Abstract
Extensive studies in rodents have established the role of neural pathways that are activated during thermoregulation. However, few studies have been conducted in humans to assess the complex, hierarchically organized thermoregulatory network in the CNS that maintains thermal homeostasis, especially as it pertains to cold exposure. To study the human thermoregulatory network during whole body cold exposure, we have used functional MRI to characterize changes in the BOLD signal within the constituents of the thermoregulatory network in 20 young adult controls during non‐noxious cooling and rewarming of the skin by a water‐perfused body suit. Our results indicate significant decreases of BOLD signal during innocuous whole body cooling stimuli in the midbrain, the right anterior insula, the right anterior cingulate, and the right inferior parietal lobe. Whereas brain activation in these areas decreased during cold exposure, brain activation increased significantly in the bilateral orbitofrontal cortex during this period. The BOLD signal time series derived from significant activation sites in the orbitofrontal cortex showed opposed phase to those observed in the other brain regions, suggesting complementary processing mechanisms during mild hypothermia. The significance of our findings lies in the recognition that whole body cooling evokes a response in a hierarchically organized thermoregulatory network that distinguishes between cold and warm stimuli. This network seems to generate a highly resolved interoceptive representation of the body's condition that provides input to the orbitofrontal cortex, where higher‐order integration takes place and invests internal states with emotional significance that motivate behavior. Hum Brain Mapp 37:3188–3202, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: thermoregulation, cold stress, midbrain, insula, orbitofrontal cortex, fMRI
INTRODUCTION
Thermoregulation is an essential autonomic response preserved across all genera [Terrien et al., 2011], but particularly in mammalian species who have extensive and dynamic homeothermic requirements. Detailed work in mammalian (and particularly rodent) models suggests that the hierarchically organized spinobrachiopreoptic pathway is essential in sub‐serving thermoregulatory autonomic defenses [Cerri et al., 2013; Morrison, 2011], which exist in parallel with the spinothalamocortical somatosensory pathway that mediates temperature perception [Nakamura and Morrison, 2008]. With respect to autonomic sympathetic thermoregulation, lateral parabrachial neurons in the midbrain region receive afferents from spinal neurons, in turn transmitting thermosensory signals to control regions of the hypothalamus including the preoptic area [Morrison et al., 2008; Nakamura and Morrison, 2008] mediating both heat‐ and cold‐related thermoregulatory defenses [Nakamura and Morrison, 2011].
Because thermoregulatory requirements are closely shaped by evolutionary pressures (that differ across species) [Angilletta, 2009], extension of animal studies to humans is essential. Limited in vivo neuroimaging studies exist, but the few conducted suggest that some of the same autonomous reflex arcs form a central facet of the human brain's thermoregulatory system. For example, during endogenous heat challenges, such as in menopausal hot flashes [Diwadkar et al., 2014], brain stem responses are closely associated with the onset of hot flashes. In comparison, responses in regions such as the prefrontal cortex, cingulate cortex and insula trail increases in body temperature. These latter interoceptive responses appear to reflect neural responses to changes in physiological states [Craig, 2002]. As such, interoceptive responses are general, resulting not only from endogenous thermal events such as hot flashes but also during the application of exogenously applied temperature sensation, including heat [Davis et al., 1998; Kubina et al., 2010; Kwan et al., 2000] and cold [Kwan et al., 2000; McAllen et al., 2006]. Processes associated with whole body skin cooling engage thermoregulatory defense mechanisms, associated with both responses to cooling and responses to (relatively) prolonged exposure to cold; however, these have rarely been studied with fMRI. fMRI is an invaluable method for estimating task‐induced hemodynamic changes in a priori identified brain regions [Logothetis, 2008]. Recent suggestions have advocated the value of fMRI in elucidating responses within (or connectivity between) theoretically constrained networks of brain regions: the choice of these regions are motivated by aspects of task‐ or paradigm‐relevant processing [Diwadkar, 2015; Friston, 2011; Stephan and Roebroeck, 2012] and follows the principle of relative specialization of brain function [Friston, 2005], an organizing tenet of the brain. Thus, just as behavioral/cognitive domains are characterized by specific distributed architectures [Mesulam, 1998; Park and Friston, 2013], thermoregulatory mechanisms within the mammalian central nervous system (CNS) are characterized by distributed architectures. Small diameter primary afferents from the periphery converge first on homeostatic nuclei within the brainstem that mediate CNS responses to these peripheral inputs [Satinoff, 1978]. The afferents from the periphery induce autonomous thermoregulatory responses in medullary nuclei that are relayed to autonomic control centers in the preoptic area of the hypothalamus [Terrien et al., 2011], but in the case of human thermoregulation, these signals are also forwarded to multiple cortical areas, including the insular cortex, the anterior cingulate, the posterior parietal somatosensory cortex and the orbitofrontal cortex, where they give rise to subjective feelings. Multiple lines of evidence across mammalian studies imply that these subcortical and cortical regions constitute a value‐generating network of regions that collectively represent sub‐processes associated with thermoregulatory defenses that subsequently guides behavior. These subprocess include autonomous homeostatic responses (midbrain, hypothalamus, and insula) [Satinoff, 1978], top–down modulation of thermoregulatory control (parietal lobe) [Gallace et al., 2014], interoceptive assessment of internal physiological states (insula and anterior cingulate cortex) [Craig, 2002; Diwadkar et al., 2014] and affective codes associated with the pleasantness or the unpleasantness of those states (orbitofrontal cortex) [Rolls, 2010]. This hypothesized network was entered as the a priori focus of effect discovery in this inquiry.
To elucidate responses in these network constituents, we concurrently acquired body temperature and fMRI signals while normal healthy volunteers were exposed to a carefully manipulated oscillating whole body temperature challenge. The challenge was designed to induce periods of mild hypothermia interspersed by periods of return to basal core body temperature. A notable feature of our analyses was the focus on multiple temporal windows within the paradigm: (1) First, we identified fMRI correlates associated with skin temperature gradients, both cooling (i.e., as temperature decreased) and warming (i.e., as temperature returned toward basal levels), and (2) Second, we also explored fMRI correlates associated with (relatively) prolonged exposure to cold, relative to prolonged states of basal temperature. These analyses within the same study explored potentially separable or overlapping CNS correlates of cooling (or warming) and cold (or warmth).
Hypothermia is the condition that results when the body's core body temperature falls below a value that can be metabolically sustained. Mild hypothermia engages sympathetic physiologic responses, with the aim of efficiently preserving body heat; these include shivering, tachychardia, vasoconstricton as well as potential activation of brown adipose tissue via sympathetic innervation [Gonzalez‐Alonso, 2012]. Without intervention, mild hypothermia transitions to a severe stage resulting in failure of critical physiologic systems. Despite the putative complexity of CNS mechanisms in thermoregulatory defenses to mild hypothermia and the accepted clinical benefits of the latter in preserving brain function following trauma, the neural correlates of mild hypothermia in humans remain obscure. Therefore, our results provide evidence that the process of inducing controlled mild‐hypothermia (using a whole‐body temperature challenge) induces a complex and heterogeneous pattern of both positive and negative fMRI‐estimated neuronal responses. We show that these neuronal responses exhibit systematic linear relationships to contemporaneously monitored skin temperature (itself related to core body temperature [Xu et al., 2013]). Moreover, we also show that changes in temperature, but not prolonged stable periods of cold and warm temperature, are more predictive of CNS responses. This evidence implies that CNS responses are most sensitive to dynamic thermoregulatory defense, rather than adaptation to a new and transient temperature following thermoregulatory challenge. This contemporaneous application of experimentally induced skin temperature challenges provides an effective framework for investigating differential neural correlates of mild hypothermia within a network of regions that includes the anterior insula, midbrain and the orbitofrontal cortex.
