Abstract
Morality is defined as prescriptive norms regarding how people should treat one another, and includes concepts of fairness, justice, and rights. One recent study with moral dilemmas suggested that testosterone administration increases utilitarian judgments, which depends on second‐to‐fourth (2D: 4D) digit ratio, as a proxy of prenatal priming. However, the neural mechanism by which acute testosterone modulates moral reasoning remains to be determined. Using a placebo‐controlled within‐subject design, the current study examined the neuromodulatory effect of testosterone in young females by combining moral dilemmas, 2D: 4D, functional magnetic resonance imaging (fMRI), and subjective ratings of morally laden scenarios. Results showed that testosterone administration elicited more utilitarian responses to evitable dilemmas. The high 2D: 4D group scored more punishments for moral evaluation, whereas the low 2D: 4D group did the opposite. The activity in the amygdala, anterior insular cortex, and dorsolateral prefrontal cortex (dlPFC) was increased when participants evaluated morally unorthodox actions (intentional harm). The activity in the posterior superior temporal sulcus/temporoparietal junction (pSTS/TPJ) to accidental harm was decreased, specific to the high 2D: 4D group. The functional connectivity between the amygdala and dlPFC was reduced. The activity in the pSTS/TPJ to perceived agency predicted utilitarian responses to evitable dilemmas. The findings demonstrate the acute effect of testosterone on neural responses associated with moral judgment, and provide evidence to support that prenatal sex‐hormones priming could be important for early neurodevelopment, which plays a crucial role in the neural and behavioral manifestations of testosterone on adult moral reasoning. Hum Brain Mapp 37:3417–3430, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: moral reasoning, testosterone, utilitarian judgments, fMRI, 2D: 4D
Abbreviations
- AIC
Anterior insular cortex
- dlPFC
Dorsolateral prefrontal cortex
- dmPFC
Dorsomedial prefrontal cortex
- FDR
False discovery rate
- mPFC
Medial prefrontal cortex
- PANAS
Positive affect and negative affect schedule
- PPI
Psychophysiological interaction
- TPJ
Temporoparietal junction
- vmPFC
Ventromedial prefrontal cortex
INTRODUCTION
Neuromodulators such as hormones modify neuronal dynamics, excitability, and synaptic functions and, as a result, influence moral cognition in humans [Crockett and Rini, 2015]. Amongst hormones, testosterone plays an important role in moral cognition [Crockett and Fehr, 2013]. Several studies have examined neuromodulatory effects of testosterone indicate an influence on moral judgments through socio‐emotional processing [Crockett and Rini, 2015; Montoya et al., 2013]. However, most of these studies are limited by the use of the same small set of moral dilemmas [Christensen and Gomila, 2012]. In addition, whether socio‐emotional processing is a source of moral judgment or as a result of it remains controversial [Decety and Cacioppo, 2012; Decety et al., 2012; Huebner et al., 2009]. The current study was designed to determine the neural mechanisms by which testosterone modulates moral cognition by combining functional magnetic resonance imaging (fMRI) and behavioral evaluation of morally laden scenarios.
Progress has been made in demonstrating how testosterone impacts moral judgments at the behavioral level. Testosterone is thought to be involved in the pursuit of social dominance [Eisenegger et al., 2011]. Baseline testosterone positively predicts reactive aggression and state dominance [Denson et al.,, 2013a]. Individuals high in baseline testosterone are more likely to make utilitarian decisions [Carney and Mason, 2010]. When confronting hypothetical moral dilemmas in which participants must decide whether to sacrifice the life of one person in order to save the lives of a greater number, individuals that endorse or reject this specific type of harm are said to be making utilitarian or deontological judgments, respectively [Greene et al., 2001]. Through the sublingual administration method developed by Tuiten and coworkers [2000], recent studies have poured efforts into investigating the acute effect of testosterone on socio‐emotional cognition in human females [Bos et al., 2012; Montoya et al., 2013; van Honk et al., 2012; van Honk and Schutter, 2007]. Particularly, Montoya and her colleagues [2013] enrolled 20 young females and demonstrated that the second‐ to fourth‐digit ratio (2D: 4D) predicted 44% of the variance in the effects of testosterone administration on moral judgments. Subjects who had higher than average 2D: 4D would shift moral judgments from a deontological to a utilitarian view following testosterone administration. The 2D: 4D ratio is a lifelong signature of prenatal hormonal exposure. Inactivation of androgen receptor or addition of estrogen decreases growth of digit 4, causing a higher 2D: 4D ratio, whereas inactivation of estrogen receptor alpha or addition of androgen increases growth of digit 4, leading to a lower 2D: 4D ratio [Zheng and Cohn, 2011].
Testosterone administration increases generosity only when the recipient has the option to punish another, suggesting that testosterone influences strategic social concerns [Eisenegger et al., 2010]. A dose of testosterone has shown to increase cooperation, but only among individuals with lower prenatal testosterone exposure [van Honk et al., 2012]. It is worth noting that there has been no work on how testosterone administration modulates with the neural mechanisms involved in moral judgments.
Neuroscience investigations, including those using fMRI and lesion studies, have begun to characterize the neural mechanisms underpinning moral cognition [de Oliveira‐Souza et al., 2015; Molenberghs et al., 2014; Sobhani and Bechara, 2011; Taber‐Thomas et al., 2014]. Scholars generally agree that moral judgments arise from the integration of cognitive, affective, and motivational systems, which involve the posterior superior temporal sulcus/temporoparietal junction (pSTS/TPJ), amygdala, anterior insular cortex (AIC), ventromedial prefrontal cortex (vmPFC), dorsolateral prefrontal cortex (dlPFC), dorsomedial prefrontal cortex (dmPFC), and medial prefrontal cortex (mPFC) [Buckholtz and Marois, 2012; Decety and Cowell, 2014; Decety et al., 2012; Greene et al., 2004; Moll et al., 2005; Young et al., 2010a; Young and Koenigs, 2007]. These systems are not specific to morality, but support more domain‐general processing, such as affective arousal, attention, intention understanding, and decision‐making [Young and Dungan, 2012]. Some of these regions overlap with the salience network anchored by orbital fronto‐insula and dorsal anterior cingulate cortex associated with orienting attention toward and facilitating the processing of personal and motivational salient social information [Harsay et al., 2012]. Recently, visual scenarios depicting intentional versus accidental harm to people versus objects has been used to examine the neural underpinnings of morality [Decety et al., 2012; Hesse et al., 2016; Yoder and Decety, 2014a; Yoder and Decety, 2014b]. Intentionality is the decisive cue in determining whether an action was malicious or not [Cushman, 2008; Killen et al., 2011]. The sense of agency is important in relation to morality in determining that people can be held responsible for their actions [Blakemore and Decety, 2001; Decety and Porges, 2011; Kahn, 1992; Moll et al., 2007].
To clarify how acute testosterone interacts with perceived agency, the current study employed the Tuiten method, and assessed behavioral evaluations and neuro‐hemodynamic responses to morally salient behavior after testosterone administration. Generally testosterone is associated with antisocial, egoistic, or even aggressive behaviors [Eisenegger et al., 2010]. Recent studies support the notion that testosterone administration may blunt cognitive empathy and shift moral decision from deontological to utilitarian responses, depending on 2D: 4D digit ratio [Montoya et al., 2013; van Honk et al., 2011]. The ratio of baseline testosterone to cortisol reactivity predicts individual differences in psychopathic traits [Glenn et al., 2011; Welker et al., 2014], as characterized by impaired moral sensitivity and blunted affective empathy [Decety et al., 2013, 2015]. Alternatively, many scholars argued that testosterone is primarily involved in status‐related behaviors in challenging social interactions [Eisenegger et al., 2010; van Honk et al., 2012]. Testosterone administration could elaborate the processing of socially relevant stimuli [Bos et al., 2013; Chen et al., 2015]. Endogenous testosterone is correlated with the involvement of the prefrontal cortex and amygdala in socio‐emotional processing [Derntl et al., 2009; Mehta and Beer, 2010; Stanton et al., 2009; van Honk et al., 2012; Volman et al., 2011]. The observation of scenarios depicting moral transgressions would activate the neuro‐hemodynamic responses involved in moral judgments and mental‐state reasoning [Yoder and Decety, 2014a]. Thus, we hypothesized that testosterone relative to placebo administration would elicit stronger neuro‐hemodynamic response and increased functional connectivity in the neural circuits underpinning moral evaluations, including the amygdala, AIC, pSTS/TPJ, dlPFC, dmPFC, and vmPFC. The altered neuro‐hemodynamic reactivity induced by testosterone would be associated with moral utilitarian decisions. Considering the 2D: 4D as a proxy for prenatal sex‐hormone (testosterone‐versus‐estradiol) priming [Hönekopp et al., 2007; Zheng and Cohn, 2011], we would analyze the 2D: 4D effect on the neural correlates of moral evaluations if there is an interaction between 2D: 4D and testosterone administration.
