Abstract
Neurofeedback training (NFT) of the alpha rhythm has been used for several decades but is still controversial in regards to its trainability and effects on working memory. Alpha rhythm of the frontoparietal region are associated with either the intelligence or memory of healthy subjects and are also related to pathological states. In this study, alpha NFT effects on memory performances were explored. Fifty healthy participants were recruited and randomly assigned into a group receiving a 8–12‐Hz amplitude (Alpha) or a group receiving a random 4‐Hz amplitude from the range of 7 to 20 Hz (Ctrl). Three NFT sessions per week were conducted for 4 weeks. Working memory was assessed by both a backward digit span task and an operation span task, and episodic memory was assessed using a word pair task. Four questionnaires were used to assess anxiety, depression, insomnia, and cognitive function. The Ctrl group had no change in alpha amplitude and duration. In contrast, the Alpha group showed a progressive significant increase in the alpha amplitude and total alpha duration of the frontoparietal region. Accuracies of both working and episodic memories were significantly improved in a large proportion of participants of the Alpha group, particularly for those with remarkable alpha‐amplitude increases. Scores of four questionnaires fell in a normal range before and after NFT. The current study provided supporting evidence for alpha trainability within a small session number compared with that of therapy. The findings suggested the enhancement of working and episodic memory through alpha NFT. Hum Brain Mapp 37:2662–2675, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: neurofeedback, alpha rhythm, working memory, episodic memory, frontoparietal
INTRODUCTION
The alpha rhythm of a scalp electroencephalogram (EEG) falls in the frequency range of 8–12 Hz, which is measured in the occipital lobe with eyes closed. A classical functional hypothesis of the alpha rhythm is that it is considered to be related to cortical inhibition [Klimesch et al., 2007]. Currently, the functional roles of alpha rhythm in different brain regions have been extensively discussed. Abnormal or asymmetric alpha brain activity is considered to be associated with mental disorders, for example, anxiety [Heller et al., 1997] or depression [Jaimchariyatam et al., 2011]. Increased alpha power is seen in insomnia patients [Cervena et al., 2014]. In contrast, the alpha activity of the frontoparietal region is highly associated with intelligence [Anokhin and Vogel, 1996; Doppelmayr et al., 2005] and memory performance [Jensen et al., 2002; Klimesch, 1999; Sauseng et al., 2005]. To further characterize its functional role, the alpha rhythm has been increasingly discussed in the protocol of neurofeedback training (NFT) of cognitive performance [Gruzelier, 2014; Palva and Palva, 2007].
NFT is an operant conditioning technique using the feedback of particular brain rhythm(s) via video or audio interfaces; participants learn how to tune brain activities on demand. It has been considered a valuable intervention for ameliorating pathological symptoms, for example, anxiety [Hammond, 2005], depression [Hammond, 2000], or insomnia [Cortoos et al., 2010]. Previous studies have reported that the enhancement of alpha amplitude through NFT is accompanied by an increase in working memory [Hanslmayr et al., 2005; Nan et al., 2012; Zoefel et al., 2011]. However, several studies have indicated that working memory shows no change in the alpha NFT group even with a significant increase in the alpha amplitude [Juhel, 2011]. NFT caused no change in alpha amplitude and working memory performance [Boynton, 2001]. These controversial results from previous studies raise a reliability issue for the application of alpha NFT. A possible reason for these controversial findings may be due to different experimental designs, for example, only a single experimental group without a control group [Cho et al., 2008; Hanslmayr et al., 2005] or an inadequate control group with no comparable training events [Nan et al., 2012; Zoefel et al., 2011] or with yoga practice [Juhel, 2011]. An identical exposure time for the training apparatus and the protocol between the control and experimental groups is important for the identification of a possible experimental effect. To reduce the possible selection bias of participants and related internal validity, previous studies [Wang and Hsieh, 2013] have introduced a sham control group with feedback of the EEG amplitude within a random frequency range in an NFT using the same equipment with equivalent training durations. The experimental design with a sham control group may be beneficial to determine the effect of NFT.
Another possible reason for the controversial results regarding alpha NFTs may be due to diverse memory tasks, for example, the digit span task [Nan et al., 2012], word span task [Escolano et al., 2011] or the mental rotation task [Escolano et al., 2014; Zoefel et al., 2011]. Event‐related alpha activity is associated with performance of both working memory and episodic memory [Klimesch et al., 2006a, b]. To our knowledge, there is no study discussing the effect of alpha NFT on episodic memory. Moreover, systematic evaluations of multidimensional memory tasks within NFT are lacking.
To further understand the effect of alpha NFT on memory, the present study evaluated the performance of two working memory tasks (a backward digit span task and an operational span task) and a word pair task of episodic memory before and after NFT. Moreover, we enrolled a sham group with the same exposure time to the training apparatus and protocol of the experimental group. The current study proposed a trainability of alpha NFT in terms of changes in both alpha amplitude and alpha duration. We further hypothesized that the alpha NFT, particular for well‐trained “Responder,” had significant increase on the accuracies of all memory tasks.
MATERIALS AND METHODS
Participants
In the present study, 50 healthy participants (range: 19–29 years) were recruited from the National Cheng Kung University and randomly assigned into two age‐ and gender‐matched groups. The experimental procedure was reviewed and approved by a local research ethics committee. Informed consent was provided and signed for all participants before the experiment. All participants were right‐handed and had not participated in an NFT study in the past. Twenty‐five participants (15 female and 10 male, mean age of 20.96 ± 2.85 years) were allocated to the group with feedback of alpha amplitude (alpha), and the twenty‐five participants (15 female and 10 male, mean age of 21.64 ± 2.40 years) in the control group (Ctrl) received amplitude feedback in random frequency bands (described below). No significant difference was observed in the factors for age (t = 0.913, P = 0.674), education (t = 0.913, P = 0.674), and gender (χ 2 = 0.083, P = 0.773) between the two groups.
Neurofeedback Training and Processing
Brain activities were recorded from six active Ag/AgCl electrodes located at 2.5 cm anterior and posterior to C3, Cz, and C4, respectively (C3a, C3p, Cza, Czp, C3a, and C4p). All EEGs were converted into a bipolar montage by calculating the difference for the electrodes of interest (C3 = C3a–C3p, Cz = Cza–Czp, C4 = C4a–C4p) [Neuper et al., 2006]. Bipolar recording was beneficial to reduce the possible artifacts of motion or eye‐blink. A ground electrode was placed on the right mastoid. The EEG signals (0.3–80 Hz) were amplified through a homemade multichannel amplifier with batteries [Shaw et al., 2002], which avoided potential 60‐Hz electromagnetic interference. Three bipolar EEGs were digitized by an analog‐to‐digital converter (USB6009, National Instruments, TX). The sampling rate was 500 Hz. All of the data acquisition and feedback processing were performed in the LabVIEW environment (National Instruments, TX). All raw EEG data were saved for advanced off‐line processes.
During the training phase of NFT, the amplitude spectra of bipolar EEGs were calculated using an FFT algorithm with a Hamming window on a second‐by‐second basis. In the Alpha group, 8–12‐Hz amplitudes of three bipolar EEGs were averaged as the feedback for every 1‐s episode. For the Ctrl group, amplitudes of three bipolar EEGs within a particular 4‐Hz bandwidth were calculated and averaged as the feedback for every 1‐s episode. The 4‐Hz bandwidth was randomly selected from the range of 7–20 Hz. Thus, the Ctrl group received various kinds of 4‐Hz amplitudes during a session.