MATERIALS AND METHODS
Subjects
MRI studies were performed in 20 young adults (10M/10F, mean age 25.1 ± 3.4 years, age range 20–31 years) with a BMI in the normal range (22.7 + 2.1 kg/m2) and a body fat percentage of 24.0 ± 4.3%. Participants were not taking any medication, and had no history of neurological or psychiatric disorder. All subjects had a normal structural MRI scan. The Human Investigation Committee of Wayne State University authorized the study and informed written consent was obtained from all participants.
MRI Procedure
Gradient echo EPI fMRI data acquisition was conducted on a 3T Siemens Verio system using a 12‐channel volume head coil (TR: 2.6 s, TE: 29 ms, FOV: 256 × 256 mm2, acquisition matrix: 128 × 128, 36 axial slices, voxel dimensions: 2 × 2 × 3 mm3). In addition, a 3D T1‐weighted anatomical MRI image was acquired (TR: 2,200 ms, TI: 778 ms, TE: 3 ms, flip‐angle = 13°, FOV: 256 × 256 mm2, 256 axial slices of thickness = 1.0 mm, matrix = 256 × 256, scan‐time = 5 min 22 s). These parameters allow acquisition of fMRI data with high in vivo spatial resolution. The total study time was approximately 1 h.
fMRI Cold Exposure Paradigm
Thermoregulatory challenge was applied using a specialized whole‐body garment through which subjects were exposed to either a neutral or cold temperature stimulus. The garment incorporates a network of small‐diameter plastic tubing (Allen Vangard, Ottawa, CA) (Fig. 1A) through which temperature‐controlled neutral (31–34°C) or cold water (2–4°C) was circulated from two separate water reservoirs located outside the scanner room. The effects of these exogenous temperature stressors on body temperature was monitored using an MRI‐compatible GaAs crystal sensor located at the tip of an optical fiber cable (OpSense, Quebec City, CA). This approach relies on the temperature dependence of the energy band gap of a GaAs semiconductor crystal. The GaAs sensor is opaque for wavelengths below the bandgap and transparent for wavelengths above the energy band gap. The sensor was taped to the skin at the location of the left rib cage, the location selected on the basis of proximity to important anatomical features (close to the pulmonary blood vessels which are possibly the most representative sites for core body temperature) and the ability to consistently place the sensors based on those anatomical landmarks. Previous studies [Xu et al., 2013; Yamakage and Namiki, 2003] have shown a strong correlation (R 2 = 0.70) between this location and core body temperature. The skin temperature was recorded (30‐s intervals) during the experimental paradigm that was blocked into five 5‐min epochs alternating between the neutral and cold stimulus (Fig. 1B).
Figure 1.
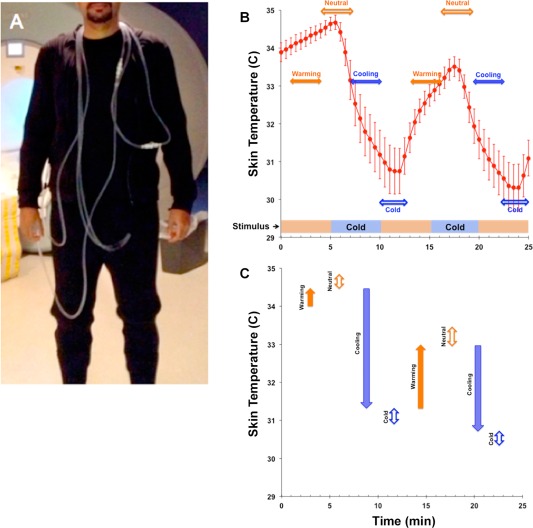
(A) Subject dressed in the tube suit covering the arms to the wrists, the legs to the ankles and the torso. (B) The bar at the base of the graph depicts the stimulus (study paradigm) consisting of two 5‐min cooling periods (blue/dark) interspersed between neutral temperature background (orange/light), resulting in average skin temperature oscillations (error bars ± s.d.). From the temperature curve, we derived two classes of epoch windows (horizontal arrows). The filled arrows depict temporal windows characterized by warming (orange/light) or cooling (blue/dark). Complementary temporal windows (open arrows) assessed fMRI responses for neutral (orange/light) or cold (blue/dark) periods. These periods were characterized by different ranges of skin temperature. (C) The vertical arrows depict the range (and direction of change) of skin temperature during warming or cooling, and neutral or cold temporal windows. Color/Shading conventions are maintained from (B). The figure clearly indicates that periods of warming and cooling were associated with more dynamic changes in skin temperature than periods of neutral or cold. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
As seen, the alternating stimulus induced skin temperature oscillations in an approximate 4°C (∼7°F) range, and this decrease from baseline (which was determined as 34 ± 1.3°C during the time when the structural T1‐weighted image was acquired and prior to water being circulated through the tube suit), is notable given the relatively short duration of cold exposure (5 min). Moreover, the temperature curve can be classified into two broadly distinct regimes: (a) A dynamic gradient associated with cooling and re‐warming (or return to neutral) reflecting high rates of skin temperature change in response to the stimulus and (b) periods when skin temperature remains relatively stable (cold or neutral) plausibly reflecting adaptation. The temporal width of these is denoted in Figure 1B, and the temperature ranges within each of the windows are depicted in Figure 1C.
These distinct regimes constituted separable physiological predictors of the BOLD response and were used to construct epochs of interest for fMRI analyses. Each epoch was modeled with a temporal radius of 1.5 min centered at either (a) points of the highest rates of skin temperature change (in the negative or positive direction) or (b) at the points of relatively skin temperature (at both neutral and cold condition). Thus, in each participant these first level models estimate neuronal responses during (relatively) rapid skin temperature transitions when thermoregulatory demands are maximal separately from periods of relatively stable skin temperature, presumably reflecting adaptation following temporary relaxation of the stimulus.
We specifically avoided collecting subjective ratings of unpleasantness during the fMRI scan to eliminate conscious deliberation of changes in internal body states. However, post‐experimental debriefing indicated that all subjects perceived the maximum stimulus as “very cold,” although all participants denied pain or substantial discomfort.
Statistical Analysis
The fMRI images were analyzed using SPM8 (Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK). In all analyses, the first four images were discarded to account for EPI equilibration effects. The remaining images in the sequence were realigned to correct for head movements, corrected for slice timing, and subsequently spatially normalized according to the transformation matrix derived between the coregistered (to the mean EPI sequence image) T1‐weighted image volume and the MNI template brain. The images were then smoothed spatially with a 3D Gaussian kernel of 6 mm FWHM and re‐sampled (2 × 2 × 2 mm3). A high‐pass filter (cutoff 1/128 s) was used to remove low‐frequency signal drifts. The data were modeled voxel‐wise, applying a general linear model based on a boxcar waveform (based on the previously described epochs modeled from skin temperature data) and convolved with the canonical hemodynamic response function. The confounding effect of global signal intensity was removed using proportional scaling. The first‐level analysis included correction for within‐scanner motion by means of 6 realignment parameters as regressors, which were derived from the initial realignment step.
