Abstract
Prior research shows that after making a choice, decision makers shift their attitudes in a choice‐congruous direction. Although this post‐choice attitude change effect is robust, the neural mechanisms underlying it are poorly understood. Here, we tested the hypothesis that decision makers elaborate on their choice in reference to self‐knowledge to justify the choice they have made. This self‐referential processing of the choice is thought to play a pivotal role in the post‐choice attitude change. Twenty‐four young American adults made a series of choices. They also rated their attitudes toward the choice options before and after the choices. In support of the current hypothesis, we found that changes in functional connectivity between two putative self‐regions (medial prefrontal cortex and posterior cingulate cortex/precuneus]) during the post‐choice (vs. pre‐choice) rating of the chosen options predicted the post‐choice shift of the attitudes toward the chosen options. This finding is the first to suggest that cognitive integration of various self‐relevant cognitions is instrumental in fostering post‐choice attitude change. Hum Brain Mapp 37:3810–3820, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: attitude change, decision making, functional connectivity, self
INTRODUCTION
When people make a choice, they often change their attitudes in a choice‐consistent direction [Brehm, 1956; Steele, 1988]. Specifically, they will increase their preference for the option they chose and decrease their preference for the option that they rejected. Although this effect of post‐choice attitude change is well established and thought to justify the choice, exactly how it might occur is unclear [Chen and Risen, 2010; Harmon‐Jones et al., 2009; Kitayama and Tompson, 2015; Steele, 1988].
In the current work, we addressed this gap using functional magnetic resonance imaging (fMRI) to examine the possibility that the decision maker justifies her choice by linking the choice to various aspects of self‐knowledge. Although often discussed in social psychological analyses of choice justification [Aronson, 1969; Gawronski et al., 2007; Kitayama et al., 2004; Steele and Liu, 1983], this hypothesis is difficult to test with behavioral measures alone as these measures can only capture down‐stream, often distal outcomes of the psychological mechanisms under discussion. Using fMRI, it may be possible to obtain more direct evidence for the self‐referential processing hypothesis. Motivated by a recent network‐oriented thinking in the field [Poldrack, 2012; Rogers, Morgan et al., 2007], we hypothesized that the post‐choice attitude change should be tracked by the post‐choice recruitment of self‐relevant knowledge as indexed by the functional connectivity between putative self‐processing areas (i.e., medial prefrontal cortex [mPFC] and posterior cingulate cortex/precuneus [PCC/Pcu]) [Denny et al., 2012; Feyers et al., 2010; Morel et al., 2014; Ries et al., 2012; van der Meer et al., 2010].
POST‐CHOICE ATTITUDE CHANGE
In an attempt to understand the mechanisms underlying post‐choice attitude change, it is important to consider (a) what might happen during the choice and (b) what might subsequently occur once the choice has been made. Researchers using behavioral measures have typically focused on the processes that occur once a choice has been made (post‐choice processes), but recent work has begun to unpack how mechanisms during the choice might also contribute to post‐choice attitude change [Jarcho et al., 2011; Kitayama et al., 2013]. Hence, we discuss the in‐choice mechanism first although the primary focus of the current work is the post‐choice mechanism.
In‐Choice Mechanism: Search for Positive Incentives
Kitayama and Tompson [Kitayama et al., 2013; Kitayama and Tompson, 2015] have proposed that when making a choice, the decision maker will look for positive features in one of available choice options. These features would allow her to choose between the options. This hypothesis implies that before a choice is made, the decision maker may develop a new attitude toward one of the options and, moreover, this new attitude may enable her to choose that option. When the attitudes toward the relevant options are assessed at a later time, the chosen option will be liked more than the rejected option. Although this attitude change is assessed after the choice and, thus, is seen as mediated entirely by post‐choice mechanisms, it may well take place, at least in part, before the choice has been made.
If the in‐choice feature search is instrumental in fostering post‐choice attitude change, one may expect that certain brain signals indicating the identification of positive features during the choice will predict a positive shift of the attitude toward the chosen option. Ventral striatum (vSTR) is a subcortical structure thought to track subjective value and reward [Bartra et al., 2013] and has been shown to track changes in subjective value as a function of situational and contextual factors [Varnum et al., 2014] and thus vSTR should be integral in facilitating the in‐choice feature search and updating value of the various choice options to foster post‐choice attitude change. In support of the in‐choice feature search hypothesis, research consistently finds a trial‐by‐trial change in vSTR reliably tracks post‐choice attitude change [Jarcho et al., 2011; Kitayama et al., 2013], suggesting that the decision maker chooses an option if during the choice he/she identifies positive features in it that activate the vSTR.1
Post‐Choice Mechanism: Recruitment of Self‐Knowledge
Although the in‐choice search mechanism plays an important role in fostering the post‐choice attitude change, it is unlikely that it is the only mechanism involved in this effect. In fact, even after having made a choice, the decision maker may continue to elaborate on information that is relevant to the choice. According to cognitive dissonance theory [Festinger, 1957] and subsequent elaborations of the theory [Aronson, 1969; Gawronski et al., 2007; Steele, 1988], the decision maker may do so to justify the choice. Consistent with these theories, we propose that the justification of a choice that has been made is accomplished through active recruitment of an assortment of cognitions that link the chosen option to the self. These cognitions may include: an image of the self as smart and as a good decision maker, autobiographic memories involving the chosen option, and/or future plans with it. These diverse self‐referential cognitions will be linked and integrated to justify the choice. For example, the image of the self as a good decision maker might be tied to some memory of having enjoyed using the chosen object in the past and/or certain plans to use it on some special occasions in the future. It is this integrated representation of the chosen option that reinforces the value of the choice and, thus, justifies it.
