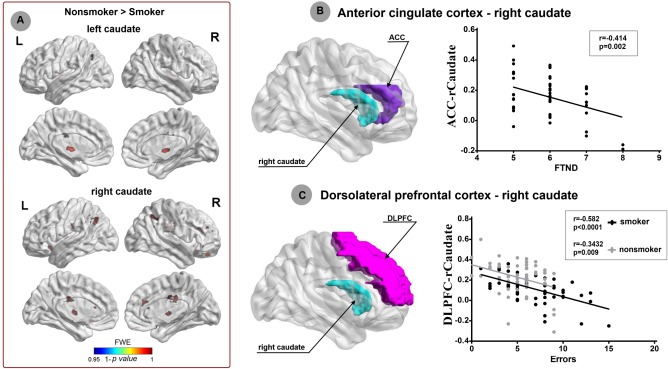
Striatum networks patterns and smoking behaviors. We revealed reduced RSFC between right caudate and several regions in young smokers (P < 0.05, FWE corrected), i.e., bilateral OFC, ACC, thalamus, angular gyrus, right DLPFC, and hippocampus. In addition, the left caudate showed decreased RSFC with bilateral ACC, thalamus, and right superior frontal gyrus (P < 0.05, FWE corrected). Significant negative correlation (r = −0.414; P = 0.002) was found between the ACC‐right caudate RSFC and FTND‐the severity of nicotine addiction in young smokers. Additionally, the incongruent response errors during Stroop task were negatively correlated with the right DLPFC‐caudate RSFC strength in young smokers (r = −0.582; P < 0.0001) and nonsmokers (r = −0.3432; P = 0.009). The results suggested that the ACC‐right caudate RSFC and right DLPFC‐caudate RSFC strength could reflect the severity of nicotine addiction and cognitive control deficits in young smokers, respectively. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Brain–Behavior Correlation Results
The purposes of this part were to assess the relationship between the brain findings (abnormal volume and RSFC of striatum) and task performances (RD and incongruent errors) as well as smoking variables (FTND, QSU, pack_years, age of onset) in young smoker, which generated 6 behavioral measurements. For the structural results, there are 6 subsets of striatum and derived 6 × 6 = 36 correlation analyses. With regard to RSFC results, there were 10 and 5 regions showed abnormal RSFC with right and left caudate, respectively, which derived (10 + 5) × 6 = 90 correlation analyses. Then, we got 36 + 90 = 126 correction analyses in smokers. For the nonsmokers, the smoking behaviors were non‐absent, so we only got 6 × 2 + 15 × 4 = 72 correction analyses. In total, we had 126 + 72 = 198 correction analyses. If the Bonfferroni correction was used, the adjusted p value for the correlation results should be 0.05/198 = 0.00025. According to these criteria, correlation analysis revealed significant positive correlation between the right caudate volume and the errors (r = 0.5055, P < 0.0001) during incongruent condition in Stroop task in young smokers (Fig. 3). Additionally, the incongruent response errors during Stroop task were negatively correlated with the right DLPFC‐caudate RSFC strength in both young smokers (r = −0.582, P < 0.0001) (Fig. 5). Several other correlations were significant (p < 0.05), but did not survive the multiple correction, which were the correlation between the right caudate volume and QSU (r = 0.270, P = 0.037) in young smokers, the correlation between the ACC‐right caudate RSFC and FTND (r = −0.414, P = 0.002) in young smokers, and the correlation between the right DLPFC‐caudate RSFC strength and the incongruent response errors during Stroop task in nonsmokers (r = −0.343, P = 0.009) (Figs. 3 and 5). No other significant results were detected between the neuroimaging findings and behavioral data.
Figure 3.
