Abstract
There are an increasing number of neuroimaging studies that allow a better understanding of symptoms, neural correlates and associated conditions of fibromyalgia. However, the results of these studies are difficult to compare, as they include a heterogeneous group of patients, use different stimulation paradigms, tasks, and the statistical evaluation of neuroimaging data shows high variability. Therefore, this meta‐analytic approach aimed at evaluating potential alterations in neuronal brain activity or structure related to pain processing in fibromyalgia syndrome (FMS) patients, using quantitative coordinate‐based “activation likelihood estimation” (ALE) meta‐analysis. 37 FMS papers met the inclusion criteria for an ALE analysis (1,264 subjects, 274 activation foci). A pooled ALE analysis of different modalities of neuroimaging and additional analyses according functional and structural changes indicated differences between FMS patients and controls in the insula, amygdala, anterior/mid cingulate cortex, superior temporal gyrus, the primary and secondary somatosensory cortex, and lingual gyrus. Our analysis showed consistent results across FMS studies with potential abnormalities especially in pain‐related brain areas. Given that similar alterations have already been demonstrated in patients with other chronic pain conditions and the lack of adequate control groups of chronic pain subjects in most FMS studies, it is not clear however, whether these findings are associated with chronic pain in general or are unique features of patients with FMS. Hum Brain Mapp 37:1749–1758, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: fibromyalgia, pain, descending pain modulation system, meta‐analysis, brain imaging, activation likelihood analysis
Abbreviations
- ACC
Anterior cingulate cortex
- ALE
Activation likelihood estimation
- DMN
Default mode network
- FMS
Fibromyalgia syndrome
- FWHM
Full width at half maximum
- MNI
Montreal Neurological Institute
- PAG
Periaqueductal gray
- ROI
Regions of interest
- STG
Superior temporal gyrus
- VOI
Volume of interest
INTRODUCTION
Patients with fibromyalgia syndrome (FMS) suffer from chronic widespread pain in the musculoskeletal system and associated symptoms such as fatigue, sleep disturbance, and cognitive dysfunctions [Schmidt‐Wilcke and Daniel J. Clauw, 2011]. The prevalence of fibromyalgia ranges from 2 to 8% and women are more affected with a 2:1 female to male ratio [Clauw, 2014].
The underlying etiopathogenesis is still unknown, but it has been hypothesized that fibromyalgia is a disorder of pain processing and pain modulation in the central nervous system due to dysfunctions of central pain inhibitory or intrinsic brain networks [Jensen et al., 2009]. Many studies have used neuroimaging to investigate neural activity in FMS patients. These studies showed differences in activation in response to experimentally applied pain stimuli in pain‐related brain areas such as the insula, anterior and posterior cingulate, inferior parietal lobe, thalamus, cerebellum and primary and secondary somatosensory cortex (SI, SII) when compared with studies of pain processing in healthy humans [Apkarian et al., 2005]. There are further studies pointing to the presence of a significant imbalance of the intrinsic brain connectivity within the pain network and disrupted intrinsic connectivity within the default mode network (DMN) as well as hyperconnectivity between pain processing regions [Ichesco et al., 2014; Napadow et al., 2010].
However, despite the growing number of studies assessing brain activation no clear picture emerged from the existing literature considering the involvement of cortical and subcortical regions. The findings of FMS neuroimaging studies are inconsistent and hard to compare, as they included a heterogeneous group of patients, used different nociceptive stimuli, and different experimental designs which led to a variety of methods employed and obvious difficulties in generalization of the results. Given the inconsistencies in the studies, we aimed to clarify disease relevant brain alterations by performing a statistical coordinate‐based meta‐analysis of FMS neuroimaging studies using the “activation likelihood estimation” (ALE) method to investigate whether conclusions from existing reviews from different modalities can be corroborated. This meta‐analytic tool enables detecting effects that may be weak, and hence went unnoticed in the original studies because they did not seem to be interesting according to the hypothesis, but are consistent across experiments [Eickhoff et al., 2009; Eickhoff et al., 2012]. The purpose of this study was therefore to test, in an exploratory fashion, which brain areas known to be important for pain processing in FMS were altered.
METHODS
Identification of FMS Brain Imaging Studies
A literature search was conducted using PubMed to identify relevant studies for inclusion in the ALE meta‐analysis. All included articles were published in English or German language prior to March 2015. The search input was a combination of main keywords according to the disease terminology of fibromyalgia and either one of the general neuroimaging techniques [(Fibromyalgia [Mesh] OR Fibromyalgia OR Fibrositis) AND (imaging OR Brain) AND (MRI OR Magnetic Resonance Imaging OR PET OR Positron Emission Tomography OR SPECT OR Single Photon Emission Computed Tomography)] or the additional combination of specific terms according evoked pain paradigms [AND (activation OR stimulation OR evoked)], resting or baseline brain activity [AND (resting state OR baseline)], structural brain imaging [AND (vbm OR voxel based morphometry OR dti OR diffusion tensor imaging OR tractography OR brain morphology OR cortical thickness)], spectroscopy studies [AND (proton magnetic resonance spectroscopy OR Spectroscopy OR glutamate OR GABA OR Glx OR NAA OR neurotransmitter)], and electroencephalographic studies [AND (EEG OR EP OR electroencephalography OR electroencephalogram OR evoked potential OR MEG OR magnetoencephalography OR magnetoencephalogram)].
Five‐hundred studies including review articles were identified and were checked for the following criteria: (1) Articles must include results of brain imaging studies. (2) Single case reports were excluded. (3) Only papers reporting results from their own studies were included. The reference lists of identified review articles were screened manually for additional citations and one additional relevant publication was identified.