Our self‐referential processing hypothesis of choice justification implies that when a chosen option is presented after the choice, this option will recruit the self‐relevant cognitions that were generated during the post‐choice processing of choice‐relevant information, as well as the brain regions underlying these cognitions. Previous studies have found that the mPFC and PCC/Pcu are linked to self‐referential processing [Chua et al., 2011; Denny et al., 2012; Northoff et al., 2006; van der Meer et al., 2010]. We may therefore anticipate that these putative self‐regions will be activated more by the chosen (vs. rejected) options when they are presented at a later point. In contrast, the rejected items are unlikely to be linked to the self‐knowledge, as they do not belong to the self.
The self‐referential processing hypothesis offers another important prediction. The diverse self‐cognitions that are recruited during the post‐choice processing of the choice (e.g., self‐images, episodes remembered, and plans made) are likely to be differentially represented in mPFC and PCC/Pcu. For example, numerous theorists have argued that abstract, trait‐like representations of the self are likely to be represented predominantly in the mPFC region, whereas more episodic representations of the self might be relatively more dominant in posterior regions of the brain including PCC/Pcu [Qin et al., 2012; Sajonz et al., 2010; van der Meer et al., 2010]. Moreover, recent fMRI research has used psychophysiological interaction (PPI) analyses to investigate how task‐dependent connectivity between brain regions is involved in thinking about and evaluating the self. Researchers have found that connectivity between mPFC and PCC/Pcu is greater when people think about and evaluate the self than when thinking about and evaluating others [Feyers et al., 2010; Morel et al., 2014; Ries et al., 2012]. mPFC‐PCC connectivity is also greater when encoding self‐referential information and predicts subsequent recall of self‐referential information [Morel et al., 2014]. Furthermore, mPFC‐PCC/Pcu connectivity is greater when evaluating whether trait words describe the self (self‐judgments) than when evaluating whether trait words are positive or negative (valence judgments) [Feyers et al., 2010] and the strength of this connectivity is positively correlated with autobiographical memory accuracy [Ries et al., 2012]. Thus, connectivity between mPFC and PCC/Pcu is thought to be involved in thinking about and evaluating the self as well as recalling self‐relevant memories.
Thus, the hypothesis that these self‐cognitions are integrated to justify a choice implies that there should be increases in the functional connectivity between mPFC and PCC/Pcu after the choice. Importantly, the self‐referential processing hypothesis suggests that this integration of the diverse self‐cognitions is instrumental in justifying the choice. As a result, we may anticipate that increases in the functional connectivity between mPFC and PCC/Pcu should predict increases in the post‐choice (vs. pre‐choice) attitude toward the chosen option. The same might not apply to the rejected options, insofar as the decision maker is unlikely to devote much effort in linking the rejected options to self‐knowledge.
Evidence for the self‐referential processing hypothesis is currently scant. In addition to the two fMRI studies reviewed earlier [Jarcho et al., 2011; Kitayama et al., 2013], there are three additional fMRI studies on post‐choice attitude change [Izuma et al., 2010; Qin et al., 2011; Sharot et al., 2009]. These studies focused on neural activations during the pre‐ and post‐choice rating periods rather than in‐choice activations. Given the self‐referential processing hypothesis, the activation of mPFC and PCC/Pcu and the functional connectivity between the two regions should predict changes in attitudes toward the choice options. Thus far, only average activation has been tested. In two of the three relevant studies, hypothetical choices were used and, thus, the procedure was not incentive compatible with real values [Izuma et al., 2010; Sharot et al., 2009]. It is likely that when the choice is hypothetical, it does not recruit any self‐knowledge. It is, therefore, not surprising that these studies did not find any evidence for the self‐referential processing hypothesis. The remaining study [Qin et al., 2011] used an incentive compatible procedure where participants received one of the music CDs they chose and found that the activation of both dorsal and ventral regions of the mPFC during the post‐choice rating of chosen options predicts the post‐choice attitude change for them. Importantly, none of the above studies tested functional connectivity during the pre‐choice or post‐choice rating tasks and it is, therefore, unclear whether functional connectivity might be involved in facilitating post‐choice attitude change.
PRESENT RESEARCH
Guided by the literature review discussed above, the current work tested implications of the self‐referential processing hypothesis using data from the Kitayama et al. [2013] study that had not previously been analyzed. Unlike the Kitayama et al. [2013] study, which focused on in‐choice brain activation and tested whether this activation would track subsequent attitude change on a trial‐by‐trial basis, the current work utilized data on neural activation from both pre‐choice and post‐choice rating periods and tested between‐subjects associations between measures of the recruitment of self‐knowledge and post‐choice attitude change.
We had three primary predictions. First, for the post‐choice (vs. pre‐choice) rating, the putative self‐regions of the brain (mPFC and PCC/Pcu) would be activated relatively more by chosen options as compared to rejected options. Second, the functional connectivity between the two regions during the post‐choice (vs. pre‐choice) rating period should be larger for the chosen options, but no corresponding increases would be expected for the rejected options. Third, increases in the functional connectivity between the two regions for the chosen options at the post‐choice (vs. pre‐choice) rating should predict post‐choice (vs. pre‐choice) attitude toward the chosen but not rejected options in choice‐consistent directions.
Our secondary goal was to explore individual differences in the degree to which self‐processing increases from pre‐choice to post‐choice. Previous evidence suggests that individuals with independent self‐construal are more likely to consider personal choices to be relevant to the self Na and Kitayama, 2012; Savani et al., 2008, 2010]. Extending recent work in cultural neuroscience [Han et al., 2013; Hyde, Tompson et al., 2015] that has found that culture and self‐construal influence how the brain makes social and cognitive judgments [Gutchess et al., 2006; Ma et al., 2014; Park et al., 2016; Varnum et al., 2014], we investigated whether the neural activation of putative self‐regions (i.e., mPFC and PCC/Pcu), as well as the connectivity between them, during the post‐choice (vs. pre‐choice) rating period might increase as a function of independent self‐construal.