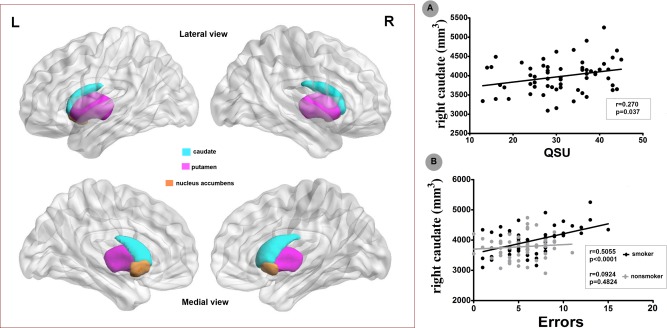
Striatum volume and incongruent condition errors of Stroop task. Correlation analysis revealed significant positive correlations between the right caudate volume and QSU‐the measurement of craving state (r = 0.27, P = 0.037) as well as the errors (r = 0.5055, P < 0.0001) during incongruent condition in Stroop task in young smokers. With regard to the nonsmokers, no significant correlations were detected between the striatum morphometry and Stroop task performances. These results suggested that the volume of caudate could reflect the craving state and cognitive control deficits in young smokers. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Mediation Analysis
The finding that caudate volume was correlated with DLPFC‐caudate RSFC strength in young smokers (r = −0.3827, p = 0.0036), together with the correlation with Stroop task performance, demonstrated that the requirements for mediation modeling were fulfilled [Baron and Kenny, 1986]. Mediation analysis was applicable among caudate volume, cognitive control deficits, and DLPFC‐caudate RSFC in young smokers. As expected, we revealed that the DLPFC‐caudate RSFC z values mediated the relationship between caudate volume and incongruent condition errors in the Stroop task (a × b = 0.0013 (0.0006), P < 0.05; c′ = 0.0025(0.0009), P < 0.05) (Fig. 6).
Figure 6.
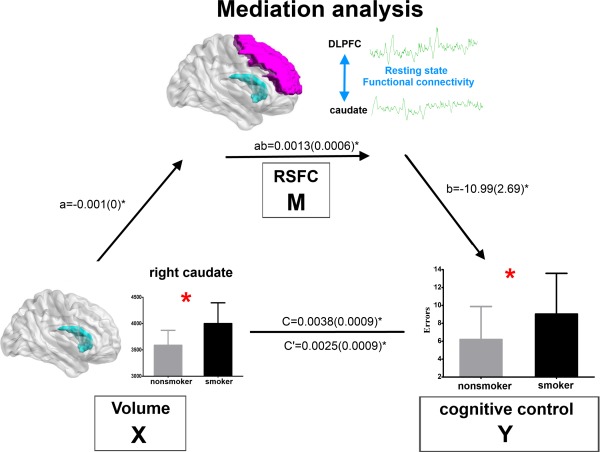
Mediation analysis. The frontostriatal RSFC z value (i.e., DLPFC‐caudate) mediated the relationship between caudate volume and committed errors during incongruent condition in the Stroop task (a × b = 0.0013 (0.006), P < 0.05; c′ = 0.0025 (0.0009), P < 0.05). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
DISCUSSION
This study clearly revealed the enlarged striatum volume (i.e., bilateral caudate) (Fig. 2) and reduced RSFC between right caudate and PFC (DLPFC, OFC) as well as limbic regions (ACC, thalamus, and hippocampus) in young smokers (Fig. 5). The cognitive control impairments were observed in young smokers by showing the more response errors during incongruent condition, which were correlated with pack_years of young smokers (Fig. 1). More importantly, the volume of right caudate was correlated with the craving measured by QSU in young smokers (Fig. 3). The right caudate‐ACC RSFC strength was correlated with the FTND in young smokers (Fig. 5). These results suggested that the striatum morphormetry and frontostriatal circuits RSFC strength could reflect the craving and severity of young smokers, respectively. Finally, the incongruent errors in Stroop task were correlated with volume of right caudate only in young smokers (Fig. 3), but correlated with caudate‐DLPFC in both groups (Fig. 5). Mediation analysis revealed that the DLPFC‐caudate RSFC strength mediated the relationship between caudate volume and incongruent errors during Stroop task in young smokers (Fig. 6). It is hoped that our study focusing on the frontostriatal circuits could shed new insights into the neural correlates of smoking behaviors in young smokers.