The 114 resulting articles were filtered for necessary inclusion criteria for ALE in a second step (Step 2): (1) Only studies which performed a statistical comparison were included. This implies that either patients with one or more control group or patients before and after treatment of any kind were statistically compared. (2) Studies needed to perform whole‐brain analyses to also cover brain areas which were not in the subjective focus of the authors. Therefore, studies with only restricted regions of interest (ROI) or volume of interest (VOI) analyses were excluded. In studies that have also conducted ROI analyses in addition to a whole‐brain analysis, only the results of the whole‐brain analyses were included. Furthermore, functional connectivity studies of a priori defined seed regions covering the whole brain or widespread cortical areas were included as well. (3) The results needed to be reported in a normalized standard stereotactic space, i.e., Talairach or Montreal Neurological Institute (MNI). The 114 studies and the respective reason for exclusion are listed in Supporting Information Table SI.
These criteria identified 37 papers for inclusion into the meta‐analysis (Fig. 1, Table 1). From these studies, the necessary parameters for an ALE analysis were extracted (Number of subjects, coordinates). We performed four independent ALE analyses:
Figure 1.
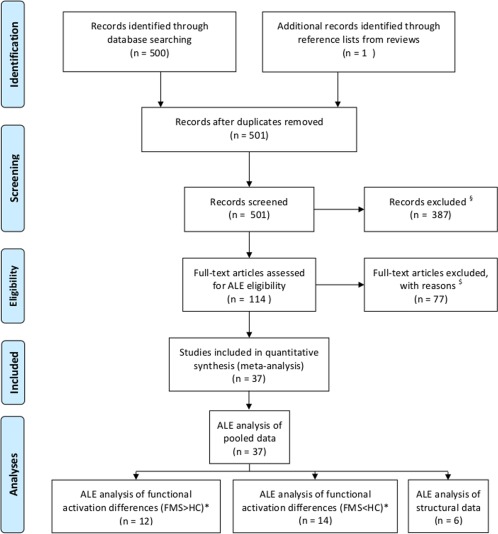
Show the sequence of the literature search and the process of inclusion or exclusion of articles according the PRISMA statement (see http://www.equator-network.org/reporting-guidelines/prisma/). §exclusion criteria = no brain imaging, single case reports, reviews. $exclusion criteria = no statistical comparison of groups, no whole‐brain analysis, no standard stereotactic space coordinates (Talairach, MNI). * some articles show results of clusters with hyperactivation or hypoactivation. For each subanalysis these clusters were integrated separately. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 1.
List of 37 studies included in the ALE analysis
| Paper | Imaging method | Statistical comparison | Stat. threshold | n of included clusters |
|---|---|---|---|---|
| Functional studies FMS vs controls | ||||
| Brown et al. Eur. J. Neurosci. 2014;39:663–672. | EEG | 16 FMS, 15 HC, 16 OA | corr. 0.05 | 14 |
| Burgmer et al. Psychosom. Med. 2011;73:751–759. | fMRI | 12 FMS, 14 HC | corr. 0.05 | 2 |
| Burgmer et al. Eur. J. Pain 2012;16:636–647. | fMRI | 17 FMS, 17 HC | corr. 0.05 | 3 |
| Glass et al. J. Pain 2011;12:1219–1229. | fMRI | 18 FMS, 14 HC | corr. 0.05 | 10 |
| Gracely et al. Arthritis Rheum. 2002;46:1333–1343. | fMRI | 16 FMS, 16 HC | corr. 0.05 | 14 |
| Guedj et al. Eur. J. Nucl. Med. Mol. Imaging 2007a;34:130–134. | SPECT | 18 FMS, 10 HC | corr. 0.05 | 18 |
| Harris et al. J. Neurosci. 2007;27:10000–10006. | PET | 17 FMS, 17 HC | corr. 0.05 | 4 |
| Jensen et al. Pain 2009;144:95–100. | fMRI | 16 FMS, 16 HC | uncorr. 0.005 | 2 |
| Jensen et al. Arthritis Rheum. 2013;65:3293–3303.a | fMRI | 26 FMS, 13 HC | corr. 0.05 | 1 |
| Kim et al. PLoS One 2013;8:e74099. | fMRI | 21 FMS, 11 HC | corr. 0.05 | 8 |
| Loggia et al. Arthritis Rheumatol. 2014;66:203–212. | fMRI | 31 FMS, 14 HC | corr. 0.05 | 25 |
| Lopez‐Sola et al. Arthritis Rheumatol. 2014;66:3200–3209. | fMRI | 35 FMS, 25 HC | corr. 0.05 | 8 |
| Maestu et al. Clin. Neurophysiol. 2013;124:752–760. | MEG | 9 FMS, 9 HC | corr. 0.01 | 9 |
| Martinsen et al. PLoS One 2014;9:e108637. | fMRI | 23 FMS, 28 HC | uncorr. 0.001 | 7 |
| Pujol et al. PLoS One 2009;4:e5224. | fMRI | 9 FMS, 9 HC | corr. 0.05 | 14 |
| Usui et al. Arthritis Res. Ther. 2010;12:R64. | SPECT | 29 FMS, 10 HC | corr. 0.05 | 10 |
| Wik et al. Neuroreport 2003;14:619–621. | PET | 8 FMS, 8 HC | corr. | 3 |
| Wood et al. J. Pain 2007;8:51–58. | PET | 6 FMS, 8 HC | uncorr. 0.01 | 14 |
| Functional studies within FMS | ||||
| Boyer et al. Neurology 2014;82:1231–1238. | PET | 16 FMS, 13 FMS | uncorr. 0.001 | 1 |
| Guedj et al. Eur. J. Nucl. Med. Mol. Imaging 2007c;34:2115–2119. | SPECT | 11 FMS, 6 FMS | uncorr. 0.001 | 3 |
| Harris et al. Neuroimage 2009;47:1077–1085. | PET | 10 FMS, 10 FMS | corr. 0.05 | 2 |
| Jensen et al. Pain 2012;153:1495–1503. | fMRI | 19 FMS, 15 FMS | corr. 0.05 | 1 |
| Jensen et al. J. Pain 2014;15:1328–1337. | fMRI | 21 FMS, 16 FMS | corr. 0.05 | 1 |
| Schmidt‐Wilcke et al. Pain Med. 2014;15:1346–1358. | fMRI | 8 FMS, 8 FMS | corr. 0.05 | 1 |