METHOD
Participants
Twenty‐five undergraduates at the University of Michigan participated in the study. One participant was excluded from analysis due to excessive head movement. Analysis was performed on the remaining 24 participants (10 males and 14 females, mean age = 19.71, SD = 1.43). All participants were born and raised in the United States, had normal or corrected‐to‐normal vision and had no history of head injury or psychiatric illness. Participants received $50. At the end of the study, they were offered the option of either keeping one of the CDs they chose or receiving eight more dollars instead of the CD. All participants opted for the extra cash. All participants gave written consent and the Institutional Review Board at the University of Michigan approved the procedure.
Procedure and Materials
Following the procedure from previous behavioral studies on choice [e.g., Kitayama et al., 2004], we used popular music CDs as stimuli. We sampled 160 CDs from the Billboard Top 100 music CDs and the Apple iTunes Top 100 music CDs from September 2009 to November 2009 and conducted the study during the first half of 2010.
Approximately 1.5 weeks prior to the experiment, participants filled out a survey packet that included the Singelis self‐construal scale [Singelis, 1994]. On arrival at the fMRI center, participants performed three tasks inside the scanner. First, they completed two runs of a pre‐choice rating task. They were shown the covers for 120 popular music CDs one at a time in randomized order (60 CDs in each run), and asked to rate how much they liked each CD on a 5‐point scale (1 = least likeable, 5 = most likeable). The cover of each CD was displayed for 3 s along with the artist and album title, with an average inter‐stimulus interval of 4 s (2, 4, or 6 s, jittered). The second fMRI task involved a single run of the choice task. Participants were presented with 60 pairs of CD covers (30 easy pairs and 30 difficult pairs as determined by how similar or different they rated the items during the pre‐choice rating task). Participants selected the CD they wanted in each pair. It was explained that one of the 60 CDs chosen by the participant would be randomly selected and given to the participant at the end of the session; so the choices were incentive compatible with real values. Third, participants repeated two runs of the rating task described above. This study focuses on brain activation patterns during the pre‐choice and post‐choice rating tasks.
Singelis Scale
We used an abbreviated 20‐item version of the Singelis self‐construal scale [Singelis, 1994] to assess independent and interdependent self‐construal (See Appendix for specific items used). This version of the scale consists of two subscales (10 items each). Independent self‐construal was assessed by averaging each participant's responses to 10 items pertaining to one's uniqueness and autonomy (e.g., I enjoy being unique and different from others in many respects), whereas interdependent self‐construal was assessed by averaging each participant's response to 10 items concerning one's interpersonal relatedness and values placed on social harmony (e.g., I will sacrifice my self‐interest for the benefit of the group I am in). Participants rated how much each item described the self on a 5‐point scale (1 = does not describe me at all, 5 = describes me very much). The reliabilities were comparable to those obtained in previous work (Cronbach's αs = 0.76 and 0.59, for independence and interdependence, respectively).
Participants reported slightly higher independent self‐construal (M = 3.74, SD = 0.55) than interdependent self‐construal (M = 3.56, SD = 0.47), although this difference was not statistically significant (t(23) = 1.01, P = 0.323). There was a significant negative correlation between independent and interdependent self‐construal (r = −0.46, P = 0.025), such that individuals who reported higher independent self‐construal were also more likely to report lower interdependent self‐construal.
fMRI Data Acquisition
Participants were tested in a GE 3T Signa Excite 2 scanner (Milwaukee, Wisconsin). We first acquired a standard T1 structural image for alignment (TR = 250, TE = 3.7, FA = 75, FOV = 220, 43 oblique axial slices, matrix 256 × 256, slice thickness = 3.5, 0 skip). During the experimental task, T2*‐weighted, spiral‐in acquisition sequence were acquired (gradient echo, TR = 2000, TE = 30, FA = 90, FOV = 220, 43 oblique axial slices, matrix 64 × 64, slice thickness 3.0 mm, 0 skip). Four initial volumes were discarded at the beginning of each run to allow for stabilization of the MRI signal. Finally, a high‐resolution T1 anatomical scan was obtained (three‐dimensional spoiled‐gradient echo [SPGR] with inversion recovery prep, time of inversion = 400 ms, TR = 9.0, TE = 1.8, FA = 15, FOV = 260, 128 slices, matrix 256 × 256, 1.2 mm slice, 0 skip).
fMRI Data Analysis
Data were analyzed using the statistical parametric mapping software package, SPM8 (Welcome Department of Cognitive Neurology, London, UK). Functional volumes were slice time corrected using MCFLIRT [Jenkinson et al., 2002] to account for temporal differences in slice acquisition time, realigned to correct for head motion, and spatially normalized to a standard template based on the Montreal Neurological Institute (MNI) reference brain using VBM8 toolbox and DARTEL high dimensional warping, and spatially smoothed using a 5‐mm Gaussian kernel.
Data for the pre‐choice and post‐choice rating tasks were modeled using an event‐related design and a modified general linear model [Worsley et al., 1992, 1997]. First‐level models included a boxcar function for each 3‐s trial, with separate regressors for time (pre‐choice vs. post‐choice), choice (chosen vs. rejected), and difficulty (difficulty vs. easy, defined by the pre‐choice difference in preference ratings for the CDs in each pair).2 Movement parameters were included as covariates for all models.
We used a large‐scale study on self‐referential processing [Chua et al., 2011] to define a set of a priori functional regions of interest (ROIs) in mPFC and PCC/Pcu. Using SPM8 and MarsBaR, we created 10mm sphere ROIs around the peak voxels in the mPFC (3,60,12) and PCC/Pcu (−3,−51,33) regions that have been linked to thinking about and evaluating the self (relative to a valence judgment; FWE‐corrected, P < 0.05, cluster threshold of k > 50; see Fig. 1). All coordinates are reported in MNI space. We ran two sets of analyses using our a priori mPFC and PCC/Pcu ROIs focusing on (i) the activations of the mPFC and PCC/Pcu ROIs and (ii) the functional connectivity between the two ROIs.