Cognitive Control Impairments in Young Smokers
While acute nicotine consumption confers short‐term cognitive improvement, chronic smoking is generally associated with cognitive impairments [Jasinska et al., 2014] although the data vary widely across studies [Feng et al., 2015; Poorthuis et al., 2009; Wagner et al., 2013]. In this study, we revealed the cognitive control deficits in nondeprived young smokers (i.e., more incongruent response errors of Stroop task) (Fig. 1). The association between pack_years and response errors during incongruent condition of Stroop task (Fig. 1) in young smokers also shed some lights on the chronic effect of nicotine on the cognitive control among young smokers. Nicotine binds with highest affinity to neuronal nicotinic acetylcholine receptors (nAChRs), which are present both pre‐ and postsynaptically on different neuronal subtypes and have a neuromodulatory function [Jasinska et al., 2014]. This property permits nicotine to have a secondary effect on virtually all neurotransmitter/neuromodulator systems within brain. Thus, it is rational that nicotine's influence encompasses a wide range of cognitive processes including sensory, motor, attention, executive, learning, and memory function. Emerging evidence had demonstrated that nicotine is highly toxic to the developing brain and chronic smoking may cause cognitive deficits through the neurotoxic effects [Swan and Lessov‐Schlaggar, 2007]. Additionally, smoker neuroimaging studies had discovered structural abnormalities in smokers, such as reduced gray matter volume/density in DLPFC, OFC, and ACC [Jasinska et al., 2014], which were associated with cognitive control activities [Matsumoto and Tanaka, 2004]. Revealing the more response errors during incongruent condition in Stroop task, we provided partial evidence for the cognitive control deficits in young smokers.
Increased Volume of Right Caudate in Young Smokers
Consistent with our previous study [Li et al., 2015], the increased right caudate volume was detected in young smokers (Fig. 2), which was also in accord with previous addiction findings including cocaine [Jacobsen et al., 2001], methamphetamine [Chang et al., 2007], pathological gambling [Koehler et al., 2015], and internet gaming disorder [Cai et al., 2015]. The right caudate volume of young smokers was correlated with the craving score measured by QSU (Fig. 3), which had been reported by one previous study [Janes et al., 2015]. Mounting evidence demonstrated that smoking induced caudate dopamine release in smokers, which were significantly correlated with craving ratings and suggested an important role of caudate in craving of smokers [Barrett et al., 2004; Brody et al., 2004, 2006]. In addition, the caudate regulated nicotine seeking following smoking abstinence and regulated craving provoked by smoking cues [McClernon et al., 2009; Sweitzer et al., 2013; Wang et al., 2007]. Taken together, the findings in this study, i.e., the increased volume of right caudate and its association with QSU, provided morphometric evidence for the role of caudate in craving in young smokers.
Reduced RSFC Within Frontostriatal Circuits in Young Smokers
Anatomically, striatum receives projections from multiple cortical and midbrain regions, which can be divided into dorsal striatum (DS) and ventral striatum (VS) [Alexander et al., 1986]. In detail, the VS (mainly NAc) receives major projections from OFC, medial prefrontal cortex (mPFC), ACC, and temporal and limbic structures [Alexander et al., 1986]. The DS (mainly caudate and putamen) receives projections primarily from association cortex (mainly dorsolateral PFC‐DLPFC), sensory and motor areas [Alexander et al., 1986]. Due to the neuroanatomical connections, frontostriatal circuits play critical roles in reward and cognitive control, which are both closely related to addiction [Casey et al., 2007; Liston et al., 2006; Tomasi and Volkow, 2013]. Previous neuroimaging studies had revealed the structural and functional differences between SUD and healthy controls [Feil et al., 2010; Motzkin et al., 2014]. However, the implication of frontostriatal circuits in neural mechanisms of young smokers remains unclear. Our findings contribute toward filling this gap by showing the reduced RSFC between bilateral caudate and PFC (OFC and right DLPFC) as well as ACC in young smokers (Fig. 5).