| Wik et al. Eur. J. Pain 1999;3:7–12. | PET | 8 FMS (pre/post) | uncorr. 0.00 | 10 |
| Structural studies | ||||
| Ceko et al. Neuroimage Clin. 2013;3:249–260. | VBM | 27 FMS, 26 HC | corr. 0.05 | 9 |
| Fallon et al. Neuroimage Clin. 2013;3:163–170. | VBM | 16 FMS, 15 HC | corr. 0.05 | 4 |
| Hsu et al. Pain 2009;143:262–267. | VBM | 29 FMS, 29 HC | corr. 0.05 | 0 |
| Jensen et al. Arthritis Rheum. 2013;65:3293–3303.a | cortical thickness | 26 FMS, 13 HC | corr. 0.05 | 7 |
| Kim et al. Arthritis Rheumatol. 2014;66:3190–3199. | DTI | 19 FMS, 18 HC | corr. 0.05 | 1 |
| Schmidt‐Wilcke et al. Pain 2007;132 Suppl 1:S109–S116. | VBM | 20 FMS, 22 HC | corr. 0.05 | 3 |
| Connectivity studies | ||||
| Flodin et al. Brain Connect. 2014;4:587–594. | rsMRI | 16 FMS, 22 HC | corr. 0.05 | 6 |
| Harris et al. Anesthesiology 2013;119:1453–1464. | rsMRI | 14 FMS (pre/post) | uncorr. 0.05 | 6 |
| Ichesco et al. J. Pain 2014;15:815–826.e1. | rsMRI | 18 FMS, 18 HC | corr. 0.05 | 8 |
| Jensen et al. Mol. Pain 2012;8:32–8069‐8‐32. | fMRI | 28 FMS, 14 HC | corr. 0.05 | 4 |
| Napadow et al. Arthritis Rheum. 2010;62:2545–2555. | rsMRI | 18 FMS, 18 HC | corr. 0.05 | 5 |
| Napadow et al. Arthritis Rheum. 2012;64:2398–2403. | fMRI | 17 FMS (pre/post) | corr. 0.05 | 2 |
| Pujol et al. Pain 2014;155:1492–1503. | rsMRI | 40 FMS, 36 HC | corr. 0.05 | 34 |
aStudy of Jensen included functional and structural analyses.
SPECT = Single Photon Emission Computed Tomography, PET = Positron Emission Tomography, VBM = Voxel‐based morphometry, DTI = diffusion tensor imaging, fMRI = functional Magnetic Resonance Imaging, rsMRI = resting‐state MRI, EEG = Electroencephalography, MEG = Magnetoencephalography, sr = seed regression, ICN = intrinsic connectivity network.
We pooled data of different modalities [functional (fMRT, PET, SPECT, EEG), structural (VBM, DTI)] into one analysis. This approach gives information about the global changes in FMS independent of the implemented neuroimaging paradigm since each imaging modality, study design and data processing might bias the results in different directions. Therefore, a pooled functional and structural analysis compensates for such method‐induced variance of data.
We analyzed the 18 functional studies only to provide information on the direction of activation changes of altered brain regions in FMS in (2a) an analysis with all brain regions showing greater activation (n = 68) in FMS in contrast to controls and (2b) a separate analysis with all brain regions showing less activation (n = 98) in FMS in contrast to controls.
For purpose of testing the impact of structural changes in FMS we performed an additional explaratory analysis using clusters of structural studies (n = 24).
ALE Meta‐Analysis
Statistical analysis of the studies was conducted using the revised ALE algorithm [Eickhoff et al., 2009] for coordinate‐based analyses [Turkeltaub et al., 2002]. At first, a whole‐brain modeled activation map (MA‐map) describing the convergence of the assessed experiments is obtained by estimating activation probabilities for each voxel in the brain. The reported activation foci are treated as centers of a 3D Gaussian probability distribution, the width reflects an estimate of the spatial uncertainty of the foci of a given map and sample size of each experiment [Turkeltaub et al., 2012]. The full width at half maximum (FWHM) value and the null distribution of each voxel has been empirically determined. In the next step, a permutation test is used to distinguish true convergence of foci across different experiments from random spatial association (i.e., noise) by comparing the ALE scores to an empirical null distribution. The histogram of the ALE scores obtained under the permutation distribution is then used to assign P‐values to compute the ALE map threshold [Eickhoff et al., 2012]. The resulting ALE maps were determined at a cluster‐level Family Wise Error (FWE) rate‐corrected threshold of P < 0.05 (cluster‐forming threshold at voxel‐level P < 0.001). For illustration, the ALE maps were imported into MRICron as overlay on a standardized anatomical MNI‐normalized template (Colin_27_T1).
RESULTS
The pooled functional and structural meta‐analysis included 37 studies with a total number of 1,264 subjects and 274 brain foci. Brain clusters in the right insula, areas of the transition between the parietal lobe and superior temporal gyrus (STG) to the insula, and the right STG were found (yellow clusters, Fig. 2).