Figure 1.
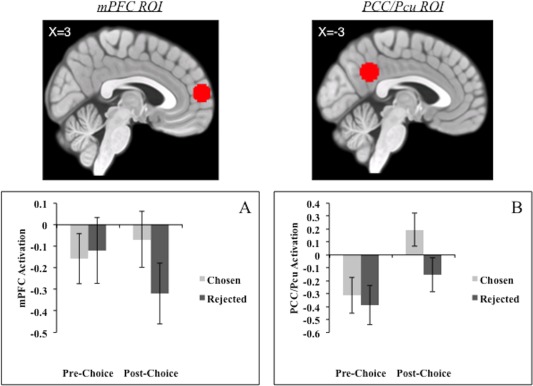
Two ROIs and the activity in the ROIs as a function of choice: specific regions of the mPFC and the PCC/precuneus (Pcu) were identified on the basis of Chua et al. [2011] (top images). Each ROI showed a systematic change in activity as a function of choice (A and B, respectively). All activation is relative to an implicit fixation baseline. Error bars are standard error. [Color figure can be viewed at http://wileyonlinelibrary.com.]
Activation of the mPFC and PCC/Pcu ROIs
A series of three analyses were performed. (i) The average activation of each ROI was analyzed in a 2 (Choice) × 2 (Time) repeated‐measures ANOVA. Follow‐up simple effects analyses were performed with two‐tailed t‐tests, with significance threshold set to 0.05. (ii) We used multiple linear regression analyses to test whether the mPFC and PCC/Pcu activations during the post‐choice (vs. in‐choice) rating period of chosen options would predict the post‐choice attitude change for these options. This same analysis was repeated for rejected options. (iii) Furthermore, we used linear regression analysis to explore whether independent or interdependent self‐construals would predict the mPFC and PCC/Pcu activations during the post‐choice (vs. pre‐choice) rating of chosen options. We repeated the same analysis for the rejected options.
Functional connectivity between mPFC and PCC/Pcu
To examine the functional connectivity between mPFC and PCC/Pcu, we used a generalized psycho‐physiological interaction (gPPI) analysis with the mPFC ROI as the seed [McLaren et al., 2012]. Similar to standard psychophysiological interaction (sPPI), gPPI identifies an association between the time courses of activation for two regions first and then tests whether the magnitude of this association will vary as a function of a psychological variable(s). If the association between the two regions should vary as a function of this psychological variable(s), this PPI would constitute evidence for the functional connectivity between the two regions. Unlike sPPI, gPPI identifies the strength of this association in each condition separately (i.e., identifies a separate regressor for each condition and the interaction between the time course for each condition and the time course of the neural activation). This allows researchers more flexibility to run multiple contrasts to test whether this relationship differs for one condition versus another (or vs. multiple other conditions), especially when there are more than two conditions [McLaren et al., 2012].
We used the same set of analyses that were used to examine average activation within mPFC and PCC/Pcu to examine the functional connectivity between mPFC and PCC/Pcu. For every voxel outside of the mPFC ROI, we first computed the task‐related association between each voxel and the mPFC ROI (i.e., computed the PPI regressor). This was done separately for each of the four conditions defined by Time (pre‐ vs. post‐choice) and Choice (chosen vs. rejected). We then extracted the average beta value in the PCC/Pcu ROI in each of the four conditions to get a measure of the task‐related association between mPFC and PCC/Pcu in each condition.
(i) The average beta values for mPFC‐PCC/Pcu functional connectivity were submitted to a 2 (Choice) × 2 (Time) repeated‐measures ANOVA. Follow‐up simple effects analyses were performed with two‐tailed t‐tests, with significance threshold set to 0.05. (ii) For the chosen options, we tested whether increases in the functional connectivity between mPFC and PCC/Pcu during the post‐choice (vs. pre‐choice) rating period would predict post‐choice attitude change in a regression analysis. We ran the same set of analyses for rejected options. (iii) Furthermore, we explored whether independent or interdependent self‐construals would predict the changes in the functional connectivity between mPFC and PCC/Pcu during the post‐choice (vs. pre‐choice) rating of the chosen options. We repeated the same analysis for the rejected options.
RESULTS
Behavioral Results
We first analyzed preference ratings of the options. We found a significant 2‐way interaction between choice (chosen vs. rejected) and time (pre‐choice vs. post‐choice; F(1,23) = 8.26, P = 0.009). Preference for chosen options increased from pre‐choice (M = 3.16, SD = 0.37) to post‐choice (M = 3.25, SD = 0.41; t(23) = 7.01, P < 0.001), whereas preference for rejected options decreased from pre‐choice (M = 2.13, SD = 0.38) to post‐choice (M = 2.03, SD = 0.34; t(23) = −4.15, P < 0.001). There was no correlation between the behavioral ratings and either independent self‐construal or interdependent self‐construal.
fMRI Results
mPFC and PCC/Pcu activations
The mPFC activations in the relevant conditions are shown in Figure 1A. The Choice × Time interaction was significant (F(1,23) = 5.32, P = 0.030). Subsequent simple effect tests showed that the activation was no different between the chosen and the rejected options at the pre‐choice rating period (t (23) = −0.45, P = 0.657), but it was significantly greater for the chosen options than for the rejected options during the post‐choice rating (t(23) = 3.07, P = 0.005). The pattern for the PCC/Pcu activation, shown in Figure 1B, also showed a significant interaction between Choice and Time (F(1,23) = 6.74, P = 0.016). The PCC/Pcu activation was no different between the chosen and the rejected options at the pre‐choice rating (t(23) = 0.96, P = 0.349), but it was significantly greater for the chosen options than for the rejected options during the post‐choice rating period (t(23) = 4.21, P < 0.001).