Structural and functional neuroimaging studies had demonstrated that abnormalities of OFC (involved in salience attribution and goal‐directed behaviors), DLPFC (involved in higher cognitive operations and decision making), and ACC (involved in inhibitory control and awareness) were associated with addiction processes (executive, inhibitory, and decision making) in smokers [Feil et al., 2010] and other SUD studies [Volkow et al., 2013]. Recent SUD studies had suggested patterns of abnormal connectivity between the reward regions and cognitive control regions played important roles in the pathology of SUD [Motzkin et al., 2014; Tomasi and Volkow, 2013]. Our findings, i.e., the dorsal ACC‐right caudate RSFC was negatively correlated with FTND in young smokers, suggested the frontostriatal circuits RSFC strength could be the biomarker of nicotine addiction severity. Additionally, several brain regions showed reduced RSFC with caudate, such as thalamus, hippocampus, angular gyrus, and superior prefrontal gyrus (Fig. 5). As primarily responsible for the smoking addictive property, nicotine binds with highest affinity to nAChRs, which were rich in thalamus, striatum, and cerebral cortex [Das et al., 2012]. Activation of thalamus, hippocampus, angular gyrus, and superior prefrontal gyrus induced by smoking cue had been demonstrated in previous neuroimaging studies, which were correlated with subjective craving [Engelmann et al., 2012]. Moreover, cerebral blood flow increases of thalamus and hippocampus predicted abstinence‐induced cravings changes in smokers [Wang et al., 2007]. Our RSFC results suggested that the information communication between the striatum and PFC as well as limbic regions (thalamus, hippocampus) in young smokers were different from nonsmokers. We failed to find the association of the abnormal RSFC between striatum and the regions mentioned above with craving score, which may be due to the sensitivity of RSFC method and the small sample size. Evidently, accurate roles of these findings should be investigated in the future by employing more sensitive method and complicated experiment design.
Frontostriatal Circuits RSFC Mediates the Relationship between Striatum Morphometry and Cognitive Control Impairments in Young Smokers
Previous SUD findings, i.e., impaired DA function in striatum (decreases in D2R and reduced DA release) were associated with reduced baseline glucose metabolism in PFC in addicts [Tomasi and Volkow, 2013], which suggested that the improper regulation by DA of reward regions in addicted subjects probably modulate the prefrontal control system function [Volkow et al., 2011]. Similar to other SUD, cigarette smoking was associated with altered reward‐related dorsal and ventral striatum activities, such as decreased striatal DA transporter [Newberg et al., 2007] and increased DA activity [Salokangas et al., 2000]. Theoretically, the caudate might modulate cortical areas to regulate addiction related behaviors. More importantly, we noticed that right caudate volume was correlated with Stroop task performances only in young smokers (Fig. 3). In contrast, the right caudate‐DLPFC RSFC strength was correlated with Stroop task performances in both young smokers and nonsmokers (Fig. 5). The findings, i.e., caudate volume was also correlated with the DLPFC‐caudate RSFC in young smokers, together with previous two correlation results, confirmed that mediation analysis was applicable [Kober et al., 2010]. Rationally, we proposed that frontostriatal RSFC z value (i.e., DLPFC‐caudate) mediated the relationship between caudate volume and committed errors during incongruent condition in the Stroop task, which was confirmed by our mediation analysis results (Fig. 6). We suggested that cognitive control deficits in young smokers could be regulated via the effects of increased striatum morphometry on reduced frontostriatal RSFC. Evidently, the hypothesis of the mediator of frontostriatal circuits RSFC should be investigated in larger sample by using more comprehensive experiment design in future.
Limitation
We did not observe any group differences in RSFC between NAc or putamen seeds and any region of cerebral cortex. The absence of group differences in connectivity with these regions may be associated with the participants in this study. We employed the young smokers; the duration of nicotine dependence and pack_years were relative small compared with the middle‐aged adults smokers. The severity and cumulative effect was not enough to detect significant RSFC findings of NAc and putamen. Whether the putamen and NAc RSFC patterns are not different remains to be determined between more severe young smokers and nonsmokers. Whereas our sample size is comparable to prior studies of nicotine dependence, research involving larger sample sizes may help address some of these additional questions. The cross‐sectional nature of this study cannot make causal association conclusions about smoking and cognitive control. Longitudinal studies should be employed in future to assess the longer term effects of smoking on cognitive control and striatum changes in young smokers.