Variations in fMRI responses under the different regimes from the skin temperature curve were modeled at the first level using pair‐wise directional contrasts. Separate contrasts identified fMRI responses associated with cooling relative to warming, and periods of cold relative to periods of neutral skin temperature (explicitly defined in Fig. 1B,C).
These individual contrast images were submitted to a second‐level random‐effects analysis [Turner et al., 1998], to assess group‐based activation during the temporal windows of interest. All analyses were constrained respecting the relative homogeneity of function within regions of interest that constitute our hypothesized a priori thermoregulatory network, introduced above (Table 1). Significant clusters within each region were subsequently identified using AlphaSim [Ward, 2000], by estimating the minimum cluster extent for activated clusters to be rejected as false positive (noise‐only) clusters.
Table 1.
Hypothesized brain regions of the a priori network implicated in thermoregulatory control
| Anatomical label | Center of gravity (MNI) | Region size (cm3/voxels) | Reference | |
|---|---|---|---|---|
| Midbrain | 0/9/42 | 39.2/4878 | Tzourio‐Mazoyer (2002) | |
| Insula | R | −43/21/20 | 14.2/1770 | Tzourio‐Mazoyer (2002) |
| L | 43/21/20 | 14.2/1770 | Tzourio‐Mazoyer (2002) | |
| ACC | 0/53/12 | 21.7/2713 | Tzourio‐Mazoyer (2002) | |
| InfPariet | R | −52/−18/−17 | 23.6/2953 | Tzourio‐Mazoyer (2002) |
| L | 52/−18/−17 | 23.6/2953 | Tzourio‐Mazoyer (2002) | |
| OFC | R | −31/60/32 | 29.8/3719 | Tzourio‐Mazoyer (2002) |
| L | 31/60/32 | 29.8/3719 | Tzourio‐Mazoyer (2002) | |
See [Tzourio‐Mazoyer, 2002]. See also Supporting Information Figure 1.
SPM analysis was constrained to these constituents.
ACC: anterior cingulate cortex; OFC: orbito‐frontal cortex; InfPariet: inferior parietal lobule.
This chosen approach performs a Monte Carlo alpha probability simulation, thus computing the probability of a random field of noise (after taking into account the spatial correlations of voxels based on the image smoothness within each region of interest estimated directly from the data set) to produce a cluster of a given size, after the noise is thresholded at a given level. Thus, instead of using the individual voxel probability threshold alone in achieving the desired overall significance level, the method uses a combination of both probability thresholding and minimum cluster size thresholding. The underlying principle is that true regions of activation will tend to occur over contiguous voxels within a region of relative functional homogeneity, whereas noise has much less of a tendency to form clusters of activated voxels. Activations were assessed in the previously motivated thermoregulatory‐interoceptive network that included the brainstem, insula, anterior cingulate cortex, orbitofrontal cortex, posterior parietal cortex, and the hypothalamus. To report activation peaks, voxel coordinates in MNI space were transformed into Talairach space using a previously established algorithm [Lancaster et al., 2007], and Brodmann areas were reported where appropriate [Lancaster et al., 2000].
RESULTS
Skin Temperature
Cold water from a reservoir filled with ice slush was circulated through the tube suit for two 5‐min periods, during which the skin temperature fell from ∼34°C to ∼30°C (P < 0.001 between cold and neutral stimulus blocks, see Fig. 1C). The relatively short time duration of cold exposure (5 min) allows the perception of a “cold” stimulus in the absence of pain. In post‐experimental interviews, all subjects perceived the maximum stimulus as “very cold,” although none considered it “painful,” and all denied shivering.
fMRI Analysis
Bidirectional contrasts identified multiple clusters in core thermoregulatory and interoceptive regions. These clusters revealed complementary responses to body temperature changes during cooling and warming. Cooling resulted in significant decreases in fMRI measured neuronal responses in core thermoregulatory regions including the midbrain (Fig. 2). These decreases generalized to the anterior insula (Fig. 3), the anterior cingulate cortex and the inferior parietal cortex (see Table 2). The decreases in fMRI activity during cooling were complemented by significantly increased activity in only one structure: the bilateral orbitofrontal cortex (Fig. 4).
Figure 2.
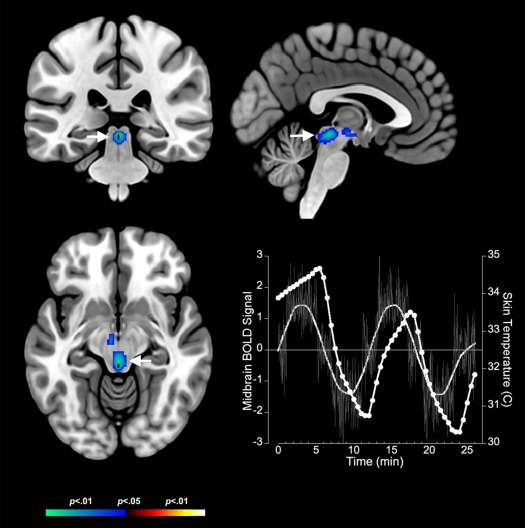
Negative BOLD responses to cold stress in the midbrain are depicted on coronal, axial and sagittal views (arrows). The adjoining graph depicts the BOLD response (no symbols) derived as the eigenvariate at the location of the midbrain activation juxtaposed against fluctuations in skin temperature (circles) in response to cold stress. Error bars are ± s.d. The BOLD response in the midbrain is proximate in phase to skin temperature responses. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 3.
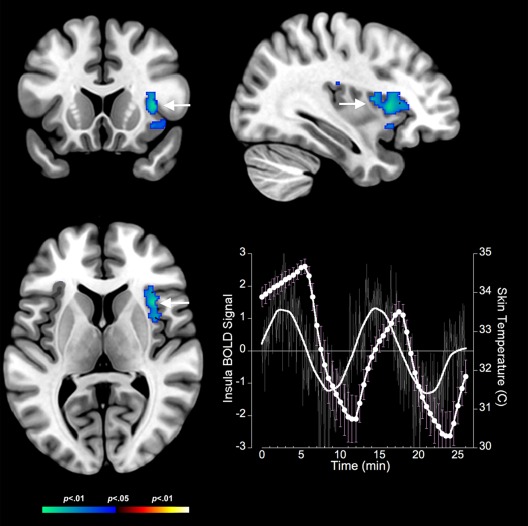
Negative BOLD responses to cold stress in the insula are depicted on coronal, axial, and sagittal views (arrows). The adjoining graph depicts the BOLD response (no symbols) derived as the eigenvariate at the location of the insula activation juxtaposed against fluctuations in skin temperature (circles) in response to cold stress. Error bars are ± s.d. As with the midbrain, the insula BOLD response is approximately phase locked to the fluctuations in skin temperature induced by cold stress. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 2.