Subsequent regression analyses showed that the changes in the activation of mPFC and PCC/Pcu did not predict post‐choice attitude change. The mPFC activation during the post‐choice (vs. pre‐choice) rating of the chosen options had no effect on the post‐choice (vs. pre‐choice) attitude for the chosen options (β = −0.33, P = 0.13). It also had no effect on the post‐choice (vs. pre‐choice) attitude for the rejected options (β = −0.10, P = 0.654). The PCC/Pcu activation during the post‐choice (vs. pre‐choice) rating of the chosen options had no effect on the post‐choice (vs. pre‐choice) attitude of these options (β = −0.01, P = 0.987). It also had no effect on the post‐choice (vs. pre‐choice) attitude for the rejected options (β = −0.08, P = 0.701, respectively).
Further regression analyses tested the effects of independent and interdependent self‐construals. We found that increases in independent self‐construal predicted increased activations in mPFC (β = 0.50, P = 0.012) and PCC/Pcu (β = 0.47, P = 0.020) from pre‐choice to post‐choice for chosen options. There was neither effect of independent self‐construal on neural activation for rejected options nor was there a relationship between interdependent self‐construal and neural activation for chosen or rejected options.3
Functional connectivity between mPFC and PCC/Pcu
When the task‐related association between the two putative self‐regions (mPFC and PCC/Pcu) was analyzed as a function of Choice and Time, the interaction between these two factors did not achieve statistical significance (F(23) = 1.88, P = 0.184). Nevertheless, this association did increase, albeit marginally, during the post‐choice (vs. pre‐choice) rating of the chosen options (t(23) = 1.77, P = 0.091). In contrast, there was no change from pre‐choice to post‐choice in the functional connectivity between the two regions during the rating of the rejected options (t(23) = 0.55, P = 0.587; see Fig. 2A). This pattern suggests a slight increase of the functional connectivity between the two regions during the rating of the chosen options.
Figure 2.
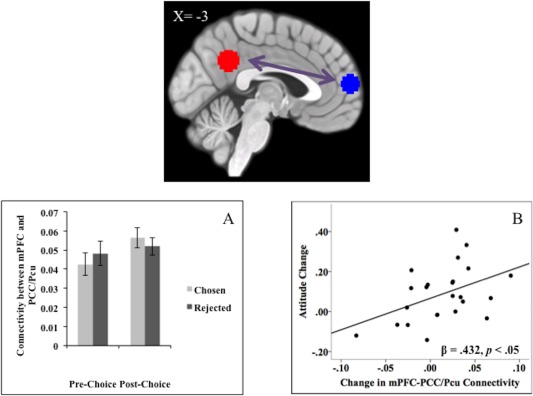
Functional connectivity between mPFC and PCC/Pcu ROIs: connectivity increased for chosen options and predicted changes in attitudes toward the chosen options after the choice. (A) The figure represents activation relative to implicit fixation baseline, with error bars representing standard error for each condition. (B) The figure shows change in appraisals of the chosen options as a function of the change in connectivity between mPFC and PCC/Pcu. [Color figure can be viewed at http://wileyonlinelibrary.com.]
Subsequent regression analyses tested whether increases in the functional connectivity between the two regions during the post‐choice (vs. pre‐choice) rating of the chosen options would predict the post‐choice (vs. pre‐choice) attitudes toward these options. As shown in Figure 2B, we found that the increases in this association significantly predicted the attitude change for the chosen options (β = 0.43, P = 0.035). This is consistent with the hypothesis that increased functional connectivity between the two putative self‐regions in the processing of the chosen options reinforces the positive attitude toward them. There was no comparable effect for the rejected options (β = 0.01, P = 0.956).
Further regression analyses tested the effects of independent and interdependent self‐construals on the functional connectivity between the two putative self‐regions (mPFC and PCC/Pcu). There was no effect of either independent self‐construal or interdependent self‐construal on this association at the post‐choice (vs. pre‐choice) rating for the chosen options (β = 0.02, P = 0.934; β = −0.20, P = 0.344; respectively). Nor did we find any effect of either independent self‐construal or interdependent self‐construal on this association at the post‐choice (vs. pre‐choice) rating of the rejected options (β = 0.07, P = 0.735; β = −0.03, P = 0.904; respectively).
DISCUSSION
The Self‐Referential Processing Hypothesis of Post‐Choice Attitude Change
The self‐referential processing hypothesis of post‐choice attitude change holds that after making a choice, individuals link the choice to their self‐knowledge so as to justify it. The hypothesis is consistent with the existing social psychological theories of post‐choice attitude change [Aronson, 1969; Kitayama and Tompson, 2015; Steele, 1988; Stone and Cooper, 2001]. Moreover, it offers clear predictions about the brain mechanisms that are likely to be recruited to produce choice justification. The current work provided the first neural evidence that lends support to some critical implications of this hypothesis.
First, we observed that during the post‐choice (vs. pre‐choice) rating of the chosen (vs. rejected) options there was an increased activation in both mPFC and PCC/Pcu (the two primary regions putatively linked to self‐referential processing). Second, there was a marginal increase in the connectivity between the two regions during the post‐choice (vs. pre‐choice) rating of the chosen options. No comparable effect was evident for the rejected options. Third and most importantly, the increased functional connectivity between the two putative self‐regions during the post‐choice (vs. pre‐choice) rating of the chosen options reliably predicted the post‐choice attitude change of the chosen options. There was no effect of the functional connectivity for the rejected options. Nor was there any effect of the activation of either mPFC or PCC/Pcu assessed separately on post‐choice attitude change. Taken together, the evidence is consistent with the hypothesis that the cognitive integration of diverse self‐referential cognitions (e.g., self‐images, episodes, and plans) and the functional connectivity of the separate regions that are involved, rather than separate activations of these cognitions and the corresponding regions, are instrumental in forging choice justification.