MATERIALS AND METHODS
Participants
Twenty healthy right‐handed females, aged between 20 and 30 years (24 ± 1.9 years) were included in the study after providing written informed consent, and received monetary compensation. Females were enrolled because the quantity and time course of testosterone's effects have previously been objectively quantified [Tuiten et al., 2000]. As a result of poor fMRI quality, a total of 19 participants were finally included in the data analysis. All had normal menstruation cycles and were nonsmokers. All participants were right‐handed without hearing or visual impairments. They had no neurological, endocrinal, and psychiatric disorders, nor were they taking any medication at the time of study. None of them were taking oral contraceptives. This study was approved by the Ethics Committee from Taipei City Hospital and conducted in accordance with the Declaration of Helsinki.
Testosterone Administration
In a double‐blind, crossover, within‐subject design, participants received a single dose of 0.5 mg of testosterone on one day and a single dose of placebo on another day. A crossover study is a longitudinal study in which subjects receive a sequence of different treatments. The testosterone samples consisted of 0.5 mg of testosterone, 5 mg of the carrier cyclodextrine, 5 mg of ethanol, and 5 ml of water. Testosterone was omitted from the placebo samples, and both testosterone and placebo were administered sublingually. The sequence of placebo and testosterone administration was counter‐balanced between subjects. Half of participants were first going through the placebo session and half of them were first going through the testosterone session. We randomly assigned participants into two different experimental sequences. The pharmacokinetics of testosterone administration in women indicated that this method leads to a 10‐ to 17‐fold increase in testosterone plasma levels, returning to baseline within 15 min, and that the behavioral effects peak at 4 h after intake [Tuiten et al., 2000; van Rooij et al., 2012]. The current study thus applied the same interval of 4 h. The interval between two administrations, 48 h, was used to enable exogenous testosterone to metabolize [Slater et al., 2001]. We ran the two administrations within 10 days after participants' menstruation to ensure low endogenous hormone levels.
Digit Ratio Measurement
The digit ratio (2D: 4D) was measured using the scanned images of the right hand of each participant, which is a valid method for measuring finger lengths [van Honk et al., 2011]. When scanning the images, we ensured that details of major creases could be clearly seen. Adobe Photoshop was used to measure the lengths of the second and fourth digits from the ventral proximal crease of the digit to the fingertip. If a band of creases existed at the base of the digit, the measurement was obtained from the most proximal crease.
Salivary Testosterone Measurement
The level of salivary testosterone indicates the free, unbound, or biologically available portion of testosterone in circulation. We used a method that has been reliably applied to measure free testosterone levels in humans [Arregger et al., 2007]. Saliva was collected within 15 min before and after the administration of both testosterone and placebo.
Procedures
The experimental design was placebo‐controlled, within‐subject, double‐masked, and a crossover (Fig. 1). We sublingually administered either 0.5 mg of testosterone or a placebo to participants on two separate days. To minimize the influence of fluctuations due to the diurnal effect of hormones, drug administration always took place in the morning.
Figure 1.
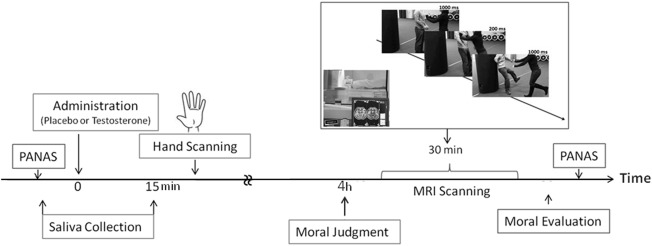
Experimental procedures. The design is placebo‐controlled, randomly assigned, double‐masked, and crossover. We sublingually administered either testosterone or placebo to participants on two separate days. Participant filled out the PANAS. Saliva was collected before and after administration. Hand scanning was employed to measure the 2D: 4D digit ratio. Participants read and responded to moral dilemmas at their own pace. During fMRI scanning, participants were presented with the stimuli depicting moral transgressions [agency (intentional vs. unintentional) and target (people vs. objects). After fMRI scanning, participants were requested to respond to questions probing moral reasoning.
To control for the potential secondary mood‐generated effects of testosterone on morality, we used the positive affect and negative affect schedule (PANAS) [Watson et al., 1988] for analyzing the participants who received the placebo and testosterone administrations. Positive Affect reflects the extent to which a person feels enthusiastic, alert, and active. Negative Affect is a general dimension of subjective distress and unpleasurable engagement that subsumes a variety of aversive mood states.
Utilitarian Judgments on Moral Dilemmas
Based on previous work [Greene et al., 2009; Greene et al., 2004; Greene et al., 2001; Huebner et al., 2011] (http://www.cell.com/neuron/supplemental/S0896‐6273(04)00634‐8), forty‐eight moral dilemmas were selected in order to make two versions of the moral judgment task balanced on emotional intensity [Koenigs et al., 2007]. Each version consists of twelve dilemmas with six personal and six impersonal dilemmas. Personal dilemmas included harm through direct physical contact (pushing the stranger) such as the Trolley dilemma, whereas the impersonal dilemmas involved indirect harm (e.g., flipping a switch). The personal dilemmas are further divided into dilemmas in which the death of or harm to the victim is inevitable or evitable. Moral permissibility judgments are higher for actions that propose inevitable harm in comparison with evitable harm. Dilemmas were translated from English to Chinese, and then translated back from Chinese to English and checked for consistency by a native English speaker. Participants read and responded to the dilemmas at their own pace.
To examine the effect of testosterone on moral dilemmas, endorsement scores were subjected to ANOVA with respect to the administration (testosterone or placebo) and dilemma type (nonmoral, impersonal, evitable, or inevitable) as the repeated‐measure factors. To test if 2D: 4D mediates the effect on moral judgment, the 2D: 4D was further added as a covariate to re‐run the repeated‐measures ANOVA. In addition, the correlations between 2D: 4D and endorsement scores of each dilemma type were tested.
Visual Stimuli
A total of 96 stimuli consisting of animations (2.2 s) depicting moral transgressions were presented to participants [Decety et al., 2012; Yoder and Decety, 2014a; Yoder and Decety, 2014b for behavioral and fMRI validation]. The stimuli belonged to one of 4 categories in a 2 by 2 factorial design [agency (intentional vs. unintentional) and target (people vs. objects)] and portrayed the following: (1) A person is shown hurting another person intentionally (people intentional, PI); (2) A person is shown hurting another unintentionally (people unintentional, PU); (3) A person is shown breaking an object intentionally (objects intentional, OI); and (4) A person is shown breaking an object unintentionally (objects unintentional, OU). One additional baseline stimulus category depicted people in everyday social interactions without any infliction of pain or damage (e.g., a person giving another individual a notebook). The clips showed situations of varying degrees of intensity, portrayed people of multiple races and ethnic groups, as well as various ages. Importantly, the faces of the protagonists were not visible, thus there was no emotional reaction visible to participants.
Functional MRI Scanning
Participants underwent two sessions of fMRI scanning (placebo and testosterone) in different days. Stimuli were presented with the E‐prime software (Psychology Software Tools, Inc., Pittsburgh, PA) and a MRI compatible goggle (VisualStim Controller, Resonance Technology Inc). A blocked design paradigm was used with a total of 20 baseline blocks (duration 17.6 s each) during which a fixation cross was presented and 20 active blocks (duration 26.4 s each) during which stimuli from one of the 5 categories were presented. The active blocks were randomized by agency (intentional/unintentional) and target (people/objects). The presentation order was counterbalanced across runs and across participants. Each active block consisted of 6 stimuli (2.2 s each) with a jittered interstimulus interval between 1.10 s and 3.4 s, during which a black fixation cross was presented against a gray background. Each session had four run. Each run had five active blocks, lasting 4 min. To avoid confounding motor‐related activation in the anterior cingulate cortex and supplementary motor area, no overt response was required. Participants were instructed to watch the stimuli carefully.
Scanning was performed on a 3T Siemens Magnetom Trio‐Tim magnet. High‐resolution structural T1‐weighted images were acquired using a 3D MPRAGE sequence (TR/TE = 2530 ms/3.5 ms, FOV = 256 × 224 mm, flip angle = 7°, slice thickness = 1 mm, no gap, matrix = 224 × 256). For functional images, 3‐mm‐thick transverse slices oriented along the AC − PC line were continuously collected using an EPI sequence (TR/TE = 2200/30 ms; flip angle = 90°; FOV = 220 mm; matrix = 64 × 64).