Brain areas displaying significantly increased/decreased brain activation pattern during the cold exposure paradigm in the whole study group (N = 20)
| Anatomical ROI | Critical cluster extent | Individual cluster extent | Uncorrected P‐value (T‐value) | Voxel peak (Tal) |
|---|---|---|---|---|
| Cooling < Warming (Figs. 2, 3, 4) | ||||
| Midbrain | 260 | 350 | < 0.001 (4.38) | (2, −30, −8) |
| Insula R | 160 | 480 | 0.002 (3.74) | (38,15,6; BA13) |
| ACC R | 191 | 543 | 0.002 (3.26) | (6, 27, 19; BA24) |
| InfPariet R | 165 | 176 | 0.003 (3.47) | (46,−28,30; BA40) |
| Cooling > Warming (Figs. 2, 3, 4) | ||||
| OFC R | 119 | 379 | 0.003 (3.20) | (16,45,−15;BA11) |
| OFC L | 119 | 227 | 0.006 (2.80) | (−36,30,−23,BA47) |
| Cold < Neutral (Fig. 5) | ||||
| InfPariet R | 99 | 141 | 0.002 (3.08) | (44,−40,33; BA40) |
| Cold > Neutral (Fig. 5) | ||||
| OFC R | 171 | 240 | 0.001 (3.28) | (34,21,−17;BA47) |
| OFC L | 171 | 347 | < 0.001 (4.35) | (−20,32,−14,BA11) |
Abbreviations: ACC, anterior cingulate cortex; OFC, orbito‐frontal cortex; InfPariet, inferior parietal lobule; R, right; L, left.
Figure 4.
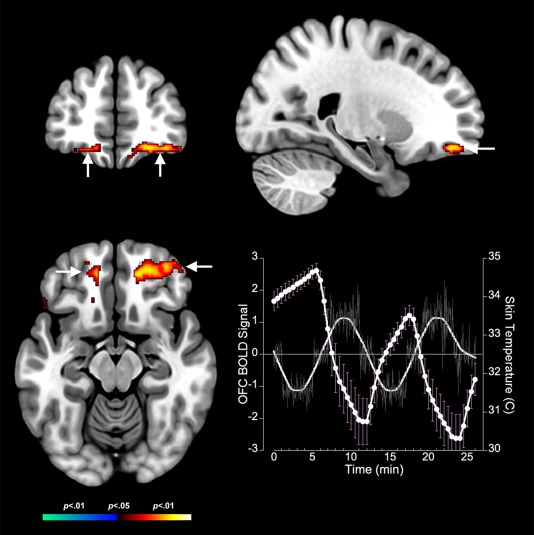
Positive BOLD responses to cold stress in the orbitofrontal cortex are depicted on coronal, axial, and sagittal views (arrows). The adjoining graph depicts the BOLD response (no symbols) derived as the eigenvariate at the location of the orbitofrontal cortex activation juxtaposed against fluctuations in skin temperature (circles). Error bars are ± s.d. Unlike the midbrain and the insula, OFC responses are in phase opposition to fluctuations in skin temperature induced by cold stress. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
The signatures of cooling were far more widespread than those seen associated with prolonged experience of cold skin temperature. Prolonged periods of cold skin temperature resulted in only two significant clusters of brain activations. First, we observed significant deactivation in the right inferior parietal cortex, but significant activation of the bilateral orbitofrontal cortex during the warm phase (see Table 2, Fig. 5). Both these activation loci were similarly positioned to those observed during the dynamic process of cooling.
Figure 5.
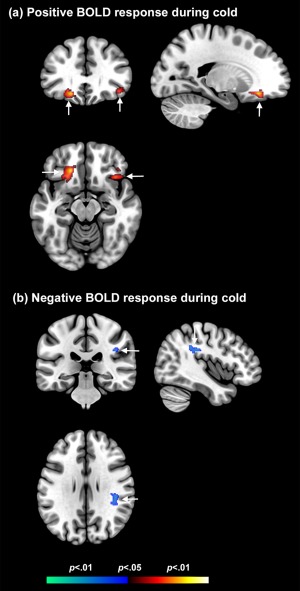
Prolonged periods of cold result in (a) positive BOLD responses in the orbitofrontal cortex but (b) negative BOLD responses in the parietal cortex (arrows). These effects can be distinguished from fMRI correlates of cooling and warming (Figs. 2, 3, 4, and 6). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
The temporal course of the responses to cooling, and their statistical relationship to changes in body temperature were investigated in further analyses.
Regional Time Series Analysis
Additional analyses were performed to relate changes in the fMRI estimated neuronal signal with dynamic changes in body temperature (resulting from thermoregulatory challenge). After significant activation clusters were identified in the second‐level analysis, these activation clusters were subsequently used as masks to extract the fMRI responses at each sampled time point across the whole study in each subject. For each activation mask and subject, the first eigenvariate from the modeled fMRI responses time sequence was extracted and then averaged over the sample. In further analyses, these values were correlated with the contemporaneously acquired skin temperature values. In these analyses, the skin temperature values were normalized to fluctuations from the subjects' mean across the experiment. The resulting correlation coefficients were tested for significance using t‐tests; moreover Fisher's test was used to determine significant differences between correlations obtained from the various brain regions. A two‐sided P value of less than 0.05 was considered as significant.
The correlation analyses further elaborated the activation effects (Fig. 6). Significant positive relationships between the normalized skin temperature and the BOLD response were observed in the midbrain, right insula, right anterior cingulate, and the right inferior parietal cortex. In contrast, a significant negative relationship was observed with the orbitofrontal cortex (Table 3). The absolute values of the correlation coefficients ranged from 0.88 to 0.92 and all correlation coefficients were highly significant (P < 0.001). Finally, no significant difference was determined among the positive correlation coefficients and among the negative correlation coefficients.
Figure 6.
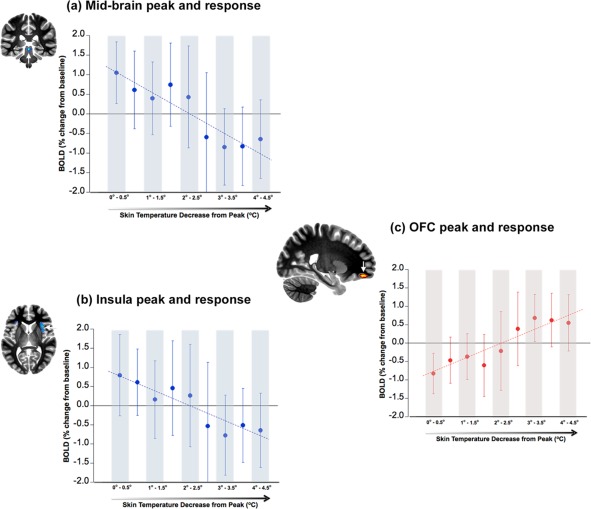
The three panels represent BOLD as a function of decreases in skin temperature that results from our cold stress paradigm. Changes in skin temperature are represented relative to decreases from the peak (x‐axis: left to right), and the BOLD data are summarized in 0.5°C bin widths. Adjoining each graph is an image of the cluster peaks from which the BOLD responses were derived (arrows). These images are for negative BOLD in the (a) midbrain (coronal slice), (b) insula (axial slice) and for positive BOLD in the (c) orbitofrontal cortex (OFC, sagittal slice). The significant decreases in BOLD in the midbrain (a) and the insula (b) as a function of decreases in skin temperature are clearly seen (R 2 = 0.82 and R 2 = 0.83, respectively). In comparison, the OFC shows a significant increase in BOLD as a function of decreases in skin temperature (R 2 = 0.85). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 3.
Correlation analysis between skin temperature and regional BOLD fMRI time series
| Anatomical ROI | Correlation coeff. R | P‐value |
|---|---|---|
| Cooling < Warming (Fig. 6) | ||
| Midbrain | 0.90 | < 0.001 |
| Insula R | 0.91 | < 0.001 |
| ACC R | 0.89 | < 0.001 |
| InfPariet R | 0.89 | < 0.001 |
| Cooling > Warming (Fig. 6) | ||
| OFC R + L | −0.92 | < 0.001 |
| Cold < Neutral | ||
| InfPariet R | 0.88 | < 0.001 |
| Cold > Neutral | ||
| OFC R+L | −0.90 | < 0.001 |
Abbreviations: ACC, anterior cingulate cortex; InfPariet, inferior parietal lobule; OFC, orbito‐frontal cortex; R, right; L, left.