One additional finding is potentially important. We assessed independent and interdependent self‐construal and found that independent self‐construal reliably predicted changes in neural activation within mPFC and PCC/Pcu for chosen options. This finding lends some tentative support to the prediction that individuals who view the self as independent from others take their choices more seriously and thus expend more effort to justify them. In the current study, however, the increased activation of mPFC and PCC/Pcu was not related either to greater connectivity between mPFC and PCC/Pcu or to post‐choice attitude change. Thus, we found no evidence that independent self‐construal is linked to a greater likelihood of choice justification.
Altogether, our work goes beyond previous work that has examined post‐choice mechanisms of choice justification [Izuma et al., 2010; Qin et al., 2011; Sharot et al., 2009]. Unlike Izuma et al. [2010] and Sharot et al. [2009], we used an incentive compatible procedure where participants received one of the options they chose. This could explain why neither Izuma et al. nor Sharot et al. found any evidence for the self‐referential processing hypothesis, but we did. Unlike Qin et al. [2011], we used a region of interest analysis, which enabled us to disambiguate the functional significance of our brain activation data. Most importantly, none of the previous studies examined post‐choice functional connectivity, and our study was the first to test whether functional connectivity predicts post‐choice attitude change. This work, therefore, expands on previous work by showing that networks of brain regions are important in promoting and facilitating post‐choice attitude change.
Interestingly, our data suggest that the functional connectivity between brain regions that are putatively involved in self‐processing is empirically distinct from the activation of each of the two regions assessed separately. Moreover, it is the functional connectivity rather than the separate activation of each region that may be more directly linked to integrative or schematic cognitions that are thought to underlie choice justification. This more dynamic facet of brain network must be further capitalized on in future work to advance our understanding of the neural mechanisms underlying how people think about the self.
Integrating the in‐Choice and Post‐Choice Mechanisms
Post‐choice attitude change is likely to be mediated by both in‐choice mechanisms and post‐choice mechanisms. Our earlier work tested in‐choice mechanisms including a search of positive features in one choice option [Kitayama et al., 2013], whereas the current work tested post‐choice mechanisms including self‐referential processing. Although the two mechanisms are conceptually distinct, they might in fact be interconnected and the entire process might be far more recursive than our discussion so far might imply [Kitayama and Tompson, 2015].
For example, when two equally attractive options are presented for a choice, the decision maker is likely to seek positive features that are uniquely linked to one of the options. These features are unlikely to be purely perceptual. To the contrary, more often than not, substantial cognitive processing including retrieval of prior episodes and future plans may be required to recognize certain features as positive. Hence, it is likely that certain degrees of self‐referential processing are involved during the in‐choice processing. Consistent with this line of analysis, prior evidence suggests that in‐choice activation of PCC/Pcu (a region closely linked to self‐referential processing) tracks post‐choice attitude change [Jarcho et al., 2011; Kitayama et al., 2013] (see Footnote 1). Hence, higher‐order self‐referential cognitions may be already implicated very early on during the choice processes.
Moreover, once one of the options has been chosen, individuals may be expected to expend further self‐referential processing to justify the choice. Yet, it is conceivable that this self‐referential processing reinforces the affective value of the chosen option as represented in the subcortical reward processing regions of the brain including vSTR [Kitayama and Tompson, 2015]. Hence, it is plausible that at least under certain conditions, post‐choice activation of vSTR tracks post‐choice attitude change. Evidence for this prediction exists in the literature [Izuma et al., 2010; Sharot et al., 2009] although we did not find any evidence for it in an additional exploratory whole‐brain analysis.
At present, we have no analytic tools at hand to analyze the feedback/feed‐forward links between the in‐choice and the post‐choice mechanisms discussed above. Future work would nonetheless benefit from a more concerted analysis of this issue.
Is Post‐Choice Attitude Change No More than a Statistical Artifact?
Using neuroimaging to examine underlying mechanisms, our work addressed a recent criticism that post‐choice attitude change is no more than a statistical artifact. Chen and Risen [2010] have argued that measurement error obscures differences in true preferences for the two choice options. They argue that even when two choice options are initially rated as equally preferred, it is likely that the individual's true preference for one option is actually greater than the other. This has two consequences. First, the individual will choose the option that is actually more preferred (according to the individual's true preferences) even though the initial ratings suggested that they were equally preferred. Second, because the true preference for the chosen option was actually greater than the true preference for the rejected option, the second, post‐choice rating of the chosen option is likely to increase, whereas the second post‐choice rating of the rejected option is likely to decrease. Following this line of reasoning, Chen and Risen have argued that the measurement errors associated with the initial preference rating might be solely responsible for the post‐choice attitude change and thus psychological processes (e.g., cognitive dissonance, self‐referential processing, etc.) are not required for this attitude change to occur.
Although certain mathematical details involved in the original analysis have as been called into question [Alós‐Ferrer and Shi, 2015], the statistical artifact implied by the Chen and Risen [2010] argument stands as a logical possibility that could increase post‐choice attitude change. However, because measurement of attitude change and measurement of neural activation are independent, the artifact described by Chen and Risen is unlikely to lead to any systematic changes in the activation in any brain regions, connectivity among them, or the association between these brain signals and attitude change [Kitayama et al., 2014]. If post‐choice attitude change is predicted by neural activation, then we can conclude that some meaningful percentage of this attitude change is independent of the statistical artifact described by Chen and Risen. Hence, our findings underscore the veracity of post‐choice attitude change as a psychological phenomenon.
CONCLUDING REMARKS
The most important contribution of the current work is to show that connectivity between brain regions during post‐choice evaluation of the choice options contributes to attitude change. These findings extend previous research that hypothesized self‐referential processing to account for post‐choice attitude change without directly assessing this processing [Aronson, 1969; Gawronski et al., 2007; Steele, 1988; Stone and Cooper, 2001]. In addition to contributing to the current understanding of brain mechanisms underlying choice justification, our findings also underscore the potential of a network‐based analysis focusing on functional connectivity in testing social psychological theories.