Subjective Evaluations on Morally Laden Scenarios
After the fMRI scanning, participants were presented with the same stimuli that they saw in the scanner. They were asked to respond to five questions probing moral reasoning using computer‐based visual analogue scales. The questions were designed to assess intentionality of the protagonists, empathic concern for the victim, personal distress, understanding of the agent's mental state, and moral evaluation. The following questions were asked: “Was this action done on purpose?” “How sad are you for the person/object that was hurt/broken?” “Was it wrong to do this?” “How mean was the person who did this?” and “How much would you punish the person who did this action?”
Data Analysis
Functional MRI data was processed with SPM8 (Wellcome Department of Imaging Neuroscience, London, UK) in MATLAB 7.0 (MathWorks Inc., Sherborn, MA). Structural scans were coregistered to the SPM8 T1 template, and a skull‐stripped image was created from the segmented gray matter, white matter, and CSF images. These segmented images were combined to create a subject‐specific brain template. EPI images were realigned and filtered (128 s cutoff), then coregistered to these brain templates, normalized to MNI space, and smoothed (8 mm FWHM). The voxel size used in the functional analysis was 3.4 mm × 3.4 mm × 3 mm with the 8 mm smoothed voxels when counting the cluster size. All participants who completed scanning had less than 1 voxels of in‐plane motion. A two‐level approach for block‐design fMRI data was adopted. A voxel‐by‐voxel multiple regression analysis of expected signal changes for each of the five block categories, which were constructed using the hemodynamic response function, was applied to the preprocessed images for each participant. Individual subject data were analyzed using a fixed‐effects model. Boxcar regressors representing the occurrence of each of the five block categories modeled condition effects at the subject level. Movement parameters from the realignment output were included as regressors of no interest. The resulting first‐level contrast images were then entered into analysis of variance (ANOVA): 2 (Administration: testosterone vs. placebo) × 2 (Agency: intentional vs. unintentional) × 2 (Target: people vs. objects). Whole brain activations are reported at P < 0.05, corrected for multiple comparisons across the whole volume to control the whole‐brain false discovery rate (FDR) rate at P < 0.05.
In addition, activities in specific regions of interest (ROIs) were analyzed including: amygdala (x −22, y −2, z −24), AIC (−30, 20, 4), vmPFC (10, 42, −18), pSTS/TPJ (56, −50, 18), dlPFC (42, 30, 26), and dmPFC (0. 54, 36). Data extraction for the ROI analyses was performed using the MarsBaR toolbox (http://marsbar.sourceforge.net/) implemented in SPM8. ROIs were defined as a 5‐mm spherical region centered on the coordinates determined on the basis of neuroanatomical atlases as well as meta‐analyses [Bzdok et al., 2012; Lamm et al., 2011] and recent fMRI studies of moral evaluations using the same stimuli [Decety et al., 2012; Yoder and Decety, 2014a]. ROI data are reported for significant contrast image peaks within 10 mm of these a priori coordinates. The individual mean parameter estimates (beta values) were then subject to ANOVA for repeated measures to test for main effects of administration, agency, and target as well as administration‐by‐agency, administration‐by‐target, and agency‐by‐target interactions. For statistical analyses of the ROI data, SPSS was used.
Functional Connectivity
Psychophysiological interaction (PPI) analysis was seeded in the left amygdala (−22, −2, −24) to estimate how testosterone administration altered the functional connectivity of amygdala during the viewing of moral versus non‐moral actions (intentional vs. unintentional: PI + OI vs. PU + OU). The time series of the first eigenvariates of the BOLD signal were temporally filtered, mean corrected, and deconvolved to generate the time series of the neuronal signal for the source region—the left amygdala—as the physiological variable in the PPI. PPI analysis assesses the hypothesis that the activity in one brain region can be explained by an interaction between cognitive process and hemodynamic activity in another brain regions. Being selected as the PPI source region, the physiological regressor was denoted by the activity in the left amygdala. Agency condition (intentional vs. unintentional) was the psychological regressor. The interaction between the first and second regressors represented the third regressor. The psychological variable was used as a vector coding for the specific task (1 for PI and OI, −1 for PU and OU) convolved with the hemodynamic response function. The individual time series for the left amygdala was obtained by extracting the first principle component from all raw voxel time series in a sphere (5 mm radius) centered on the coordinates of the subject‐specific amygdala activations. These time series were mean‐corrected and high‐pass filtered to remove low‐frequency signal drifts. The physiological factor was then multiplied with the psychological factor to constitute the interaction term. PPI analyses were then carried out for each subject involving the creation of a design matrix with the interaction term, the psychological factor, and the physiological factor as regressors. Subject‐specific contrast images were then entered into random effects analyses (thresholded at P < 0.01, uncorrected, k = 25). PPI analyses were conducted at each administration separately (testosterone and placebo), in order to identify brain regions showing significant changes in functional coupling with the amygdala during moral relative to non‐moral harm in relation to testosterone administration.
RESULTS
Mood Measurement
The PANAS was employed to indicate the possible effects of testosterone on affects (mean ± SE). The Wilcoxon signed‐rank test on positive and negative affects revealed no significant differences between before and after the administration of placebo (positive: 32.45 ± 1.11 vs. 32.05 ± 1.24, P = .10; negative: 21.80 ± 0.88 vs. 20.95 ± 0.87, P = 0.12) and testosterone (32.80 ± 1.11 vs. 32.25 ± 1.17, P = 0.18; 20.75 ± 1.03 vs. 20.20 ± 1.18, P = 0.22). Given that testosterone administration did not impact participants' affect states, the observed effects of testosterone on moral dilemmas and moral evaluations cannot be attributed to secondary mood‐generated response biases.
Salivary Testosterone
Testosterone levels were negatively skewed and therefore log transformed (Supporting Information materials). Mean baseline testosterone levels did not differ between testosterone and placebo conditions (LOG [testosterone] mean ± SD: 2.41 ± 0.26, LOG [placebo]: 2.40 ± 0.30, t(18) = −0.20, P = 0.84). The salivary testosterone level significantly increased after testosterone administration (LOG [before]: mean ± SD: 2.41 ± 0.26, LOG [after]: 3.51 ± 0.15, t(18) = −16.11, P < 0.001], but did not change after placebo administration (LOG [before]: mean ± SD: 2.40 ± 0.30, LOG [after]: 2.41 ± 0.30, t(18) = −0.33, P = 0.74]. In this cohort, baseline testosterone levels were correlated with the 2D: 4D (r = 0.54, P = 0.018). Baseline testosterone levels were positively related to impersonal moral permissibility judgments (r = 0.52, P = 0.024), but not to personal moral permissibility judgments on dilemmas involving inevitable harm (r = −0.03, P = 0.90) or evitable harm (r = −0.12, P = 0.65).
Utilitarian Judgments on Moral Dilemmas
The dilemma type (nonmoral, impersonal, evitable, or inevitable) produced a main effect [F(3, 54) = 29.84, P < 0.001, η 2 = 0.65], but the administration (testosterone vs. placebo) did not [F(1, 18) = 0.57, P = 0.46, η 2 = 0.03]. The nonmoral (mean ± SE: 62.82 ± 2.92), impersonal (62.35 ± 3.59) and the inevitable dilemmas (46.47 ± 6.91) were judged as more morally endorsed than did the dilemmas with evitable harm (20.29 ± 2.34). In addition, an interaction occurred between the administration and dilemma type [F(3, 54) = 4.11, P = 0.01, η 2 = 0.20] (Fig. 2). Post hoc analyses indicated that the administration effect existed for only the dilemmas with evitable harm ([testosterone]: mean ± SD: 26.35 ± 11.57, [placebo]: 14.24 ± 11.79, P = 0.002), rather than other dilemma types (P = 0.09; P = 0.18; P = 0.13). The inclusion of PANAS as covariates did not influence the statistical significance (all P > 0.05).
Figure 2.
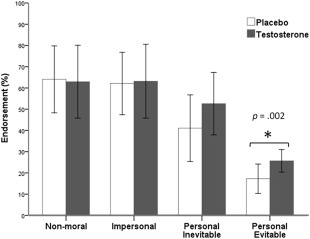
Utilitarian judgments on moral dilemmas after testosterone administration. The y‐axis indicates proportions of 'yes' judgments. Error bar represents the 95% confidence intervals. There were four dilemma types: non‐moral, impersonal, personal‐inevitable, and personal‐evitable moral dilemmas. On personal‐evitable moral dilemmas, testosterone relative to placebo administration significantly increased the frequency of endorsing 'yes' responses (P = 0.002).