DISCUSSION
Here, we probed the neuronal correlates of mild hypothermia through the contemporaneous acquisition of skin temperature and fMRI data during an oscillatory thermoregulatory challenge. The challenge induced skin temperature changes that were characterized by dynamic oscillations in cooling and warming interspersed with periods when skin temperature remained relatively stable for more prolonged periods (as the applied paradigm shifted from cold to neutral temperature water).
Complementary phase patterns of decreasing and increasing BOLD signal in response to skin temperature changes induced by the mild hypothermic challenge were observed, segregated by region. Cooling was associated with (a) Significant decreases in BOLD in the midbrain, the right anterior insula, the right anterior cingulate, and the right inferior parietal lobe but (b) significant increase in BOLD that was confined to the bilateral orbitofrontal cortex. Moreover, these fMRI estimated neuronal changes were tightly and intricately coupled with observed changes in skin temperature: In each of the midbrain, insula, anterior cingulate, and inferior parietal lobe, decreases in skin temperature predicted decreases in fMRI responses. In comparison, in the orbitofrontal cortex, decreases in skin temperature predicted increases in fMRI responses. Our cumulative results suggest that thermoregulatory and interoceptive structures may be more sensitive to temperature dynamics, than to adaptation/habituation to prolonged periods of skin temperature in narrow temperature bands.
Plausible Neurophysiological Correlates of Observed Deactivation
Co‐localization of fMRI and electrophysiological data has related deactivation in the fMRI signal to decreases in neuronal activity [Shmuel et al., 2006]; in turn neurometabolic coupling has been closely linked to synaptic activity [Viswanathan and Freeman, 2007]. Therefore, the observed decreases in fMRI responses in the mid‐brain and other putative interoceptive targets, may reflect a “turning down” of metabolic load as body temperature changes signal the onset of mild hypothermia. A logical speculation is that this reduction reflects efficient neuronal principles of energy conservation that are early signatures of CNS responses to non‐threatening core‐body temperature decreases. Moreover, the observed decrease of the fMRI signal in interoceptive brain regions (that is contemporaneous with skin cooling) in the right insula corresponds well with previous reports that postulate that this structure contains a sensory representation of small‐diameter afferent activity that relates to the physiological condition of the entire body, making discriminative thermal sensation possible [Craig et al., 2000; Craig, 2002]. The discriminatory thermal function of the insula is also supported by our previous findings that showed, during endogenous thermal events such as menopausal hot flash episodes, an increase of the fMRI signal in the bilateral insula [Diwadkar et al., 2014].
The complementary response of the OFC is notable both for its functional significance, and for its distinct positive pattern. This distinction suggests that efficient neuronal responses may be specific to thermoregulatory and interoceptive systems. The OFC is the principle exteroceptive region of the brain [Bechara et al., 2000; Petrides, 2007]. Extent theories imply that the OFC receives interoceptive “images” of internal process, and is highly responsive to the noxiousness elicited by the image. The OFC has been shown to correlate strongly with subjective thermal perception and is associated with the discrimination of positive and negative rewards, or hedonic valence [Craig et al., 2000; Rolls et al., 2003, 2008]. Thus, in our experiment, temperature‐sensitive representations (i.e., mildly noxious changes in body temperature) may be forwarded, via the colossal pathway, from the insula to the OFC where hedonic valence is attached to the subjective feeling of distress. It has been speculated that energy‐efficient maintenance of the body state (i.e., homeostasis) is achieved through an ordered set of neural constructs that are re‐represented at various integration levels, starting in the midbrain and then progressing to the posterior, middle and finally anterior insula [Craig, 2009]. During this process, the mid‐insula integrates these homeostatic re‐representations with activity that is associated with emotionally salient environmental stimuli. In this sense, the opposite phase of the insular and orbitofrontal activations observed in this study can be regarded as a temperature differentiation process that is subsequently converted into a subjective evaluation of the stimulus.
Of particular interest is the observation that periods of dynamic skin temperature are more evocative of pronounced responses in thermoregulatory brain regions than periods of relatively stable skin temperature. Our analyses of time series confirmed that the activation/deactivation of brain areas was closely predicted by the skin temperature gradient, that is, with the time when the change in skin temperature is maximal. As noted earlier, this suggests that thermoregulatory brain centers are highly sensitive to the degree of heat loss and less sensitive to states when skin temperature remains nearly constant, independent from the absolute temperature level. Although the exact mechanisms are unknown, this observation may constitute CNS correlates of peripheral systems. For example, temperature‐activated transient receptor potential (TRP) ion channels are expressed in free nerve endings in all layers of the skin [Patapoutian et al., 2003] and function as versatile polymodal cellular sensors that sense and are modulated by a wide array of inputs, including temperature, pressure, pH, voltage, chemicals, lipids, and other proteins. Several classes have been identified (TRPv1 – TRPv8), with channel activities dependent on specific temperature ranges. TRPM8 is the primary cold sensor in higher organisms, and several studies have shown that TRPM8 regulates body temperature [Almeida et al., 2012; Gavva et al., 2012], although it is also implicated in a wide variety of other physiologically important roles [Hilton et al., 2015]. As a result, it is conceivable that the activity of this channel is most sensitive to rapid changes in skin temperature and less so to relatively stable temperature, an inference that our observations conform to.
In addition to core thermoregulatory brain regions, we observed de‐activations in the area of the inferior parietal cortex. This finding is consistent with a recent model that suggests that a network of brain areas, including the posterior parietal and the insular cortices, might play a crucial role in maintaining the integrity of the body at both the homeostatic (i.e., thermoregulation) and psychological (i.e., in terms of perception and the sense of body ownership) levels [Moseley et al., 2012]. Within this structure, named the “body matrix,” multisensory information regarding the body and the space around it, is constantly integrated. This notion is based on recent reports showing that a reversible functional interference of the general area of the posterior parietal cortex via regional transcranial stimulation is able to disrupt thermoregulatory control [Gallace et al., 2014]. Our data extends these results and suggests that the inferior parietal cortex might also contribute to a top–down modulation of thermoregulatory control. In this sense, the inferior parietal cortex might be involved in both processing incoming signals regarding a variation of body temperature, as well as in affecting the functioning of those efferent systems responsible for modulating such homeostatic variable. These mechanisms appear to be in play during both types of skin temperature regimes that we addressed.