Figure 2.
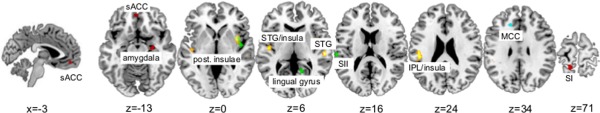
Depicts the results of the ALE‐analyses on the Colin27_T1_seg_MNI template. Clusters of the pooled analysis are shown in yellow, clusters of the functional analysis are shown in green (FMS hyperactivation) and red (FMS hypoactivation), and the cluster of the structural analysis is shown in cyan. sACC = subgenual anterior cingulate cortex, STG = superior temporal gyrus, SI = primary somatosensoric cortex, SII = secondary somatosensoric cortex, IPL = inferior parietal lobe, MCC = middle cingulate cortex. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
The additional meta‐analyses taking into account the direction of activation changes in FMS showed six brain regions. Patients with FMS presented hyperactivation in the right insula, the left postcentral gyrus (SII), and the right lingual gyrus (green clusters, Fig. 2). Hypoactivation in FMS was seen in the left postcentral gyrus (SI), the subgenual region (area 32) of left anterior cingulate cortex, and the right amygdala (red clusters, Fig. 2).
To test for the impact of structural alterations in FMS we performed an explaratory meta‐analysis of the six studies investigating structural brain changes in FMS. An alteration was detected in the midcingulate gyrus around Brodmann area 32 (cyan cluster, Fig. 2).
Table 2 provides the respective coordinates, cluster sizes, and number of contributing foci of the related papers of all four analyses.
Table 2.
Results of the global ALE analysis of FMS different modalities studies and ALE analyses of the functional studies with hyperactivation and with hypoactivation in FMS in contrast to controls
| Label | Cluster size | Coordinates (MNI) | N foci contrib. | ||
|---|---|---|---|---|---|
| mm3 | X | y | z | ||
| Global analysis (274 foci, 1264 subjects) | |||||
| Right insula | 784 | 39 | 4 | 1 | 4 |
| Left IPL/insula | 888 | −48 | −27 | 24 | 6 |
| Left STG/insula | 672 | −46 | −12 | 5 | 4 |
| Right STG | 448 | 56 | −23 | 5 | 3 |
| FMS hyperactivation (68 foci, 383 subjects) | |||||
| Right insula | 480 | 43 | −2 | 1 | 3 |
| Left SII | 280 | −62 | −25 | 17 | 2 |
| Right lingual gyrus | 272 | 13 | −55 | 7 | 2 |
| FMS hypoactivation (98 foci, 503 subjects) | |||||
| Left SI | 608 | −15 | −48 | 72 | 3 |
| Left subgenual ACC | 328 | −2 | 48 | −12 | 2 |
| Right amygdala | 304 | 27 | −12 | −15 | 2 |
| Structural analysis (24 foci, 202 subjects) | |||||
| Left midcingulate gyrus | 400 | −17 | 30 | 31 | 2 |
Cluster‐forming value = P uncorr.<0.001, cluster‐level inference = P corr. <0.05, N foci contrib. = number of foci of the included studies which contributed to resulting cluster.
To control for a possible impact of the within‐FMS studies (pre versus post intervention) on the result of the first pooled analysis, because these studies might represent the neural correlates of the treatment effect (in the context of FMS) but not the neural correlates of FMS itself, we performed an additional meta‐analysis of the pooled data without these seven studies. This analysis confirmed our prior findings with little changes according the cluster localizations and sizes (see Supporting Information Table SII).
DISCUSSION
The aim of this ALE meta‐analysis was to evaluate the potential structural or functional brain alterations in patients with FMS to gain a more detailed insight into the underlying pathophysiology of this chronic pain syndrome. Our analyses revealed differences in several brain regions critically involved in pain processing, including insula, amygdala, STG, lingual gyrus, and anterior/mid cingulate cortex. Because of the low number of included studies and foci (n = 24) in our structral analysis the obtained result should not be given the same weight as our other analyses und should be regarded with major caution. However, the mid cingulate cortex has been also reported in other pain and FMS studies [Amanzio et al., 2013; Ichesco et al., 2014; Schmidt‐Wilcke et al., 2014].
In the following section, these regions will be discussed according to their specific functions in pain processing, as well as according to their potential involvement in a broader brain network for pain processing in FMS.
Insula
It has been recognized that the insula can be distinguished on the basis of anatomical and functional criteria. The role of the insula has been especially related to self‐awareness, regulating emotion, and sensory motor and interoceptive processing as it occurs in pain. The anterior part of the insula is thought to coordinate the affective and emotional aspects of pain, whereas the posterior insula may play a greater role in sensory discriminative aspects of pain [Kurth et al., 2010]. Moreover, it has been described that chronic pain is associated with activation of the anterior insula, whereas experimental pain induces posterior insula activation, as in our results [Friebel et al., 2011]. Regardless of the stimulation technique used to elicit pain, neuroimaging studies have consistently shown a significant involvement of the insula [Apkarian et al., 2005]. In our meta‐analysis of pooled data activation foci in the insula were detected mainly in the posterior part, which is in line with posterior insula activation in studies implementing experimental pain and the greater pain transmission in FMS.