Based on the Shapiro‐Wilk test (P = .21) [Razali and Wah, 2011; Shapiro and Wilk, 1965], a normal Q‐Q plot indicated that the 2D: 4D was approximately normal distributed with a skewness of −0.763 (SE = 0.524) and a kurtosis of −0.048 (SE = 1.014). To test if the 2D: 4D mediated the testosterone effect on moral judgments, we added the 2D: 4D as a covariate to run a repeated‐measures ANOVA with respect to the administration (testosterone or placebo) and dilemma type (nonmoral, impersonal, evitable, or inevitable). A significant interaction occurred in the 2D: 4D, administration, and dilemma type [F(3, 54) = 3.93, P = 0.014, η 2 = 0.21] as well as the adminstraton and dilemma type [F(3, 54) = 3.55, P = 0.022, η 2 = 0.19]. Follow‐up comparisons showed that the interaction between 2D: 4D and administration approached significance in the evitable [F(1, 18) = 4.29, P = 0.056, η 2 = 0.22] and impersonal dilemmas [F(1, 18) = 4.02, P = 0.061, η 2 = 0.19], whereas no interactions were found for the dilemmas with inevitable harm [F(1, 18) = 0.01, P = 0.92, η 2 < 0.01].
In addition, the 2D: 4D positively explained 22% of the variance in the effect of testosterone administration on the moral endorsement of evitable dilemmas (r = 0.47, P = 0.056) but negatively explained 27% of the variance on impersonal (r = −0.52., P = 0.032) and 23% on nonmoral dilemmas (r = −0.48., P = 0.05). The inevitable dilemmas had no such a correlation (r = 0.03, P = 0.92). Fisher r‐to‐z transformation confirmed significantly different correlation coefficients between evitable and impersonal dilemmas (Δz = 3.07, P = 0.002), as well as between evitable and nonmoral dilemmas (Δz = 2.92, P = 0.004).
Following one recent study [Montoya, et al., 2013], we further divided groups of relatively low and high 2D: 4D based on median split (Mean ± SD = 0.957 ± 0.042; Median = 0.96). Non‐parametric Wilconxon tests indicated that the high 2D: 4D group (n = 9) had a trend that approached significance (Mean rank [testosteron]: 6; Mean rank [placebo]: 4.29, Z = −1.73, P = 0.08), whereas the low 2D: 4D group (n = 10) did not show an effect of testosterone on impersonal dilemma (Mean rank [testosteron]: 3.8; Mean rank [placebo]: 4.5, Z = 0.85, P = 0.40). Testosterone relative to placebo administration tended to reduce impersonal permissibility judgements in subjects with high 2D: 4D.
Subjective Evaluations on Morally Laden Scenarios
In parallel with one recent fMRI study on the same stimuli [Decety, et al., 2012], there were main effects for agency and target (all P < 0.001). Participants rated intentional harm relative to unintentional (accidental) harm with higher empathic concerns and punishments, whereas harm to objects was rated as less wrong than harm to people. However, administration did not produce any effect regardless of the designed questions to assess intentionality, empathic concern, understanding agency, personal distress, and moral evaluation [F(1, 18) = 0.12, P = 0.73, η 2 < 0.01; F(1, 18) < 0.01, P = 0.95, η 2 < 0.01; F(1, 18) = 0.27, P = .61, η 2 = 0.02; F(1, 18) = 0.8, P = 0.38, η 2 = 0.04; F(1, 18) = 0.01, P = 0.95, η 2 < 0.01).
Furthermore, we divided groups of relatively low and high 2D: 4D. With regards to moral evaluation, there was a marginal interaction between group (high vs. low 2D: 4D) and administration (testosterone vs. placebo) [F(1, 18) = 4.2, P = 0.05, η 2 = 0.20]. Post hoc analyses indicated that the high 2D: 4D group scored higher punishments after administration of testosterone (15.21 ± 0.92) relative to placebo (14.39 ± 0.82), whereas the low 2D: 4D group did the opposite (13.4 ± 0.97; 14.41 ± 0.86).
fMRI Results
Table 1 lists the brain regions showing a significant hemodynamic change to perceived agency and target after placebo and testosterone administration. In response to perceived agency, the testosterone administration showed the activation in the amygdala, vmPFC, superior temporal gyrus, caudate nucleus, anterior and posterior cingulate cortex, whereas the placebo showed the activation in the vmPFC (Fig. 3a). To perceived target, the testosterone showed the activation in the AIC and pSTS/TPJ, whereas the placebo showed the activation in the amygdala, vmPFC, AIC, and pSTS/TPJ along with dlPFC, inferior frontal gyrus, supramarginal gyrus, and precentral gyrus (Fig. 3b).
Table 1.
Brain regions showing significant hemodynamic changes to perceived agency and target after placebo and testosterone administration
| MNI coordinates | ||||||
|---|---|---|---|---|---|---|
| Brain region | side | x | y | z | t‐value | k |
| Intentional vs. Unintentional | Placebo | ||||||
| vmPFC | – | 0 | 54 | −6 | 2.94* | 93 |
| Intentional vs. Unintentional | Testosterone | ||||||
| Amygdala | R | 26 | 2 | −16 | 2.42* | 50 |
| Amygdala | L | −24 | 0 | −14 | 2.76* | 10 |
| vmPFC | R | 2 | 46 | −10 | 3.93 | 250 |
| Superior temporal gyrus | L | −58 | 2 | −2 | 2.84 | 90 |
| Caudate nucleus | L | −12 | 20 | 2 | 3.30 | 186 |
| Anterior cingulate cortex | – | 0 | 48 | 4 | 2.80 | 265 |
| Posterior cingulate cortex | L | −4 | −40 | 18 | 3.90 | 298 |
| People vs. Objects | Placebo | ||||||
| Amygdala | L | −28 | −4 | −22 | 2.05* | 27 |
| vmPFC | R | 2 | 42 | −20 | 3.04* | 67 |
| Amygdala | R | 18 | −2 | −14 | 1.86* | 13 |
| Inferior frontal gyrus | L | −52 | 38 | 0 | 3.18 | 245 |
| AIC | R | 38 | 30 | 4 | 2.22* | 97 |
| pSTS/TPJ | L | −48 | −52 | 13 | 3.59 | 224 |
| pSTS/TPJ | R | 44 | −60 | 20 | 3.39 | 144 |
| dlPFC | R | 54 | 28 | 30 | 3.92 | 202 |
| Supramarginal gyrus | L | −56 | −44 | 32 | 2.95 | 44 |
| Precentral gyrus | R | 54 | 14 | 42 | 3.60 | 36 |
| People vs. Objects | Testosterone | ||||||
| AIC | R | 36 | 20 | 12 | 1.84* | 9 |
| pSTS/TPJ | L | −50 | −60 | 14 | 2.84* | 74 |
| pSTS/TPJ | R | 52 | −62 | 16 | 2.48* | 356 |
Pooled group results for all participants (n = 19).
All clusters are significant at FDR‐corrected P < 0.05, and only clusters of 10 or more contiguous voxels are reported, except those marked with a asterisk, which are taken from predefined ROIs and significant at uncorrected P < 0.05.
Abbreviations: R, right; L, left; AIC, anterior insular cortex; vmPFC, ventromedial prefrontal cortex; dlPFC, dorsolateral prefrontal cortex; pSTS/TPJ, posterior superior temporal sulcus/temporoparietal junction.
Figure 3.
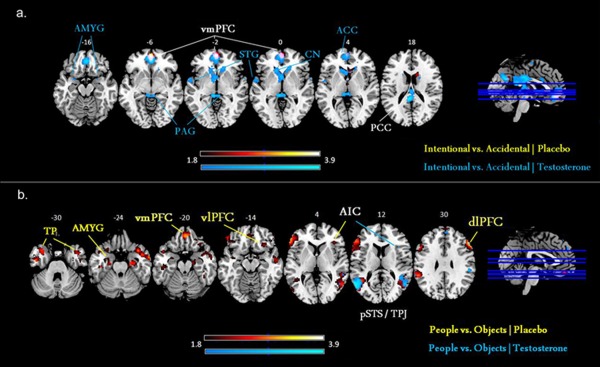
Response to perceived agency and target after testosterone administration. (a) To perceived agency (intentional vs. unintentional harm), the testosterone administration increased the activity in the amygdala (AMYG), periacqueductal gray (PAG), ventromedial prefrontal cortex (vmPFC), superior temporal gyrus (STG), caudate nucleus (CN), anterior cingulate cortex (ACC), and posterior cingulate cortex (PCC). Instead, the placebo was associated with the activity in the vmPFC only (thresholded at P < 0.01, uncorrected, k = 25 for visual purposes). (b) To perceived target (people vs. objects), the testosterone increased the activity in the anterior insular cortex (AIC) and posterior superior temporal sulcus/temporoparietal junction (pSTS/TPJ), whereas the placebo was associated with the activity in the amygdala (AMYG), ventromedial (vmPFC), dorsolateral (dlPFC), and ventrolateral prefrontal cortex (vlPFC) along with temporal pole (TP) and pSTS/TPJ (thresholded at P < 0.01, uncorrected, k = 25 for visual purposes).