Relationship to Previous Findings
The involvement of the insula in the interoception of thermoregulatory processes has been extensively demonstrated in the literature [Craig et al., 2000; Fechir et al., 2010; James et al., 2013], yet to our knowledge, this is the first study to specifically assessing cooling, and therefore, the first report of de‐activation in this region during cooling‐related body temperature decreases. Previous imaging studies have typically applied short thermal stimuli to small skin areas and have focused on thermal sensory responses that are inextricably associated with pain perception [Rolls et al., 2008]. The only other previous study that applied similar methodology to ours was by McAllen et al. [2006]. They focused on, and elegantly demonstrated highly specific medullary raphe activations on a rostral slice of the medullary system, chosen for being closest in comparative anatomy to the rodent. Our study complements this work: Our fMRI acquisition was motivated by focus on a wider thermoregulatory and thermoreceptive network. Such a focus demanded more extensive sub‐cortical and cortical fMRI coverage from the mid‐brain to superior brain regions. Thus, our resultant slice prescriptions did not consistently capture fMRI responses in the rostral portions of the brain stem identified by McAllen et al. However, the loci of fMRI deactivation in the mid‐brain reported here are highly consistent with our recently reported evidence of increased activations during endogenously generated hyper‐thermic events [Diwadkar et al., 2014]. Specifically, mid‐brain regions (and regions across the interoceptive network including the insula and anterior cingulate) are positively activated when symptomatic menopausal women experience hot flashes, that is, intense heat surges in the body. Thus, mid‐brain loci appear to respond differently in response to hypo‐ and hyper‐thermic challenges.
The response of the thermoregulatory network to cold exposure has also been studied using positron emission tomography (PET) imaging, a method that uses F18‐labeled deoxyglucose (FDG) to measure cerebral glucose metabolism during brain activation. Because glucose uptake is a relatively slow process (glucose uptake is 90% complete 30 min after injection of the FDG tracer), FDG PET imaging represents the average glucose metabolism over an extended time period (30–45min) and provides complementary information to the faster blood flow changes measured with BOLD fMRI. Nevertheless, FDG PET studies using a similar methodology as ours showed right insular deactivation during whole body cooling [Fechir et al., 2010], in agreement with our findings. The authors interpreted this result as a release of inhibitory control by higher‐order brain regions on autonomic centers located in the brainstem. Such a mechanism might provide a reasonable explanation for sympathetic hyperactivity, which occurs after hemispheric stroke [Pellecchia et al., 2003; Riedl et al., 2001].
In addition to the anterior insula, areas in both the anterior cingulate cortex and midbrain were co‐activated during cold exposure with similar valence exhibiting temperature discriminatory function. Activation of the anterior cingulate cortex is observed in imaging studies of emotion [Gressens et al., 2008; Gunn et al., 1997] consistent with the fact that an emotion is both a feeling and a motivation. For example, an imaging study of placebo analgesia found concomitant activation of both the anterior cingulate cortex and the right anterior insula [Xie et al., 2007], supporting the notion that the feeling associated with the internal homeostatic representation is accompanied by activation in brain areas that modulate behavior. Moreover, co‐activations observed in the midbrain areas are likely associated with low‐level control of homeostasis including cardiovascular and cardiorespiratory regulation. The similarity of the observed activation pattern in the insula, anterior cingulate cortex and midbrain points toward a vertically integrated system ranging from sub‐conscious homeostatic regulatory mechanisms to abstract meta‐representations of the physiological state of the body that triggers a conscious behavioral response.
Further Considerations and Limitations
In the absence of direct measurements of temperature from within the body itself, we are careful to note that our CNS effects can only be related to the skin temperature changes that we directly measured, and it is unclear how the relatively short cooling periods (2 × 5min) might have affected (if at all) core body temperature. It has been argued that skin temperature represents only an auxiliary feedback signal to the main thermoregulatory system, reducing the system's response time and making core body temperature more stable [Romanovsky, 2007, 2014]. Consistent with this model is the observation that skin temperature is relatively more important for driving most (but not all) thermoregulatory behaviors [Roberts, 1988], whereas core body temperature is relatively more important for triggering autonomic responses [Jessen, 1981; Sakurada et al., 1993]. Such an organization reflects the fact that behavioral responses are often aimed at escaping impeding thermal insults.
We also note that our observed fMRI patterns were in evidence despite potential challenges to sensitivity as temporal changes in the temperature stressor overlapped with the phase of scanner drift. As a result, portions of the BOLD signals in regions of interest might have been removed by the applied high‐pass filter potentially decreasing the statistical power of our results. Nevertheless, the observation of low‐frequency oscillations of regional BOLD signal with both opposite phase and similar amplitude suggests that scanner drift corrections were almost exclusively driven by global changes in BOLD signal, thus preserving local oscillations. Moreover, mild hypothermia is associated with physiological reactions like shivering, tachycardia, and vasoconstriction. Although skin temperature was monitored throughout the study and the observed skin temperature oscillations were found to be comparable across subjects, there might potentially exist differences in both temperature perception as well as in physiological responses to the periodic cooling and warming paradigm, even in the studied highly homogenous group of young lean subjects (age range 20–31 years, BMI 20–25 kg/m2). Thus, these effects on activation cannot be excluded, despite the fact that post‐experimental debriefing indicated that subjects perceived the maximum stimulus as “very cold.”
Our study's generalizability may be restricted by some temporal and spatial limitations inherent in fMRI. Although our spatial resolution was high relative to many studies (2 × 2 mm in‐plane) precise anatomical location remains challenging because of variations in the intrinsic spatial resolution of cortical regions and sub‐cortical nuclei. Cellular differences in midbrain nuclei are not easily distinguishable using conventional imaging methods (explaining the absence of well resolved anatomical masks). Thus, the designation of the exact anatomical location with respect to the observed significant deactivation in the midbrain region is challenging and one can only speculate with respect to the underlying mechanisms. One possibility is that our loci represent a subpopulation of neurons in the dorsal raphe nucleus, based on the work by Lowry et al. [Lowry et al., 2009]. According to this model, neurons within lamina I of the spinal cord project (via fiber tracts in the ventrolateral funiculus) to the midline raphe magnus nucleus, from where ascending projections innervate the medial reticular formation and strongly innervate the region lateral and ventral to the medial longitudinal fasciculi in the interfascicular part of the dorsal raphe nucleus [Bobillier et al., 1976]. This is in line with observations indicating that the interfascicular part of the dorsal raphe region is a critical part of afferent pathways regulating thermoregulatory function [Consolazione et al., 1984; Gottschlich and Werner, 1985; Werner and Bienek, 1985, 1990], but serotonergic neurons in the dorsal raphe nucleus also project to forebrain limbic structures regulating emotional behavior [Lowry et al., 2008].
A region that was notably silent was the hypothalamus, which has been shown to be heavily implicated in thermoregulatory control based on rodent literature. However, the hypothalamus has been only infrequently identified in human fMRI studies of thermoregulation (Freedman et al. 2006; Diwadkar et al. 2014). This absence [Diwadkar et al., 2014; Kochanek and Safar, 2003] may be attributed to partial volume effects associated with the small structure of the hypothalamic nuclei and/or cross‐species distinctions in evolutionary endowed thermoregulatory mechanisms of the structure. This is an open question, itself worthy of systematic inquiry.
Finally, application of fMRI to investigate brainstem responses is beset by several methodological challenges. In addition to magnetic susceptibility artifacts associated with signal originating from regions close to the brain‐CSF interfaces, there is mixing of signal as a consequence of voxel resampling during image preprocessing, such as motion correction and spatial normalization. Moreover, no suitable neuroimaging brainstem atlas exists to aid in the registration and warping to a common template. As a result the alignment of brainstem structures might be suboptimal, decreasing the statistical power of the analysis, especially given the small size of the underlying nuclei.