The feedback contained two parts: the instantaneous information of an 1‐s averaged amplitude and the cumulative waveform of all 1‐s averaged amplitudes. The instantaneous amplitude was given by the means of a horizontal color bar. If the EEG amplitude increased, the bar moved to the right. Otherwise, a decreased EEG amplitude drove the bar left. Participants were instructed to move the bar to a rightmost position and to hold it there as long as possible. During the resting period between two consecutive training blocks, researchers identified occurred timestamps of high alpha amplitudes from a cumulative waveform then asked participants to recall what strategy they used to gain a high alpha amplitude. This process seemed to be greatly useful for the development of strategy. Participants were informed that eye closure was not a valid strategy during the training phase. A digital camera was used to rule out the effects of possible behavioral artifacts, for example, falling asleep/drowsiness, paying less attention during the training, or an inadequate strategy involving body movement.
To ascertain the training effect of an NFT, off‐line data processing of EEGs was performed. Alpha amplitude of 1‐s EEG was calculated using an FFT algorithm with a Hamming window for both baseline and training blocks. To eliminate possible artifacts, several steps were performed. For automatic artifact removal, two steps were executed. First, the mean and standard deviation (SD) of 1–30 Hz amplitudes of 1‐s EEGs within a 6‐min block were calculated. Second, the artifact was marked if the amplitude of a 1‐s EEG exceeded the mean plus 2.5 × SD. This automatic artifact removal worked for rejecting head or body motion artifacts. Subsequently, the original EEGs were visualized and manually marked for other contaminations such as eye blink.
In the present study, several parameters were used to indicate training performance. The relative alpha amplitude was defined by the amplitude of 8–12 Hz normalized by the average 8–12‐Hz amplitude of all 1‐s baseline EEGs, as shown below.
The relative alpha amplitude of all 1‐s EEGs within a session was averaged to obtain an index of the mean relative alpha amplitude. The mean relative alpha amplitude throughout 12 sessions was used to reflect the dynamic changes of alpha amplitude within an NFT. When the relative alpha amplitude of 1‐s EEG was higher than 1.5, thus the 1‐s EEG segment was considered a successful event. All successful 1‐s events within a session were cumulated as an index of the total alpha duration of successful alpha events. To evaluate the continuity of successful alpha events within an NFT, we counted the occurrence frequency of episodes of continuous successful alpha events. The occurrence frequency of duration of these successful alpha episodes was transferred into a probability density function to reflect the changes in alpha continuity throughout the training sessions.
The present study further identified the well‐trained “Responder” to evaluate alpha NFT on memory performance and spatial distribution of the training‐induced alpha enhancement. Due to no obvious change among the first three sessions in terms of alpha amplitude or duration (see results shown in Fig. 5A,B), the “Responder” was identified as a participant who had the total alpha duration of the 12th session greater than 95% confidence interval of the total alpha durations of the first three sessions.
Figure 5.
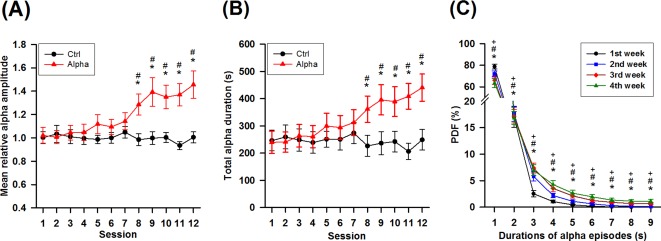
Dynamic changes of alpha rhythm throughout NFT. (A) Changes of mean relative alpha amplitude throughout 12 sessions in the two groups. *P < 0.05 versus 1st session, # P < 0.05 versus Ctrl. (B) Changes in the total alpha duration throughout 12 sessions in the two groups. *P < 0.05 versus 1st session, # P < 0.05 versus Ctrl. (C) Changes in the probability density function (PDF) of durations of alpha episodes per week in the Alpha group. *P < 0.05 2nd week versus 1st week. # P < 0.05 3rd week versus 1st week. + P < 0.05 4th week versus 1st week. All multiple comparisons are Bonferroni corrected. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
EEG Mapping
To verify the spatial distribution of the training‐induced alpha enhancement, whole‐head EEG recording and mapping were conducted for before the pre‐test and after the post‐test phases. EEG was recorded using 32‐Ag/AgCl electrode Neuroscan™ Quik‐Cap system, NuAmps amplifier and Scan 4.3 acquisition software (Neuroscan, Inc.). Electrode arrangement was performed according to the international 10–20 system and referenced to link bilateral mastoid processes [(A1 + A2)/2]. The impedance of each electrode was kept below 5 kΩ. EEG data were sampled at 500 Hz. The raw data were band‐pass filtered between 0.5 and 30 Hz. The Alpha amplitude for EEG mapping was calculated for each second using a FFT algorithm with a Hamming window by MATLAB software and EEGLAB toolbox (available at http://www.sccn.ucsd.edu/eeglab).
Memory Tasks
In the current study, the effects of an NFT on working memory and episodic memory were assessed. A backward digit span task was used to test the capacity of working memory storage. An operation span task was used to assess the storage and process of working memory. The word pair task tested two stages of episodic memory, that is, learning and recall. All memory tasks were presented in two sets used in the pre‐test and post‐test periods, respectively.
Backward digit span task
The backward digit span task is a typical test of memory span and is involved in the conversion and reorganization of working memory storage capacity [Jensen and Figueroa, 1975]. Thirty trials were performed. Each trial contained four to eight digits and each digit lasted for 1 s. A fixation (1 s) appeared before each trial. Participants were asked to enter the digits in reverse order (Fig. 1A). Each correct digit was awarded 1 point, and the maximum score for this task was 180.
Figure 1.
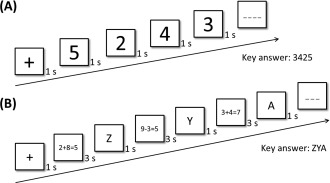
Examples of the backward digit span task (A) and the operation span task (B).
Operation span task
The operation span task is a test of memory span and involves the maintenance and processing of working memory under divided attention [Turner and Engle, 1989]. There were 20 trials. A fixation (1 s) appeared before each trial. In the present study, each trial contained three unrelated letters in a forward manner (such as ZYA, 1 s for each letter) and three addition/subtraction mathematical operations (such as 2 + 8 = 5, 3 s for each equation) that were interleaved. Participants were asked to answer “yes” or “no” in response to a mathematical equation within 3 s. At the end of each trial, participants needed to type the letters sequentially (Fig. 1B). Each correct letter or answer to a mathematical operation was awarded 1 point; the maximum score for this task was 120.
Word pair task
The word pair task [Plihal and Born, 1997], an episodic memory task, contained two stages, that is, learning and recall (Fig. 2). The present study used 80 pairs of Chinese words. In the learning stage, each trial started with a fixation (1,000 ms) followed by a pair of Chinese words (1,500 ms). Afterward, a blank picture (3,500 ms) was displayed before the next trial. In the recall stage, there were 80 retest trials. Each retest trial started with a fixation (1,000 ms) followed by a word shown in the learning stage (6,500 ms). Participants were asked to report the paired word. The interval between the learning and recall stages of the word pair task was 20 min or more [Wang and Zhou, 2002], which can preclude reliance on working memory rehearsal. In the present study, participants had to finish four questionnaires between the learning and recall stages, which took 30 min. Each correct answer in the word pair task was awarded one point, with a maximum score of 80.
Figure 2.