Regarding the ROI results, significant interactions between testosterone administration and perceived agency were observed in the amygdala [F(1, 18) = 6.11, P = 0.024, η 2 = 0.25], AIC [F(1, 18) = 6.30, P = 0.022, η 2 = 0.26], and dlPFC [F(1, 18) = 4.47, P = 0.049, η 2 = 0.20], but none in the pSTS/TPJ [F(1, 18) = 0.47, P = 0.50, η 2 = 0.03] and dmPFC [F(1, 18) = 0.13, P = 0.73, η 2 < 0.01]. The vmPFC had a marginal effect with a medium to large effect size [F(1, 18) = 4.25, P = 0.054, η 2 = 0.19]. The follow‐up analyses indicated that the administration effect in the amygdala, AIC, dlPFC, and vmPFC had opposite directions depending on the factor of agency. Acute testosterone administration increased the amygdala, AIC, dlPFC, and vmPFC activity when viewing intentional harm (mean ± SE: 0.46 ± 0.67; 0.48 ± 0.61; 0.08 ± 0.34; 1.93 ± 1.08), whereas it decreased their activity upon witnessing accidental harm (−2.13 ± 0.92; −2.09 ± 1.00; −0.85 ± 0.38; −0.58 ± 0.98). On the other hand, none of these regions achieved any interaction between administration and target.
Interestingly, there was an interaction of group (high vs. low 2D: 4D) × administration (testosterone vs. placebo) × agency (intentional vs. unintentional) × target (people vs. objects) in the pSTS/TPJ [F(1, 18) = 5.19, P = 0.036, η 2 = 0.23]. The 2D: 4D did not affect testosterone modulation in the amygdala [F(1, 18) = 0.01, P = 0.94, η 2 < 0.01], AIC [F(1, 18) < 0.01, P = 0.99, η 2 < 0.01], dlPFC [F(1, 18) = 0.5, P = 0.49, η 2 = 0.03], dmPFC [F(1, 18) = 1.48, P = 0.24, η 2 = 0.08], and vmPFC [F(1, 18) = .68, P = 0.42, η 2 = 0.04]. Post hoc analyses indicated that harm to objects exerted an interaction of administration x agency x group [F(1, 18) = 7.88, P = 0.012, η 2 = 0.32], whereas harm to people did not have this effect [F(1, 18) = .73, P = 0.4, η 2 = 0.04]. After administration of testosterone relative to placebo, the high 2D: 4D group showed a trend that approached a significant decrease in the pSTS/TPJ activity during the observation of accidental harm [testosterone vs. placebo: 3.1 ± 0.74 vs. 4.56 ± 0.8; t(8) = 1.87, P = 0.09], whereas the low 2D: 4D had no such effect [4.61 ± 0.81 vs. 3.39 ± 0.55; t(9) = −1.84, P > 0.1] (Fig. 4a).
Figure 4.
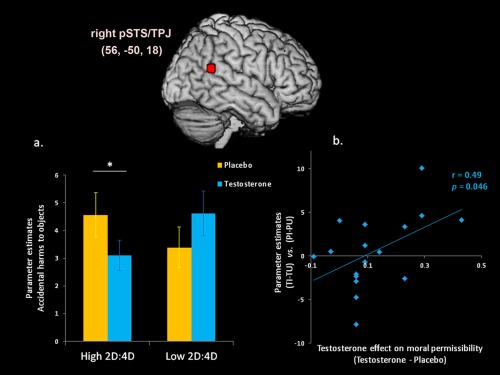
Testosterone modulation on moral evaluations in the right pSTS/TPJ. (a) Neuro‐hemodynamic response to unintentional (accidental) harm to objects was reduced by testosterone administration, depending on the 2D: 4D. Testosterone relative to placebo administration decreased activity in the pSTS/TPJ in the high 2D: 4D group (testosterone vs. placebo: 3.1 ± 0.74 vs. 4.56 ± 0.8), but not in the low 2D: 4D group (4.61 ± 0.81 vs. 3.39 ± 0.55). Asteroid indicated P < 0.05, one‐tailed. (b) As a result of testosterone administration, the activity in the pSTS/TPJ in response to moral evaluations predicted moral utilitarian decisions. The endorsement of evitable dilemmas was positively correlated with the activity in the pSTS/TPJ to perceived agency. Abbreviations: TI, testosterone/intentional harm; TU, testosterone/unintentional harm; PI, placebo/intentional harm; PU, placebo/unintentional harm.
Regarding the relationship between endorsement scores to moral dilemmas and hemodynamic responses to moral evaluations (Fig. 4b), we found that the endorsement of evitable dilemmas was positively correlated with the pSTS/TPJ activity to perceived agency as a result of testosterone administration (r = 0.49, P = 0.046).
Functional Connectivity
Testosterone administration triggered the distinct patterns in functional coupling. After the placebo, the PPI analysis seeded in the left amygdala (−22, −2, −24) showed a significant increase in coupling with the left rostral dlPFC (−58, 18, 18), supramarginal cortex (−62, −42, 30), and middle temporal gyrus (−66, −26, −18) along with right supramarginal cortex (64, −44, 32) and inferior parietal cortex (56, −38, 52). After testosterone administration, the left amygdala showed significantly negative connectivity with the left dmPFC (−14, 36, 22) and superior temporal gyrus (−64, −18, 4) along with right rostal dlPFC (50, 6, 22), dlPFC (16, 8, 70), and dmPFC (6, 52, 42). Importantly, testosterone relative to placebo administration significantly decreased the coupling of the left amygdala with bilateral rostral dlPFC (58, 18, 22; −26, 58, 22), right dlPFC (20, 6, 70), and left dmPFC (−2, 42, 52) (Fig. 5).
Figure 5.
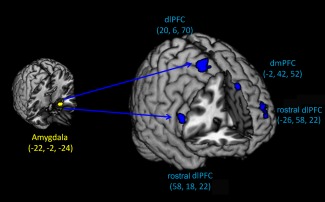
Functional connectivity after testosterone administration. Testosterone relative to placebo administration significantly decreased the functional connectivity of the amygdala with the rostral dorsolateral (dlPFC) and dorsomedial prefrontal cortex (dmPFC).
DISCUSSION
While it has been suggested that testosterone plays a role in moral cognition and judgment, the available empirical evidence is, at best, contradictory. Examining the neural mechanisms by which testosterone administration modulates behavioral evaluations and hemodynamic responses to morally laden behavior can contribute to a better understanding of the neural computations underlying moral reasoning.
In line with the work of Montoya et al. [2013], we found that testosterone administration elicited greater utilitarian response, in relation with 2D: 4D. Despite their effect driven by inevitable dilemmas, our exogenously administered testosterone produced higher endorsement of evitable dilemmas. Interestingly, the 2D: 4D predicted acute testosterone effect on personal and impersonal dilemmas in opposite directions. Positive correlations between the 2D: 4D and personal evitable dilemmas was in accordance with the work of Montoya et al. [2013], indicating that testosterone administration would shift moral decision‐making from deontotogical to utilitarian responses, especially in participants with higher 2D: 4D. On the other hand, the 2D: 4D had negative correlations with impersonal and nonmoral dilemmas. This dissociation between personal and impersonal or between moral and nonmoral dilemmas might reflect the energy re‐allocation, in which testosterone administration may elaborate the processing of significant social signals and reduce the processing of non‐relevant information at the same time [Bos et al., 2013; Chen et al., 2015]. Recently, one pharmacological fMRI study demonstrated that testosterone administration increased amygdala responses during threat approach, but decreased amygdala responses during threat avoidance, suggesting the motivation‐specific effects of testosterone on amygdala tuning [Radke et al., 2015]. This result is supported by our fMRI results. Indeed, while participants evaluated scenarios depicting moral transgressions, testosterone administration increased the amygdala, AIC, dlPFC, and vmPFC activities when viewing intentional harm, whereas it decreased their activity upon observing accidental harm. In the same vein, testosterone decreased the pSTS/TPJ reactivity to accidental harm to objects (i.e., non‐relevant stimuli), specific to subjects with high 2D: 4D.