Abbreviated 20‐Item Version of Singelis [1994] Self‐Construal Scale
| Item | Subscale |
|---|---|
| 1. I always try to have my own opinions. | Independence |
| 2. I am comfortable with being singled out for praise or rewards. | Independence |
| 3. The best decisions for me are the ones I made by myself. | Independence |
| 4. In general I make my own decisions. | Independence |
| 5. I act the same way no matter who I am with. | Independence |
| 6. I am not concerned if my ideas or behavior are different from those of other people. | Independence |
| 7. I always express my opinions clearly. | Independence |
| 8. Being able to take care of myself is a primary concern for me. | Independence |
| 9. I enjoy being unique and different from others in many respects. | Independence |
| 10. I do my own thing, regardless of what others think. | Independence |
| 11. I am concerned about what people think of me. | Interdependence |
| 12. In my own personal relationships I am concerned about the other person's status compared to me and the nature of our relationship. | Interdependence |
| 13. I think it is important to keep good relations among one's acquaintances. | Interdependence |
| 14. I avoid having conflicts with members of my group. | Interdependence |
| 15. When my opinion is in conflict with that of another person's, I often accept the other opinion. | Interdependence |
| 16. I respect people who are modest about themselves. | Interdependence |
| 17. I will sacrifice my self‐interest for the benefit of the group I am in. | Interdependence |
| 18. I often have the feeling that my relationships with others are more important than my own accomplishment. | Interdependence |
| 19. I feel my fate is intertwined with the fate of those around me. | Interdependence |
| 20. Depending on the situation and the people that are present, I will sometimes change my attitude and behavior. | Interdependence |
Author Contributions: Steven Tompson (S.T.), Hannah Faye Chua (H.F.C.), and Shinobu Kitayama (S.K.) conceived of and designed the study. S.T. collected, analyzed, and interpreted the data with oversight from H.F.C. and S.K. S.T. drafted the manuscript with critical revisions from H.F.C. and S.K. All authors have read and approved the final version of this manuscript.
Footnotes
Another finding that is consistently obtained in both studies concerns PCC/Pcu. A trial‐by‐trial change in the in‐choice activation of this region also reliably tracked post‐choice attitude change. As reviewed later in the article, PCC/Pcu is often involved in self‐referential processing. Hence, the activation of PCC/Pcu could suggest some self‐processing was recruited to identify positive features in one of the options. In addition, Jarcho et al. identified several other regions (e.g., mPFC and IFG) as well. However, these regions were not replicated in the Kitayama et al. study.
No main effects or interactions were observed for Difficulty and so subsequent analyses focused on the effects of Choice and Time.
In addition, we tested whether brain activation for any of the analyses above would be moderated by choice difficulty. There were no significant differences between difficult and easy choices for any of the analyses noted above.
REFERENCES
- Alós‐Ferrer C, Shi F (2015): Choice‐induced preference change and the free‐choice paradigm: A clarification. Judgm Decis Mak 10:34–49. [Google Scholar]
- Aronson E (1969): The theory of cognitive dissonance: A current perspective. Adv Exp Soc Psychol 4:1–34. [Google Scholar]
- Bartra O, McGuire JT, Kable JW (2013): The valuation system: A coordinate‐based meta‐analysis of BOLD fMRI experiments examining neural correlates of subjective value. NeuroImage 76:412–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm JW (1956): Postdecision changes in the desirability of alternatives. J Abnorm Soc Psychol 52:384–389. [DOI] [PubMed] [Google Scholar]
- Chen MK, Risen JL (2010): How choice affects and reflects preferences: Revisiting the free‐choice paradigm. J Pers Soc Psychol 99:573–594. [DOI] [PubMed] [Google Scholar]
- Chua HF, Ho SS, Jasinska AJ, Polk TA, Welsh RC, Liberzon I, Strecher VJ (2011): Self‐related neural response to tailored smoking‐cessation messages predicts quitting. Nat Neurosci 14:426–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny BT, Kober H, Wager TD, Ochsner KN (2012): A meta‐analysis of functional neuroimaging studies of self‐ and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. J Cogn Neurosci 24:1742–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festinger L (1957): A Theory of Cognitive Dissonance. New York: Row, Peterson & Co. [Google Scholar]
- Feyers D, Collette F, D'Argembeau A, Majerus S, Salmon E (2010): Neural networks involved in self‐judgement in young and elderly adults. NeuroImage 53:341–347. [DOI] [PubMed] [Google Scholar]
- Gawronski B, Bodenhausen GV, Becker AP (2007): I like it, because I like myself: Associative self‐anchoring and post‐decisional change of implicit evaluations. J Exp Soc Psychol 43:221–232. [Google Scholar]
- Gutchess AH, Welsh RC, Boduroglu A, Park DC (2006): Cultural differences in neural function associated with object processing. Cogn Affect Behav Neurosci 6:102–109. [DOI] [PubMed] [Google Scholar]
- Han S, Northoff G, Vogeley K, Wexler BE, Kitayama S, Varnum MEW (2013): A cultural neuroscience approach to the biosocial nature of the human brain. Annu Rev Psychol 64:335–359. [DOI] [PubMed] [Google Scholar]
- Harmon‐Jones E, Amodio DM, Harmon‐Jones C (2009): Action‐based model of dissonance. A review, integration, and expansion of conceptions of cognitive conflict. In: Advances in Experimental Social Psychology, 1st ed., Vol. 41, Chapter 3. Elsevier Inc. Available at: http://www.apastyle.org/learn/faqs/when-include-retrieval-date.aspx
- Hyde LW, Tompson S, Creswell JD, Falk EB (2015): Cultural neuroscience: New directions as the field matures. Cult Brain 3:75–92. [Google Scholar]
- Izuma K, Matsumoto M, Murayama K, Samejima K, Sadato N, Matsumoto K (2010): Neural correlates of cognitive dissonance and choice‐induced preference change. Proc Natl Acad Sci USA 107:22014–22019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarcho JM, Berkman ET, Lieberman MD (2011): The neural basis of rationalization: Cognitive dissonance reduction during decision‐making. Soc Cogn Affect Neurosci 6:460–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S (2002): Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage 17:825–841. [DOI] [PubMed] [Google Scholar]
- Kitayama S, Chua HF, Tompson S, Han S (2013): Neural mechanisms of dissonance: An fMRI investigation of choice justification. NeuroImage 69:206–212. [DOI] [PubMed] [Google Scholar]
- Kitayama S, Snibbe AC, Markus HR, Suzuki T (2004): Is there any “free” choice? Self and dissonance in two cultures. Psychol Sci 15:527–533. [DOI] [PubMed] [Google Scholar]
- Kitayama S, Tompson S (2015): A biosocial model of affective decision making: Implications for dissonance, motivation, and culture. Adv Exp Soc Psychol 52:71–137. [Google Scholar]
- Kitayama S, Tompson S, Chua HF (2014): Cultural neuroscience of choice justification. Control within: Motivation and its regulation, 313–330.