CONCLUSION
We revealed reduced RSFC within frontostriatal circuits and the correlation with smoking behavior and cognitive control impairments in young smokers. More importantly, the caudate‐DLPFC RSFC strength mediated the relationship between the caudate volume and Stroop task response errors in young smokers. It is hoped that our findings may shed new insights into the neural mechanisms of young smokers. This study may provide new clues for the young smoking treatment in the future, e.g., transcranial direct current stimulation on PFC can be used to enhance cognitive control by modulating the function of frontostriatal circuits.
Supporting information
Supporting Information Table S1
Supporting Information Table S2
Supporting Information Table S3
Supporting Information Table S4
Supporting Information Table S5
Supporting Information Table S6
ACKNOWLEDGMENTS
The authors report no biomedical financial interests or potential conflicts of interest. Conflict of interest: The authors declare that we have no conflict of interest.
REFERENCES
- Alexander GE, DeLong MR, Strick PL (1986): Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neuroscience 9:357–381. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA (1986): The moderator–mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Personality Social Psychol 51:1173 [DOI] [PubMed] [Google Scholar]
- Barrett SP, Boileau I, Okker J, Pihl RO, Dagher A (2004): The hedonic response to cigarette smoking is proportional to dopamine release in the human striatum as measured by positron emission tomography and [11C] raclopride. Synapse 54:65–71. [DOI] [PubMed] [Google Scholar]
- Bauer UE, Briss PA, Goodman RA, Bowman BA (2014): Prevention of chronic disease in the 21st century: Elimination of the leading preventable causes of premature death and disability in the USA. Lancet 384:45–52. [DOI] [PubMed] [Google Scholar]
- Y Bi, K Yuan, Y Guan, J Cheng, Y Zhang, Y Li, D Yu, W Qin, J Tian (2016): Altered resting state functional connectivity of anterior insula in young smokers. Brain Imag Behav DOI: 10.1007/s11682-016-9511-z. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Scheibal D, Hahn E, Shiraga S, Zamora‐Paja E, Farahi J, Saxena S, London ED (2006): Gene variants of brain dopamine pathways and smoking‐induced dopamine release in the ventral caudate/nucleus accumbens. Arch Gen Psychiatry 63:808–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Olmstead RE, London ED, Farahi J, Meyer JH, Grossman P, Lee GS, Huang J, Hahn EL, Mandelkern MA (2004): Smoking‐induced ventral striatum dopamine release. Am J Psychiatry 161:1211–1218. [DOI] [PubMed] [Google Scholar]
- Cai C, Yuan K, Yin J, Feng D, Bi Y, Li Y, Yu D, Jin C, Qin W, Tian J (2015): Striatum morphometry is associated with cognitive control deficits and symptom severity in internet gaming disorder. Brain Imag Behav 1–9, DOI: 10.1007/s11682-015-9358-8. [DOI] [PubMed] [Google Scholar]
- Caramori G, Kirkham P, Barczyk A, Di Stefano A, Adcock I (2015): Molecular pathogenesis of cigarette smoking–induced stable COPD. Ann N Y Acad Sci 1340:55–64. [DOI] [PubMed] [Google Scholar]
- Casey B, Epstein J, Buhle J, Liston C, Davidson M, Tonev S, Spicer J, Niogi S, Millner A, Reiss A (2007): Frontostriatal connectivity and its role in cognitive control in parent‐child dyads with ADHD. Am J Psychiatry 164:1729–1736. [DOI] [PubMed] [Google Scholar]
- Chang L, Alicata D, Ernst T, Volkow N (2007): Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction 102:16–32. [DOI] [PubMed] [Google Scholar]