Notably, testosterone administration exhibits differential effects in high and low 2D: 4D groups. The 2D: 4D positively predicted evitable dilemmas but negatively predicted impersonal dilemmas in terms of the testosterone effect on moral acceptability. Testosterone decreased impersonal permissibility ratings, specific to subjects with high 2D: 4D. Testosterone elicited greater punishement ratings for moral evaluation in the high 2D: 4D, but less in the low 2D: 4D. It also reduces fearfulness [Hermans et al.,, 2007, 2006a] and affective empathy [Hermans et al., 2006b], but does not influence cognitive empathy [van Honk et al., 2011], and enhances inevitable permissibility [Montoya et al., 2013] in high 2D: 4D subjects. The results of the current study corroborate previous findings that showed a larger effect of testosterone administration on social cognitions in the cohort with higher 2D: 4D. According to the dual‐process theory [Greene et al., 2001, 2004; Haidt, 2007], it is reasonable to assume that acute testosterone administration in subjects with high 2D: 4D might intuitively inhibit prepotent emotional responses arising from dilemmas, but could instrumentally enhance cognitive overriding in order to endorse utilitarian judgments. Considering that 2D: 4D is a proxy for prenatal sex‐hormone (testosterone‐versus‐estradiol) priming [Zheng and Cohn, 2011], these findings might support the notion that early neurodevelopmental effects of sex steroids play a crucial role in the neural and behavioral manifestations of testosterone on moral reasoning later in life.
As a result of testosterone administration, participants with stronger activity in the pSTS/TPJ to perceived agency were more prone to endorse utilitarian choices on evitable dilemmas. The pSTS/TPJ plays a critical role in the sense of agency [Decety and Lamm, 2007; Jackson and Decety, 2004], involved in perceiving and understanding the intentions and beliefs of others when making moral appraisals of a situation [Decety and Sommerville, 2003; Funk and Gazzaniga, 2009; Lamm et al., 2007]. Increased neuro‐hemodynamic activity in the pSTS/TPJ was reported for strangers compared to friends when inferring the pain of others [Cheng et al., 2010], and when making reward choices on their behalf [Braams et al., 2014]. When the right pSTS/TPJ functioning is disrupted by transcranial magnetic stimulation, participants would rate attempted harms as less morally impermissible than controls [Young et al., 2010b]. The present study demonstrated that testosterone administration increased permissibility for evitable harm, which, in turn, had a positive coupling with the activity in the pSTS/TPJ in response to perceived agency.
Testosterone relative to placebo administration was associated with distinct hemodynamic changes associated with moral evaluations (see Fig. 3). It is worth noting that this is the first study to address how testosterone administration modulates the neural correlates involved in moral evaluations. In spite of showing no effect for subjective measures, testosterone administration elicited significant neuro‐hemodynamic changes, which depends on the agency and 2D: 4D. Specifically, the administration effect produced the interaction with perceived agency in the amygdala and AIC, dlPFC, and vmPFC, but not with perceived target in any brain region. Sublingual testosterone enhanced the amygdala activity to socially relevant stimuli [Bos et al., 2013; Goetz et al., 2014] as well as heightened the activity in the AIC when perceiving crying infants [Bos et al., 2010]. Endogenous testosterone levels have been associated with the amygdala and vmPFC activity while viewing angry faces [Stanton et al., 2009] and the amygdala‐dlPFC connectivity during emotional control [Denson et al., 2013b]. Here, the functional connectivity of the amygdala with the dlPFC during moral evaluations was reduced by sublingual testosterone. In parallel, testosterone administration shifted amygdala output away from the orbiotofrontal cortex towards the thalamus and rapidly reduced functional coupling of the amygdala with the orbitofrontal cortex [van Wingen et al., 2010]. Given the amygdala − dlPFC connectivity as the top‐down control network [Banks et al., 2007; Yoder and Decety, 2014a], we proposed that the shift in functional coupling or energy reallocation induced by testosterone administration could regulate emotional processing, which, in turn, would heighten reactivity to social provocation.
For the subjective measures of morally laden scenarios, the administration of testosterone did not produce any effect regardless of the questions designed to assess moral judgment. This could be attributed to the concern of ceiling effects that might mask the efficacy of testosterone on moral evaluations among healthy participants. Using the same stimuli as the work of Decety et al., [2012], we reproduced the main effects for agency and target that match previous validations from healthy participants. Intentional relative to accidental harm triggered higher empathic concern and greater deserved punishments. The literature suggests that acute testosterone administrations should take place outside of the realm of the consciously reportable [Hermans et al., 2007, 2006a; van Honk et al., 2005; van Honk and Schutter, 2007]. Sublingual administration of testosterone in healthy participants did not show a consistent relation between physiological and self‐reported variables [Schutter and van Honk, 2004; Tuiten et al., 2002]. As had previously been argued, steroid hormones, including testosterone, mainly influence the limbic system [Wood, 1996], whereas self‐reported measures imply higher cortical functions [Hermans et al., 2006b]. The physiological changes of affective processing induced by testosterone cannot always be reliably captured by subjective ratings [Gray et al., 1991]. Supposedly, testosterone administration might not affect moral evaluations in young healthy females.
Here, the baseline testosterone levels, i.e., endogenous testosterone, were positively correlated with moral permissibility judgments to impersonal dilemmas. In parallel, individuals with higher basal testosterone would be more likely to make utilitarian decisions [Carney and Mason, 2010]. Alternatively, the baseline testosterone levels were related to the moral permissibility to personal dilemmas [Montoya et al., 2013]. These mixed findings could be attributed to the method of assessing moral permissibility as well as the characteristics of study participants. Montoya and her coworkers [2013] used a visual analog scale from −100 to 0 to 100, whereas we followed previous methods [Koenigs et al., 2007] to apply yes/no questions for moral dilemmas. We also observed an association between baseline testosterone levels and 2D: 4D, whereas Muller et al., [2011] and Montoya et al., [2013] found this relation non‐significant. Hence, the interplay between acute testosterone effect on moral evaluations, baseline testosterone levels, and prenatal hormone priming need to be considered in future research. Additionally, regarding a sample size of 19 women, the generalizability of the results is limited. There was only a small to medium effect size in terms of hemodynamic and behavioral changes after testosterone administration. Most of the correlation analyses should be treated as pilot results for future investigation.
CONCLUSION
Taken together, testosterone administration was associated with distinct neuro‐hemodynamic reactivity during moral evaluations. The activity in the pSTS/TPJ was reduced, specifically in participants from the high 2D: 4D group. Subjects from this latter group gave greater punishment ratings in moral evaluation, whereas the low 2D: 4D group did less. The 2D: 4D explained 22% of the variance in the testosterone effect on the moral endorsement of evitable dilemmas. Testosterone reduced the amygdala − dlPFC functional connectivity when viewing morally laden scenarios, whereas it elicited stronger pSTS/TPJ activity in relation with utilitarian responses to evitable dilemmas. In line with previous literature [Hermans et al., 2006a,b; 2007; Montoya et al., 2013; van Honk et al., 2011], the present findings support the notion that prenatal sex hormones in priming neurodevelopment play a crucial role in the neural and behavioral manifestations of testosterone on adult moral reasoning.