The process of functional brain network discovery using neuroimaging data is fundamentally challenging [Friston et al., 2012]. These challenges relate in part to lack of specific representation of neuronal events in fMRI signals, the hemodynamic bases of which agglomerate neural events across multiple spatial and temporal scales [Logothetis, 2008; Singh, 2012]. Moreover, the generative neuronal drivers of the fMRI signal can only be estimated from the overt signals themselves [Stephan, 2004]. These collective considerations exercise limits on the interpretive possibilities of fMRI data regardless of the conditions under which they are acquired and we acknowledge our inability with this paradigm to clearly isolate specific functional differences. Moreover, the current iteration of our work does not delineate patterns of functional integration across networks. This remains an important future extension of our work, given that integration of diverse functional modules (as opposed to the relative specialization of such modules assessed here) is a parallel organizing principle of brain function [Friston, 2005].
CONCLUSION
Whole body skin cooling clearly evokes systematic responses in a hierarchically organized thermoregulatory network. This network seems to generate a highly resolved interoceptive representation of the body's condition that provides input to the orbitofrontal cortex, where higher‐order integration may invest emotional significance to external stimuli to the body, subsequently motivating behavior [Rolls, 2010]. These novel results begin to elucidate cortical and sub‐cortical responses to thermoregulatory challenge. A validated framework for assessing CNS effects of thermoregulatory challenge in vivo is valuable as impaired thermoregulation has been implicated in a host of metabolic and endocrine syndromes. We hope that our work can contribute to the creation of a putative framework for linking peripheral and CNS measures in large cohort‐based studies.
Supporting information
Supporting Information Figure 1.
ACKNOWLEDGMENT
We thank Dalal Khatib for assistance in collecting the data. The authors declare no competing financial interests.
REFERENCES
- Almeida MC, Hew‐Butler T, Soriano RN, Rao S, Wang W, Wang J, Tamayo N, Oliveira DL, Nucci TB, Aryal P, Garami A, Bautista D, Gavva NR, Romanovsky AA (2012): Pharmacological blockade of the cold receptor TRPM8 attenuates autonomic and behavioral cold defenses and decreases deep body temperature. J Neurosci 32:2086–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angilletta MJ (2009) Thermal Adaptation: A Theoretical and Empirical Synthesis. Oxford: Oxford University Press. [Google Scholar]
- A Bechara, H Damasio, AR Damasio (2000): Emotion, decision making and the orbitofrontal cortex. Cereb Cortex; 10(3): 295–307. Review. [DOI] [PubMed] [Google Scholar]
- Bobillier P, Seguin S, Petitjean F, Salvert D, Touret M, Jouvet M (1976): The raphe nuclei of the cat brain stem: A topographical atlas of their efferent projections as revealed by autoradiography. Brain Res 113:449–486. [DOI] [PubMed] [Google Scholar]
- Cerri M, Mastrotto M, Tupone D, Martelli D, Luppi M, Perez E, Zamboni G, Amici R (2013): The inhibition of neurons in the central nervous pathways for thermoregulatory cold defense induces a suspended animation state in the rat. J Neurosci 33:2984–2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consolazione A, Priestley JV, Cuello AC (1984): Serotonin‐containing projections to the thalamus in the rat revealed by a horseradish peroxidase and peroxidase antiperoxidase double‐ staining technique. Brain Res 322:233–243. [DOI] [PubMed] [Google Scholar]
- Craig AD, Chen K, Bandy D, Reiman EM (2000): Thermosensory activation of insular cortex. Nat Neurosci 3:184–190. [DOI] [PubMed] [Google Scholar]
- Craig AD (2002): How do you feel? Interoception: The sense of the physiological condition of the body. Nat Rev Neurosci 3:655–666. [DOI] [PubMed] [Google Scholar]
- AD Craig (2009): How do you feel–now? The anterior insula and human awareness. Nat Rev Neurosci; 10(1): 59–70 [DOI] [PubMed] [Google Scholar]
- Davis KD, Kwan CL, Crawley AP, Mikulis DJ (1998): Functional MRI study of thalamic and cortical activations evoked by cutaneous heat, cold, and tactile stimuli. J Neurophysio 80:1533–1546. [DOI] [PubMed] [Google Scholar]
- Diwadkar VA (2015): Critical perspectives on causality and inference in brain networks: Allusions, illusions, solutions?: Comment on: “Foundational perspectives on causality in large‐scale brain networks” by M. Mannino and S.L. Bressler. Phys Life Rev 15:141–144. [DOI] [PubMed] [Google Scholar]
- Diwadkar VA, Murphy ER, Freedman RR (2014): Temporal sequencing of brain activations during naturally occurring thermoregulatory events. Cereb Cortex 24:3006–3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fechir M, Klega A, Buchholz HG, Pfeifer N, Balon S, Schlereth T, Geber C, Breimhorst M, Maihöfner C, Birklein F, Schreckenberger M (2010): Cortical control of thermo‐regulatory sympathetic activation. Eur J Neurosci 31:2101–2111. [DOI] [PubMed] [Google Scholar]
- Freedman RR, Benton MD, Genik RJ 2nd, Graydon FX (2006): Cortical activation during menopausal hot flashes. Fertil Steri 85:674–678. [DOI] [PubMed] [Google Scholar]
- Friston KJ (2005): Models of brain function in neuroimaging. Annu Rev Psychol 56:57–87. [DOI] [PubMed] [Google Scholar]
- Friston KJ (2011): Functional and effective connectivity: A review. Brain Connectivity 1:13–36. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Li B, Daunizeau J, Stephan KE (2012): Network discovery with DCM. NeuroImage 56:1202–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallace A, Soravia G, Cattaneo Z, Moseley L, Vallar G (2014): Temporary interference over the posterior parietal cortices disrupts thermoregulatory control in humans. PLoS One 9:e88209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavva NR, Davis C, Sonya GL, Rao S, Wang W, Zhu DX (2012): Transient receptor potential melastatin 8 (TRPM8) channels are involved in body temperature regulation. Mol Pain 8:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez‐Alonso J (2012): Human thermoregulation and the cardiovascular system. Exp Physiol 97:340–346. [DOI] [PubMed] [Google Scholar]
- Gottschlich KW, Werner J (1985): Effects of medial midbrain lesions on thermo‐responsive neurons in the thalamus of the rat. Exp Brain Res 57:355–361. [DOI] [PubMed] [Google Scholar]
- Gressens P, Dingley J, Plaisant F, Porter H, Schwendimann L, Verney C, Tooley J, Thoresen M (2008): Analysis of neuronal, glial, endothelial, axonal and apoptotic markers following moderate therapeutic hypothermia and anesthesia in the developing piglet brain. Brain Pathol 18:10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn AJ, Gunn TR, de Haan HH, Williams CE, Gluckman PD (1997): Dramatic neuronal rescue with prolonged selective head cooling after ischemia in fetal lambs. J Clin Invest 99:248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton JK, Rath P, Helsell CV, Beckstein O, Van Horn WD (2015): Understanding thermosensitive transient receptor potential channels as versatile polymodal cellular sensors. Biochemistry 54:2401–2413. [DOI] [PubMed] [Google Scholar]
- James C, Henderson L, Macefield VG (2013): Real‐time imaging of brain areas involved in the generation of spontaneous skin sympathetic nerve activity at rest. Neuroimage 74:188–194. [DOI] [PubMed] [Google Scholar]
- Jessen C (1981): Independent clamps of peripheral and central temperatures and their effects on heat production in the goat. J Physiol 311:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanek PM, Safar PJ (2003): Therapeutic hypothermia for severe traumatic brain Injury. JAMA 2893:007–3009. [DOI] [PubMed] [Google Scholar]