The Anterior Cingulate Cortex (ACC)
The ACC plays a key role in regulatory and executive processes, and is responsible for expressing emotions especially associated with enhanced attention directed towards salient stimuli. Several studies have revealed a functional dissociation of this region during emotional processing [Bush et al., 2002; Paus, 2001]. Thus, it has been suggested that dorsal ACC is activated during experimental tasks eliciting a cognitive interference with non‐emotional stimuli, whereas ventral ACC is rather involved in the modulation of emotional responses [Kanske and S. A. Kotz, 2011]. Cytoarchitectonically, the subgenual region of the ACC (sACC), as it was found in our functional meta‐analysis showing hypoactivation in FMS, is more heterogeneous and seems to be particularly more related to cognitive and affective experiences than the pregenual (pACC) subregion [Palomero‐Gallagher et al., 2015].
The ACC is involved in the processing of the affective component of pain, encoding unpleasantness and emotional memories, and regulating endogenous pain modulation [Fuchs et al., 2014]. In chronic pain, ACC hyperactivation has been interpreted as a deficit in emotional modulation and considered a possible underlying mechanism for the chronification [Kamping et al., 2013]. This view is also consistent with the hypothesis that opioidergic and neurotransmitter dysregulation of the ACC could play a significant role in insufficient pain inhibition [Martikainen et al., 2013].
Amygdala
The amygdala plays a central role in emotional learning, acquisition of emotional memories and emotional processing of sensory stimuli, especially fear and defensive behavior [Costafreda et al., 2008]. Neuroimaging studies have revealed a crucial role for the amygdala in the evaluation and the emotional processing of pain [Simons et al., 2014]. The pain modulatory role of amygdala is based on its projections to descending pain regions in the brain [Simons et al., 2014; Tracey and P. W. Mantyh, 2007], as well as on the fact that it is a relay station for both emotional‐affective and cognitive processing of nociceptive and antinociceptive inputs including memories and expectations for pain [Bingel et al., 2006; Simons et al., 2014], which might be reason for the decreased activation in FMS in our functional contrast analysis.
The Superior Temporal Gyrus (STG)
The STG is typically associated with auditory perception and contains the primary auditory cortex and auditory association areas. Previous studies indicate that this region is also involved in the production, interpretation and self‐monitoring of language, in the processing of social information, and in higher cognitive functioning [Howard et al., 2000; Pearlson, 1997]. Previous neuroimaging studies have suggested that the STG plays a role in the processing of pain‐related unpleasantness and that its function seems to be affected in patients with chronic pain [Becerra et al., 1999; Duerden and M. C. Albanese, 2013; Smallwood et al., 2013]. However, the relation between STG activity, as it was found in our pooled analysis in FMS patients, and pain is so far not clear. Still, a number of recently published studies indicated that in FMS not only the somatosensory system is affected, but that also other modalities are possibly involved. Some authors even hypothesize that FMS and other musculoskeletal diseases might be related to abnormal multimodal intergataion. As such the STG and the auditory cortex may also be of importance in abnormal pain processing [Lopez‐Sola et al., 2014].
The Lingual Gyrus
The lingual gyrus is a part of the visual association cortex and plays a relevant role in the analysis of visual memories [Bogousslavsky et al., 1987]. Studies with depressed patients have also shown functional and structural abnormalities in the lingual gyrus [Veer et al., 2010]. The lingual gyrus is also considered as part of the so‐called DMN, a network playing a central role in emotional self‐awareness, social cognition, creativity, and ethical decision making [Boyatzis et al., 2014]. Moreover, hyperactivation of lingual gyrus has been associated with augmented sensitivity to pain [Loggia et al., 2011], as it was found in our functional meta‐analysis.
The Postcentral Gyrus (SI and SII)
The SI has been implicated in the spatial coding and sensory‐discriminitive aspects of pain such as anticipation, quality, intensity and localization processing [Apkarian et al., 2005; Bushnell et al., 1999; Schnitzler and M. Ploner, 2000]. The SII seems to be involved in relaying nociceptive information to the temporal lobe limbic structures. Functionally, the SII has been involved in the detection, recognition, learning, and memory of painful stimuli [Schnitzler and M. Ploner, 2000]. Studies have shown significant decreases in gray matter of the somatosensory cortex of chronic pain patients [Schmidt‐Wilcke et al., 2006]. Chronic pain patients show reduced brain processing of the physical properties of somatosensory information (e.g. SI, SII), together with an enhanced activation of brain regions involved in the processing of cognitive, emotional and introspective aspects of pain [Apkarian et al., 2005; Williams and R. H. Gracely, 2006].
HOW DO WE EVALUATE ABNORMALITIES IN PAIN REGIONS IN FMS?
The ALE‐findings seem to indicate that FMS might be associated with structural or functional changes in brain regions which are altered in other chronic pain disorders as well. Previous studies in FMS have explored different hypotheses to explain these alterations in FMS, such as pathological pain augmentation responses to experimental pain stimuli [Gracely et al., 2002], alteration of neurotransmitter function [Harris et al., 2007], impairment of the descending pain inhibitory network [Jensen et al., 2009], or altered resting state networks [Napadow et al., 2010].
One of the most discussed mechanisms in FMS has been an impaired descending pain inhibition [Jensen et al., 2009]. It is known that the periaqueductal gray (PAG), as part of the descending inhibitory pathway, receives inputs from cortical and subcortical regions such as the PFC, ACC, insula, and amygdala, thus modulating nociceptive information processing within the rostroventral medulla and the dorsal horn of the spinal cord [Tracey and P. W. Mantyh, 2007]. Moreover, the ACC and the insula are key regions involved in processing of affective pain components [Cifre et al., 2012]. Affective and cognitive factors, such as anticipation, attention, anxiety, or depressive states could modulate pain perception by alterating the normal function of these areas. Thus, the observed hypoactivation of the ACC and amygdala in FMS in our functional meta‐analysis, together with hyperactivation of the insula might support the idea of a dysfunction of the system of descending pain modulation in FMS [Jensen et al., 2012]. However, in the context of these ALE meta‐analyses, it cannot be answered whether the interaction of these brain regions is altered as a network or if the alterations might occure independend of their network function.