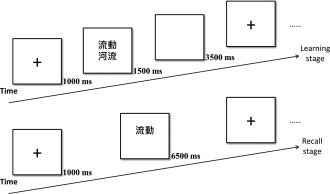
Schematic flowchart of the word pair task. There are two stages, that is, learning (top panel) and recall (bottom panel). The flow ( ) and river (
) and river ( ) items are paired.
) items are paired.
Questionnaires
Mini‐mental status examination (MMSE)
MMSE is commonly used to assess the cognitive function of participants. The MMSE score ranges from 0 to 30 points. A score of ≥24 is considered to indicate a participant with normal cognition. The MMSE score is used to ascertain whether a participant has severe (≤9 points), moderate (10–18 points), or mild (19–24 points) cognitive impairment. The reliability and validity of the MMSE has been verified [Folstein and McHugh, 1975].
Beck depression inventory‐second edition (BDI)
The BDI is a self‐reported questionnaire that contains 21 items to assess the intensity of depression. Each question has four choices (0–3 points). The BDI score is used to determine whether a participant has minimal (0–9 points), mild (10–18 points), moderate (19–29 points), or severe (30–63 points) depression. The reliability and validity of the BDI has been verified [Beck et al., 1996].
Beck anxiety inventory (BAI)
BAI is a self‐reported questionnaire which contains 21 questions about how the participant has felt in the last week. Each question has four choices (0–3 points). The BAI score indicates whether a participant has minimal (0–7 points), mild (8–15 points), moderate (16–25 points), or severe (26–63 points) anxiety. The reliability and validity of the BAI has been verified [Fydrich et al., 1992].
Pittsburgh sleep quality index (PSQI)
PSQI is a self‐reported questionnaire to measure the quality and patterns of sleep. Seven domains related to sleep are measured: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction over the last month. A PSQI score of ≤5 is considered to be that of a normal sleeper. The reliability and validity of the PSQI has been verified [Backhaus et al., 2002]
Experimental Procedure
The experimental procedure contained three phases (Fig. 3A): pre‐test, training, and post‐test. During the training phase, twelve sessions were carried out within 4 weeks (three sessions per week). Each session contained a block of a 2‐min EEG baseline recording followed by six training blocks of 6 min with a 1‐min break for resting [Dempster and Vernon, 2009]. In a baseline block, participants were asked to keep their eyes open and relax. During the resting period, researchers used the 6‐min tendency of alpha amplitude to help identify the situation of increased high alpha amplitude and to reinforce them to develop a strategy to produce selected brain activity.
Figure 3.
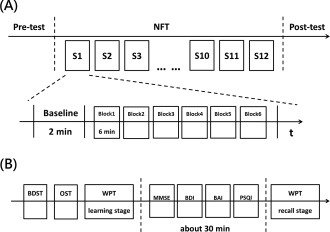
Experimental procedure of neurofeedback training (NFT). (A) An NFT contained three phases, that is, pre‐test, training, and post‐test. The training phase contained 12 sessions. Each session contained a 2‐min baseline followed by six blocks of 6‐min training. (B) During the pre‐test and post‐test phases, two working memory tasks (BDST and OST), an episodic memory task (WPT) and four questionnaires (MMSE, BDI, BAI, and PSQI) were performed. BAI, Beck anxiety inventory; BDI, Beck depression inventory; BDST, backward digit span task; MMSE, mini‐mental status examination; OST, operation span task; PSQI, Pittsburgh sleep quality index; WPT, word pair task.
During the phases of the pre‐test and post‐test, several questionnaires and cognitive tasks were performed. Two working memory tasks, that is, the backward digit span task and the operation span task, were performed, followed by the learning stage of the word pair task. Subsequently, four questionnaires, that is, the MMSE, PSQI, BAI, and BDI, were carried out. The questionnaires took approximately 30 min. Afterward, the pairs of words were recalled and evaluated. A schematic diagram for the entire experiment is illustrated in Figure 3.
Statistical Analysis
A chi‐squared test and Student's t‐test were used to assess the demographic characteristics of the two groups. Two‐factor mixed analysis of variance (ANOVA) followed by post hoc comparisons were conducted on the mean relative alpha amplitude, total alpha duration, and alpha amplitude of the baseline block. Two‐way repeated measured ANOVAs were conducted for the probability density function of alpha duration. All pairwise comparisons were conducted with Bonferroni correction. Amplitudes of the delta (1–3 Hz), theta (4–7 Hz), alpha (8–12 Hz), and beta (13–30 Hz) bands in the 1st and 12th sessions of both groups were compared using paired t‐test.
Accuracies of memory tasks before and after NFT were assessed by two‐factor mixed ANOVA. All pairwise comparisons were conducted with Bonferroni correction. Improved accuracy was defined as a post‐test accuracy of an individual being greater than its pre‐test accuracy. The improved accuracy was evaluated by Student's t‐test. The chi‐squared test was further used to examine the proportion of Alpha group and “Responder” with improved accuracy compared with 50% of a random level and compared with that of the Ctrl group. All statistical analyses were performed by Statistical Package for Social Sciences version 17.0 software (SPSS, Chicago, Illinois). Data were expressed as the mean ± standard error of the mean. A two‐tailed significance level was set at P < 0.05.
RESULTS
During the training, we often encouraged all participants to try their best to induce high brain amplitude at the 1‐min rest period between training blocks. All participants in both groups did not complain or become frustrated throughout the training. Although the Ctrl group seemed to be uneasy about controlling their brain activities, they reported no problems in continuing and completing the training.
EEG Data
The original EEG and its time‐frequency plot of a participant in the Alpha group are shown in Figure 4. Time‐frequency analysis of the EEG was performed using the short‐time FFT with a Hamming window of 1‐s EEG under 90% temporal overlap. No obvious artifact was observed in the EEG trace. At the beginning of the training session, a great low‐frequency activity (<6 Hz) accompanied by sparse alpha activity was recorded. In the middle of the training session, a transient alpha amplitude occurred at several segments. At the end of the training session, a continuous alpha rhythm was consistently recorded. In this participant, the amplitude and duration of the alpha rhythm were progressively and remarkably elevated throughout the training.
Figure 4.
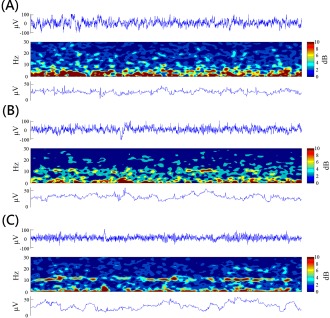
Original EEG trace and its time‐frequency plot in the 1st (A), 6th (B), and 12th (C) session of a participant in the Alpha group.
For the averaged alpha amplitude of baseline blocks throughout 12 sessions, there was no significant difference in the factors of group (F 1,48 = 0.052, P = 0.82), session (F 11,528 = 0.592, P = 0.836), or their interaction (F 11,528 = 0.572, P = 0.852). The mean relative alpha amplitude showed no obvious change in the first three sessions then had progressively increased throughout the training (Fig. 5A). Participants reported trial‐and‐error process to build up a strategy at the beginning. The mean relative alpha amplitude had a significant difference in the factors of group (F 1,48 = 6.334, P = 0.015), session (F 11,528 = 5.371, P < 0.001), and their interaction (F 11,528 = 7.047, P < 0.001). The mean relative alpha amplitude showed no significant difference in the Ctrl group throughout the training. In contrast, the mean relative alpha amplitudes of the 8th–12th sessions in the Alpha group showed significant differences compared with those of its first session, and they also significantly differed from those of the Ctrl group. Moreover, change of the mean absolute alpha amplitude revealed a similar development as did the mean relative alpha amplitude throughout the training (Fig. S1 in Supporting Information).