- Kubina B, Ristic D, Weber J, Stracke CP, Forster C, Ellrich J (2010): Bilateral brainstem activation by thermal stimulation of the face in healthy volunteers. J Neurol 257:271–280. [DOI] [PubMed] [Google Scholar]
- Kwan CL, Crawley AP, Mikulis DJ, Davis KD (2000): An fMRI study of the anterior cingulate cortex and surrounding medial wall activations evoked by noxious cutaneous heat and cold stimuli. Pain 85:359–374. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT (2000): Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp 10:120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas‐Gutiérrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT (2007): Bias between MNI and Talairach coordinates analyzed using the ICBM‐152 brain template. Hum Brain Mapp 28:1194–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK (2008): What we can do and what we cannot do with fMRI. Nature 453:869–878. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Evans AK, Gasser PJ, Hale MW, Staub DR, Shekhar A (2008) Topographical organization and chemoarchitecture of the dorsal raphe nucleus and the median raphe nucleus In: Monti JM, Pandi‐Perumal BL, Jacobs BL, Nutt DL, editors. Serotonin and Sleep: Molecular, Functional and Clinical Aspects. Basel: Birkhauser, pp 25–68. [Google Scholar]
- Lowry CA, Lightman SL, Nutt DJ (2009): That warm fuzzy feeling: Brain serotonergic neurons and the regulation of emotion. J Psychopharmacol 23:392–400. [DOI] [PubMed] [Google Scholar]
- McAllen RM, Farrell M, Johnson JM, Trevaks D, Cole L, McKinley MJ, Jackson G, Denton DA, Egan GF (2006): Human medullary responses to cooling and rewarming the skin: A functional MRI study. Proc Natl Acad Sci USA 103:809–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM (1998): From sensation to cognition. Brain 121:1013–1052. [DOI] [PubMed] [Google Scholar]
- Morrison SF (2011): The 2010 carl ludwig distinguished lectureship of the APS neural control and autonomic regulation section: Central neural pathways for thermo‐regulatory cold defense. J Appl Physiol 110:1137–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF, Nakamura K, Madden CJ (2008): Central control of thermogenesis in mammals. Exp Physiol 93:773–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley GL, Gallace A, Iannetti GD (2012): Spatially defined modulation of skin temperature and hand ownership of both hands in patients with unilateral complex regional pain syndrome. Brain 135:3676–3686. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF (2008): A thermosensory pathway that controls body temperature. Nat Neurosci 11:62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF (2011): Central efferent pathways for cold‐defensive and febrile shivering. J Physiol 589:3641–3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, Friston K (2013): Structural and functional brain networks: From connections to cognition. Science (New York, NY) 342:1238411. [DOI] [PubMed] [Google Scholar]
- Patapoutian A, Peier AM, Story GM, Viswanath V (2003): ThermoTRP channels and beyond: Mechanisms of temperature sensation. Nat Rev Neurosci 4:529–539. [DOI] [PubMed] [Google Scholar]
- Pellecchia MT, Criscuolo C, De Joanna G, D'Amico A, Santoro L, Barone P (2003): Pure unilateral hyperhidrosis after pontine infarct. Neurology 61:1305. [DOI] [PubMed] [Google Scholar]
- Petrides M (2007): Deviation from Expectation and Memory. Ann N Y Acad Sci 1121:33–53. [DOI] [PubMed] [Google Scholar]
- Riedl B, Beckmann T, Neundörfer B, Handwerker HO, Birklein F (2001): Autonomic failure after stroke–is it indicative for pathophysiology of complex regional pain syndrome? Acta Neurol Scand 103:27–34. [DOI] [PubMed] [Google Scholar]
- Roberts WW (1988): Differential thermosensor control of thermoregulatory grooming, locomotion, and relaxed postural extension. Ann N Y Acad Sci 525:363–734. [DOI] [PubMed] [Google Scholar]
- Rolls ET (2010): The affective and cognitive processing of touch, oral texture, and temperature in the brain. Neurosci Biobehav Rev 34:237–245. [DOI] [PubMed] [Google Scholar]
- Rolls ET, O'Doherty J, Kringelbach ML, Francis S, Bowtell R, McGlone F (2003): Representations of pleasant and painful touch in the human orbitofrontal and cingulate cortices. Cereb Cortex 13:308–317. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Grabenhorst F, Parris BA (2008): Warm pleasant feelings in the brain. NeuroImage 41:1504–1513. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA (2007): Thermoregulation: Some concepts have changed. Functional architecture of the thermoregulatory system. Am J Physiol Regul Integr Comp Physiol 292:R37–R46. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA (2014): Skin temperature: Its role in thermoregulation. Acta Physiol (Oxf) 210:498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurada S, Shido O, Fujikake K, Nagasaka T (1993): Relationship between body core and peripheral temperatures at the onset of thermoregulatory responses in rats. Jpn J Physiol 43:659–667. [DOI] [PubMed] [Google Scholar]
- Satinoff E (1978): Neural organization and evolution of thermal regulation in mammals. Science (New York, NY) 201:16–22. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Augath M, Oeltermann A, Logothetis NK (2006): Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat Neurosci 9:569–577. [DOI] [PubMed] [Google Scholar]
- Singh KD (2012): Which “neural activity” do you mean? fMRI, MEG, oscillations and neurotransmitters. NeuroImage 62:1121–1130. [DOI] [PubMed] [Google Scholar]
- Stephan KE (2004): On the role of general system theory for functional neuroimaging. J Anat 205:443–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Roebroeck A (2012): A short history of causal modeling of fMRI data. NeuroImage 62:856–863. [DOI] [PubMed] [Google Scholar]
- Terrien J, Perret M, Aujard F (2011): Behavioral thermoregulation in mammals: A review. Frontiers Biosci 16:1428–1444. [DOI] [PubMed] [Google Scholar]
- Turner R, Howseman A, Rees GE, Josephs O, Friston K (1998): Functional magnetic resonance imaging of the human brain: Data acquisition and analysis. Exp Brain Res 123:5–12. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15:273–289. [DOI] [PubMed] [Google Scholar]
- Viswanathan A, Freeman RD (2007): Neurometabolic coupling in cerebral cortex reflects synaptic more than spiking activity. Nat Neurosci 10:1308–1312. [DOI] [PubMed] [Google Scholar]
- Ward BD 2000. Simultaneous inference for fMRI data. Milwaukee, WI: Medical College of Wisconsin. [Google Scholar]
- Werner J, Bienek A (1985): The significance of nucleus raphe dorsalis and centralis for thermoafferent signal transmission to the preoptic area of the rat. Exp Brain Res 59:543–547. [DOI] [PubMed] [Google Scholar]
- Werner J, Bienek A (1990): Loss and restoration of preoptic thermo‐ reactiveness after lesions of the rostral raphe nuclei. Exp Brain Res 80:429–435. [DOI] [PubMed] [Google Scholar]
- Xie YC, Li CY, Li T, Nie DY, Ye F (2007): Effect of mild hypothermia on angiogenesis in rats with focal cerebral ischemia. Neurosci Lett 422:87–90. [DOI] [PubMed] [Google Scholar]
- Xu X, Karis AJ, Buller MJ, Santee WR (2013): Relationship between core temperature, skin temperature, and heat flux during exercise in heat. Eur J Appl Physiol 113:2381–2389. [DOI] [PubMed] [Google Scholar]
- Yamakage M, Namiki A (2003): Deep temperature monitoring using a zero‐heat‐flow method. J Anesth 7:108–115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figure 1.