Another interesting perspective in the study of brain dysfunction in FMS is provided by the dynamics of resting‐state brain networks. In this context, the observed hyperactivation in FMS of the lingual gyrus in our functional meta‐analysis, as part of a so‐called DMN are highly relevant [Boyatzis et al., 2014]. This network is typically deactivated during a variety of externally focused tasks [Buckner et al., 2008]. Whereas DMN deactivation is induced during acute pain in healthy subjects, distrupted DMN connectivity or DMN‐insula have been observed in multiple chronic pain conditions [Baliki et al., 2008; Napadow et al., 2010; Seminowicz and K. D. Davis, 2007]. It is speculated that an altered intrinsic DMN connectivity may characterize a common mechanism in chronic pain, rather than being specific to FMS. In this sense, these alterations of the DMN should be interpreted as a consequence of the chronification of pain. However, these brain regions have also other functions and are involved in other brain networks. Our pooled structural and functional ALE meta‐analysis does not consequently imply that an associated brain network is specifically dysfunctional or even involved in FMS.
There have been other meta‐analyses utilizing the ALE approach in order to decipher commonly activated brain areas of the pain matrix and its modulation. In chronic neuropathic pain increased activation in the left secondary somatosensory cortex, ACC, and right caudal anterior insula was shown when compared to experimentally induced pain [Friebel et al., 2011]. Another meta‐analysis examined brain activation in response to different types of painful stimuli in healthy volunteers and thereby provided positive evidence for the involvement of SI, SII, ACC, insula, prefrontal cortex (PFC), thalamus, and basal ganglia in processing of nociceptive stimuli [Duerden and M. C. Albanese, 2013]. Moreover, another ALE analysis suggest that there are distinct, but overlapping neuronal networks, such as in the insula, ACC, PFC, SII and thalamus in different types of stimulus‐evoked pain (hyperalgesia, allodynia), clinical neuropathic and experimental pain [Lanz et al., 2011]. During placebo analgesia in paradigms using experimental noxious stimulation, increased activity in the ACC, insula, thalamus and hypothalamus as well as in the PAG was observed. Results were interpreted in favor of a true antinociceptive effect underlying placebo analgesia, in addition to assumed modulation of cognitive evaluation of pain intensity [Amanzio et al., 2013]. Results from all these meta‐analyses [Amanzio et al., 2013; Duerden and M. C. Albanese, 2013; Friebel et al., 2011; Lanz et al., 2011] are compatible with our findings, i.e., they show different activation patterns of commonly activated regions underlying nociception.
ARE THE REPORTED REGIONS THE MAIN AREAS THAT FUTURE STUDIES IN FMS SHOULD FOCUS ON?
Comparable alterations in pain processing areas have also been observed in patients with other chronic pain conditions including low back pain, and irritable bowel syndrome [Apkarian et al., 2005]. Therefore, it remains unclear whether brain alterations of our results apply only to patients with FMS, or might reflect a general brain alteration linked to chronic pain itself. In our opinion, one of the major problems of the existing neuroimaging literature in FMS is the lack of adequate comparison groups with chronic pain or other relevant comorbid conditions, like depression. In our pooled meta‐analysis only one study included a pain control group. Therefore, it is clearly possible that FMS and other chronic pain disorders share overlapping central mechanisms, and show similar features and common neural correlates of pain. Our meta‐analysis summarizes previous findings and indicates possible differences of identified areas in patients with FMS. Further studies are needed to provide both a specific and general view on dysfunction of pain processing in this disease. From our perspective future studies should keep the following recommendations in mind, to improve the quality of neuroimaging research in FMS:
To avoid false positive or negative results or a reporting bias every study should always include a whole‐brain analysis and report the corresponding results. A restricted analysis of special ROI like areas of the pain network should be the second choice of analysis only and must be reserved to test restricted hypotheses or to explore detailed statistical information like the temporal characteristics, particular sizes and directions of effects, or correlation of effects with behavioural data. But it is important that the whole‐brain approach is the only basis to give better and unbiased information on the possible underlying neurobiological processes in FMS.
To control the specifity of results, studies must integrate a group of patients suffering from a chronic pain disorder with an underlying and identifiable organic reason as a control population. Otherwise results cannot beviewed as being specific to FMS.
LIMITATIONS
It should be also noted that besides studies during resting state conditions, which indirectly investigated clinical pain in FMS as well, no direct contrast between clinical and experimental pain conditions has been examined in FMS so far. The central representation of experimentally induced and spontaneously ongoing FMS pain might be different such that the current analyses do not provide the clinically relevant abnormalities and neuronal correlates of spontaneous clinical pain in FMS. Therefore, results of these studies should be generalized with some caution in understanding the pathophysiology of FMS.
Coordinate‐based meta‐analyses include studies with different statistical thresholds and there is no possibility (other than excluding studies) to control this possible bias. As shown in table I, only seven studies with 43 clusters of our analysis reported results with uncorrected statistical thresholds. In face of 274 analyzed clusters in total we feel safe that including these studies with a certain risk of false positive results is more appropriate than neglecting them altogether.
Another limitation of our pooled ALE‐analysis might that even with strict inclusion criteria, the included studies still varied on a large number of variables. The data were derived from heterogeneous paradigms (resting state or task‐oriented), experimental methods (functional or structural modalities), different statistical power and control comparisons (patients vs. healthy controls or after vs. before treatment with different treatment methods). By using the statistical ALE‐approach however, the risk of false positive results is greatly reduced and results which are not typical for the majority of the studies will not show significant results. The current pooled analysis aimed to investigate consistent regions of alteration in FMS across modalities and type of task, rather than identifying correlates of alteration in specific tasks and modalities.