In addition to the mean relative alpha amplitude, total alpha duration showed no change at the first three sessions then had progressive increase throughout the training (Fig. 5B). The total alpha duration had significantly different in the factors of session (F 11,599 = 4.108, P < 0.001) and the interaction between group and session (F 11,599 = 6.254, P < 0.001). There was no significant difference in the Ctrl group throughout 12 sessions. In contrast, the total alpha duration of the 8th–12th sessions in the Alpha group showed significant differences compared with that of the first session, and they also significantly differed from those of the Ctrl group.
To further characterize the continuity of alpha rhythm throughout an NFT, continuous episodes of alpha rhythm were counted in terms of probability. In the present study, the probability density function per week showed a significant difference in the factors of week (F 3,576 = 2,661.420, P < 0.001), alpha duration (F 3,576 = 6.998, P < 0.001), and their interaction (F 3,576 = 431.594, P < 0.001). Alpha episodes of 1–9 s in the 4th week significantly differed from those of the 1st week (Fig. 5C). These progressive changes indicated that the duration of alpha episodes increased progressively in the Alpha group.
To further characterize changes in the amplitude spectra of both groups, the amplitude spectra between the 1st and 12th sessions were illustrated in Figure 6. In the Ctrl group, there was no significant difference in their spectra between the 1st and 12th sessions. In contrast, there was significantly different in the alpha band (t = −2.876, P = 0.008) but not in other bands (delta, P = 0.789; theta, P = 0.162; beta, P = 0.301) of the Alpha group. In particular, amplitudes of 10, 11, and 12 Hz at the 12th session were significantly higher than those of the first session in the Alpha group.
Figure 6.
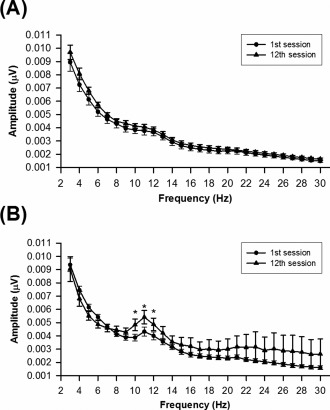
Amplitude spectra of the 1st and 12th sessions in the Ctrl group (A) and Alpha group (B). *P < 0.05 versus 1st session with Bonferroni correction.
EEG Mapping
Several aspects of alpha rhythm showed progressive enhancement throughout the training sessions. We further characterized a successful trainer as a “Responder” in the Alpha group. Twenty participants (80%) were identified as “Responder” in the present study.
The averaged absolute alpha amplitude of baseline at the 1st session for “Responder” (n = 20) and non‐responder (n = 5) of Alpha group were 0.0219 ± 0.002 and 0.0226 ± 0.003 μV, respectively. There was no significant difference in the alpha amplitude between “Responder” and non‐responder (t = 0.185, P = 0.855, Student's t‐test). Our results indicated that the alpha amplitude of “Responder” before NFT didn't differ from that of non‐responder. Moreover, the non‐responder showed a slight increase in amplitude but revealed a great variance throughout the training. Both the non‐responder and the Ctrl group had no significant difference in the alpha amplitude (F 1,28 = 1.256, P = 0.25) or total alpha duration (F 1,28 = 0.608, P = 0.822) throughout training sessions.
We further characterized the spatial distribution of alpha rhythm in 20 “Responders” of the Alpha group. In the raw EEG data (5 s) for a “Responder” (Fig. 7), alpha activity was not obvious before NFT. The distribution of alpha rhythm in all “Responders” was not identified. In contrast, a dramatic enhancement of alpha rhythm was seen over the frontal and parietal cortices after NFT.
Figure 7.
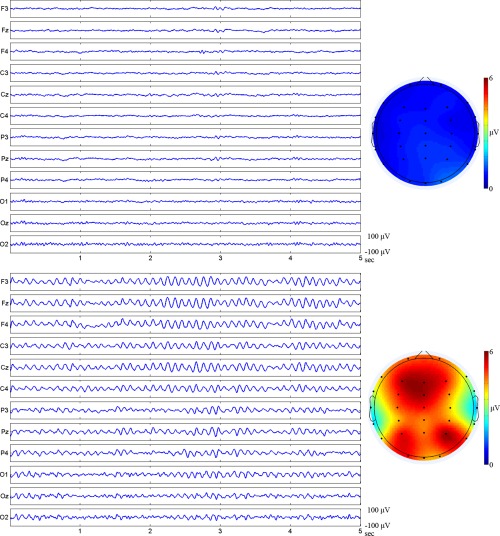
Raw EEG data of a selected “Responder” of the Alpha group (left column) and averaged whole‐head EEG mapping (right column) for 20 “Responders” before (top panel) and after NFT (bottom panel). Enhanced alpha amplitude is obviously shown in the frontal and parietal cortices after NFT.
Memory Performance
Backward digit span task
Figure 8A shows the performance of the backward digit span task in the two groups before and after NFT. Accuracy of the backward digit span task showed a significant difference in the factor of time (F 1,48 = 16.911, P < 0.001), but not in the factors of group (F 1,48 = 2.585, P = 0.114) and their interaction (F 1,48 = 0.429, P = 0.516). Both groups had significant increases on accuracy after training.
Figure 8.
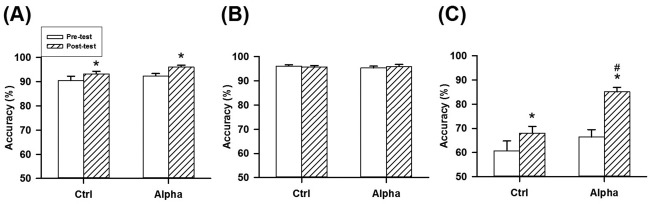
Changes in the accuracies of the backward digit span task (A), operation span task (B), and word pair task (C) before and after NFT in the two groups. *P < 0.05 versus pre‐test, # P < 0.05 versus Ctrl with Bonferroni correction.
The Alpha group (3.75 ± 0.68, range: −0.56 to 14.44) showed slightly higher improvement of accuracy than the Ctrl group (2.72 ± 1.41, range: −7.78 to 18.33; t = 0.655, P = 0.516). We further analyzed the proportion of participants that gained improvement in accuracy. The proportion of the Alpha group with improved accuracy significantly differed from a chance level of 50% (P < 0.001) and was also higher than that of the Ctrl group (P < 0.001, Table 1).
Table 1.
Proportion of participants with improved memory performance in the two training groups
| Group | Backward digit span task | Operation span task | Word pair task |
|---|---|---|---|
| Ctrl | 52% | 60% | 68%[Link] |
| Alpha | 96%[Link],[Link] | 76%[Link] | 100%[Link],[Link] |
Ctrl, control group;
P < 0.05 vs. Ctrl,
P < 0.05 vs. 50% chance by chi‐squared test.
In consideration of “Responders” in the Alpha group, accuracy of the backward digit span task showed significantly different in the factors of time (F 1,43 = 14.759, P < 0.001) and group (F 1,43 = 4.473, P = 0.040), but not in their interaction (F 1,43 = 0.531, P = 0.470). The post‐test accuracy of “Responders” in the Alpha group was significantly higher than that of Ctrl group. All “Responders” (100%) showed improved accuracy. The proportion of the “Responder” with improved accuracy significantly differed from a chance level of 50% (P < 0.001) and was also higher than that of the Ctrl group (P < 0.001).