Supporting information
Supporting Information
REFERENCES
- Arregger AL, Contreras LN, Tumilasci OR, Aquilano DR, Cardoso EM (2007): Salivary testosterone: A reliable approach to the diagnosis of male hypogonadism. Clin Endocrinol (Oxf) 67:656–662. [DOI] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL (2007): Amygdala‐frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci 2:303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Decety J (2001): From the perception of action to the understanding of intention. Nat Rev Neurosci 2:561–567. [DOI] [PubMed] [Google Scholar]
- Bos PA, Hermans EJ, Montoya ER, Ramsey NF, van Honk J (2010): Testosterone administration modulates neural responses to crying infants in young females. Psychoneuroendocrinology 35:114–121. [DOI] [PubMed] [Google Scholar]
- Bos PA, Panksepp J, Bluthe RM, van Honk J (2012): Acute effects of steroid hormones and neuropeptides on human social‐emotional behavior: A review of single administration studies. Front Neuroendocrinol 33:17–35. [DOI] [PubMed] [Google Scholar]
- Bos PA, van Honk J, Ramsey NF, Stein DJ, Hermans EJ (2013): Testosterone administration in women increases amygdala responses to fearful and happy faces. Psychoneuroendocrinology 38:808–817. [DOI] [PubMed] [Google Scholar]
- Braams BR, Peters S, Peper JS, Guroglu B, Crone EA (2014): Gambling for self, friends, and antagonists: Differential contributions of affective and social brain regions on adolescent reward processing. Neuroimage 100:281–289. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Marois R (2012): The roots of modern justice: Cognitive and neural foundations of social norms and their enforcement. Nat Neurosci 15:655–661. [DOI] [PubMed] [Google Scholar]
- Bzdok D, Schilbach L, Vogeley K, Schneider K, Laird AR, Langner R, Eickhoff SB (2012): Parsing the neural correlates of moral cognition: ALE meta‐analysis on morality, theory of mind, and empathy. Brain Struct Funct 217:783–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney DR, Mason MF (2010): Decision making and testosterone: When the ends justify the means. J Exp Soc Psychol 46:668–671. [Google Scholar]
- Chen C, Chen CY, Yang CY, Lin CH, Cheng Y (2015): Testosterone modulates preattentive sensory processing and involuntary attention switches to emotional voices. J Neurophysiol 113:1842–1849. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Chen C, Lin CP, Chou KH, Decety J (2010): Love hurts: An fMRI study. Neuroimage 51:923–929. [DOI] [PubMed] [Google Scholar]
- Christensen JF, Gomila A (2012): Moral dilemmas in cognitive neuroscience of moral decision‐making: A principled review. Neurosci Biobehav Rev 36:1249–1264. [DOI] [PubMed] [Google Scholar]
- Crockett MJ, Fehr E (2013): Pharmacology of economic and social decision making In: Glimcher P, Fehr E, editors. Neuroeconomics: Decision making and the brain. New York: Academic Press; p 255–275. [Google Scholar]
- Crockett MJ, Rini RA (2015): Neuromodulation and the (in)stability of moral cognition In: Decety J, Wheatley T, editors. The Moral Brain: A Multidisciplinary Perspective. Cambridge, MA: MIT Press; p 221–236. [Google Scholar]
- Cushman F (2008): Crime and punishment: Distinguishing the roles of causal and intentional analyses in moral judgment. Cognition 108:353–380. [DOI] [PubMed] [Google Scholar]
- de Oliveira‐Souza R, Zahn R, Moll J (2015): Neural correlates of human morality: An overview In: Decety J, Wheatley T, editors. The Moral Brain—An Multidisciplinary Perspective. Cambridge: MIT press; p 183–195. [Google Scholar]
- Decety J, Cacioppo S (2012): The speed of morality: A high‐density electrical neuroimaging study. J Neurophysiol 108:3068–3072. [DOI] [PubMed] [Google Scholar]
- Decety J, Chen C, Harenski C, Kiehl KA (2013): An fMRI study of affective perspective taking in individuals with psychopathy: Imagining another in pain does not evoke empathy. Front Hum Neurosci 7:489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Chen C, Harenski CL, Kiehl KA (2015): Socioemotional processing of morally‐laden behavior and their consequences on others in forensic psychopaths. Hum Brain Mapp 36:2015–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Lamm C (2007): The role of the right temporoparietal junction in social interaction: How low‐level computational processes contribute to meta‐cognition. Neuroscientist 13:580–593. [DOI] [PubMed] [Google Scholar]
- Decety J, Cowell JM (2014): Friends or foes: Is empathy necessary for moral behavior? Perspect Psychol Sci 9:525–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Porges EC (2011): Imagining being the agent of actions that carry different moral consequences: An fMRI study. Neuropsychologia 49:2994–3001. [DOI] [PubMed] [Google Scholar]
- Decety J, Sommerville JA (2003): Shared representations between self and other: A social cognitive neuroscience view. Trends Cogn Sci 7:527–533. [DOI] [PubMed] [Google Scholar]
- Decety J, Michalska KJ, Kinzler KD (2012): The contribution of emotion and cognition to moral sensitivity: A neurodevelopmental study. Cereb Cortex 22:209–220. [DOI] [PubMed] [Google Scholar]
- Denson TF, Mehta PH, Ho Tan D (2013a): Endogenous testosterone and cortisol jointly influence reactive aggression in women. Psychoneuroendocrinology 38:416–424. [DOI] [PubMed] [Google Scholar]
- Denson TF, Ronay R, von Hippel W, Schira MM (2013b): Endogenous testosterone and cortisol modulate neural responses during induced anger control. Soc Neurosci 8:165–177. [DOI] [PubMed] [Google Scholar]
- Derntl B, Windischberger C, Robinson S, Kryspin‐Exner I, Gur RC, Moser E, Habel U (2009): Amygdala activity to fear and anger in healthy young males is associated with testosterone. Psychoneuroendocrinology 34:687–693. [DOI] [PubMed] [Google Scholar]
- Eisenegger C, Naef M, Snozzi R, Heinrichs M, Fehr E (2010): Prejudice and truth about the effect of testosterone on human bargaining behaviour. Nature 463:356–359. [DOI] [PubMed] [Google Scholar]
- Eisenegger C, Haushofer J, Fehr E (2011): The role of testosterone in social interaction. Trends Cogn Sci 15:263–271. [DOI] [PubMed] [Google Scholar]
- Funk CM, Gazzaniga MS (2009): The functional brain architecture of human morality. Curr Opin Neurobiol 19:678–681. [DOI] [PubMed] [Google Scholar]
- Glenn AL, Raine A, Schug RA, Gao Y, Granger DA (2011): Increased testosterone‐to‐cortisol ratio in psychopathy. J Abnorm Psychol 120:389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz SM, Tang L, Thomason ME, Diamond MP, Hariri AR, Carre JM (2014): Testosterone rapidly increases neural reactivity to threat in healthy men: A novel two‐step pharmacological challenge paradigm. Biol Psychiatry 76:324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray A, Jackson DN, McKinlay JB (1991): The relation between dominance, anger, and hormones in normally aging men: Results from the Massachusetts Male Aging Study. Psychosom Med 53:375–385. [DOI] [PubMed] [Google Scholar]
- Greene JD, Sommerville RB, Nystrom LE, Darley JM, Cohen JD (2001): An fMRI investigation of emotional engagement in moral judgment. Science 293:2105–2108. [DOI] [PubMed] [Google Scholar]
- Greene JD, Nystrom LE, Engell AD, Darley JM, Cohen JD (2004): The neural bases of cognitive conflict and control in moral judgment. Neuron 44:389–400. [DOI] [PubMed] [Google Scholar]
- Greene JD, Cushman FA, Stewart LE, Lowenberg K, Nystrom LE, Cohen JD (2009): Pushing moral buttons: The interaction between personal force and intention in moral judgment. Cognition 111:364–371. [DOI] [PubMed] [Google Scholar]
- Haidt J (2007): The new synthesis in moral psychology. Science 316:998–1002. [DOI] [PubMed] [Google Scholar]
- Harsay HA, Spaan M, Wijnen JG, Ridderinkhof KR (2012): Error awareness and salience processing in the oddball task: Shared neural mechanisms. Front Hum Neurosci 6:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans EJ, Putman P, Baas JM, Gecks NM, Kenemans JL, van Honk J (2007): Exogenous testosterone attenuates the integrated central stress response in healthy young women. Psychoneuroendocrinology 32:1052–1061. [DOI] [PubMed] [Google Scholar]
- Hermans EJ, Putman P, Baas JM, Koppeschaar HP, van Honk J (2006a): A single administration of testosterone reduces fear‐potentiated startle in humans. Biol Psychiatry 59:872–874. [DOI] [PubMed] [Google Scholar]
- Hermans EJ, Putman P, van Honk J (2006b): Testosterone administration reduces empathetic behavior: A facial mimicry study. Psychoneuroendocrinology 31:859–866. [DOI] [PubMed] [Google Scholar]
- Hesse E, Mikulan E, Decety J, Sigman M, del Carmen Garcia M, Silva W, Ciraolo C, Vaucheret E, Baglivo F, Huepe D, Lopez V, Manes F, Bekinschtein TA, Ibanez A (2016): Early detection of intentional harm in the human amygdala. Brain 139:54–61. [DOI] [PubMed] [Google Scholar]