- Ma Y, Bang D, Wang C, Allen M, Frith C, Roepstorff A, Han S (2014): Sociocultural patterning of neural activity during self‐reflection. Social Cognitive and Affective Neuroscience, 9:3–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, Johnson SC (2012): A generalized form of context‐dependent psychophysiological interactions (gPPI): A comparison to standard approaches. NeuroImage 61:1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel N, Villain N, Rauchs G, Gaubert M, Piolino P, Landeau B, Mézenge F, Desgranges B, Eustache F, Chételat G (2014): Brain activity and functional coupling changes associated with self‐reference effect during both encoding and retrieval. PLoS One 9:e90488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na J, Kitayama S (2012): Will people work hard on a task they choose? Social‐eyes priming in different cultural contexts. J Exp Soc Psychol 48:284–290. [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J (2006): Self‐referential processing in our brain‐A meta‐analysis of imaging studies on the self. NeuroImage 31:440–457. [DOI] [PubMed] [Google Scholar]
- Park B, Tsai JL, Chim L, Blevins E, Knutson B (2016): Neural evidence for cultural differences in the valuation of positive facial expressions. Soc Cogn Affect Neurosci 11:243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA (2012): The future of fMRI in cognitive neuroscience. NeuroImage 62:1216–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Kimel S, Kitayama S, Wang X, Yang X, Han S (2011): How choice modifies preference: Neural correlates of choice justification. NeuroImage 55:240–246. [DOI] [PubMed] [Google Scholar]
- Qin P, Liu Y, Shi J, Wang Y, Duncan N, Gong Q, Weng X, Northoff G (2012): Dissociation between anterior and posterior cortical regions during self‐specificity and familiarity: A combined fMRI‐meta‐analytic study. Hum Brain Mapp 33:154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries ML, McLaren DG, Bendlin BB, Guofanxu Rowley HA, Birn R, Kastman EK, Sager MA, Asthana S, Johnson SC (2012): Medial prefrontal functional connectivity–relation to memory self‐appraisal accuracy in older adults with and without memory disorders. Neuropsychologia 50:603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers BP, Morgan VL, Newton AT, Gore JC (2007): Assessing functional connectivity in the human brain by fMRI. Magn Reson Imaging 25:1347–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajonz B, Kahnt T, Margulies DS, Park SQ, Wittmann A, Stoy M, Ströhle A, Heinz A, Northoff G, Bermpohl F (2010): Delineating self‐referential processing from episodic memory retrieval: Common and dissociable networks. NeuroImage 50:1606–1617. [DOI] [PubMed] [Google Scholar]
- Savani K, Markus HR, Conner AL (2008): Let your preference be your guide? Preferences and choices are more tightly linked for North Americans than for Indians. J Pers Soc Psychol 95:861–876. [DOI] [PubMed] [Google Scholar]
- Savani K, Markus HR, Naidu NVR, Kumar S, Berlia NV (2010): What counts as a choice? U.S. Americans are more likely than Indians to construe actions as choices. Psychol Sci 21:391–398. [DOI] [PubMed] [Google Scholar]
- Sharot T, De Martino B, Dolan RJ (2009): How choice reveals and shapes expected hedonic outcome. J Neurosci 29:3760–3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singelis TM (1994): The measurement of independent and interdependent self‐construals. Pers Soc Psychol Bull 20:580–591. [Google Scholar]
- Steele CM (1988): The psychology of self‐affirmation: Sustaining the integrity of the self. Adv Exp Soc Psychol 21:261–302. [Google Scholar]
- Steele CM, Liu TJ (1983): Dissonance processes as self‐affirmation. J Pers Soc Psychol 45:5–19. [Google Scholar]
- Stone J, Cooper J (2001): A self‐standards model of cognitive dissonance. J Exp Soc Psychol 37:228–243. [Google Scholar]
- van der Meer L, Costafreda S, Aleman A, David AS (2010): Self‐reflection and the brain: A theoretical review and meta‐analysis of neuroimaging studies with implications for schizophrenia. Neurosci Biobehav Rev 34:935–946. [DOI] [PubMed] [Google Scholar]
- Varnum MEW, Shi Z, Chen A, Qiu J, Han S (2014): When “Your” reward is the same as “My” reward: Self‐construal priming shifts neural responses to own vs. friends' rewards. NeuroImage 87:164–169. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, Neelin P (1992): A three‐dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab 12:900–918. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Poline JB, Friston KJ, Evans AC (1997): Characterizing the response of PET and fMRI data using multivariate linear models. NeuroImage 6:305–319. [DOI] [PubMed] [Google Scholar]