Operation span task
Figure 8B shows the performance of the operation span task in the two groups before and after NFT. Accuracy of the operation span task showed no significant difference in the factors of time (F 1,48 = 0.054, P = 0.817), group (F 1,48 =0.073, P = 0.788), and their interaction (F 1,48 = 1.126, P = 0.294). Accuracies of the pre‐test and post‐test phases in the operation span task were high (>95%).
The Alpha group (0.57 ± 0.73, range: −12.50 to 9.17) showed slightly higher improvement of accuracy than the Ctrl group (−0.36 ± 0.48, range: −6.30 to 4.17; t = 1.061, P = 0.294). The proportion of Alpha group with improved accuracy significantly differed from a chance of 50% (P < 0.04, Table 1). The number of participants with improved accuracy in the Alpha group was slightly higher than that of the Ctrl group (P = 0.363).
For “Responders” in the Alpha group, the accuracy of the operation span task showed a significant difference in the group‐time interaction (F 1,43 = 7.023, P = 0.011), but not in the factors of group (F 1,43 = 0.107, P = 0.745) and time (F 1,43 = 2.688, P = 0.108). The post‐test accuracy of the “Responder” was significantly higher than the pre‐test accuracy. Improved accuracy of the “Responder” (1.54 ± 0.53, range: −2.5 to 9.17) showed significantly higher than that of the Ctrl group (t = 2.650, P = 0.011). The proportion of the “Responder” with improved accuracy was 85%, which significantly differed from a chance level of 50% (P < 0.001) and almost attained a significant level compared with that of the Ctrl group (P = 0.064).
Word pair task
Figure 8C shows the performance of the word pair task in the two groups before and after NFT. Accuracy of the word pair task was significantly different in the factors of group (F 1,48 = 9.442, P = 0.003), time (F 1,48 = 32.449, P < 0.001), and their interaction (F 1,48 = 6.098, P = 0.017). Both groups had a significant increase in accuracy after NFT. Furthermore, the post‐test accuracy of the Alpha group was significantly higher than that of the Ctrl group.
Based on improvement of accuracy, the Alpha group (18.65 ± 2.49, range: 3.75–50.00) as significantly higher than the Ctrl group (7.37 ± 3.83, range: −38.75 to 51.25; t = 2.469, P = 0.017). Interestingly, all participants in the Alpha group gained increased accuracy (Table 1). The proportion of the Alpha group with improved accuracy was significantly higher than a chance of 50% (P < 0.001). The percentage of participants with increased accuracy was significantly different in the two groups (P < 0.001).
For “Responders” in the Alpha group, accuracy of the word pair task showed significant difference in the factors of time (F 1,43 = 33.195, P < 0.001), group (F 1,43 = 5.812, P = 0.020) and their interaction (F 1,43 = 7.699, P = 0.008). The post‐test accuracy of the “Responder” was significantly higher than that of the Ctrl group. Improved accuracy of the “Responder” (21.06 ± 2.73, range: 6.25–50.00) was significantly higher than that of the Ctrl group (t = 2.775, P = 0.008). All “Responders” revealed improved accuracy.
Questionnaires
MMSE
MMSE scores of the Ctrl group in the pre‐ and post‐test phases were 29.24 ± 1.17 and 29.68 ± 0.63, respectively. MMSE scores of the Alpha group in the pre‐ and post‐test phases were 29.24 ± 1.09 and 29.80 ± 0.50, respectively. All participants had a normal score (≥24) before and after NFT.
BDI
BDI scores of the Ctrl group in the pre‐ and post‐test phases were 5.52 ± 4.84 and 5.08 ± 4.58, respectively. BDI scores of the Alpha group in the pre‐ and post‐test phases were 6.56 ± 4.65 and 4.76 ± 4.08, respectively. BDI scores of all participants were in the normal range (≤13) before and after NFT.
BAI
BAI scores of the Ctrl group in the pre‐ and post‐test phases were 4.52 ± 4.28 and 3.20 ± 3.58, respectively. BAI scores of the Alpha group in the pre‐ and post‐test phases were 4.76 ± 3.41 and 3.04 ± 2.51, respectively. BAI scores of all participants were in the normal range (≤7) before and after NFT.
PSQI
PSQI scores of the Ctrl group for the pre‐ and post‐test were 4.20 ± 2.18 and 4.04 ± 2.23, respectively. PSQI scores of the Alpha group for pre‐ and post‐test were 3.96 ± 1.31 and 3.92 ± 1.15, respectively. PSQI scores of all participants were in the normal range (≤5) after NFT.
DISCUSSION
In the present study, successful training of the frontoparietal alpha rhythm was demonstrated in terms of progressive changes in the mean relative alpha amplitude, the total alpha duration, and the probability density function of continuous alpha episodes throughout 12 sessions in the Alpha group exclusively. The Alpha group (particularly for “Responder”) showed a higher proportion of participants with significant enhancement in both working memory and episodic memory compared with that of the Ctrl group. Three questionnaires dealing with anxiety, depression, and insomnia revealed normal scores before and after NFT. Our findings suggest that alpha NFT has a great benefit on memory enhancement with little effect on anxious, depressive, or insomnia symptom.
Previous studies have raised the issue of trainability in alpha rhythm [Boynton, 2001; Cho et al., 2008; Juhel, 2011; Zoefel et al., 2011]. In the present study, 80% of the 25 participants showed significant training success throughout 12 sessions of six 6‐min training blocks. This is remarkable because in therapy, there is typically a 3‐ to 10‐fold higher number of sessions being utilized [Holtmann et al., 2011; Monastra et al., 2006].
In the training course of alpha NFT, there was no obvious change in the mean relative alpha amplitude and total alpha duration at the beginning. This may indicate a potential focus of the participants' attention on trying to produce a right bar movement. This may lead participants to initially drive their attention on the feedback information. Such an issue of attention could lead to an interference in the genesis of alpha rhythm, which is indicated in previous studies [Aftanas and Golocheikine, 2001; Cooper et al., 2003]. This attention process may be partially supported by the early stage with theta activation (Figs. 4 and 6), which is related to the attention process [Klimesch, 1999; Wang and Hsieh, 2013]. Afterward, a slight increase in the mean relative alpha amplitude was seen in the 5th–7th sessions followed by a dramatic increase in the mean relative alpha amplitude after the 8th–12th sessions. This pattern of increased alpha amplitude may reflect the latent learning of a trial‐and‐error process. The trial‐and‐error operation is a crucial process for biofeedback/neurofeedback [Gruzelier, 2014].
Our findings on the progress in the amplitude of upper alpha frequency bandwidth differ from a previous report that showed a linear increase of upper‐alpha amplitude [Zoefel et al., 2011]. The discrepancies between these two studies may arise from differences in training design (every day for 5 days [Zoefel et al., 2011] vs. 3 days per week for 4 weeks) or in feedback context (color gradient [Zoefel et al., 2011] vs. two‐step feedback [instantaneous color bar and cumulative information of each 6‐min block]).
In addition to changes in alpha amplitude and total alpha duration, the length of each individual alpha episode was progressively increased when the session number increased. From participants' reports, they can devise a strategy to produce a stable alpha rhythm in the frontoparietal region. In regards to the reliability issue of an NFT, the phenomenon of a long and stable successful event episode may be an important index to assess successful training [Dempster and Vernon, 2009]. Thus, the present study provided valuable information about the trainability of alpha rhythm in terms of probability density function of durations of successful alpha episodes.