- Counotte DS, Spijker S, Van de Burgwal LH, Hogenboom F, Schoffelmeer AN, De Vries TJ, Smit AB, Pattij T (2009): Long‐lasting cognitive deficits resulting from adolescent nicotine exposure in rats. Neuropsychopharmacology 34:299–306. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG (2001): Evaluation of the brief questionnaire of smoking urges (QSU‐brief) in laboratory and clinical settings. Nicotine Tob Res 3:7–16. [DOI] [PubMed] [Google Scholar]
- Das D, Cherbuin N, Anstey KJ, Sachdev PS, Easteal S (2012): Lifetime cigarette smoking is associated with striatal volume measures. Addict Biol 17:817–825. [DOI] [PubMed] [Google Scholar]
- Engelmann JM, Versace F, Robinson JD, Minnix JA, Lam CY, Cui Y, Brown VL, Cinciripini PM (2012): Neural substrates of smoking cue reactivity: A meta‐analysis of fMRI studies. Neuroimage 60:252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil J, Sheppard D, Fitzgerald PB, Yücel M, Lubman DI, Bradshaw JL (2010): Addiction, compulsive drug seeking, and the role of frontostriatal mechanisms in regulating inhibitory control. Neurosci Biobehav Revs 35:248–275. [DOI] [PubMed] [Google Scholar]
- Feng D, Yuan K, Li Y, Cai C, Yin J, Bi Y, Cheng J, Guan Y, Shi S, Yu D, C Jin, X Lu, W Qin, J Tian (2015): Intra‐regional and inter‐regional abnormalities and cognitive control deficits in young adult smokers. Brain Imag Behav 1–11, DOI: 10.1007/s11682-015-9427-z. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, FAGERSTROM KO (1991): The Fagerström test for nicotine dependence: A revision of the Fagerstrom Tolerance Questionnaire. Brit J Addict 86:1119–1127. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Giedd JN, Gottschalk C, Kosten TR, Krystal JH (2001): Quantitative morphology of the caudate and putamen in patients with cocaine dependence. Am J Psychiatry 158:486–489. [DOI] [PubMed] [Google Scholar]
- Janes AC, Park MTM, Farmer S, Chakravarty MM (2015): Striatal morphology is associated with tobacco cigarette craving. Neuropsychopharmacology 40:406–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Japuntich SJ, Eilers MA, Shenhav S, Park ER, Winickoff JP, Benowitz NL, Rigotti NA (2015): Secondhand tobacco smoke exposure among hospitalized nonsmokers with coronary heart disease. JAMA Int Med 175:133–136. [DOI] [PubMed] [Google Scholar]
- Jasinska AJ, Zorick T, Brody AL, Stein EA (2014): Dual role of nicotine in addiction and cognition: A review of neuroimaging studies in humans. Neuropharmacology 84:111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Zhang T, Cai C, Bi Y, Li Y, Yu D, Zhang M, Yuan K (2015): Abnormal prefrontal cortex resting state functional connectivity and severity of internet gaming disorder. Brain Imag Behav, DOI: 10.1007/s11682-015-9439-8. [DOI] [PubMed] [Google Scholar]
- Kaufmann L, Koppelstaetter F, Delazer M, Siedentopf C, Rhomberg P, Golaszewski S, Felber S, Ischebeck A (2005): Neural correlates of distance and congruity effects in a numerical Stroop task: An event‐related fMRI study. Neuroimage 25:888–898. [DOI] [PubMed] [Google Scholar]
- Kober H, Mende‐Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, Ochsner KN (2010): Prefrontal–striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci USA 107:14811–14816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler S, Hasselmann E, Wüstenberg T, Heinz A, Romanczuk‐Seiferth N (2015): Higher volume of ventral striatum and right prefrontal cortex in pathological gambling. Brain Struct Funct 220:469–477. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Guranda M, Wilson AA, Houle S, Rusjan PM, Wing VC, Zawertailo L, Busto U, Selby P, Brody AL (2014): Elevation of dopamine induced by cigarette smoking: Novel insights from a [ 11C]‐(+)‐PHNO PET study in humans. Neuropsychopharmacology 39:415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Yuan K, Cai C, Feng D, Yin J, Bi Y, Shi S, Yu D, Jin C, von Deneen KM, W Qin, J Tian (2015): Reduced frontal cortical thickness and increased caudate volume within fronto‐striatal circuits in young adult smokers. Drug Alcohol Depend 151:211–219. [DOI] [PubMed] [Google Scholar]