CONCLUSION
The aim of this meta‐analysis was to explore brain alterations in FMS patients using the statistical power of the ALE‐meta‐analytic approach. The prevailing hypothesis in previous literature that FMS is characterized by brain abnormalities of pain processing is consistent with the present ALE analysis. Nevertheless, since these alterations have been also demonstrated in patients with other chronic pain conditions (e.g. irritable bowel syndrome, low back pain), the specifity of these findings must be regarded with some caution. The lack of positive control groups of patients with other chronic pain conditions in almost all of the reviewed studies makes it difficult to assess whether these changes are associated with chronic pain in general or are unique features of patients with FMS. Therefore, further neuroimaging studies are required comparing FMS and other chronic pain disorders to answer the question of disorder specificity.
Supporting information
Supporting Information Figure 1.
REFERENCES
- Amanzio M, Benedetti F, Porro CA, Palermo S, Cauda F (2013): Activation likelihood estimation meta‐analysis of brain correlates of placebo analgesia in human experimental pain. Hum Brain Mapp 34:738–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede R, Zubieta J (2005): Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 9:463–484. [DOI] [PubMed] [Google Scholar]
- Baliki MN, Geha PY, Apkarian AV, Chialvo DR (2008): Beyond feeling: Chronic pain hurts the brain, disrupting the default‐mode network dynamics. J Neurosci 28:1398–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra LR, Breiter HC, Stojanovic M, Fishman S, Edwards A, Comite AR, Gonzalez RG, Borsook D (1999): Human brain activation under controlled thermal stimulation and habituation to noxious heat: An fMRI study. Magn Reson Med 41:1044–1057. [DOI] [PubMed] [Google Scholar]
- Bingel U, Lorenz J, Schoell E, Weiller C, Buchel C (2006): Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain 120:8–15. [DOI] [PubMed] [Google Scholar]
- Bogousslavsky J, Miklossy J, Deruaz JP, Assal G, Regli F (1987): Lingual and fusiform gyri in visual processing: A clinico‐pathologic study of superior altitudinal hemianopia. J Neurol Neurosurg Psychiatry 50:607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyatzis RE, Rochford K, Jack AI (2014): Antagonistic neural networks underlying differentiated leadership roles. Front Hum Neurosci 8:114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews‐Hanna JR, Schacter DL (2008): The brain's default network: Anatomy, function, and relevance to disease. Ann New York Acad Sci 1124:1–38. [DOI] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR (2002): Dorsal anterior cingulate cortex: A role in reward‐based decision making. Proc Natl Acad Sci USA 99:523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell MC, Duncan GH, Hofbauer RK, Ha B, Chen JI, Carrier B (1999): Pain perception: Is there a role for primary somatosensory cortex? Proc Natl Acad Sci USA 96:7705–7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifre I, Sitges C, Fraiman D, Munoz MA, Balenzuela P, Gonzalez‐Roldan A, Martinez‐Jauand M, Birbaumer N, Chialvo DR, Montoya P (2012): Disrupted functional connectivity of the pain network in fibromyalgia. Psychosom Med 74:55–62. [DOI] [PubMed] [Google Scholar]
- Clauw DJ (2014): Fibromyalgia: A clinical review. JAMA 311:1547–1555. [DOI] [PubMed] [Google Scholar]
- Costafreda SG, Brammer MJ, David AS, Fu CH (2008): Predictors of amygdala activation during the processing of emotional stimuli: A meta‐analysis of 385 PET and fMRI studies. Brain Res Rev 58:57–70. [DOI] [PubMed] [Google Scholar]
- Duerden EG, Albanese MC (2013): Localization of pain‐related brain activation: A meta‐analysis of neuroimaging data. Hum Brain Mapp 34:109–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT (2009): Coordinate‐based activation likelihood estimation meta‐analysis of neuroimaging data: A random‐effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp 30:2907–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT (2012): Activation likelihood estimation meta‐analysis revisited. Neuroimage 59:2349–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friebel U, Eickhoff SB, Lotze M (2011): Coordinate‐based meta‐analysis of experimentally induced and chronic persistent neuropathic pain. Neuroimage 58:1070–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs PN, Peng YB, Boyette‐Davis JA, Uhelski ML (2014): The anterior cingulate cortex and pain processing. Front Integr Neurosci 8:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracely RH, Petzke F, Wolf JM, Clauw DJ (2002): Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum 46:1333–1343. [DOI] [PubMed] [Google Scholar]
- Harris RE, Clauw DJ, Scott DJ, McLean SA, Gracely RH, Zubieta J (2007): Decreased central μ‐opioid receptor availability in fibromyalgia. J Neurosci 27:10000–10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard MA, Volkov IO, Mirsky R, Garell PC, Noh MD, Granner M, Damasio H, Steinschneider M, Reale RA, Hind JE, Brugge JF (2000): Auditory cortex on the human posterior superior temporal gyrus. J Comp Neurol 416:79–92. [DOI] [PubMed] [Google Scholar]
- Ichesco E, Schmidt‐Wilcke T, Bhavsar R, Clauw DJ, Peltier SJ, Kim J, Napadow V, Hampson JP, Kairys AE, Williams DA, Harris RE (2014): Altered resting state connectivity of the insular cortex in individuals with fibromyalgia. J Pain 15:815–826.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KB, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, Williams SC, Choy E, Giesecke T, Mainguy Y, Gracely R, Ingvar M (2009): Evidence of dysfunctional pain inhibition in Fibromyalgia reflected in rACC during provoked pain. Pain 144:95–100. [DOI] [PubMed] [Google Scholar]