In the present study, a specific enhancement of the alpha rhythm but no other frequency bands was observed (Fig. 6). The phenomenon is consistent with previous studies [Cho et al., 2008; Zoefel et al., 2011]. Thus, NFT effects were restricted to a particular trained frequency band, reflecting independence. An NFT can be conducted by focusing on the alpha frequency component in isolation from other components of the EEG [Cho et al., 2008] or by incorporating the surrounding frequencies of theta or beta bands into the NFT protocol [Egner et al., 2004]. It would be interesting to see whether different training paradigms elicit different results.
The alpha rhythm reveals age‐related changes [Klimesch, 1999]. Young adults show an alpha peak at a relatively higher frequency (≥10 Hz), and children or elders show their peak at a relatively lower frequency (<10 Hz). Many strategies have been proposed for alpha NFT, including feedback of alpha peak amplitude [Angelakis et al., 2007] and entire alpha amplitude [Cho et al., 2008; this study]. Moreover, some studies focused on individual differences in alpha rhythm; thus, they proposed to train the upper band of an individual alpha frequency [Hanslmayr et al., 2005; Zoefel et al., 2011]. Although many strategies have been proposed for alpha trainings, the upper alpha band is often emphasized for memory performance [Doppelmayr et al., 2002; Klimesch et al., 2006a, b]. The present study provided the 8–12‐Hz amplitude as feedback in the Alpha group, and the amplitude had significant elevation exclusively in the upper alpha band (10–12 Hz). However, there was no change in amplitude spectra of the Ctrl group. The phenomenon of enhanced amplitude in the upper alpha band of the Alpha group is an objective‐oriented training effect and not an artifact. It remains to be determined why most participants in this study revealed a trend toward elevating the amplitude of the upper alpha band. Young adults were recruited and enrolled in the present study. Young adults were used to present alpha amplitudes in the upper alpha frequency band [Klimesch, 1999]. The age‐related issue may explain the tendency toward a specific training effect on the upper alpha band.
Although previous studies indicated that a proper control‐group design cannot be fully realized [Kotchoubey et al., 2001; Vernon et al., 2003], numerous studies have introduced several types of control groups such as a no comparable training event [Nan et al., 2012; Zoefel et al., 2011] or taking yoga practice [Juhel, 2011]. The present study used the same apparatus to provide feedback of a 4‐Hz amplitude in the range of 7–20 Hz randomly in the sham Ctrl group. Ideally, a 4‐Hz amplitude in the Ctrl group can be selected from a broad frequency band (e.g., 0–80 Hz). However, a relatively high amplitude existed in the low‐frequency range (<6 Hz), and a low amplitude was shown in the high‐frequency range (>20 Hz, Fig. 4). In our pilot study, feedback of a 4‐Hz amplitude in the broad band caused a great fluctuation compared with that used in the Ctrl group here. This large fluctuation usually caused great instability in feedback that resulted in participant frustration about learning an operant task. Thus, we selected a 4‐Hz amplitude in the range of 7–20 Hz. To reduce possible frustration and fatigue, a brief 6‐min training block accompanied by a 1‐min resting period was designed. Researchers indicated time points of successful events from the cumulative waveform to help participants develop a strategy and also encouraged them during the resting period. As we observed in this study, there was no frustration reported throughout the trainings in both groups. The Ctrl group had equivalent days and times as the Alpha group, and there was no significant change in the alpha amplitude and total alpha duration (Fig. 5) as well as its amplitude spectra (Fig. 6). Moreover, the proportion of participants with improved accuracy showed no difference compared with a chance of 50% in terms of working memory tasks (Table 1). These results may, at least in part, support that the sham Ctrl group may be reasonable and adequate.
Controversial results among previous NFT studies may be greatly due to different experimental designs and diverse memory tasks. In addition, selective data analysis for successful well‐trained “Responder” may be critical. Previous studies reported that 50%–75% of participants successfully enhanced their alpha amplitudes after alpha NFT and revealed improved performance on cognitive functions [Angelakis et al., 2007; Hanslmayr et al., 2005; Zoefel et al., 2011]. The Alpha group revealed significant elevation on the accuracy of the word pair task compared with the Ctrl group. At this moment, the Alpha group contained “Responder” and non‐responder. The “Responder” analysis can strengthen specific effect of NFT on cognitive functions. In the present study, “Responders” were identified from successful training in terms of significant elevation in total alpha durations throughout the alpha NFT. We found 80% of participants as “Responder” in the Alpha group. When we considered the “Responders” exclusively, there were significant increases in accuracies of all memory tasks after NFT. The accuracies of both the word pair task and backward digit span task after NFT were significantly higher in the “Responder” than the Ctrl group. Our results indicate a specific effect of the alpha NFT on memory. Taken together, “Responder” should be considered in evaluation of an NFT.
Alpha trainings cause different results in cognitive performance. A previous study with an NFT of alpha amplitude reported no effect on memory performance compared with that of a yoga group in elderly people [Juhel, 2011]. Most studies training the upper alpha band showed significant enhancement in short‐term memory or working memory (mental rotation, digit span and semantic span tasks) [Hanslmayr et al., 2005; Nan et al., 2012; Zoefel et al., 2011]. The present study revealed significant improvement in accuracy of working memory using the backward digit span task and operation span task, particularly for “Responder” of the Alpha group. Interestingly, all participants of the Alpha group gained improvement in the word pair task of episodic memory. The accuracy of the word pair task was significantly higher than that of the Ctrl group. The present study extends our knowledge about the advantages of alpha training not only on working memory but also on episodic memory.
In the present study, we designed a sham control group proposed in the study of Wang and Hsieh [2013]. Some similarities exist in the two studies, for example, a sham control experimental design, no change of amplitude of trained bandwidth in the control group, and performing working memory on young adults. However, there are numerous differences between the two studies. First, training bandwidths of EEGs are different (increased theta/inhibited delta & high beta [Wang and Hsieh, 2013] vs. increased alpha of this study). Second, different training protocols are used for sham controls. Wang and Hsieh [2013] randomly selected a bandwidth from a set of 10–13, 13–16, 16–20, or 20–25 Hz and used the selected bandwidth as the feedback during a session. In contrast, we selected a 4‐Hz amplitude randomly from the range of 7–20 Hz in every 1‐s episode. Thus, we did not use a fixed bandwidth feedback during a session. Third, Wang and Hsieh [2013] reported a change of baseline theta activation after training. We did not see baseline alpha changes throughout the training. Finally, Wang and Hsieh [2013] did not observe significant improvement of working memory in young adults even though their participants had significant increased theta amplitude. In a sharp contrast, we observed significant improvement on accuracies of both working memory and episodic memory, particularly for alpha “Responders.”
The Ctrl group after NFT had significant increases in the accuracies of the backward digit span task and word pair task. Similar phenomenon has been reported in the control group of numerous neurofeedback studies [Escolano et al., 2014; Nan et al., 2012; Juhel, 2011]. Indeed, participants are more familiar with a cognitive task at the second run than the first test. They can usually get a better post‐test performance because of learning. The learning effect due to repeated measures of a cognitive task may explain improvement of the Ctrl group.