- Hönekopp J, Bartholdt L, Beier L, Liebert A (2007): Second to fourth digit length ratio (2D:4D) and adult sex hormone levels: New data and a meta‐analytic review. Psychoneuroendocrinology 32:313–321. [DOI] [PubMed] [Google Scholar]
- Huebner B, Dwyer S, Hauser M (2009): The role of emotion in moral psychology. Trends Cogn Sci 13:1–6. [DOI] [PubMed] [Google Scholar]
- Huebner B, Hauser M, Pettit P (2011): How the source, inevitability and means of bringing about harm interact in folk‐moral judgments. Mind Language 26:210–233. [Google Scholar]
- Jackson PL, Decety J (2004): Motor cognition: A new paradigm to study self‐other interactions. Curr Opin Neurobiol 14:259–263. [DOI] [PubMed] [Google Scholar]
- Kahn PH Jr (1992): Children's obligatory and discretionary moral judgments. Child Dev 63:416–430. [PubMed] [Google Scholar]
- Killen M, Lynn Mulvey K, Richardson C, Jampol N, Woodward A (2011): The accidental transgressor: Morally‐relevant theory of mind. Cognition 119:197–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Young L, Adolphs R, Tranel D, Cushman F, Hauser M, Damasio A (2007): Damage to the prefrontal cortex increases utilitarian moral judgements. Nature 446:908–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Batson CD, Decety J (2007): The neural substrate of human empathy: Effects of perspective‐taking and cognitive appraisal. J Cogn Neurosci 19:42–58. [DOI] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T (2011): Meta‐analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage 54:2492–2502. [DOI] [PubMed] [Google Scholar]
- Mehta PH, Beer J (2010): Neural mechanisms of the testosterone‐aggression relation: The role of orbitofrontal cortex. J Cogn Neurosci 22:2357–2368. [DOI] [PubMed] [Google Scholar]
- Molenberghs P, Bosworth R, Nott Z, Louis WR, Smith JR, Amiot CE, Vohs KD, Decety J (2014): The influence of group membership and individual differences in psychopathy and perspective taking on neural responses when punishing and rewarding others. Hum Brain Mapp 35:4989–4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J, Zahn R, de Oliveira‐Souza R, Krueger F, Grafman J (2005): The neural basis of human moral cognition. Nat Rev Neurosci 6:799–809. [DOI] [PubMed] [Google Scholar]
- Moll J, de Oliviera‐Souza R, Garrido GJ, Bramati IE, Caparelli‐Daquer EMA, Paiva ML, Zahn R, Grafman J (2007): The self as a moral agent: Linking the neural bases of social agency and moral sensitivity. Soc Neurosci 2:336–352. [DOI] [PubMed] [Google Scholar]
- Montoya ER, Terburg D, Bos PA, Will GJ, Buskens V, Raub W, van Honk J (2013): Testosterone administration modulates moral judgments depending on second‐to‐fourth digit ratio. Psychoneuroendocrinology 38:1362–1369. [DOI] [PubMed] [Google Scholar]
- Muller DC, Giles GG, Bassett J, Morris HA, Manning JT, Hopper JL, English DR, Severi G (2011): Second to fourth digit ratio (2D:4D) and concentrations of circulating sex hormones in adulthood. Reprod Biol Endocrinol 9:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke S, Volman I, Mehta P, van Son V, Enter D, Sanfey A, Toni I, de Bruijn ER, Roelofs K (2015): Testosterone biases the amygdala toward social threat approach. Sci Adv 1:e1400074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razali NM, Wah YB (2011): Power comparisons of Shapiro‐Wik, Kolmogorov‐Smirnov, Lilliefors and Anderson‐Darling tests. J Stat Model Analytics 2:21–33. [Google Scholar]
- Schutter DJ, van Honk J (2004): Decoupling of midfrontal delta‐beta oscillations after testosterone administration. Int J Psychophysiol 53:71–73. [DOI] [PubMed] [Google Scholar]
- Shapiro SS, Wilk MB (1965): An analysis of variance test for normality (complete samples). Biometrika 52:591–611. [Google Scholar]
- Slater CC, Souter I, Zhang C, Guan C, Stanczyk FZ, Mishell DR (2001): Pharmacokinetics of testosterone after percutaneous gel or buccal administration. Fertil Steril 76:32–37. [DOI] [PubMed] [Google Scholar]
- Sobhani M, Bechara A (2011): A somatic marker perspective of immoral and corrupt behavior. Soc Neurosci 6:640–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton SJ, Wirth MM, Waugh CE, Schultheiss OC (2009): Endogenous testosterone levels are associated with amygdala and ventromedial prefrontal cortex responses to anger faces in men but not women. Biol Psychol 81:118–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber‐Thomas BC, Asp EW, Koenigs M, Sutterer M, Anderson SW, Tranel D (2014): Arrested development: Early prefrontal lesions impair the maturation of moral judgement. Brain 137:1254–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuiten A, Van Honk J, Koppeschaar H, Bernaards C, Thijssen J, Verbaten R (2000): Time course of effects of testosterone administration on sexual arousal in women. Arch Gen Psychiatry 57:149–153. discussion 155‐6. [DOI] [PubMed] [Google Scholar]
- Tuiten A, van Honk J, Verbaten R, Laan E, Everaerd W, Stam H (2002): Can sublingual testosterone increase subjective and physiological measures of laboratory‐induced sexual arousal? Arch Gen Psychiatry 59:465–466. [DOI] [PubMed] [Google Scholar]
- van Honk J, Schutter DJ (2007): Testosterone reduces conscious detection of signals serving social correction: Implications for antisocial behavior. Psychol Sci 18:663–667. [DOI] [PubMed] [Google Scholar]
- van Honk J, Peper JS, Schutter DJ (2005): Testosterone reduces unconscious fear but not consciously experienced anxiety: Implications for the disorders of fear and anxiety. Biol Psychiatry 58:218–225. [DOI] [PubMed] [Google Scholar]
- van Wingen G, Mattern C, Verkes RJ, Buitelaar J, Fernandez G (2010): Testosterone reduces amygdala‐orbitofrontal cortex coupling. Psychoneuroendocrinology 35:105–113. [DOI] [PubMed] [Google Scholar]
- van Honk J, Schutter DJ, Bos PA, Kruijt AW, Lentjes EG, Baron‐Cohen S (2011): Testosterone administration impairs cognitive empathy in women depending on second‐to‐fourth digit ratio. Proc Natl Acad Sci USA 108:3448–3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij K, Bloemers J, de Leede L, Goldstein I, Lentjes E, Koppeschaar H, Olivier B, Tuiten A (2012): Pharmacokinetics of three doses of sublingual testosterone in healthy premenopausal women. Psychoneuroendocrinology 37:773–781. [DOI] [PubMed] [Google Scholar]
- van Honk J, Montoya ER, Bos PA, van Vugt M, Terburg D (2012): New evidence on testosterone and cooperation. Nature 485: E4–E5; discussion E5–E6. [DOI] [PubMed] [Google Scholar]
- Volman I, Toni I, Verhagen L, Roelofs K (2011): Endogenous testosterone modulates prefrontal‐amygdala connectivity during social emotional behavior. Cereb Cortex 21:2282–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A (1988): Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol 54:1063–1070. [DOI] [PubMed] [Google Scholar]
- Welker KM, Lozoya E, Campbell JA, Neumann CS, Carre JM (2014): Testosterone, cortisol, and psychopathic traits in men and women. Physiol Behav 129:230–236. [DOI] [PubMed] [Google Scholar]
- Wood RI (1996): Functions of the steroid‐responsive neural network in the control of male hamster sexual behavior. Trends Endocrinol Metab 7:338–344. [DOI] [PubMed] [Google Scholar]
- Yoder KJ, Decety J (2014a): The Good, the bad, and the just: Justice sensitivity predicts neural response during moral evaluation of actions performed by others. J Neurosci 34:4161–4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KJ, Decety J (2014b): Spatiotemporal neural dynamics of moral judgment: A high‐density ERP study. Neuropsychologia 60:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L, Koenigs M (2007): Investigating emotion in moral cognition: A review of evidence from functional neuroimaging and neuropsychology. Br Med Bull 84:69–79. [DOI] [PubMed] [Google Scholar]
- Young L, Dungan J (2012): Where in the brain is morality? Everywhere and maybe nowhere. Soc Neurosci 7:1–10. [DOI] [PubMed] [Google Scholar]
- Young L, Bechara A, Tranel D, Damasio H, Hauser M, Damasio A (2010a): Damage to ventromedial prefrontal cortex impairs judgment of harmful intent. Neuron 65:845–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L, Camprodon JA, Hauser M, Pascual‐Leone A, Saxe R (2010b): Disruption of the right temporoparietal junction with transcranial magnetic stimulation reduces the role of beliefs in moral judgments. Proc Natl Acad Sci U S A 107:6753–6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Cohn MJ (2011): Developmental basis of sexually dimorphic digit ratios. Proc Natl Acad Sci USA 108:16289–16294. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