- Liston C, Watts R, Tottenham N, Davidson MC, Niogi S, Ulug AM, Casey B (2006): Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cereb Cortex 16:553–560. [DOI] [PubMed] [Google Scholar]
- Liu X, Banich MT, Jacobson BL, Tanabe JL (2004): Common and distinct neural substrates of attentional control in an integrated Simon and spatial Stroop task as assessed by event‐related fMRI. Neuroimage 22:1097–1106. [DOI] [PubMed] [Google Scholar]
- Ma N, Liu Y, Li N, Wang CX, Zhang H, Jiang XF, Xu HS, Fu XM, Hu X, Zhang DR (2010): Addiction related alteration in resting‐state brain connectivity. Neuroimage 49:738–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Tanaka K (2004): Conflict and cognitive control. Psychol Sci 303:969–970. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Kozink RV, Lutz AM, Rose JE (2009): 24‐h smoking abstinence potentiates fMRI‐BOLD activation to smoking cues in cerebral cortex and dorsal striatum. Psychopharmacology 204:25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzkin JC, Baskin‐Sommers A, Newman JP, Kiehl KA, Koenigs M (2014): Neural correlates of substance abuse: Reduced functional connectivity between areas underlying reward and cognitive control. Hum Brain Mapp 35:4282–4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberg A, Lerman C, Wintering N, Ploessl K, Mozley PD (2007): Dopamine transporter binding in smokers and nonsmokers. Clin Nucl Med 32:452–455. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Patel AX, Kundu P, Rubinov M, Jones PS, Vértes PE, Ersche KD, Suckling J, Bullmore ET (2014): A wavelet method for modeling and despiking motion artifacts from resting‐state fMRI time series. NeuroImage 95:287–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorthuis RB, Goriounova NA, Couey JJ, Mansvelder HD (2009): Nicotinic actions on neuronal networks for cognition: General principles and long‐term consequences. Biochem Pharmacol 78:668–676. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF (2004): SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput 36:717–731. [DOI] [PubMed] [Google Scholar]
- Salokangas RK, Vilkman H, Ilonen T, Taiminen T, Haaparanta M, Solin O, Alanen A, Hietala J (2000): High levels of dopamine activity in the basal ganglia of cigarette smokers. Am J Psychiatry 157:632–634. [DOI] [PubMed] [Google Scholar]
- Swan GE, Lessov‐Schlaggar CN (2007): The effects of tobacco smoke and nicotine on cognition and the brain. Neuropsychology Rev 17:259–273. [DOI] [PubMed] [Google Scholar]
- Sweitzer MM, Geier CF, Joel DL, McGurrin P, Denlinger RL, Forbes EE, Donny EC (2013): Dissociated effects of anticipating smoking versus monetary reward in the caudate as a function of smoking abstinence. Biol Psychiatry 76:681–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND (2013): Striatocortical pathway dysfunction in addiction and obesity: Differences and similarities. Crit Rev Biochem Mol Biol 48:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink M, Zandbelt BB, Gladwin T, Hillegers M, Hoogendam JM, den Wildenberg WP, Du Plessis S, Kahn RS (2014): Frontostriatal activity and connectivity increase during proactive inhibition across adolescence and early adulthood. Hum Brain Mapp 35:4415–4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F (2011): Addiction: Beyond dopamine reward circuitry. Proc Natl Acad Sci USA 108:15037–15042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Tomasi D, Baler RD (2013): Unbalanced neuronal circuits in addiction. Curr Opin Neurobiol 23:639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M, Schulze‐Rauschenbach S, Petrovsky N, Brinkmeyer J, von der Goltz C, Gründer G, Spreckelmeyer KN, Wienker T, Diaz‐Lacava A, Mobascher A (2013): Neurocognitive impairments in non‐deprived smokers—results from a population‐based multi‐center study on smoking‐related behavior. Addict Biol 18:752–761. [DOI] [PubMed] [Google Scholar]