The amplitude of alpha oscillations reflects a level of cortical inhibition [Palva and Palva, 2007]. In general, high‐amplitude alpha oscillations reflect the inhibition and disengagement of task‐irrelevant cortical areas [Klimesch, 1999]. Why can the alpha NFT create a potential benefit for memory? Several lines of evidence may provide plausible clues to explain this phenomenon. During the analysis of event‐related potentials, the amplitude of the alpha band was enhanced during the retention phase of short‐term memory, working memory, and episodic memory [Jensen et al., 2002; Klimesch et al., 2006a, b; Sauseng et al., 2005]. It has been proposed that alpha oscillations in the frontoparietal network during the memory retention period are essential for the network activity that sustains the neuronal representations of memory items [Klimesch et al., 2007; Palva and Palva, 2007]. The retrieval‐associated alpha suppression reflects, in part, the termination of the memory process itself [Jensen et al., 2002]. Moreover, alpha rhythm play an active role to inhibit task‐irrelevant processes and also manipulate the timing of cortical processing [Klimesch et al., 2007], which may create a better signal‐to‐noise ratio and improve efficiency. These views are supported by a positive correlation between the alpha amplitude with the working memory load [Jensen et al., 2002] and task difficulty [Sauseng et al., 2005]. These findings suggest that endogenous alpha rhythm by NFT may engage with process of working and episodic memories.
In addition to event‐related memory processing of alpha rhythm, higher alpha activity of the frontoparietal region, particularly in the upper alpha band, has been demonstrated to reflect better memory and intelligence [Doppelmayr et al., 2002; Klimesch, 1999]. The present study showed participants had a great ability to control the frontoparietal alpha rhythm and revealed great memory enhancement. Thus, participants in the Alpha group may have a propensity to display alpha rhythm and show a better memory performance.
Both perception and attention are crucial for learning and further memory enhancement [Nissen and Bullemer, 1987]. The alpha‐frequency band synchrony is also important in attention and perception. For example, intercortical alpha‐frequency band synchrony is prominent during “Go” trials of the Go/No‐Go task [von Stein et al., 2000]. In addition, the alpha‐frequency amplitude of the sensorimotor and parietal cortices can reflect attentional efforts or optimal filter properties for detecting weak incoming stimuli in a psychophysical task [Linkenkaer‐Hansen et al., 2004]. An increase in prefrontal alpha amplitudes enables a tight functional coupling within prefrontal cortical areas and thereby allows the control of the executive processes in primary visual cortices under a visuospatial task [Sauseng et al., 2005]. These data support a direct role for alpha‐frequency band oscillations in the neuronal mechanisms of top‐down modulation and attention. Recently, the sensorimotor alpha rhythm of a single NFT session was used to facilitate the early acquisition of a procedural motor task [Ros et al., 2014]. This evidence may suggest that a substantial top‐down network is modulated by alpha NFT to potentiate learning and memory.
Memory improvement has been reported in the present study and previous alpha NFT studies [Hanslmayr et al., 2005; Nan et al., 2012; Zoefel et al., 2011]. Similar phenomena about enhanced memory are also observed in numerous NFT studies using different feedback rhythms, such as sensorimotor, theta, or gamma rhythm [see review, Gruzelier, 2014]. One may argue that memory improvement caused by NFT is non‐related to brain rhythms or non‐specific. The argument may suggest a common neural substrate or network for NFT on memory enhancement. Increased memory performance may be associated with improving perception, attention, or essential elements of memory (including encoding, consolidation, or retrieve). Indeed, theta rhythm in hippocampo‐cortical network reflect the encoding of new information on memory, whereas upper alpha rhythm in thalamo‐cortical network reflect retrieval processes of memory [Klimesch, 1999]. According to these findings, NFT of different brain rhythms may have distinct variation on memory processes and lead to a better memory outcome.
In contrast to the correlation between alpha amplitude and memory, the amplitude of alpha oscillations has been proposed to be related to anxiety [Heller et al., 1997], depression [Jaimchariyatam et al., 2011], or insomnia [Cervena et al., 2014] in patients. In the present study, all participants had a normal score and did not show a worsening in psychiatric measures (measured by BAI, BDI, and PSQI) and cognitive function (MMSE). Both groups had no significant difference in alpha amplitudes of baseline throughout the training. In our follow‐up questionnaires 3 months later, participants had no complaints about these psychiatric problems (data not shown). These results suggest that alpha NFT may not cause symptom of anxiety, depression, or insomnia.
In the present study, accuracy of the operation span task was approximately 95%, which implies very easy to most participants. If a task has extremely high accuracy, it is difficult to have good differentiability. Fortunately, “Responder” of the Alpha group showed significant improvement after the NFT, and the Ctrl group had no difference. In order to boost differentiability of the operation span task, a task difficulty, for example, chance level or complexity of mathematical calculation, should be tested before the experiment.
CONCLUSIONS
Our study provided promising results using an NFT of 8–12‐Hz amplitude to demonstrate the trainability of frontoparietal alpha rhythm and their functional correlations with working memory and episodic memory. Design of a sham control group and “Responder” analysis provided valuable information to strengthen effect of the alpha NFT on memory. These findings may trigger further validation on the relationship between the frontoparietal alpha rhythm of NFT and cognitive performance.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The authors thank the Mind Research and Imaging Center, National Cheng Kung University, for instrument availability.
REFERENCES
- Aftanas LI, Golocheikine SA (2001): Human anterior and frontal midline theta and lower alpha reflect emotionally positive state and internalized attention: High‐resolution EEG investigation of meditation. Neurosci Lett 310:57–60. [DOI] [PubMed] [Google Scholar]
- Angelakis E, Stathopoulou S, Frymiare JL, Green DL, Lubar JF, Kounios J (2007): EEG neurofeedback: A brief overview and an example of peak alpha frequency training for cognitive enhancement in the elderly. Clin Neuropsychol 21:110–129. [DOI] [PubMed] [Google Scholar]
- Anokhin A, Vogel F (1996): EEG alpha rhythm frequency and intelligence in normal adults. Intelligence 23:1–14. [Google Scholar]
- Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F (2002): Test–retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res 53:737–740. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK (1996): Manual for the BDI‐I. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Boynton T (2001): Applied research using alpha/theta training for enhancing creativity and well‐being. J Neurother 5:5–18. [Google Scholar]
- Cervena K, Espa F, Perogamvros L, Perrig S, Merica H, Ibanez V (2014): Spectral analysis of the sleep onset period in primary insomnia. Clin Neurophysiol 125:979–987. [DOI] [PubMed] [Google Scholar]
- Cho MK, Jang HS, Jeong SH, Jang IS, Choi BJ, Lee MT (2008): Alpha neurofeedback improves the maintaining ability of alpha activity. Neuroreport 19:315–317. [DOI] [PubMed] [Google Scholar]