- Jensen KB, Loitoile R, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, Williams SC, Choy E, Mainguy Y, Vitton O, Gracely RH, Gollub R, Ingvar M, Kong J (2012): Patients with fibromyalgia display less functional connectivity in the brain's pain inhibitory network. Mol Pain 8:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamping S, Bomba IC, Kanske P, Diesch E, Flor H (2013): Deficient modulation of pain by a positive emotional context in fibromyalgia patients. Pain 154:1846–1855. [DOI] [PubMed] [Google Scholar]
- Kanske P, Kotz SA (2011): Emotion triggers executive attention: Anterior cingulate cortex and amygdala responses to emotional words in a conflict task. Hum Brain Mapp 32:198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB (2010): A link between the systems: Functional differentiation and integration within the human insula revealed by meta‐analysis. Brain Struct Funct 214:519–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanz S, Seifert F, Maihofner C (2011): Brain activity associated with pain, hyperalgesia and allodynia: An ALE meta‐analysis. J Neural Transm (Vienna) 118:1139–1154. [DOI] [PubMed] [Google Scholar]
- Loggia ML, Jensen K, Gollub RL, Wasan AD, Edwards RR, Kong J (2011): The catechol‐O‐methyltransferase (COMT) val158met polymorphism affects brain responses to repeated painful stimuli. PLoS One 6:e27764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez‐Sola M, Pujol J, Wager TD, Garcia‐Fontanals A, Blanco‐Hinojo L, Garcia‐Blanco S, Poca‐Dias V, Harrison BJ, Contreras‐Rodriguez O, Monfort J, Garcia‐Fructuoso F, Deus J (2014): Altered functional magnetic resonance imaging responses to nonpainful sensory stimulation in fibromyalgia patients. Arthritis Rheumatol 66:3200–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martikainen IK, Pecina M, Love TM, Nuechterlein EB, Cummiford CM, Green CR, Harris RE, Stohler CS, Zubieta JK (2013): Alterations in endogenous opioid functional measures in chronic back pain. J Neurosci 33:14729–14737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napadow V, LaCount L, Park K, As‐Sanie S, Clauw DJ, Harris RE (2010): Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum 62:2545–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomero‐Gallagher N, Eickhoff SB, Hoffstaedter F, Schleicher A, Mohlberg H, Vogt BA, Amunts K, Zilles K (2015): Functional organization of human subgenual cortical areas: Relationship between architectonical segregation and connectional heterogeneity. Neuroimage 115:177–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T (2001): Primate anterior cingulate cortex: Where motor control, drive and cognition interface. Nat Rev Neurosci 2:417–424. [DOI] [PubMed] [Google Scholar]
- Pearlson GD (1997): Superior temporal gyrus and planum temporale in schizophrenia: A selective review. Prog Neuro‐Psychopharmacol Biol Psychiatry 21:1203–1229. [DOI] [PubMed] [Google Scholar]
- Schmidt‐Wilcke T, Clauw DJ (2011): Fibromyalgia: From pathophysiology to therapy. Nat Rev Rheumatol 7:518–527. [DOI] [PubMed] [Google Scholar]
- Schmidt‐Wilcke T, Leinisch E, Ganssbauer S, Draganski B, Bogdahn U, Altmeppen J, May A (2006): Affective components and intensity of pain correlate with structural differences in gray matter in chronic back pain patients. Pain 125:89–97. [DOI] [PubMed] [Google Scholar]
- Schmidt‐Wilcke T, Kairys A, Ichesco E, Fernandez‐Sanchez ML, Barjola P, Heitzeg M, Harris RE, Clauw DJ, Glass J, Williams DA (2014): Changes in clinical pain in fibromyalgia patients correlate with changes in brain activation in the cingulate cortex in a response inhibition task. Pain Med 15:1346–1358. [DOI] [PubMed] [Google Scholar]
- Schnitzler A, Ploner M (2000): Neurophysiology and functional neuroanatomy of pain perception. J Clin Neurophysiol 17:592–603. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Davis KD (2007): Pain enhances functional connectivity of a brain network evoked by performance of a cognitive task. J Neurophysiol 97:3651–3659. [DOI] [PubMed] [Google Scholar]
- Simons LE, Moulton EA, Linnman C, Carpino E, Becerra L, Borsook D (2014): The human amygdala and pain: Evidence from neuroimaging. Hum Brain Mapp 35:527–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood RF, Laird AR, Ramage AE, Parkinson AL, Lewis J, Clauw DJ, Williams DA, Schmidt‐Wilcke T, Farrell MJ, Eickhoff SB, Robin DA (2013): Structural brain anomalies and chronic pain: A quantitative meta‐analysis of gray matter volume. J Pain 14:663–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey I, Mantyh PW (2007): The cerebral signature for pain perception and its modulation. Neuron 55:377–391. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA (2002): Meta‐analysis of the functional neuroanatomy of single‐word reading: Method and validation. Neuroimage 16:765–780. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P (2012): Minimizing within‐experiment and within‐group effects in Activation Likelihood Estimation meta‐analyses. Hum Brain Mapp 33:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veer IM, Beckmann CF, van Tol MJ, Ferrarini L, Milles J, Veltman DJ, Aleman A, van Buchem MA, van der Wee NJ, Rombouts SA (2010): Whole brain resting‐state analysis reveals decreased functional connectivity in major depression. Front Syst Neurosci 4. pii: 41. doi: 10.3389/fnsys.2010.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DA, Gracely RH (2006): Biology and therapy of fibromyalgia. Functional magnetic resonance imaging findings in fibromyalgia. Arthritis Res Ther 8:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figure 1.