- Wang Z, Faith M, Patterson F, Tang K, Kerrin K, Wileyto EP, Detre JA, Lerman C (2007): Neural substrates of abstinence‐induced cigarette cravings in chronic smokers. J Neurosci 27:14035–14040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White HR, Bray BC, Fleming CB, Catalano RF (2009): Transitions into and out of light and intermittent smoking during emerging adulthood. Nicotine Tob Res 11:211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing L, Yuan K, Bi Y, Yin J, Cai C, Feng D, Li Y, Song M, Wang H, Yu D, T Xue, C Jin, W Qin, J Tian (2014): Reduced fiber integrity and cognitive control in adolescents with internet gaming disorder. Brain Res 1586:109–117. [DOI] [PubMed] [Google Scholar]
- Yin J, Yuan K, Feng D, Cheng J, Li Y, Cai C, Bi Y, Sha S, Shen X, Zhang B, T Xue, W Qin, D Yu, X Lu, J Tian (2015): Inhibition control impairments in adolescent smokers: Electrophysiological evidence from a Go/NoGo study. Brain Imag Behav, DOI: 10.1007/s11682-015-9418-0. [DOI] [PubMed] [Google Scholar]
- Yu D, Yuan K, Zhang B, Liu J, Dong M, Jin C, Luo L, Zhai J, Zhao L, Zhao Y, Y Gu, T Xue, X Liu, X Lu, W Qin, J Tian (2015): White matter integrity in young smokers: A tract‐based spatial statistics study. Addict Biol, DOI: 10.1111/adb.12237. [DOI] [PubMed] [Google Scholar]
- Yu R, Zhao L, Lu L (2011): Regional grey and white matter changes in heavy male smokers. PloS One 6:e27440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R, Zhao L, Tian J, Qin W, Wang W, Yuan K, Li Q, Lu L (2013): Regional homogeneity changes in heavy male smokers: A resting‐state functional magnetic resonance imaging study. Addict Biol 18:729–731. [DOI] [PubMed] [Google Scholar]
- Yuan K, Qin W, Dong M, Liu J, Sun J, Liu P, Zhang Y, Wang W, Wang Y, Li Q, L Zhao, KM von Deneen, Y Liu, MS Gold, J Tian (2010): Gray matter deficits and resting‐state abnormalities in abstinent heroin‐dependent individuals. Neurosci Lett 482:101–105. [DOI] [PubMed] [Google Scholar]
- Yuan K, Qin W, Yu D, Bi Y, Xing L, Jin C, Tian J (2015a): Core brain networks interactions and cognitive control in internet gaming disorder individuals in late adolescence/early adulthood. Brain Struct Funct, DOI: 10.1007/s00429-014-0982-7. [DOI] [PubMed] [Google Scholar]
- Yuan K, Yu D, Cai C, Feng D, Li Y, Bi Y, Liu J, Zhang Y, Jin C, Li L, W Qin, J Tian (2015b): Frontostriatal circuits, resting state functional connectivity and cognitive control in internet gaming disorder. Addict Biol, DOI: 10.1111/adb.12348. [DOI] [PubMed] [Google Scholar]
- Yuan K, Zhao L, Cheng P, Yu D, Zhao L, Dong T, Xing L, Bi Y, Yang X, von Deneen KM, F Liang, Q Gong, W Qin, J Tian (2013): Altered structure and resting state functional connectivity of the basal ganglia in migraine patients without aura. J Pain 14:836–844. [DOI] [PubMed] [Google Scholar]
- Zhao LY, Tian J, Wang W, Qin W, Shi J, Li Q, Yuan K, Dong MH, Yang WC, Wang YR, LL Sun, L Lu (2012): The role of dorsal anterior cingulate cortex in the regulation of craving by reappraisal in smokers. PloS One 7:e43598. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Table S1
Supporting Information Table S2
Supporting Information Table S3
Supporting Information Table S4
Supporting Information Table S5
Supporting Information Table S6