- Cooper NR, Croft RJ, Dominey SJJ, Burgess AP, Gruzelier JH (2003): Paradox lost? Exploring the role of alpha oscillations during externally vs. internally directed attention and the implications for idling and inhibition hypotheses. Int J Psychophysiol 47:65–74. [DOI] [PubMed] [Google Scholar]
- Cortoos A, De Valck E, Arns M, Breteler MHM, Cluydts R (2010): An exploratory study on the effects of tele‐neurofeedback and tele‐biofeedback on objective and subjective sleep in patients with primary insomnia. Appl Psychophys Biof 35:125–134. [DOI] [PubMed] [Google Scholar]
- Dempster T, Vernon D (2009): Identifying indices of learning for alpha neurofeedback training. Appl Psychophys Biof 34:309–318. [DOI] [PubMed] [Google Scholar]
- Doppelmayr M, Klimesch W, Stadler W, Pöllhuber D, Heine C (2002): EEG alpha power and intelligence. Intelligence 30:289–302. [Google Scholar]
- Doppelmayr M, Klimesch W, Hödlmoser K, Sauseng P, Gruber W (2005): Intelligence related upper alpha desynchronization in a semantic memory task. Brain Res Bull 66:171–177. [DOI] [PubMed] [Google Scholar]
- Egner T, Zech TF, Gruzelier JH (2004): The effects of neurofeedback training on the spectral topography of the electroencephalogram. Clin Neurophysiol 115:2452–2460. [DOI] [PubMed] [Google Scholar]
- Escolano C, Aguilar M, Minguez J (2011): EEG‐based upper alpha neurofeedback training improves working memory performance. 33rd Ann Int Conf of IEEE Eng Med Biol Soc. Boston, USA, pp 2327–2330. [DOI] [PubMed]
- Escolano C, Navarro‐Gil M, Garcia‐Campayo J, Minguez J (2014): The effects of a single session of upper alpha neurofeedback for cognitive enhancement: A sham‐controlled study. Appl Psychophys Biof 39:227–236. [DOI] [PubMed] [Google Scholar]
- Folstein SE, McHugh PR (1975): “Mini‐Mental State”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198. [DOI] [PubMed] [Google Scholar]
- Fydrich T, Dowdall D, Chambless DL (1992): Reliability and validity of the Beck Anxiety Inventory. J Anxiety Disord 6:55–61. [Google Scholar]
- Gruzelier JH (2014): EEG‐neurofeedback for optimising performance. I: A review of cognitive and affective outcome in healthy participants. Neurosci Biobehav R 44:124–141. [DOI] [PubMed] [Google Scholar]
- Hammond DC (2000): Neurofeedback treatment of depression with the Roshi. J Neurother 4:45–56. [Google Scholar]
- Hammond DC (2005): Neurofeedback treatment of depression and anxiety. J Adult Dev 12:131–137. [Google Scholar]
- Hanslmayr S, Sauseng P, Doppelmayr M, Schabus M, Klimesch W (2005): Increasing individual upper alpha power by neurofeedback improves cognitive performance in human subjects. Appl Psychophys Biof 30:1–10. [DOI] [PubMed] [Google Scholar]
- Heller W, Nitschke JB, Etienne MA, Miller GA (1997): Patterns of regional brain activity differentiate types of anxiety. J Abnorm Psychol 106:376–385. [DOI] [PubMed] [Google Scholar]
- Holtmann M, Steiner S, Hohmann S, Poustka L, Banaschewski T, Boelte S (2011): Neurofeedback in autism spectrum disorders. Dev Med Child Neurol 53:986–993. [DOI] [PubMed] [Google Scholar]
- Jaimchariyatam N, Rodriguez CL, Budur K (2011): Prevalence and correlates of alpha‐delta sleep in major depressive disorders. Innov Clin Neurosci 8:35–49. [PMC free article] [PubMed] [Google Scholar]
- Jensen AR, Figueroa RA (1975): Forward and backward digit span interaction with race and IQ: Predictions from Jensen's theory. J Educ Psychol 67:882–893. [PubMed] [Google Scholar]
- Jensen O, Gelfand J, Kounios J, Lisman JE (2002): Oscillations in the alpha band (9–12 Hz) increase with memory load during retention in a short‐term memory task. Cereb Cortex 12:877–882. [DOI] [PubMed] [Google Scholar]
- Juhel J (2011): The effects of neurofeedback training on memory performance in elderly subjects. Psychology 2:846–852. [Google Scholar]
- Klimesch W (1999): EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res Rev 29:169–195. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Hanslmayr S (2006a): Upper alpha ERD and absolute power: Their meaning for memory performance. Prog Brain Res 159:151–165. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Hanslmayr S, Sauseng P, Gruber W, Brozinsky CJ, Kroll NEA, Yonelinas AP, Doppelmayr M (2006b): Oscillatory EEG correlates of episodic trace decay. Cereb Cortex 16:280–290. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S (2007): EEG alpha oscillations: The inhibition–timing hypothesis. Brain Res Rev 53:63–88. [DOI] [PubMed] [Google Scholar]
- Kotchoubey B, Strehl U, Uhlmann C, Holzapfel S, König M, Fröscher W, Blankenhorn V, Birbaumer N (2001): Modification of slow cortical potentials in patients with refractory epilepsy: A controlled outcome study. Epilepsia 42:406–416. [DOI] [PubMed] [Google Scholar]
- K Linkenkaer‐Hansen, VV Nikulin, S Palva, RJ Ilmoniemi, JM Palva (2004): Prestimulus oscillations enhance psychophysical performance in humans. J Neurosci 24:10186–10190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monastra VJ, Lynn S, Linden M, Lubar JF, Gruzelier JH, La Vaque TJ (2006): Electroencephalographic biofeedback in the treatment of attention‐deficit/hyperactivity disorder. Appl Psychophys Biof 9:5–34. [DOI] [PubMed] [Google Scholar]
- Nan W, Rodrigues JP, Ma J, Qu X, Wan F, Mak PI, Mak PU, Vai MI, Rosa A (2012): Individual alpha neurofeedback training effect on short term memory. Int J Psychophysiol 86:83–87. [DOI] [PubMed] [Google Scholar]
- Neuper C, Wörtz M, Pfurtscheller G (2006): ERD/ERS patterns reflecting sensorimotor activation and deactivation. Prog Brain Res 159:211–222. [DOI] [PubMed] [Google Scholar]
- Nissen MJ, Bullemer P (1987): Attentional requirements of learning: Evidence from performance measures. Cognitive Psychol 19:1–32. [Google Scholar]
- Palva S, Palva JM (2007): New vistas for α‐frequency band oscillations. Trends Neurosci 30:150–158. [DOI] [PubMed] [Google Scholar]
- Plihal W, Born J (1997): Effects of early and late nocturnal sleep on declarative and procedural memory. J Cognitive Neurosci 9:534–547. [DOI] [PubMed] [Google Scholar]
- Ros T, Munneke MAM, Parkinson LA, Gruzelier JH (2014): Neurofeedback facilitation of implicit motor learning. Biol Psychol 95:54–58. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Doppelmayr M, Pecherstorfer T, Freunberger R, Hanslmayr S (2005): EEG alpha synchronization and functional coupling during top‐down processing in a working memory task. Hum Brain Mapp 26:148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw FZ, Lai CJ, Chiu TH (2002): A low‐noise flexible integrated system for recording and analysis of multiple electrical signals during sleep–wake states in rats. J Neurosci Methods 118:77–87. [DOI] [PubMed] [Google Scholar]
- Turner ML, Engle RW (1989): Is working memory capacity task dependent? J Mem Lang 28:127–154. [Google Scholar]
- Vernon D, Egner T, Cooper N, Compton T, Neilands C, Sheri A, Gruzelier JH (2003): The effect of training distinct neurofeedback protocols on aspects of cognitive performance. Int J Psychophysiol 47:75–85. [DOI] [PubMed] [Google Scholar]
- Von Stein A, Chiang C, Konig P (2000): Top‐down processing mediated by interareal synchronization. Proc Natl Acad Sci USA 97:14748–14753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JR, Hsieh S (2013): Neurofeedback training improves attention and working memory performance. Clin Neurophysiol 124:2406–2420. [DOI] [PubMed] [Google Scholar]
- Wang QS, Zhou JN (2002): Retrieval and encoding of episodic memory in normal aging and patients with mild cognitive impairment. Brain Res 924:113–115. [DOI] [PubMed] [Google Scholar]
- Zoefel B, Huster RJ, Herrmann CS (2011): Neurofeedback training of the upper alpha frequency band in EEG improves cognitive performance. Neuroimage 54:1427–1431. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


